Abstract
A substantial intracellular localization of matrix metalloproteinase 2 (MMP2) has been reported in cardiomyocytes, where it plays a role in the degradation of the contractile apparatus following ischemia-reperfusion injury. Whether MMP2 may have a similar function in skeletal muscle is unknown. This study determined that the absolute amount of MMP2 is similar in rat skeletal and cardiac muscle and human muscle (~10–18 nmol/kg muscle wet wt) but is ~50- to 100-fold less than the amount of calpain-1. We compared mechanically skinned muscle fibers, where the extracellular matrix (ECM) is completely removed, with intact fiber segments and found that ~30% of total MMP2 was associated with the ECM, whereas ~70% was inside the muscle fibers. Concordant with whole muscle fractionation, further separation of skinned fiber segments into cytosolic, membranous, and cytoskeletal and nuclear compartments indicated that ~57% of the intracellular MMP2 was freely diffusible, ~6% was associated with the membrane, and ~37% was bound within the fiber. Under native zymography conditions, only 10% of MMP2 became active upon prolonged (17 h) exposure to 20 μM Ca2+, a concentration that would fully activate calpain-1 in seconds to minutes; full activation of MMP2 would require ~1 mM Ca2+. Given the prevalence of intracellular MMP2 in skeletal muscle, it is necessary to investigate its function using physiological conditions, including isolation of any potential functional relevance of MMP2 from that of the abundant protease calpain-1.
Keywords: calpain-1, gelatinolytic activity, MMP2, single fibers, subcellular distribution, zymography
INTRODUCTION
Matrix metalloproteinases (MMPs), a family of zinc-containing enzymes widely found in plants and animals, are implicated in degradation and remodeling of components of the extracellular matrix (ECM) (4, 6, 34). On the basis of their domain organization and substrate preference, MMPs are categorized into six groups: collagenases, gelatinases, stromelysins, matrilysins, and membrane-type and other MMPs (39). Each MMP is synthesized as an inactive zymogen called a proenzyme, or pro-MMP. The activity of the MMPs is precisely regulated through activation of the proenzymes and by inhibition by endogenous inhibitors known as tissue inhibitors of metalloproteinases (39). Most studies of the function of MMPs have focused on the ECM, in which MMPs have been viewed solely as secreted or membrane-associated proteases acting extracellularly (11). However, since the identification of subcellular localization signals in specific MMPs, accumulating evidence has pointed to possible intracellular actions and substrates of MMPs (2, 11, 17, 18, 27, 51).
MMP2, or gelatinase A, is produced by skeletal muscle fibers and has been shown to play critical roles in differentiation, regeneration, and repair of skeletal muscle (12, 38, 61). An intracellular role of MMP2 has been investigated in cardiac muscle subjected to ischemia-reperfusion. A substantial proportion of the total MMP2 in cardiomyocytes is found in the cytosol (1, 3), and MMP2 was seen to associate with the contractile apparatus following ischemia-reperfusion (57). It has been proposed that intracellular MMP2 in cardiomyocytes is responsible for the proteolysis of troponin I (57), myosin light chain-1 (MLC-1) (49), and titin (2) occurring with ischemia-reperfusion injury. It has also been shown that peroxynitrite treatment of cardiac cells activated MMP2 and resulted in degradation of α-actinin and desmin (55). Although troponin I was suggested as an intracellular substrate of MMP2 in cardiomyocytes with ischemia-reperfusion injury (57), it is more generally thought to be degraded by calpains (28), which are Ca2+-dependent cysteine proteases involved in the proteolysis of sarcomeric proteins in both cardiac and skeletal muscle (43). The Ca2+ sensitivity of MMP2 activation has not been reported, and little is known about the cellular localization and possible intracellular actions of MMP2 in skeletal muscle. One study on fixed skeletal muscle fibers reported colocalization of intracellular MMP2 staining and Ca2+-stimulated gelatinolytic activity, with accompanying immunogold electron microscopy indicating that the bound MMP2 was concentrated at the Z lines of the sarcomeres, in the nuclear membrane, and in mitochondria (22). However, it is unclear how much of the MMP2 is located intracellularly in unfixed muscle cells, whether this MMP2 is predominantly diffusible or bound, and, most importantly, whether MMP2 is proteolytically active at normal intracellular Ca2+ concentrations ([Ca2+]).
Gelatin in-gel zymography is commonly used to detect the activity of gelatinases such as MMP2 and MMP9 (52). However, standard gelatin zymography does not actually indicate the true in situ activity of MMP2 and MMP9; during SDS-PAGE, MMP2 and MMP9 undergo nonphysiological activation, because SDS allows release of the propeptide from the catalytic domain (11, 44, 53, 56). After renaturation, when the SDS is washed away, pro-MMP2 and pro-MMP9 remain active and degrade gelatin and, thus, display gelatinolytic activity on zymography. Thus, to gauge the actual in situ activity of the MMPs, the substrate zymography must be performed under native conditions and, importantly, with normal physiological intracellular [Ca2+].
Here, we used quantitative Western blotting to detect MMP2 in skeletal muscle, using a carefully validated antibody. By comparing the signals with those of known amounts of recombinant MMP2 pure protein, we were able to quantify the absolute amount of MMP2 in skeletal and cardiac muscle. In addition, by comparing MMP2 levels in intact muscle fibers with MMP2 levels in mechanically skinned fibers, which completely lack ECM, we were able to determine the proportions of MMP2 located intracellularly and extracellularly. Furthermore, the proportion of the MMP2 that was freely diffusible within the freshly skinned fibers could be readily determined simply by bathing the skinned fibers in a physiological solution for a set time and examining the amount of MMP2 that diffuses into the bathing solution. Finally, the gelatinolytic activity of MMP2 in human and rat muscle was detected by gelatin zymography: the standard SDS methodology was compared with zymography under native conditions, and the effects of different [Ca2+] were examined to determine whether the MMP2 degrades the substrate at normal intracellular [Ca2+].
MATERIALS AND METHODS
Materials and antibodies.
All chemicals were purchased from Sigma (Sydney, NSW, Australia) unless otherwise stated. Antibodies probed on Western blotting include anti-MMP2 (no. 4022, Cell Signaling Technology, Danvers, MA; 1:1,000 dilution), anti-MMP2 (Ab92536, Abcam, Cambridge, UK; 1:2,000 dilution), anti-MMP2 (Ab37150, Abcam, Cambridge, UK; 1:1,000 dilution), anti-MMP2 (Ab19015, Chemicon, Temecula, CA; 1:1,000 dilution), anti-calpain-1 (catalog no. C0355, Sigma Aldrich, St. Louis, MO; 1:1,000 dilution), anti-GAPDH (Ab8245, Abcam, Cambridge, UK; 1:1,000 dilution), anti-SERCA2a (sarcoplasmic reticulum Ca2+ pumps) (catalog no. A010-20, Badrilla, Leeds, UK; 1:2,000 dilution), and anti-laminin (catalog no. L9393, Sigma, St. Louis, MO; 1:1,000 dilution). Human recombinant pro-MMP2 (catalog no. PF037; 72 kDa) and human recombinant active MMP2 (catalog no. PF023; 64 kDa) were obtained from Millipore (Darmstadt, Germany), recombinant mouse/rat MMP2 (catalog no. 924-MP-010; 72 kDa) from R&D Systems (Minneapolis, MN), and calpain-1 from human erythrocytes (catalog no. 208713; 80 kDa) from Calbiochem (Darmstadt, Germany).
Animal experiments and ethics.
All animal procedures were approved by the La Trobe University Animal Ethics Committee. Male Sprague-Dawley rats (4–6 mo old, n = 10) were euthanized by overdose of isoflurane (4% vol/vol), and the heart, extensor digitorum longus (EDL) and soleus muscles, brain, kidney, thymus, diaphragm, spleen, and liver were excised, snap-frozen in liquid nitrogen, and stored at −80°C for sample preparation, and in the case of skeletal muscle samples, a portion was placed in paraffin oil.
Human experiments and ethics.
Biopsy samples from the vastus lateralis muscle were obtained from three young adult male volunteers. All human protocols and procedures were approved by the Human Research Ethics Committees at Victoria University and La Trobe University. Informed consent was obtained in writing from all subjects, and the studies conformed to the standards set by the Declaration of Helsinki. The subjects were healthy, and most participated in regular physical activity but were not specifically trained in any sport. After injection of a local anesthetic [1% lidocaine (lignocaine)] into the skin and fascia, a small incision was made in the middle third of the vastus lateralis muscle of each subject, and a muscle sample was taken using a Bergstrom biopsy needle. An experienced medical practitioner took all biopsies at approximately constant depth. The excised muscle sample was rapidly blotted on filter paper to remove excess blood and placed in paraffin oil.
Sample preparation.
Portions of human muscle and rat tissues [1:40 (wt/vol)] in an ice-cold solution containing 50 mM Tris·HCl, 150 mM NaCl, and 10 mM CaCl2 (pH 7.5) were homogenized three times at maximum speed for ~8 s using a Polytron (model PT 1200 E, Kinematica, Lucerne, Switzerland); tissues were placed on ice between each burst. Half of the homogenate was transferred to another centrifuge tube, to which 3× SDS loading buffer [1:2 (vol/vol)] containing 125 mM Tris·HCl (pH 6.8), 4% SDS, 10% glycerol, 400 mM urea, 10% β-mercaptoethanol, and 0.001% bromophenol blue was added for subsequent Western blotting. The remaining homogenate was mixed with 4× nonreducing loading buffer [1:3 (vol/vol)] containing 400 mM Tris·HCl (pH 6.8), 4% SDS, 20% glycerol, and 0.005% bromophenol blue for detection of gelatinolytic activity on zymography.
p-Aminophenylmercuric acetate activation.
Muscle samples were homogenized as described above (see Sample preparation) and treated with 1 mM p-aminophenylmercuric acetate (APMA) for 1 h at 37°C (54). Half of the homogenate was transferred to another centrifuge tube to which 3× SDS loading buffer was added for detection of MMP2 on Western blotting. Nonreducing loading buffer (4×) was added to the remaining homogenate [1:3 (vol/vol)], as described above, for detection of gelatinolytic activity on zymography.
Dissection and skinning of single muscle fibers.
To investigate the amount of MMP2 localized on the inside of skeletal muscle fibers, segments of skinned and intact (i.e., nonskinned) fibers were isolated from human vastus lateralis and rat soleus muscles, as described previously (31, 36). Briefly, the muscles or muscle biopsies were pinned onto a Sylgard-coated dish and immersed in paraffin oil for dissection of single fibers under a microscope. Jeweler’s forceps were used to skin a ~2-mm-long fiber segment: some myofibrils on one side were pulled away, causing the sarcolemma to roll back along the length of the fiber segment. Groups of 15 intact or skinned fiber segments were collected for Western blotting in 30 µl of a K+-based physiological buffer [in mM: 126 K+, 36 Na+, 8.5 total Mg, 90 HEPES, 8 ATP, and 10 creatine phosphate, with free Ca2+ weakly buffered with 50 mM N-hydroxyethylenediamine triacetic acid (HDTA; Fluka, Buchs, Switzerland)] to which 3× SDS loading buffer (15 µl) was added.
Subcellular distribution of MMP2 in skinned muscle fibers.
To examine the distribution and diffusibility of MMP2 inside muscle fibers, groups of freshly skinned fibers (10–15 fiber segments tied together with silk suture, total fiber volume ~0.15 µl) were placed in 30 µl of K+-based physiological buffer (see Dissection and skinning of single muscle fibers) for 10 min. The tied fiber segments were then transferred to another centrifuge tube containing the same amount of the buffer and 1% (vol/vol) Triton X-100 and washed again for 10 min. The treated fiber segments were collected in another tube in the same amount of buffer (see Ref. 59 for illustration of overall procedure). The skinned fibers were vortexed periodically during each exposure period. Finally, 3× SDS (15 μl) was added to each of the three tubes (i.e., initial wash solution, wash solution with Triton X-100, and skinned fibers in the final solution), and these samples were run side-by-side in subsequent Western blotting. In a subset of cases, the procedure was carried out using a buffer solution with 50 mM EGTA2−, instead of 50 mM HDTA2−, to strongly buffer the free Ca2+ at a very low level (~1 nM); this appeared to aid in dispersal of the membrane-associated SERCA proteins during treatment with 1% Triton X-100 (see results).
Crude fractionation of muscle homogenate.
To further confirm the distribution of MMP2 within skeletal muscle, whole muscle homogenates were fractionated to separate the subcellular compartments. Rat and human muscles were homogenized as described above (see Sample preparation). A 100-µl portion of unfractionated rat muscle homogenate to which 50 μl of 3× SDS loading buffer was added was used as a control in the Western blotting. A similar amount (100 μl) of the unfractionated homogenate was centrifuged at 14,000 g for 10 min at 4°C. The supernatant (~100 µl), containing cytosolic proteins, was transferred to a centrifuge tube, and 50 μl of 3× SDS loading buffer was added. The pellet was resuspended in 100 µl of the buffer solution used for homogenization, and 3× SDS loading buffer (50 μl) was added. All samples were incubated at room temperature for 1 h and subsequently stored at −80°C for analysis. When equal volumes of supernatant, pellet, and whole muscle preparations are run on a gel and examined by Western blotting, the sum of the densities of a given band in the supernatant and pellet fractions should approximately equal the density of that band in the whole muscle sample (see Fig. 4C) (59).
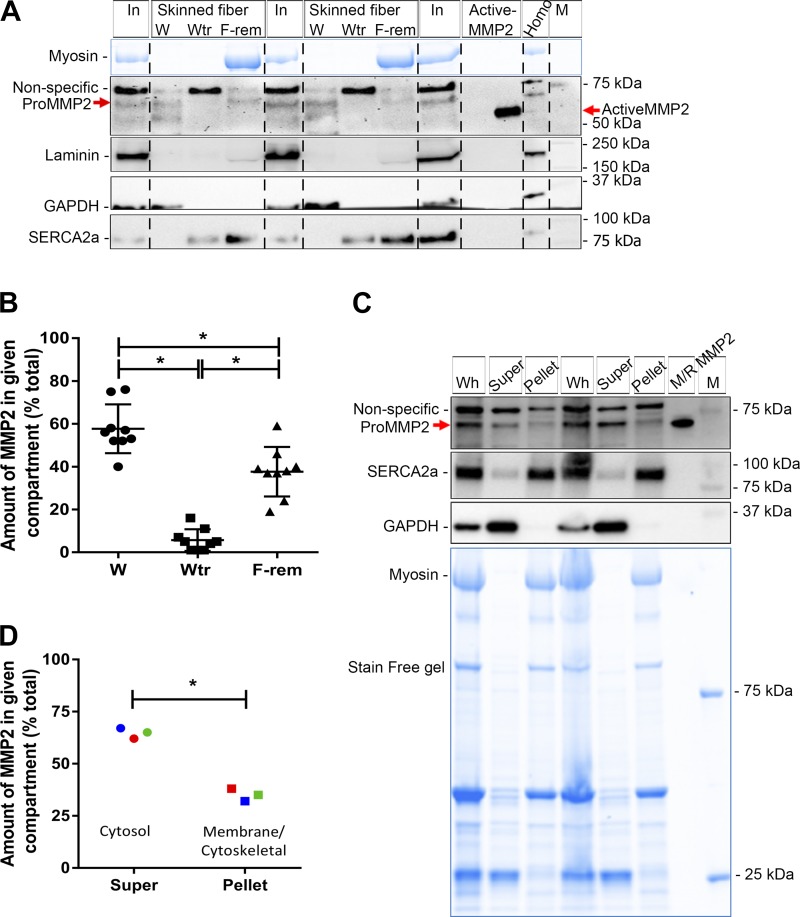
Fig. 4.
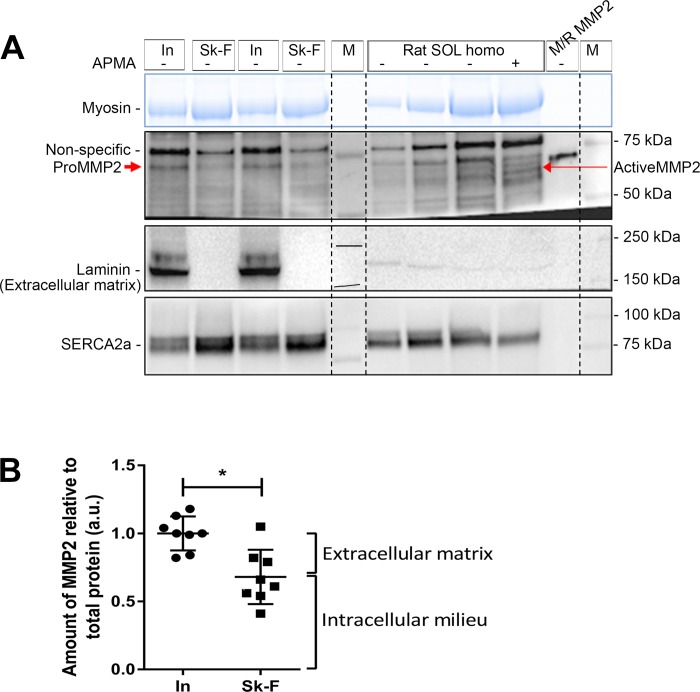
Subcellular distribution of matrix metalloproteinase 2 (MMP2) in rat soleus (SOL) muscle fibers. A: Western blot of MMP2 in intact (In) and skinned soleus fibers (segments of 10–15 fibers in each). Skinned fiber segments were further divided into 3 fractions: 1) fraction diffusing into the bathing solution with a 10-min wash (W), 2) fraction removed by a further 10-min wash with addition of Triton X-100 (Wtr), and 3) fraction remaining in fiber segments (F-rem). Active MMP2 pure protein and soleus homogenate (Homo) were run on the same gel. Western blots for laminin (a marker of extracellular matrix), GAPDH (cytosol), and SERCA2a [sarcoplasmic reticulum (SR) membrane] are also shown. Stain-Free (blue) gel for myosin was used as a loading control; absence of myosin in W and Wtr fractions confirms that there are no contractile proteins in these fractions. Vertical dashed lines were added for ease of distinguishing sets of skinned fiber fractions (W, Wtr, and F-rem) from intact fibers. Nonspecific bands and molecular mass markers (M) are indicated. B: percentage of MMP2 in skinned fibers in W, Wtr, and F-rem fractions. Data are individual values in 9 sets of fibers and means ± SD. *P < 0.05 (by 1-way ANOVA and Tukey’s post hoc multiple-comparisons test). C: Western blot of soleus muscle, showing both the whole homogenate (Wh) and the portion separated into supernatant (Super) and pellet, and mouse/rat (M/R) pro-MMP2, probed for MMP2, SERCA2a (membrane), and GAPDH (cytosol). Stain-Free (blue) gel indicates the presence of myosin only in whole muscle and pellet fractions. D: proportion of MMP2 in soleus muscle homogenate in cytosol [supernatant (super)] and membrane-cytoskeletal compartment (pellet). *P < 0.05 (by paired Student’s t-test). Different-color symbols indicate different rats (n = 3).
The procedure for crude fractionation of the human muscle homogenates into three subfractions was similar to that of Wette et al. (59). Briefly, a 100-μl unfractionated sample of the human muscle homogenate, to which 3× SDS loading buffer (50 µl) was added, was used as the whole muscle sample. A similar amount (100 μl) of the same human muscle homogenate was centrifuged at 14,000 g for 10 min at 4°C. The supernatant (~100 μl), containing cytosolic proteins, with addition of 50 μl of 3× SDS loading buffer, was denoted the “cytosolic fraction.” The pellet was resuspended in 100 μl of the solution used for homogenization, to which 1% Triton X-100 was added, incubated for 10 min on ice, and then centrifuged at 14,000 g for 10 min at 4°C. The supernatant (~100 μl), containing membrane-bound proteins, was removed, and 3× SDS loading buffer (50 μl) was added; this was denoted the “membranous fraction.” Lastly, the pellet, including nuclei and cytoskeletal proteins, was resuspended in 100 μl of the solution used for homogenization, and 3× SDS loading buffer (50 μl) was added; this fraction was denoted the “cytoskeletal and nuclear fraction” (see Fig. 5C).
Fig. 5.
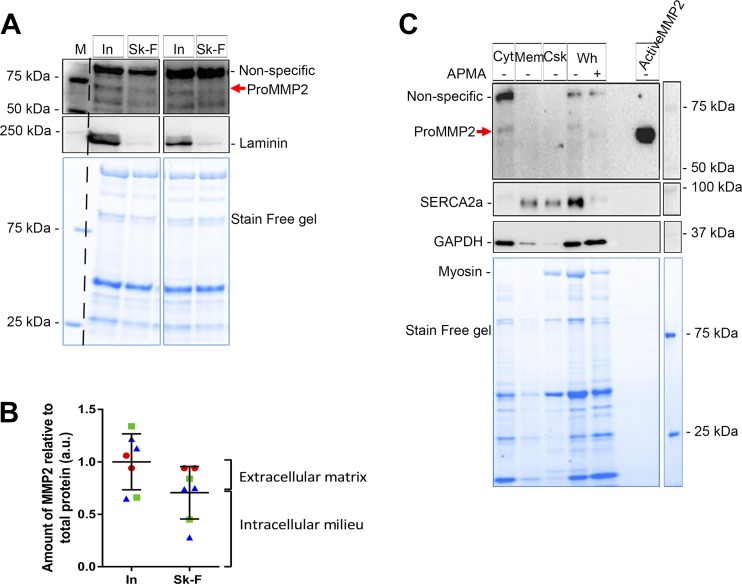
Subcellular distribution of matrix metalloproteinase 2 (MMP2) in human muscle fibers. A: human intact (In) and skinned (Sk-F) fibers (segments of 15 fibers in each collected from 1 subject) probed for MMP2 (using Ab92536) and laminin. B: relative amount [arbitrary units (au)] of MMP2 in intact and skinned fibers (7 sets of fibers from 3 subjects, each represented by different-color symbol). Data are individual values and means ± SD. Although the 2 sets of values were not significantly different (P > 0.05, by unpaired Student’s t-test), mean levels are a guide to proportions of total MMP2 associated with the extracellular matrix and intracellular milieu, as in Fig. 3B. C: representative Western blot of human vastus lateralis muscle whole homogenate [Wh, with and without p-aminophenylmercuric acetate (APMA) treatment] and its fractionation into cytosolic (Cyt), membrane (Mem), and cytoskeletal (Csk) compartments, probed for MMP2, SERCA2a, and GAPDH. Most of the MMP2 was cytosolic in the muscle of both human subjects (each muscle sample was examined twice). Stain-Free (blue) gel shows total protein.
Western blotting.
The abundance of MMP2 in muscle samples was detected by quantitative Western blotting, which was performed as described previously (35, 59). Briefly, prepared samples were separated on 4–15% Criterion Stain-Free gels (Bio-Rad, Hercules, CA) at 200 V for 45 min. Total proteins on Stain-Free gels were imaged (Bio-Rad Stain-Free Imager) and transferred to a nitrocellulose membrane at 100 V for 30 min. After the membranes were blocked for ~2 h at room temperature, they were probed for MMP2, GAPDH, SERCA2a, and laminin (see Materials and antibodies) overnight at 4°C. Membranes were exposed to goat anti-mouse (catalog no. PIE31430, Thermo Fisher Scientific; 1:20,000 dilution) or anti-rabbit (catalog no. PIE31460, Thermo Fisher Scientific; 1:60,000 dilution) horseradish peroxidase for 1 h at room temperature. After exposure to chemiluminescence substrate (Thermo Scientific, SuperSignal West Femto), membranes were imaged using a charge-coupled device camera attached to a ChemiDoc MP imaging system (Bio-Rad), and densitometry was performed using Image Lab software (version 6.0, Bio-Rad). For analysis of values from Western blotting, the density of a given band was adjusted to a calibration curve (generated by loading a range of amounts of an unfractionated homogenate mixture of muscles from independent individuals; not shown) and then normalized to the amount of total protein in that lane obtained from the Stain-Free gel (35).
Gelatin zymography.
Gelatin zymography was used to investigate the gelatinolytic activity of MMP2 in muscle and other tissues. Samples prepared in nonreducing loading buffer (see Sample preparation) were run on 8% SDS-polyacrylamide gels copolymerized with 1.0 mg/mL gelatin (Bio-Rad, Gladesville, NSW, Australia) (56). After electrophoresis, gels were washed twice for 40 min each in a buffer containing 2.5% Triton X-100, 50 mM Tris·HCl, and 5 mM CaCl2 (pH 7.6) to remove SDS. The washed gels were transferred to a solution containing 50 mM Tris·HCl and 5 mM CaCl2 (pH 7.6), washed twice for 15 min each, and incubated overnight (~17 h) at 37°C in a buffer containing 50 mM Tris·HCl, 150 mM NaCl, 0–10 mM CaCl2, 1 μM ZnCl2, and 0.002% NaN3 (pH 7.5). The gels were stained in a solution including 0.05% Coomassie R 250, 30% methanol, and 10% acetic acid for 3 h and destained with 30% methanol and 10% acetic acid in water for 3 h, and gelatinase activity was visualized against a dark-blue background. Zymography gels were imaged using a charge-coupled device camera attached to a ChemiDoc MP imaging system (Bio-Rad). The white areas against a dark background (see Fig. 6) were generated by degradation of gelatin by MMP2. Densitometry was performed using Image Lab software (version 6.0, Bio-Rad) after the signal was inverted from white to black.
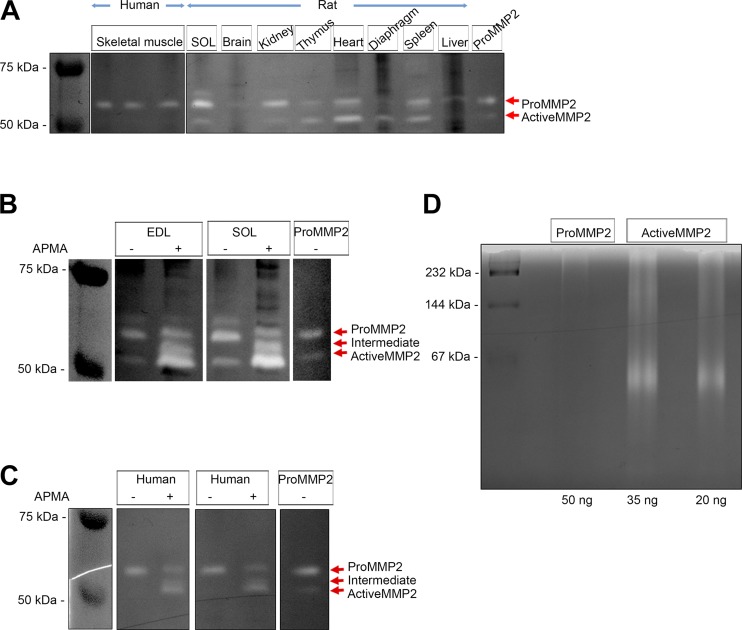
Fig. 6.
Gelatinolytic activity of matrix metalloproteinase 2 (MMP2) detected by the standard procedure and under native conditions. A: standard SDS-based zymogram indicating gelatinolytic activity of pro-MMP2 in human vastus lateralis muscle (n = 3 subjects) and rat soleus (SOL) muscle, brain, kidney, thymus, heart, diaphragm, spleen, and liver (see materials and methods). Pro-MMP2 (20 pg) was used as positive control. B and C: similar standard zymogram detection of gelatinolytic activity of MMP2 in rat extensor digitorum longus (EDL) and soleus muscle (B) and human vastus lateralis muscle (C), with or without p-aminophenylmercuric acetate (APMA) treatment. D: zymography of pro-MMP2 (50 ng) and active MMP2 (35 and 20 ng) pure proteins under native conditions (see materials and methods). White bands (A–C) or regions (D) indicate gelatinolytic activity of MMP2. Gelatinolytic activity under denatured (A–C) and native (D) conditions was examined in the presence of 50 mM Tris·HCl, 150 mM NaCl, 10 mM CaCl2, 1 µM ZnSO4, and 0.002% NaN3 (pH 7.5).
Zymography under native conditions.
Gelatinolytic activities of human recombinant pro- and active MMP2 under native conditions were determined on blue native gel copolymerized with gelatin (1 mg/mL). The preparation of blue native gel and electrophoresis were based on the method of Schagger and von Jagow (50). Pro- and active MMP2 pure proteins were prepared in a loading dye containing 5% Coomassie Blue G, 500 mM ε-amino n-caproic acid, and 100 mM Bis-Tris (pH 7.0) and loaded onto 8% blue native gels copolymerized with gelatin and run for 45 min at 150 V and 25 min at 250 V. After electrophoresis, the gels were incubated overnight (~17 h) at 37°C in a buffer containing 50 mM Tris·HCl, 150 mM NaCl, 0–10 mM CaCl2, 1 μM ZnCl2, and 0.002% NaN3 (pH 7.5) and destained in double-distilled water. Then the gels were imaged and analyzed (see Gelatin zymography).
Statistics.
Unpaired Student’s t-tests were used to compare the relative amounts of MMP2 in EDL and soleus muscles and in intact and skinned muscle fibers. A paired Student’s t-test was used to analyze the relative amounts of MMP2 in the supernatant and pellet following whole muscle fractionation and a 1-way ANOVA with Tukey’s post hoc multiple-comparisons test to compare between skinned fiber distributions. Data are presented as means ± SD, unless otherwise stated. Significance was set at P < 0.05. All statistics were performed using GraphPad Prism 6.
RESULTS
Antibody validation.
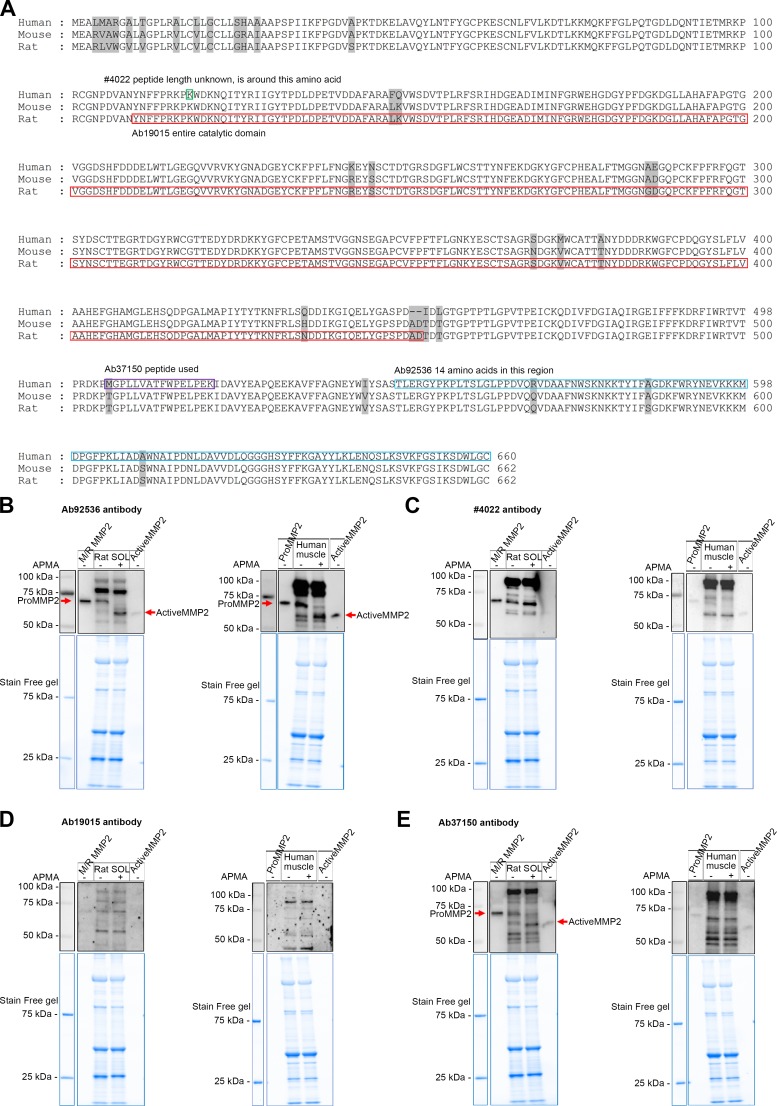
Basic local alignment search tool (BLAST) similarity analysis of the protein sequences of human, mouse, and rat MMP2 indicates that rat and mouse MMP2 are 99% homologous and both share 97% homologous sequence with human MMP2 (Fig. 1A). The regions of the protein sequence used for production of various anti-MMP2 antibodies are also shown (Fig. 1A).
Fig. 1.
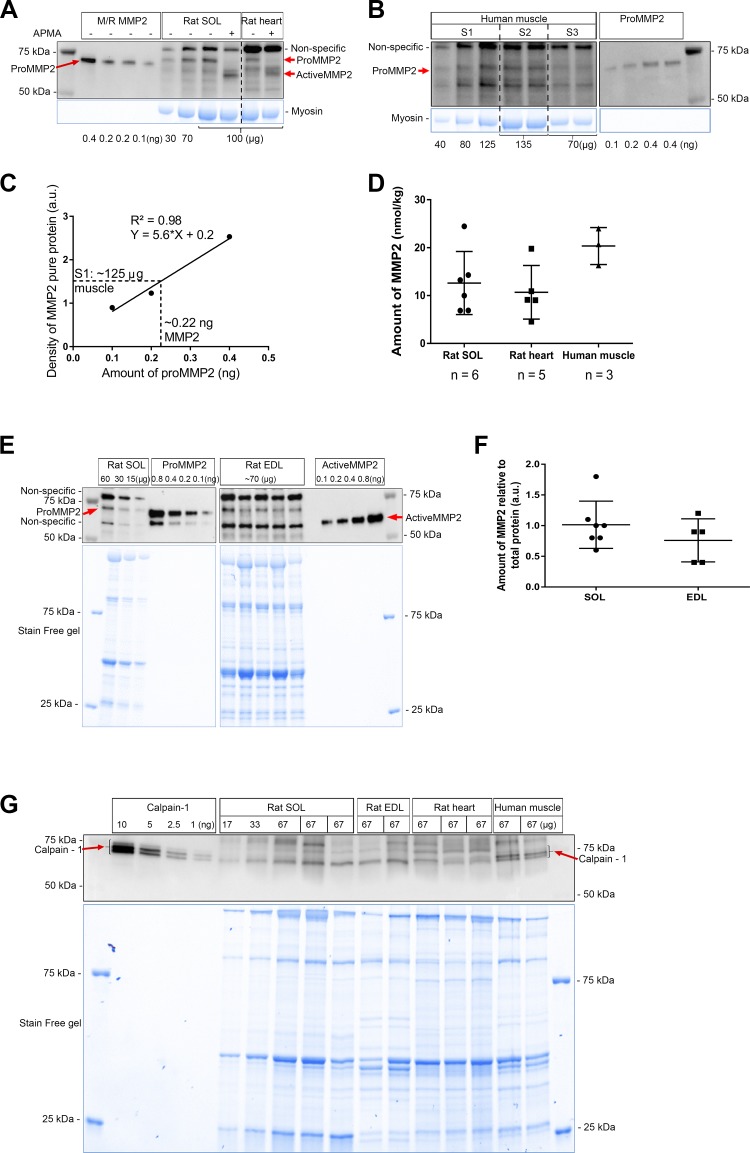
Testing the appropriateness of matrix metalloproteinase 2 (MMP2) antibodies in Western blotting. A: sequence alignment for immunogens of the MMP2 antibodies using basic local alignment search tool (BLAST) similarity analysis of MMP2 in human, mouse, and rat species. The antigen of the antibody no. 4022 is around amino acid 116 (green rectangle), the catalytic domain of rat MMP2 is in the red rectangle, and the antibody Ab19015 uses an antigen of this full region; the antibody Ab37150 peptide is in the purple rectangle and the antibody Ab92536 peptide is in the blue rectangle. The number of amino acids is shown at right. Nonconserved amino acids of MMP2 from 3 species are highlighted in gray. B–E: p-aminophenylmercuric acetate (APMA)-treated (37°C for 1 h) or untreated rat soleus (SOL) and human vastus lateralis muscle homogenates (100 μg muscle wet wt), along with mouse/rat pure pro-MMP2 protein (M/R MMP2) and human pro- and active MMP2 pure proteins (0.2 ng) probed for MMP2 with Ab92536 (B), no. 4022 (C), Ab19015 (D), and Ab37150 (E); molecular mass markers are shown at left. Stain-Free (blue) gels indicate total protein loaded. B: specific bands of pro- and active MMP2 (red arrows) were detected with Ab92536 in APMA-treated and untreated rat soleus and human vastus lateralis muscle homogenates. C and D: no. 4022 and Ab19015 failed to detect pro- and active MMP2 in rat soleus and human vastus lateralis muscle homogenates. E: Ab37150 detected pro- and active MMP2 (red arrows) in APMA-treated and untreated rat soleus muscle homogenate; many nonspecific bands were detected, suggesting that Ab37150 may not be suitable for use in immunofluorescence studies; Ab37150 failed to detect pro- and active MMP2 in human vastus lateralis muscle. Aliquots of the same muscle samples were used in B–E. In addition to specfic bands (B and E), all antibodies detected nonspecific bands.
Four different antibodies for MMP2 were tested by Western blotting (Fig. 1). APMA treatment, which activates pro-MMP2 (72 kDa), leading to its autocatalysis and generation of active MMP2 (64 kDa) (11, 29, 44), was used to help identify true MMP2 bands. Ab92536 detected mouse/rat MMP2 pure protein, migrating at ~72 kDa, as well as human recombinant pro- and active MMP2 pure proteins, migrating at ~72 and ~64 kDa, respectively (Fig. 1B). Importantly, it also detected pro- and active MMP2 in rat soleus muscle; APMA treatment resulted in >90% conversion of pro-MMP2 to its active form, allowing the MMP2 bands to be clearly distinguished from other nonspecific bands (Fig. 1B). APMA treatment also caused similar conversion of pro-MMP2 to active MMP2 in human skeletal muscle (Fig. 1B).
In contrast, two other MMP2 antibodies, no. 4022 and Ab19015, were not useful (Fig. 1, C and D). Although each antibody was able to detect isolated MMP2 pure proteins, they did not detect MMP2 in skeletal muscle preparations; the antibodies labeled many bands, but none of these were evidently MMP2, because they were completely unaltered by the APMA treatment, which was shown by Ab92536 to cause autocatalysis of pro-MMP2 to the active form in exactly the same treated muscle homogenate preparation (Fig. 1B). The findings with all the antibodies were confirmed in at least three independent preparations. A fourth antibody, Ab37150, was also examined, because it had been used in an earlier immunofluorescence study of mouse and human skeletal muscle (22). Although this examination was under denaturing conditions and this antibody detected pure pro- and active MMP2 proteins, many bands were detected in rat and human muscle (Fig. 1E), casting doubt on its use for immunofluorescence examination.
Quantification of MMP2 in muscle.
The absolute amounts of MMP2 in samples of rat skeletal and cardiac muscle and human skeletal muscle were quantified by comparison with known amounts of the respective MMP2 pure protein run on the same gels (Fig. 2, A and B). For each gel, a “standard curve” for the relevant pure MMP2 protein was generated by plotting the Western blot band density of each protein aliquot vs. the amount loaded (Fig. 2C). The amount of MMP2 in each of the muscle samples run on that same gel could then be determined by comparison with the relevant standard curve, as illustrated in Fig. 2C; in this example, the 125-µg sample of skeletal muscle from subject S1 on that gel contained ~0.22 ng of MMP2. This procedure was repeated for each muscle sample on at least two independent gels. The amount of MMP2 in rat soleus muscle was determined to be 12.6 ± 6.6 nmol/kg wet wt (n = 6 rats; Fig. 2D). Moreover, a similar absolute amount of MMP2 in rat soleus muscle was obtained when Ab92536 and Ab37150 were used (Fig. 1, B and E). A broadly similar absolute amount of MMP2 was also found in rat heart [total heart (10.7 ± 5.6 nmol/kg wet wt, n = 5 rats)] and human vastus lateralis muscle (20.3 ± 3.8 nmol/kg wet wt, n = 3 subjects, repeated over 2 gels in duplicate or triplicate) (Fig. 2D). Comparison between rat soleus and EDL muscles showed no significant difference (P > 0.05, by unpaired Student’s t-test; Fig. 2, E and F).
Fig. 2.
Quantification of matrix metalloproteinase 2 (MMP2) protein in rat skeletal [soleus (SOL) and extensor digitorum longus (EDL)] and cardiac muscle and human skeletal muscle. A: detection of mouse/rat (M/R) MMP2 pure protein (0.4, 0.2, and 0.1 ng) and rat soleus (30, 70, and 100 µg) and heart (100 µg) homogenates, with or without p-aminophenylmercuric acetate (APMA) treatment, with the antibody Ab92536. B: human vastus lateralis muscle homogenates loaded in duplicate or triplicate (40, 80, and 125 µg) and human pro-MMP2 pure protein (0.1, 0.2, and 0.4 ng). Vertical dashed lines demarcate lanes of subjects S1–S3. In A and B, Stain-Free (blue) gels for myosin, nonspecific bands, and molecular mass markers are shown. C: standard curve of MMP2 pure protein in B, derived by plotting the amount of pure pro-MMP2 (0.1, 0.2, 0.4 ng; x-axis) vs. respective relative band density of Western blot (y-axis). [Where the protein amount was examined in duplicate (0.4 ng), the average of Western band densities was plotted.] Dashed line illustrates how the band density of the 125-µg human muscle sample from subject S1 on the same gel shows it contained ~0.22 ng of MMP2, corresponding to ~24 nmol/kg muscle wet wt. au, Arbitrary units. D: individual values and means ± SD of absolute amount of MMP2 in rat soleus (n = 6), rat heart (n = 5), and human vastus lateralis (n = 3) muscle; n indicates number of independent animals/subjects, with human samples each analyzed on ≥2 separate Western blots. E: representative Western blot of MMP2 in homogenates of slow-twitch muscle (soleus; 60, 30, and 15 µg) and fast-twitch muscle (EDL; ~70 µg) from 5 different rats, along with pro- and active MMP2 pure proteins (0.1, 0.2, 0.4, and 0.8 ng), probed using Ab92536. Nonspecific bands, molecular mass markers, and Stain-Free (blue) gel are shown. F: relative amount of MMP2 in soleus (n = 7 rats) and EDL (n = 5 rats) muscle. Values are pooled data and means ± SD. Values are not significantly different (P > 0.05, by unpaired Student’s t-test). G: calpain-1 pure protein (10, 5, 2.5, and 1 ng) and rat soleus (n = 3), EDL (n = 2), and heart (n = 3) muscle and human muscle homogenates (n = 2 subjects) detected for calpain-1.
The amount of calpain-1, an important intracellular Ca2+-dependent protease, was similarly measured in rat and human skeletal and rat cardiac muscle by comparison with human calpain-1 pure protein, with each tissue sample assessed at least three times. The absolute amount of calpain-1 was 1.0 ± 0.2 µmol/kg (n = 7 rats) in rat skeletal muscle, 0.6 ± 0.1 µmol/kg in rat cardiac muscle (n = 4 rats), and 1.3 ± 0.2 μmol/kg (n = 2 subjects) in human vastus lateralis muscle (Fig. 2G).
Localization of MMP2 in rat muscle fibers.
The crude localization of MMP2 in skeletal muscle fibers was investigated by removal of the ECM and sarcolemma by microdissection under paraffin oil, to separate the ECM from the intracellular contents. Segments of skinned and intact fibers of rat soleus muscle were run side-by-side on gels and examined by Western blotting (Fig. 3A). Laminin, a protein marker for the ECM, was completely removed by the fiber-skinning process in all cases (Fig. 3A). The amount of MMP2 in the skinned fiber segments was ~70% of that in the intact fibers, indicating that ~30% of the total MMP2 was associated with the ECM and the remaining ~70% was associated with the intracellular milieu (Fig. 3B). To ascertain if active MMP2 was present in the intact or skinned fibers, APMA-treated and untreated soleus muscle samples were run on the same gels to positively identify activated MMP2. No active MMP2 was seen in intact or skinned fiber segments (Fig. 3A).
Fig. 3.
Amounts of matrix metalloproteinase 2 (MMP2) in intact and skinned rat muscle fibers. A: Western blot of MMP2, probed with Ab92536, and antibodies against the extracellular matrix protein laminin, and SERCA2a in segments of intact (In) and skinned (Sk-F) soleus (SOL) fibers (15 fiber segments in each). SERCA2a, along with myosin on the Stain-Free (blue) gel, reflects loading amounts of total protein. Soleus homogenate (homo) with or without p-aminophenylmercuric acetate (APMA) treatment and M/R pro-MMP2 pure protein were loaded on the same gel. Pro- and active MMP2, nonspecific bands, and molecular weight markers (M) are indicated. B: relative amount [arbitrary units (au)] of MMP2 in intact and skinned fibers. Values are pooled data and means ± SD; n = 8 sets of fiber segments in each. *P < 0.05 (by unpaired Student’s t-test). Labels on right denote MMP2 that can be apportioned to extracellular matrix and intracellular milieu, using the mean values.
Subcellular distribution of MMP2 within rat skeletal muscle fibers.
Additional skinned fiber segments from rat soleus muscle were washed in a K+-based physiological intracellular solution for 10 min to allow any freely diffusible contents in the cytosol to diffuse into the bathing solution. The fibers were then removed from the bathing solution and washed again for 10 min in a similar solution to which 1% Triton X-100 was added to dissociate sarcoplasmic reticulum (SR) and similar internal membranes before the fibers were removed for separate analysis. Subsequent Western blot analysis of these fractions (Fig. 4A) demonstrated that, on average, ~57% of the MMP2, along with most of the GAPDH, in the skinned fibers was freely diffusible in the cytosol, ~6% of the MMP2 dissociated with the subsequent 10-min Triton X-100 treatment, and ~37% of the MMP2 remained in the skinned fiber (Fig. 4, A and B). Treatment with Triton X-100 caused washout of only some of the SERCA2a proteins in the SR membrane. This outcome differed from our previous study of Triton X-100-treated skinned fibers (36), where ~90% of the SERCA washed out, most likely because the free Ca2+ was not strongly buffered at a low level in the present experiments, allowing the fibers to contract when the Triton X-100 lysed the SR. Additional experiments with Ca2+ buffered with 50 mM EGTA resulted in near-complete washout of the SERCA2a with Triton X-100 treatment (not shown), but the proportion of MMP2 washed out was still similar to that in Fig. 4B. In line with the subcellular distribution of MMP2 in the skinned muscle fibers, crude fractionation of rat soleus muscle homogenate into supernatant and pellet also indicated that ~65% of the MMP2 was present in the cytosol (supernatant), where all the GAPDH was found, and ~35% of the MMP2 was found in the pellet (Fig. 4, C and D).
Subcellular distribution of MMP2 in human muscle fibers.
MMP2 localization was also examined using segments of intact and skinned fibers from human vastus lateralis muscle. As with the rat fibers, the fiber-skinning procedure removed all the ECM, as indicated by the absence of virtually all laminin in the skinned fibers (Fig. 5A), and ~70% of the total MMP2 was found inside the muscle fibers (Fig. 5B). Fractionation of whole homogenates of human muscle into cytosolic, membrane, and cytoskeletal fractions also indicated that the majority of the MMP2 in human fibers is diffusible in the cytosol, with little associated with intracellular membranes or present in the cytoskeletal fraction (n = 2 subjects; Fig. 5C).
Gelatinolytic activity of MMP2 detected by gelatin zymography.
The enzymatic activity of MMP2 in human muscle and rat tissues was first examined using standard SDS-based gelatin zymography (Fig. 6A). The single white band against the dark background in the zymogram of human vastus lateralis muscle indicates gelatinolytic activity of pro-MMP2, with very little activity of active MMP2 (Fig. 6A). In rat soleus muscle and other tissues, pro- and active MMP2 showed gelatinase activity, as well as a higher band that has been reported previously to represent a rodent-specific glycosylated MMP2 (20, 23). However, we did not verify this. APMA promotes activation of pro-MMP2 and its autocatalysis to active MMP2, which substantially increased total gelatinase activity in rat EDL and soleus muscle (Fig. 6B) and human vastus lateralis muscle (Fig. 6C), with a very noticeable increase in activity of active MMP2, as well as an intermediate isoform, particularly in the rat muscle. However, it is been reported that the SDS reagent utilized in the standard gelatin zymography process can cause pro-MMP2 to show gelatinase activity (11, 44, 53, 56). This was, indeed, evidently the case here, because recombinant pro-MMP2 pure protein displayed gelatinase activity when examined with the standard zymographic procedure (Fig. 6, A and C). It has previously been suggested that pro-MMP2 may indeed be active (11), but it did not show any such activity when the zymography was performed under native (nondenaturing) conditions without SDS, where only the active MMP2 isoform degraded gelatin (Fig. 6D). Attempts were also made to examine MMP2 activity in muscle homogenates under native conditions, but gelatinase activity was poorly resolved on such gels.
Influence of [Ca2+] on gelatinolytic activity of MMP2.
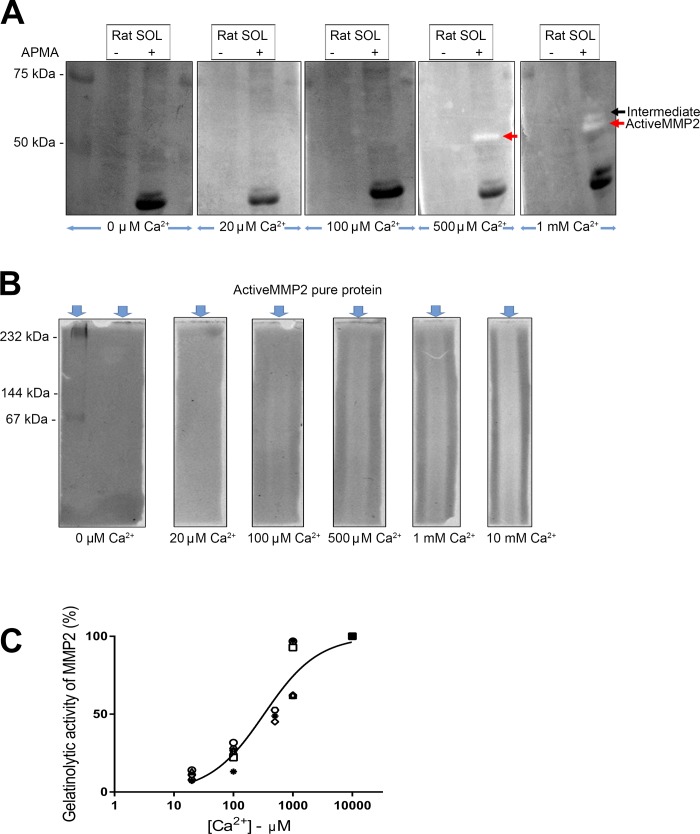
The gelatinase activity described above, for both the standard and native zymography conditions, was detected with incubation in 10 mM Ca2+, which is three to four orders of magnitude higher than the intracellular [Ca2+] during normal muscle activity (~0.1–20 µM). To examine the effect of the incubating [Ca2+] on the level of gelatinase activity, standard zymography was carried out on soleus muscle homogenates with 0–10 mM Ca2+ and all other conditions remaining the same (Fig. 7A). In the control, non-APMA-treated, homogenate, no gelatinase activity was apparent at <1 mM Ca2+. In the APMA-treated homogenate, gelatinase activity was still not apparent at <100 µM Ca2+ and became appreciable only at ≥500 µM Ca2+ (Fig. 7A). The zymograms of the APMA-treated homogenates also showed marked dark bands (e.g., at ~20 kDa), which were most likely proteolytic products of myosin and other muscle proteins produced during the APMA treatment period in 10 mM Ca2+, possibly by activated MMP2 (46) or calpains (21) or other proteases.
Fig. 7.
Influence of Ca2+ on gelatinolytic activity of matrix metalloproteinase 2 (MMP2). A: representative zymography for detection of gelatinolytic activity of MMP2 in rat soleus (SOL) muscle, with or without p-aminophenylmercuric acetate (APMA) treatment, incubated in different free [Ca2+] from 0 µM to 1 mM, and 50 mM Tris·HCl, 150 mM NaCl, 1 µM ZnSO4, and 0.002% NaN3 (pH 7.5). White bands indicate gelatinolytic activity of pro- and active MMP2 in muscle. A similar pattern of MMP2 activity was observed in 4 independent experiments. B: gelatinolytic activity of active MMP2 pure protein (25 ng) under native conditions, with incubation in 0 µM to 10 mM free Ca2+ and other constituents described as in A. White regions indicate gelatinolytic activity of active MMP2. For A and B, zymography gels incubated in different Ca2+ concentrations were imaged together. C: relative gelatinolytic activity of active MMP2 detected by zymography under native conditions with different calcium concentrations ([Ca2+]). Values from 6 independent experiments are shown as different symbols. The y-axis shows activity relative to that observed with 10 mM Ca2+ (all values expressed relative to 10 mM Ca2+), and x-axis shows free [Ca2+] on log10 scale. Hill curve was fitted to data.
To examine whether MMP2 can proteolyze gelatin at normal intracellular [Ca2+] in the absence of any effect of SDS, zymography was also performed under native conditions using recombinant active MMP2 pure protein (Fig. 7B). Although the MMP2 did not run well in the gel, it was evident from the degradation of gelatin (white regions) that the MMP2 was proteolytically active to only a relatively small extent at 20 μM Ca2+ and showed progressively greater activity at higher [Ca2+], reaching ~90% of maximal activity at 1 mM Ca2+ (Fig. 7C).
DISCUSSION
To correctly identify the amount and location of MMP2 in skeletal muscle, it was first necessary to validate the MMP2 antibody that could be most reliably used (Fig. 1). Of the four antibodies examined, Ab92536 was found to accurately identify MMP2 by Western blotting of rat and human skeletal muscle, with pro- and active MMP2 discerned from nonspecific bands by the use of APMA treatment to trigger autolysis of the 72-kDa pro-MMP2 to its 64-kDa active form (11, 29, 44). We provide evidence that the other three MMP2 antibodies (no. 4022, Ab19015, and Ab37150) either did not correctly identify MMP2 in human skeletal muscle or labeled specific, as well as nonspecific, proteins in rat skeletal muscle. In addition, it is evident that all four MMP2 antibodies labeled various non-MMP2 proteins in rat and human skeletal muscle (Fig. 1).
Research into the changes in MMP2 protein levels and gene expression during differentiation and regeneration of skeletal muscle is increasing (33, 40, 61). Furthermore, although MMP2 knockout mice do not show gross abnormalities, their growth rate is significantly (~15%) slower than that of their wild-type littermates, supporting a role for MMP2 in development (25). Nevertheless, the absolute amount of MMP2 has not been ascertained in either skeletal or cardiac muscle, even though this information is clearly important for a full understanding of the significance of MMP2 in cellular function. This study established the presence of only ~13 nmol of MMP2 per kilogram of muscle in the soleus (slow-twitch) muscle of the rat (Fig. 2D) and a similar absolute amount of MMP2 also in both rat cardiac muscle (~11 nmol/kg) and human vastus lateralis muscle (~20 nmol/kg), which consists of ~50% slow-twitch and ~50% fast-twitch fibers. In contrast, the absolute amount of calpain-1 in rat skeletal and cardiac muscle and human vastus lateralis was ~50- to 100-fold higher (~600–1,300 nmol/kg; see results). We used only the MMP2 bands that shifted in molecular weight with APMA treatment, but we cannot exclude the possibility that the antibodies may detect a stable inhibitory complex of MMP2 and that the values obtained may be underestimated. With this in mind, the functional role of MMP2 in the development of skeletal muscle will be ascertained only if a MMP2-specific target is identified that would explain the slower growth rate (25) and if this role of MMP2 could not be performed by other, more abundant, proteases.
Localization of MMP2.
By physical removal of the sarcolemma and all the ECM around a muscle fiber by microdissection (36) (Fig. 3A), it was further shown in both rat and human skeletal muscle that only ~30% of the total MMP2 is associated with the ECM; instead, the majority of the MMP2 is located inside the muscle fibers (Figs. 3 and 5). Moreover, it was found that the majority of the MMP2 inside the muscle fibers is freely diffusible, as shown by its rapid washout when freshly skinned fibers are bathed briefly in a comparatively large volume of a solution mimicking the normal intracellular conditions (Fig. 4, A and B). The latter finding was further verified by fractionation of homogenates of skeletal muscle into diffusible (cytosolic) and nondiffusible components (Fig. 4, C and D, and Fig. 5C), although it cannot be excluded that the ECM or another extracellular site was the source of some of the diffusible MMP2 in the fractionation experiments. The findings are comparable with those seen with fractionation of cardiac muscle, where approximately half of the total MMP2 partitioned into the diffusible fraction (3). The relatively low proportion of the total MMP2 associating with the ECM seems, at first, to be at odds with immunolocalization studies of MMP2 in skeletal muscle; these studies show the most intense labeling around the periphery of fibers (22, 61). However, this is simply because 1) most of the intracellular MMP2 inside the muscle fibers is freely diffusible (see above) and would have been lost during the washing procedures involved in preparing the sections for immunolocalization examination and 2) the signal emanating from the MMP2 inside the fiber is spread out over a comparatively large area compared with the MMP2 in the ECM, which is relatively confined near the periphery of the fiber.
Approximately 40% of the intracellular MMP2 in skeletal muscle fibers (i.e., ~28% of the total MMP2) is nondiffusible and, presumably, bound to structural and/or contractile proteins or associated with the nuclei, because it was not liberated by disruption of the SR and similar intracellular membranes by 10 min of treatment with 1% Triton X-100 (Fig. 4). Such detergent treatment typically causes almost complete dispersal of the SERCA pumps in the SR (36) (see results) but is not sufficient to disrupt nuclear membranes (59). Previous immunolocalization and electron microscopy studies of cardiac (2, 3, 14, 24, 30, 49, 57) and skeletal (22) muscle have shown MMP2 binding at the Z line and also more broadly across the thick and thin contractile filaments, as well as within mitochondria and the nuclei, although, as mentioned above, there must be some concern about the specificity of the MMP2 antibodies used in all immunolocalization and electron microscopy studies. MMP2 has been found to proteolyze titin (2) and desmin (55) in vitro and also to proteolyze troponin I (57), α-actinin (55), and MLC-1 (49) in cardiac muscle cells exposed to ischemia-reperfusion or treated with peroxynitrite, which indicates that MMP2 can interact with each of these proteins, although it does not identify which, if any, of these proteins represents the main MMP2 binding sites in muscle.
Importantly, MMP2 has also been observed to colocalize with caveolin (Cav)-3 at the Z line in cardiac cells and with both Cav-3 and Cav-1 at the sarcolemma (14); other evidence further indicates that MMP2 directly interacts with at least Cav-1 (16). It seems likely that at least some of the MMP2 at the Z lines in cardiac muscle (14) is actually associated with the transverse (T)-tubule system, rather than Z line structural proteins, because the T tubules in cardiac muscle are positioned at the Z line and the Cav-3 inside both cardiac and skeletal muscle cells is associated with the T tubules (8, 36, 41, 45, 60). This is also strongly indicated by the finding, by close examination of the pattern of in situ gelatinase activity in transverse sections of skeletal muscle fibers (see Fig. 3E in Ref. 22), that the activity, which is thought to indicate MMP2 localization, appears as small ring-like shapes encircling the individual myofibrils, exactly as observed previously for Cav-3 (36) and the T tubules in such transverse sections of muscle fibers (26). Thus it appears that MMP2 is located not only around the surface of each muscle fiber, but also deep inside each fiber in association with the network of T tubules, which are, in effect, deep contiguous invaginations of the surface membrane filled with high-[Ca2+] extracellular fluid. This last point may be crucial for the function of this pool of MMP2, as discussed below.
Gelatinolytic activity of MMP2 in rat and human muscle.
Gelatin zymography is a simple and sensitive technique widely used to analyze the gelatinolytic activity of MMP2 in biological samples (52). In the present study, standard gelatinase zymography indicated that the gelatinase activity of MMP2 in quiescent skeletal muscle was attributable primarily or almost exclusively to the full-length proenzyme form of MMP2 in both rat and human muscle (Fig. 6, A–C), in accord with previous findings in mouse (29) and human (47, 48) muscle. However, this does not indicate that MMP2 is proteolytically active in quiescent muscle, because the activity resulted purely from the presence of SDS, causing artifactual activation of the pro-MMP2 (11, 44, 53), as demonstrated by our finding that pro-MMP2 does not display gelatinase activity when assayed under native conditions in the absence of SDS (Fig. 6D) but does display gelatinase activity when examined by standard SDS-based zymography (Fig. 6C). The results of the standard gelatinase zymography, nevertheless, clearly show that most of the MMP2 in quiescent skeletal muscle is present in its full-length, nonactive, proenzyme form (Fig. 6), in good agreement with our Western blot findings using Ab92536 (Figs. 1C and 2), with the ability of APMA to trigger proteolytic cleavage of pro-MMP2 to its smaller active form similarly apparent in both the Western blot and gelatinase zymography data.
Importantly, it was further found that MMP2 displays little or no gelatinase activity at normal intracellular [Ca2+] (Fig. 7). The intracellular [Ca2+] in a resting skeletal muscle fiber is only ~0.1 µM, and the peak level, on average, in the cytoplasm during muscle contractions is only ~2–20 µM (5, 58). All previous studies in skeletal or cardiac muscle by either standard zymography or in situ methodology (3, 13, 15, 19, 22, 29, 47–49, 57) have examined gelatinase activity in the presence of 5–10 mM applied Ca2+, which is three to five orders of magnitude higher than normal intracellular levels. The gelatinase activity of active MMP2 assayed under native conditions was progressively reduced when the activating [Ca2+] was decreased to <1 mM, with the activity being only ~10% of the peak level in the presence of 20 µM Ca2+ (Fig. 7C); this is comparable to findings in cultured neurons, where MMP2 showed very little gelatinase activity at <100 µM Ca2+ (42). This Ca2+ dependence immediately raises the following question: is the MMP2 inside skeletal muscle cells actually proteolytically active to any appreciable extent under normal circumstances? Certainly, the majority of MMP2 in muscle is found in its full-length proenzyme form (22, 29, 47, 48) (Fig. 6), even though MMP2 is thought to readily proteolyze itself if activated (44). MMP2 did show some proteolytic activity at 20 µM Ca2+, the upper limit that Ca2+ normally reached in the cytoplasm, but the assay involved Ca2+ exposure for a very prolonged period (17 h), casting doubt about whether anything comparable would happen with normal muscle activity. However, as noted in Localization of MMP2, it seems likely that a portion of the MMP2 identified as “intracellular” in muscle is closely associated with the T-tubule system. If that pool of MMP2 is actually located within the T tubule, it would be exposed to the normal millimolar extracellular [Ca2+] and, consequently, could be expected to show proteolytic activity if activated by a suitable stimulus (e.g., oxidation or another MMP or protease) (11, 44). Even if the MMP was, instead, located intracellularly just beneath the T-tubule membrane within the triad junction, it could still be exposed to [Ca2+] reaching 100 µM or more upon opening of the adjacent SR Ca2+ release channels (9).
Finally, the identity of any proteolytic targets of intracellular MMP2 in skeletal muscle remains unclear. It has been reported that MMP2 can proteolyze cardiac titin (2) and desmin (55), but these studies used proteins in vitro in the presence of 5 mM Ca2+. MMP2 has also been reported to proteolyze troponin I (57) and MLC-1 (49) in cardiac cells subjected to ischemia-reperfusion damage. However, troponin I and MLC-1, as well as desmin and titin, are also proteolyzed by calpain-1 (21, 32). This study shows >50 times more calpain-1 than MMP2 in cardiac cells. Consequently, it seems quite likely that some of the proteolysis of troponin I and MLC-1 and other proteins in cardiac cells exposed to ischemia-reperfusion was due to the action of calpain-1, rather than MMP2, particularly given the comparatively low rate of proteolysis by active MMP2 at the intracellular [Ca2+] reached in cardiac cells during and after ischemia-reperfusion (~2 µM) (7, 10) (Fig. 7C), whereas calpain-1, once activated, displays close to its maximal rate of proteolysis at 2 µM Ca2+ (37). In any experiments exploring MMP2 activity, it is clearly necessary to isolate the activity of calpain-1 from the proteolytic activity of MMP2; therefore, optimal, physiologically relevant conditions for MMP2 are required. MMP2 knockout mice would be useful in these circumstances to ascertain if the proteolysis of potential MMP2 targets occurred with ischemia-reperfusion injury. Any MMP2 within the T tubule lumen would be restricted to interacting with proteins located there. Any MMP2 bound on the intracellular side of the T tubules would have limited access to most structural and regulatory proteins, whereas the large pool of diffusible MMP2 inside muscle cells (Figs. 4 and 5) should be able to access most intracellular proteins, although its primary targets remain undefined.
Conclusions.
The present study has shown that the absolute amount of MMP2 is similar in rat cardiac and skeletal muscle and human muscle. By showing that only 30% of the total MMP2 is localized to the ECM and the majority of MMP2 is localized inside skeletal muscle fibers, we provide a new perspective for the functional role of MMP2 in a physiologically relevant setting. Potential roles of MMP2 have been studied previously, but only when [Ca2+] is several orders of magnitude greater than would ever be present inside cells. We have shown that MMP2 could substantially proteolyze gelatin only when [Ca2+] reaches ~20 μM, the maximum [Ca2+] reached in the cytoplasm during muscle contraction, although this was over a prolonged period and, therefore, does not represent a physiologically relevant environment. We further found that most of the intracellular MMP2 (~40% of the total MMP2) is freely diffusible in the cytosol, very little is associated with intracellular membranes (~4%), and the rest is bound to the cytoskeletal compartment (~28% of the total MMP2). In quiescent skeletal muscle, MMP2 is mainly in the full-length, nonactive pro-MMP2 state. Given that we now have a much better understanding of where MMP2 resides inside skeletal muscle and what is required for its physiological activation, future work must utilize relevant conditions to further understand the physiological role of intracellular MMP2, which should reveal its intracellular target(s) and the interventions that may cause the activation of pro-MMP2 to MMP2.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.R., G.D.L., and R.M.M. conceived and designed research; X.R. performed experiments; X.R. and R.M.M. analyzed data; X.R., G.D.L., and R.M.M. interpreted results of experiments; X.R. prepared figures; X.R. and G.D.L. drafted manuscript; X.R., G.D.L., and R.M.M. edited and revised manuscript; X.R., G.D.L., and R.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Cedric Lamboley, who helped obtain the human tissue, and Maria Cellini, who provided technical assistance.
REFERENCES
- 1.Ali MA, Chow AK, Kandasamy AD, Fan X, West LJ, Crawford BD, Simmen T, Schulz R. Mechanisms of cytosolic targeting of matrix metalloproteinase-2. J Cell Physiol 227: 3397–3404, 2012. doi: 10.1002/jcp.24040. [DOI] [PubMed] [Google Scholar]
- 2.Ali MAM, Cho WJ, Hudson B, Kassiri Z, Granzier H, Schulz R. Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion injury. Circulation 122: 2039–2047, 2010. doi: 10.1161/CIRCULATIONAHA.109.930222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baghirova S, Hughes BG, Poirier M, Kondo MY, Schulz R. Nuclear matrix metalloproteinase-2 in the cardiomyocyte and the ischemic-reperfused heart. J Mol Cell Cardiol 94: 153–161, 2016. doi: 10.1016/j.yjmcc.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Balcerzak D, Querengesser L, Dixon WT, Baracos VE. Coordinate expression of matrix-degrading proteinases and their activators and inhibitors in bovine skeletal muscle. J Anim Sci 79: 94–107, 2001. doi: 10.2527/2001.79194x. [DOI] [PubMed] [Google Scholar]
- 5.Baylor SM, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol 551: 125–138, 2003. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol 7: 728–735, 1995. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 7.Bolli R, Marbán E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79: 609–634, 1999. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 8.Bryant SM, Kong CHT, Watson JJ, Gadeberg HC, Roth DM, Patel HH, Cannell MB, James AF, Orchard CH. Caveolin-3 KO disrupts T-tubule structure and decreases T-tubular ICa density in mouse ventricular myocytes. Am J Physiol Heart Circ Physiol 315: H1101–H1111, 2018. doi: 10.1152/ajpheart.00209.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannell MB, Kong CHT, Imtiaz MS, Laver DR. Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination. Biophys J 104: 2149–2159, 2013. doi: 10.1016/j.bpj.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrozza JP Jr, Bentivegna LA, Williams CP, Kuntz RE, Grossman W, Morgan JP. Decreased myofilament responsiveness in myocardial stunning follows transient calcium overload during ischemia and reperfusion. Circ Res 71: 1334–1340, 1992. doi: 10.1161/01.RES.71.6.1334. [DOI] [PubMed] [Google Scholar]
- 11.Cauwe B, Opdenakker G. Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol 45: 351–423, 2010. doi: 10.3109/10409238.2010.501783. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adhes Migr 3: 337–341, 2009. doi: 10.4161/cam.3.4.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 101: 1833–1839, 2000. doi: 10.1161/01.CIR.101.15.1833. [DOI] [PubMed] [Google Scholar]
- 14.Cho WJ, Chow AK, Schulz R, Daniel EE. Matrix metalloproteinase-2, caveolins, focal adhesion kinase and c-Kit in cells of the mouse myocardium. J Cell Mol Med 11: 1069–1086, 2007. doi: 10.1111/j.1582-4934.2007.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YC, Dalakas MC. Expression of matrix metalloproteinases in the muscle of patients with inflammatory myopathies. Neurology 54: 65–71, 2000. doi: 10.1212/WNL.54.1.65. [DOI] [PubMed] [Google Scholar]
- 16.Chow AK, Cena J, El-Yazbi AF, Crawford BD, Holt A, Cho WJ, Daniel EE, Schulz R. Caveolin-1 inhibits matrix metalloproteinase-2 activity in the heart. J Mol Cell Cardiol 42: 896–901, 2007. doi: 10.1016/j.yjmcc.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 17.DeCoux A, Lindsey ML, Villarreal F, Garcia RA, Schulz R. Myocardial matrix metalloproteinase-2: inside out and upside down. J Mol Cell Cardiol 77: 64–72, 2014. doi: 10.1016/j.yjmcc.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eguchi T, Kubota S, Kawata K, Mukudai Y, Uehara J, Ohgawara T, Ibaragi S, Sasaki A, Kuboki T, Takigawa M. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Mol Cell Biol 28: 2391–2413, 2008. doi: 10.1128/MCB.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisdal E, Teiger E, Lefaucheur JP, Adnot S, Planus E, Lafuma C, D’ortho MP. Increased expression of gelatinases and alteration of basement membrane in rat soleus muscle following femoral artery ligation. Neuropathol Appl Neurobiol 26: 11–21, 2000. doi: 10.1046/j.1365-2990.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- 20.Gao CQ, Sawicki G, Suarez-Pinzon WL, Csont T, Wozniak M, Ferdinandy P, Schulz R. Matrix metalloproteinase-2 mediates cytokine-induced myocardial contractile dysfunction. Cardiovasc Res 57: 426–433, 2003. doi: 10.1016/S0008-6363(02)00719-8. [DOI] [PubMed] [Google Scholar]
- 21.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 83: 731–801, 2003. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hadler-Olsen E, Solli AI, Hafstad A, Winberg JO, Uhlin-Hansen L. Intracellular MMP-2 activity in skeletal muscle is associated with type II fibers. J Cell Physiol 230: 160–169, 2015. doi: 10.1002/jcp.24694. [DOI] [PubMed] [Google Scholar]
- 23.Hessel MHM, Atsma DE, van der Valk EJM, Bax WH, Schalij MJ, van der Laarse A. Release of cardiac troponin I from viable cardiomyocytes is mediated by integrin stimulation. Pflugers Arch 455: 979–986, 2008. doi: 10.1007/s00424-007-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes BG, Fan X, Cho WJ, Schulz R. MMP-2 is localized to the mitochondria-associated membrane of the heart. Am J Physiol Heart Circ Physiol 306: H764–H770, 2014. doi: 10.1152/ajpheart.00909.2013. [DOI] [PubMed] [Google Scholar]
- 25.Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of β-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem 272: 22389–22392, 1997. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- 26.Jayasinghe ID, Launikonis BS. Three-dimensional reconstruction and analysis of the tubular system of vertebrate skeletal muscle. J Cell Sci 126: 4048–4058, 2013. doi: 10.1242/jcs.131565. [DOI] [PubMed] [Google Scholar]
- 27.Jobin PG, Butler GS, Overall CM. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim Biophys Acta Mol Cell Res 1864: 2043–2055, 2017. doi: 10.1016/j.bbamcr.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Ke L, Qi XY, Dijkhuis AJ, Chartier D, Nattel S, Henning RH, Kampinga HH, Brundel BJM. Calpain mediates cardiac troponin degradation and contractile dysfunction in atrial fibrillation. J Mol Cell Cardiol 45: 685–693, 2008. doi: 10.1016/j.yjmcc.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdière-Sahuqué M, Fardeau M, Alameddine HS. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol 205: 158–170, 1999. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]
- 30.Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J 18: 690–692, 2004. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 31.Lamb GD, Stephenson DG. Measurement of force and calcium release using mechanically skinned fibers from mammalian skeletal muscle. J Appl Physiol (1985) 125: 1105–1127, 2018. doi: 10.1152/japplphysiol.00445.2018. [DOI] [PubMed] [Google Scholar]
- 32.Lametsch R, Roepstorff P, Møller HS, Bendixen E. Identification of myofibrillar substrates for μ-calpain. Meat Sci 68: 515–521, 2004. doi: 10.1016/j.meatsci.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Lluri G, Jaworski DM. Regulation of TIMP-2, MT1-MMP, and MMP-2 expression during C2C12 differentiation. Muscle Nerve 32: 492–499, 2005. doi: 10.1002/mus.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J 12: 1075–1095, 1998. doi: 10.1096/fasebj.12.12.1075. [DOI] [PubMed] [Google Scholar]
- 35.Mollica JP, Oakhill JS, Lamb GD, Murphy RM. Are genuine changes in protein expression being overlooked? Reassessing Western blotting. Anal Biochem 386: 270–275, 2009. doi: 10.1016/j.ab.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Murphy RM, Mollica JP, Lamb GD. Plasma membrane removal in rat skeletal muscle fibers reveals caveolin-3 hot-spots at the necks of transverse tubules. Exp Cell Res 315: 1015–1028, 2009. doi: 10.1016/j.yexcr.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Murphy RM, Verburg E, Lamb GD. Ca2+ activation of diffusible and bound pools of μ-calpain in rat skeletal muscle. J Physiol 576: 595–612, 2006. doi: 10.1113/jphysiol.2006.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69: 562–573, 2006. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem 274: 21491–21494, 1999. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 40.Nowak E, Gawor M, Ciemerych MA, Zimowska M. Silencing of gelatinase expression delays myoblast differentiation in vitro. Cell Biol Int 42: 373–382, 2018. doi: 10.1002/cbin.10914. [DOI] [PubMed] [Google Scholar]
- 41.Parton RG, Way M, Zorzi N, Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol 136: 137–154, 1997. doi: 10.1083/jcb.136.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittman RN. Release of plasminogen activator and a calcium-dependent metalloprotease from cultured sympathetic and sensory neurons. Dev Biol 110: 91–101, 1985. doi: 10.1016/0012-1606(85)90067-3. [DOI] [PubMed] [Google Scholar]
- 43.Portbury AL, Willis MS, Patterson C. Tearin’ up my heart: proteolysis in the cardiac sarcomere. J Biol Chem 286: 9929–9934, 2011. doi: 10.1074/jbc.R110.170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol 26: 587–596, 2007. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ralston E, Ploug T. Caveolin-3 is associated with the T-tubules of mature skeletal muscle fibers. Exp Cell Res 246: 510–515, 1999. doi: 10.1006/excr.1998.4305. [DOI] [PubMed] [Google Scholar]
- 46.Rouet-Benzineb P, Buhler JM, Dreyfus P, Delcourt A, Dorent R, Perennec J, Crozatier B, Harf A, Lafuma C. Altered balance between matrix gelatinases (MMP-2 and MMP-9) and their tissue inhibitors in human dilated cardiomyopathy: potential role of MMP-9 in myosin-heavy chain degradation. Eur J Heart Fail 1: 337–352, 1999. doi: 10.1016/S1388-9842(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 47.Rullman E, Norrbom J, Strömberg A, Wågsäter D, Rundqvist H, Haas T, Gustafsson T. Endurance exercise activates matrix metalloproteinases in human skeletal muscle. J Appl Physiol (1985) 106: 804–812, 2009. doi: 10.1152/japplphysiol.90872.2008. [DOI] [PubMed] [Google Scholar]
- 48.Rullman E, Rundqvist H, Wågsäter D, Fischer H, Eriksson P, Sundberg CJ, Jansson E, Gustafsson T. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol (1985) 102: 2346–2351, 2007. doi: 10.1152/japplphysiol.00822.2006. [DOI] [PubMed] [Google Scholar]
- 49.Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D, Schulz R. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2. Circulation 112: 544–552, 2005. doi: 10.1161/CIRCULATIONAHA.104.531616. [DOI] [PubMed] [Google Scholar]
- 50.Schägger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199: 223–231, 1991. doi: 10.1016/0003-2697(91)90094-A. [DOI] [PubMed] [Google Scholar]
- 51.Si-Tayeb K, Monvoisin A, Mazzocco C, Lepreux S, Decossas M, Cubel G, Taras D, Blanc JF, Robinson DR, Rosenbaum J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am J Pathol 169: 1390–1401, 2006. doi: 10.2353/ajpath.2006.060005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snoek-van Beurden PAM, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 38: 73–83, 2005. doi: 10.2144/05381RV01. [DOI] [PubMed] [Google Scholar]
- 53.Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci USA 87: 364–368, 1990. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stetler-Stevenson WG, Krutzsch HC, Wacher MP, Margulies IMK, Liotta LA. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem 264: 1353–1356, 1989. [PubMed] [Google Scholar]
- 55.Sung MM, Schulz CG, Wang W, Sawicki G, Bautista-López NL, Schulz R. Matrix metalloproteinase-2 degrades the cytoskeletal protein α-actinin in peroxynitrite mediated myocardial injury. J Mol Cell Cardiol 43: 429–436, 2007. doi: 10.1016/j.yjmcc.2007.07.055. [DOI] [PubMed] [Google Scholar]
- 56.Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol 48: 222–272, 2013. doi: 10.3109/10409238.2013.770819. [DOI] [PubMed] [Google Scholar]
- 57.Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JRB, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 106: 1543–1549, 2002. doi: 10.1161/01.CIR.0000028818.33488.7B. [DOI] [PubMed] [Google Scholar]
- 58.Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol 98: 615–635, 1991. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wette SG, Smith HK, Lamb GD, Murphy RM. Characterization of muscle ankyrin repeat proteins in human skeletal muscle. Am J Physiol Cell Physiol 313: C327–C339, 2017. doi: 10.1152/ajpcell.00077.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong J, Baddeley D, Bushong EA, Yu Z, Ellisman MH, Hoshijima M, Soeller C. Nanoscale distribution of ryanodine receptors and caveolin-3 in mouse ventricular myocytes: dilation of T-tubules near junctions. Biophys J 104: L22–L24, 2013. doi: 10.1016/j.bpj.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimowska M, Brzoska E, Swierczynska M, Streminska W, Moraczewski J. Distinct patterns of MMP-9 and MMP-2 activity in slow and fast twitch skeletal muscle regeneration in vivo. Int J Dev Biol 52: 307–314, 2008. doi: 10.1387/ijdb.072331mz. [DOI] [PubMed] [Google Scholar]