Abstract
Progressive tubulointerstitial fibrosis may occur after acute kidney injury due to persistent inflammation. Purinergic signaling by 5′-ectonucleotidase, CD73, an enzyme that converts AMP to adenosine on the extracellular surface, can suppress inflammation. The role of CD73 in progressive kidney fibrosis has not been elucidated. We evaluated the effect of deletion of CD73 from kidney perivascular cells (including pericytes and/or fibroblasts of the Foxd1+ lineage) on fibrosis. Perivascular cell expression of CD73 was necessary to suppress inflammation and prevent kidney fibrosis in Foxd1CreCD73fl/fl mice evaluated 14 days after unilateral ischemia-reperfusion injury or folic acid treatment (250 mg/kg). Kidneys of Foxd1CreCD73fl/fl mice had greater collagen deposition, expression of proinflammatory markers (including various macrophage markers), and platelet-derived growth factor recepetor-β immunoreactivity than CD73fl/fl mice. Kidney dysfunction and fibrosis were rescued by administration of soluble CD73 or by macrophage deletion. Isolated CD73−/− kidney pericytes displayed an activated phenotype (increased proliferation and α-smooth muscle actin mRNA expression) compared with wild-type controls. In conclusion, CD73 in perivascular cells may act to suppress myofibroblast transformation and influence macrophages to promote a wound healing response. These results suggest that the purinergic signaling pathway in the kidney interstitial microenvironment orchestrates perivascular cells and macrophages to suppress inflammation and prevent progressive fibrosis.
Keywords: adenosine, ectonucleotidase, fibroblast, folic acid, ischemia-reperfusion, macrophage, pericyte
INTRODUCTION
Chronic kidney disease (CKD) remains a major public health problem, and the available treatments are limited. Interstitial fibrosis, a hallmark of CKD, may result in organ dysfunction and kidney failure (26). Novel therapeutic strategies depend on a critical understanding of the molecular mechanisms that occur after injury and contribute to the development of fibrosis.
Interstitial fibrosis is characterized by extracellular collagen deposition, tubular loss, chronic inflammation, chronic hypoxia, and capillary rarefaction (3, 16, 60, 66). Important pathogenic mechanisms in CKD progression include vascular and endothelial cell damage (2), hypoxia/hypoxia-inducible factor (22), innate and adaptive immunity (65), cell cycle arrest (72), and epigenetic mechanisms (5, 68). While some injured tubules undergo repair and regeneration, injury may also be accompanied by inflammation, maturation, and proliferation of fibroblasts and extracellular matrix deposition as part of the process of fibrosis. Inflammation contributes to tissue fibrosis (69). Although T helper (Th)2 cytokines may induce fibrosis, Th1 cytokines such as IL-12 or interferon-γ inhibit pulmonary (21, 35) and renal (1, 49) fibrosis. The source of fibroblasts in the injured kidney (fibrocytes, epithelial-to-mesenchymal transition, intrinsic fibroblasts, or pericytes) remains controversial (27, 42). Normal pericyte function becomes abnormal after the transformation to activated myofibroblasts, which leads to loss of microvascular stability, ineffective angiogenesis, increased permeability, and capillary rarefaction, processes leading to fibrosis.
ATP can be released in an uncontrolled fashion by cell necrosis or through specific mechanisms, e.g., activation of pannexin channels (31, 32, 47), and can function as a danger-associated molecular pattern by activating the immune system (28). Alternatively, CD39 can dephosphorylate ATP and produce ADP/AMP; ADP can bind to P2 purinergic receptors and initiate cellular responses, including proliferation, differentiation, apoptosis, metabolism, secretion, and cell migration (15). CD73, a membrane-anchored 5′-ectonucleotidase that converts AMP to adenosine (47), is highly expressed on interstitial fibroblasts/pericytes and the brush-border membrane of proximal tubule epithelial cells (12) and is found on rodent mesangial cells and intercalated cells (41). Adenosine has many affects in the kidney and signals through four G protein-coupled receptors (4). Adenosine A1 receptors (A1ARs) in renal tubules and endothelial cells (43, 44), adenosine A2a receptors (A2aARs) on hematopoietic cells (12, 13), adenosine A2b receptors (A2bARs) on renal parenchymal cells (19), and adenosine A3 receptors (A3ARs) (54) have been shown to modulate initial kidney injury, yet their role in kidney fibrosis is not well understood.
Kidney pericytes and resident fibroblasts, platelet-derived growth factor receptor-β (PDGFRβ)+ cells of the Foxd1+ lineage as shown by fate mapping studies (18, 27), express CD73 (59) and contribute to the myofibroblast pool in murine models of progressive kidney fibrosis (38, 39). To test the hypothesis that CD73 expression on PDGFRβ+ cells regulates kidney fibrosis, we used a lineage-specific CD73 knockout (KO) mouse (Foxd1CreCD73fl/fl), which we have previously characterized (59). We identified a cell intrinsic role for CD73 on PDGFRβ+ cells and PDGFRβ+ cell/macrophage cross talk in the protection against fibrosis. These results suggest that adenosine production by CD73 in the local kidney microenvironment is necessary to prevent fibrosis.
METHODS
Mice.
Male mice (8−12 old, 18–25 g) were used unless otherwise indicated. Foxd1CreCD73fl/fl (on a mixed background) and littermate control CD73fl/fl mice have been previously described (59). Immunofluorescence localization of CD73 and other cell-specific markers in the kidney has been previously used to validate deletion of CD73 in perivascular cells (Foxd1CreCD73fl/fl mice) (59). Briefly, these prior studies demonstrated that PDGFRβ+ cells (fibroblasts or pericytes) expressed CD73 in wild-type (WT) but not KO (Foxd1CreCD73fl/fl) kidneys while CD73 expression in proximal tubules of WT kidneys was not altered in KO kidneys. B6.129S1-Nt5etm1Lft/J (CD73−/−) mice (on the C57BL/6J background) were provided by Linda Thompson (Oklahoma Medical Research Foundation, Oklahoma City, OK) and are also available from Jackson Laboratories; Jackson C57BL/6J WT mice were used as controls.
Osmotic mini-pumps (Alzet Osmotic Pumps, Cupertino, CA) were implanted subcutaneously on days 2 or 4 after unilateral ischemia-reperfusion injury (IRI) and delivered soluble CD73 [5′-nucleotidase (5′-NT), Enzo Life Sciences, Farmingdale, NY] or PBS vehicle until day 14. Neutral clodronate liposomes or control liposomes (FormuMAX Scientific, Palo Alto, CA) were injected intraperitoneally to Foxd1CreCD73fl/fl mice (200 μl on day 2 and 100 μl on days 6 and 10 after unilateral IRI).
Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the University of Virginia Animal Care and Use Committee (Animal Protocol no. 3830). Animals housed in the same cage were randomized to different treatement groups. Mice were maintained in standard vivarium housing with a 12:12-h light-dark cycle on a chow diet, and water was freely available. Blood was collected under anesthesia, mice were euthanized while anesthetized by cervical dislocation, and kidneys were collected for analysis.
In vivo models of fibrosis.
Unilateral IRI surgery (ischemia followed by 1 or 14 days of reperfusion) was performed as previously described (51) except with 20 min of ischemia to produce equivalent initial injury in Foxd1CreCD73fl/fl and littermate control CD73fl/fl mice [no significant differences between the two groups in plasma creatinine, neutrophil gelatinase-associated lipocalin (Ngal), or kidney injury molecule-1 (Kim1) levels]. To assess kidney function after unilateral IRI, when renal reserve would mask changes in the injured kidney, the contralateral (unoperated) kidney was removed (nephrectomy) 1 day before necropsy and saved as the control kidney.
In a second model, mice were injected with folic acid (250 mg/kg ip in a vehicle of 0.3 M sodium bicarbonate) and euthanized 14 days later.
Assessment of kidney function and histology.
Plasma creatinine was measured by enzymatic assay according to the manufacturer’s instructions (Diazyme Laboratories, Poway, CA) but using twice the recommended sample volume; the accuracy of this method has been verified by LC-MS (29, 36). Blood urea nitrogen (BUN) was measured using the DetectX Urea Nitrogen (BUN) Detection kit (Arbor Assays, Ann Arbor, MI). Collagen, the most abundant extracellular matrix protein, was detected using Masson’s trichrome stain (which does not discriminate collagen subtypes) and picrosirius red stain (with polarized microscopy used to view birefringence of collagen fibers; mostly mature, thicker, densely packed collagen bundles; smaller bundles are weakly birefringent). Masson’s trichrome staining and quantification were performed using stereological methods (StereoInvestigator software, MBF Bioscience, Williston, VT) as previously described (1) and as described below. Picrosirus red staining and quantification with ImageJ was performed as previously described (51).
Quantitative stereological assessment of kidney histology.
The extent of kidney tubulointerstitial fibrosis revealed by trichrome staining was assessed in an unbiased, systematic manner using design-based stereology to achieve statistically accurate random sampling of kidney sections. The investigator was blinded to the experimental identity of the sections. Sections were imaged using a Zeiss Axio Imager Z2/Apotome Microscope fitted with motorized focus drives and motorized XYZ microscope stage (MBF Bioscience) and integrated to a workstation running StereoInvestigator software (version 11.06.2, MBF Bioscience). The area fraction fractionator probe (StereoInvestigator) was used for stereological analysis of the fractional area (percentage of the total surface area) of the section occupied by tubulointerstitial collagen deposition (Masson’s trichrome stain). The following parameters were defined: counting frame, 400 × 400 μm; sample grid, 800 × 800 μm; and grid spacing, 85 μm. These values were determined empirically such that adequate numbers of sample sites were visited and adequate numbers of markers (a binary system of marking for presence of blue stain, indicating extracellular deposition of collagen, or absence at the marker locus) were acquired, as determined by the software, in keeping with accepted counting rules for stereology. A total of ~575 grid sites were evaluated per section; the sampling fraction was 25% of a total average area of 4.9 × 106 μm2 for each kidney section.
Using the same Zeiss AxioImager Z1 and MBF Bioscience system, virtual tissue section images were acquired using StereoInvestigator software. Parameters were defined in this application of the software so that images of kidney sections acquired with a ×5 or ×10 objective were automatically stitched together to produce a full view of the kidney section (e.g., as shown in Supplemental Fig. S1, available online at https://doi.org/10.6084/m9.figshare.8869256.v2) that could be magnified from the image with preservation of the original digital resolution.
Quantitative real-time PCR.
Total RNA was isolated and reverse transcribed to cDNA, and RT-PCR was performed as previously described (1). The primers sequences for the following transcripts were as cited: Ngal (52), Kim1 (52), α-smooth muscle actin (α-SMA; Acta) (52), collagen type III-α1 (Col3a1) (52), IL-1β (IL1b) (52), IL-6 (Il6) (52), interferon-γ (Ifng) (29), TNF-α (Tnfa) (52), nitric oxide synthase 2 (Nos2) (29), arginase 1 (Arg1) (29), mannose receptor, C type 1 (Mrc1) (29), and macrophage scavenger receptor 1 (Msr1) (29). Additional primer sequences are shown in Table 1. Each gene was normalized to the housekeeping gene and then to the control group (ΔΔCq) except for adenosine receptors, which were only normalized to the housekeeping gene (ΔCq).
Table 1.
Primer sequences for real-time quantitative PCR
| Transcript | Sequence ID | Forward | Reverse |
|---|---|---|---|
| Csf1 | NM_001113530 | 5′-ATGAGCAGGAGTATTGCCAAGG-3′ | 5′-TCCATTCCCAATCATGTGGCTA-3′ |
| Nt5e (Cd73) | NM_011851 | 5′-GTCACTGAGACTCAAATCCCACAC-3′ | 5′-CTGTACAGTGCTGACTATGCTCAC-3′ |
| 18S rRNA | NR_003278 | 5′-CGGCTACCACATCCAAGGAA-3′ | 5′-AGCTGGAATTACCGCGGC-3′ |
| Pdgfrb | NM_008809 | 5′-ACCCTCTCCATTCCCCTGATT-3′ | 5′-CAACTGGCCTCTGAGGACTAAA-3′ |
| Adora1 | NM_001008533.3 | 5′-CTCTGAAGAGATGCCATGGAACAG-3′ | 5′-CTCAGAGAACAGCCAGGAATGATG-3′ |
| Adora2a | NM_001331095.1 | 5′-TGGCTTGGTGACGGGTATG-3′ | 5′-CGCAGGTCTTTGTGGAGTTC-3′ |
| Adora2b | NM_007413.4 | 5′-CTGGGACACGAGCGAGAG-3′ | 5′-GCTGGTGGCACTGTCTTTAC-3′ |
| Adora3 | NM_009631.4 | 5′-GTGAGTTCTCTGGACTGTTGTGAC-3′ | 5′-GATGTAGGTGATGTTCAGCCAGTC-3′ |
Csf1, colony stimulating factor 1; Nt5e, 5′-ectonucleotidase; Pdgfrb, platelet-derived growth factor receptor-β; Adora1, Adora2a, Adora2b, and Adora3, adenosine A1, A2a, A2b, and A3 receptor, respectively.
Hydroxyproline assay of tissue collagen content.
Hydroxyproline content in the kidney was measured by the color development of Ehrlich’s reagent with furans derived from hydroxyproline oxidation essentially as previlously described (67) but with minor modifications. Kidneys (approximately one-half of a whole kidney) were dried overnight at 110°C, weighed, and then hydrolyzed in 1 ml of 6 M HCl at 110°C for 24 h. Debris was removed by centrifuging, and Amberlite MB-1 resin (8 mg, Sigma-Aldrich) was added to the supernatant to neutralize samples, which were then mixed on a tube rotator for 30 min at room temperature followed by centrifugation for 30 min at 12,000 rpm at room temperature. A 25-µl aliquot of the supernatant was mixed with 500 µl of 0.05 M chloramine-T (sodium p-toluenesulfonchloramide) in citrate-acetate buffer (0.88 M sodium acetate, 0.24 M citric acid, 0.21 M glacial acetic acid, 0.85 M sodium hydroxide, and 1 mM EDTA, pH 6.0) and incubated for 20 min at room temperature to oxidize the kidney hydrolyzate. The reaction was terminated by the addition of 500 μl of 3.15 M HClO4 (prepared by diluting 27 ml of 70% HClO4 to 100 ml final with water), and samples were mixed thoroughly followed by incubation for 5 min at room temperature. Next, 500 μl of Ehrlich’s solution [prepared by the addition of 15 ml of 50% p-dimethylaminobenzaldehyde in 60% (wt/vol) perchloric acid to 65 ml isopropanol] were added, and samples were mixed thoroughly and incubated for 20 min at 60°C to allow for color development. Immediately after incubation, samples were cooled in an ice bath for 5 min, and absorbance was measured at 550 nm. Samples were compared with a standard curve of hydroxyproline standards.
Primary cell culture.
Pericytes were isolated from kidneys of global CD73−/− and Jackson C57BL/6J WT mice by positive selection of PDGFRβ-positive cells using a modification of previously published methods (37). A single cell suspension was obtained by treating minced mouse kidneys with DMEM-F-12 containing 1% penicillin-streptomycin, 0.5 mg/ml liberase DL, and 100 U/l DNase I. Digestion was inactivated with DMEM-F-12 containing 10% FBS. The suspension was then passed through a 40-μm cell strainer to remove glomeruli and multicellular debris. To deplete tubular cells, the cell suspension was treated with CD326 EpCAM Microbeads (Miltenyi Biotec, Auburn, CA) and separated with LS magnetic columns (Miltenyi) where the flow through was collected. Cells were plated on a 6-cm culture dish and incubated overnight at 37°C with 5% CO2 to allow for recovery of PDGFRβ expression on cell membranes.
Both nonattached (the majority of cells) and attached (if present; recovered by trypsinization) cells were harvested. To obtain PDGFRβ+ cells, cells were incubated with rat anti-mouse CD140b clone APB5 (eBioscience/ThermoFisher) in MACS buffer followed by incubation with goat anti-rat IgG Microbeads (Myltenyi), and separation was performed using LS columns (Myltenyi). Cells were plated on 0.2% gelatin-coated tissue culture dishes in DMEM-F-12 with 1% penicillin-streptomycin, 1% insulin-transferrin-selenium (GIBCO/ThermoFisher), and 10% FBS and incubated at 37°C in a humidified atmosphere with 5% CO2. Cultures from passages 3–8 were used for all experiments. Cell phenotype was verified by demonstrating expression of α-SMA (Fig. 3) and PDGFRβ (Supplemental Fig. S1, available online at https://doi.org/10.6084/m9.figshare.8869256.v2) and, for KO cultures, absence of Cd73 (Fig. 3).
Fig. 3.
CD73 suppresses a myofibroblast phenotype in pericytes/fibroblasts. Platelet-derived growth factor receptor-β (PDGFRβ)+ cells were isolated from kidneys of wild-type (WT) and global CD73−/− [knockout (KO)] mice. A: transcript levels of Cd73 [5′-ectonucleotidase (Nt5e)] and α-smooth muscle actin (α-SMA; Acta) by RT-PCR. n = 5 WT mice and 6 CD73−/− mice. B: CD73 and α-SMA detection by immunofluorescence. C and D: proliferation of WT and KO pericytes decreased with treatment with increasing doses of soluble CD73 [5′-nucleotidase (5′-NT); C] and was higher in CD73−/− pericytes than in WT pericytes (D). OD540, optical density at a wavelength of 540 nm. The statistical comparisons shown in C are vehicle versus all doses of 5′-NT except as shown in KO graph at day 7. Individual data points (C and D) are shown in dot plots in Supplemental Fig. S3 (available online at https://doi.org/10.6084/m9.figshare.8869256.v2). Data were normalized to WT day 1 and are representative of 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by unpaired t-test in A and two-way ANOVA in C and D. Scale bar = 50 µm.
Assessment of cell viability.
WT mouse primary perivascular cells were seeded on six-well plates coated with 0.2% gelatin. Once confluent, cells were treated with 5′-NT (10 U/ml) for 24 h and stained with FITC-annexin V and 7-amino-actinomycin D (7-AAD; to discriminate live/dead cells using an eBioscience Annexin V-FITC Apoptosis Detection kit, no. 88-8005-74, ThermalFisher Scientific) according to the manufacturer’s protocol. Briefly, cells were trypsinized and centrifuged followed by a wash with annexin V binding buffer (AVBB). Floating cells from the media were pooled together with adherent cells for the analysis. Cells were incubated with 5 μl FITC-annexin V in 100 µl AVBB for 15 min in the dark followed by the addition of 7-AAD to a final concentration of 1 µg/ml in AVBB. Cells were kept on ice and immediately subjected to flow cytometry analysis; data were collected using a BD FACS Calibur cytometer (Becton Dickinson, Franklin Lakes, NJ) with Cytek 8 color upgrade (Cytek Development). Data analysis was performed using FlowJo 10.5.3 (TreeStar). Annexin V or 7-AAD single stained and unstained cells were used for correction of spillover fluorescence compensation.
Immunofluorescent labeling of kidney sections.
Kidney samples were prepared and analyzed as previously described (51). Rabbit anti-α-SMA-Cy3 (clone 1A4, Sigma-Aldrich), rat anti-PDGFRβ-A647 (clone APB, BioLegend, San Diego, CA), and anti-F4/80-A555 (clone BM8, Invitrogen/ThermoFisher) were used. In addition, anti-F4/80 (CI-A3-1, Bio-X-Cell) monoclonal antibody was conjugated with A488 monoclonal antibody labeling kits (Invitrogen). Similarly, goat anti-mouse macrophage mannose receptor (CD206, R&D Systems) was conjugated with A555. These latter two conjugated antibodies were used for the sections shown in Supplemental Fig. S4 (available online at https://doi.org/10.6084/m9.figshare.8869256.v2). All antibodies were used at 2 µg/ml.
Immunofluorescent labeling of cultured pericytes.
Cultured pericytes were plated on gelatin-coated chamber slides or coverslips and incubated overnight to achieve a confluent monolayer. Confluent monolayers were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer for 15–30 min. Cells were labeled with sheep anti-porcine CD73 [2 μg/ml, AF4488, R&D Systems, direct tagged using the Alexa Fluor 555 or Alexa Fluor 647 Antibody/Protein Labeling Kit (ThermoFisher) per the manufacturer’s instructions], mouse anti-α-SMA-Cy3 (1:500, 1A4, Sigma; Fig. 3), or mouse anti-α-SMA (1:500, A488, Santa Cruz Biotechnology; data not shown).
MTT cell proliferation assay.
Cultured pericytes were plated (4,000 cells/well) in 96-well flat bottom plates with DMEM-F-12 plus 1% penicillin-streptomycin and 10% FBS but without phenol red. Proliferation was determined using the Vybrant MTT Cell Proliferation kit (ThermoFisher Scientific). After the addition of MTT, the concentration of solubilized formazen product was determined by measuring absorbance at 540 nm on days 0, 1, 2, and 4 or days 0, 1, 4, and 7.
Flow cytometry.
Kidneys were digested, and single cell suspensions were prepared, labeled, and analyzed as previously described (52). Antibodies against CD11b (M170), CD11c (418), CD45 (30-F11), F4/80 (BM8), Ly6C (HK1.4), and Ly6G (IA8) were purchased from Biolegend. Data were acquired on a FACS Calibur cytometer (Becton Dickinson, Franklin Lakes, NJ) with the Cytek 8 color flow cytometry upgrade (Cytek Development) and analyzed using FlowJo (version 10) software (FlowJo). Visualization of data was first performed using T-distributed stochastic neighbor embedding (tSNE), and the resulting clustering of cell populations determined in an unbiased fashion by the algorithm was used to inform subsequent gating strategies for myeloid population subsetting from which cell numbers were gathered.
Statistics.
Data are presented as means ± SE. Comparison of two groups was performed using an unpaired Student’s t-test. For multiple-group comparisons with two variables, two-way ANOVA followed by a Tukey's multiple-comparisons test was performed. P values of <0.05 were considered statistically significant. All tests were performed with Prism version 6.0 software (GraphPad Software, La Jolla, CA).
RESULTS
Loss of CD73 in Foxd1+ cells induces kidney dysfunction and fibrosis.
After acute kidney injury (AKI), kidneys can undergo repair and recover function or maladaptive repair and lose function, resulting in CKD (62, 63). To assess the role of CD73 on Foxd1+ cells in a model of kidney recovery after unilateral IRI, we established an ischemic time that produced equivalent initial injury in all groups. Based on prior results using these Foxd1CreCD73fl/fl mice (59), mice in the present study were subjected to 20 min of unilateral kidney ischemia. Twenty-four hours later, plasma creatinine (Fig. 1A) and biomarkers of kidney injury, Kim1 and Ngal, in IRI kidneys (Fig. 1B) were similarly increased in Foxd1CreCD73fl/fl and CD73fl/fl control mice compared with their respective sham-operated controls. In separate groups of mice (and all subsequent experiments), the function of the ischemic kidney (20-min ischemia) was measured at 14 days of reperfusion, 1 day after nephrectomy of the contralateral (uninjured) kidney. Plasma creatinine levels were increased in Foxd1CreCD73fl/fl mice (Fig. 1C), suggesting that while CD73fl/fl mice were able to recover kidney function by 14 days, Foxd1CreCD73fl/fl mice exhibited continued kidney dysfunction.
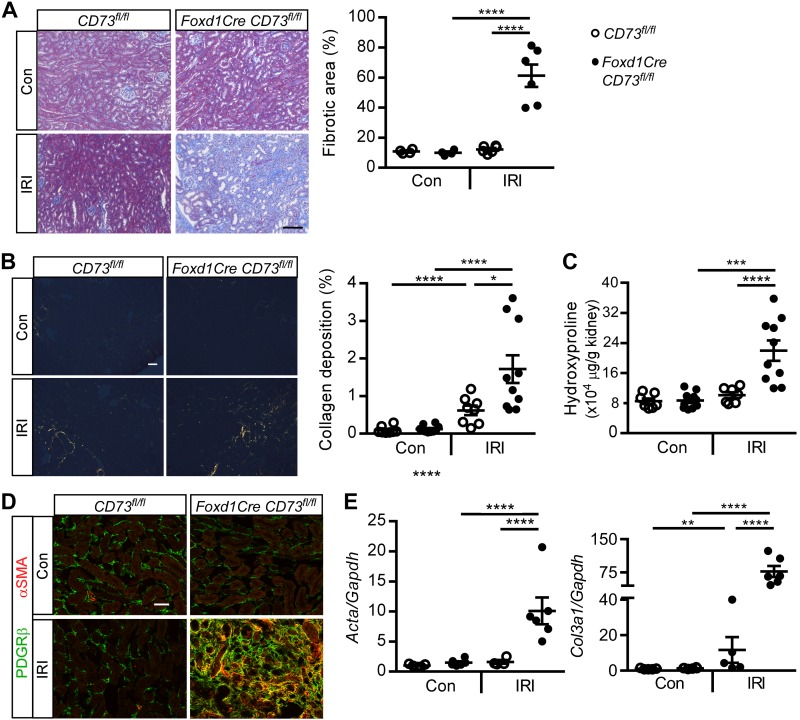
Fig. 1.
Loss of CD73 on pericytes/fibroblasts prevents recovery of kidney function after ischemia-reperfusion injury (IRI). Unilateral IRI was performed on kidneys from Foxd1Cre CD73fl/fl (n = 9) and littermate control (Con; n = 9) mice, and plasma creatinine (PCr; A) as well as kidney transcript levels of kidney injury molecule-1 (Kim1; B, left) and neutrophil gelatinase-associated lipocalin (Ngal; B, right) relative to Gapdh (relative mRNA levels, fold changes) were measured 24 h later. In a separate group of mice, unilateral IRI was performed on kidneys from Foxd1Cre CD73fl/fl (n = 9) and littermate Con (n = 10) mice, a nephrectomy of the contralateral Con kidney was performed on day 13, and PCr was measured on day 14 (C). ***P < 0.001 by and unpaired t-test.
IRI kidneys from Foxd1CreCD73fl/fl mice had increased collagen deposition as assessed by Masson’s trichrome stain (Fig. 2A), picrosirius red stain (Fig. 2B), and hydroxyproline content (Fig. 2C) compared with CD73fl/fl mice and contralateral control kidneys. This degree of fibrosis is similar to prior findings with this injury model (1, 27, 51, 52). In CD73fl/fl mice, IRI kidneys had a small increase in interstitial collagen deposition, suggesting that there may have been activation of a wound healing response in mice that recovered from IRI. PDGFRβ+, α-SMA+ myofibroblast density was increased in IRI kidneys from Foxd1CreCD73fl/fl mice compared with CD73fl/fl mice (Fig. 2D); this increased PDGFRβ immunoreactivity has been previously observed in kidney fibrosis (31, 52). Transcript levels of fibrosis markers, Acta (encoding α-SMA) and Col3a1 (encoding collagen type III), were increased in IRI kidneys from Foxd1Cre CD73fl/fl mice compared with controls (Fig. 2E). Kidneys of Foxd1CreCD73fl/fl mice had more fibrosis and higher BUN (comparable to increased fibrosis and plasma creatinine after IRI) than CD73fl/fl mice 14 days after folic acid injection (another fibrotic model; Supplemental Fig. S1, available online at https://doi.org/10.6084/m9.figshare.8869256.v2). Taken together, the data indicate that CD73 expression by Foxd1+ cells is necessary to promote kidney recovery from AKI and prevent progressive fibrosis. Consistent with this conclusion is the finding that the weight of IRI kidneys decreased significantly (~30%) compared with contralateral control kidneys from Foxd1CreCD73fl/fl mice but no significant decrease was found between IRI and contralateral kidneys from CD73fl/fl mice (Supplemental Fig. S1D).
Fig. 2.
Foxd1Cre CD73fl/fl mice have increased kidney fibrosis. The experimental setup was as described in Fig. 1C. A and B: representative images and quantification of kidney collagen deposition by Masson’s trichrome stain (fibrotic area expressed as a percentage of the kidney tissue surface area occupied by blue trichrome stain; A) and interstitial collagen deposition (orange/yellow) as assessed by picrosirus red staining and collagen birefringence under polarized microscopy (percentage of the kidney tissue surface area occupied by orange/yellow pixels; B). C: quantification of kidney hydroxyproline (in μg hydroxyproline/g kidney tissue dry wt). D: myofibroblasts (orange/yellow) detected by colocalization of platelet-derived growth factor receptor-β (PDGFRβ; green) and α-smooth muscle actin (α-SMA; red) by immunofluorescence in kidneys. E: kidney α-SMA (Acta) and collagen type III-α1 (Col3a1) relative to Gapdh transcript levels (relative mRNA levels, fold changes). Con, contralateral control (unoperated) kidney; IRI, ischemia-reperfusion kidney. *P < 0.05, ***P < 0.001, and ****P < 0.0001 by two-way ANOVA. n = 4 CD73fl/fl mice and 6 Foxd1Cre CD73fl/fl mice in A, n = 8 CD73fl/fl mice and 10 Foxd1Cre CD73fl/fl mice in B, 7 CD73fl/fl mice and 10 Foxd1Cre CD73fl/fl mice in C, n = 5 CD73fl/fl mice and 6 Foxd1Cre CD73fl/fl mice in D. Scale bars = 100 µm in A and 50 µm in B and D.
CD73 suppresses a myofibroblast phenotype in perivascular PDGFRβ+ cells.
Fibroblasts and pericytes contribute to the myofibroblast pool during kidney fibrosis (14, 27). PDGFRβ+ cells (56, 71) were isolated from the kidney and will be referred to here as pericytes (18, 71), while recognizing that specific pericyte and fibroblast markers are lacking in the kidney. Transcript and protein levels of CD73 confirmed deletion of CD73 in CD73−/− pericytes (Fig. 3, A and B). Pericytes, but not tubule, endothelial, or macrophage cell lines, expressed Pdgfr transcripts (Supplemental Fig. S2, available online at https://doi.org/10.6084/m9.figshare.8869256.v2). WT and CD73−/− pericytes expressed very low levels of A1AR, A2aAR, and A2bAR transcripts and approximately threefold higher levels of A3ARs (A3AR > A1AR ~ A2aAR ~ A2bAR; data not shown), thus supporting the idea that CD73-generated adenosine could act in an autocrine fashion. Characteristic of myofibroblasts, CD73−/− pericytes had increased expression of α-SMA by RT-PCR. Addition of 5′-NT to CD73−/− pericytes did not alter α-SMA production (data not shown), suggesting that acutely generated adenosine does not regulate α-SMA production. However, increasing doses of 5′-NT decreased proliferation in WT and CD73−/− pericytes (Fig. 3C), and CD73−/− pericytes proliferated faster than WT pericytes (Fig. 3D). It is unlikely that 5′-NT affected cell viability. In a separate experiment, WT pericytes were exposed to vehicle or the highest concentration of 5′-NT (10 U/ml) for 24 h. Cells were then trypsinized and labeled with 7-AAD and FITC-annexin V. The percent viability was 89.3 (n = 2) and 90.1 (n = 3) for vehicle and 5′-NT, respectively. These data suggest that CD73 deletion may increase the pericyte to myofibroblast transition in a cell intrinsic manner.
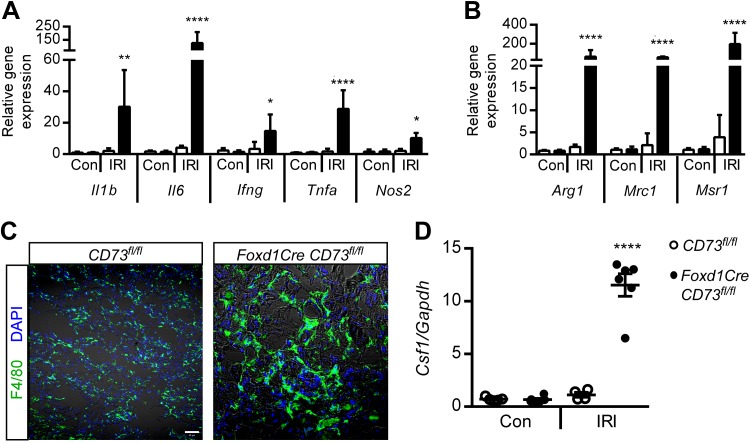
Loss of CD73 in Foxd1+ cells reduces chronic inflammation.
Resolution of inflammation is necessary for kidney recovery, and chronic inflammation can promote fibrosis (73). Macrophages can dictate the balance between wound healing and progressive fibrosis. Transcript levels of proinflammatory cytokines (Tnfa, Il1b, Il6, and Ifng; Fig. 4A), which can be produced by many cell types including macrophages, as well as transcript levels of markers for proinflammatory macrophages (e.g., Nos2) and profibrotic macrophages (Arg1, Mrc1, and Msr1; Fig. 4B) [dot plots for Fig. 4, A and B, are provided in Supplemental Fig. S3, available online at https://doi.org/10.6084/m9.figshare.8869256.v2) were elevated in IRI kidneys from Foxd1CreCD73fl/fl mice compared with control mice. Tissue macrophage density and gene expression for a macrophage growth factor, colony stimulating factor 1 (Csf1), were increased in IRI kidneys from Foxd1CreCD73fl/fl mice (Fig. 4, C and D). Macrophages upregulate F4/80 in inflamed tissues, including kidney tissue (data not shown), which could make the cells appear larger (Fig. 4C), although flow cytometry showed no change in size (data not shown). Immunofluorescence labeling revealed increased immunoreactivity for macrophage mannose receptor (aka CD206 or MRC1, M2 marker) in IRI kidneys from Foxd1CreCD73fl/fl mice (Supplemental Fig. S4, available online at https://doi.org/10.6084/m9.figshare.8869256.v2), consistent with RT-PCR data.
Fig. 4.
Loss of CD73 on pericytes/fibroblasts increases ischemia-reperfusion injury (IRI)-induced macrophage-mediated inflammation. The experimental setup was as shown in Fig. 1C. A and B: kidney transcript levels of proinflammatory cytokines IL-1β (Il1b), IL-6 (Il6), interferon-γ (Ifng), and TNF-α (Tnfa) and macrophage marker nitric oxide synthase 2 (Nos2) (A) as well as profibrotic/wound healing macrophage markers arginase 1 (Arg1), mannose receptor, C type 1 (Mrc1), and macrophage scavenger receptor 1 (Msr1) (B) relative to Gapdh. Con, contralateral control (unoperated) kidney; IRI, ischemia-reperfused kidney. Individual data points in A and B are shown in dot plots in Supplemental Fig. S3 (available online at https://doi.org/10.6084/m9.figshare.8869256.v2). C: detection of F4/80+ macrophages by immunofluorescence microscopy. D: kidney transcript levels of colony stimulating factor 1 (Csf1) relative to Gapdh. n = 5 CD73fl/fl mice and 6 Foxd1Cre CD73fl/fl mice. *P < 0.05, **P < 0.01, and ****P < 0.0001 by two-way ANOVA. Scale bar = 50 µm.
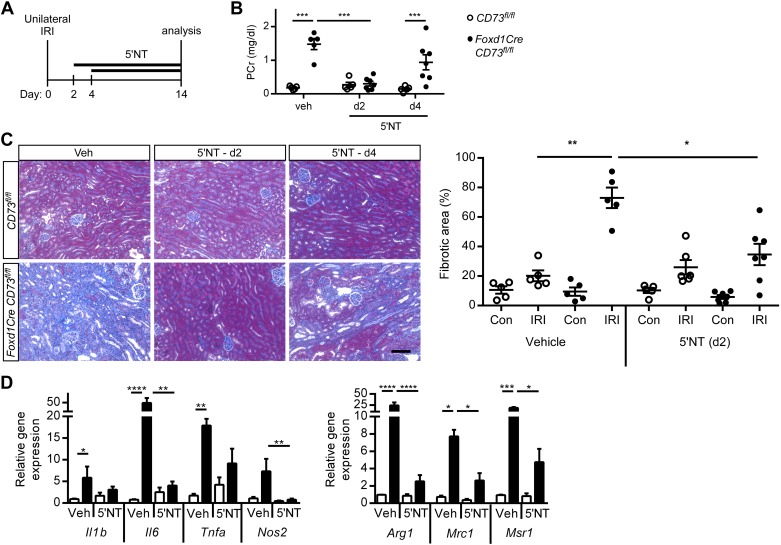
Soluble CD73 rescues Foxd1CreCD73fl/fl mice and prevents kidney dysfunction, inflammation, and fibrosis.
During the first 2–4 days after AKI, a switch from proinflammatory to wound healing macrophages is necessary for tissue repair. 5′-NT or vehicle was administered continuously from days 2 or 4 after unilateral IRI until day 14 (Fig. 5A). The increase in plasma creatinine (Fig. 5B) and fibrotic area (Fig. 5C) in vehicle-treated Foxd1CreCD73fl/fl mice was abrogated when 5′-NT administration began on day 2. Increased transcript levels of the proinflammatory cytokine Il6 and markers of proinflammatory (Nos2) or profibrotic (Arg1, Mrc1, and Msr1) macrophages were suppressed in IRI kidneys from 5′-NT-treated Foxd1CreCD73fl/fl mice (Fig. 5D) (dot plots for Fig. 5D are shown in Supplemental Fig. S3, available online at https://doi.org/10.6084/m9.figshare.8869256.v2). These data suggest that day 2 is a crucial window for CD73 expression in suppressing a proinflammatory, profibrotic phenotype and promoting wound healing processes.
Fig. 5.
Soluble CD73 [5′-nucleotidase (5′-NT)] rescues kidney function, suppresses the induction of macrophage markers, and reduces fibrosis. A: Foxd1Cre CD73fl/fl (filled circles and bars) and CD73fl/fl control (open circles and bars) littermate mice were treated with vehicle (Veh) or 5′-NT (5 U/day sc per mouse in osmotic minipumps) starting at day 2 (d2) or day 4 (d4) after unilateral ischemia-reperfusion injury (IRI), and samples were harvested on day 14. B: plasma creatinine (PCr). C: kidney fibrosis (collagen deposition) in IRI and contralateral (unoperated) control (Con) kidneys was evaluated by Masson’s trichrome (left, images; right, quantification of fibrotic area expressed as the percentage of the kidney tissue surface area occupied by blue trichrome stain). D: transcript levels of profibrotic and proinflammatory macrophage markers by real-time PCR were measured in IRI kidneys from vehicle- and 5′-NT (day 2)-treated mice. Il1b, IL-1β; Il6, IL-6; Tnfa, TNF-α; Nos2, nitric oxide synthase 2; Arg1, arginase 1; Mrc1, mannose receptor, C type 1; Msr1, macrophage scavenger receptor 1. Individual data points in D are shown in dot plots in Supplemental Fig. S3 (available online at https://doi.org/10.6084/m9.figshare.8869256.v2). n = 5 CD73fl/fl mice and 5–7 Foxd1Cre CD73fl/fl mice. *P < 0.05, **P < 0.01, ***P < 0.001, and **** P < 0.0001 by two-way ANOVA. Scale bar = 100 µm.
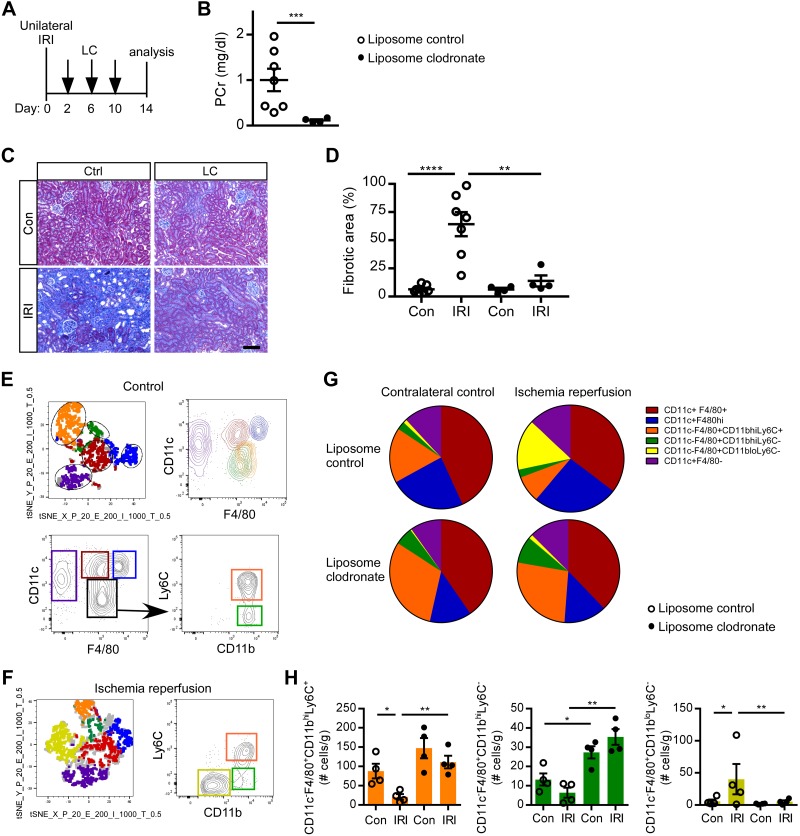
Monocyte/macrophage depletion promotes kidney recovery from IRI in Foxd1CreCD73fl/fl mice.
Phagocytic monocyte/macrophages were depleted with liposome clodronate beginning on day 2 (Fig. 6A). Plasma creatinine (Fig. 6B) and fibrosis (Fig. 6, C and D) were decreased in IRI kidneys from macrophage-depleted Foxd1CreCD73fl/fl mice. To examine the monocyte/macrophage phenotype, we used a flow cytometry gating strategy to quantify kidney myeloid populations in an unbiased manner. tSNE analysis was performed on concatenated samples from control kidneys pregated on live, CD45+, singlet, Ly6G−, and CD11b+ cells. tSNE plots of liposome control, contralateral control kidneys from CD73fl/fl mice revealed five distinct populations using CD11b, CD11c, F4/80, and Ly6C (Fig. 6E) and a sixth population in liposome control, ischemia-reperfused kidneys from Foxd1CreCD73fl/fl mice (Fig. 6 F and G). Quantification of myeloid populations revealed a decrease in the CD11c−F4/80+CD11bhiLy6C+ (orange) subset and an increase in the CD11c−F4/80+CD11bloLy6C- (yellow) population in liposome control IRI kidneys compared with contralateral controls and IRI kidneys from liposome clodronate-treated Foxd1CreCD73fl/f mice. There was no statistically significant difference in CD11c+F4/80+, CD11c+F4/80hi, CD11c+F4/80− populations (data not shown). Furthermore, in IRI kidneys that were protected from progression to fibrosis after liposome clondronate, CD11c−F4/80+CD11bhiLy6C+ (orange) and CD11c−F4/80+CD11bhiLy6C− (green) populations increased ~7–10-fold compared with liposome control IRI kidneys, whereas the CD11c−F4/80+CD11bloLy6C- (yellow) subset declined, thus suggesting (but requiring future functional characterization of subsets) that a shift in macrophage populations contributes to susceptibility to fibrotic progression after IRI. Taken together, these results clearly demonstrate a role for macrophages in promoting the profibrotic phenotype observed in IRI kidneys of Foxd1CreCD73fl/fl mice.
Fig. 6.
Deletion of macrophages rescues kidney function and reduces fibrosis in Foxd1Cre CD73fl/fl mice. A: Foxd1Cre CD73fl/fl mice were administered control liposomes (Ctrl; n = 7) or liposome clodronate (LC; n = 4) on days 2, 6, and 8 after unilateral ischemia-reperfusion injury (IRI). B: on day 14, kidney function was evaluated by measuring plasma creatinine (PCr). C and D: kidney fibrosis (collagen deposition) as revealed by Masson’s trichrome (representative images; C) was quantified in contralateral control (Con) and IRI kidneys (fibrotic area expressed as a percentage of the kidney tissue surface area occupied by blue trichrome stain; D). E−H: flow cytometry analysis. Samples were pregated on live, CD45+, singlet, Ly6G−, and CD11b+ cells. E: T-distributed stochastic neighbor embedding (tSNE) plot (top left) of concatenated samples from an unoperated control kidney in the liposome control group of CD73fl/fl mice. Gates were drawn on the five different groups and applied to the CD11c by F4/80 plot (top right) to determine myeloid populations by surface phenotype in an unbiased manner. Bottom plots show the gating strategy as determined by tSNE analysis. Colored gates were then applied back on the tSNE plot, demonstrating the gating strategy corresponds with the five clusters. F: representative tSNE plot and CD11clo F4/80+ population (black gate) further gated for Ly6C and Cd11b and displaying an additional population (yellow) in liposome control-treated IRI kidneys of Foxd1Cre CD73fl/fl mice. G and H: pie chart (G) and quantification (H) of myeloid populations from IRI and contralateral control kidneys of liposome control- and LC-treated Foxd1Cre CD73fl/fl mice. *P < 0.05, **P < 0.01, and ****P < 0.0001 by an unpaired t-test (B) or two-way ANOVA (D and H). Scale bar = 100 µm.
DISCUSSION
Our results suggest a key role for purinergic signaling in the kidney microenvironment to suppress inflammation and promote wound healing processes that minimize fibrosis. We demonstrated that CD73 expression by Foxd1+ perivascular pericytes/fibroblasts is necessary to suppress inflammation by attenuating macrophage infiltration in kidneys subjected to IRI. In the absence of CD73 on perivascular pericyte/fibroblasts, persistent inflammation was associated with marked tissue infiltration of macrophages that bore molecular signatures consistent with proinflammatory and wound healing type macrophages. Cultured perivascular pericytes/fibroblasts lacking CD73 had augmented proliferation and mRNA expression of α-SMA consistent with their transformation into myofibroblasts. Thus, CD73-generated adenosine produced locally by perivascular fibroblasts/pericytes may act on adenosine receptors in an autocrine and/or paracrine fashion, attenuate macrophage infiltration, and thereby regulate resolution of inflammation and fibrosis (Fig. 7).
Fig. 7.
Perivascular CD73 is necessary to attenuate progressive fibrosis after acute kidney injury. CD73 expression by pericytes/fibroblasts suppresses progressive kidney fibrosis in wild-type mice (CD73fl/fl control mice in the present study). CD73-generated adenosine produced locally by perivascular fibroblasts/pericytes may act on adenosine receptors (ARs) in an autocrine and/or paracrine fashion, attenuate macrophage (M⌀) infiltration and profibrotic properties, and thereby regulate resolution of inflammation and fibrosis. In the absence of perivascular CD73, acute kidney injury progresses to fibrosis. The results suggest a key role for purinergic signaling in the kidney microenvironment in coordinating intercellular and intracellular communication to suppress inflammation and subsequent fibrosis.
Endothelial activation, epithelial cell death, and inflammation occur early in fibrosis (6, 57). If inflammation resolves and tubules regenerate, an effective wound healing response occurs and kidneys can recover (62). However, if maladaptive processes occur, such as persistent inflammation and myofibroblast transformation, progressive fibrotic processes persist (16, 62, 63). Attenuating inflammation is a potential strategy to mitigate fibrosis, and improved strategies may derive from understanding the specific cellular and molecular mechanisms of the AKI to CKD transition.
Kidney 5′-ectonucleotidase expression and regulation (41) in mice is low in the cortical part of the proximal tubule (although it is expressed in mesangial cells) but increases significantly at the corticomedullary junction, reaching a maximum intensity in the S3 segment of the outer medulla (33, 41, 59). Brush-border CD73 likely acts on nucleotide substrates that gain access to the urine by filtration or by release from proximal tubule cells (41) to produce adenosine whose action, due to its rapid metabolism, is likely limited to the luminal side, unlikely to reach the peritubular interstitium (40, 41) and limited to the site of CD73 expression (55, 61). Perivascular pericytes and fibroblasts abundantly express CD73 (33, 41), which likely acts on nucleotide substrates released by tubule cells (7, 8, 11) during normal homeostasis to control local vascular tone (23) and regulate erythropoietin production (50). In our injury model, mRNA expression of CD73 was transiently higher (though not significantly) in kidneys 24 h after 20 min of ischemia compared with contralateral uninjured controls, but there was no difference in expression at 14 days (Supplemental Fig. S5, available online at https://doi.org/10.6084/m9.figshare.8869256.v2). Increased CD73 has also been observed in kidneys in an ischemic preconditioning model (20).
Our results identified the critical role that Foxd1+ cells contribute to the local production of adenosine by CD73 in fibrosis. Foxd1 progenitor cells give rise to a population of stromal cells that mature to form vascular smooth muscle cells, glomerular mesangial cells, pericytes, and fibroblasts (18). In disease states, Foxd1 lineage perivascular cells detach from the capillary walls, migrate to the interstitial space, and proliferate while expressing collagen type I, PDGFRβ, PDGFRα, and CD248 (10, 58). Although there may be ample production of adenosine by CD73 in proximal tubules in Foxd1CreCD73fl/fl mice, its compartmentalized localization and metabolism near the brush border (17) limits its local action to the tubule lumen (40) and would appear to make it inaccessible to the interstitial microenvironment. As proximal tubule cell expression of CD73 is necessary to prevent AKI, proximal tubule-generated adenosine may be necessary to limit injury during the early phase (59), while pericyte/fibroblast-generated adenosine may be necessary in the recovery phase to suppress chronic inflammation, myofibroblast transformation, and fibrosis, as shown in the present study. This suggests that the adenosine produced by CD73 in the lumen of proximal tubules does not contribute to limiting inflammation in the interstitium. Perivascular purinergic signaling, however, suppresses interstitial inflammation and coordinates the complex interplay in the AKI to CKD transition. In the absence of CD73 on perivascular pericytes/fibroblasts, there is an abundance of macrophage infiltration after IRI, primarily in the kidney outer medulla, that has the molecular signature consistent with both proinflammatory (Il1b, Ifng, Tnfa, and Nos2) and wound healing macrophages (Arg1, Mrc1, and Msr1). Although we have previously shown that chemokine (C-C motif) receptor 2 and chemokine (C-X3-C motif) receptor 1 contribute to monocyte influx into the kidney (46), the increased expression of kidney Csf1 (Fig. 4D) likely also contributes to the infiltration of macrophages (9, 73). Furthermore, CSF1 may contribute to M2 macrophage polarization from resident or bone marrow-derived macrophages (25, 45, 64).
The persistence of inflammation in the absence of perivascular pericyte/fibroblast CD73 leads to progressive fibrosis. Although inflammation may have an important role in the elimination of dead cells and foreign organisms, it may also have a critical early role in the regeneration of injured tissues (34). Thus, inflammation may be necessary early after injury to initiate repair processes; however, unopposed or persistent inflammation may lead to fibrosis (70). Recently, it has been shown in chondrocytes that IL-1 and TNF-α decrease expression of Sox9, a transcription factor important for regeneration (48). Thus, perivascular pericyte/fibroblast CD73 may be necessary to attenuate inflammation to allow tubule regeneration. Whether the absence of perivascular pericyte/fibroblast CD73 inhibits regenerative mechanisms has not been evaluated.
Although germline deletion of CD73 may lead to other compensatory changes, our reconstitution experiments with 5′-NT provide additional support for the role of CD73 in mitigating inflammation and fibrosis. In these experiments, we initiated treatment of Foxd1CreCD73fl/fl mice with soluble 5′-NT beginning on day 2, which resulted in decreased M1 and M2 cytokines and fibrosis at day 14. These results are qualitatively similar to the kidney cytokine profile after unilateral IRI in WT mice with sufficient CD73. Interestingly, soluble 5′-NT initiated on day 4 was less effective than 5′-NT administered on day 2, suggesting that unless early inflammation is attenuated, progressive fibrosis may develop. This suggests that an effective therapeutic window for exogenous CD73 may be shortly after IRI. The tissue-specific site of action for 5′-NT is still unknown. 5′-NT may enter the interstitium in the IRI kidney in Foxd1CreCD73fl/fl mice due to increased endothelial permeability in kidney injury. While 5′-NT, and therefore presumably adenosine production in the microenvironment, was associated with reducing macrophage markers and fibrosis, adenosine generation may also regulate other pathways, such as transforming growth factor-β, a central mediator of fibrosis, and vascular responses (24), in mediating recovery.
The fibrosis that ensues after unilateral IRI in Foxd1CreCD73fl/fl mice requires the presence of macrophages. Depletion of phagocytic macrophages beginning on day 2 after IRI in Foxd1CreCD73fl/fl mice, which reduced fibrosis, allows for a proper wound healing response and prevention of chronic inflammation. This study and others have identified a role for macrophages as an important switch in the AKI to CKD progression (45, 73). While Arg1, Mrc1, and Msr1 markers have been associated with wound healing (25), it is possible that without resolution of inflammation or in the presence of a profibrotic microenvironment, this heterogeneous population of macrophages can also promote fibrosis (66). With the protocol used in this study, highly phagocytic myeloid cells are depleted and cells begin to repopulate 3 days after clondronate (13). This may result in depletion of a proinflammatory, profibrotic population and repopulation of a protective population that promotes wound healing. Likely a delicate balance dictates a wound healing response versus progressive fibrosis, and defining the roles of specific macrophage subpopulations will require single cell analysis of phenotype and function (53).
Our study suggests that purinergic signaling in kidney pericytes/fibroblasts regulates myofibroblast transformation. Generation of adenosine in the interstitial microenvironment may signal in an autocrine or paracrine manner to adenosine receptors to regulate fibrosis (17). Extracellular ATP and activation of P2 receptors promotes lung fibroblast proliferation, extracellular matrix deposition, and myofibroblast transformation (17), while A2AR stimulation promotes retinal pericyte proliferation (30). In the heart, A2bAR activation reduces fibroblast production of collagen (17). In in vitro experiments, we found that the absence of perivascular pericyte/fibroblast CD73 was associated with an increase in α-SMA expression and proliferation in Foxd1+ cells. Whether this is due to increased nucleotide binding to P2 receptors, decreased adenosine binding to adenosine receptors (via autocrine signaling), or loss of a nonenzymatic function of CD73 has yet to be determined.
In summary, this study identified Foxd1+ cells as crucial players in mediating inter- and intracellular purinergic signaling during kidney fibrosis. As CD73 is still expressed on other cells besides pericytes in Foxd1CreCD73fl/fl mice, the data suggest a role for local paracrine signaling between pericytes/fibroblasts and macrophages. Adenosine generation by proximal tubules during the early injury phase may be necessary to limit inflammation (59); then, as kidneys enter the recovery phase, adenosine generation by pericytes/fibroblasts is necessary to suppress chronic inflammation, myofibroblast transformation, and fibrosis. Altogether, purinergic signaling coordinates the complex interplay of the kidney microenvironment during the initiation of injury and recovery.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants F32-DK-108563 (to H. M. Perry), T32-DK-72922, R01-DK-062324, and R01-DK-085259 (to M. D. Okusa), and DK-091444 and DK-107941 (to A. Bajwa), by the American Society of Nephrology Ben Lipps Fellowship Program (to H. M. Perry), by International Research Training Group Grant IRTG1902 (“Intra- and interorgan communication of the cardiovascular system”) funded by the Deutsche Forschungsgemeinschaft (to J. Schrader), and by the Ministerium für Kultur und Wissenschaft des Landes Nordrhein-Westfalen Landesprogramm für geschlechtergerechte Hochschulen (to N. Görldt). The stereology data described here were gathered on an “MBF Bioscience and Zeiss microscope system for stereology and tissue morphology” funded by NIH Grant 1-S10-RR-026799-01 (to M. D. Okusa).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
M. D. Okusa has a research grant from Pfizer and has equity in Adenosine Therapeutics. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
H.M.P., N.G., S.-s.J.S., K.P.R., A.B., N.P., J.Y., D.L.R., J.S., and M.D.O. conceived and designed research; H.M.P., N.G., L.H., K.P.R., I.M.E., S.T., N.P., J.Y., and D.L.R. performed experiments; H.M.P., N.G., S.-s.J.S., L.H., K.P.R., I.M.E., S.T., N.P., J.Y., D.L.R., and M.D.O. analyzed data; H.M.P., N.G., S.-s.J.S., K.P.R., S.T., N.P., J.Y., D.L.R., J.S., and M.D.O. interpreted results of experiments; H.M.P., N.G., K.P.R., and D.L.R. prepared figures; H.M.P., N.G., D.L.R., J.S., and M.D.O. drafted manuscript; H.M.P., N.G., S.-s.J.S., K.P.R., S.T., J.Y., D.L.R., J.S., and M.D.O. edited and revised manuscript; H.M.P., N.G., S.-s.J.S., L.H., K.P.R., I.M.E., A.B., S.T., N.P., J.Y., D.L.R., J.S., and M.D.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the University of Virginia Research Histology Core for the assistance with the preparation of histology slides.
REFERENCES
- 1.Bajwa A, Huang L, Kurmaeva E, Ye H, Dondeti KR, Chroscicki P, Foley LS, Balogun ZA, Alexander KJ, Park H, Lynch KR, Rosin DL, Okusa MD. Sphingosine kinase 2 deficiency attenuates kidney fibrosis via IFN-γ. J Am Soc Nephrol 28: 1145–1161, 2017. doi: 10.1681/ASN.2016030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151–156, 2007. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 3.Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, Kellum JA, Ronco C; ADQI XIII Work Group . Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 27: 687–697, 2016. doi: 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol 22: 14–20, 2011. doi: 10.1681/ASN.2009121217. [DOI] [PubMed] [Google Scholar]
- 5.Bechtel W, McGoohan S, Zeisberg EM, Müller GA, Kalbacher H, Salant DJ, Müller CA, Kalluri R, Zeisberg M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 16: 544–550, 2010. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broadus AE, Kaminsky NI, Hardman JG, Sutherland EW, Liddle GW. Kinetic parameters and renal clearances of plasma adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate in man. J Clin Invest 49: 2222–2236, 1970. doi: 10.1172/JCI106441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butlen D, Jard S. Renal handling of 3′-5′-cyclic AMP in the rat. The possible role of luminal 3′-5′-cyclic AMP in the tubular reabsorption of phosphate. Pflugers Arch 331: 172–190, 1972. doi: 10.1007/BF00587260. [DOI] [PubMed] [Google Scholar]
- 9.Cao Q, Harris DC, Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 30: 183–194, 2015. doi: 10.1152/physiol.00046.2014. [DOI] [PubMed] [Google Scholar]
- 10.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, Duffield JS, Lin SL. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80: 1170–1181, 2011. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 11.Coulson R. Metabolism and excretion of exogenous adenosine 3′:5′-monophosphate and guanosine 3′:5′-monophosphate. Studies in the isolated perfused rat kidney and in the intact rat. J Biol Chem 251: 4958–4967, 1976. [PubMed] [Google Scholar]
- 12.Day Y-J, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest 112: 883–891, 2003. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- 14.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflugers Arch 452: 552–562, 2006. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- 16.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11: 264–276, 2015. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari D, Gambari R, Idzko M, Müller T, Albanesi C, Pastore S, La Manna G, Robson SC, Cronstein B. Purinergic signaling in scarring. FASEB J 30: 3–12, 2016. doi: 10.1096/fj.15-274563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez IG, Duffield JS. The FOXD1 lineage of kidney perivascular cells and myofibroblasts: functions and responses to injury. Kidney Int Suppl 4: 26–33, 2014. doi: 10.1038/kisup.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5: e137, 2008. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Köhle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 18: 833–845, 2007. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 21.Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-beta and procollagen I and III gene expression. Exp Lung Res 21: 791–808, 1995. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- 22.Haase VH. Pathophysiological consequences of HIF activation: HIF as a modulator of fibrosis. Ann N Y Acad Sci 1177: 57–65, 2009. doi: 10.1111/j.1749-6632.2009.05030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holz FG, Steinhausen M. Renovascular effects of adenosine receptor agonists. Ren Physiol 10: 272–282, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Huang DY, Vallon V, Zimmermann H, Koszalka P, Schrader J, Osswald H. Ecto-5′-nucleotidase (cd73)-dependent and -independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol 291: F282–F288, 2006. doi: 10.1152/ajprenal.00113.2005. [DOI] [PubMed] [Google Scholar]
- 25.Huen SC, Cantley LG. Macrophages in renal injury and repair. Annu Rev Physiol 79: 449–469, 2017. doi: 10.1146/annurev-physiol-022516-034219. [DOI] [PubMed] [Google Scholar]
- 26.Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol 80: 309–326, 2018. doi: 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- 27.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 509: 310–317, 2014. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, Ye H, Rosin DL, Guyenet PG, Okusa MD. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J Clin Invest 126: 1939–1952, 2016. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson JA, Carlson EC. Inhibition of bovine retinal microvascular pericyte proliferation in vitro by adenosine. Am J Physiol Heart Circ Physiol 263: H634–H640, 1992. doi: 10.1152/ajpheart.1992.263.2.H634. [DOI] [PubMed] [Google Scholar]
- 31.Jankowski J, Perry HM, Medina CB, Huang L, Yao J, Bajwa A, Lorenz UM, Rosin DL, Ravichandran KS, Isakson BE, Okusa MD. Epithelial and endothelial pannexin1 channels mediate AKI. J Am Soc Nephrol 29: 1887–1899, 2018. doi: 10.1681/ASN.2017121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11: 201–212, 2011. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaissling B, Le Hir M. The renal cortical interstitium: morphological and functional aspects. Histochem Cell Biol 130: 247–262, 2008. doi: 10.1007/s00418-008-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature 529: 307–315, 2016. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keane MP, Belperio JA, Burdick MD, Strieter RM. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 281: L92–L97, 2001. doi: 10.1152/ajplung.2001.281.1.L92. [DOI] [PubMed] [Google Scholar]
- 36.Keppler A, Gretz N, Schmidt R, Kloetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int 71: 74–78, 2007. doi: 10.1038/sj.ki.5001988. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi H, Liu Q, Binns TC, Urrutia AA, Davidoff O, Kapitsinou PP, Pfaff AS, Olauson H, Wernerson A, Fogo AB, Fong GH, Gross KW, Haase VH. Distinct subpopulations of FOXD1 stroma-derived cells regulate renal erythropoietin. J Clin Invest 126: 1926–1938, 2016. doi: 10.1172/JCI83551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramann R, Humphreys BD. Kidney pericytes: roles in regeneration and fibrosis. Semin Nephrol 34: 374–383, 2014. doi: 10.1016/j.semnephrol.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD. Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J Am Soc Nephrol 28: 776–784, 2017. doi: 10.1681/ASN.2016030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Hir M, Dubach UC. Sodium gradient-energized concentrative transport of adenosine in renal brush border vesicles. Pflugers Arch 401: 58–63, 1984. doi: 10.1007/BF00581533. [DOI] [PubMed] [Google Scholar]
- 41.Le Hir M, Kaissling B. Distribution and regulation of renal ecto-5′-nucleotidase: implications for physiological functions of adenosine. Am J Physiol Renal Physiol 264: F377–F387, 1993. doi: 10.1152/ajprenal.1993.264.3.F377. [DOI] [PubMed] [Google Scholar]
- 42.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15: 102–111, 2004. doi: 10.1097/01.ASN.0000102474.68613.AE. [DOI] [PubMed] [Google Scholar]
- 44.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol 286: F298–F306, 2004. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE Jr, Lobo PI, Okusa MD. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menzies RI, Tam FW, Unwin RJ, Bailey MA. Purinergic signaling in kidney disease. Kidney Int 91: 315–323, 2017. doi: 10.1016/j.kint.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 48.Murakami S, Lefebvre V, de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J Biol Chem 275: 3687–3692, 2000. doi: 10.1074/jbc.275.5.3687. [DOI] [PubMed] [Google Scholar]
- 49.Oldroyd SD, Thomas GL, Gabbiani G, El Nahas AM. Interferon-gamma inhibits experimental renal fibrosis. Kidney Int 56: 2116–2127, 1999. doi: 10.1046/j.1523-1755.1999.00775.x. [DOI] [PubMed] [Google Scholar]
- 50.Paul P, Rothmann SA, Meagher RC. Modulation of erythropoietin production by adenosine. J Lab Clin Med 112: 168–173, 1988. [PubMed] [Google Scholar]
- 51.Perry HM, Huang L, Wilson RJ, Bajwa A, Sesaki H, Yan Z, Rosin DL, Kashatus DF, Okusa MD. Dynamin-related protein 1 deficiency promotes recovery from AKI. J Am Soc Nephrol 29: 194–206, 2018. doi: 10.1681/ASN.2017060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perry HM, Huang L, Ye H, Liu C, Sung SJ, Lynch KR, Rosin DL, Bajwa A, Okusa MD. Endothelial sphingosine 1-phosphate receptor-1 mediates protection and recovery from acute kidney injury. J Am Soc Nephrol 27: 3383–3393, 2016. doi: 10.1681/ASN.2015080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry HM, Okusa MD. Driving change: kidney proximal tubule CSF-1 polarizes macrophages. Kidney Int 88: 1219–1221, 2015. doi: 10.1038/ki.2015.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabadi MM, Lee HT. Adenosine receptors and renal ischaemia reperfusion injury. Acta Physiol (Oxf) 213: 222–231, 2015. doi: 10.1111/apha.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schrader J, Berne RM, Rubio R. Uptake and metabolism of adenosine by human erythrocyte ghosts. Am J Physiol 223: 159–166, 1972. doi: 10.1152/ajplegacy.1972.223.1.159. [DOI] [PubMed] [Google Scholar]
- 56.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens 20: 297–305, 2011. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 57.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 58.Smith SW, Schrimpf C, Parekh DJ, Venkatachalam M, Duffield JS. Kidney pericytes: a novel therapeutic target in interstitial fibrosis. Histol Histopathol 27: 1503–1514, 2012. [DOI] [PubMed] [Google Scholar]
- 59.Sung SJ, Li L, Huang L, Lawler J, Ye H, Rosin DL, Vincent IS, Le TH, Yu J, Görldt N, Schrader J, Okusa MD. Proximal tubule CD73 is critical in renal ischemia-reperfusion injury protection. J Am Soc Nephrol 28: 888–902, 2017. doi: 10.1681/ASN.2016020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 307: F1187–F1195, 2014. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 61.Thompson CI, Sparks HV, Spielman WS. Renal handling and production of plasma and urinary adenosine. Am J Physiol Renal Physiol 248: F545–F551, 1985. doi: 10.1152/ajprenal.1985.248.4.F545. [DOI] [PubMed] [Google Scholar]
- 62.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26: 1765–1776, 2015. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Chang J, Yao B, Niu A, Kelly E, Breeggemann MC, Abboud Werner SL, Harris RC, Zhang MZ. Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells, and recovery in acute kidney injury. Kidney Int 88: 1274–1282, 2015. doi: 10.1038/ki.2015.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, Sgonc R. The immunology of fibrosis: innate and adaptive responses. Trends Immunol 31: 110–119, 2010. doi: 10.1016/j.it.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wick G, Grundtman C, Mayerl C, Wimpissinger TF, Feichtinger J, Zelger B, Sgonc R, Wolfram D. The immunology of fibrosis. Annu Rev Immunol 31: 107–135, 2013. doi: 10.1146/annurev-immunol-032712-095937. [DOI] [PubMed] [Google Scholar]
- 67.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93: 440–447, 1961. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 68.Wynn TA. Fibrosis under arrest. Nat Med 16: 523–525, 2010. doi: 10.1038/nm0510-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040, 2012. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44: 450–462, 2016. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xavier S, Sahu RK, Landes SG, Yu J, Taylor RP, Ayyadevara S, Megyesi J, Stallcup WB, Duffield JS, Reis ES, Lambris JD, Portilla D. Pericytes and immune cells contribute to complement activation in tubulointerstitial fibrosis. Am J Physiol Renal Physiol 312: F516–F532, 2017. doi: 10.1152/ajprenal.00604.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]