Abstract
The products and by‐products of Olea europaea L.: olive fruits (primary agricultural product), oils (primary agro‐industrial product), pomaces (agro‐industrial processing by‐product), and leaves (agricultural practices by‐product), are promising sources of bioactive compounds. In the present study, qualitative and quantitative analyses of selected bioactive components in olive fruits, oils, and pomaces were performed. Total polyphenol content and antioxidant activity were analyzed in all samples (humid pomaces 2015: TPP, 26.0 ± 1.5–43.7 ± 3.0 g(GAEq)/kg DW; TEAC/ABTS, 189.5 ± 3.7–388.1 ± 12.0 mmol(Trx)kg DW). Radical (DPPH) quenching potential was analyzed via photometric and EPR methods, obtaining Vis/EPR signal ratio by 1.05 ± 0.45 and 1.66 ± 0.39 for fruits and pomaces, respectively. Through HPLC‐UV and HPLC‐MS/MS techniques, oleuropein and hydroxytyrosol, as well as selected hydroxycinnamic acids and flavonoids, were identified and quantified in olive fruits and pomaces. The main components were rutin, luteolin, and chlorogenic acid. Cytotoxic assay on fibroblast cells revealed toxic effects for selected extracts at highest tested concentrations (5%).
Keywords: Antioxidant, by‐products, Olea europaea L., pomace
1. INTRODUCTION
Olea europaea L. is one of the most diffuse plants in the Mediterranean area, from which the extra‐virgin olive oil (EVOO) is traditionally obtained by cold pressing of olive fruits. EVOO represents the most used dressing in the Mediterranean diet, and many studies report its beneficial effects on human health (Battino et al., 2018; Buckland & Gonzalez, 2015; Covas, Torre, & Fitó, 2015; Delgado‐Lista et al., 2016; Estruch et al., 2018; Finicelli et al., 2018; Guasch‐Ferré et al., 2014; Khalatbary, 2013; Konstantinidou et al., 2010; Pérez‐Martínez, García‐Ríos, Delgado‐Lista, Pérez‐Jiménez, & López‐Miranda, 2011). This last aspect has received increased interest by the consumers of functional foods (containing biologically active minor components; Bonechi et al., 2017; Bonechi et al., 2018). The quality standards for EVOO are currently based on the combined evaluation of raw agricultural product, varietal and geographic characterization, organoleptic properties and sensory consumer expectations, and health‐related characteristics, such as high concentration of oleic acid, and content of bioactive components (at low percentage). The oxidative stability of EVOO is directly related to the presence of minor antioxidant components, which are also responsible for its main organoleptic properties (such as the spice, bitter taste, distinguishing the freshly milled product), as well as its health‐related properties, as preventing agents for cardiovascular diseases, atherosclerosis, and heart attacks, and antitumoural activities against colon and breast cancer (Batarseh & Kaddoumi, 2018; Bulotta et al., 2014; Rigacci & Stefani, 2016). Recently, olive polyphenols have been recognized as “health claims” by the EFSA, EU (Source: www.efsa.europa.eu). To this extent, it is very important to underline that antioxidant species, like carotenoids, tocopherols, and vitamins, can be found in many different foods (i.e., vegetables, cereals; Tamasi et al., 2015; Tamasi et al., 2019; Van Hung, 2016), whereas specific hydrophilic (poly)phenolic compounds (i.e., iridoids and secoiridoids, such as tyrosol, hydroxytyrosol, oleuropein, and ligstroside) are present, in great amount, only in EVOO, and related by‐products of olive oil production.
The production of olive oil presents a challenge to agro‐industrial waste management. Humid pomace, dry pomace, and mill wastewaters are produced in different quantities, based on specific milling technology. Mediterranean countries produce about 30 × 106 m3 of mill waste. These by‐products are pathogen‐free and very rich in organic matter, in nutrients, and are also characterized by high levels of bioactive molecules (particularly, polyphenols), showing strong antimicrobial and phytotoxic activities and not easily biodegradable. For those bioactive properties, these by‐products can be recovered and reused for the production of functional foods for human or animal consumption, as well as for diet supplements and cosmetics formulations (Gullón et al., 2018; Herrero et al., 2011; Kishikawa et al., 2015; Di Nunzio et al., 2018; Romero, Medina, Mateo, & Brenes, 2018; Sousa, Costa, Alexandre, & Prata, 2019; Vitali Čepo et al., 2018). For that reason, the use of the phenolic compounds extracted from olive by‐products represents a great opportunity for the circular economy. Particular attention has been recently devoted to optimize nonconventional extraction procedures able to produce high‐quality phytocomplexes by using nontoxic solvents. These protocols are usually assisted by ultrasound, microwave, or supercritical fluid extraction (SFE) using carbon dioxide as solvent (Chanioti & Tzia, 2018; Herrero, Pilar Sánchez‐Camargo, Cifuentes, & Ibáñez, 2015; Xie et al., 2019). Several studies also indicate the possibility to increase the stability and the bioavailability of antioxidant and natural bioactive molecules using new carrier systems, like liposomes or polymeric micelles’ formulations (Bonechi et al., 2018; Leone et al., 2018, 2016; Zhang, Huang, & Li, 2014).
Given this opportunity, the present study explored the chemical and nutraceutical characterization of products and by‐products of O. europaea L.: olive fruits (primary agricultural products), EVVOs (primary agro‐industrial products), pomaces (by‐products from agro‐industrial processing), collected at the harvestings in 2014–2015. Particular attention was devoted to the evaluation of radical scavenging activity, via Trolox‐equivalent antioxidant capacity assays (TEAC) following the quenching of two different radicals, ABTS cation, through UV‐Vis spectrophotometry, and neutral DPPH, through UV‐Vis and EPR techniques (Polovka, 2006; Prior, Wu, & Schaich, 2005; Thaipong, Boonprakob, Crosby, Cisneros‐Zevallos, & Byrne, 2006; Yu & Cheng, 2008). EPR spectroscopy is a straightforward tool for the radical determination, and it has been previously applied for the determination of antioxidant activity of crude extracts and galloyl quinic derivatives, as well as antioxidant defense and oxidative damage processes evaluation against stress conditions (Baratto et al., 2003; Fini et al., 2014; Gori et al., 2016; Megar et al., 2009). Selected polyphenols have been also identified and quantified through HPLC‐UV and HPLC‐MS/MS techniques, optimizing the analytical protocols on the basis of the chemical properties of the matrix and analytes.
The approach presented in this study focuses on the valorization of primary and secondary products from O. europaea L., highlighting the possibility to utilize the pomaces as source of bioactive molecules. This represents a challenge and a great opportunity from both environmental and economical points of view, building a model to increase the sustainability of agricultural and agro‐industrial productions.
2. MATERIALS AND METHODS
2.1. Reagents, standards, and solvents
All reagents and standards were analytical grade, and were used as purchased from Sigma‐Aldrich: Folin–Ciocalteu's phenol reagent, sodium carbonate, gallic acid, potassium persulfate, ABTS (2,2'‐azino‐bis(3‐ethylbenzthiazoline‐6‐sulphonic acid)), DPPH (2,2‐diphenyl‐1‐picrylhydrazyl), Trolox (6‐hydroxy‐2,5,7,8‐tetramethylchroman‐2‐carboxylic acid), tyrosol, hydroxytyrosol, oleuropein, caffeic acid, ferulic acid, p‐Coumaric acid, chlorogenic acid, luteolin, luteolin‐7‐O‐rutinoside, quercetin dihydrate, rutin trihydrate, naringenin, genistein, and resveratrol. All solvents were gradient HPLC grade: methanol, ethanol, acetonitrile, formic acid, acetic acid, diethyl oxide, cyclohexane, and n‐hexane. Bi‐distilled water was produced by Acquinity P/7 distiller (MembraPure GmbH).
2.2. Sample collection and storage
All samples were collected at harvesting/milling time in 2014 and 2015, from oil milling plants in southwest Tuscany (names are not reported for privacy reasons; Table S1). The samples of olive fruits, olive oil (extra‐virgin), and pomace, coded as F1x‐Y, EVOO1x‐Y, and P1x‐Y, were related to the same farm/oil mill. All the oil milling plants were based on two‐phase technology (olive oil and humid pomace), except for samples 15‐A and 15‐C that were from three‐phase systems (olive oil, dry pomace, and vegetation water). In addition, the groups of samples (14‐A, 15‐D, 15‐E) and (15‐F, 15‐G, 15‐H, 15‐I, 15‐J) were collected from two distinct oil milling plants. EVOO samples were stored in the dark at −20 ± 1°C, until pretreatment or direct analyses. Olive fruits and pomace samples were freeze‐dried within 24 hr after collection (5Pascal Lio‐5P; usual working condition: condenser, −51 ± 2°C; pump pressure 1.2 ± 0.5 mbar; 72 hr) and stored (darkness, −20 ± 1°C) until subsequent pretreatment.
2.3. Sample pretreatments: extraction of antioxidant components
2.3.1. Extra‐virgin olive oils (EVOOs)
Aliquots of 2.50 g of sample (analytically weighed, Radwag AC220/C/2, max capacity 100 g, readability 0.0001 g; Radom, Poland) were diluted by 12.5 ml of n‐hexane and then extracted by 5 ml of hydroalcoholic mixture (EtOH/H2O, 80/20%, v/v). The extraction was ultrasound assisted (10 min, 21 ± 2°C; power, 120 Watt; sound frequency, 35 kHz; ultrasonic bath Sonorex Bandelin), and the suspension was centrifuged (5 min, 1,882 g; Centrifuge Thermo Electron Corporation PK 110). The procedure was repeated two additional times, using 5 ml hydroalcoholic medium each (total volume extract, 15 ml). The extract was used as such.
2.3.2. Olive fruits and pomaces
Aliquots of 0.250 g of each sample (analytically weighed) were defatted by 7 ml of n‐hexane (twice), and the liquid phase was discarded. The residual solid phase was then extracted (ultrasound assisted) by an hydroalcoholic mixture (EtOH/H2O, 80/20%, v/v; first extraction 5 ml, second and third extractions 2.5 ml; total volume extract, 10 ml). The extract was used as such, or dried under nitrogen flow (overnight) and then lyophilized. The dry extract was stored (darkness, −20 ± 1°C). Before HPLC analyses, the dry extract was reconstituted in 2 ml of solvent. In case of pomace samples, preliminary analyses comparing extracts with and without defatting process were carried out, obtaining results within 3% difference. Following this analysis, pomaces were usually extracted without previous defatting process.
2.4. Antioxidant activity assays
2.4.1. Acidity analysis for EVOO samples
Total acidity for EVOOs was determined following the 1991 2568/1991 and 1830/2015 procedure. An aliquot by 3.0 g of oil was diluted by Et2O/EtOH mixture (75/25%, v/v) and was titrated by KOH 0.010 M ethanolic solution (previously standardized by standard HCl 0.010 M), using phenolphthalein as indicator. The results were expressed as oleic acid equivalent percentage (%OAEq).
2.4.2. Folin–Ciocalteu assay: total polyphenol content (TPP)
Total polyphenols (TPP) were determined by spectrophotometric Folin–Ciocalteu method (Singleton, Orthofer, & Lamuela‐Raventós, 1999), with some modifications (Tamasi et al., 2019). UV‐Vis spectrophotometer used was a dual‐beam Perkin Elmer Lambda EZ 201, equipped with software PESSW 1.2 (Perkin Elmer). The instrumental spectra range was 190–1100 nm, and PMMA/UV grade cuvettes (Kartell; 10 mm optical pathway) were used. Briefly, 3.5 ml of filtered and diluted (if necessary) hydroalcoholic extracts was added to 0.1 ml of Folin–Ciocalteu reagent and 0.4 ml of Na2CO3 water solution (35%, w/v). The mixture was incubated for 30 min in the dark at 21 ± 2°C. Finally, the absorbance at 750 nm (Abs750) was recorded, against water. The calibration curves were recorded by using standard solutions of gallic acid in the linear range, 0.25–10.00 mg/L, (R 2 > 0.990 were accepted for analyses; Figure S1a). The results were expressed as mg gallic acid equivalent per kg of dried sample (mg(GAEq)/kg DW).
2.4.3. Trolox‐Equivalent antioxidant capacity (TEAC) assays
Antioxidant activity was assayed by following the radical scavenger activity of free radicals ABTS•+ and DPPH• according to procedures previously reported (Brand‐Williams, Cuvelier, & Berset, 1995; Re et al., 1999) with some modifications (Tamasi et al., 2019). The calibration curves were recorded by using standard solutions of Trolox, in the linear range, 0.20–20.00 µM (from a 0.55 mM mother solution in EtOH; R 2 > 0.990 were accepted for analyses; Figure S1b,c). The quenching percentage was calculated as (ABTS•+, ΔAbs734%; DPPH•, ΔAbs517%):
where AbsTrolox/Sample is the absorbance of the radical solution treated with standards or samples, and AbsBlank is the absorbance of the radical solution as such (ABTS•+or DPPH• not treated solutions).
The results of TEAC were expressed as mmol Trolox equivalent per kg of dried sample (mmol(TrxEq)/kg DW).
2.4.4. TEAC/ABTS assay
The ABTS•+ radical cation was prepared by incubation of a solution of ABTS (7 mM in EtOH) with a K2S2O8 solution (140 mM in water) overnight (darkness, 4 ± 1°C) and dilution in EtOH before use. A known volume of this solution was treated with Trolox standard solutions or a known amount of extract (diluted, if necessary). After 30 min of incubation in the dark, at 21 ± 2°C, the adsorption at 734 nm was recorded, against EtOH.
2.4.5. TEAC/DPPH assays
A stock solution of DPPH• (0.10 mM in MeOH) was freshly prepared and used within 4 hr. A known volume of DPPH• solution was treated with Trolox standard solutions or a known amount of extract (diluted, if necessary). After 15 min of incubation in the dark, at 21 ± 2°C, the adsorption at 517 nm was recorded, against MeOH. In case of olive fruits and pomaces, the same experiment was carried out reading the DPPH• solution (blank and treated) via EPR spectroscopy. EPR spectra were acquired on continuous‐wave X‐band (CW, 9GHz) using a Bruker E500 ELEXSYS Series spectrometer (Bruker, Italy), with the ER 4,122 SHQE cavity. EPR measurements were performed at 21 ± 1°C, 9.8 GHz microwave frequency, 0.1 mT modulation amplitude, and 4 mW microwave power. The sample was placed into a 3.0 mm ID × 4.0 mm OD, suprasil tube. In this case, stock solution of DPPH• was prepared (1.0 mM in MeOH) and the final concentration of radicals in each sample was 0.45 mM. The acquisition of EPR signal was carried out 15 min after the addition of the antioxidant (Trolox or extract; darkness, 21 ± 2°C; Figure S1d), and the antioxidant activity was calculated by the relative decrease in area (instead of absorbance). The area of the EPR spectra was calculated by the double integral of the DPPH signal.
2.5. Chromatography analyses
Liquid chromatography was conducted for the identification and quantification of tyrosol, hydroxytyrosol, and oleuropein, by using the isocratic HPLC Varian ProStar 210 machine equipped with UV 9050 detector, managed by Varian Workstation software (Varian, Inc). For other hydroxycinnamic acids and flavonoid analysis, the HPLC‐MS method was optimized by using a HPLC Agilent 1,200 Series (Agilent Technologies) coupled with a mass spectrometer TSQ Quantum Access (Thermo Scientific), equipped with electrospray ion source (ESI) and triple quadrupole analyzer. The Xcalibur software (Thermo Scientific) managed the instrument and collected the data.
2.5.1. HPLC‐UV: identification and quantification of tyrosol, hydroxytyrosol, and oleuropein
The chromatographic separation was carried out following Tamasi et al. (2015) with some modifications on the basis of the matrix/analytes. A reverse‐phase column (Phenomenex Luna C18, 5U, 250 × 4.6 mm, 5 μm particles, 100 Å pores) with safeguard precolumn (Phenomenex C18, 4 × 3.0 mm) was used, and the elution was isocratic with H2O (CH3COOH, 0.2%)/CH3CN (70:30, v/v) eluent, at 0.5 ml/min flow rate (21 ± 2°C). The injection volume was 20 µl, and the UV detector was set at 280 nm. The analytical determination was carried out via external calibration method, using resveratrol as internal standard and standard solutions of hydroxytyrosol and oleuropein in MeOH; retention times: R t, 6.46 min (hydroxytyrosol) and 10.60 min (oleuropein) (Figure S2). The linear calibration ranges (R 2 > 0.990) were 15–230 (hydroxytyrosol) and 30–900 mg/L (oleuropein). The tyrosol (R t, 7.5 min) was also identified, through standard additions, but not quantified, as it was present in trace concentrations. The limit of quantification (LOQ) and limit of detection (LOD) were 10 and 3 mg/L for hydroxytyrosol, and 15 and 5 mg/L for oleuropein.
2.5.2. HPLC‐MS: identification and quantification of hydroxycinnamic acids and flavonoids
A reverse‐phase column (Phenomenex Kinetex biphenyl, 10 × 2.1 mm, 5 μm particles, 100 Å pore, shell‐core), with safeguard precolumn (Phenomenex Phenyl, 4 × 2.0 mm), thermostated at 35 ± 1°C, was used (Tamasi et al., 2019). The eluents were as follows: (A) H2O/HCOOH 0.1% (v/v) and (B) MeOH/HCOOH 0.1% (v/v), and the separation was performed through a linear gradient: 0–1.0 min, 10% B (isocratic); 1.0–11.0 min, from 10% to 90% B (linear); 11.0–14.5 min, 90% B (isocratic); 14.5–15.0 min, from 90% to 10% B (linear); 15.0–18.0 min, 90% B (isocratic). The elution flow rate was 0.5 ml/min, and the injection volume was 5 µl. The peaks were analyzed by ESI‐MS detector having a triple quadrupole analyzer. The ESI conditions were optimized for negative ion current mode, based on a standard solution of quercetin: nebulizer gas (N2) inlet pressure, 30 psi; temperature, 270°C; capillary voltage, 4,000 V; collision energy, 30 V. Full scan (Total Ion Current, TIC mode) data were acquired by scanning m/z, 150–1,000, to identify the analytes. The quantification of selected species was carried out via SIM (single ion monitoring) and SRM (selected reaction monitoring) methods. Selected MS/MS method parameters are reported in Table 1 and Figure S3. The analytical determination was carried out via external calibration, by using genistein as internal standard. Calibration showing correlation factors R 2 > 0.990 was accepted for analyses. The values for LOQ and LOD were also defined (Table 1).
Table 1.
Selected HPLC‐MS/MS and calibration method parameters for hydroxycinnamic acids and flavonoid identification and quantification
| R t (min) | PM | [M‐H]‐ (m/z) | MS2 (m/z) | Mode | Calibration range (mg/ml) | LOQ/LOD (mg/ml) | |
|---|---|---|---|---|---|---|---|
| Hydroxycinnamic acids | |||||||
| Caffeic acid | 4.60 | 180.2 | 179 | 135 | SRM | 0.01–9.00 | 0.010//0.003 |
| Chlorogenic acid | 4.73 | 354.3 | 353 | 179; 191 | MRM | 0.05–10.00 | 0.020//0.007 |
| p‐Coumaric acid | 6.07 | 164.2 | 163 | 119 | SRM | 0.10–5.00 | 0.030//0.010 |
| Ferulic acid | 6.90 | 194.2 | 193 | 134 | SRM | 0.10–5.00 | 0.030//0.010 |
| Flavonoids | |||||||
| Rutin | 7.16 | 610.5 | 609 | 301 | SRM | 0.02–9.00 | 0.010//0.003 |
| Quercetin | 8.55 | 302.2 | 301 | 151 | SRM | 0.02–2.00 | 0.010//0.003 |
| Luteolin | 8.96 | 286.2 | 285 | – | SIM | 0.02–9.00 | 0.010//0.003 |
| Naringenin | 9.50 | 272.2 | 271 | 151 | SRM | 0.02–9.00 | 0.010//0.004 |
| Genistein (IS) | 9.43 | 270.2 | 269 | – | SIM | ||
2.6. Cytotoxicity assay
Hydroalcoholic (EtOH/H2O, 80/20%, v/v) extracts of EVOOs, olive fruits, and pomaces 2014 and 2015 were tested in vitro for cytotoxicity, on mouse fibroblast NIH3T3 cells, following the ISO 10995‐5:2009 protocol. Standard solutions of pure rutin, quercetin, and pure EtOH were also tested, for comparison reasons. Details on cell culture and cell viability procedures were those reported in Bonechi et al. (2018). Briefly, the fibroblasts NIH3T3 were propagated in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal calf serum (FCS), 1% L‐glutamine–penicillin–streptomycin, and 1% MEM nonessential amino acid, and maintained at 37°C, in a humidified atmosphere (5% CO2). When at confluence, cells were washed with phosphate‐buffered saline solution (0.1 M, PBS) and separated using a trypsin–EDTA solution and centrifuged (118 g, 5 min). The pellet was resuspended and diluted in medium solution and added by different concentrations (0.5, 1.0, and 5.0%, v/v) of tested solutions (standards or extracts). After 24 hr of incubation, cell viability was evaluated by neutral red uptake. The incubation medium was removed, and cells were washed with prewarmed PBS; then, the neutral red medium (NR, 0.33 g in 100 ml sterile water, then diluted 100 times) was added and the samples were further incubated at 37°C, 95% humidity, 5.0% CO2 for 3 hr. After incubation, the cells were rinsed with prewarmed PBS, NR desorbing solution (glacial acetic acid:ethanol:H2O, 1:50:49%, v/v) was added and samples were shaked (20–45 min). Finally, the absorbance of each solution was recorded at 540 nm, within 5 min.
2.7. Statistical data treatment
Three samples were collected for each type, pretreatments (extracts) were performed in triplicate for each sample, and triplicate analyses were performed for all measurements in each extract. The results were reported as mean values and estimated standard deviations (esd, n = 27). Calculation was made by using Microsoft Office Excel 2007, implemented with regression analysis subroutine, and Origin Pro8 SR2, (v.0891, B891, OriginLab Corporation). The analysis of variance and Tukey's test were carried out to determine significant differences, and data showing p‐values < 0.05 were considered statistically significant. The linear regression studies were based on the Pearson correlation matrix, and p‐value was calculated at 95% confidence interval. A multivariate statistical analysis was also performed by using the Unscrambler X version 10.4 (Camo software), and principal component analysis (PCA) was computed on auto‐scaled variables for olive fruits and pomace samples.
3. RESULTS AND DISCUSSION
3.1. Antioxidant activity characterization of EVOOs, olive fruits, and pomaces
3.1.1. EVOO samples
The total acidity values for EVOOs‐14 (Table 2) were relatively high. In two cases, they were higher than the maximum value for “extra‐virgin” (0.8% OAEq) defined by 1991 (2568/1991; 1830/2015). The 2014 harvest was influenced by infestation of Bactrocera oleae (olive fly). Better results were obtained for EVOOs‐15, for which the total acidity averaged 0.314 ± 0.070% OAEq.
Table 2.
Values of total acidity %(OAEq), TPP mg(GAEq)/kg FW, and TEAC/ABTS mmol(TrxEq)/kg FW, for EVOO hydroalcoholic (EtOH/H2O, 80/20%, v/v) extracts
| Acidity | TPP | TEAC/ABTS | |
|---|---|---|---|
| 2014 | |||
| EVOO14‐A | 0.29 ± 0.01a | 517 ± 35a | 1.61 ± 0.04a |
| EVOO14‐B | 1.10 ± 0.01b | 252 ± 15b | 0.93 ± 0.05b |
| EVOO14‐C | 1.01 ± 0.01b | 268 ± 9b | 0.89 ± 0.05b |
| 2015 | |||
| EVOO15‐A | 0.300 ± 0.005a | 452 ± 7c | 1.43 ± 0.04a |
| EVOO15‐B | 0.385 ± 0.007c | 516 ± 10a | 2.10 ± 0.10c |
| EVOO15‐C | 0.374 ± 0.004c | 427 ± 8d | 1.32 ± 0.09a |
| EVOO15‐D | 0.383 ± 0.005c | 576 ± 6e | 2.37 ± 0.15d |
| EVOO15‐E | 0.380 ± 0.009c | 600 ± 15f | 2.50 ± 0.11d |
| EVOO15‐F | 0.239 ± 0.005d | 514 ± 5a | 1.90 ± 0.05c |
| EVOO15‐G | 0.213 ± 0.007e | 558 ± 9e | 2.24 ± 0.10d |
| EVOO15‐H | 0.217 ± 0.003e | 489 ± 11g | 1.78 ± 0.07a |
| EVOO15‐I | 0.340 ± 0.003f | 496 ± 8g | 1.85 ± 0.05 a |
| EVOO15‐J | 0.310 ± 0.004 a | 503 ± 4 a | 2.04 ± 0.08 c |
The values are reported as average ± esd, and different letters in the same column indicate significant differences (p < .05, Tukey's test).
Parameters of polyphenol content (TPP) and antioxidant capacity (TEAC/ABTS) followed similar year difference. The TPP parameters ranged from 452 ± 7 to 600 ± 15 mg(GAEq)/kg FW, with exception for EVOO14‐B/‐C, that showed lower values by 252 ± 15–268 ± 9 mg(GAEq)/kg FW (Table 2). A similar trend was revealed for TEAC/ABTS parameters that ranged 1.32 ± 0.09–2.50 ± 0.11 mmol(TrxEq)/kg FW for EVOOs‐15 and well compared with other studies (range 1.5–2.7 mmol(TrxEq)/kg FW; Pellegrini et al., 2003; Samaniego Sánchez et al., 2007). It is interestingly to note that reported vales were relevant to oils that were diluted without any preliminary extraction; thus, the TEAC values include the fat‐soluble antioxidant components. The TPP values were also well compared with data previously published (247–537 mg(GAEq)/kg, Šarolić et al., 2014; Galvano et al., 2007). A linear correlation between TPP and TEAC/ABTS parameters (Figure 1a) was found (y = 0.0045x‐0.3890; R 2 = 0.883; p < .001, at 95% confidence interval).
Figure 1.
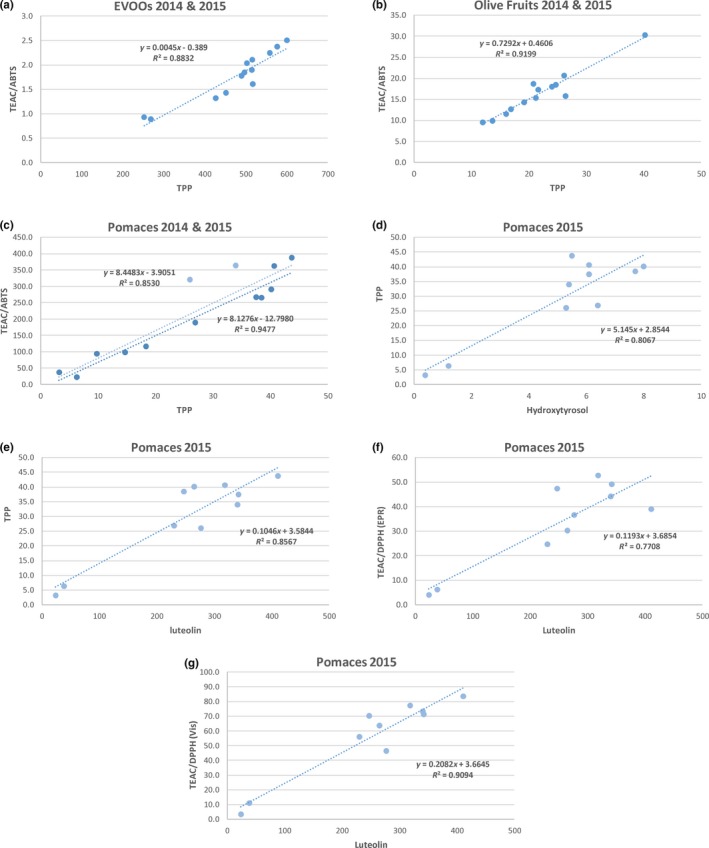
Linear correlation between antioxidant parameters and selected antioxidant components (hydroxytyrosol and luteolin). Analysis of significance showed p < .001 (95% confidence interval) for all data sets
3.1.2. Olive fruit samples
TPP values for olive fruits ranged 12.0 ± 0.9–40.2 ± 1.2 g(GAEq)/kg DW (Table 3), in agreement with previously reported studies of Tuscan cultivars (Frantoio, Rossellino, Ciliegino, Cuoricino, Grossolana; Romani, Mulinacci, Pinelli, Vincieri, & Cimato, 1999) and the Turkish Sarıulak variety (Arslan & Ozcan, 2011). Many factors can contribute to polyphenol content in fruits including variety, harvesting time, soil, and climatic conditions. As already mentioned, 2014 was an unusual year, with a Bactrocera oleae infestation in central Italy, and unusual weather conditions. The lower summer temperature and the higher summer humidity maximum ca. 22°C and 80%, respectively, (Figure S4) strongly influenced the fruit ripening process, producing a marked decrease in final quantity. On the other hand, warmer winter temperatures allowed higher quantity of insects to survive and lay eggs inside the fruits (Rice, 2000). On the contrary, the 2015 summer was hot and dry, and followed by a colder and dryer winter, leading to an increase in production and fruits’ quality.
Table 3.
Values of TPP g(GAEq)/kg DW, TEAC/ABTS, and TEAC/DPPH mmol(Trx)/kg DW (for both photometric and EPR measurements) for olive fruit and pomace hydroalcoholic (EtOH/H2O, 80/20%, v/v) extracts
| TPP | TEAC/ABTS | TEAC/DPPH(Vis) | TEAC/DPPH(EPR) | |
|---|---|---|---|---|
| Olive fruits | ||||
| F14‐A | 19.2 ± 0.7a | 14.3 ± 0.5a | 55.5 ± 1.9a | 62.5 ± 2.3a |
| F14‐B | 12.0 ± 0.9b | 9.5 ± 0.8b | 16.0 ± 0.7b | 14.2 ± 0.9b |
| F14‐C | 13.7 ± 0.5b | 9.9 ± 0.6b | 14.7 ± 0.5b | 37.1 ± 1.5c |
| F15‐A | 16.9 ± 0.7c | 12.6 ± 0.5c | 50.2 ± 2.1a | 44.5 ± 1.1d |
| F15‐B | 26.1 ± 0.7d | 20.6 ± 0.6d | 78.4 ± 4.1c | 57.2 ± 1.1e |
| F15‐C | 21.2 ± 0.4a | 15.3 ± 0.7a | 62.7 ± 0.4d | 45.5 ± 2.6d |
| F15‐D | 16.0 ± 0.3c | 11.5 ± 0.2c | 40.4 ± 1.7e | 23.0 ± 1.8f |
| F15‐E | 24.7 ± 0.9d | 18.4 ± 0.5e | 55.2 ± 0.3a | 36.5 ± 0.9c |
| F15‐F | 40.2 ± 1.2e | 30.3 ± 1.1f | 60.6 ± 1.1d | 121.1 ± 1.2g |
| F15‐G | 24.0 ± 0.8 d | 18.0 ± 0.8 e | 75.8 ± 2.9 c | 53.5 ± 1.1 e |
| F15‐H | 21.6 ± 0.6a | 17.3 ± 0.5e | 67.3 ± 6.0f | 85.3 ± 9.8hr |
| F15‐I | 20.8 ± 0.2a | 18.7 ± 0.1e | 74.7 ± 0.6c | 69.4 ± 0.8hr |
| F15‐J | 26.4 ± 0.5d | 15.8 ± 0.3a | 38.0 ± 0.1e | 117.6 ± 6.2g |
| Pomaces | ||||
| P14‐A | 18.3 ± 0.6a | 116.2 ± 8.6a | 63.3 ± 2.0a | 42.2 ± 1.7a |
| P14‐B | 14.7 ± 1.0a | 98.0 ± 3.1b | 71.3 ± 1.7b | 37.5 ± 2.4a |
| P14‐C | 9.8 ± 0.6b | 93.6 ± 1.3b | 69.2 ± 2.1b | 40.7 ± 2.1a |
| P15‐A | 6.3 ± 0.5c | 23.0 ± 2.8c | 11.0 ± 0.2c | 6.2 ± 0.2b |
| P15‐B | 26.9 ± 1.2d | 189.5 ± 3.7d | 55.9 ± 1.6d | 24.7 ± 0.3c |
| P15‐C | 3.2 ± 0.5e | 37.2 ± 2.7e | 3.2 ± 0.1e | 3.9 ± 0.5d |
| P15‐D | 38.4 ± 3.3f | 265.4 ± 10.1f | 70.3 ± 0.9f | 47.3 ± 1.6e |
| P15‐E | 40.1 ± 2.2f | 290.5 ± 9.2g | 63.6 ± 3.0g | 30.3 ± 3.2f |
| P15‐F | 37.5 ± 1.9f | 267.4 ± 19.3f | 71.3 ± 5.5f | 49.1 ± 0.7e |
| P15‐G | 40.6 ± 0.5f | 362.0 ± 18.2hr | 77.1 ± 3.8hr | 52.8 ± 3.4e |
| P15‐H | 43.7 ± 3.0g | 388.1 ± 12.0hr | 83.4 ± 7.3hr | 39.0 ± 1.0g |
| P15‐I | 33.9 ± 0.1hr | 364.5 ± 9.5hr | 73.3 ± 1.0f | 44.2 ± 4.9hr |
| P15‐J | 26.0 ± 1.5d | 321.2 ± 15.4g | 46.4 ± 0.1i | 36.6 ± 0.3g |
The values are reported as average ± esd (n = 27), and different letters in the same column (among the same matrix) indicate significant differences (p < .05, Tukey's test).
A similar trend was found in the TEAC/ABTS and TEAC/DPPH data. TEAC/ABTS values ranged 9.5 ± 0.8–30.3 ± 1.1 mmol(TrxEq)/kg DW, resulting from two to six times higher with respect to values reported for a Turkish variety (4.7 mmol(TrxEq)/kg DW; Arslan & Ozcan, 2011; considering experimental 56% of water content). Also in this case, a significant correlation between TPP and TEAC/ABTS occurred (y = 0.7292x + 0.4606; R 2 = 0.920; p < .001, at 95% confidence interval; Figure 1b). Finally, as regards the TEAC/DPPH analyses, data ranged 14.7 ± 0.5–78.4 ± 4.1, and 14.2 ± 0.9–121.1 ± 1.2 mmol(TrxEq)/kg DW, for photometric and EPR measurements, respectively, the two methods being in quite good agreement showing a ratio (visible/EPR) by 1.05 ± 0.45.
3.1.3. Pomace samples
Particular attention was paid to pomace material, as a by‐product of olive oil production and as a potential source of antioxidant molecules. The pomaces showed very high antioxidant activities, particularly in samples P15‐D/‐J, that ranged 26.0 ± 1.5–43.7 ± 3.0 g(GAEq)/kg DW (TPP), and 265.4 ± 10.1–388.1 ± 12.0 mmol(TrxEq)/kg DW (TEAC/ABTS; Table 3). In addition to fruits’ quality, the other important factor which strongly affects the content of polyphenols in olive pomace is olive oil production technology. The usage of hot water in three‐phase mill systems brings about a lower antioxidant activity and polyphenol content, as revealed by two samples from the year 2015 that were very dry (P15‐A and P15‐C). This could be reasonably explained suggesting that, the added hot water, works as extragent, moving the polyphenols and other antioxidant species, to waste wasters. Other impact production process can be related to the possible seed removal, which also is a source of antioxidant compounds. Leaving seeds in production process could cause higher antioxidant activity for pomace than for fruits, as seeds were removed from fruits before their analysis.
Finally, it was confirmed an acceptable agreement between TEAC/DPPH analyses, via photometric and EPR measurements, the ratio (visible/EPR) being 1.66 ± 0.39. Moreover, these data confirm that TEAC values measured via the ABTS•+ radical cation quenching are usually higher than values measured via the DPPH• radical quenching (Floegel, Kim, Chung, Koo, & Chun, 2011), this being not revealed in olive fruit samples. Furthermore, also in this case, TEAC/ABTS and TPP parameters showed a very good linear correlation in 2014 and 2015 pomace samples R 2 = 0.853 (y = 8.4483x‐3.9051), improving to R 2 = 0.948 (y = 8.1276x‐12.7980) when samples P15‐I and P15‐J were excluded (p < .001, at 95% confidence interval, in both cases; Figure 1c). These latter samples were from geographical areas different from that of the other samples.
3.2. Chromatographic characterization of selected antioxidant components in olive fruits and pomaces
3.2.1. HPLC‐UV: identification and quantification of hydroxytyrosol and oleuropein in olive fruit and pomace samples
The hydroalcoholic (EtOH/H2O, 80/20%, v/v) extracts of olive fruits from 2015 harvest showed contents of hydroxytyrosol and oleuropein of the same order of magnitude, ranging 2.4 ± 0.2–6.8 ± 0.3, and 0.7 ± 0.1–9.8 ± 0.4 g/kg DW, respectively (Table 4 and Figure S5). The minimum value for both analytes was found for sample F15‐D (2.4 ± 0.2 g/kg DW). It is interesting to note that sample F15‐J showed a very low content of oleuropein (trace, <0.5 g/kg DW). Found values resulted in great agreement with data previously published for Portuguese varieties (hydroxytyrosol, 1.48–15.76 g/kg DW; oleuropein, 0.34–21.70 g/kg DW; Vinha et al., 2005) and about two/three times higher with respect to Turkish varieties (hydroxytyrosol, 0.04–3.60 g/kg DW; oleuropein, 0.25–3.00 g/kg DW; Arslan & Ozcan, 2011).
Table 4.
Values of hydroxytyrosol and oleuropein (g/kg DW) in olive fruit and pomace hydroalcoholic (EtOH/H2O, 80/20%, v/v) extracts
| Olive fruits | Hydroxytyrosol | Oleuropein | Pomaces | Hydroxytyrosol |
|---|---|---|---|---|
| F15‐A | 3.5 ± 0.2a | 0.9 ± 0.1a | P15‐A | 1.2 ± 0.1a |
| F15‐B | 4.2 ± 0.1b | 9.8 ± 0.4b | P15‐B | 6.4 ± 0.3b |
| F15‐C | 6.8 ± 0.3c | 2.0 ± 0.1c | P15‐C | 0.4 ± 0.1c |
| F15‐D | 2.4 ± 0.2d | 0.7 ± 0.1a | P15‐D | 7.7 ± 0.2d |
| F15‐E | 4.7 ± 0.2b | 7.5 ± 0.2d | P15‐E | 8.0 ± 0.3d |
| F15‐F | 4.3 ± 0.3b | 9.7 ± 0.3b | P15‐F | 6.1 ± 0.3b |
| F15‐G | 4.3 ± 0.1b | 1.4 ± 0.1a | P15‐G | 6.1 ± 0.1b |
| F15‐H | 3.3 ± 0.2a | 1.0 ± 0.1a | P15‐H | 5.5 ± 0.2e |
| F15‐I | 6.6 ± 0.3c | 2.0 ± 0.2c | P15‐I | 5.4 ± 0.2e |
| F15‐J | 4.2 ± 0.2b | Tracee | P15‐J | 5.3 ± 0.2e |
The values are reported as average ± esd (n = 27), and different letters in the same column indicate significant differences (p < .05, Tukey's test).
Regarding the olive pomaces from 2015, oleuropein was not quantifiable (trace, <0.5 g/kg DW), whereas hydroxytyrosol ranged 5.3 ± 0.2–8.0 ± 0.3 g/kg DW, excluding the two dry pomace samples (P15‐A and P15‐C), for which the values were much lower (1.2 ± 0.1 and 0.4 ± 0.1 g/kg DW, respectively). The hydroxytyrosol contents correlate quite well with general antioxidant parameters (particularly with TPP; y = 5.1450x + 2.8544; R 2 = 0.807; p < .001, at 95% confidence interval, Figure 1d). Studies indicate that many factors, such as cultivar, geographic origin, pedo‐climatic conditions, agronomical cultivation protocols (i.e., irrigation, fertilization, plant and soil treatments), ripening stage, and postharvest processing, strongly affect the phenolic profile of olive fruits, oil product, and pomace (Uylaşer & Yildiz, 2014). In particular, higher contents of oleuropein, related to the bitter taste to the drupes, have been mainly found in the skin of the fruit, and were reported as related to its ripening stage. Oleuropein undergoes enzymatic processes, by hydrolases and oxidases producing hydroxytyrosol and/or the quinone derivative (Scheme S1). These oxidative reactions also occur during the oil production process (malaxation stage), bringing about oleoside derivatives in olive pomace (Cardoso et al., 2005; Marsilio, 2001; Romero et al., 2004).
3.2.2. HPLC‐MS: identification and quantification of hydroxycinnamic acids and flavonoids in olive fruit and pomace samples
The main components revealed in hydroalcoholic (EtOH/H2O, 80/20%, v/v) extracts of olive fruits were chlorogenic acid, rutin, and luteolin, and ranged 3.6 ± 0.5–60.1 ± 2.8, 36.7 ± 4.4–583.9 ± 10.2, and 20.9 ± 1.6–121.0 ± 6.2 mg/kg DW, respectively (Table 5). Quercetin and luteolin‐7‐O‐rutinoside were one or two order(s) of magnitude lower with respect to the analogous just mentioned. The concentrations were in agreement with contents reported in previous studies on different varieties: chlorogenic acid, trace – 76.6 mg/kg DW; luteolin, 4.2–269.5 mg/kg DW; rutin, 22.9–242.8 mg/kg DW.
Table 5.
Values of selected hydroxycinnamic acids and flavonoids (mg/kg DW) for olive fruit and pomace hydroalcoholic (EtOH/H2O, 80/20%, v/v) extracts (trace, <LOQ; nd, not determined < LOD; na, not analyzed)
| Hydroxycinnamic acids | Flavonoids | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Caffeic | Chlorogenic | Ferulic | p‐Coumaric | Rutin | Quercetin | Luteolin | Luteolin−7‐O‐Rutinoside* | Naringenin | |
| Olive fruits | |||||||||
| F14‐A | Tracea | 18.3 ± 1.9a | nd | Trace | 41.6 ± 5.2a | 0.8 ± 0.1a | 57.6 ± 4.3a | na | Tracea |
| F14‐B | Tracea | 7.4 ± 0.9b | nd | Trace | 72.6 ± 6.2b | 2.6 ± 0.2b | 35.9 ± 3.1b | na | Tracea |
| F14‐C | Tracea | 3.6 ± 0.5c | nd | Trace | 36.7 ± 4.4a | 2.7 ± 0.3b | 55.1 ± 4.6a | na | Tracea |
| F15‐A | 0.5 ± 0.1b | 22.6 ± 1.4a | nd | Trace | 66.6 ± 4.3a | 1.8 ± 0.1b | 20.9 ± 1.6c | 1.6 ± 0.1a | Tracea |
| F15‐B | 0.5 ± 0.1b | 60.1 ± 2.8d | nd | Trace | 410.2 ± 13.4c | 2.0 ± 0.2b | 93.6 ± 1.2d | 8.7 ± 0.1b | 1.3 ± 0.1b |
| F15‐C | 0.6 ± 0.1b | 25.8 ± 0.9a | Trace | Trace | 310.1 ± 14.0d | 2.2 ± 0.3b | 21.7 ± 2.1c | 2.6 ± 0.1c | Tracea |
| F15‐D | 0.7 ± 0.1b | 18.3 ± 2.1a | nd | Trace | 178.0 ± 9.1e | 0.5 ± 0.1a | 75.0 ± 0.5e | 3.2 ± 0.3c | Tracea |
| F15‐E | 1.0 ± 0.1b | 33.6 ± 0.2e | nd | Trace | 428.9 ± 2.4c | 0.9 ± 0.1a | 86.3 ± 3.7d | 3.9 ± 0.1d | Tracea |
| F15‐F | Tracea | 29.5 ± 0.1e | Trace | Trace | 249.3 ± 7.5f | 0.6 ± 0.1a | 52.5 ± 0.4a | 2.3 ± 0.4c | Tracea |
| F15‐G | 0.9 ± 0.1b | 23.7 ± 1.5a | Trace | Trace | 575.6 ± 62.1 g | 0.8 ± 0.1a | 71.1 ± 2.8e | 6.5 ± 0.9b | 1.1 ± 0.1b |
| F15‐H | 0.6 ± 0.1b | 39.9 ± 1.3f | nd | Trace | 583.9 ± 10.2g | 0.9 ± 0.1a | 121.0 ± 6.2d | 7.8 ± 0.7b | 1.1 ± 0.1b |
| F15‐I | 1.0 ± 0.1b | 19.1 ± 0.1a | nd | Trace | 374.0 ± 23.7hr | 1.0 ± 0.1a | 52.1 ± 11.2a | 7.2 ± 0.3b | 1.3 ± 0.1b |
| F15‐J | 0.8 ± 0.1b | 12.2 ± 0.3g | nd | Trace | 149.9 ± 2.8e | 1.0 ± 0.1a | 78.0 ± 0.3e | 2.3 ± 0.1c | 1.3 ± 0.1b |
| Pomaces | |||||||||
| P14‐A | 451.5 ± 22.3a | 9.7 ± 1.1a | 13.3 ± 1.1a | 33.0 ± 2.9a | 7.4 ± 1.1a | 26.2 ± 0.9a | 57.6 ± 2.1a | na | 0.9 ± 0.1a |
| P14‐B | 523.2 ± 32.1b | Traceb | 34.6 ± 2.1 b | 67.1 ± 5.2 b | 1.9 ± 0.2b | 19.7 ± 0.8 b | 32.9 ± 1.3b | na | 0.9 ± 0.1a |
| P14‐C | 876.2 ± 45.2c | Traceb | 31.5 ± 1.9 b | 31.3 ± 3.1 a | 1.3 ± 0.1b | 33.2 ± 0.5 c | 55.1 ± 2.1a | na | 1.4 ± 0.1b |
| P15‐A | 0.7 ± 0.1d | Traceb | Tracec | Tracec | 4.6 ± 0.5c | 1.2 ± 0.1d | 38.1 ± 2.4b | Tracea | 1.7 ± 0.1b |
| P15‐B | 53.2 ± 4.6e | 22.1 ± 1.6c | Tracec | 16.8 ± 1.9 d | 9.4 ± 2.6a | 32.8 ± 3.1 c | 230.4 ± 3.0 c | 5.0 ± 0.1b | 1.6 ± 0.1b |
| P15‐C | Tracef | Traceb | Tracec | Tracec | 5.3 ± 0.2c | 0.5 ± 0.1d | 23.6 ± 0.1d | Tracea | 1.0 ± 0.1a |
| P15‐D | 214.0 ± 3.9g | 16.2 ± 0.2d | 10.0 ± 2.1 a | 28.9 ± 1.1 a | 10.7 ± 1.0a | 33.9 ± 1.6 c | 246.7 ± 4.8 e | 4.6 ± 0.3b | 1.5 ± 0.2b |
| P15‐E | 227.2 ± 18.0g | 16.8 ± 1.1 d | 16.6 ± 1.1 d | 34.3 ± 1.5 a | 10.6 ± 1.1a | 36.6 ± 1.9 c | 264.9 ± 8.0 f | 3.7 ± 0.4b | 1.8 ± 0.1b |
| P15‐F | 35.0 ± 0.1hr | 20.4 ± 0.5 c | 6.1 ± 0.9 d | 13.3 ± 0.3 e | 161.8 ± 15.2 d | 23.0 ± 0.3 a | 341.9 ± 3.3 g | 6.9 ± 0.7c | 2.5 ± 0.1c |
| P15‐G | 22.5 ± 0.8i | 39.8 ± 3.5e | Tracec | 7.4 ± 0.4f | 211.9 ± 12.3e | 11.9 ± 0.1 e | 318.4 ± 3.0 hr | 6.1 ± 0.5 c | 2.5 ± 0.3c |
| P15‐H | 8.6 ± 0.4j | 47.7 ± 2.6e | Tracec | 8.0 ± 0.8f | 354.2 ± 24.0 f | 9.2 ± 1.2 e | 410.9 ± 23.9i | 8.0 ± 0.9d | 2.5 ± 0.1c |
| P15‐I | 48.9 ± 0.4k | 25.4 ± 0.5f | Tracec | 18.9 ± 1.2 d | 178.9 ± 3.9d | 21.0 ± 1.7 a | 340.0 ± 6.5 g | 6.1 ± 0.8c | 3.3 ± 0.2d |
| P15‐J | 37.4 ± 0.5 hr | 11.8 ± 0.4g | 7.2 ± 0.7e | 13.4 ± 0.6 e | 84.2 ± 6.6g | 25.6 ± 2.1 a | 276.9 ± 2.3 f | 4.1 ± 0.2b | 2.2 ± 0.1c |
The values are reported as average ± esd (n = 27), and different letters in the same column (among the same matrix) indicate significant differences (p < .05, Tukey's test).
Luteolin‐7‐O‐rutinoside was quantified as luteolin equivalent (mg/kg DW).
The hydroalcoholic (EtOH/H2O, 80/20%, v/v) extracts of pomaces confirmed that the contents of hydroxycinnamic acids and flavonoid are related to the production technology. Samples P15‐A and P15‐C (dry pomaces) revealed very low content of all the analyzed species, the highest being the concentration of luteolin (38.1 ± 2.4 and 23.6 ± 0.1 mg/kg DW). Luteolin was the most abundant flavonoid in all the samples of 2015 ranging 230.4 ± 3.0–410.9 ± 23.9 mg/kg DW, two to ten times higher than that of the relevant fruits. The luteolin contents correlate very well with all the antioxidant parameters (TPP: y = 0.1046x + 3.5844; R 2 = 0.857; TEAC/DPPH(EPR): y = 0.1193x + 3.6854; R 2 = 0.771; TEAC/DPPH(Vis): y = 5.1450x + 2.8544; R 2 = 0.807; y = 0.2082x + 3.6645; R 2 = 0.909; p < .001, for all data sets, Figure 1e‐g). A second major component was rutin, ranging 84.2–354.2 mg/kg DW, in samples P15‐F/‐J, relevant to the same milling factory, but not the same primary producer. Looking at the contents of hydroxycinnamic acids, it is possible to identify a synergism between caffeic, chlorogenic, and ferulic acids. Considering that caffeic acid is the precursor of chlorogenic acid (the ester of caffeic acid and quinic acid; Scheme S2a), it appears that chlorogenic acid undergoes enzymatic hydrolysis, bringing about caffeic acid and ferulic acid as metabolites (Scheme S2b). Interestingly, data from 2014 were in good agreement with data from 2015, excluding chlorogenic acid. Studies show that secondary metabolites with high antioxidant capacity play an important role in the protection of plants under oxidative stress (Yan, Cui, Zhao, Chen, & Tang, 2016).
3.2.3. Principal component analysis
The data just above commented were confirmed, at a great extent, by the principal component analysis (PCA). The PCA score and loading plots for olive fruits samples were reported in a biplot (Figure 2a) to highlight the correlation between variables and samples. First PC explained the 48% of the total variance. The olive fruits from 2014 harvest appeared separated with higher quercetin contents with respect to the other samples that revealed higher values of TEAC/DPPH, TPP, luteolin, and rutin. The second PC explained 17% of the total variance and separated sample F15‐F, because of its antioxidant parameters values (TPP, TEAC/DPPH, TEAC/ABTS) and oleuropein content.
Figure 2.
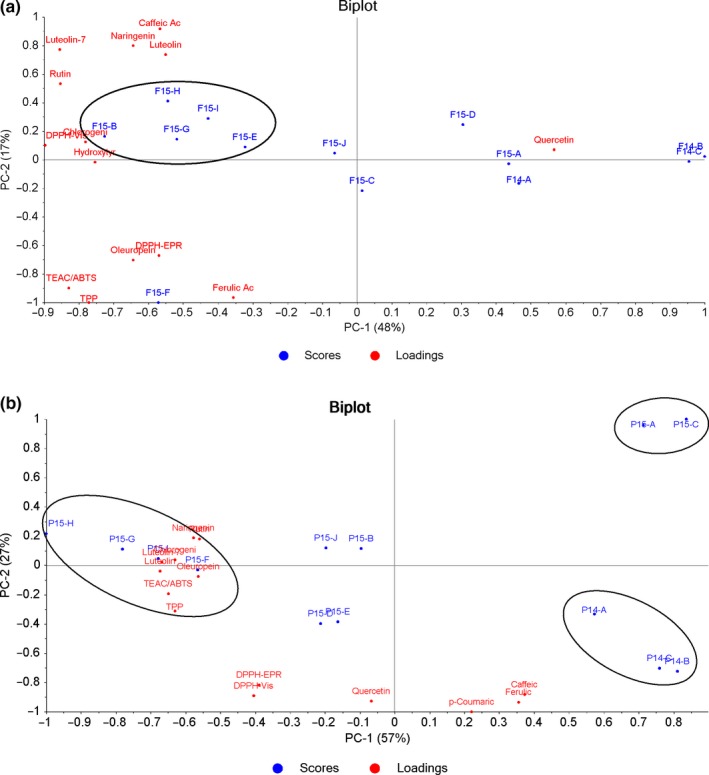
Principal component analysis (PCA) for (a) olive sample and (b) pomace sample data
Pomace samples were also analyzed for the same variables, and the PCA biplot is reported in Figure 2b. It showed that the first and second PCs explained the 57% and 27% of the total variance, respectively. Several groups of samples can be observed revealing a separation with respect to the harvest year and milling technology. The samples 2014 were well separated from samples 2015, on the basis of the higher concentrations of caffeic acid and ferulic acid, both being anticorrelated to the chlorogenic acid. The two samples P15‐A and P15‐C, relevant to dry pomaces from a three‐phase milling process, were also separated because of the low values of TEAC/DPPH. Finally, P15‐F/‐G/‐H/‐I were grouped on the basis of high values of TPP, luteolin, and rutin, and were from the same olive milling factory (using a two‐phase milling process and producing humid pomace). It is noteworthy that olive samples F15‐B and F15‐E were very close to F15‐F/‐G/‐H/‐I from the olive's biplot (Figure 2a), but the first two underwent different treatments at oil phase production and resulted separated in the pomace biplot (Figure 2b): The pressing procedure affected the total antioxidant properties, as well as the chlorogenic acid, luteolin, rutin, and naringenin contents. The multivariate analysis also confirmed the data above commented as regards correlation analysis (TPP vs. TEAC/ABTS, luteolin vs. TPP, luteolin vs. TEAC/ABTS, luteolin vs. TEAC/DPPH).
3.2.4. Cytotoxicity assay: vitality test on NIH3T3 fibroblasts cells
The hydroalcoholic (EtOH/H2O, 80/20%, v/v) extracts of olive fruits, EVOOs, and pomaces from the 2014 and 2015 harvests were tested for toxicity on NIH3T3 fibroblasts cells, and compared with pure EtOH and rutin and quercetin standard solutions, at the same extract concentrations (0.001 to 0.1 μM). Quercetin, rutin, and pomace samples from 2014 did not affect NIH3T3 viability, compared to the control at all the tested concentrations (Figure 3a). On the contrary, fruits and EVOOs 2014 revealed toxic effect at 5% (v/v), with a major effect from EVOOs with respect to fruits. For the 2015 harvest samples, none of the tested extracts were toxic at 0.5 and 1% (v/v) concentrations (Figure 3b and Figure S6), whereas 5% (v/v) treated cells showed a great decrease of viability. It was previously reported that quercetin and rutin modified mouse fibroblasts NIH3T3 viability at higher concentrations than those present in the extracts tested in this study (Araújo, de M.B. Costa, Pazini, Valadares, & de Oliveira, 2013; Bonechi et al., 2018). However, this outcome may be reasonably explained as a result of the cumulative and synergic effects of several components and their metabolites.
Figure 3.
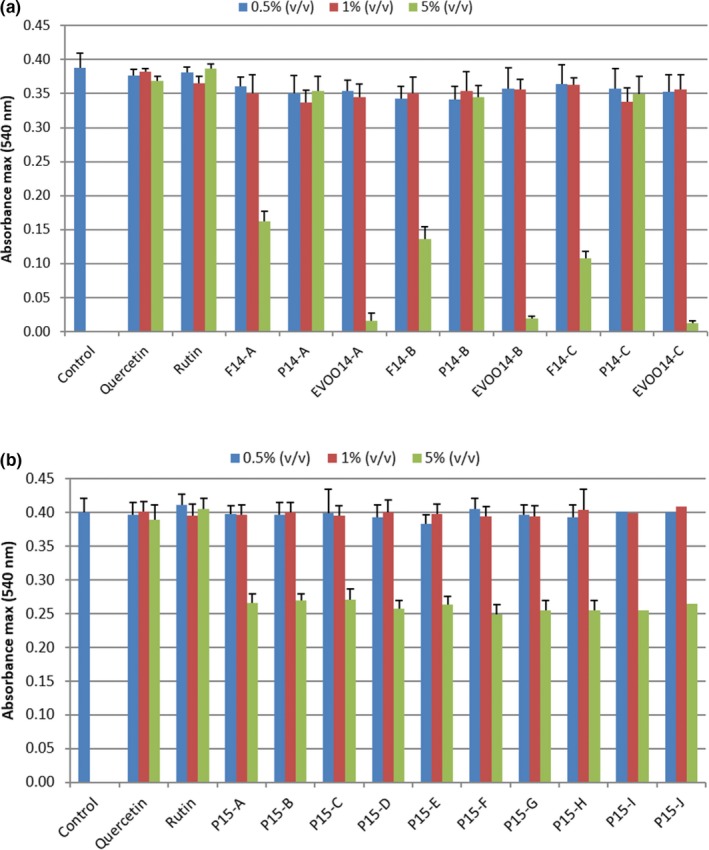
Fibroblast NIH3T3 viability (24 hr) after treatment by hydroalcoholic (EtOH/H2O, 80/20%, v/v) extracts of (a) samples 2014 (0.5, 1.0, and 5.0%, v/v), and rutin and quercetin standard solutions (0.001–0.1 μM), and (b) pomaces 2015 (0.5, 1.0 and 5.0%, v/v). The values are reported as average ± esd (six replicates)
4. CONCLUSIONS
Qualitative and quantitative analyses of olive fruits, olive oils (primary product), and olive pomaces (by‐product from technology) showed multiple factors that influenced the antioxidant properties and polyphenol components. These include genetic factors, fruit maturation stage, agronomical practices, geographical and pedo‐climatic conditions, as well as production technologies (dry and humid pomaces). The results showed that pomace, in particular the humid by‐product, is a promising source of bioactive and antioxidant compounds, without cytotoxic properties. Taking into account the human health benefits of antioxidant polyphenols and considering the importance of olive oil production in the Mediterranean basin, the possibility to utilize olive pomaces as source of nutraceuticals should be a priority. These materials, usually considered as waste products, could be used for the formulation of novel diet supplements and food fortifiers, as well as for applications in cosmetics. This approach allows the valorization of primary and secondary products from O. europaea L. and could be considered a model for other agriculture productions (e.g., viticulture, horticulture, cereal crops) to increase the sustainability of agricultural activities.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL STATEMENT
This study does not involve neither human nor animal testing.
Supporting information
ACKNOWLEDGMENTS
Tuscan Regional Administration is acknowledged for funding the project NUTRIforOIL. The authors gratefully acknowledge factory partners that kindly gifted samples of EVOOs, olive fruits, and pomaces. Toscana Life Sciences Foundation is acknowledged for the use of HPLC‐MS instrument.
Tamasi G, Baratto MC, Bonechi C, et al. Chemical characterization and antioxidant properties of products and by‐products from Olea europaea L.. Food Sci Nutr. 2019;7:2907–2920. 10.1002/fsn3.1142
Contributor Information
Gabriella Tamasi, Email: gabriella.tamasi@unisi.it.
Claudia Bonechi, Email: claudia.bonechi@unisi.it.
REFERENCES
- Araújo, K. C. F. , de M.B. Costa, E. M. , Pazini, F. , Valadares, M. C. , & de Oliveira, V. (2013). Bioconversion of quercetin and rutin and the cytotoxicity activities of the transformed products. Food Chemistry Toxicology, 51, 93–96. 10.1016/j.fct.2012.09.015 [DOI] [PubMed] [Google Scholar]
- Arslan, D. , & Ozcan, M. M. (2011). Phenolic profile and antioxidant activity of olive fruits of the Turkish variety “Sarıulak” from different locations. Grasas Y Aceites, 62, 453–461. 10.3989/gya.034311 [DOI] [Google Scholar]
- Baratto, M. C. , Tattini, M. , Galardi, C. , Pinelli, P. , Romani, A. , Visioli, F. , … Pogni, R. (2003). Antioxidant activity of galloyl quinic derivatives isolated from Pistacia lentiscus leaves. Free Radical Research, 37, 405–412. [DOI] [PubMed] [Google Scholar]
- Batarseh, Y. S. , & Kaddoumi, A. (2018). Oleocanthal‐rich extra‐virgin olive oil enhances donepezil effect by reducing amyloid‐β load and related toxicity in a mouse model of Alzheimer's disease. Journal of Nutritional Biochemistry, 55, 113–123. 10.1016/j.jnutbio.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battino, M. , Forbes‐Hernández, T. Y. , Gasparrini, M. , Afrin, S. , Cianciosi, D. , Zhang, J. , … Giampieri, F. (2018). Relevance of functional foods in the Mediterranean diet: The role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Critical Reviews in Food Science and Nutrition, 59(6), 893–920. 10.1080/10408398.2018.1526165 [DOI] [PubMed] [Google Scholar]
- Bonechi, C. , Donati, A. , Tamasi, G. , Leone, G. , Consumi, M. , Rossi, C. , … Magnani, A. (2018). Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophysical Chemistry, 233, 55–63. 10.1016/j.bpc.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Bonechi, C. , Lamponi, S. , Donati, A. , Tamasi, G. , Consumi, M. , Leone, G. , … Magnani, A. (2017). Effect of resveratrol on platelet aggregation by fibrinogen protection. Biophysical Chemistry, 222, 41–48. 10.1016/j.bpc.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Brand‐Williams, W. , Cuvelier, M. E. , & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT‐Food Science and Technology, 28, 23–30. 10.1016/S0023-6438(95)80008-5 [DOI] [Google Scholar]
- Buckland, G. , & Gonzalez, C. A. (2015). The role of olive oil in disease prevention: A focus on the recent epidemiological evidence from cohort studies and dietary intervention trials. British Journal of Nutrition, 113(S2), S94–S101. 10.1017/S0007114514003936 [DOI] [PubMed] [Google Scholar]
- Bulotta, S. , Celano, M. , Lepore, S. M. , Montalcini, T. , Pujia, A. , & Russo, D. (2014). Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. Journal of Translational Medicine, 12, 219–227. 10.1186/s12967-014-0219-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, S. M. , Guyot, S. , Marnet, N. , Lopes‐da‐Silva, J. A. , Renard, C. M. G. C. , & Coimbra, M. A. (2005). Characterisation of phenolic extracts from olive pulp and olive pomace by electrospray mass spectrometry. Journal of the Science of Food and Agriculture, 85, 21–32. 10.1002/jsfa.1925 [DOI] [Google Scholar]
- Chanioti, S. , & Tzia, C. (2018). Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innovative Food Science & Emerging Technologies, 48, 228–239. 10.1016/j.ifset.2018.07.001 [DOI] [Google Scholar]
- Covas, M. I. , de la Torre, R. , & Fitó, M. (2015). Virgin olive oil: A key food for cardiovascular risk protection. British Journal of Nutrition, 113 . Suppl, 2, S19–28. 10.1017/S0007114515000136 [DOI] [PubMed] [Google Scholar]
- Delgado‐Lista, J. , Perez‐Martinez, P. , Garcia‐Rios, A. , Alcala‐Diaz, J. F. , Perez‐Caballero, A. I. , Gomez‐Delgado, F. , … Perez‐Jimenez, F. (2016). CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (the CORDIOPREV study): Rationale, methods, and baseline characteristics: A clinical trial comparing the efficacy of a Mediterranean diet rich in olive oil versus a low‐fat diet on cardiovascular disease in coronary patients. American Heart Journal, 177, 42–50. 10.1016/j.ahj.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nunzio, M. , Picone, G. , Pasini, F. , Caboni, M. F. , Gianotti, A. , Bordoni, A. , & Capozzi, F. (2018). Olive oil industry by‐products. Effects of a polyphenol‐rich extract on the metabolome and response to inflammation in cultured intestinal cell. Food Research International, 113, 392–400. 10.1016/j.foodres.2018.07.025 [DOI] [PubMed] [Google Scholar]
- Estruch, R. , Ros, E. , Salas‐Salvadó, J. , Covas, M. I. , Corella, D. , Arós, F. , … PREDIMED Study Investigators (2018). Primary prevention of cardiovascular disease with a Mediterranean Diet supplemented with extra‐virgin olive oil or nuts. The New England Journal of Medicine, 378, e34(1)‐e34(14) 10.1056/NEJMoa1800389 [DOI] [PubMed] [Google Scholar]
- European Commission Regulation 2568/1991 (1991). on the characteristics of olive oil and olive‐residue oil and on the relevant methods of analysis; and its amending 1830/2015 (8 July 2015).
- Fini, A. , Guidi, L. , Giordano, C. , Baratto, M. C. , Ferrini, F. , Brunetti, C. , … Tattini, M. (2014). Salinity stress constrains photosynthesis in Fraxinus ornus more when growing in partial shading than in full sunlight: Consequences for the antioxidant defense system. Annals of Botany, 114, 525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finicelli, M. , Squillaro, T. , Di Cristo, F. , Di Salle, A. , Melone, M. A. B. , Galderisi, U. , & Peluso, G. (2018). Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. Journal of Cellular Physiology, 234(5), 5807–5826. 10.1002/jcp.27506 [DOI] [PubMed] [Google Scholar]
- Floegel, A. , Kim, D.‐O. , Chung, S.‐J. , Koo, S. I. , & Chun, O. K. (2011). Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant‐rich US foods. Journal of Food Composition and Analysis, 24, 1043–1048. 10.1016/j.jfca.2011.01.008 [DOI] [Google Scholar]
- Galvano, F. , La Fauci, L. , Graziani, G. , Ferracane, R. , Masella, R. , Di Giacomo, C. , … Galvano, G. (2007). Phenolic compounds and antioxidant activity of Italian extra virgin olive oil Monti Iblei. Journal of Medicinal Food, 10, 650–656. 10.1089/jmf.2007.409 [DOI] [PubMed] [Google Scholar]
- Gori, A. , Ferrini, F. , Marzano, M. C. , Tattini, M. , Centritto, M. , Baratto, M. C. , … Brunetti, C. (2016). Characterization and antioxidant activity of crude extract and polyphenolic rich fractions from C. Incanus leaves. International Journal of Molecular Science, 17, 1344–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch‐Ferré, M. , Hu, F. B. , Martínez‐González, M. A. , Fitó, M. , Bulló, M. , Estruch, R. , … Salas‐Salvadó, J. (2014). Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Medicine, 12, 78–88. 10.1186/1741-7015-12-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullón, B. , Gullón, P. , Eibes, G. , Cara, C. , De Torres, A. , López‐Linares, J. C. , … Castro, E. (2018). Valorisation of olive agro‐industrial by‐products as a source of bioactive compounds. Science of the Total Environment, 645, 533–542. 10.1016/j.scitotenv.2018.07.155 [DOI] [PubMed] [Google Scholar]
- Herrero, M. , del Pilar Sánchez‐Camargo, A. , Cifuentes, A. , & Ibáñez, E. (2015). Plants, seaweeds, microalgae and food by‐products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. Trends in Analytical Chemistry, 71, 26–38. 10.1016/j.trac.2015.01.018 [DOI] [Google Scholar]
- Herrero, M. , Temirzod, T. N. , Segura‐Carretero, A. , Quirantes, R. , Plaza, M. , & Ibañez, E. (2011). New possibilities for the valorization of olive oil by‐products. Journal of Chromatography A, 1218, 7511–7520. 10.1016/j.chroma.2011.04.053 [DOI] [PubMed] [Google Scholar]
- Khalatbary, A. R. (2013). Olive oil phenols and neuroprotection. Nutritional Neuroscience, 16, 243–249. 10.1179/1476830513Y.0000000052 [DOI] [PubMed] [Google Scholar]
- Kishikawa, A. , Ashour, A. , Zhu, Q. , Yasuda, M. , Ishikawa, H. , & Shimizu, K. (2015). Multiple biological effects of olive oil by‐products such as leaves, stems, flowers, olive milled waste, fruit pulp, and seeds of the olive plant on skin. Phytotherapy Research, 29, 877–886. 10.1002/ptr.5326 [DOI] [PubMed] [Google Scholar]
- Konstantinidou, V. , Covas, M. I. , Muñoz‐Aguayo, D. , Khymenets, O. , de la Torre, R. , Saez, G. , … Fitó, M. (2010). In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB Journal, 24, 2546–2557. [DOI] [PubMed] [Google Scholar]
- Leone, G. , Consumi, M. , Franzi, C. , Tamasi, G. , Lamponi, S. , Donati, A. , … Bonechi, C. (2018). Development of liposomal formulations to potentiate natural lovastatin inhibitory activity towards 3‐hydroxy‐3‐methyl‐glutaryl coenzyme A (HMG‐CoA) reductase. Journal of Drug Delivery Science and Technology, 43, 107–112. 10.1016/j.jddst.2017.09.019 [DOI] [Google Scholar]
- Leone, G. , Consumi, M. , Pepi, S. , Lamponi, S. , Bonechi, C. , Tamasi, G. , … Magnani, A. (2016). New formulations to enhance lovastatin release from Red Yeast Rice (RYR). Journal of Drug Delivery Science and Technology, 36, 110–119. 10.1016/j.jddst.2016.10.001 [DOI] [Google Scholar]
- Marsilio, V. , Campestre, C. , & Lanza, B. (2001). Phenolic compounds change during California‐style ripe olive processing. Food Chemistry, 74, 55–60. 10.1016/S0308-8146(00)00338-1 [DOI] [Google Scholar]
- Megar, J. C. , Guidi, L. , Remorini, D. , Agati, G. , Degl'Innocenti, E. , Castelli, S. , … Tattini, M. (2009). Antioxidant defenses and oxidative damage in salt‐treated olive plants under contrasting sunlight irradiance. Tree Physiology, 29, 187–1198. [DOI] [PubMed] [Google Scholar]
- Pellegrini, N. , Serafini, M. , Colombi, B. , Del Rio, D. , Salvatore, S. , Bianchi, M. , & Brighenti, F. (2003). Total antioxidant capacity of plant foods, beverages and oils consume in Italy assessed by three different in vitro assays. Journal of Nutrition, 133, 2812–2819. [DOI] [PubMed] [Google Scholar]
- Pérez‐Martínez, P. , García‐Ríos, A. , Delgado‐Lista, J. , Pérez‐Jiménez, F. , & López‐Miranda, J. (2011). Mediterranean diet rich in olive oil and obesity, metabolic syndrome and diabetes mellitus. Current Pharmaceutical Design, 17, 769–777. 10.2174/138161211795428948 [DOI] [PubMed] [Google Scholar]
- Polovka, M. (2006). EPR spectroscopy: A tool to characterise stability and antioxidant properties of foods. Journal of Food and Nutrition Research, 45, 1–11. [Google Scholar]
- Prior, R. L. , Wu, X. , & Schaich, K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in food and dietary supplements. Journal of Agriculture and Food Chemistry, 53, 4290–4302. [DOI] [PubMed] [Google Scholar]
- Re, R. , Pellegrini, N. , Proteggente, A. , Pannala, A. , Yang, M. , & Rice‐Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26, 1231–1237. 10.1016/S0891-5849(98)00315-3 [DOI] [PubMed] [Google Scholar]
- Rice, R. (2000). Bionomics of the olive fruit fly bactrocera (dacus) oleae. UC Plant Protection Quarterly, 10, 1–5. [Google Scholar]
- Rigacci, S. , & Stefani, M. (2016). Nutraceutical properties of olive oil polyphenols. An Itinerary from cultured cells through animal models to humans. International Journal of Molecular Science, 17, 843–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani, A. , Mulinacci, N. , Pinelli, P. , Vincieri, F. F. , & Cimato, A. (1999). Polyphenolic content in five tuscany cultivars of Olea europaea L. Journal of Agriculture and Food Chemistry, 47, 964–967. [DOI] [PubMed] [Google Scholar]
- Romero, C. , Brenes, M. , Yousfi, K. , García, P. , García, A. , & Garrido, A. (2004). Effect of cultivar and processing method on the contents of polyphenols in table olives. Journal of Agriculture and Food Chemistry, 52, 479–484. 10.1021/jf030525l [DOI] [PubMed] [Google Scholar]
- Romero, C. , Medina, E. , Mateo, M. A. , & Brenes, M. (2018). New by‐products rich in bioactive substances from the olive oil mill processing. Journal of the Science of Food and Agriculture, 98, 225–230. 10.1002/jsfa.8460 [DOI] [PubMed] [Google Scholar]
- Samaniego Sánchez, C. , Troncoso González, A. M. , García‐Parrilla, M. C. , Quesada Granados, J. J. , García, L. , de la Serrana, H. , & López Martínez, M. C. (2007). Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Analitica Chimica Acta, 593, 103–107. 10.1016/j.aca.2007.04.037 [DOI] [PubMed] [Google Scholar]
- Šarolić, M. , Gugić, M. , Tuberoso, C. I. G. , Jerković, I. , Šuste, M. , Marijanović, Z. , & Kuś, P. M. (2014). Volatile profile, phytochemicals and antioxidant activity of virgin olive oils from Croatian autochthonous varieties Mašnjača and Krvavica in comparison with Italian variety Leccino. Molecules, 19, 881–895. 10.3390/molecules19010881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton, V. L. , Orthofer, R. , & Lamuela‐Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin‐Ciocalteu reagent. Methods in Enzymology, 299, 152–178. [Google Scholar]
- Sousa, D. A. , Costa, A. I. , Alexandre, M. R. , & Prata, J. V. (2019). How an environmental issue could turn into useful high‐valued products: The olive mill wastewater case. Science of the Total Environment, 647, 1097–1105. 10.1016/j.scitotenv.2018.08.060 [DOI] [PubMed] [Google Scholar]
- Tamasi, G. , Cambi, M. , Gaggelli, N. , Autino, A. , Cresti, M. , & Cini, R. (2015). The content of selected minerals and vitamin C for potatoes (Solanum tuberosum L.) from the high Tiber Valley area, Southeast Tuscany. Journal of Food Composition and Analysis, 41, 157–164. [Google Scholar]
- Tamasi, G. , Pardini, A. , Bonechi, C. , Donati, A. , Pessina, F. , Marcolongo, P. , … Rossi, C. (2019). Characterization of nutraceutical components in tomatoes pulp, skin and locular gel. European Food Research and Technology, 245, 907–918. [Google Scholar]
- Thaipong, K. , Boonprakob, U. , Crosby, K. , Cisneros‐Zevallos, L. , & Byrne, D. H. (2006). Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis, 19, 669–675. 10.1016/j.jfca.2006.01.003 [DOI] [Google Scholar]
- Uylaşer, V. , & Yildiz, G. (2014). The historical development and nutritional importance of olive and olive oil constituted an important part of the Mediterranean diet. Critical Reviews in Food Science and Nutrition, 54, 1092–1101. 10.1080/10408398.2011.626874 [DOI] [PubMed] [Google Scholar]
- Van Hung, P. (2016). Phenolic compounds of cereals and their antioxidant capacity. Critical Reviews in Food Science and Nutrition, 56, 25–35. 10.1080/10408398.2012.708909 [DOI] [PubMed] [Google Scholar]
- Vinha, A. F. , Ferreres, F. , Silva, B. M. , Valentão, P. , Gonçalves, A. , Pereira, J. A. , … Andrade, P. B. (2005). Phenolic profiles of Portuguese olive fruits (Olea europaea L.): Influences of cultivar and geographical origin. Food Chemistry, 89, 561–568. 10.1016/j.foodchem.2004.03.012 [DOI] [Google Scholar]
- Vitali Čepo, D. , Radić, K. , Jurmanović, S. , Jug, M. , Grdić Rajković, M. , Pedisić, S. , … Albahari, P. (2018). Valorization of olive pomace‐based nutraceuticals as antioxidants in chemical, food, and biological models. Molecules, 23, E2070–E2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, P. , Huang, L. , Zhang, C. , Deng, Y. , Wang, X. , & Cheng, J. (2019). Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: Process parameters, kinetics and thermodynamics, and greenness assessment. Food Chemistry, 276, 662–674. 10.1016/j.foodchem.2018.10.079 [DOI] [PubMed] [Google Scholar]
- Yan, K. , Cui, M. , Zhao, S. , Chen, X. , & Tang, X. (2016). Salinity stress is beneficial to the accumulation of chlorogenic acids in honeysuckle (Lonicera japonica Thunb.). Frontiers in Plant Science, 7, 1563–1572. 10.3389/fpls.2016.01563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, L. L. , & Cheng, Z. (2008). Application of electron spin resonance (ESR) spectrometry in nutraceutical and food research. Molecular Nutrition & Food Research, 52, 62–78. 10.1002/mnfr.200700395 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Huang, Y. , & Li, S. (2014). Polymeric micelles: Nanocarriers for cancer‐targeted drug delivery. An Official Journal of the American Association of Pharmaceutical Scientists, 15, 862–871. 10.1208/s12249-014-0113-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


