Abstract
Transplanted kidneys usually experience several episodes of ischemia, including cold ischemia during allograft storage in preservation solution. However, previous studies focusing on cold renal ischemia were only carried out in vitro or ex vivo. In the present study, we developed and characterized an in vivo mouse model of renal ischemia-reperfusion injury (IRI) induced exclusively by cold ischemia. C57BL/6 mice underwent right kidney nephrectomy, and the left kidney was kept cool with circulating cold saline in a kidney cup, while body temperature was maintained at 37°C. We clamped the renal pedicle and flushed out the blood inside the kidney with cold saline via an opening on the renal vein. The severity of renal IRI was examined with different ischemic durations. We found that the mice with <2 h of cold ischemia exhibited no significant changes in renal function or histopathology; animals with 3 or 4 h of cold ischemia developed into mild to moderate acute kidney injury with characteristic features, including the elevation in plasma creatinine concentration and reduction in glomerular filtration rate and tubular necrosis, followed by a subsequent recovery. However, mice with 5 h of cold ischemia died in a few days with severe acute kidney injury. In summary, we generated a mouse model of renal IRI induced exclusively by cold ischemia, which mimics graft cold storage in preservation solution, and renal function can be evaluated in vivo.
Keywords: cold ischemia, ischemia-reperfusion injury, kidney transplantation
INTRODUCTION
Kidney transplantation is the best therapeutic option for patients with end-stage renal disease. Not only is renal replacement therapy a life-saving procedure, but it also significantly improves the patient’s quality of life (9, 11, 20, 47). Ischemia-reperfusion injury (IRI) is one of the most serious pitfalls in kidney transplantation and considered as the most critical nonspecific factor for both early and late allograft dysfunction (6, 21, 48).
Ischemia and reperfusion are natural steps during kidney procurement and transplantation. Transplanted organs normally undergo several episodes of ischemia, including warm ischemia during harvesting operations and anastomosis of the graft and cold ischemia during organ storage in preservation solutions (5, 8, 25, 33, 39). Renal ischemia and reperfusion temporarily interrupts the supply of oxygen and nutrient to the kidney, initiating a cascade of deleterious cellular and molecular responses primarily in tubular epithelial cells (1, 3, 30). The pathophysiology of renal IRI, including cold ischemia-induced IRI, has been extensively studied in both experimental animal models and clinical trials (27, 28, 32, 38). However, the mechanisms underlying renal IRI have not been fully elucidated.
Animal models of warm ischemia-induced IRI generated by temporary unilateral or bilateral clamping of renal pedicles or renal arteries have been well-established and widely used in experimental studies (7, 44, 46). However, to our knowledge, most of the studies on renal cold ischemia were carried out using in vitro or ex vivo models (10, 22, 28, 32, 38) in which the graft functions are unable to be assessed in these models, whereas the in vivo models unavoidably include different durations of warm ischemia during procurement and vascular anastomosis. In the present study, we developed a new mouse model of renal IRI that is induced exclusively by cold ischemia, which mimics graft cold storage in preservation solution, and renal function can be evaluated in vivo.
METHODS
Animals.
C57BL/6 mice (male, 10–12 wk old) were purchased from Jackson Laboratory and housed in the University of South Florida Animal Facility. All protocols were approved by the Institutional Animal Care and Use Committee at the University of South Florida College of Medicine.
Cold ischemia-reperfusion model.
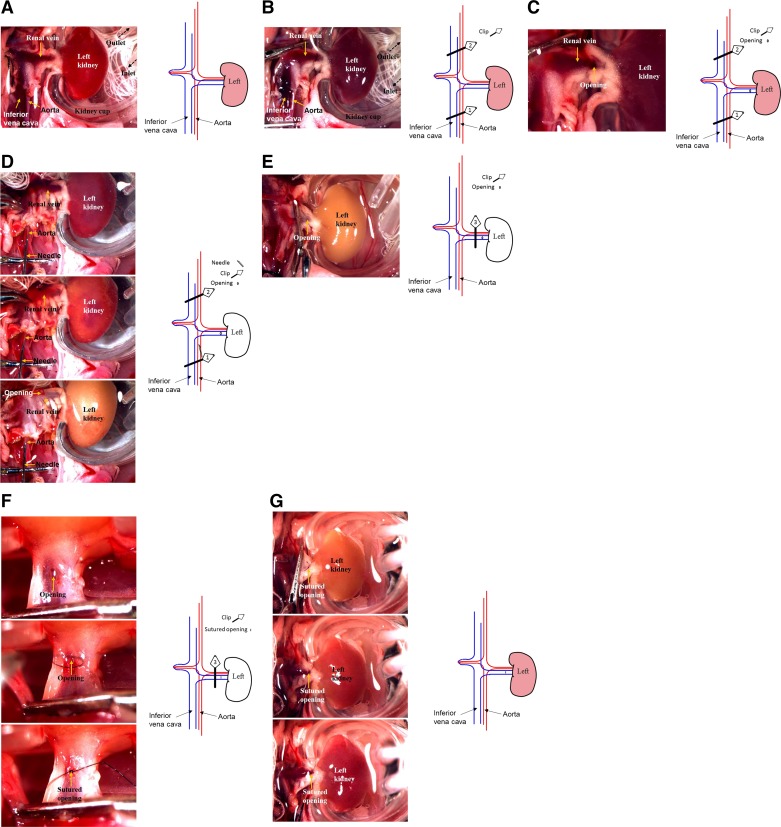
C57BL/6 mice were anesthetized with pentobarbital sodium (50 mg/ml ip), and their body temperature was maintained at 36.8–37.2°C during surgery with a temperature-controlled operating table (03-02, Vestavia Scientific). A midline abdominal incision was performed. The right pedicle was ligated, and the right kidney was removed. The left kidney, aorta, and inferior vena cava were exposed. Next, the left kidney was placed in a kidney cup (03-11, Vestavia Scientific) filled with saline at 4 ± 0.5°C by circulating sterilized solution from a saline reservoir on ice with a pump (P720, Instech Laboratories) (Fig. 1A). Three 5-mm microvascular miniclips (FE690K, AESCULAP) were used to prevent bleeding and induce ischemia in the following steps: 1) the inferior vena cava and aorta below the renal arteries were clamped together (clip 1) and the inferior vena cava and aorta above the renal arteries were then clamped (clip 2; Fig. 1B); 2) a small opening was cut on the left renal vein (Fig. 1C); 3) the aorta between the left renal artery and clip 1 was injected with 1–1.5 ml of 4°C 0.9% sterilized saline using a 30-gauge needle until the solution from the opening on the renal vein cleared and the color of the left kidney became white (Fig. 1D); 4) the left renal pedicle between the opening and the aorta was clamped (clip 3) and the two clips (clips 1 and 2) on the vena cava and aorta were removed, sequentially (Fig. 1E); and 5) the small opening was sutured with 10-0 suture (BV75-4, ETHICON; Fig. 1F). The ischemic durations were 1, 2, 3, 4, and 5 h (cold IRI-1 h, IRI-2 h, IRI-3 h, IRI-4 h, and IRI-5 h groups, respectively). The time of ischemia started from clamping the aorta with clip 2. The clip on the renal pedicle (clip 3) and kidney cup were removed at the end of the respective ischemic times. Renal blood flow was restored, and the wound was sutured. Mice were recovered in an incubator until fully awake. Sham-operated mice underwent identical surgical procedures, and the left kidney was incubated with 4 ± 0.5°C sterilized saline for 5 h without ischemia induction as a control group (cold IRI-0 h group).
Fig. 1.
Typical procedure images and schematic illustration of the cold ischemia-reperfusion injury (IRI) model. A: the left kidney was placed in a kidney cup and incubated with 4°C saline. B: the inferior vena cava and aorta below and above the renal arteries were clamped (clips 1 and 2, respectively). C: a small opening was cut on the left renal vein. D: the aorta between the left renal artery and clip 1 was injected with sterilized saline using a needle until the color of the left kidney became white. E: the left renal pedicle between the opening and the aorta was clamped (clip 3), and the two clips (clips 1 and 2) on the vena cava and aorta were removed. F: the small opening was sutured. G: renal blood flow was restored.
Plasma creatinine measurement.
Mice were anesthetized again with isoflurane 24 h and 7, 14, and 28 days after cold IRI. Blood samples (50 µl each) were collected through the retroorbital venous sinus and centrifuged at 8,000 rpm for 5 min at 4°C. Plasma (25 µl each) was obtained for creatinine measurement. Plasma creatinine concentration (PCr) was measured by HPLC at the O’Brien Center Core of the University of Alabama at Birmingham.
Glomerular filtration rate measurement.
Glomerular filtration rate (GFR) was measured by plasma FITC-sinistrin clearance with a single bolus injection in conscious mice before cold IRI (baseline with two kidneys at day −1) and 7, 14, and 28 days after cold IRI, as we have previously described (14, 42, 45). Briefly, mice were injected with FITC-sinistrin solution (5.6 mg/100 g body wt) through the retroorbital venous sinus. Blood (<10 µl) was collected in heparinized capillary tubes through the tail vein at 3, 7, 10, 15, 35, 55, and 75 min after sinistrin injection. Blood samples were centrifuged at 8,000 rpm for 5 min at 4°C. Plasma (1 µl) was collected from each sample. The FITC-sinistrin concentration of the plasma was measured using a plate reader (Cytation3, BioTek) with 485-nm excitation and 538-nm emission. GFR was calculated with GraphPad Prism 7 (GraphPad Software, San Diego, CA).
Morphological evaluation with light microscopy.
Kidneys were harvested and fixed in 4% paraformaldehyde solution 24 h or 28 days after cold IRI as previously described (44, 45). Fixed kidney tissues were embedded in paraffin, and 2-µm kidney tissue slices were cut and treated with periodic acid-Schiff (PAS) stain or Masson’s trichrome stain. In PAS-stained slices, 10 randomly chosen fields per section were captured under ×400 magnification from the corticomedullary region. The percentage of either necrotic tubules or obstructed tubules by casts in each image was quantified as previously reported (19, 23). In Masson’s trichrome-stained slices, 10 randomly chosen fields per section were captured under ×400 magnification. The percentage of interstitial fibrotic area was quantified as we previously described (45). All morphometric analyses were performed in a blinded manner.
Western blot analysis.
Protein extracts from kidney tissue (50 μg/lane) were separated on a 7.5% SDS-PAGE gel as we have previously described (14, 49). After blockade for 1 h at room temperature with 5% skim milk, membranes were incubated overnight at 4°C with a vascular endothelial cadherin (VE-cadherin) antibody (rabbit polyclonal IgG, 1:600, Abcam, Cambridge, UK). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit, IgG, 1:300,000, Bio-Rad, Hercules, CA). Immunoreactive bands were revealed by the ChemiDoc System (Bio-Rad). For protein normalization, membranes were striped with Restore Western Blot Stripping Buffer (Fisher Scientific, Waltham, MA) for 15 min to remove VE-cadherin antibody and secondary antibody and incubated overnight at 4°C with an antibody of β-actin (mouse monoclonal IgG, 1:5,000, Sigma, St. Louis, MO). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (goat anti-mouse IgG, 1:300,000, Bio-Rad). Immunoreactive bands were revealed by the ChemiDoc System (Bio-Rad) and qualified by Image Laboratory software (Bio-Rad).
Statistical analysis.
Effects of interest were tested using a Student’s paired t-tests or two-factor ANOVA with repeated measures when appropriate. Data are presented as means ± SE. Changes were considered to be significant if the P value was <0.05.
RESULTS
Plasma creatinine.
PCr had no significant change in the cold IRI-0 h, IRI-1 h, and IRI-2 h groups throughout the experiment compared with baseline at 24 h before surgery. PCr in the cold IRI-3 h group increased to 0.34 ± 0.06 mg/dl at 24 h after surgery and was restored to the basal level within 14 days after surgery. PCr in the cold IRI-4 h group increased to 1.14 ± 0.21 mg/dl at 24 h after surgery and was restored to the basal level within 28 days after surgery (Fig. 2). All mice in the cold IRI-5 h group died within 3 days after surgery.
Fig. 2.
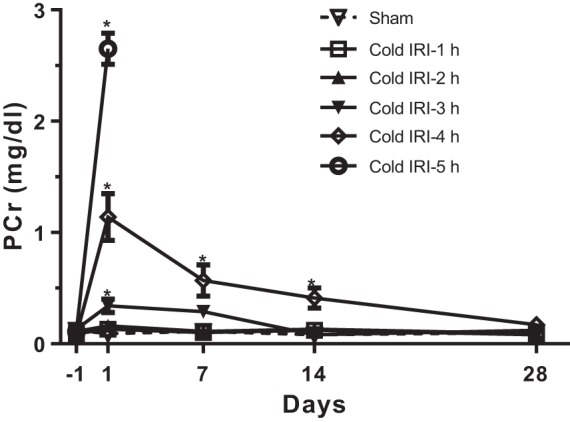
Plasma creatinine concentration (PCr) of the cold ischemia-reperfusion injury (IRI) model. PCr was measured for 28 days after cold IRI. All mice in the cold IRI-5 h group died within 3 days after surgery. Cold IRI induced significant increases in PCr of cold IRI-3 h and cold IRI-4 h groups at 24 h after surgery. PCr in the cold IRI-3 h and cold IRI-4 h groups recovered to basal levels within 14 and 28 days after surgery, respectively. n = 15–17/group. *P < 0.01 vs. the cold IRI-0 h group.
GFR in conscious mice.
Basal GFR were similar in all groups of mice. GFR in the cold IRI-0 h group was decreased by ~25% (from 233.6 ± 7.2 to 175.2 ± 15.1 μl/min) because of uninephrectomy and maintained at this level until the end of experiment. GFR had no significant difference in the cold IRI-1 h and cold IRI-2 h groups compared with the cold IRI-0 h group. GFR in the cold IRI-3 h group decreased to 142.9 ± 11.5 µl/min at 24 h after surgery and recovered to a level similar to the cold IRI-0 h group within 14 days after surgery. GFR in the cold IRI-4 h group decreased to 101.5 ± 9.8 µl/min at 24 h after surgery and recovered to a level similar to the cold IRI-0 h group within 28 days after surgery (Fig. 3).
Fig. 3.
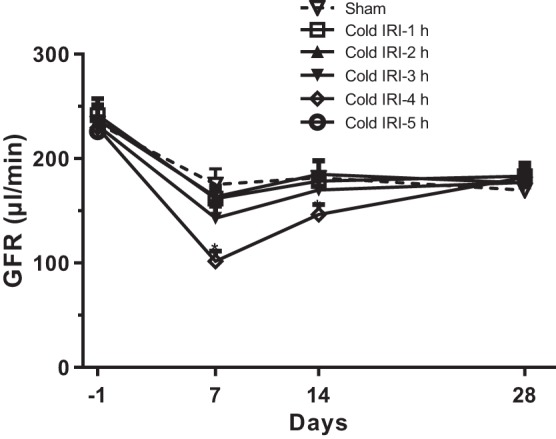
Glomerular filtration rate (GFR) of the cold ischemia-reperfusion injury (IRI) model. GFR was measured for 28 days after cold IRI. Cold IRI induced significant decreases in GFR of the cold IRI-3 h and cold IRI-4 h groups at 7 days after surgery. GFR in cold IRI-3 h and cold IRI-4 h groups were restored to similar levels to the cold IRI-0 min group within 14 and 28 days after surgery, respectively. n = 15–17/group. *P < 0.01 vs. the cold IRI-0 h group.
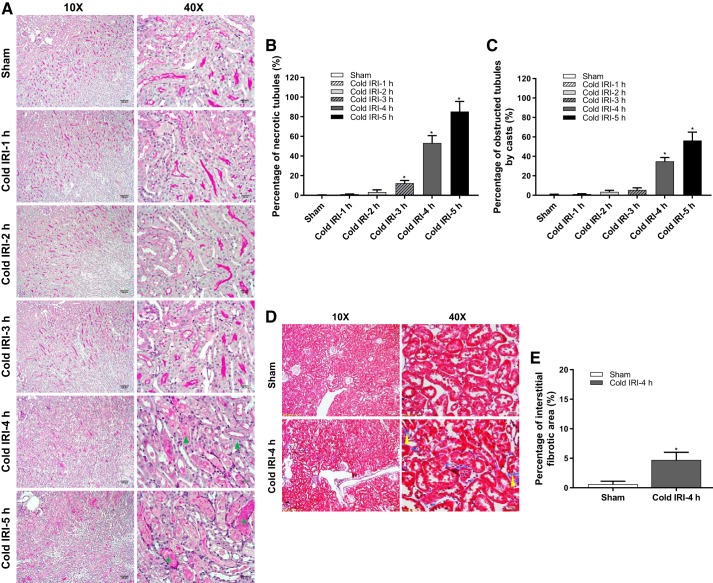
Histopathological analysis.
Renal histological analysis with PAS staining showed the most severe tubular injury, with the largest incidence of tubular necrosis and tubular casts in the kidney slices of the cold IRI-5 h group followed by the cold IRI-4 h group and cold IRI-3 h group at 24 h after surgery. These typical histopathological features of acute kidney injury were not observed in the kidney slices of cold IRI-0 h, cold IRI-1 h, or cold IRI-2 h groups (Fig. 4, A–C). The histological analysis with Masson’s trichrome staining showed a 4.7 ± 1.3% interstitial fibrotic area in the kidney slices of the cold IRI-4 h group, which was significantly larger than the cold IRI-0 h group (0.6 ± 0.5%) at 28 days after surgery (Fig. 4, D and E).
Fig. 4.
Histological analysis of the cold ischemia-reperfusion injury (IRI) model. A: periodic acid-Schiff (PAS) staining showed the typical histopathological features of acute kidney injury (AKI), including necrotic tubule (blue triangle) and obstructed tubules by casts (green star). Mice in the cold IRI-5 h group showed the most severe tubular injury, with the largest incidence of tubular necrosis (B) and tubular casts (C), followed by the cold IRI-4 h group and the cold IRI-3 h group. n = 11–14/group. *P < 0.01 vs. the cold IRI-0 h group. D and E: Masson’s trichrome staining showed significant interstitial fibrosis (yellow arrowhead) in the cold IRI-4 h group at 28 days after surgery. n = 5/group. *P < 0.01 vs. the cold IRI-0 h group.
Western blot analysis.
To assess vascular endothelium damage, protein levels of VE-cadherin in the kidney were measured and compared between cold IRI-0 h and cold IRI-4 h groups at 24 h after surgery. Renal VE-cadherin expression was 54.6 ± 17.3% lower in the cold IRI-4 h group compared with the cold IRI-0 h group (Fig. 5, A and B).
Fig. 5.
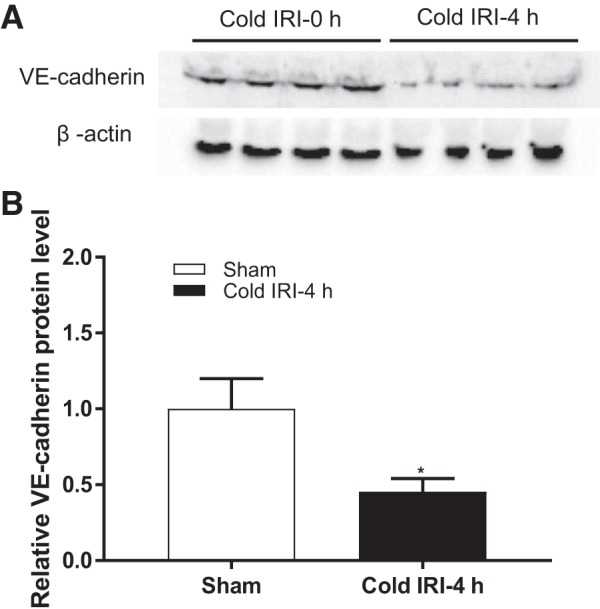
Protein level of vascular-endothelial (VE-)cadherin. A: immunoblots of VE-cadherin and the loading control of β-actin for protein normalization. B: protein level of VE-cadherin in the kidney was significantly decreased in the cold IRI-4 h group compared with the cold IRI-0 h group. n = 4. *P < 0.01 vs. the cold IRI-0 h group.
DISCUSSION
In the present study, we developed and characterized a novel in vivo model of renal IRI induced exclusively by cold ischemia. In this model, the kidney was kept in a kidney cup filled with circulating cold saline while the body temperature was maintained at 37°C. The blood inside the kidney was first flushed out with cold saline via a tiny opening on the renal vein, and renal ischemia was then generated by clamping of the renal pedicle. Before the clip on the renal pedicle was released, the opening on the renal vein was sutured to achieve the subsequent kidney reperfusion and animal survival. These surgical procedures mimic the episode of cold ischemia during graft storage in kidney transplantation and avoid any warm ischemia, which usually occurred during procurement of donor kidney and vascular anastomosis of the graft.
To mimic the conditions of graft storage in preservation solution, we flushed out the blood from the kidney with saline during cold ischemia, as previously reported by us (40, 41) and other groups (26, 35, 37) in mouse kidney transplants. The University of Wisconsin organ preservation solution (UW solution) is one of the most popular preservation solutions for kidney cold storage in human transplants (2, 17, 34), and several preservation solutions, including normal saline (16, 26, 35), PBS (15), Ringer solution (43) and UW solution (36), have been used in mouse kidney transplantation. Recently, we compared the protective effect between saline and UW solution on graft function in mouse kidney transplantation. We found that the UW solution exhibited better protective effect against ischemic tolerance in kidney transplantation, with 30% less renal injury than that preserved in saline (42). Accordingly, we speculate that flushing and preserving the kidney with cold UW solution instead of saline should result in less renal injury in this model. However, because of the surgical procedures, we had to use saline in the present study.
Cellular metabolism is closely correlated with temperature, which is an important factor for the severity of IRI (3, 18, 46). Therefore, the duration for cold ischemia is expected to be much longer than warm ischemia to induce a similar degree of IRI. We found that mice with <2 h of cold renal ischemia survived well and exhibited no significant changes in clearance function or histopathology. Animals of the models with 3 or 4 h of cold renal ischemia developed into mild to moderate acute kidney injury with the characteristic features, including an elevation in PCr and reductions in GFR and tubular necrosis followed by a subsequent recovery in structural integrity and kidney function. However, mice with 5 h of cold renal ischemia died in a few days, suggesting the renal injury was too severe to recover. Thus, these results indicated that the severity of IRI is closely correlated with cold ischemic duration. These experimental results are consistent with the clinical situation that the prolonged cold storage time is associated with a higher risk of delayed graft function and a lower graft survival rate (12, 24, 29). In addition, animals of a model with 4 h of cold renal ischemia exhibited significant vascular endothelium damage as well as interstitial fibrosis over the longer term, which are also consistent with the complications of longer cold ischemia time in humans (4, 13, 31).
In summary, we developed an in vivo experimental model of renal IRI that is induced exclusively by cold ischemia. This model mimics the episode of cold ischemia during graft storage in preservation solution. This animal model may provide a new tool to investigate the differences and similarities in pathophysiological mechanisms, including in cellular and molecular signal pathways for renal IRI induced by warm or cold ischemia. Additionally, it should be a useful model to test new or modified preservation solutions while avoiding the confounding effect of warm ischemia.
GRANTS
This work was supported by the American Society of Nephrology Ben J. Lipps Research Fellowship Awards (to J. Zhang and J. Wei), American Heart Association Career Development Award 18CDA34110441 (to L. Wang), and National Institutes of Health Grants DK-099276, DK-098582, and HL-137987 (to R. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W. and R.L. conceived and designed research; J.W. and J.Z. performed experiments; J.W., Y.W., and L.F. analyzed data; J.Z. prepared figures; J.W. and J.Z. drafted manuscript; J.W., Y.W., L.W., B.J.C., J.B., and R.L. edited and revised manuscript; J.W., Y.W., J.Z., L.W., L.F., B.J.C., J.B., and R.L. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of Y. Wang: Affiliated Linzi Hospital of Binzhou Medical College, Zibo City, Shandong 255400, China.
REFERENCES
- 1.Agarwal A, Dong Z, Harris R, Murray P, Parikh SM, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative XIII Working Group . Cellular and molecular mechanisms of AKI. J Am Soc Nephrol 27: 1288–1299, 2016. doi: 10.1681/ASN.2015070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belzer FO, D’Alessandro AM, Hoffmann RM, Knechtle SJ, Reed A, Pirsch JD, Kalayoglu M, Sollinger HW. The use of UW solution in clinical transplantation. A 4-year experience. Ann Surg 215: 579–583, 1992. doi: 10.1097/00000658-199206000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardinal H, Dieudé M, Hébert MJ. Endothelial dysfunction in kidney transplantation. Front Immunol 9: 1130, 2018. doi: 10.3389/fimmu.2018.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation. Initial perfusion and 30 hours’ ice storage. Lancet 2: 1219–1222, 1969. doi: 10.1016/S0140-6736(69)90753-3. [DOI] [PubMed] [Google Scholar]
- 6.Halloran PF, Aprile MA, Farewell V, Ludwin D, Smith EK, Tsai SY, Bear RA, Cole EH, Fenton SS, Cattran DC. Early function as the principal correlate of graft survival. A multivariate analysis of 200 cadaveric renal transplants treated with a protocol incorporating antilymphocyte globulin and cyclosporine. Transplantation 46: 223–228, 1988. doi: 10.1097/00007890-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hesketh EE, Czopek A, Clay M, Borthwick G, Ferenbach D, Kluth D, Hughes J. Renal ischaemia reperfusion injury: a mouse model of injury and regeneration. J Vis Exp (88): 2014. doi: 10.3791/51816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homer-Vanniasinkam S, Crinnion JN, Gough MJ. Post-ischaemic organ dysfunction: a review. Eur J Vasc Endovasc Surg 14: 195–203, 1997. doi: 10.1016/S1078-5884(97)80191-8. [DOI] [PubMed] [Google Scholar]
- 9.Hricik DE, Halbert RJ, Barr ML, Helderman JH, Matas AJ, Pirsch JD, Schenkel FA, Siegal B, Ferguson RM. Life satisfaction in renal transplant recipients: preliminary results from the transplant learning center. Am J Kidney Dis 38: 580–587, 2001. doi: 10.1053/ajkd.2001.26884. [DOI] [PubMed] [Google Scholar]
- 10.Ishitsuka Y, Fukumoto Y, Kondo Y, Irikura M, Kadowaki D, Narita Y, Hirata S, Moriuchi H, Maruyama T, Hamasaki N, Irie T. Comparative effects of phosphoenolpyruvate, a glycolytic intermediate, as an organ preservation agent with glucose and N-acetylcysteine against organ damage during cold storage of mouse liver and kidney. ISRN Pharmacol 2013: 375825, 2013. doi: 10.1155/2013/375825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2: 970–974, 2002. doi: 10.1034/j.1600-6143.2002.21015.x. [DOI] [PubMed] [Google Scholar]
- 12.Kayler LK, Magliocca J, Zendejas I, Srinivas TR, Schold JD. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant 11: 2647–2656, 2011. doi: 10.1111/j.1600-6143.2011.03741.x. [DOI] [PubMed] [Google Scholar]
- 13.Kayler LK, Srinivas TR, Schold JD. Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant 11: 2657–2664, 2011. doi: 10.1111/j.1600-6143.2011.03817.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, Liu EY, Zhang J, Hansen PB, Fan F, Juncos LA, Wang L, Pollock J, Huang PL, Fu Y, Wang S, Liu R. Macula densa nitric oxide synthase 1β protects against salt-sensitive hypertension. J Am Soc Nephrol 27: 2346–2356, 2016. doi: 10.1681/ASN.2015050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutz J, Lu R, Strobl M, Huang H, Deng M, Wang M, Ouyang N, Heemann U. ICOS/B7RP-1 interference in mouse kidney transplantation. Transplantation 84: 223–230, 2007. doi: 10.1097/01.tp.0000267439.15439.61. [DOI] [PubMed] [Google Scholar]
- 16.Martins PN. Technique of kidney transplantation in mice with anti-reflux urinary reconstruction. Int Braz J Urol 32: 713–718, 2006. doi: 10.1590/S1677-55382006000600013. [DOI] [PubMed] [Google Scholar]
- 17.Mühlbacher F, Langer F, Mittermayer C. Preservation solutions for transplantation. Transplant Proc 31: 2069–2070, 1999. doi: 10.1016/S0041-1345(99)00265-1. [DOI] [PubMed] [Google Scholar]
- 18.Munshi R, Hsu C, Himmelfarb J. Advances in understanding ischemic acute kidney injury. BMC Med 9: 11, 2011. doi: 10.1186/1741-7015-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muroya Y, Fan F, Regner KR, Falck JR, Garrett MR, Juncos LA, Roman RJ. Deficiency in the formation of 20-hydroxyeicosatetraenoic acid enhances renal ischemia-reperfusion injury. J Am Soc Nephrol 26: 2460–2469, 2015. doi: 10.1681/ASN.2014090868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. Ann Plast Surg 12: 70–83, 1984. doi: 10.1097/00000637-198401000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Nehus EJ, Devarajan P. Acute kidney injury: AKI in kidney transplant recipients−here to stay. Nat Rev Nephrol 8: 198–199, 2012. doi: 10.1038/nrneph.2012.40. [DOI] [PubMed] [Google Scholar]
- 22.O’Callaghan JM, Knight SR, Morgan RD, Morris PJ. Preservation solutions for static cold storage of kidney allografts: a systematic review and meta-analysis. Am J Transplant 12: 896–906, 2012. doi: 10.1111/j.1600-6143.2011.03908.x. [DOI] [PubMed] [Google Scholar]
- 23.Pegues MA, McCrory MA, Zarjou A, Szalai AJ. C-reactive protein exacerbates renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 304: F1358–F1365, 2013. doi: 10.1152/ajprenal.00476.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez Valdivia MA, Gentil MA, Toro M, Cabello M, Rodríguez-Benot A, Mazuecos A, Osuna A, Alonso M. Impact of cold ischemia time on initial graft function and survival rates in renal transplants from deceased donors performed in Andalusia. Transplant Proc 43: 2174–2176, 2011. doi: 10.1016/j.transproceed.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 25.Ploeg RJ, Vreugdenhil P, Goossens D, McAnulty JF, Southard JH, Belzer FO. Effect of pharmacologic agents on the function of the hypothermically preserved dog kidney during normothermic reperfusion. Surgery 103: 676–683, 1988. [PubMed] [Google Scholar]
- 26.Rong S, Lewis AG, Kunter U, Haller H, Gueler F. A knotless technique for kidney transplantation in the mouse. J Transplant 2012: 127215, 2012. doi: 10.1155/2012/127215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saat TC, van den Akker EK, IJzermans JN, Dor FJ, de Bruin RW. Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: lost in translation? J Transl Med 14: 20, 2016. doi: 10.1186/s12967-016-0767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salahudeen AK. Cold ischemic injury of transplanted kidneys: new insights from experimental studies. Am J Physiol Renal Physiol 287: F181–F187, 2004. doi: 10.1152/ajprenal.00098.2004. [DOI] [PubMed] [Google Scholar]
- 29.Sert I, Colak H, Tugmen C, Dogan SM, Karaca C. The effect of cold ischemia time on delayed graft function and acute rejection in kidney transplantation. Saudi J Kidney Dis Transpl 25: 960–966, 2014. doi: 10.4103/1319-2442.139865. [DOI] [PubMed] [Google Scholar]
- 30.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 31.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant 11: 2279–2296, 2011. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen I, Rong S, Susnik N, Gueler F, Shushakova N, Albrecht M, Dittrich AM, von Vietinghoff S, Becker JU, Melk A, Bohlmann A, Reingruber S, Petzelbauer P, Haller H, Schmitt R. Bbeta(15–42) attenuates the effect of ischemia-reperfusion injury in renal transplantation. J Am Soc Nephrol 22: 1887–1896, 2011. doi: 10.1681/ASN.2011010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Southard JH, Rice MJ, Ametani MS, Belzer FO. Effects of short-term hypothermic perfusion and cold storage on function of the isolated-perfused dog kidney. Cryobiology 22: 147–155, 1985. doi: 10.1016/0011-2240(85)90168-3. [DOI] [PubMed] [Google Scholar]
- 34.Southard JH, van Gulik TM, Ametani MS, Vreugdenhil PK, Lindell SL, Pienaar BL, Belzer FO. Important components of the UW solution. Transplantation 49: 251–257, 1990. doi: 10.1097/00007890-199002000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Tian Y, Chen J, Gaspert A, Segerer S, Clavien PA, Wüthrich RP, Fehr T. Kidney transplantation in mice using left and right kidney grafts. J Surg Res 163: e91–e97, 2010. doi: 10.1016/j.jss.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Tse GH, Hesketh EE, Clay M, Borthwick G, Hughes J, Marson LP. Mouse kidney transplantation: models of allograft rejection. J Vis Exp 92: e52163, 2014. doi: 10.3791/52163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tse GH, Hughes J, Marson LP. Systematic review of mouse kidney transplantation. Transpl Int 26: 1149–1160, 2013. doi: 10.1111/tri.12129. [DOI] [PubMed] [Google Scholar]
- 38.Turkmen K, Martin J, Akcay A, Nguyen Q, Ravichandran K, Faubel S, Pacic A, Ljubanović D, Edelstein CL, Jani A. Apoptosis and autophagy in cold preservation ischemia. Transplantation 91: 1192–1197, 2011. doi: 10.1097/TP.0b013e31821ab9c8. [DOI] [PubMed] [Google Scholar]
- 39.Wahlberg JA, Love R, Landegaard L, Southard JH, Belzer FO. 72-Hour preservation of the canine pancreas. Transplantation 43: 5–8, 1987. doi: 10.1097/00007890-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Song J, Wang S, Buggs J, Chen R, Zhang J, Wang L, Rong S, Li W, Wei J, Liu R. Cross-sex transplantation alters gene expression and enhances inflammatory response in the transplanted kidneys. Am J Physiol Renal Physiol 313: F326–F338, 2017. doi: 10.1152/ajprenal.00039.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Wang X, Jiang S, Wei J, Buggs J, Fu L, Zhang J, Liu R. Graft function assessment in mouse models of single- and dual-kidney transplantation. Am J Physiol Renal Physiol 315: F628–F636, 2018. doi: 10.1152/ajprenal.00068.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Wei J, Jiang S, Li HH, Fu L, Zhang J, Liu R. Effects of different storage solutions on renal ischemia tolerance after kidney transplantation in mice. Am J Physiol Renal Physiol 314: F381–F387, 2018. doi: 10.1152/ajprenal.00475.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, Bai J, Baumann M, Heemann U. New model for simultaneous heart and kidney transplantation in mice. Microsurgery 23: 164–168, 2003. doi: 10.1002/micr.10111. [DOI] [PubMed] [Google Scholar]
- 44.Wei J, Song J, Jiang S, Zhang G, Wheeler D, Zhang J, Wang S, Lai EY, Wang L, Buggs J, Liu R. Role of intratubular pressure during the ischemic phase in acute kidney injury. Am J Physiol Renal Physiol 312: F1158–F1165, 2017. doi: 10.1152/ajprenal.00527.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei J, Zhang J, Wang L, Cha BJ, Jiang S, Liu R. A new low-nephron CKD model with hypertension, progressive decline of renal function, and enhanced inflammation in C57BL/6 mice. Am J Physiol Renal Physiol 314: F1008–F1019, 2018. doi: 10.1152/ajprenal.00574.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol 303: F1487–F1494, 2012. doi: 10.1152/ajprenal.00352.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 48.Womer KL, Vella JP, Sayegh MH. Chronic allograft dysfunction: mechanisms and new approaches to therapy. Semin Nephrol 20: 126–147, 2000. [PubMed] [Google Scholar]
- 49.Zhang J, Wei J, Jiang S, Xu L, Wang L, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R. Macula Densa SGLT1-NOS1-Tubuloglomerular Feedback Pathway, a New Mechanism for Glomerular Hyperfiltration during Hyperglycemia. J Am Soc Nephrol 30: 578–593, 2019. doi: 10.1681/ASN.2018080844. [DOI] [PMC free article] [PubMed] [Google Scholar]