Abstract
Hypertension (HTN) affects one in three adults in the United States and is a major risk factor for cardiovascular disease and kidney failure. There is emerging evidence that more intense blood pressure lowering reduces mortality in patients with kidney disease who are at risk of cardiovascular disease and progression to end-stage renal disease. However, the ideal blood pressure threshold for patients with kidney disease remains a question of debate. Novel tools to more precisely diagnose HTN, tailor treatment, and predict the risk of end-organ damage such as kidney disease are needed. Analysis of circulating and urinary extracellular vesicles (EVs) and their cargo (protein and RNA) has the potential to identify novel noninvasive biomarkers that can also reflect a specific pathological mechanism of different HTN phenotypes. We will discuss the use of extracellular vesicles as markers of HTN severity and explain their profile change with antihypertensive medicine and potential to detect early end-organ damage. However, more studies with enhanced rigor in this field are needed to define the blood pressure threshold to prevent or delay kidney disease progression and decrease cardiovascular risk.
Keywords: biomarker, blood pressure threshold, extracellular vesicles, hypertension, kidney damage
INTRODUCTION
Hypertension (HTN), a silent disease affecting over 100 million Americans (29), is a major modifiable risk factor for cardiovascular disease (CVD) (31) and kidney failure (27). The national burden of chronic kidney disease (CKD) in the United States is high and rising (4). In particular, death due to CKD rose by 58% from 2002 to 2016 and was substantial for younger adults from 20 to 54 yr of age. High blood pressure (BP) is a third major risk factor for this development beside diabetes and obesity. There is emerging evidence supporting that more intensive BP control reduces mortality in patients with CKD who are at high risk of CVD and progression to end-stage renal disease (ESRD) (19, 24); however, this benefit is mostly seen in patients with proteinuria. Clearly novel tools and approaches are needed to identify the ideal BP threshold and mechanism of disease in patients with HTN with kidney disease or at risk of kidney disease. This article will briefly summarize the current controversy about BP thresholds in patients with kidney disease. We will then outline novel areas of research that have the potential to better define the BP threshold in this patient population and provide novel insights into the pathophysiology of HTN and its management.
CONTROVERSIES IN THE BP THRESHOLD
For almost 4 decades, the Joint National Committee on Detection, Evaluation, and Treatment for High Blood pressure (JNC) developed guidelines for BP thresholds for optimal treatment of HTN. Improvement of our knowledge of HTN and availability of more effective treatments led to several changes of JNC recommendations, some with significant controversy. The most recent, JNC 8 (2014), changed the threshold of treatment initiation for patients > 60 yr old from 140/90 to 150/90 mmHg. However, a subsequent meta-analysis of four trials in over 10,000 elderly individuals with HTN demonstrated that reduction of systolic BP to <140 mmHg lowered cardiovascular mortality, stroke, myocardial infarction, and heart failure (3). Of significance, JNC 8 and the 2013 European Society of Hypertension/European Society of Cardiology Task Force proposed the BP target of <140/90 mmHg for individuals with CKD but made no distinction based on the albuminuria level. The Kidney Disease Improving Global Outcomes BP guidelines (KDIGO) (15a) from 2012, however, recommended a BP goal of <130/80 mmHg for individuals with CKD and moderate or severe albuminuria and <140/90 mmHg for those with CKD and albuminuria of <30 mg/day. Two major BP goal trials of patients with CKD [The Modification of Diet in Renal Disease (16) and African American Study of Kidney Disease and Hypertension (47)] failed initially to demonstrate the benefits of BP lowering to delay CKD progression, but they were likely underpowered to address CVD and mortality. However, long-term followup of 14–19 yr of those patients with proteinuria revealed a benefit of lower BP target on the incidence of ESRD (19, 24). Due to varying BP goals, different degrees of proteinuria, and different patient populations (i.e., diabetic vs. nondiabetic) in studies in patients with kidney disease, many questions remain unanswered.
The recent landmark Systolic Blood Pressure Intervention Trial (SPRINT) (48) has generated new and important data from over 9,000 patients with HTN without diabetes and with high CVD risk. Approximately 30% of the participants had CKD [estimated glomerular filtration rate (eGFR): 20–59 ml/min]. SPRINT found a substantially lower CVD risk and lower all-cause mortality risk in participants treated to a systolic BP target of <120 mmHg (intensive treatment) compared with <140 mmHg (standard treatment). Although renal outcomes were not different in patients with CKD, the cardiovascular benefit of intensive treatment was also observed in this subgroup. However, patients in the intensive treatment group had a significant increase in acute kidney injury (AKI; reduction of eGFR) events within the first 6 mo, possibly due to a renal hemodynamic effect, the significance of which on a long-term clinical outcome is unknown. Since AKI can lead to CKD, this certainly raises concerns that, for some patients, intensive treatment may cause too low a BP level, leading to a J-curve phenomenon. SPRINT data significantly influenced the latest 2017 American College of Cardiology (ACC)/American Heart Association (AHA) (45) guidelines to change the target systolic BP from <140/80 mmHg to 130/80 mmHg. Other international societies, such as the European Society of Cardiology/European Society of Hypertension and Kidney Disease Outcomes Quality Initiative (KDIGO), have also updated their guidelines for the management of HTN (17, 46). Interestingly, European Society of Cardiology/European Society of Hypertension guidelines define normal BP at 120–129 mmHg, whereas AHA guidelines define normal BP as <120 mmHg, illustrating an ongoing controversy about the BP threshold (46). KDIGO also provided a commentary on the 2017 ACC/AHA HTN guidelines in April 2019 (17). All individuals with CKD stage 3 with or without albuminuria >300 mg/day albumin-to-creatinine ratio and all patients with CKD stages 1 and 2 with albuminuria should be treated to maintain a BP goal of <130/80 mmHg. The new BP threshold guideline for patients with CKD without proteinuria was also lower compared with the <140/80-mmHg threshold set in 2012 by KDIGO (17).
An important and new aspect of the latest AHA/ACC (45) recommendation is that a patient’s cardiovascular risk is used to guide the BP target. However, there is varying susceptibility to end-organ damage. In this regard, despite the high prevalence of HTN, only ~1% of patients with HTN develop kidney failure. Thus, risk factors for kidney disease development and/or progression need to be better characterized and should be included in the assessment.
Clinicians are still faced with many unanswered questions: What is a good BP target for my patients with kidney disease? When is BP too low or too high? Which BP level is protective against the development of CKD and progression to ESRD? Are there different BP targets for diabetic versus nondiabetic proteinuric diseases? How can more precise and personalized medicine be provided to patients with HTN and kidney disease? Are there specific pathological mechanism(s) that can be targeted in this patient population beyond blockade of the renin-angiotensin-aldosterone system?
NEED FOR NOVEL BIOMARKERS IN THE DIAGNOSIS AND TREATMENT OF HTN
For over a century, the indirect Riva-Rocci and Korotkoff methods have been used to measure BP to diagnose HTN, which is defined by manometric unit of millimeter of mercury. Much has been debated about the accuracy and predictive value of the timing of the manometric approach to BP measurements: ambulatory versus office, seated and resting but for how long, and daytime versus night time (2). Novel tools to enhance the diagnosis of clinically significant high BP are critically needed. Ideally, these new tools should be noninvasive (e.g., urine) or minimally invasive (e.g., blood) and able to inform the clinician about the etiology of HTN and its effect on the end organ in an individual patient. Biomarkers, in particular circulating and urinary extracellular vesicles (EVs), have the potential to be such a tool (8).
EXTRACELLULAR VESICLES
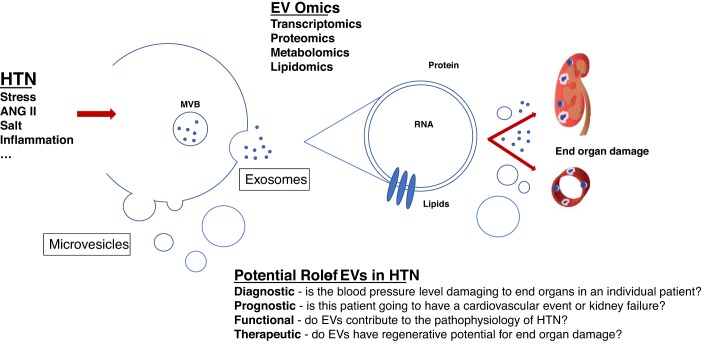
EVs are a heterogeneous population of small membrane fragments shed from various cell types (Figs. 1 and 2). These vesicles are <1 (1) μm in size (those smaller than <100 nm are called exosomes; those larger than >100 nm are called microvesicles) and carry markers of the parent cell including protein, lipids, and nucleic acid (43). EVs are released from each cell in the body into various body fluids (e.g., urine and plasma) upon various stimuli, from inflammation to oxidative and shear stress (43). Analysis of urinary EVs may serve as a logical and novel diagnostic approach for the nephrologist because these vesicles can derive from the kidney and urinary tract (8). Nevertheless, the accurate measurement of these vesicles is challenged by size heterogeneity, low refractive index, and lack of dynamic measurement range for most of the available technologies. To fully develop the potential of EVs as a diagnostic tool, more rigorous and standardized quantitation and characterization of their properties need to be established to determine their role in health and disease (6, 8, 26). To date, many published studies have not thoroughly characterized EVs nor followed the latest guidelines set by the EV societies (40, 42) and have described only a limited range of EVs (>500 nm), primarily due to technical limitations (7, 41).
Fig. 1.
Upon various stimuli (shear and oxidative stress, inflammation, salt, and ANG II), extracellular vesicles (EVs) can be released from all cells in the body, e.g., endothelial cell-derived EVs are secreted into the circulation or kidney-derived EVs, such as EVs of podocyte origin, into the urine. Smaller EVs (<100 nm, also called exosomes) are released from multivesicular bodies (MVBs) when these fuse with the outer membrane; larger EVs (100–1,000 nm, also called microvesicles) are secreted through a blebbing process. These EVs carry proteins/markers of their parent cell, RNA (mRNA and microRNA), carbohydrates, and different types of lipids. Their role has been described as diagnostic, prognostic, functional, and/or therapeutic. HTN, hypertension.
Fig. 2.
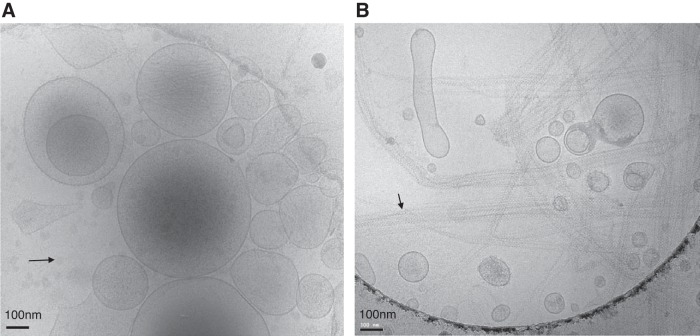
Cryo-electron microscopy of extracellular vesicles (EVs) from platelet-poor plasma (A) and urine (B) from two healthy controls (Institutional Review Board-approved protocol no. 17192 at the University of Virginia) after enrichment with differential centrifugation (20,000 g), which is one of the most commonly used EV enrichment techniques. Images show a heterogeneous population of small submicron vesicles of various sizes and densities. However, the bilipid layer morphology is the same for EVs from both body fluids. Of note, EV isolation procedures such as differential centrifugation (e.g., 20,000 g, as used here) can also lead to co-sedimentation and enrichment of non-EV proteins or lipids. The black arrow in A indicates some lipid droplets without a lipid bilayer in the plasma sample. The black arrow in B indicate polymers of Tamm-Horsfall proteins in the urine sample, which are also coprecipitated and may entrap some EVs.
EV PROTEIN CARGO
EV protein cargo has already gained significant attention as potential new surrogate biomarkers and bioactivators for endothelial dysfunction and vascular damage in HTN (6, 14, 23, 37, 39, 44). Most patients with essential HTN were found to have higher levels of circulating EVs carrying proteins of endothelial origin compared with healthy controls. These EV levels also correlate with the degree of HTN and vascular dysfunction (14, 37, 39, 44, 50). These data support the use of EV protein cargo as markers of HTN severity. However, these studies varied in the testing of different endothelial protein markers (e.g., CD31, CD105, CD144, and CD62E) and the application of different EV detection techniques. It remains to be determined which specific subtype(s) of endothelium-derived EVs (e.g., CD31+, CD144+, CD105+, and CD62E+) correlates best with HTN severity. Due to the use of different EV detection techniques and technical limitations, some studies have likely tested only larger EVs of >500 nm in size and without further detailed characterization (7, 9, 41). The whole spectrum of EVs should be studied, including size, from small (e.g., exosomes) to large (e.g., microvesicles), content (surface protein, EV and non-EV proteins, RNA, and lipids), and morphology (image) (40, 42). Transparent reporting should be standardized (42). In addition, other EV subtypes in the circulation, such as platelet- and leukocyte-derived EVs, need to be evaluated to gain insights into their diagnostic and functional properties. For example, Zu et al. (50) showed that leukocyte-derived EVs, and not endothelium-derived EVs (the most studied EVs in HTN), were elevated in their cohort of 25 patients with HTN. These data remain to be confirmed but highlight the need of a more comprehensive analysis of EV origin.
A few groups have tested the effects of antihypertensive therapy on EV count and protein content to identify the BP threshold to initiate treatment. Ideally, the BP threshold should be established when treatment of HTN normalizes EV levels to those seen in the normotensive condition. For this reason, serial measurements of EVs in the same animal or human before and after HTN correction should be measured. Zu et al. (50) observed an increase in circulating endothelial EVs (CD144+) over 24 wk in 25 humans treated with amlodipine, a Ca2+ channel blocker, whereas leukocyte-derived EVs remained unaffected over this time period. This work indicates that different subtypes of EVs are differently affected after normalization of BP. Nomura et al. (28) reported that treatment of 24 patients with HTN on hemodialysis with aliskiren, a direct renin inhibitor, for 12 wk did not improve BP control; however, it improved levels of platelet-derived microparticles (EVs) and flow-mediated dilation. This study suggested that EVs can be used as a biomarker of improved vascular endothelial function due to treatment with aliskiren. Another group (10) showed that irbesartan, an angiotensin receptor antagonist, therapeutically decreased annexin V-positive EV infiltration into the vascular wall of hypertensive-hypercholesterolemic hamsters, indicating a mechanistic role of EVs in HTN pathology. More studies are needed to dissect if different drugs have different effects on EV subtypes and how EVs contribute to the pathogenesis of HTN. To our knowledge, only one study has tested if subtypes of EVs have either a beneficial (e.g., regenerative) or harmful (e.g., profibrotic) effect in a model of essential HTN. Otani et al. (32) isolated EVs from Wistar-Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs) and injected these EV preps intraperitoneally for 6 wk into WKY rats and SHRs. BP levels in SHRs could be lowered with EVs from WKY rats, whereas BP levels of WKY rats were increased by EVs derived from SHRs. Less collagen deposition was also observed in SHRs that received WKY EVs. These experiments indicate that EVs have the potential to alter systemic BP and have likely a therapeutic or regenerative effect on end-organ damage in HTN.
EVs are seen as novel messengers for cell-to-cell and organ-to-organ communication. For comprehensive discussion of EVs as novel communicators in cardiovascular and renal pathology, we will refer the reader to other review papers that address these topics (8, 15, 36).
Another important question is whether circulating or urinary EVs can be used to detect early hypertensive kidney damage. To address this, a group of investigators studied 125 patients with essential HTN, 36% of those had micro-and macroalbuminuria. By multivariate analysis, CD31+/annexin V-positive circulating EV levels in the circulation were an independent predictor of urinary albumin excretion in patients with HTN (14). Other studies have examined urinary EVs derived from podocytes as an early marker for hypertensive renal damage. Nephrin- and podocalyxin-positive EV protein markers were elevated in 31 patients with renovascular HTN (20). This study also showed an inverse correlation with renal blood flow; yet, there was no correlation with mean arterial pressure and albuminuria. However, because of technical limitations, this study might have missed the characterization of very small EVs (13). To increase the potential predictive and/or diagnostic value before symptom development of hypertensive kidney disease, and to gain mechanistic insights that can be informed by EVs, further characterization of all subgroups of EVs with sizing, imaging, and protein and RNA content should be performed in a long-term prospective study. Urinary podocyte-derived EVs have already been identified in other disease processes as early markers in prealbuminuric diabetic glomerular injury in a mouse model of diabetes (5) and in humans with preeclampsia associated altered podocyte protein expression (11). In addition, it should be emphasized that each type of EVs, either deriving from the circulation or urinary tract, may reflect different pathological events. A link between circulating and urinary EVs as novel biomarkers or bioactivators in HTN has not been established. It is not clear whether circulating EVs cause kidney damage or simply are markers of vascular damage. Efforts are underway in the EV research community to determine if circulating EVs can cross the glomerular filtration barrier and if urinary EVs are primarily secreted or filtered. It should be noted that large amount of protein in the urine can be challenging for the purification of urinary EVs (26).
EV RNA CARGO
EV RNA cargo in arterial HTN is another area of intense studies. In particular, microRNAs have been found to be packaged and protected in EVs from proteases and RNAses; however, some circulating miRNAs are packaged in other circulating proteins (e.g., argonaute protein) (49). In general, miRNAs are a class of small noncoding RNAs (18–22 nt in length) that act as a modulator of gene expression by regulating specific mRNA. These miRNAs have many functions, including inflammation, angiogenesis, and senescence-associate miRNAs. miRNAs have already been found to have regulatory function in endothelial cells, in the renin-aldosterone-angiotensin system, vascular smooth muscle cells, and renal tubular cells (21, 30).
EV RNA cargo may serve a dual purpose as biomarker and therapeutic target, elucidating novel pathways of hypertensive kidney damage. In a study by Liu et al. (22), miRNA profiling of circulating EVs in SHRs and control WKY rats revealed that 27 miRNAs were significantly differentially expressed, including 23 miRNAs that were upregulated and 4 miRNAs that were downregulated in SHR circulating EVs compared with WKY circulating EVs. This proof-of-concept study demonstrated that miRNA cargo in circulating EVs in HTN can reveal potential targets for diagnosis, prognosis, and treatment. Perez-Hernandez et al. (35) studied 52 patients with HTN with and without albuminuria and 20 healthy volunteers as a control group to determine if miRNA from urinary EVs can assess risk for hypertensive kidney damage. High albuminuria was associated with 2.5-fold less miR-146a in the urinary exosome fraction; however, the miR-146a level did not change in the microvesicle fraction. miR-146 in urinary exosomes may thus be a potentially useful tool for studying early renal injury in HTN before symptom development (35). Ideally specific miRNAs need to be identified that can detect hypertensive damage before the development of symptoms such as albuminuria. This study also highlights the use of urine as an ideal noninvasive body fluid to identify novel biomarkers and bioactivators in HTN with kidney disease. Additionally, Gildea et al. (12) studied the miRNAome of urinary EVs and found 45 miRNAs likely to be potential biomarkers that correlate with salt sensitivity. Some of these miRNAs regulate signaling pathways associated with Na+ handling in HTN (12).
Other groups have studied circulating miRNAs that are not specifically detected in enriched EV preparations but are detected in cells or cell-free urine and blood in patients with HTN. Parthenakis et al. (34) tested miRNA expression levels in peripheral blood mononuclear cells and found that miR-208b and miR-133a were independently correlated with 24-h urinary albumin excretion in untreated patients with newly diagnosed essential HTN, illustrating that these miRNAs have the potential to be used as biomarkers for monitoring end-organ damage in HTN. Another study performed a correlation study of blood miRNA levels and arterial stiffness in 95 patients with well-controlled essential HTN (33). Only miR-21 was strongly associated with an improvement in arterial stiffness in patients with well-controlled essential HTN, independently of their BP. This suggests a functional role of miR-21 in vascular remodeling and its potential as a prognostic biomarker and therapeutic target (33). The same group found that miR-22 and miR-223 derived from platelet-rich plasma were reduced in patients with HTN and correlated negatively with systolic BP levels. In addition, these platelet-derived miRNA levels were lower in patients who had cardiovascular disease, suggesting that platelet miRNAs can serve as novel biomarkers of atherothrombotic risk in patients with HTN (25).
miRNAs can be also used to reveal the pathogenetic mechanisms in HTN and, therefore, to characterize different phenotypes of HTN. For example, miRNAs profiled from cortical collecting ducts and proximal tubules have been found to regulate 11b-hydroxysteroid dehydrogenase type 2; its diminished activity is associated with salt-sensitive HTN (38). miRNAs are also potentially therapeutic. Anti-miR-21 treatment targeting genes involved in HTN, such as NADPH oxidase 4 mRNA, lowered BP in mice fed with a 4% salt diet (18). Whether these same miRNAs are contained in EVs and maintain the same characteristic or functional properties as those in the circulation needs to be determined.
EV biomarkers also have the potential to be multiplexed with other molecular markers or screening modalities to develop integrated molecular-base computational tools and to be used for multi-omics (including transcriptomics, metabolomics, and proteomics). This “omic approach” has the potential to offer a more complete and precise picture of an organism’s molecular structure and potentially a personalized antihypertensive therapy (1). The pathophysiology of HTN is very complex and involves multiple pathways. So far, proteomic and metabolomic studies in HTN are limited but have started to emerge. For all “omic domains,” independent replication and functional followup studies with rigor and reproducibility are needed. In addition, these approaches pose challenges in big data management. However, they can provide a system biology approach to predict, stratify, diagnose, guide, and monitor treatment. It should be emphasized, however, that EVs are not yet obtainable on a large scale for clinical use. EV cargo analysis might serve as a screening tool for biomarker research. Once a biomarker is identified, easier point-of-care tools have to be developed.
CONCLUSIONS
Novel approaches are needed to personalize and more precisely diagnose HTN, tailor treatment, and predict risks of end-organ damage. Analysis of EVs and their cargo (protein and RNA) may offer a noninvasive opportunity. Promising data indicate that EVs correlate with the severity of HTN and kidney damage, and their profile changes with antihypertensive treatment. More studies with enhanced rigor are needed to demonstrate that EVs can be used to define the BP threshold and detect early organ damage in advance of symptom development, to prevent or delay kidney disease progression and decrease cardiovascular risks.
GRANTS
This work was supported in part by National Institutes of Health Grants K23-HL-126101 (to U. Erdbrügger) and RO1-DK-113632 and RO1-DK-094907 (to T. H. Le).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
U.E. prepared figures; U.E. and T.H.L. drafted manuscript; U.E. and T.H.L. edited and revised manuscript; U.E. and T.H.L. approved final version of manuscript.
REFERENCES
- 1.Arnett DK, Claas SA. Omics of blood pressure and hypertension. Circ Res 122: 1409–1419, 2018. doi: 10.1161/CIRCRESAHA.118.311342. [DOI] [PubMed] [Google Scholar]
- 2.Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 134: 904–905, 2016. doi: 10.1161/CIRCULATIONAHA.116.022536. [DOI] [PubMed] [Google Scholar]
- 3.Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol 69: 486–493, 2017. doi: 10.1016/j.jacc.2016.10.077. [DOI] [PubMed] [Google Scholar]
- 4.Bowe B, Xie Y, Li T, Mokdad AH, Xian H, Yan Y, Maddukuri G, Al-Aly Z. Changes in the US burden of chronic kidney disease from 2002 to 2016: an analysis of the global burden of disease study. JAMA Netw Open 1: e184412, 2018. doi: 10.1001/jamanetworkopen.2018.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger D, Thibodeau JF, Holterman CE, Burns KD, Touyz RM, Kennedy CR. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol 25: 1401–1407, 2014. doi: 10.1681/ASN.2013070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickhout A, Koenen RR. Extracellular vesicles as biomarkers in cardiovascular disease; chances and risks. Front Cardiovasc Med 5: 113, 2018. doi: 10.3389/fcvm.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erdbrügger U, Lannigan J. Analytical challenges of extracellular vesicle detection: a comparison of different techniques. Cytometry A 89: 123–134, 2016. doi: 10.1002/cyto.a.22795. [DOI] [PubMed] [Google Scholar]
- 8.Erdbrügger U, Le TH. Extracellular vesicles in renal diseases: more than novel biomarkers? J Am Soc Nephrol 27: 12–26, 2016. doi: 10.1681/ASN.2015010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdbrügger U, Rudy CK, Etter ME, Dryden KA, Yeager M, Klibanov AL, Lannigan J. Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytometry A 85: 756–770, 2014. doi: 10.1002/cyto.a.22494. [DOI] [PubMed] [Google Scholar]
- 10.Georgescu A, Alexandru N, Nemecz M, Titorencu I, Popov D. Irbesartan administration therapeutically influences circulating endothelial progenitor cell and microparticle mobilization by involvement of pro-inflammatory cytokines. Eur J Pharmacol 711: 27–35, 2013. doi: 10.1016/j.ejphar.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Gilani SI, Anderson UD, Jayachandran M, Weissgerber TL, Zand L, White WM, Milic N, Suarez MLG, Vallapureddy RR, Nääv Å, Erlandsson L, Lieske JC, Grande JP, Nath KA, Hansson SR, Garovic VD. Urinary extracellular vesicles of podocyte origin and renal injury in preeclampsia. J Am Soc Nephrol 28: 3363–3372, 2017. doi: 10.1681/ASN.2016111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gildea JJ, Carlson JM, Schoeffel CD, Carey RM, Felder RA. Urinary exosome miRNome analysis and its applications to salt sensitivity of blood pressure. Clin Biochem 46: 1131–1134, 2013. doi: 10.1016/j.clinbiochem.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Görgens A, Bremer M, Ferrer-Tur R, Murke F, Tertel T, Horn PA, Thalmann S, Welsh JA, Probst C, Guerin C, Boulanger CM, Jones JC, Hanenberg H, Erdbrügger U, Lannigan J, Ricklefs FL, El-Andaloussi S, Giebel B. Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material. J Extracell Vesicles 8: 1587567, 2019. doi: 10.1080/20013078.2019.1587567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang PH, Huang SS, Chen YH, Lin CP, Chiang KH, Chen JS, Tsai HY, Lin FY, Chen JW, Lin SJ. Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J Hypertens 28: 1655–1665, 2010. doi: 10.1097/HJH.0b013e32833a4d0a. [DOI] [PubMed] [Google Scholar]
- 15.Jansen F, Li Q, Pfeifer A, Werner N. Endothelial- and immune cell-derived extracellular vesicles in the regulation of cardiovascular health and disease. JACC Basic Transl Sci 2: 790–807, 2017. doi: 10.1016/j.jacbts.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.KDIGO Group KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2: 337–414, 2012. [Google Scholar]
- 16.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group . The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 17.Kramer HJ, Townsend RR, Griffin K, Flynn JT, Weiner DE, Rocco MV, Choi MJ, Weir MR, Chang TI, Agarwal R, Beddhu S. KDOQI US Commentary on the 2017 ACC/AHA Hypertension Guideline. Am J Kidney Dis 73: 437–458, 2019. doi: 10.1053/j.ajkd.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kriegel AJ, Baker MA, Liu Y, Liu P, Cowley AW Jr., Liang M. Endogenous microRNAs in human microvascular endothelial cells regulate mRNAs encoded by hypertension-related genes. Hypertension 66: 793–799, 2015. doi: 10.1161/hypertensionaha.115.05645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ku E, Gassman J, Appel LJ, Smogorzewski M, Sarnak MJ, Glidden DV, Bakris G, Gutiérrez OM, Hebert LA, Ix JH, Lea J, Lipkowitz MS, Norris K, Ploth D, Pogue VA, Rostand SG, Siew ED, Sika M, Tisher CC, Toto R, Wright JT Jr, Wyatt C, Hsu CY. BP control and long-term risk of ESRD and mortality. J Am Soc Nephrol 28: 671–677, 2017. doi: 10.1681/ASN.2016030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon SH, Woollard JR, Saad A, Garovic VD, Zand L, Jordan KL, Textor SC, Lerman LO. Elevated urinary podocyte-derived extracellular microvesicles in renovascular hypertensive patients. Nephrol Dial Transplant 32: 800–807, 2017. doi: 10.1093/ndt/gfw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leimena C, Qiu H. Non-coding RNA in the pathogenesis, progression and treatment of hypertension. Int J Mol Sci 19: E927, 2018. doi: 10.3390/ijms19040927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Yuan W, Yang L, Li J, Cai J. miRNA profiling of exosomes from spontaneous hypertensive rats using next-generation sequencing. J Cardiovasc Transl Res 12: 75-83, 2019. doi: 10.1007/s12265-017-9784-7. [DOI] [PubMed] [Google Scholar]
- 23.Lovren F, Verma S. Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clin Chem 59: 1166–1174, 2013. doi: 10.1373/clinchem.2012.199711. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra R, Nguyen HA, Benavente O, Mete M, Howard BV, Mant J, Odden MC, Peralta CA, Cheung AK, Nadkarni GN, Coleman RL, Holman RR, Zanchetti A, Peters R, Beckett N, Staessen JA, Ix JH. Association between more intensive vs less intensive blood pressure lowering and risk of mortality in chronic kidney disease stages 3 to 5: a systematic review and meta-analysis. JAMA Intern Med 177: 1498–1505, 2017. doi: 10.1001/jamainternmed.2017.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marketou M, Kontaraki J, Papadakis J, Kochiadakis G, Vrentzos G, Maragkoudakis S, Fragkiadakis K, Katsouli E, Plataki M, Patrianakos A, Chlouverakis G, Papanikolaou K, Vardas P, Parthenakis F. Platelet microRNAs in hypertensive patients with and without cardiovascular disease. J Hum Hypertens 33: 149−156, 2019. doi: 10.1038/s41371-018-0123-5. [DOI] [PubMed] [Google Scholar]
- 26.Merchant ML, Rood IM, Deegens JKJ, Klein JB. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat Rev Nephrol 13: 731–749, 2017. doi: 10.1038/nrneph.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet 370: 591–603, 2007. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- 28.Moriya H, Kobayashi S, Ohtake T, Tutumi D, Mochida Y, Ishioka K, Oka M, Maesato K, Hidaka S, Nomura S. Aliskiren, a direct renin inhibitor, improves vascular endothelial function in patients on hemodialysis independent of antihypertensive effect approximately a pilot study approximately. Kidney Blood Press Res 37: 190–198, 2013. doi: 10.1159/000350144. [DOI] [PubMed] [Google Scholar]
- 29.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 137: 109–118, 2018. doi: 10.1161/CIRCULATIONAHA.117.032582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murakami K. Non-coding RNAs and hypertension-unveiling unexpected mechanisms of hypertension by the dark matter of the genome. Curr Hypertens Rev 11: 80–90, 2015. doi: 10.2174/1573402111666150401105317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP, Hering D, López-Jaramillo P, Martinez F, Perkovic V, Rietzschel ER, Schillaci G, Schutte AE, Scuteri A, Sharman JE, Wachtell K, Wang JG. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 388: 2665–2712, 2016. doi: 10.1016/S0140-6736(16)31134-5. [DOI] [PubMed] [Google Scholar]
- 32.Otani K, Yokoya M, Kodama T, Hori K, Matsumoto K, Okada M, Yamawaki H. Plasma exosomes regulate systemic blood pressure in rats. Biochem Biophys Res Commun 503: 776–783, 2018. doi: 10.1016/j.bbrc.2018.06.075. [DOI] [PubMed] [Google Scholar]
- 33.Parthenakis F, Marketou M, Kontaraki J, Patrianakos A, Nakou H, Touloupaki M, Vernardos M, Kochiadakis G, Chlouverakis G, Vardas P. Low levels of microRNA-21 are a marker of reduced arterial stiffness in well-controlled hypertension. J Clin Hypertens (Greenwich) 19: 235–240, 2017. doi: 10.1111/jch.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parthenakis FI, Marketou ME, Kontaraki JE, Maragoudakis F, Maragkoudakis S, Nakou H, Roufas K, Patrianakos A, Chlouverakis G, Malliaraki N, Vardas PE. Comparative microRNA profiling in relation to urinary albumin excretion in newly diagnosed hypertensive patients. J Hum Hypertens 30: 685–689, 2016. doi: 10.1038/jhh.2016.15. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Hernandez J, Olivares D, Forner MJ, Ortega A, Solaz E, Martinez F, Chaves FJ, Redon J, Cortes R. Urinary exosome miR-146a is a potential marker of albuminuria in essential hypertension. J Transl Med 16: 228, 2018. doi: 10.1186/s12967-018-1604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pomatto MAC, Gai C, Bussolati B, Camussi G. Extracellular vesicles in renal pathophysiology. Front Mol Biosci 4: 37, 2017. doi: 10.3389/fmolb.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 41: 211–217, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Rezaei M, Andrieu T, Neuenschwander S, Bruggmann R, Mordasini D, Frey FJ, Vogt B, Frey BM. Regulation of 11beta-hydroxysteroid dehydrogenase type 2 by microRNA. Hypertension 64: 860–866, 2014. doi: 10.1161/hypertensionaha.114.00002. [DOI] [PubMed] [Google Scholar]
- 39.Sansone R, Baaken M, Horn P, Schuler D, Westenfeld R, Amabile N, Kelm M, Heiss C. Endothelial microparticles and vascular parameters in subjects with and without arterial hypertension and coronary artery disease. Data Brief 19: 495–500, 2018. doi: 10.1016/j.dib.2018.04.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750, 2018. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Pol E, Sturk A, van Leeuwen T, Nieuwland R, Coumans F, Mobarrez F, Arkesteijn G, Wauben M, Siljander PR-M, Sánchez-López V, et al.; ISTH-SSC-VB Working group Standardization of extracellular vesicle measurements by flow cytometry through vesicle diameter approximation. J Thromb Haemost 16: 1236–1245, 2018. doi: 10.1111/jth.14009. [DOI] [PubMed] [Google Scholar]
- 42.Van Deun J, Mestdagh P, Agostinis P, Akay Ö, Anand S, Anckaert J, Martinez ZA, Baetens T, Beghein E, Bertier L, et al.; EV-TRACK Consortium EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods 14: 228–232, 2017. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 43.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19: 213–228, 2018. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 44.Wang JM, Su C, Wang Y, Huang YJ, Yang Z, Chen L, Wu F, Xu SY, Tao J. Elevated circulating endothelial microparticles and brachial-ankle pulse wave velocity in well-controlled hypertensive patients. J Hum Hypertens 23: 307–315, 2009. doi: 10.1038/jhh.2008.137. [DOI] [PubMed] [Google Scholar]
- 45.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Soc Hypertension 12: 579.e1−e73, 2018. doi: 10.1016/j.jash.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Group ESCSD; ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 39: 3021–3104, 2018. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 47.Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG; African American Study of Kidney Disease and Hypertension Study Group . Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 288: 2421–2431, 2002. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 48.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang HN, Xu QQ, Thakur A, Alfred MO, Chakraborty M, Ghosh A, Yu XB. Endothelial dysfunction in diabetes and hypertension: role of microRNAs and long non-coding RNAs. Life Sci 213: 258–268, 2018. doi: 10.1016/j.lfs.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 50.Zu L, Ren C, Pan B, Zhou B, Zhou E, Niu C, Wang X, Zhao M, Gao W, Guo L, Zheng L. Endothelial microparticles after antihypertensive and lipid-lowering therapy inhibit the adhesion of monocytes to endothelial cells. Int J Cardiol 202: 756–759, 2016. doi: 10.1016/j.ijcard.2015.10.035. [DOI] [PubMed] [Google Scholar]