Abstract
A murine line haploinsufficient in the cardiac sodium channel has been used to model human Brugada syndrome: a disease causing sudden cardiac death due to lethal ventricular arrhythmias. We explored the effects of cholinergic tone on electrophysiological parameters in wild-type and genetically modified, heterozygous, Scn5a+/− knockout mice. Scn5a+/− ventricular slices showed longer refractory periods than wild-type both at baseline and during isoprenaline challenge. Scn5a+/− hearts also showed lower conduction velocities and increased mean increase in delay than did littermate controls at baseline and blunted responses to isoprenaline challenge. Carbachol exerted limited effects but reversed the effects of isoprenaline with coapplication. Scn5a+/− mice showed a reduction in conduction reserve in that isoprenaline no longer increased conduction velocity, and this was not antagonized by muscarinic agonists.
Keywords: autonomic nervous system, Brugada syndrome, conduction, SCN5A haploinsufficiency
INTRODUCTION
Brugada syndrome is recognized by a triad of right bundle branch block, coved ST elevation in the right precordial leads, and lethal ventricular arrhythmias (6, 25). It may be responsible for up to a fifth of cases of sudden cardiac death in the young (25). Where a mutation is identified, this most commonly occurs in the main cardiac sodium channel isoform SCN5A (4), although in many patients no obvious mutations are found. The main pathophysiological feature is the presence of significant cardiac conduction delays particularly in the right ventricular outflow tract, and these contribute to the ECG pattern and arrhythmic predisposition (15). Furthermore, it is well known that sodium channel density is an important determinant of conduction velocity in the heart (14).
An interesting feature of Brugada syndrome is that ventricular arrhythmia occurs at night when the patient is sleeping, and this can be accompanied by accentuation of the characteristic ECG pattern (21, 23). The autonomic nervous system is well known to modulate ventricular excitability; however, in many other channelopathies it is exercise or stress that precipitates ventricular tachycardia and/or fibrillation and thus this observation is intriguing (9). During rest, vagal activity is predominant in contrast to the situation in exercise where vagal activity is reduced and sympathetic drive predominates, although the detailed picture may be more complex than this standard interpretation (18). In this study, we explore this question in a model of Brugada syndrome, namely the SCN5A-haploinsufficient mouse (Scn5a+/−). This model recapitulates a number of the features of Brugada syndrome seen in patients (11, 12, 19, 24) and provides a route to exploring the autonomic modulation of the electrophysiological substrate.
METHODS
Murine breeding and genotyping.
Mice were maintained in an animal core facility under UK Home Office guidelines relating to animal welfare. All mice were kept in individually ventilated, pathogen-free, temperature-controlled cages (21–23°C) with 12:12-h day-night light cycles. Animals had free access to standard rodent chow and water. Mice of both sexes were studied between 12 and 24 wk of age under standardized conditions for tissue slice analysis. Whole heart studies were performed at between 9 mo and 1 yr of age in mice of both sexes. The generation of the Scn5a+/− heterozygotic mice has been previously described (24). Genotyping was performed at 6 wk of age. Littermate controls were used throughout, and experiments and analysis were performed blinded to genotype (MF, VV). The work was carried out under UK Home Office project licenses PPL-6732 and PPL-7665.
Cardiac excision.
Mice were euthanized by cervical dislocation. The thorax was immediately dissected, and the heart was exposed. Cardioplegia was induced by applying 10 ml of ice-cold, oxygenated (bubbled with 95% O2-5% CO2) Ca2+-free Krebs solution (in mM: 119 NaCl, 4 KCl, MgCl2, 1.2 KH2PO4, 25 NaHCO3, 10 glucose, 2 Na pyruvate; pH 7.4) directly onto the epicardial surface. The heart and great vessels were removed within 20 s and placed into a dissection dish containing oxygenated ice-cold Ca2+-free Krebs solution. A 23-gauge cannula was inserted into the aorta and secured under light microscopy. For tissue slicing, cold retrograde perfusion was commenced with oxygenated, ice-cold, Ca2+-free Krebs solution at a flow rate of 16.5 ml/min. Whole heart recording perfusion used oxygenated Krebs solution (containing 1.4 mM Ca2+) at room temperature. The time taken from cervical dislocation to establishment of Langendorff perfusion was <3 min.
Preparation of cardiac tissue slices.
After perfusion for 2 min, the perfusate was altered to an ice-cold, oxygenated Ca2+-free Krebs solution containing high K+ (20 mM KCl) and blebbistatin (20 µM; Cambridge Bioscience, Cambs, UK) for 3 min. The hearts were then removed from the Langendorff perfusion rig and placed again in a dissection dish containing cold perfusate. Ventricular tissue was dissected from atrial tissue under light microscopy. The dissected ventricles were then embedded in low-melt agarose (4% low-melting point agarose in Ca2+-free Krebs solution) and then rapidly chilled on ice. The agarose block was oriented and fixed onto a magnetic stage using cyanoacrylate glue and placed in the cutting chamber of a Vibratome (Campden Instruments, London, UK). This chamber was maintained at 4°C using external ice. It was filled with cold, modified oxygenated Krebs solution (containing 0.6 mM Ca2+, 10 mM K+). Tissue sections were obtained from apex to base at an interval thickness of 200 µm. The vibrating PTFE-coated steel blade (Wilkinson Sword, Bucks, UK) was advanced at <200 µm/s. The x-axis vibration was applied at an amplitude of 2 mm and frequency of 80 Hz. The z-axis deviation was calibrated before every use to be <1 µm. The cut sections were immediately placed in oxygenated low-Ca2+ Krebs solution (4 mM KCl, 0.6 mM Ca2+) containing 10 mM blebbistatin maintained at room temperature. After 25 min, the samples were transferred to standard oxygenated Krebs solution (4 mM KCl, 1.1 mM Ca2+) and kept at room temperature until electrophysiological studies were performed.
Cardiac slice electrophysiological studies.
Stimulation was generated by a stimulus isolation unit (DS-2A, Warner Instruments) with signal timing driven by an Arduino Uno microcontroller (Arduino, Italy). Stimulation was applied using a silver chloride bipolar electrode. The sections were placed in the recording chamber and carefully positioned manually so that left ventricular tissue overlaid the measurement electrodes. A 1-cm-diameter metal ring with an overlying nylon mesh (Harp-slice grid, Micro Control Instruments, East Sussex, UK) was used to hold the tissue flat in place on the electrode grid and ensured adequate tissue electrode contact. The recording chamber was mounted in the head stage, and perfusion commenced at 2 ml/min. Tissue was allowed to settle to a steady state over 1 min before electrode placement. The bipolar stimulating electrode was carefully lowered to just contact the tissue slice on the left ventricular tissue, but not to move the slice on the array. Stimulation was started at a frequency of 5 Hz using a monophasic 1-ms duration pulse for real-time recording of electrical activity. The stimulus voltage was increased until electrical capture was achieved. The stimulus voltage was then reduced until electrical capture was lost: the lowest voltage stimulus that could reliably achieve capture was then taken as the stimulus threshold. Cardiac signals were recorded at a sampling frequency of 10 kHz. A simulation protocol was performed with steady-state pacing at an interstimulus interval of 200 ms, with stimulation during recording applied at an amplitude of twice threshold. Around 30 s of pacing activity were recorded for each state for each slice. A perfusate solution containing drugs was washed in over 30 s at 20 ml/min. Slices were then stimulated at 5 Hz for 2 min before threshold was determined and recording commenced.
Ex vivo whole heart recordings.
For whole heart recordings, hearts were retrogradely perfused at 16.5 ml/min with normal oxygenated Krebs solution (1.4 mM Ca2+). A unipolar silver chloride stimulation electrode and flexible 32-pole multielectrode array (MEA; FlexMEA, Multielectrode Systems) were placed on the ventricular epicardium, and an S1S2 decremental stimulation protocol was performed to determine the ventricular effective refractory period (VERP). Stimulation was performed with a biphasic pulse of amplitude 2 V and duration of 0.5 ms, with S1S2 intervals reduced from 150 ms by decrements of 5–100 ms and thereafter by decrements of 2 ms until tissue refractoriness was reached. Arrhythmogenicity was further tested for by applying stimulating trains of 100 beats at coupling intervals progressively reduced from 100 ms. Ventricular tachycardia was defined as a ventricular arrhythmia persisting more than 2 s.
Analysis of electrograms.
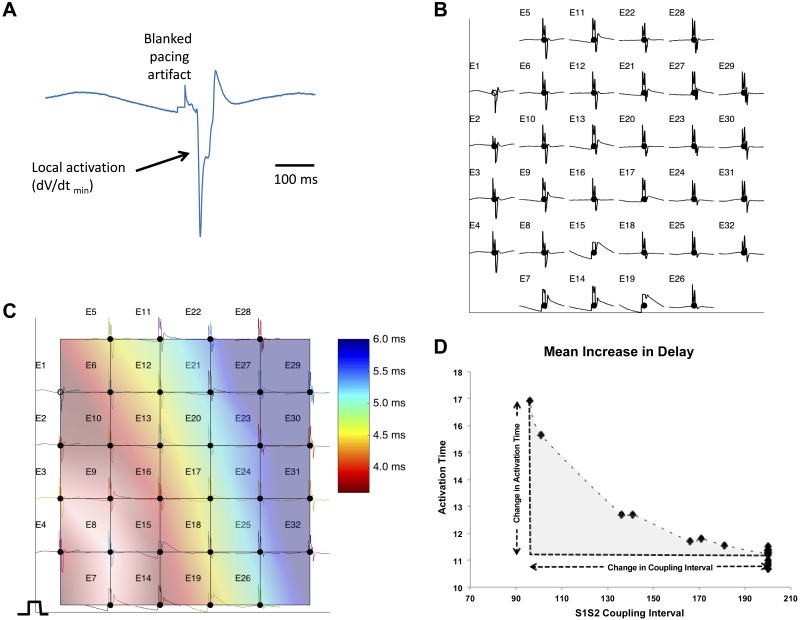
All analysis of murine electrophysiology was performed using custom software running in Matlab, v2014b (Mathworks). The time point of local activation was taken at the steepest negative gradient of the unipolar electrogram. Conduction velocities were determined using a gradient method, with conduction velocity defined as the inverse of the gradient in activation times across the array (Fig. 1, A–C). Electrodes with significant noise were excluded, and all electrograms and time points were checked manually. The mean increase in delay (MID), a well-validated measure of inducibility of conduction delay, was calculated by determining the area under the conduction-delay curve (15) and according to the equation
where S1 CI is the coupling interval of steady-state pacing (S1), and minS2 CI is the minimum coupling interval above ERP. The integral was calculated using the trapz function in Matlab.
Fig. 1.
Measurement of conduction velocity and mean increase in delay from multielectrode array. A: example electrogram from a single electrode of a multielectrode array. The local activation time is taken as the dV/dt min (arrow); during calculation, the pacing artifact is blanked. B: each electrogram and activation time are assigned to a 2D coordinate on a grid reflecting the geometry of the multielectrode array. Local activation time is determined for all the electrodes on the grid. Electrode numbers are given as E1, E2, etc. C: the gradient of activation times is then determined by interpolation (shown as a colormap). The inverse of this “activation time gradient” can be determined to give the conduction velocity. The median conduction velocity over the multielectrode array was used for measurement. D: determination of mean increase in delay. The activation time is plotted for a series of S1S2 coupling intervals. The mean change in activation time over this interval (the mean increase in delay) is calculated by determining the area of the activation time change with coupling interval change (shaded area). This area is divided by the change in coupling interval to give the unit mean increase in delay. The mean increase in delay allows the susceptibility to conduction slowing with coupling interval changes to be compared.
The mean timing of the activation time of all recording electrodes was used for each measurement of conduction delay, and the MID was defined as the unit increase in conduction delay per unit reduction in S1S2 coupling interval (ms/ms). Stimulation protocols were performed in normal Krebs solution in baseline (control) conditions or with 100 nM isoprenaline and/or 10 µM carbachol.
Immunofluorescence of cardiac sections.
Mouse hearts were rinsed in PBS and cut longitudinally with a blade and tissue holder. The hearts were fixed in 10% formalin (Sigma) for at least 24 h followed by two PBS washes and stored in 70% ethanol before paraffin embedding. Paraffin-embedded hearts were cut to 5-μm-thick sections and mounted on Superfrost plus microscope slides. Sections were then deparaffinized with xylene and rehydrated with four ethanol washes (from 100 to 50%) before heat-mediated antigen retrieval with citrate buffer (pH 6.0). Following antigen retrieval, sections were washed several times in PBS, permeabilized with 0.1% Triton X-100 for 15 min, and blocked with 5% goat serum in PBS for 1 h at room temperature. Sections were then incubated with primary antibodies with 1% goat serum overnight at 4°C: mouse monoclonal Cx43 clone 4E6.2 (MAB3067, Millipore) and rabbit polyclonal N-cadherin antibody (SC-7939, Santa Cruz). The sections were incubated with fluorescently labeled secondary antibodies Alexa Fluor goat anti-mouse 488 and goat anti-rabbit 555 secondary antibodies (Invitrogen) for 1 h at room temperature in the dark. Sections were washed several times with PBS and costained with 4′,6-diamidino-2-phenylindole (DAPI; nuclear stain) and stored in the dark until further analysis. The samples were analyzed using confocal microscopy (LSM 510, Carl Zeiss with DAPI: excitation 405 nm, emission LP420; FITC: excitation 488 nm, emission BP 505–550; and Cy3: excitation 543 nm, emission LP 560. Images were acquired sequentially. Quantitative analysis of the images was performed using ImageJ. Thresholding was applied to the images, and they were then converted to binary files. The Cx43-stained image was subtracted by the N-cadherin-stained image and represented “Cx43 not located at the intercalated disk.
Statistical analysis.
All statistical analysis was performed using R software [The Comprehensive R Archive Network (CRAN)]. Continuous parametric data are presented as means ± standard deviations (SD) or, in the case of significance derived from regression models, mean with (95% confidence interval), unless otherwise specified. Comparisons in which a single measurement was taken for each subject, e.g., VERP, dispersion of repolarization time, were made using Student’s t-test with post hoc correction for multiple comparisons. Continuous parametric data derived from electrogram data were modeled using mixed-effects linear regression [software: Linear and Nonlinear Mixed Effects (NLME) package running in R version 2.14], and statistical significance was inferred from the model. Quartile regression with bootstrapping [Quantile Regression Description Estimation and inference (QUANTREG) package] was used to compare nonparametric continuous data. Statistical significance is indicated by *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
There are conflicting reports of the effects of autonomic modulation on electrophysiological parameters in ventricular tissue such as conduction velocity (7). We accordingly investigated the effects of autonomic challenge on indices representing activation and recovery in littermate normal murine hearts and Scn5a-haploinsufficient heterozygous (Scn5a+/−) mice. We used two approaches to examine tissue-level electrophysiology: tissue slices from juvenile animals (aged 2–3 mo) placed on a MEA system and ex vivo Langendorff perfused hearts from mature animals (aged 9 mo to 1 yr) studied using an externally applied electrode array. Previous studies in older Langendorff-perfused hearts had associated arrhythmic phenotypes with the Scn5a+/− genotype, but we were unable to obtain viable tissue slices from older animals for multielectrode array studies. We applied isoprenaline as a β-adrenoreceptor activator to mimic sympathetic activation and the muscarinic receptor agonist carbachol to approximate vagal activation.
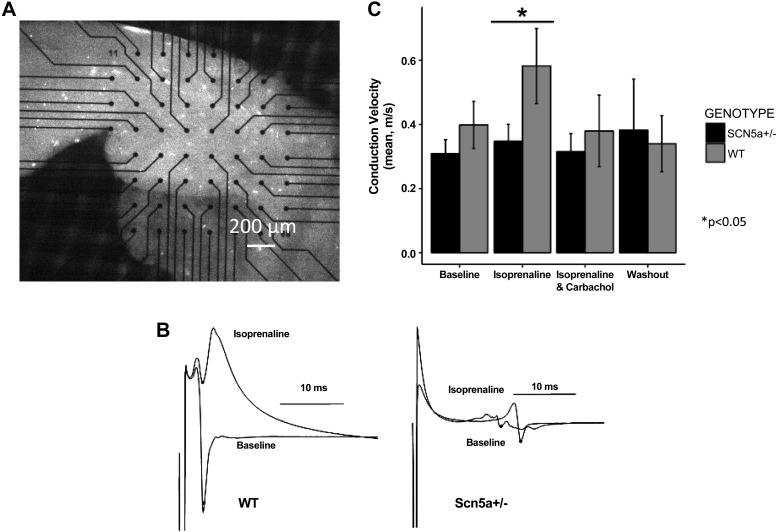
Ventricular slices obtained from juvenile Scn5a+/− mice required higher stimulus voltages than did wild-type (WT; 4.0 ± 0.7 vs. 2.7 ± 0.4 V at baseline, P < 0.01) for consistent capture both before and following all the different pharmacological manipulations. Untreated slices from Scn5a+/− hearts showed a trend toward lower conduction velocities than WT hearts (0.31 ± 0.04 vs. 0.40 ± 0.7 m/s), but this was not statistically significant. Isoprenaline (100 nM) challenge increased conduction velocity in slices from WT (0.58 ± 0.11 m/s) but not in Scn5a+/− heart slices (0.34 ± 0.05 m/s, P < 0.05). The increase in conduction velocity was reversed with carbachol coapplication (Fig. 2). Tissue slices from Scn5a+/− hearts showed consistently longer effective refractory periods both before (means ± SE: Scn5a+/− 79 ± 4 vs. WT 63 ± 4 ms, P < 0.001) and during isoprenaline challenge (73 ± 7 vs. 52 ± 7 ms). Carbachol markedly shortened the VERP in slices from WT but not Scn5a+/− hearts (Scn5a+/−: 76 ± 7 ms, WT: 41 ± 5 ms, P < 0.01).
Fig. 2.
Murine tissue slice conduction velocity (CV) measurements. A: light microscope image of ventricular slice section on multielectrode array. B: example electrograms acquired from wild-type (WT) and haploinsufficient (Scn5a+/−) mice hearts under baseline and isoprenaline challenge. C: effects of isoprenaline and the ACh agonist carbachol in the 2 murine groups. A significant increase in CV is seen in response to isoprenaline, which is absent in the Scn5a+/− mice. This effect is abolished by carbachol. Asterisks indicate statistical significances from comparisons between Scn5a+/− and WT (*P < 0.05).
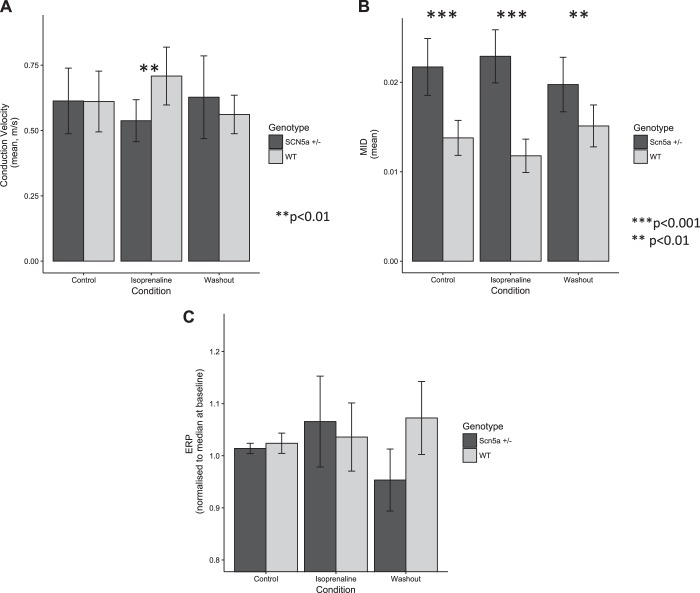
In Langendorff-perfused isolated hearts, S1S2 decremental protocols were attempted before pharmacological challenge and in the presence of 100 nM isoprenaline and/or 10 µM carbachol. However, pacing capture became inconsistent during carbachol administration in six of eight Scn5a+/− hearts despite attempts at increasing stimulation amplitude. Data from Langendorff-perfused Scn5a+/− hearts were therefore formally analyzed for isoprenaline effects only (n = 11) and were compared with littermate WT hearts (n = 10). Washout (5 min) was performed between drug challenges. Scn5a+/− hearts showed blunted responses to isoprenaline, mimicking the data from the cardiac tissue slice preparations (Fig. 3). Notably, a 16% increase in conduction velocity observed in WT hearts in response to isoprenaline was again blunted in Scn5a+/− hearts (normalized conduction velocity control vs. Scn5a−/− response to isoprenaline, P < 0.01), which exhibited a marginal decrease in conduction velocity. No consistent changes in electrophysiological parameters were observed during carbachol administration in the WT group.
Fig. 3.
Electrophysiological properties of Langendorff-perfused hearts from Scn5a+/− and WT mice under isoprenaline challenge. A: conduction velocity dynamics mirrored those seen in tissue slice preparations, with paradoxical responses to isoprenaline challenge in the heterozygous mouse hearts. B: the mean increase in delay (MID) of Scn5a+/− hearts was markedly greater than those of WT animals. C: unlike tissue slices, only marginal differences in effective refractory period (ERP) were observed in whole heart preparations. Asterisks indicate statistical significances from comparisons between Scn5a+/− and WT (**P < 0.01, ***P < 0.001).
Induced conduction delay in response to premature extrastimuli was also investigated. The mean increase in delay was almost double in Scn5a+/− hearts compared with WT controls (P < 0.001). Controls and Scn5a+/− murine hearts studied through these procedures did not show differences in arrhythmia inducibility under control conditions, or 100 nM isoprenaline or 10 µM carbachol challenge (arrhythmic phenomena shown in 1/7 hearts in both drug challenges, with no sustained arrhythmias in either Scn5a −/+ or controls). Little consistent change in VERP with drug challenge was observed in whole heart preparations (Fig. 3C).
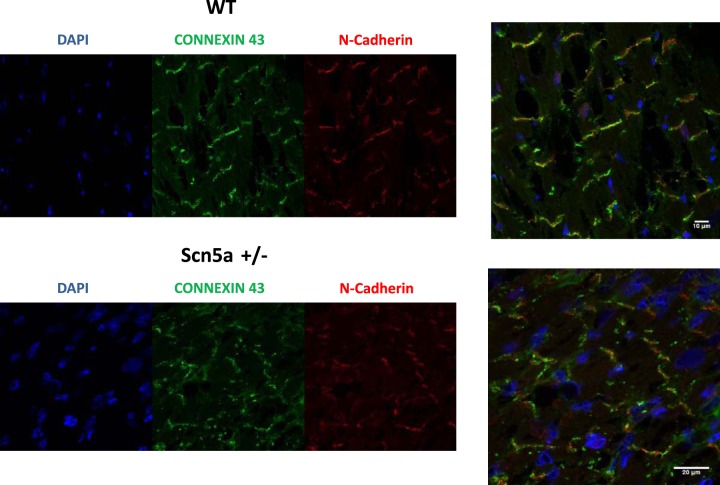
Finally, we investigated a possible relationship between haploinsufficiency of Scn5a and the localization and expression of Cx43 in the juvenile animals. The expression of Cx43 was not grossly changed, but there was an impression of localization away from the intercalated disk (marked by N-cadherin staining) and into the cytosol (Fig. 4). We confirmed this by quantifying the redistribution as detailed in methods. We expressed the localization as a ratio of the amount of Cx43 not at the intercalated disk divided by the total expression of Cx43. In WT mice, this number was 53.7 ± 3.9% and in Scn5a+/− mice 71.6 ± 2.7% (n = 5 both groups, P < 0.01).
Fig. 4.
Localization of Cx43 in Scn5a+/− and WT mice using laser scanning confocal microscopy. Representative heart sections are shown, with N-cadherin staining marking the intercalated disk and 4′,6-diamidino-2-phenylindole (DAPI) the nucleus. Cx43 is expressed and present at the intercalated disk; however, there is an impression of mislocalization of Cx43 away from the intercalated disk. This is confirmed using a numerical approach as detailed in the text. The sections are representative of a number of sections from a single mouse, and the experiment was repeated in an additional littermate control and Scn5a+/− mouse (n = 5 total sections in each group).
DISCUSSION
In this study, we have investigated the tissue-level electrophysiological properties of ventricles from Scn5a haploinsufficient mice and compared these with littermate controls. Our main finding is that there is an impairment of the response of conduction velocity to autonomic challenge in Scn5a+/− mice. In hearts from control mice, isoproterenol increased conduction velocity and decreased mean propagation delays. Scn5a+/− hearts demonstrated a reduced response of conduction velocity with increased mean propagation delays in response to isoprenaline. This effect was reversed by further challenge with carbachol, a muscarinic agonist, although carbachol alone did not have prominent effects. This is consistent with previous reports that sympathetic activation increases conduction velocity in normal hearts (2, 7). In heart slice preparations in juvenile animals, ERP was prolonged in Scn5a+/− hearts while in older animals there was no significant difference when studied in Langendorff-perfused intact hearts.
The findings in murine hearts may correlate with previous reports that β-adrenoreceptor activation increases sodium currents and therefore action potential conduction velocity through a protein kinase A-dependent mechanism (20); reduced effects of isoprenaline could then be associated with the Na+ channel haploinsufficiency in Scn5a+/− hearts. There is also the possibility that signaling pathways downstream of the β-adrenergic signaling pathway may also modulate gap junction density at the intercalated disk (7, 16). We also saw a potential mislocalization of Cx43 away from the intercalated disk in sodium channel haploinsufficient mice, and this may contribute to the conduction slowing. It is plausible that some form of interaction with scaffolding or other proteins may be responsible for maintaining stoichiometry of Cx43 and Scn5a at the intercalated disk. A number of interacting sodium channel proteins are known, and Cx43 can influence the trafficking of Scn5a (1, 27). This is a topic for investigation in future studies. We did not see prominent responses to carbachol in tissue-level electrophysiological parameters after washout of isoprenaline. However, combined application of both isoprenaline and carbachol in WT tissue slices reversed the effect of isoprenaline on conduction velocity. This likely involves receptor activation of M2 muscarinic receptors coupled to inhibitory G proteins that directly antagonize the response at the level of adenylate cyclase (10). The Gi2 isoform seems to be central to muscarinic receptor signaling: L-type calcium channel modulation in ventricular myocytes is known to be abolished in Gi2 knockout mice (8). The slow conduction may be proarrhythmic through promotion of reentry and wavebreak. Importantly, the increases in MID observed in Scn5a+/− hearts provide evidence of the propensity for this group to have greater induced (rather than fixed) conduction velocity slowing. The implication therefore is that the consistency of conduction may become destablized when challenged by premature extrastimuli in the context of autonomic modulation, while in the steady-state or resting condition these hearts exhibit conduction velocity dynamics similar to those in WT littermates.
The marked differences in baseline conduction velocities between the tissue slice preparations and the whole heart Langendorff preparations may result from the involved tissue preparation techniques required to obtain viable slices. Even limited tissue disruptions that may have occurred during slice preparation could lead to reductions in cell-to-cell coupling, and thus accentuate preexisting conduction delays.
In understanding the human Brugada syndrome, two main hypotheses have been advanced (3, 15, 22): either that conduction is slowed and thus activation delayed or that repolarization occurs prematurely. More specifically, coved ST segment elevation in ECG leads V1–V3, often taken as the hallmark of the syndrome, equates to either delayed depolarization from the right ventricular body to the right ventricular outflow tract or a shortened action potential in the epicardium, leading to repolarization gradients across the right ventricular wall. The present findings in this mouse model show that autonomic activation has the potential to significantly modulate conduction delays, particularly in the context of preexisting conduction deficiency. Our major finding is that adrenergic increases in conduction velocity are impaired in the Scn5a+/− mouse. This process in the normal murine heart is antagonized by muscarinic receptor activation but lost in the sodium channel haploinsufficient mouse. In the intact animal, there will be a degree of autonomic balance and even at rest vagal tone will be modified by a degree of sympathetic drive. The absolute slowing may then be greatest when high vagal tone is combined with cardiac sodium channel haploinsufficiency. We have recently completed a study in humans examining the effects of edrophonium on endocardial and epicardial right ventricular electrophysiology (5). We demonstrated that edrophonium appears to modulate both conduction and repolarization in patients with Brugada syndrome, particularly delaying activation and repolarization in the right ventricular epicardium, in line with these presented results.
Although the use of isolated tissue from transgenic mice permits study of tissue-level phenomena, this approach does have limitations which are reflected in this study. The relatively small anatomy and thin ventricular walls did not permit detailed examination of differential epicardial and endocardial characteristics or selective studies of the right ventricular outflow tract. However, with some adaptation of the array technology this may be possible in the future. In a previous study, only a trend to an increase in conduction velocity with isoprenaline was observed which did not reach statistical significance (17). Variations in animal lines may explain such variance. Sodium channel mutations leading to phenotypic disease can occur in families where Brugada syndrome is inherited in an autosomal dominant fashion. In general, these mutations lead to a loss of sodium channel function and the disease is generated by SCN5A haploinsufficiency (13, 26). However, in the majority of patients no mutation is obvious, the disease occurs sporadically and may have a different pathogenesis (13, 26). Caution must therefore be exercised in applying the results of animal studies to this human syndrome.
In conclusion, we have examined the influence of autonomic regulation on tissue-level cardiac electrophysiology in a mouse model of Brugada syndrome. Haploinsufficiency of Scn5a leads to impairment of conduction velocity reserve with blunting of sympathetically mediated increases and reversal by muscarinic receptor activation compared with littermate control mice.
GRANTS
J. Bhar-Amato was funded by a Heart Research UK Fellowship; M. Orini is funded by a Marie Curie Fellowship; D. Santos is funded by an MRC CASE Studentship; P. D. Lambiase is supported by the University College London Hospitals Biomedicine National Institute of Health Research (NIHR), Barts Biomedical Research Centre, and the Stephen Lyness Memorial Fund; and C. L.-H. Huang is funded by the British Heart Foundation. A. Tinker is supported by the British Heart Foundation (RG/15/15/31742). A. Tinker and M. Finlay were facilitated in this project by the NIHR Biomedical Research Centre at Barts.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.F., J.B.-A., K.-E.N., D.S., M.O., V.V., P.T., A.A.G., C.L.-H.H., P.D.L., and A.T. conceived and designed research; M.F., J.B.-A., K.-E.N., V.V., P.T., and A.A.G. performed experiments; M.F., J.B.-A., K.-E.N., M.O., V.V., P.T., and A.A.G. analyzed data; M.F., J.B.-A., V.V., P.T., A.A.G., C.L.-H.H., P.D.L., and A.T. interpreted results of experiments; M.F., J.B.-A., and K.-E.N. prepared figures; M.F., J.B.-A., D.S., M.O., V.V., P.T., A.A.G., C.L.-H.H., P.D.L., and A.T. drafted manuscript; M.F., J.B.-A., A.A.G., C.L.-H.H., P.D.L., and A.T. edited and revised manuscript; M.F., J.B.-A., K.-E.N., D.S., M.O., V.V., P.T., A.A.G., C.L.-H.H., P.D.L., and A.T. approved final version of manuscript.
REFERENCES
- 1.Agullo-Pascual E, Lin X, Leo-Macias A, Zhang M, Liang FX, Li Z, Pfenniger A, Lübkemeier I, Keegan S, Fenyö D, Willecke K, Rothenberg E, Delmar M. Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and NaV1.5 localization at the intercalated disc. Cardiovasc Res 104: 371–381, 2014. doi: 10.1093/cvr/cvu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajijola OA, Lux RL, Khahera A, Kwon O, Aliotta E, Ennis DB, Fishbein MC, Ardell JL, Shivkumar K. Sympathetic modulation of electrical activation in normal and infarcted myocardium: implications for arrhythmogenesis. Am J Physiol Heart Circ Physiol 312: H608–H621, 2017. doi: 10.1152/ajpheart.00575.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antzelevitch C. Genetic, molecular and cellular mechanisms underlying the J wave syndromes. Circ J 76: 1054–1065, 2012. doi: 10.1253/circj.CJ-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezzina CR, Lahrouchi N, Priori SG. Genetics of sudden cardiac death. Circ Res 116: 1919–1936, 2015. doi: 10.1161/CIRCRESAHA.116.304030. [DOI] [PubMed] [Google Scholar]
- 5.Bhar-Amato J, Finlay M, Santos D, Orini M, Chaubey S, Vyas V, Taggart P, Grace AA, Huang CL, Ben Simon R, Tinker A, Lambiase PD. Pharmacological modulation of right ventricular endocardial-epicardial gradients in Brugada syndrome. Circ Arrhythm Electrophysiol 11: e006330, 2018. doi: 10.1161/CIRCEP.118.006330. [DOI] [PubMed] [Google Scholar]
- 6.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: A multicenter report. J Am Coll Cardiol 20: 1391–1396, 1992. doi: 10.1016/0735-1097(92)90253-J. [DOI] [PubMed] [Google Scholar]
- 7.Campbell AS, Johnstone SR, Baillie GS, Smith G. β-Adrenergic modulation of myocardial conduction velocity: connexins vs. sodium current. J Mol Cell Cardiol 77: 147–154, 2014. doi: 10.1016/j.yjmcc.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Spicher K, Jiang M, Birnbaumer L, Wetzel GT. Lack of muscarinic regulation of Ca2+ channels in Gi2α gene knockout mouse hearts. Am J Physiol Heart Circ Physiol 280: H1989–H1995, 2001. doi: 10.1152/ajpheart.2001.280.5.H1989. [DOI] [PubMed] [Google Scholar]
- 9.Finlay M, Harmer SC, Tinker A. The control of cardiac ventricular excitability by autonomic pathways. Pharmacol Ther 174: 97–111, 2017. doi: 10.1016/j.pharmthera.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Gomeza J, Zhang L, Kostenis E, Felder CC, Bymaster FP, Brodkin J, Shannon H, Xia B, Duttaroy A, Deng CX, Wess J. Generation and pharmacological analysis of M2 and M4 muscarinic receptor knockout mice. Life Sci 68: 2457–2466, 2001. doi: 10.1016/S0024-3205(01)01039-6. [DOI] [PubMed] [Google Scholar]
- 11.Jeevaratnam K, Guzadhur L, Goh YM, Grace AA, Huang CL. Sodium channel haploinsufficiency and structural change in ventricular arrhythmogenesis. Acta Physiol (Oxf) 216: 186–202, 2016. doi: 10.1111/apha.12577. [DOI] [PubMed] [Google Scholar]
- 12.Jeevaratnam K, Poh Tee S, Zhang Y, Rewbury R, Guzadhur L, Duehmke R, Grace AA, Lei M, Huang CL. Delayed conduction and its implications in murine Scn5a(+/-) hearts: independent and interacting effects of genotype, age, and sex. Pflügers Arch 461: 29–44, 2011. doi: 10.1007/s00424-010-0906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto Y, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze-Bahr E, Antzelevitch C, Salisbury BA, Guicheney P, Wilde AA, Brugada R, Schott JJ, Ackerman MJ. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm 7: 33–46, 2010. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King JH, Huang CL, Fraser JA. Determinants of myocardial conduction velocity: implications for arrhythmogenesis. Front Physiol 4: 154, 2013. doi: 10.3389/fphys.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambiase PD, Ahmed AK, Ciaccio EJ, Brugada R, Lizotte E, Chaubey S, Ben-Simon R, Chow AW, Lowe MD, McKenna WJ. High-density substrate mapping in Brugada syndrome: combined role of conduction and repolarization heterogeneities in arrhythmogenesis. Circulation 120: 106–117, 2009. doi: 10.1161/CIRCULATIONAHA.108.771401. [DOI] [PubMed] [Google Scholar]
- 16.Lambiase PD, Tinker A. Connexins in the heart. Cell Tissue Res 360: 675–684, 2015. doi: 10.1007/s00441-014-2020-8. [DOI] [PubMed] [Google Scholar]
- 17.Lane JD, Montaigne D, Tinker A. Tissue-level cardiac electrophysiology studied in murine myocardium using a microelectrode array: autonomic and thermal modulation. J Membr Biol 250: 471–481, 2017. doi: 10.1007/s00232-017-9973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machhada A, Trapp S, Marina N, Stephens RCM, Whittle J, Lythgoe MF, Kasparov S, Ackland GL, Gourine AV. Vagal determinants of exercise capacity. Nat Commun 8: 15097, 2017. doi: 10.1038/ncomms15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin CA, Siedlecka U, Kemmerich K, Lawrence J, Cartledge J, Guzadhur L, Brice N, Grace AA, Schwiening C, Terracciano CM, Huang CL. Reduced Na+ and higher K+ channel expression and function contribute to right ventricular origin of arrhythmias in Scn5a+/- mice. Open Biol 2: 120072, 2012. doi: 10.1098/rsob.120072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda JJ, Lee H, Shibata EF. Enhancement of rabbit cardiac sodium channels by beta-adrenergic stimulation. Circ Res 70: 199–207, 1992. doi: 10.1161/01.RES.70.1.199. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo K, Kurita T, Inagaki M, Kakishita M, Aihara N, Shimizu W, Taguchi A, Suyama K, Kamakura S, Shimomura K. The circadian pattern of the development of ventricular fibrillation in patients with Brugada syndrome. Eur Heart J 20: 465–470, 1999. doi: 10.1053/euhj.1998.1332. [DOI] [PubMed] [Google Scholar]
- 22.Meregalli PG, Wilde AA, Tan HL. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc Res 67: 367–378, 2005. doi: 10.1016/j.cardiores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Mizumaki K, Fujiki A, Tsuneda T, Sakabe M, Nishida K, Sugao M, Inoue H. Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada syndrome. J Cardiovasc Electrophysiol 15: 667–673, 2004. doi: 10.1046/j.1540-8167.2004.03601.x. [DOI] [PubMed] [Google Scholar]
- 24.Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, Colledge WH, Grace AA. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci USA 99: 6210–6215, 2002. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C, Ackerman M, Belhassen B, Estes NA III, Fatkin D, Kalman J, Kaufman E, Kirchhof P, Schulze-Bahr E, Wolpert C, Vohra J, Refaat M, Etheridge SP, Campbell RM, Martin ET, Quek SC; Document Reviewers; Heart Rhythm Society; European Heart Rhythm Association; Asia Pacific Heart Rhythm Society . Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 15: 1389–1406, 2013. doi: 10.1093/europace/eut272. [DOI] [PubMed] [Google Scholar]
- 26.Sarquella-Brugada G, Campuzano O, Arbelo E, Brugada J, Brugada R. Brugada syndrome: clinical and genetic findings. Genet Med 18: 3–12, 2016. doi: 10.1038/gim.2015.35. [DOI] [PubMed] [Google Scholar]
- 27.Shy D, Gillet L, Abriel H. Cardiac sodium channel NaV1.5 distribution in myocytes via interacting proteins: the multiple pool model. Biochim Biophys Acta 1833: 886–894, 2013. doi: 10.1016/j.bbamcr.2012.10.026. [DOI] [PubMed] [Google Scholar]