Abstract
Endothelin-1 (ET-1) is a potent vasoconstrictor and proinflammatory peptide that is upregulated in obesity. Herein, we tested the hypothesis that ET-1 signaling promotes visceral adipose tissue (AT) inflammation and disrupts glucose homeostasis. We also tested if reduced ET-1 is a required mechanism by which exercise ameliorates AT inflammation and improves glycemic control in obesity. We found that 1) diet-induced obesity, AT inflammation, and glycemic dysregulation were not accompanied by significantly increased levels of ET-1 in AT or circulation in wild-type mice and that endothelial overexpression of ET-1 and consequently increased ET-1 levels did not cause AT inflammation yet impaired glucose tolerance; 2) reduced AT inflammation and improved glucose tolerance with voluntary wheel running was not associated with decreased levels of ET-1 in AT or circulation in obese mice nor did endothelial overexpression of ET-1 impede such exercise-induced metabolic adaptations; 3) chronic pharmacological blockade of ET-1 receptors did not suppress AT inflammation in obese mice but improved glucose tolerance; and 4) in a cohort of human subjects with a wide range of body mass indexes, ET-1 levels in AT, or circulation were not correlated with markers of inflammation in AT. In aggregate, we conclude that ET-1 signaling is not implicated in the development of visceral AT inflammation but promotes glucose intolerance, thus representing an important therapeutic target for glycemic dysregulation in conditions characterized by hyperendothelinemia. Furthermore, we show that the salutary effects of exercise on AT and systemic metabolic function are not contingent on the suppression of ET-1 signaling.
Keywords: adipose tissue, endothelin-1, exercise, glucose control, inflammation
INTRODUCTION
Adipose tissue (AT) is an active immunological organ that contributes to whole body metabolism through endocrine mechanisms (20, 32). Visceral AT inflammation plays a key pathogenic role in the development of metabolic complications in obesity, including dysregulation of glucose homeostasis (51). Unabated adipocyte hypertrophy initiates a cellular stress response, which triggers the infiltration of circulating immune cells, contributing to the inflammatory state of AT and consequent production of cytokines that are linked with impairments in glucose regulation (33, 38, 47, 51, 58). As such, a better understanding of the molecular transducers and lifestyle factors implicated in the induction of inflamed AT and associated glucose intolerance is critical for the prevention and treatment of obesity-related metabolic diseases.
A hypothesis gaining traction (56) is that impaired microvascular function in AT is a precipitating factor in AT dysfunction and inflammation. In this regard, upregulation of endothelin-1 (ET-1), a peptide primarily expressed by and secreted from endothelial cells, is a common molecular signature of obesity and insulin resistance in both rodents and humans (9, 10, 22, 45, 52, 57). Aside from its known vasoconstrictive effects, overexpression of ET-1 in endothelial cells prompts the expression of adhesion molecules and vascular inflammation (2, 3, 13, 14, 34, 35, 42, 44, 48). Secreted ET-1 induces autocrine/paracrine effects via the activation of its receptors, endothelin receptor type-A (ETA) and type-B (ETB), which are constitutively expressed in many cell types, including adipocytes (7, 28). Notably, ET-1 signaling has been reported to induce lipolysis (7, 15, 28, 29) and decrease insulin sensitivity in adipocytes (7, 11), whereas inhibition of ET-1 signaling via ETA blockade restores adipocyte insulin action (11). Furthermore, circulating ET-1 concentrations are increased in obesity (16) and are associated with impaired glucose tolerance in humans (43). Therefore, it is conceivable that, in obesity, upregulation of ET-1 is a causal factor for the development of visceral AT inflammation and glycemic dysregulation.
It is well established that exercise training improves systemic glucose tolerance and reduces inflammation systemically and in AT (5, 6, 8, 12, 31, 59, 61, 66), though the mechanisms underlying these effects are not fully understood. We previously ruled out a role of fibroblast growth factor 21 and estrogen rector alpha as necessary mediators for exercise-induced improvements in AT inflammation (49, 64). Importantly, a critical observation has been made that aerobic exercise decreases plasma ET-1 concentrations in conjunction with improvements in glucose homeostasis in individuals with impaired glucose tolerance (30, 40). Whether a reduction in ET-1 is required for exercise-induced improvements in AT inflammation and glycemic control remains unknown.
Accordingly, a series of experiments was performed to test the following hypotheses: 1) endothelial cell-specific overexpression of ET-1 and resultant hyperendothelinemia causes visceral AT inflammation and glucose intolerance in lean mice and that these effects are recapitulated by diet-induced obesity; 2) forced expression of ET-1 limits exercise-induced reduction in AT inflammation and improvement in glucose control in obese mice; 3) blockade of ET-1 receptors suppresses AT inflammation and improves systemic glucose control in obese mice; and 4) circulating and AT levels of ET-1 are positively correlated with markers of AT inflammation in humans with a wide range of adiposity.
METHODS
Ethics and Approvals
All animal study procedures received prior approval by the University of Missouri Institutional Animal Care and Use Committee. The University of Missouri is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All human study procedures conformed to the Declaration of Helsinki and were approved by the University of Missouri Institutional Review Board. Written informed consent was obtained from all subjects before surgery.
Endothelial Cell-Specific Human Prepro-ET-1 Breeding Protocol, Diet, and Housing Conditions
Female C57BL/6NHSD mice purchased from Envigo Laboratories (Indianapolis, IN) were bred with male transgenic endothelial cell-specific human prepro-ET-1 (eET-1) mice on the C57BL/6NHSD background as previously reported (2). Breeding took place at the University of Missouri College of Veterinary Medicine to produce both eET-1 transgenic and nontransgenic (i.e., wild-type) littermates. Females were utilized in experimental protocol 1, whereas male littermates were utilized in experimental protocol 2. All animals were given ad libitum access to either chow diet (3.35 kcal/g food, Laboratory Rodent Diet 5001*, Laboratory Diet) or a Western diet (4.73 kcal/g food, Test Diet modified 58Y1, 5APC, Test Diet) and water for the duration of the study. Mice were housed in an environmentally controlled animal facility maintained at 28°C on a 12:12-h light-dark cycle from 0700 to 1900. Mice were kept at thermoneutrality, as this is considered optimal for human translation (17, 21).
Experimental Protocol 1
Female eET-1 and wild-type littermates (n = 40) were housed by genotype, 2 or 3 per cage. At 6 wk of age, mice were randomized to either maintain a chow diet or begin a Western diet for 21 wk, thus creating 4 groups (n = 9–11/group): wild-type chow-fed, eET-1 chow-fed, wild-type Western diet-fed, and eET-1 Western diet-fed. Energy expenditure assessment was conducted via indirect calorimetry using metabolic chambers in a subset of mice at week 15 of the intervention. Glucose tolerance testing (GTT) was performed at week 19 of intervention.
Experimental Protocol 2
eET-1 male and wild-type littermates (n = 37) at 6 wk of age were randomized to a cage (i.e., individually housed) with or without access to a running wheel for 12 wk, creating 4 groups (n = 7–12/ group): wild-type sedentary, eET-1 sedentary, wild-type exercise, and eET-1 exercise. All groups were fed a Western diet. Wheel running distances were recorded weekly by the Sigma Sport BC 800 Bicycle Monitoring System (Cherry Creek Cyclery, Forster Falls, VA), as previously described (46). GTT was performed at week 10 of the intervention.
Experimental Protocol 3
Male low-density lipoprotein receptor knockout mice (LDLr KO, B6.129S7-Ldlrtm1Her/J on C57BL/6J background) (n = 18) were individually housed and placed on a Western diet at 12–13 wk of age. LDLr KO mice were included to test the hypothesis that blockade of ET-1 signaling mitigates AT inflammation and metabolic dysfunction in a documented model of accelerated obesity and hyperlipidemia (41). LDLr KO mice were administered either ~54 mg of reduced-fat peanut butter (6.7 kcal/g food, JIF peanut butter, The J.M. Smucker Company, Orrville, OH) with bosentan, a dual ET-1 receptor antagonist (100 mg·kg−1·day−1; cat. no. HY-A0013, MedchemExpress, Monmouth Junction, NJ), or vehicle control (i.e., peanut butter) daily for 8 wk. Peanut butter contributed to ~3% daily caloric intake. Male C57BL/6J mice (n = 10) were purchased from Jackson Laboratory (Bar Harbor, ME), housed 2–3/cage, and fed a chow diet serving as lean healthy controls. GTT was performed during week 6 of the intervention.
Experimental Protocol 4
Omental AT and blood samples were obtained from human patients [n = 66; 83% females; 89% Caucasian; 53% hypertensive; 21% diabetic (HbA1C 5.8%–10.3%); age = 49.0 ± 1.6 yr (21–80 yr); body mass index (BMI) = 43.0 ± 1.2 kg/m2 (22.0–66.7 kg/m2)] undergoing planned and emergency abdominal surgery at the University of Missouri Hospital. Plasma and omental AT samples were kept frozen until analysis. Patient characteristics and blood parameters were obtained from patients medical history.
Experimental Procedures
Glucose tolerance testing.
After a 5-h fast, blood glucose was measured from mice via tail vein. The tail was nicked, and blood was sampled by a handheld glucometer (AlphaTrak 2, Abbott Laboratories, Abbott Park, IL). A baseline measure of blood glucose was determined before giving a sterile solution of 50% dextrose (2 g/kg body wt) via intraperitoneal injection, as previously performed (23, 64). Glucose measures were determined at 15, 30, 45, 60, and 120 min after the glucose injection, and glucose area under the curve (AUC) was calculated using the trapezoidal rule.
Metabolic chambers.
Using a metabolic monitoring system (Promethion, Sable Systems, Las Vegas, NV), energy expenditure and spontaneous physical activity during 12-h light and 12-h dark cycles were determined by monitoring oxygen consumption and carbon dioxide production over a 3-day period in a subset of mice in experimental protocol 1, as previously described (60). Energy expenditure is presented as kcal/24 h.
Body composition.
Mouse body fat and lean mass were measured by a nuclear magnetic resonance imaging whole body composition analyzer (EchoMRI, 4in1/1100; Echo Medical Systems, Houston, TX) within 1 wk of euthanasia.
ET-1 concentrations in plasma and AT.
ET-1 concentrations in plasma and AT homogenates were determined via a commercially available quantikine ELISA kit as per the manufacturer’s instructions (cat. no. DET100, R&D Systems, Minneapolis, MN). AT ET-1 concentrations were normalized to total protein.
Fasting blood determinants.
Quantification of plasma glucose, insulin, cholesterol, HDL, LDL, triglycerides, and nonesterified fatty acids was performed by a commercial laboratory (Comparative Clinical Pathology Services, Columbia, MO) on an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea, CA) using assays per manufacturer’s guidelines.
Skeletal muscle citrate synthase activity.
Citrate synthase activity was determined in experimental protocol 2 using methods from Srere (55) and Bass et al. (4). Briefly, quadriceps skeletal muscle homogenate was incubated in the presence of oxaloacetates, acetyl-CoA, and DTNB. Spectrophotometric detection of reduced DTNB at a wavelength of 405 nm served as an index of enzyme activity, as previously described (50).
Gene expression.
The following visceral AT depots from mice and human patients were excised and processed given their appreciated role in obesity-associated inflammation and metabolic disease (33, 38, 47, 51, 58): mouse perigonadal and epididymal and human omental AT. AT was homogenized in TRIzol reagent (TissueLyser LT, Qiagen, Valencia, CA), and total RNA was isolated using Qiagen’s RNeasy lipid tissue homogenization kit per manufacturer’s instructions. RNA concentration and purity were assessed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). First-strand cDNA was synthesized from total RNA using the 5x iScript RT supermix (Bio-Rad, Hercules, CA). Quantitative real-time PCR was performed as previously described using the ABI StepOne Plus sequence detection system (Applied Biosystems) (45, 54). Primers were purchased from Integrated DNA Technologies (Coralville, IA) or Sigma Aldrich (St. Louis, MO). The list of gene primer sequences is provided in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 18S were used as the housekeeping control genes for experimental protocols 1 and 2 and protocol 3, respectively, whereas B-tubuliln was used for protocol 4. mRNA expression values were calculated by the -∆∆CT method.
Table 1.
PCR primer sequences
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Species |
|---|---|---|---|
| IL-1β | AAATGGAGACCTGGAATC | CCTCTTACTTCACTGTCT | Mouse |
| TNFα | CTATGTCTCAGCCTCTTC | CATTTGGGAACTTCTCAT | Mouse |
| MCP-1 | CAAGATGATCCCAATGAG | TTGGTGACAAAAACTACA | Mouse |
| CD68 | TGTTCAGCTCCAAGCCCAAA | GTACCGTCACAACCTCCCTG | Mouse |
| EdnrA | AAATTGTTTTCAGTCCTGCC | ACTGGATACTCGTTCCATTC | Mouse |
| EdnrB | AGCTTCTGAGCTTTTTGTTG | AATACAGAGCGATTGGATTG | Mouse |
| 18S | TCAAGAACGAAAGTCGGAGG | GGACATCTAAGGGCATCAC | Mouse |
| GAPDH | CCAGCTACTCGCGGCTTTA | GAGGGCTGCAGTCCGTATTT | Mouse |
| CD68 | CAACTGGTGCAGACAGCCTA | ACAGTCATTCCCTGTCCCCT | Human |
| TNFα | GACAAGCCTGTAGCCCATGT | GGAGGTTGACCTTGGTCTGG | Human |
| IL-1β | TCGCCAGTGAAATGATGGCT | GGTCGGAGATTCGTAGCTGG | Human |
| β-Tubulin | GCGTCTACTACAACGGAGG | ACTCTGACCAAAGATGAA | Human |
GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
Histological assessments.
Excised mouse perigonadal and epididymal AT were placed in 10% formalin, paraffin embedded, and 5-µm sections were stained with macrophage marker MAC-2 antibody (MAC-2) (1:1,000, Cedar Lane) or CAT hemaoxylin (1:10, Biocare Medical). Tissue sections were imaged under an Olympus BX34 photomicroscope (Olympus, Melville, NY) and taken via an Olympus SC30 Optical Microscope Accessory CMOS color camera. Adipocyte size (area µm2) was assessed and calculated from the average of ~100 adipocytes per animal from at least 3 different captured images (i.e., fields of view). Cross-sectional areas and distribution of adipocytes per discrete cell size were examined as previously described (23, 63). Immune cell infiltration via MAC-2 staining was quantified via Image J (National Institutes of Health public domain, National Institutes of Health, Bethesda, MD). All procedures were performed by an investigator who was blinded to the experimental groups.
Statistical analysis.
GraphPad Prism (version 7.0, GraphPad Prism Software, La Jolla, CA) was used for statistical analyses. A two-way ANOVA was used for experimental protocol 1 (diet and genotype as factors) and experimental protocol 2 (exercise and genotype as factors), with Fisher’s least-significant difference post hoc test for pairwise comparisons. In addition, a repeated measures two-way ANOVA was used to assess group × time interactions for weekly body weight. Experimental protocol 3 was analyzed via one-way ANOVA. Pearson correlations were performed for experimental protocol 4, assessing the relationships between BMI and inflammatory gene expression with ET-1 concentrations. Values are expressed as means ± SE. For all statistical tests, significance was accepted when P < 0.05.
RESULTS
Overexpression of ET-1 impairs glucose tolerance but does not cause visceral AT inflammation in female mice.
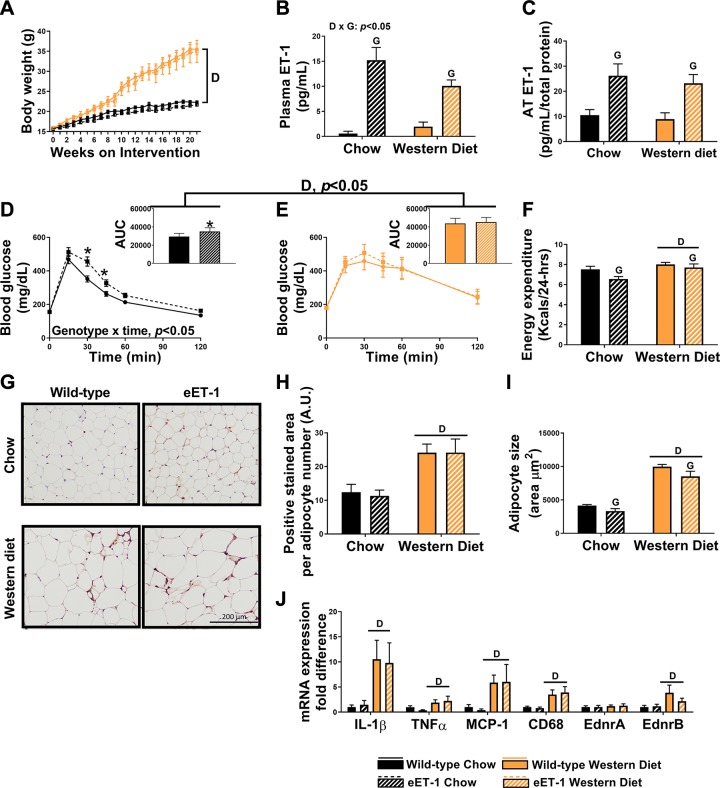
Body weight gain was greater in Western diet-fed female mice regardless of genotype (Fig. 1A). Percent body fat was elevated in Western diet-fed mice compared with chow-fed mice (wild-type chow: 14 ± 1%, eET-1 chow: 12 ± 1%, wild-type Western diet: 44 ± 1%, eET-1 Western diet: 43 ± 3%, P < 0.05). Percent lean mass was declined in the Western diet-fed mice compared with chow-fed mice (wild-type chow: 80 ± 1%, eET-1 chow: 80 ± 2%, wild-type Western diet: 52 ± 1%, eET-1 Western diet: 54 ± 3%, P < 0.05). In accordance with our model, plasma and AT ET-1 concentrations were markedly increased in eET-1 compared with wild-type animals (Fig. 1, B and C, P < 0.05). Relative to wild-type controls, glucose concentrations in response to an intraperitoneal glucose load were higher in eET-1 mice on a chow diet (Fig. 1D, P < 0.05). Western diet-fed animals had greater glucose AUC than chow-fed mice, but it was not exacerbated in eET-1 mice (Fig. 1E, P < 0.05). Twenty-four-hour energy expenditure was lower in eET-1 compared with wild-type mice regardless of diet, whereas, as expected, Western diet-fed mice from both genotypes had higher energy expenditure than their chow-fed counterparts (Fig. 1F, P < 0.05). Fasting lipids, except nonesterified fatty acids, glucose, and insulin were elevated in Western diet-fed mice compared with chow-fed mice but were not different between wild-type and eET-1 mice (Table 2, P < 0.05). AT-positive staining for macrophage infiltration with MAC-2 was significantly greater in Western diet-fed mice compared with their chow-fed counterparts, with no differences between eET-1 and wild-type mice (Fig. 1, G and H, P < 0.05). Adipocyte size was also greater in Western diet-fed mice; however, eET-1 mice had smaller adipocytes than diet-matched controls (Fig. 1I, P < 0.05). Gene expression markers of inflammation and immune cell infiltration in AT were elevated in the Western diet-fed mice compared with chow-fed mice (Fig. 1J, P < 0.05), but no genotype effects were noted. EdnrA and EdnrB, mRNA transcripts for ETA and ETB receptors, respectively, were unaltered by diet or genotype (Fig. 1J).
Fig. 1.
Overexpression of endothelin-1 (ET-1) impairs glucose tolerance but does not cause visceral adipose tissue (AT) inflammation in female mice. A: body weight growth curves over 21 wk of diet intervention. B: plasma ET-1 concentrations. C: ET-1 concentrations in perigonadal AT. D: glucose tolerance test (GTT) with area under the curve (AUC) conducted at 19 wk of intervention in chow-fed mice. E: GTT with an AUC at 19 wk of intervention in Western diet-fed mice. F: energy expenditure throughout 24 h. G: macrophage marker MAC-2 immunostained perigonadal adipose sections. Magnification ×20 with ×10 objective. H: average MAC-2-positive stained area. I: average adipocyte size per 100 cells. J: expression of inflammatory genes in perigonadal AT, represented as fold differences from wild-type chow. Values are means ± SE; n = 9–11/group. “D” indicates P < 0.05, main effect of diet. “G” indicates P < 0.05, main effect of genotype. “D × G” indicates P < 0.05, diet × genotype interaction. *P < 0.05 vs. wild-type counterpart. A two-way ANOVA (diet and genotype as factors) with Fisher’s least-significant difference post hoc for pairwise comparisons was used. In addition, a repeated measures two-way ANOVA was used to assess group × time interactions for weekly body weight. A.U., arbitrary units.
Table 2.
Fasting blood parameters from experimental protocol 1
| Fasting Blood Parameter | Wild-Type Chow | eET-1 Chow | Wild-Type WD | eET-1 WD | ANOVA |
|---|---|---|---|---|---|
| Cholesterol, mg/dL | 43.4 ± 2.1 | 44.5 ± 1.9 | 101.0 ± 7.3 | 93.1 ± 6.8 | G × D: NS G: NS D: P < 0.05 |
| Triglycerides, mg/dL | 124.5 ± 4.7 | 140.5 ± 9.4 | 121.4 ± 5.4 | 133.8 ± 7.5 | G × D: NS G: NS D: NS |
| HDL, mg/dL | 23.8 ± 1.0 | 24.6 ± 1.0 | 40.9 ± 1.8 | 37.1 ± 1.8 | G × D: NS G: NS D: P < 0.05 |
| LDL, mg/dL | 4.0 ± 0.3 | 4.1 ± 0.3 | 8.5 ± 0.6 | 7.6 ± 0.9 | G × D: NS G: NS D: P < 0.05 |
| NEFA, mmol/L | 0.40 ± 0.03 | 0.30 ± 0.05 | 0.29 ± 0.03 | 0.28 ± 0.05 | G × D: NS G: NS D: NS |
| Insulin, g/dL | 0.52 ± 0.04 | 0.44 ± 0.04 | 1.68 ± 0.13 | 1.67 ± 0.26 | G × D: NS G: NS D: P < 0.05 |
| Glucose, mg/dL | 206.6 ± 8.0 | 178.0 ± 11.9 | 269.3 ± 14.8 | 225.3 ± 25.7 | G × D: NS G: NS D: P < 0.05 |
Values are means ± SE. Bold values indicate statistical significance. D, main effect of diet; eET-1, endothelial cell-specific human prepro-endothelin-1; G, main effect of genotype; G × D; genotype × diet interaction; NEFA, nonesterified fatty acid; NS, nonsignificant (P > 0.05); WD, Western diet.
Downregulation of ET-1 is not compulsory for exercise-induced amelioration of visceral AT inflammation and improvements in glucose control in male mice.
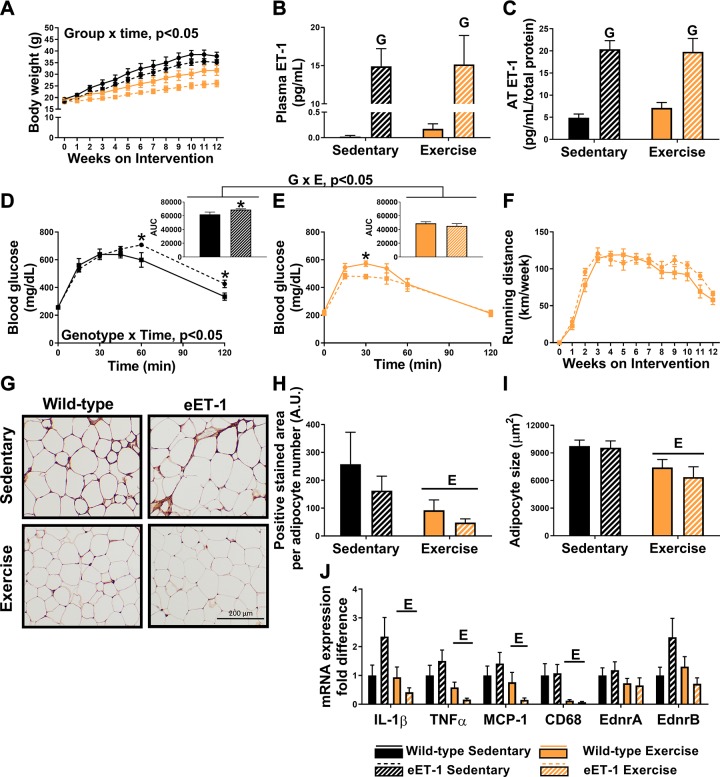
As expected, exercising male mice had decreased rates of weight gain, and this effect was amplified in eET-1 mice (Fig. 2A). Similarly, percent body fat was elevated in sedentary mice compared with exercise-trained mice (wild-type sedentary: 38 ± 1%, eET-1 sedentary: 37 ± 1%, wild-type exercise: 24 ± 2%, eET-1 exercise: 20 ± 3%, P < 0.05). Plasma and AT ET-1 concentrations were significantly elevated in eET-1 compared with their wild-type counterparts, regardless of training status (Fig. 2, B and C, P < 0.05). Glucose concentrations during the GTT were elevated to a greater extent in eET-1 sedentary mice compared with wild-type sedentary mice (Fig. 2E, genotype × time interaction, P < 0.05), and compared with sedentary animals, exercise-trained mice had improved glucose tolerance (Fig. 2, D and E). In fact, the exercise-induced decrease in glucose AUC was greater in eET-1 than wild-type mice (Fig. 2, D and E, genotype × exercise interaction, P < 0.05). Fasting lipids and insulin concentrations were significantly decreased in exercise-trained mice compared with sedentary mice, with no differences between eET-1 and wild-type animals (Table 3, P < 0.05). Weekly running distance was similar between eET-1 and wild-type mice (Fig. 2F, P > 0.05). Skeletal muscle citrate synthase activity was significantly elevated in the exercise-trained mice compared with sedentary mice (wild-type sedentary: 87.1 ± 8.2 μmol·min−1·mg−1 protein, eET-1 sedentary: 92.5 ± 6.7 μmol·min−1·mg−1 protein, wild-type exercise: 107.9 ± 9.2 μmol·min−1·mg−1 protein, eET-1 exercise: 124.4 ± 10.2 μmol·min−1·mg−1 protein, P < 0.05), regardless of genotype, which is indicative of classic training adaptations (19). AT-positive staining for macrophage infiltration with MAC-2 and adipocyte size was significantly decreased in exercise-trained animals compared with their sedentary counterparts with no genotype effects (Fig. 1, G, H, and I). Likewise, expression of inflammatory genes in AT was significantly decreased with exercise training, and no genotype effects were noted (Fig. 2J, P < 0.05). EdnrA and EdnrB were not significantly altered by exercise or genotype (Fig. 2J).
Fig. 2.
Downregulation of endothelin-1 (ET-1) is not compulsory for exercise-induced amelioration of visceral adipose tissue (AT) inflammation and improvements in glucose control. A: body weight growth curves over 12 wk of the intervention. B: plasma ET-1 concentrations. C: ET-1 concentrations in epididymal AT. D and E: glucose tolerance test with area under the curve (AUC) conducted at 10 wk of intervention in sedentary and exercise-trained male mice, respectively. F: mean wheel running distance throughout the 12-wk intervention. G: macrophage marker MAC-2 immunostained epididymal adipose sections. Magnification ×20 with ×10 objective. H: average positive MAC-2-stained area. I: average adipocyte size per 100 cells. J: expression of inflammatory genes in epididymal AT (exercise × genotype interaction P values: IL-1β, P = 0.064; TNFα, P = 0.165; MCP-1, P = 0.146; CD68, P = 0.8186; EdnrA, P = 0.621; EdnrB, P = 0.051). Values are means ± SE; n = 7–12/group. “E” indicates P < 0.05, main effect of exercise. “G” indicates P < 0.05, main effect of genotype. “G × E” indicates P < 0.05, genotype × exercise interaction. *P < 0.05 vs. wild-type. A two-way ANOVA (exercise and genotype as factors) with Fisher’s least-significant difference post hoc for pairwise comparisons was used. In addition, a repeated measures two-way ANOVA was used to assess group × time interactions for weekly body weight.
Table 3.
Fasting blood parameters from experimental protocol 2
| Fasting Blood Parameter | Wild-Type Sedentary | eET-1 Sedentary | Wild-Type Exercise | eET-1 Exercise | ANOVA |
|---|---|---|---|---|---|
| Cholesterol, mg/dL | 152.9 ± 15.3 | 130.8 ± 7.4 | 94.3 ± 4.8 | 103.1 ± 8.0 | G × E: NS G: NS E: P < 0.05 |
| Triglycerides, mg/dL | 138.2 ± 7.7 | 131.3 ± 3.7 | 115.7 ± 6.5 | 125.6 ± 8.3 | G × E: NS G: NS E: P < 0.05 |
| HDL, mg/dL | 61.7 ± 2.7 | 58.8 ± 2.1 | 49.6 ± 2.0 | 53.7 ± 3.5 | G × E: NS G: NS E: P < 0.05 |
| LDL, mg/dL | 91.1 ± 12.9 | 72.1 ± 5.8 | 44.7 ± 3.2 | 49.4 ± 4.7 | G × E: NS G: NS E: P < 0.05 |
| NEFA, mmol/L | 0.35 ± 0.06 | 0.29 ± 0.03 | 0.27 ± 0.04 | 0.27 ± 0.03 | G × E: NS G: NS E: NS |
| Insulin, ng/dL | 2.9 ± 0.3 | 3.1 ± 0.3 | 1.7 ± 0.2 | 2.0 ± 0.3 | G × E: NS G: NS E: P < 0.05 |
| Glucose, mg/dL | 277.1 ± 43.8 | 247.8 ± 11.7 | 216.2 ± 15.9 | 220.1 ± 23.8 | G × E: NS G: NS E: NS |
Values are means ± SE. Bold values indicate statistical significance. E, main effect of exercise; eET-1, endothelial cell-specific human prepro-endothelin-1; G, main effect of genotype; G × E; genotype × exercise interaction; NEFA, nonesterified fatty acid; NS, nonsignificant (P > 0.05).
Inhibition of ET-1 signaling via oral bosentan administration improves glycemic control but does not reduce visceral AT inflammation.
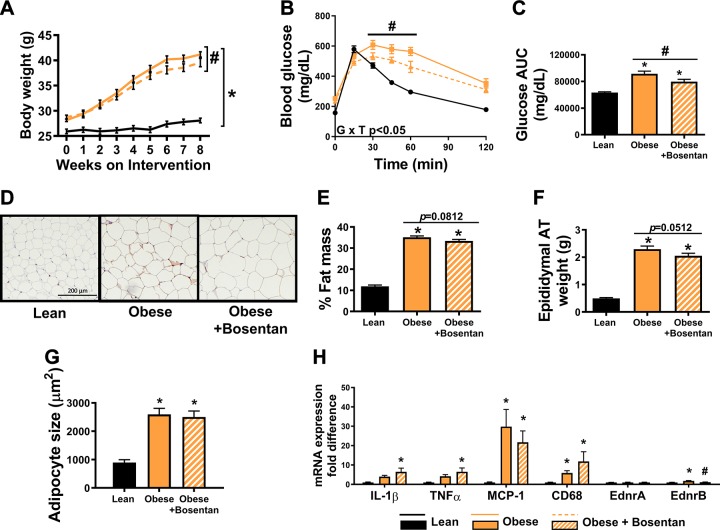
Next, we sought to determine whether inhibition of ET-1 signaling via oral bosentan administration improves metabolic outcomes in the LDLr KO mouse model of obesity and dyslipidemia. Bosentan-treated male LDLr KO mice gained slightly less body mass compared with untreated counterparts (Fig. 3A, P < 0.05). Fasting plasma parameters are summarized in Table 4. Blood glucose levels during a GTT were decreased in bosentan-treated mice (Fig. 3, B and C, P < 0.05) compared with untreated mice. There was no significant difference in fat mass percentage between bosentan-treated and untreated mice (Fig. 3E, P = 0.08), though bosentan-treated mice tended to have decreased epididymal AT mass compared with obese controls (Fig. 3F, P = 0.051) despite no reduction in adipocyte size (Fig. 3G, P < 0.05). Bosentan treatment did not decrease markers of immune cell infiltration or inflammation (Fig. 3H, P > 0.05).
Fig. 3.
Inhibition of endothelin-1 signaling via oral bosentan administration improves glycemic control but does not reduce visceral adipose tissue (AT) inflammation in male LDL receptor knockout mice. A: body weight growth curves over the 8-wk intervention. B: glucose tolerance test (GTT) conducted at 6 wk of the intervention. C: glucose area under the curve (AUC) during GTT. D: macrophage marker MAC-2-immunostained epididymal adipose sections. Magnification ×20 with ×10 objective. E: percent body fat as determined by EchoMRI. F: epididymal AT weight. G: average adipocyte size per 100 cells. H: mRNA expression in epididymal AT. Values are means ± SE; n = 7–12/group. *P < 0.05 vs. lean. #P < 0.05 vs. obese untreated. A one-way ANOVA with Fisher’s least-significant difference post hoc for pairwise comparisons was used.
Table 4.
Fasting blood parameters from experimental protocol 3
| Fasting Blood Parameter | Lean | Obese | Obese + Bosentan |
|---|---|---|---|
| Cholesterol, mg/dL | 71.3 ± 1.8 | 870.4 ± 38.7* | 817.4 ± 66.0* |
| Triglycerides, mg/dL | 181.6 ± 20.2 | 584.5 ± 60.6* | 664.0 ± 97.1* |
| HDL, mg/dL | 45.6 ± 1.2 | 88.5 ± 5.0* | 90.3 ± 5.7* |
| LDL, mg/dL | 3.1 ± 0.1 | 270.9 ± 14.2* | 254.3 ± 22.7* |
| NEFA, mmol/L | 0.73 ± 0.05 | 0.78 ± 0.04 | 0.82 ± 0.06 |
| Insulin, ng/dL | 0.6 ± 0.1 | 3.1 ± 0.4* | 2.5 ± 0.5* |
| Glucose, mg/dL | 241.1 ± 11.0 | 332.0 ± 13.1* | 307.3 ± 11.5* |
Values are means ± SE. NEFA, nonesterified fatty acid.
P < 0.05 vs. lean.
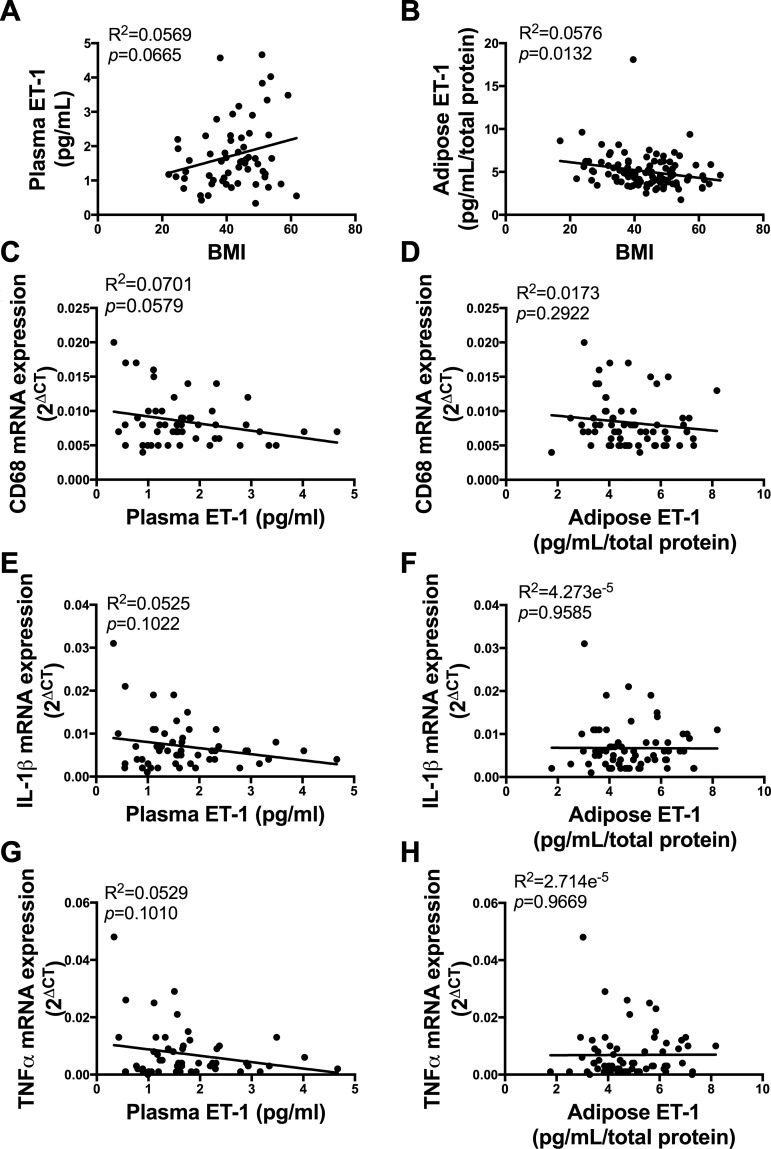
ET-1 levels in circulation or visceral AT do not correlate with markers of inflammation in AT of humans with a wide range of BMIs.
BMI was not correlated with plasma ET-1 (Fig. 4A, P > 0.05) but was negatively correlated with AT ET-1 (Fig. 4B, P < 0.05). ET-1 concentrations in plasma or AT were not correlated with gene expression markers of AT inflammation [Fig. 4, C, E, and G (plasma ET-1) and Fig. 4, D, F, and H, P > 0.05]. Furthermore, plasma ET-1 concentrations and AT inflammatory markers (CD68, IL-1β, and TNFα) did not exhibit a curvilinear relationship (all R2 < 0.09, data not shown).
Fig. 4.
Endothelin-1 (ET-1) levels in circulation or visceral adipose tissue (AT) do not correlate with markers of inflammation in AT of humans with a wide range of body mass indexes (BMIs). A: relationship between BMI and plasma ET-1 concentrations. B: relationship between BMI and AT ET-1 concentrations. C, E, and G: relationship between plasma ET-1 concentrations and inflammatory gene expression. D, F, and H: relationship between AT ET-1 concentrations and inflammatory gene expression. Pearson correlations were performed to regress BMI and inflammatory gene expression with ET-1 concentrations; n = 55–66.
DISCUSSION
Counter to our hypothesis, we found that increased ET-1 expression and signaling are not determinants in the development of visceral AT inflammation associated with obesity, nor does excess ET-1 limit exercise-induced anti-inflammatory effects in AT. Several lines of evidence presented here are in support of this conclusion, including: 1) diet-induced obesity and associated AT inflammation was not accompanied by significantly increased ET-1 levels in circulation or AT in wild-type mice, nor did endothelial ET-1 overexpression and consequent increased levels of ET-1 cause AT inflammation; 2) reduced AT inflammation with voluntary wheel running was not associated with decreased levels of ET-1 in AT or circulation in obese mice, nor did endothelial overexpression of ET-1 impede such exercise-induced reductions in AT inflammation; 3) chronic pharmacological blockade of ET-1 receptors did not suppress AT inflammation in obese mice; and 4) in a cohort of human subjects with a wide range of BMIs, ET-1 levels in AT or circulation were not correlated with markers of inflammation in AT. In contrast, we found that elevated ET-1 signaling promotes glucose intolerance, identifying an important mechanism contributing to glycemic dysregulation in obesity and likely other conditions associated with hyperendothelinemia.
AT inflammation is an underlying cause of obesity-associated insulin resistance and cardiovascular disease (25). Indeed, with caloric excess, aggregates of immune cells surround and clear dying adipocytes. This process triggers the production of proinflammatory cytokines and chemoattractants that perpetuate a low-grade inflammatory state (62, 65). A greater understanding of the factors and molecular mechanisms that control this low-grade inflammation in AT is necessary for the development of novel therapeutic targets to treat AT dysfunction and other obesity-associated pathologies. Here, we tested the hypothesis that ET-1 is implicated in the regulation of AT inflammation in obesity and, furthermore, that a reduction in ET-1 production mediates exercise-induced amelioration of AT inflammation. This hypothesis was largely founded on previous research demonstrating heightened ET-1 signaling in obesity and its proinflammatory effects in vascular cells and adjacent perivascular AT (1, 3, 13, 14, 26, 27, 35, 39), as well as evidence indicating that physical activity reduces plasma ET-1 concentrations (37). However, contrary to our postulate, we found that in our mouse model of diet-induced obesity, increased visceral AT inflammation was not accompanied by significant increases of ET-1 in circulation or AT. Similarly, exercise-induced amelioration of obesity-associated AT inflammation did not parallel reductions in ET-1 levels in circulation or AT. Furthermore, overexpression of ET-1 in endothelial cells and consequent increased presence of ET-1 in AT and circulation did not promote AT inflammation in either lean or obese mice, nor did it limit the lessening of AT inflammation resulting from exercise in obese mice. Additional support for the lack of a link between ET-1 signaling and visceral AT inflammation came from the finding that chronic blockade of ET-1 receptors with oral administration of bosentan did not suppress epidydimal AT inflammation in a mouse model of obesity and dyslipidemia. Moreover, further validating these abovementioned observations, we report that circulating and omental AT levels of ET-1 are not correlated with markers of inflammation in AT obtained from humans with a wide range of adiposity. Taken together, data from the present investigation argue against the hypothesis that ET-1 signaling contributes to visceral AT inflammation. An interesting observation that should be noted is that previous studies using this ET-1 transgenic mouse model have documented evidence of inflammation in perivascular AT depots (26, 39). It is reasonable to speculate that fat depots adjacent to vascular beds are more vulnerable to inflammation as a result of their amplified exposure to endothelium-derived ET-1.
Despite the apparent disconnect between ET-1 and visceral AT inflammation, we provide the first evidence that endothelial overexpression of ET-1 impairs glucose tolerance, an effect that is most notable in lean mice and that is independent of changes in body composition. These findings support existing data from a longitudinal study demonstrating that elevated ET-1 predicts development of glucose intolerance in women (43). Further supporting the role of ET-1 in regulating glucose homeostasis, we found that chronic inhibition of ET-1 signaling via bosentan treatment improved glucose tolerance in obese and dyslipidemic mice, an effect that was also largely independent of changes in body weight and body composition. This finding implies that blockade of ET-1 signaling may have therapeutic utility in the absence of exercise. The mechanisms by which ET-1 signaling regulates systemic glycemic control are not entirely clear; however, we have ruled out AT inflammation as a vital link. It is possible that ET-1 signaling regulates glucose control by its direct effects on skeletal muscle. Consistent with this notion, existing data from in vitro and in vivo experiments demonstrate that ET-1 reduces glucose uptake in human skeletal muscle (24, 53, 54). Likewise, elevated ET-1 may also limit insulin-stimulated vasodilation (36) and reduce glucose uptake (53, 54) by minimizing the delivery of insulin and glucose to skeletal muscle.
Another important finding reported herein is that excess ET-1 did not hinder exercise-induced improvements in glucose control in the setting of obesity. Exercise improved glucose tolerance in wild-type mice, and to a greater extent in ET-1-overexpressing mice, such that glucose tolerance was normalized and the genotype effect abrogated. Stated differently, exercise-trained mice were “resistant” to the deleterious effects of a Western diet and/or ET-1 overexpression on glycemic control. This finding, in combination with the observation that exercise did not reduce ET-1 levels in wild-type mice, counters the hypothesis that reduced ET-1 is a required mechanism by which exercise improves glycemic control and highlights the powerful role of physical activity in preserving normal glucose tolerance.
Within the context of our findings, some considerations may be required. Overexpression of ET-1 impaired glucose tolerance without exacerbating AT inflammation in both male and female mice. However, because of differences in experimental protocols (i.e., study duration and age), we were unable to determine whether an interaction between ET-1, glycemic control, and sex exists. Furthermore, it should be noted that LDLr KO mice treated with bosentan are on a different background (BL/6J) than eET-1 mice (BL/6N). Although there are known substrain differences in glucose homeostasis and insulin action between BL/6J and BL/6N mice (18), we show that ET-1 is implicated in the regulation of glucose metabolism, regardless of background strain. Given the finding that ET-1-overexpressing mice were glucose intolerant, it was surprising that fasting insulin was not elevated. It is plausible that in response to the intraperitoneal glucose challenge, eET-1 mice had enhanced insulin secretion/hyperinsulinemia relative to WT mice, indicative of systemic insulin resistance; however, we were unable to measure insulin during the GTT to confirm this in our current data set.
Collectively, we present evidence against the hypothesis that ET-1 signaling is implicated in the development of visceral AT inflammation associated with obesity while supporting a role for ET-1 in promoting glucose intolerance. Furthermore, we provide evidence that the salutary effects of exercise on AT and metabolic function are not reliant on the suppression of ET-1 signaling.
GRANTS
This work was supported in part by the NIH Grants R01-HL-137769 (to J. Padilla), K01-HL-125503 (to J. Padilla), R01-HL-136746 (to W. P. Fay), and R01-HL-088105 (to L. A. Martinez-Lemus) and by American Heart Association Grant 17GRNT33671082 (to W. P. Fay). P. J. Fadel was supported by NIH Grant R01-HL-127071. J. R. Ball was supported by the NIH Initiative for Maximizing Student Diversity EXPRESS Fellows Program Grant R25-GM-056901. N. C. Winn is supported by NIH Grant T32-DK-07563. The work of E. L. Schiffrin and P. Paradis was supported by a Canadian Institutes of Health (CIHR) First Pilot Foundation Grant 143348, a Canada Research Chair (CRC) on Hypertension and Vascular Research by the CRC Government of Canada/CIHR Program, and by the Canada Fund for Innovation, all to E. L. Schiffrin. Bosentan was purchased with funds provided by the graduate research grant from the Sports, Cardiovascular, and Wellness Nutrition Dietetic practice group of the Academy of Nutrition and Dietetics (Z. I. Grunewald).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.L.S., V.J.V.-P., P.J.F., L.A.M.-L., and J.P. conceived and designed research; T.J.J., Z.I.G., M.L.W., J.R.B., T.N.S., A.W., A.L.R., K.F.S.-O., and Y.J. performed experiments; T.J.J., Z.I.G., M.L.W., N.C.W., J.R.B., and T.N.S. analyzed data; T.J.J., Z.I.G., N.C.W., W.P.F., P.P., E.L.S., V.J.V.-P., P.J.F., L.A.M.-L., and J.P. interpreted results of experiments; T.J.J., Z.I.G., and N.C.W. prepared figures; T.J.J. and J.P. drafted manuscript; T.J.J., Z.I.G., M.L.W., N.C.W., J.R.B., T.N.S., A.W., A.L.R., K.F.S.-O., Y.J., W.P.F., P.P., E.L.S., V.J.V.-P., P.J.F., L.A.M.-L., and J.P. edited and revised manuscript; T.J.J., Z.I.G., M.L.W., N.C.W., J.R.B., T.N.S., A.W., A.L.R., K.F.S.-O., Y.J., W.P.F., P.P., E.L.S., V.J.V.-P., P.J.F., L.A.M.-L., and J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Mariana Quinones-Morales and Francisco Ramirez-Perez for collection of human adipose tissue biopsies.
REFERENCES
- 1.Amiri F, Paradis P, Reudelhuber TL, Schiffrin EL. Vascular inflammation in absence of blood pressure elevation in transgenic murine model overexpressing endothelin-1 in endothelial cells. J Hypertens 26: 1102–1109, 2008. doi: 10.1097/HJH.0b013e3282fc2184. [DOI] [PubMed] [Google Scholar]
- 2.Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TL, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation 110: 2233–2240, 2004. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- 3.Barhoumi T, Briet M, Kasal DA, Fraulob-Aquino JC, Idris-Khodja N, Laurant P, Paradis P, Schiffrin EL. Erythropoietin-induced hypertension and vascular injury in mice overexpressing human endothelin-1: exercise attenuated hypertension, oxidative stress, inflammation and immune response. J Hypertens 32: 784–794, 2014. doi: 10.1097/HJH.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 4.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 5.Baynard T, Vieira-Potter VJ, Valentine RJ, Woods JA. Exercise training effects on inflammatory gene expression in white adipose tissue of young mice. Mediators Inflamm 2012: 767953, 2012. doi: 10.1155/2012/767953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab 295: E586–E594, 2008. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briançon-Marjollet A, Monneret D, Henri M, Hazane-Puch F, Pepin JL, Faure P, Godin-Ribuot D. Endothelin regulates intermittent hypoxia-induced lipolytic remodelling of adipose tissue and phosphorylation of hormone-sensitive lipase. J Physiol 594: 1727–1740, 2016. doi: 10.1113/JP271321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 290: E961–E967, 2006. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 9.Campia U, Tesauro M, Di Daniele N, Cardillo C. The vascular endothelin system in obesity and type 2 diabetes: pathophysiology and therapeutic implications. Life Sci 118: 149–155, 2014. doi: 10.1016/j.lfs.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 106: 1783–1787, 2002. doi: 10.1161/01.CIR.0000032260.01569.64. [DOI] [PubMed] [Google Scholar]
- 11.Chien Y, Lai YH, Kwok CF, Ho LT. Endothelin-1 suppresses long-chain fatty acid uptake and glucose uptake via distinct mechanisms in 3T3-L1 adipocytes. Obesity (Silver Spring) 19: 6–12, 2011. doi: 10.1038/oby.2010.124. [DOI] [PubMed] [Google Scholar]
- 12.Crissey JM, Jenkins NT, Lansford KA, Thorne PK, Bayless DS, Vieira-Potter VJ, Rector RS, Thyfault JP, Laughlin MH, Padilla J. Adipose tissue and vascular phenotypic modulation by voluntary physical activity and dietary restriction in obese insulin-resistant OLETF rats. Am J Physiol Regul Integr Comp Physiol 306: R596–R606, 2014. doi: 10.1152/ajpregu.00493.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong F, Zhang X, Wold LE, Ren Q, Zhang Z, Ren J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol 145: 323–333, 2005. doi: 10.1038/sj.bjp.0706193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duerrschmidt N, Wippich N, Goettsch W, Broemme HJ, Morawietz H. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem Biophys Res Commun 269: 713–717, 2000. doi: 10.1006/bbrc.2000.2354. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson AK, van Harmelen V, Stenson BM, Aström G, Wåhlén K, Laurencikiene J, Rydén M. Endothelin-1 stimulates human adipocyte lipolysis through the ET A receptor. Int J Obes 33: 67–74, 2009. doi: 10.1038/ijo.2008.212. [DOI] [PubMed] [Google Scholar]
- 16.Ferri C, Bellini C, Desideri G, Baldoncini R, Properzi G, Santucci A, De Mattia G. Circulating endothelin-1 levels in obese patients with the metabolic syndrome. Exp Clin Endocrinol Diabetes 105, Suppl 2: 38–40, 1997. doi: 10.1055/s-0029-1211794. [DOI] [PubMed] [Google Scholar]
- 17.Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol Metab 7: 161–170, 2018. doi: 10.1016/j.molmet.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher-Wellman KH, Ryan TE, Smith CD, Gilliam LA, Lin CT, Reese LR, Torres MJ, Neufer PD. A direct comparison of metabolic responses to high-fat diet in C57BL/6J and C57BL/6NJ mice. Diabetes 65: 3249–3261, 2016. doi: 10.2337/db16-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitts RH, Booth FW, Winder WW, Holloszy JO. Skeletal muscle respiratory capacity, endurance, and glycogen utilization. Am J Physiol 228: 1029–1033, 1975. doi: 10.1152/ajplegacy.1975.228.4.1029. [DOI] [PubMed] [Google Scholar]
- 20.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316: 129–139, 2010. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Ganeshan K, Chawla A. Warming the mouse to model human diseases. Nat Rev Endocrinol 13: 458–465, 2017. doi: 10.1038/nrendo.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes 58: 2238–2245, 2009. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunewald ZI, Winn NC, Gastecki ML, Woodford ML, Ball JR, Hansen SA, Sacks HS, Vieira-Potter VJ, Padilla J. Removal of interscapular brown adipose tissue increases aortic stiffness despite normal systemic glucose metabolism in mice. Am J Physiol Regul Integr Comp Physiol 314: R584–R597, 2018. doi: 10.1152/ajpregu.00332.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horinouchi T, Hoshi A, Harada T, Higa T, Karki S, Terada K, Higashi T, Mai Y, Nepal P, Mazaki Y, Miwa S. Endothelin-1 suppresses insulin-stimulated Akt phosphorylation and glucose uptake via GPCR kinase 2 in skeletal muscle cells. Br J Pharmacol 173: 1018–1032, 2016. doi: 10.1111/bph.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 542: 177–185, 2017. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 26.Idris-Khodja N, Ouerd S, Trindade M, Gornitsky J, Rehman A, Barhoumi T, Offermanns S, Gonzalez FJ, Neves MF, Paradis P, Schiffrin EL. Vascular smooth muscle cell peroxisome proliferator-activated receptor γ protects against endothelin-1-induced oxidative stress and inflammation. J Hypertens 35: 1390–1401, 2017. doi: 10.1097/HJH.0000000000001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javeshghani D, Barhoumi T, Idris-Khodja N, Paradis P, Schiffrin EL. Reduced macrophage-dependent inflammation improves endothelin-1-induced vascular injury. Hypertension 62: 112–117, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01298. [DOI] [PubMed] [Google Scholar]
- 28.Juan CC, Chang CL, Lai YH, Ho LT. Endothelin-1 induces lipolysis in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 288: E1146–E1152, 2005. doi: 10.1152/ajpendo.00481.2004. [DOI] [PubMed] [Google Scholar]
- 29.Juan CC, Chang LW, Huang SW, Chang CL, Lee CY, Chien Y, Hsu YP, Ho PH, Chen YC, Ho LT. Effect of endothelin-1 on lipolysis in rat adipocytes. Obesity (Silver Spring) 14: 398–404, 2006. doi: 10.1038/oby.2006.53. [DOI] [PubMed] [Google Scholar]
- 30.Kasımay O, Ergen N, Bilsel S, Kaçar O, Deyneli O, Gogas D, Akalın S, Yeğen BC, Kurtel H. Diet-supported aerobic exercise reduces blood endothelin-1 and nitric oxide levels in individuals with impaired glucose tolerance. J Clin Lipidol 4: 427–434, 2010. doi: 10.1016/j.jacl.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Kawanishi N, Mizokami T, Yano H, Suzuki K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med Sci Sports Exerc 45: 1684–1693, 2013. doi: 10.1249/MSS.0b013e31828ff9c6. [DOI] [PubMed] [Google Scholar]
- 32.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548–2556, 2004. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 33.Kohlgruber AC, LaMarche NM, Lynch L. Adipose tissue at the nexus of systemic and cellular immunometabolism. Semin Immunol 28: 431–440, 2016. doi: 10.1016/j.smim.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Leung JW, Wong WT, Koon HW, Mo FM, Tam S, Huang Y, Vanhoutte PM, Chung SS, Chung SK. Transgenic mice over-expressing ET-1 in the endothelial cells develop systemic hypertension with altered vascular reactivity. PLoS One 6: e26994, 2011. doi: 10.1371/journal.pone.0026994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li MW, Mian MO, Barhoumi T, Rehman A, Mann K, Paradis P, Schiffrin EL. Endothelin-1 overexpression exacerbates atherosclerosis and induces aortic aneurysms in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 33: 2306–2315, 2013. doi: 10.1161/ATVBAHA.113.302028. [DOI] [PubMed] [Google Scholar]
- 36.Lteif A, Vaishnava P, Baron AD, Mather KJ. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes 56: 728–734, 2007. doi: 10.2337/db06-1406. [DOI] [PubMed] [Google Scholar]
- 37.Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol (1985) 95: 336–341, 2003. doi: 10.1152/japplphysiol.01016.2002. [DOI] [PubMed] [Google Scholar]
- 38.Man K, Kutyavin VI, Chawla A. Tissue immunometabolism: development, physiology, and pathobiology. Cell Metab 25: 11–26, 2017. doi: 10.1016/j.cmet.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchesi C, Rehman A, Rautureau Y, Kasal DA, Briet M, Leibowitz A, Simeone SM, Ebrahimian T, Neves MF, Offermanns S, Gonzalez FJ, Paradis P, Schiffrin EL. Protective role of vascular smooth muscle cell PPARγ in angiotensin II-induced vascular disease. Cardiovasc Res 97: 562–570, 2013. doi: 10.1093/cvr/cvs362. [DOI] [PubMed] [Google Scholar]
- 40.Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes 51: 3517–3523, 2002. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- 41.Neuhofer A, Wernly B, Leitner L, Sarabi A, Sommer NG, Staffler G, Zeyda M, Stulnig TM. An accelerated mouse model for atherosclerosis and adipose tissue inflammation. Cardiovasc Diabetol 13: 23, 2014. doi: 10.1186/1475-2840-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishiyama SK, Zhao J, Wray DW, Richardson RS. Vascular function and endothelin-1: tipping the balance between vasodilation and vasoconstriction. J Appl Physiol (1985) 122: 354–360, 2017. doi: 10.1152/japplphysiol.00772.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olausson J, Daka B, Hellgren MI, Larsson CA, Petzold M, Lindblad U, Jansson PA. Endothelin-1 as a predictor of impaired glucose tolerance and type 2 diabetes—A longitudinal study in the Vara-Skövde Cohort. Diabetes Res Clin Pract 113: 33–37, 2016. doi: 10.1016/j.diabres.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 44.Padilla J, Carpenter AJ, Das NA, Kandikattu HK, López-Ongil S, Martinez-Lemus LA, Siebenlist U, DeMarco VG, Chandrasekar B. TRAF3IP2 mediates high glucose-induced endothelin-1 production as well as endothelin-1-induced inflammation in endothelial cells. Am J Physiol Heart Circ Physiol 314: H52–H64, 2018. doi: 10.1152/ajpheart.00478.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padilla J, Olver TD, Thyfault JP, Fadel PJ. Role of habitual physical activity in modulating vascular actions of insulin. Exp Physiol 100: 759–771, 2015. doi: 10.1113/EP085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park YM, Padilla J, Kanaley JA, Zidon TM, Welly RJ, Britton SL, Koch LG, Thyfault JP, Booth FW, Vieira-Potter VJ. Voluntary running attenuates metabolic dysfunction in ovariectomized low-fit rats. Med Sci Sports Exerc 49: 254–264, 2017. doi: 10.1249/MSS.0000000000001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez LM, Pareja-Galeano H, Sanchis-Gomar F, Emanuele E, Lucia A, Gálvez BG. ‘Adipaging’: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J Physiol 594: 3187–3207, 2016. doi: 10.1113/JP271691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pernow J, Shemyakin A, Böhm F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci 91: 507–516, 2012. doi: 10.1016/j.lfs.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 49.Porter JW, Rowles JL III, Fletcher JA, Zidon TM, Winn NC, McCabe LT, Park YM, Perfield JW II, Thyfault JP, Rector RS, Padilla J, Vieira-Potter VJ. Anti-inflammatory effects of exercise training in adipose tissue do not require FGF21. J Endocrinol 235: 97–109, 2017. doi: 10.1530/JOE-17-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 51.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 13: 633–643, 2017. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds LJ, Credeur DP, Manrique C, Padilla J, Fadel PJ, Thyfault JP. Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. J Appl Physiol (1985) 122: 38–47, 2017. doi: 10.1152/japplphysiol.00286.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shemyakin A, Salehzadeh F, Böhm F, Al-Khalili L, Gonon A, Wagner H, Efendic S, Krook A, Pernow J. Regulation of glucose uptake by endothelin-1 in human skeletal muscle in vivo and in vitro. J Clin Endocrinol Metab 95: 2359–2366, 2010. doi: 10.1210/jc.2009-1506. [DOI] [PubMed] [Google Scholar]
- 54.Shemyakin A, Salehzadeh F, Esteves Duque-Guimaraes D, Böhm F, Rullman E, Gustafsson T, Pernow J, Krook A. Endothelin-1 reduces glucose uptake in human skeletal muscle in vivo and in vitro. Diabetes 60: 2061–2067, 2011. doi: 10.2337/db10-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srere PA. Citrate synthase [EC 4.1.3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. In: Methods in Enzymology, edited by Lowenstein JM. Academic Press, 1969, vol. 13, p. 3–11. [Google Scholar]
- 56.Stehouwer CDA. Microvascular dysfunction and hyperglycemia: a vicious cycle with widespread consequences. Diabetes 67: 1729–1741, 2018. doi: 10.2337/dbi17-0044. [DOI] [PubMed] [Google Scholar]
- 57.Tang ST, Su H, Zhang Q, Tang HQ, Wang CJ, Zhou Q, Wei W, Zhu HQ, Wang Y. Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-κB/IκBα system through the activation of AMP-activated protein kinase. Int J Mol Med 37: 1558–1566, 2016. doi: 10.3892/ijmm.2016.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trim W, Turner JE, Thompson D. Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front Immunol 9: 169, 2018. doi: 10.3389/fimmu.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab 296: E1164–E1171, 2009. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vieira-Potter VJ, Padilla J, Park YM, Welly RJ, Scroggins RJ, Britton SL, Koch LG, Jenkins NT, Crissey JM, Zidon T, Morris EM, Meers GM, Thyfault JP. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 308: R530–R542, 2015. doi: 10.1152/ajpregu.00401.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wainright KS, Fleming NJ, Rowles JL, Welly RJ, Zidon TM, Park YM, Gaines TL, Scroggins RJ, Anderson-Baucum EK, Hasty AH, Vieira-Potter VJ, Padilla J. Retention of sedentary obese visceral white adipose tissue phenotype with intermittent physical activity despite reduced adiposity. Am J Physiol Regul Integr Comp Physiol 309: R594–R602, 2015. doi: 10.1152/ajpregu.00042.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winn NC, Grunewald ZI, Gastecki ML, Woodford ML, Welly RJ, Clookey SL, Ball JR, Gaines TL, Karasseva NG, Kanaley JA, Sacks HS, Vieira-Potter VJ, Padilla J. Deletion of UCP1 enhances ex vivo aortic vasomotor function in female but not male mice despite similar susceptibility to metabolic dysfunction. Am J Physiol Endocrinol Metab 313: E402–E412, 2017. doi: 10.1152/ajpendo.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winn NC, Jurrissen TJ, Grunewald ZI, Cunningham RP, Woodford ML, Kanaley JA, Lubahn DB, Manrique-Acevedo C, Rector RS, Vieira-Potter VJ, Padilla J. Estrogen receptor-α signaling maintains immunometabolic function in males and is obligatory for exercise-induced amelioration of nonalcoholic fatty liver. Am J Physiol Endocrinol Metab 316: E156–E167, 2019. doi: 10.1152/ajpendo.00259.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zidon TM, Park YM, Welly RJ, Woodford ML, Scroggins RJ, Britton SL, Koch LG, Booth FW, Padilla J, Kanaley JA, Vieira-Potter VJ. Voluntary wheel running improves adipose tissue immunometabolism in ovariectomized low-fit rats. Adipocyte 7: 20–34, 2018. doi: 10.1080/21623945.2017.1402991. [DOI] [PMC free article] [PubMed] [Google Scholar]