Abstract
Branched-chain amino acid (BCAAs: leucine, isoleucine, and valine) contribute to the development of obesity-associated insulin resistance in the context of consumption of a high-fat diet (HFD) in humans and rodents. Maternal diet is a major determinant of offspring health, and there is strong evidence that maternal HFD alters hypothalamic developmental programming and disrupts offspring energy homeostasis in rodents. In this study, we exposed pregnant and lactating C57BL/6JB female mice to either HFD, HFD with supplemented BCAA (HFD+BCAA), or standard diet (SC), and we studied offspring metabolic phenotypes. Both maternal HFD and HFD supplemented with BCAA had similar effect rendering the offspring metabolic imbalance and impairing their ability to cope with HFD when challenged during aging. The metabolic effects of HFD challenge were more profound in females, worsening female offspring ability to cope with an HFD challenge by activating hypothalamic inflammation in aging. Moreover, the sex differences in hypothalamic estrogen receptor α (ER-α) expression levels were lost in female offspring upon HFD challenge, supporting a link between ER-α levels and hypothalamic inflammation in offspring and highlighting the programming potential of hypothalamic inflammatory responses and maternal nutrition.
Keywords: BCAA, hypothalamic inflammation, maternal nutrition, metabolic reprogramming
INTRODUCTION
Imbalanced maternal nutrition during pregnancy is now recognized as a major determinant of adult health, and late-onset metabolic syndrome in the offspring (21). Despite this well-established association between poor maternal dietary choices and detrimental long-term consequences for the health of the offspring, there is no consensus on nutritional components in maternal diet that mostly contribute to these phenotypes (35), and the mechanisms underlying this association are poorly defined.
The Western-type diet, defined as an overconsumption of animal protein, is overabundant in branched-chain amino acids (BCAA), leucine (Leu), isoleucine (Ile), and valine (Val), which must be obtained exogenously via dietary intake (19). Human and animal studies performed on male rats demonstrated that a high intake of BCAA, when associated with high-fat intake, results in insulin resistance and glucose intolerance, leading to metabolic syndrome (9, 18). Similarly, high levels of BCAA in maternal breast milk are associated with a higher risk of insulin resistance in children from obese women (34). Thus, increased levels of BCAA concentration may potentially predict the long-term risk of developing a metabolic imbalance.
The mechanisms linking maternal overnutrition to metabolic syndrome in offspring are complex. However, obesity has been strongly associated with systemic low-grade inflammation, which is implicated in numerous disease processes (28). Moreover, there is strong evidence linking inflammatory responses due to maternal diet or obesity to impaired fetal brain development (31, 36). Activation of the systemic maternal immune system due to overnutrition increases cytokine expression within the offspring brain at birth and results in long-term changes in neuroimmune function in different brain regions that are associated with memory and behavior.
In the hypothalamus, the inflammatory state triggered by diet-induced obesity (DIO) disrupts its ability to sense metabolic abnormalities, affecting energy balance and glucose metabolism, leading to obesity and insulin resistance (29). Previous studies demonstrated that DIO stimulates accumulation of reactive, proinflammatory microglia and astrocytes in the hypothalamus of rodents, in a sexually dimorphic manner, affecting male, but not female, mice from the onset of high-fat diet (HFD) feeding (6). Similarly, old male mice exhibit stronger age-associated hypothalamic gliosis than old females (25), suggesting the role of the hypothalamus in the sex differences in susceptibility to age-associated metabolic dysregulation.
In this study, we aimed at exploring the role of supplementation of a HFD with BCAA in maternal nutrition, similar to a Western-type diet, on metabolic parameters in offspring and to determine the consequences of late-life HFD challenge on sex- and age-dependent differences in hypothalamic gliosis and metabolic regulation.
METHODS
Composition of diets.
Females were randomly placed on one of three customized diets. Composition of all custom-formulated diets (Research Diets, New Brunswick, NJ): HFD, cat. no. D06011802 (45% of kcal fat, 19% of kcal protein, and 35% of kcal carbohydrate), HFD+BCAA, cat. no. D06050807 (43% of kcal fat, 23% of kcal protein, and 34% of kcal carbohydrate), and control standard diet (SC), cat. no. D07010502 diet (10% of kcal fat, 19% of kcal protein, and 71% of kcal carbohydrate) was used as detailed previously (18). As previously stated, in the HFD+BCAA group, the increase in bulk amino acids was obtained by increasing leucine, isoleucine, and valine by 150% relative to the HFD (18). The complete nutritional profiles of each diet are available online (https://www.researchdiets.com).
Animals.
Females were fed ad libitum throughout pregnancy and lactation. Dams were singly housed once visibly pregnant and were allowed to give birth naturally. At the age of 28 days, when nursing is finished in mice, pups were weaned onto a control diet. We used litters from at least 4 or 5 dams for each experiment. At the age of 12-mo, mice were fed a HFD cat. no. D06011802 for the period of 2 wk. All procedures and experiments were carried out in accordance with the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Miami Institutional Animal Care and Use Committee. Most of the data presented here relate to both male and female mice unless otherwise stated.
Metabolic analysis.
Intraperitoneal glucose tolerance tests were performed on mice fasted for 16 h overnight. Blood glucose levels were measured on randomly fed or overnight-fasted animals in mouse-tail blood using Glucometer Elite (Bayer), and serum samples were collected for the insulin measurements. Animals were then injected intraperitoneally with d-glucose (2 g/kg), and blood glucose levels were measured (27). For insulin tolerance tests, mice fasted for a 4-h period in the light cycle before intraperitoneal injections of insulin (Humulin R; 0.8 U/kg) diluted in sterile saline. Blood glucose concentrations were measured at the indicated time points. Blood insulin and leptin levels were determined on serum from tail vein bleed using a mouse insulin ELISA kit and mouse leptin ELISA kit (Crystal Chem, Elk Grove Village, IL). Lean and fat body mass was assessed by dual-energy X-ray absorptiometry (DEXA) (GE Lunar, Madison, WI).
Perfusion and immunolabeling.
Mice were anesthetized with an overdose of intraperitoneal pentobarbital sodium and transcardially perfused with phosphate-buffered saline (PBS; pH 7.5) followed by 4% paraformaldehyde. Brains were postfixed, dehydrated, and then sectioned coronally (30 µm) using a sliding microtome followed by immunofluorescent analysis, as previously described (20). Brain sections were washed with PBS several times, blocked for 2 h in 0.3% Triton X-100 with 3% normal donkey serum in PBS, and then stained with the following primary antibodies overnight: rabbit anti-glial fibrillary acidic protein (GFAP; 1:1,000; Millipore, Temecula, CA), mouse anti-TNF-α (1:100; Abcam, Cambridge, MA), and rabbit anti-ionized calcium-binding adapter molecule (1Iba1; 1:1,000; Wako, Richmond, VA). For rabbit anti-ER-α (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) immunostaining, sections were pretreated for 20 min in 0.5% NaOH and 0.5% H2O2 in PBS, followed by immersion in 0.3% glycine for 10 min. Sections were then placed in 0.03% SDS for 10 min and placed in 4% normal serum plus 0.4% Triton X-100 plus 1% BSA for 20 min and then stained with primary antibodies overnight. All floating brain sections were washed with PBS several times and incubated with Alexa Fluor-conjugated secondary antibodies for 2 h (Invitrogen, Carlsbad, CA), as previously published (4, 17, 26, 32, 33). Sections were mounted onto Superfrost Plus slides (Fisher Scientific, Hudson, NH) and coverslipped with ProLong Antifade mounting medium (Invitrogen, Carlsbad, CA). For the specificity of the staining, the immunohistochemical experiments were also performed with brain sections in which the primary antibody was omitted and substituted with serum. Microscopic images were obtained using a Nikon 800 fluorescent microscope equipped with Nikon imaging DS-R12 color-cooled SCMOS, version 5.00.
Quantification analysis.
For quantification of immunoreactive positive cells, pictures of matched brain areas were taken from at least three sections containing the arcuate nucleus of the hypothalamus (ARC) for each brain between bregma −1.58 mm to −1.94 mm (according to the Franklin mouse brain atlas). Serial brain sections across the mediobasal hypothalamus (MBH) were made at 30 μm thickness, and every five sections were represented by one section with staining and cell counting. All sections were arranged from rostral to caudal to examine the distribution of labeled cells. The images were quantified with ImageJ, using the function spot to count nuclei and surface to measure the area of the different objects. The number of positive cells was presented as means ± SE.
Statistical analysis.
Data sets were analyzed for statistical significance using Statistica software (version 10). An ANOVA with repeated measures was used to analyze glucose tolerance test (GTT) and insulin tolerance test (ITT) test, and other parameters were analyzed by two-way ANOVA, followed by Newman-Keuls test. Additionally, an analysis for a two-tail unpaired Student’s t-test was used when two groups were compared. The level of significance (α) was set at 5%.
RESULTS
Maternal diets high in fat and BCAA generate obesity and insulin resistance in male offspring.
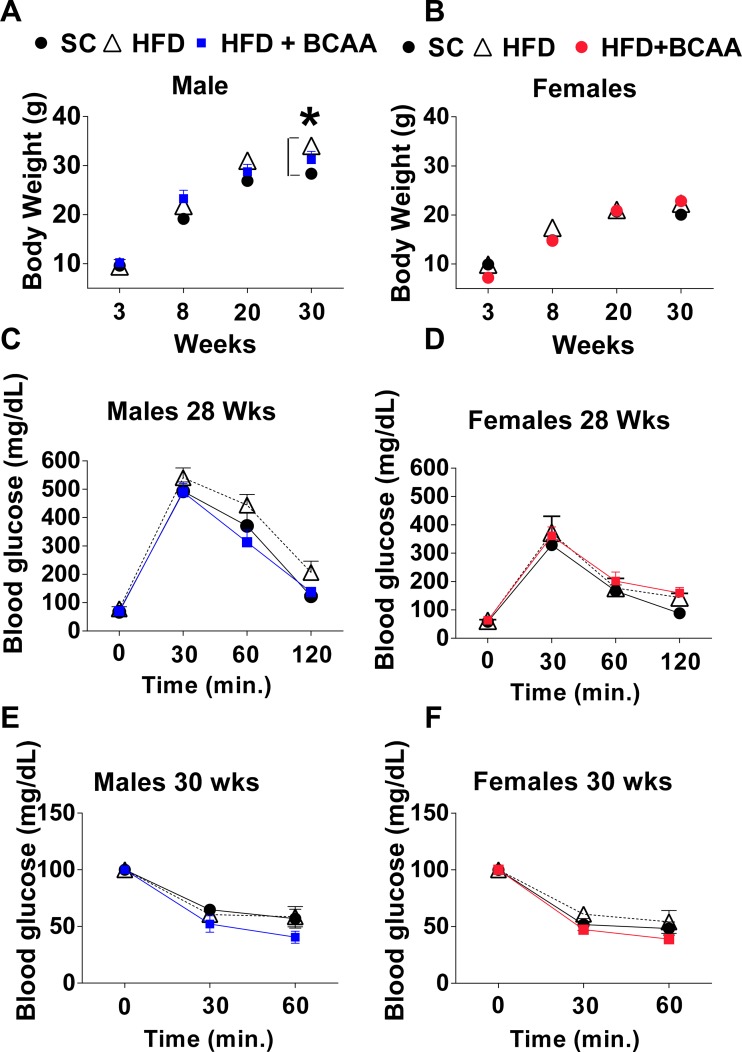
Maternal HFD feeding or supplementation of HFD with BCAA did not significantly affect male and female offspring body mass at weaning; however, differences in male offspring body weight appeared at 30 wk (ANOVA, P < 0.01 control vs. HFD and HFD+BCAA) (Fig. 1A). Female offspring body weight was not affected by maternal nutrition, and by 30 wk of age, it was similar among all the groups (Fig. 1B). Both male and female offspring glucose tolerance was not significantly affected by maternal nutrition at the age of 28 wk (Fig. 1, C and D). By this age, no significant differences in insulin tolerance appeared in male and female offspring of HFD or HFD+BCAA dams, as demonstrated by the insulin tolerance test (Fig. 1, E and F).
Fig. 1.
Offspring metabolic parameters on the control diet. Body mass of male (A) and female (B) offspring. Glucose tolerance tests (GTTs) of 28-wk-old male (C) and female (D) offspring. Insulin tolerance tests (ITTs) of 30-wk-old male (E) and female (F) offspring (n = 6/group for males, n = 6–8/group for females). Data are expressed as means ± SE. *Significant effect (P < 0.05) of maternal diet [control diet vs. high-fat diet (HFD) or vs. HFD+ branched-chain amino acid (BCAA)], as assessed with ANOVA with repeated measures, followed by Newman-Keuls test.
Maternal Western-diet predisposes to a metabolic imbalance in HFD-fed aged mice.
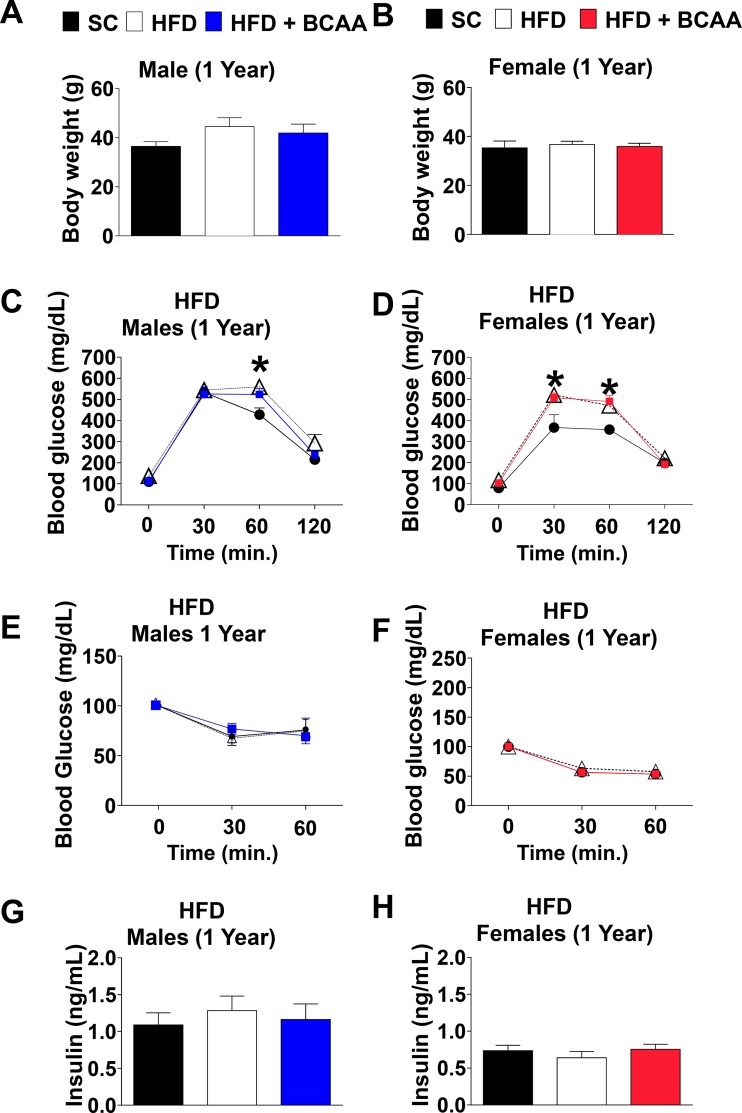
Two weeks of HFD challenge of aged 12-mo-old male mice slightly, but not significantly, increased body mass in HFD and HFD+BCAA groups (Fig. 2A). On the other hand, short-term HFD challenge did not magnify age-associated weight gain among female offspring (Fig. 2B). HFD challenge of aged mice significantly impaired glucose tolerance in both male and female offspring of HFD and HFD+BCAA dams (Fig. 2, C and D). This effect was more profound in female offspring (ANOVA, P < 0.001 control vs. HFD and HFD+BCAA) (Fig. 2D), suggesting that maternal nutrition contributes to age-associated hyperglycemia triggered by HFD in old age. While insulin tolerance in aged male offspring of HFD or HFD+BCAA dams was not further exacerbated by HFD challenge (Fig. 2E), only female offspring of HFD dams demonstrated resistance to insulin tolerance test upon HFD challenge. Insulin responses between aged female offspring of SC and HFD+BCAA dams were comparable after HFD challenge (Fig. 2F). Fasted insulin levels were unchanged in both male and female offspring (Fig. 1, G and H).
Fig. 2.
Aged offspring metabolic parameters challenged with high-fat diet (HFD). Body mass of 12-mo-old male (A) and female (B) offspring challenged with HFD for the period of 2 wk. Glucose tolerance tests (GTT) of 12-mo-old male (C) and female (D) offspring challenged with HFD for the period of 2 wk. Insulin tolerance tests (ITTs) of 12-mo-old male (E) and female (F) offspring challenged with HFD for the period of 2 wk. Fasted insulin levels of 12-mo-old male (G) and female (H) offspring challenged with HFD for the period of 2 wk. Data are presented as means ± SE; n = 6/group for males, and n = 6–8/group for females. *Significant effect (P < 0.05) of maternal diet [control diet vs. HFD or vs. HFD+ branched-chain amino acid (BCAA)], as assessed by one-way ANOVA with repeated measures for the glucose tolerance test (GTT) and ITT, followed by Newman-Keuls test.
Maternal Western diet predisposes to hypothalamic inflammation in HFD-fed aged female mice.
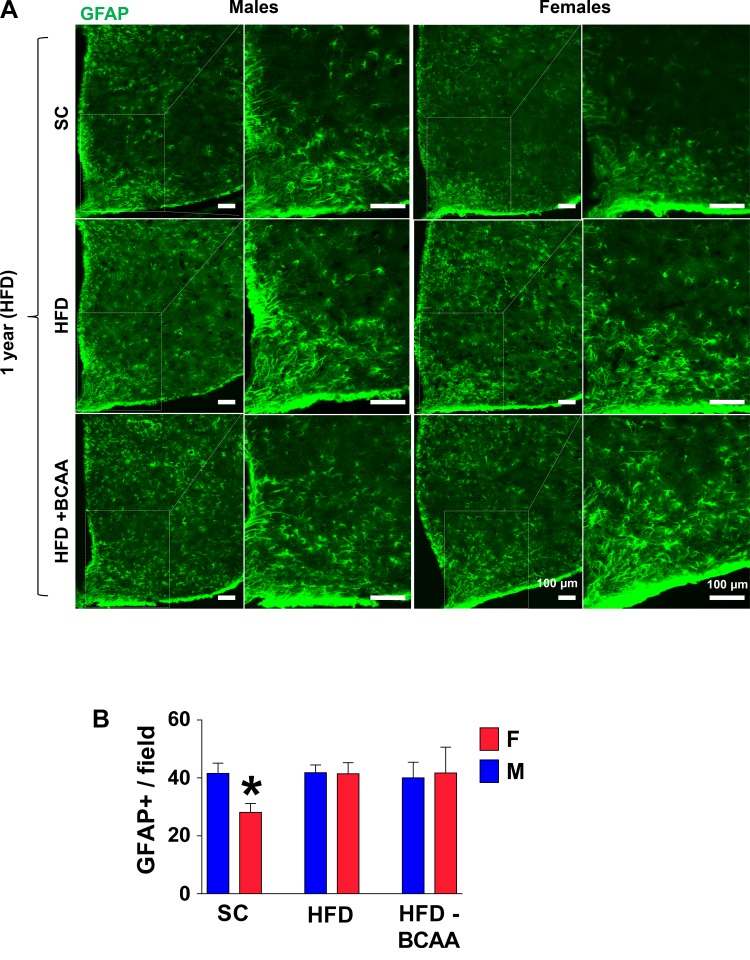
The hypothalamus is the control center sensitive to nutrient changes and metabolic imbalance and is a major mediator of the process of aging of the entire body (33). The increased neuroinflammatory reaction is frequently observed in the hypothalamus during normal brain aging (25). To assess the effect of age and diet on hypothalamic inflammation in offspring of HFD and HFD+BCAA dams, we examined hypothalamic inflammatory responses in aged 12-mo-old offspring challenged with HFD for 2 wk. Astrogliosis with advancing age or with HFD feeding is correlated with increased expression of the GFAP (11, 30). We have previously demonstrated that the number of GFAP-positive astrocytes in aged hypothalamus is higher in male than in female mice (25); moreover, diet-induced obesity is associated with hypothalamic gliosis in male, but not in female, mice (15, 16). Consistent with this, HFD-fed 12-mo-old SC females demonstrate a lower number of immunostained GFAP+ astrocytes in the MBH compared to SC male mice (P = 0.02) (Fig. 3, A and B). Interestingly, aged female offspring of HFD or of HFD+BCAA dams demonstrated similar numbers of GFAP+ astrocytes as in male offspring (Fig. 3, A and B), suggesting that maternal diet completely eliminated the protective sex effect associated with diet-induced hypothalamic inflammation.
Fig. 3.
Hypothalamic astrogliosis in aged high-fat diet (HFD)-fed offspring. A: representative images of astrocytes identified by immunofluorescent detection of glial fibrillary acidic protein (GFAP) protein in coronal sections of hypothalamus obtained from 12-mo-old male and female offspring of HFD or HFD+BCAA dams. Scale bar: 100 µm. B: quantification of GFAP-staining intensity (error bars reflect means ± SE) in the arcuate nucleus of the hypothalamus (ARC) from male and female offspring (n = 6/group). *P < 0.001 control vs. HFD and HFD+BCAA), as assessed by two-way ANOVA followed by Newman-Keuls test. BCAA, branched-chain amino acid.
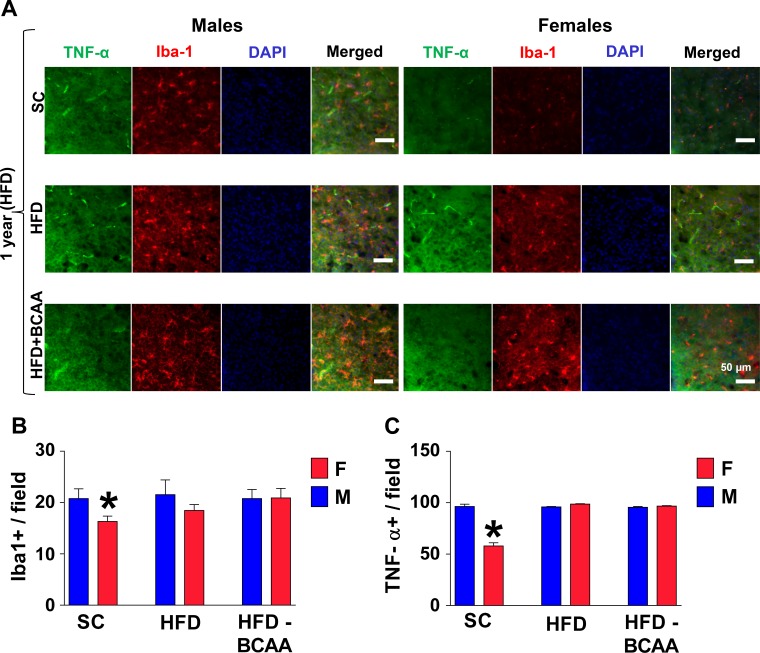
Next, we evaluated the numbers of microglia in the hypothalamus of aged HFD-challenged offspring mice. Using immunostaining for the microglia-specific Iba1 marker, we found that in the SC group, the numbers of microglial cells in the MBH are about 20% lower in aged HFD-fed female mice, as compared to HFD-fed male mice (P < 0.004) (Fig. 4, A and B). The proportion of Iba1+ microglia that produce tumor necrosis factor-α (TNF-α), as an indication of their inflammatory status was significantly reduced (P < 0.0001) in HFD-fed aged SC females compared to age-matched SC males (Fig. 4, A and C). On the other hand, maternal HFD or HFD+BCAA nutrition predisposed aged female offspring to HFD-induced microgliosis to levels similar to male mice. Together, our data, thus, document long-term hypothalamic changes as a result of maternal nutrition, changes that worsen specifically the female offspring’s ability to cope with an HFD challenge.
Fig. 4.
Hypothalamic microgliosis in aged high-fat diet (HFD)-fed offspring. Brain sections of 12-mo-old male and female offspring challenged with HFD for 2 wk were analyzed for hypothalamic microglia and TNF-α. A: images of immunostaining in the arcuate nucleus of the hypothalamus (ARC). Scale bars: 50 µm. B: numbers of cells immunoreactive for ionized calcium-binding adapter molecule 1 (Iba-1), or TNF-α (C) in the ARC (across the confocal microscopic field of serial sections) from male and female offspring of standard diet (SC), HFD, or HFD+BCAA dams (n = 6/group). Error bars reflect means ± SE. *P < 0.01 vs. SC for Iba-1, and P < 0.0001 for TNF-α, as assessed by two-way ANOVA followed by Newman-Keuls test. BCAA, branched-chain amino acid.
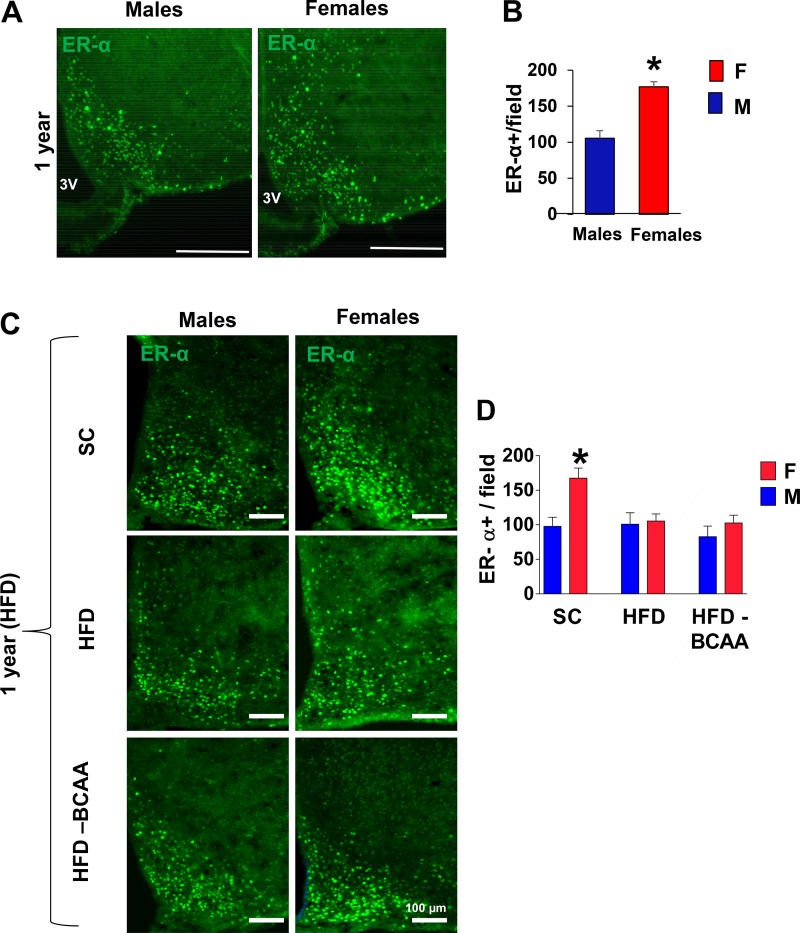
Maternal Western-diet eliminates the protective effect of ER-α on hypothalamic inflammation in HFD-fed aged female mice.
Estrogen receptor α (ER-α) protects female mice from the metabolic complications of diet-induced hypothalamic inflammation (16), while decreased ER-α levels can facilitate inflammatory responses and are significantly reduced with advanced age (8). The levels of ER-α expression appear to be sexually dimorphic, with higher expression of ER-α in aged SC-fed females than in males (P < 0.01) (Fig. 5, A and B). In accord with previous studies, ER-α expression was significantly lower in the ARC of aged SC male offspring, but not SC female offspring, following HFD exposure (Fig. 5, C and D). However, the sex differences in hypothalamic ER-α expression levels upon HFD challenge were lost in female offspring of HFD or HFD+BCAA dams and were comparable to HFD-fed male mice (Fig. 5, C and D). These findings further confirm a correlation between decreased ER-α and higher hypothalamic inflammation observed in these animals.
Fig. 5.
Estrogen receptor α (ERα) immunoreactivity in aged high-fat diet (HFD)-fed offspring. A: representative images of ER-α immunoreactivity in the mediobasal hypothalamus (MBH) subregion of 12-mo-old male and female mice. Scale bars: 100 µm. B: numbers of cells immunoreactive for ER-α in the arcuate nucleus of the hypothalamus (ARC) from 12-mo-old male and female mice (Student’s t-test). C: representative images of ER-α immunoreactivity in the ARC of 12-mo-old male and female offspring challenged with HFD for 2 wk. D: numbers of cells immunoreactive for ER-α in the ARC from male and female offspring of standard diet (SC), HFD, or HFD+BCAA dams (n = 6/group), error bars reflect means ± SE. *P < 0.01 vs. SC, as assessed by two-way ANOVA followed by Newman-Keuls test. BCAA, branched-chain amino acid.
DISCUSSION
These data are the first to document that metabolic programming in offspring exposed to maternal HFD enriched with BCAA mostly resemble offspring born to mothers solely fed HFD and demonstrate the potent sex-biased programming role of maternal nutrition in hypothalamic inflammation triggered by HFD-induced insulin resistance in aging. Maternal HFD nutrition alone or enriched with BCAA impairs offspring glycemic control at adulthood and worsens the ability of females to cope with HFD challenge by activating hypothalamic inflammation in aging.
The current study is the first to directly test the role of BCAA in the maternal diet on the metabolic and neuroinflammatory outcomes in adult or aged offspring. Increased levels of BCAA are associated with insulin resistance in both human and rodents (9, 18), while reduced BCAA diet modestly improves glucose tolerance and slows fat mass gain (5). The mature breast milk of obese mothers contained more BCAA than breast milk of lean mothers, suggesting that altered breast milk profile could affect metabolic risk in the breast-fed child (13). Indeed, increased plasma BCAA concentrations are found in obese children, with a positive correlation with body mass index (BMI) (7). Moreover, in adult rats, supplementation with BCAA, combined with HFD, was shown to be associated with a rise in plasma BCAA concentration (18). Other studies, however, did not find a correlation between dietary BCAA intake and plasma BCAA concentrations in adult humans (3, 22). While little is known about the effect of maternal BCAA intake on children health, we demonstrate that supplementation of BCAA in maternal HFD does not contribute to a higher risk for metabolic alterations in adult offspring. On the contrary, BCAA supplementation in maternal diet prevented age- and diet-induced insulin intolerance in a sex-specific manner, protecting female offspring of HFD+BCAA dams. While studies show that hypothalamic inflammation can directly impact systemic insulin resistance (14), a more systematic evaluation of this finding will be required, on a tissue-specific level, before solid recommendations can be made.
Our data are broadly in accordance with previous work showing HFD is linked with microglial changes and central inflammation (2, 30). Males, when compared with female mice, have higher levels of inflammatory markers in the hypothalamus following exposure to HFD. The increase in markers of inflammation in male mice is possibly due to the reductions in proliferator-activated receptor γ coactivator 1α (PGC-1α) and estrogen receptor-α (ER-α), which is not found in female mice (16). Our current findings indicate that maternal HFD or HFD supplemented with BCAA, predisposes female offspring to hypothalamic inflammation when being challenged with HFD. Consistently, hypothalamic inflammation is induced and ER-α is reduced in female HFD-challenged offspring, thus suggesting the role of maternal nutrition during pregnancy in ER-α-mediated regulation of metabolic homeostasis and hypothalamic inflammation. In support, maternal HFD impairs weight, glucose tolerance, and inflammatory cytokines of female offspring lacking ER-α (24).
In our study, maternal HFD or HFD+BCAA nutrition did not massively affect offspring weight. Similarly, maternal HFD feeding does not universally induce alterations in offspring body mass at adulthood when feeding a chow diet (1, 12), suggesting that metabolic phenotype induced by maternal programming may remain concealed. Likewise, maternal HFD or HFD supplemented with BCAA did not alter glucose tolerance in both male and female offspring. However, both male and female offspring from HFD-fed dams, and male offspring from HFD+BCAA-fed dams were severely insulin intolerant, with elevated fasting blood glucose levels, indicating that maternal nutrition does induce insulin resistance in the peripheral organs involved in glucose clearance. An HFD challenge, imposed later in life, can unveil the programmed alterations (29). Indeed, maternal HFD or HFD+BCAA predisposed aged, 1-yr-old offspring of both sexes to hyperglycemia upon short HFD challenge, regardless of relatively comparable body weights. HFD-fed female offspring were more susceptible to metabolic imbalance, further emphasizing the potential role of reduced ER-α signaling in the hypothalamus when discussing the effects of maternal HFD on insulin and glucose homeostasis.
Sexual dimorphism is common in studies of maternal programming; however, the general direction of effects is still not clear. Here, we observed a more pronounced phenotype among female offspring, rendering them more susceptible to immune activation in the hypothalamus upon a HFD challenge even as aged adults. Our data are consistent with previously published results showing that male mice have higher levels of hypothalamic glia than females (23, 25). Similarly, a recent study demonstrated that throughout the adult life span, the typical female brain is more youthful, i.e., metabolically neotenous, than the male brain (10). These changes may reflect early developmental effects rather than the effects of aging and are consistent with the possibility of a threshold effect underlying female programming by maternal nutrition.
To our knowledge, this is the first direct demonstration that in the context of metabolic programming and maternal high-fat diet consumption, supplementation with BCAA does not make an independent contribution to the development of metabolic syndrome in offspring. Maternal poor nutrition worsens female offspring’s ability to cope with an HFD challenge by activating hypothalamic inflammation in aging. The underlying mechanisms seem to include the regulation of ER-α in the hypothalamus by maternal nutrition, the functional implications of which remain to be elucidated. Additional investigations in rodent models are needed to understand the long-term effects of BCAA supplementation and to clarify associations between prenatal nutritional BCAA supplementation in the maternal diet on brain developmental outcomes in both children and animal offspring.
GRANTS
This project was supported by National Institutes of Health Grant R01-DK-084236, R01-DK-073716, and Merit Review Award IBX002728A from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Program for E. Bernal-Mizrachi. Additional funding includes American Diabetes Association Grant 1-lB-IDF-063 and a Feasibility Grant from the Michigan Diabetes Research Center (P30DK020572) for M. Sadagurski.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S. and E.B.-M. conceived and designed research; M.S., L.D., J.P.W.-dC., A.A.A., and T.A.B. analyzed data; M.S., L.D., T.A.B., and E.B.-M. interpreted results of experiments; M.S. and L.D. prepared figures; M.S. drafted manuscript; M.S. and E.B.-M. edited and revised manuscript; M.S., L.D., J.P.W.-dC., A.A.A., T.A.B., and E.B.-M. approved final version of manuscript; L.D., J.P.W.-dC., A.A.A., and T.A.B. performed experiments.
ACKNOWLEDGMENTS
The authors thank Gillian Cady for technical assistance.
REFERENCES
- 1.Alfaradhi MZ, Kusinski LC, Fernandez-Twinn DS, Pantaleão LC, Carr SK, Ferland-McCollough D, Yeo GS, Bushell M, Ozanne SE. Maternal obesity in pregnancy developmentally programs adipose tissue inflammation in young, lean male mice offspring. Endocrinology 157: 4246–4256, 2016. doi: 10.1210/en.2016-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 24: 2104–2115, 2010. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 3.Boulet MM, Chevrier G, Grenier-Larouche T, Pelletier M, Nadeau M, Scarpa J, Prehn C, Marette A, Adamski J, Tchernof A. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am J Physiol Endocrinol Metab 309: E736–E746, 2015. doi: 10.1152/ajpendo.00231.2015. [DOI] [PubMed] [Google Scholar]
- 4.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7: 179–185, 2008. doi: 10.1016/j.cmet.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, Poudel C, Sherman DS, Yu D, Arriola Apelo SI, Cottrell SE, Geiger G, Barnes ME, Wisinski JA, Fenske RJ, Matkowskyj KA, Kimple ME, Alexander CM, Merrins MJ, Lamming DW. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol 596: 623–645, 2018. doi: 10.1113/JP275075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorfman MD, Krull JE, Douglass JD, Fasnacht R, Lara-Lince F, Meek TH, Shi X, Damian V, Nguyen HT, Matsen ME, Morton GJ, Thaler JP. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat Commun 8: 14556, 2017. doi: 10.1038/ncomms14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flores-Guerrero JL, Osté MCJ, Kieneker LM, Gruppen EG, Wolak-Dinsmore J, Otvos JD, Connelly MA, Bakker SJL, Dullaart RPF. Plasma branched-chain amino acids and risk of incident type 2 diabetes: results from the PREVEND prospective cohort study. J Clin Med 7: 513, 2018. doi: 10.3390/jcm7120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster TC. Role of estrogen receptor α and β expression and signaling on cognitive function during aging. Hippocampus 22: 656–669, 2012. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goffredo M, Santoro N, Tricò D, Giannini C, D’Adamo E, Zhao H, Peng G, Yu X, Lam TT, Pierpont B, Caprio S, Herzog RI. A branched-chain amino acid-related metabolic signature characterizes obese adolescents with non-alcoholic fatty liver disease. Nutrients 9: 642, 2017. doi: 10.3390/nu9070642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal MS, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, Benzinger TL, Morris JC, Raichle ME, Vlassenko AG. Persistent metabolic youth in the aging female brain. Proc Natl Acad Sci USA 116: 3251–3255, 2019. [Erratum in Proc Natl Acad Sci USA 116: 5198, 2019.]. doi: 10.1073/pnas.1815917116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyenet SJ, Nguyen HT, Hwang BH, Schwartz MW, Baskin DG, Thaler JP. High-fat diet feeding causes rapid, non-apoptotic cleavage of caspase-3 in astrocytes. Brain Res 1512: 97–105, 2013. doi: 10.1016/j.brainres.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruse M, Seki Y, Vuguin PM, Du XQ, Fiallo A, Glenn AS, Singer S, Breuhahn K, Katz EB, Charron MJ. High-fat intake during pregnancy and lactation exacerbates high-fat diet-induced complications in male offspring in mice. Endocrinology 154: 3565–3576, 2013. doi: 10.1210/en.2012-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melnik BC. Excessive leucine-mTORC1-signalling of cow milk-based infant formula: the missing link to understand early childhood obesity. J Obes 2012: 197653, 2012. doi: 10.1155/2012/197653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, Roman EA, Romanatto T, Pascoal LB, Caricilli AM, Torsoni MA, Prada PO, Saad MJ, Velloso LA. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes 61: 1455–1462, 2012. doi: 10.2337/db11-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morselli E, Frank AP, Palmer BF, Rodriguez-Navas C, Criollo A, Clegg DJ. A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int J Obes 40: 206–209, 2016. doi: 10.1038/ijo.2015.114. [DOI] [PubMed] [Google Scholar]
- 16.Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP, Li DL, Gao Y, Yi CX, Hahner L, Palmer BF, Tschöp MH, Clegg DJ. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep 9: 633–645, 2014. doi: 10.1016/j.celrep.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 18.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9: 311–326, 2009. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie C, He T, Zhang W, Zhang G, Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci 19: 954, 2018. doi: 10.3390/ijms19040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson CM, Bouret SG, Park S, Irani BG, Dunn-Meynell AA, Levin BE. Large litter rearing enhances leptin sensitivity and protects selectively bred diet-induced obese rats from becoming obese. Endocrinology 151: 4270–4279, 2010. doi: 10.1210/en.2010-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penfold NC, Ozanne SE. Developmental programming by maternal obesity in 2015: Outcomes, mechanisms, and potential interventions. Horm Behav 76: 143–152, 2015. doi: 10.1016/j.yhbeh.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Piccolo BD, Comerford KB, Karakas SE, Knotts TA, Fiehn O, Adams SH. Whey protein supplementation does not alter plasma branched-chained amino acid profiles but results in unique metabolomics patterns in obese women enrolled in an 8-week weight loss trial. J Nutr 145: 691–700, 2015. doi: 10.3945/jn.114.203943. [DOI] [PubMed] [Google Scholar]
- 23.Roberts DE, Killiany RJ, Rosene DL. Neuron numbers in the hypothalamus of the normal aging rhesus monkey: stability across the adult lifespan and between the sexes. J Comp Neurol 520: 1181–1197, 2012. doi: 10.1002/cne.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roepke TA, Yasrebi A, Villalobos A, Krumm EA, Yang JA, Mamounis KJ. Loss of ERα partially reverses the effects of maternal high-fat diet on energy homeostasis in female mice. Sci Rep 7: 6381, 2017. doi: 10.1038/s41598-017-06560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadagurski M, Cady G, Miller RA. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell 16: 652–660, 2017. doi: 10.1111/acel.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadagurski M, Leshan RL, Patterson C, Rozzo A, Kuznetsova A, Skorupski J, Jones JC, Depinho RA, Myers MG Jr, White MF. IRS2 signaling in LepR-b neurons suppresses FoxO1 to control energy balance independently of leptin action. Cell Metab 15: 703–712, 2012. doi: 10.1016/j.cmet.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadagurski M, Norquay L, Farhang J, D’Aquino K, Copps K, White MF. Human IL6 enhances leptin action in mice. Diabetologia 53: 525–535, 2010. doi: 10.1007/s00125-009-1580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiegelman BM, Hotamisligil GS. Through thick and thin: wasting, obesity, and TNF α. Cell 73: 625–627, 1993. doi: 10.1016/0092-8674(93)90243-J. [DOI] [PubMed] [Google Scholar]
- 29.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson JR, Gustafsson HC, DeCapo M, Takahashi DL, Bagley JL, Dean TA, Kievit P, Fair DA, Sullivan EL. Maternal diet, metabolic state, and inflammatory response exert unique and long-lasting influences on offspring behavior in non-human primates. Front Endocrinol (Lausanne) 9: 161, 2018. doi: 10.3389/fendo.2018.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogt MC, Paeger L, Hess S, Steculorum SM, Awazawa M, Hampel B, Neupert S, Nicholls HT, Mauer J, Hausen AC, Predel R, Kloppenburg P, Horvath TL, Brüning JC. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 156: 495–509, 2014. doi: 10.1016/j.cell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497: 211–216, 2013. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, Wolpin BM, Hu FB, Qi L. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol 45: 1482–1492, 2016. doi: 10.1093/ije/dyw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Y, Hedderson MM, Sridhar S, Xu F, Feng J, Ferrara A. Poor diet quality in pregnancy is associated with increased risk of excess fetal growth: a prospective multi-racial/ethnic cohort study. Int J Epidemiol In press. doi: 10.1093/ije/dyy285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziko I, De Luca S, Dinan T, Barwood JM, Sominsky L, Cai G, Kenny R, Stokes L, Jenkins TA, Spencer SJ. Neonatal overfeeding alters hypothalamic microglial profiles and central responses to immune challenge long-term. Brain Behav Immun 41: 32–43, 2014. doi: 10.1016/j.bbi.2014.06.014. [DOI] [PubMed] [Google Scholar]