Abstract
The transient receptor potential mucolipin 1 (TRPML1) channel has been reported to mediate lysosomal Ca2+ release that is involved in Ca2+-dependent lysosome trafficking and autophagic flux. However, this regulatory mechanism of lysosomal TRPML1 channel activity in podocytes remains poorly understood. In the present study, we tested whether the TRPML1 channel in podocytes mediates lysosome trafficking, which is essential for multivesicular body (MVB) degradation by lysosomes. We first demonstrated the abundant expression of TRPML1 channel in podocytes. By GCaMP3 Ca2+ imaging, we characterized the lysosomal specificity of TRPML1 channel-mediated Ca2+ release in podocytes. Given the important role of acid ceramidase (AC) in lysosome function and podocyte injury, we tested whether AC regulates this TRPML1 channel-mediated Ca2+ release and consequent lysosome-dependent MVB degradation in podocytes. Pharmacologically, it was found that TRPML1 channel activity was remarkably attenuated by the AC inhibitor carmofur. Sphingosine, as an AC product, was demonstrated to induce TRPML1-mediated Ca2+ release, which was inhibited by a TRPML1 blocker, verapamil. Using a Port-a-Patch planar patch-clamp system, we found that AC-associated sphingolipids, sphingomyelin, ceramide, and sphingosine had different effects on TRPML1 channel activity in podocytes. Functionally, the inhibition of AC or blockade of TRPML1 channels was found to suppress the interaction of lysosomes and MVBs, leading to increased exosome release from podocytes. These results suggest that AC is critical for TRPML1 channel-mediated Ca2+ release, which controls lysosome-MVB interaction and exosome release in podocytes.
Keywords: acid ceramidase, exosome, lysosome, podocyte, sphingolipids, transient receptor potential channels
INTRODUCTION
It is well known that podocyte dysfunction and depletion may lead to podocytopathy. However, it remains poorly understood how podocytopathy occurs under different pathological conditions. In particular, it remains unclear how podocytes as a terminally differentiated cell type maintain their normal function and respond to different pathological challenges. In this regard, previous studies have highlighted the particular importance of lysosome function as a key homeostatic mechanism not only under physiological but also under stress conditions (12, 18, 26). With their intracellular digestion function, lysosomes serve as the major degradative compartments for endocytic, phagocytic, and autophagic materials targeted for destruction (16, 34, 36). Beyond intracellular digestion, lysosomes may also importantly participate in the physiological regulation of cell functions or activities in many tissues or cells (4, 15, 45), including podocytes (28, 33, 48). It has been found that deletion of lysosome-associated genes results in podocyte degeneration, foot process effacement, and depletion leading to focal segmental glomerular sclerosis (FSGS) or global glomerulosclerosis (8, 58). Recently, lysosome function has also been implicated in the regulation of the multivesicular body (MVB) fate that determines the excretion of exosomes, one of the extracellular vesicles (EVs) (23). These EVs have been known as a regulator of cell-to-cell communications or signaling. Increases in exosome release have been shown to produce podocyte injury and ultimate podocytopathy (24, 56). According to these previous findings, the lysosome dysfunction-induced exosome release may be a mechanism leading to podocyte injury and glomerular damage under pathological conditions.
Based on previous studies, the fusion of MVBs and lysosomes is essential for the degradation of MVB by lysosome (20, 23). This fusion process may be dependent on the regular lysosome trafficking, which is mediated by lysosomal Ca2+ release (45, 59). Under the resting condition, Ca2+ enters lysosomal compartment by H+/Ca2+ exchange and is released through transient receptor potential-mucolipin-1 (TRPML1) channels in response to endogenously produced NAADP (11, 21, 64, 66) or other factors like PIPs [PI(3,5)P2] and ions (14, 15, 31). The mucolipin family of transient receptor potential proteins are ion channels expressed in intracellular endosomes and lysosomes. Mutations of human TRPML1 cause type IV mucolipidosis, a childhood neurodegenerative disease. TRPML1 is ubiquitously expressed in every tissue of mammals with the highest levels of expression in the brain, kidney, spleen, liver, and heart (47). Associated with the loss of TRPML1, the enlarged vacuole phenotype is observed in all cell types of ML4 patients and TRPML1 knockout mice (46, 53). Recently, it has been found that the regulation of lysosomal TRPML1 channel activity by sphingolipids is a key mechanism determining lysosome trafficking (38, 45). As the central core in sphingolipid metabolism, ceramide (Cer) is mainly produced by acid sphingomyelinase (ASM)-mediated hydrolysis of sphingomyelin (SM) and metabolized into sphingosine (Sph) via acid ceramidase (AC) in lysosomes. Mutations in the AC gene (ASAH1) or deficiency of lysosomal AC activity in human cells was found to be a major genetic or pathogenic mechanism for the development of Farber disease and partially for juvenile idiopathic arthritis that were shown to develop membranous nephropathy, FSGS, and minimal change disease (40, 49, 54). Therefore, we wondered whether AC-mediated sphingolipid signaling might gate lysosomal TRPML1 channels in podocytes to control Ca2+-dependent lysosome trafficking and fusion to MVBs that govern exosome excretion, which may explain the mechanism by which AC gene mutation induces podocyte injury and glomerular damage.
In the present study, the expression of TRPML1 was characterized in glomeruli and cultured murine podocytes. Then, we investigated the TRPML1 channel activity and addressed the regulatory role of AC on the activity of this channel in cultured murine podocytes. It is demonstrated that activation of TRPML1 channel by Sph, an AC product, contributes to lysosome trafficking and fusion to MVBs, which may result in MVB degradation and reduction of exosome release from podocytes.
MATERIALS AND METHODS
Cell culture.
Conditionally immortalized mouse podocytes were cultured and maintained as described previously (1). Briefly, they were grown at the permissive temperature (33°C) on collagen I-coated flasks or plates in RPMI 1640 medium supplemented with 10% fetal bovine serum and 10 U/mL recombinant mouse interferon-γ. The podocytes were then passaged and allowed to differentiate at 37°C for 10–14 days without interferon-γ before use in the experiments described below.
Nucleofection.
Podocytes were transfected with the plasmid of GCaMP3-ML1, a single-wavelength genetically encoded Ca2+ indicator GCaMP3 fused to the NH2-terminus of TRPML1, directly to the nucleus via nucleofector technology developed by Lonza (Basel, Switzerland). GCaMP3 contains an engineered Ca2+ binding calmodulin (CaM) variant and CaM-binding peptide fused to a single circularly permuted green fluorescent protein (cpGFP). The nucleofection involves the temporary creation of small pores in the membrane through electrical impulses together with cell-specific solutions to deliver substrates through the cytoplasm and into the nuclear membrane. Podocytes were trypsinized and counted, and 1 × 106 cells were gently centrifuged at 90 g for 10 min at room temperature. Cells were resuspended in SF Cell Line nucleofector solution containing 2 μg plasmid DNA and then transferred into a certified cuvette. The cuvette was placed into the nucleofector system and subjected to cell-type specific program DS-150, chosen based on optimization experiments. Nucleofected cells were resuspended with prewarmed medium and transferred to cultured plates for use in experiments.
GCaMP3 Ca2+ imaging.
At 18–24 h after nucleofection with GCaMP3-ML1, podocytes were used for experiments. The fluorescence intensity at 470 nm (F470) was recorded with a digital camera (Nikon Diaphoto TMD Inverted Microscope). Metafluor imaging and analysis software were used to acquire, digitize, and store the images for offline processing and statistical analysis (Universal Imaging, Bedford Hills, NY). Lysosomal Ca2+ release was measured under a “low” external Ca2+ solution, which contained 145 mM NaCl, 5 mM KCl, 3 mM MgCl2, 10 mM glucose, 1 mM EGTA, and 20 mM HEPES (pH 7.4). Glycyl-l-phenylalanine 2-naphthylamide (GPN; Cayman Chemical, Ann Arbor, MI) was used as positive control to induce Ca2+ release from lysosomes in podocytes. ML-SA1 (Sigma-Aldrich Chemicals, St. Louis, MO) was used as a potent TRPML channel agonist. Verapamil (Cayman Chemical, Ann Arbor, MI) was used as a TRPML channel blocker. SN-2 (Tocris Bioscience, Bristol, UK) was used as a selective TRPML3 activator.
Isolation of lysosomes from podocytes.
After treatment with vacuolin-1 (1 µM) for 2 h, isolation of lysosomes was performed (43). After podocytes were washed with precooled PBS, precooled homogenization buffer (0.25 M sucrose and 10 mM Tris, pH 7.4) was used for detachment of podocytes by a cell scraper. Cell suspension in glass-grinding vessel was homogenized using a Teflon pestle operated at 900 rotations per minute (rpm). The homogenate was then transferred to a 1.5-ml microfuge test tube and centrifuged at 14,000 g for 15 min at 4°C. After centrifuge, the middle part of supernatant was transferred to a 10-ml polycarbonate centrifuge tube. An equal volume of 16 mM CaCl2 was added to precipitate lysosomes. After being shaken on a rotary shaker at 100 rpm for 5 min at 4°C, the supernatant was centrifuged at 25,000g for 15 min at 4°C in an ultracentrifuge. The supernatant was discarded and the pellet was resuspended in one volume of ice-cold washing buffer (150 mM KCl and 10 mM Tris, pH 7.4). The suspension was centrifuged at 25,000 g for 15 min at 4°C in an ultracentrifuge. After the supernatant was discarded, the pellet containing lysosomes was resuspended in 40 μl of washing buffer, which was used for whole lysosome patch-clamp recording. All steps were performed on ice to minimize the activation of damaging from intracellular phospholipases and proteases. Lysosomes were used for electrophysiological recordings within 3 h of isolation to keep lysosomes fresh. To confirm the purity of isolated lysosomes, the CytoPainter lysosome staining kit (Abcam, Cambridge, UK) was used to stain the lysosomes in podocytes before lysosome isolation. The dye working solution was added to the culture medium and incubated at 37°C for 2 h. After lysosome isolation, the green fluorescence at excitation/emmission = 490/525 nm of samples was detected using Guava Easycyte Mini Flow Cytometry System (Guava Technologies, Hayward, CA). The data were analyzed using Guava acquisition and analysis software (Guava Technologies).
Whole lysosome patch-clamp recording.
Podocytes were treated with vacuolin-1 (1 µM) for 2 h to increase the size of lysosomes. With the use of a planar patch-clamp system, Port-a-Patch (Nanion Technologies), the whole lysosome electrophysiology was performed on isolated lysosomes from podocytes (43). The Port-a-Patch planar patch-clamping system combines with a pressure control system and microstructured glass recording chips containing an aperture of ~1-μm diameter. During the experiments, solutions and podocytes were added onto the recording chip, where a single podocyte was automatically captured and sealed by suction using a computer controlled pump as shown by formation of 10–15 MΩ electrical resistance in the circuit. The salt-agar bridges were used to connect the reference Ag/AgCl wire to the bath solution to minimize voltage offsets. Ag/AgCl-coated electrodes were chlorinated in bleach solution ~15 min until a black AgCl-layer was obvious on the silver wire. The liquid junction potential was calculated and corrected as described previously (6, 25). The bath solution contained the following (in mM): 60 KMSA, 60 KF, and 10 HEPES (pH 7.2, 2 mM CaMSA was added immediately before starting the measurements to avoid precipitation of CaF2). The luminal solution contained the following (in mM): 70 KMSA, 60 CaMSA, 2 MgCl2, and 10 HEPES (pH 4.6). Currents were recorded using an EPC-10 patch-clamp amplifier and PatchMaster acquisition software (HEKA). Data were digitized at 40 kHz and filtered at 2.8 kHz. The membrane potential was held at −60 mV, and 500-ms voltage ramps from −200 to +100 mV were applied every 5 s. All recordings were obtained at room temperature (21–23°C), and all recordings were analyzed using PatchMaster (HEKA) and Origin 6.1 (OriginLab).
Real-time reverse transcription polymerase chain reaction.
Total RNA was isolated from podocytes, reverse transcribed to cDNA, and subjected to PCR amplification according to the procedures described previously (61). Primers were synthesized by OriGene (Rockville, MD) with the following sequences: Mcoln1, sense GTCGGTGTCATTCGCTACCTGA and antisense GAACGATCCAGCCACAGAAGCA; Mcoln2, sense TACGTCCTGGTCACTATCAGCG and antisense GAGCAAGATGCTGCACACGTCA; and Mcoln3, sense CTACAGGCAAGGAACCATCTGC and antisense TGTGGAAGTCCAGGCTCAGGTT.
Western blot analysis.
Western blot analysis was performed as described previously (27). In brief, homogenates of murine podocytes were prepared using sucrose buffer containing protease inhibitors. After boiling for 5 min at 95°C in a 5 × loading buffer, total proteins (20 μg) were subjected to SDS-PAGE, transferred onto a PVDF membrane, and blocked by a solution with dry milk. Then, the membrane was probed with rabbit anti-TRPML1 (1:1,000; Abcam Biotechnology) or anti-β-actin (1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C followed by incubation with horseradish peroxidase-labeled IgG (1:5,000). The immunoreactive bands were detected by chemiluminescence methods and visualized on Kodak Omat X-ray films. Densitometric analysis of the images obtained from X-ray films was performed using the ImageJ software (NIH, Bethesda, MD). Mouse TRPML1 synthetic peptide (Thermo Fisher Scientific, Waltham, MA) was used to validate the specificity of TRPML1 antibody.
Animals.
Eight-week-old C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were euthanized and their kidneys were harvested for the characterization of TRPML1-3 in glomeruli. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Confocal microscopy.
Double-immunofluorescence staining was performed using cultured podocytes grown on collagen-coated glass coverslips (35, 60). Briefly, after fixation, the cells were incubated with rabbit anti-VPS16 (1:200; Proteintech, Rosemont, IL) and rat anti-Lamp-1 (1:200; Abcam Biotechnology, Cambridge, UK) overnight at 4°C. Then, Alexa 488-labeled anti-rabbit secondary antibody (1:200; Life Technologies) and Alexa 555-labeled anti-rat secondary antibody (1:200; Life Technologies) were added to the cell slides and incubated for 1 h at room temperature. Slides were then washed, mounted, and observed using a confocal laser scanning microscope (Fluoview FV1000; Olympus), and Image Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD) was employed to analyze colocalization, expressed as the Pearson correlation coefficient.
Immunohistochemistry.
Kidneys were embedded with paraffin, and 5-mm sections were cut from the embedded blocks. After heat-induced antigen retrieval, washing with 3% H2O2, and 30 min blocking with fetal bovine serum, slides were incubated with primary antibody diluted in PBS with 4% fetal bovine serum. Rabbit anti-TRPML1 (1:200; Abcam Biotechnology), rabbit anti-TRPML2 (1:200; Alomone Laboratories, Hadassah Ein Kerem, Jerusalem, Israel), and rabbit anti-TRPML3 (1:200; Thermo Fisher Scientific) were used as primary antibodies in this study. After incubation with the primary antibody overnight at 4°C, the sections were washed in PBS and incubated with biotinylated IgG (1:200) for 1 h and then with streptavidin-horseradish peroxidase for 30 min at room temperature. 3,3′-Diaminobenzidine (50 µl) was added to each kidney section and stained for 1 min. After sections were washed, the slides were counterstained with hematoxylin for 5 min. The slides were then mounted, observed under a microscope, and photomicrographed for analysis (57). Images of 20 glomeruli on the kidney section of each mice were taken under light microscope, and Image Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD) was employed to analyze the percentage of staining area. To measure the percentage of TRPML positive area, nucleus stain (purple color), TRPML stain (brown color), and background (white color) were manually selected for calculation [TRPM-positive area percentage = TRPML stain/(nucleus stain + TRPML stain + background) × 100%]. The area percentages of brown color in range statistics were used to quantify the percentage of TRPML-positive area in glomeruli.
Nanoparticle tracking analysis.
Nanoparticle tracking analysis (NTA) measurements were performed with a NanoSight NTA3.2 Dev Build 3.2.16 (Malvern Instruments), equipped with a sample chamber with a 638-nm laser and a Viton fluoroelastomer O-ring. The samples were injected in the sample chamber with sterile syringes (BD) until the liquid reached the tip of the nozzle. All measurements were performed at room temperature. The screen gain and camera level was 10 and 13, respectively. Each sample was measured using standard protocols, 30 s with manual shutter, and gain adjustments. Three measurements of the each sample was performed. Three-dimensional (3-D) figures were exported from the software. Particles sized between 50 and 100 nm were calculated (3).
Statistical analysis.
All of the values are expressed as means ± SE. Significant differences among multiple groups were examined using one-way or two-way ANOVA followed by a Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
RESULTS
Expression of TRPML1 in podocytes.
We first characterized the expression of TRPML1 in murine podocytes in vivo and in vitro. By immunohistochemistry, abundant expression of TRPML1 was shown in renal glomeruli of C57BL/6J wild-type (WT) mice, which was significantly higher than TRPML2 and TRPML3 (Fig. 1A). Using confocal microscopy, we found that red fluorescence of TRPML1 (Alexa 555) was highly colocalized with green fluorescence of nephrin (Alexa 488), a podocyte marker, or Lamp-1 (Alexa 488), a lysosome marker, in renal glomeruli of C57BL/6J WT mice (Fig. 1, B and C). RT-PCR results showed that the expression level of TRPML1 was remarkably higher than TRPML2 and -3 in cultured murine podocytes (Fig. 1D). To further confirm our finding, Western blot analysis demonstrated that TRPML1 (64 kDa) was highly expressed in cultured murine podocytes (Fig. 1E). Mouse TRPML1 synthetic peptide was found to block the binding of antibody to the target protein, which validated the specificity of this antibody (Fig. 1F). These experiments provided the first evidence showing the expression of TRPML1 in murine podocytes.
Fig. 1.
Expression of transient receptor potential mucolipin 1 (TRPML1) in podocytes. A: representative images and summarized data showing the immunohistochemical staining of TRPML1–3 in mouse glomeruli (n = 4 mice, 20 glomeruli counted from each mouse). Scale bars = 50 µm. B: representative image of confocal microscopy showing the colocalization of TRPML1 with nephrin in mouse renal glomeruli (n = 6). Scale bars = 50 µm. C: representative images of confocal microscopy showing the colocalization of TRPML1 with Lamp-1 in mouse renal glomeruli (n = 6). Scale bars = 50 µm. D: summarized data showing relative mRNA levels of TRPML1–3 in murine podocytes detected by RT-PCR (n = 4). E: Western blot gel documents showing the expression of TRPML1 in cultured murine podocytes (n = 4). F: failure to detect TRPML1 with anti-TRPML1 antibody preincubated with mouse TRPML1 synthetic peptide (n = 4). *P < 0.05 vs. TRPML1.
Characterization of TRPML1 channel activity in podocytes using GCaMP3-ML1.
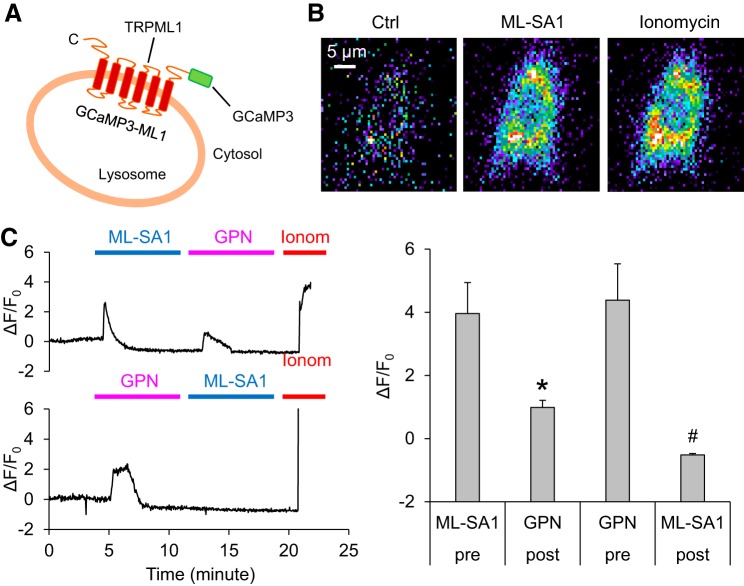
Next, we characterized TRPML1-mediated lysosomal Ca2+ release in intact podocytes using GCaMP3, a single-wavelength genetically encoded Ca2+ indicator, to the cytoplasmic amino terminus of TRPML1 (Fig. 2A). To determine the specificity of the GCaMP3-ML1 fluorescence signal, we tested the response of transfected podocytes to the potent TRPML channel agonist ML-SA1 or GPN. As a cathepsin C substrate that induces osmotic lysis of lysosomes, GPN can be used to test whether emptying lysosome Ca2+ storage can reduce or abolish GCaMP3 detected Ca2+ signals. A fluorescent microscopic imaging system was used to dynamically and continuously monitor the GCaMP3 fluorescence signal (F470) in podocytes. The representative images of GCaMP3 fluorescence signal in podocytes are shown in Fig. 2B. It was found that early addition of ML-SA1 induced a rapid increase in GCaMP3 fluorescence and that the following addition of GPN only induced a small GCaMP3 fluorescence signal in podocytes (Fig. 2C). On the other hand, the early addition of GPN triggered a similar increase in GCaMP3 fluorescence in podocytes. The pretreatment with GPN totally blocked GCaMP3 fluorescence signal induced by the late addition of ML-SA1. These data indicate that the Ca2+ release detected by GCaMP3 is derived from lysosomes in podocytes, which is mainly through the TRPML1 channels on the lysosome membrane.
Fig. 2.
Characterization of transient receptor potential mucolipin 1 (TRPML1) channel activity in podocytes using GCaMP3-ML1. A: the strategy of GCaMP3-TRPML1 (GCaMP3-ML1) fusion. GCaMP3 is fused to the NH2 terminus of TRPML1. B: representative micrographs showing the changes of GCaMP3 fluorescence upon bath application of ML-SA1 and ionomycin (Ionom) to GCaMP3-ML1-transfected podocytes. C: rapid increases in GCaMP3 fluorescence was induced by ML-SA1 (measured as the change of GCaMP3 fluorescence ∆F over basal fluorescence F0; ∆F/F0) in podocytes transfected with GCaMP3-ML1. Subsequent application of glycyl-l-phenylalanine 2-naphthylamide (GPN; 200 µM) induced smaller responses than ML-SA1. On the other hand, ML-SA1 induced small responses in GCaMP3-ML1-expressing podocytes that had received an application of GPN. The application of ionomycin (1 µM) induced maximal responses (n = 7 batches of cultures with total 22 cells). *P < 0.05 vs. pretreatment with ML-SA1. #P < 0.05 vs. pretreatment with GPN.
To confirm whether the TRPML2 channel was involved in the ML-SA1-induced lysosomal Ca2+ release, we inhibited TRPML2 channel expression using TRPML2 siRNA before GCaMP3 Ca2+ imaging. The inhibition of TRPML2 by siRNA was confirmed using Western blot analysis (Supplemental Fig. S1; Supplemental Data: https://doi.org/10.6084/m9.figshare.8055731.v1). It was found that TRPML2 siRNA had no effects on ML-SA1-induced Ca2+ release from lysosomes, which indicate the potential for less involvement of TRPML2 in such Ca2+ release response (Supplemental Fig. S2). Also, the possibility of that the TRPML3 channel contributed to ML-SA1-induced lysosomal Ca2+ release was tested. Podocytes were treated with SN-2, a selective TRPML3 activator, during GCaMP3 Ca2+ imaging, and no enhancement of lysosomal Ca2+ release was observed. These results suggest that the TRPML3 channel may not be a major contributor to ML-SA1-induced Ca2+ release from lysosomes (Supplemental Fig. S3).
Role of AC in TRPML1 channel activity.
Given that sphingolipids have been reported to regulate TRPML1 channel activity, we tested whether the opening of TRPML1 channels in podocytes can be altered by acid ceramidase (AC), an important lysosomal enzyme that catalyzes the degradation of ceramide into sphingosine and fatty acid under normal condition. As shown in Fig. 3A, pretreatment with carmofur, a selective AC inhibitor, remarkably decreased Ca2+ release through the TRPML1 channel induced by ML-SA1 compared with the vehicle condition. The quantification of GCaMP3 fluorescence signal in podocytes is summarized below the representative curves (Fig. 3B). As the product of AC-dependent metabolism of ceramide, the effect of sphingosine on TRPML1 channel activity was also tested. It was found that Sph markedly induced TRPML1 channel activity, which was similar to ML-SA1. However, pretreatment with verapamil, a TRPML1 blocker, totally blocked Sph-induced activation of TRPML1 channel in podocytes, suggesting some TRPML1 specificity of Ca2+ release induced by Sph (Fig. 4A). The quantification of Ca2+ release through TRPML1 channel measured by GCaMP3 is shown below the representative curves (Fig. 4B).
Fig. 3.

Inhibition of transient receptor potential mucolipin 1 (TRPML1) channel activity by acid ceramidase inhibitor. A: ML-SA1 (20 µM) induced remarkable increases in GCaMP3 fluorescence in podocytes transfected with GCaMP3-ML1. On the other hand, ML-SA1 had no effects on GCaMP3 fluorescence in GCaMP3-ML1-expressing podocytes after pretreatment with carmofur (Carm; 1 µM) for 30 min. The application of ionomycin (Ionom; 1 µM) induced maximal responses. B: summarized data showing responses in podocytes transfected with GCaMP3-ML1 induced by ML-SA1 (n = 6 batches of cultures with total 21 cells). *P < 0.05 vs. control (Ctrl)-vehicle (Vehl). #P < 0.05 vs. ML-SA1-Vehl.
Fig. 4.

Activation of transient receptor potential mucolipin 1 (TRPML1) channel by sphingosine (Sph). A: Sph (20 µM) induced remarkable increases in GCaMP3 fluorescence in podocytes transfected with GCaMP3-ML1. On the other hand, Sph had no effects on GCaMP3 fluorescence in GCaMP3-ML1-expressing podocytes after pretreatment with verapamil (Vera; 20 µM). The application of ionomycin (1 µM) induced maximal responses. B: summarized data showing responses in podocytes transfected with GCaMP3-ML1 induced by Sph (n = 6 batches of cultures with total 25 cells). *P < 0.05 vs. control (Ctrl)-vehicle (Vehl). #P < 0.05 vs. Sph-Vehl.
Moreover, the effects of ML-SA1 on TRPML1 channels in podocytes transfected with AC CRISPR activation plasmid (activation) or AC CRISPR/cas9 KO plasmid (KO) were detected. Western blot analysis was performed to confirm that the expression of AC was significantly enhanced by AC CRISPR activation plasmid, while AC CRISPR/cas9 KO plasmid remarkably reduced the expression of AC in podocytes (Fig. 5A). As shown in Fig. 5B, lysosomal Ca2+ release induced by ML-SA1 was significantly enhanced by AC CRISPR activation plasmid in podocytes. On the other hand, ML-SA1 failed to induce Ca2+ release through TRPML1 channel in AC CRISPR/cas9 KO plasmid-transfected cells compared with control cells. However, as the product of ceramide metabolism by AC, Sph was found to induce remarkable Ca2+ release in both control cells and AC CRISPR/cas9 KO plasmid-transfected cells, which indicates that exogenous Sph can overcome the inhibitory effect of AC deficiency on TRPML1 channel (Fig. 5C). These results revealed that the normal function of AC is essential for TRPML1 channel-mediated Ca2+ release in podocytes.
Fig. 5.

Regulation of transient receptor potential mucolipin 1 (TRPML1) channel activity by acid ceramidase (AC) gene editing. A: representative Western blot images and summarized data showing the expression of AC in control, AC CRISPR activation, and AC CRISPR/cas9 KO podocytes. B: ML-SA1 (20 µM) induced remarkable increases in GCaMP3 fluorescence in podocytes transfected with GCaMP3-ML1. ML-SA1-induced lysosomal Ca2+ release was enhanced by AC CRISPR activation. ML-SA1 had no effects on GCaMP3 fluorescence in AC CRISPR/cas9 KO podocytes transfected with GCaMP3-ML1 (n = 5 batches of cultures with total 26 cells). C: sphingosine (Sph; 20 µM) induced remarkable increases in GCaMP3 fluorescence in podocytes transfected with GCaMP3-ML1. Also, Sph produced similar effects on GCaMP3 fluorescence in AC CRISPR/cas9 KO podocytes transfected with GCaMP3-ML1 (n = 5 batches of cultures with total 20 cells). *P < 0.05 vs. control Ctrl or Ctrl-Vehl. #P < 0.05 vs. ML-SA1-Vehl. Activation, AC CRISPR activation plasmid; Ctrl, control plasmid; KO, AC CRISPR/cas9 knockout (KO) plasmid; Vehl, vehicle.
Effects of different sphingolipids on TRPML1 channel currents.
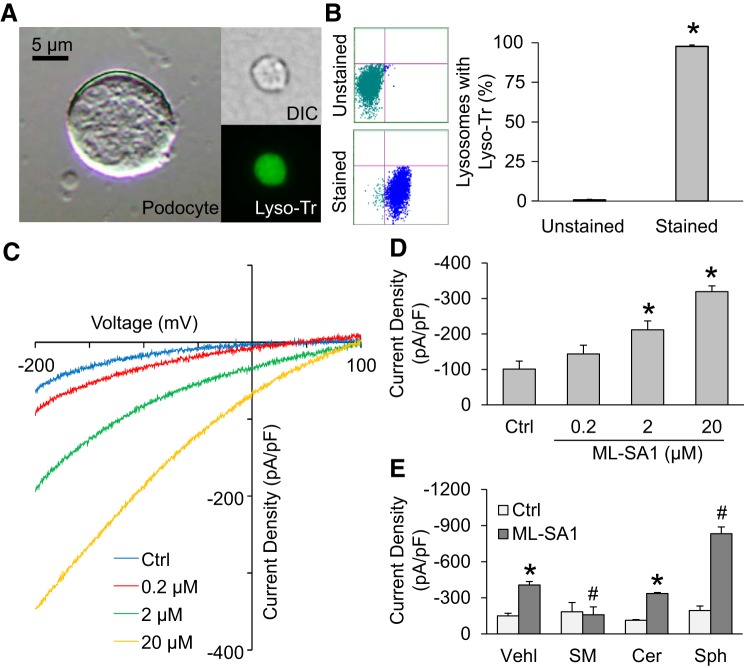
To further confirm the role of AC in TRPML1 channel-mediated Ca2+ release from lysosomes, the effects of sphingolipids associated with AC, sphingomyelin (SM), ceramide (Cer), and sphingosine (Sph) on TRPML1 channel currents were tested by whole lysosome patch-clamp recording. As shown in Fig. 6A, a lysosome isolated from podocytes was confirmed by differential interference contrast microscopy compared with podocytes in size. Isolated lysosomes loaded with LysoTracker were also confirmed with green fluorescence that indicates the low pH of lysosome lumen. Also, the purity of isolated lysosomes was further shown by flow cytometry. Figure 6B showed that isolated lysosomes loaded with LysoTracker shifted to the area with high fluorescence intensity. Quantification of the flow cytometry data indicated the very high purity of isolated lysosomes.
Fig. 6.
Effects of different sphingolipids on transient receptor potential mucolipin 1 (TRPML1) channel currents. A: representative images of 1) differential interference contrast (DIC) picture of suspended podocyte, 2) DIC picture of the isolated lysosome, and 3) isolated lysosome stained with LysoTracker. B: the purity of isolated lysosomes detected by flow cytometry (n = 5). C: representative whole lysosome currents enhanced by ML-SA1. D: ML-SA1 enhanced TRPML1 channel activity in a concentration-dependent manner (n = 5). E: summarized data of the effects of various sphingolipids on whole lysosome currents (n = 5). *P < 0.05 vs. unstained lysosomes, control (Ctrl), or vehicle (Vehl)-Ctrl. #P < 0.05 vs. Vehl-ML-SA1. SM, sphingomyelin; Cer, ceramide; Sph, sphingosine.
After demonstration of integrity and purity of isolated lysosomes, the whole lysosome patch-clamp approach was adopted to characterize the properties of TRPML1 channel currents in isolated lysosomes. As shown in Fig. 6, C and D, bath application of ML-SA1 induced Ca2+ release from lysosome in a dose-dependent manner. In the presence of 20 µM ML-SA1 in the bath solution, the whole lysosome current was −333.18 ± 18.83 pA at −200 mV with positive reversal potentials. These data demonstrated the normal function of the TRPML1 channel in isolated lysosomes from podocytes. Next, we tested the effects of sphingolipids on TRPML1 channel of isolated lysosomes. As shown in Fig. 6E, we found that ML-SA1-induced Ca2+ release through TRPML1 channels was blocked by sphingomyelin. Ceramide had no effects on TRPML1 channel-mediated Ca2+ currents induced by ML-SA1. Sphingosine, the AC product of ceramide, was found to enhance TRPML1 channel activation in the presence of ML-SA1. These results imply that inhibition of AC may reduce sphingosine production, thereby decreasing TRPML1 channel activity. It is also possible that the reduction of AC activity induces ceramide accumulation in lysosomes, which in turn results in sphingomyelin increase, thereby suppressing Ca2+ release through TRPML1 channel.
Interaction of lysosomes and MVBs regulated by AC-TRPML1 signaling pathway.
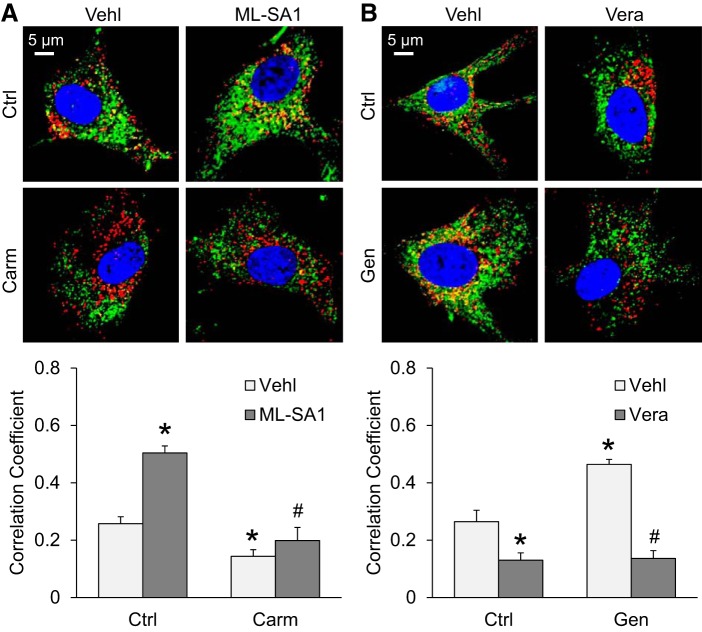
We also observed the interaction of lysosomes and MVBs in podocytes using confocal microscopy, which is the evidence of lysosome trafficking and fusion to MVBs. Podocytes were treated with ML-SA1 (10 µM) or verapamil (10 µM) for 12 h. Carmofur (1 µM) or genistein (20 µM) was used 30 min before treatment with ML-SA1 or verapamil. As shown in Fig. 7A, colocalization of the MVB marker VPS16 (Alex 488, green fluorescence) and the lysosome marker Lamp-1 (Alex 555, red fluorescence) yielded yellow puncta, indicating the interaction of lysosome and MVB. Compared with the vehicle group, TRPML1 channel activation by ML-SA1 markedly increased the colocalization of VPS16 and Lamp-1 in podocytes. However, such enhancement was reversed by pretreatment of podocytes with the AC inhibitor carmofur. To further confirm our findings, the effect of genistein, an AC inducer, on the interaction of lysosomes and MVBs was also tested. As shown in Fig. 7B, genistein markedly increased the colocalization of VPS16 and Lamp-1 in podocytes, indicating that activation of AC enhanced MVB-lysosome interaction in podocytes. Such enhancement was reversed by verapamil, a TRPML1 blocker. These results indicate that enhancement of TRPML1 channel-mediated Ca2+ release or AC activity may promote the fusion of lysosomes and MVBs, leading to the degradation of MVBs and reduction of exosome release from podocytes.
Fig. 7.
Interaction of lysosomes and multivesicular bodies (MVBs) regulated by the acid ceramidase-transient receptor potential mucolipin 1 (TRPML1) signaling pathway. A: representative images and summarized data of the colocalization of VPS16 and Lamp-1 in podocytes before and after treatment with ML-SA1 and carmofur (Carm; n = 6 batches of cultures with total 24 cells). B: representative images and summarized data of the colocalization of VPS16 and Lamp-1 in podocytes before and after treatment with verapamil and genistein (n = 6 batches of cultures with total 24 cells). *P < 0.05 vs. control (Ctrl) vehicle (Vehl). #P < 0.05 vs. Ctrl-ML-SA1 or genistein (Gen)-vehicle (Vehl).
Exosome release from podocytes controlled by AC-TRPML1 signaling pathway.
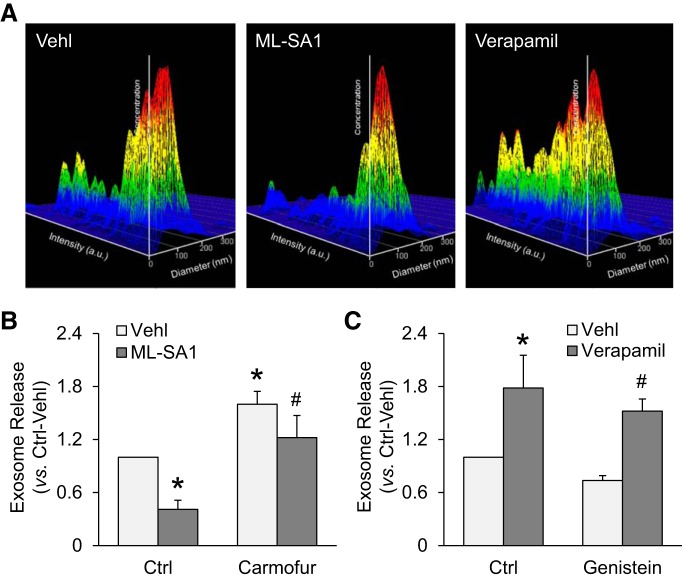
To test whether AC-TRPML1 signaling pathway controls MVB fate and exosome release from podocytes, we performed nanoparticle tracking analysis (NTA) to measure the number of exosomes released from podocytes treated with ML-SA1, verapamil, carmofur, and genistein. Podocytes were treated with ML-SA1 (10 µM) or verapamil (10 µM) for 24 h. Carmofur (1 µM) or genistein (20 µM) was used 30 min before treatment with ML-SA1 or verapamil. The representative 3-D histograms of vesicles showed the decreased release of vesicles (40–140 nm) from podocytes treated with ML-SA1 and enhanced exosome release from podocytes treated with verapamil (Fig. 8A). As shown in Fig. 8B, it was found that ML-SA1 significantly reduced the number of exosomes released from podocytes. On the contrary, inhibition of AC activity by carmofur remarkably enhanced exosome release from podocytes under the vehicle condition and reversed the inhibition of exosome release by ML-SA1. Moreover, blockade of TRPML1 channel by verapamil markedly elevated exosome release from podocytes under the vehicle condition and reversed the reduction of exosome release by genistein (Fig. 8C). These results provide further evidence suggesting that both TRPML1 channel-mediated Ca2+ release and AC activity contribute to lysosome trafficking and fusion to MVBs, leading to degradation of MVBs and inhibition of exosome release from podocytes.
Fig. 8.
Exosome release from podocytes controlled by the acid ceramidase-transient receptor potential mucolipin 1 (TRPML1) signaling pathway. A: representative images showing that ML-SA1 (20 µM) reduced exosome release from podocytes and verapamil enhanced exosome release from podocytes. B: summarized data showing that ML-SA1 (20 µM) reduced podocyte-derived exosome release which was prevented by carmofur (n = 5 batches of cultures). C: summarized data showing that verapamil (20 µM) enhanced exosome release from podocytes and reversed the reduction of podocyte-derived exosome release by genistein (n = 5 batches of cultures). Carm, carmofur. *P < 0.05 vs. control (Ctrl)-vehicle (Vehl). #P < 0.05 vs. Ctrl-ML-SA1 or genistein-vehicle (Vehl).
DISCUSSION
In the present study, we tested whether TRPML1 channel-mediated Ca2+ release contributes to lysosome trafficking and fusion to MVBs in an AC-dependent manner in podocytes. After confirmation of TRPML channel proteins, in particular, TRPLML1, in glomerular podocyte and cultured conditionally immortalized mouse podocytes, we demonstrated that activation of TRPML1 channel significantly enhanced lysosome trafficking and fusion to MVBs in cultured podocytes, which led to inhibition of exosome release from podocytes. It was also found that the activation of TRPML1 channel was attributed to the normal function of AC and consequent production of sphingosine. Inhibition of AC blocked TRPML1 channel activity and attenuated lysosome trafficking and fusion to MVBs, leading to enhancement of exosome release from podocytes. These results together provide the first evidence that AC-dependent TRPML1 channel activation is an important mechanism that mediates lysosome trafficking and fusion to MVBs in podocytes, which controls MVB fate and exosome release from podocytes.
Recent studies in our laboratory have demonstrated that the TRPML1 channel is a lysosomal NAADP-sensitive Ca2+ channel that controls Ca2+ release from the sarcoplasmic reticulum, apoptosis, and endosome-lysosome interaction (62, 63, 65). The lysosome trafficking depends on the TRPML1 channel-mediated Ca2+ release from lysosomes. This Ca2+-dependent lysosome trafficking was observed in different cells including podocytes (15, 31, 45, 58), which is essential for various lysosome-dependent functions, such as phagocytosis, exocytosis, and autophagy (41, 52). Therefore, we wondered whether the TRPML1 channel also played a significant role in the regulation of lysosome function in podocytes. We first characterized the expression and activity of the TRPML1 channel in murine podocytes. It was found that, among TRPMLs, only TRPML1 was abundantly expressed in murine podocytes. Using GCaMP3-ML1 Ca2+ imaging and whole lysosome patch-clamping, we found that the lysosomal Ca2+ release through TRPML1 channel was induced by a potent TRPML agonist, ML-SA1. GPN, as a lysosome Ca2+ storage-depleting chemical, remarkably attenuated ML-SA1-induced Ca2+ release. The recorded channel was defined as TRPML1 by these pharmacological characteristics. To our knowledge, these results for the first time demonstrated the presence of TRPML1 in podocytes and its functional activity as a Ca2+ channel in the lysosome membrane of these kidney cells. In previous studies, these features of TRPML1 channels have also been shown in many other cell types such as arterial myocytes, myoblasts, fibroblasts, and macrophages (10, 31, 62).
It has been reported that lysosomes and other intracellular vehicles traffic along microtubules to encounter, kiss, and fuse together (9, 20, 37). These intracellular vesicle interactions have been reported within many different cell types (2, 30, 32). However, so far little is known how lysosome trafficking and fusion are activated and regulated actively. In this regard, there is evidence that the sphingolipid ceramide may be involved in lysosome fusion to cell plasma membrane, endosomes, phagosomes, MVBs, and other organelles (5, 22, 39, 44). Via acid ceramidase (AC) in lysosomes, ceramide is metabolized into sphingosine that may be further converted to sphingosine-1-phosphate via sphingosine kinase (7, 19). In recent studies, Sph has been reported to enhance lysosome functions in a TRPML1 channel-dependent manner to improve cell function in Niemann-Pick disease due to acid sphingomyelinase (ASM) gene mutations (45). Therefore, we tested whether AC regulates TRPML1 channel activity and determines lysosome trafficking and cellular function in podocytes. By GCaMP3 Ca2+ imaging and whole lysosome patch clamping, we demonstrated that both pharmacological and genetic inhibitions of AC activity blocked TRPML1 channel activity in podocytes, suggesting that the normal function of AC is essential for TRPML1 channel opening, controlling Ca2+ release from lysosomes. AC-associated sphingolipids, SM, Cer, and Sph had different effects on TRPML1 channel activity in podocytes, with inhibition by SM, no effect from Cer, but enhancement by Sph. In AC CRISPR/cas9 KO podocytes, Sph remained to increase TRPML1 channel activity, suggesting that AC gene deletion does not affect its downstream product if exogenously administrated. These results imply that the AC deficiency may reduce Sph production and thereby decrease TRPML1 channel activity. Also, the AC gene defect may induce Cer accumulation in lysosomes. Given the unique feature of Cer metabolism that works reciprocally, Cer accumulation during Asah1 gene defect may also result in an SM increase suppressing TRPML1 channel activity. In this regard, it has been reported that TRPML1-mediated lysosomal Ca2+ release is dramatically reduced in Niemann-Pick disease cells, which have an insufficient activity of ASM. The inhibition of TRPML1 channel activity is due to distinctively accumulated sphingomyelins (SMs) in NP disease cells; ceramide has no effects on TRPML1 channel-mediated Ca2+ release; and sphingosine potentiates TRPML1 channel activation induced by an agonist (45). However, there is evidence that ceramide may trigger and enhance autophagy including autophagosome formation and degradation (42), and it is also involved in lysosome fusion to cell plasma membrane, endosomes, phagosomes, MVBs and other organelles (5, 42, 44, 51). The present study did not attempt to define the mechanisms by which sphingolipids regulate TRPML1 channel activity. However, previous studies have shown that some regulatory mechanisms of TRPML1 channel activity may be associated with the regulation of TRPML1 channel by AC. In the lysosome, the highly acidic environment has been proposed to modulate the activity of TRPML1 channels (13). A recent study revealed that the acidic pH favors the release of Ca2+ from the lumen through TRPML1 activation, whereas, on the plasma membrane, the high pH of the extracellular milieu inhibits Ca2+ influx (29). Correspondingly, defective lysosomal acidification has been found to result in an imbalance between ASM cleavage of sphingomyelin to ceramide and AC consumption of ceramide, resulting in the higher levels of ceramide and consequent cystic fibrosis (50). These findings indicate that alteration of sphingolipid metabolism may explain the TRPML1 channel inhibition by defective lysosomal acidification.
Previous studies have revealed that lysosome function is involved in the regulation of MVB fate and the excretion of exosomes (2, 17, 23). Lysosome trafficking and fusion to MVBs are essential for the regulation of exosome excretion. This fusion of lysosome with other vesicles was found to occur near the microtubule-organizing center. MVBs and lysosomes traffic along microtubules to encounter, kiss, and finally fuse (9, 20, 37, 55). However, the mechanisms mediating this lysosome active trafficking or fusion are far from clear. In the present study, it was demonstrated that lysosome-MVB interaction was markedly increased by activation of TRPML1 channel or AC in podocytes. On the contrary, inhibition of TRPML1 channel or AC substantially decreased the interaction of lysosomes and MVBs in podocytes. Correspondingly, enhancement of activity of TRPML1 channel or AC significantly inhibited exosome release from podocytes, while inhibition of the activity of TRPML1 channel or AC remarkably increased exosome release from these cells. These findings suggest that lack of Sph and accumulation of SM due to AC deficiency inhibit TRPML1 channel-mediated Ca2+ release and lysosome trafficking, leading to MVB accumulation and exosome release from podocytes. This enhanced exosome release due to AC deficiency may contribute to podocyte injury and glomerular damage through cell-to-cell communication. In this regard, mutations in the AC gene (ASAH1) or deficiency of lysosomal AC activity in human cells were found to be a major genetic or pathogenic mechanism for the development of Farber disease and partially for juvenile idiopathic arthritis that were shown to develop membranous nephropathy, FSGS, and minimal change disease (40, 49, 54). Also, it has been reported that Sph, the product of ceramide metabolism by AC, enhances lysosome functions in a TRPML1 channel-dependent manner to improve cell function in Niemann-Pick disease due to ASM gene mutations (45).
In summary, the present study demonstrated that TRPML1 channel was an abundant form of TRPMLs in podocytes and that this lysosomal channel mediates Ca2+ release across the lysosomal membrane to regulate lysosome trafficking. The normal function of AC was found to be essential for TRPML1 channel activity and lysosome trafficking. The AC-TRPML1 signaling pathway controls lysosome trafficking, lysosome-dependent MVB degradation, and exosome release from podocytes. These findings indicate that inhibition of TRPML1 channel activity by AC deficiency under pathological conditions may lead to podocyte-derived exosome release and consequent podocyte injury and glomerular damage.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-054927 and DK-102539 from National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.L., D.H., and P.-L.L. conceived and designed research; G.L., D.H., J.H., O.M.B., and X.Y. performed experiments; G.L., D.H., J.H., O.M.B., and X.Y. analyzed data; G.L. interpreted results of experiments; G.L. prepared figures; G.L. drafted manuscript; G.L. and P.-L.L. edited and revised manuscript; G.L., D.H., J.H., O.M.B., X.Y., and P.-L.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The conditionally immortalized mouse podocytes were kindly provided by Dr. Paul E. Klotman (Baylor College of Medicine, Houston, TX). The GCaMP3-ML1 plasmid was kindly provided by Dr. Haoxing Xu (Department of Molecular, Cellular, and Developmental Biology, University of Michigan, Ann Arbor, MI).
REFERENCES
- 1.Abais JM, Zhang C, Xia M, Liu Q, Gehr TW, Boini KM, Li PL. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid Redox Signal 18: 1537–1548, 2013. doi: 10.1089/ars.2012.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 42: 360–367, 2011. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antes TJ, Middleton RC, Luther KM, Ijichi T, Peck KA, Liu WJ, Valle J, Echavez AK, Marbán E. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnology 16: 61, 2018. doi: 10.1186/s12951-018-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach G. Mucolipidosis type IV. Mol Genet Metab 73: 197–203, 2001. doi: 10.1006/mgme.2001.3195. [DOI] [PubMed] [Google Scholar]
- 5.Bao JX, Jin S, Zhang F, Wang ZC, Li N, Li PL. Activation of membrane NADPH oxidase associated with lysosome-targeted acid sphingomyelinase in coronary endothelial cells. Antioxid Redox Signal 12: 703–712, 2010. doi: 10.1089/ars.2009.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51: 107–116, 1994. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 7.Bhat OM, Yuan X, Li G, Lee R, Li PL. Sphingolipids and redox signaling in renal regulation and chronic kidney diseases. Antioxid Redox Signal 28: 1008–1026, 2018. doi: 10.1089/ars.2017.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boini KM, Xia M, Xiong J, Li C, Payne LP, Li PL. Implication of CD38 gene in podocyte epithelial-to-mesenchymal transition and glomerular sclerosis. J Cell Mol Med 16: 1674–1685, 2012. doi: 10.1111/j.1582-4934.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bright NA, Reaves BJ, Mullock BM, Luzio JP. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J Cell Sci 110: 2027–2040, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Cheng X, Zhang X, Gao Q, Ali Samie M, Azar M, Tsang WL, Dong L, Sahoo N, Li X, Zhuo Y, Garrity AG, Wang X, Ferrer M, Dowling J, Xu L, Han R, Xu H. The intracellular Ca2+ channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat Med 20: 1187–1192, 2014. doi: 10.1038/nm.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111: 703–708, 2002. doi: 10.1016/S0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 12.De Rechter S, Decuypere JP, Ivanova E, van den Heuvel LP, De Smedt H, Levtchenko E, Mekahli D. Autophagy in renal diseases. Pediatr Nephrol 31: 737–752, 2016. doi: 10.1007/s00467-015-3134-2. [DOI] [PubMed] [Google Scholar]
- 13.Di Paola S, Scotto-Rosato A, Medina DL. TRPML1: The Ca2+ retaker of the lysosome. Cell Calcium 69: 112–121, 2018. doi: 10.1016/j.ceca.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455: 992–996, 2008. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun 1: 38, 2010. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn WA., Jr Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol 4: 139–143, 1994. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 17.Eitan E, Suire C, Zhang S, Mattson MP. Impact of lysosome status on extracellular vesicle content and release. Ageing Res Rev 32: 65–74, 2016. doi: 10.1016/j.arr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fougeray S, Pallet N. Mechanisms and biological functions of autophagy in diseased and ageing kidneys. Nat Rev Nephrol 11: 34–45, 2015. doi: 10.1038/nrneph.2014.201. [DOI] [PubMed] [Google Scholar]
- 19.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep 5: 777–782, 2004. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol 132: 1011–1023, 1996. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galione A. NAADP, a new intracellular messenger that mobilizes Ca2+ from acidic stores. Biochem Soc Trans 34: 922–926, 2006. doi: 10.1042/BST0340922. [DOI] [PubMed] [Google Scholar]
- 22.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol 5: 317–323, 2004. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 23.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75: 193–208, 2018. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichii O, Otsuka-Kanazawa S, Horino T, Kimura J, Nakamura T, Matsumoto M, Toi M, Kon Y. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS One 9: e110383, 2014. doi: 10.1371/journal.pone.0110383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee GY, You DG, Lee HR, Hwang SW, Lee CJ, Yoo YD. Romo1 is a mitochondrial nonselective cation channel with viroporin-like characteristics. J Cell Biol 217: 2059–2071, 2018. doi: 10.1083/jcb.201709001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenoir O, Tharaux PL, Huber TB. Autophagy in kidney disease and aging: lessons from rodent models. Kidney Int 90: 950–964, 2016. doi: 10.1016/j.kint.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Li CX, Xia M, Han WQ, Li XX, Zhang C, Boini KM, Liu XC, Li PL. Reversal by growth hormone of homocysteine-induced epithelial-to-mesenchymal transition through membrane raft-redox signaling in podocytes. Cell Physiol Biochem 27: 691–702, 2011. doi: 10.1159/000330078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, Li CX, Xia M, Ritter JK, Gehr TW, Boini K, Li PL. Enhanced epithelial-to-mesenchymal transition associated with lysosome dysfunction in podocytes: role of p62/Sequestosome 1 as a signaling hub. Cell Physiol Biochem 35: 1773–1786, 2015. doi: 10.1159/000373989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Zhang WK, Benvin NM, Zhou X, Su D, Li H, Wang S, Michailidis IE, Tong L, Li X, Yang J. Structural basis of dual Ca2+/pH regulation of the endolysosomal TRPML1 channel. Nat Struct Mol Biol 24: 205–213, 2017. doi: 10.1038/nsmb.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li PL, Zhang Y, Abais JM, Ritter JK, Zhang F. Cyclic ADP-ribose and NAADP in vascular regulation and diseases. Messenger (Los Angel) 2: 63–85, 2013. doi: 10.1166/msr.2013.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Rydzewski N, Hider A, Zhang X, Yang J, Wang W, Gao Q, Cheng X, Xu H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol 18: 404–417, 2016. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liebau MC, Braun F, Höpker K, Weitbrecht C, Bartels V, Müller RU, Brodesser S, Saleem MA, Benzing T, Schermer B, Cybulla M, Kurschat CE. Dysregulated autophagy contributes to podocyte damage in Fabry’s disease. PLoS One 8: e63506, 2013. doi: 10.1371/journal.pone.0063506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu WJ, Li ZH, Chen XC, Zhao XL, Zhong Z, Yang C, Wu HL, An N, Li WY, Liu HF. Blockage of the lysosome-dependent autophagic pathway contributes to complement membrane attack complex-induced podocyte injury in idiopathic membranous nephropathy. Sci Rep 7: 8643, 2017. doi: 10.1038/s41598-017-07889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8: 622–632, 2007. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 35.Maciel W, Lopes WD, Cruz B, Teixeira W, Felippelli G, Sakamoto CA, Fávero FC, Buzzulini C, Soares V, Gomes LV, Bichuette M, da Costa AJ. Effects of Haematobia irritans infestation on weight gain of Nelore calves assessed with different antiparasitic treatment schemes. Prev Vet Med 118: 182–186, 2015. doi: 10.1016/j.prevetmed.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Mullins C, Bonifacino JS. The molecular machinery for lysosome biogenesis. BioEssays 23: 333–343, 2001. doi: 10.1002/bies.1048. [DOI] [PubMed] [Google Scholar]
- 37.Mullock BM, Bright NA, Fearon CW, Gray SR, Luzio JP. Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J Cell Biol 140: 591–601, 1998. doi: 10.1083/jcb.140.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccoli E, Nadai M, Caretta CM, Bergonzini V, Del Vecchio C, Ha HR, Bigler L, Dal Zoppo D, Faggin E, Pettenazzo A, Orlando R, Salata C, Calistri A, Palù G, Baritussio A. Amiodarone impairs trafficking through late endosomes inducing a Niemann-Pick C-like phenotype. Biochem Pharmacol 82: 1234–1249, 2011. doi: 10.1016/j.bcp.2011.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 23: 519–547, 2007. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha A, Pais P, Iyengar AA, Abraham AK. Proteinuria in children with juvenile idiopathic arthritis: Making the case for early urinary screening. Saudi J Kidney Dis Transpl 28: 1408–1411, 2017. doi: 10.4103/1319-2442.220854. [DOI] [PubMed] [Google Scholar]
- 41.Samie M, Wang X, Zhang X, Goschka A, Li X, Cheng X, Gregg E, Azar M, Zhuo Y, Garrity AG, Gao Q, Slaugenhaupt S, Pickel J, Zolov SN, Weisman LS, Lenk GM, Titus S, Bryant-Genevier M, Southall N, Juan M, Ferrer M, Xu H. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev Cell 26: 511–524, 2013. doi: 10.1016/j.devcel.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, Ghidoni R, Codogno P. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem 279: 18384–18391, 2004. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- 43.Schieder M, Rötzer K, Brüggemann A, Biel M, Wahl-Schott C. Planar patch clamp approach to characterize ionic currents from intact lysosomes. Sci Signal 3: pl3, 2010. doi: 10.1126/scisignal.3151pl3. [DOI] [PubMed] [Google Scholar]
- 44.Schramm M, Herz J, Haas A, Krönke M, Utermöhlen O. Acid sphingomyelinase is required for efficient phago-lysosomal fusion. Cell Microbiol 10: 1839–1853, 2008. doi: 10.1111/j.1462-5822.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 45.Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, Xu H. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun 3: 731, 2012. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slaugenhaupt SA. The molecular basis of mucolipidosis type IV. Curr Mol Med 2: 445–450, 2002. doi: 10.2174/1566524023362276. [DOI] [PubMed] [Google Scholar]
- 47.Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS Jr, Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet 9: 2471–2478, 2000. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 48.Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, Chin-Kanasaki M, Araki H, Araki S, Koya D, Asanuma K, Kim EH, Haneda M, Kajiwara N, Hayashi K, Ohashi H, Ugi S, Maegawa H, Uzu T. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes 65: 755–767, 2016. doi: 10.2337/db15-0473. [DOI] [PubMed] [Google Scholar]
- 49.Takeyama J, Umebayashi H, Inagaki T. Renal involvement in a patient with juvenile idiopathic arthritis presenting after treatment for Hodgkin lymphoma. J Pediatr Hematol Oncol 29: 347, 2007. doi: 10.1097/MPH.0b013e318054714a. [DOI] [PubMed] [Google Scholar]
- 50.Teichgräber V, Ulrich M, Endlich N, Riethmüller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kürthy G, Schmid KW, Weller M, Tümmler B, Lang F, Grassme H, Döring G, Gulbins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 14: 382–391, 2008. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 51.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247, 2008. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 52.Venkatachalam K, Wong CO, Zhu MX. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium 58: 48–56, 2015. doi: 10.1016/j.ceca.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venugopal B, Browning MF, Curcio-Morelli C, Varro A, Michaud N, Nanthakumar N, Walkley SU, Pickel J, Slaugenhaupt SA. Neurologic, gastric, and opthalmologic pathologies in a murine model of mucolipidosis type IV. Am J Hum Genet 81: 1070–1083, 2007. doi: 10.1086/521954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voyer LE, Alvarado C, Cuttica RJ, Balestracci A, Zardini M, Lago N. Nephrotic syndrome due to immunoglobulin M mesangial glomerulonephritis preceding juvenile idiopathic arthritis. Iran J Kidney Dis 7: 231–234, 2013. [PubMed] [Google Scholar]
- 55.Ward DM, Pevsner J, Scullion MA, Vaughn M, Kaplan J. Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages. Mol Biol Cell 11: 2327–2333, 2000. doi: 10.1091/mbc.11.7.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X, Gao Y, Xu L, Dang W, Yan H, Zou D, Zhu Z, Luo L, Tian N, Wang X, Tong Y, Han Z. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci Rep 7: 9371, 2017. doi: 10.1038/s41598-017-09907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia M, Conley SM, Li G, Li PL, Boini KM. Inhibition of hyperhomocysteinemia-induced inflammasome activation and glomerular sclerosis by NLRP3 gene deletion. Cell Physiol Biochem 34: 829–841, 2014. doi: 10.1159/000363046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong J, Xia M, Xu M, Zhang Y, Abais JM, Li G, Riebling CR, Ritter JK, Boini KM, Li PL. Autophagy maturation associated with CD38-mediated regulation of lysosome function in mouse glomerular podocytes. J Cell Mol Med 17: 1598–1607, 2013. doi: 10.1111/jcmm.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu M, Li X, Walsh SW, Zhang Y, Abais JM, Boini KM, Li PL. Intracellular two-phase Ca2+ release and apoptosis controlled by TRP-ML1 channel activity in coronary arterial myocytes. Am J Physiol Cell Physiol 304: C458–C466, 2013. doi: 10.1152/ajpcell.00342.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang C, Boini KM, Xia M, Abais JM, Li X, Liu Q, Li PL. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension 60: 154–162, 2012. doi: 10.1161/HYPERTENSIONAHA.111.189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang C, Xia M, Boini KM, Li CX, Abais JM, Li XX, Laperle LA, Li PL. Epithelial-to-mesenchymal transition in podocytes mediated by activation of NADPH oxidase in hyperhomocysteinemia. Pflugers Arch 462: 455–467, 2011. doi: 10.1007/s00424-011-0981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F, Jin S, Yi F, Li PL. TRP-ML1 functions as a lysosomal NAADP-sensitive Ca2+ release channel in coronary arterial myocytes. J Cell Mol Med 13, 9B: 3174–3185, 2009. doi: 10.1111/j.1582-4934.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang F, Li PL. Reconstitution and characterization of a nicotinic acid adenine dinucleotide phosphate (NAADP)-sensitive Ca2+ release channel from liver lysosomes of rats. J Biol Chem 282: 25259–25269, 2007. doi: 10.1074/jbc.M701614200. [DOI] [PubMed] [Google Scholar]
- 64.Zhang F, Xia M, Li PL. Lysosome-dependent Ca(2+) release response to Fas activation in coronary arterial myocytes through NAADP: evidence from CD38 gene knockouts. Am J Physiol Cell Physiol 298: C1209–C1216, 2010. doi: 10.1152/ajpcell.00533.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang F, Xu M, Han WQ, Li PL. Reconstitution of lysosomal NAADP-TRP-ML1 signaling pathway and its function in TRP-ML1(-/-) cells. Am J Physiol Cell Physiol 301: C421–C430, 2011. doi: 10.1152/ajpcell.00393.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang F, Zhang G, Zhang AY, Koeberl MJ, Wallander E, Li PL. Production of NAADP and its role in Ca2+ mobilization associated with lysosomes in coronary arterial myocytes. Am J Physiol Heart Circ Physiol 291: H274–H282, 2006. doi: 10.1152/ajpheart.01064.2005. [DOI] [PubMed] [Google Scholar]