Abstract
Interleukin-1β (IL1) is a sleep regulatory substance. The IL1/IL1 type 1 receptor complex requires a receptor accessory protein (AcP) to signal. There are three isoforms of AcP. In the current experiments, mice lacking a neuron-specific isoform, called AcPb knockout (AcPb KO), or mice lacking AcP + AcPb isoforms (AcP KO) or wild-type (WT) mice were used. Spontaneous sleep and sleep responses to sleep deprivation (SD) between zeitgeber time (ZT) 20–ZT4 and ZT8–ZT16 were characterized. Furthermore, somatosensory cortical protein extracts were examined for phosphorylated (p) proto-oncogene tyrosine-protein kinase sarcoma (Src) and p38MAPK levels at ZT4 and ZT16 and after SD. Spontaneous sleep was similar in the three strains, except rapid eye movement sleep (REMS) duration between ZT12–ZT16 was greater in AcP KO than WT mice. After SD at ZT4, only WT mice had non-REMS (NREMS) rebounds. All mouse strains lacked an NREMS rebound after SD at ZT16. All strains after both SD periods had REMS rebounds. AcPb KO mice, but not AcP KO mice, had greater EEG delta wave (0.5–4 Hz) power during NREMS than WT mice. p-Src was very low at ZT16 but high at ZT4, whereas p-p38MAPK was low at ZT4 and high at ZT16. p-p38MAPK levels were not sensitive to SD. In contrast, p-Src levels were less after SD at the P = 0.08 level of significance in the strains lacking AcPb. We conclude that AcPb is required for NREMS responses to sleep loss, but not for SD-induced EEG delta wave or REMS responses.
NEW & NOTEWORTHY Interleukin-1β (IL1), a well-characterized sleep regulatory substance, requires an IL1 receptor accessory protein (AcP); one of its isoforms is neuron-specific (called AcPb). We showed that in mice, AcPb is required for nonrapid eye movement sleep responses following 8 h of sleep loss ending 4 h after daybreak but did not affect rapid eye movement sleep rebound. Sleep loss reduced phosphorylation of proto-oncogene tyrosine-protein kinase sarcoma but not of the less sensitive p38MAPK, downstream IL1 signaling molecules.
Keywords: cytokine, interleukin-1 signaling, sleep loss
INTRODUCTION
Interleukin-1β (IL1) is a well-characterized sleep regulatory substance in health and disease (12, 15, 18–23, 25, 33, 36, 43, 49, 51). For example, lower doses of IL1, given centrally or systemically, enhance duration of non-rapid eye movement sleep (NREMS) and electroencephalogram (EEG) delta wave (0.5–4Hz) power (an index of sleep intensity). Conditions that enhance endogenous production of IL1, e.g., excessive food intake (13) or inflammation (37), promote NREMS (21, 23). Conversely, inhibition of endogenous IL1, e.g., using the IL1 receptor antagonist, inhibits spontaneous sleep and sleep rebound after sleep deprivation (SD) (35, 36, 48–50). Brain levels of IL1 mRNA (45, 46) and IL1 protein (1a) and plasma levels of IL1 (31) vary with the sleep-wake cycle, with the highest levels correlating with high sleep propensity. IL1 injections into sleep regulatory brain areas, including the brain locus coeruleus (7), the dorsal raphe (30), or the anterior hypothalamus (1), promote NREMS. Furthermore, local injections onto the surface of the somatosensory cortex enhance local EEG delta power (52, 53).
IL1 signals through its type I receptor (R) (10). The IL1 type I R is responsible for IL1-enhanced NREMS; mice lacking this receptor do not respond to IL1, and their spontaneous NREMS is less than strain controls (8). There is also a type 2 IL1 R, but it lacks an intracellular signaling domain. The IL1 type 2 R, along with IL1 soluble Rs (the cleaved extracellular domains of IL1Rs), are involved in IL1 regulation (10, 50). IL1Rs are found in neurons and astrocytes, and both cells mediate IL1-altered sleep (16).
The IL1/IL1 type 1 R complex requires an accessory protein (AcP) to signal (42). There are three isoforms of AcP. In neurons, there is an alternatively processed form of AcP called neuron-specific AcP (AcPb). Another lower molecular weight AcP isoform is secreted into the extracellular fluid; it is called soluble AcP (sAcP). The sAcP binds to the IL1 type 2 receptor to increase its affinity to IL1, playing an anti-inflammatory role. In rats, somatosensory cortical levels of AcPb mRNA vary across the day in parallel with sleep propensity peaking at zeitgeber time (ZT) 0 (47). Somatosensory AcPb mRNA, but not AcP mRNA, also increases after SD. Furthermore, low somnogenic doses of IL1 induce AcPb but not AcP. IL1 mRNA values are lowest around ZT10–ZT20, then peak 4 h later at ZT0, the end of the rat active period (47). If wild-type (WT) and AcPb knockout (KO) mice are SD from ZT10–ZT20, NREMS and rapid eye movement sleep (REMS) rebounds in AcPb KO mice are greatly attenuated compared with the responses observed in WT mice (6). However, our prior study did not include use of AcP KO mice; herein we expand upon that study by including AcP KO mice in baseline and SD experiments. We show that AcP KO mice, which lack both AcP and AcPb, and AcPb KO mice, which lack only AcPb, lack NREMS responses to SD between ZT20–ZT4, suggesting that AcPb is required for sleep homeostasis. Furthermore, WT, AcP KO, and AcPb KO mice lack NREMS rebound if sleep deprived from ZT8–ZT16, indicating that sleep homeostatic rebound is highly circadian rhythm-dependent.
Low doses of IL1 enhance proto-oncogene tyrosine-protein kinase sarcoma (Src) phosphorylation in neurons; this action is absent in AcPb KO mice (14). Src family kinases have multiple actions in the brain, including regulation of excitatory and inhibitory transmission, learning, memory, depressive behavior, and responses to alcohol (34). In this report, we focus on phosphorylated Src (p-Src) and show that it has measurable levels in the somatosensory cortex at ZT4, the middle of the mouse sleep phase, but at ZT16, the middle of the mouse wake phase, p-Src was very low. At much higher IL1 levels, IL1 also signals via p38 MAPK phosphorylation in both neurons and astrocytes with the involvement of AcP (14). In contrast to p-Src, p-p38MAPK levels are low during the sleep phase and high during the wake phase (dark hours). Herein we also show that neither p-Src nor p-p38 MAPK is affected by SD, although in mice lacking AcPb (i.e., AcP KO and AcPb KO mice) there was an SD-linked reduction in Src phosphorylation.
MATERIALS AND METHODS
Animals
Breeding pairs of IL1 receptor AcP KO mice and AcPb KO mice (experimental strains) were purchased from Jackson Laboratories (Bar Harbor, ME) under the auspices of an agreement with Amgen Inc. (Thousand Oaks, CA). Breeding pairs of C57BL6J mice (control strain, called WT throughout) were also purchased from Jackson Laboratories. All mice were bred for 9–11 generations at Washington State University and were tail snipped and genotyped by Transnetyx (Cordova, TN). Breeding mice and pups were on a 14:10-h light-dark cycle until male pups were weaned at 21 days of age. After that, the weaned male mice were on a 12:12 light-dark cycle (light onset = ZT0). Mice lived in plastic filter-top cages with their male siblings until the time of surgery. Food (Teklad Global Diets, 2018 18% Protein Rodent Diet, Envigo, South Kent, WA) and water were available ad libitum. Experimental protocols were approved by the Washington State University Animal Care and Use Committee and conformed to National Institutes of Health guidelines. Although we did not quantitatively characterize mouse behavior other than sleep, AcP KO mice awoke from anesthesia more quickly, as judged by reactions to toe/tail pinch during surgery, than AcPb KO or WT strains. Thus, AcP KO mice had to receive an additional booster shot of 87 mg/kg ketamine (ip) to maintain an appropriate level of anesthesia. This sensitivity to the anesthetic was independently recognized by three different surgeons. No other overt behavioral differences were noted between the strains.
Surgery
At the time of surgery, the average body weight of the WT, AcPb KO, and AcP KO mice was 25.6 g, 25.9 g, and 30.8 g, respectively. The average age of the WT, AcPb KO, and AcP KO mice was 9.9 wk, 10.5 wk, and 12.7 wk, respectively. Mice were anesthetized with an injection of ketamine-xylazine (87 mg/kg ip and 13 mg/kg ip, respectively), which was followed by a subcutaneous injection of carprofen (5 mg/kg) and sterile saline (1 mL) before surgical implantation of EEG electrodes. Electrodes (Plastics One, Roanoke, VA, cat. no. 8IE3631SPCXE) were implanted over the left and right frontal cortex (rostral to bregma) and over the left and right parietal cortex (caudal to bregma), and a fifth electrode was implanted slightly to the right of the intersection of the lambdoid suture and the sagittal suture. The electrodes were fixed to the calvarium with visible light cure composite (Prime-Dent Flowable, Chicago IL). An electromyogram (EMG) electrode was implanted in the nuchal muscle. The leads from both the EEG and EMG electrodes were inserted into a plastic pedestal (Plastics One, MS363 Pedestal 2298 6-Pin White Del) and arranged so that the frontal and parietal electrodes of each respective hemisphere would reference each other across the hippocampal plane. The EEG signal with the least amount of noise was used for fast Fourier transformation (FFT) extractions during data analyses. Mice were then transferred into separate plastic filter-top cages placed on heated water blankets and given MediGel CPF Carprofen (ClearH20). Mice were monitored and weighed daily postoperatively for 3 days and were allowed at least 1 wk to recover from the surgery before the start of the acclimation phase of the experiment.
Following recovery and before SD, mice were transferred to experimental cages (6 × 9.5 in., 7 in. tall) and put in sound-attenuated experimental chambers (Campden Instruments, Loughborough, England). The temperature was maintained at 23°C–25°C, and the relative humidity ranged from 33% to 41%. Once the mice were tethered to recording cables, they were checked daily to ensure habituation to the experimental conditions. Only male mice were used in this study to avoid potential estrus cycle effects on sleep.
Recording and Analyses
Recordings and analyses were done as previously described (6, 54). Briefly, the recording cables were connected to commutators that were connected to a 15A54 Quad Amplifier (Grass Technologies, Quincy, MA) and then digitized at 128 Hz. EEG signals were filtered below 0.1 Hz and above 100 Hz. EMG signals were filtered below 100 Hz and above 3,000 Hz. Sleep Sign for Animal software (Kissei Comtec, Nagano, Japan) was used to extract sleep-wake activity. Raw data (time in stage, episode length and frequency, and FFT values) for each mouse were determined for the recovery periods starting immediately following SD and ending at either light onset or offset 32 h later, depending on if SD ended at ZT16 or ZT4, respectively. Raw data from a 24-h baseline file for each mouse was extracted, which corresponded with the same time period as the recovery files, except as noted (cohort 1, ZT20–ZT4 baseline). Vigilance states (waking, NREMS, REMS) were determined in 10-s epochs by visually using EEG wave forms and FFT power spectrum graphs. This was done with a page view corresponding to three separate 1-min recording periods so that transitional periods were more easily identified. Waking periods were identified by EMG activity and less regular low-amplitude EEG waveforms with FFT power occurring in a variety of frequencies. NREMS periods were characterized by high-amplitude EEG slow waves and predominant FFT activity in the delta range (0.5–4 Hz) and low EMG activity. REMS periods were characterized by very regular low-amplitude waves with predominant theta (6–9 Hz) activity and a lack of EMG activity. Each 10-s epoch’s FFT output was then averaged within groups (wake, NREMS, REMS) in 0.5-Hz bins from 0 to 20 Hz for each hour. NREMS EEG slow-wave activity (0.5–4Hz) was determined in each epoch by FFT using a Hanning window. EEG slow-wave activity values from the control recordings were averaged for each individual animal to use as a reference value for normalization of data collected across a 24-h time block both for baseline and experimental values. Delta power was calculated by adding values (µV2) from 0.5- to 4-Hz range for each animal for each 1-h time bin and was expressed as a percentage of the 24-h reference value as previously described (24). Power spectral analysis was also calculated for the first and last 4 h post-SD in 0.5-Hz bin sizes in the 1.0–20-Hz frequency range. The 17.0–17.5-Hz values from each animal were used to normalize baseline or experimental values because values from all 3 strains converged from 17 to 20 Hz. Two-way ANOVAs were used to determine if there were significant differences between treatments (baseline vs. SD) within each strain.
All scoring was done by the same individual over a 2-mo period. Because of poor EEG quality that did not allow consistent scoring, two WT and AcPb KO mice were excluded, and one AcPb KO mouse was excluded. Therefore, sleep and wake activity were analyzed for a total of 34 mice (WT, n = 10; AcPb KO, n = 11; AcP KO, n = 13).
Experiments: Sleep Recordings and SDs
Four separate cohorts of mice were used for baseline sleep determinations and for SD from ZT8–ZT16 and ZT20–ZT4 on separate days. There were 6 to 7 days of recovery between SDs. The mouse strain composition of each cohort of 9 to 10 mice was dependent upon availability of roughly age-matched mice from 2 or more strains. The recordings took place over 6 mo.
Mice were acclimated for at least 48 h before a 48-h baseline recording. The baseline recordings ended immediately before the start of SD. SD was performed by two teams each composed of two individuals, which enabled each team to get an hour-long break every other hour. During SD, mice were gently handled and aroused via disturbing their nesting material with a soft paint brush, tapping on the cage, or gently nudging the mice as a last resort measure. The mice remained tethered throughout the SD period, and recordings were taken for 24 h after SD ended without disturbing them. Two different SDs (ZT8–ZT16 and ZT20–ZT4) were performed at least 1 wk apart on each cohort of mice. Separate 48-h baselines were obtained immediately before the start of each SD, except for cohort 1, in which the 48-h baseline recorded before the ZT8–ZT16 SD was used as a baseline for the ZT20–ZT4 cohort, as human error prevented the recording of a ZT20–ZT4 baseline.
Repeated measures two-way ANOVAs, performed in Statistica (TIBCO Software, Inc.), were used to assess factors of time (in 2-h bins) and treatment (baseline vs. post-SD) for each strain (WT, AcPb KO, and AcP KO), 8-h period of SD (ZT8–ZT16 and ZT20–ZT4), and sleep state (NREMS and REMS). Post hoc comparisons were made using Fisher’s least-significant difference (LSD) tests when appropriate. A significance level of P < 0.05 was accepted.
Western Blot Methods
Immediately on completion of two 8-h SD treatments ending at ZT16 or at ZT4, tissue samples from the somatosensory cortex were harvested from WT (n = 28), AcP KO (n = 27), and AcPb KO (n = 28) mice. SD methods used were those described above for the SD experiments. However, the mice used for protein determinations were independent from those used for sleep experiments. Samples were represented equally in each of the treatment conditions (non-SD and SD) and time points. Samples were flash frozen in liquid nitrogen, stored at −80°C, and were placed in ice cold lysis buffer prepared using ready-to-use radioimmunoprecipitation assay buffer (Sigma-Aldrich, St. Louis, MO) supplemented with protease/phosphatase inhibitor cocktail (Cell Signaling Technology, Danvers, MA). Tissue was homogenized and centrifuged (20 min, 16,000 g, 4°C). The supernatant was collected for protein quantification using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA) as per manufacturer instructions.
Equal amounts of protein (20 μg) were applied to hand-cast sodium dodecyl sulfate polyacrylamide 10% gels and transferred to nitrocellulose membranes. As it was not possible to run all samples on the same blot, samples from each experiment were run in parallel and duplicated. To minimize nonspecific antibody binding, blots were blocked on a rocker for 1 h at room temperature. Blots for phosphorylated proteins were blocked in 3% bovine serum albumin in tris-buffered saline with 0.05% Tween. Blots for total proteins were blocked in 5% milk in tris-buffered saline with Tween. Blots were incubated with primary antibody prepared in either 3% bovine serum albumin or 5% milk overnight on a rocker at 4°C. The primary antibodies for phosphorylated and total proteins included anti-p38MAPK, anti-Src, anti-phospho-p38MAPK (Thr180/Tyr182), anti-phospho-Src (Tyr416) (1:1,000; cat. nos. 2110, 9212, 2101, and 9215; Cell Signaling Technology), and anti-β-Tubulin (1:5,000; cat. no. MA5–16308; Thermo Fisher Scientific). Blots were thrice washed with tris-buffered saline with Tween and incubated for 1 h at room temperature with secondary antibody labeled with horseradish peroxidase (1:4,000; cat. no. P 0447, goat anti-mouse; cat. no. P 0448, goat anti-rabbit; Dako Agilent Pathology Solutions, Santa Clara, CA). Membranes were washed in tris-buffered saline with Tween another three times before development with Pierce ECL Western Blotting Substrates (cat. no. 32106; Thermo Fisher Scientific) and subsequent visualization with ImageQuant LAS 4000 system software (GE Healthcare). After visualization, membranes were stripped of phosphorylated proteins using a buffer comprised of 2.5 mM Tris-HCl pH 6.8, 2% sodium dodecyl sulfate, and 100 mM β-mercaptoethanol at 50°C for 30 min. Stripped membranes were washed 10 times in tris-buffered saline with Tween, reblocked, and probed with anti-p38MAPK, anti-Src, or positive-control anti-β-Tubulin antibodies.
Quantification for all protein, strains, and time points was performed in ImageJ software (National Institutes of Health, Bethesda, MD) using imported immunoblot images produced in ImageQuant. Within ImageJ, a region of interest was determined for the most prominent band on each individual blot for each protein of interest. All other bands were measured against that region of interest. The same region of interest was used to determine background densities for each protein, either above or below the protein band, depending on space availability. Densities calculated by ImageJ were imported into Excel, where the background density for each sample was subtracted from its associated protein band to derive a net protein band density value for each protein of interest within each strain and treatment condition on each blot. For each treatment condition within each strain, the loading control (β-Tubulin) with the highest net density value was used as a divisor for the remaining densities to calculate a relative normalizing control value, i.e., the highest density’s relative normalizing control value was set equal to one with all other relative normalizing control values less than one. Normalized protein values were calculated for each protein by multiplying the net density value (i.e., protein band density less background density) by the relative normalizing control value that was determined for each sample. Normalized values for each of the duplicated samples were averaged and then used for subsequent calculations. Phosphorylated protein fold changes were calculated by dividing the normalized phosphorylated protein values by the normalized total protein values for each sample within each strain and each treatment condition. Similarly, fold changes after SD were calculated by dividing by the non-SD normalized values for each phosphorylated protein.
A factorial ANOVA tested protein fold-change significance for the following factors: strain, treatment condition, SD time point, protein, and all factor interactions. Post hoc testing to determine specific significant differences was completed with Tukey’s honestly significant difference multiple comparison of means using a 95% family-wise confidence level. Statistical tests were completed in the freely available software R [version 3.5.0 (2018–04–23)]. All results were graphically visualized mean ± SE.
RESULTS
Baseline Sleep
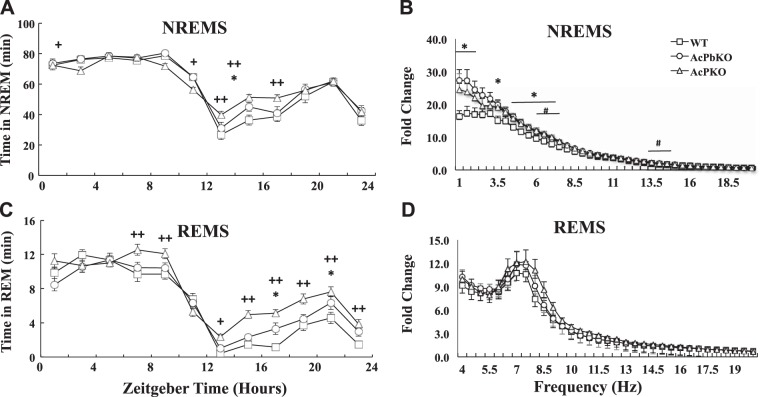
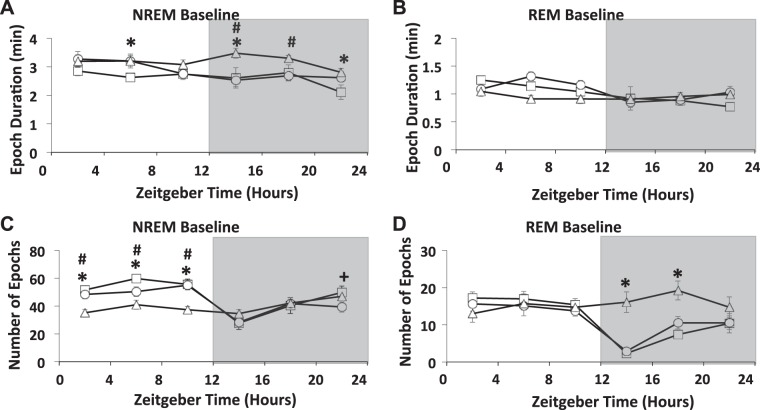
The three strains of mice used in this study exhibited typical daily NREMS and REMS rhythms, with more sleep occurring during daylight hours (ZT0–ZT12) than during the dark phase (Fig. 1, A and C). Furthermore, all strains had a late-evening increase in NREMS and REMS that is typical of C57BL/6 mice (9, 47). There were small NREMS duration differences between the strains in the light hours, with AcP KO mice having slightly less NREMS during that time. During the light phase, the longer AcP KO NREMS bout durations (Fig. 2A) did not result in greater NREMS because of the decrease in the number of NREMS bouts (epochs) in AcP KO mice compared with WT mice during daylight hours (Fig. 2C). In contrast, the higher NREMS duration during the dark hours in AcP KO mice compared with WT mice was due to an increase in NREMS bout duration in AcP KO mice compared with WT mice, which was not accompanied by a decrease in the number of bouts (Fig. 2A). During ZT12–ZT24, dark hours, both AcP KO and AcPb KO mice had slightly more NREMS than WT mice Fig. 1A). Differences in REMS durations between strains were also evident (Fig. 1C). Thus, AcP KO mice had substantially more REMS during the dark phase than WT mice and slightly more than AcPb KO mice. The increases in REMS occurring in AcP KO mice during the dark phase were the result of an increased number of REMS epochs (Fig. 2D). There were no differences in REMS duration between WT and AcPb KO mice (Fig. 1C).
Fig. 1.
Interleukin-1 receptor accessory protein (AcP) knockout (KO) mice have distinct baseline sleep and electroencephalogram (EEG) power spectra compared with wild-type (WT) or brain-specific AcP (AcPb) KO mice. Time spent in non-rapid eye movement sleep (NREMS), during baseline recordings (A) and time spent in rapid eye movement sleep (REMS) (C) are different between strains throughout the dark period [zeitgeber time (ZT)12–ZT24] (*P < 0.05 WT vs. AcPb KO; +P < 0.05, ++P < 0.01 WT vs. AcP KO). NREMS EEG spectral power (1–20 Hz) (B) and REMS EEG spectral power (4–20 Hz) (D) during light period (ZT0–ZT12) in AcPb KO (○), AcP KO (Δ), and WT (□) mice. Data (B and D) are fold changes from power in the (17–17.5 Hz) frequency bin; in this frequency bin, absolute power from all 3 strains was very close (*P < 0.05, AcPb KO vs. WT mice; #P < 0.05, AcP KO vs. WT). Graphs show means ± SE.
Fig. 2.
The mean ± SE for epochs and epoch durations were calculated using 4-h time blocks over a 24-h period for wild-type (WT), brain-specific interleukin-1 receptor accessory protein (AcPb) KO, and AcP KO mice. Average non-rapid eye movement sleep (NREMS) and rapid eye movement sleep (REMS) epoch durations were calculated in minutes, and there were strain differences during the dark period during NREMS but not in REMS (A and B). The number of NREMS epochs occurring during the light period were different among strains (A and C). During the first 8 h of the dark period, the number of REMS epochs differed between WT vs. AcP KO mice (D). *P > 0.05, WT vs. AcP; +P > 0.05, WT vs. AcPb; #P > 0.05, AcP vs. AcPb.
EEG FFT power spectra obtained from all three strains during ZT0–ZT12 are shown in Fig. 1, B and D. During NREMS, AcPb KO mice had significantly more power in the lower frequency ranges than WT mice. NREMS EEG FFT values from AcP KO mice were not different from WT or AcPb KO mice values. During REMS, there were no differences in power between strains. However, during REMS, theta EEG FFT values were evident, thereby providing reassurance of REMS scoring accuracy. Similar power spectra occurred during the active phase, ZT12–ZT23, although the variance was higher, likely because of more movement occurring while awake (data not shown; see Fig. 7 for another example of similar power spectra).
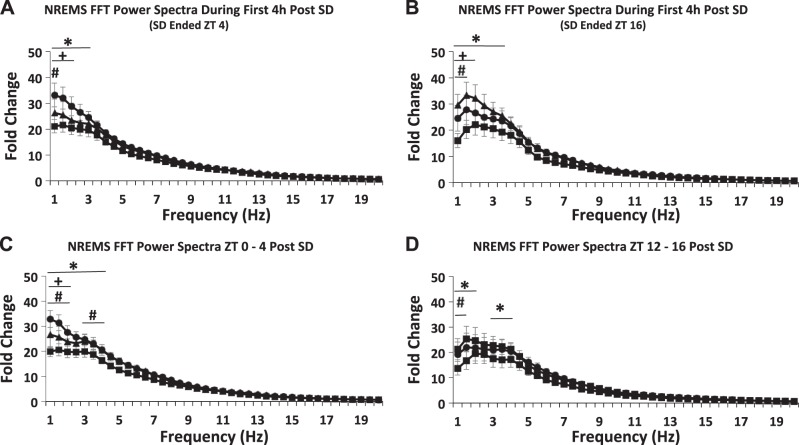
Fig. 7.
Power spectra of the first (A and B) and last (C and D) 4-h time blocks post-sleep deprivation (SD). Zeitgeber time (ZT)20–ZT4 (A and C) and ZT8–ZT16 (B and D) were calculated for 0.5-Hz bins from 1.0- to 20-Hz frequency range; values were normalized to values between 17- and 17.5-Hz bin as in Fig. 1B. Means ± SE are shown. Differences between strains were found in the lower frequency bands (A through D). *P < 0.05, AcPb knockout (KO) vs. wild-type (WT); #P < 0.05, AcP KO vs. WT; +P < 0.05, AcPb KO vs. AcP KO. AcP, interleukin-1 receptor accessory protein; AcPb, brain-specific AcP; FFT, fast Fourier transformation; NREMS, non-rapid eye movement sleep.
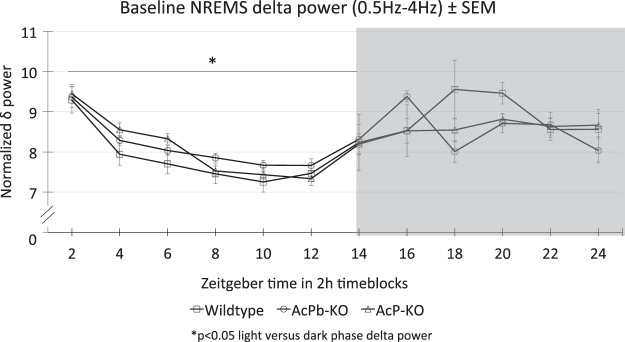
The distribution of mouse delta power during NREMS (Fig. 3), normalized to total delta power over 24 h, with slightly higher values during nighttime than daytime, was similar to that previously reported (24, 47). However, there were not significant differences between strains.
Fig. 3.
Diurnal rhythm of electroencephalogram delta power within non-rapid eye movement sleep (NREMS) epochs. Delta power fast Fourier transformation values for each 2-h block were divided by the sum of the 12 2-h blocks across the 24-h day to give a fractional percent of power within each 2-h block. Values shown are means ± SE. There were no significant strain differences; collectively, power was lower during daylight hours [zeitgeber time (ZT)0–ZT12] than during night hours as previously described for C57BL/6 mice (24, 47). *P < 0.05, light versus dark phase delta power.
In summary, all three strains shared similarities, e.g., diurnal variations typical of mice and power spectra typical of NREMS and REMS. There were, however, significant differences in durations of REMS with AcP KO and in power spectra with AcPb KO mice, having higher values, respectively, than WT mice.
Sleep After SD
NREMS after 8 h of SD.
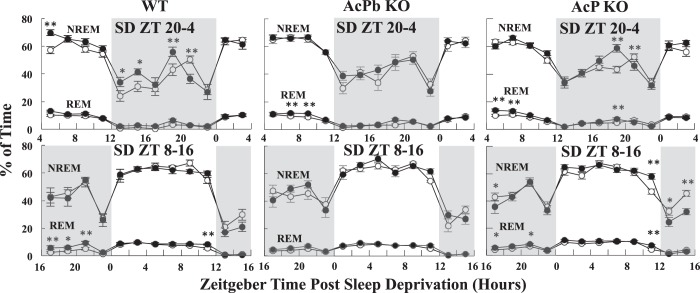
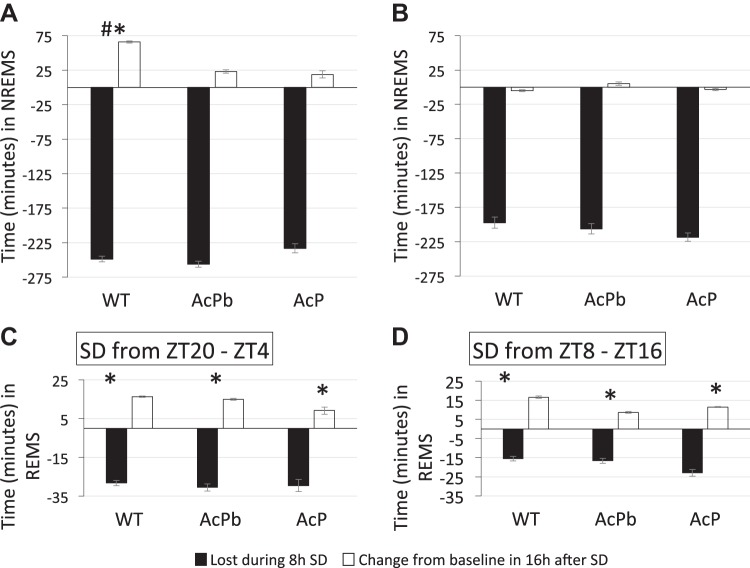
NREMS differed significantly from corresponding baseline values for WT [time × treatment: F11,88 = 3.24, P < 0.01] and AcP KO [time × treatment: F11,132 = 3.35, P < 0.01] after SD from ZT20–ZT4. During the first 2 h post-SD, ZT4–ZT6, NREMS increased above the corresponding baseline in WT mice (Fisher’s LSD, P < 0.01) but not in AcPb KO or AcP KO mice (Fig. 4). Looking at longer post-SD periods (Fig. 5), WT mice had a significant NREMS rebound during the first 16 h post-SD. Small increases in NREMS occurred during that period in AcP KO and AcPb KO mice, but these changes were not significant. The rebound in duration of NREMS did not make up for the NREMS lost during the 8-h SD period (Fig. 5). During the 8 h of SD, the mice lost around 4 h of NREMS, but during the first 16 h post-SD, ZT4–ZT20, they gained only ~1 h of sleep above their normal baseline levels. During the 8-h period (ZT20–ZT4), following the first 16 h post-SD, there were sporadic significant changes in duration of NREMS (Fig. 4), but for these 8 h (ZT20–ZT4 post-SD block), there were no significant differences in any of the changes (data not shown for the 8-h blocks for any of the strains used).
Fig. 4.
Baseline (open circles) and post-sleep deprivation (post-SD) (closed circles) time in nonrapid eye movement sleep (NREMS) and rapid eye movement sleep (REMS) for wild-type (WT), brain-specific interleukin-1 receptor accessory protein (AcPb) knockout (KO), and AcP KO mice for the first 24 h post-SD. SDs occurred between zeitgeber time (ZT)20–ZT4 (top) and ZT8–ZT16 (bottom), expressed as a percent of total minutes in each 2-h bin. Each data point is a 2-h average ± SE. Time in the ZT4–ZT6 bin is a percent of 102 min due to the loss of the first 18 min of recovery for 9 AcP KO subjects from the ZT20–ZT4 SD. Gray background indicates lights off. *P < 0.05 and **P < 0.01, baseline vs. post-SD.
Fig. 5.
Sleep lost during sleep deprivation (SD) vs. sleep rebound during the first 16 h after SD. Black bars show sleep lost during the respective SD periods; losses were calculated from baseline values for duration of (A and B) non-rapid eye movement sleep (NREMS) and (C and D) rapid eye movement sleep (REMS) (see Fig. 1). More sleep was lost during the SD occurring during zeitgeber time (ZT)20–ZT4 (A and C) because mice sleep more during that period than during ZT8–ZT16. After SD during ZT20–ZT4, only wild-type (WT) mice had a NREMS rebound occurring between ZT4–ZT20 (A); values occurring after SD were compared with control values from the same mice (differences between those values are shown in the black bars). No NREMS rebound occurred after SD between ZT8–ZT16 (B). All strains showed a REMS rebound after both SD conditions (C and D). Values shown are means ± SE. #P < 0.05 ZT4 vs. ZT16; *P < 0.05 baseline vs. post-SD recovery. AcP, interleukin-1 receptor accessory protein; AcPb, brain-specific AcP.
After SD between ZT8 and ZT16, there were no long-term significant differences in duration of NREMS in any of the strains (Fig. 5). However, during the first 2 h post-SD (ZT16–ZT18) (Fig. 4, bottom graphs), all strains (WT, AcPb KO, and AcP KO) had less NREMS than their corresponding baseline values, and this effect was larger in AcPb KO and AcP KO mice than WT mice. However, this decrease was only statistically significant in AcP KO mice (Fisher’s LSD, P < 0.05).
REMS after SD.
After SD between ZT20 and ZT4, REMS differed significantly from baseline values in AcPb KO mice [time × treatment: F11,110 = 2.90, P < 0.01] and AcP KO mice [time × treatment: F11,132 = 3.35, P < 0.01]. The significant changes in REMS durations occurred sporadically in various 2-h blocks (Fig. 4) in the various strains. However, if the analyses are restricted to the first 16-h post-SD block, ZT4–ZT20, all three strains increase REMS duration [treatment (SD vs. non-SD), F1,1 = 396.49, P = 0.03, post hoc t-tests adjusted P < 0.05] (Fig. 5). There were no differences in REMS duration between strains during the 8-h post-SD ZT20–ZT4 period (data not shown).
After 8 h of SD from ZT8–ZT16, REMS increased in WT mice [time × treatment: F11,99 = 2.42, P < 0.05] and AcP KO mice [time × treatment: F11,132 = 2.08, P < 0.05] from the corresponding strain control values (Fig. 4). During the first 16-h post-SD period, ZT16–ZT8, all strains exhibited significant REMS rebounds (Fig. 5). There were no differences in REMS duration between strains during the post-SD ZT8–ZT16 period (data not shown).
EEG NREMS delta power and power spectra after SD.
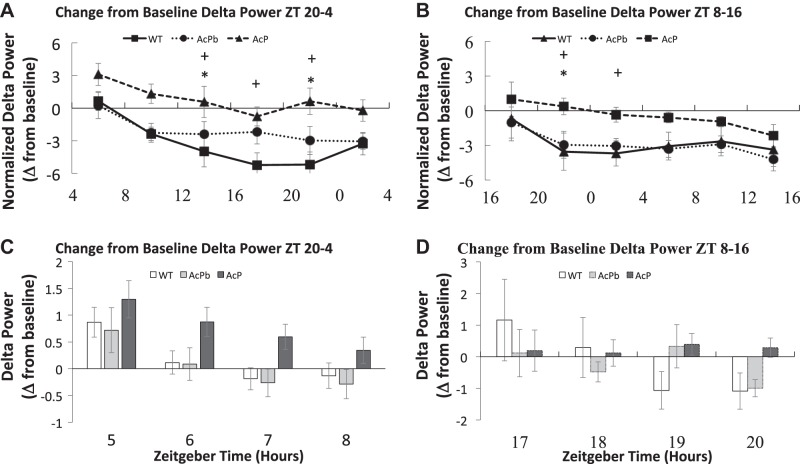
During the first 4 h after either SD period (Fig. 6, A and B), AcP KO mice increased their NREMS delta power compared with their baseline values. During the next 20 h post-SD, AcP KO mice had NREMS delta power that was close to their baseline values. In AcP KO mice, the post-SD from ZT8–ZT16 SD delta wave power was smaller, albeit not significantly, during the first 4 h post-SD than compared with the 4 h post ZT20–ZT4 SD (Fig. 6B). A strain effect was present for ZT20–ZT4 SD [strain – F2,180 = 21.928, P ≤ 0.01] and ZT8–ZT16 [strain – F2,186 = 10.769, P ≤ 0.01]. WT and AcPb KO mice exhibited small nonsignificant increases in EEG delta power if SD occurred between ZT20 and ZT4 or decreases if SD occurred during ZT8–ZT16 (Fig. 6, A and B). Regardless, as previously reported for WT mice deprived during a similar time period (6), WT EEG NREMS delta power during the first hour post-SD increased then subsequently decreased during the next 3 h post-SD, although these differences were not significant (Fig. 6, C and D). After the first 4 h post-SD, both WT and AcPb KO mice had a prolonged reduction in EEG delta power during NREMS for the remainder of the recording period (Fig. 6, A and B). In contrast, AcP KO mice lacked this negative delta power rebound, indicating that AcP is required for the manifestation of the negative rebound.
Fig. 6.
Sleep deprivation (SD)-induced changes in nonrapid eye movement sleep delta power were dependent upon the time of day SD was terminated and upon the presence of interleukin-1 receptor accessory protein (AcP) (A and B). Mean ± SE values were calculated by dividing delta power post-SD and by its respective baseline value during the same time period in 4-h blocks (C and D). The initial 4-h post-SD period is further broken down into 1-h blocks (C and D). AcPb, brain-specific AcP; WT, wild-type; ZT, zeitgeber time. +P ≤ 0.05, AcP KO versus WT; *P ≤ 0.05, AcP KO versus AcPb KO.
Power spectral analyses of the first and last 4-h time blocks post-SD indicated differences between the three strains. [Fig. 7A, strain – F2,1230 = 14.481, P ≤ 0.01; Fig. 7C, strain – F2,1230 = 22.712, P ≤ 0.01; Fig. 7B, strain – F2,1271 = 17.43, P ≤ 0.01; Fig. 7D, strain – F2,1271 = 16.107, P ≤ 0.01]. During both time blocks, AcPb KO mice had greater delta power than the other two strains as was found under baseline conditions (Fig. 1B).
Western Blot Results
Relative levels of p-p38MAPK and p-Src somatosensory proteins were determined in control non-SD samples and after SD between ZT20 and ZT4 and between ZT8 and ZT16 at two time points, ZT16 and ZT4. SD failed to alter significantly phosphorylation of p-38MAPK or Src with respect to non-SD in any of the strains at either time point. Furthermore, there were no differences between p-p38MAPK and p-Src when expressed as a ratio between SD/non-SD; those values were all close to 100% (Table 1). Thus, SD in and of itself, from ZT8 to ZT16, or from ZT20 to ZT4, had no effect on the phosphorylation of Src or p38MAPK. However, because both AcP KO and AcPb KO mice lack AcPb, and to gain statistical power, we combined results from AcP KO and AcPb KO mice and compared them with results from WT mice. There was a reduction in p-Src in the absence of AcPb (t-test, P = 0.08). Although not quite reaching the traditional value considered significant, we propose that they are biologically significant (see Discussion).
Table 1.
Fold change: phosphorylated proteins relative to total protein and after sleep deprivation relative to non-sleep deprivation
| WT, % | AcPb, % | AcP, % | |
|---|---|---|---|
| p-p38:total p38 | |||
| ZT16 nSD | 65.03 | 57.18 | 58.53 |
| ZT16 SD | 64.94 | 60.19 | 58.66 |
| ZT4 nSD | 46.86 | 33.37 | 54.86 |
| ZT4 SD | 54.19 | 32.71 | 51.52 |
| ZT16 SD/nSD | 99.86 | 105.25 | 100.21 |
| ZT4 SD/nSD | 115.66 | 98.04 | 93.91 |
| p-Src:total Src | |||
| ZT4 nSD | 26.85 | 33.67 | 28.12 |
| ZT4 SD | 26.46 | 29.10 | 23.02 |
| ZT4 SD/nSD | 98.56 | 86.41 | 81.87 |
AcP, interleukin-1 receptor accessory protein; AcPb, neuron-specific AcP; nSD, non-sleep deprived; p-Scr, phosphorylated proto-oncogene tyrosine-protein kinase; SD, sleep deprivation; WT, wild-type; ZT, zeitgeber time.
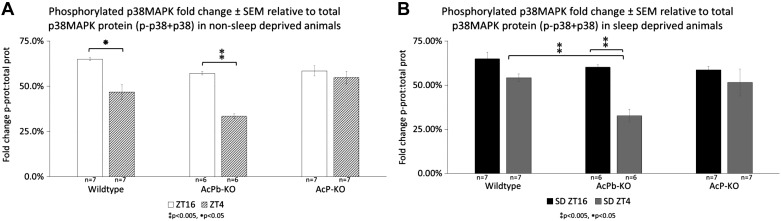
Within the non-SD treatment condition (Table 1, Fig. 8), WT mice at ZT16 had significantly higher p-p38MAPK relative to total p38MAPK than their ZT4 counterparts [strain × time × treatment F11,70 = 9.636, P < 0.005; Tukey’s honestly significant difference adjusted P value < 0.05]. This was also reflected in AcPb KO animals (adjusted P value < 0.005) but not in AcP KO animals. However, at ZT4, AcP KO animals had significantly higher p-p38MAPK-total p38MAPK fold change when compared with AcPb KO animals (adjusted P value < 0.005). These significant differences were also mirrored in the SD treatment condition (WT vs. AcPb KO, adjusted P value < 0.005; AcP KO vs. AcPb KO, adjusted P value < 0.05; AcPb KO ZT4 vs. AcPb KO ZT16, adjusted P value < 0.005) (Fig. 8). Significant differences between fold changes of phosphorylated proteins [strain × protein × treatment F11,70 = 8.623, P < 0.005] were present at ZT4, with sleep deprived WT (adjusted P value < 0.005) and AcP KO (adjusted P value < 0.005) animals having higher fold change of p-p38MAPK than p-Src. This protein difference was also the case for non-sleep deprived AcP KO animals (WT, adjusted P value < 0.05; AcPb KO adjusted P value < 0.005) but not for AcPb KO animals in either treatment condition. These results suggest that ZT-dependent phosphorylation of p38MAPK, whether mice are SD or not, requires the presence of AcP.
Fig. 8.
Graphic representation ± SE of quantified phosphorylated (p)-p38MAPK with respect to total p38MAPK in non-sleep deprived (non-SD) animals (A) and SD animals (B). Wild-type animals at zeitgeber time (ZT)16 had significantly more p-p38MAPK than their ZT4 counterparts (Tukey adjusted P value < 0.05). This was also reflected in brain-specific interleukin-1 receptor accessory protein (AcPb) knockout (KO) animals (Tukey adjusted P value < 0.005) but not in AcP KO animals. However, at ZT4, AcP KO animals had significantly more p-p38MAPK when compared with AcPb KO animals (Tukey adjusted P value < 0.005). These significant differences were mirrored after sleep deprivation (C). **P < 0.005, *P < 0.05.
Although Src was present at both time points, p-Src was detected only at ZT4. At the ZT4 time point, the fold change of p-Src relative to total Src protein was insignificant between strains and treatment conditions. This suggests that, like p38MAPK, Src phosphorylation is dependent on the time of SD.
DISCUSSION
A major finding reported herein is that when subjected to SD across the transition from dark to light phases (ZT20–ZT4), WT, but not AcP KO or AcPb KO, mice exhibit a sleep rebound. This finding is consistent with our earlier work showing that AcPb KO mice, if SD was from ZT22 to ZT8, have less sleep rebound than WT mice during the post-SD period, although the SD period previously used was longer and AcP KO mice were not used (6). Those and current data suggest that AcP isoforms are important for homeostatic NREMS responses. Furthermore, because none of the strains exhibited a homeostatic NREMS response to SD carried out between ZT8 and ZT16, current data also indicate strong interactions of NREMS homeostatic regulatory mechanisms with circadian rhythm mechanisms. Thus, NREMS rebound in WT mice SD between ZT20 and ZT4, although significant, was substantially less than if WT mice are deprived of sleep during their normal sleep period (e.g., ZT6–ZT12) (44). These data are consistent with prior reports in rats that SD at different times of the day can result in reduced time in NREMS because of an interaction with circadian rhythm mechanisms (11). One such mechanism may be the inhibitory action IL1 has on the CLOCK Circadian Regulator/Brain And Muscle Aryl Hydrocarbon Receptor Nuclear Translocator Like (Clock/BMAL) complex, a key element in the regulation of circadian rhythms (3).
A second major finding reported herein is that the roles that AcP isoforms play in REMS regulation are substantially different than their roles in NREMS. Thus, spontaneous REMS in mice lacking AcP and AcPb (AcP KO mice) is substantially greater during dark hours (ZT12–ZT18) than that occurring in AcPb KO mice or WT mice (Fig. 1). An increase in spontaneous REMS duration in AcPb KO mice, although smaller than that occurring in AcP KO mice, was previously observed (47). In contrast, mice lacking the IL1 type I receptor, a component of the IL1/IL1R/IL1AcP signaling complex, have less spontaneous REMS during the murine active phase (ZT12–ZT24) (8). The reasons for this difference remain unknown but could reflect possible IL1 triggering another IL1 family receptor such as the IL36 receptor. Another possibility is that the third isoform of AcP, the secreted sAcP, interacts with the soluble IL1 type 2 receptor to enhance its affinity for IL1, thereby effectively reducing the extracellular IL1 concentration (41). In the AcPb KO mice, the sAcP mRNA isoform is elevated ~7-fold in AcPb KO mice relative to WT mice, but it is not affected by SD in either strain (unpublished RNA-sequencing results). Regardless of the exact mechanism, current results clearly indicate that the absence of AcPb and AcP isoforms is associated with enhanced REMS duration during the dark phase.
In contrast to spontaneous REMS, REMS rebound after SD occurs whether SD took place during ZT20–ZT4 or during ZT8–ZT16 in the three strains of mice used herein. This suggests that the mechanism responsible for the SD-induced REMS rebound is independent of IL1. Furthermore, even though AcP KO mice lost more REMS during SD from ZT8 to ZT16, their rebound was slightly less than that occurring in WT and AcPb KO mice (Fig. 5). One possibility for this apparent discrepancy is that prolactin, a stress hormone, increases with SD (32, 39) and likely drives, in part, SD-induced REMS responses. There are multiple additional interactions between IL1, prolactin, sleep, and stress, e.g., IL1 can induce pituitary release of prolactin (40), and both are upregulated by stressors. Regardless of such links, we conclude that AcP isoforms have clear roles in NREMS homeostatic responses to SD and in spontaneous REMS duration. However, their involvement in spontaneous NREMS duration and REMS homeostatic rebound is minimal.
A third major finding described herein is the confirmation of the earlier conclusion that the NREMS duration regulatory mechanisms and the NREMS delta wave power mechanisms is, in part, separate from each other, although they are often linked to each other (5, 9). Thus, whereas duration of spontaneous NREMS was similar in WT, AcPb KO, and AcP KO mice, AcPb KO mice had greater NREMS delta wave power than WT mice, whether during spontaneous NREMS (Fig. 1B) or during NREMS after SD, e.g., after SD was performed from ZT20 to ZT4 (when WT mice, but not the other two strains, had an NREMS rebound) (Fig. 7, A and C). The absolute levels of delta power are greater in AcPb KO mice than AcP KO or WT mice under baseline conditions (Fig. 1) or after SD (Fig. 7, A and C). This is consistent with AcPb causing an attenuation of delta wave power. In contrast, after SD, the relative changes in delta wave power occurring within a strain are higher in AcP KO mice compared with AcPb KO mice or WT mice (Fig. 6, A–D). AcP KO mice had greater increases in delta power post-SD compared with their own controls (Fig. 6, A and B). Furthermore, they lacked the delayed, prolonged, negative NREMS delta power that both AcPb KO or WT mice exhibited, suggesting that both AcP and AcPb are required for this delayed effect.
A fourth finding reported herein is related to a prior finding that low doses of IL1 enhance Src phosphorylation in neurons via AcPb (14). The dose of IL1 needed to enhance p-Src in hippocampal neurons is ~1,000-fold less than that needed to activate inflammatory responses (14). How that observation is related to IL1-related sleep responses reported herein remains unknown. However, current data are consistent with the hypothesis that under physiological conditions, brain levels of IL1 are sufficient to activate, via AcPb, the p-Src pathway but are insufficient to activate the p-p38MAPK pathway. We posit that AcPb/p-Src dampens EEG delta power. Furthermore, after SD or injection of exogenous IL1, IL1 levels reach sufficient levels to activate, via AcP, p-p38MAPK, and, in turn, the AcP/p-p38MAPK enhances delta power. Thus, under physiological conditions, the role of AcPb-p-Src signaling would dominate, and mice lacking AcPb, i.e., both AcP KO and AcPb KO mice, would have higher delta power (Fig. 1). In contrast, during pathological and developmental conditions, including after SD, or excessive neuronal activation with higher IL1 levels, or during brain development when IL1 levels are high (17, 28, 29, 46), the p-p38MAPK pathway would dominate. Injection of exogenous low doses of IL1 in vivo promote EEG delta power, suggesting both p-Src and p-p38MAPK pathways are activated (38). Furthermore, low doses of IL1 promote NREMS whether given during light or dark hours. If high doses are given, IL1 inhibits sleep whether in day or night hours. How those observations relate to phosphorylation of either Src or p38MAPK and their time of day variations in the brain is unknown. However, it seems likely that the promotion of sleep by low IL1 doses is mediated via p-Src, e.g., p-Src is present at ZT4 during the sleep period but much lower at ZT16, which is in the middle of the active period. In contrast, high IL1 doses inhibit sleep via p-p38MAPK, which is present during the active phase at ZT16 and lower during the sleep (light) phase (Fig. 8).
There are, however, additional IL1 signaling pathways, e.g., NFkB also plays a role in sleep (2, 26). Although IL1-NFkB signaling enhances both sleep and delta power (26), in the current study we focused on IL1-mediated phosphorylation of Src and p38MAPK because they involve neurons, whereas IL1-NFkB signaling is astrocytic (14). Furthermore, Src family kinases affect multiple brain processes that in turn affect sleep. They include regulation of excitatory and inhibitory transmission, learning, memory, depressive behavior, and responses to alcohol (34). MAPKs are also implicated in sleep regulation, especially as related to sleep apnea hypoxia (4). In addition, IL1 is part of a much larger cytokine network involved in sleep regulation, although to date only extensive evidence exists for IL1 and TNFα in sleep regulation (15, 19, 33). Other sleep-promoting cytokines include IL2, IL6, IL8, IL15, IL18, acidic fibroblast growth factor, and interferons (15, 19, 21, 23). In contrast, other cytokines inhibit NREMS, including the IL1 receptor antagonist, IL4, IL10, IL13, insulin-like growth factor, and the soluble IL1 and TNF receptors (15, 19, 33, 48, 50). Antisomnogenic cytokines, e.g., IL4, IL13, and IL37, act in part by inhibiting production of prosomnogenic cytokines (23). Regardless of such complexities, current results indicate a major role for the IL1 family of molecules in sleep regulation.
GRANTS
This work was supported by National Institutes of Health Grant NS-025378.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.K. conceived and designed research; J.N., C.M.G., C.D.-A., R.E., and P.T. performed experiments; J.N., C.M.G., C.D.-A., R.E., K.M.S.K., and P.T. analyzed data; J.N., C.D.-A., and J.M.K. interpreted results of experiments; J.N., C.M.G., C.D.-A., and R.E. prepared figures; J.N. and C.D.-A. drafted manuscript; C.M.G., K.M.S.K., and J.M.K. edited and revised manuscript; J.N., C.M.G., C.D.-A., R.E., P.T., and J.M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dirk Smith, Insight BioConsulting, LLC, for advice on this project. We also thank Dr. Charles A. Dinarello, University of Colorado-Denver, for comments and for long-term collaboration in the IL1 sleep project. We thank Dr. Mark Opp, University of Colorado-Boulder, who also has been a long-term IL1 sleep project collaborator and a Co-Investigator in NS025378.
REFERENCES
- 1.Alam MN, McGinty D, Bashir T, Kumar S, Imeri L, Opp MR, Szymusiak R. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur J Neurosci 20: 207–216, 2004. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 1a.Alue F, Bail M, Jephthah-Ochola J, Carayanniotis K, Gorczynski R, Moldofsky H. Sleep and cerebrospinal fluid interleukin-1-like activity in the cat. Int J Neurosci 42: 179–183, 1988. doi: 10.3109/00207458808991595. [DOI] [PubMed] [Google Scholar]
- 2.Brandt JA, Churchill L, Rehman A, Ellis G, Mémet S, Israël A, Krueger JM. Sleep deprivation increases the activation of nuclear factor kappa B in lateral hypothalamic cells. Brain Res 1004: 91–97, 2004. doi: 10.1016/j.brainres.2003.11.079. [DOI] [PubMed] [Google Scholar]
- 3.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci USA 104: 12843–12848, 2007. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuang LP, Chen NH, Lin SW, Chang YL, Liao HR, Lin YS, Chao IJ, Lin Y, Pang JH. Increased C-C chemokine receptor 2 gene expression in monocytes of severe obstructive sleep apnea patients and under intermittent hypoxia. PLoS One 9: e113304, 2014. doi: 10.1371/journal.pone.0113304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon? J Clin Sleep Med 7, Suppl: S16–S18, 2011. doi: 10.5664/jcsm.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis CJ, Dunbrasky D, Oonk M, Taishi P, Opp MR, Krueger JM. The neuron-specific interleukin-1 receptor accessory protein is required for homeostatic sleep and sleep responses to influenza viral challenge in mice. Brain Behav Immun 47: 35–43, 2015. doi: 10.1016/j.bbi.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Sarro G, Gareri P, Sinopoli VA, David E, Rotiroti D. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci 60: 555–564, 1997. doi: 10.1016/S0024-3205(96)00692-3. [DOI] [PubMed] [Google Scholar]
- 8.Fang J, Wang Y, Krueger JM. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol Regul Integr Physiol 274: R655–R660, 1998. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- 9.Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol 275: R1127–R1137, 1998. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- 10.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity 39: 1003–1018, 2013. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grønli J, Meerlo P, Pedersen TT, Pallesen S, Skrede S, Marti AR, Wisor JP, Murison R, Henriksen TE, Rempe MJ, Mrdalj J. A rodent model of night-shift work induces short-term and enduring sleep and electroencephalographic disturbances. J Biol Rhythms 32: 48–63, 2017. doi: 10.1177/0748730416675460. [DOI] [PubMed] [Google Scholar]
- 12.Hallett H, Churchill L, Taishi P, De A, Krueger JM. Whisker stimulation increases expression of nerve growth factor- and interleukin-1beta-immunoreactivity in the rat somatosensory cortex. Brain Res 1333: 48–56, 2010. doi: 10.1016/j.brainres.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen MK, Taishi P, Chen Z, Krueger JM. Cafeteria feeding induces interleukin-1beta mRNA expression in rat liver and brain. Am J Physiol Regul Integr Physiol 274: R1734–R1739, 1998. doi: 10.1152/ajpregu.1998.274.6.R1734. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Smith DE, Ibáñez-Sandoval O, Sims JE, Friedman WJ. Neuron-specific effects of interleukin-1β are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci 31: 18048–18059, 2011. doi: 10.1523/JNEUROSCI.4067-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci 10: 199–210, 2009. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingiosi AM, Raymond RM Jr, Pavlova MN, Opp MR. Selective contributions of neuronal and astroglial interleukin-1 receptor 1 to the regulation of sleep. Brain Behav Immun 48: 244–257, 2015. doi: 10.1016/j.bbi.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Jewett KA, Taishi P, Sengupta P, Roy S, Davis CJ, Krueger JM. Tumor necrosis factor enhances the sleep-like state and electrical stimulation induces a wake-like state in co-cultures of neurons and glia. Eur J Neurosci 42: 2078–2090, 2015. doi: 10.1111/ejn.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapsimalis F, Richardson G, Opp MR, Kryger M. Cytokines and normal sleep. Curr Opin Pulm Med 11: 481–484, 2005. doi: 10.1097/01.mcp.0000183062.98665.6b. [DOI] [PubMed] [Google Scholar]
- 19.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des 14: 3408–3416, 2008. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krueger JM. Translation of brain activity into sleep. Hirosaki Igaku 63, Suppl: S1–S16, 2012. [PMC free article] [PubMed] [Google Scholar]
- 21.Krueger JM, Huang YH, Rector DM, Buysse DJ. Sleep: a synchrony of cell activity-driven small network states. Eur J Neurosci 38: 2199–2209, 2013. doi: 10.1111/ejn.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krueger JM, Rector DM, Churchill L. Sleep and cytokines. Sleep Med Clin 2: 161–169, 2007. doi: 10.1016/j.jsmc.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci 9: 910–919, 2008. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krueger JM, Taishi P, De A, Davis CJ, Winters BD, Clinton J, Szentirmai E, Zielinski MR. ATP and the purine type 2 X7 receptor affect sleep. J Appl Physiol (1985) 109: 1318–1327, 2010. doi: 10.1152/japplphysiol.00586.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol Regul Integr Physiol 246: R994–R999, 1984. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- 26.Kubota T, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB inhibitor peptide inhibits spontaneous and interleukin-1beta-induced sleep. Am J Physiol Regul Integr Comp Physiol 279: R404–R413, 2000. doi: 10.1152/ajpregu.2000.279.2.R404. [DOI] [PubMed] [Google Scholar]
- 28.Ma L, Li XW, Zhang SJ, Yang F, Zhu GM, Yuan XB, Jiang W. Interleukin-1 beta guides the migration of cortical neurons. J Neuroinflammation 11: 114, 2014. doi: 10.1186/1742-2094-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackiewicz M, Sollars PJ, Ogilvie MD, Pack AI. Modulation of IL-1 beta gene expression in the rat CNS during sleep deprivation. Neuroreport 7: 529–533, 1996. doi: 10.1097/00001756-199601310-00037. [DOI] [PubMed] [Google Scholar]
- 30.Manfridi A, Brambilla D, Bianchi S, Mariotti M, Opp MR, Imeri L. Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci 18: 1041–1049, 2003. doi: 10.1046/j.1460-9568.2003.02836.x. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci 18: 2239–2246, 1998. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obál F Jr, Garcia-Garcia F, Kacsóh B, Taishi P, Bohnet S, Horseman ND, Krueger JM. Rapid eye movement sleep is reduced in prolactin-deficient mice. J Neurosci 25: 10282–10289, 2005. doi: 10.1523/JNEUROSCI.2572-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obal F Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci 8: d520–d550, 2003. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 34.Ohnishi H, Murata Y, Okazawa H, Matozaki T. Src family kinases: modulators of neurotransmitter receptor function and behavior. Trends Neurosci 34: 629–637, 2011. doi: 10.1016/j.tins.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Opp MR, Krueger JM. Anti-interleukin-1 beta reduces sleep and sleep rebound after sleep deprivation in rats. Am J Physiol 266: R688–R695, 1994. doi: 10.1152/ajpregu.1994.266.3.R688. [DOI] [PubMed] [Google Scholar]
- 36.Opp MR, Krueger JM. Interleukin 1-receptor antagonist blocks interleukin 1-induced sleep and fever. Am J Physiol 260: R453–R457, 1991. doi: 10.1152/ajpregu.1991.260.2.R453. [DOI] [PubMed] [Google Scholar]
- 37.Opp MR, Krueger JM. Interleukin-1 is involved in responses to sleep deprivation in the rabbit. Brain Res 639: 57–65, 1994. doi: 10.1016/0006-8993(94)91764-7. [DOI] [PubMed] [Google Scholar]
- 38.Opp MR, Obal F Jr, Krueger JM. Interleukin 1 alters rat sleep: temporal and dose-related effects. Am J Physiol 260: R52–R58, 1991. doi: 10.1152/ajpregu.1991.260.1.R52. [DOI] [PubMed] [Google Scholar]
- 39.Roky R, Obál F Jr, Valatx JL, Bredow S, Fang J, Pagano LP, Krueger JM. Prolactin and rapid eye movement sleep regulation. Sleep 18: 536–542, 1995. [PubMed] [Google Scholar]
- 40.Skurlova M, Stofkova A, Jurcovicova J. Exogenous IL-1beta induces its own expression, but not that of IL-6 in the hypothalamus and activates HPA axis and prolactin release. Endocr Regul 40: 125–128, 2006. [PubMed] [Google Scholar]
- 41.Smith DE, Hanna R, Della Friend, Moore H, Chen H, Farese AM, MacVittie TJ, Virca GD, Sims JE. The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity 18: 87–96, 2003. doi: 10.1016/S1074-7613(02)00514-9. [DOI] [PubMed] [Google Scholar]
- 42.Smith DE, Lipsky BP, Russell C, Ketchem RR, Kirchner J, Hensley K, Huang Y, Friedman WJ, Boissonneault V, Plante MM, Rivest S, Sims JE. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity 30: 817–831, 2009. doi: 10.1016/j.immuni.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Susić V, Totić S. “Recovery” function of sleep: effects of purified human interleukin-1 on the sleep and febrile response of cats. Metab Brain Dis 4: 73–80, 1989. doi: 10.1007/BF00999497. [DOI] [PubMed] [Google Scholar]
- 44.Szentirmai E, Kapás L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol Regul Integr Comp Physiol 292: R575–R585, 2007. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- 45.Taishi P, Bredow S, Guha-Thakurta N, Obál F Jr, Krueger JM. Diurnal variations of interleukin-1 beta mRNA and beta-actin mRNA in rat brain. J Neuroimmunol 75: 69–74, 1997. doi: 10.1016/S0165-5728(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 46.Taishi P, Chen Z, Obál F Jr, Hansen MK, Zhang J, Fang J, Krueger JM. Sleep-associated changes in interleukin-1beta mRNA in the brain. J Interferon Cytokine Res 18: 793–798, 1998. doi: 10.1089/jir.1998.18.793. [DOI] [PubMed] [Google Scholar]
- 47.Taishi P, Davis CJ, Bayomy O, Zielinski MR, Liao F, Clinton JM, Smith DE, Krueger JM. Brain-specific interleukin-1 receptor accessory protein in sleep regulation. J Appl Physiol (1985) 112: 1015–1022, 2012. doi: 10.1152/japplphysiol.01307.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi S, Fang J, Kapás L, Wang Y, Krueger JM. Inhibition of brain interleukin-1 attenuates sleep rebound after sleep deprivation in rabbits. Am J Physiol 273: R677–R682, 1997. doi: 10.1152/ajpregu.1997.273.2.R677. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi S, Kapás L, Fang J, Krueger JM. Somnogenic relationships between tumor necrosis factor and interleukin-1. Am J Physiol 276: R1132–R1140, 1999. doi: 10.1152/ajpregu.1999.276.4.R1132. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi SK, Hansen M, Zhang J, Seyer JM, Krueger JM. An interleukin-1 (IL-1) soluble receptor fragment inhibits IL-1 beta-induced sleep and non-rapid-eye-movement-sleep rebound after sleep deprivation in rabbits. Sleep Res 24A: 457, 1996. [Google Scholar]
- 51.Tobler I, Borbély AA, Schwyzer M, Fontana A. Interleukin-1 derived from astrocytes enhances slow wave activity in sleep EEG of the rat. Eur J Pharmacol 104: 191–192, 1984. doi: 10.1016/0014-2999(84)90391-1. [DOI] [PubMed] [Google Scholar]
- 52.Yasuda K, Churchill L, Yasuda T, Blindheim K, Falter M, Krueger JM. Unilateral cortical application of interleukin-1beta (IL1beta) induces asymmetry in fos, IL1beta and nerve growth factor immunoreactivity: implications for sleep regulation. Brain Res 1131: 44–59, 2007. doi: 10.1016/j.brainres.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 53.Yasuda T, Yoshida H, Garcia-Garcia F, Kay D, Krueger JM. Interleukin-1beta has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep 28: 177–186, 2005. doi: 10.1093/sleep/28.2.177. [DOI] [PubMed] [Google Scholar]
- 54.Zielinski MR, Taishi P, Clinton JM, Krueger JM. 5′-Ectonucleotidase-knockout mice lack non-REM sleep responses to sleep deprivation. Eur J Neurosci 35: 1789–1798, 2012. doi: 10.1111/j.1460-9568.2012.08112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]