Abstract
Recent recognition that TGF-β signaling disruption is involved in the development of aortic aneurysms has led to renewed investigations into the role of TGF-β biology in the aortic wall. We previously found that the type I receptor of TGF-β (TGFBR2) receptor contributes to formation of ascending aortic aneurysms and dissections (AADs) induced by smooth muscle cell (SMC)-specific, postnatal deletion of Tgfbr1 (Tgfbr1iko). Here, we aimed to decipher the mechanistic signaling pathway underlying the pathogenic effects of TGFBR2 in this context. Gene expression profiling demonstrated that Tgfbr1iko triggers an acute inflammatory response in developing AADs, and Tgfbr1iko SMCs express an inflammatory phenotype in culture. Comparative proteomics profiling and mass spectrometry revealed that Tgfbr1iko SMCs respond to TGF-β1 stimulation via robust up-regulation of cyclophilin A (CypA). This up-regulation is abrogated by inhibition of TGFBR2 kinase activity, small interfering RNA silencing of Tgfbr2 expression, or inhibition of SMAD3 activation. In mice, Tgfbr1iko rapidly promotes CypA production in SMCs of developing AADs, whereas treatment with a CypA inhibitor attenuates aortic dilation by 56% (P = 0.003) and ameliorates aneurysmal degeneration (P = 0.016). These protective effects are associated with reduced aneurysm-promoting inflammation. Collectively, these results suggest a novel mechanism, wherein loss of type I receptor of TGF-β triggers promiscuous, proinflammatory TGFBR2 signaling in SMCs, thereby promoting AAD formation.—Zhou, G., Liao, M., Wang, F., Qi, X., Yang, P., Berceli, S. A., Sharma, A. K., Upchurch, G. R., Jr., Jiang, Z. Cyclophilin A contributes to aortopathy induced by postnatal loss of smooth muscle TGFBR1.
Keywords: AAD, receptor, growth factor
TGF-β is essential for vasculogenesis during embryonic development (1). However, in mature vascular beds, particularly peripheral arteries, TGF-β can also promote aberrant profibrotic effects in chronic cardiovascular conditions, such as atherosclerosis and vascular restenosis (2, 3). This protein further plays a critical role in maintaining vascular homeostasis, a function that has recently become the subject of renewed interest with the discovery that dysregulation of TGF-β signaling causes syndromic (named Loeys-Dietz syndrome) and familial thoracic aortic aneurysms and dissections (TAADs) (4, 5). These groundbreaking studies have inspired the field to revisit the role of TGF-β biology in the aortic wall.
TGF-β propagates its signal through a cascade of mediators consisting of type I receptor of TGF-β (TGFBR1; encoded by Tgfbr1 in mice), TGF-β type II receptor (TGFBR2; encoded by Tgfbr2 in mice), SMAD2/3, and SMAD4, with the readout of this pathway negatively regulated by endogenous inhibitors, such as the proto-oncogene, SKI (1). Clinical investigations have revealed that mutations of any of these TGF-β mediators can lead to the development of TAAD in patients (6). To date, TGF-β mediator mutations identified in this condition have exclusively consisted of missense or nonsense mutations, which would theoretically reduce TGF-β signaling activity in the affected cells (7). Mechanistic investigations, however, have shown mixed effects on TGF-β signaling activity. For example, some studies reported that these mutations result in excessive TGF-β signaling that, in turn, drives TAAD formation (8–10), whereas others have indicated that enhanced SMAD2 signaling is the result of epigenetic up-regulation of SMAD2 transcription (11, 12). Not surprisingly, systemic blockage of TGF-β signaling had either deleterious effects on aortic dilation and rupture or no obvious impact, depending on the animal model used (13–15) and the timing of TGF-β inhibition (16).
These controversies and inconsistencies underscore the well-known phenomenon by which TGF-β functions in a context-dependent fashion and further indicate that TGF-β activity must be finely tuned, with deviation in either direction having potentially detrimental effects on the homeostasis of the aortic wall. However, the key question as to which signaling pathways are responsible for the distinct effects of TGF-β on TAAD development remains unresolved. This issue is even more confusing at the receptor level, given that signaling disruption resulting from the heterozygous mutation of either TGF-β receptor subunit is more complex than the predicted haploinsufficiency (14).
Using a Cre-loxP–mediated, inducible gene-deletion system, multiple groups have shown that deletion of smooth muscle cell (SMC) Tgfbr2 (Tgfbr2iko) in immature mice (6 wk of age) results in the rapid formation of ascending aortic aneurysms and dissections (AADs) (17, 18). Although these studies indicate a physiologic function for the SMC TGF-β in aortic maturation, we demonstrated that intact TGF-β signaling in SMCs is essential for maintaining the architecture of mature aortas in mice older than 9 wk. Specifically, we found that deletion of Tgfbr1 in SMCs leads to AAD formation with a pathology characterized by intimal/medial tears, dissections, and medial thinning. Additionally, we compared the aortic phenotype induced by Tgfbr1iko and Tgfbr2iko and found that these disruptions have differential impacts on the aortic wall, with Tgfbr1iko causing more severe AADs than Tgfbr2iko in adult mice (19). This is consistent with the observed differences in TAAD presentation in patients with TGFBR1 and TGFBR2 mutations (20). These studies not only establish the importance of TGF-β in SMCs under physiologic conditions, but also indicate that the consequences of TGF-β signaling disruption surpass the predictions made by the current TGF-β signaling dogma. Thus, given this revised role of TGF-β signaling in the aortic wall, further studies are needed to elucidate the mechanisms through which TGF-β governs molecular homeostasis in SMCs and, conversely, how disruption of TGF-β signaling leads to AAD formation.
In this study, we aimed to elucidate the cellular and molecular mechanisms by which Tgfbr1iko induces AAD formation. We have previously shown that AAD onset occurs rapidly in Tgfbr1iko mice (19). Given the extremely slow rate of matrix turnover in normal aortas, we reasoned that acute collapse of the wall architecture must result from active tissue destruction rather than insufficient matrix synthesis due to the TGF-β deficiency. Therefore, we tested the hypothesis that loss of Tgfbr1 in SMCs induces AAD formation via the induction of an acute inflammatory response in the aortic wall.
MATERIALS AND METHODS
Animals
This study conforms to the rules established in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA), and the protocol was approved by the Institutional Animal Care and Use Committee of the University of Florida. An inducible Cre-loxP system, driven by the myosin heavy chain 11 (Myh11) promoter, was used for mutant construction. Briefly, the Tgfbr1f/f (Tgfbr1-floxed) strain was crossed with a Y-linked Myh11-CreER strain. Male offspring with the Tgfbr1f/f.Myh11-CreER+/ genotype were analyzed at an age of 9–11 wk. This system allows inducible, SMC-specific deletion of the Tgfbr1 gene (Tgfbr1iko) due to the restricted expression of the Cre recombinase in SMCs (21). Wild-type (WT) controls [i.e., male mice not carrying the Cre allele (Tgfbr1f/f)] were produced by mating male breeders that carry an X-linked Myh11-CreER+/0 allele (22) with female breeders that do not carry the Cre allele. All strains were generated on a C57BL/6 background. Although we have recently developed an X-linked Myh11-CreER+/0 mouse line (22), this colony had only just been established when the current study was completed. Therefore, only male mice were used in this analysis.
Experimental treatments and sample collection
Tgfbr1iko was induced via daily intraperitoneal injection of tamoxifen (25 mg/ml in corn oil, 2.5 mg/mouse, T5648; MilliporeSigma, Burlington, MA, USA) for 5 consecutive days, as previously reported in refs. 19 and 21. The day of the first tamoxifen injection was defined as d 0. Animals in the Tgfbr1f/f control group received the same treatment. Ascending thoracic aortas (ATAs) were collected on d 7, 14, or 28; aortic tissues were either snap frozen in liquid nitrogen for mRNA and protein assays or perfusion fixed with 10% neutral-buffered formalin for histologic assessments. Cyclosporine A (CsA) oral solution (100 mg/ml, 78930518; Patterson Veterinary, Greeley, CO, USA) was diluted in saline and administrated once daily by oral gavage (25 mg/kg body weight in 0.1 ml volume). Treatment control mice were administered the same volume of saline (vehicle). Treatments were initiated 1 wk prior to tamoxifen injection and continued until d 28. At the time of tissue collection, ATAs were longitudinally divided into 2 pieces. One piece was snap frozen in liquid nitrogen for protein assays, and the other piece was fixed with 10% neutral-buffered formalin for histologic evaluation.
Ultrasound imaging
ATA dilation was monitored using long-axis B-mode ultrasound imaging, as previously described by Schmit et al. (21). Briefly, mice were anesthetized via inhalation of 1.5% isoflurane and secured in a supine position on a heated platform. Right parasternal B-mode images were acquired on a high-resolution Vevo 2100 Imaging System, with a MS550D (central frequency: 40 MHz) linear array transducer (VisualSonics, Toronto, ON, Canada). Using live B-mode imaging, the transducer position was carefully adjusted so that the ultrasound beam was aligned with the longitudinal centerline of the ascending aorta. Then, cineloops were recorded and reviewed frame by frame to select the one displaying the widest ATA. On this frame, the diameter of the ATA was measured at the site corresponding to the 11-o’clock position on the ring of the pulmonary artery.
Histology
A series of cross-sections (5.0 µm) was collected at distances of 0, 100, and 200 µm from the proximal end of the ATA samples. Point 0 was defined as the plane where a full- or half-circle (in cases where ATAs were cut longitudinally during tissue collection) cross section was first observed during the serial sectioning. This set of sections was stained using Masson’s trichrome staining protocol, as previously described in refs. 2 and 23. A blinded observer with expertise in vascular histology evaluated the pathology of each specimen and applied a 5-point scale system, as previously described by Yang et al. (19) to score the pathology of intimal/medial tears, intramural hematomas, and medial thinning/depletion. The scores assigned to each specimen were summed, and a mean of the sums obtained at each of the 3 locations was calculated to represent the degree of aneurysmal degeneration of the ATA.
Flow cytometry
Single-cell ATA solutions were prepared by enzymatic digestion of the aortic tissue. The enzyme cocktail contained a mixture of collagenase type XI (125 U/ml, LS004196; Worthington Biochemical, Lakewood, NJ, USA), hyaluronidase type 1-s (60 U/ml, LS005477; Worthington Biochemical), and collagenase type I (450 U/ml, LS004176; Worthington Biochemical) in PBS. After a 60-min digestion at room temperature, the cell solution was filtered through a 70-µm strainer, followed by centrifugation at 400 g for 5 min. Cells were then resuspended in PBS containing 2% fetal bovine serum (FBS). Blood samples (∼100 µl) were collected via tail vein bleeding before and after CsA treatment, and these were lysed (lysis buffer, 000-4300-54; Thermo Fisher Scientific, Waltham, MA, USA) to remove red blood cells.
Lysed blood samples were centrifuged at 200 g for 5 min, and white cell pellets were resuspended in PBS containing 2% FBS. The samples were blocked with a rat anti-CD16/CD32 antibody, and then each resuspended cell solution was divided into 2 aliquots and stained with antibody (rat IgG) cocktails containing APC-CD45, PerCP-Cy5-CD3, PE-Cy7-CD4, and APC-Cy7-CD8 and APC-CD45, FITC-CD19, PE-Cy7-Ly6C, and PE-Ly6G. All antibodies were purchased from BioLegend (San Diego, CA, USA). The antibody cocktails were prepared in PBS, containing 1% bovine serum albumin, using a predetermined dilution factor for each antibody (Table 1). Staining was evaluated by flow cytometry (LSR II; BD Biosciences), and the data were analyzed using FACSDiva software (BD Biosciences).
TABLE 1.
Antibodies used in the flow cytometry analysis
| Antibody | Dilution | Host | Catalog no. | Vendor |
|---|---|---|---|---|
| APC-CD45 | 1:100 | Rat | 103112 | BioLegend |
| PerCP-Cy5-CD3 | 1:300 | Rat | 560527 | BD Biosciences |
| PE-Cy7-CD4 | 1:100 | Rat | 100422 | BioLegend |
| APC-Cy7-CD8 | 1:100 | Rat | 557654 | BD Biosciences |
| FITC-CD19 | 1:100 | Rat | 152404 | BioLegend |
| PE-Cy7-Ly6C | 1:100 | Rat | 128018 | BioLegend |
| PE-Ly6G | 1:100 | Rat | 127608 | BioLegend |
Immunohistochemistry and immunofluorescence staining
All assays were performed on formalin-fixed, paraffin-embedded sections. Leukocyte infiltrates were detected using an antibody against the pan-leukocyte antigen CD45 (1:100, Rat anti-mouse IgG2b, 30-F11; BD Biosciences), and cyclophilin A (CypA) expression was assessed using a rabbit anti-CypA (1:100, ab126738; Abcam, Cambridge, MA, USA). The levels of CypA in SMCs were evaluated using immunofluorescence double-labeling for α-actin (a Cy3-conjugated anti-α-actin, C6198; MilliporeSigma) and CypA (1:100, ab126738; Abcam); nuclei were counterstained with DAPI (D9542; MilliporeSigma). A rat isotype IgG2b (1:100, MAB0061; R&D Systems, Minneapolis, MN, USA) and a nonimmunized rabbit IgG (1:100, NBP2- 24891; Novus Biologicals, Centennial, CO, USA) were used as negative controls. Antigen-specific signals were detected with Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:200, A11008; Thermo Fisher Scientific) or a biotin-conjugated goat anti-rabbit-IgG (1:200, BA1000; Vector Laboratories, Burlingame, CA, USA), followed by amplification with the ABC Kit (PK6100; Vector Laboratories) and visualization with the DAB Detection Kit (SK-4100; Vector Laboratories).
CD45 staining was imaged using a monochrome camera to record CD45+ cells in 1 channel and autofluorescence of the specimen in another channel. Images with merged channels were analyzed using Zen Lite 2012 software (Carl Zeiss GmbH, Oberkochen, Germany) to quantify the CD45+ cells lining the inner surface of the internal elastic lamina (IEL) and the outer surface of the external elastic lamina (EEL). Data were normalized to the length of the IEL or EEL, followed by calibration to the mean of Tgfbr1f/f control ATAs. The immunofluorescence double-labeling assays were evaluated using confocal microscopy.
Cell culture
Primary aortic SMCs were explanted from the thoracic aortas of Tgfbr1iko and Tgfbr1f/f mice, as previously described by Fu et al. (24). Cells at passages 8–9 were seeded at a density of 1 × 105 cells/cm2, cultured to ∼80% confluence, and serum-starved (2% FBS) for 24 h before use in experiments. Transfection of murine Tgfbr1-specific small interfering RNA (siRNA) or scramble siRNA oligos (1320001 and 4390843; Thermo Fisher Scientific) was performed using Lipofectamine 2000 Transfection Reagent (11668019; Thermo Fisher Scientific), according to the manufacturer’s instructions. Cells were stimulated with TGF-β1 (1.0 ng/ml, 240-B-002; R&D Systems), growth differentiation factor 15 (GDF15; 20 ng/ml, 957-GD-025; R&D Systems), or vehicle control for 24 h. Treatments with LY2109761 (a dual TGF-β receptor inhibitor, 10.0 µM; Santa Cruz Biotechnology, Dallas, TX, USA), pan-TGF-β neutralizing antibody (10.0 µg/ml, BE0057; Bio X Cell, West Lebanon, NH, USA), or SIS3 (a SMAD3-specific inhibitor, 10.0 µM; MilliporeSigma) were initiated 30 min before TGF-β1 or GDF15 stimulation. All treatments were performed in triplicate, with vehicle medium included as controls.
Two-dimensional difference gel electrophoresis and liquid chromatography–tandem mass spectrometry
Tgfbr1iko SMCs were treated with 1.0, 5.0, or 10.0 ng/ml TGF-β1 (240-B-002; R&D Systems) for 24 h; vehicle-treated SMCs were included as a control. Two-dimensional (2D) difference gel electrophoresis (DIGE) protein expression profiling and liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis of cell lysates was performed by Applied Biomics (Hayward, CA, USA). Briefly, total proteins (100 µg) were separated by 2D electrophoresis. Gels were stained with a CyDye (Cy2), and an image for each gel was acquired with a Typhoon image scanner. The spots were visually compared, and those with intensity differentials ranked in the top 10 were collected for further determination of their amino acid sequences with LC-MS/MS.
NanoString analysis
Total RNA was extracted from Tgfbr1f/f and Tgfbr1iko ATAs and primary SMCs using the RNeasy Mini Kit (74134; Qiagen, Hilden, Germany), according to the manufacturer’s instructions. For each sample, 150 ng of total RNA was reverse-transcribed to cDNAs. We then used the nCounter GX Mouse Inflammation Kit (XT-GXA-Min2; NanoString Technologies, Seattle, WA, USA), which covers 179 inflammation-related genes, to label the cDNAs generated from aortic tissues. A newer version of the kit (XT-CSO-Min2; NanoString Technologies), which covers 248 inflammation-related genes, was used to label the cDNAs obtained from primary SMCs. Labeled cDNA templates for each gene were counted using a NanoString system (NanoString Technologies), and the raw data were processed using nSolver Analysis software (v.3.0; https://www.nanostring.com/products/analysis-software/nsolver). For this analysis, the quality controls were set with the default parameters recommended for gene expression. After subtracting the counts of the negative controls, the data were normalized to the geometric mean of the positive controls. Gene expression data were analyzed using BRB-Array Tools (24). Because the data set includes only inflammation-related genes, some genes were expressed at very low levels in Tgfbr1f/f controls but up-regulated in Tgfbr1iko ATAs and SMCs. Therefore, we set the filters to exclude genes: 1) with fewer than 4 counts, 2) with <10% of expression values having at least a 1.5-fold change in either direction from the gene’s median values, and 3) whose percent missing exceeded 5%. Comparison between groups was performed with the significance level set at P < 0.01. Genes differentially expressed between the Tgfbr1f/f and Tgfbr1iko groups were then uploaded to Ingenuity Pathway Analysis (IPA) and analyzed using the core analysis module.
Gel and in situ zymography
Gel and in situ gelatin zymography were performed to assess the activity of matrix metalloproteinase (MMP) 2 and MMP9 in ATAs. For gel zymography, ATA homogenates (5.0 µg/sample) were separated on 10% zymography gels (EC6175; Thermo Fisher Scientific) under denaturing conditions. The gels were then renatured for 30 min in renaturing buffer (LC2670; Thermo Fisher Scientific) and incubated in developing buffer (LC2671; Thermo Fisher Scientific) for 12 h at 37°C. Gels were stained using the Colloidal Blue Staining Kit (LC6025; Thermo Fisher Scientific), and the density of each band was quantified using Bio-Rad Image Lab Software v.4.0 (Bio-Rad, Hercules, CA, USA). For in situ zymography, frozen sections (5 μm) were covered with a mixture containing 1% agarose and 0.1 mg/ml DQ gelatin (D12054; Thermo Fisher Scientific) in the reaction buffer. Sections pretreated with 1,10 phenanthroline (0.5 mM; Thermo Fisher Scientific), an MMP inhibitor, were included as negative controls. After a 12-h incubation at room temperature, the assays were evaluated under a fluorescence microscope.
Western blot
Total protein was extracted from SMCs and ATAs with RIPA buffer (89900; Thermo Fisher Scientific), supplemented with protease and phosphatase inhibitor cocktails (1861281; Thermo Fisher Scientific). Protein concentrations were determined using the Pierce BCA protein Assay kit (23225; Thermo Fisher Scientific), and 10 μg total protein from each sample was separated on SDS-PAGE gels. Gels were transferred to PVDF membranes, and rabbit mAb were used to detect CypA (1:500, ab126738; Abcam), total RelA (1:1000, 3034; Cell Signaling Technology, Danvers, MA, USA), and phospho-RelA (1:1000, 3033; Cell Signaling Technology). The immunoblots were developed with chemiluminescent substrate (TMA-6; Lumigen, Southfield, MI, USA) and quantified for total intensity using the Bio-Rad ChemiDoc MP Imaging System (Bio-Rad). Data were expressed as intensity normalized to the levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (19).
Statistical analysis
All data are expressed as means ± sem. Statistical analyses were performed using Sigma Plot v.14.0. (San Jose, CA, USA), and the data sets were evaluated using normality and equivalence variance testing. For those failing this evaluation, logarithmic and exponential transformations were performed to meet these requirements. Student’s t tests, 2-way ANOVA, and 2-way repeated measures ANOVA were performed, when appropriate, with Holm-Sidak analysis used for post hoc tests. In all cases, P < 0.05 was considered statistically significant.
RESULTS
Tgfbr1iko induces an acute innate immune response in the aortic wall
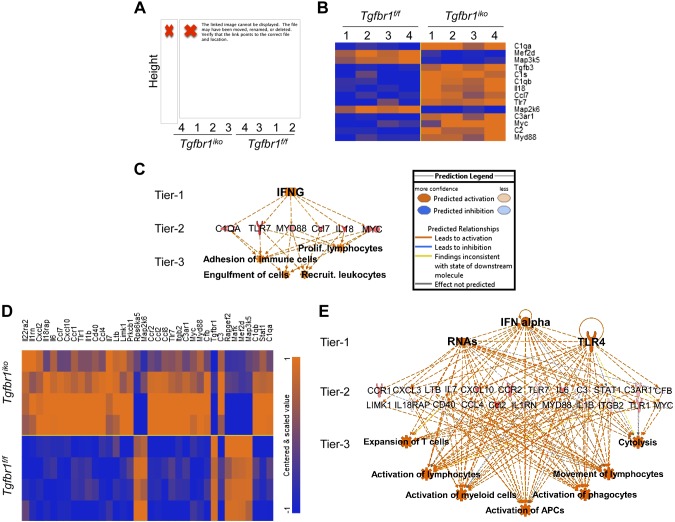
To determine the inflammatory response resulting from SMCs-specific, postnatal deletion of Tgfbr1, we profiled expression of inflammation-related genes in ATAs from Tgfbr1iko and Tgfbr1f/f mice, collected 7 and 14 d after the first dose of tamoxifen (n = 4/ genotype at each time point). The nCounter GX Mouse Inflammation Kit, which contains multiplexed probes, specific for 179 inflammatory genes, was used for this analysis, and the data were analyzed using BRB-Array Tools and IPA. Unsupervised clustering revealed that the inflammation-related gene signature separated ATAs from Tgfbr1iko and Tgfbr1f/f animals into 2 distinct clusters as early as d 7, when the induction of Tgfbr1 deletion was just completed (Fig. 1A). Two-way ANOVA analysis using BRB-Array Tools, with a significance cutoff of P < 0.01, identified 48 and 46 genes with variable expression depending on genotype and time, respectively. Further, ad hoc comparisons revealed that 14 genes were differentially expressed in Tgfbr1iko and Tgfbr1f/f ATAs on d 7 (P < 0.01), with the majority of these up-regulated in Tgfbr1iko ATAs (Fig. 1B). Based on IPA analysis for regulator effects, genes in this set are predicted to share IFN-γ as a master upstream regulator and function to activate immune cells through multiple biologic processes (Fig. 1C).
Figure 1.
Loss of Tgfbr1 in SMCs activates innate immune responses in the aortic wall. A) Unsupervised clustering of immune signatures in ATAs from mice with SMCs-specific, postnatal Tgfbr1 deletion (Tgfbr1iko) and WT controls (Tgfbr1f/f) collected on d 7 after induction of Tgfbr1 deletion. Height represents the correlation distance between the branches/clusters, and the numbers represent identifiers for samples in the indicated group. B) Heat map of genes differentially expressed in Tgfbr1iko and Tgfbr1f/f ATAs with the identification numbers specified in A. Expression levels are color coded, with orange and blue representing higher and lower expression, respectively, in Tgfbr1iko vs. Tgfbr1f/f ATAs. C) Top regulatory effects predicted by IPA for the gene set listed in B. Tier 1: predicted upstream regulators (P < 0.01, z score >2.0); Tier 2: target genes in the data set; Tier 3: predicted biologic effects (P < 0.01, z score >2.0). The prediction legend depicts the various regulatory effects shown in the network. D) Heat map of genes differentially expressed in Tgfbr1iko and Tgfbr1f/f ATAs by d 14 following induction of the Tgfbr1 deletion. Color coding is the same as described in B. E) The top regulatory effects predicted by IPA analysis for the gene set listed in D. Tiers are the same as those described in C.
The inflammatory gene signature further discriminated Tgfbr1iko ATAs from their WT counterparts (Tgfbr1f/f) at d 14 (Supplemental Fig. S1A, B). At this time point, 29 out of 179 inflammatory genes were found to be significantly up-regulated in Tgfbr1iko ATAs, as compared to Tgfbr1f/f controls (Fig. 1D). Based on IPA analysis, RNA and TLR-4 were predicted as the 2 major upstream regulators for these genes, and this gene network was proposed activate several cellular events that are critical for the innate immune response (Fig. 1E). These results demonstrate that Tgfbr1iko induces an acute inflammatory response in ATAs, which may occur due to the activation of RNA- and TLR4-mediated danger signals.
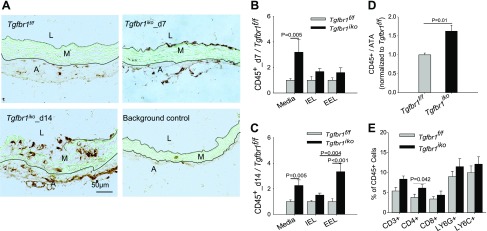
Given these data showing the up-regulation of gene networks that are predicted to promote innate immune responses in Tgfbr1iko ATAs, we next investigated the infiltration of immune cells in ATA tissue from Tgfbr1iko and Tgfbr1f/f mice. Immunohistochemistry (IHC) assays showed that, in d 7 Tgfbr1iko ATAs, CD45+ cells clustered on the luminal surface and near the outer boarder of the medial layer and then invaded into the deeper layer of the tunica media by d 14 (Fig. 2A). This indicates immune cell migration into the tunica media from both the innermost and outermost surfaces. Quantitative analysis further revealed that Tgfbr1iko ATAs contained significantly more CD45+ cells than Tgfbr1f/f ATAs on d 7 (n = 6–7/group, P = 0.003, 2-way ANOVA, Fig. 2B) and d 14 (n = 5/group, P < 0.001, 2-way ANOVA, Fig. 2C). Although this difference was dominated by infiltrates within the medial layer on d 7 (media, P = 0.005, Fig. 2B), infiltrates became more prominent on the outermost surface (EEL, P < 0.001) and deep layers of the tunica media (media, P = 0.005) by d 14 (Fig. 2C). Additionally, we found that CD45+ infiltrates on the EEL surface of Tgfbr1iko ATAs increased from 1.6-fold (P = NS, Fig. 2B) to 3.4-fold (Fig. 2C) higher than the counts inTgfbr1f/f control ATAs (P < 0.001), indicating accumulation of CD45+ cells in the media-adventitia border region during TAAD development.
Figure 2.
Tgfbr1iko ATAs contain increased inflammatory infiltrates relative to Tgfbr1f/f ATAs. A) Immunohistochemical staining of CD45 in Tgfbr1f/f and Tgfbr1iko ATAs. Brown: CD45+ cells; green: autofluorescence; black dashed lines: black solid lines: A, adventitia; L, lumen; M, media. B, C) Quantification of CD45+ cells in ATAs collected on d 7 (B) and 14 (C) after induction of Tgfbr1 deletion. Data are expressed as the fraction of CD45+ area in the media and on the inner surface of the IEL or the outer surface of EEL (n = 5–7/genotype, per time point), normalized to the corresponding Tgfbr1f/f control group, and analyzed using 2-way ANOVA and Holm-Sidak tests. Genotype-dependent differences (P < 0.001) on d 7 and 14. D) CD45+ cells detected by flow cytometry analysis in ATAs collected on d 7. Data were normalized to the mean of Tgfbr1f/f controls and analyzed using unpaired Student’s t tests. E) Immune cell subsets in d 7 Tgfbr1iko ATAs; P = 0.007 for genotype-dependent difference in percentage of CD4+ T cells, as determined by 2-way ANOVA.
Because of the semiquantitative nature of the IHC staining, we further quantified and phenotyped the CD45+ population in Tgfbr1iko (d 7, n = 8) and Tgfbr1f/f (d 7, n = 5) ATAs using flow cytometry analysis. As expected, a greater number of CD45+ cells were detected in Tgfbr1iko than Tgfbr1f/f ATAs (Fig. 2D, P = 0.01). Interestingly, our data also reveal a significantly higher percentage of CD4+ T cells in Tgfbr1iko than in Tgfbr1f/f ATAs (2-way ANOVA, P = 0.007, Fig. 2E), indicating that these immune cell subsets are the major cellular responders to the cytokine milieu elaborated by SMCs-specific, postnatal deletion of Tgfbr1 in the aortic wall.
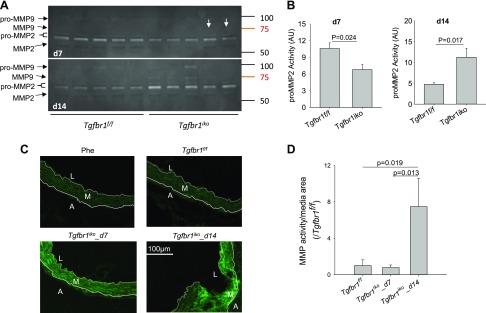
Tgfbr1iko increases MMP2 activity in the aortic wall
MMPs, particularly MMP2 and MMP9, are proteolytic enzymes that break down the aortic architectures during aortic aneurysm formation (25). Because of the rapid onset of intimal/medial tears in Tgfbr1iko ATAs, we measured activity and production of MMP2 and MMP9 in these tissues. Zymogram assays showed a significantly lower level of pro-MMP2 (P = 0.024) in Tgfbr1iko (n = 5) ATAs than in Tgfbr1f/f control ATAs (n = 5) on d 7 (Fig. 3A, B). However, 2 out of 5 Tgfbr1iko ATAs displayed a faint, but visible, active MMP9 band (Fig. 3A, arrows). By d 14, Tgfbr1iko ATAs (n = 5) produced a significantly higher level of pro-MMP2 (P = 0.017) than the controls (n = 5). Pro-MMP9 was also produced, but it was more readily detectable in the Tgfbr1iko ATAs, for which intramural hematoma was noted during tissue collection. Additionally, active MMP2 and MMP9 were present in most of the Tgfbr1iko ATAs, whereas neither of these enzymes was discernible in Tgfbr1f/f controls (Fig. 3A). In situ zymography assays further showed that Tgfbr1f/f controls had a detectable baseline level of gelatinolytic activity in the medial layer. However, in Tgfbr1iko ATAs, gelatinolytic activity was also detected in the medial layer and was more pronounced in regions with intimal/medial tears (Fig. 3C). Quantitatively, when compared with Tgfbr1f/f controls, Tgfbr1iko ATAs exhibited an initial trend of decreased gelatinolytic activity, followed by a significant increase (P = 0.019) (Fig. 3D), a pattern that correlates nicely with data from gel-based zymogram assays.
Figure 3.
Tgfbr1iko promotes MMP activity in ATAs. A) Zymogram assays to measure MMP activity in ATAs collected on d 7 and 14 after induction of Tgfbr1 deletion. Arrows indicate faint bands corresponding to active MMP9. B) Quantification of pro-MMP2 activity. Data are expressed as band intensity, using arbitrary units (AUs), and the significance was analyzed using unpaired Student’s t tests. C) In situ zymography of MMP activity (green) in Tgfbr1f/f and Tgfbr1iko ATAs. White dashed lines: IEL; white solid lines: EEL. D) Quantification of gelatinolytic activity in the medial layer of d 7 and 14 ATAs. Phe, 1,10 phenanthroline, an MMP inhibitor. Time-dependent effect: P = 0.019, 1-way ANOVA; Tgfbr1f/f vs. Tgfbr1iko: P = 0.013.
Based on the observed changes in MMP2 and MMP9 activity, we next measured the production of these enzymes using IHC. From these assays, we detected a higher level of both MMP2 (P = 0.018) and MMP9 (P = 0.027) in the medial layer of Tgfbr1iko ATAs, as compared to ATAs from Tgfbr1f/f animals, by d 14 (Supplemental Fig. S2A, B, D). In the adventitial layer, low levels of MMP2 and MMP9 were detected in d 7 and 14 Tgfbr1f/f ATAs. In contrast, strong MMP2 and MMP9 staining was observed in the area near the formation of intimal/medial tears in Tgfbr1iko ATAs by d 14 (Supplemental Fig. S2A, C, E). Notably, the up-regulation of these enzymes was also confirmed by Western blotting assays (Supplemental Fig. S2F–I).
SMCs acquire an inflammatory phenotype after loss of Tgfbr1
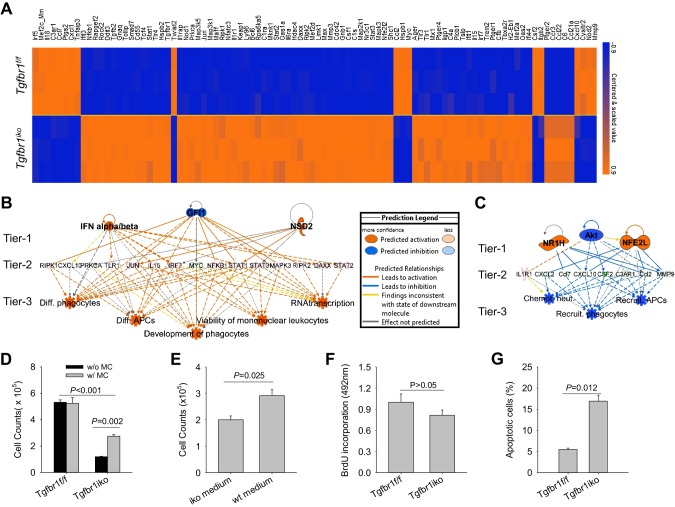
TGF-β is a potent regulator of SMC-contractile protein expression, and previous studies have shown that inhibition of TGF-β reduces the production of both α-actin and smooth muscle myosin heavy chain (26). We therefore hypothesize that acute deletion of Tgfbr1 will affect the function of the SMC-contractile apparatus, leading to altered mechanosensing and activation of an inflammatory response in the aortic wall. In addition, SMCs can acquire a secretory phenotype, in which they gain the ability to produce a large panel of inflammatory mediators (27). Therefore, it is possible that loss of Tgfbr1 may disrupt the molecular homeostasis of SMCs and cause phenotypic switching from a contractile to a secretory state. To test this hypothesis, we profiled the expression of 248 inflammation-related genes in primary Tgfbr1iko and Tgfbr1f/f SMCs using NanoString assays. Deletion of Tgfbr1 was verified using RT-PCR with primers flanking the target exon (forward: 5′-TCCTCGAGACAGGCCATTTG-3′, reverse: 5′-TGCCTCTCGGAACCATGAAC-3′) and Western blot assays with an antibody to TGFBR1. RT-PCR results confirmed that Tgfbr1iko SMCs express a mutated Tgfbr1 mRNA that is not translated to TGFBR1 protein, and a WT Tgfbr1 was not detected in Tgfbr1iko SMC cultures (Supplemental Fig. S3).
BRB-Array Tools software was then employed to analyze results from NanoString assays and compare gene expression in Tgfbr1f/f and Tgfbr1iko SMCs. Using a significance cutoff of P < 0.001, we found that 76 inflammation-related genes were up-regulated in Tgfbr1iko SMCs, whereas 18 genes were inhibited in Tgfbr1iko SMCs, as compared to WT Tgfbr1f/f SMCs (Fig. 4A). IPA further identified pattern recognition and PPARα/RXRα as the top canonical pathways represented by the up- and down-regulated genes, respectively. Analysis for “regulator effects” predicted increased IFN-α/β, decreased growth factor independent 1 (GFI1), and/or increased nuclear receptor binding SET domain protein 2 (NSD2) as the possible upstream regulators for the network in which the up-regulated genes were enriched to the greatest extent (Fig. 4B). In contrast, AKT inhibition, NR1H activation, and/or NFE2L (nuclear factor, erythroid 2 like 1) activation were predicted as upstream effectors for the network in which the down-regulated genes were enriched to the greatest extent (Fig. 4C). Notably, however, both networks are predicted to regulate multiple biologic processes key to the innate immune response (Fig. 4B, C). These results demonstrate that loss of Tgfbr1 results in SMCs acquiring an inflammatory phenotype. This is also consistent with the observation that the up-regulation of inflammatory genes and activation of immune responses were detected during the early stage of AAD formation in Tgfbr1iko aortas (Fig. 1C, E). Thus, the agreement between in vivo and in vitro observations suggests that inflammatory SMCs act as an initiator for the activation of inflammation in ATAs after SMC-specific Tgfbr1 deletion.
Figure 4.
SMCs acquire an inflammatory phenotype after loss of Tgfbr1. A) Heat map of differentially expressed, inflammation-related genes in Tgfbr1iko and Tgfbr1f/f SMCs under regular culture conditions. Levels of gene expression are color coded, with the scale bar shown on the right. B, C) Top regulatory effects predicted by IPA analysis for genes up-regulated (B) and down-regulated (C) in Tgfbr1iko SMCs relative to Tgfbr1f/f SMCs. The prediction legend depicts the various regulatory effects shown in the 2 networks. Tier 1: predicted upstream regulators (P < 0.01, z score >2.0); Tier 2: target genes in the data set; Tier 3: predicted biologic effects (P < 0.01, z score >2.0). D) Population expansion of SMCs after 72 h growth with (w/) and without (w/o) daily medium change (MC). E) Population expansion of Tgfbr1f/f SMCs treated with conditioned medium collected from Tgfbr1iko (iko medium) or Tgfbr1f/f (WT medium) SMCs for 72 h. F) SMC proliferation determined by the rate of BrdU incorporation in a 24-h culture period with complete media. G) SMC apoptosis determined by Annexin V expression under complete media culture condition. Significance values were calculated using unpaired Student’s t tests.
To further characterize the phenotype of Tgfbr1iko SMCs, we evaluated their growth and survival under complete media culture condition. We found that the population of Tgfbr1iko SMCs expands much more slowly than Tgfbr1f/f SMCs. Interestingly, daily refreshment of the culture medium doubled the growth of Tgfbr1iko SMCs (P = 0.002) but had little impact on Tgfbr1f/f SMCs (Fig. 4D), suggesting that the secretome of Tgfbr1iko SMCs negatively affects their growth. To test this hypothesis, we treated Tgfbr1f/f SMCs with conditioned medium collected from Tgfbr1iko and Tgfbr1f/f SMCs. As expected, SMCs treated with Tgfbr1iko medium yielded a 33% smaller population (P = 0.025, Fig. 4E) than those treated with Tgfbr1f/f medium, suggesting that Tgfbr1iko SMCs can affect growth of neighboring cells via a paracrine effect. Additionally, BrdU incorporation assays and Annexin V expression revealed that Tgfbr1iko SMCs not only proliferate at a slower rate (Fig. 4F) but they also die from apoptosis at a much higher rate than Tgfbr1f/f SMCs (P = 0.012, Fig. 4G). Therefore, in addition to acquisition of an inflammatory phenotype, Tgfbr1iko SMCs show impaired survival and decreased proliferation.
Loss of Tgfbr1 triggers promiscuous TGFBR2 signaling and promotes CypA expression in SMCs
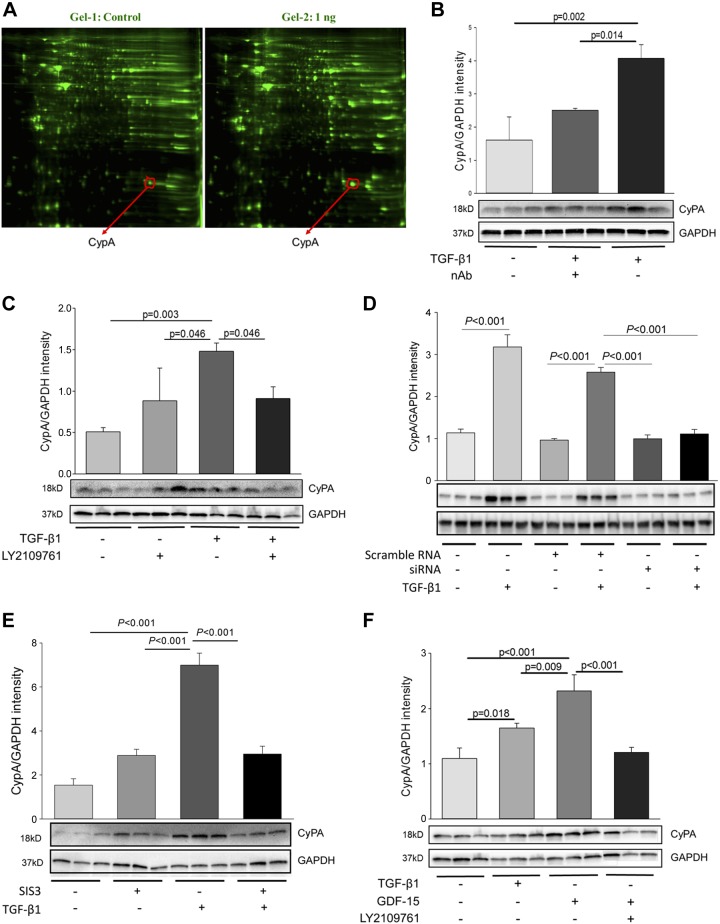
We have previously reported that deletion of SMC Tgfbr2 (Tgfbr2iko) attenuates AAD formation in Tgfbr1iko mice (19). This finding suggests that in the absence of Tgfbr1, Tgfbr2 becomes detrimental to SMCs; however, the specific signaling pathways involved in this process were not defined. In order to investigate this, we treated Tgfbr1iko SMCs with TGF-β1, the primary ligand for TGFRB2, at concentrations of 0.0, 1.0, 5.0, and 10.0 ng/ml. We then performed 2D DIGE, followed by LC-MS/MS to determine the identity of proteins differentially expressed in treated vs. untreated cells and determined the complete amino acid sequences for the 10 most highly induced proteins. A top protein on this list, peptidyl-prolyl cis-trans isomerase A (also known as CypA; Fig. 5A), has distinct biologic functions depending on its subcellular location. On the cell surface, it functions as a potent proinflammatory stimulator via binding to CD147 (28). Genetic deletion of CypA has also been shown to attenuate murine abdominal aortic aneurysms (AAAs) and atherosclerosis (29, 30), suggesting it may also play a role in AAD formation.
Figure 5.
Deletion of Tgfbr1 promotes aberrant TGFBR2 signaling and induction of CypA production in Tgfbr1iko SMCs. A) Two-dimensional DIGE was used to profile proteins extracted from Tgfbr1iko SMCs with or without TGF-β1 treatment (1.0 ng/ml, 24 h). Proteins differentially expressed in treated vs. untreated cells were identified by LC-MS/MS, and 1 highly induced protein was found to be CypA. B) CypA production in Tgfbr1iko SMCs after TGF-β1 stimulation. C) CypA production in Tgfbr1iko SMCs after treatment with TGF-β1, LY2109761 (a TGFBR2 kinase inhibitor), or LY2109761 and TGF-β1. D) CypA production in Tgfbr1iko SMCs after treatment with GDF15, LY2109761, or LY2109761 and GDF15. E) Effect of Tgfbr2 siRNA silencing on CypA production in Tgfbr1iko SMCs after TGF-β1 stimulation. F) TGF-β1–induced CypA production in Tgfbr1iko SMCs pretreated with SIS3 (a SMAD3 inhibitor). Expression data (B–F) were obtained by Western blot analysis. Data are expressed as band intensity normalized to the levels of GAPDH, and significance was analyzed using 1-way ANOVA.
In order to test this possibility and evaluate the importance of CypA in AAD formation, we first verified our CypA results from 2D DIGE and LC-MS/MS analyses using Western blotting analysis. These data showed that treatment with 1.0 ng/ml TGF-β1 promotes CypA production in Tgfbr1iko SMCs by more than 2-fold as compared to untreated controls, whereas preincubation of the TGF-β1 solution with a pan-TGF-β neutralizing antibody abolishes this response (Fig. 5B). This confirms that the observed CypA induction occurs as a result of TGF-β1 signaling. To determine whether this signaling is propagated through TGFBR2, we first performed assays in which we inhibited the TGFBR2 kinase activity with the inhibitor, Ly2109761. As expected, treatment of Tgfbr1iko SMCs with Ly2109761, but not the vehicle control, significantly inhibits the induction of CypA by TGF-β1 (Fig. 5C). We then used siRNA to silence the expression of TGFBR2; siRNA transfection decreased Tgfbr2 expression by more than 95% (data not shown). Notably, CypA induction was observed in TGF-β1–treated cells transfected with scramble siRNA control and in those treated with transfection reagent alone. However, this response was almost completely abolished in cells transfected with the Tgfbr2-specific siRNAs (Fig. 5D). These results suggest that a functional TGFBR2 is required for the TGF-β1–mediated induction of CypA expression in Tgfbr1iko SMCs.
We previously determined that TGF-β1 cannot activate the SMAD2 and MAPK pathways in Tgfbr1iko SMCs (19). This suggests the intriguing hypothesis that SMAD3 mediates TGF-β1–induced CypA production in Tgfbr1iko SMCs. To test this, we performed experiments to determine whether inhibition of SMAD3 activation suppresses CypA up-regulation. Our data showed that whereas Tgfbr1iko SMCs reproducibly increase production of CypA in response to TGF-β1 stimulation, treatment of these cells with SIS3, a SMAD3-specific inhibitor, abolishes TGF-β1–mediated CypA induction (Fig. 5E). Taken together, the results presented above suggest a model in which, in the absence of TGFBR1, TGFR2 recognizes TGF-β1, leading to SMAD3 activation and CypA induction.
Although TGF-β isoforms are the primary ligands for TGFBR2 under physiologic conditions, other ligands in the TGF-β superfamily, such as GDF15 (31, 32), may bind to TGFBR2, triggering promiscuous TGFBR2 signaling under pathologic conditions. We therefore determined whether GDF15 can signal through TGFBR2 in Tgfbr1iko SMCs. From these experiments, we found that treatment with GDF15 (20 ng/ml) for 24 h can induce CypA production to a greater degree than what is elaborated by TGF-β1 stimulation (P = 0.009). Notably, pretreatment with Ly2109761, a TGFBR2 kinase inhibitor, abolishes the GDF15-mediate CypA induction (P < 0.01, Fig. 5F), suggesting that GDF15 is another ligand capable of signaling through TGFBR2 to induce CypA production in Tgfbr1iko SMCs.
Lastly, we examined whether Tgfbr1iko SMCs secrete CypA as an extracellular ligand. To this end, we measured levels of CypA in the culture medium of Tgfbr1iko SMCs after treatment with TGF-β1 (1.0 ng/ml) for 24 h. We found that, compared with the vehicle control, treatment with TGF-β1 significantly increases CypA levels in the culture medium. Neutralization of TGF-β1 with a pan-TGF-β antibody, however, abrogates this induction (Supplemental Fig. S4). Therefore, these data suggest that Tgfbr1iko SMCs induce CypA production and release this protein into the extracellular space in response to promiscuous TGFBR2 signaling, which enables CypA to stimulate inflammatory responses in both an autocrine and paracrine fashion.
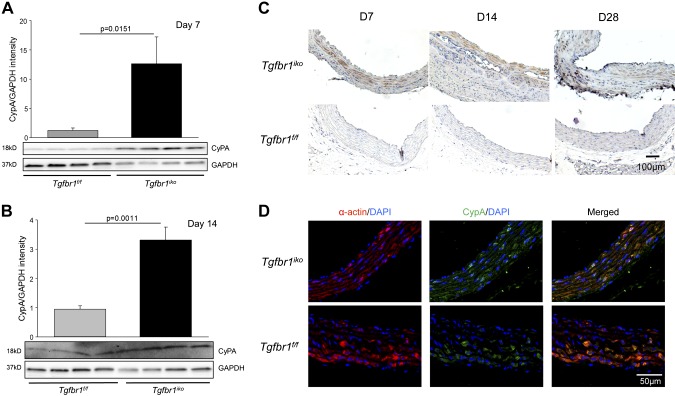
CypA is up-regulated in aortic SMCs deficient in Tgfbr1 in vivo
To determine whether CypA is involved in AAD formation in Tgfbr1iko mice, we used Western blotting assays to evaluate its production in developing AADs at d 7 (n = 4) and d 14 (n = 4) after initiation of Tgfbr1 deletion. We found that, compared with Tgfbr1f/f controls, CypA abundance in Tgfbr1iko ATAs was significantly increased at d 7 (P = 0.015, Fig. 6A) and d 14 (P = 0.001, Fig. 6B). IHC staining revealed that cells located in the medial layer of the ATAs (Fig. 6C, top panels) produce CypA protein. Immunofluorescence double-labeling of CypA and α-actin on Tgfbr1iko ATAs collected on d 7 further showed that the CypA signals primarily colocalize with cells expressing α-actin (Fig. 6D, top panels), indicating that SMCs are the major cell type producing CypA in early-stage AADs. In addition, a weak CypA signal was also detected in α-actin–positive cells from Tgfbr1f/f ATAs (Fig. 6D, lower panels), suggesting that SMCs in healthy aortas produce a baseline level of CypA.
Figure 6.
CypA is up-regulated in SMCs from early-stage ascending AADs induced by Tgfbr1iko. A, B) Western blot of CypA expression in Tgfbr1iko ATAs on d 7 (A) and d 14 (B) following induction of Tgfbr1 deletion. Data are expressed as band intensity normalized to the levels GAPDH, and significance was analyzed using unpaired Student’s t tests. C) IHC assays for CypA production in Tgfbr1iko and Tgfbr1f/f ATAs collected at the indicated time points after initiation of Tgfbr1 deletion. D) Double immunofluorescence staining of α-actin (red) and CypA (green) in Tgfbr1iko and Tgfbr1f/f ATAs. Blue, DAPI nuclear counterstain.
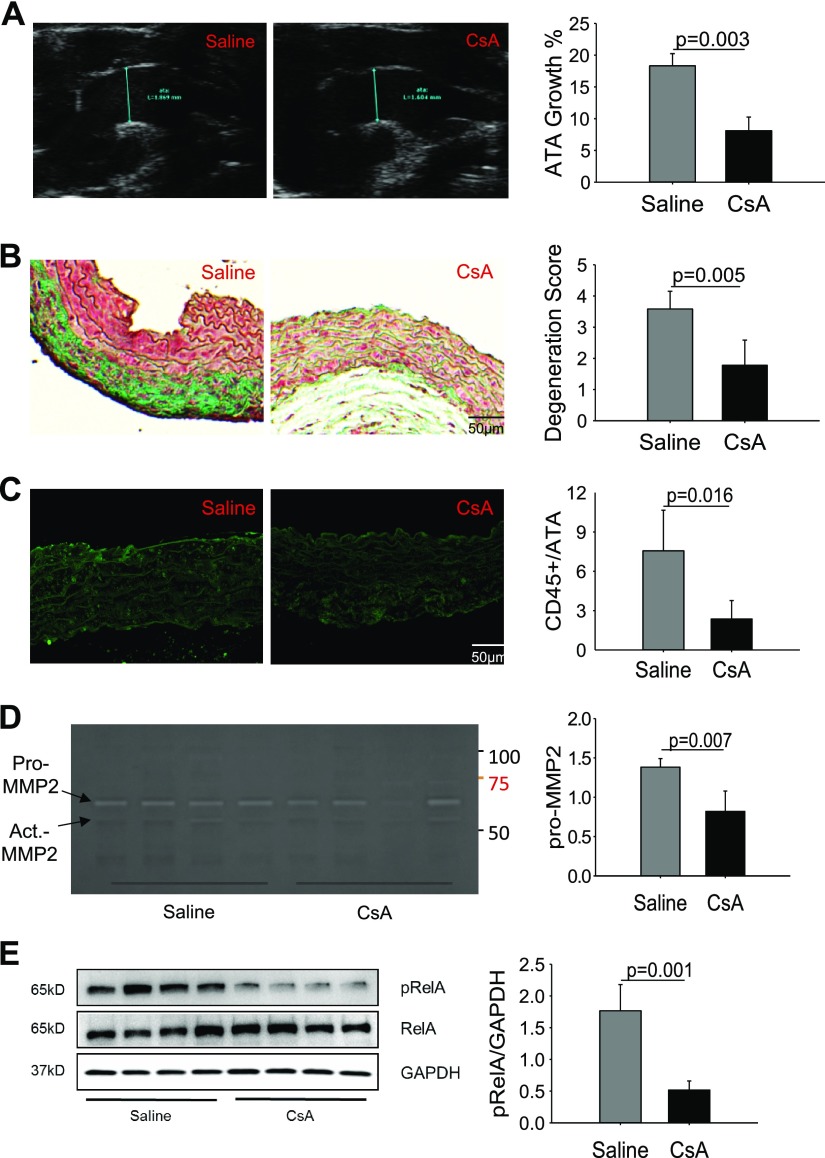
Inhibition of CypA attenuates AAD formation in Tgfbr1iko mice
The early up-regulation of CypA observed during AAD formation led us to test whether this correlation represents a true cause-and-effect relationship. Therefore, we treated Tgfbr1iko mice with CsA (n = 6), a potent CypA inhibitor (33), or saline control (n = 5), starting at 1 wk prior to initiation of Tgfbr1iko deletion and continuing for 4 wk. Ultrasound imaging showed that treatment with CsA inhibited ATA dilation by 13% (P = 0.003, Fig. 7A) in 4 wk. ATAs treated with CsA also displayed an improved histologic structure, with attenuation in 1 or more evaluated pathologies, as compared with those treated with saline. Accordingly, the degeneration score was significantly lower for ATAs treated with CsA than those treated with saline (P = 0.005, Fig. 7B). Consistent with the improved histology, CsA treatment also inhibited accumulation of inflammatory infiltrates in the aortic wall, as evidenced by fewer CD45+ cells in the medial layer of ATAs treated with CsA than those treated with saline (P = 0.016, Fig. 7C). In addition, ATAs treated with CsA displayed a lower level of pro-MMP2 activity (P = 0.007, Fig. 7D) and RelA activation (P = 0.001, Fig. 7E) than those treated with saline. Overall, these results show that treatment with the CypA inhibitor, CsA, prevents AAD formation in Tgfbr1iko mice, and the protective effects are associated with reduced inflammation in the aortic wall.
Figure 7.
Inhibition of CypA attenuates aneurysmal degeneration of Tgfbr1iko ATAs. A) Ultrasound imaging of ATAs from mice treated with saline or the CypA inhibitor, CsA, on d 28 following induction of Tgfbr1 deletion. Data are expressed as percent increase of the ATA diameter. B) Histologic evaluation of aneurysmal degeneration of ATAs collected on d 28. Representative images of Masson’s staining of ATAs from groups subjected to the indicated treatments are shown in the left 2 panels, and the assigned degeneration score for each group is plotted in the bar graph. C) Immunofluorescence labeling of CD45+ cells in Tgfbr1iko ATAs treated with saline or CsA. Data are expressed as the fractional CD45+ area in the medial layer of the ATAs. D) Zymogram gel analysis of gelatinolytic activity in Tgfbr1iko ATAs treated with CsA or saline control. E) Phospho-RelA expression in the Tgfbr1iko ATAs treated with CsA or saline control, measured by Western blot. Data are expressed as band intensity normalized to the levels of GAPDH, and significance was analyzed using the unpaired Student’s t test.
CsA is an immunosuppressant that inhibits proliferation of T cells and affects the function B cells. Therefore, we next examined the systemic effects of CsA treatment on the subpopulation of circulating lymphocytes in Tgfbr1iko mice. In the saline-treated control, we observed a significant increase in the percentage of B cells during AAD development (P < 0.01, Supplemental Fig. S5A), suggesting that inflammation in ATAs can lead to systemic immune activation. Conversely, treatment with CsA dampened this increase to such a degree that the difference was no longer statistically significant (P = NS, Supplemental Fig. S5B). These results are consistent with the inhibition of the proinflammatory effects of CypA by the CsA treatment.
DISCUSSION
We previously reported that individual disruption of SMC-specific Tgfbr1 and Tgfbr2 results in AAD formation at a differential penetrance in adult male mice and that the addition of the Tgfbr2 deletion ameliorates Tgfbr1 deletion-induced AAD formation (19). In the current study, we have expanded upon these results and generated several novel insights into the biology of TGF-β in the aortic wall. Specifically, we show that in the absence of TGFBR1, TGFBR2 recognizes not only its physiologic ligand (TGF-β1) but also the nonphysiologic partner (GDF15), triggering a promiscuous TGFBR2 signaling activity. We then show that this promiscuous TGFBR2 signaling induces production of CypA, a potent proinflammatory cytokine (28), in Tgfbr1iko SMCs, likely via SMAD3 activation. The aberrant TGFBR2 signaling, acting via CypA, also exacerbates AAD formation induced by Tgfbr1iko, which provides a mechanistic explanation for our previous observation that the addition of Tgfbr2iko attenuates AAD formation in Tgfbr1iko mice (19). Lastly, we demonstrate that loss of Tgfbr1 promotes expression of inflammation-related genes in primary SMCs and developing AADs, indicating that elimination of TGFBR1 disrupts the molecular homeostasis of SMCs, which ultimately leads to activation of the inflammatory response and immune injury to the aortic wall.
Unlike AAAs, for which the etiology is quite complex, nearly 25% of TAADs are attributable to genetic predisposition (34). Because the genes linked to TAAD formation are either direct components or upstream regulators of the SMC-contractile apparatus, altered mechanosensing and disruption of SMC force generation have been postulated as mechanisms that drive SMC phenotypic switching, proteolytic matrix destruction, and ultimately TAAD formation (35). These hypotheses provide a plausible explanation for how genetic defects trigger aneurysm-promoting signals in SMCs. The subsequent medial degeneration, however, may involve a series of deleterious processes. One of these is the inflammatory response, and indeed, inflammasome activation has been observed in SMCs from human TAADs (36). Further, both syndromic and sporadic TAADs contain an increased number of inflammatory infiltrates, particularly various subsets of T cells and macrophages (37, 38).
Previous studies have proposed enhanced redox signaling and TLR4 signaling as possible mechanisms leading to activation of inflammatory responses in the aortic wall (39, 40). Consistent with these studies, we found that, in vitro, Tgfbr1iko SMCs express an inflammatory phenotype characterized by an increased rate of cell death and activation of innate immune responses. Furthermore, our in vivo results show that increased inflammatory cell infiltration during AAD initiation correlates with an early gene signature, characterized by enhanced inflammatory gene expression in the aortic wall. These findings suggest that, in SMCs, TGFBR1 is essential for maintaining molecular homeostasis, and loss of TGFBR1 can activate immune responses in the aortic wall via cell-autonomous effects. Moreover, analysis of regulator effects with IPA predicts that RNA and TLR4 are the major drivers for inflammatory gene induction, indicating the activation of danger signals in Tgfbr1iko ATAs. Because inflammatory infiltrates are detected in AADs at the time during which aortic tissues were collected for gene expression profiling, future investigations of cell-type specific activation of danger signals will help elucidate the mechanisms that trigger the early inflammatory response in Tgfbr1iko aortas.
Within the TGF-β superfamily, type II receptors have the ability to complex with multiple different type I receptors in order to recognize various types of ligands. This receptor-receptor and ligand-receptor promiscuity allows individual ligands to regulate distinct biologic processes (41). Under pathologic conditions, however, this flexibility may also provide an opportunity for TGF-β to induce deleterious effects. For example, TGFBR2 normally complexes with TGFBR1, and this complex is only bound by TGF-β isoforms (namely TGF-β1–3) (42). However, during atherosclerosis, the TGFBR1/TGFBR2 complex recognizes GDF15, which promotes macrophage chemotaxis and reduces the stability of the atherosclerotic plaque (32). Similarly, in hypothalamic neurons, TGFBR2 binds to GDF15, and this signaling pathway contributes to the progression of cancer-associated wasting syndrome (31).
Consistent with these studies, we previously showed that deletion of SMC Tgfbr1 triggers aberrant TGFBR2 activity, which has deleterious effects on AAD formation (19). Here, we further demonstrate that SMCs lacking Tgfbr1 respond to TGF-β1 or GDF15 stimulation by up-regulating CypA, a critical mediator implicated in AAA formation (29), in a TGFBR2-dependent fashion. This finding suggests that loss of Tgfbr1 not only leads to the loss of physiologic TGF-β function but also results in a gain of pathogenic, promiscuous TGFBR2 signaling in SMCs. This conclusion is consistent with a previous study showing that loss of TGFBR2 triggers a promiscuous signaling through the TGFBR1/TGFBR3 complex, which contributes to formation of craniofacial defects (43). It is well established that without the ability to complex with a type I receptor, TGFBR2 alone cannot signal (1, 44). Although 1 study demonstrated that TGFBR2 is able to phosphorylate Par6c in epithelial cells, TGFBR1 is still required to bring Par6c in physical contact with the kinase domain of the TGFBR2 (45). Therefore, the current model of TGF-β signaling predicts that at least 1 type of I receptor member, other than TGFBR1, is involved in propagating the promiscuous TGFBR2 signaling in Tgfbr1iko SMCs.
When signaling through the TGFBR1/TGFBR2 complex, TGF-β can activate both SMAD-dependent and SMAD-independent pathways (46). We previously showed that TGF-β1 cannot activate the SMAD2 and ERK pathways in primary, Tgfbr1-deficient SMCs (19). Interestingly, in this study, we found that TGF-β1 induces CypA production in the same SMC cell line, and this induction is abolished by inhibition of SMAD3. These results suggest that TGFBR1 is dispensable for TGF-β1–mediated activation of the SMAD3 pathway in SMCs. However, the identity of the type I receptor that functions with TGFBR2 to relay the TGF-β1 signal in this pathway and the mechanisms by which TGF-β1 selectively activates SMAD3 to induce CypA expression remain unclear. Answers to these questions will provide deeper insights into how disruption at the receptor level affects the TGF-β signaling in SMCs.
CypA is a peptidyl-prolyl isomerase that is constitutively expressed as a cytosolic protein in the endothelium, SMCs, and immune cells. Upon exposure to inflammatory stimuli, these cells can secrete cytosolic CypA into the extracellular space (47). Recent studies have demonstrated that CypA itself is a potent proinflammatory mediator that promotes expression of cytokines and radical oxygen species, as well as MMP2 and MMP9 via both intra- and extracellular mechanisms. Accordingly, CypA has emerged as a promising target for chronic cardiovascular conditions (28, 47). In the present study, we found that CypA is up-regulated in early-stage AADs, and this enhanced CypA production is associated with an up-regulation of a large panel of proinflammatory cytokines. These data therefore suggest that CypA acts as a node of the inflammatory network in developing AADs. Furthermore, we show that inhibition of CypA function by CsA treatment attenuates aneurysmal degeneration. Notably, this protective effect is associated with a decreased inflammatory response, evidenced by reductions in CD45+ cell infiltration, MMP2 activity, and NF-κB activation. These data therefore suggest that CypA contributes to AAD formation via its proinflammatory activities.
Our finding that CypA contributes to AAD formation is consistent with previous studies that have demonstrated a critical role for this protein in promoting production of MMPs and AAA formation (29, 48). The current understanding of the signaling pathways through which CypA stimulates inflammatory responses remains incomplete. The most well-studied pathway involves the recognition of extracellular CypA by the cell surface receptor, CD147 (28); however, the proinflammatory effects of CypA may not limited to its ability to signal through CD147. A recent study showed that intracellular CypA acts as a positive regulator of danger signaling that is mediated by the pathogen recognition receptor, retinoic acid-inducible gene I (RIG-I) (49). RIG-I is an intracellular RNA sensor that is able to recognize self-RNAs (50). It is interesting to note that our IPA analysis predicts RNA as an upstream regulator for the inflammatory genes that are induced in AADs. Further studies are required, however, to delineate the relationship between CypA induction and the RNA-mediated danger signals in SMCs.
We note that the present study has several limitations. First, the mouse model used in this investigation was created through a postnatal homozygous Tgfbr1 deletion, which is different from the heterozygous TGFBR1 mutation in patients with TAAD (20). Nevertheless, the Tgfbr1iko model is able to recapitulate the major pathologies of human TAADs, including intimal/medial tears, intramural hematoma, and aortic rupture (19). Because it does not require any artificial insults, the cellular and molecular events occurring in this model are physiologically relevant to TAAD formation. Therefore, it represents a viable alternative to models that are widely accepted in the field, such as those involving the chronic infusion of angiotensin II (36, 51) or the local application of elastase (52) and calcium chloride (53). We further note that CsA is not a specific CypA inhibitor, and this compound can inhibit a number of other biologic processes, including T- and B-cell proliferation (54). Thus, although our results show that treatment with CsA inhibits medial degeneration of AADs, we cannot rule out the contribution of immunosuppression to the treatment effect. Further studies using non-immunosuppressive CypA inhibitors (28) may help to resolve this issue.
In summary, we previously demonstrated that deletion of Tgfbr1 promotes AAD formation via both loss- and gain-of-function mechanisms (19). In the current study, we provide evidence demonstrating that loss of Tgfbr1 causes SMCs to acquire an inflammatory phenotype, and that gain of a promiscuous Tgfbr2 signaling activity underlies the deleterious effects of Tgfbr1 deletion (Supplemental Fig. S6). An unresolved question in the field of aortic TGF-β biology has been the readout of TGF-β when it signals through receptor members with loss-of-function mutations (7, 14). Thus, it will be intriguing to test whether promiscuous TGF-β signaling plays a role in this process.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors express gratitude to Ricky F. Ungaro (University of Florida College of Medicine) for help with the NanoString Assays. This study was supported by grants from the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute (RO1HL105764 to Z.J.), and Florida Health James and Esther King Biomedical Research Program (7JK05 to Z.J.). The authors declare no conflicts of interest.
Glossary
- 2D
2-dimensional
- AAA
abdominal aortic aneurysm
- AAD
ascending aortic aneurysm and dissection
- ATA
ascending thoracic aorta
- CsA
cyclosporine A
- CypA
cyclophilin A
- DIGE
difference gel electrophoresis
- EEL
external elastic lamina
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GDF15
growth differentiation factor 15
- IEL
internal elastic lamina
- IHC
immunohistochemistry
- IPA
Ingenuity Pathway Analysis
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- MMP
matrix metalloproteinase
- Myh11
myosin heavy chain 11
- siRNA
small interfering RNA
- SMC
smooth muscle cell
- TAAD
thoracic aortic aneurysm and dissection
- TGFBR1
type I receptor of TGF-β
- Tgfbr1iko
SMC-specific postnatal deletion of Tgfbr1
- TGFBR2
type II receptor of TGF-β
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
G. Zhou, M. Liao, S. A. Berceli, A. K. Sharma, G. R. Upchurch Jr., and Z. Jiang designed the research; G. Zhou, M. Liao, F. Wang, X. Qi, and P. Yang performed the research; G. Zhou and M. Liao analyzed the data; and G. Zhou and Z. Jiang wrote the manuscript.
REFERENCES
- 1.Massagué J. (2012) TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 13, 616–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Z., Tao M., Omalley K. A., Wang D., Ozaki C. K., Berceli S. A. (2009) Established neointimal hyperplasia in vein grafts expands via TGF-beta-mediated progressive fibrosis. Am. J. Physiol. Heart Circ. Physiol. 297, H1200–H1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan T. H., Huang X. Q., Tan H. M. (2013) Vascular fibrosis in atherosclerosis. Cardiovasc. Pathol. 22, 401–407 [DOI] [PubMed] [Google Scholar]
- 4.Loeys B. L., Schwarze U., Holm T., Callewaert B. L., Thomas G. H., Pannu H., De Backer J. F., Oswald G. L., Symoens S., Manouvrier S., Roberts A. E., Faravelli F., Greco M. A., Pyeritz R. E., Milewicz D. M., Coucke P. J., Cameron D. E., Braverman A. C., Byers P. H., De Paepe A. M., Dietz H. C. (2006) Aneurysm syndromes caused by mutations in the TGF-beta receptor. N. Engl. J. Med. 355, 788–798 [DOI] [PubMed] [Google Scholar]
- 5.Tran-Fadulu V., Pannu H., Kim D. H., Vick G. W., III, Lonsford C. M., Lafont A. L., Boccalandro C., Smart S., Peterson K. L., Hain J. Z., Willing M. C., Coselli J. S., LeMaire S. A., Ahn C., Byers P. H., Milewicz D. M. (2009) Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J. Med. Genet. 46, 607–613 [DOI] [PubMed] [Google Scholar]
- 6.Gillis E., Van Laer L., Loeys B. L. (2013) Genetics of thoracic aortic aneurysm: at the crossroad of transforming growth factor-β signaling and vascular smooth muscle cell contractility. Circ. Res. 113, 327–340 [DOI] [PubMed] [Google Scholar]
- 7.Isselbacher E. M., Lino Cardenas C. L., Lindsay M. E. (2016) Hereditary influence in thoracic aortic aneurysm and dissection. Circulation 133, 2516–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsay M. E., Schepers D., Bolar N. A., Doyle J. J., Gallo E., Fert-Bober J., Kempers M. J., Fishman E. K., Chen Y., Myers L., Bjeda D., Oswald G., Elias A. F., Levy H. P., Anderlid B. M., Yang M. H., Bongers E. M., Timmermans J., Braverman A. C., Canham N., Mortier G. R., Brunner H. G., Byers P. H., Van Eyk J., Van Laer L., Dietz H. C., Loeys B. L. (2012) Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat. Genet. 44, 922–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle A. J., Doyle J. J., Bessling S. L., Maragh S., Lindsay M. E., Schepers D., Gillis E., Mortier G., Homfray T., Sauls K., Norris R. A., Huso N. D., Leahy D., Mohr D. W., Caulfield M. J., Scott A. F., Destrée A., Hennekam R. C., Arn P. H., Curry C. J., Van Laer L., McCallion A. S., Loeys B. L., Dietz H. C. (2012) Mutations in the TGF-β repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat. Genet. 44, 1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holm T. M., Habashi J. P., Doyle J. J., Bedja D., Chen Y., van Erp C., Lindsay M. E., Kim D., Schoenhoff F., Cohn R. D., Loeys B. L., Thomas C. J., Patnaik S., Marugan J. J., Judge D. P., Dietz H. C. (2011) Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332, 358–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez D., Coyet A., Ollivier V., Jeunemaitre X., Jondeau G., Michel J. B., Vranckx R. (2011) Epigenetic control of vascular smooth muscle cells in Marfan and non-Marfan thoracic aortic aneurysms. Cardiovasc. Res. 89, 446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez D., Kessler K., Michel J. B., Vranckx R. (2013) Modifications of chromatin dynamics control Smad2 pathway activation in aneurysmal smooth muscle cells. Circ. Res. 113, 881–890 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Ait-Oufella H., Herbin O., Bonnin P., Ramkhelawon B., Taleb S., Huang J., Offenstadt G., Combadière C., Rénia L., Johnson J. L., Tharaux P. L., Tedgui A., Mallat Z. (2010) TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Invest. 120, 422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo E. M., Loch D. C., Habashi J. P., Calderon J. F., Chen Y., Bedja D., van Erp C., Gerber E. E., Parker S. J., Sauls K., Judge D. P., Cooke S. K., Lindsay M. E., Rouf R., Myers L., ap Rhys C. M., Kent K. C., Norris R. A., Huso D. L., Dietz H. C. (2014) Angiotensin II-dependent TGF-β signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J. Clin. Invest. 124, 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X., Rateri D. L., Howatt D. A., Balakrishnan A., Moorleghen J. J., Cassis L. A., Daugherty A. (2016) TGF-β neutralization enhances AngII-induced aortic rupture and aneurysm in both thoracic and abdominal regions. PLoS One 11, e0153811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook J. R., Clayton N. P., Carta L., Galatioto J., Chiu E., Smaldone S., Nelson C. A., Cheng S. H., Wentworth B. M., Ramirez F. (2015) Dimorphic effects of transforming growth factor-β signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 35, 911–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W., Li Q., Jiao Y., Qin L., Ali R., Zhou J., Ferruzzi J., Kim R. W., Geirsson A., Dietz H. C., Offermanns S., Humphrey J. D., Tellides G. (2014) Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J. Clin. Invest. 124, 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J. H., Wei H., Jaffe M., Airhart N., Du L., Angelov S. N., Yan J., Allen J. K., Kang I., Wight T. N., Fox K., Smith A., Enstrom R., Dichek D. A. (2015) Postnatal deletion of the type II transforming growth factor-β receptor in smooth muscle cells causes severe aortopathy in mice. Arterioscler. Thromb. Vasc. Biol. 35, 2647–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang P., Schmit B. M., Fu C., DeSart K., Oh S. P., Berceli S. A., Jiang Z. (2016) Smooth muscle cell-specific Tgfbr1 deficiency promotes aortic aneurysm formation by stimulating multiple signaling events. Sci. Rep. 6, 35444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jondeau G., Ropers J., Regalado E., Braverman A., Evangelista A., Teixedo G., De Backer J., Muiño-Mosquera L., Naudion S., Zordan C., Morisaki T., Morisaki H., Von Kodolitsch Y., Dupuis-Girod S., Morris S. A., Jeremy R., Odent S., Adès L. C., Bakshi M., Holman K., LeMaire S., Milleron O., Langeois M., Spentchian M., Aubart M., Boileau C., Pyeritz R., Milewicz D. M.; Montalcino Aortic Consortium (2016) International registry of patients carrying TGFBR1 or TGFBR2 mutations: results of the MAC (Montalcino Aortic Consortium). Circ. Cardiovasc. Genet. 9, 548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmit B. M., Yang P., Fu C., DeSart K., Berceli S. A., Jiang Z. (2015) Hypertension overrides the protective effect of female hormones on the development of aortic aneurysm secondary to Alk5 deficiency via ERK activation. Am. J. Physiol. Heart Circ. Physiol. 308, H115–H125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao M., Zhou J., Wang F., Ali Y. H., Chan K. L., Zou F., Offermanns S., Jiang Z., Jiang Z. (2017) An X-linked Myh11-CreERT2 mouse line resulting from Y to X chromosome-translocation of the Cre allele. Genesis 55, 23054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Z., Wu L., Miller B. L., Goldman D. R., Fernandez C. M., Abouhamze Z. S., Ozaki C. K., Berceli S. A. (2004) A novel vein graft model: adaptation to differential flow environments. Am. J. Physiol. Heart Circ. Physiol. 286, H240–H245 [DOI] [PubMed] [Google Scholar]
- 24.Fu C., Yu P., Tao M., Gupta T., Moldawer L. L., Berceli S. A., Jiang Z. (2012) Monocyte chemoattractant protein-1/CCR2 axis promotes vein graft neointimal hyperplasia through its signaling in graft-extrinsic cell populations. Arterioscler. Thromb. Vasc. Biol. 32, 2418–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Hamamsy I., Yacoub M. H. (2009) Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat. Rev. Cardiol. 6, 771–786 [DOI] [PubMed] [Google Scholar]
- 26.Bobik A. (2006) Transforming growth factor-betas and vascular disorders. Arterioscler. Thromb. Vasc. Biol. 26, 1712–1720 [DOI] [PubMed] [Google Scholar]
- 27.Orr A. W., Hastings N. E., Blackman B. R., Wamhoff B. R. (2010) Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J. Vasc. Res. 47, 168–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue C., Sowden M. P., Berk B. C. (2018) Extracellular and intracellular cyclophilin A, native and post-translationally modified, show diverse and specific pathological roles in diseases. Arterioscler. Thromb. Vasc. Biol. 38, 986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh K., Nigro P., Matoba T., O’Dell M. R., Cui Z., Shi X., Mohan A., Yan C., Abe J., Illig K. A., Berk B. C. (2009) Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat. Med. 15, 649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nigro P., Satoh K., O’Dell M. R., Soe N. N., Cui Z., Mohan A., Abe J., Alexis J. D., Sparks J. D., Berk B. C. (2011) Cyclophilin A is an inflammatory mediator that promotes atherosclerosis in apolipoprotein E-deficient mice. J. Exp. Med. 208, 53–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnen H., Lin S., Kuffner T., Brown D. A., Tsai V. W., Bauskin A. R., Wu L., Pankhurst G., Jiang L., Junankar S., Hunter M., Fairlie W. D., Lee N. J., Enriquez R. F., Baldock P. A., Corey E., Apple F. S., Murakami M. M., Lin E. J., Wang C., During M. J., Sainsbury A., Herzog H., Breit S. N. (2007) Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 13, 1333–1340 [DOI] [PubMed] [Google Scholar]
- 32.De Jager S. C., Bermúdez B., Bot I., Koenen R. R., Bot M., Kavelaars A., de Waard V., Heijnen C. J., Muriana F. J., Weber C., van Berkel T. J., Kuiper J., Lee S. J., Abia R., Biessen E. A. (2011) Growth differentiation factor 15 deficiency protects against atherosclerosis by attenuating CCR2-mediated macrophage chemotaxis. J. Exp. Med. 208, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kockx M., Jessup W., Kritharides L. (2010) Cyclosporin A and atherosclerosis--cellular pathways in atherogenesis. Pharmacol. Ther. 128, 106–118 [DOI] [PubMed] [Google Scholar]
- 34.LeMaire S. A., Russell L. (2011) Epidemiology of thoracic aortic dissection. Nat. Rev. Cardiol. 8, 103–113 [DOI] [PubMed] [Google Scholar]
- 35.Humphrey J. D., Milewicz D. M., Tellides G., Schwartz M. A. (2014) Cell biology. Dysfunctional mechanosensing in aneurysms. Science 344, 477–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu D., Ren P., Zheng Y., Zhang L., Xu G., Xie W., Lloyd E. E., Zhang S., Zhang Q., Curci J. A., Coselli J. S., Milewicz D. M., Shen Y. H., LeMaire S. A. (2017) NLRP3 (nucleotide oligomerization domain-like receptor family, pyrin domain containing 3)-caspase-1 inflammasome degrades contractile proteins: implications for aortic biomechanical dysfunction and aneurysm and dissection formation. Arterioscler. Thromb. Vasc. Biol. 37, 694–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He R., Guo D. C., Sun W., Papke C. L., Duraisamy S., Estrera A. L., Safi H. J., Ahn C., Buja L. M., Arnett F. C., Zhang J., Geng Y. J., Milewicz D. M. (2008) Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J. Thorac. Cardiovasc. Surg. 136, 922–929, 929.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang P. C., Yakimov A. O., Teesdale M. A., Coady M. A., Dardik A., Elefteriades J. A., Tellides G. (2005) Transmural inflammation by interferon-gamma-producing T cells correlates with outward vascular remodeling and intimal expansion of ascending thoracic aortic aneurysms. FASEB J. 19, 1528–1530 [DOI] [PubMed] [Google Scholar]
- 39.Wu J., Saleh M. A., Kirabo A., Itani H. A., Montaniel K. R., Xiao L., Chen W., Mernaugh R. L., Cai H., Bernstein K. E., Goronzy J. J., Weyand C. M., Curci J. A., Barbaro N. R., Moreno H., Davies S. S., Roberts L. J., II, Madhur M. S., Harrison D. G. (2016) Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J. Clin. Invest. 126, 50–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruvolo G., Pisano C., Candore G., Lio D., Palmeri C., Maresi E., Balistreri C. R. (2014) Can the TLR-4-mediated signaling pathway be “a key inflammatory promoter for sporadic TAA”? Mediators Inflamm. 2014, 349476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller T. D., Nickel J. (2012) Promiscuity and specificity in BMP receptor activation. FEBS Lett. 586, 1846–1859 [DOI] [PubMed] [Google Scholar]
- 42.Shi Y., Massagué J. (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- 43.Iwata J., Hacia J. G., Suzuki A., Sanchez-Lara P. A., Urata M., Chai Y. (2012) Modulation of noncanonical TGF-β signaling prevents cleft palate in Tgfbr2 mutant mice. J. Clin. Invest. 122, 873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. (1992) TGF beta signals through a heteromeric protein kinase receptor complex. Cell 71, 1003–1014 [DOI] [PubMed] [Google Scholar]
- 45.Ozdamar B., Bose R., Barrios-Rodiles M., Wang H. R., Zhang Y., Wrana J. L. (2005) Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307, 1603–1609 [DOI] [PubMed] [Google Scholar]
- 46.Moustakas A., Heldin C. H. (2009) The regulation of TGFbeta signal transduction. Development 136, 3699–3714 [DOI] [PubMed] [Google Scholar]
- 47.Nigro P., Pompilio G., Capogrossi M. C. (2013) Cyclophilin A: a key player for human disease. Cell Death Dis. 4, e888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai J., Michineau S., Franck G., Desgranges P., Becquemin J.-P., Gervais M., Allaire E. (2011) Long term stabilization of expanding aortic aneurysms by a short course of cyclosporine A through transforming growth factor-beta induction. PLoS One 6, e28903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W., Li J., Zheng W., Shang Y., Zhao Z., Wang S., Bi Y., Zhang S., Xu C., Duan Z., Zhang L., Wang Y. L., Jiang Z., Liu W., Sun L. (2017) Cyclophilin A-regulated ubiquitination is critical for RIG-I-mediated antiviral immune responses. Elife 6, e24425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu C., MacDougall M. (2017) RIG-I-like receptor signaling in Singleton-Merten syndrome. Front. Genet. 8, 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daugherty A., Rateri D. L., Charo I. F., Owens A. P., Howatt D. A., Cassis L. A. (2010) Angiotensin II infusion promotes ascending aortic aneurysms: attenuation by CCR2 deficiency in apoE-/- mice. Clin. Sci. (Lond.) 118, 681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnston W. F., Salmon M., Pope N. H., Meher A., Su G., Stone M. L., Lu G., Owens G. K., Upchurch G. R., Jr., Ailawadi G. (2014) Inhibition of interleukin-1β decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation 130, S51–S59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikonomidis J. S., Gibson W. C., Gardner J., Sweterlitsch S., Thompson R. P., Mukherjee R., Spinale F. G. (2003) A murine model of thoracic aortic aneurysms. J. Surg. Res. 115, 157–163 [DOI] [PubMed] [Google Scholar]
- 54.Hannam-Harris A. C., Taylor D. S., Nowell P. C. (1985) Cyclosporin A directly inhibits human B-cell proliferation by more than a single mechanism. J. Leukoc. Biol. 38, 231–239 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.