Abstract
In vitro, cell cultures are essential tools in the study of intestinal function and disease. For the past few decades, monolayer cellular cultures, such as cancer cell lines or immortalized cell lines, have been widely applied in gastrointestinal research. Recently, the development of three-dimensional cultures known as organoids has permitted the growth of normal crypt-villus units that recapitulate many aspects of intestinal physiology. Organoid culturing has also been applied to study gastrointestinal diseases, intestinal-microbe interactions, and colorectal cancer. These models are amenable to CRISPR gene editing and drug treatments, including high-throughput small-molecule testing. Three-dimensional intestinal cultures have been transplanted into mice to develop versatile in vivo models of intestinal disease, particularly cancer. Limitations of currently available organoid models include cost and challenges in modeling nonepithelial intestinal cells, such as immune cells and the microbiota. Here, we describe the development of organoid models of intestinal biology and the applications of organoids for study of the pathophysiology of intestinal diseases and cancer.
Keywords: colon cancer, genetic editing, gut physiology, intestinal organoids, organoid culture
HISTORY OF ORGANOID TECHNOLOGY: INTRODUCTION
Immortalized cancer cell lines have been widely used to study cell physiology and cancer since the report of HeLa cervical cancer cells in 1953 (100). They are relatively inexpensive, easy to maintain, and amenable to genetic manipulation. However, cancer cell lines are limited in several important ways: 1) they grow in two dimensions, and, therefore, fail to recapitulate the three-dimensional architecture of the normal and malignant intestine; 2) cell lines can be successfully derived from only a small fraction of patient cancer specimens, mostly likely because of the challenges in growing tissue on a flat plastic surface; 3) normal tissue typically cannot be grown long-term in standard tissue culture plates without genetic modification; and 4) the genetic profile of immortalized cancer cell lines changes in culture, according to growth conditions, and affects proliferation and response to therapies (3, 66).
Recent advances in stem cell biology have led to the successful three-dimensional culture of tissue in vitro, also known as “organoids”, or three-dimensional organ-like structures composed of functional, live cells that can self-renew and spatially organize. Organoids overcome many of the limitations of standard cell lines: they reproduce the three-dimensional architecture of human tissue and can be derived from almost any normal intestinal or cancer sample for long-term propagation. These features have made organoid cultures essential tools for basic and translational research in intestinal biology and cancer. To date, organoid cultures have been developed for a wide range of mouse and human tissues, including esophagus, intestine, colon (Fig. 1), kidney, liver, breast, and brain, which have provided valuable insights into mammalian physiology. In this review, we summarize the history of seminal developments in organoid technology, applications of organoids for the study of gastrointestinal physiology in health and disease, limitations to organoids, and alternative in vitro models of intestinal physiology.
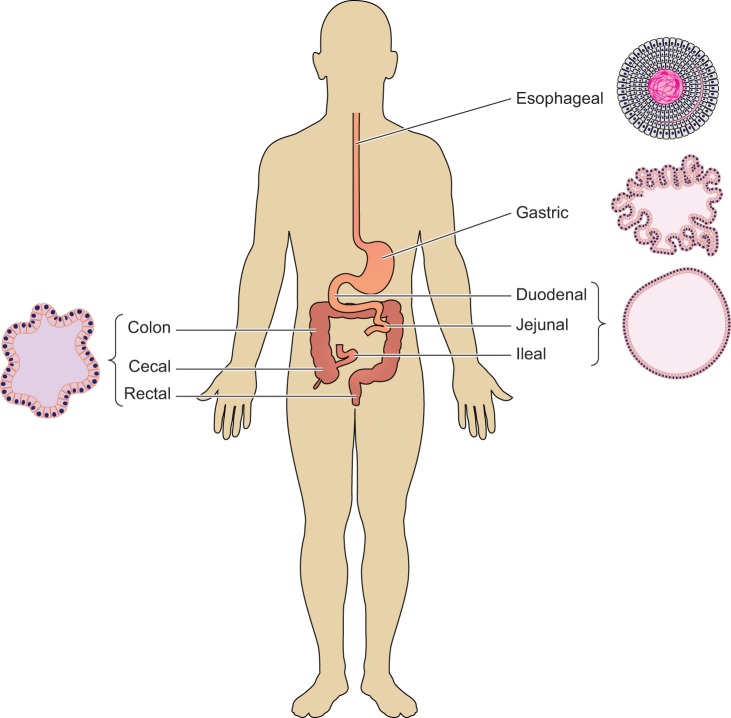
Fig. 1.
Origin of gastrointestinal organoids. Organoids can be derived from different segments of the gastrointestinal tract, including esophagus (20), stomach (87), small intestine (72, 74, 116), and colon (18, 97).
NOMENCLATURE FOR THREE-DIMENSIONAL INTESTINAL CULTURES
The term “organoid” was first used in 1987 to describe in vitro cultures derived from neuroblastomas (46) and lung (134). Short-term intestinal cultures were first described by Evans et al. (25), who defined an intestinal organoid as a structure of crypts and villi growing in vitro. Sato and Clevers described three-dimensional cultures of intestinal stem cells (ISCs) in 2009 as “organoids” and have continued to refer to purely epithelial cultures as organoids (95a). In the same year, Ootani and Kuo reported three-dimensional cultures of intestinal fragments, which they also referred to organoids (65). In 2012, the Intestinal Stem Cell Consortium published nomenclature guidelines to distinguish between types of intestinal cultures (109). The authors of this work suggest the term “organoid” for cultures that contain multiple cell types, including epithelial and mesenchyme, while cultures of pure epithelial populations are designated as “enteroids” or “colonoids,” if derived from the small intestine or colon, respectively. The term “spheroid” has also been used to denote epithelial three-dimensional cultures (76). In this review, all three-dimensional organ structures are referred to as “organoids”, and qualified by tissue location, species, and type of tissue (e.g., “colon tumor organoid”).
ORGANOID MODELS OF INTESTINAL PHYSIOLOGY
Before the advent of in vitro organoid cultures, many attempts were made to develop long-term cultures of intestinal tissue that reproduce the crypt-villus structure of intestine (8, 55, 83). The crypt base columnar (CBC) cell was first described as a rapidly cycling cell at the bottom of the crypt by Cheng and Leblond in 1974 (14). Thus, Leblond and others focused on isolating and culturing crypts from the intestinal epithelium (88). However, isolated intestinal crypts rapidly undergo apoptosis upon detachment from the basement membrane, a phenomenon referred to as “anoikis”, typically within 4 hours (35, 110). Several lines of evidence suggested that specific growth factors are required for long-term culture of intestinal crypts: 1) anoikis occurs even if crypts are embedded in a collagen-like matrix (110); 2) isolated crypts survive in standard cell culture media only for up to 10 days (8, 11, 55); 3) embryonic three-dimensional organoid cultures are also limited to 14 days of growth (1); and 4) human or rat crypts develop epithelial, differentiated structures in vivo when transplanted into immunodeficient mice or syngeneic rats (10, 58). Subsequent research showed that paracrine Wnt and Notch signals (which are presumably present in in vivo transplant models) are required for intestinal regeneration (34, 61, 86, 117a). A milestone in the development of organoid culture was the identification of leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) as a marker of crypt-base stem cells (2). Other groups have identified markers of cells located above the uppermost Paneth cell (i.e., +4 cell) with stem cell activity, including Bmi1 (95), Tert (12, 73), Hopx (112), and Lrig1 (89). These studies permitted the direct study of ISC function in in vivo and in vitro systems.
In 2009, Calvin Kuo’s group circumvented the challenges of defining specific culture conditions for murine intestinal crypts by embedding epithelial and mesenchymal tissues from the neonatal small intestine, colon, and stomach into a collagen-like matrix on an air-liquid interface (ALI) (80). An important feature of this system is that epithelial-mesenchymal interactions are preserved, which permits long-term culture of organoid units that exhibit proliferation and multilineage differentiation for at least 1 year. The authors also demonstrated that Wnt signals, particularly the Wnt agonist R-spondin 1, and Notch signals, are essential to long-term organoid culture.
The most widely used model of organoid culture was described by Sato and Clevers, also in 2009 (95a). In this system, isolated intestinal crypts are cultured in a collagen matrix (i.e., Matrigel, produced by Corning) with media containing the Wnt agonist R-spondin 1, epidermal growth factor (EGF), and Noggin (i.e., WNR media). Under these conditions, the crypts form sealed structures with apoptotic cells in the lumen. These organoids reproduce intestinal crypt-like units, which contain Lgr5+ ISCs, as well as villus-like units, which contain all differentiated intestinal cell types, including Paneth cells (antimicrobial and niche supportive functions), absorptive enterocytes or colonocytes, goblet cells (mucous production), and enteroendocrine cells (hormone production) (Fig. 2) (97). Organoids grown from FACS-sorted Lgr5+ ISCs are indistinguishable from organoids cultured from crypts. In a follow up study, the Clevers lab demonstrated that niche Paneth cells support stem cell function in vitro by supplying Wnt3a, Egf, and Dll4; coculture of Paneth cells with ISCs markedly increases organoid formation (96). Subsequent work from the Clevers laboratory demonstrated the application of their organoid model for culture of mouse colon organoids, which unlike mouse small intestinal organoids, require exogenous Wnt3a for long-term culture and expansion (97). These findings underscore both the value and drawbacks to intestinal organoid models: Paneth cells are dispensable for stem cell function in vivo, most likely due to Rspondin-1 and Wnt signals from the stromal niche (22, 43, 54, 59, 105).
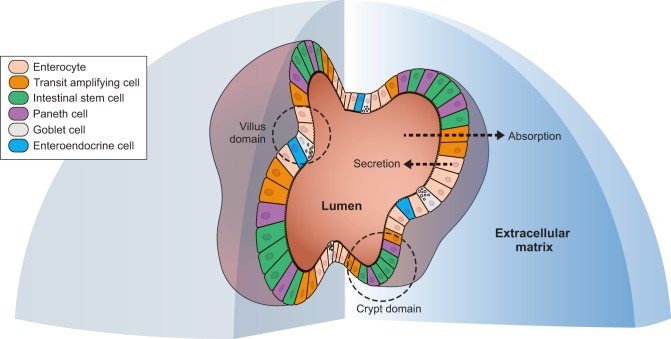
Fig. 2.
Anatomy of an intestinal organoid. A three-dimensional section of an intestinal organoid embedded into extracellular matrix in vitro. The organoid consists of spatially arranged cells, with the luminal side facing the inside of the organoid and the apical side facing the extracellular matrix. Intestinal organoids comprise all of the intestinal cellular types, including stem cells, Paneth cells, transit amplifying cells, enterocytes, goblet cells, and enteroendocrine cells. The architecture of the organoid includes a crypt domain where stem cells reside and a villus domain containing differentiated cells.
An important advance in organoid technology was the ability to differentiate intestinal organoids into defined lineages. The addition of Wnt3a to culture media results in stem-like, undifferentiated organoids, and subsequent removal of Wnt3a induces differentiation of organoid cells and formation of crypt-like buds (97). The self-renewal and differentiation of ISCs are regulated by the Wnt and Notch signaling pathways. CHIR99021, a (GSK3-β inhibitor and Wnt activator) and valproic acid (a histone deacetylase inhibitor and Notch activator) synergistically maintain self-renewal of Lgr5+ mouse ISCs. Various combinations of Wnt activators, Notch activators, Notch inhibitors, and ROCK inhibitors can be used to induce differentiation into the enterocyte, goblet cell, Paneth cell, and enteroendocrine lineages (4, 85, 131). Intestinal organoids were recently used to delineate the differentiation of single stem cells into Paneth cells, which is driven by YAP1 and DLL1 activation (102) (Figs. 2 and 3).
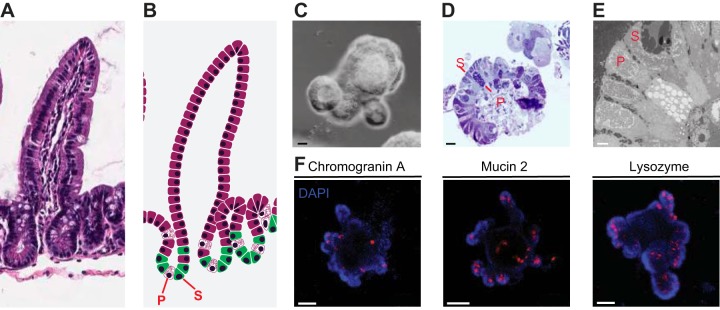
Fig. 3.
Imaging of mouse small intestinal organoids. A: light microscopy of the small intestine. B: intestinal epithelium organization from crypt to villus with stem cells (S) and Paneth cells (P) at the bottom of the crypt. C: light microscopy image of a mouse small intestinal organoid. D: one-micron section of mouse small intestinal organoids counterstained with Toluidine Blue. E: electron microscopy image of mouse small intestinal organoids. F: immunofluorescence for chromogranin A, mucin 2, and lysozyme in mouse small intestinal organoids (blue = DAPI, red = cell-specific antibody). C and D scale bars = 20 μm; E scale bar = 2 μm, F scale bar = 50 μm. [Reproduced from Beyaz et al. (5), with permission].
Human intestinal and colon crypts can also be cultured as organoids (Fig. 4), with similar media components as used for mouse crypt culture. Isolated human intestinal crypts can be grown long-term as organoids in media containing Egf, Noggin, R-spondin 1, Wnt3a, nicotinamide, a small molecule inhibitor of Alk, and a p53 MAPK inhibitor (97). Alternatively, patient-derived intestinal and colonic crypts can be cultured using Wnt3a, Rspondin-1, and Noggin conditioned media supplemented with fetal bovine serum, a ROCK inhibitor, and a TGFBR1 inhibitor (117). Supplementation with gastrin, p38 MAPK inhibition, and PGE2 supports growth of organoids from human colon crypts (53). The addition of insulin-like growth factor 1 (IGF-1) and fibroblast growth factor 2 (FGF-2) to human intestinal culture media facilitates long-term passage and multilineage differentiation of human intestinal organoids (36). Dissociated human small intestinal and colonic organoids have been seeded into monolayers on collagen IV-coated Transwell filters, which permits studies of intestinal barrier function, host-microbe interaction, and apical cell function (51, 52).
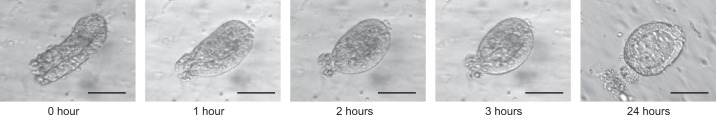
Fig. 4.
Evolution of a human intestinal crypt in culture. Human colonic crypts embedded in Matrigel and cultured in media containing Wnt3a, Rspondin-1, Noggin, and other growth factors form organoids within 24 h. Scale bar = 50 μm.
ORGANOIDS TO STUDY INTESTINAL PHYSIOLOGY AND DISEASE
Intestinal organoid technology has been applied to study the embryonic development of the intestine. Mouse embryonic “early” intestine (~E10–E15.5) is pseudostratified and largely undifferentiated, whereas embryonic “late”-stage (~E15.5–E18) intestinal epithelial cells rapidly expand along a single layer together with an underlying muscle layer (57). Mustata et al. (76) found that early prenatal cells grow in culture as undifferentiated spherical organoids, without the budding structures that signify differentiation. These spheroids do not express the adult stem cell marker Lgr5 and do not require Wnt pathway ligands for maintenance. Fordam et al. (30) largely reproduced these findings in human fetal (i.e., gestational age of 10 weeks) intestinal epithelium. The authors also demonstrated that mouse-derived fetal organoids (i.e., E16, corresponding to human gestational age of 10 weeks) can be engrafted onto injured colon and form differentiated epithelium in vivo, with potential applications for regenerative therapy. Choi et al. (15) developed one of the earliest small intestinal whole-organ models using organoids by transplanting cultured organoids onto a polymer scaffolds. The scaffold-organoid units were then transplanted into the omentum of rats and showed cellular differentiation with preserved histologic cytologic features. This demonstrated the potential use of organoids in small intestinal transplants aimed toward diseases, such as short bowel syndrome (15).
One exciting application of organoid technology is the generation of tissues from pluripotent stem cells (PSCs) and embryonic stem cells (ESCs) to model normal intestinal development and to potentially treat intestinal disease with a personalized source of intestinal cells. Spence et al. (108) used sequential growth factor modifications that mimic embryonic intestinal development to establish human intestinal organoids from iPSCs. The Spence lab later showed that induced organoids, like human intestine-derived organoids, recapitulate the crypt-villus structure and stem cell hierarchy of the intestinal epithelium. Human embryonic stem cell-derived intestinal organoids are directed into organoids resembling human duodenum or ileum with exposure to FGF4 and a GSK3-β inhibitor (114). Recently, the Wells group demonstrated differentiation of human pluripotent stem cells into colonic organoids by activation of BMP signaling (75). Human iPSC-derived colonic organoids have also been engrafted in vivo into the renal capsule of recipient mice (123) or in scaffolds of decellularized porcine intestinal matrices, which demonstrates the potential of these organoids for regenerative medicine applications (27). Human iPSC-derived organoids cultured with human PSC-derived neural crest cells differentiate into neurons and glial cells, and reproduce key features of the enteric nervous system, such as structures that resemble the myenteric and submucosal plexus (128). These findings demonstrate the potential of pluripotent stem cells to study both adult intestinal tissue and embryonic intestinal development.
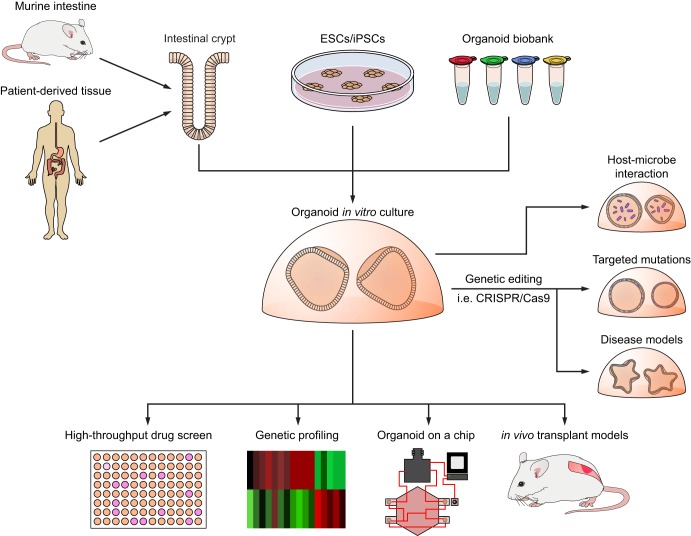
Intestinal organoid cultures have been applied to study many aspects of intestinal biology and disease (Fig. 5 and Table 1). The physiology of electrolyte transport with membrane transporters, such as NHE3, DRA, CFTR, BLM, KCC1, and sodium potassium ATPase, have been investigated using differentiated human small intestinal organoids (31). These transporters are differentially expressed based on the organoid’s tissue site of origin, which is reflected in their in vivo function. For example, only organoids derived from the distal ileum express apical sodium-dependent bile acid transporter (ASBT) and basolateral organic solute transporter β subunit (OSTB), whose function is bile acid uptake. Conversely, these ileum-derived organoids lack GATA4, which is primarily expressed in the proximal small intestine (32). An important development in the study of intestinal transporters was the ability to seed intestinal organoids as monolayers. Monolayer culturing permits analysis of intestinal barrier function and distinguishes the apical from basolateral surfaces. This system was used to study Na+/H+ exchanger 3 activity in human small intestinal organoids (32). Mouse jejunal organoids have been used to examine the tissue-intrinsic circadian rhythm of intestinal tissue in vitro in response to growth factors (74). Small intestinal organoids had been also applied to study cell death and apoptosis in response to cisplatin in organoids deficient in the proapoptotic protein BIM compared with wild-type intestinal organoids (42).
Fig. 5.
Applications of organoid culture. Organoid cultures are established from murine or human intestinal crypts, repurposed embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), or frozen organoids. Potential applications of organoid culture include use in drug discovery by high-throughput screening, characterizing tissue by genetic profiling, exploring host-microbe interaction by coculturing with pathogens, inducing somatic mutations using genetic editing techniques such as CRISPR-Cas9 to target specific mutations, creating models of intestinal diseases, implementing multiorgan cultures in an organoid-on-a-chip model, and modeling intestinal diseases and cancer through in vivo transplantation.
Table 1.
Different utility of intestinal organoid culture in different fields
| Category | Organism | Tissue of Origin | Type of Experiment | Physiology Studied | Ref. No. |
|---|---|---|---|---|---|
| Cellular proteins | Human/mouse | Duodenum, jejunem, ileum | Gene expression analysis | Organoids differentially express proteins such as GATA4, lactase, ferroprotein, and ASBT relative to their origin (duodenum vs. jejunum vs. ileum) | (71) |
| Human/mouse | Small intestinal mouse, rectal human | Organoid CFTR-dependent, Forskolin-induced swelling | Cystic fibrosis organoids do not respond to forskolin; defect can be reversed in vitro with CRISPR-Cas9 | (19, 102) | |
| Human | Small intestine | Organoid culture with the presence of cyclic AMP | Characterization of sodium-bicarbonate cotransporter 1 in secretory diarrheas | (31) | |
| Mouse | Small intestine | Organoid culture | Reg4 as a novel marker for enteroendocrine cells within the intestinal epithelium | (44) | |
| Host-microbe | Human | induced human intestinal organoids | Viral coculture with organoids | Mechanism of rotaviral infection using organoids as surrogate for in vivo pathophysiology; demonstrated STO-609 activity against rotaviral infectivity by genomic analysis | (28) |
| Human | Small intestinal | EHEC coculture | Exposed organoids to EHEC EspP causing micropinocytosis and leading to Shiga toxin absorption | (31) | |
| Human | Stomach | Organoid proliferative assay coculture with H. pylori | Study of effects of CagA negative H. pylori on gastric organoid proliferation. | (129) | |
| Human | Small intestine | Coculture organoids with human rotavirus | Evaluation of viral infectivity in vivo with affinity to differentiated cells, and rotaviral mechanism of diarrhea. | (98) | |
| Human | Small intestine | Coculture organoids with C. difficile | Characterization of toxin A as the driving force of C. difficile associated diarrhea via disruption of epithelial barrier. | (64) | |
| Human | Human iPSCs organoids | Microinjection of S. enterica into organoids | Pathogenesis of S. enterica invasion of the epithelial barrier | (29) | |
| Human | Small intestine | Coculturing organoids with norovirus | Successful viral cultivation and replication assays in organoids | (24) | |
| Immunity | Human/Mouse | Small intestine and colon tumors | Culturing tumor organoids with their native immune cells such as T lymphocytes | Studying tumor-immune microenvironment and modeling tumor immune-escape mechanisms | (77) |
| Mouse | Small intestine | Coculture organoids with T-lymphocytes | The effects of activated T cells on the morphology and proliferation of organoid | (92) | |
| Mouse | Small intestine | Coculturing with intraepithelial lymphocytes | Characterization of the motility of intraepithelial lymphocytes in organoid culture to study their interactions | (79) | |
| Diet | Mouse | Small intestine | Organoid culture with fatty acids | Effect of fatty acids on proliferation of intestinal epithelium is PPAR-δ-driven | (5) |
Intestinal organoid cultures offer the opportunity to study host-microbe interactions due to the presence of an apical epithelial surface in the lumen of the organoid, which constitutes the main attachment site for many intestinal pathogens. For example, the mechanisms of secretory diarrhea caused by Vibrio cholerae have been studied using organoids grown from human duodenal and jejunal biopsies; cholera toxin induces large-volume diarrhea by inhibiting the NHE3 transporter and stimulating fluid secretion (31). Human organoids are substantially more permissive to rotavirus infection than mouse organoids, produce infectious viral particles, and respond to treatment with interferon-γ, ribavirin, or a calcium/calmodulin kinase 2 (CAMKK2) inhibitor (98, 132). A recent publication investigated Clostridioides difficile infection pathogenesis by microinjecting human iPSC-derived organoids with C. difficile toxins, which showed that the pathology associated with C. difficile infection is attributed to in situ toxin production (64). Another investigation examined the role of the Wnt receptor Frizzled in C. difficile pathogenesis using human intestinal organoids. The authors found that organoids deficient in Frizzled were not sensitive to C. difficile Toxin B, but wild-type organoids demonstrated decreased viability in response to this toxin (113). Finally, the pathogenesis of Salmonella typhimurium and noroviral infection has been studied in human intestinal organoids derived from iPSCs (24, 29).
Mucosal barrier function is believed to be regulated by interactions between epithelial cells and intraepithelial immune cells such as lymphocytes. However, study of the functions of intraepithelial lymphocytes has been limited by lack of adequate in vitro models (47). T cells have been cocultured with organoids to study immune-epithelial interactions (7, 92). Supplementation of growth media with interleukin-2, interleukin-7, or interleukin-15 promotes the viability of T cells in organoid culture (79). Coculture of intestinal organoids with macrophages has also been successfully performed to study mucosal barrier function after bacterial infection (78).
Organoid cultures have been applied to study heterogeneity of cell types in the intestine. Single-cell RNA sequencing analysis of hundreds of cells from murine intestinal organoids confirmed that organoid buds harbor stem cells that differentiate to form progenitor cells, enterocytes, enteroendocrine, and secretory cells. The authors then explored heterogeneity within the enteroendocrine cells and defined a novel subpopulation of endocrine cells that express Reg4 (44). The existence of these cells has been confirmed by a recent single-cell RNA sequencing analysis of mouse small intestinal epithelial cells directly isolated from mice (45). The authors also found that Lgr5+ stem cells in organoids are a relatively uniform population mixed with rare Paneth and enteroendocrine cells. Together, these findings demonstrate that intestinal organoids recapitulate the stem cell lineage of the in vivo intestine, including rare cell types (44).
An important feature of intestinal organoids is the ability to assess the organoid-forming capacity of FACS-sorted intestinal stem cells, alone or together with niche Paneth cells, as a proxy of their in vivo function (97). We used the organoid coculture system to study the effects of calorie restriction on intestinal cell function. We and others found that calorie restriction enhances intestinal stem cell function by inhibiting mechanistic target of rapamycin complex 1 (mTORC1) signaling in niche Paneth cells, but not in intestinal stem cells. The effects of calorie restriction and mTORC1 inhibition on stem cell function are recapitulated by the paracrine factor cyclic ADP ribose in Paneth cells (130). We subsequently used intestinal organoid models to study the effects of a high-fat diet on stem cell function. This led to the discovery that a high-fat diet, or an agonist of the peroxisomal proliferator-activated receptor delta (PPAR-δ) pathway, enhanced the niche-independent function of intestinal stem cells in an organoid assay. Furthermore, these effects were recapitulated by exogenous administration of fatty acids to mouse and human intestinal organoids (5).
Organoids are potentially useful as in vitro models of patient-specific disease for translational and clinical applications. Cystic fibrosis is a hereditary condition that is caused by dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR). Administration of forskolin to intestinal organoids induces CFTR-dependent swelling of the organoids by increasing cAMP levels. This response is defective in colonic organoids from cystic fibrosis patients. CRISPR-Cas9 gene editing has been used to repair the CFTR defect in organoids derived from cystic fibrosis patients (119). Subsequent research has shown that the in vitro forskolin test in colonic organoids predicts clinical response in cystic fibrosis patients (18). In another example, van Rijn et al. (117b) recently reported 10 patients with congenital diarrheal disorders who were found to harbor mutations in the DGAT1 gene, which led to aberrant lipid metabolism. Analysis of patient-derived intestinal organoids, as well as intestinal organoids engineered with DGAT1 mutations using CRISPR-Cas9 demonstrated that these mutations cause fat intolerance (117b).
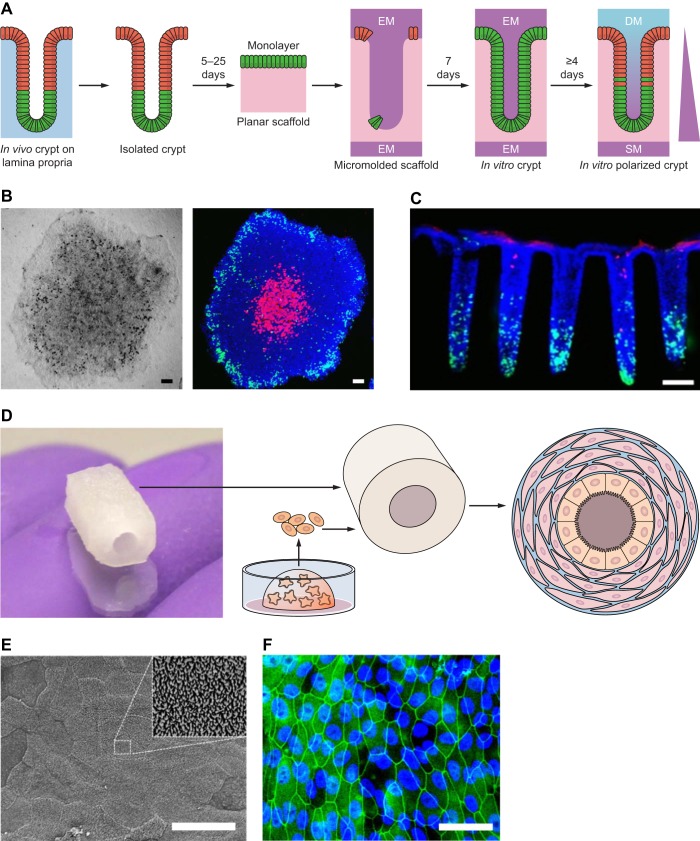
Microscaffold systems were used to study the effects of microbiota-produced compounds on the intestinal epithelium and to study intestinal absorption of drugs (103, 122). Chen et al. (13) embedded intestinal organoid cells onto a 3D silk scaffold, and then introduced myofibroblasts to the culture. The investigators then studied the effects of Escherichia coli infection of the intestinal epithelium. Recently developed organoid-on-a-chip platforms add continuously flowing fluid systems to organoid cultures. The chips are typically made of polymers that are cast into molds with microchannels and consist of two polymer layers separated by a permeable membrane (49). This microenvironment allows for cellular monolayers to be cultured with preservation of the luminal and basolateral aspects, as well as monitoring of parameters, such as mechanical stress, oxygenation, and interaction between different tissues by means of circulating fluid (127). Mouse colon organoids have been used in a three-dimensional printed chip to study intestinal microbiome physiology (126). These technologies have the potential to transform intestinal research by recapitulating many complex features of the human intestine, such as micronutrient availability and signaling gradients. However, they currently remain highly specialized and expensive tools and are not widely used. These newer organoid culture methods are presented in Fig. 6.
Fig. 6.
Novel organoid culture models. A: isolation of intestinal crypt and culturing onto planer scaffold yields an organoid monolayer that can be further modeled into a scaffold that resembles in vivo architecture of the crypts. EM, expansion medium; SM, stem medium; DM, differentiation medium. B: light microscopy of the organoid monolayers (left) and fluorescent microscopy stained for EdU (green), Muc2 (red), and nuclei (blue). C: fluorescent microscopic side sections stained for EdU pulse (green), ALP stain (red), and Hoechst-DNA labeling (blue) (121, 122). D: intestinal organoids embedded in Matrigel are digested and embedded onto a three-dimensional silk scaffold alongside myofibroblasts, forming a polarized culture along the silk scaffold. E: scanning electron microscopy showing the microvilli brush border on the apical surface of the scaffold. F: fluorescent microscopy of the apical surface staining for zonula occludens-1 (green) and DAPI (blue) (13). B and C scale bars =100 μm. E and F scale bars = 250 μm.
ROLE OF ORGANOIDS IN MODELING COLORECTAL CANCER
For decades, colorectal cancer (CRC) research has been performed using two-dimensional cell lines that are limited by a low rate of successful culture from primary cancers, contamination, culture-induced mutations, and selection for growth properties that may not reflect the pathophysiology of the original malignancy (67, 68). Transplanted CRC cell lines form poorly differentiated tumors in vivo that do not resemble the histology of human cancers. In contrast, CRC organoid cultures histologically and genetically resemble their parental tumor after transplantation and can be derived from most tumors (6, 60, 63). The Clevers and Sato laboratories reported human CRC organoids derived from cohorts of patient tissues, with successful organoid formation from over 90% of tumors and strong genetic and molecular correlation to their original tumors (124). For example, patient-derived KRAS mutant CRC organoids retain many features of patient tumors, including response to targeted therapies, such as cetuximab (63, 118). CRC organoids can also be derived from approximately 70% of patients with liver metastases (37, 124). CRC organoids can be used to study the genetic landscape in cancer. This was recently used to show that chromosomal instability in CRC organoids is common and could provide means for predicting response to therapy and potentially clinical outcomes (9).
Patient-derived organoids have been a resource for drug screening applications (33, 37, 125). For example, a subset of patient-derived CRC organoids demonstrated sensitivity to porcupine inhibitors and carried mutations in the negative Wnt regulator RNF43 (125). These drug screening methods with the aid of cell lines and patient-derived xenografts have been characterized and used to predict outcomes of clinical trials (39). A recent report of a cohort of patient-derived cancer organoids suggests that in vitro testing of therapeutic agents such as carboplatin and paclitaxel in organoids may reveal therapeutic targets that are not identified by other approaches such as whole genome sequencing (84). Interestingly, response to therapy was similar in CRC patients and organoid lines derived from their tumors (120). These reports demonstrate the importance of CRC models using organoid cultures as a bridge between preclinical research and clinical trials.
The combination of three-dimensional organoid cultures and CRISPR-Cas genome editing technology permits the study of genetically defined cultures from mice of any genetic background or from human specimens. The Clevers lab first reported editing of the APC tumor suppressor gene in mouse and human intestinal organoids by transfection of plasmids containing single-guide RNAs and the Cas9 endonuclease, followed by selection in media lacking Wnt agonists (102). They later sequentially engineered APC, KRASG12D, TP53, and SMAD4 mutations in human small intestinal and colon organoids using CRISPR-Cas9 technology to recapitulate the stepwise progression of driver mutations in human adenomas and CRCs (21). In a concurrent paper, Toshiro Sato’s group similarly used CRISPR-Cas9 methods to generate APC, KRASG12V, PIK3CAE545K, TP53, and SMAD4 mutations in human intestinal organoids (69). Both groups modified culture conditions to select for CRISPR-edited organoids. One significant finding from these efforts revealed that loss of the APC and TP53 tumor suppressor genes was sufficient to induce aneuploidy, a common feature of advanced cancers.
Cancer stem cells have been studied in patient-derived cancer organoids. The Sato laboratory first selected a set of patient-derived cancer organoid lines that maintain a stem cell hierarchy, as defined by expression of both LGR5 (a stem cell marker) and KRT20 (a differentiation marker). They then inserted GFP into the LGR5 or KRT20 alleles using CRISPR-Cas9 technology to visualize and lineage trace stem and differentiated cells. As previously demonstrated in mouse models by our group and others (19a, 93, 99), LGR5 tumor cells self-renew and differentiate, and ablation of LGR5+ cells results in tumor regression. Interestingly, as the KRT20+ differentiated cells continuously diminished over time, they were able to regenerate tumor upon loss of LGR5+ (GFP+) stem cells. These findings illustrate the potentially broad application of patient-derived cancer organoids for studying human disease (104).
Organoids have proven to be useful in studying genetic and phenotypic tumor heterogeneity at the single-cell level. Roerink et al. (91) cultured and expanded cancer cells from four to six regions in three cancers, as well as adjacent normal tissue. The authors demonstrated substantial mutagenesis in cancers compared with normal cells, as well as remarkable genetic heterogeneity within individual tumors. They also showed that tumors from different individuals exhibit divergent epigenetic (i.e., methylation) states, whereas methylation states in normal stem cells are similar between patients. Interestingly, tumor organoid clones showed differential response to cancer therapies (i.e., up to 1,000-fold differences in IC50), some of which could not be explained by genetic diversity (91).
Kuo and colleagues (77) demonstrated the use of patient-derived organoids with preserved immune cells in studying cancer immune microenvironment. Tumor samples were derived from 19 different distinct tissues, including small intestinal, gastric, and colorectal tumors, and were propagated using an ALI. This allowed the preservation of endogenous immune cells such as T lymphocytes and, thus, the tumor-immune response interactions. Importantly, T lymphocytes in such cultures recapitulated the same spectrum of T-cell receptors in vivo and their tumor interactions. Immune checkpoint blockade therapy using anti-PD-1/PD-L1 monoclonal antibodies were shown to be effective in inducing tumor cytotoxicity in these organoid cultures. This system allows for the evaluation of the different mechanisms by which tumor cells evade the immune system and permits in vitro studies with immunotherapy (77). With the rise of more therapeutic options against cancer cells, chimeric antigen receptor (CAR) lymphocytes are gaining increasing attention. A recent organoid CRC model with cocultured CAR lymphocytes targeted against the Frizzled receptor were shown to be an effective model for screening CAR lymphocyte therapy for CRC (101).
Intestinal organoids can be used to study in vivo physiology using transplantation mouse models that reproduce the structure and functions of the human colon. Normal mouse intestinal organoids have been successfully engrafted into the mouse colon with dextran sodium sulfate (DSS)-induced colitis via rectal enema (133), while human intestinal organoids have been orthotopically transplanted following EDTA-induced denuding of the colonic epithelium (111). Alternatively, we transplanted normal organoids into the lamina propria of recipient mice via colonoscopy-guided injection (93). Using these methods, the organoids integrate into the recipient colon epithelium. Organoids are also employed to investigate genetic requirements of evasion and metastasis. CRISPR-Cas9-engineered human CRC organoids have been engrafted into the subcutaneous space (21), renal capsule (69), and cecum of immunodeficient mice (38) and together, these efforts confirm that concurrent oncogenic mutations in the Wnt, EGFR, P53, and TGF-β signaling pathways promote metastasis. Yet, a key caveat of these studies is that the tumors are not located in the native microenvironment of the colonic mucosa (41). Three reports describe orthotopic engraftment of mouse and patient-derived CRC organoids into syngeneic and immunodeficient recipient mice. O’Rourke et al. (82) introduced DSS colitis into mice, and then delivered CRC organoids via rectal enema. The organoids engrafted into the mucosa of recipient mice to form adenomas that can be longitudinally monitored with optical colonoscopy (82). We used a colonoscopy-guided injection approach to deliver tumor organoids into the laminal propria of recipient mice, which also efficiently formed adenomas (93). Both groups reported invasion of the muscularis and liver metastasis in the setting of oncogenic Kras and Trp53 mutations. Both reports also describe efficient primary tumor formation in the colon from patient-derived tumor organoids that progresses to liver metastasis. Mouse cancer organoids with advanced mutations (i.e., Apc, Kras, Trp53, Smad4) have been engrafted into prolapsed rectal mucosa to model liver metastasis and Lgr5+ cancer stem cell function (19a). Together, these findings highlight the application of orthotopic transplantation techniques to efficiently integrate intestinal organoid models with mouse models to recapitulate human biology and disease, particularly cancer.
LIMITATIONS OF ORGANOID CULTURES
Advantages and disadvantages of organoid culture in comparison to other models of intestinal disease are described in Table 2. One limitation of most organoid cultures is the use of a three-dimensional matrix (i.e., Matrigel). Matrigel, as the most commonly used extracellular matrix to support the organoid cultures, is a poorly defined heterogeneous protein mixture (106). This adds unknown variables not accounted for while culturing organoids, which may affect their abilities to accurately recapitulate in vivo physiology (48). Further, as Matrigel is an animal-derived extraction, it creates an unnatural niche for embedded human organoids. Matrigel can actively alter the architecture of xenografted human cancer organoids. Recently developed synthetic hydrogels may overcome these inherent limitations of Matrigel, but they have yet to be tested in the wider scientific community (16, 17, 40). Despite the advent of conditioned media that contain Wnt3a, Rspondin1, and Noggin (72), these media require fetal bovine serum, which further adds potential variability and cost. One alternative is to use defined media with purified components. The accumulation of shed debris and secreted components into the intestinal organoid lumen requires serial passaging and limits the study of the luminal surface of the intestine. Microinjection of organoids and use of intestinal monolayers partially overcome this problem, but they are labor-intensive and require specialized equipment (107). Wnt, BMP, and Notch signaling gradients in the intestine are not reproduced in organoid culture and are essential to driving differentiation of intestinal cell types. Thus, many features of the crypt-villus unit are not captured by organoid models (4).
Table 2.
Advantages and disadvantages of in vitro cultures and in vivo models of intestinal physiology and disease
| Model Type | Advantages | Disadvantages |
|---|---|---|
| Cell line | Accessible Grow quickly Minimal care required Inexpensive Genetically engineered to study disease |
Lack cell-matrix interactions Lack cellular architecture Lack of microenvironment Genetic or phenotypic drift with passaging |
| Organoid | Preserves three-dimensional tissue architecture Grow quickly Reflect cell-matrix and cell-cell interactions Genetically engineered to study disease Preserved cellular genetics over time |
Costly reagents (i.e., collagen matrix, growth factors) Accessing the apical (luminal) surface is difficult Does not recapitulate the polarity of signaling gradients, such as Wnt and Notch Does not reproduce the three-dimensional structure of the crypt-villus axis Extracellular matrix used contains undetermined active components Challenging to model immune system and stroma Challenging to establish high-throughput screening Does not reproduce the intestinal stroma, which is essential for intestinal stem cell function |
| Genetically engineered mice | Accessible Recapitulates in vivo histology and physiology of human disease |
Time-consuming Costly |
| Transplantation | Reflects in vivo physiology Well-defined and localized Reflects normal physiology, disease, and cancer Faster than genetically engineered models Permit study of human tissues in vivo Ability to engraft is a proxy for stem cell function |
Requires specific set of skills Costly Subcutaneous transplants do not provide a correct microenvironment Orthotopic transplant may be technically challenging or have low engraftment rate |
| Organoid on a chip | Represents a wide variety of tissue/organs Contains fluid flow resembling in vivo fluid dynamics Can be used to study cell kinetics Can grow with little-to-no additional media components |
Expensive Currently inaccessible and under development |
Organoids are typically purely epithelial, unless the air liquid interface is used. Commonly used organoid cultures fail to integrate the immune system, stroma, vasculature, neural network, musculature, and microbiome that are essential components of normal intestinal physiology. Moreover, organoids do not reproduce interactions with the immune system that are known to be essential in the pathophysiology of infectious, neoplastic, and inherited processes in the gut. Coculturing organoids with various cell types such as intraepithelial lymphocytes (79) and microbiota-derived factors (115) has been reported. Although only embryonic tissue can be used in the ALI model, it is the only reported organoid system that recapitulates some aspects of the immune microenvironment (80). However, important limitations of this model are that long-term cultures are limited to neonatal murine tissues, and cultures cannot be expanded by passaging (80). As a result, other methods have been explored to coculture organoids with fibroblast-conditioned media to create a more accurate replica of in vivo stem cell-niche interactions (26, 50, 62). Another major limitation of organoid models is that they do not reproduce the signaling gradients and vascular flow surrounding the intestinal crypt. To overcome this problem, organoid monolayers can be seeded onto a microscaffold, to create invaginations that resemble crypt surfaces, which permit signaling gradients across the length of the scaffold (103, 122).
CONCLUSIONS AND FUTURE DIRECTIONS
Intestinal organoids are three-dimensional structures that reproduce the hierarchy of the crypt-villus unit. Organoid models have proven to be more accurate models of intestinal biology than standard two-dimensional cell cultures. Organoids have been applied for diverse applications, including studies in host-pathogen interactions, colorectal cancer biology, and drug development. Recent advances include the ability to direct pluripotent stem cells into intestinal or colonic lineages, orthotopic transplantation models of CRC organoids into recipient mouse colons, the study of intestinal transporters and luminal microbes using organoid monolayers, CRISPR-Cas-mediated repair of disease, and high-throughput drug screening. Future advances in organoid technology will most likely be in modeling the complex microenvironment surrounding the intestinal epithelium. Ultimately, a major goal of organoid technology is for regenerative medicine applications to replace or repair defective intestinal tissues in patients.
GRANTS
Research conducted in the authors’ laboratories was supported by the NIH (Grant K08 CA198002, to J. Roper; Grants R00 AG045144, R01 CA211184, and R01 CA034992, to Ö. H. Yilmaz); Department of Defense (PRCRP Career Development Award CA120198, to J. Roper); the V Foundation V Scholar Award (J. Roper and Ö. H. Yilmaz); the Sidney Kimmel Scholar Award (Ö. H. Yilmaz); the Pew-Stewart Trust Scholar Award (Ö. H. Yilmaz); the Koch Institute Frontier Research Program through the Kathy and Curt Marble Cancer Research Fund (Ö. H. Yilmaz); and by the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A. and J.R. prepared figures; M.A., M.D.M., J.R., and Ö.H.Y. drafted manuscript; M.A., M.D.M., J.R., and Ö.H.Y. edited and revised manuscript; M.A., M.D.M., J.R., and Ö.H.Y. approved final version of manuscript.
REFERENCES
- 1.Abud HE, Watson N, Heath JK. Growth of intestinal epithelium in organ culture is dependent on EGF signalling. Exp Cell Res 303: 252–262, 2005. doi: 10.1016/j.yexcr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 3.Ben-David U, Siranosian B, Ha G, Tang H, Oren Y, Hinohara K, Strathdee CA, Dempster J, Lyons NJ, Burns R, Nag A, Kugener G, Cimini B, Tsvetkov P, Maruvka YE, O’Rourke R, Garrity A, Tubelli AA, Bandopadhayay P, Tsherniak A, Vazquez F, Wong B, Birger C, Ghandi M, Thorner AR, Bittker JA, Meyerson M, Getz G, Beroukhim R, Golub TR. Genetic and transcriptional evolution alters cancer cell line drug response. Nature 560: 325–330, 2018. doi: 10.1038/s41586-018-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beumer J, Artegiani B, Post Y, Reimann F, Gribble F, Nguyen TN, Zeng H, Van den Born M, Van Es JH, Clevers H. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat Cell Biol 20: 909–916, 2018. doi: 10.1038/s41556-018-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong S-J, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta N, Ferrone CR, Deshpande V, Yuan G-C, Orkin SH, Sabatini DM, Yilmaz ÖH. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531: 53–58, 2016. [Erratum in Nature 560: E26, 2018.] doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro—a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol 15: 405–412, 2005. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, Ashenberg O, Su C-W, Smillie C, Shekhar K, Chen Z, Wu C, Ordovas-Montanes J, Alvarez D, Herbst RH, Zhang M, Tirosh I, Dionne D, Nguyen LT, Xifaras ME, Shalek AK, von Andrian UH, Graham DB, Rozenblatt-Rosen O, Shi HN, Kuchroo V, Yilmaz OH, Regev A, Xavier RJ. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 175: 1307–1320.e22, 2018. doi: 10.1016/j.cell.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjerknes M, Cheng H. Intestinal epithelial stem cells and progenitors. Methods Enzymol 419: 337–383, 2006. doi: 10.1016/S0076-6879(06)19014-X. [DOI] [PubMed] [Google Scholar]
- 9.Bolhaqueiro ACF, Ponsioen B, Bakker B, Klaasen SJ, Kucukkose E, van Jaarsveld RH, Vivié J, Verlaan-Klink I, Hami N, Spierings DCJ, Sasaki N, Dutta D, Boj SF, Vries RGJ, Lansdorp PM, van de Wetering M, van Oudenaarden A, Clevers H, Kranenburg O, Foijer F, Snippert HJG, Kops GJPL. Ongoing chromosomal instability and karyotype evolution in human colorectal cancer organoids. Nat Genet 51: 824–834, 2019. doi: 10.1038/s41588-019-0399-6. [DOI] [PubMed] [Google Scholar]
- 10.Booth C, O’Shea JA, Potten CS. Maintenance of functional stem cells in isolated and cultured adult intestinal epithelium. Exp Cell Res 249: 359–366, 1999. doi: 10.1006/excr.1999.4483. [DOI] [PubMed] [Google Scholar]
- 11.Booth C, Patel S, Bennion GR, Potten CS. The isolation and culture of adult mouse colonic epithelium. Epithelial Cell Biol 4: 76–86, 1995. [PubMed] [Google Scholar]
- 12.Breault DT, Min IM, Carlone DL, Farilla LG, Ambruzs DM, Henderson DE, Algra S, Montgomery RK, Wagers AJ, Hole N. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci USA 105: 10420–10425, 2008. doi: 10.1073/pnas.0804800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Zhou W, Roh T, Estes MK, Kaplan DL. In vitro enteroid-derived three-dimensional tissue model of human small intestinal epithelium with innate immune responses. PLoS One 12: e0187880, 2017. doi: 10.1371/journal.pone.0187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141: 537–561, 1974. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 15.Choi RS, Vacanti JP. Preliminary studies of tissue-engineered intestine using isolated epithelial organoid units on tubular synthetic biodegradable scaffolds. Transplant Proc 29: 848–851, 1997. doi: 10.1016/S0041-1345(96)00164-9. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Acuña R, Quirós M, Farkas AE, Dedhia PH, Huang S, Siuda D, García-Hernández V, Miller AJ, Spence JR, Nusrat A, García AJ. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol 19: 1326–1335, 2017. doi: 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz-Acuña R, Quirós M, Huang S, Siuda D, Spence JR, Nusrat A, García AJ. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat Protoc 13: 2102–2119, 2018. [Erratum in Nat Protoc 14: 2258, 2019.] doi: 10.1038/s41596-018-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EES, Houwen RHJ, Vleggaar FP, Escher JC, de Rijke YB, Majoor CJ, Heijerman HGM, de Winter-de Groot KM, Clevers H, van der Ent CK, Beekman JM. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med 8: 344ra84, 2016. doi: 10.1126/scitranslmed.aad8278. [DOI] [PubMed] [Google Scholar]
- 19.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NWM, Bijvelds MJC, Scholte BJ, Nieuwenhuis EES, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19: 939–945, 2013. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 19a.de Sousa e Melo F. Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z, Koeppen H, Dijkgraaf GJP, Piskol R, de Sauvage FJ. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543: 676–680, 2017. doi: 10.1038/nature21713. [DOI] [PubMed] [Google Scholar]
- 20.DeWard AD, Cramer J, Lagasse E. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep 9: 701–711, 2014. doi: 10.1016/j.celrep.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H, Korving J, van de Wetering M, Schwank G, Logtenberg M, Cuppen E, Snippert HJ, Medema JP, Kops GJPL, Clevers H. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521: 43–47, 2015. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- 22.Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci USA 109: 8965–8970, 2012. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng X-L, Qu L, Kou B, Opekun AR, Burrin D, Graham DY, Ramani S, Atmar RL, Estes MK. Replication of human noroviruses in stem cell-derived human enteroids. Science 353: 1387–1393, 2016. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci 101: 219–231, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143: 1518–1529.e7, 2012. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 27.Finkbeiner SR, Freeman JJ, Wieck MM, El-Nachef W, Altheim CH, Tsai Y-H, Huang S, Dyal R, White ES, Grikscheit TC, Teitelbaum DH, Spence JR. Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol Open 4: 1462–1472, 2015. doi: 10.1242/bio.013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkbeiner SR, Zeng X-L, Utama B, Atmar RL, Shroyer NF, Estes MK. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. MBio 3: e00159-12, 2012. doi: 10.1128/mBio.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forbester JL, Goulding D, Vallier L, Hannan N, Hale C, Pickard D, Mukhopadhyay S, Dougan G. Interaction of Salmonella enterica Serovar Typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect Immun 83: 2926–2934, 2015. doi: 10.1128/IAI.00161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fordham RP, Yui S, Hannan NRF, Soendergaard C, Madgwick A, Schweiger PJ, Nielsen OH, Vallier L, Pedersen RA, Nakamura T, Watanabe M, Jensen KB. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13: 734–744, 2013. doi: 10.1016/j.stem.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foulke-Abel J, In J, Kovbasnjuk O, Zachos NC, Ettayebi K, Blutt SE, Hyser JM, Zeng X-L, Crawford SE, Broughman JR, Estes MK, Donowitz M. Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp Biol Med (Maywood) 239: 1124–1134, 2014. doi: 10.1177/1535370214529398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foulke-Abel J, In J, Yin J, Zachos NC, Kovbasnjuk O, Estes MK, de Jonge H, Donowitz M. Human enteroids as a model of upper small intestinal ion transport physiology and pathophysiology. Gastroenterology 150: 638–649.e8, 2016. doi: 10.1053/j.gastro.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francies HE, Barthorpe A, McLaren-Douglas A, Barendt WJ, Garnett MJ. Drug sensitivity assays of human cancer organoid cultures. In: Methods in Molecular Biology. New York: Humana, 2016. doi: 10.1007/7651_2016_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435: 964–968, 2005. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 35.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124: 619–626, 1994. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii M, Matano M, Toshimitsu K, Takano A, Mikami Y, Nishikori S, Sugimoto S, Sato T. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell 23: 787–793.e6, 2018. doi: 10.1016/j.stem.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, Ohta Y, Toshimitsu K, Nakazato Y, Kawasaki K, Uraoka T, Watanabe T, Kanai T, Sato T. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18: 827–838, 2016. doi: 10.1016/j.stem.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Fumagalli A, Drost J, Suijkerbuijk SJE, van Boxtel R, de Ligt J, Offerhaus GJ, Begthel H, Beerling E, Tan EH, Sansom OJ, Cuppen E, Clevers H, van Rheenen J. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc Natl Acad Sci USA 114: E2357–E2364, 2017. doi: 10.1073/pnas.1701219114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, Zhang C, Schnell C, Yang G, Zhang Y, Balbin OA, Barbe S, Cai H, Casey F, Chatterjee S, Chiang DY, Chuai S, Cogan SM, Collins SD, Dammassa E, Ebel N, Embry M, Green J, Kauffmann A, Kowal C, Leary RJ, Lehar J, Liang Y, Loo A, Lorenzana E, Robert McDonald E III, McLaughlin ME, Merkin J, Meyer R, Naylor TL, Patawaran M, Reddy A, Röelli C, Ruddy DA, Salangsang F, Santacroce F, Singh AP, Tang Y, Tinetto W, Tobler S, Velazquez R, Venkatesan K, Von Arx F, Wang HQ, Wang Z, Wiesmann M, Wyss D, Xu F, Bitter H, Atadja P, Lees E, Hofmann F, Li E, Keen N, Cozens R, Jensen MR, Pryer NK, Williams JA, Sellers WR. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med 21: 1318–1325, 2015. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- 40.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature 539: 560–564, 2016. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 41.Golovko D, Kedrin D, Yilmaz ÖH, Roper J. Colorectal cancer models for novel drug discovery. Expert Opin Drug Discov 10: 1217–1229, 2015. doi: 10.1517/17460441.2015.1079618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grabinger T, Luks L, Kostadinova F, Zimberlin C, Medema JP, Leist M, Brunner T. Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis 5: e1228, 2014. doi: 10.1038/cddis.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greicius G, Kabiri Z, Sigmundsson K, Liang C, Bunte R, Singh MK, Virshup DM. PDGFRα + pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc Natl Acad Sci USA 115: E3173–E3181, 2018. doi: 10.1073/pnas.1713510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grün D, Lyubimova A, Kester L, Wiebrands K, Basak O, Sasaki N, Clevers H, van Oudenaarden A. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 525: 251–255, 2015. doi: 10.1038/nature14966. [DOI] [PubMed] [Google Scholar]
- 45.Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, Regev A. A single-cell survey of the small intestinal epithelium. Nature 551: 333–339, 2017. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hachitanda Y, Tsuneyoshi M. Neuroblastoma with a distinct organoid pattern: a clinicopathologic, immunohistochemical, and ultrastructural study. Hum Pathol 25: 67–72, 1994. doi: 10.1016/0046-8177(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 47.Hohwieler M, Illing A, Hermann PC, Mayer T, Stockmann M, Perkhofer L, Eiseler T, Antony JS, Müller M, Renz S, Kuo C-C, Lin Q, Sendler M, Breunig M, Kleiderman SM, Lechel A, Zenker M, Leichsenring M, Rosendahl J, Zenke M, Sainz B Jr, Mayerle J, Costa IG, Seufferlein T, Kormann M, Wagner M, Liebau S, Kleger A. Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut 66: 473–486, 2017. doi: 10.1136/gutjnl-2016-312423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10: 1886–1890, 2010. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 49.Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, Hamilton GA, Ingber DE. Microfabrication of human organs-on-chips. Nat Protoc 8: 2135–2157, 2013. doi: 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- 50.Hynds RE, Giangreco A. Concise review: the relevance of human stem cell-derived organoid models for epithelial translational medicine. Stem Cells 31: 417–422, 2013. doi: 10.1002/stem.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.In J, Foulke-Abel J, Zachos NC, Hansen A-M, Kaper JB, Bernstein HD, Halushka M, Blutt S, Estes MK, Donowitz M, Kovbasnjuk O. Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell Mol Gastroenterol Hepatol 2: 48–62.e3, 2016. doi: 10.1016/j.jcmgh.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.In J, Lukyanenko V, Foulke-Abel J, Hubbard AL, Delannoy M, Hansen A-M, Kaper JB, Boisen N, Nataro JP, Zhu C, Boedeker EC, Girón JA, Kovbasnjuk O. Serine protease EspP from enterohemorrhagic Escherichia coli is sufficient to induce shiga toxin macropinocytosis in intestinal epithelium. PLoS One 8: e69196, 2013. doi: 10.1371/journal.pone.0069196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung P, Sato T, Merlos-Suárez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, Clevers H, Batlle E. Isolation and in vitro expansion of human colonic stem cells. Nat Med 17: 1225–1227, 2011. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 54.Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison, Aliyev J, Wu Y, Bunte R, Williams BO, Rossant J, Virshup DM. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141: 2206–2215, 2014. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 55.Kaeffer B. Mammalian intestinal epithelial cells in primary culture: a mini-review. In Vitro Cell Dev Biol Anim 38: 123–134, 2002. doi:. [DOI] [PubMed] [Google Scholar]
- 57.Kaufman MH. (). The Atlas of Mouse Development. Amsterdam: Elsevier Academic Press, 2010. [Google Scholar]
- 58.Kim SS, Kaihara S, Benvenuto MS, Choi RS, Kim BS, Mooney DJ, Vacanti JP. Effects of anastomosis of tissue-engineered neointestine to native small bowel. J Surg Res 87: 6–13, 1999. doi: 10.1006/jsre.1999.5743. [DOI] [PubMed] [Google Scholar]
- 59.Kim T-H, Escudero S, Shivdasani RA. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci USA 109: 3932–3937, 2012. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kondo J, Endo H, Okuyama H, Ishikawa O, Iishi H, Tsujii M, Ohue M, Inoue M. Retaining cell-cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci USA 108: 6235–6240, 2011. doi: 10.1073/pnas.1015938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhnert F, Davis CR, Wang H-T, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 101: 266–271, 2004. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lahar N, Lei NY, Wang J, Jabaji Z, Tung SC, Joshi V, Lewis M, Stelzner M, Martín MG, Dunn JCY. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS One 6: e26898, 2011. doi: 10.1371/journal.pone.0026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee S-H, Hong JH, Park HK, Park JS, Kim B-K, Lee J-Y, Jeong JY, Yoon GS, Inoue M, Choi G-S, Lee I-K. Colorectal cancer-derived tumor spheroids retain the characteristics of original tumors. Cancer Lett 367: 34–42, 2015. doi: 10.1016/j.canlet.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 64.Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, Spence JR. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect Immun 83: 138–145, 2015. doi: 10.1128/IAI.02561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Ootani A, Kuo C. An air-liquid interface culture system for 3D organoid culture of diverse primary gastrointestinal tissues. Methods Mol Biol 1422: 33–40, 2016. doi: 10.1007/978-1-4939-3603-8_4. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Mi Y, Mueller T, Kreibich S, Williams EG, Van Drogen A, Borel C, Frank M, Germain PL, Bludau I, Mehnert M, Seifert M, Emmenlauer M, Sorg I, Bezrukov F, Bena FS, Zhou H, Dehio C, Testa G, Saez-Rodriguez J, Antonarakis SE, Hardt WD, Aebersold R. Multi-omic measurements of heterogeneity in HeLa cells across laboratories. Nat Biotechnol 37: 314–322, 2019. doi: 10.1038/s41587-019-0037-y. [DOI] [PubMed] [Google Scholar]
- 67.Marx V. Cell-line authentication demystified. Nat Methods 11: 483–488, 2014. doi: 10.1038/nmeth.2932. [DOI] [PubMed] [Google Scholar]
- 68.Masters JR, Stacey GN. Changing medium and passaging cell lines. Nat Protoc 2: 2276–2284, 2007. doi: 10.1038/nprot.2007.319. [DOI] [PubMed] [Google Scholar]
- 69.Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med 21: 256–262, 2015. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 71.Middendorp S, Schneeberger K, Wiegerinck CL, Mokry M, Akkerman RDL, van Wijngaarden S, Clevers H, Nieuwenhuis EES. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells 32: 1083–1091, 2014. doi: 10.1002/stem.1655. [DOI] [PubMed] [Google Scholar]
- 72.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc 8: 2471–2482, 2013. doi: 10.1038/nprot.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk MEG, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA 108: 179–184, 2011. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moore SR, Pruszka J, Vallance J, Aihara E, Matsuura T, Montrose MH, Shroyer NF, Hong CI. Robust circadian rhythms in organoid cultures from PERIOD2:LUCIFERASE mouse small intestine. Dis Model Mech 7: 1123–1130, 2014. doi: 10.1242/dmm.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Múnera JO, Sundaram N, Rankin SA, Hill D, Watson C, Mahe M, Vallance JE, Shroyer NF, Sinagoga KL, Zarzoso-Lacoste A, Hudson JR, Howell JC, Chatuvedi P, Spence JR, Shannon JM, Zorn AM, Helmrath MA, Wells JM. Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell 21: 51–64.e6, 2017. [Erratum in Cell Stem Cell 24: 829, 2019.] doi: 10.1016/j.stem.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mustata RC, Vasile G, Fernandez-Vallone V, Strollo S, Lefort A, Libert F, Monteyne D, Pérez-Morga D, Vassart G, Garcia M-I. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep 5: 421–432, 2013. doi: 10.1016/j.celrep.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, Liu IH, Chiou S-H, Salahudeen AA, Smith AR, Deutsch BC, Liao L, Zemek AJ, Zhao F, Karlsson K, Schultz LM, Metzner TJ, Nadauld LD, Tseng Y-Y, Alkhairy S, Oh C, Keskula P, Mendoza-Villanueva D, De La Vega FM, Kunz PL, Liao JC, Leppert JT, Sunwoo JB, Sabatti C, Boehm JS, Hahn WC, Zheng GXY, Davis MM, Kuo CJ. Organoid modeling of the tumor immune microenvironment. Cell 175: 1972–1988.e16, 2018. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, Zachos NC. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep 7: 45270, 2017. [Erratum in Sci Rep 7: 46790, 2017.] doi: 10.1038/srep45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nozaki K, Mochizuki W, Matsumoto Y, Matsumoto T, Fukuda M, Mizutani T, Watanabe M, Nakamura T. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J Gastroenterol 51: 206–213, 2016. doi: 10.1007/s00535-016-1170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15: 701–706, 2009. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Rourke KP, Loizou E, Livshits G, Schatoff EM, Baslan T, Manchado E, Simon J, Romesser PB, Leach B, Han T, Pauli C, Beltran H, Rubin MA, Dow LE, Lowe SW. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat Biotechnol 35: 577–582, 2017. doi: 10.1038/nbt.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pageot LP, Perreault N, Basora N, Francoeur C, Magny P, Beaulieu JF. Human cell models to study small intestinal functions: recapitulation of the crypt-villus axis. Microsc Res Tech 49: 394–406, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 84.Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, Sailer V, Augello M, Puca L, Rosati R, McNary TJ, Churakova Y, Cheung C, Triscott J, Pisapia D, Rao R, Mosquera JM, Robinson B, Faltas BM, Emerling BE, Gadi VK, Bernard B, Elemento O, Beltran H, Demichelis F, Kemp CJ, Grandori C, Cantley LC, Rubin MA. Personalized In Vitro and In Vivo cancer models to guide precision medicine. Cancer Discov 7: 462–477, 2017. doi: 10.1158/2159-8290.CD-16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petersen N, Frimurer TM, Terndrup Pedersen M, Egerod KL, Wewer Albrechtsen NJ, Holst JJ, Grapin-Botton A, Jensen KB, Schwartz TW. Inhibiting RHOA signaling in mice increases glucose tolerance and numbers of enteroendocrine and other secretory cells in the intestine. Gastroenterology 155: 1164–1176.e2, 2018. doi: 10.1053/j.gastro.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 86.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713, 2003. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pompaiah M, Bartfeld S. Gastric organoids: an emerging model system to study Helicobacter pylori pathogenesis. Curr Top Microbiol Immunol 400: 149–168, 2017. doi: 10.1007/978-3-319-50520-6_7. [DOI] [PubMed] [Google Scholar]
- 88.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110: 1001–1020, 1990. [DOI] [PubMed] [Google Scholar]
- 89.Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL, Coffey RJ. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149: 146–158, 2012. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roerink SF, Sasaki N, Lee-Six H, Young MD, Alexandrov LB, Behjati S, Mitchell TJ, Grossmann S, Lightfoot H, Egan DA, Pronk A, Smakman N, van Gorp J, Anderson E, Gamble SJ, Alder C, van de Wetering M, Campbell PJ, Stratton MR, Clevers H. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 556: 457–462, 2018. doi: 10.1038/s41586-018-0024-3. [DOI] [PubMed] [Google Scholar]
- 92.Rogoz A, Reis BS, Karssemeijer RA, Mucida D. A 3-D enteroid-based model to study T-cell and epithelial cell interaction. J Immunol Methods 421: 89–95, 2015. doi: 10.1016/j.jim.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roper J, Tammela T, Cetinbas NM, Akkad A, Roghanian A, Rickelt S, Almeqdadi M, Wu K, Oberli MA, Sánchez-Rivera FJ, Park YK, Liang X, Eng G, Taylor MS, Azimi R, Kedrin D, Neupane R, Beyaz S, Sicinska ET, Suarez Y, Yoo J, Chen L, Zukerberg L, Katajisto P, Deshpande V, Bass AJ, Tsichlis PN, Lees J, Langer R, Hynes RO, Chen J, Bhutkar A, Jacks T, Yilmaz ÖH. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat Biotechnol 35: 569–576, 2017. doi: 10.1038/nbt.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920, 2008. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95a.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265. 2009. doi: 10.1038/nature0793595a. [DOI] [PubMed] [Google Scholar]
- 96.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sato T, Stange DE, Ferrante M, Vries RGJ, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 98.Saxena K, Blutt SE, Ettayebi K, Zeng X-L, Broughman JR, Crawford SE, Karandikar UC, Sastri NP, Conner ME, Opekun AR, Graham DY, Qureshi W, Sherman V, Foulke-Abel J, In J, Kovbasnjuk O, Zachos NC, Donowitz M, Estes MK. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol 90: 43–56, 2016. doi: 10.1128/JVI.01930-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337: 730–735, 2012. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 100.Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med 97: 695–710, 1953. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schnalzger TE, de Groot MH, Zhang C, Mosa MH, Michels BE, Röder J, Darvishi T, Wels WS, Farin HF. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J 38: e100928, 2019. doi: 10.15252/embj.2018100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schwank G, Koo B-K, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EES, Beekman JM, Clevers H. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13: 653–658, 2013. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 103.Shim K-Y, Lee D, Han J, Nguyen N-T, Park S, Sung JH. Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed Microdevices 19: 37, 2017. doi: 10.1007/s10544-017-0179-y. [DOI] [PubMed] [Google Scholar]
- 104.Shimokawa M, Ohta Y, Nishikori S, Matano M, Takano A, Fujii M, Date S, Sugimoto S, Kanai T, Sato T. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature 545: 187–192, 2017. doi: 10.1038/nature22081. [DOI] [PubMed] [Google Scholar]
- 105.Shoshkes-Carmel M, Wang YJ, Wangensteen KJ, Tóth B, Kondo A, Massasa EE, Itzkovitz S, Kaestner KH. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 557: 242–246, 2018. [Erratum in Nature 560: E29, 2018.] doi: 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shuhendler AJ, Prasad P, Cai P, Hui KKW, Henderson JT, Rauth AM, Wu XY. Matrigel alters the pathophysiology of orthotopic human breast adenocarcinoma xenografts with implications for nanomedicine evaluation. Nanomedicine (Lond) 9: 795–805, 2013. doi: 10.1016/j.nano.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 107.Spence JR. Taming the Wild West of organoids, enteroids, and mini-guts. Cell Mol Gastroenterol Hepatol 5: 159–160, 2018. doi: 10.1016/j.jcmgh.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470: 105–109, 2011. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stelzner M, Helmrath M, Dunn JCY, Henning SJ, Houchen CW, Kuo C, Lynch J, Li L, Magness ST, Martin MG, Wong MH, Yu J; NIH Intestinal Stem Cell Consortium . A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol 302: G1359–G1363, 2012. doi: 10.1152/ajpgi.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sträter J, Wedding U, Barth TF, Koretz K, Elsing C, Möller P. Rapid onset of apoptosis in vitro follows disruption of beta 1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology 110: 1776–1784, 1996. doi: 10.1053/gast.1996.v110.pm8964403. [DOI] [PubMed] [Google Scholar]
- 111.Sugimoto S, Ohta Y, Fujii M, Matano M, Shimokawa M, Nanki K, Date S, Nishikori S, Nakazato Y, Nakamura T, Kanai T, Sato T. Reconstruction of the human colon epithelium in vivo. Cell Stem Cell 22: 171–176.e5, 2018. doi: 10.1016/j.stem.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 112.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science 334: 1420–1424, 2011. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tao L, Zhang J, Meraner P, Tovaglieri A, Wu X, Gerhard R, Zhang X, Stallcup WB, Miao J, He X, Hurdle JG, Breault DT, Brass AL, Dong M. Frizzled proteins are colonic epithelial receptors for C. difficile toxin B. Nature 538: 350–355, 2016. doi: 10.1038/nature19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsai Y-H, Nattiv R, Dedhia PH, Nagy MS, Chin AM, Thomson M, Klein OD, Spence JR. In vitro patterning of pluripotent stem cell-derived intestine recapitulates in vivo human development. Development 144: 1045–1055, 2017. doi: 10.1242/dev.138453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsuruta T, Saito S, Osaki Y, Hamada A, Aoki-Yoshida A, Sonoyama K. Organoids as an ex vivo model for studying the serotonin system in the murine small intestine and colon epithelium. Biochem Biophys Res Commun 474: 161–167, 2016. doi: 10.1016/j.bbrc.2016.03.165. [DOI] [PubMed] [Google Scholar]
- 116.Tüysüz N, van Bloois L, van den Brink S, Begthel H, Verstegen MM, Cruz LJ, Hui L, van der Laan LJ, de Jonge J, Vries R, Braakman E, Mastrobattista E, Cornelissen JJ, Clevers H, Ten Berge D. Lipid-mediated Wnt protein stabilization enables serum-free culture of human organ stem cells. Nat Commun 8: 14578, 2017. doi: 10.1038/ncomms14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA, Stappenbeck TS. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64: 911–920, 2015. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117a.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963, 2005. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 117b.van Rijn JM, Ardy RC, Kuloğlu Z, Härter B, van Haaften-Visser DY, van der Doef HPJ, van Hoesel M, Kansu A, van Vugt AHM, Thian M, Kokke FTM, Krolo A, Başaran MK, Kaya NG, Aksu AÜ, Dalgıç B, Ozcay F, Baris Z, Kain R, Stigter ECA, Lichtenbelt KD, Massink MPG, Duran KJ, Verheij JBGM, Lugtenberg D, Nikkels PGJ, Brouwer HGF, Verkade HJ, Scheenstra R, Spee B, Nieuwenhuis EES, Coffer PJ, Janecke AR, van Haaften G, Houwen RHJ, Müller T, Middendorp S, Boztug K. Intestinal failure and aberrant lipid metabolism in patients with DGAT1 deficiency. Gastroenterology 155: 130–143.e15, 2018. doi: 10.1053/j.gastro.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Verissimo CS, Overmeer RM, Ponsioen B, Drost J, Mertens S, Verlaan-Klink I, Gerwen BV, van der Ven M, Wetering MV, Egan DA, Bernards R, Clevers H, Bos JL, Snippert HJ. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. eLife 5: e18489, 2016. doi: 10.7554/eLife.18489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vidović D, Carlon MS, da Cunha MF, Dekkers JF, Hollenhorst MI, Bijvelds MJC, Ramalho AS, Van den Haute C, Ferrante M, Baekelandt V, Janssens HM, De Boeck K, Sermet-Gaudelus I, de Jonge HR, Gijsbers R, Beekman JM, Edelman A, Debyser Z. rAAV-CFTRΔR rescues the cystic fibrosis phenotype in human intestinal organoids and cystic fibrosis mice. Am J Respir Crit Care Med 193: 288–298, 2016. doi: 10.1164/rccm.201505-0914OC. [DOI] [PubMed] [Google Scholar]
- 120.Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, Rata M, Koh D-M, Tunariu N, Collins D, Hulkki-Wilson S, Ragulan C, Spiteri I, Moorcraft SY, Chau I, Rao S, Watkins D, Fotiadis N, Bali M, Darvish-Damavandi M, Lote H, Eltahir Z, Smyth EC, Begum R, Clarke PA, Hahne JC, Dowsett M, de Bono J, Workman P, Sadanandam A, Fassan M, Sansom OJ, Eccles S, Starling N, Braconi C, Sottoriva A, Robinson SP, Cunningham D, Valeri N. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359: 920–926, 2018. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y, DiSalvo M, Gunasekara DB, Dutton J, Proctor A, Lebhar MS, Williamson IA, Speer J, Howard RL, Smiddy NM, Bultman SJ, Sims CE, Magness ST, Allbritton NL. Self-renewing monolayer of primary colonic or rectal epithelial cells. Cell Mol Gastroenterol Hepatol 4: 165–182.e7, 2017. doi: 10.1016/j.jcmgh.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y, Kim R, Gunasekara DB, Reed MI, DiSalvo M, Nguyen DL, Bultman SJ, Sims CE, Magness ST, Allbritton NL. Formation of human colonic crypt array by application of chemical gradients across a shaped epithelial monolayer. Cell Mol Gastroenterol Hepatol 5: 113–130, 2018. doi: 10.1016/j.jcmgh.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Watson CL, Mahe MM, Múnera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, Grabowski G, Finkbeiner SR, Spence JR, Shroyer NF, Wells JM, Helmrath MA. An in vivo model of human small intestine using pluripotent stem cells. Nat Med 20: 1310–1314, 2014. doi: 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weeber F, van de Wetering M, Hoogstraat M, Dijkstra KK, Krijgsman O, Kuilman T, Gadellaa-van Hooijdonk CGM, van der Velden DL, Peeper DS, Cuppen EPJG, Vries RG, Clevers H, Voest EE. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci USA 112: 13308–13311, 2015. doi: 10.1073/pnas.1516689112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, McLaren-Douglas A, Blokker J, Jaksani S, Bartfeld S, Volckman R, van Sluis P, Li VSW, Seepo S, Sekhar Pedamallu C, Cibulskis K, Carter SL, McKenna A, Lawrence MS, Lichtenstein L, Stewart C, Koster J, Versteeg R, van Oudenaarden A, Saez-Rodriguez J, Vries RGJ, Getz G, Wessels L, Stratton MR, McDermott U, Meyerson M, Garnett MJ, Clevers H. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161: 933–945, 2015. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Williamson IA, Arnold JW, Samsa LA, Gaynor L, DiSalvo M, Cocchiaro JL, Carroll I, Azcarate-Peril MA, Rawls JF, Allbritton NL, Magness ST. A high-throughput organoid microinjection platform to study gastrointestinal microbiota and luminal physiology. Cell Mol Gastroenterol Hepatol 6: 301–319, 2018. doi: 10.1016/j.jcmgh.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Workman MJ, Gleeson JP, Troisi EJ, Estrada HQ, Kerns SJ, Hinojosa CD, Hamilton GA, Targan SR, Svendsen CN, Barrett RJ. Enhanced utilization of induced pluripotent stem cell-derived human intestinal organoids using microengineered chips. Cell Mol Gastroenterol Hepatol 5: 669–677.e2, 2018. doi: 10.1016/j.jcmgh.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang C-F, Schiesser J, Aubert P, Stanley EG, Elefanty AG, Miyaoka Y, Mandegar MA, Conklin BR, Neunlist M, Brugmann SA, Helmrath MA, Wells JM. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 23: 49–59, 2017. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wroblewski LE, Piazuelo MB, Chaturvedi R, Schumacher M, Aihara E, Feng R, Noto JM, Delgado A, Israel DA, Zavros Y, Montrose MH, Shroyer N, Correa P, Wilson KT, Peek RM Jr. Helicobacter pylori targets cancer-associated apical-junctional constituents in gastroids and gastric epithelial cells. Gut 64: 720–730, 2015. doi: 10.1136/gutjnl-2014-307650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino-Kenudson M, Zukerberg LR, Bhan AK, Deshpande V, Sabatini DM. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486: 490–495, 2012. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods 11: 106–112, 2014. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yin Y, Bijvelds M, Dang W, Xu L, van der Eijk AA, Knipping K, Tuysuz N, Dekkers JF, Wang Y, de Jonge J, Sprengers D, van der Laan LJW, Beekman JM, Ten Berge D, Metselaar HJ, de Jonge H, Koopmans MPG, Peppelenbosch MP, Pan Q. Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antiviral Res 123: 120–131, 2015. doi: 10.1016/j.antiviral.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 133.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18: 618–623, 2012. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 134.Zimmermann B. Lung organoid culture. Differentiation 36: 86–109, 1987. doi: 10.1111/j.1432-0436.1987.tb00183.x. [DOI] [PubMed] [Google Scholar]