Abstract
CB1 receptor (CB1R) antagonism improves the deleterious effects of a high-fat diet (HFD) by reducing body fat mass and adipocyte cell size. Previous studies demonstrated that the beneficial effects of the CB1R antagonist rimonabant (RIM) in white adipose tissue (WAT) are partially due to an increase of mitochondria numbers and upregulation thermogenesis markers, suggesting an induction of WAT beiging. However, the molecular mechanism by which CB1R antagonism induces weight loss and WAT beiging is unclear. In this study, we probed for genes associated with beiging and explored longitudinal molecular mechanisms by which the beiging process occurs. HFD dogs received either RIM (HFD+RIM) or placebo (PL) (HFD+PL) for 16 wk. Several genes involved in beiging were increased in HFD+RIM compared with pre-fat, HFD, and HFD+PL. We evaluated lipolysis and its regulators including natriuretic peptide (NP) and its receptors (NPRs), β-1 and β-3 adrenergic receptor (β1R, β3R) genes. These genes were increased in WAT depots, accompanied by an increase in lipolysis in HFD+RIM. In addition, RIM decreased markers of inflammation and increased adiponectin receptors in WAT. We observed a small but significant increase in UCP1; therefore, we evaluated the newly discovered UCP1-independent thermogenesis pathway. We confirmed that SERCA2b and RYR2, the two key genes involved in this pathway, were upregulated in the WAT. Our data suggest that the upregulation of NPRs, β-1R and β-3R, lipolysis, and SERCA2b and RYR2 may be one of the mechanisms by which RIM promotes beiging and overall the improvement of metabolic homeostasis induced by RIM.
Keywords: adipose tissue beiging, CB1R antagonist, dogs, insulin resistance
INTRODUCTION
Obesity results when energy intake exceeds energy expenditure. The excess energy is stored in adipocytes, which go through hypertrophic and hyperplastic expansion during overfeeding. In addition, long-term consumption of a high-fat diet (HFD) induces inflammation within the adipose tissue, leading to insulin resistance and the metabolic syndrome (18). It is well known that white adipose tissue (WAT) is the main regulator of energy storage, whereas brown adipose tissue (BAT) is a main site of adaptive thermogenesis and is associated with increased energy expenditure and weight loss (51). Recently, it has been appreciated that WAT has the capacity to promote thermogenesis through a process that has now been termed “beiging.” Beige adipocytes, like BAT, contain a high content of mitochondria and uncoupling protein 1 (UCP1) and peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α) (23). Beiging can be induced by cold exposure, through nonshivering thermogenesis (7), and activation of β-1 adrenergic receptor (β1R) and β3R and is linked to diet-induced thermogenesis (46, 57). Inability to induce beiging of WAT in times of overfeeding has been speculated to contribute to obesity (3, 23).
The endocannabinoid system plays an important role in controlling body weight and energy homeostasis. Endocannabinoids are generated in the cell membrane from phospholipid precursors and activate the cannabinoid receptor subtypes CB1R and CB2R (20). CB1R has previously been demonstrated to be involved in feeding, energy expenditure, reward pathways, and metabolic homeostasis (54). Chronic treatment with the CB1R antagonist rimonabant (RIM) leads to weight loss and improved insulin sensitivity in obese rodents (16, 45), canines (29, 47), and humans (58). In addition to improvements in cardiometabolic risk factors, patients with abdominal obesity given CB1R antagonists had significant reductions in both intra-abdominal and liver fat (14). All metabolic improvements, including weight loss and reduction in adiposity, have been linked to upregulation of adiponectin and downregulation of cytokines within WAT (4, 17, 33, 55). We have previously shown that RIM increases plasma adiponectin and adiponectin gene expression in the subcutaneous (SC) and visceral (VIS) fat depots in the HFD dog (29). The increase in adiponectin following RIM treatment correlates with reductions in weight and fat mass (47). The reduced fat mass is, in part, due to a reduction in adipocyte cell size compared with adipocyte size in lean animals, despite maintenance on an HFD (30). These data correlate with a recent study demonstrating that RIM reduced the lipid content of BAT and increased activation of thermogenesis and energy expenditure (2).
Studies have shown that a nonbrain-penetrating CB1R antagonist induced BAT thermogenesis in mice (6). In addition, RIM increased UCP1 and PGC1α expressions and mitochondrial biogenesis in immortalized murine WAT (44), suggesting an induction of WAT beiging. More recently, another study demonstrated the important role of CB1R in WAT remodeling. CB1R knockout mice had alternatively activated macrophages and increased sympathetic tone in WAT that coincided with beiging, as well as protection from HFD-induced obesity (49). However, to date, most studies examining adipose tissue beiging have been conducted in cell lines and rodents [rodent fat depots are not similar to those in humans (11)] or data were derived from cross-sectional analysis and mostly limited to SC fat tissue. The canine model used in our laboratory facilitated the longitudinal characterization of the physiological and molecular mechanisms underlying the beneficial effects of RIM in adipose tissue. We showed in previous studies that after 16 wk of RIM treatment, body weight and abdominal fat were reduced in canines (30, 47). However, body weight changes were not associated with changes in either basal resting metabolic rate or food intake, and rectal temperature did not change throughout the study (47). We hypothesized that one of the mechanisms by which RIM improves metabolic function is by enhancing adipose tissue metabolism through adipose tissue beiging. The present study analyzed longitudinal effects of CB1R antagonism treatment on WAT beiging and the molecular mechanisms through which the beiging is induced in HFD canines. Therefore, we probed genes involved in WAT beiging (61), such as PGC1α, PR domain containing 16 (Prdm16), UCP1, fibroblast growth factor 21 (FGF21), cyclooxygenase 2 (Cox2), fatty acid elongase 3 (Elovl3), type II iodothyronine deiodinase (Dio2), and cell death activator (Cidea). We also investigated the mechanisms by which RIM could alter markers of beiging. It has been demonstrated that RIM can potentiate the release of cardiac natriuretic peptides (NPs) (48), which can induce beiging and increase lipolysis of WAT (21). Specifically, NPs are able to activate lipolysis via activation of their own receptors (34). NPs have been demonstrated to induce adipose tissue lipolysis via a cGMP-dependent pathway (34). In this pathway, atrial NP receptor 1 (NPR1) activation increases production of cGMP to activate protein kinase G, which is thought to stimulate lipolysis through the phosphorylation of hormone-sensitive lipase (HSL) and the lipid droplet-associated protein perilipin (53). In addition, adipose triglyceride lipase (ATGL) coordinates with HSL to catabolize lipids in adipose tissue (63). Therefore, we also measured NPRs, HSL, and ATGL in addition to ex vivo lipolysis. It has been demonstrated that atrial NP (ANP) inhibits the secretion of factors involved in inflammation adipocyte insulin resistance (46). Therefore, we further tested the hypothesis that RIM-induced improvements in insulin sensitivity observed in our canine model may be due to improvements in adipose tissue inflammation. Together, we demonstrate longitudinally how CB1R antagonism plays a crucial role in adipose tissue beiging and improvements in metabolic homeostasis in an HFD model.
MATERIALS AND METHODS
Animals and diet.
Twenty male mongrel 1-yr-old dogs (body weight: 29 ± 0.9 kg) were housed in the vivarium of the Keck School of Medicine, University of Southern California, under controlled kennel conditions (12:12-h light-dark cycle) at room temperature (RT), as described previously (33, 47). All research experiments were performed following ethical standards for animal research, including full compliance with the approved protocol by the Institutional Animal Care and Use Committee of the University of Southern California, and all surgeries were performed under 3% inhaled isoflurane.
Upon arrival, dogs underwent a period of acclimation for 3 wk, during which time they were fed a standard diet to ensure weight stabilization before any experiments were conducted. After 3 wk, dogs were fed a hypercaloric HFD that consisted of ~5,527 kcal/day (28% carbohydrates, 53% lipids, and 19% proteins), representing an approximate 20% increase in calories from fat (30, 32, 47). Previous studies have demonstrated that 6 wk of fat feeding is sufficient to promote a consistent increase of adiposity in the VIS and SC depot, rendering dogs insulin resistant (31). In our study, the HFD was maintained for 9 ± 2 wk before RIM treatment. Data about food intake, body weight, resting energy expenditure, and some biochemical parameters have been published elsewhere (47). Briefly, meals were presented daily for 3 h, from 9:00 AM to 12:00 PM. Meals were semisolid (melted lard was mixed well with the control dry chow diet to create the HFD) and were weighed before presentation. After the 3-h feeding, all leftover food was collected, including any spillage, and the bowl was weighed again. The difference between the weights of total food presented (based on each dog’s body weight to achieve a total daily calorie meal of 3,885 kcal) and the weight of leftover food represented the absolute amount of food eaten by each animal each day.
Assessment of basal resting metabolic rate and body temperature.
In a subset of dogs [RIM, n = 7; placebo (PL), n = 5], we assessed resting metabolic rate by indirect calorimetry. All animals underwent metabolic assessments in random order at three time periods: before fat feeding (pre-fat), after 6 wk of an initial fat feeding period to presumably induce insulin resistance (HFD), and after an additional 16 wk of fat feeding concomitant with HFD+PL or HFD+RIM. Dogs were fasted for at least 12 h before each experiment.
Basal resting metabolic rate, which in humans accounts for more than 50% of the daily energy expenditure (36, 40), was measured as described previously (47). The calorimetric chamber used consisted of a standard dog kennel adapted for the experiments. We also assessed a single core body temperature measurement before each in vivo experiment by measuring dog rectal temperature as described by Greer et al. (19). We did not measure 24-h total energy expenditure because of technical difficulties related to the use of large animals in our study.
Treatment.
After HFD initiation, dogs were randomly assigned to 1 of 2 groups while being maintained on the HFD for an additional 16 wk: 1 group received RIM (HFD+RIM, n = 11) and the other group received PL (HFD+PL, n = 9). Each animal was on 22 wk of an HFD by the end of the study and 16 wk on RIM or PL treatments. RIM (Sanofi-Aventis, Paris, France) was encapsulated (AMC Pharmacy, Burbank, CA) and administered orally at 1.25 mg·kg−1·day−1. PL-treated animals received inert capsules (47).
Tissue biopsies.
Unique to the canine model is that biopsies can be taken during the longitudinal physiological assessments of the animal, which is hard to get consent for in humans and not possible to perform in rodents. All biopsies were obtained under general inhalant anesthesia and under sterile conditions in a surgery room at the University of Southern California. All biopsies were performed by two surgeons. Animals were fasted overnight (~12 h), sedated with acepromazine maleate (0.22 mg/kg; Prom-Ace, Aueco, Fort Dodge, IA) and atropine sulfate (Western Medical, Arcadia, CA; 0.11 mL/kg) before endotracheal intubation. Anesthesia was induced with sodium pentobarbital (0.5 mL/kg; Western Medical) or propofol (6 mg/kg; Western Medical) and maintained with 3% isoflurane (Western Medical). Blood pressure (cuff on hind leg), heart rate, O2 saturation, and CO2 were monitored continuously. Bupivacaine hydrochloride, a local anesthetic, was administered subcutaneously before surgical incisions. Biopsies were taken from SC fat depots at the level of the anterior upper trunk using a midline incision. Biopsies of VIS fat were collected from the fat located between the VIS peritoneum and the small intestine. Biopsies of SC fat were collected from under the skin on the ventral trunk. To avoid the SC scar tissue, subsequent biopsies were taken 0.5 cm anterior to the previous biopsy. All animals that underwent surgery were followed up until full recovery. All biopsies were collected at 3 time periods before fat feeding (pre-fat), after 6 wk of an initial fat feeding period to presumably induce insulin resistance (HFD), and after an additional 16 wk of fat feeding concomitant with HFD+PL or HFD+RIM. Biopsies were collected swiftly and ~500 mg of tissue was excised and placed immediately in RNAse-free conditions in liquid nitrogen. The tissues were stored at −80°C until processing.
Total RNA isolation and real-time PCR.
RNA was extracted from biopsied tissues using the Tri-Reagent Kit (Molecular Research Center, Cincinnati, OH). First-strand cDNA was synthesized per the manufacturer’s protocol from 1 µg total RNA obtained from the adipose tissue biopsies using Superscript II (Invitrogen, Carlsbad, CA). Real-time PCR was performed using a LightCycler 4.8 instrument (Roche Applied Science, Indianapolis, IN). cDNA was amplified using the “universal probe system” on a Roche microplate with a final volume of 10 µL reaction mix containing 2.5 µL, 100-fold diluted cDNA, 7 µL LightCycler TaqMan Master Mix buffer (Roche Probes Master kit, Roche Applied Science), 1 µM specific forward-reverse primers, and 0.5µL specific universal probes. Primers and universal probes are shown in Table 1. For sarcoendoplasmic reticulum calcium (SERCA2b) and ryanodine receptor 2 (RYR2) gene expressions, we used TaqMan probes Mm01201431-m1 and Cf02674741-m1, respectively (Thermo Fisher Scientific, Canoga Park, CA). The specificity of amplification was determined by melting curve analysis. The results were analyzed by relative quantification, the ΔΔC method.
Table 1.
List of primers and universal probes used in the real-time PCR
| Gene | Forward | Reverse | UPL |
|---|---|---|---|
| PGC1α | CACACACAATCGCAGTCTCA | GGGTCATTTGGTGACTCTGG | 6 |
| Prdm16 | CCGAGTGTGGGAAGACATTT | AAGGTTTGACGGTGCTGTG | 88 |
| FGF21 | ACTGTGGGTCCCTGTGCT | GGAGTCAGGGATCGGATGT | 11 |
| Cidea | GCAGGCTCCTCCCAAGTC | CCCTGCAGCTTGCCTATCT | 50 |
| Cox2 | CCCTCTTTAACCGTGAAAACA | GGGATTATGTAGGAGTCAAAGTTT | 49 |
| Elovl3 | ACTGGATGGTTTGCCAGCTA | AGGATGATGAAGGCCGTGT | 14 |
| Dio2 | TGACTCGGTCATTCTCCTCA | CAGTTTCACCTGCTTGTAGGC | 69 |
| UCP1 | GGATCTCCGCTGGTGTAATG | CCTCAGTGGGTTGCCCTAT | 52 |
| NPR1 | CAACGGGACTTCCCAAGAG | TGGGGATGTCAGGAGGTG | 83 |
| NPR2 | TTTCCGTCCTTACAGAAAGCTG | ATGCGCCACAACATGCTA | 17 |
| NPR3 | TCGCTATGACAGACCCAGAA | CGCATTTCAAAACGACCTTC | 79 |
| ADR1 | TCCCCTGGCTCTATTACTCCT | CAGGACACAGACGATGGAGA | 76 |
| ADR2 | CCCAAACATCTCCTTTGTGG | GCCCCCAAGAAGAATAATCC | 2 |
| IL-6 | CTCCACAAGCGCCTTCTC | CGGGGTAGGGAAAGCAGTA | 9 |
| TNFα | GCCGTCTCCTACCAGACAAA | GGGTCTCCCTTTGGCAAG | 53 |
| β1R | GTCCTTCCTGCCCATCCT | AGCACTTGGGGTCGTTCT | 141 |
| β3R | CTTCGTGGCCAACGTGAT | GCGTAGCCCAGCCAGTTA | 79 |
| ATGL | GCCATGATGGTGCCCTAC | CCAGCAAGCGGATGGTAA | 34 |
β1R, β-1 adrenergic receptor; β3R, β-3 adrenergic receptor; ADR, adiponectin receptor; ATGL, adipose triglyceride lipase; Cidea, cell death activator; Cox2, cyclooxygenase; Dio2, type II iodothyronine deiodinase; Elovl3, fatty acid elongase 3; FGF21, fibroblast growth factor; NPR, natriuretic peptide receptor; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1α; Prdm16, PR domain containing 16; UCP1, uncoupling protein 1.
Western blot.
Protein lysates were prepared by homogenizing 100 mg of adipose tissue in Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA) supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The homogenate was centrifuged at 13,000 revolutions/min for 30 min at 4°C. The supernatant was collected, and total protein concentration in the supernatant was determined using a BCA Protein Assay kit (Thermo Fischer Scientific). Equal amounts of proteins were separated on a 10% Mini-Protean TGX precast SDS-PAGE gel (Bio-Rad Laboratories, Irvine, CA) by electrophoresis, transferred onto a nitrocellulose membrane (Bio-Rad Laboratories) using a Trans-Blot SD Semi Dry Transfer Cell (Bio-Rad Laboratories). Membranes were blocked with 5% nonfat dry milk in 1× Tris-buffered saline with Tween 20 for 1 h at RT and then incubated overnight at 4°C with 1:500 diluted polyclonal primary antibodies (Cell Signaling Technology). The membranes were then washed and incubated with 1:5,000 diluted Alexa Fluor 680 anti-rabbit secondary antibodies (Life Technology) for 1 h at RT. Signals were detected and quantitated with the Odyssey Infrared Imaging System (Li-COR, Lincoln, NE) and accompanying Li-COR software. The band densities of HSL and phosphorylated HSL (HSL-P) were normalized to that of β-actin.
Adipose tissue immunohistochemistry.
Formalin-fixed paraffin-embedded SC and VIS adipose tissue sections were deparaffinized and subsequently washed with distilled water, ethanol, and xylene and then counterstained with Harris hematoxylin (Histology Laboratory in Liver Center at the University of Southern California). For antigen staining, formalin-fixed paraffin-embedded sections were deparaffinized and rehydrated before antigen unmasking by boiling in 10 mM sodium citrate, pH 6.0, supplemented with 0.1% Tween. CD68 immunostaining was done using avidin-biotin complex method. Samples were blocked with 10% horse serum for 1 h at RT, incubated for 1 h at RT with mouse monoclonal anti-CD68 (1:100; Dako Laboratories), and labeled with biotinylated horse anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA) for 30 min at RT. Target antigen signal was amplified with avidin-biotin peroxidase complexes (Vector Laboratories) and 3,3-diaminobenzidine tetrachloride chromogen reagent (Vector Laboratories). For each slide, the number of CD68-positive crown-like structured macrophages were counted per 100 cells using light microscopy. For UCP1, fixed adipose tissues were processed by the Histology Core at Cedars-Sinai Medical Center. UCP1 staining was done with a rabbit polyclonal anti-UCP1 at dilution 1:500 (Abcam, Cambridge, MA). Images were captured using Olympus Camera and analyzed using Image J software (National Institutes of Health, Bethesda, MD).
Lipolysis.
Adipocytes from SC and VIS were isolated per the methods previously described (30). In vitro lipolysis was performed per Castro et al. (8). Isolated adipocyte suspensions were incubated for 2 h at 37°C in Krebs-Ringer buffer, with or without β-adrenergic receptor agonist isoproterenol at differing concentrations (1 × 10−8, 6.25 × 10−8, 1.25 × 10−7, 2.1 × 10−7, 2.5 × 10−7, 5.1 × 10−7, 5.1 × 10−6, and 1 × 10−5 mol/L). Lipolysis was estimated as the rate of glycerol released into the medium, measured in a 100-μL aliquot, using the Glycerol-Free Reagent kit (Sigma-Aldrich). Cell number was counted in three 10-μL aliquots, each at 4× using Image-Pro Express. Lipolysis results were expressed in μmol·106 cells−1·2 h−1.
Biochemical parameters.
Plasma IL-6 and TNFα were measured using canine ELISA kits (R&D systems, Minneapolis, MN). Plasma ANPs were analyzed by an ELISA kit (Sigma-Aldrich). Biochemical parameter measurements, including glucose, insulin, free fatty acids (FFAs), and adiponectin, were processed as previously published (33, 47).
Statistical analysis.
All experimental data presented in this study are expressed as means ± SE unless otherwise indicated. Normally distributed data were analyzed using repeated measures ANOVA, and nonnormally distributed data were analyzed using the Wilcoxon signed ranked test. As multiple post hoc statistical tests were performed simultaneously on a single data set, Bonferroni corrections were applied. P < 0.05 was considered statistically significant. All statistical analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
RIM decreased body weight and trunk fat despite no change in food intake, resting energy expenditure, and body temperature.
The main metabolic changes induced by the HFD in this model have been previously published. By week 16 of treatment, the HFD+RIM group lost 2.5% of their body weight (HFD: 31.7 ± 1.3 kg; HFD+RIM: 30.8 ± 1.1 kg; P < 0.001), whereas the HFD+PL group increased their body weight by 6.9% compared with HFD (33.9 ± 1.9 kg, P < 0.001). Detailed food intake and energy expenditure data were published previously (47). Briefly, food intake that was normalized to body weight showed a significant difference between groups only at week 2; however, we did not observe future changes in food intake throughout the study (47). Basal resting metabolic rate did not differ between HFD+RIM and HFD+PL groups by week 16, the time of study completion (47). At the end of the study, rectal temperature also remained unchanged between groups (pre-fat: 38.82 ± 0.21°C; HFD: 38.64 ± 0.20°C, HFD+PL; 38.44 ± 0.10°C; HFD+RIM: 39.03 ± 0.22°C). HFD+RIM reduced SC fat by 21% compared with HFD+PL (HFD: 374 ± 75 cm3; HFD+RIM: 379 ± 48 cm3; HFD+PL: 480 ± 97 cm3; P < 0.05), whereas HFD+RIM decreased the VIS depot by 13% compared with HFD+PL (HFD: 506 ± 70 cm3; HFD+RIM: 555 ± 64 cm3; HFD+PL: 641 ± 103 cm3; P < 0.05) (30). Thus, RIM decreased both SC and VIS fat depots compared with PL.
Markers of beiging are increased in VIS and SC adipose tissues following RIM treatment.
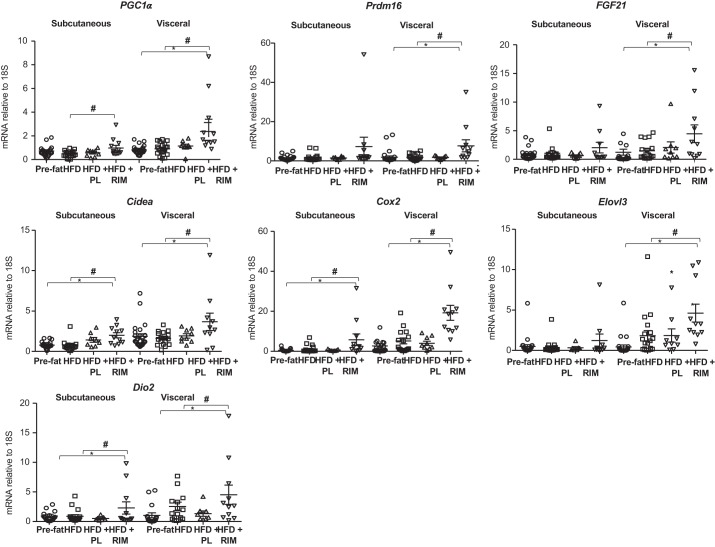
To explore whether the improvements in metabolic function and reductions in fat mass were secondary to beiging of adipose tissue in the fat-fed dog, we probed the adipose tissue for markers of adipose tissue beiging (Fig. 1). The VIS depot of HFD+RIM, compared with pre-fat and HFD periods, had significantly increased expression of markers associated with beiging, including PGC1α (P < 0.01), Prdm16 (P < 0.05), FGF21 (P < 0.05), Cidea (P < 0.01), Cox2 (P < 0.01), Elovl3 (P < 0.05), and Dio2 (P < 0.05). Similar results were seen in the SC depot, with increases in the HFD+RIM group in PGC1α (P < 0.05), Cidea (P < 0.05), Cox2 (P < 0.05), and Dio2 (P < 0.05) compared with pre-fat and HFD periods. Prdm16, FGF21, and Elovl3 were not significantly elevated in HFD+RIM SC tissues. No change in any of these markers of beiging were seen in either HFD or HFD+PL groups compared with the pre-fat and pre-fat or HFD time points, respectively. For the first time, we demonstrate the upregulation of several beiging genes following chronic CB1R antagonist treatment in a canine model.
Fig. 1.
Peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α), PR domain containing 16 (Prdm16), fibroblast growth factor (FGF21), cell death activator (Cidea), cyclooxygenase (Cox2), fatty acid elongase 3 (Elovl3), and type II iodothyronine deiodinase (Dio2) gene expressions in the subcutaneous and visceral adipose tissue. Placebo (PL), n = 9; rimonabant (RIM), n = 11. P values vs. pre-fat period (*P < 0.05) and vs. high-fat diet (HFD) (#P < 0.05). Data are means ± SE.
UCP1-independent thermogenesis and glucose uptake genes are increased following treatment with RIM.
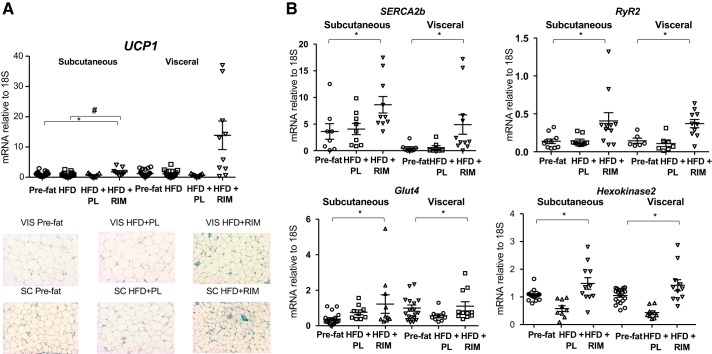
We measured UCP1 gene expression and UCP1 staining in both fat depots. Unexpectedly, RIM treatment did not induce a statistically significant increase in UCP1 gene expression in the VIS, but it did result in a statistically significant increase in the SC depot (P < 0.05, Fig. 2A). Consistent with these results, immunostaining of UCP1 showed less UCP1 staining in both SC and VIS (Fig. 2A).
Fig. 2.
A: effect of rimonabant (RIM) on uncoupling protein 1 (UCP1) gene expression in the subcutaneous (SC) and visceral (VIS) adipose tissue with representative immunohistochemistry images of UCP1 protein in the SC and VIS adipose tissue. B: sarcoendoplasmic reticulum calcium ATPase 2b (SERCA2b), ryanodine receptor 2 (RyR2), glucose transporter4 (Glut4), and hexokinase 2 gene expressions. Placebo (PL), n = 9; RIM, n = 11. P values vs. pre-fat period (*P < 0.05) and vs. high-fat diet (HFD) (#P < 0.05). Data are means ± SE.
Given the small increase in UCP1 found in our samples, we measured both RyR2 and SERCA2b in the SC and VIS depots (Fig. 2B). Expression of both genes increased significantly in the HFD+RIM SC (RyR2: P < 0.05; SERCA2b: P < 0.05) and VIS depots (RyR2: P < 0.01; SERCA2b: P < 0.005) compared with pre-fat and HFD groups. In addition, we measured genes involved in glucose uptake in the SC and VIS depots; glucose transporter 4 (Glut4) gene expression increased by 3.7-fold (P < 0.05) in the SC and 1.8-fold (P < 0.05) in the VIS depot. Consistent with that, hexokinase 2 gene expression increased by 2.8-fold in the SC (P < 0.001) and 3.3-fold (P < 0.001) in the VIS depot (Fig. 2B). These data show that decreases in adipose tissue inflammation, possibly via RIM-induced increases in NPs, and increases in glucose disposal activity, possibly because of RIM-induced beiging, are associated with improvements in insulin sensitivity in animals treated with a CB1R antagonist for 16 wk.
Taken together with the previously reported weight loss and glucose homeostasis results (47) and the upregulation of the Ca2+ cycling genes, we suggest that RIM promotes beiging through UCP1-independent pathways.
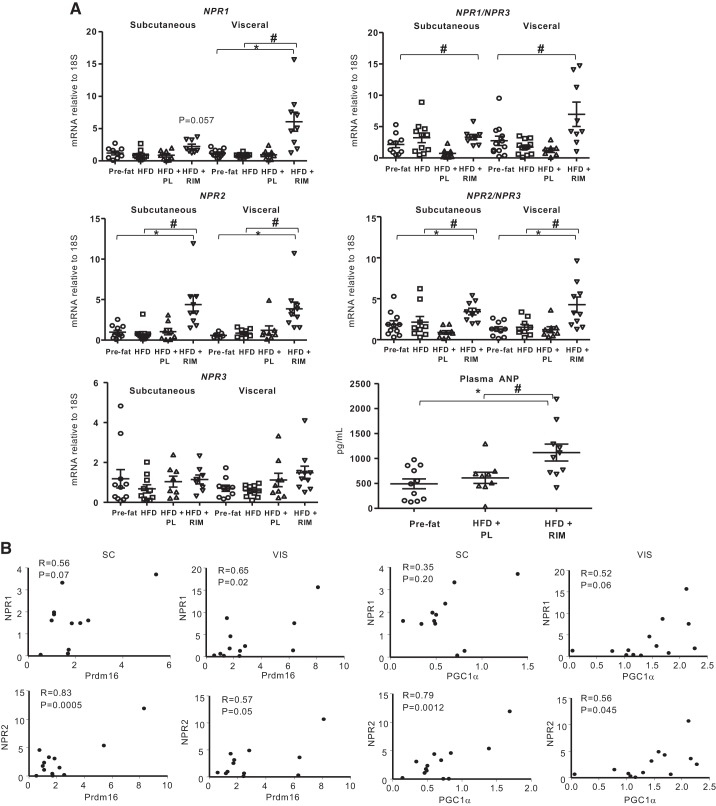
RIM increases NP signaling in WAT that is associated with increased markers of beiging.
It has been demonstrated that RIM can potentiate the release of cardiac NPs, which can induce beiging and increase lipolysis of WAT via activation of their own receptors (22, 52). Therefore, we measured NPR1 and NPR2, respectively, as well as the clearance receptor NPR3 in the SC and VIS depots. As shown in Fig. 3A, expression of NPR1 showed a tendency to increase (P = 0.057) and NPR2 expression significantly increased (P < 0.05) in the SC depot of HFD+RIM compared with pre-fat and HFD periods. NPR1 and NPR2 expression significantly increased in VIS (NPR1: P < 0.001; NPR2: P < 0.05) in the HFD+RIM group compared with HFD but not in the PL group. Importantly, NPR3 gene expression remained unchanged following HFD+RIM and HFD+PL. The increase in NPR1/NPR3 and NPR2/NPR3 following RIM treatment appears to be a result of an increase in NP secretion and not because of an increase in NP degradation. In addition, we measured plasma ANP in the subset of dogs from pre-fat (n = 10), HFD+PL (n = 8), and HFD+RIM (n = 8) groups. Plasma ANP increased two times in the HFD+RIM group compared with HFD+PL groups (P < 0.05) (Fig. 3A).
Fig. 3.
A: natriuretic peptide receptor (NPR)1, 2, and 3 gene expressions as well as NPR1/NPR3, NPR2/NPR3 in the subcutaneous (SC) and visceral (VIS) adipose tissue and plasma arterial natriuretic peptide (ANP). B: correlations between NPR1 and NPR2 with PR domain containing 16 (Prdm16) and peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α). Placebo (PL), n = 9; rimonabant (RIM), n = 11. P values vs. pre-fat period (*P < 0.05) and vs. high-fat diet (HFD) (#P < 0.05). Data are means ± SE.
The increased availability of NPs in the fat depot may contribute to beiging of WAT. We correlated the NPR expression with two beiging genes (Fig. 3B). In the SC depot, NPR1 showed a tendency to correlate with Prdm16 (r = 0.56, P = 0.07). In the VIS depot, NPR1 expression correlated with Prdm16 expression (r = 0.65, P < 0.05) and showed a tendency to correlate with PCG1α (r = 052, P = 0.06). The correlations were stronger between NPR2 and the beiging genes. NPR2 expression significantly correlated with Prdm16 and PGC1α expression in both depots (SC: r = 0.83, P < 0.001; r = 0.79, P < 0.01, respectively; VIS: r = 0.57, P = 0.05; r = 0.56, P < 0.05, respectively), producing a strong association between NPs and the expression of genes associated with beiging and suggesting that RIM treatment may also increase adipocyte lipolysis.
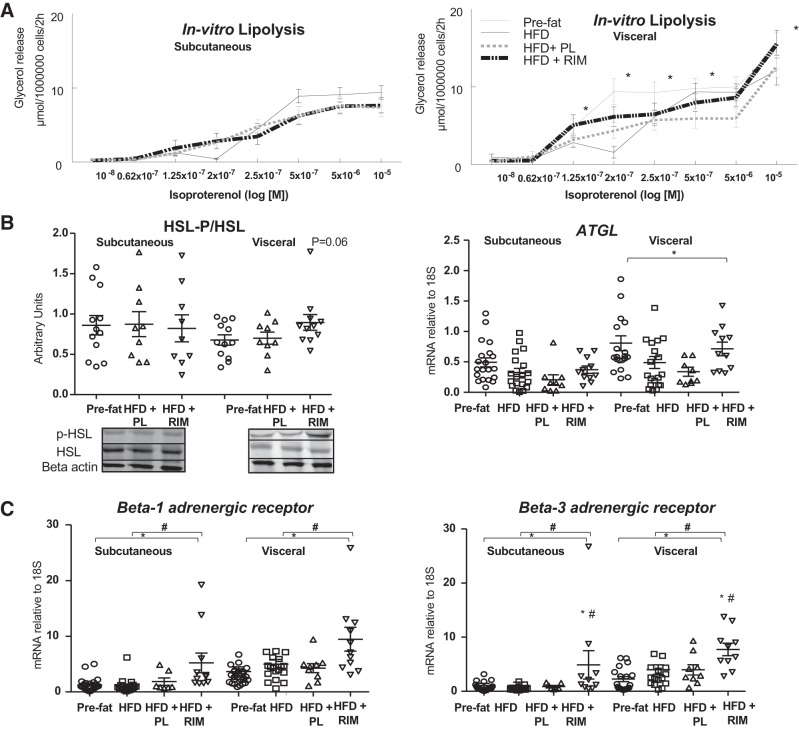
RIM induces lipolysis in adipose tissue, possibly through increased NP and β-adrenergic signaling.
We measured in vitro lipolysis in isolated adipocytes from SC and VIS depots in each group. Basal lipolysis was higher in the VIS depot (pre-fat: 0.41 ± 0.06, HFD: 0.60 ± 0.10, HFD+PL: 0.33 ± 0.05, HFD+RIM: 0.39 ± 0.06 per 106cells/2 h) compared with the SC depot (pre-fat: 0.21 ± 0.02, HFD: 0.32 ± 0.06, HFD+PL; 0.28 ± 0.05, HFD+RIM: 0.16 ± 0.03 μmol per 106 cells/2 h) in all groups. However, basal lipolysis was not significantly altered by exposure to the HFD or to the different treatments. Despite no change in basal lipolysis, isoproterenol-stimulated lipolysis was increased following RIM treatment in the VIS depot when compared with the HFD+PL group (P < 0.05; Fig. 4A). We also measured HSL-P/HSL protein by Western blot analysis and ATGL gene expression in the SC and VIS depots. Following treatment with RIM, there was a trend for HSL-P/HSL to increase in the VIS depot (P = 0.06) and ATGL gene expression significantly increased in both depots with RIM treatment compared with pre-fat and HFD period (P < 0.05) (Fig. 4B). Given that isoproterenol works through activation of the adrenergic receptors, we next examined β1R and β3R.
Fig. 4.
A: effect of rimonabant (RIM) of the subcutaneous and visceral adipose tissue on lipolysis in vitro. Concentration-response curves to isoproterenol; lipolysis was determined as the rate of glycerol release. B: protein expression of phosphorylated hormone-sensitive lipase (HSL-P)/HSL and adipose triglyceride lipase (ATGL) gene expression in the SC and VIS adipose tissue. Images of blots were cropped from the same gel. C: β-1 adrenergic receptor (β1R) and β3R gene expressions. P values vs. pre-fat period [placebo (PL), n = 9; rimonabant (RIM), n = 11; *P < 0.05] and vs. high-fat diet (HFD) (#P < 0.05). Data are means ± SE.
β1R and β3R activation and upregulation has previously been associated with thermogenesis and beiging of WAT (46, 57). Recently, Liu et al. (38) demonstrated that that the activation of mammalian target of rapamycin complex 1 (mTORC1) is necessary for NP-induced browning of adipocytes. In addition, activation of mTORC1 is essential for β-adrenergic stimulation of adipose tissue browning (37). We measured β1R and β3R expression in SC and VIS tissues and found it to be significantly upregulated in both depots in the HFD+RIM compared with pre-fat and HFD groups (P < 0.05, Fig. 4C). It is well established that β3R activation increases lipolysis via the cyclic AMP-protein kinase A pathway (21). The upregulation of the β1R and β3R pathway and the NPR pathway following RIM treatment appears to lead to enhanced lipolysis, reducing fat cell size and fat mass as a function of the beiging of WAT.
RIM-induced increases in adiponectin and its receptors may contribute to adipose tissue beiging.
As previously reported, fasting plasma glucose, glucagon, FFA, and adiponectin remained unchanged following 6 wk of an HFD. At the end of the 16-wk treatment period, fasting glucose, insulin, and FFA remained unchanged between the groups. However, adiponectin levels increased by 80% with HFD+RIM treatment compared with HFD+PL (P < 0.001) (33).
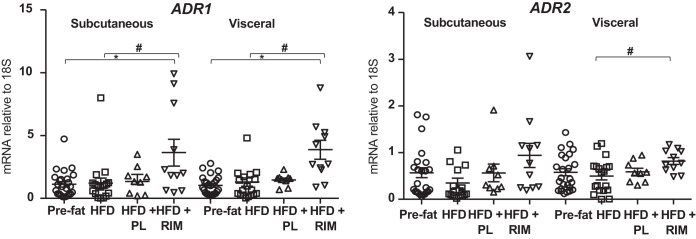
NPs increase adiponectin production in human adipocytes (5, 56). An increase in adiponectin has also been associated with beiging of adipose tissues (26). We previously demonstrated that adiponectin secretion and adiponectin gene expression are both significantly increased in HFD+RIM compared with both HFD and HFD+PL (30). Here, we further explored the association between NPs and adiponectin by measuring adiponectin receptors ADR1 and ADR2 in the SC and VIS depots. The expression of both receptors was increased following HFD+RIM in both fat depots (P < 0.05; Fig. 5). These data together suggest that adiponectin levels and activity are increased, likely because of an RIM-induced increase in NPs, and that both adiponectin and NPs are linked to the beiging properties of RIM.
Fig. 5.
Adiponectin receptor (ADR)1 and 2 gene expressions in the subcutaneous and visceral adipose tissue. P values vs. pre-fat period (*P < 0.05) and vs. high-fat diet (HFD) (#P < 0.05). Data are means ± SE. PL, placebo; RIM, rimonabant.
Reductions in inflammation and increases in glucose uptake are associated with RIM treatment and improvements in systemic insulin sensitivity.
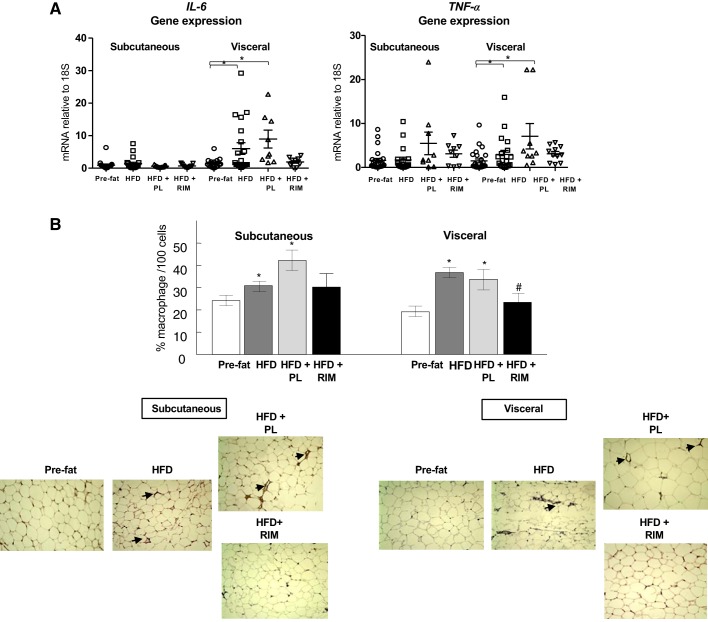
We have shown that inflammatory markers such as IL-6 and TNFα decreased in liver tissue, and plasma IL-6 and TNFα increased following the HFD and HFD+PL when compared with the pre-fat period, whereas in HFD+RIM plasma, cytokines decreased compared with HFD+PL (29). Consistent with these data, we found that in the VIS fat, exposure to the HFD increased IL-6 expression (HFD: P < 0.05) compared with the pre-fat period, and the increase was maintained during continuation of the HFD (HFD+PL: P < 0.05) compared with the pre-fat group (Fig. 6A). On the contrary, RIM reduced the expression of IL-6 back to the pre-fat level. TNFα showed similar results. In addition to the proinflammatory cytokines, we examined macrophage infiltration in the adipose tissue. We quantified the crown-like macrophage structures in the SC and VIS depots by staining for the macrophage marker CD68. HFD increased crown-like macrophages in both depots, which was exacerbated by continued HFD in the HFD+PL group. As expected, RIM treatment decreased the CD68 marker significantly in the HFD+RIM group compared with HFD+PL (P < 0.05) (Fig. 6B).
Fig. 6.
A: IL-6 and TNFα gene expression in the subcutaneous and visceral adipose tissue. P values vs. pre-fat period (*P < 0.05) and vs. high-fat diet (HFD) (#P < 0.05). Placebo (PL), n = 9; rimonabant (RIM), n = 11. Data are means ± SE. B: effect of RIM on CD68-positive (% macrophages per 100 cells) adipocytes (PL, n = 9; RIM, n = 11). Representative Immunohistochemistry images of CD68 protein in the SC and VIS depot. P values vs. pre-fat period (*P < 0.05) and vs. HFD (#P < 0.05). Data are means ± SE.
DISCUSSION
The CB1R antagonist RIM reduces body weight and body fat storage and improves insulin sensitivity in rodents and humans (13, 45). We have previously confirmed these findings in obese, insulin-resistant dogs, demonstrating that RIM significantly reduces body weight, SC, and VIS fat depots with transient changes in food intake, despite maintenance on an HFD. Additionally, we reported a profound effect of CB1R blockade to reverse the effects of an HFD on adipocyte size and size distribution that lead to improvements in insulin sensitivity (30). The mechanisms by which RIM reversed the effects of HFD on fat accumulation are not well understood. In a recent study, CB1R antagonism was implicated in beiging of WAT that occurred alongside metabolic improvements (49). We therefore studied potential molecular mechanisms underlying the beneficial effects of RIM in the SC and VIS depots to contribute to weight loss and improvements in glucose metabolism. We found increases of NPRs that drove a host of changes, ultimately leading to increased beiging and lipolysis. This study advances the field of adipose tissue beiging by suggesting a link between CB1R antagonist and improvements in adipose tissue function mediated through NPRs and a UCP1-independent pathway.
Following exposure to RIM, there were significant increases in PGC1α, Cidea, Cox2, Dio2, and UCP1 in the SC depot and in PGC1α, Prdm16, FGF21, Cidea, Cox2, Elovl3, Dio2, and UCP1 in the VIS depot. Prdm16 is thought to be a main regulator of adipose tissue beiging (23), and although it was only significantly increased in the VIS tissue, it is associated with the loss of tissue mass in that depot. PGC1α, upregulated in both adipose tissue depots, regulates oxidative metabolism by increasing mitochondrial gene expression and function (62). The beiging gene program also included Dio2, which has been shown to promote adaptive thermogenesis (10), and Elovl3 and Cidea, regulators of lipid droplet remodeling (1, 59). FGF21, also upregulated in the VIS depot, plays a role in regulating chronic adaptive thermogenesis and the induction of WAT beiging (15). We observed that WAT beiging is more pronounced in the VIS than SC depots. Most of the studies performed in rodents showed that WAT beiging occurred in the SC depots (12, 50). However, recent studies demonstrated the susceptibility of different WAT depots to beiging in a context of chronic obesity is different between rodents and humans. These data suggest that the expression of beiging genes is higher in VIS than in SC WAT in humans, a pattern that is opposite to what is observed in rodents (64). In addition, fat deposition in the canine model is very similar to humans (30, 47); therefore, WAT beiging is more distinct in the VIS depot.
It is generally thought that UCP1 is the lynchpin of the thermogenic activity in BAT and beige cells; however, other mechanisms of beiging have been more recently discovered, including creatine-dependent substrate cycling, calcium-dependent ATP hydrolysis, and lipid cycling (9). Recently, Ikeda et al. demonstrated that adipose tissue beiging primarily occurs through a UCP1-independent and SERCA2b-dependent pathway. They showed that the fuel used by beige cells to direct glucose into the TCA cycle is derived from a Ca2+-dependent ATPase (27). Upon sympathetic activation by α1- and/or β3Rs, SERCA2b and RyR2 move Ca2+ in and out of the sarcoendoplasmic reticulum, respectively, producing heat. This futile cycling leads to an increase in thermogenesis, independent of UCP1, and glucose uptake by the beige cells that effectively act as a glucose sink, improving glucose tolerance.
Although we found a significant increase in UCP1 gene expression only in SC, the increase was relatively small. Recent work from Ikeda et al. (27) suggests that in beige cells, increased adrenergic activity can initiate a pathway that directs the ATP produced during glycolysis and TCA cycle to SERCA2b. SERCA2b, in conjunction with RyR2, uses ATP in a futile cycle to shuttle Ca2+ in and out of the sarcoendoplasmic reticulum and thus generating heat independent of UCP1. Our data show that both SERCA2b and RyR2 genes are upregulated in the SC and VIS depots following treatment with RIM, suggesting uncoupling via a UCP1-independent pathway similar to a previous study in mice (28). Our study is the first to link this futile Ca2+ cycling to beiging in a large-animal model. This also explains the mechanism by which CB1R antagonism increases beiging of WAT, despite such a small increase in UCP1. However, future mechanistic studies should be conducted to precisely demonstrate the role of CB1R antagonist on metabolic improvement mediated by UCP1 and UCP1-independent pathway, such as effect of RIM on SERCA2b knockout mice.
The futile Ca2+ cycling also acts as a glucose sink and may help explain the increased insulin sensitivity previously reported in our model (33). Glut4 and hexokinase 2 (24, 60) expressions were increased in both fat depots. In our study, the major link between treatment with RIM and the metabolic outcomes is an increase in plasma NP and NPR(s) in the WAT. Consistent with previous data showing that secretion of NP is potentiated by acute RIM treatment (48), our data demonstrate that the chronic RIM treatment increases the expression of NPR1 and NPR2 in the SC and VIS depots. Importantly, we did not observe any changes in the clearance receptor, NPR3. Our data, therefore, suggest an increase in NP activity with no change in NP degradation. Previous work has suggested that receptor concentration is the mediator of NP activity, at least in mice (41). In addition, we measured ANP in the plasma from a subset of dogs from pre-fat, HFD+PL, and HFD+RIM groups. We demonstrate for the first time that ANP was higher in the HFD+RIM group. In addition, to evaluate the direct effect of NP in adipocyte lipolysis, we added different amounts of ANP to isolated adipocytes from control dogs (data not shown, n = 3). ANP stimulated in vitro lipolysis by 50% in the SC and VIS depots compared with isoproterenol. Thus, chronic elevated ANP could stimulate lipolysis at the cellular level.
We were able to show increases in expression of genes associated with beiging that correlated with increased NPR1 and NPR2 expression. Our data are similar to the reports from Neinast et al. and others (22, 41) in which they demonstrated that the increased ratio of NPR1/NPR3 and NPR2/NPR3 is associated with Roux-en-Y gastric bypass-induced beiging in female mice.
FGF21 promotes the expression and secretion of adiponectin in adipocytes (25) and has been associated with beiging of adipose tissues (15). NPs have also been shown to increase adiponectin production in human adipocytes (56). We have previously shown that adiponectin secretion (33) and adiponectin gene expression (29) are increased with HFD+RIM and that the expression of adiponectin receptors ADR1 and ADR2 were increased in the liver (29). As mentioned above, FGF21 was increased in the VIS depot following HFD+RIM, suggesting an increase in adiponectin activity. ADR1 and ADR2 expression were increased in both depots.
Although various markers of beiging were increased in the SC and VIS fat tissues, our gene expression data are corroborated by increased lipolysis. Activation of adrenergic receptors, such as β1R and β3R, stimulates beiging of WAT through lipolysis (35, 39). In response to HFD+RIM, β1R and β3R expression significantly increased. NP and Dio2 are also powerful lipolytic agents in human adipose tissue in situ and in isolated fat cells in vitro (39, 52) and were increased in the present study. ATGL expression significantly increased, and HSL-P/HSL protein showed a trend to increase. In addition, in vitro lipolysis was enhanced by RIM treatment in the VIS depot. Together, these data show that treatment with RIM increased lipolysis, which contributed to the beiging of the WAT. Our data suggest that an increase in adrenergic receptors and NP expression may be the drivers of increased lipolysis, especially given that NP and adrenergic-induced beiging is driven through mTORC1 (37, 38).
It is noteworthy that the SC depot was less responsive to both basal and stimulated lipolysis. The duration of treatment (in vitro) may not have been enough to see changes in lipolysis in the SC depot, and a more prolonged RIM treatment period may elicit a greater adrenergic response in the SC depot. In the present study, one limitation is the use of only male dogs. The literature suggests that males have a higher number of adrenergic receptors and more receptor activation in the VIS depot than in the SC depot. Furthermore, these data are also consistent with Hsiao et al. (8), where they demonstrated that a new peripheral CB1R antagonist, BPR0921, promoted UCP1 and HSL expression; however, the mechanism by which this peripheral CB1R antagonist exerted these effects was not fully explored.
Furthermore, NPs and adiponectin have both been linked to decreases in inflammation in adipose tissue, with decreased inflammation associated with improved insulin sensitivity as well. ANP, either by direct action on adipocytes and macrophages or through activation of HSL, inhibits the secretion of factors involved in inflammation and adipose tissue insulin resistance (46). The anti-inflammatory properties of adiponectin have also been extensively studied and demonstrated to show that plasma adiponectin levels are inversely correlated with proinflammatory cytokines (42, 43). In this study, we found decreased expression of the inflammatory markers IL-6 and TNFα in VIS following HFD+RIM treatment. In addition, measurement of IL-6 and TNFα were decreased in the plasma following HFD+RIM. Furthermore, we found a corresponding reduction in crown-like structures in the adipose tissue. Taken together, these data suggest that RIM decreases inflammation via adiponectin, NPs, and their receptors and that in conjunction with the glucose sink activity, these changes improve insulin sensitivity in HFD dogs.
Other alternative mechanisms for the reduction in body adiposity and weight loss may be attributed to changes in energy expenditure. Total energy expenditure consists of three components: basal energy expenditure, diet-induced thermogenesis, and activity-induced thermogenesis. However, one limitation of this study was a lack of 24-h total energy expenditure, 24-h change in whole body temperature, or monitoring of physical activity (and consequently thermogenesis measurements), which is very difficult to obtain in large animals compared with rodents. We acknowledge that other components of total energy expenditure might explain the body weight loss and beneficial metabolic effects seen in our study, such as physical activity or adaptive thermogenesis.
In spite of its several beneficial effects, RIM was withdrawn from the market because of psychotropic side effects. The development of new, nonbrain-penetrating CB1R antagonists has recently been the focus of many studies and may provide an exciting therapeutic opportunity to influence metabolic benefits without the detrimental side effects. Studies focusing on the efficacy of a nonbrain-penetrating CB1R antagonist will be important to address the role of the central nervous system in coordination with the endocannabinoid system to facilitate adipose tissue beiging.
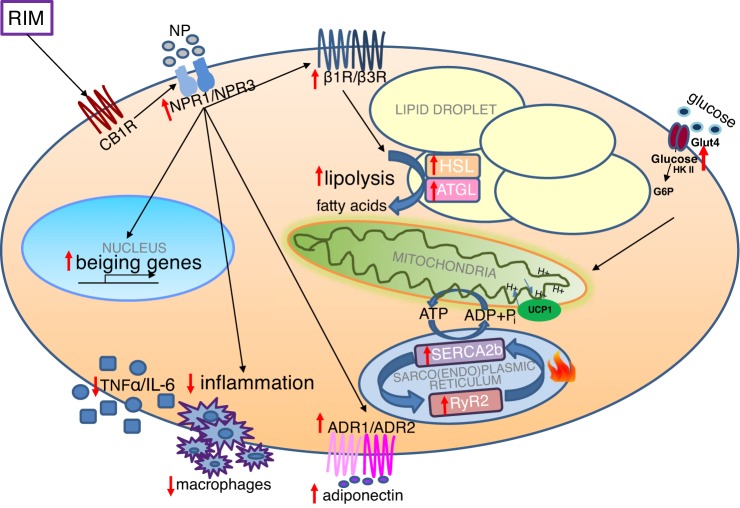
In conclusion, our findings suggest that the upregulation of markers of WAT beiging (including NPRs, β1R, and β3R), lipolysis, and SERCA2b and RYR2 may be one of the mechanisms by which the CB1R antagonist RIM promotes beiging and overall the improvement of metabolic homeostasis. These results suggest an important role for the endocannabinoid system in mediating adipose tissue beiging and thereby improving metabolic homeostasis, at least in male dogs (Fig. 7).
Fig. 7.
The proposed mechanism by which CB1 receptor (CB1R) antagonist promotes beiging in adipose tissue. CB1R antagonist binds to CB1R and promotes beiging of adipose tissue via upregulation of β-3 adrenergic receptor (β3R), natriuretic peptide receptors (NPRs), lipolysis, and adiponectin receptors (ADRs) and activates uncoupling protein 1 (UCP1)-independent thermogenic pathway. CB1R antagonist decreased inflammation in adipocytes. All the above-mentioned would dissipate energy as heat and promote adipocyte beiging by CB1R. CB1R could have an important implication in the regulation of fat mass and energy homeostasis. ATGL, adipose triglyceride lipase; Glut4, glucose transporter 4; HSL, hormone-sensitive lipase.
GRANTS
This work has been supported by Sanofi-Aventis and grants from the National Institutes of Health (DK-029867 and DK-27619).
DISCLOSURES
R. N. Bergman is supported by grants from AstraZeneca and Janssen Research and Development and is an advisory board member of Novo Nordisk. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
R.N.B., D.J.C., and M.K. conceived and designed research; M.S.I., R.L.P., I.A.B., Q.W., and M.K. performed experiments; M.S.I., R.L.P., D.S., and M.K. analyzed data; M.S.I., R.L.P., and M.K. interpreted results of experiments; M.S.I., R.L.P., and M.K. prepared figures; M.K., R.L.P., and M.S.I. drafted the manuscript; M.S.I., R.L.P., R.N.B., J.M.R., O.O.W., S.P.K., C.M.K., D.J.C., and M.K. edited and revised manuscript; M.S.I., R.L.P., R.N.B., J.M.R., O.O.W., I.A.-B., Q.W., S.P.K., D.S., C.M.K., D.J.C., and M.K. approved final version of manuscript.
REFERENCES
- 1.Abreu-Vieira G, Fischer AW, Mattsson C, de Jong JM, Shabalina IG, Rydén M, Laurencikiene J, Arner P, Cannon B, Nedergaard J, Petrovic N. Cidea improves the metabolic profile through expansion of adipose tissue. Nat Commun 6: 7433, 2015. [Corrigendum in Nat Commun 7: 12395, 2016.] doi: 10.1038/ncomms8433. [DOI] [PubMed] [Google Scholar]
- 2.Bajzer M, Olivieri M, Haas MK, Pfluger PT, Magrisso IJ, Foster MT, Tschöp MH, Krawczewski-Carhuatanta KA, Cota D, Obici S. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia 54: 3121–3131, 2011. doi: 10.1007/s00125-011-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada J, Dieguez C, Lopez M, Frühbeck G, Nogueiras R. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63: 3346–3358, 2014. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 4.Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrié P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 63: 908–914, 2003. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- 5.Birkenfeld AL, Boschmann M, Engeli S, Moro C, Arafat AM, Luft FC, Jordan J. Atrial natriuretic peptide and adiponectin interactions in man. PLoS One 7: e43238, 2012. doi: 10.1371/journal.pone.0043238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boon MR, Kooijman S, van Dam AD, Pelgrom LR, Berbée JF, Visseren CA, van Aggele RC, van den Hoek AM, Sips HC, Lombès M, Havekes LM, Tamsma JT, Guigas B, Meijer OC, Jukema JW, Rensen PC. Peripheral cannabinoid 1 receptor blockade activates brown adipose tissue and diminishes dyslipidemia and obesity. FASEB J 28: 5361–5375, 2014. doi: 10.1096/fj.13-247643. [DOI] [PubMed] [Google Scholar]
- 7.Calvani R, Leeuwenburgh C, Marzetti E. Brown adipose tissue and the cold war against obesity. Diabetes 63: 3998–4000, 2014. doi: 10.2337/db14-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro AV, Woolcott OO, Iyer MS, Kabir M, Ionut V, Stefanovski D, Kolka CM, Szczepaniak LS, Szczepaniak EW, Asare-Bediako I, Paszkiewicz RL, Broussard JL, Kim SP, Kirkman EL, Rios HC, Mkrtchyan H, Wu Q, Ader M, Bergman RN. Increase in visceral fat per se does not induce insulin resistance in the canine model. Obesity (Silver Spring) 23: 105–111, 2015. doi: 10.1002/oby.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chouchani ET, Kazak L, Spiegelman BM. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab 29: 27–37, 2019. doi: 10.1016/j.cmet.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, Rabelo R, Curcio C, Martins L, Kimura ET, Bianco AC. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes 53: 577–584, 2004. doi: 10.2337/diabetes.53.3.577. [DOI] [PubMed] [Google Scholar]
- 11.Chusyd DE, Wang D, Huffman DM, Nagy TR. Relationships between rodent white adipose fat pads and human white adipose fat depots. Front Nutr 3: 10, 2016. doi: 10.3389/fnut.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JP, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156: 304–316, 2014. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Després JP, Golay A, Sjöström L; Rimonabant in Obesity-Lipids Study Group . Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353: 2121–2134, 2005. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 14.Després JP, Ross R, Boka G, Alméras N, Lemieux I; ADAGIO-Lipids Investigators . Effect of rimonabant on the high-triglyceride/ low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: the ADAGIO-Lipids trial. Arterioscler Thromb Vasc Biol 29: 416–423, 2009. doi: 10.1161/ATVBAHA.108.176362. [DOI] [PubMed] [Google Scholar]
- 15.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26: 271–281, 2012. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, Chabbert M, Cruccioli N, Pfersdorff C, Roque C, Arnone M, Croci T, Soubrié P, Oury-Donat F, Maffrand JP, Scatton B, Lacheretz F, Le Fur G, Herbert JM, Bensaid M. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology 46: 122–129, 2007. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- 17.Ge Q, Maury E, Rycken L, Gérard J, Noël L, Detry R, Navez B, Brichard SM. Endocannabinoids regulate adipokine production and the immune balance of omental adipose tissue in human obesity. Int J Obes 37: 874–880, 2013. doi: 10.1038/ijo.2012.123. [DOI] [PubMed] [Google Scholar]
- 18.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab 15: 635–645, 2012. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greer RJ, Cohn LA, Dodam JR, Wagner-Mann CC, Mann FA. Comparison of three methods of temperature measurement in hypothermic, euthermic, and hyperthermic dogs. J Am Vet Med Assoc 230: 1841–1848, 2007. doi: 10.2460/javma.230.12.1841. [DOI] [PubMed] [Google Scholar]
- 20.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets 8: 403–421, 2009. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankir MK. Loading and firing the brown adipocyte. Adipocyte 7: 4–11, 2018. doi: 10.1080/21623945.2017.1405879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hankir MK, Bronisch F, Hintschich C, Krügel U, Seyfried F, Fenske WK. Differential effects of Roux-en-Y gastric bypass surgery on brown and beige adipose tissue thermogenesis. Metabolism 64: 1240–1249, 2015. doi: 10.1016/j.metabol.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 19: 1252–1263, 2013. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 24.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 5: 237–252, 2007. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Hui X, Feng T, Liu Q, Gao Y, Xu A. The FGF21-adiponectin axis in controlling energy and vascular homeostasis. J Mol Cell Biol 8: 110–119, 2016. doi: 10.1093/jmcb/mjw013. [DOI] [PubMed] [Google Scholar]
- 26.Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, Feng T, Zhong C, Wang Y, Lam KS, Xu A. adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab 22: 279–290, 2015. doi: 10.1016/j.cmet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P, Tajima K, Ajuwon KM, Soga T, Kajimura S. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23: 1454–1465, 2017. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Péleraux A, Pénarier G, Soubrié P, Le Fur G, Galiègue S, Casellas P. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 19: 1567–1569, 2005. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- 29.Kabir M, Iyer MS, Richey JM, Woolcott OO, Asare Bediako I, Wu Q, Kim SP, Stefanovski D, Kolka CM, Hsu IR, Catalano KJ, Chiu JD, Ionut V, Bergman RN. CB1R antagonist increases hepatic insulin clearance in fat-fed dogs likely via upregulation of liver adiponectin receptors. Am J Physiol Endocrinol Metab 309: E747–E758, 2015. doi: 10.1152/ajpendo.00196.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabir M, Stefanovski D, Hsu IR, Iyer M, Woolcott OO, Zheng D, Catalano KJ, Chiu JD, Kim SP, Harrison LN, Ionut V, Lottati M, Bergman RN, Richey JM. Large size cells in the visceral adipose depot predict insulin resistance in the canine model. Obesity (Silver Spring) 19: 2121–2129, 2011. doi: 10.1038/oby.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab 292: E1590–E1598, 2007. doi: 10.1152/ajpendo.00669.2006. [DOI] [PubMed] [Google Scholar]
- 32.Kim SP, Ellmerer M, Van Citters GW, Bergman RN. Primacy of hepatic insulin resistance in the development of the metabolic syndrome induced by an isocaloric moderate-fat diet in the dog. Diabetes 52: 2453–2460, 2003. doi: 10.2337/diabetes.52.10.2453. [DOI] [PubMed] [Google Scholar]
- 33.Kim SP, Woolcott OO, Hsu IR, Stefanoski D, Harrison LN, Zheng D, Lottati M, Kolka C, Catalano KJ, Chiu JD, Kabir M, Ionut V, Bergman RN, Richey JM. CB(1) antagonism restores hepatic insulin sensitivity without normalization of adiposity in diet-induced obese dogs. Am J Physiol Endocrinol Metab 302: E1261–E1268, 2012. doi: 10.1152/ajpendo.00496.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lafontan M. Advances in adipose tissue metabolism. Int J Obes 32, Suppl 7: S39–S51, 2008. doi: 10.1038/ijo.2008.237. [DOI] [PubMed] [Google Scholar]
- 35.Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res 34: 1057–1091, 1993. [PubMed] [Google Scholar]
- 36.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science 307: 584–586, 2005. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Bordicchia M, Zhang C, Fang H, Wei W, Li JL, Guilherme A, Guntur K, Czech MP, Collins S. Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. J Clin Invest 126: 1704–1716, 2016. doi: 10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu D, Ceddia RP, Collins S. Cardiac natriuretic peptides promote adipose ‘browning’ through mTOR complex-1. Mol Metab 9: 192–198, 2018. doi: 10.1016/j.molmet.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louis SN, Jackman GP, Nero TL, Iakovidis D, Louis WJ. Role of beta-adrenergic receptor subtypes in lipolysis. Cardiovasc Drugs Ther 14: 565–577, 2000. doi: 10.1023/A:1007838125152. [DOI] [PubMed] [Google Scholar]
- 40.McMurray RG, Soares J, Caspersen CJ, McCurdy T. Examining variations of resting metabolic rate of adults: a public health perspective. Med Sci Sports Exerc 46: 1352–1358, 2014. doi: 10.1249/MSS.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neinast MD, Frank AP, Zechner JF, Li Q, Vishvanath L, Palmer BF, Aguirre V, Gupta RK, Clegg DJ. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol Metab 4: 427–436, 2015. doi: 10.1016/j.molmet.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation 107: 671–674, 2003. doi: 10.1161/01.CIR.0000055188.83694.B3. [DOI] [PubMed] [Google Scholar]
- 43.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta 380: 24–30, 2007. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perwitz N, Wenzel J, Wagner I, Büning J, Drenckhan M, Zarse K, Ristow M, Lilienthal W, Lehnert H, Klein J. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes Metab 12: 158–166, 2010. doi: 10.1111/j.1463-1326.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 45.Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284: R345–R353, 2003. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- 46.Razzoli M, Frontini A, Gurney A, Mondini E, Cubuk C, Katz LS, Cero C, Bolan PJ, Dopazo J, Vidal-Puig A, Cinti S, Bartolomucci A. Stress-induced activation of brown adipose tissue prevents obesity in conditions of low adaptive thermogenesis. Mol Metab 5: 19–33, 2016. doi: 10.1016/j.molmet.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richey JM, Woolcott OO, Stefanovski D, Harrison LN, Zheng D, Lottati M, Hsu IR, Kim SP, Kabir M, Catalano KJ, Chiu JD, Ionut V, Kolka C, Mooradian V, Bergman RN. Rimonabant prevents additional accumulation of visceral and subcutaneous fat during high-fat feeding in dogs. Am J Physiol Endocrinol Metab 296: E1311–E1318, 2009. doi: 10.1152/ajpendo.90972.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruginsk SG, Uchoa ET, Elias LL, Antunes-Rodrigues J. Cannabinoid CB1 receptor mediates glucocorticoid effects on hormone secretion induced by volume and osmotic changes. Clin Exp Pharmacol Physiol 39: 151–154, 2012. doi: 10.1111/j.1440-1681.2011.05658.x. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz de Azua I, Mancini G, Srivastava RK, Rey AA, Cardinal P, Tedesco L, Zingaretti CM, Sassmann A, Quarta C, Schwitter C, Conrad A, Wettschureck N, Vemuri VK, Makriyannis A, Hartwig J, Mendez-Lago M, Bindila L, Monory K, Giordano A, Cinti S, Marsicano G, Offermanns S, Nisoli E, Pagotto U, Cota D, Lutz B. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J Clin Invest 127: 4148–4162, 2017. doi: 10.1172/JCI83626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121: 96–105, 2011. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seale P, Lazar MA. Brown fat in humans: turning up the heat on obesity. Diabetes 58: 1482–1484, 2009. doi: 10.2337/db09-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sengenès C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J 14: 1345–1351, 2000. doi: 10.1096/fasebj.14.10.1345. [DOI] [PubMed] [Google Scholar]
- 53.Sengenès C, Bouloumie A, Hauner H, Berlan M, Busse R, Lafontan M, Galitzky J. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem 278: 48617–48626, 2003. doi: 10.1074/jbc.M303713200. [DOI] [PubMed] [Google Scholar]
- 54.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 17: 475–490, 2013. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow JS, Innis RB, Cheng K, Rice KC, Deschamps JR, Chorvat RJ, McElroy JF, Kunos G. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab 16: 167–179, 2012. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, Okazaki H, Asai M, Nagamachi Y, Maeda N, Shintani Y, Minamino T, Asakura M, Kishimoto I, Funahashi T, Tomoike H, Kitakaze M. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol 53: 2070–2077, 2009. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 57.Ueta CB, Fernandes GW, Capelo LP, Fonseca TL, Maculan FD, Gouveia CH, Brum PC, Christoffolete MA, Aoki MS, Lancellotti CL, Kim B, Bianco AC, Ribeiro MO. β(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. J Endocrinol 214: 359–365, 2012. doi: 10.1530/JOE-12-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S; RIO-Europe Study Group . Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365: 1389–1397, 2005. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 59.Westerberg R, Månsson JE, Golozoubova V, Shabalina IG, Backlund EC, Tvrdik P, Retterstøl K, Capecchi MR, Jacobsson A. ELOVL3 is an important component for early onset of lipid recruitment in brown adipose tissue. J Biol Chem 281: 4958–4968, 2006. doi: 10.1074/jbc.M511588200. [DOI] [PubMed] [Google Scholar]
- 60.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol 206: 2049–2057, 2003. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 61.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 27: 234–250, 2013. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 63.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306: 1383–1386, 2004. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 64.Zuriaga MA, Fuster JJ, Gokce N, Walsh K. Humans and mice display opposing patterns of “Browning” gene expression in visceral and subcutaneous white adipose tissue depots. Front Cardiovasc Med 4: 27, 2017. doi: 10.3389/fcvm.2017.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]