Abstract
Mesenchymal stromal cells (MSCs) are multipotent stem cells that participate in tissue repair and possess considerable immunomodulatory potential. MSCs have been shown to promote allograft survival, yet the mechanisms behind this phenomenon have not been fully defined. Here, we investigate the capacity of MSCs to suppress the allogeneic immune response by secreting the pleiotropic molecule hepatocyte growth factor (HGF). Using an in vivo mouse model of corneal transplantation, we report that MSCs promote graft survival in an HGF‐dependent manner. Moreover, our data indicate that topically administered recombinant HGF (a) suppresses antigen‐presenting cell maturation in draining lymphoid tissue, (b) limits T‐helper type‐1 cell generation, (c) decreases inflammatory cell infiltration into grafted tissue, and (d) is itself sufficient to promote transplant survival. These findings have potential translational implications for the development of HGF‐based therapeutics. stem cells translational medicine 2019;8:1030–1040
Keywords: Cornea, Transplantation, Mesenchymal stromal cells, Hepatocyte growth factor
Significance Statement.
Corneal transplantation is the most common form of tissue grafting, with more than 45,000 surgeries performed in the United States alone each year. Graft recipients often have ocular inflammation and increased corneal blood vessels, and these features are associated with >50% transplant failure despite maximal treatment with immunosuppressive therapy. Mesenchymal stem cells have substantial immunoregulatory and tissue reparative functions at the ocular surface, but cell‐based therapeutics are currently limited by the regulatory and financial challenges of manufacturing clinical‐grade cells. Here, the authors show that mesenchymal stem cells' promotion of corneal graft survival depends on their expression of hepatocyte growth factor. Furthermore, the study demonstrates that hepatocyte growth factor alone is sufficient to limit the alloimmune response and promote corneal graft survival.
Introduction
Corneal transplantation is the definitive treatment for a variety of end‐stage ocular surface diseases 1. In clinical practice, graft recipients commonly exhibit inflamed and vascularized host beds, in which case failure rates exceed 50% despite maximal treatment with nonspecific immunosuppressive medications 2, 3. Given that conventional immunosuppressive agents are associated with severe ocular side effects (including cataract, glaucoma, and increased susceptibility to infections) 4, there is an unmet need for novel immunomodulatory strategies to promote corneal transplant survival.
Corneal graft rejection occurs due to the activation of proinflammatory immune pathways directed against specific alloantigens 5. Following transplantation, the localized upregulation of proinflammatory cytokines promotes antigen‐presenting cell (APC) maturation (i.e., acquisition of major histocompatibility complex (MHC) class II and other costimulatory molecules) 6. Subsequently, APCs migrate via lymphatic vessels to the draining lymph nodes (DLNs), where they prime naïve T cells by presenting alloantigens, which differentiate and proliferate into interferon‐γ (IFNγ)‐secreting CD4+ T helper type 1 (Th1) cells 5. The efferent arm of the alloimmune response involves the egress of Th1 cells from the lymph nodes and their migration via blood vessels toward the graft site along a chemokine gradient 7. At the cornea, Th1 cells promote graft rejection through the production of proinflammatory cytokines 5. Importantly, the alloimmune response is limited by cellular and molecular immunoregulatory mechanisms that suppress the effector response and foster graft tolerance 8.
Mesenchymal stromal cells (MSCs) are multipotent stem cells that are capable of differentiating into a variety of cell types 9. MSCs also have substantial immunoregulatory potential and have been reported to modulate both innate and adaptive immune responses 9, 10. Previous work from our group, using a murine model of corneal transplantation, has established that systemically administered MSCs delivered postoperatively specifically home to the inflamed ocular tissues 11. Moreover, we have demonstrated that MSCs promote allograft survival through the inhibition of APC maturation and suppression of alloreactive T‐cell induction 11. However, the exact mechanisms by which MSCs modulate the immune response in allogeneic tissue transplantation have not been fully delineated.
Hepatocyte growth factor (HGF) is a pleiotropic growth factor that acts via the receptor c‐met 12. Renowned for promoting epithelial motility and proliferation, HGF is also recognized as an important immunoregulatory factor in both acute and chronic inflammation 13. In a previous study, we established that corneal injury induces an inflammatory milieu that promotes the secretion of high levels of HGF by MSCs 14. Furthermore, we have shown that topical administration of HGF following corneal injury suppresses ocular inflammation 15. In this study, we systematically investigate the capacity of HGF to promote corneal transplant survival by suppressing generation of the alloimmune Th1 effector response and decreasing inflammatory cell infiltration. In our well‐characterized murine model of corneal transplantation, approximately 50% of grafts are rejected within the first three postoperative weeks, with the remaining grafts surviving indefinitely 16, 17, 18. Unlike other models of transplantation, this system allows for continuous assessment of the alloimmune response (i.e., the evaluation of graft opacity) without necessitating sacrifice of the recipient.
Our data demonstrate that MSCs promote corneal transplant survival in an HGF‐dependent manner. Moreover, we establish that topical application of HGF limits the generation of Th1 cells, inhibits graft infiltration of immune cells, and is itself sufficient to increase corneal allograft survival.
Materials and Methods
Animals
Seven‐ to eight‐week‐old BALB/c and C57BL/6 wild‐type male mice were purchased from Charles River Laboratories (Wilmington, MA). The mice were housed in the animal vivarium of the Schepens Eye Research Institute. The mice were treated in accordance with the Association of Research in Vision and Ophthalmology's “Statement for the Use of Animals in Ophthalmic and Vision Research.” The Institutional Animal Care and Use Committee of the Schepens Eye Research Institute reviewed and approved all animal procedures.
Corneal Transplantation
Allogeneic corneal transplantation was performed as described previously 19, 20. Briefly, 2‐mm‐diameter corneal buttons were excised from the central cornea of C57BL/6 mice and were grafted onto similarly prepared 1.5‐mm host beds of BALB/c mice using eight interrupted 11‐0 nylon sutures. Recipient eyelids were closed for 3 days postoperatively using a single 8‐0 nylon suture. Corneal sutures were removed 7 days after surgery. Syngeneic corneal transplantation was performed in a similar fashion but using BALB/c donors and BALB/c hosts.
Evaluation of Corneal Graft Survival
Graft opacity was graded using a slit‐lamp microscope on a weekly basis for 8 weeks postoperatively, according to an established 0–5+ scale 21, 22. Allograft rejection was determined according to corneal opacity scores, with scores of >2 (i.e., a level of opacity that conceals iris details) for more than two consecutive weeks considered to be immune rejected. Eyes that became opaque in the first two postoperative weeks and never became clear were excluded from the analysis, as were eyes that underwent either intraoperative or postoperative complications (e.g., hemorrhage, infection, cataract, or synechia).
MSC and HGF Administration
For experiments investigating the effects of MSC administration, in vitro expanded and characterized MSCs were treated with either HGF‐specific small interfering RNA (siRNA) or control siRNA and injected into the tail veins of mice at 3 hours post‐transplantation (0.5 × 106 cells suspended in 100 μl of sterile saline) as previously described 11, 14, 23. For experiments investigating the effects of HGF administration, 5 μl of either 0.1% murine recombinant HGF protein (R&D systems, Minneapolis, MN) in phosphate‐buffered saline (PBS) or protein control (mouse serum, Sigma‐Aldrich, St. Louis, MO) was applied topically to the ocular surface twice daily for 14 days following corneal transplantation.
MSC Purification and Expansion
Bone marrow was harvested from the femurs of euthanized BALB/c mice. MSCs were phenotypically and functionally characterized according the criteria set forth by The International Society for Cellular Therapy 24. The previously described plastic adherence technique of MSC cultivation was used 25, 26. Bone marrow cells were cultured in murine MSC‐specific MesenCult medium with supplement (STEMCELL Technologies, Vancouver, Canada). Nonadherent cells were removed every 48 hours by changing the medium. MSCs were harvested at passage 2 for use in experiments. Detailed characterizations of MSCs were performed prior to using them in experiments, as previously described 11, 14, 23.
Cell Culture Assays
For our in vitro experiments, dendritic cells (DCs) were differentiated from bone marrow cells harvested from the femurs of BALB/c mice using a protocol described previously 27. Briefly, bone marrow cells were cultured in media containing granulocyte‐macrophage colony‐stimulating factor (20 ng/ml) for 6 days. At this time, cultures routinely contain >85% CD11chigh cells. For the coculture assays, DCs were cultured alone or on a monolayer of MSCs either in the presence or absence of IL1β (100 ng/ml) for 24 hours. In our HGF neutralization assays, cocultures were treated with soluble HGF receptor (s‐HGFR) at the doses of 1 and 10 μg/ml. Two mice were used in each experiment, and each experiment was repeated three times.
siRNA Transfection
MSCs (1.5 × 106 cells) were plated and incubated in a 75‐cm2 culture flask for 18–24 hours in order to reach 60%–70% confluence. Subsequently, the cells were washed and transfected with 4.8 μg of Hgf‐specific or nonspecific control siRNA using transfection reagent in siRNA transfection medium as per the manufacturer's protocol (Santa Cruz Biotechnology, Dallas, TX). Following overnight incubation, the transfection medium was replaced with standard MSC growth culture medium, and the cells were cultured for an additional 48 hours. Real‐time polymerase chain reaction (PCR) using Hgf‐specific primers was used to validate the knockdown efficiency of siRNA after 3 days of transfection.
Real‐Time PCR
A commercially available kit was used to isolate total RNA (RNeasy Micro Kit; Qiagen, Valencia, CA). Reverse transcriptase (Superscript III; Invitrogen, Carlsbad, CA) was used to reverse transcribe RNA into cDNA. Following this, quantitative real‐time PCR was performed using preformulated Taqman‐based probes for murine HGF (Hgf, Mm01135184_m1) and glyceraldehyde‐3‐phosphate dehydrogenase (Gapdh, Mm99999915_gl; Taqman Universal PCR Mastermix, Thermo Fisher Scientific, Waltham, MA) in a Mastercycler Realplex2 (Eppendorf, Hamburg, Germany). The results were analyzed by the comparative threshold cycle method and normalized to GAPDH as an internal control.
Flow Cytometry
Corneal single cell suspensions were prepared as previously described 23. In brief, corneas were digested in Roswell Park Memorial Institute (RPMI)‐ 1640 media (Lonza, Walkersville, MD) containing 2 mg/ml collagenase type IV (Sigma Aldrich, St. Louis, MO) and 2 mg/ml DNase I (Roche, Basel, Switzerland) for a duration of 45 minutes at a temperature of 37°C. Following this, cells were filtered through a 70 μl cell strainer. Single‐cell suspensions were stained with fluorochrome‐conjugated monoclonal antibodies. Cell surface staining was conducted using antibodies against CD11c, MHC class II, CD80, CD4, CD45, CD3, and CD11b. To evaluate the intracellular expression of IFNγ in Th1 cells, single cell suspensions derived from lymph nodes were stimulated with phorbol 12‐myristate 13‐acetate (20 ng/ml; Sigma‐Aldrich) and ionomycin (1ug/ml; Sigma‐Aldrich) for a duration of 4 hours in the presence of Golgistop (0.1 μl/100 μl media; BD Biosciences, San Jose, CA) at 37°C, as described previously 19, 28. Intracellular staining was performed in order to evaluate the expression of IFNγ in Th1 cells and Foxp3 in CD4+ cells (regulatory T cell [Tregs]). Antibodies and isotype controls were sourced from Biolegend (San Diego, CA). Stained cells were analyzed using an LSR II flow cytometer (BD Biosciences) and Summit software (Dako Colorado Inc., Fort Collins, CO).
Enzyme‐Linked Immunosorbent Assay
Protein levels of HGF in MSC culture supernatants and corneas were analyzed using commercially available murine enzyme‐linked immunosorbent assay (ELISA) kits (R&D Systems) according to the manufacturer's instructions. Levels of HGF in culture supernatants were normalized according to the number of MSCs (100,000 cells/200 μl) included in each group.
Statistical Analysis
Unpaired two‐tailed Student's t tests were used to compare means between two groups, and p values <.05 were considered statistically significant. Survival curves were constructed using Kaplan‐Meier analysis, and the log‐rank test was used to compare rates of corneal graft survival. Data are presented as mean ± SD of mean of three independent experiments. Samples sizes were estimated on the basis of previous corneal transplantation studies 29, 30.
Results
MSC‐Derived HGF Suppresses the Maturation of APCs
For the efficient presentation of antigens to naïve T cells, APCs undergo a process of maturation with increased surface expression of MHCII and CD80 11, 27. In view of our previously published data demonstrating that MSCs suppress allosensitization 11, we first sought to clarify whether MSCs suppress the activation and maturation of APCs in the setting of corneal transplantation. To this aim, DCs were cocultured with MSCs, and the acquisition of MHCII and CD80 expression by DCs was evaluated by flow cytometry. Given the limited number of MSCs and DCs at the ocular surface, murine bone marrow was harvested for both the isolation of MSCs and the generation of CD11c+ DCs. Detailed characterization of MSCs was performed as described previously 11, 14, 23, with flow cytometric analysis of MSCs revealing positive expression of the mesenchymal cell marker CD29 and negative expression of hematopoietic cell markers CD45 and CD34 (Fig. 1A). Increased expression of HGF by MSCs was observed in response to stimulation with the inflammatory cytokine interleukin‐1β (IL1β) (p = .008; Fig. 1B).
Figure 1.
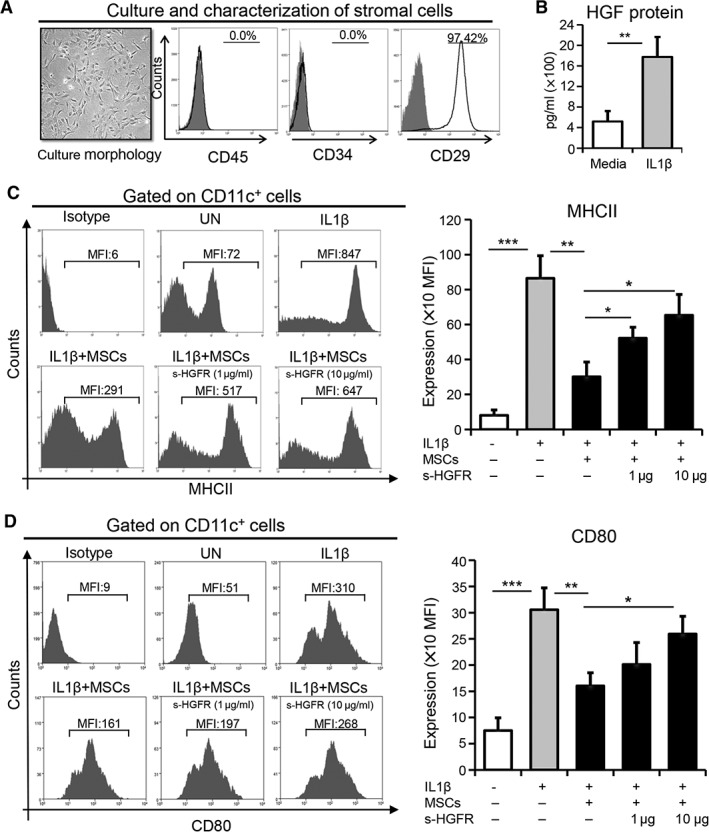
MSC‐derived HGF suppresses the maturation of antigen presenting cells. (A): MSCs were purified from bone marrow and expanded in vitro using MSC‐conditioned culture media via the plastic adherence method. Representative microscopic image exhibiting the morphology of expanded MSCs at passage two (left). Flow cytometry histograms showing negative expression of CD45 and CD34, as well as positive expression of CD29 (right). (B): Protein levels of HGF in the supernatants of MSCs cultured in media or stimulated with IL1β for 24 hours using enzyme‐linked immunosorbent assay. Representative flow cytometry plots (left) and quantitative bar charts (right) demonstrating the expression of (C) MHCII and (D) CD80 on CD11c+ cells cocultured with MSCs in the presence or absence of IL1β (100 ng/ml) with or without s‐HGFR (1 or 10 μg/ml) for 24 hours. Each experiment was repeated three times. Data are represented as mean ± SD. t test: *, p < .05; **, p < .01; ***, p < .001. Abbreviations: HGF, hepatocyte growth factor; MSC, mesenchymal stromal cell; UN, untreated; s‐HGFR, soluble hepatocyte growth factor receptor; MFI, mean fluorescence intensity.
Subsequently, DCs were stimulated with IL1β, with or without MSCs, for 24 hours. Unstimulated DCs exhibited low expression of MHCII and CD80, but stimulation with IL1β dramatically increased the expression of MHCII and CD80 by DCs (p < .001 for both MHCII and CD80; Fig. 1C, 1D). Notably, this increased expression was significantly abrogated by MSCs (p = .003 for MHCII, p = .006 for CD80), suggesting that MSCs suppress APC maturation. In view of our result showing increased HGF expression by MSCs in inflammatory conditions (Fig. 1B), we next investigated whether MSC‐derived HGF could itself inhibit IL1β‐induced DC maturation. To evaluate this, we neutralized HGF using s‐HGFR and showed that neutralization of HGF abrogated the suppressive potential of MSCs (Fig. 1C, 1D). Moreover, this effect was confirmed at two different dosages of s‐HGFR: 1 μg/ml (p = .021 for MHCII, p = not significant for CD80) and 10 μg/ml (p = .014 for MHCII, p = .015 for CD80). Our results clearly demonstrate that MSCs inhibit the maturation of APCs by the secretion of HGF.
MSCs Inhibit Corneal Leukocyte Infiltration and Maturation of APCs, and Promote Allograft Survival in an HGF‐Dependent Manner
Next, we investigated whether in vivo administration of MSCs promotes increased levels of HGF at the graft site. Transplant recipients received i.v. injection of MSCs at 3 hours post‐transplantation. Corneas were harvested from MSC‐treated and untreated hosts on day 7 and day 14 following transplantation. ELISA analysis demonstrated significant, two‐fold increases in the level of HGF in corneal lysates sourced from MSC‐treated hosts relative to untreated hosts at both day 7 and 14 post‐transplantation (Fig. 2A).
Figure 2.
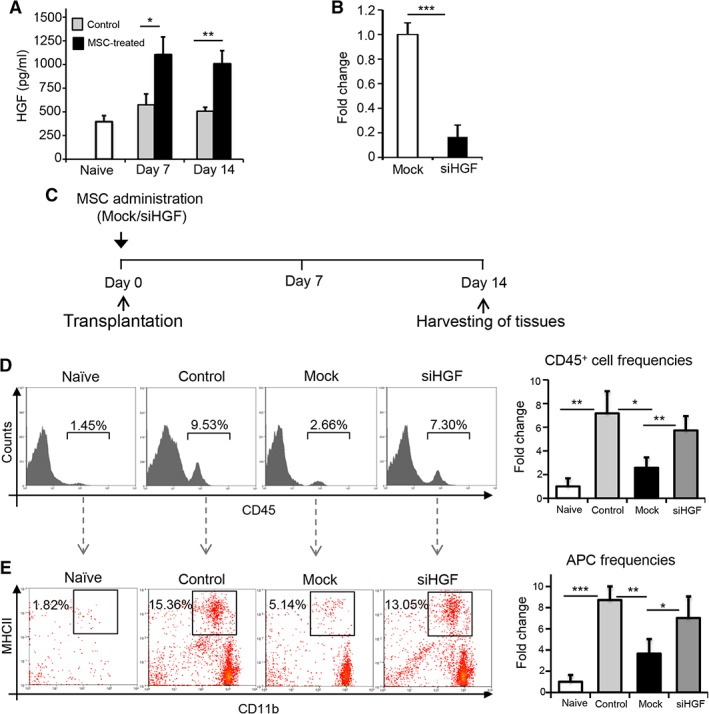
MSCs inhibit corneal infiltration of leukocytes and mature APCs in an HGF‐dependent manner. (A): Corneas were harvested from untreated and MSC‐treated allograft recipients at day 7 and day 14 post‐transplantation. Bar chart depicts enzyme‐linked immunosorbent assay data of HGF expression in corneal lysates. (B): Real‐time analysis showing reduced expression of Hgf mRNA at 72 hours after treatment of MSCs with siHGF compared with control small interfering RNA (siRNA; Mock). (C): Schematic of experimental design showing time points for MSC administration and tissue harvesting for flow cytometry. (D): Representative flow cytometric histograms (left) and cumulative bar chart (right) showing the relative frequencies of CD45+ immune cells in the corneas of naïve mice, untreated allograft recipients (Control), allograft recipients injected with control siRNA‐treated MSCs (Mock), or Hgf‐silenced MSCs at 2 weeks post‐transplantation. (E): Representative flow cytometric histograms (left) and cumulative bar chart (right) showing the relative frequencies of mature APCs (CD11b+MHCII+; gated on CD45+ cells) in the corneas of naïve mice, untreated allograft recipients (Control), allograft recipients injected with control siRNA‐treated MSCs (Mock), or Hgf‐silenced MSCs at 2 weeks post‐transplantation. Experiment was repeated two times, with four animals per group in each experiment. Data are represented as mean ± SD. t test: *, p < .05; **, p < .01; ***, p < .001. Abbreviations: APC, antigen presenting cell; HGF, hepatocyte growth factor; MSC, mesenchymal stromal cell; siHGF, HGF‐specific small interfering RNA.
To determine whether MSC‐derived HGF regulates alloimmune responses at the graft site, HGF expression was silenced using siRNA, which reduced expression of HGF by MSCs by approximately 80% (p < .001; Fig. 2B). Corneal transplantation was performed, and recipient mice were either not treated with MSCs or received i.v. injection of either Hgf‐silenced MSCs or control siRNA‐treated MSCs (Mock; Fig. 2C). Corneas were harvested at 14 days post‐transplantation, and single‐cell suspensions were prepared and flow cytometry was performed. Our data show that administration of control siRNA‐treated MSCs reduced corneal infiltration of CD45+ cells by ∼65% relative to untreated allograft recipients (p = .018), but administering Hgf‐silenced MSCs substantially reduced this effect (p = .007; Fig. 2D). Consistent with this observation, flow cytometry analysis of corneal single cell suspensions demonstrated significantly reduced frequencies of CD11b+MHCII+ APCs in hosts that were administered control siRNA‐treated MSCs compared with untreated hosts (p = .009), yet this effect was abrogated in hosts that received Hgf‐silenced MSCs (p = .031; Fig. 2E). These data show that HGF expressed by MSCs inhibits the alloimmune response following corneal transplantation.
Next, we sought to determine whether suppression of HGF expression by MSCs reduces their capacity to promote graft survival 11. Following corneal transplantation, graft survival was compared in four treatment groups: (a) allogeneic graft recipients i.v. injected with control siRNA‐treated (Mock) MSCs; (b) allogeneic graft recipients i.v. injected with Hgf‐silenced MSCs; (c) untreated allogeneic graft recipients; and (d) untreated syngeneic graft recipients. In order to assess transplant survival, grafts were examined by slit lamp biomicroscopy once weekly for 6 weeks following transplantation (Fig. 3A, 3B). Our data demonstrate a two‐fold increase in graft survival in recipients i.v. injected with control siRNA‐treated MSCs (78%) compared with untreated controls (Fig. 3C). However, recipients i.v. injected with Hgf‐silenced MSCs showed reduced survival (48%) compared with recipients that were administered control siRNA‐treated MSCs. These results clearly demonstrate that HGF expressed by MSCs promotes graft survival following corneal transplantation.
Figure 3.
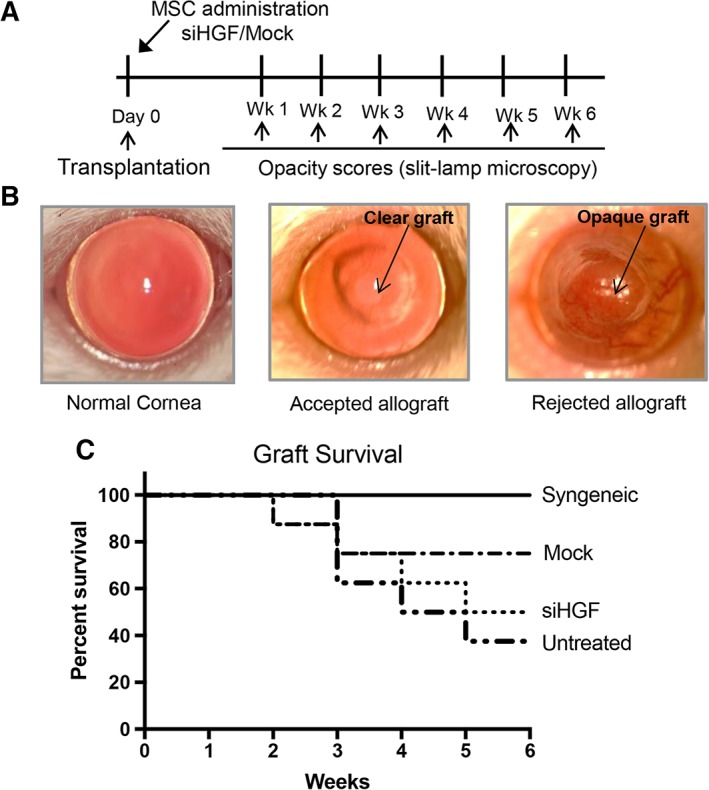
MSCs promote corneal graft survival in an hepatocyte growth factor (HGF)‐dependent manner. (A): Schematic of experimental design detailing the time points of MSC administration as well as graft opacity evaluation via slit‐lamp biomicroscopy. (B): Representative slit lamp photographs of a normal murine cornea, an accepted corneal allograft, and a rejected corneal allograft. (C): Line diagram showing Kaplan‐Meier graft survival analysis of (i) untreated syngeneic graft recipients, (ii) allogeneic graft recipients i.v. injected with control small interfering RNA‐treated (Mock) MSCs, (iii) allogeneic graft recipients i.v. injected with Hgf‐silenced MSCs, or (iv) untreated allogeneic graft recipients (n = 8–10 in each group; log‐rank test; p = .026). Abbreviations: MSC, mesenchymal stromal cell; siHGF, HGF‐specific small interfering RNA.
Treatment with HGF Alone Suppresses APC Activation and Limits the Generation of Th1 Cells in Allograft Recipients
To investigate whether HGF alone can inhibit the allogeneic immune response, we evaluated the in vivo effect of HGF on APC maturation and the generation of IFNγ‐producing CD4+ T cells (Th1 cells) in the DLNs. Five microliters of HGF in PBS (0.1%) or protein control was applied topically to host eyes, twice daily for 2 weeks postoperatively (Fig. 4A). For our flow cytometry analyses, DLNs were harvested at day 14 following transplantation, and single‐cell suspensions were prepared. Our data revealed significantly increased frequencies of CD11c+MHCII+ mature APCs in control‐treated transplant recipients compared with naïve controls (p = .015; Fig. 4B). However, treatment with HGF significantly reduced frequencies of CD11c+MHCII+ mature APCs relative to the control‐treated group (p = .006). A six‐fold increase in CD4+IFNγ+ Th1 cell frequencies was observed in the DLNs of vehicle‐treated recipients after corneal transplantation, relative to the naïve group (p = .002; Fig. 4C). However, this increase was substantially reduced following HGF treatment. Specifically, HGF‐treated mice showed a significant 41% decrease in the generation of IFNγ‐secreting Th1 cells compared with control‐treated controls (p = .033; Fig. 4C). As CD4+Foxp3+ Treg have been shown to inhibit the development of the Th1 immune response, we investigated whether HGF treatment affects Treg frequencies following transplantation and observed a moderate increase in the frequencies of CD4+Foxp3+ Tregs in draining lymphoid tissues (Fig. 4D). Collectively, our results demonstrate that HGF suppresses the alloimmune response primarily by inhibiting APC maturation and subsequent Th1 generation.
Figure 4.
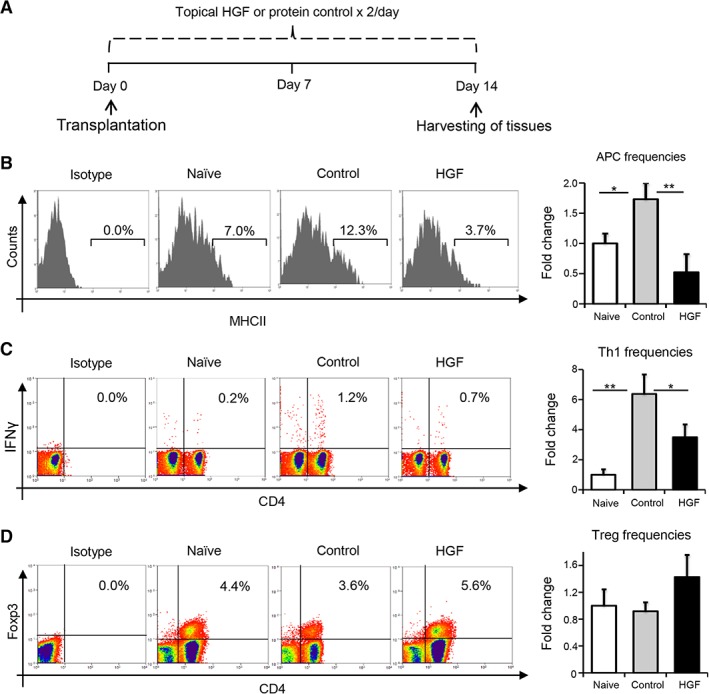
HGF treatment suppresses induction of alloimmunity in transplant recipients. (A): Schematic of experiment detailing the time points of topical administration of HGF (0.1%) or protein control twice daily for 2 weeks post‐transplantation. Following week 2, ipsilateral draining lymph nodes were harvested to prepare single cells suspension for flow cytometry analysis. (B): Representative flow cytometric histograms (left) and cumulative bar chart (right) showing the relative frequencies of CD11c+MHCII+ mature APCs in the draining lymph nodes of naïve, control‐treated, and HGF‐treated groups at 2 weeks post‐transplantation. (C): Representative flow cytometric dot plots (left) and quantification chart (right) of frequencies of IFNγ+CD4+ Th1 cells in the draining lymph nodes of naive, protein control, and HGF‐treated groups at 2 weeks post‐transplantation. (D): Representative flow cytometric analysis (left) and cumulative bar chart (right) showing the relative frequencies of CD4+Foxp3+ Tregs in the draining lymph nodes of naïve, control‐treated, and HGF‐treated groups at 2 weeks post‐transplantation. Representative data from four independent experiments are shown, and each experiment consisted of four to six animals. Data are represented as mean ± SD (error bar). t test: *, p < .05; **, p < .01. Abbreviations: APC, antigen presenting cell; HGF, hepatocyte growth factor.
Topical Application of HGF Inhibits Immune Cell Infiltration into Corneal Grafts
Having observed HGF‐mediated inhibition of APC maturation and Th1 cell generation in the DLNs, we evaluated the alloimmune effector response at the graft site. Following transplantation, hosts were administered topical HGF or control eye drops as described previously (Fig. 4A). Corneas were harvested at day 14 following transplantation, and the infiltration of CD45+ inflammatory cells, CD3+ T cells, and CD11b+ macrophages was evaluated using flow cytometry. Our data demonstrate an eight‐fold increase in the infiltration of CD45+ cells into the cornea in the control‐treated allogeneic group compared with the naïve group (p = .005; Fig. 5A). Notably, frequencies of CD45+ inflammatory cells were significantly reduced in the HGF‐treated group relative to the control‐treated group (52%; p = .026). Relative to control‐treated graft recipients, HGF‐treated hosts exhibited decreased corneal infiltration of CD3+ T cells by 48% (p = .016; Fig. 5B) and CD11b+ cells by 73% (p = .018; Fig. 5C). Taken together, these results demonstrate that HGF inhibits the alloimmune effector response at the graft site.
Figure 5.
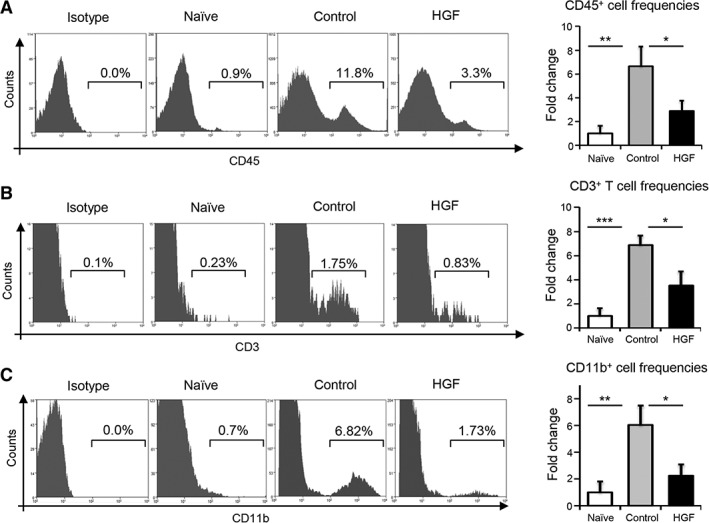
Topical application of HGF inhibits immune cell infiltration into corneal grafts. At 2 weeks post‐transplantation, corneal grafts were harvested and digested to prepare single cell suspensions. (A): Representative flow cytometry histograms and quantitative bar charts showing the infiltration of CD45+ inflammatory cells into corneal grafts of control‐ and HGF‐treated groups, relative to naïve controls. (B): Histogram plot (left) and bar graphs (right) demonstrating the frequencies of CD3+ T cells in corneas sourced from naïve mice and transplant recipients treated with either protein control or HGF. (C): Representative plots (left) and frequency data (right) of CD11b+ macrophages in corneal grafts of indicated groups at 2 weeks post‐transplantation. Four independent experiments were performed, with each experiment consisting of four to six animals. Data are represented as mean ± SD (error bar). t test: *, p < .05; **, p < .01; ***,p < .001. Abbreviation: HGF, hepatocyte growth factor.
HGF Alone Is Sufficient to Promote Graft Survival
Finally, taking account of the observed in vivo effect of HGF on the suppression of alloimmune responses, we evaluated whether topical HGF administration promotes corneal allograft survival. To determine this, once weekly slit‐lamp biomicroscopy was performed to evaluate graft survival in four groups: (a) allogeneic grafts treated with HGF; (b) allogeneic grafts treated with protein control; (c) untreated allogeneic grafts; and finally (d) untreated syngeneic grafts. Treatment was administered for 2 weeks postoperatively (Fig. 6A). Control‐treated and untreated mice exhibited approximately 40% graft survival at 6 weeks (Fig. 6B). However, HGF treatment increased graft survival, as indicated by 67% survival at week 6. Syngeneic controls exhibited 100% survival at week 6. Thus, in addition to inhibiting the alloimmune response, our data indicate that topical HGF increases graft survival following corneal transplantation.
Figure 6.
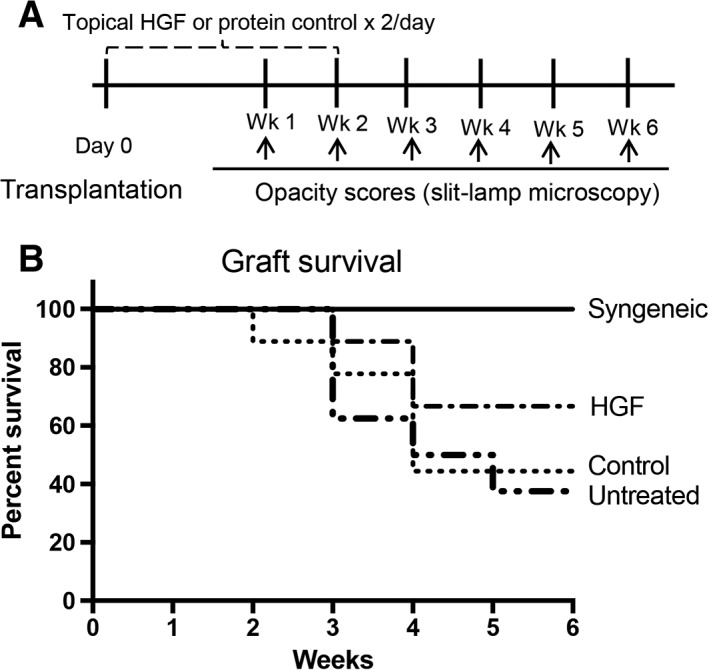
HGF alone is sufficient to prolong graft survival. (A): Schematic of experimental design. After transplantation, grafts were treated with HGF eye drops (0.1%) or control eye drops twice daily for 2 weeks. Slit‐lamp microscopy was used to evaluate the corneal opacity of naïve, control‐treated, and HGF‐treated mice. (B): Kaplan‐Meier analysis showing graft survival of HGF‐treated allogeneic graft recipients, control‐treated allogeneic graft recipients, untreated allogeneic graft recipients, and untreated syngeneic graft recipients (n = 8–10 in each group; log‐rank test; p = .022). Abbreviation: HGF, hepatocyte growth factor.
Discussion
Current research suggests that there is considerable plasticity in the immunosuppressive capacity of MSCs and that the immunoregulatory factors produced by MSCs are markedly influenced by the local inflammatory microenvironment 9. The current study deepens our understanding of the immunoregulatory mechanisms of MSCs in the setting of corneal allotransplantation. Specifically, our data demonstrate that: (a) HGF expression by MSCs is upregulated in an inflammatory milieu; (b) the secretion of HGF by MSCs suppresses corneal leukocyte infiltration and APC maturation and prolongs corneal graft survival; (c) topical HGF treatment limits APC maturation, Th1 generation, and inflammatory cell infiltration of the graft site; and finally (d) topical HGF alone (in the absence of MSCs) promotes corneal allograft survival.
The immunosuppressive function of MSCs is enhanced in the presence of inflammatory cytokines 31. Our data indicate that stimulation of MSCs with IL1β substantially increases expression of HGF, corroborating our results from a previous study 14. Prior to initiating the alloimmune response, APCs undergo a maturation process with the acquisition of MHCII and other costimulatory molecules including CD80 5, 32. The capacity of MSCs to inhibit APC maturation has previously been established 33, 34. Indeed, Oh and colleagues have reported that peritransplant i.v. infusion of MSCs prevents the rejection of allogeneic corneal transplants and observed reduced APC activation 35. Our data, obtained using a soluble HGF receptor acting as an HGF “sink,” indicate that the suppression of APC maturation by MSCs is mediated primarily via secretion of HGF. Moreover, our findings demonstrate that the promotion of corneal graft survival by MSCs is dependent on their secretion of HGF. Hosts that received i.v. injection of MSCs transfected with control siRNA exhibited 75% graft survival at 6 weeks compared with 37.5% in the untreated group. However, this survival benefit with MSC therapy was diminished when MSCs transfected with Hgf siRNA were administered, with a survival rate of only 50%. These data elucidate an immunoregulatory mechanism underlying our previous observations of suppressed allosensitization and improved corneal allograft survival in MSC‐treated mice 11. Specifically, our previous study established that GFP+ MSCs i.v. injected at 3 hours post‐transplantation home to the inflamed ocular surface, resulting in decreased APC maturation and reduced frequencies of IFNγ‐secreting Th1 cells 11. Of note, we have previously shown that systemically administered GFP+ MSCs specifically home to inflamed corneal tissue, where they exhibit long‐term survival (i.e., more than 50 days) 25.
HGF has been shown to be protective against a variety of inflammatory and autoimmune diseases 13. In a murine model of inflammatory bowel disease, Oh and colleagues transfected mice intramuscularly with human HGF cDNA and demonstrated that HGF treatment suppressed inflammation 36. The authors reported decreased intestinal mRNA expression of Th1 cytokines (including IL1β, IFNγ, and tumor necrosis factor‐α [TNF‐α]), as well as decreased CD4+ T cells and decreased neutrophils in the intestinal epithelium 36. In a rat model of experimental autoimmune myocarditis, Futamatsu and colleagues injected human HGF gene with hemagglutinating virus of the Japan‐envelope vector directly into the myocardium and demonstrated reduced antigen‐specific T‐cell proliferation and production of IFNγ by CD4+ T cells isolated from mice treated with HGF 37. The immunomodulatory properties of HGF have also been reported in murine studies of allergic airway disease 38 and collagen‐induced arthritis 39.
In the field of transplantation, HGF has been reported to promote the survival of heterotopic cardiac allografts in a murine model 40. Yamaura and colleagues demonstrated that treatment with HGF decreased cellular infiltration of the cardiac graft, reduced myocardial necrosis, and suppressed IFNγ expression 40. The authors attribute the enhanced allograft survival to the immunomodulative potency of HGF. Decreased IFNγ and TNF‐α expression has been reported in the liver and intestinal mucosa of mice transfected with HGF cDNA following bone marrow transplantation in a model of acute graft‐versus‐host disease (GVHD), with decreased donor T‐cell infiltration observed in the host liver 41. In a model of chronic GVHD, HGF gene transfer has been shown to suppress MHC class II expression by host B cells and downregulate DC immunogenicity, resulting in the amelioration of murine lupus, cholangitis, and sialoadenitis 42. Although these studies have indicated the translational potential of HGF in transplantation, further research is required to formally establish the precise immunoregulatory mechanisms of HGF in this setting.
In our study, we demonstrate that HGF inhibits APC maturation both in vitro and in vivo. We show that topical application of HGF suppresses Th1 generation in the DLNs. Given that the fate of an allograft is determined by the interactions between proinflammatory and immunoregulatory arms of the immune response 5, it is interesting to note that our data also indicate a moderate (but not significant) increase in the frequencies of Tregs following topical application of HGF.
Conclusion
The tissue regenerative and immunomodulatory activities of MSCs have been established 43, 44, 45, yet as a cell‐based therapy, their therapeutic potential is constrained by the technical challenge of consistently producing clinical‐grade cells, as well as the expense (both financial and temporal) of meeting high regulatory standards. MSCs are known to exert their immunoregulatory activity via a diverse array of mechanisms, with the majority of these involving the secretion of soluble factors 46. In this study, we demonstrate the immunomodulatory function of MSC‐derived HGF, showing that the promotion of corneal graft survival by MSCs is partly dependent on their expression of HGF. Furthermore, we establish that topical HGF suppresses APC maturation and the generation of a Th1 immune response in the lymphoid tissue, limits immune cell infiltration into corneal grafts, and is itself sufficient to promote graft survival. In doing so, our work suggests the translational potential of topical HGF in the setting of corneal transplantation, offering an alternative to cell‐based therapeutic approaches.
Author Contributions
S.K.M.: conception/design, collection of data, data analysis and interpretation, manuscript writing; W.F.: data analysis and interpretation, manuscript writing; S.S., E.E., M.O.: collection of data, data analysis and interpretation, revising the manuscript; S.K.C.: conception/design, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This work was supported in part by the National Institutes of Health (EY024602 to S.K.C. and Core Grant P30EY003790) and the Eye Bank Association of America.
References
- 1. Dana MR, Qian Y, Hamrah P. Twenty‐five‐year panorama of corneal immunology: Emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 2000;19:625–643. [DOI] [PubMed] [Google Scholar]
- 2. Maguire MG, Stark WJ, Gottsch JD et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Ophthalmology 1994;101:1536–1547. [DOI] [PubMed] [Google Scholar]
- 3. The Collaborative Corneal Transplantation Studies Research Group . The collaborative corneal transplantation studies (CCTS): Effectiveness of histocompatibility matching in high‐risk corneal transplantation. Arch Ophthalmol 1992;110:1392. [PubMed] [Google Scholar]
- 4. Abud TB, Di Zazzo A, Kheirkhah A et al. Systemic immunomodulatory strategies in high‐risk corneal transplantation. J Ophthalmic Vis Res 2017;12:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amouzegar A, Chauhan SK, Dana R. Alloimmunity and tolerance in corneal transplantation. J Immunol 2016;196:3983–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huq S, Liu Y, Benichou G et al. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol 2004;173:4464–4469. [DOI] [PubMed] [Google Scholar]
- 7. Amouzegar A, Chauhan SK. Effector and regulatory T cell trafficking in corneal allograft rejection. Mediators Inflamm 2017;2017:8670280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foulsham W, Marmalidou A, Amouzegar A et al. Review: The function of regulatory T cells at the ocular surface. Ocul Surf 2017;15:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Chen X, Cao W et al. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol 2014;15:1009–1016. [DOI] [PubMed] [Google Scholar]
- 10. Gao F, Chiu SM, Motan DAL et al. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis 2016;7:e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Omoto M, Katikireddy KR, Rezazadeh A et al. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Invest Ophthalmol Vis Sci 2014;55:6631–6638. [DOI] [PubMed] [Google Scholar]
- 12. Ilangumaran S, Villalobos‐Hernandez A, Bobbala D et al. The hepatocyte growth factor (HGF)–MET receptor tyrosine kinase signaling pathway: Diverse roles in modulating immune cell functions. Cytokine 2016;82:125–139. [DOI] [PubMed] [Google Scholar]
- 13. Molnarfi N, Benkhoucha M, Funakoshi H et al. Hepatocyte growth factor: A regulator of inflammation and autoimmunity. Autoimmun Rev 2015;14:293–303. [DOI] [PubMed] [Google Scholar]
- 14. Mittal SK, Omoto M, Amouzegar A et al. Restoration of corneal transparency by mesenchymal stem cells. Stem Cell Rep 2016;7:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Omoto M, Suri K, Amouzegar A et al. Hepatocyte growth factor suppresses inflammation and promotes epithelium repair in corneal injury. Mol Ther 2017;25:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inomata T, Mashaghi A, Di Zazzo A et al. Ocular surgical models for immune and angiogenic responses. J Biol Methods 2015;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tahvildari M, Omoto M, Chen Y et al. In vivo expansion of regulatory T cells by low‐dose interleukin‐2 treatment increases allograft survival in corneal transplantation. Transplantation 2016;100:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Zazzo A, Tahvildari M, Subbarayal B et al. Proangiogenic function of T cells in corneal transplantation. Transplantation 2017;101:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li M, Mittal SK, Foulsham W et al. Mast cells contribute to the induction of ocular mucosal alloimmunity. Am J Transplant 2019;19:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hua J, Inomata T, Chen Y et al. Pathological conversion of regulatory T cells is associated with loss of allotolerance. Sci Rep 2018;8:7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dohlman TH, Omoto M, Hua J et al. VEGF‐trap aflibercept significantly improves long‐term graft survival in high‐risk corneal transplantation. Transplantation 2015;99:678–686. [DOI] [PubMed] [Google Scholar]
- 22. Hua J, Jin Y, Chen Y et al. The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Investig Opthalmol Vis Sci 2014;55:5944–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amouzegar A, Mittal SK, Sahu A et al. Mesenchymal stem cells modulate differentiation of myeloid progenitor cells during inflammation. Stem Cells 2017;35:1532–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 25. Lan Y, Kodati S, Lee HS et al. Kinetics and function of mesenchymal stem cells in corneal injury. Invest Ophthalmol Vis Sci 2012;53:3638–3644. [DOI] [PubMed] [Google Scholar]
- 26. Lee RH, Yu JM, Foskett AM et al. TSG‐6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci USA 2014;111:16766–16771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mittal SK, Cho K‐J, Ishido S et al. Interleukin 10 (IL‐10)‐mediated immunosuppression: March‐1 induction regulates antigen presentation by macrophages but not dendritic cells. J Biol Chem 2015;290:27158–27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chauhan SK, El Annan J, Ecoiffier T et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol 2009;182:1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dohlman TH, Di Zazzo A, Omoto M et al. E‐selectin mediates immune cell trafficking in corneal transplantation. Transplantation 2016;100:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chauhan SK, Saban DR, Dohlman TH et al. CCL‐21 conditioned regulatory T cells induce allotolerance through enhanced homing to lymphoid tissue. J Immunol 2014;192:817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ren G, Zhang L, Zhao X et al. Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008;2:141–150. [DOI] [PubMed] [Google Scholar]
- 32. Lutz MB, Schuler G. Immature, semi‐mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol 2002;23:445–449. [DOI] [PubMed] [Google Scholar]
- 33. English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett 2008;115:50–58. [DOI] [PubMed] [Google Scholar]
- 34. Jacobs SA, Roobrouck VD, Verfaillie CM et al. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol 2013;91:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oh JY, Lee RH, Yu JM et al. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther 2012;20:2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oh K, Iimuro Y, Takeuchi M et al. Ameliorating effect of hepatocyte growth factor on inflammatory bowel disease in a murine model. Am J Physiol Liver Physiol 2005;288:G729–G735. [DOI] [PubMed] [Google Scholar]
- 37. Futamatsu H, Suzuki J, Mizuno S et al. Hepatocyte Growth factor ameliorates the progression of experimental autoimmune myocarditis. Circ Res 2005;96:823–830. [DOI] [PubMed] [Google Scholar]
- 38. Okunishi K, Dohi M, Nakagome K et al. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol 2005;175:4745–4753. [DOI] [PubMed] [Google Scholar]
- 39. Okunishi K, Dohi M, Fujio K et al. Hepatocyte growth factor significantly suppresses collagen‐induced arthritis in mice. J Immunol 2007;179:5504–5513. [DOI] [PubMed] [Google Scholar]
- 40. Yamaura K, Ito K, Tsukioka K et al. Suppression of acute and chronic rejection by hepatocyte growth factor in a murine model of cardiac transplantation: Induction of tolerance and prevention of cardiac allograft vasculopathy. Circulation 2004;110:1650–1657. [DOI] [PubMed] [Google Scholar]
- 41. Kuroiwa T, Kakishita E, Hamano T et al. Hepatocyte growth factor ameliorates acute graft‐versus‐host disease and promotes hematopoietic function. J Clin Invest 2001;107:1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuroiwa T, Iwasaki T, Imado T et al. Hepatocyte growth factor prevents lupus nephritis in a murine lupus model of chronic graft‐versus‐host disease. Arthritis Res Ther 2006;8:R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma S, Xie N, Li W et al. Immunobiology of mesenchymal stem cells. Cell Death Differ 2014;21:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shi Y, Su J, Roberts AI et al. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol 2012;33:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Knaän‐Shanzer S. Concise Review: The Immune Status of Mesenchymal Stem Cells and Its Relevance for Therapeutic Application. Stem Cells 2014;32:603–608. [DOI] [PubMed] [Google Scholar]
- 46. English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol 2013;91:19–26. [DOI] [PubMed] [Google Scholar]


