Abstract
Casein kinase 2 (CK2) is a tetrameric protein kinase composed of 2 catalytic (α and α′) and 2 regulatory β subunits. Our study provides the first molecular and cellular characterization of the different CK2 subunits, highlighting their individual roles in skeletal muscle specification and differentiation. Analysis of C2C12 cell knockout for each CK2 subunit reveals that: 1) CK2β is mandatory for the expression of the muscle master regulator myogenic differentiation 1 in proliferating myoblasts, thus controlling both myogenic commitment and subsequent muscle-specific gene expression and myotube formation; 2) CK2α is involved in the activation of the muscle-specific gene program; and 3) CK2α′ activity regulates myoblast fusion by mediating plasma membrane translocation of fusogenic proteins essential for membrane coalescence, like myomixer. Accordingly, CK2α′ overexpression in C2C12 cells and in mouse regenerating muscle is sufficient to increase myofiber size and myonuclei content via enhanced satellite cell fusion. Consistent with these results, pharmacological inhibition of CK2 activity substantially blocks the expression of myogenic markers and muscle cell fusion both in vitro in C2C12 and primary myoblasts and in vivo in mouse regenerating muscle and zebrafish development. Overall, our work describes the specific and coordinated functions of CK2 subunits in orchestrating muscle differentiation and fusogenic activity, highlighting CK2 relevance in the physiopathology of skeletal muscle tissue.—Salizzato, V., Zanin, S., Borgo, C., Lidron, E., Salvi, M., Rizzuto, R., Pallafacchina, G., Donella-Deana, A. Protein kinase CK2 subunits exert specific and coordinated functions in skeletal muscle differentiation and fusogenic activity.
Keywords: myoblast differentiation, myoblast fusion, MyoD expression, muscle development, fusogenic proteins
Protein kinase casein kinase 2 (CK2) is a ubiquitous and highly conserved serine-threonine kinase endowed with constitutive activity (1–3). It is usually present as a heterotetrameric holoenzyme composed of 2 catalytic (α and α′) and 2 regulatory β subunits (4), which stabilize the kinase holoform, enhance its activity, and mediate its substrate specificity (5). However, several lines of evidence have been provided for different expression levels and independent functions of each of the 3 CK2 subunits (6–9). CK2 is involved in fundamental cellular processes (1, 10), and the dysregulation of its signaling is associated with a wide spectrum of human diseases (2, 3). CK2 is also implicated in cell differentiation in various tissues, including skeletal muscle (1, 10, 11). Skeletal muscle differentiation is an irreversible and tightly orchestrated process, initiated by the activity of key transcription factors of the myogenic regulatory factor (MRF) family, such as myogenic differentiation 1 (MyoD) and myogenic factor 5 (Myf-5). These MRFs trigger the expression of later myogenic transcription factors (such as myogenin and myocyte enhancer factor 2) (12, 13) and subsequently that of muscle-specific structural genes (troponins and myosin heavy chains) (14, 15) and genes involved in myoblast fusion (caveolin-3, myomixer, and myomaker) (16–20). Recently, myomaker (20) together with myomixer (17), also named myomerger (18) or minion (19), have been demonstrated to act as the crucial molecular pair necessary and sufficient for the activation of cytoskeletal remodeling, membrane rearrangement, and coalescence required for myoblast fusion (17–23).
CK2 kinase activity has been described to target members of both the MRF and paired box (Pax)3 and 7 families via either direct phosphorylation or phosphorylation of their transcriptional regulators and protein partners (24–31) and to be mainly involved in promoting muscle precursor cell proliferation (27, 28, 30–32). However, the role of CK2 in the progression of the myogenic program and skeletal muscle formation is still to be investigated. Moreover, little is known about the relative contribution of each CK2 subunit to the myogenic process because CK2α- and CK2β-null mouse models display early embryonic lethality precluding further studies (33, 34), and CK2α′-null mice are viable with no evident muscular phenotype (35, 36). The only 2 studies investigating the in vivo muscle-specific function of CK2 subunits show that CK2α′-null mice, despite showing a muscular architecture similar to wild-type (WT) animals, display reduced size of regenerating fibers after muscle injury (36), and mice with CK2β deletion in differentiated myofibers develop a myoasthenic phenotype as a result of impaired muscle endplate structure and function (37).
In the present study, for the first time, we address the specific role of each CK2 subunit in skeletal muscle differentiation by clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated protein 9 (Cas9) gene editing in C2C12 myoblasts and by ectopic expression of individual CK2 protein in C2C12 cells and in regenerating mouse muscle. Moreover, we analyze the contribution of CK2 activity in muscle differentiation by using the CK2-specific inhibitor 5-(3-ethynylphenylamino)pyrimido[4,5-c]quinoline-8-carboxylic acid (CX-5011) (38) both in vitro in C2C12 and primary muscle cells and in vivo in mouse muscle regeneration and during zebrafish muscle development.
MATERIALS AND METHODS
C2C12 cell culture
Murine myogenic WT C2C12 cells [European Collection of Authenticated Cell Cultures (ECACC), Salisbury, United Kingdom] and CK2-knockout C2C12 cells were maintained at 37°C and 5% CO2 in DMEM (MilliporeSigma, Burlington, MA, USA) supplemented with 10% fetal bovine serum (MilliporeSigma), 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin (MilliporeSigma). Differentiation was induced in cells plated on gelatin-coated 6-well plates in the presence of differentiation medium containing DMEM supplemented with 2% horse serum (Thermo Fisher Scientific, Waltham, MA, USA) and 2 mM l-glutamine. When indicated, the vehicle (DMSO; MilliporeSigma) or CX-5011 (Glixx Laboratories, Hopkinton, MA, USA) were included in the growth or differentiation medium.
Primary muscle precursor cells
Primary muscle precursor cells were obtained from newborn C57BL/6 mice (postnatal d 1–2) following the procedure described by Brini et al. (39) with some modifications. Briefly, posterior hind limb muscles were removed, washed twice in PBS, minced in small pieces with scissors, and then incubated in a solution containing 0.07% trypsin (MilliporeSigma) in PBS for 40 min at 37°C with gentle shaking. The cell-containing supernatant was collected and filtered twice with 100 μm Cell Strainer (Thermo Fisher Scientific), and cells were pelleted by centrifugation for 10 min at 800 g. The cell pellet was resuspended in 1:1 mixture of Ham’s–F12 medium (Euroclone, Pero, Italy) supplemented with 20% fetal bovine serum and 2 mM l-glutamine (MilliporeSigma). Cells were plated at a density of 6 × 104/cm2 and incubated for 14 min at 37°C in order to let fibroblasts adhere. Following this preplating, adherent cells were discarded, whereas nonadherent cells containing the muscle precursor–enriched cellular fraction were collected and seeded onto gelatin-coated 6-well plates at a density of 5 × 104/cm2 and then maintained at 37°C and 5% CO2. The day after, muscle precursor cells were induced to differentiate replacing the growth medium with the differentiation medium. When indicated, the vehicle (DMSO) or CX-5011 were included in the differentiation medium.
Mice
Adult CD1 male mice (3 mo of age) were maintained in a temperature- and light-controlled environment with free access to food and water. All animal experiments were carried out according to the regulation of the Italian Ministry of Health (D.LGS. 26/2014 art. 31, authorization n. 82/2017-PR prot. D2748.20 obtained on November 16, 2016).
Zebrafish
Adult zebrafish were maintained in the facility of University of Padua at 28.5°C according to standard protocols and were kept in a 14-h light–10-h dark cycle. In vivo experiments were performed in accordance with the regulation of the Italian Ministry of Health [D.LGS. 26/2014, with the authorization of the local Organism Responsible for Animal Welfare, Organismo Preposto al Benessere degli Animali (OPBA)] of the University of Padua.
Antibodies
The antibodies against the following targets were used: CK2α/CK2α′ (MCA3031Z; Bio-Rad, Hercules, CA, USA); CK2α′, myogenin, p21, p27, lactate dehydrogenase (6481, 12732, 817, 1641, 33781; Santa Cruz Biotechnology, Dallas, TX, USA), Myf-5 (129925; GeneTex, Irvine, CA, USA), MyoD (M3512; Agilent Technologies, Santa Clara, CA, USA), myomixer (AF4580; R&D Systems, Minneapolis, MN, USA), caveolin-3 (610421; BD Biosciences, San Jose, CA, USA), poly (ADP-ribose) polymerase 1 (PARP-1), CK2β, phospho (p)-Akt1 (S129) and plasma membrane13 calcium ATPase (PMCA) (1078-1, 76025, 133458, 2825; Abcam, Cambridge, United Kingdom), Ki67 (M3062; Roche, Basel, Switzerland), Akt1, troponin I (2938, 7137; Cell Signaling Technology, Danvers, MA, USA), β-actin, α-tubulin, laminin, troponin T, sarcomeric tropomyosin (A5441, T9026, L9393, T6277, T9283 MilliporeSigma), embryonic myosin heavy chain (emb-MyHC), sarcomeric myosins (MF20), Pax7 (BF-G6, MF 20, 528428; Developmental Study Hybridoma Bank, Iowa City, IA, USA). Anti-CK2α antibody was raised in rabbits as described by Sarno et al. (40).
Generation of C2C12-knockout cells by CRISPR-Cas9 technology
Individual CK2 subunit knockout was performed as previously detailed by Borgo et al. (41). Briefly, all-in-one plasmids expressing Cas9–Dasher green fluorescent protein (GFP) and the single guide RNA (sgRNA) guide (CMV-Cas9-2A-GFP, Cas9-ElecD) to target the specific CK2 subunits were purchased from ATUM (Newark, CA, USA). The sgRNA guide sequences are 5′-CCTGGATTATTGTCACAGCA-3′ for casein kinase II (Csnk2a1)a1, 5′-AACTGGTTCGAAAACTTGGT-3′ for Csnk2a2, and 5′-TCCTGGTTCTGTGGGCTCCG-3′ for Csnk2b. Sequences have been chosen using the CRISPR MultiTargeter tool (42), and off targets have been excluded using GT-Scan tools (43).
C2C12 cells were cultured in 6-well dishes to 70–80% confluence. Cells were cotransfected with 1 μg of sgRNA plasmid and Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. At 48 h post-transfection, cells were pelleted in PBS, and GFP-positive cells were sorted in 96-well plates using fluorescence-activated cell sorting with a FACSAria II Cell Sorter (BD BioSciences). Sorted cells were expanded to obtain individual clones, which were selected by immunoblotting analysis using 2 different antibodies specific for each CK2 subunit in order to verify the complete absence of target protein expression. Sanger sequence analysis of a single CK2α−/−, CK2α′−/−, and CK2β−/− clone of those used in our study has been previously reported in Supplemental Figs. S1A, S2D, and S3, respectively, by Borgo et al. (41).
Cell viability assay
Cell viability was detected using the thiazolyl blue tetrazolium bromide (MTT) assay. C2C12 cells were seeded (4 × 103) in a 96-well plate, and after 1 d, the growth medium was replaced with 100 μl of differentiation medium containing vehicle or increasing concentrations of CX-5011. Each assay was performed in triplicate. After 6 d, 10 µl of thiazolyl blue tetrazolium bromide solution (5 mg/ml in PBS; MilliporeSigma) were added to each well. After 1 h, incubation was stopped by addition of 20 µl of a buffer (pH 4.7) containing 20% (w/v) SDS, 50% (v/v) N,N-dimethylformamide, 2% (v/v) acetic acid, and 25 mM HCl. Plates were read for optical density at λ 540 in a Titertek Multiskan Plus Plate Reader (Flow Laboratories, Sunnyvale, CA, USA).
Immunoblot analysis and immunoprecipitation experiments
C2C12 cells and primary myoblasts were lysed by scraping in ice-cold lysis buffer containing 20 mM Tris-HCl (pH 7.5), 1% Triton X-100, 10% glycerol, 1 mM EDTA, 150 mM NaCl, protease inhibitor cocktail (MilliporeSigma), and phosphatase inhibitor cocktails 2 and 3 (MilliporeSigma). Following 1 h incubation on ice, the cell lysates were centrifuged for 15 min at 14,000 g at 4°C.
For skeletal muscle lysis, mouse tibialis anterior (TA) cryosections (n = 30 sections, 20-μm thick, each muscle) were homogenized in lysis buffer for 3 min at 30/s frequency using the Tissue Lyser (Qiagen, Hilden, Germany). Muscle lysates were centrifuged for 15 min at 14,000 g at 4°C.
For zebrafish lysis, ≥20 fish per sample were used. Lysis was performed on ice in cold RIPA buffer containing 125 mM NaCl, 25 mM Tris-Cl (pH 7.4), 1 mM EGTA-Tris (pH 7.4), 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and Complete EDTA-free protease inhibitor cocktail (Roche) for 30 min, and crude extracts were cleared by centrifugation for 15 min at 14,000 g.
Supernatant cellular lysates, muscle, and zebrafish extracts were then collected, and protein concentration was determined by Bradford method. Proteins (15 μg for cell lysates, 20 μg for muscle extracts, and 50 μg for zebrafish extracts) were subjected to 7.5, 11, or 15% SDS-PAGE, blotted on Immobilon-P membranes (MilliporeSigma), incubated with the indicated antibodies, and revealed by enhanced chemiluminescent detection system (Thermo Fisher Scientific). Immunostained bands were quantified using a 4000 mm Pro Kodak Image Station (Kodak, Rochester, NY, USA), followed by analysis with Carestream Molecular Imaging software (Carestream, Rochester, NY, USA).
For immunoprecipitation experiments, C2C12 cell lysates (300 µg) or nuclear fractions prepared as described below (200 µg) were immunoprecipitated overnight at 4°C with the indicated antibodies, followed by addition of protein A-Sepharose (MilliporeSigma) for 1 h. The immunocomplexes were washed 3 times with 50 mM Tris-HCl (pH 7.5) and analyzed by immunoblotting.
Cellular fractionation
Nuclear and cytosolic fractions of C2C12 cells were separated using the NE-PER nuclear and cytoplasmic extraction reagent (Thermo Fisher Scientific) following the manufacturer’s recommendations with few modifications. Briefly, ∼2 × 106 C2C12 cells were harvested with trypsin-EDTA (MilliporeSigma) and centrifuged at 500 g for 5 min at 4°C. After 3 washes with PBS, the pellet was resuspended in 200 μl of ice-cold cytoplasmic extraction reagent I containing the protease inhibitor cocktail (Calbiochem) and phosphatase inhibitor cocktails 2 and 3, stirred for 15 s, and incubated on ice for 10 min. Ice-cold cytoplasmic extraction reagent II was then added to the sample, which was stirred for 5 s, placed on ice for 1 min, vortexed for 5 s, and then centrifuged for 5 min at 16,000 g at 4°C. The supernatant, representing the cytoplasmic fraction, was immediately placed on ice. The pellet containing the nuclei was resuspended with 100 μl of ice-cold nuclear buffer (20 mM Tris-HCl pH 7.5, 0.5% Triton X-100, 10% glycerol, 1 mM EDTA, 50 mM NaCl). After 1 h on ice, the sample was centrifuged at 16,000 g for 15 min at 4°C and then used for the immunoprecipitation experiments.
Isolation of the cellular plasma membranes
Plasma membranes were isolated from vehicle- and CX-5011–treated WT and CK2α′−/− C2C12 cells differentiated for 4 d using the Plasma Membrane Protein Extraction Kit (Abcam) following the manufacturer’s recommendations. Plasma membrane proteins were resuspended in lysis buffer containing 20 mM Tris-HCl (pH 7.5), 1% Triton X-100, 10% glycerol, 1 mM EDTA, 150 mM NaCl, protease inhibitor cocktail, and phosphatase inhibitor cocktails 2 and 3.
CK2 kinase activity assay
Cell lysates, mouse muscle, and zebrafish protein extracts were prepared as described above. Cell lysates (1–2 μg) or mouse muscle and zebrafish extracts (2–4 μg) were incubated for 10 min at 30°C in 25 μl of a phosphorylation medium containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 12 mM MgCl2 and 20 µM [γ-33P]ATP (Hartmann Analytic, Braunschweig, Germany) (∼1000 cpm/pmol). Kinase activity was tested against 400 μM eukaryotic initiation factor 2β (eIF2β) (1–22) synthetic peptide (MSGDEMIFDPTMSK8P), which is a substrate only of CK2 holoform, or 400 μM CK2tide (RRRADDSDDDDD), which is a substrate of both CK2 holoform and monomeric CK2 catalytic subunits [peptides were kindly provided by Prof. Oriano Marin, University of Padua, Italy (44)]. Assays were stopped by absorption onto P81 phosphocellulose filters (Reaction Biology, Malvern, PA, USA). Filters were washed 4 times in 75 mM phosphoric acid and analyzed by a Scintillation Counter (PerkinElmer, Waltham, MA, USA). CK2 activity was expressed as percentage of controls.
Real-time PCR analysis
Total RNA was isolated from C2C12 cells using Trizol Reagent (Thermo Fisher Scientific) following the manufacture’s procedures. cDNA was generated from 500 ng RNA with SuperScript II Reverse Transcriptase (Thermo Fisher Scientific), and real-time PCR was performed using iQ SYBR Green Supermix and the iQ5 iCycler (Bio-Rad). The oligonucleotides used for amplification are: Myod1: forward, 5′-CTCTGATGGCATGATGGATT-3′; reverse, 5′-GTGGAGATGCGCTCCACTAT-3′; myogenin gene (Myog): forward, 5′-CAACCAGGAGGAGCGCGATCTCCG-3′; reverse, 5′-AGGCGCTGTGGGAGTTGCATTCACT-3′; Myf-5: forward, 5′-GAGCTGCTGAGGGAACAGGTGGAGA-3′; reverse, 5′-GTTCTTTCGGGACCAGACAGGGCTG-3′; myoferlin gene (Myof): forward, 5′-ACCTTCAGCTCCACATCCAA-3′; reverse, 5′-GGCCTCTGACTATGCCATCT-3′; myomaker gene (Tmem8c): forward, 5′-TATACTCCGGTCCCATAGGC-3′; reverse, 5′-ATGCTCTTGTCGGGGTACAG-3′; β-actin gene (Actb): forward, 5′-CAAACATCCCCCAAAGTTCTAC-3′; reverse, 5′-TGAGGGACTTCCTGTAACCACT-3′.
RNA interference
C2C12 cells (1 × 105) were seeded in a 6-well plate, and the day after, transfected with 50 nM CK2β specific small interfering RNA (siRNA) (sequence target: 5′-GCCATGGTGAAGCTCTACT-3′) or scrambled siRNA siControl FISC-Free #1 (Dharmacon, Lafayette, CO, USA) as control, using the GenMute siRNA Transfection Reagent (SignaGen Laboratories, Rockville, MD, USA) according to the manufacturer’s recommendations. Cells were lysed after 2 or 6 d from transfection by scraping in the lysis buffer and analyzed by immunoblotting. When indicated, the vehicle (DMSO) or 2 μM CX-5011 were added in the growing medium at 2 d post-transfection.
Creatine kinase assay
Creatine kinase activity was determined using the Creatine Kinase Activity Assay Kit (MilliporeSigma) according to the manufacturer’s instructions. Briefly, 100 µl of reconstituted reagent was added to 1.5 µg of cell lysates into a 96-well plate. Samples were incubated at room temperature, and absorbance at 340 nm (A340) was measured after 20 and 40 min using a Titertek Multiskan Plus plate reader. Creatine kinase activity (U/L) was calculated by the following equation: [(A340)40min−(A340)20min]/[(A340)calibrator−(A340)blank] × 150. Each assay was performed in triplicate.
Wound healing assay
C2C12 cells were seeded at a density of 4 × 104 cells on each side of an Ibidi culture insert (Ibidi, Gräfelfing, Germany) into a 6-well plate. After appropriate cell attachment, the culture insert was gently removed to form a scratch into the cell monolayer. Cells were allowed to migrate for additional 14–18 h, and wound areas were examined after 0, 14, and 18 h using a Leica DM IRB microscope equipped with a Leica DFC300 FX camera and Leica PH 1 ×10/0.25 objective (Leica Microsystems, Wetzlar, Germany) and measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA) (45). Images were captured with Leica IM50 Image Manager software and presented without any further manipulation.
Immunofluorescence, hematoxylin and eosin staining, and bright-field image acquisition
Cell immunofluorescence
C2C12 cells and primary muscle cells were seeded on 13-mm gelatin-coated coverslips and fixed with 4% paraformaldehyde, permeabilized, and blocked with a solution containing 0.3% Triton X-100 and 5% bovine serum albumin (BSA) (MilliporeSigma) in PBS for 1 h at room temperature. Cells were then incubated with troponin T antibody in blocking solution overnight at 4°C and, after 3 washes, with Alexa Fluor 488–conjugated secondary antibody (Thermo Fisher Scientific). Hoechst (MilliporeSigma) was used to stain nuclei. Alexa Fluor 555–conjugated Phalloidin (Thermo Fisher Scientific) was used to stain α-actin filaments. The coverslips were mounted with Mowiol (Calbiochem), images were acquired with Leica DMR epifluorescence microscope, provided with a Leica DFC300 FX camera and equipped with an HC PL Fluotar ×20/0.5 objective (Leica Microsystems) using Micro-Manager v.1.4.20 software (University of California, San Francisco, CA, USA).
Muscle section immunofluorescence
Freshly prepared TA cryosections (12 μm thick) from the midportion of the muscle belly were blocked in Mouse on Mouse IgG blocking reagent (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions and then incubated with primary antibodies in PBS containing 2% goat serum and 0.5% BSA overnight at 4°C. After 3 PBS washes, sections were incubated with Alexa Fluor 488- or Alexa Fluor 555–conjugated secondary antibodies (Thermo Fisher Scientific) for 1 h at room temperature. Hoechst was used to stain nuclei. Images were acquired with a Leica DMR epifluorescence microscope, provided with a Leica DFC300 FX camera, and equipped with an HC PL Fluotar ×20/0.5 objective using Micro-Manager v.1.4.20 software. The brightness and contrast of the acquisitions were adjusted, and median filter (1-pixel radius) was applied for a better rendering using Fiji software (46). The number of nuclei per fiber was calculated as the mean of the number of nuclei detectable within the basal lamina of each single myofiber present in the analyzed fields of the muscle cross sections.
Muscle section immunohistochemistry
Freshly prepared TA cryosections (12 μm thick) were stained with a hematoxylin and eosin kit (Optika, Ponteranica, Italy) according to the manufacturer’s instructions.
Zebrafish immunofluorescence
Developing zebrafish at 48 and 96 h post fertilization (hpf) were fixed in 4% paraformaldehyde overnight at 4°C, washed with PBS, and then kept in 100% methanol at −20°C until rehydrated in a gradient of methanol-PBS, permeabilized with acetone, and blocked with 10% goat serum in PBS, 2% BSA, and 1% Triton X-100 for 2 h at room temperature, followed by primary antibody incubation overnight at 4°C. Fish were washed in PBS, 2% BSA, and 1% Triton X-100 and then incubated with secondary antibody Alexa Fluor–conjugated secondary antibodies overnight at 4°C. Hoechst was used to stain nuclei. Fish were finally mounted on slides with agarose at room temperature, and images were acquired with a Leica SP5 confocal microscope equipped with a Leica HCX PL APO ×40/1.25–0.75 objective. Images were presented as the maximum projection of z stacks. The brightness and contrast of the acquisitions were adjusted, and median filter (1-pixel radius) applied for a better rendering using Fiji software (46).
Bright-field images
C2C12 and primary myoblasts were captured live with a Leica DM IRB microscope provided with a Leica DFC300 FX camera and equipped with a Leica PH 1 ×10/0.25 objective. Images were captured with Leica IM50 Image Manager software, and they were presented without any further manipulation.
Cardiotoxin injury
Cardiotoxin (CTX) injury of TA muscles was performed as described by Pallafacchina et al. (47). In brief, adult CD1 male mice were anesthetized with an intraperitoneal injection of a solution containing tiletamine + zolazepam (40 mg/kg) and xilazine (5 mg/kg). The TA muscle was exposed from the lower limb and injected intramuscularly with 50 µl CTX from the Naja naja snake (10 µM; Latoxan, Portes-lès-Valence, France) dissolved in 0.9% NaCl saline solution.
The pharmacological CK2 inhibition treatment was obtained by intramuscular injection of regenerating TA muscles with 50 µl CX-5011 (90 μg) dissolved in 0.9% NaCl (saline solution) or vehicle (saline solution) for control starting at 3 d of regeneration. The treatment was repeated every 3 d. Mice were euthanized at 4, 7, and 14 d after injury, TA muscles were collected and quickly frozen in liquid nitrogen–cooled isopentane (MilliporeSigma) for further analysis.
Ectopic expression of CK2 subunits
Plasmids
For cells and muscle transfection, the following plasmid constructs were used: pcDNA3.1 (Thermo Fisher Scientific), pEGFP-N1 (Takara Bio Kusatsu, Japan), pcDNA3.1-CK2β-GFP (GenScript, Piscataway, NJ, USA), pCMV6-CK2α′-myc-DDK (Origene Technologies, Rockville, MD, USA), and pRcCMV2-CK2α′-HA [kindly provided by David W. Litchfield, University of Western Ontario, London, ON, Canada (48)].
Cell transfection
Subconfluent C2C12 myoblasts were transfected with pEGFP-N1 alone and pEGFP-N1 + pcDNA3.1 plasmids for empty vector samples, or with pcDNA3.1-CK2β-GFP and pEGFP-N1 + pRcCMV2-CK2α′-HA constructs for CK2β- and CK2α′-overexpressing samples, respectively, using Lipofectamine 2000 according to the manufacturer’s instructions. Cells were incubated with Lipofectamine 2000–DNA complexes for 4–5 h, and then the transfection medium was replaced with fresh growing medium. After additional 2–3 h, cells were induced to differentiate by replacing growing medium with differentiation medium. Myotubes were fixed or lysed 48 h after transfection for further analyses.
In vivo muscle electroporation
DNA transfection in regenerating muscle was achieved by intramuscular injection of plasmid DNA followed by electroporation according to the protocol described by Peng et al. (49). Briefly, adult male mice were anesthetized by intraperitoneal injection of a solution containing tiletammine + zolazepam (40 mg/kg) and xilazine (5 mg/kg). TA muscles were exposed and injected with 20 μg plasmid DNA (pEGFP-N1 + pcDNA3.1 plasmids for empty vector control samples or pEGFP-N1 + pCMV6-CK2α′-myc-DDK plasmids for CK2α′-overexpressing samples) in 20 μl sterile saline (0.9% NaCl) using a 30-gauge needle. Immediately after the injection, electroporation was applied through a pair of stainless-steel spatula electrodes placed on each side of the isolated muscle belly covering the injection site. The electroporation parameters were the following: 6 electric pulses at a voltage of 55 V (i.e., 200 V/cm), 50-ms duration each, and a 1-s delay between pulses. The skin was surgically sutured, and the mice were placed back in the cage to recover. After 10 d, transfected muscles were dissected and quickly frozen in liquid nitrogen–cooled isopentane for further analysis.
Zebrafish treatment
Fertilized eggs were collected, washed with fish water (0.5 mM NaH2PO4, 0.5 mM NaHPO4, 0.2 mg/L methylene blue), and maintained at 28.5°C in fish water with 3 mg/L Instant Ocean (Instant Ocean, Blacksburg, VA, USA). Embryos were staged as described by Kimmel et al. (50). For all the described experiments, embryos were dechorionated at 18–20 hpf and treated with 1 µM CX-5011 dissolved in fish water or vehicle (fish water) starting from 20 hpf. Developing fish were then collected and analyzed at 48 and 96 hpf.
Zebrafish touch-evoked escape response
Locomotor activity of zebrafish embryos was assessed at 48 hpf by placing individual embryos at the center of a 10-cm diameter Petri dish filled with clean fish water. The mechanosensory stimulus was applied to the tail of the embryo using a blunt pipette tip, and the embryo escape response to a gentle touch was recorded and classified as follows: “normal” when embryos swim away in a straight direction, “altered” when embryos move to a lesser extent or in an abnormal way, or “none” when embryos do not move.
Birefringence assay
Muscle birefringence was analyzed by taking advantage of muscle fiber anisotropy. Embryos were anesthetized with 0.016% (w/v) tricaine methanesulfonate (MilliporeSigma) at room temperature and mounted in 1% methylcellulose on a glass slide, and muscle light refraction was analyzed using 2 polarizing filters. The first filter produces the polarized light to illuminate the sample, and the second filter, called the analyzer, restricts the detection to the refracted light coming from the structurally organized muscle fibers. For image acquisition, a Leica M165FC stereomicroscope provided with a DFC420 C camera and equipped with a Leica Plan APO ×1.0 objective using Leica Application Suite v.4.6 imaging software was used. The integrated area of birefringence was calculated using ImageJ software.
Quantification and statistical analysis
Data are presented as means ± sem, and statistical significance was calculated by a 2-tailed Student’s t test. Values of P < 0.05 were defined as statistically significant, as indicated in the figure legends. In the case of the quantification of CK2 proteins and activity in C2C12-knockout cells, statistical significance was calculated by 2-way ANOVA, followed by Bonferroni’s multiple comparison test. All graphs were produced using Prism (GraphPad Software, La Jolla, CA, USA) software.
RESULTS
Characterization of C2C12 myoblasts deleted for the individual CK2 subunits
To investigate the specific contributions of the different CK2 subunits to myogenic differentiation, we generated stable clones deleted for the individual Csnk2a1, Csnk2a2, or Csnk2b genes (coding for CK2α, CK2α′, and CK2β subunits, respectively) by CRISPR-Cas9 technology in the murine C2C12 myoblast cell line. At least 2 independent clones for each gene deletion were examined in the study.
The characterization of the CK2-knockout myoblasts reveals that the expression of the 3 subunits is differently regulated in the various C2C12 genotypes (Fig. 1A and Supplemental Fig. S1A). CK2α−/− cells show a substantial down-regulation of CK2β, most likely due to its instability when not associated with catalytic subunits (41, 51). In contrast, CK2α′−/− cells exhibit CK2α and CK2β levels similar to those of controls, whereas CK2β−/− clones up-regulate CK2α and down-regulate CK2α′. As expected, the detection of CK2 activity in the different clones demonstrates that the deletion of a single catalytic subunit only partially hampers the phosphorylation of the CK2-specific intracellular target Akt1-Ser129 (52) (Fig. 1A and Supplemental Fig. S1B). Conversely, CK2β ablation, which prevents the CK2 tetramer formation, abrogates the phosphorylation of this target. Similar data were obtained in in vitro CK2 kinase assay performed with cellular lysates using a CK2 peptide substrate (Supplemental Fig. S1C).
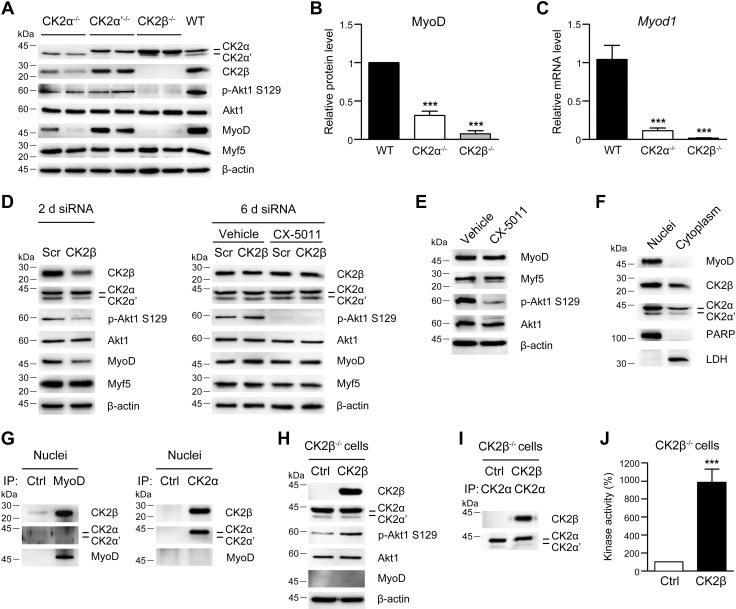
Figure 1.
Biochemical characterization of C2C12 myoblasts deleted for the individual CK2 subunits. A) Immunoblotting analysis of cellular lysates from WT C2C12 cells and cells deleted for Csnk2a1 (CK2α−/−), Csnk2a2 (CK2α′−/−), or Csnk2b (CK2β−/−) using the indicated antibodies. β-Actin was used as loading control. A total of 2 independent clones for each gene knockout were examined. B) Densitometric quantification of immunostained MyoD bands obtained as in A normalized to β-actin and relative to WT cells (n = 8 independent experiments; means ± sem). C) Myod1 mRNA level determined by quantitative real-time PCR in WT, CK2α−/−, and CK2β−/− C2C12 cells normalized to β-actin (Actb) mRNA and relative to WT cells (n = 3; means ± sem). D) RNA silencing in WT C2C12 cells transfected with scramble siRNA (Scr) or CK2β-specific siRNA for 2 d (left panel) and 6 d (right panel). Where indicated, cells were treated with vehicle or 2 μM CX-5011 starting at 2 d post-transfection. Cell lysates were analyzed by immunoblotting (n = 4). E) Immunoblotting analysis of WT C2C12 cells treated with vehicle or 2 μM CX-5011 for 4 d (n = 3). F) Immunoblotting analysis of nuclear and cytoplasmic fractions (15 µg each) from WT C2C12 cells. Poly (ADP-ribose) polymerase/p116 and lactate dehydrogenase (LDH) were used as nuclear and cytosolic markers, respectively. G) Immunostaining of immunoprecipitates (IPs) from the nuclear fraction (F) obtained with antibodies toward an unrelated protein (ubiquitin fusion degradation-2 ubiquitin ligase; Ctrl) and MyoD (left panel) or with preimmune serum (Ctrl) and anti‐CK2α antibody (right panel) (n = 4). H–J) CK2β−/− C2C12 cells were transfected with plasmids coding for GFP (Ctrl) or CK2β-GFP and analyzed at 48 h after transfection. H) Immunoblotting analysis of cellular lysates. I) Immunoblotting analysis of IPs using anti‐CK2α antibody (n = 4). J) CK2 kinase activity tested in vitro toward eIF2β (1–22) peptide substrate and expressed as percentage relative to Ctrl. Ctrl, control; p-Akt, phosphorylated Akt. ***P < 0.0001.
CK2β is required for MyoD expression in C2C12 cells
Analysis of the myogenic properties of C2C12-knockout clones reveals that the expression of the muscle master regulator MyoD is strongly reduced in CK2α−/− and almost abrogated in CK2β−/− myoblasts (Fig. 1A, B), whereas the amount of the other key myogenic factor Myf-5 is unchanged in the different clones (Fig. 1A). The decrease of MyoD protein in CK2α−/− and CK2β−/− clones is accompanied by a parallel reduction of Myod1 mRNA (Fig. 1C). Considering that CK2α-deleted cells exhibit a substantial decrease of CK2β (Fig. 1A and Supplemental Fig. S1A), we can hypothesize a specific role of the CK2β subunit in the transcriptional regulation of Myod1. This hypothesis is supported by the finding that the transient CK2β silencing in C2C12 myoblasts leads to a parallel down-regulation of MyoD, CK2α′, and CK2 holoform activity at 2 d post-transfection despite the presence of CK2α levels similar to those in controls (Fig. 1D, left panel and Supplemental Fig. S1D, left panel). This outcome also rules out the possibility that the MyoD reduction in CK2β−/− cells might be a secondary effect of clone selection. In addition, the rescue of CK2β protein amount at 6 d postsilencing is accompanied by the concomitant up-regulation of MyoD, CK2α′, and CK2 holoform activity. Notably, MyoD recovery at 6 d occurs even if CK2 kinase activity is inhibited by treating CK2β-silenced myoblasts with the CK2-specific inhibitor CX-5011 (38), which almost abrogates the phosphorylation of the CK2 cellular target Akt1-Ser129 (Fig. 1D, right panel and Supplemental Fig. S1D, right panel) without affecting the cell proliferation rate (unpublished results). MyoD amount is not affected by pharmacological inhibition of CK2 activity even if CX-5011 inhibitor is added to WT proliferating C2C12 cells for 4 d (Fig. 1E and Supplemental Fig. S1E). These results clearly point to a primary role of CK2β subunit in modulating MyoD expression and, importantly, suggest that the CK2β-mediated regulation of MyoD expression is not dependent on the activity of the CK2 tetrameric holoform. Finally, we performed subcellular fractionation and coimmunoprecipitation experiments showing that CK2β is an interacting partner of MyoD in myoblast nuclei, whereas CK2α or CK2α′, which are also present in the nucleus, are not (Fig. 1F, G). This close interaction suggests that MyoD transcriptional activity on the promoters of target genes, including its own promoter (53, 54), might be regulated by CK2β.
We next ectopically expressed CK2β in CK2β−/− cells. Although the reintroduction of CK2β effectively restores CK2β expression (Fig. 1H), tetrameric holoenzyme complex formation (Fig. 1I), and its kinase activity (Fig. 1J), it is not able to induce MyoD protein expression in CK2β−/− cells at 48 h post-transfection (Fig. 1H). This suggests that CK2β is not sufficient to activate Myod1 de novo transcription in C2C12 myoblasts.
Individual CK2 subunits play distinct and coordinated roles in C2C12 myoblast differentiation
The differentiation potential of the C2C12-knockout clones was investigated along 6 d of differentiation. WT cells exhibit an efficient myotube formation (Fig. 2A) associated with a decreased MyoD amount and a gradual increase in the expression of muscle-specific structural (troponins and tropomyosins) (55, 56) and fusion (caveolin-3, myomixer, and myomaker) (16–20) markers (Fig. 2B). Accordingly, the activity of the muscle-specific differentiation marker creatine kinase (57, 58) augments (Fig. 2C), and the clustering of acetylcholine receptors (AchRs) at plasma membrane (59, 60) becomes evident (Supplemental Fig. S2A). Notably, the protein level of the 3 CK2 subunits is differently regulated during differentiation: CK2α and CK2α′ do not significantly change, whereas CK2β increases up to 2-fold in differentiated compared with undifferentiated WT cells (Fig. 2D and Supplemental Fig. S2B). Analysis of the potential association of this additional CK2β with free catalytic subunits to form new tetrameric enzyme demonstrates that each CK2 subunit coimmunoprecipitates with comparable amount of the tetrameric partners in proliferating and differentiating myoblasts (Supplemental Fig. S2C), and the CK2 kinase activity does not significantly change during differentiation as indicated by the extent of Akt1-Ser129 phosphorylation (ratio phosphorylated Akt1–Ser129:Akt1) (Fig. 2D and Supplemental Fig. S2D) and by in vitro kinase assay (Supplemental Fig. S2E). These data suggest that the CK2β increase occurring during myotube maturation is not involved in the regulation of CK2 holoenzyme activity. However, the potential role of this additional CK2β in differentiated muscle cells is still to be clarified.
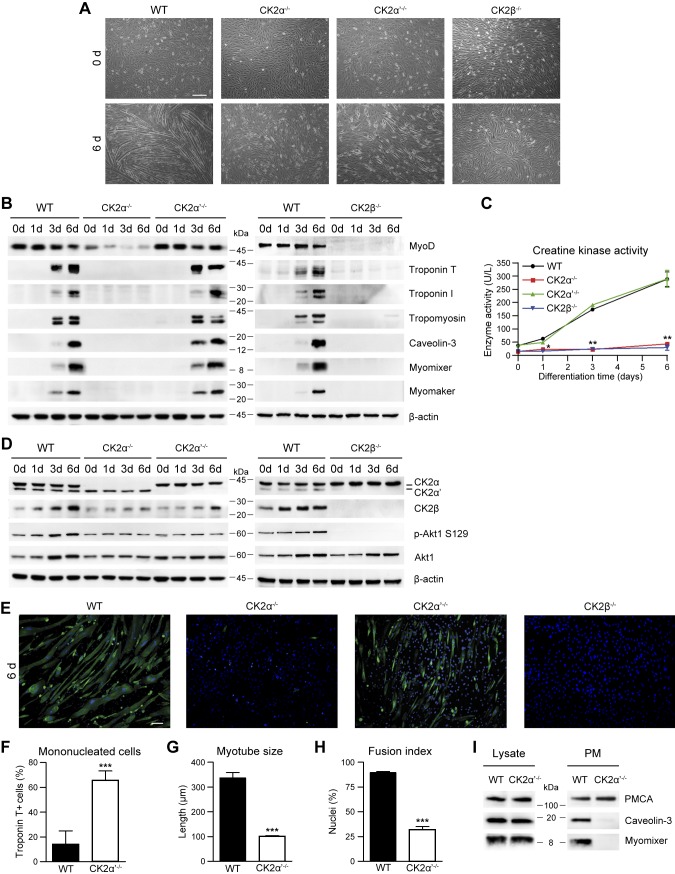
Figure 2.
The knockout of individual CK2 subunits differently affects the myogenic differentiation of C2C12 cells. A–D) Differentiating WT, CK2α−/−, CK2α′−/−, or CK2β−/− C2C12 cells were analyzed at the indicated time points. A) Cellular morphology assessed by bright-field microscopy (n = 5). Scale bar, 200 μm. B) Representative immunoblotting of cellular lysates (n = 5). C) Creatine kinase activity (n = 3; means ± sem). *P < 0.05, **P < 0.01 vs. WT cells. D) Representative immunoblotting (n = 5). E) Immunofluorescence of troponin T (green) in WT, CK2α−/−, CK2α′−/−, or CK2β−/− C2C12 cells at 6 d of differentiation. Hoechst (blue) stains nuclei (n = 3). Scale bar, 50 μm. F) Number of mononucleated WT and CK2α′−/− troponin T–positive cells from images obtained as in E. Data are percentages relative to the total troponin T–positive cells (n = 3; means ± sem). G) Troponin T–positive myotube length from images obtained as in E (n = 3; means ± sem). H) Fusion index, defined as the percentage of nuclei present in troponin T–positive myotubes from images obtained as in E of which cells containing ≥3 nuclei are considered myotubes (n = 3; means ± sem). I) Immunoblotting analysis of total (Lysate; 10 µg) and plasma membrane (PM; 5 µg) proteins from WT and CK2α′−/− C2C12 cells at 4 d of differentiation. PMCA was used as a PM marker (n = 4). p-Akt, phosphorylated Akt. ***P < 0.0001.
Morphologic analysis of differentiating C2C12-knockout myoblasts reveals a dramatic impairment of myotube formation in CK2α−/− and CK2β−/− cells (Fig. 2A, E). This is accompanied by the absence of muscle-specific markers (Fig. 2B), creatine kinase activity (Fig. 2C), and AchR clustering (Supplemental Fig. S2A), similar to what was already reported in Myod1−/− myoblasts (61–64). In contrast, in CK2α′−/− myoblasts, the differentiation gene program is unaffected compared with controls, as demonstrated by the expression of MyoD and muscle-specific markers (Fig. 2B, C and Supplemental Fig. S2B). However, the differentiated troponin T–positive CK2α′−/− cells are mostly mononucleated (Fig. 2A, E, F), and the few visible myotubes are shorter, with a reduced fusion index (Fig. 2G, H) and limited AchR clustering (Supplemental Fig. S2A) compared with controls.
To better understand the CK2α′ function in myoblast fusion, we analyzed the effect of this subunit deletion on cell migration and plasma membrane translocation of fusogenic proteins in CK2α′−/− myoblasts compared with WT cells. Wound healing assay and actin cytoskeleton staining in proliferating myoblasts suggest that CK2α′ is not required for cell migration (Supplemental Fig. S3A, B) and cytoskeletal remodeling (Supplemental Fig. S3C). Notably, we show that CK2α′ controls the subcellular distribution of key components of the membrane fusion machinery in differentiating myoblasts. In fact, the plasma membrane translocation of the fusogenic proteins caveolin-3 and myomixer (16–19) is extremely reduced in CK2α′−/− compared with WT C2C12 cells assessed at 4 d of differentiation, when cell fusion is ongoing (Fig. 2I and Supplemental Fig. S3D).
Taken together, these results provide evidence that the CK2α′ subunit is dispensable for C2C12 cell myogenic commitment, activation of differentiation, and expression of the late muscle-specific markers but fundamental in the regulation of C2C12 myoblast fusogenic activity through the modulation of intracellular protein sorting.
Inhibition of CK2 activity impairs C2C12 myoblast differentiation
To further explore the role of CK2 in myogenesis, we analyzed the contribution of its kinase activity to myoblast differentiation by treating C2C12 cells with subtoxic concentration of the CK2 inhibitor CX-5011 (Supplemental Fig. S4A–D). CX-5011 treatment causes a substantial and dose-dependent inhibition of CK2 activity, as revealed in cells by the extent of Akt1-Ser129 phosphorylation (Fig. 3A and Supplemental Fig. S4A, F) and in vitro by CK2 kinase assay (Supplemental Fig. S4B, G), with negligible effect on cell viability (Supplemental Fig. S4C). CX-5011 addition during myoblast differentiation affects neither the constant amount of CK2α and CK2α′ nor the increase of CK2β (Fig. 3A and Supplemental Fig. S4E). Notably, CX-5011 treatment dramatically impaired myoblast differentiation, inhibiting the formation of myotubes in a dose-dependent manner (Fig. 3B and Supplemental Fig. S4D). This is accompanied by a significant down-regulation of muscle-specific marker expression as for cell cycle exit [cyclin-dependent kinase inhibitor 1 and 2 (p21CIP1 and p27KIP1)], differentiation structural (troponins and tropomyosins) and fusion markers (caveolin-3, myomixer, myomaker, and myoferlin), and muscle creatine kinase activity (Fig. 3C–E and Supplemental Fig. S4A, H). In contrast, early myogenic factors (MyoD, Myog, and Myf-5) are not significantly reduced by CK2 inhibition (Fig. 3C, D and Supplemental Fig. S4A, I).
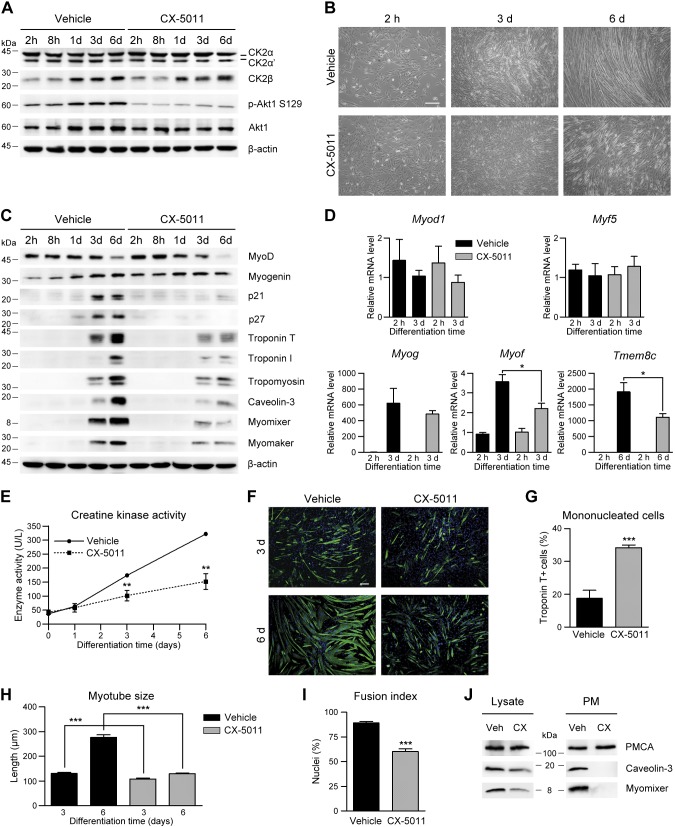
Figure 3.
Inhibition of CK2 activity impairs C2C12 myoblast differentiation. A–E) WT C2C12 cells were differentiated in the presence of vehicle or 2 μM CX-5011 and then analyzed at the indicated differentiation times starting from 2 h, when the effect of the inhibitor on CK2 activity was detectable. A) Representative immunoblotting of cellular lysates (n = 5). B) Cellular morphology assessed by bright-field microscopy (n = 5). Scale bar, 200 μm. C) Representative immunoblotting (n = 5). D) Real-time PCR quantification of MyoD (Myod1), Myf5 (Myf-5), myogenin (Myog), myoferlin (Myof), and Tmem8c (myomaker) mRNA level in differentiating vehicle- or CX-5011–treated cells, normalized to Actb mRNA amount and relative to WT cells at 2 h of differentiation (n = 3; means ± sem). *P < 0.05. E) Creatine kinase activity (n = 4; means ± sem). **P < 0.01 vs. vehicle-treated cells. F) Immunofluorescence of troponin T (green) in WT C2C12 cells differentiated for 3 and 6 d in the presence of vehicle or 2 μM CX-5011. Hoechst (blue) stains nuclei (n = 3). Scale bar, 50 μm. G) Quantification of mononucleated troponin T–positive cells from images obtained as in F. Data are percentages relative to the total troponin T–positive cells (n = 3; means ± sem). H) Troponin T–positive myotube length from images obtained as in F (n = 3; means ± sem). I) Fusion index, defined as the percentage of nuclei present in troponin T–positive myotubes from images obtained as in F of which cells containing ≥3 nuclei are considered myotubes. (n = 3; means ± sem). J) Immunoblotting analysis of total (Lysate, 10 µg) and plasma membrane (PM; 5 µg) proteins from WT C2C12 cells treated with vehicle (Veh) or 2 µM CX-5011 (CX) and differentiated for 4 d. PMCA was used as plasma membrane marker (n = 3). p-Akt, phosphorylated Akt. ***P < 0.0001.
The finding that both CK2α deletion and CK2 activity inhibition prevent muscle-specific gene expression in C2C12 cells (Figs. 2B, C and 3C–E) suggests that CK2α activity might participate in the activation of the myogenic transcriptional program. To verify this hypothesis, differentiating CK2α′−/− myoblasts, which contain only the CK2α catalytic subunit, were treated with CX-5011 (Supplemental Fig. S5). As expected, CK2α inhibition leads to a dramatic reduction of muscle-specific gene expression (troponin T, tropomyosins, caveolin-3, and myomixer) but does not significantly affect the early myogenic marker MyoD.
Morphometric analysis of differentiating myoblasts showed a marked increase of mononucleated cells accompanied by a concomitant decrease of myotube size in CX-5011–treated compared with vehicle-treated C2C12 cells after 3 and 6 d of differentiation, consistent with a significant reduction in the fusion index (Fig. 3F–I).
We then analyzed whether the impairment of cell fusion observed after CX-5011 treatment is caused by the reduced expression of the fusogenic markers (Fig. 3C, E) or by the inhibition of the activity of CK2α′, which mediates the fusogenic protein translocation to the plasma membrane (Fig. 2I). Subcellular fractionation of vehicle- and CX-5011–treated WT cells was performed at 4 d of differentiation, when fusion is ongoing and caveolin-3 and myomixer are only partially down-regulated by CX-5011 treatment in whole cellular lysates (Fig. 3J, left panel). The finding that both fusogenic proteins are almost undetectable in the enriched plasma membrane fraction of CX-5011–treated myoblasts (Fig. 3J, right panel and Supplemental Fig. S4J) drives us to conclude that the kinase activity of CK2α′ subunit is responsible for myoblast fusion.
Finally, CX-5011 treatment of C2C12 myoblasts for different times demonstrates that CK2 activity is required for proper myotube maturation in a restricted time window; namely, between 1 and 3 d after switching to differentiation medium (Supplemental Fig. S4K), indicating once again that CK2 activity is not required for triggering myoblast differentiation but necessary for the subsequent myoblast fusion and myotube maturation.
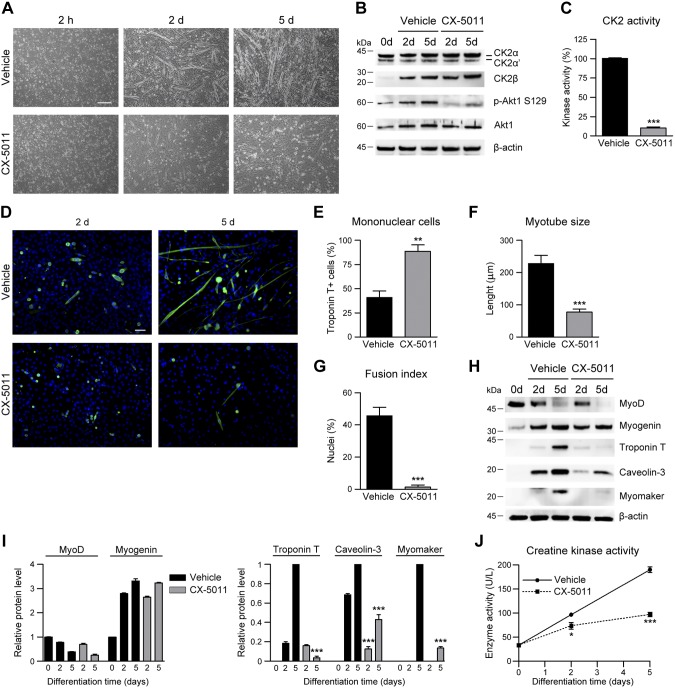
Inhibition of CK2 activity impairs myogenic differentiation of primary muscle cells
To assess a more general relevance of CK2 activity in the myogenic process, we analyzed primary mouse muscle precursor cells differentiated in the absence or presence of CX-5011 (Fig. 4). As found in C2C12 myoblasts, CK2β level increases during muscle precursor differentiation, and the almost complete inhibition of CK2 activity by CX-5011 treatment (Fig. 4B, C) greatly impairs myotube formation, as revealed by the increased number of mononucleated cells and the concomitant reduction of the myotube size and fusion index at 5 d of differentiation (Fig. 4A, D–G). Consistently, the expression of the early differentiation markers MyoD and myogenin is not significantly affected by CK2 inhibition, whereas late structural and fusion markers (troponin T, caveolin-3, and myomaker) and creatine kinase activity are substantially reduced (Fig. 4H–J). These results confirm that CX-5011 treatment impairs myogenic differentiation and cell fusion also in primary muscle precursor cells.
Figure 4.
Inhibition of CK2 activity impairs myoblast differentiation in primary muscle precursor cells. Muscle precursor cells derived from newborn C57BL/6 mice differentiated in the presence of vehicle or 2 μM CX-5011 were analyzed at the indicated times. A) Cell morphology assessed by bright-field microscopy (n = 3). Scale bar, 200 μm. B) Representative immunoblotting (n = 3). C) In vitro CK2 kinase activity tested in cellular lysates toward eIF2β (1–22) peptide substrate and expressed as percentage relative to controls (n = 3; means ± sem). D) Immunofluorescence of troponin T (green) in muscle precursor cells after 2 and 5 d in differentiating medium in the presence of vehicle or 2 μM CX-5011. Hoechst (blue) stains nuclei (n = 3). Scale bar, 50 μm. E) Quantification of mononucleated troponin T–positive cells at 5 d of differentiation and treated as in D. Data are expressed as percentages relative to the total troponin T–positive cells (n = 3; means ± sem). F) Quantification of troponin T–positive primary myotube length at 5 d of differentiation from images obtained as in D (n = 3; means ± sem). G) Fusion index measured in images obtained as in D, defined as the percentage of nuclei present in troponin T–positive primary myotubes at 5 d of differentiation of which cells containing ≥3 nuclei are considered myotubes (n = 3; means ± sem). H–J) Differentiating primary muscle cells were treated with 2 μM CX-5011 and compared with vehicle-treated cells at each indicated time point. H) Representative immunoblotting (n = 3). I) Densitometric quantification of the immunostained bands obtained as in H. Values were normalized to β-actin amount and relative to undifferentiated cells (for MyoD and myogenin) or to 5-d differentiated cells (for troponin T, caveolin-3, and myomaker. n = 3; means ± sem. J) Creatine kinase activity (n = 3; means ± sem). p-Akt, phosphorylated Akt. *P < 0.05, **P < 0.01, ***P < 0.0001 vs. vehicle treated primary muscle cells.
Inhibition of CK2 activity impairs skeletal muscle differentiation in vivo
To test the CK2 contribution to myogenic differentiation in a more physiologic context, we assayed the effect of CK2 inhibition by CX-5011 in 2 in vivo systems, mouse skeletal muscle regeneration and zebrafish embryonic muscle development.
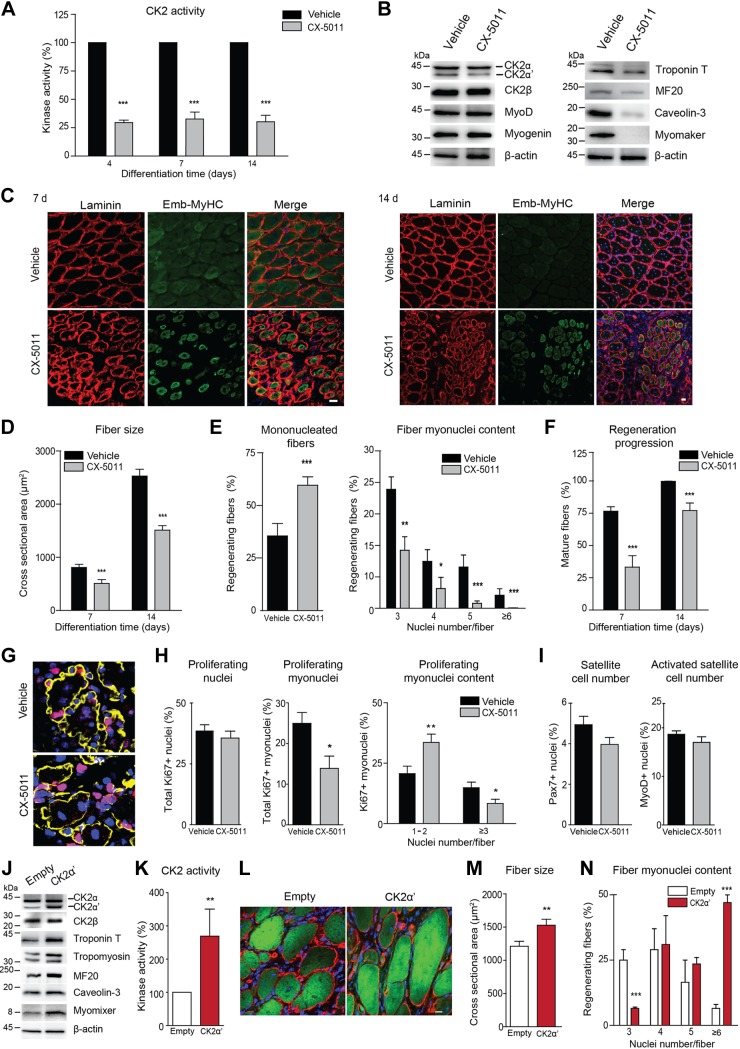
TA muscles of CD1 adult mice were injured by CTX and locally treated with vehicle or 90 μg CX-5011 starting at 3 d, and the treatment was subsequently repeated every 3 d. Regenerating muscles were then analyzed at different time points after injury. As expected, CX-5011 treatment induces a consistent reduction of CK2 activity (∼70%, Fig. 5A) in regenerating muscles up to 14 d without affecting the expression of the CK2 subunits (Fig. 5B). CK2 inhibition is associated with a hampered expression of the late muscle-specific markers analyzed at 7 d postinjury (Fig. 5B and Supplemental Fig. S6A) and a dramatic impairment of myofiber growth compared with vehicle-treated contralateral muscles during regeneration (Fig. 5C, D and Supplemental Fig. S6B). The reduction of fiber size in CX-5011–treated regenerating muscles is accompanied by decreased myonuclei content compared with vehicle-treated fibers (Fig. 5E). This outcome fits in with the fact that inhibition of CK2 catalytic activity, in particular that of CK2α′ (Figs. 2E–I and 3J), affects myoblast fusion in culture and demonstrates that it also controls the incorporation of myonuclei into nascent regenerating fibers in vivo.
Figure 5.
Inhibition of CK2 activity impairs in vivo skeletal muscle regeneration in mouse TA muscle. TA muscles of adult CD1 mice injected with CTX were treated with vehicle (0.9% NaCl) or CX-5011 (90 μg/muscle) every 3 d starting at 3 d and analyzed at the indicated time points of regeneration. A) In vitro CK2 kinase activity tested toward CK2tide-substrate in vehicle- and CX-5011–treated muscle extracts at 4, 7, and 14 d of regeneration and expressed as percentage relative to vehicle-treated samples (n = ≥4 mice/group, means ± sem). B) Representative immunoblotting of muscle extracts at 7 d of regeneration (n = 7 mice). C) Representative images of muscle transverse sections immunostained with anti-laminin antibody (red) to detect basal membrane and with anti–emb-MyHC antibody to detect nascent regenerating fibers (green) at 7 and 14 d of regeneration. Hoechst (blue) stains nuclei (n = ≥4 mice/group). Scale bar, 20 μm. D) Measurement of fiber cross-sectional area in regenerating CX-5011- vs. vehicle-treated muscles obtained as in C (≥4 mice and ≥2000 fibers from 5 different fields of each muscle were measured; means ± sem). E) Left graph: quantification of mononucleated muscle fibers at 14 d of regeneration and expressed as percentage over the total regenerating fibers, identified by the presence of central nuclei. Right graph: quantification of muscle fiber myonuclei content at 14 d of regeneration from images obtained as in C. Data are expressed as percentage of regenerating fibers with the indicated number of nuclei over the total regenerating fibers in the analyzed cross section fields; only fibers with ≥3 nuclei were considered (4 mice and ≥800 fibers from 7 different fields were measured; means ± sem). F) The progression of regeneration was quantified as the extent of embryonic-to-adult fiber-type switching in muscles treated as in C and expressed as the percentage of mature regenerating fibers (identified by the presence of central nuclei and by a negative emb-MyHC labeling) over the total number of regenerating fibers in each field. (≥4 mice and ≥2000 fibers from 7 different fields muscle were measured; means ± sem). G) Muscle transverse sections immunostained with anti-laminin antibody to detect basal membrane (yellow) and anti-Ki67 antibody to detect proliferating cells (red) at 4 d of regeneration. Hoechst (blue) stains nuclei (n = 4 mice). Scale bar, 20 μm. H) Quantification of proliferating nuclei in muscle cross sections treated as in G. Left graph: percentage of Ki67-positive nuclei over the total nuclei number. Middle graph: percentage of Ki67-positive myonuclei within basal lamina over the total number of Ki67-positive nuclei. Right graph, proliferating myonuclei distribution in fibers with <3 or ≥3 nuclei assessed as percentage of Ki67-positive myonuclei over the total myonuclei number (n = 4 mice; ≥3 different fields muscle section were analyzed; means ± sem). I) Quantification of the total or activated satellite cell number assessed as the percentage of Pax7-positive (left graph) or MyoD-positive (right graph) nuclei, respectively, over the total number of nuclei in muscle sections at 4 d of regeneration treated as in Supplemental Fig. S6C. J–N) Muscles were cotransfected with pEGFP-N1 and pcDNA3.1 empty vector (empty) or pEGFP-N1 and pCMV6-CK2α′-myc-DDK (CK2α′) plasmids by high-voltage electroporation, which concomitantly causes damage-induced regeneration. Transfected muscles were analyzed at 10 d of regeneration. J) Representative immunoblotting analysis of muscle extracts. K) CK2 kinase activity tested in vitro toward the CK2tide substrate and expressed as percentage relative to control (n = 3 mice; means ± sem). L) Representative images of muscle sections stained with anti-laminin antibody (red) to detect basal membrane. GFP fluorescence of transfected fibers is shown in green. Hoechst (blue) stains nuclei (n = 3 mice). Scale bar, 50 μm. M) Measurement of transfected fiber cross-sectional area in regenerating muscles treated as in L (n = 3 mice; ≥150 fibers from 5 different fields/muscle section were measured; means ± sem). N) Quantification of the myonuclei content in images obtained as in L. Data are expressed as percentage of fibers with the indicated number of nuclei over the total number of transfected fibers in the cross sections. Only fibers with ≥3 nuclei were considered (n = 4 mice; ≥800 fibers from 7 different fields muscle were measured; means ± sem). *P < 0.05, **P < 0.01, ***P < 0.0001.
As expected, the progression of regenerating fiber maturation is also significantly impaired by CK2 inhibition. Indeed, CX-5011 treatment inhibits the regenerating fiber-type switching, as revealed by the decreased number of mature regenerating fibers (negative for embryonic myosin staining but still presenting central nuclei) due to the persistence of immature fibers (expressing embryonic myosin) at both 7 and 14 d postinjury (Fig. 5F). We then assessed whether the effect of CK2 inhibition on regenerating fiber growth and maturation can be ascribed to a defective proliferation of satellite cells or to a block of satellite cell fusion to nascent myofibers. To this purpose, the extent of proliferation was determined in the whole tissue by measuring the number of Ki67-positive nuclei in sections of regenerating muscles treated with vehicle or CX-5011 at 4 d of regeneration (Fig. 5G, H, left graph). Concomitantly, we assessed the amount of satellite cells newly incorporated into nascent regenerating fibers by counting the number and distribution of proliferating Ki67-positive myonuclei within myofibers (Fig. 5G, H, middle and right graphs). Our data clearly indicate that CX-5011 treatment does not affect general cell proliferation (Fig. 5H, left graph) but significantly impairs the fusion of activated satellite cells to regenerating fibers. This is revealed by the global reduction of proliferating myonuclei (i.e., Ki67-positive nuclei within fiber basal lamina) (Fig. 5H, middle graph) and by their predominance in fibers with few nuclei (Fig. 5H, right graph). In addition, total (Pax7-positive) and activated (MyoD-positive) satellite cell number is not significantly affected by CX-5011 treatment (Fig. 5I and Supplemental Fig. S6C).
Finally, we ectopically expressed the CK2α′ catalytic subunit in regenerating TA fibers in vivo (Fig. 5J–N) and in WT C2C12 myoblasts in culture (Supplemental Fig. S6E–G). Expression of CK2α′ coding plasmid in the regenerating fibers of TA muscle after electroporation-induced damage greatly increases CK2α′ protein level (Fig. 5J and Supplemental Fig. S6D) and CK2 activity (Fig. 5K) and concomitantly up-regulates the level of muscle-specific markers (Fig. 5J and Supplemental Fig. S6D). Notably, myofibers and myotubes overexpressing CK2α′ show increased size and myonuclei number compared with cells expressing empty vectors (Fig. 5L–N and Supplemental Fig. S6E–G). These results confirm in vivo the specific role of CK2α′ in promoting myofiber regeneration and growth via the stimulation of myoblast fusion.
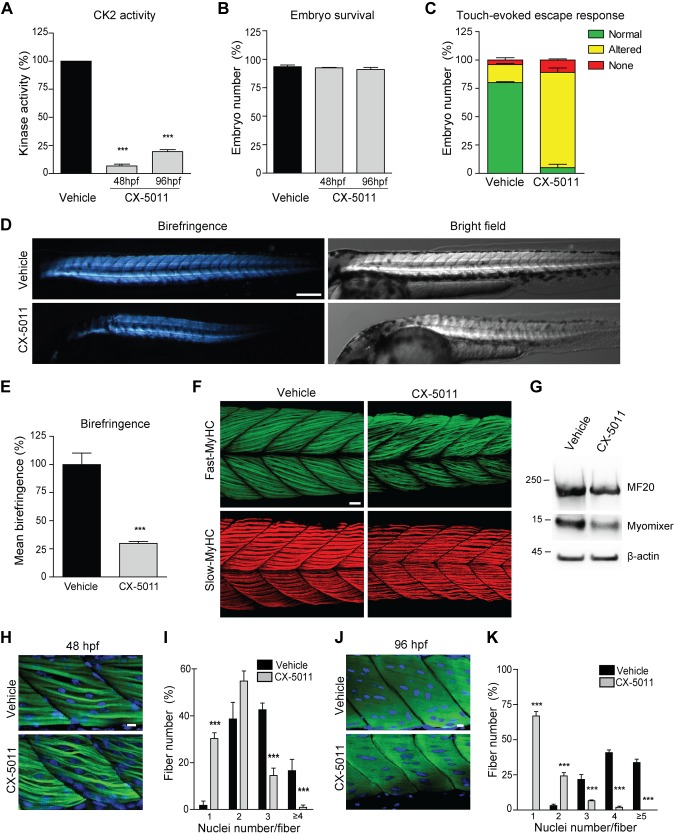
To investigate the role of CK2 in vertebrate muscle formation during development, zebrafish embryos were treated with CX-5011 starting from 20 hpf, a time point when embryonic muscle precursor cells are undergoing terminal differentiation and fusing to form mature somitic muscle fibers (50, 65). Interestingly, 20 hpf is also the time at which the expression of the fusogenic protein pair myomixer and myomaker is maximal in developing zebrafish somites (66, 67). CX-5011 treatment for 28 and 76 h strongly decreases the CK2 activity in zebrafish extracts (Fig. 6A) without affecting animal survival (Fig. 6B). Moreover, CK2 inhibition significantly impairs locomotor activity and skeletal muscle morphology, assessed at 48 hpf by touch-evoked escape response (Fig. 6C), birefringence (Fig. 6D, E), myosin-specific antibody staining (Fig. 6F), and expression of late muscle-specific markers (Fig. 6G). Notably, fast muscle fibers are more affected than slow fibers by CX-5011 treatment (Fig. 6F). In addition, CK2 inhibition significantly reduces the percentage of multinucleated fast fibers in the muscles of trunk (Fig. 6H–K) and tail (Supplemental Fig. S6H–K) somites of developing fish at both 48 and 96 hpf, which is in line with the notion that only fast muscle cells undergo fusion in zebrafish (65). The similarity between the phenotype of CX-5011–treated zebrafish and that of the recently reported myomixer- or myomaker-deficient zebrafish (66, 67) confirms that CK2 activity is required for myoblast fusion in vivo by acting through the regulation of fusogenic proteins, including the myomixer-myomaker pair, in zebrafish development as well.
Figure 6.
Inhibition of CK2 activity impairs in vivo skeletal muscle development in zebrafish. Developing zebrafish were treated with vehicle (fish water) or 1 μM CX-5011 at 20 hpf and analyzed at 48 and 96 hpf. A) In vitro CK2 activity tested toward CK2tide substrate in fish extracts and expressed as percentage relative to vehicle-treated samples (n = 30 fish/group; means ± sem). B) Zebrafish viability assessed as the percentage of living animals and expressed as percentage relative to the initial number of fertilized eggs (n = 200 vehicle-treated and n = 250 CX-5011–treated animals from 3 independent experiments). C) Zebrafish locomotor activity assessed by touch-evoked escape response test at 48 hpf. The embryo response to a gentle touch of the tail was classified as follows: “normal” when embryos swim away (green), “altered” when embryos move to a lesser extent or in abnormal way (yellow), or “none” when embryos do not move (red). Results are expressed as percentage of embryos showing the indicated class of response (n = 100 vehicle-treated and n = 80 CX-5011–treated embryos from 3 independent experiments; means ± sem). D) Representative birefringence (left panels) and bright-field (right panels) images of zebrafish embryos at 48 hpf. Scale bar, 250 μm. E) Quantification of muscle birefringence in zebrafish embryos obtained as in D. Data are expressed as percentage of vehicle-treated zebrafish mean birefringence value (n = 30 vehicle-treated and n = 40 CX-5011–treated embryos stained as in H; means ± sem). F) Maximum intensity confocal projections of zebrafish midtrunk somites (lateral view, anterior to the left) at 48 hpf, immunostained for fast (F310 antibody, green) or slow (F59 antibody, red) fiber type–specific myosin heavy chain (MyHC). Scale bar, 20 μm. G) Representative immunoblotting analysis of zebrafish embryo extracts at 48 hpf (n = 30 embryos/group from 3 independent experiments). H) Confocal images of 48-hpf zebrafish midtrunk somites (lateral view, anterior to the left) immunostained for fast fiber type–specific myosin (F310 antibody; green) and counterstained with Hoechst (blue) to detect nuclei. Scale bar, 10 μm. I) Number of nuclei per muscle fiber in 48 hpf zebrafish midtrunk somites stained as in H. Data are presented as percentage of fibers with the indicated number of nuclei in CX-5011- (n = 294 fibers) vs. vehicle-treated embryos (n = 230 fibers). Means ± sem. J) Confocal images of 96-hpf zebrafish midtrunk somites (lateral view, anterior to the left) immunostained for fast fiber type–specific myosin (F310 antibody; green) and counterstained with Hoechst (blue) to detect nuclei. Scale bar, 10 μm. K) Number of nuclei per muscle fiber in 96-hpf zebrafish midtrunk somites stained as in J. Data are presented as percentage of fibers with the indicated number of nuclei in CX-5011- (n = 357 fibers) vs. vehicle-treated embryos (n = 290 fibers). Means ± sem. ***P < 0.0001.
DISCUSSION
In this study, we investigate the role of protein kinase CK2 in skeletal muscle differentiation, highlighting, for the first time, the specific contribution of each CK2 subunit in the commitment, differentiation, and fusogenic activity of muscle cells using multiple approaches both in vitro and in vivo.
Pharmacologic inhibition of CK2 using CX-5011 demonstrates that the activity of this kinase directly governs 2 fundamental aspects of muscle biology: namely, the activation of muscle-specific gene expression and the maturation of myoblasts into multinucleated myotubes. The results of CK2 inhibition indicate that CK2 activity is dispensable for the maintenance of the myogenic identity and the expression of early myogenic markers (MyoD and myogenin) (Figs. 3C, D, 4H, I, and 5B) but required for the progression of myogenesis in differentiating muscle cells, as revealed by the impaired expression of the late muscle-specific markers (such as troponins, caveolin-3, myomixer, and creatine kinase) both in vitro and in vivo (Figs. 3C–E, 4H–J, 5B, and 6G).
CX-5011 treatment also reveals the crucial role of CK2 in promoting myotube formation in vitro and myofiber enlargement in vivo. We demonstrate that CK2 inhibition causes a significant decrease of myotube length and fusion index in C2C12 cells and primary muscle precursors and fiber cross-sectional area in regenerating mouse muscles. This latter effect is due to the reduced incorporation of myonuclei in nascent CX-5011–treated myofibers as a result of deficient satellite cell fusion, whereas satellite cell number and proliferation seem unaffected (Fig. 5G–I). Accordingly, CK2 inhibition compromises muscle architecture and function in developing zebrafish by blocking myonuclei incorporation in muscle fibers and leading to impaired locomotor activity (Fig. 6 and Supplemental Fig. S6H–K). Interestingly, the fusion defects of fast muscle cells found in our CX-5011–treated zebrafish resemble very closely the phenotype of myomixer- and myomaker-deleted zebrafish embryos (66, 67). These findings support our hypothesis of a hierarchical regulation of myomixer-myomaker pair fusogenicity by CK2 catalytic activity and underline the conserved role of protein kinase CK2 in myogenesis across species.
Furthermore, we provide a significant insight into the complex molecular mechanism of myoblast fusion by demonstrating that CK2 kinase activity is required for the plasma membrane translocation of the fusogenic proteins caveolin-3 and myomixer (Fig. 3J), consistent with previous reports describing CK2 involvement in cell trafficking (68, 69). Caveolin-3 is well known to be associated with myoblast membranes during differentiation and fusion (16, 70, 71), whereas myomixer has been recently demonstrated to act together with myomaker to constitute a minimal program necessary and sufficient to induce muscle cell fusion (17–19, 21, 22). Here, we show, for the first time, that myomixer also requires a dynamic plasma membrane translocation to exert its fusogenic activity at cell surface (22), as shown for myomaker (21), and that this translocation is mediated by CK2 (Fig. 3J). Given the ubiquitous expression and constitutive activity of CK2 (1, 10), we can reasonably envisage that this kinase might play a more general role in the control of the plasma membrane translocation of other fusogenic proteins promoting the membrane fusion also in additional cell types (as osteoclasts, regenerating hepatocytes, or cancer cells). Future investigations on this topic will clarify the potential contribution of CK2 to membrane fusion in different contexts.
Another relevant and original achievement of our study is the functional characterization of cells selectively deleted for CK2α, CK2α′, or CK2β, revealing their specific and coordinated roles in myogenesis. In particular, we demonstrate that CK2β is mandatory for the expression of the muscle master regulator MyoD by controlling its gene transcription (Fig. 1A–D). As a consequence, CK2β deletion prevents also the appearance of the later muscle differentiation markers and the formation of myotubes (Fig. 2A–C, E), similarly to the phenotype previously described in Myod1−/− cells (61, 62, 64). Notably, the CK2β effect on MyoD expression seems to be independent of the CK2 catalytic subunits and holoenzyme activity based on the following pieces of evidence: 1) changes in MyoD protein level parallel those of CK2β (Fig. 1A–D), whereas MyoD amount does not correlate with variation or inhibition of CK2 activity in CK2α′−/− cells (Fig. 1A) and in CX-5011–treated C2C12 myoblasts (Fig. 1E); 2) MyoD expression is independent of CK2α because CK2β silencing causes a decrease of MyoD despite the presence of a CK2α amount similar to controls (Fig. 1D); 3) MyoD expression is also independent of CK2α′ because CK2α′−/− cells, which contain a normal level of CK2α and CK2β, display a MyoD amount similar to controls (Fig. 1A); and 4) CK2β, but not CK2α and CK2α′, interacts with MyoD in myoblast nuclei (Fig. 1G). Despite all these indications, however, the data obtained to date do not rule out the possibility that a minimal residual CK2 activity in CX-5011–treated samples might be involved in Myod1 expression.
Our experiments to rescue CK2β protein amount in CK2β knockdown and knockout C2C12 cells show that CK2β plays a crucial role in the maintenance of MyoD levels in cells that already express this MRF (i.e., in 6 d CK2β-silenced cells; Fig. 1D, right panel), but this CK2 subunit is not able to induce de novo Myod1 expression (i.e., in CK2β-transfected CK2β−/− cells; Fig. 1H–J). This is conceivable with the requirement of an additional, CK2-independent, epigenetic chromatin remodeling component in order to activate Myod1 transcription and is in line with the peculiar role of MyoD protein as a master myogenic gene activator capable of opening the chromatin at loci that are otherwise inaccessible (such as Myog, muscle creatine kinase, Ckm, and Myod1 itself) (72).
These results highlight a novel and specific CK2β function in the MyoD-dependent commitment to myogenic differentiation still unexplored in the context of undifferentiated myoblasts.
CK2α deletion in C2C12 cells dramatically impairs MyoD expression (Fig. 1A–C) and myogenic differentiation (Fig. 2A–C). However, the finding that CK2α−/− cells display a strong reduction of CK2β protein amount (Fig. 1A) due to their instability in the absence of catalytic subunits (41), prevents the drawing of definitive conclusions on a CK2α-specific role in myogenesis using this cell model. The function of the CK2α subunit in C2C12 cell differentiation is unambiguously revealed by the analysis of CK2α′−/− myoblasts, which exhibit an amount of CK2α, CK2β, MyoD, and all the analyzed differentiation markers similar to controls (Figs. 1A and 2B, C). This suggests that CK2α plays a major role in the regulation of the muscle gene expression program, whereas CK2α′ seems not to be involved in this context. Our hypothesis is confirmed by the reduced expression of the late muscle-specific markers, but not MyoD, in both differentiating CX-5011-treated WT myoblasts and, importantly, CX-5011-treated CK2α′−/− cells, which contain only CK2α catalytic subunit (Fig. 3C–E and Supplemental Fig. S5).
Regarding the contribution of CK2α′ in myogenesis, our CK2α′−/− cell model clearly shows that this CK2 subunit is dispensable for the commitment of C2C12 cells to the myogenic lineage (Fig. 1A) and for the expression of the late muscle-specific genes (Fig. 2B, C). However, we identify a novel and crucial role of CK2α′ catalytic activity in mediating the CK2-dependent regulation of myoblast fusion during myotube maturation. This role is revealed by the reduced number and size of CK2α′−/− myotubes (Fig. 2A, E–H), which might explain the muscle regeneration defects reported in CK2α′-null mice (36), and, importantly, by the impaired translocation of caveolin-3 and myomixer to the plasma membrane of differentiating CK2α′−/− myoblasts (Fig. 2I). Notably, the fusion defect found in differentiating CK2α′−/− cells is not accompanied by impairment of differentiation marker expression (Fig. 2A–C), similar to the phenotype of myomixer-deficient cells (18, 19). This sets CK2α′ and myomixer on the same molecular pathway triggering cell fusion. Remarkably, we demonstrate that CK2α′ is not only required but also sufficient to induce skeletal muscle cell fusion both in vitro and in vivo. Indeed, the ectopic expression of CK2α′ in C2C12 cells and in regenerating mouse muscle increases fusion index and myotube and myofiber size (Fig. 5L–N and Supplemental Fig. S6E–G). These effects clearly depend on the enhanced incorporation of myonuclei due to the greater fusogenic activity of differentiating cells overexpressing CK2α′ (Fig. 5N and Supplemental Fig. S6E–G). In all, our findings provide a novel crucial piece to the fusion-machinery puzzle, identifying CK2α′ as a molecular regulator of fusogenic protein targeting to the cell surface. The clear functional difference highlighted between CK2α and CK2α′ proves that 1 catalytic subunit does not compensate for the other in C2C12 myoblast differentiation, which is in line with the remarkably distinct phenotypes reported for CK2α- and CK2α′-knockout mice (33, 35).
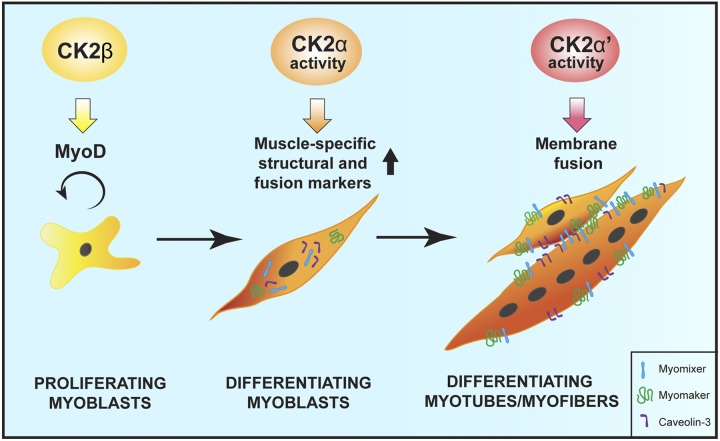
Altogether, our data demonstrate that CK2 subunits play specific and fundamental roles in skeletal muscle differentiation through distinct but coordinated actions as proposed in the model depicted in Fig. 7. In particular, we show that CK2β is required for the maintenance of myogenic identity through the regulation of Myod1 expression, CK2α mediates the activation of the late muscle-specific gene expression, and CK2α′ controls myoblast fusion through the regulation of fusogenic protein plasma membrane translocation (Fig. 7). Although our study does not provide evidence for the direct partners and targets acting with CK2 subunits, it gives the foundation for further exploration of the complex molecular pathways upstream and downstream of CK2 in myogenesis.
Figure 7.
Coordinated actions of CK2 subunits during skeletal muscle differentiation. Schematic representation of the specific role of each CK2 subunit in differentiating myoblasts: CK2β is required for the maintenance of myogenic identity through the regulation of Myod1 expression, CK2α mediates the activation of the late muscle-specific gene transcription, and CK2α′ promotes cell fusion by controlling the targeting of fusogenic proteins to the plasma membrane.
Considering the relevance of skeletal muscle trophism in a number of muscular and nonmuscular pathologies as well as in physiologic aging, the discovery of new molecules and mechanisms is crucial for the development of innovative therapeutic strategies to counteract muscle waste and protect human health. In this scenario, our work identifies the CK2 subunits and their activity as important players that hierarchically control critical steps of myogenesis and provide potentially targetable checkpoints to be considered in muscle dysfunction.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. D. W. Litchfield (University of Western Ontario, London, ON, Canada) for kindly sharing the pRcCMV2-CK2α′-HA construct, Dr. D. P. Millay (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA) and Dr. O. Marin (Department of Biomedical Sciences, University of Padua) for kindly providing anti-myomaker antibody and the eIF2β(1–22) peptide, respectively. This work was supported by the University of Padua (PrAt-CPDA130310 to A.D.-D.), the Italian Ministry of Education, University and Research (FIRB; to R.R.), and U.S. National Institutes of Health, National Institute on Aging Grant 1P01AG025532-01A1(to R.R.). The authors declare no conflicts of interest.
Glossary
- AchR
acetylcholine receptor
- Akt1
AKT serine-threonine kinase 1
- BSA
bovine serum albumin
- Cas9
CRISPR-associated protein 9
- CK2
casein kinase 2
- CRISPR
clustered regularly interspaced short palindromic repeats
- Csnk2
casein kinase II
- CTX
cardiotoxin
- CX-5011
5-(3-ethynylphenylamino)pyrimido[4,5-c]quinoline-8-carboxylic acid
- eIF2β
eukaryotic initiation factor 2β
- emb-MyHC
embryonic myosin heavy chain
- GFP
green fluorescent protein
- hpf
hour post fertilization
- MRF
myogenic regulatory factor
- Myf-5
myogenic factor 5
- MyoD
myogenic differentiation 1
- Myof
myoferlin
- Myog
myogenin gene
- Pax
paired box
- PMCA
plasma membrane calcium ATPase
- sgRNA
single guide RNA
- siRNA
small interfering RNA
- TA
tibialis anterior
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
V. Salizzato and S. Zanin performed most of the experiments and contributed to data interpretation; C. Borgo performed RNA silencing and immunoprecipitation experiments; E. Lidron performed zebrafish in vivo experiments; C. Borgo and M. Salvi generated the knockout cells; R. Rizzuto contributed to discussion and data interpretation; G. Pallafacchina and A. Donella-Deana conceived and designed the experiments and wrote the manuscript with input from all authors; and all authors read and approved the manuscript.
REFERENCES
- 1.Meggio F., Pinna L. A. (2003) One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17, 349–368 [DOI] [PubMed] [Google Scholar]
- 2.St-Denis N. A., Litchfield D. W. (2009) Protein kinase CK2 in health and disease: from birth to death: the role of protein kinase CK2 in the regulation of cell proliferation and survival. Cell. Mol. Life Sci. 66, 1817–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trembley J. H., Wang G., Unger G., Slaton J., Ahmed K. (2009) Protein kinase CK2 in health and disease: CK2: a key player in cancer biology. Cell. Mol. Life Sci. 66, 1858–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niefind K., Guerra B., Ermakowa I., Issinger O.-G. (2001) Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. EMBO J. 20, 5320–5331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibby A. C., Litchfield D. W. (2005) The multiple personalities of the regulatory subunit of protein kinase CK2: CK2 dependent and CK2 independent roles reveal a secret identity for CK2beta. Int. J. Biol. Sci. 1, 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerra B., Issinger O.-G. (1999) Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 20, 391–408 [DOI] [PubMed] [Google Scholar]
- 7.Pinna L. A., Meggio F. (1997) Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog. Cell Cycle Res. 3, 77–97 [DOI] [PubMed] [Google Scholar]
- 8.Messenger M. M., Saulnier R. B., Gilchrist A. D., Diamond P., Gorbsky G. J., Litchfield D. W. (2002) Interactions between protein kinase CK2 and Pin1. Evidence for phosphorylation-dependent interactions. J. Biol. Chem. 277, 23054–23064 [DOI] [PubMed] [Google Scholar]
- 9.Filhol O., Martiel J.-L., Cochet C. (2004) Protein kinase CK2: a new view of an old molecular complex. EMBO Rep. 5, 351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvi M., Sarno S., Cesaro L., Nakamura H., Pinna L. A. (2009) Extraordinary pleiotropy of protein kinase CK2 revealed by weblogo phosphoproteome analysis. Biochim. Biophys. Acta 1793, 847–859 [DOI] [PubMed] [Google Scholar]
- 11.Götz C., Montenarh M. (2017) Protein kinase CK2 in development and differentiation. Biomed. Rep. 6, 127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentzinger C. F., Wang Y. X., Rudnicki M. A. (2012) Building muscle: molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 4, a008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckingham M., Rigby P. W. J. (2014) Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 28, 225–238 [DOI] [PubMed] [Google Scholar]
- 14.Brown D. M., Parr T., Brameld J. M. (2012) Myosin heavy chain mRNA isoforms are expressed in two distinct cohorts during C2C12 myogenesis. J. Muscle Res. Cell Motil. 32, 383–390 [DOI] [PubMed] [Google Scholar]
- 15.Bucher E. A., Maisonpierre P. C., Konieczny S. F., Emerson C. P., Jr (1988) Expression of the troponin complex genes: transcriptional coactivation during myoblast differentiation and independent control in heart and skeletal muscles. Mol. Cell. Biol. 8, 4134–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galbiati F., Volonte D., Engelman J. A., Scherer P. E., Lisanti M. P. (1999) Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2C12 myoblasts. Transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube formation. J. Biol. Chem. 274, 30315–30321 [DOI] [PubMed] [Google Scholar]
- 17.Bi P., Ramirez-Martinez A., Li H., Cannavino J., McAnally J. R., Shelton J. M., Sánchez-Ortiz E., Bassel-Duby R., Olson E. N. (2017) Control of muscle formation by the fusogenic micropeptide myomixer. Science 356, 323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn M. E., Goh Q., Kurosaka M., Gamage D. G., Petrany M. J., Prasad V., Millay D. P. (2017) Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 8, 15665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q., Vashisht A. A., O’Rourke J., Corbel S. Y., Moran R., Romero A., Miraglia L., Zhang J., Durrant E., Schmedt C., Sampath S. C., Sampath S. C. (2017) The microprotein Minion controls cell fusion and muscle formation. Nat. Commun. 8, 15664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millay D. P., O’Rourke J. R., Sutherland L. B., Bezprozvannaya S., Shelton J. M., Bassel-Duby R., Olson E. N. (2013) Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 499, 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamage D. G., Leikina E., Quinn M. E., Ratinov A., Chernomordik L. V., Millay D. P. (2017) Insights into the localization and function of myomaker during myoblast fusion. J. Biol. Chem. 292, 17272–17289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leikina E., Gamage D. G., Prasad V., Goykhberg J., Crowe M., Diao J., Kozlov M. M., Chernomordik L. V., Millay D. P. (2018) Myomaker and myomerger work independently to control distinct steps of membrane remodeling during myoblast fusion. Dev. Cell 46, 767–780.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh Q., Millay D. P. (2017) Requirement of myomaker-mediated stem cell fusion for skeletal muscle hypertrophy. Elife 6, e20007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padilla-Benavides T., Nasipak B. T., Paskavitz A. L., Haokip D. T., Schnabl J. M., Nickerson J. A., Imbalzano A. N. (2017) Casein kinase 2-mediated phosphorylation of Brahma-related gene 1 controls myoblast proliferation and contributes to SWI/SNF complex composition. J. Biol. Chem. 292, 18592–18607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winter B., Kautzner I., Issinger O. G., Arnold H. H. (1997) Two putative protein kinase CK2 phosphorylation sites are important for Myf-5 activity. Biol. Chem. 378, 1445–1456 [DOI] [PubMed] [Google Scholar]
- 26.Johnson S. E., Wang X., Hardy S., Taparowsky E. J., Konieczny S. F. (1996) Casein kinase II increases the transcriptional activities of MRF4 and MyoD independently of their direct phosphorylation. Mol. Cell. Biol. 16, 1604–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietz K. N., Miller P. J., Hollenbach A. D. (2009) Phosphorylation of serine 205 by the protein kinase CK2 persists on Pax3-FOXO1, but not Pax3, throughout early myogenic differentiation. Biochemistry 48, 11786–11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietz K. N., Miller P. J., Iyengar A. S., Loupe J. M., Hollenbach A. D. (2011) Identification of serines 201 and 209 as sites of Pax3 phosphorylation and the altered phosphorylation status of Pax3-FOXO1 during early myogenic differentiation. Int. J. Biochem. Cell Biol. 43, 936–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyengar A. S., Loupe J. M., Miller P. J., Hollenbach A. D. (2012) Identification of CK2 as the kinase that phosphorylates Pax3 at Ser209 in early myogenic differentiation. Biochem. Biophys. Res. Commun. 428, 24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dick S. A., Chang N. C., Dumont N. A., Bell R. A. V., Putinski C., Kawabe Y., Litchfield D. W., Rudnicki M. A., Megeney L. A. (2015) Caspase 3 cleavage of Pax7 inhibits self-renewal of satellite cells. Proc. Natl. Acad. Sci. USA 112, E5246–E5252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González N., Moresco J. J., Cabezas F., de la Vega E., Bustos F., Yates J. R., III, Olguín H. C. (2016) Ck2-dependent phosphorylation is required to maintain Pax7 protein levels in proliferating muscle progenitors. PLoS One 11, e0154919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller P. J., Dietz K. N., Hollenbach A. D. (2008) Identification of serine 205 as a site of phosphorylation on Pax3 in proliferating but not differentiating primary myoblasts. Protein Sci. 17, 1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lou D. Y., Dominguez I., Toselli P., Landesman-Bollag E., O’Brien C., Seldin D. C. (2008) The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol. Cell. Biol. 28, 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchou T., Vernet M., Blond O., Jensen H. H., Pointu H., Olsen B. B., Cochet C., Issinger O.-G., Boldyreff B. (2003) Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol. Cell. Biol. 23, 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X., Toselli P. A., Russell L. D., Seldin D. C. (1999) Globozoospermia in mice lacking the casein kinase II alpha’ catalytic subunit. Nat. Genet. 23, 118–121 [DOI] [PubMed] [Google Scholar]
- 36.Shi X., Seldin D. C. C., Garry D. J. J. (2012) Foxk1 recruits the Sds3 complex and represses gene expression in myogenic progenitors. Biochem. J. 446, 349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheusova T., Khan M. A., Schubert S. W., Gavin A.-C., Buchou T., Jacob G., Sticht H., Allende J., Boldyreff B., Brenner H. R., Hashemolhosseini S. (2006) Casein kinase 2-dependent serine phosphorylation of MuSK regulates acetylcholine receptor aggregation at the neuromuscular junction. Genes Dev. 20, 1800–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Battistutta R., Cozza G., Pierre F., Papinutto E., Lolli G., Sarno S., O’Brien S. E., Siddiqui-Jain A., Haddach M., Anderes K., Ryckman D. M., Meggio F., Pinna L. A. (2011) Unprecedented selectivity and structural determinants of a new class of protein kinase CK2 inhibitors in clinical trials for the treatment of cancer. Biochemistry 50, 8478–8488 [DOI] [PubMed] [Google Scholar]
- 39.Brini M., De Giorgi F., Murgia M., Marsault R., Massimino M. L., Cantini M., Rizzuto R., Pozzan T. (1997) Subcellular analysis of Ca2+ homeostasis in primary cultures of skeletal muscle myotubes. Mol. Biol. Cell 8, 129–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarno S., Vaglio P., Meggio F., Issinger O.-G., Pinna L. A. (1996) Protein kinase CK2 mutants defective in substrate recognition. Purification and kinetic analysis. J. Biol. Chem. 271, 10595–10601 [DOI] [PubMed] [Google Scholar]
- 41.Borgo C., Franchin C., Scalco S., Bosello-Travain V., Donella-Deana A., Arrigoni G., Salvi M., Pinna L. A. (2017) Generation and quantitative proteomics analysis of CK2α/α′(-/-) cells. Sci. Rep. 7, 42409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prykhozhij S. V., Rajan V., Gaston D., Berman J. N. (2015) CRISPR multitargeter: a web tool to find common and unique CRISPR single guide RNA targets in a set of similar sequences. PLoS One 10, e0119372; erratum: e0138634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Brien A., Bailey T. L. (2014) GT-Scan: identifying unique genomic targets. Bioinformatics 30, 2673–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salvi M., Sarno S., Marin O., Meggio F., Itarte E., Pinna L. A. (2006) Discrimination between the activity of protein kinase CK2 holoenzyme and its catalytic subunits. FEBS Lett. 580, 3948–3952 [DOI] [PubMed] [Google Scholar]
- 45.Schneider C. A., Rasband W. S., Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pallafacchina G., François S., Regnault B., Czarny B., Dive V., Cumano A., Montarras D., Buckingham M. (2010) An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res. (Amst.) 4, 77–91 [DOI] [PubMed] [Google Scholar]
- 48.Vilk G., Saulnier R. B., St Pierre R., Litchfield D. W. (1999) Inducible expression of protein kinase CK2 in mammalian cells. Evidence for functional specialization of CK2 isoforms. J. Biol. Chem. 274, 14406–14414 [DOI] [PubMed] [Google Scholar]
- 49.Peng B., Zhao Y., Lu H., Pang W., Xu Y. (2005) In vivo plasmid DNA electroporation resulted in transfection of satellite cells and lasting transgene expression in regenerated muscle fibers. Biochem. Biophys. Res. Commun. 338, 1490–1498 [DOI] [PubMed] [Google Scholar]
- 50.Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995) Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- 51.Lüscher B., Litchfield D. W. (1994) Biosynthesis of casein kinase II in lymphoid cell lines. Eur. J. Biochem. 220, 521–526 [DOI] [PubMed] [Google Scholar]
- 52.Girardi C., James P., Zanin S., Pinna L. A., Ruzzene M. (2014) Differential phosphorylation of Akt1 and Akt2 by protein kinase CK2 may account for isoform specific functions. Biochim. Biophys. Acta 1843, 1865–1874 [DOI] [PubMed] [Google Scholar]
- 53.Braun T., Bober E., Buschhausen-Denker G., Kohtz S., Grzeschik K. H., Arnold H. H. (1989) Differential expression of myogenic determination genes in muscle cells: possible autoactivation by the Myf gene products. EMBO J. 8, 3617–3625; erratum: 4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weintraub H. (1993) The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75, 1241–1244 [DOI] [PubMed] [Google Scholar]
- 55.Wade R., Sutherland C., Gahlmann R., Kedes L., Hardeman E., Gunning P. (1990) Regulation of contractile protein gene family mRNA pool sizes during myogenesis. Dev. Biol. 142, 270–282 [DOI] [PubMed] [Google Scholar]
- 56.Braun T., Gautel M. (2011) Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 12, 349–361 [DOI] [PubMed] [Google Scholar]
- 57.Shainberg A., Yagil G., Yaffe D. (1971) Alterations of enzymatic activities during muscle differentiation in vitro. Dev. Biol. 25, 1–29 [DOI] [PubMed] [Google Scholar]
- 58.Zalin R. (1972) Creatine kinase activity in cultures of differentiating myoblasts. Biochem. J. 130, 79P [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruegg M. A., Bixby J. L. (1998) Agrin orchestrates synaptic differentiation at the vertebrate neuromuscular junction. Trends Neurosci. 21, 22–27 [DOI] [PubMed] [Google Scholar]
- 60.Sanes J. R., Lichtman J. W. (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791–805 [DOI] [PubMed] [Google Scholar]
- 61.Sabourin L. A., Girgis-Gabardo A., Seale P., Asakura A., Rudnicki M. A. (1999) Reduced differentiation potential of primary MyoD-/- myogenic cells derived from adult skeletal muscle. J. Cell Biol. 144, 631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cornelison D. D. W., Olwin B. B., Rudnicki M. A., Wold B. J. (2000) MyoD(-/-) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev. Biol. 224, 122–137 [DOI] [PubMed] [Google Scholar]
- 63.Dedieu S., Mazères G., Cottin P., Brustis J.-J. (2002) Involvement of myogenic regulator factors during fusion in the cell line C2C12. Int. J. Dev. Biol. 46, 235–241 [PubMed] [Google Scholar]
- 64.Wang C., Liu W., Nie Y., Qaher M., Horton H. E., Yue F., Asakura A., Kuang S. (2017) Loss of MyoD promotes fate transdifferentiation of myoblasts into brown adipocytes. EBioMedicine 16, 212–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srinivas B. P., Woo J., Leong W. Y., Roy S. (2007) A conserved molecular pathway mediates myoblast fusion in insects and vertebrates. Nat. Genet. 39, 781–786 [DOI] [PubMed] [Google Scholar]
- 66.Shi J., Bi P., Pei J., Li H., Grishin N. V., Bassel-Duby R., Chen E. H., Olson E. N. (2017) Requirement of the fusogenic micropeptide myomixer for muscle formation in zebrafish. Proc. Natl. Acad. Sci. USA 114, 11950–11955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W., Roy S. (2017) Myomaker is required for the fusion of fast-twitch myocytes in the zebrafish embryo. Dev. Biol. 423, 24–33 [DOI] [PubMed] [Google Scholar]
- 68.Koreishi M., Yu S., Oda M., Honjo Y., Satoh A. (2013) CK2 phosphorylates Sec31 and regulates ER-To-Golgi trafficking. PLoS One 8, e54382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J., Wang Y., Wang F., Yang J., Gao M., Li C., Liu Y., Liu Y., Yamaji N., Ma J. F., Paz-Ares J., Nussaume L., Zhang S., Yi K., Wu Z., Wu P. (2015) The rice CK2 kinase regulates trafficking of phosphate transporters in response to phosphate levels. Plant Cell 27, 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ralston E., Ploug T. (1999) Caveolin-3 is associated with the T-tubules of mature skeletal muscle fibers. Exp. Cell Res. 246, 510–515 [DOI] [PubMed] [Google Scholar]
- 71.Galbiati F., Engelman J. A., Volonte D., Zhang X. L., Minetti C., Li M., Hou H., Jr., Kneitz B., Edelmann W., Lisanti M. P. (2001) Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J. Biol. Chem. 276, 21425–21433 [DOI] [PubMed] [Google Scholar]
- 72.Gerber A. N., Klesert T. R., Bergstrom D. A., Tapscott S. J. (1997) Two domains of MyoD mediate transcriptional activation of genes in repressive chromatin: a mechanism for lineage determination in myogenesis. Genes Dev. 11, 436–450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.