Abstract
Bone infections are a frequent cause for large bony defects with a reduced healing capacity. In previous findings, we could already show diminished healing capacity after bone infections, despite the absence of the causing agent, Staphylococcus aureus. Moreover, these bony defects showed reduced osteoblastogenesis and increased osteoclastogenesis, meaning elevated bone resorption ongoing with an elevated B‐cell activity. To overcome the negative effects of this postinfectious inflammatory state, we tried to use the regenerative capacity of mesenchymal stem cells derived from adipose tissue (adipose‐derived stem cells [ASCs]) to improve bone regeneration and moreover were curious about immunomodulation of applicated stem cells in this setting. Therefore, we used our established murine animal model and applicated ASCs locally after sufficient debridement of infected bones. Bone regeneration and resorption as well as immunological markers were investigated via histology, immunohistochemistry, Western blot, and fluorescence‐activated cell scanning (FACS) analysis and μ‐computed tomography (CT) analysis. Interestingly, ASCs were able to restore bone healing via elevation of osteoblastogenesis and downregulation of osteoclasts. Surprisingly, stem cells showed an impact on the innate immune system, downregulating B‐cell population. In summary, these data provide a fascinating new and innovative approach, supporting bone healing after bacterial infections and moreover gain insights into the complex ceremony of stem cell interaction in terms of bone infection and regeneration. stem cells translational medicine 2019;8:1084–1091
Keywords: Post‐traumatic osteomyelitis, Bone regeneration, Stem cells, B‐cells, Osteoimmunology
Significance Statement.
This study focused on reduced bone regeneration after severe bone infections. To restore bone healing in this context, stem cells gained from fat tissue (adipose‐derived stem cells) were transferred to bony defects right after surgical treatment of infected bones in mice. The gained study results could help to improve therapy of bone infections and moreover contribute to the understanding of stem cell interaction in bone in an inflammatory state.
Introduction
Post‐traumatic osteomyelitis is a severe complication especially after open fractures, with reported incidences between 5% and 30%, depending on the severity of the fracture and soft tissue injury as well as patient related comorbidities 1. Besides antibiotic treatment, the gold standard treating bone infections is an adequate surgical debridement, removing all infected purulent bone tissue. The presence of infected bone material reduces the effectiveness of antibiotic treatment by 103 as remaining bacteria can form biofilms 2. In our previous work, we could show a highly impaired bone regeneration capacity after bone infections in a murine tibia defect model 3. Besides that, we could underline the importance of surgical debridement versus antibiotic treatment alone for the therapy of post‐traumatic osteomyelitis. Despite virtually complete removal of all bacteria, remaining bony defects showed a markedly decreased bone regeneration capacity and moreover elevated bone resorption as a result of increased osteoclast activity in comparison to the control group (debrided but not infected) in our previously established mouse model. This was mediated by an inflammatory reaction, regulating various cytokines and elevated B‐cells 4. Thus, depending on the extent of the bone infection a large bony defect can remain, showing a markedly reduced bone healing capacity 3.
Bone regeneration is a highly complex regulated process containing bone resorption and bone formation. A distinct feature of bone infections, especially by Staphylococcus aureus, cause bone resorption. In detail, proliferation and mineralization of infected osteoblasts is altered and osteoclast activity is increased in a receptor activator of NF‐κB ligand (RANKL)‐dependent manner 5, 6, 7, 8, 9.
The regenerative capacity of mesenchymal stem cells (MSCs), derived from adipose tissue (adipose‐derived stem cells [ASCs]) or bone marrow‐derived stem cells (BMSCs) gained a lot of attention in recent years 10. Besides successful clinical use enhancing regenerative processes 11, ASCs can be easily harvested during a minor invasive liposuction procedure and moreover medical devices processing lipoaspirate into ASCs in real‐time are already fully licensed 12.
In the setting of bone infections, studies investigating the effects of MSCs are sparse. Subsequently, we sought to improve bone healing after post‐traumatic osteomyelitis in mice via stem cell application. Besides the regenerative capacity of MSCs, improving bone formation, this study aimed to investigate the beneficial effects on the highly dysregulated immune system.
For this purpose, ASCs were administered to debrided bony defects after bone infection to increase new bone formation and decrease bone resorption.
Materials and Methods
Mouse Osteomyelitis Model
All experiments were performed in adherence to the National Institutes of Health guidelines for the use of experimental animals and after approval by the German legislation. The protocol was approved by the Landesamt für Natur, Umwelt und Verbraucherschutz (NRW, Germany; permit‐number: 84‐02.04.2014.A044). Animals were housed and caged individually with free access to water and food under specific pathogen free conditions.
C57BL/6J male and female mice, 12 weeks old with an average weight of 25 g, were used for this project. Surgical steps were performed, as previously described in our established mouse model, showing decreased bone formation after debridement following bacterial infection 3. Briefly, after placement of a skin incision over the proximal medial tibia, a hole (1 mm in diameter) was drilled into the proximal medial tibia. Then S. aureus was injected into the medullary cavity of the tibia. Thereafter, the muscle was reapproximated and the wound closed.
Two weeks after bone infection, second surgery was performed. Placing the skin incision once again over the proximal medial tibia the bone defect was exposed. Then, infected bone tissue was debrided, followed by rinsing of debrided defects with isotone sodium chloride solution.
After sufficient debridement, ASCs (105 cells in 1 ml) were administered into the bony defect on a collagen sponge. As a control, 1 ml of phosphate‐buffered saline (PBS) was administered.
After debridement, the wound was closed and animals were monitored until recovery from anesthesia. Mice were sacrificed 3 and 7 days after debridement.
ASC Isolation
ASCs were isolated and cultivated from inguinal subcutaneous fat pads harvested from C57Bl6 mice. After thoroughly washing the fat pads in sterile PBS, tissue was minced with sterile scissors. Thereafter, 0.075% collagenase type II was administered and incubated at 37°C. Collagenase enzyme reaction was stopped with culture medium. After centrifugation (1,000 rpm, 5 minutes), adipocytes and supernatant were removed and remaining cell pellet was resuspended in culture medium. Cells were then seeded on cells culture flask and passaged later on according to proliferation of cells.
Protein Isolation
Tibiae were quick‐frozen after harvest and stored at −80°C. After pestling in liquid nitrogen, bone fragments were collected and homogenized in lysis buffer, containing protease inhibitors until lysis was completed. Cellular debris was removed by centrifugation and isolated protein was stored for further experiments.
Western Blot
Isolated protein was combined and mixed with Laemmli sample buffer. After denaturation at 95°C, samples were kept on ice until loading of the SDS‐PAGE. Fifteen percent of polyacrylamide gels were used for electrophoresis of 30 μg total protein per lane. Protein was transferred to a nitrocellulose membrane using wet transfer method before membranes were blocked with 3% bovine serum albumin to prevent unspecific binding. After washing, membranes were incubated with primary antibodies against RANKL, runt‐related transcription factor 2 (RUNX2), CD19, interferon‐γ (IFN‐γ), B‐cell activating factor (BAFF), and galectin‐9 (GAL9; Abcam, Cambridge, U.K.) overnight at 4°C, followed by washing and incubation with horseradish peroxidase (HRP)‐conjugated secondary antibody (Thermo Fisher Scientific, Waltham; Santa Cruz Biotechnologies, Dallas). Proteins were detected for 30–60 seconds by enhanced chemoluminescence.
Flow Cytometry
Tibiae were rinsed with phosphate buffered saline containing 10% fetal calve serum using a fine cannula to wash out bone marrow. A single cell suspension was created and cells of two tibiae per group were combined for flow cytometry analysis. To prevent nonspecific binding, Fc receptors were blocked with anti‐CD16/CD32 antibodies (BD Pharmingen, San Diego) before cells were stained with antibodies against CD3, CD19, Gr1 (eBioscience, San Diego), and CD45R (BD Bioscience, San Jose). Flow cytometry was carried out using BD LSRFortessa and corresponding software to compensate fluorescence intensity of antibodies. Further analysis and gating to final cell subpopulations was performed using FlowJo single cell analysis software. Cells were characterized for CD45R+CD19+ B cells, Gr‐1+ granulocytes, and CD3+ T cells, respectively.
Microcomputed Tomographic Analysis
Bone specimens were scanned with a μCT device (Viva CT 80; Scanco Medical AG, Brüttisellen, Switzerland) operated at 70 kVp, 114 μA, 8 W, 31.9‐mm Field‐of‐View, an integration time of 1,167 ms and 2× frame averaging. The data sets were reconstructed into 3D volumes with an isotropic nominal resolution of 15.6‐μm voxel size.
Image Processing
Further processing of the scanned images was performed using μ‐CT Evaluation Software Program V6.5 (Scanco Medical AG, Brüttisellen, Switzerland). A standardized cylindrical volume of interest with 16.84 mm in diameter was placed within the defect site. Thereafter, bone volume to total volume was assessed according to the guidelines for assessment of bone microstructures using μCT (Bouxsein, guidelines for assessment of bone microstructure in rodents using microcomputed tomography 2010).
Histology, Immunohistochemistry, and Immunofluorescence
For all histological procedures, tibiae were taken and fixed in 4% paraformaldehyde solution overnight, decalcified in 19% EDTA solution and finally paraffin embedded. Thereafter, tibiae were longitudinally sectioned at 9 μm. Following, aniline blue staining was performed as previously described 13. Moreover, TRAP‐staining was performed with TRAP Kit (Sigma–Aldrich, St. Louis) after manufacturer's instruction.
Additionally, immunohistochemical stainings with primary antibodies against osteocalcin, proliferating‐cell‐nuclear‐antigen (PCNA; Santa Cruz Biotechnologies) and platelet endothelial cell adhesion molecule 1 (PECAM‐1; BD Biosciences, Franklin Lakes) were performed using Vectastain ABC Kit (Vector Laboratories, Burlingame). After deparaffinization and rehydration, specimens were incubated with proteinase K for antigen demasking. Endogenous peroxidase activity was quenched by incubation with 3% hydrogen peroxide solution. Thereafter, specimens were blocked with normal blocking serum to prevent unspecific binding of primary antibody that was subsequently applicated and incubated overnight at 4°C. Following, secondary antibody conjugated to HRP was used and staining reaction was performed by use of NovaRED (HRP) Peroxidase Substrate Kit (Vector Laboratories, Burlingame).
For immunofluorescent stainings, primary antibodies against RUNX2 (Santa Cruz Biotechnologies) and CD19 (Abcam) were used. Initial steps were carried out similar to immunohistochemical staining until application of primary antibody. Thereafter, samples were incubated with secondary antibody conjugated to Alexa Fluor594 (Thermo Fisher Scientific). Three images per specimen were taken with Zeiss Axioplan microscope and histomorphometry of all histological stainings was performed by semiautomatic pixel quantification using Adobe Photoshop as previously been described.
Statistics
Results are presented as mean ± SEM of at least three independent experiments. Normal distribution was tested using chi square test. p values were calculated by Student's t test comparing two groups. For post hoc comparisons, Tukey's test was used. Statistical significances were set at a p‐value <.05.
Results
Application of ASCs Leads to Elevated Osteogenesis and Angiogenesis
One of our primary interests after ASC application was the evaluation of new bone formation after treatment. Aniline blue staining revealed significant promotion of osteogenesis by MSCs in comparison to PBS control at day 7 postoperatively. In fact, a 10‐fold increase of new bone formation in aniline blue stainings could be made out after stem cell application (Fig. 1). This observation was further validated by μ‐CT scans showing elevated bone volume to total volume within the defect site of ASC treated animals (Fig. 1). In this context, we could observe enhanced osteoblastogenesis and osteoblast activity in Runx2 and osteocalcin stainings (Fig. 2). These findings could be further supported by Western blot analysis targeting Runx2 (Fig. 3). Besides osteogenesis, proliferation verified by PCNA staining, could be increased by ASC treatment (Fig. 2). To verify if applicated ASCs could likewise increase angiogenesis, stainings with primary antibody against PECAM‐1 were performed, elucidating significantly increased number of vessels within the defect (Fig. 2).
Figure 1.
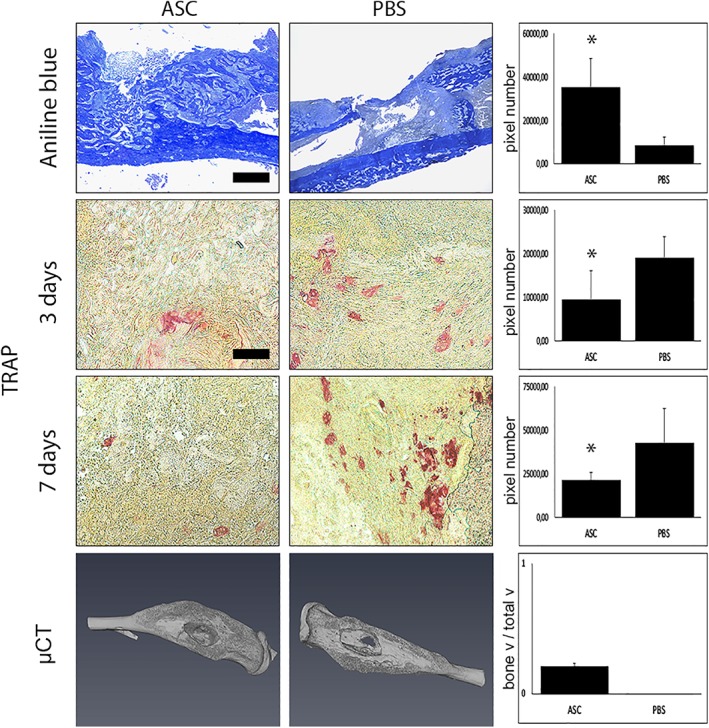
Osteogenesis is elevated and osteoclast activity reduced by adipose‐derived stem cell (ASC) treatment. Aniline blue, Tartrate‐resistant acid phosphatase (TRAP) stainings, and μCT‐analysis of animals treated with ASCs and phosphate‐buffered saline. Aniline blue stainings made after 7 days showed significantly elevated new bone formation after stem cell treatment. In accordance, μCT scans revealed markedly increased bone volume to total volume of ASC‐treated animals in comparison with control animals. Furthermore, TRAP staining indicated a downregulation of osteoclasts 3 and 7 days after surgery due to ASC treatment (seven animals/group). Scale bar: aniline blue represents 200 μm; scale bar: TRAP represents 100 μm. *, p < .05.
Figure 2.
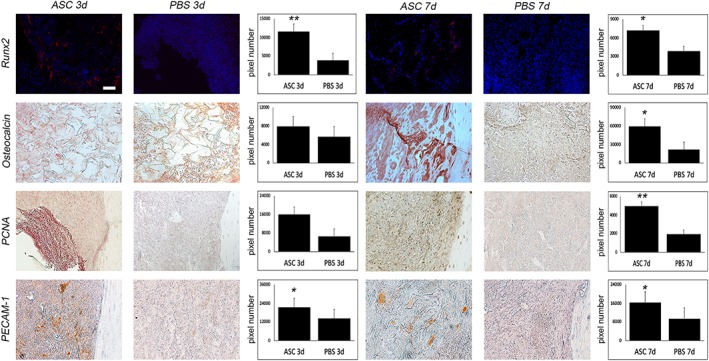
Stem cell application leads to increased osteoblastogenesis, angiogenesis, and proliferation. Immunoflourescent stainings with primary antibody against runt‐related transcription factor 2 (Runx2) and immunohistochemical stainings against osteocalcin, proliferating‐cell‐nuclear‐antigen (PCNA), and platelet endothelial cell adhesion molecule 1 (PECAM‐1). Subsequently to elevated bone formation after stem cell application, osteoblastogenesis which could be seen in Runx2 stainings was significantly elevated 3 days after surgery. In accordance, osteocalcin, a marker for mature osteoblasts showed significantly enhanced signals after 3 and 7 days. PCNA stainings, indicating cell proliferation was also upregulated after stem cell treatment. Interestingly, angiogenesis seemed to be enhanced by ASC treatment, as signals observed in PECAM‐1 stainings were significantly increased (seven animals/group). Scale bar represents 100 μm. *, p < .05; **, p < .01.
Figure 3.
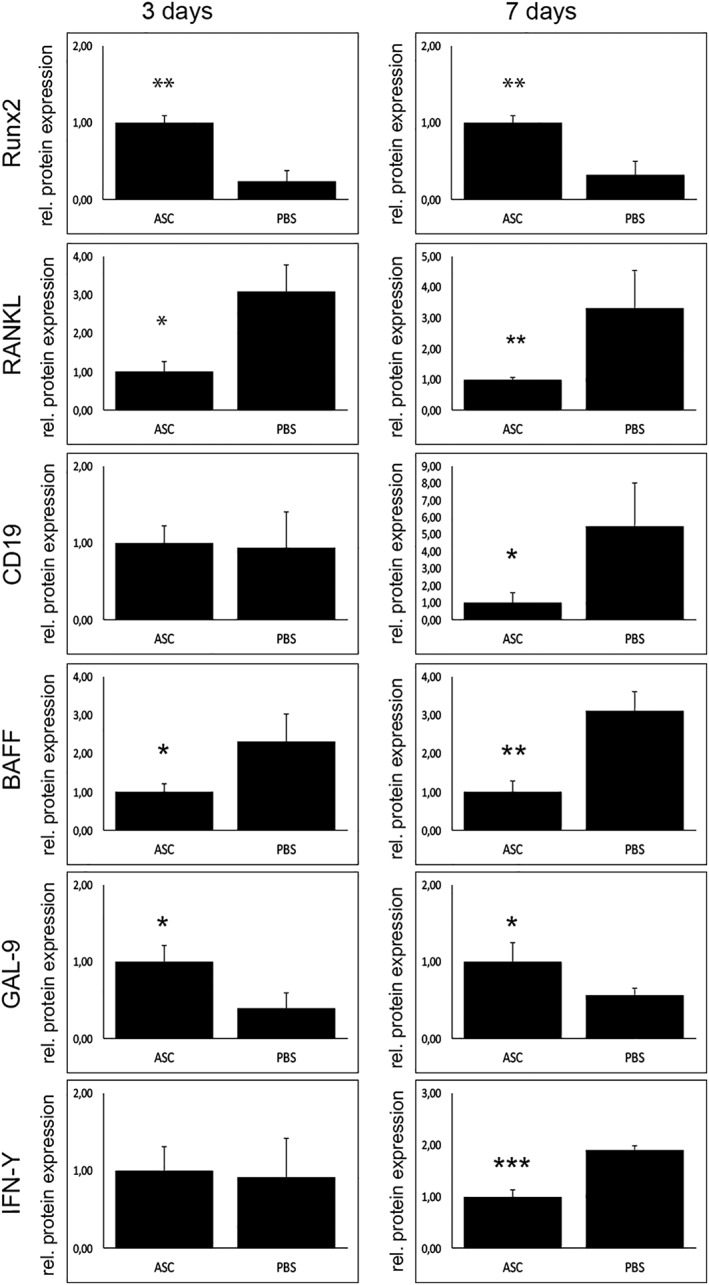
B‐cell depletion is mediated via galectin‐9 (GAL9) and B‐cell activating factor (BAFF). Western blots of markers for osteoblastogenesis, osteoclast, and B‐cell activity. In accordance to immunoflourescent stainings, protein levels of runt‐related transcription factor 2 could be raised by stem cell application. Decreased osteoclast activity seems to be downregulated in a receptor activator of NF‐κB ligand‐dependent manner as levels were significantly reduced in stem cell group. Investigating B‐cells, CD19 was downregulated in ASC group 7 days after surgery. Subsequently, GAL9 known to inhibit B‐cells is upregulated and moreover, BAFF is downregulated after stem cell application. Interestingly, interferon‐γ is decreased 7 days after surgery in stem cell group (nine animals/group). *, p < .05; **, p < .01; ***, p < 0.001.
Stem Cells Could Decrease Bone Resorption in a RANKL‐Dependent Manner
As already shown, osteoclast activity is markedly enhanced during postinfectious, inflammatory state of osteomyelitis. Importantly, applicated ASCs were capable to decrease osteoclast activity, seen in TRAP staining (Fig. 1), and thereby bone resorption. Accordingly, RANKL, essential for osteoclast function, was significantly enhanced in Western blot (Fig. 3).
ASCs Show Immunomodulation of the Adaptive Immune System
Besides studying the osteogenic potential of ASCs in this special setting, a closer look on the immune system was one of the main goals of this work. Detecting an elevated B‐cell activity in our previous work, a FACS analysis revealed the depletion of B‐cells in stem cell treated group (Fig. 4). Interestingly, this effect could be observed only 7 days after treatment. Accordingly, Western blot levels of BAFF were diminished and GAL9 elevated (Fig. 3). Interestingly, cell numbers of T‐cells and granulocytes were not affected by ASC treatment (data not shown).
Figure 4.
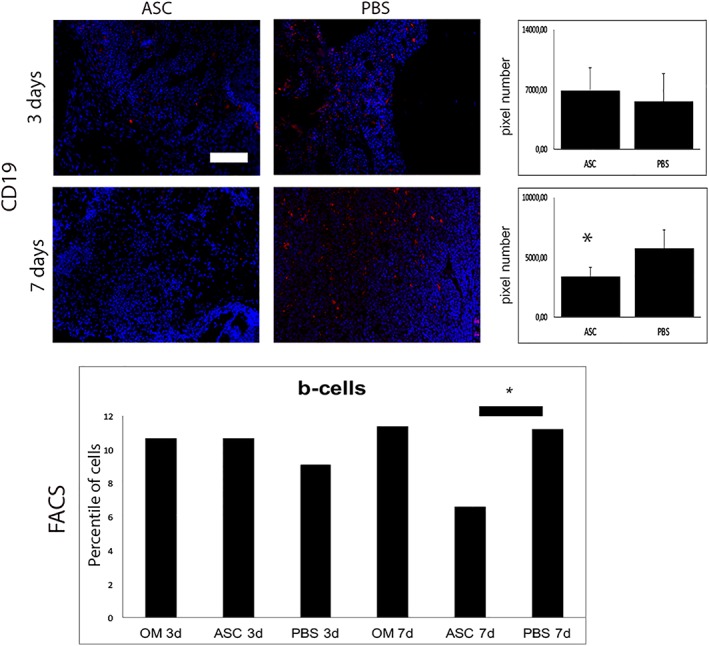
B‐cells were decreased 7 days after surgery by stem cell application. Immunofluorescent stainings with primary antibody against CD19 and FACS analysis of B‐cells. In accordance to Western blot results, immunoflourescent stainings and FACS analysis revealed B‐cell depletion by stem cell application 7 days after surgery (six animals/group). Scale bar represents 100 μm; *, p < .05.
Discussion
Decreased bone regeneration due to inflammatory processes, especially after bone infections is a common problem of orthopedic surgery 14. In our previous work, we have demonstrated decreased bone regeneration after debridement following bacterial infection as a result of a dysregulated inflammatory reaction (3 and 4). In the current study, we sought to investigate the ability of ASCs to restore the regenerative capacity of postinfectious bone defects. In addition, our investigations focused on the immunomodulatory properties of MSCs.
Interestingly, ASC application leads to dramatically enhanced bone formation after debridement of osteomyelitic bone. Accordingly, proliferation and differentiation of osteoblasts as well as angiogenesis was markedly enhanced.
In this context, many studies have already demonstrated the potential of MSCs for osteogenic differentiation 15, 16, 17. Moreover, ASCs have the capacity to differentiate into different types of tissue including cartilage, bone, muscle, vessels, and adipose tissue 10, 15.
Interestingly, our findings indicated the potential of ASCs to modulate osteoclast activity and thereby diminish bone resorption. Accordingly, stem cells could modulate RANKL/osteoprotegerin (OPG)‐axis, which was dysregulated due to bone infection. Consistent to our findings, MSCs are known to decrease cell number of osteoclasts via modulation of RANKL/OPG‐axis 18. In a murine psoriasis model Th17 cells enhanced osteoclasts in a RANKL‐dependent manner, whereas MSC application could downregulate osteoclasts via OPG production 18. Moreover, stem cell‐based therapy seemed to be promising to reduce bone resorption in rheumatoid arthritis 19.
Besides regenerative abilities, emerging data indicate immunomodulatory properties of MSCs 20. For instance, BMSCs showed the capacity to reduce the inflammatory response and developing fibrosis in a murine lung injury model, secreting interleukin (IL)‐1 receptor antagonist (IL‐1ra) 21. Furthermore, BMSC infusion in a murine myocardial infarct model led to a significant increase of anti‐inflammatory protein Tsg‐6 22. In this context, TSG‐6 application as well as human BMSCs have been shown to reduce secretion of tumor necrosis factor α (TNF‐α) and IL‐1α during inflammatory state in a peritonitis model. Further beneficial effects of BMSCs were evident in a sepsis model reducing the expression of TNF‐α and IL‐6 23. Accordingly, ASCs could also modulate the immune response of arthritic Dilute Brown Non‐Agouti/1 mice. Here, ASC treatment resulted in a decreased incidence and severity of arthritis accompanied by a significant decrease in inflammatory cytokines such as TNF‐α, Il‐1β, and IFN‐γ. In an experimental colitis and sepsis model, ASCs turned out to have a protective and anti‐inflammatory effect via secretion of IL‐10 24. Investigations concerning cystic fibrosis revealed the ability of MSCs battling bacterial infections via secretion of antimicrobial agents 25. In this context, the immunomodulatory and antibacterial abilities of MSCs could be used as a new and innovative approach treating bacterial infections and autoimmune disorders like graft versus host disease 26. More specifically, ASCs showed beneficial effects preventing and treating osteomyelitis 27 and could restore bone regeneration of osteonecrosis in maxillofacial surgery 28.
Accordingly, our data could demonstrate the modulation of the innate immune system via stem cell application, regulating B‐cells during late fracture healing. BAFF and GAL9 levels seemed to have a regulatory function in this context.
B‐cell function is essential for bone remodeling and osteoclast function. Activated B‐cells secrete RANKL and thereby increase osteoclast activity 29. However, the physiological balance of bone formation and resorption can be shifted toward bone erosion by increased B‐cell activity 29. In inflammatory musculoskeletal diseases like rheumatoid arthritis, B‐cells have a major impact on focal bone erosion 30, 31, 32. Supporting this hypothesis, B‐cell depleting therapy seems quite effective suppressing bone erosions and synovitis in rheumatoid arthritis 31. Moreover, B‐cells seem to be involved in periodontitic bone loss 33.
Recent studies elucidated a downregulation of excessive B‐cell proliferation via MSCs. Potential mechanism responsible for this is a cell contact based inhibition mediated by GAL9 34, 35. BAFF is indispensable for B‐cell maturation and enhances B‐cell survival 36. In terms of B‐cell overactivity, MSCs showed the potential to affect B‐cells via BAFF regulation 34.
Conclusion
Our investigations show how ASCs could overcome the impairment of bone regeneration after osteomyelitis in a murine animal model. Interestingly, bone formation and osteoblastogenesis could be elevated and moreover osteoclastogenesis decreased in a RANKL‐dependent manner. Besides that, immunomodulatory effects of applicated ASCs on the innate immune system were evident in a B‐cell depletion, mediated by GAL9 and BAFF.
Author Contributions
J.M.W.: conception and design, collection of data, data analysis and interpretation, manuscript writing, final approval of manuscript; F.R.: collection of data, data analysis and interpretation, manuscript writing, final approval of manuscript; C.W., M.D., J.H.: data analysis and interpretation, final approval of manuscript; S.V.S., M.D., H.J., M.B., V.D., K.B., N.R.: collection of data, data analysis and interpretation, final approval of manuscript; S.D.: collection of data, final approval of manuscript; M.L.: administrative support, final approval of manuscript; B.B.: conception and design, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgment
This work was supported by a grant of Deutsche Forschungsgemeinschaft (DFG; BE 4169/8‐1).
Data Availability Statement:The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Lew DP, Waldvogel FA. Osteomyelitis. Lancet 2004;364:369–379. [DOI] [PubMed] [Google Scholar]
- 2. Costerton JW. Biofilm theory can guide the treatment of device‐related orthopaedic infections. Clin Orthop Relat Res 2006;437:7–11. [DOI] [PubMed] [Google Scholar]
- 3. Wagner JM, Zollner H, Wallner C et al. Surgical debridement is superior to sole antibiotic therapy in a novel murine posttraumatic osteomyelitis model. PLoS One 2016;11:e0149389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagner JM, Jaurich H, Wallner C et al. Diminished bone regeneration after debridement of posttraumatic osteomyelitis is accompanied by altered cytokine levels, elevated B cell activity and increased osteoclast activity. J Orthop Res 2017;35:2425–2434. [DOI] [PubMed] [Google Scholar]
- 5. Claro T, Widaa A, O'Seaghdha M et al. Staphylococcus aureus protein A binds to osteoblasts and triggers signals that weaken bone in osteomyelitis. PLoS One 2011;6:e18748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dapunt U, Maurer S, Giese T et al. The macrophage inflammatory proteins MIP1alpha (CCL3) and MIP2alpha (CXCL2) in implant‐associated osteomyelitis: Linking inflammation to bone degradation. Mediators Inflamm 2014;2014:728619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi S, Zhang X. Interaction of Staphylococcus aureus with osteoblasts (review). Exp Ther Med 2012;3:367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Widaa A, Claro T, Foster TJ et al. Staphylococcus aureus protein A plays a critical role in mediating bone destruction and bone loss in osteomyelitis. PLoS One 2012;7:e40586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Josse J, Velard F, Gangloff SC. Staphylococcus aureus vs. osteoblast: Relationship and consequences in osteomyelitis. Front Cell Infect Microbiol 2015;5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Behr B, Ko SH, Wong VW et al. Stem cells. Plast Reconstr Surg 2010;126:1163–1171. [DOI] [PubMed] [Google Scholar]
- 11. Gimble JM. Adipose tissue‐derived therapeutics. Expert Opin Biol Ther 2003;3:705–713. [DOI] [PubMed] [Google Scholar]
- 12. Lin K, Matsubara Y, Masuda Y et al. Characterization of adipose tissue‐derived cells isolated with the Celution system. Cytotherapy 2008;10:417–426. [DOI] [PubMed] [Google Scholar]
- 13. Behr B, Leucht P, Longaker MT et al. Fgf‐9 is required for angiogenesis and osteogenesis in long bone repair. PNAS 2010;107:11853–11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt‐Rohlfing B, Lemmen SW, Pfeifer R et al. Osteomyelitis in adults. Diagnostic principles and therapeutic strategies. Unfallchirurg 2012;115:55–66. [DOI] [PubMed] [Google Scholar]
- 15. Zuk PA, Zhu M, Mizuno H et al. Multilineage cells from human adipose tissue: Implications for cell‐based therapies. Tissue Eng 2001;7:211–228. [DOI] [PubMed] [Google Scholar]
- 16. Behr B, Tang C, Germann G et al. Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose‐derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells 2011;29:286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cowan CM, Shi Y‐Y, Aalami OO et al. Adipose‐derived adult stromal cells heal critical‐size mouse calvarial defects. Nat Biotechnol 2004;22:560–567. [DOI] [PubMed] [Google Scholar]
- 18. Cho K‐A, Park M, Kim Y‐H et al. Mesenchymal stem cells inhibit RANK‐RANKL interactions between osteoclasts and Th17 cells via osteoprotegerin activity. Oncotarget 2017;8:83419–83431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka Y. Human mesenchymal stem cells as a tool for joint repair in rheumatoid arthritis. Clin Exp Rheumatol 2015;33:S58–S62. [PubMed] [Google Scholar]
- 20. Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol Ther 2012;20:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ortiz LA, Dutreil M, Fattman C et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 2007;104:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee RH, Pulin AA, Seo MJ et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti‐inflammatory protein TSG‐6. Cell Stem Cell 2009;5:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nemeth K, Leelahavanichkul A, Yuen PST et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)‐dependent reprogramming of host macrophages to increase their interleukin‐10 production. Nat Med 2009;15:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson P, Souza‐Moreira L, Morell M et al. Adipose‐derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 2013;62:1131–1141. [DOI] [PubMed] [Google Scholar]
- 25. Sutton MT, Fletcher D, Ghosh SK et al. Antimicrobial properties of mesenchymal stem cells: Therapeutic potential for cystic fibrosis infection, and treatment. Stem Cells Int 2016;2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mezey E, Nemeth K. Mesenchymal stem cells and infectious diseases: Smarter than drugs. Immunol Lett 2015;168:208–214. [DOI] [PubMed] [Google Scholar]
- 27. Mohiti‐Asli M, Molina C, Diteepeng T et al. Evaluation of silver ion‐releasing scaffolds in a 3D coculture system of MRSA and human adipose‐derived stem cells for their potential use in treatment or prevention of osteomyelitis. Tissue Eng Part A 2016;22:1258–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Handschel J, Meyer U. Infection, vascularization, remodelling—Are stem cells the answers for bone diseases of the jaws? Head Face Med 2011;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horowitz M, Fretz J, Lorenzo J. How B cells influence bone biology in health and disease. Bone 2010;47:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meednu N, Zhang H, Owen T et al. Production of RANKL by memory B cells: A link between B cells and bone erosion in rheumatoid arthritis. Arthritis Rheumatol 2016;68:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McQueen FM, Issa S. Is rheumatoid arthritis a B‐cell haematological disease with a predilection for the joints? Following the B cell thread to its logical conclusion. Med Hypotheses 2014;82:266–270. [DOI] [PubMed] [Google Scholar]
- 32. Weitzmann MN. T‐cells and B‐cells in osteoporosis. Curr Opin Endocrinol Diabetes Obes 2014;21:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hienz SA, Paliwal S, Ivanovski S. Mechanisms of bone resorption in periodontitis. J Immunol Res 2015;2015:615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fan L, Hu C, Chen J et al. Interaction between mesenchymal stem cells and B‐cells. Int J Mol Sci 2016;17:650–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ungerer C, Quade‐Lyssy P, Radeke HH et al. Galectin‐9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev 2014;23:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mackay F, Browning JL. BAFF: A fundamental survival factor for B cells. Nat Rev Immunol 2002;2:465–475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


