Abstract
Sarcopenia, the age-associated loss of skeletal muscle mass and function, is coupled with declines in physical functioning leading to subsequent higher rates of disability, frailty, morbidity, and mortality. Aging and obesity independently contribute to muscle atrophy that is assumed to be a result of the activation of mutual physiological pathways. Understanding mechanisms contributing to the induction of skeletal muscle atrophy with aging and obesity is important for determining targets that may have pivotal roles in muscle loss in these conditions. We find that aging and obesity equally induce an anabolic resistance to acute skeletal muscle contraction as observed with decreases in anabolic signaling activation after contraction. Furthermore, treatment with the sphingosine-1-phosphate analog FTY720 for 4 wk increased lean mass and strength, and the anabolic signaling response to contraction was improved in obese but not older animals. To determine the role of chronic inflammation and different fatty acids on anabolic resistance in skeletal muscle cells, we overexpressed IKKβ with and without exposure to saturated fatty acid (SFA; palmitic acid), polyunsaturated fatty acid (eicosapentaenoic acid), and monounsaturated fatty acid (oleic acid). We found that IKKβ overexpression increased inflammation markers in muscle cells, and this chronic inflammation exacerbated anabolic resistance in response to SFA. Pretreatment with FTY720 reversed the inflammatory effects of palmitic acid in the muscle cells. Taken together, these data demonstrate chronic inflammation can induce anabolic resistance, SFA aggravates these effects, and FTY720 can reverse this by decreasing ceramide accumulation in skeletal muscle.
Keywords: aging, ceramide, inflammation, obesity, skeletal muscle
INTRODUCTION
Sarcopenia, the age-associated loss of skeletal muscle mass and function, is coupled with declines in physical functioning leading to subsequent higher rates of disability, frailty, morbidity, and mortality (13). Aging and obesity independently contribute to muscle atrophy that is assumed to be a result of the activation of mutual physiological pathways (32). The infiltration of lipids in muscle is characteristic in both aging and obesity, giving rise to declines in muscle quality and strength (2, 25). In addition, obesity can dramatically increase the risk of functional decline and mobility disability in older adults, likely a result of a compounding effect of skeletal muscle decline and adipose accumulation promoting a cycle of weight gain, systemic inflammation, and an exacerbation of muscle loss (33). Although sarcopenia is a complex and multifactorial process, it is unknown whether excess adiposity or other obesity-mediated factors and pathways work synergistically to induce muscle atrophy.
We (35, 36) and others have previously reported a reduction in anabolic capacity in both obese and aged skeletal muscle. Muscle contraction is a potent anabolic stimulator in healthy skeletal muscle and is shown to have a synergistic effect on the nutrient-induced stimulation of protein synthesis (9, 20, 27). The activation of protein synthesis by resistance exercise can be observed by 1 h after exercise and can persist for up to 48 h (43). The cellular mechanisms associated with contraction-induced protein synthesis include the activation of Akt/mammalian target of rapamycin (mTOR) signaling and changes in expression of noncoding RNA like microRNA (miRNA) and can occur independent of IGF-1 receptor signaling (39). In fact, downstream activation of the mTOR pathway component S6 kinase 1 (S6K1) by high-force contractions is associated with activation of protein synthesis and muscle growth in both humans and animals (28). However, attenuated Akt/mTOR signaling and miRNA expression have been implicated in the impaired anabolic response in aging and obesity (35).
Ceramide is a bioactive lipid mediator involved in cellular stress response and is a fundamental component of sphingolipid metabolism that augments more complex structural sphingolipids and other intermediates such as sphingosine-1-phosphate (S1P; Ref. 24). Whereas increases of ceramide synthesis through the excess dietary intake of saturated fatty acids, such as palmitate, are largely documented in the development of insulin resistance, we hypothesize a central role for ceramide accumulation in muscle mass regulation, particularly in muscle atrophy with aging.
FTY720 is an S1P analog that functions as the immunosuppressive lipid second messenger that acts by downregulating S1P receptors (S1PR) or degrading sphingolipids such as ceramide (11). Recent studies have shown an interest in reducing sphingolipid accumulation as a treatment of obesity-and age-induced pathobiology (4, 38, 40). Therefore, we hypothesized the in vivo reduction of sphingolipids stored in skeletal muscle with FTY720 administration as well as the added effect of minimizing inflammation in aging and obesity could reverse anabolic resistance seen in these states.
An improved understanding of skeletal muscle response to anabolic stimulation with aging and obesity is of great clinical importance. Furthermore, investigations of the impact of the S1P analog FTY720 on aged and obese skeletal muscle will provide novel insights into the role of inflammation and lipotoxicity on the anabolic capacity to contraction and skeletal muscle health. We now report that administration of FTY720 reverses obesity from 16 wk of high-fat feeding by not only regulating myocellular ceramide accumulation, but also attenuating proinflammatory signaling activation and anabolic resistance. Although FTY720 was able to attenuate ceramide accumulation in the skeletal muscle of older animals, this had no effect on the anabolic response to contraction in these animals.
METHODS
Study Design
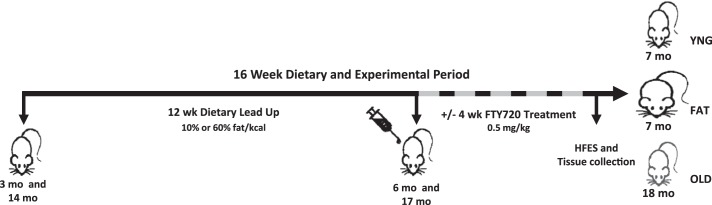
Male C57BL/6 mice aged 3 mo (n = 40) and 14 mo (OLD; n = 24) were purchased from the National Institute on Aging Aged Rodent Colony/Jackson (Bar Harbor, ME). Mice were housed in a temperature-controlled animal room (21°C) maintained on a 12:12-h light-dark cycle with free access to food and water for 4 mo. Final ages of the groups of mice when contraction experiments occurred and tissues were collected and evaluated were 7 mo, YNG and FAT, and 18 mo, OLD (Fig. 1). All animal experimentation procedures were approved by the Institutional Animal Care and Use Committee of the Jean Mayer U.S. Department of Agriculture (USDA) Human Nutrition Research Center on Aging at Tufts University.
Fig. 1.
Timeline and design of the in vivo mouse experiments. Mice aged 3 and 14 mo started their dietary lead up of 12 wk. After the dietary period, the mice now aged 6 and 17 mo were maintained on their diets and were treated with an injection of sphingosine-1-phosphate analog FTY720 every other day for 4 wk. After the treatment period, mice now aged 7 and 18 mo underwent high-frequency electrical stimulation (HFES) and tissue collection 1 h later. FAT, 7 mo, 60% fat/kcal; OLD, 18 mo, 10% fat/kcal; YNG, 7 mo, 10% fat/kcal diet.
Dietary Period
Fourteen-month-old mice were assigned a 10% fat/kcal isocaloric diet (D12450B; Research Diets, New Brunswick, NJ) and were maintained on this diet for a total of 16 wk (OLD). Three-month-old mice were randomly assigned to either a 10% fat/kcal isocaloric diet (YNG; D12450B; Research Diets) or a high-fat 60% fat/kcal isocaloric diet (FAT; D12492; Research Diets) for a total of 16 wk (YNG and FAT, respectively).
Electrical Stimulation
The high-frequency electrical stimulation (HFES) model has been previously described (16). HFES experiments were performed after the 16-wk dietary period during two separate experiments, with and without the FTY720 group (YNG, n = 16; FAT, n = 24; OLD, n = 24). Animals were anesthetized by intraperitoneal injection of sodium pentobarbital. The peroneal nerve was isolated and mounted on hook electrodes. On the HFES limb, each contraction was elicited by stimulating the experimental nerve with a 3-s train of pulses (frequency 100 Hz, duration 1 ms, at 10–12 V) by a Grass S48 stimulator (Grass Telefactor, Quincy, MA). Animals performed a total of 100 contractions grouped into 10 sets of 10 contractions. Each contraction was followed by a 10-s rest, and a 60-s rest interval separated each set, resulting in a protocol time of 30 min. This protocol results in an eccentric (lengthening) contraction of the tibialis anterior. The tibialis anterior was chosen to be analyzed because it is a mixed muscle made up of both glycolytic (approximately 80–85%, type 2b/x) and oxidative (approximately 10–15%, type 2a) fibers and contractile activity highly induces anabolic signaling in this muscle after HFES (16). Throughout the stimulation protocol, muscles were wetted with 1× PBS. The opposite limb of each animal served as the surgical sham control (SHAM). One hour following electrical stimulation, animals were euthanized by cervical dislocation, and muscles were dissected and flash-frozen in liquid nitrogen.
Cell Culture
Stock C2C12 (mouse) myoblasts (American Type Culture Collection, Manassas, VA) were maintained at 37 °C (95% O2-5% CO2) on 75-cm2 flasks in α-modified Eagle’s medium (Invitrogen) containing 10% fetal bovine serum (Sigma-Aldrich), 1% penicillin-streptomycin (Sigma-Aldrich), and 5.5 mM glucose as described (37). For experimental procedures, the myoblasts were trypsinized and seeded in 12-well plates.
Adenoviral Transduction
Adenovirus serotype 5 driving inhibitor of κB kinase-β (Ad5-IKKβ) or green fluorescent protein (Ad5-GFP) under control of the cytomegalovirus promoter was obtained from Vector Biolabs (Philadelphia, PA). All cells were infected with either Ad5-IKKβ or Ad5-GFP (cat. nos. 1487 and 1060) at a multiplicity of infection of 150 for 24 h.
In Vitro Fatty Acid Experiments
After 24 h, cells expressing Ad5-IKKβ or Ad5-GFP were serum-starved for 4 h and incubated in DMEM containing 100 μM either the saturated fatty-acid (SFA) palmitic acid (PA; 16:0), the monounsaturated fatty acid oleic acid (OA; 18:1, n-9), and the polyunsaturated fatty acid eicosapentaenoic acid (EPA; 20:5, n-3) conjugated to 2% fatty acid-free BSA or vehicle (ethanol/2% BSA) for 12 h.
In Vitro FTY720 Experiments
Cells expressing Ad5-IKKβ or Ad5-GFP were pretreated with FTY720 (100 nM) solubilized in 1% α-cyclodextrin or a vehicle control for 4 h, serum-starved for 2 h, and then incubated in PA conjugated to 2% fatty acid-free BSA or vehicle (ethanol/2% BSA) for 12 h.
In Vitro Protein Synthesis
Protein synthesis was measured by the surface sensing of translation (SUnSET) technique via immunological detection of puromycin-labeled peptides as described previously (37). Briefly, C2C12 myoblasts were serum-starved for 2 h, and puromycin (1 μM) was added to the medium of the cells 30 min before harvest.
In Vivo Inhibition of Ceramide Synthesis
After 12 wk of ad libitum feeding, 16 FAT mice, now aged 6 mo, and 16 OLD mice, now aged 17 mo, were maintained on their diets, randomized, and injected intraperitoneally every other day with the sphingosine-1-phosphate analog FTY720 (0.5 mg/kg) solubilized in 1% α-cyclodextrin or a vehicle control during the last 4-wk dietary period. In addition, 8 YNG mice, now aged 6 mo, were maintained on their 10% fat diet and were injected with the vehicle control during the last 4-wk dietary period (Fig. 1).
Neuromuscular Strength and Lean Mass
A Chatillon E-DFE Digital Force Gauge (AMETEK, Largo, FL) was used to measure grip strength. Briefly, the forelimb of the mouse was allowed to grasp onto the pull-bar assembly. The animal was then gently pulled backward by the tail leading away from the sensor until the grip was released and the maximum force attained and stored on the display. Three trials were performed for each mouse with a 10-min resting period between trials. Determination of the body composition of each animal was performed using a quantitative magnetic resonance EchoMRI-900 whole body composition analyzer (Echo Medical Systems, Houston, TX).
Lipidomics
Sphingolipids were analyzed using high-performance liquid chromatography-tandem mass spectrometry as previously described (35, 36). Briefly, lipids were extracted in isopropanol-water-ethyl acetate (30:10:60) evaporated under N2 gas stream and then resuspended in chloroform-methanol (2:1) from gastrocnemius muscle. Separation by HPLC was carried out using a C8 XBridge (150 mm) column (Waters, Milford, MA). Levels of individual lipids were quantified using spiked internal standards, including d-erythro-sphingosine-d7 (860657), d-erythro-sphingosine-1-phosphate (860492), N-palmitoyl-d-erythro-sphingosine (860516), and N-stearoyl-d-erythro-sphingosine (860518; Avanti Polar Lipids, Alabaster, AL). Mass spectrometry was carried out in an AB QTRAP 5500 mass spectrometer (Agilent Technologies, Lexington, MA) following the expected fragmentation pattern of each of the lipids under specific conditions.
Cellular Signaling
The phosphorylation and concentration of signaling proteins were quantified via Western blotting as previously (37). The tibialis anterior muscle was cut, weighed, and then homogenized at 1:10 wt/vol with ice-cold homogenization buffer [50 mM Tris·HCl (pH 7.5), 5 mM Na-pyrophosphate, 50 mM NaF, 1 mM EDTA, 1 mM EGTA, 10% glycerol (vol/vol), 1% Triton X-100, 1 mM DTT, cOmplete ULTRA (Roche Applied Science, Indianapolis, IN), and PhosSTOP inhibitors (1 tablet/10 mL; Roche Applied Science)]. Following centrifugation (10,000 g at 4°C) for 10 min, the supernatant was collected and assayed for protein content. The supernatant was solubilized in Laemmli buffer (10 mM DTT), separated by SDS-PAGE, and transferred to PVDF membranes. The membranes were then blocked (5% nonfat dry milk) and incubated overnight at 4°C with primary antibodies specific for phosphorylated Akt (phospho-Akt; cat. no. 9271; AB_329825), Akt (cat. no. 9272; AB_329827), phospho-p90 ribosomal S6 kinase (phospho-p90RSK; cat. no. 9335; AB_561151), RSK1/2/3 (cat. no. 9355; AB_659900), phospho-CAMKII (cat. no. 12716; AB_2713889), CAMKII (cat. no. 4436; AB_10545451), phospho-histone deacetylation enzyme 3 (phospho-HDAC3; cat. no. 3815; AB_2264084), HDAC3 (cat. no. 85057; AB_2800047), phospho-cAMP response element-binding protein (phospho-CREB; cat. no. 9198; AB_2561044), CREB (cat. no. 9197; AB_331277), phospho-S6K (cat. no. 9205; AB_330944), S6K1 (cat. no. 9202; AB_331676), phospho-ribosomal protein S6 (phospho-rpS6; cat. no. 2215; AB_331682), rpS6 (cat. no. 2217; AB_331355), phospho-IKKα/β (cat. no. 2697; AB_2079382), IKKβ (cat. no. 8943; AB_11024092), phospho-NF-κB (cat. no. 3033; AB_331284), and NF-κB (cat. no. 8242; AB_10859369). Membranes were probed with GAPDH (cat. no. 2118; AB_561053) or α/β-tubulin (cat. no. 2148; AB_2288042) to monitor protein loading; all antibodies were from Cell Signaling Technology (Danvers, MA), and anti-puromycin (MABE343; AB_2566826) was from MilliporeSigma (Burlington, MA). The immunoreactive proteins were detected with SuperSignal Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL), and intensities were quantified by densitometry (Bio-Rad ChemiDoc XRS+ system; San Leandro, CA) and analyzed as previously described (39).
Quantitative PCR Analysis
RNA was extracted from the tibialis anterior muscle using Aurum Total RNA Fatty and Fibrous Tissue Pack per manufacturer’s instructions (cat. no. 732-6830; Bio-Rad, Hercules, CA). mRNA concentration and purity were determined by spectrophotometry (NanoDrop 1000; Thermo Fisher Scientific, Wilmington, DE). cDNA was generated via iScript Reverse Transcription Supermix for RT-qPCR (cat. no. 170-8840; Bio-Rad). cDNA levels were measured using validated primer pairs from PrimerBank for the following genes: Casp3 (12851951a1), Crp (6681025a1), Fbox32 (13385848a1), Foxo1 (34328255a1), Fos (6753873c3), Ikbkb (33469101a1), Il1a (52669a1), Il1b (6680415a1), Il6 (13624311a1), Jun (49355797c1), Myf6 (6678984a1), Ncor1 (17467268a1), Nfkbia (6754840a1), Pax3 (26377023a1), Prkaa1 (26349431a1), Prkaa2 (26336122a1), Rela (6677709a1), S1pr1 (21687214a1), S1pr2 (4324651a1), S1pr3 (6753716a1), S1pr5 (16716489a1), Sik1 (110815829c2), Tlr2 (31981333a1), Traf6 (6678429a1), and Trim63 (21523717a1; Ref. 44). All reactions were run using a commercially available reaction mixture (iTaq Universal SYBR Green Supermix; Bio-Rad) on a CFX96 Touch (Bio-Rad). Fold change in gene expression was normalized to the geometric mean of the reference genes B2m (31981890a1), Gapdh (6679937a1), and Rpl32 (25742730a1).
Bioinformatics Analysis of miRNA
miR-26b-3p, miR-199a-3p, miR-324-3p, and miR-378a-3p were uploaded to DNA Intelligent Analysis (DIANA)-miRPath 3.0 (Alexander Fleming Biomedical Sciences Research Center, Athens, Greece; http://diana.imis.athena-innovation.gr/DianaTools/index.php) to determine potential molecular pathways that these miRNA have previously been reported to regulate. Relevant Kyoto Encyclopedia of Genes and Genomes (KEGG; https://www.genome.jp/kegg/) pathways were identified using experimentally verified targets from TarBase 7.0 (Alexander Fleming Biomedical Sciences Research Center).
Statistical Analysis
FTY720 treatment in mice and lipidomics.
Differences between groups were identified using a one-way ANOVA with Tukey post hoc test with GraphPad Prism version 5.00 for Windows (GraphPad Software; https://www.graphpad.com/). Results are expressed as means ± SE, and statistical significance was accepted at P < 0.05.
Age/dietary differences in contraction response and in vitro experiments.
Differences between groups were identified using a two-way ANOVA with Bonferroni post hoc test with GraphPad Prism 5.00 for Windows. Results are expressed as means ± SE, and statistical significance was accepted at P < 0.05.
RESULTS
Anabolic Response to Muscle Contraction is Blunted with Aging and Obesity
We mimicked resistance exercise in mice using in situ contraction of skeletal muscle via HFES. The HFES model was chosen because when contractions are adequately repeated all hindlimb muscles are recruited including the tibialis anterior analyzed in this study (36). We (16) and others have previously reported that in response to HFES, anabolic signaling, protein synthesis, and hypertrophy are increased in skeletal muscle.
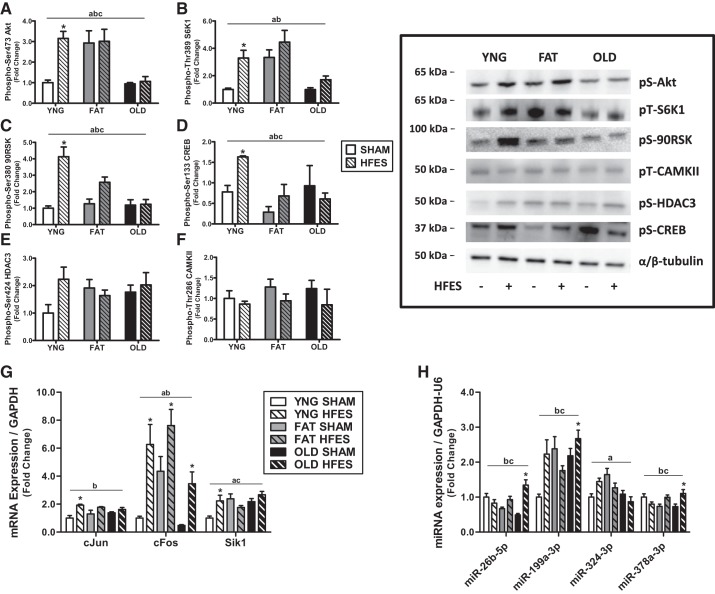
We first wanted to determine anabolic response of skeletal muscle to HFES as a consequence of obesity and aging. There was a significant main effect of age/diet, response to contraction, and an interaction between age/diet and contraction in Akt and S6K1 activation and a significant main effect of age/diet and response to contraction in p90RSK activation. Young animals had a significant three- to fourfold increase in Akt, S6K1, and p90RSK activation after the acute contraction compared with baseline with no change in obese and older animals (Fig. 2, A–C).
Fig. 2.
Anabolic response of lean (YNG), obese (FAT), and older (OLD) mice at rest (SHAM) and after contraction (high-frequency electrical stimulation, HFES) in tibialis anterior muscle. Relative protein levels of phosphorylated (Phospho-) Akt (A), S6 kinase 1 (S6K1; B), p90 ribosomal S6 kinase (90RSK; C), cAMP response element-binding protein (CREB; D), histone deacetylation enzyme 3 (HDAC3; E), and CAMKII (F) were quantified using Western blot analysis. mRNA expression of immediate early response genes (G) and microRNA (miRNA) associated with anabolic response (H) was measured by quantitative PCR. aP < 0.05 for main effect phenotype, bP < 0.05 for main effect contraction, cP < 0.05 for interaction phenotype-contraction, *P < 0.05 vs. YNG SHAM by 2-way ANOVA, n = 8/group. pS, phosphoserine; pT, phosphothreonine; Sik1, salt-inducible kinase 1; U6, U6 noncoding small nuclear RNA.
We next determined contraction-mediated signaling and myogenic transcription factor expression in these same groups of mice. There was a significant main effect of age/diet, response to contraction, and an interaction between age/diet and contraction in CREB phosphorylation. Young animals had a significant fourfold increase in CREB phosphorylation in response to acute contraction with no change in obese and older animals (P < 0.001; Fig. 2D). However, there was no significant change of CAMKII or HDAC3 phosphorylation after contraction in any of the groups (Fig. 2, E and F).
Subsequently, the expression of immediate early response genes 1 h after an acute bout of contraction was measured in the three groups of mice. There was a significant main effect of contraction response in c-Jun and c-Fos expression, a significant main effect of age/diet in c-Fos and salt-inducible kinase 1 (SIK1) expression, and an interaction between age/diet and contraction in SIK1 expression. Young animals had a significant change in c-Jun, c-Fos, and SIK1 expression in response to acute contraction, whereas obese and older animals had a significant increase in c-Fos expression after acute contraction (Fig. 2G).
We (34) have previously reported a differential miRNA expression profile in young and aged skeletal muscle after acute resistance exercise. Therefore, we chose specific miRNA that had previously been determined to be divergently expressed with age, and subsequent bioinformatic analysis established a significant role of these miRNA in anabolic regulation (Table 1). Older animals had a significant 1.5- to 3-fold increase in miR-26b-5p, miR-199a-3p, and miR-378a-3p expression in response to acute contraction, whereas there were no changes in lean or obese animals (Fig. 2H).
Table 1.
Bioinformatic identification of significant (P < 0.05) and relevant KEGG pathways associated with anabolic regulation by the 4 measured miRNAs
| KEGG Pathway | P Value | No. Genes | No. miRNAs |
|---|---|---|---|
| mTOR signaling pathway | 0.001107 | 12 | 4 |
| Arrhythmogenic right ventricular cardiomyopathy | 0.001107 | 11 | 4 |
| Phosphatidylinositol signaling system | 0.001232 | 9 | 4 |
| ErbB signaling pathway | 0.006947 | 12 | 3 |
| AMP-activated protein kinase signaling pathway | 0.033429 | 16 | 3 |
| Hypertrophic cardiomyopathy | 0.036201 | 12 | 3 |
| Gap junction | 0.036201 | 6 | 4 |
| Proteoglycans in cancer | 0.036201 | 19 | 4 |
Boldface indicates relevant pathways. ErbB, epidermal growth factor receptor; KEGG, Kyoto Encyclopedia of Genes and Genomes; miRNAs, microRNAs; mTOR, Akt/mammalian target of rapamycin.
Together, our data establish a significant blunting of anabolic regulation after contraction in the skeletal muscle of obese and older mice and suggest a role of differential miRNA expression in older animals in the attenuation of anabolism in skeletal muscle.
Saturated Fatty Acid and IKKβ Overexpression Inhibits Protein Synthesis and Anabolic Signaling, Activates NF-κB Signaling, and is Attenuated by the Inhibition of Ceramide Synthesis in Skeletal Muscle Cells
Both aging and obesity are thought to be highly associated with chronic inflammation (13). Therefore, we wanted to determine the role of various fatty acids, pretreatment with the ceramide inhibitor FTY720, and chronically elevated inflammation on skeletal muscle cells in vitro.
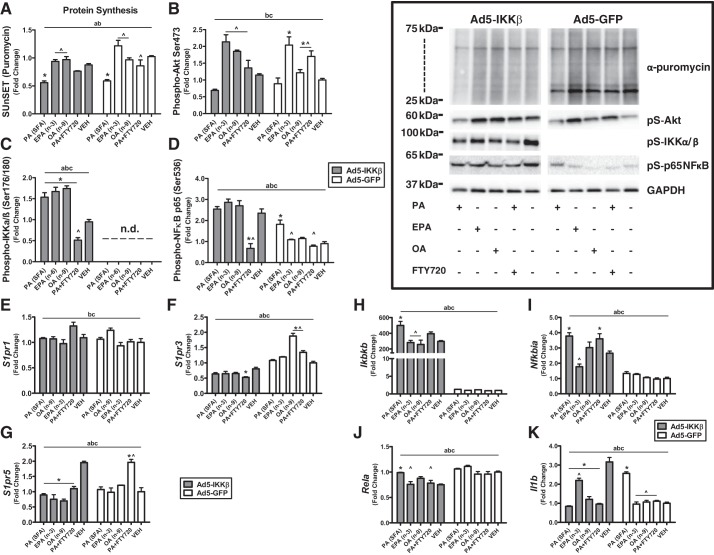
Palmitate significantly decreased protein synthesis twofold with IKKβ overexpression (P < 0.01) and in the GFP control (P < 0.0001) compared with vehicle. Pretreatment with FTY720 reversed the protein synthesis effects of palmitate on GFP control (P < 0.05) but not IKKβ overexpression. Protein synthesis after treatment with EPA and OA was significantly two- to fourfold higher from PA with IKKβ overexpression and in GFP control (Fig. 3A). When anabolic signaling was measured, EPA, OA, and FTY720 pretreatment increased Akt (P < 0.05) phosphorylation twofold compared with vehicle in GFP control but not with IKKβ overexpression. EPA, OA, and FTY720 pretreatment also increased Akt phosphorylation (P < 0.05) compared with PA in both GFP control and IKKβ overexpression (Fig. 3B). PA, EPA, and OA increased IKKα/β phosphorylation (P < 0.05) compared with vehicle with IKKβ overexpression. When the activation of proinflammatory components was measured, FTY720 pretreatment attenuated the effects of the phosphorylation of IKKα/β (P < 0.05) with IKKβ overexpression. The phosphorylation of IKKα/β was not detectable in the GFP control (Fig. 3C). The phosphorylation of NF-κB was threefold lower with FTY720 pretreatment in IKKβ overexpression (P < 0.05) compared with vehicle and PA treatment. PA increased NF-κB phosphorylation twofold in GFP control compared with vehicle and EPA (P < 0.05), and FTY720 pretreatment reversed the effects of PA (P < 0.05) in the phosphorylation of NF-κB (Fig. 3D).
Fig. 3.
Chronic inflammation and saturated fatty acids inhibit protein synthesis and anabolic signaling and are improved with inhibition of ceramide synthesis in skeletal muscle cells. A: protein synthesis measured by surface sensing of translation (SUnSET) technique via immunological detection of puromycin-labeled peptides. Relative protein levels of phosphorylated (Phospho-) Akt (B), IKKα/β (C), and NF-κB (D) were quantified using Western blot analysis. mRNA expression of sphingosine-1-phosphate receptor 1 (S1PR1; E), S1PR3 (F), S1PR5 (G), IKKβ (H), IκBα (I), p65 subunit of NF-κB (J), and IL-1β (K) was measured by quantitative PCR. aP < 0.05 for main effect phenotype, bP < 0.05 for main effect fatty acid/treatment, cinteraction phenotype-fatty acid, *P < 0.05 vs. vehicle (VEH), ^P < 0.05 vs. palmitic acid (PA) by 2-way ANOVA, n = 3/group. Ad5-GFP, adenovirus serotype 5 driving green fluorescent protein; EPA, eicosapentaenoic acid; FTY720, S1P analog; n.d., not detectable; OA, oleic acid; pS, phosphoserine; SFA, saturated fatty acid.
We next determined the effects of different fatty acids and S1P agonism on the expression of the lipid signaling S1P receptors. There were no significant changes between IKKβ overexpression and fatty acids in S1PR1 expression (Fig. 3E). S1PR3 and S1PR5 had significant differences between IKKβ overexpression and GFP controls (P < 0.05). FTY720 pretreatment in PA significantly changed S1PR3 expression compared with vehicle and PA with IKKβ overexpression and GFP control (P < 0.05). OA significantly increased S1PR3 expression twofold only in GFP control (P < 0.05) compared with vehicle (Fig. 3F). PA, EPA, OA, and FTY720 (P < 0.05) pretreatment decreased S1PR5 expression 50% in IKKβ-overexpressed cells. FTY720 pretreatment with PA increased S1PR5 expression twofold in GFP control cells (Fig. 3G).
We then determined the effects of the expression of inflammatory genes with different fatty acids and ceramide inhibition during chronic inflammation. IKKβ overexpression increased the expression of IKKβ and IκBα between 500- and 4-fold compared with GFP control. In IKKβ-overexpressed cells, PA increased (P < 0.001) IKKβ expression compared with vehicle. EPA (P < 0.0001) and OA (P < 0.001) had significantly lower IKKβ expression compared with PA in IKKβ-overexpressed cells (Fig. 3H). PA (P < 0.0001) and FTY720 pretreatment with PA (P < 0.05) increased IκBα expression compared with vehicle with IKKβ overexpression. IκBα expression was significantly lower (P < 0.0001) in EPA compared with PA with IKKβ overexpression (Fig. 3I). PA significantly increased NF-κB expression (P < 0.01) compared with vehicle with IKKβ overexpression. NF-κB expression was significantly lower with FTY720 pretreatment (P < 0.05) and EPA (P < 0.01) compared with PA in IKKβ-overexpressed cells (Fig. 3J). PA, EPA, OA, and FTY720 pretreatment decreased IL-1β expression compared with vehicle in IKKβ-overexpressed cells. However, PA increased IL-1β expression compared with vehicle in GFP control cells. EPA increased IL-1β expression compared with PA with IKKβ overexpression. EPA, OA, and FTY720 pretreatment had significantly lower (P < 0.05) IL-1β expression compared with PA in GFP control (Fig. 3K).
Inhibition of Ceramide Synthesis Attenuates Changes in Body Composition, Muscle Mass, and Function in Obesity
Functional and compositional consequences of obesity and aging with/without 4 wk of FTY720 treatment were first determined in each group. Sixteen weeks of high-fat feeding increased body mass by 68 and 58% compared with control feeding and aging, respectively. Body mass was 26% lower in obese animals treated with FTY720 for 4 wk but was unchanged in older animals. Lean mass was 35% lower in obese compared with young and older mice and was significantly increased by 15 and 13% with FTY720 treatment in obese and older animals, respectively. Gonadal fat pad mass was 250% higher in obese compared with young and older mice and was significantly lowered by 22% with FTY720 treatment in obese animals but unchanged in older animals. Grip strength is shown to be a reliable predictor of future mortality and morbidity and is related to total muscle strength. It has been recently reported that grip strength declines as early as 12 mo of age in C57BL/6, continues to decline with increasing age, and is an indicator of frailty (17, 26). We now show that grip strength is significantly lower by 42 and 31% with obesity compared with young and older mice, respectively. Four weeks of FTY720 treatment significantly increased grip strength 27% in obese but not in older animals (Table 2). Food consumption was 48% higher in obese compared with young animals and was unchanged after 4 wk of FTY720 treatment in both obese and older animals (Table 2). These data establish that with obesity there is a significant reduction in muscle mass and function as measured by grip strength and that FTY720 reversed these effects.
Table 2.
Inhibition of ceramide synthesis with FTY720 attenuates changes in body composition, muscle mass, and function
| YNG | FAT | OLD | |
|---|---|---|---|
| Body mass, g | |||
| Vehicle | 28.79 | 49.10* | 31.44† |
| FTY720 | 39.01*† | 31.79 | |
| Gonadal fat pad mass, g | |||
| Vehicle | 0.73 | 2.57* | 0.74† |
| FTY720 | 1.99*† | 0.64 | |
| Grip strength, N/g body mass | |||
| Vehicle | 5.71 | 3.32* | 4.82† |
| FTY720 | 4.54*† | 4.92 | |
| Lean mass, % | |||
| Vehicle | 84.23 | 54.13* | 83.17† |
| FTY720 | 63.09*† | 95.48*‡ | |
| Food consumption, g/day | |||
| Vehicle | 2.15 | 3.18* | 2.65 |
| FTY720 | 2.67* | 2.51 |
Values are means. FTY720, sphingosine-1-phosphate analog.
P < 0.05 vs. lean (YNG),
P < 0.05 vs. FAT vehicle,
P < 0.05 vs. OLD vehicle by 1-way ANOVA, n = 8/group.
Four Weeks of S1P Receptor Agonism Decreases Ceramide Accumulation in Skeletal Muscle of Older and Obese Mice
Sphingolipids have as of recently been revealed to act as effector molecules with essential roles in many aspects of cell biology (40). Ceramide (Cer), sphingosine (So), and sphingosine-1-phosphate (S1P) are the most studied and are shown to have opposite effects in physiology. Ceramide and sphingosine usually inhibit proliferation and promote apoptotic responses to stress stimuli, whereas S1P is known to stimulate cell growth and promote cell survival (29). FTY720 is an S1P analog that has been reported to improve anabolic signaling and muscle metabolism through ceramide, diacylglycerol, and triacylglycerol reduction in muscle. Therefore, we wanted to determine the effects of FTY720 treatment on sphingolipid accumulation in obese and older mice via lipidomic analysis.
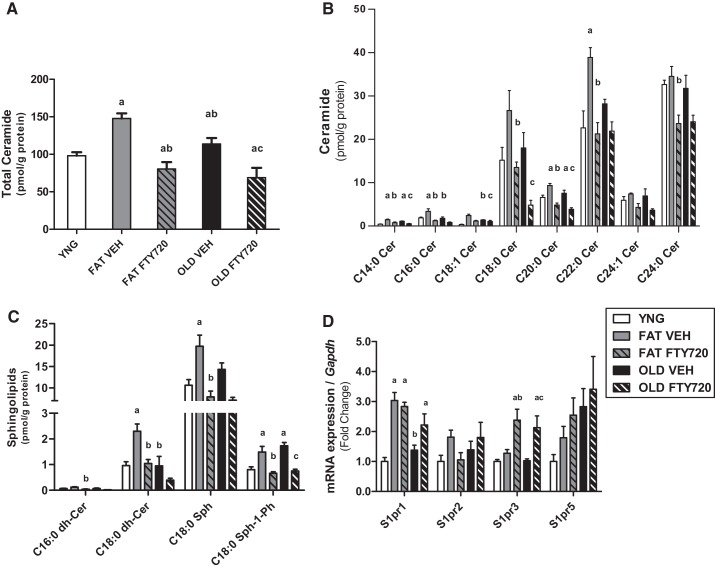
Total ceramide was significantly increased by 50 and 15% with obesity and aging compared with young, respectively (P < 0.05). FTY720 treatment reversed the increase in ceramide accumulation in the skeletal muscle of the obese and older mice by 68 and 47% with the intervention, respectively (Fig. 4A). The accumulation of individual ceramide species (C) in skeletal muscle show that C14:0 and C20:0 Cer and S1P were significantly higher (P < 0.05) in obesity and aging and were lowered after 4 wk of FTY720 treatment in both groups (Fig. 4, B and C). C16:0 and C22:0 Cer, C18:0 dihydroceramide, and C18:0 So were elevated (P < 0.05) with fat feeding and reduced after FTY720 treatment but were unaffected by age. C18:0, C18:1, and C24:0 Cer and C16:0 dihydroceramide were not altered by obesity and age but were lowered with FTY720 treatment in both groups (Fig. 4, B and C).
Fig. 4.
Sphingosine-1-phosphate (S1P) receptor agonism decreases ceramide accumulation in the skeletal muscle of aged and obese mice. Intramuscular accumulation of total ceramide (A), individual ceramide species (C; B), and other sphingolipids and ceramide metabolites (C) are shown. mRNA expression of the sphingosine-1-phosphate receptors (D) was measured by quantitative PCR. aP < 0.05 vs. lean (YNG), bP < 0.05 vs. FAT vehicle (VEH), cP < 0.05 vs. OLD VEH by 1-way ANOVA, n = 8/group. Cer, ceramide; dh-Cer, dihydroceramide; FTY720, S1P analog; Sph, sphingosine; Sph-1-Ph, sphingosine-1-phosphate; S1PR, S1P receptor.
We next wanted to determine the effect of obesity, age, and FTY720 treatment on the expression of the G protein-coupled sphingosine-1-phosphate receptors (S1PR1–5), of which S1P and FTY720 act as extracellular ligands. S1pr1 and S1pr3 expression were twofold higher after FTY720 treatment with both obesity and aging compared with vehicle and YNG. Only S1pr1 expression was increased threefold by 16 wk of fat feeding compared with young animals (P < 0.05). S1pr2 and S1pr5 were not different between groups or after treatment (Fig. 4D).
Modulation of the S1P Receptor with FTY720 Improves Anabolic Response and Chronic Inflammation in Obese Skeletal Muscle
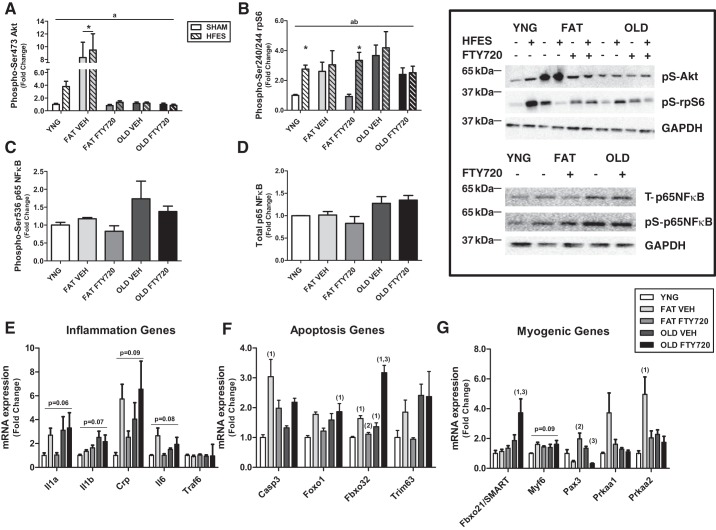
Both obesity and aging are associated with a chronic inflammatory state likely associated with a blunting in the anabolic response of skeletal muscle (1, 15). This is likely through the accumulation of more toxic, bioactive lipid intermediates like ceramide. Therefore, we wanted to determine whether inhibition of ceramide synthesis with FTY720 would reverse obesity and age-associated anabolic blunting. Obese animals had significantly eight- and threefold higher phosphorylation of Akt and rpS6 in sham and after contraction compared with lean, respectively, and were improved with FTY720 treatment (P < 0.01). Akt and rpS6 activation in older animals were unaffected by contraction and FTY720 treatment (Fig. 5, A and B). Total protein expression of NF-κB and the phosphorylation of NF-κB were not different between groups after contraction or with FTY720 treatment (Fig. 5, C and D).
Fig. 5.
Modulation of the sphingosine-1-phosphate (S1P) receptor with S1P analog FTY720 improves anabolic response and chronic inflammation in obese skeletal muscle but not older animals. Relative protein levels of phosphorylated (Phospho-) Akt (A), ribosomal protein S6 (rpS6; B), and NF-κB (C) and total NF-κB (D) were quantified using Western blot analysis. mRNA expression of inflammation (E), apoptosis (F), and myogenic genes (G) was measured by quantitative PCR. aP < 0.05 for main effect for phenotype/treatment, bP < 0.05 for main effect for contraction, *P < 0.05 vs. lean (YNG) SHAM by 2-way ANOVA, n = 8/group; 1P < 0.05 vs. YNG, 2P < 0.05 vs. FAT vehicle (VEH), 3P < 0.05 vs. OLD VEH by 1-way ANOVA, n = 8/group. HFES, high-frequency electrical stimulation; pS, phosphoserine; T, total.
We next measured the expression of key inflammatory, apoptotic, and myogenic regulatory genes. Although there tended to be differences in the expression or inflammatory mRNA targets between groups and after FTY720 treatment, none was changed significantly (Fig. 5E). Obesity increased the expression of apoptotic-associated genes caspase-3 (Casp3) and muscle atrophy F-box (MAFbx; Fbx032) 3- and 1.5-fold (P < 0.05), respectively, and FTY720 treatment reversed these increases (Fig. 5F). Aging significantly increased MAFbx expression 1.2-fold and was increased to 3-fold increase after FTY720 treatment. FTY720 treatment also increased forkhead box O1 (FoxO1) expression 1.9-fold (P < 0.05) in older animals compared with young animals (Fig. 5F). The novel E3-ligase Fbxo21 (specific of muscle atrophy and regulated by transcription, SMART) was 4-fold higher (P < 0.05) in older animals after FTY720 treatment compared with young animals (Fig. 5G). There tended to be differences in Myf6 (P = 0.09) expression between groups (Fig. 5G). Pax3 expression was increased 2-fold in obese animals after FTY720 treatment, although there was no difference in Pax3 expression after only fat feeding. In older animals, there was a 2-fold decrease in Pax3 expression after FTY720 treatment (Fig. 5G). The expression of AMP-activated protein kinase-α2 was increased 5-fold in obesity and was normalized after FTY720 treatment (Fig. 5G).
DISCUSSION
Understanding mechanisms contributing to the induction of skeletal muscle atrophy with aging and obesity is important for determining targets that may attenuate muscle loss in these conditions. We find that aging and obesity equally induce an anabolic resistance to acute skeletal muscle contraction. Furthermore, treatment with the S1P analog FTY720 for 4 wk increased lean mass and strength, and anabolic response to contraction was improved in obese but not older animals. To determine the role of chronic inflammation and differences of saturated (SFA), polyunsaturated, and monounsaturated fatty acids on anabolic resistance in skeletal muscle cells, we overexpressed IKKβ with and without exposure to SFA (PA), polyunsaturated fatty acid (EPA), and monounsaturated fatty acid (OA). We found that IKKβ overexpression increased inflammation in muscle cells and this chronic inflammation exacerbated anabolic resistance in response to SFA. Pretreatment with FTY720 reversed the inflammatory effects of PA in the muscle cells. Taken together, these data demonstrate chronic inflammation can induce anabolic resistance in obesity, SFA aggravates these effects, and FTY720 can reverse this by decreasing ceramide accumulation in skeletal muscle.
We (35–37) and others (5) have observed a reduction in anabolic capacity of skeletal muscle with aging and obesity. These effects are hypothesized to be a result of a low-grade chronic inflammation occurring in each of these states (15). Aging (6), obesity (30), and chronic bed rest or periods of disuse (10), all of which increase inflammation to varying degrees, result in an anabolic resistance to stimuli such as amino acids or insulin. Muscle contraction is an effective stimulus with the profound ability to change muscle phenotype, likely contributing in the ability of particular strength exercise training programs to induce muscle hypertrophy in young, healthy adults (42). We now report that both aging and obesity induce an anabolic resistance to muscle contraction that is likely due to increases in the accumulation of ceramide and other sphingolipids that we observed together in these conditions. The accumulation of the lipotoxic lipid ceramide was associated with increases in proinflammatory signaling activation and a disparate expression profile of genes coding for inflammatory, apoptotic, and myogenic regulators and relevant noncoding miRNA that modulate anabolic pathways after 16 wk of fat feeding or at 18 mo of age. This is consistent with previously published studies that have reported a role for ceramide in diminished anabolic capacity with age and obesity (21, 31, 41). Indeed, Hyde et al. (21) demonstrated in skeletal muscle cells that ceramide is generated after cytokine signaling and via metabolism of saturated fatty acids had profound effects on amino acid transport and protein synthesis. In addition to Hyde et al. (21), our current results provide further evidence of ceramide’s significant role in anabolic resistance in skeletal muscle in obesity and with older age.
When determining the mechanistic effects of fatty acids in vitro, we incubated skeletal muscle cells with three separate fatty acids and/or pretreatment with FTY720. In addition, chronic inflammation is thought to be a crucial regulator of age-induced anabolic resistance and aging processes. We, therefore, overexpressed IKKβ, a regulatory unit of the NF-κB pathway and critical activator of inflammation in the cell, to mimic aging in cells. We show SFA can inhibit skeletal muscle anabolism independent of the inflammatory state of the cell and this can be prevented with FTY720 treatment. This mechanism is likely through the inhibition of NF-κB signaling and decreased ceramide synthesis that has been previously observed (7, 8, 22). In addition, increasing S1P levels has also been observed to improve muscle pathologies by promoting anabolic-mediated muscle regeneration (22). These data and ours provide evidence for therapies that are able to relieve inflammation, decrease ceramide accumulation, and increase S1P levels, which could be beneficial in the prevention of age- and obesity-associated muscle loss.
To test the contribution and mechanistic influence of intramyocellular ceramide accumulation on muscle atrophy in obesity and aging, de novo ceramide synthesis was inhibited with the S1P analog FTY720 for 4 wk. Previous investigations have reported a positive treatment effect on skeletal muscle glucose metabolism with FTY720 administration (12, 23, 29). Our data now show that 4 wk of FTY720 therapy decreased the accumulation of ceramide and other sphingolipids in skeletal muscle of obese and older animals. In obese animals, this decrease in ceramide accretion was associated with lower body mass, greater grip strength, and higher lean mass. However, in older animals, decreases in ceramide were solely associated with higher lean mass, likely a result of losses of fat mass in these animals. These changes corresponded with significant improvements in the anabolic response to contraction in obese animals with no change in older mice. In older animals, our results showed markers of inflammation were upregulated after FTY720 therapy even in light of decreases in sphingolipid accumulation in muscle. These results may be partly explained by and show evidence for an “obesity paradox” that has been previously observed in aging (14). The obesity paradox has shown that overweight, older adults are at an increased risk for cardiovascular diseases but decreased mortality from these same diseases (3, 18, 19). Therefore, our results suggest that decreasing whole body sphingolipids with FTY720 in aged may not be a favorable treatment for the prevention of sarcopenia in this population.
In conclusion, our data show that the anabolic response to muscle contraction is inhibited in both aging and obesity. The anabolic resistance observed in obese and older muscle is associated with increased ceramide accumulation and inflammation. FTY720 therapy decreases ceramide and other sphingolipid species in skeletal muscle of both older and obese mice but only reverses anabolic resistance in obese animals.
GRANTS
This work was supported by National Institute on Aging Grants K01-AG-047247 and 1-P30-AG-031679 to D. A. Rivas. This material is also based on work supported by the USDA under Agreement 58-1950-4-003.
DISCLAIMERS
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.A.R. and R.A.F. conceived and designed research; D.A.R., N.P.R., Y.E., D.J.M., and B.E.C. performed experiments; D.A.R., N.P.R., Y.E., and D.J.M. analyzed data; D.A.R. and R.A.F. interpreted results of experiments; D.A.R. prepared figures; D.A.R., N.P.R., Y.E., and R.A.F. drafted manuscript; D.A.R. and R.A.F. edited and revised manuscript; D.A.R., N.P.R., Y.E., D.J.M., B.E.C., and R.A.F. approved final version of manuscript.
REFERENCES
- 1.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 11: 191–198, 2005. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 2.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 14: 513–537, 2018. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman IM. Obesity paradox during aging. Interdiscip Top Gerontol 37: 20–36, 2010. doi: 10.1159/000319992. [DOI] [PubMed] [Google Scholar]
- 4.Choi S, Snider AJ. Sphingolipids in high fat diet and obesity-related diseases. Mediators Inflamm 2015: 520618, 2015. doi: 10.1155/2015/520618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 6.Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol 8: 1045, 2017. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Larichaudy J, Zufferli A, Serra F, Isidori AM, Naro F, Dessalle K, Desgeorges M, Piraud M, Cheillan D, Vidal H, Lefai E, Némoz G. TNF-α- and tumor-induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet Muscle 2: 2, 2012. doi: 10.1186/2044-5040-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donati C, Cencetti F, Bruni P. Sphingosine 1-phosphate axis: a new leader actor in skeletal muscle biology. Front Physiol 4: 338, 2013. doi: 10.3389/fphys.2013.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol (1985) 104: 1452–1461, 2008. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MJ, Marcus RL, Lastayo PC. Targeting anabolic impairment in response to resistance exercise in older adults with mobility impairments: potential mechanisms and rehabilitation approaches. J Aging Res 2012: 486930, 2012. doi: 10.1155/2012/486930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyckman AJ. Modulators of sphingosine-1-phosphate pathway biology: recent advances of sphingosine-1-phosphate receptor 1 (S1P1) agonists and future perspectives. J Med Chem 60: 5267–5289, 2017. doi: 10.1021/acs.jmedchem.6b01575. [DOI] [PubMed] [Google Scholar]
- 12.Fayyaz S, Japtok L, Kleuser B. Divergent role of sphingosine 1-phosphate on insulin resistance. Cell Physiol Biochem 34: 134–147, 2014. doi: 10.1159/000362990. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15: 505–522, 2018. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 293: 1861–1867, 2005. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 15.Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age-related diseases. Front Immunol 8: 1745, 2017. doi: 10.3389/fimmu.2017.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funai K, Parkington JD, Carambula S, Fielding RA. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 290: R1080–R1086, 2006. doi: 10.1152/ajpregu.00277.2005. [DOI] [PubMed] [Google Scholar]
- 17.Ge X, Cho A, Ciol MA, Pettan-Brewer C, Rabinovitch P, Ladiges W. Grip strength is potentially an early indicator of age-related decline in mice. Pathobiol Aging Age Relat Dis 6: 32981, 2016. [Corrigendum in Pathobiol Aging Age Relat Dis 6: 33718, 2016.] doi: 10.3402/pba.v6.32981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, Satler LF, Lindsay J Jr. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol 39: 578–584, 2002. doi: 10.1016/S0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 19.Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care 36, Suppl 2: S276–S281, 2013. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodson N, McGlory C, Oikawa SY, Jeromson S, Song Z, Rüegg MA, Hamilton DL, Phillips SM, Philp A. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol 313: C604–C611, 2017. doi: 10.1152/ajpcell.00176.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 19: 461–463, 2005. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- 22.Ieronimakis N, Pantoja M, Hays AL, Dosey TL, Qi J, Fischer KA, Hoofnagle AN, Sadilek M, Chamberlain JS, Ruohola-Baker H, Reyes M. Increased sphingosine-1-phosphate improves muscle regeneration in acutely injured mdx mice. Skelet Muscle 3: 20, 2013. doi: 10.1186/2044-5040-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurek K, Mikłosz A, Łukaszuk B, Chabowski A, Górski J, Żendzian-Piotrowska M. Inhibition of ceramide de novo synthesis ameliorates diet induced skeletal muscles insulin resistance. J Diabetes Res 2015: 154762, 2015. doi: 10.1155/2015/154762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen PJ, Tennagels N. On ceramides, other sphingolipids and impaired glucose homeostasis. Mol Metab 3: 252–260, 2014. doi: 10.1016/j.molmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lessard SJ, Rivas DA, Chen ZP, Bonen A, Febbraio MA, Reeder DW, Kemp BE, Yaspelkis BB 3rd, Hawley JA. Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes 56: 1856–1864, 2007. doi: 10.2337/db06-1065. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Graber TG, Ferguson-Stegall L, Thompson LV. Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci 69: 1485–1491, 2014. doi: 10.1093/gerona/glt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macnaughton LS, Wardle SL, Witard OC, McGlory C, Hamilton DL, Jeromson S, Lawrence CE, Wallis GA, Tipton KD. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol Rep 4: e12893, 2016. doi: 10.14814/phy2.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marabita M, Baraldo M, Solagna F, Ceelen JJ, Sartori R, Nolte H, Nemazanyy I, Pyronnet S, Kruger M, Pende M, Blaauw B. S6K1 is required for increasing skeletal muscle force during hypertrophy. Cell Rep 17: 501–513, 2016. doi: 10.1016/j.celrep.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Moon MH, Jeong JK, Lee JH, Park YG, Lee YJ, Seol JW, Park SY. Antiobesity activity of a sphingosine 1-phosphate analogue FTY720 observed in adipocytes and obese mouse model. Exp Mol Med 44: 603–614, 2012. doi: 10.3858/emm.2012.44.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murton AJ, Marimuthu K, Mallinson JE, Selby AL, Smith K, Rennie MJ, Greenhaff PL. Obesity appears to be associated with altered muscle protein synthetic and breakdown responses to increased nutrient delivery in older men, but not reduced muscle mass or contractile function. Diabetes 64: 3160–3171, 2015. doi: 10.2337/db15-0021. [DOI] [PubMed] [Google Scholar]
- 31.Park M, Kaddai V, Ching J, Fridianto KT, Sieli RJ, Sugii S, Summers SA. A role for ceramides, but not sphingomyelins, as antagonists of insulin signaling and mitochondrial metabolism in C2C12 myotubes. J Biol Chem 291: 23978–23988, 2016. doi: 10.1074/jbc.M116.737684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parr EB, Coffey VG, Hawley JA. ‘Sarcobesity’: a metabolic conundrum. Maturitas 74: 109–113, 2013. doi: 10.1016/j.maturitas.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev 11: 671–685, 2010. doi: 10.1111/j.1467-789X.2009.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivas DA, Lessard SJ, Rice NP, Lustgarten MS, So K, Goodyear LJ, Parnell LD, Fielding RA. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J 28: 4133–4147, 2014. doi: 10.1096/fj.14-254490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivas DA, McDonald DJ, Rice NP, Haran PH, Dolnikowski GG, Fielding RA. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am J Physiol Regul Integr Comp Physiol 310: R561–R569, 2016. doi: 10.1152/ajpregu.00198.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivas DA, Morris EP, Haran PH, Pasha EP, Morais MS, Dolnikowski GG, Phillips EM, Fielding RA. Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J Appl Physiol (1985) 113: 1727–1736, 2012. doi: 10.1152/japplphysiol.00412.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivas DA, Yaspelkis BB 3rd, Hawley JA, Lessard SJ. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside. J Endocrinol 202: 441–451, 2009. doi: 10.1677/JOE-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross JS, Russo SB, Chavis GC, Cowart LA. Sphingolipid regulators of cellular dysfunction in Type 2 diabetes mellitus: a systems overview. Clin Lipidol 9: 553–569, 2014. doi: 10.2217/clp.14.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 1: 4, 2011. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Summers SA, Nelson DH. A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing’s syndrome. Diabetes 54: 591–602, 2005. doi: 10.2337/diabetes.54.3.591. [DOI] [PubMed] [Google Scholar]
- 41.Tardif N, Salles J, Guillet C, Tordjman J, Reggio S, Landrier JF, Giraudet C, Patrac V, Bertrand-Michel J, Migne C, Collin ML, Chardigny JM, Boirie Y, Walrand S. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2α activation. Aging Cell 13: 1001–1011, 2014. doi: 10.1111/acel.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tipton KD, Elliott TA, Cree MG, Aarsland AA, Sanford AP, Wolfe RR. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab 292: E71–E76, 2007. doi: 10.1152/ajpendo.00166.2006. [DOI] [PubMed] [Google Scholar]
- 43.Walker DK, Dickinson JM, Timmerman KL, Drummond MJ, Reidy PT, Fry CS, Gundermann DM, Rasmussen BB. Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med Sci Sports Exerc 43: 2249–2258, 2011. doi: 10.1249/MSS.0b013e318223b037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res 31: e154, 2003. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]