Abstract
Protein arginine methyltransferases (PRMTs) are a family of enzymes that catalyze the methylation of arginine residues on target proteins. While dysregulation of PRMTs has been documented in a number of the most prevalent diseases, our understanding of PRMT biology in human skeletal muscle is limited. This study served to address this knowledge gap by exploring PRMT expression and function in human skeletal muscle in vivo and characterizing PRMT biology in response to acute and chronic stimuli for muscle plasticity. Fourteen untrained, healthy men performed one session of sprint interval exercise (SIE) before completing four bouts of SIE per week for 6 wk as part of a sprint interval training (SIT) program. Throughout this time course, multiple muscle biopsies were collected. We found that at basal, resting conditions PRMT1, PRMT4, PRMT5, and PRMT7 were the most abundantly expressed PRMT mRNAs in human quadriceps muscle. Additionally, the broad subcellular distribution pattern of PRMTs suggests methyltransferase activity throughout human myofibers. A spectrum of PRMT-specific inductions, and decrements, in expression and activity were observed in response to acute and chronic cues for muscle plasticity. In conclusion, our findings demonstrate that PRMTs are present and active in human skeletal muscle in vivo and that there are distinct, enzyme-specific responses and adaptations in PRMT biology to acute and chronic stimuli for muscle plasticity. This work advances our understanding of this critical family of enzymes in humans.
NEW & NOTEWORTHY This is the first report of protein arginine methyltransferase (PRMT) biology in human skeletal muscle in vivo. We observed that PRMT1, -4, -5, and -7 were the most abundant PRMT mRNAs in human muscle and that PRMT proteins exhibited a broad subcellular localization that included myonuclear, cytosolic, and sarcolemmal compartments. Acute exercise and chronic training evoked PRMT-specific alterations in expression and activity. This study reveals a hitherto unknown complexity to PRMT biology in human muscle.
Keywords: exercise, fiber types, histones, in vivo
INTRODUCTION
Protein arginine methyltransferases (PRMTs) are a family of enzymes that catalyze the methylation of arginine residues on target proteins. As the occurrence of arginine methylation happens on par with that of phosphorylation and ubiquitination (44), PRMTs have emerged as critical regulators of a variety of functions including signal transduction, transcriptional activation, and repression (1, 2, 7, 53, 66). Type 1, 2, and 3 PRMTs are able to catalyze the addition of a single methyl group on a target protein to form the monomethylarginine (MMA) mark. Type 1 and 2 PRMTs perform a second methylation step, which produces the asymmetric dimethylarginine (ADMA) or symmetric dimethylarginine (SDMA) marks, respectively (1, 2, 29, 66). PRMT1, PRMT4 (also known as coactivator-associated arginine methyltransferase 1; CARM1), and PRMT5 account for the majority of cellular arginine methylation reactions (1), with PRMT1 alone being responsible for ~85% (1, 67, 74), which partly accounts for why these enzymes are the most widely studied PRMT family members. Furthermore, genetic deletion of PRMT1, CARM1, or PRMT5 leads to lethal phenotypes in mice (40, 56, 68, 76, 78). Together, these data demonstrate the vital role that these proteins play in proteome regulation, cell physiology, and survival.
An emerging area of research highlights the importance of PRMTs in the maintenance and remodeling of skeletal muscle phenotype. Early studies performed in murine muscle or myogenic cell cultures (14, 18, 35, 39), as well as subsequent in vivo rodent experiments (49, 74), characterized the expression and function of PRMT1, CARM1, and PRMT5 in skeletal muscle and demonstrated critical roles for these enzymes in myogenesis, insulin signaling, and glucose metabolism. A more recent series of elegant studies revealed that muscle progenitor cell expression of PRMT1, CARM1, PRMT5, or PRMT7 were necessary for the full execution of the muscle-regenerative program in response to injury in mice (4, 5, 38, 79). Additional experiments employing similar transgenic approaches demonstrated that PRMT7 knockout animals show a shift toward a faster, more glycolytic myogenic program, which indicates that the enzyme functions to maintain some aspects of skeletal muscle phenotype (36). Using physiological-based interventions, we recently observed that PRMT expression and function are altered during various conditions of skeletal muscle plasticity (65, 71). For example, Stouth et al. (65) demonstrated that skeletal muscle PRMT1, CARM1, and PRMT5 were modified following neurogenic muscle disuse in mice, which included the identification of a novel PRMT1-AMP-activated protein kinase (AMPK)-peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) signaling axis. This is notable since AMPK and PGC-1α govern neuromuscular determination, maintenance, and plasticity (20, 42). Furthermore, vanLieshout and colleagues (71) found that PRMT gene expression and global enzyme activities were muscle specific and also observed physical interactions between PRMT1-PGC-1α and CARM1-PGC-1α in mouse skeletal muscle during conditions of acute muscle plasticity elicited by exercise. Thus PRMTs are clearly important for the maintenance and plasticity of skeletal muscle in rodents.
It has been extensively documented that PRMT expression and function are dysregulated in some of the most prevalent human diseases, such as cancer, cardiovascular disease, and diabetes (11, 66). For example, employing a Bayesian network approach, Porta et al. (57) recently observed increased CARM1 expression in blood samples taken from individuals with type 2 diabetes, which suggests that the enzyme may impact the development or progression of the chronic disease in humans in vivo. In contrast, our understanding of PRMT biology in human skeletal muscle is limited, and to our knowledge PRMT biology in human skeletal muscle in vivo has not been previously described. Addressing this knowledge gap may aid in furthering our understanding of the molecular mechanisms that regulate muscle phenotype in humans. Therefore, the purpose of this study was twofold: first, to examine PRMT expression and function in human skeletal muscle and second, to characterize PRMT biology in response to acute and chronic stimuli for muscle plasticity in humans. We hypothesized that 1) PRMTs are expressed in human skeletal muscle in vivo at enzyme-specific levels; 2) acute exercise will alter PRMT activity; and 3) exercise training will evoke an adaptive response in PRMT content and function.
METHODS
Participants.
Fourteen healthy men volunteered to participate in the study. Participant characteristics can be found in Table 1. For at least 6 mo before study enrollment, no participant was involved in more than 3 h of aerobic exercise per week or involved in any structured training program. A preliminary screening session for each participant was held where they were briefed on the study, provided informed consent, and had their height and weight recorded. All experimental procedures performed were approved by the Health Sciences Human Research Ethics Board at Queen’s University, Kingston, ON, and conformed to the Declaration of Helsinki. Both verbal and written explanation of the experiment protocol and associated risks was provided to all participants before obtaining written informed consent. Participant characteristics have been previously published (8, 21).
Table 1.
Participant characteristics
| Characteristic | Value |
|---|---|
| Age, yr | 22.0 ± 2.4 |
| Height, cm | 178.1 ± 5.9 |
| Weight, kg | 83.6 ± 17.8 |
| BMI, kg/m2 | 26.3 ± 5.5 |
Values are means ± SD. BMI, body mass index.
Experimental design.
Participants reported to the laboratory following an overnight fast for ~12 h after consuming a standardized dinner {Stouffer’s Sauté Sensations Country Beef Pot Roast [540 kcal; 56 g carbohydrates (CHO), 20 g fat, 14 g protein]}, [Dole Fruit Cup (160 kcal; 29 g CHO, 3.5 g fat, 2 g protein)], and 500 mL of 2% milk (260 kcal; 24 g CHO, 10 g fat, 18 g protein) the night before. Upon arrival at the laboratory, participants were fed breakfast [plain bagel (190 kcal; 1 g fat, 36 g CHO, 7 g protein)] with peanut butter (110 kcal; 10 g fat, 4 g CHO, 4 g protein), and 200 mL of apple juice (90 kcal; 22 g CHO, 0 g fat, 0 g protein). One hour after completing the meal, a resting muscle biopsy (PRE) was taken. Immediately following the procedure, participants completed one session of sprint interval exercise (SIE) before resting for 3 h and having a second muscle biopsy taken (3HR) from a separate incision site on the same leg as the first biopsy. SIE consisted of 8 × 20-s intervals at ~170% of V̇o2peak work rate separated by 10 s of recovery for a total of 4 min (61). The participants then completed four bouts of SIE per week for 6 wk as part of a sprint interval training (SIT) exercise program. In total, participants completed 22 training sessions. Before each session, participants warmed up with loadless cycling at a cadence of their choosing for 1 min. It is important to note that week 3 only had two training session due to the midtraining (MID) V̇o2peak test and biopsy. This MID muscle biopsy was taken following 2 wk (i.e., 8 sessions) of SIT and was collected ~72 h (range: 65–72 h) after the completion of the previous exercise session, as per the protocol used for the PRE biopsy. The MID V̇o2peak test was completed ~24 h after the biopsy. In the following training weeks 3–6, the targeted work rate was increased to 180%. Approximately 72 h (range: 64–74 h) after the last training session, a posttraining (POST) biopsy was obtained, following the same protocol as the PRE and MID biopsies. All of this was followed by a posttraining V̇o2peak test ~24 h after the POST biopsy. Descriptions of all physiological testing and training and muscle biopsy procedures have been published previously (8, 21).
RNA extraction and quantitative real-time PCR.
RNA extraction and real-time PCR were performed as described previously (8, 21) on PRE and 3HR samples. RNA was extracted using a modified version of the single-step method by guanidinium thiocyanate-phenol-chloroform extraction (17) and quantified spectrophotometrically at 260 nm using a Take3 Plate (Biotek, Winooski, VT). Protein contamination was assessed by measuring absorbance at 280 nm. Resulting RNA was reverse transcribed using the QuantiTect Reverse Transciption Kit (Qiagen, Mississauga, ON), and samples were run in duplicate reactions comprised of cDNA, primers, and GoTaq PCR Master Mix containing SYBR Green (Promega, Madison, WI). The primers used for this study can be found in Table 2. Primers were designed using the NIH Primer-BLAST online tool. All RNA data are expressed relative to TATA-binding protein (TBP), which exhibited no difference in the raw CT values observed between PRE and 3HR samples.
Table 2.
PCR primers used in the study
| Primer | Sequence |
|---|---|
| PRMT1 | |
| Forward | GCCACCTTGGCTAATGGGAT |
| Reverse | AGTTGCGGTAAGTGAGGGTG |
| PRMT2 | |
| Forward | CACGTGGCAGGATGAAGAGT |
| Reverse | CTGCAGGATGACACTGTGGT |
| PRMT3 | |
| Forward | GAATTGCCACAACAGGGTCG |
| Reverse | TGAGGGTCACGGTGAGAGAA |
| PRMT4 | |
| Forward | AAGGTGGAGGAGGTGTCACT |
| Reverse | AGCTGTTCATCCGTGAAGGG |
| PRMT5 | |
| Forward | CACCAGAGGCTCATCTTCCG |
| Reverse | GCATTAGGTGGAGGACGGTT |
| PRMT6 | |
| Forward | ACTCGGAGATCGTTGTGCAG |
| Reverse | CAGGGAAGGTCACCTGGAAC |
| PRMT7 | |
| Forward | GCCAACATCCTGGTCACAGA |
| Reverse | AGCTTGTTCCACGACCACAT |
| PRMT9 | |
| Forward | TGTGCTGATGTTTGGGTTGC |
| Reverse | AGAGTTCCACTGGCTTGCTT |
| TBP | |
| Forward | AGACGAGTTCCAGCGCAAGG |
| Reverse | GCGTAAGGTGGCAGGCTGTT |
PRMT, protein arginine methyltransferase; TBP, TATA-binding protein.
Muscle protein extraction.
A muscle biopsy from the vastus lateralis (QUAD) was added to a sample tube with a predetermined volume of RIPA buffer (Sigma-Aldrich, St. Louis, MO; 20 μL of RIPA/1 mg muscle weight) supplemented with a protease and phosphatase inhibitor cocktail (Roche, Laval, PQ). Two stainless steel ball bearings were added to the tube (1 before the muscle was added, and 1 after). Care was taken to ensure that the samples were kept on ice throughout the entire extraction process. The tube was loaded into a precooled Phillips Homogenizer (Qiagen, Toronto, ON) and run for 3–5 bouts of 40 s at a frequency of 20.0 1/s. Samples were spun, and the resulting supernates were collected. The bicinchoninic assay (BCA; Thermo Fisher Scientific, BioTek, Toronto, ON) was performed to determine protein concentration.
Western blotting.
Protein (20–40 μg) was loaded into each lane of 10–12.5% polyacrylamide gels and subjected to SDS-PAGE, before being transferred to nitrocellulose membranes. After transfer, Ponceau S solution (Sigma, Darmstadt, Germany) was used to verify equal loading across all lanes (59). The Ponceau solution was washed off with Tris-buffered saline with 1% Tween 20 (TBS-T). Membranes were then blocked with 5% milk for 60 min before being washed for 5 × 3 min with TBS-T. Primary antibody dilutions were prepared in 5% milk or bovine serum albumin (BSA) as per the manufacturer’s recommendations. The following antibodies were used: PRMT1 (07–404, EMD Millipore, Etobicoke, ON, RRID:AB_310588); CARM1 (A300–421A, Bethyl Laboratories, Montgomery, TX, RRID:AB_420962); PRMT5 (07–405, EMD Millipore, RRID:AB_310589), PRMT7 (sc-376077, Santa Cruz, Dallas, TX, RRID:AB_10990266); PRMT9 (clone 128–29–1, EMD Millipore, RRID:AB_2801509); MMA (8015, Cell Signal, Whitby, ON, RRID:AB_2799401); ADMAGAR (13522, Cell Signal, RRID:AB_2665370); CARM1 substrate [denoted as ADMA-5CARM1 (16), was a kind gift from Dr. Mark Bedford, MD Anderson Cancer Center, University of Texas]; SDMA (13222, Cell Signal, RRID:AB_2714013); histone 4 arginine 3 (H4R3; ab129231, Abcam, Toronto, ON, RRID:AB_2801527); H3R17 (ab8284, Abcam, RRID:AB_306434); H3R8 (ab130740, Abcam, RRID:AB_2801510); H3 (ab1791, Abcam, RRID:AB_302613); and H4 (ab10158, Abcam, RRID:AB_296888). MMA, ADMAGAR, and SDMA are pan-methylation antibodies that have been extensively used in previous literature (16, 19, 30, 48, 62, 65, 71). The ADMAGAR and SDMA antibodies primarily recognize arginine methylation at glycine- and arginine-rich (GAR) motifs. The ADMA-5CARM1 reagent preferentially recognizes pan-CARM1-marked substrates. Primary antibodies were applied overnight at 4°C with gentle shaking and washed off the following morning with 5 × 3-min washes in TBS-T. Appropriate horseradish peroxidase-linked secondary antibodies were applied for 2 h at room temperature followed by 5 × 3-min washes in TBS-T. Finally, enhanced chemiluminescence substrate (Bio-Rad, Mississauga, ON) was applied to detect target proteins. Images were captured with the ChemiDoc MP Imaging System (Bio-Rad), and Image Laboratory (Bio-Rad) was employed for densitometry.
Immunofluorescence analysis.
The immunostaining procedure was carried out as described previously (20, 65). QUAD muscles stored in optimum cutting temperature compound from participants were sectioned on a cryostat (Thermo Fisher Scientific, Waltham, MA) into 5-μm slices. To obtain serial sections, an initial cut was placed on the respective PRMT slide while the next cut was placed on the myosin heavy chain (MHC) isoforms slide. PRMT slides were fixed with 4% paraformaldehyde (PFA) for 10 min. Following this, slides were washed in either 1% PBS (PRMT5) or PBS-T (PRMT1, CARM1) for 3 × 5 min. Triton X (0.1%) in 1% PBS was placed on PRMT5 slides for 10 min following this. All slides were then incubated in a blocking solution of 10% goat serum with 1% BSA for 90 min. Following another 3 × 5-min wash in either PBS or PBS-T, slides were incubated in primary antibodies. Protein expression and localization of PRMTs were examined by probing for dystrophin, to identify the sarcolemma, and PRMT antibodies as described above. The antibodies that we selected have previously been used and published for immunofluorescence techniques (34, 58, 60, 65, 73). Slides were incubated overnight at 4°C in a primary antibody solution targeting dystrophin (1:1,000, ab3149; Abcam). Following another 3 × 5-min wash in PBS or PBS-T, slides were once again incubated for 12 h at 4°C in either a PRMT1 (1:250), CARM1 (1:250), or PRMT5 (1:750) primary antibody solution (antibodies listed above). After primary antibody incubations, slides were washed for 3 × 5 min in PBS or PBS-T. Alexa Fluor-conjugated secondary antibodies (Thermo Fisher Scientific, Burlington, ON) were applied to samples for 2 h at room temperature (Alexa Fluor 594 for dystrophin and Alexa Fluor 647 for PRMTs), which was followed by another 3 × 5-min wash in PBS or PBS-T. 4′6-Diamidino-2-phenylindole dihydrochloride (DAPI; DI306; Thermo Fisher Scientific) was incubated for 10 min on slides to label myonuclei. Slides were then washed for 5 min in PBS or PBS-T, followed by a final wash for 5 min in PBS. After slides were dried, fluorescent mounting media (DAKO North American, Carpentaria, CA) was applied, and the slide was mounted with a coverslip.
Staining of the serial MHC isoforms slides were performed as previously described (6) using primary antibodies against MHC I (BA-F8, RRID:AB_10572253), MHC I and IIa (BF-35, RRID:AB_2274680), and MHC IIx (6H1, RRID:AB_1157897) (Developmental Studies Hybridoma Bank, Iowa City, IA), followed by isotype-specific fluorescent secondary antibodies (Invitrogen, Carlsbad, CA). This allowed for the identification of type I (1853872, Invitrogen), type IIa and I (1820808, Invitrogen), and type IIx (1828021, Invitrogen) fibers. All slides were viewed with the Nikon Eclipse Ti Microscope (Nikon Instruments, Mississauga, ON), equipped with a high-resolution Photometrics CoolSNAP HQ2 fluorescent camera. Images were captured and analyzed using the Nikon NIS Elements AR 3.2 software. All images were obtained with the ×20 objective.
PRMT localization was examined by creating three square regions of interest (ROIs), each representing 10% of the area of the section, thereby representing 30% of the total cross-sectional area of the muscle sample. A threshold was applied to create a binary layer to remove background fluorescence for both DAPI and dystrophin. For PRMT myonuclear localization, the percentage of PRMT measured by binary sum intensity/ROI area was taken at all three ROIs with an overlay of DAPI. Membrane localization was done similarly but with an overlay of dystrophin. Any remaining PRMT fluorescence was considered cytosolic. All three ROI values were then averaged, and PRMT localization was determined as the percentage of total cellular PRMT fluorescence. Determination of PRMT expression in myofibers of differing MHC composition was done by overlaying serial sections of MHC stains and PRMTs. A minimum of 50 myofibers was identified in the MHC stain (combination of type I and IIa), and the type of fiber was marked on the corresponding PRMT image. Binary layers were created for each of the fiber types, and binary sum intensity/binary area calculations were performed on the PRMT channel to determine PRMT levels in each MHC fiber type.
Statistical analysis.
Differences between group means were evaluated using a one-way ANOVA, two-way ANOVA, or Student’s t test, as appropriate. Any significant interactions or main effects were further analyzed using a Bonferroni post hoc analysis. Statistical tests were performed on the raw data. Statistical differences were considered significant if P < 0.05. Data in graphical summaries are means ± SD.
RESULTS
PRMT biology in human skeletal muscle in vivo.
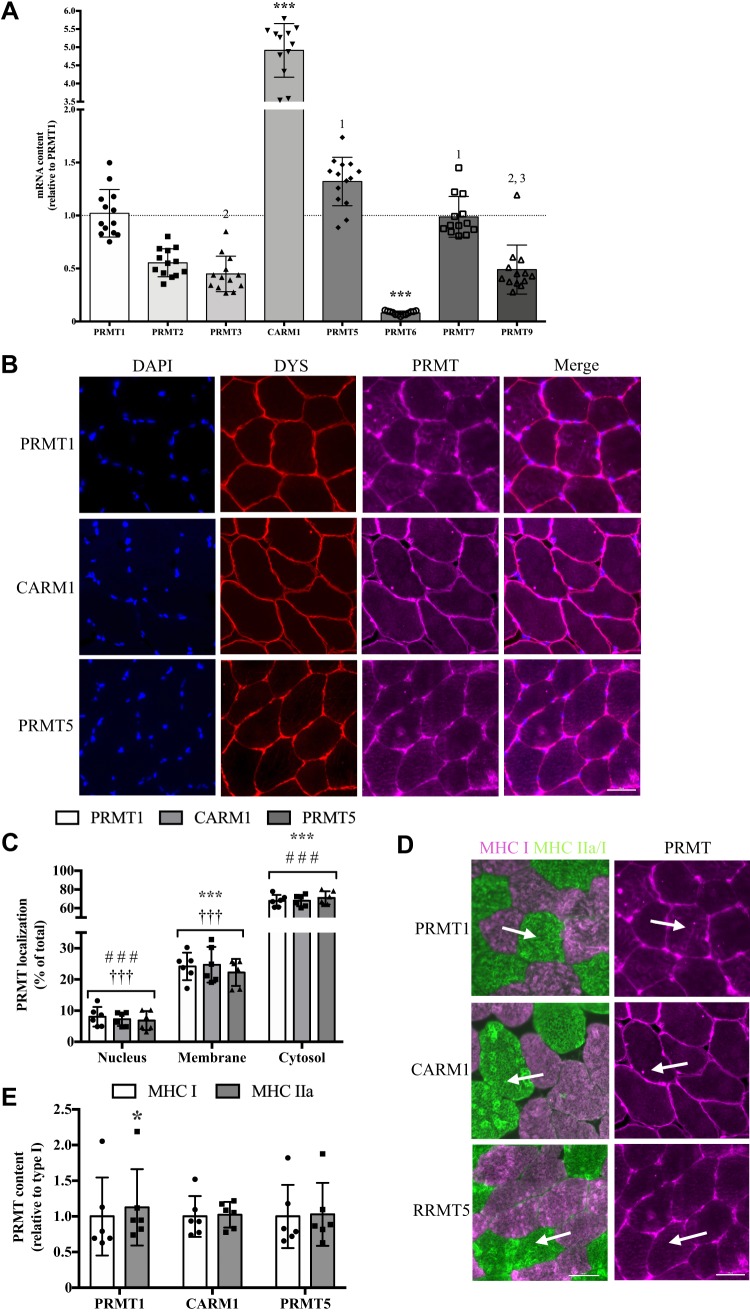
To investigate PRMT enzyme-specific amounts in human skeletal muscle in vivo, we analyzed the mRNA content of PRMT1–7 and PRMT9. It has previously been shown that PRMT8 has a narrow tissue expression, being limited mainly to the brain (1, 31, 45, 64), and as such PRMT8 was excluded from this analysis. CARM1 was expressed to the greatest extent in human quadriceps skeletal muscle, which was biopsied from young, healthy men, with levels ~5-fold higher (P < 0.001) compared with PRMT1, PRMT5, and PRMT7 transcripts, and ~7- to 60-fold greater vs. PRMT2, PRMT3, PRMT6, and PRMT9 mRNAs (Fig. 1A). Next, we examined the myocellular localization of PRMTs using an immunofluorescence microscopy approach. PRMT colocalization with dystrophin was used to identify the presence of the enzyme at the muscle membrane, while PRMT and DAPI colocalization marked its presence in the myonuclear compartment. Any extranuclear PRMT staining within the dystrophin boundary was considered cytosolic expression (20). PRMT1, CARM1, and PRMT5 were expressed in each cellular compartment, with the majority of PRMTs (~70% of total expression) found in the cytosol, and significantly lower (P < 0.001) amounts found at the sarcolemma (~25% of total expression) and within myonuclei (~5% of total expression) (Fig. 1, B and C). Finally, serial sections probed with an MHC antibody cocktail (6) were used to investigate the expression of PRMTs in a fiber type-specific manner. At the PRE time point, we found that PRMT1 was 15% higher (P < 0.05) in MHC type IIa-positive fibers compared with type I myofibers (Fig. 1, D and E). CARM1 and PRMT5 content was similar between type I and IIa fibers at the PRE time point (Fig. 1, D and E).
Fig. 1.
Human skeletal muscle protein arginine methyltransferase (PRMT) biology. A: graphical summary of PRMT mRNA content in human skeletal muscle. Results are displayed relative to PRMT1 levels. Data were analyzed using a 1-way ANOVA. ***P < 0.001 vs. all other PRMTs; number, P > 0.05 vs. the PRMT denoted; n = 13 for PRMT1, -2, -3, -6, -7, -9; n = 12 for CARM1; n = 14 for PRMT5. B: representative immunofluorescence images of human quadriceps muscle with stains for 6-diamidino-2-phenylindole dihydrochloride (DAPI) to identify myonuclei, dystrophin (DYS) to indicate the sarcolemma, specific PRMT, along with merged images. C: graphical summary of PRMT localization in the nucleus, at the muscle membrane, and within the cytosol. Analysis of data was completed using a 2-way ANOVA. ***P < 0.001 vs. nucleus. ###P < 0.001 vs. membrane. †††P < 0.01 vs. cytoplasm; n = 6 for all conditions. D: typical immunofluorescence images for serial sections of myosin heavy chains [MHC; I (pink) and IIa/I (green)] and PRMTs. The tip of the arrow identifies the same type IIa fiber in both images, while the base of the arrow depicts the same type I fiber. E: graphical summary of PRMT expression in type I and IIa myofibers. Data were analyzed using Student’s t tests. In all data summaries, bars indicate group means, points represent individual participant data, and error bars denote SD. Scale bars = 50 μM. *P < 0.05 vs. MHC I; n = 6 for all conditions.
PRMT mRNA expression after acute exercise.
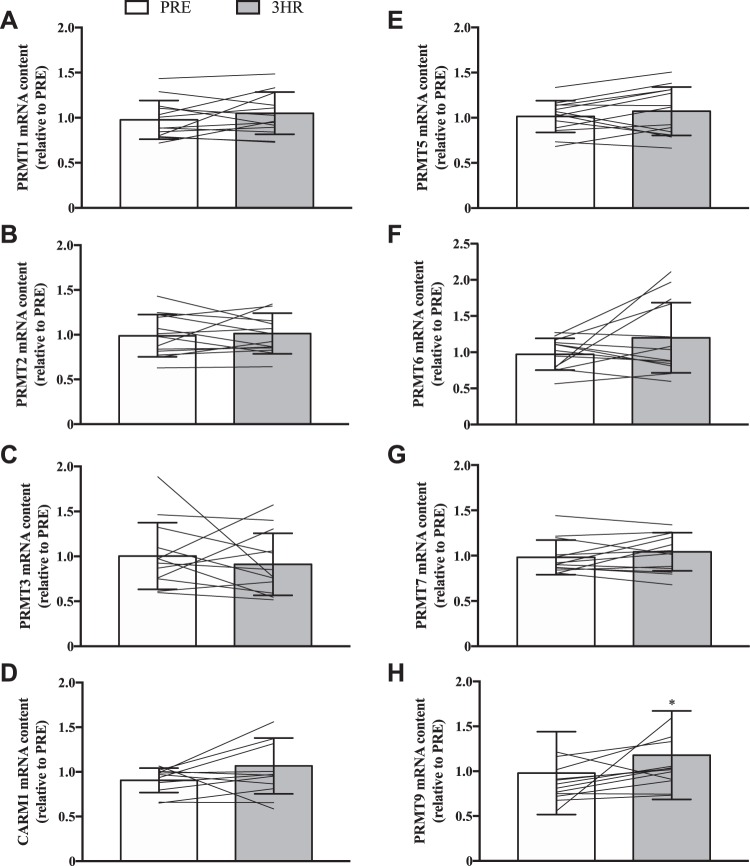
In an effort to comprehensively characterize PRMT gene expression and function in response to an acute stimulus for muscle plasticity, we first compared PRMT transcript levels PRE and 3HR after a single bout of SIE (Fig. 2, A–H). The majority of PRMT transcript levels were similar PRE vs. 3HR; however, PRMT9 mRNA increased significantly (+20%) at the 3HR time point (Fig. 2H).
Fig. 2.
PRMT mRNA levels in response to acute exercise. Graphical summaries of changes in PRMT1 (A), PRMT2 (B), PRMT3 (C), CARM1 (D), PRMT5 (E), PRMT6 (F), PRMT7 (G), and PRMT9 (H) mRNA levels following a single bout of sprint interval exercise (SIE). Data are displayed relative to levels before (PRE) a single bout of SIE. For A–H, bars are indicative of group means, while lines represent individual participant results, and error bars denote SD. Analysis of data was completed using Student’s t tests. *P < 0.05 vs. PRE; n = 13 for A, B, E, F, and G; n = 12 for C, and H; n = 11 for D.
PRMT protein expression and activity following acute exercise.
We next examined PRMT protein expression following SIE. We chose to focus on PRMT1, CARM1, PRMT5, and PRMT7 as we found these PRMTs to be the most abundantly expressed mRNAs in human skeletal muscle, as well as on PRMT9 as it was the only arginine methyltransferase with altered mRNA levels in response to acute exercise. Using previously published antibodies (4, 5, 38, 62, 71, 79), we found that the five enzymes are indeed present in human muscle (Fig. 3, A–F). The protein levels of PRMT1, CARM1, and PRMT5 all significantly increased (+15–50%) following an acute bout of SIE (Fig. 3, A–D), while PRMT7 and PRMT9 remained unchanged (Fig. 3, E and F). We then analyzed the content of skeletal muscle MMA, ADMA, and SDMA as these marks are indicative of PRMT enzymatic activity (1, 2, 65, 71). All PRMTs can catalyze the MMA reaction, while PRMT1 and CARM1 account for the majority of ADMA production, and PRMT5 proportionally dominates synthesis of SDMA (10, 19, 66). As PRMT1 preferentially methylates GAR motifs, we used an asymmetric dimethylation antibody that recognizes this site (ADMAGAR) and another, novel reagent to identify methylated arginines favored by CARM1 (ADMA-5CARM1) (3, 15, 16). Together, these methylation marks provide a measure of distinct PRMT1, CARM1, and PRMT5 enzymatic functions in vivo. MMA levels increased by +25% (P < 0.05) following SIE, whereas in contrast ADMAGAR, ADMA-5CARM1, and SDMA content were similar between PRE and 3HR time points (Fig. 4A).
Fig. 3.
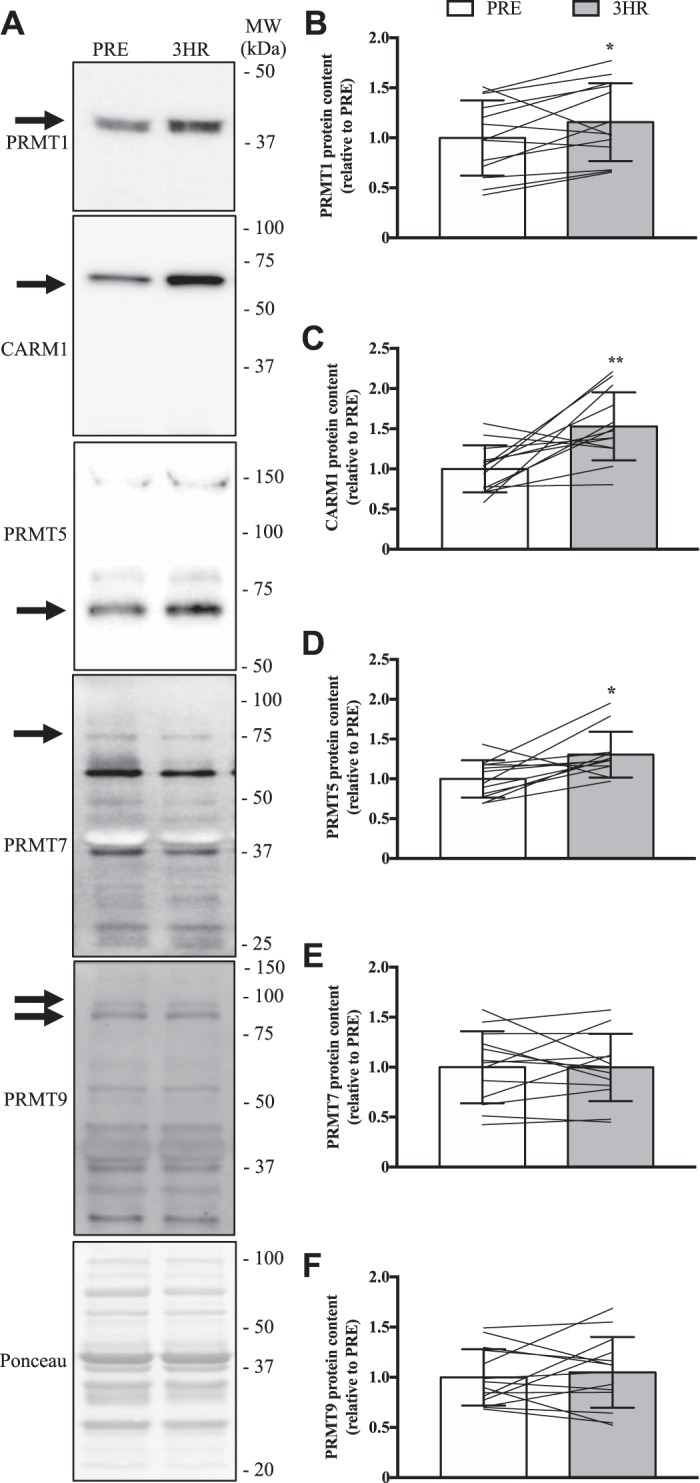
Acute exercise increases PRMT1, CARM1, and PRMT5 protein content. A: representative Western blots for PRMT1, CARM1, PRMT5, PRMT7, and PRMT9 from quadriceps muscle biopsies at PRE and 3 h (3HR) after an acute bout of SIE. The arrow on the left of each image denotes the band of interest, while the Ponceau stain at the bottom demonstrates equal loading between groups. Approximate molecular weights (MW) in kilodaltons (kDa) are indicated on the right of each image. Graphical summaries of PRMT1 (B), CARM1 (C), PRMT5 (D), PRMT7 (E), and PRMT9 (F) protein contents. Data are displayed as relative to PRE levels. Bars indicate group means, lines are individual participant data, and error bars denote SD. Data analysis was completed using Student’s t tests. *P < 0.05 vs. PRE. **P < 0.01 vs. PRE; n = 13 for B, C, and E; n = 11 for D; n = 14 for F.
Fig. 4.
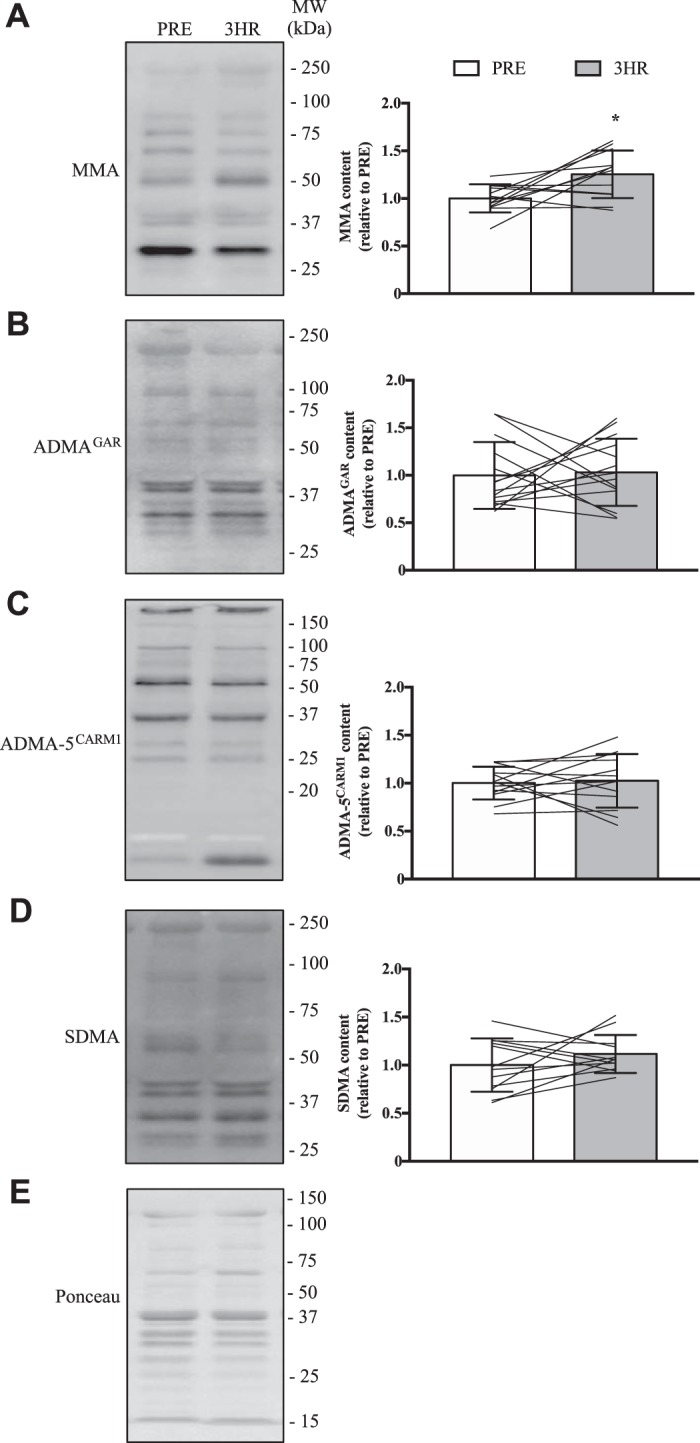
Global PRMT activity following acute exercise in human skeletal muscle. Typical immunoblots of monomethylarginine (MMA; A), glycine- and arginine-rich (GAR)-motif asymmetric dimethylarginine (ADMAGAR; B), CARM1 substrate (ADMA-5CARM1; C), and symmetric dimethylarginine (SDMA; D) marks in human quadriceps muscles at PRE and 3HR after SIE. Approximate MWs are indicated on the right of each image. Graphical summaries of the muscle MMA, ADMAGAR, ADMA-5CARM1, and SDMA content are on the right of each panel. E: an example blot of the Ponceau stain demonstrating equal loading. Bars in the graphical summaries represent group means, while lines are individual participant results, and error bars indicate SD. Analysis of data was completed using Student’s t tests. *P < 0.05 vs. PRE; n = 12 for A and D; n = 14 for B; n = 13 for C.
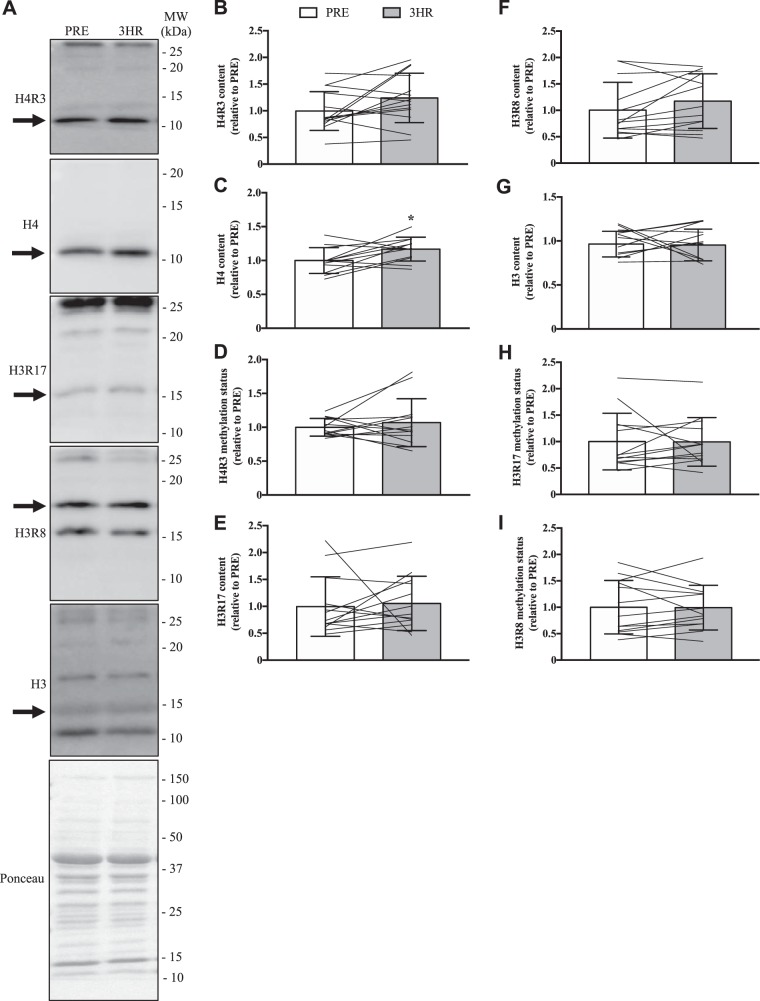
We then explored the methylation of specific PRMT targets. It is accepted that H3R17 bearing the ADMA modification (H3R17-ADMA) and H3R8-SDMA are marked exclusively by CARM1 and PRMT5, respectively (23, 75). Thus measurement of these specific methylated histone arginine residues affords a targeted and refined appraisal of PRMT enzymatic activities. While H4R3-ADMA can be marked by PRMT1, PRMT2, and PRMT6 (33, 43), the fact that PRMT2 catalytic activity of this histone mark is 800-fold less than that of PRMT1 (43), combined with the extremely low expression of PRMT6 mRNA that we observed in human skeletal muscle, provides a rationale to use this histone target as a specific measure of PRMT1 enzymatic activity. We assessed the total contents of H3 and H4, as well as the presence of H4R3, H3R17, and H3R8 by Western blotting, and then determined the methylation status of these proteins by calculating the ratio of the methylated histone relative to its total content (71). The data demonstrate that a single bout of SIE did not affect the majority of these metrics (Fig. 5). Notwithstanding, a 15% increase (P < 0.05) in H4 content after exercise was revealed (Fig. 5C).
Fig. 5.
PRMT-specific activity following acute exercise. A: representative Western blots for the ADMA mark on histone 4 arginine 3 (H4R3), H4, the ADMA-marked H3R17 (H3R17), the SDMA modification on H3R8 (H3R8), and H3 in whole muscle quadriceps samples collected before and after a single bout of SIE. The arrow to the left of each image depicts the band of interest, while the Ponceau stain demonstrates equal loading. Approximate MWs are indicated on the right of each image. Graphical summaries of the levels of H4R3 (B), H4 (C), and H4R3 (D) methylation status, calculated as the H4R3 content relative to the total amount of H4 (E), H3R17 (F), H3R8 (G), H3 (H), and H3R17 and H3R8 methylation status (I) in the 3 experimental groups. Data are displayed relative to PRE levels. For graphical summaries, bars indicate groups means, lines denote individual participant data, and error bars show SD. Data were analyzed using Student’s t tests. *P < 0.05 vs. PRE; n = 14 for B; n = 12 for C, D, and G; n = 13 for E, F, H, and I.
PRMT content and function in human muscle after chronic exercise training.
In the present study, we also sought to characterize PRMT biology in human skeletal muscle in vivo during and after an adaptive period of chronic muscle remodeling. To this end, the same participants preformed SIT and quadriceps muscle biopsies were obtained PRE SIT, MID, as well as POST. Skeletal muscle PRMT1, PRMT5, PRMT7, and PRMT9 protein levels were similar between PRE compared with MID or POST (Fig. 6, A, B, D–F). In contrast, CARM1 content was significantly elevated (+20%) at the MID time point vs. PRE (Fig. 6, A and C).
Fig. 6.
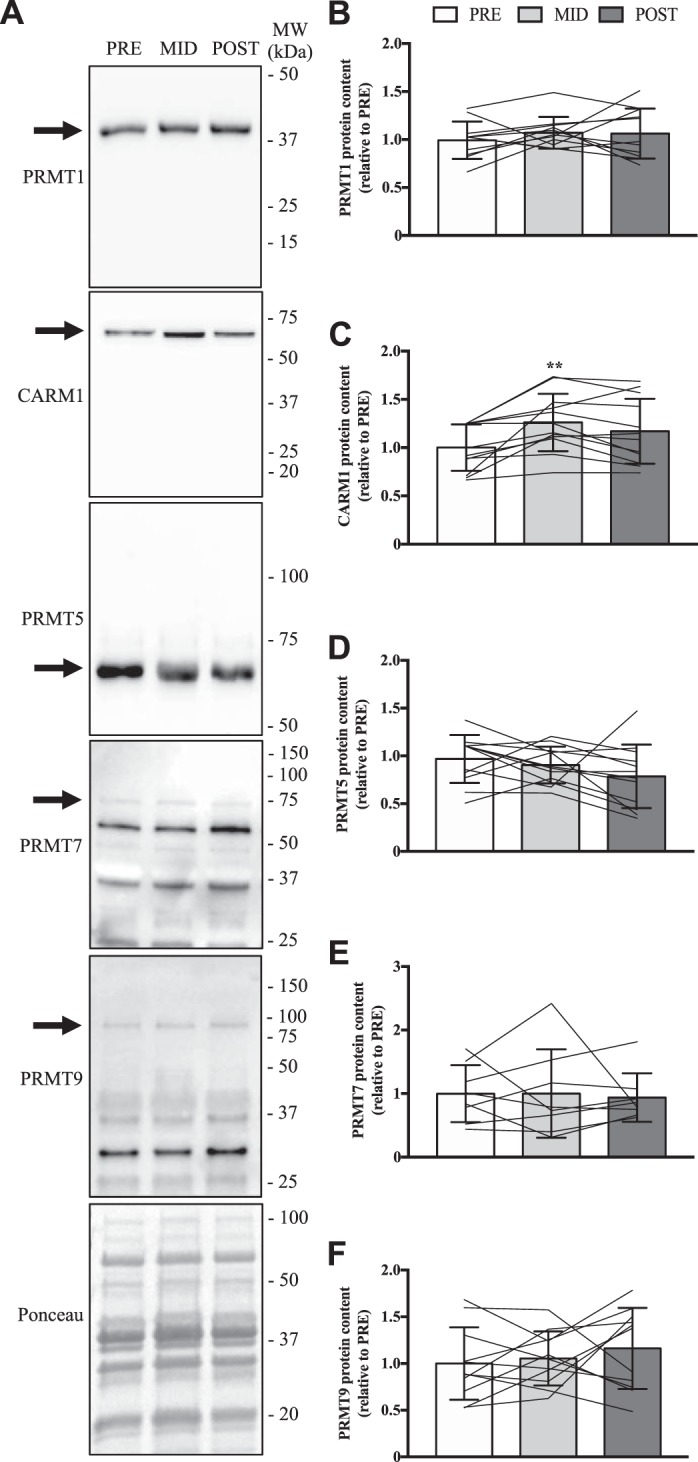
PRMT content after chronic exercise training. A: typical immunoblots of PRMT1, CARM1, PRMT5, PRMT7, and PRMT9 in human quadriceps muscle from PRE, as well as after 2 wk (MID) of sprint interval training (SIT), and following 6 wk of SIT (POST). To the left of each image is an arrow denoting the band of interest. The Ponceau stain demonstrates equal loading. Approximate MWs are indicated on the right of each image. Graphical summaries of PRMT1 (B), CARM1 (C), PRMT5 (D), PRMT7 (E), and PRMT9 (F) protein content. Data are displayed as relative to PRE levels, with bars representing group means, lines illustrating individual participant results, and error bars demonstrating SD. Analysis of data was completed using a 1-way ANOVA. **P < 0.01 vs. PRE; n = 11 for B and F; n = 12 for C and D; n = 8 for E.
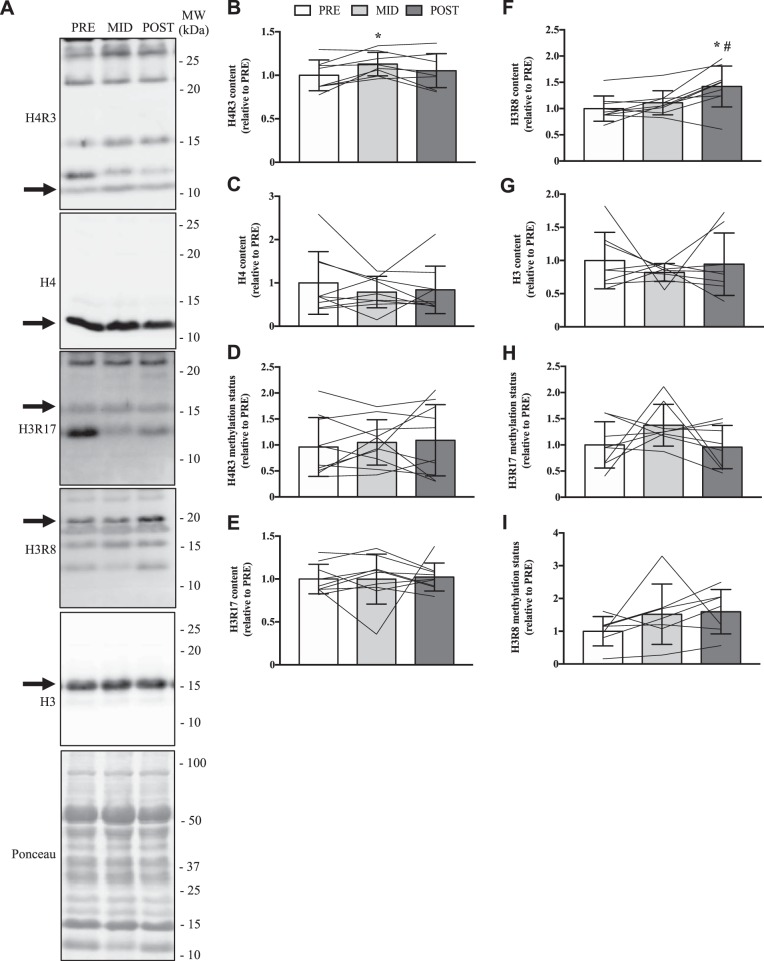
MMA levels were similar across all time points of the SIT protocol (Fig. 7A). ADMAGAR was 20% lower (P < 0.05) at POST vs. PRE (Fig. 7B), while ADMA-5CARM1 was significantly higher (+20%) at MID vs. PRE (Fig. 7C). SDMA content was 35% higher (P < 0.05) at MID compared with PRE (Fig. 7D). H4R3 content was significantly higher (+15%) at MID vs. PRE (Fig. 8B), and H3R8 content was increased 30–40% (P < 0.05) after 6 wk of SIT compared with MID and PRE (Fig. 8F). Other measurements of PRMT-specific methyltransferase activities were similar between SIT protocol time points.
Fig. 7.
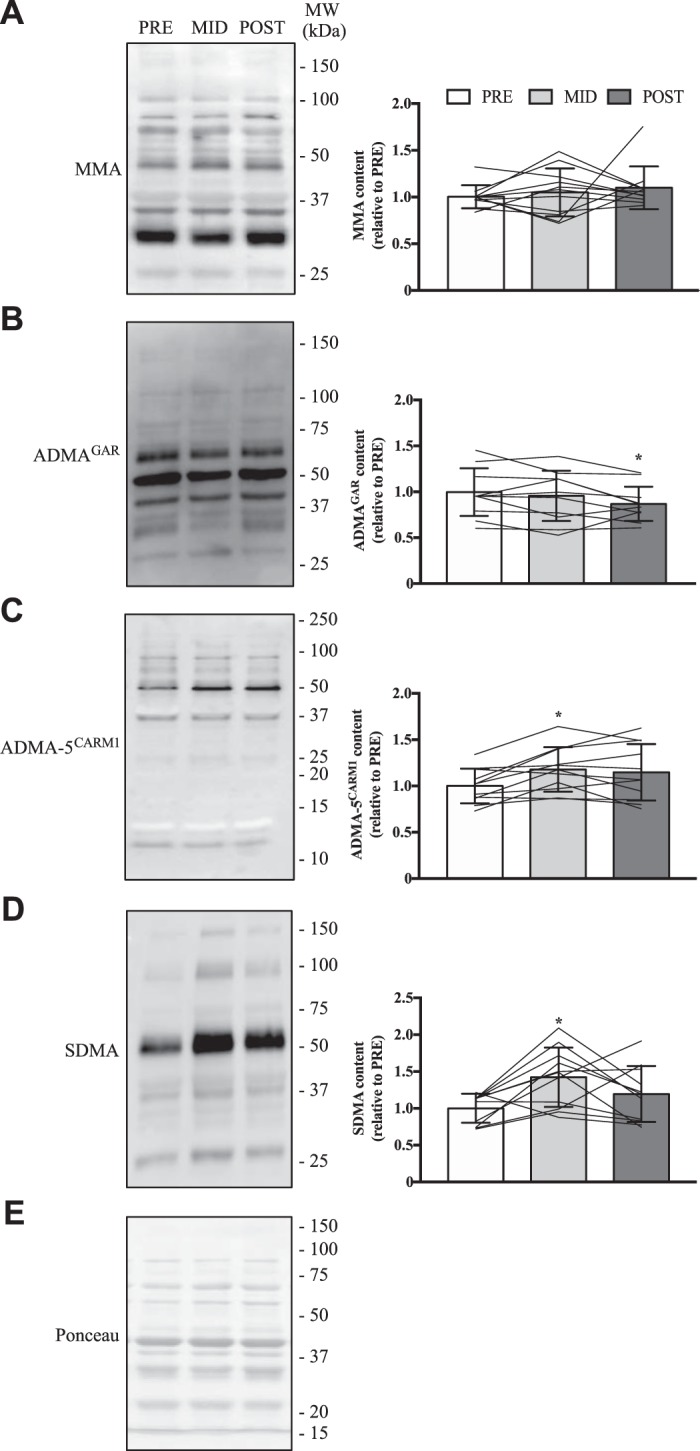
Global PRMT function in chronically trained human skeletal muscle. Typical Western blots for MMA (A), ADMAGAR (B), ADMA-5CARM1 (C) and SDMA (D) marks from human skeletal muscle at PRE, MID, and POST experimental time points. To the right of the example blot is a graphical summary of respective arginine methylation content. Approximate MWs are indicated on the right of the representative immunoblots. Data are displayed relative to PRE levels. For A–D, bars are indicative of group means, whereas lines represent individual participant data, and SD is displayed as error bars. E: typical Ponceau S stain demonstrating equal loading. Data were analyzed using a 1-way ANOVA. *P < 0.05 vs. PRE; n = 11 for all conditions.
Fig. 8.
PRMT enzyme activity following chronic exercise. A: representative immunoblots in whole muscle quadriceps samples at PRE, MID, and POST time points for H4R3, H4, H3R17, H3R8, and H3. The arrow on the left of each image denotes the band of interest, while the Ponceau stain demonstrates equal loading. Approximate MWs are indicated on the right of each image. Graphical summaries of H4R3 (B), H4 (C), H4R3 (D) methylation status, H3R17 (E), H3R8 (F), H3 (G), H3R17 (H) methylation status, and H3R8 methylation status (I) in the 3 experimental groups. Data are displayed relative to PRE levels. Bars indicate group means, lines illustrate individual participant results, and error bars show SD. Analysis of data was completed using a 1-way ANOVA. *P < 0.05 vs. PRE. #P < 0.05 vs. MID; n = 8 for B, G, and H; n = 9 for C, D, E, and F; n = 7 for I.
DISCUSSION
This is the first report to detail PRMT expression and function in human skeletal muscle in the basal, rested state, as well as during conditions of acute and chronic muscle remodeling elicited by exercise. Our data demonstrate that PRMT enzymes display distinct expression profiles in human muscle, with for example CARM1 being the most abundant mRNA and PRMT1 protein preferentially found in faster myofibers. However, the relative cellular localization of highly expressed PRMTs, including PRMT1, CARM1, and PRMT5, were nearly identical. In response to an acute cue for muscle plasticity, we observed PRMT-specific inductions in gene expression and activity, which indicate that PRMTs are differentially sensitive to exercise-evoked signals and also suggest that individual PRMTs may have unique functions in skeletal muscle. Chronic conditions of skeletal muscle remodeling elicited by exercise training also resulted in enzyme-specific alterations in content and function. Cumulatively, these results demonstrate that PRMTs are present and active in human skeletal muscle in vivo and that there are distinct enzyme-specific responses and adaptations in PRMT biology to acute and chronic stimuli for muscle plasticity. These data reveal a complexity of PRMT gene expression and function not observed previously in human muscle.
We first characterized PRMT biology in human skeletal muscle by examining transcript levels of PRMT family members. Previous research has shown that the majority of PRMTs are present ubiquitously throughout the body; however, PRMT8 expression is limited to the brain and spinal cord and so was excluded from this analysis (1, 2, 31, 45, 64, 66). Our data demonstrate that in human quadriceps muscle PRMT transcript content can be stratified into four levels: the highest being CARM1, followed next by PRMT1, -5, and -7, then PRMT2, -3, and -9, and finally PRMT6 having the lowest expression. These data are consistent with results from previous in vivo and in vitro rodent studies (74). A notable caveat here is that differences between PRMT transcripts do not necessarily forecast discrepancies at later stages of gene expression as well, for example at the protein level. Nevertheless, this relative abundance of PRMT1, CARM1, PRMT5, and PRMT7 mRNA in skeletal muscle, their recent emergence as critical factors in animal and cell culture models of muscle plasticity (36, 66), as well as their predominant methyltransferase activities in other cell types (2, 67, 70, 74), strongly suggest that these enzymes have important roles to play in myofibers. Thus we concentrated our efforts at further examining these particular enzymes for our subsequent analyses, as well as PRMT9, which was the only mRNA that was altered in response to acute exercise.
We observed that PRMT1, CARM1, and PRMT5 shared a similar subcellular protein distribution between myonuclear, cytosolic, and membrane compartments. This localization pattern matches that of previously published data in cell and animal studies (5, 65, 71). This extensive expression profile suggests that these enzymes function in multiple cellular locations in human muscle, as clearly demonstrated in previous studies of nonhuman tissues (45, 65). Alternative splicing of PRMT pre-mRNAs to produce multiple protein isoforms that display distinct subcellular localizations has been observed (26); however, whether this mechanism occurs in muscle has not yet been examined. Finally, under basal conditions, the relative expression of CARM1 and PRMT5 was similar between type I and IIa myofibers, whereas PRMT1 levels were modestly but significantly higher in IIa fibers. This fiber type-specific pattern of expression may reflect interactions between PRMT1 and putative targets that are more highly expressed in this fiber type, such as PGC-1α or p38 mitogen-activated protein kinase (24, 27, 66). Future work will address this question, as well as further detail the subcellular expression and function of PRMTs in skeletal muscle. A potential technical limitation of our study is that we were unable to examine PRMT7 and PRMT9 subcellular localization and fiber type-specific expression. Although some of these details have been previously reported elsewhere (31, 36, 77), additional characterization of PRMT7 and -9 in human skeletal muscle is clearly required.
We next examined PRMT content following an acute bout of physical activity, which is a robust stimulus to initiate early events for skeletal muscle plasticity. We observed that the majority of PRMT transcript levels remained unchanged following exercise, with the exception being PRMT9, which significantly increased after SIE. A role for PRMT9 in skeletal muscle has not yet been defined. However, given its function as an activator of protein kinase B in hepatocellular carcinoma cells (37), along with its induction in response to exercise, it is tempting to speculate that this methyltransferase might be involved in muscle remodeling. Previous rodent studies from our laboratory showed that exercise augmented PRMT mRNAs in the active muscles; however, this occurred in enzyme- and exercise intensity-specific fashions (49, 71). Clearly, species is another variable to qualify when attempting to generalize these results. Interestingly, the present study also demonstrates that while PRMT7 and PRMT9 levels did not change, PRMT1, CARM1, and PRMT5 protein content increased after the acute stimulus. This was a particularly surprising result since most studies utilizing the single exercise bout design show alterations in mRNA levels, rather than protein content, within hours after the cessation of activity. This pattern is believed to be due to the highly temporal nature of gene expression, where rapid but transient exercise-induced elevations in mRNAs precede increases in protein content that generally occur after repeated bouts of activity across a longer duration (22). However, it is also important to consider that posttranscriptional events can certainly influence protein content, independently of mRNA levels. Collectively, our mRNA and protein data therefore suggest that translation and/or stability of these PRMTs is enhanced with SIE. This assertion is supported by previous work showing that, for example, PRMT1 translation can be controlled by the microRNA miR-503 (2), which itself is influenced by exercise (54). In addition to microRNAs, the many mechanisms that regulate PRMT translation, such as alternative splicing, oxidation, methylation, and interactions with various binding partners (2), provide future avenues for inquiry to further understand PRMT gene expression during conditions of acute muscle remodeling.
Previous work has shown that global cellular PRMT function, as well as specific PRMT activity, can successfully be quantified in rodent muscle (65, 71). Using similar methods, we were able to measure arginine methyltransferase function in human skeletal muscle. MMA and SDMA content are commonly used markers of PRMT and type II PRMT activities, respectively (19). PRMT1 has been shown to preferentially methylate GAR motifs, and so we used an asymmetric dimethylation antibody that recognizes this site (ADMAGAR), while a different antibody was used to identify sites favored by CARM1 (ADMA-5CARM1) (2, 15, 16). We found that in response to an acute bout of exercise, whole myocellular MMA levels increased, while ADMAGAR and ADMA-5CARM1, as well as SDMA levels remained unchanged. These data do not rule out the possibility of changes in specific PRMT dimethylation activity. Therefore, we wanted to further examine the enzymatic activities of PRMT1, CARM1, and PRMT5 in human muscle samples by assessing dimethylation of their specific targets. PRMT1, CARM1, and PRMT5 methylate H4R3, H3R17, and H3R8 with the ADMA (H4R3, H3R17) and SDMA (H3R8) marks, respectively (2). The data show that H3R8, H3R17, and H4R3 methylation remained unchanged. The absence of altered methylation status of bona fide PRMT1, CARM1, and PRMT5 histone targets does not preclude exercise affecting alternative PRMT substrates, for instance PGC-1α, p53, E2F transcription factor 1, and receptor interacting protein 140, which are arginine methylated in other cell types (13, 32, 41, 55, 63). Additionally, PRMT function is regulated via physical interactions with, and/or posttranslational modifications by, proteins such as BTG antiproliferation factor 1, BTG antiproliferation factor 2 (BTG2), and nucleosomal methylation activator complex. Some of these enzymes, like BTG2 for example, are altered in skeletal muscle by exercise (50) and therefore may in turn affect arginine methyltransferase activities. Reputed arginine demethylases, such as jumonji domain-containing protein 6 (2, 9, 12), might also be influencing measures of PRMT function by demethylating marked proteins throughout the cell. The preceding statements are clearly speculative, with the main caveat being that these details of PRMT biology, namely their targets, regulators, and opposing enzymes, have not yet been widely identified in skeletal muscle, let alone human muscle, and thus require further study. Alternative modes of physical activity, for example low-intensity prolonged endurance-type exercise, or a heavy resistance-type movement, might yield different results on PRMT function, as intracellular signaling is well known to be dependent, in part, on the frequency, intensity, duration, and type of stimulus (22).
Following this, we examined PRMT biology during conditions of chronic muscle remodeling brought about by SIT. Growing evidence suggests that high-intensity interval training stimulates skeletal muscle physiological remodeling similar to traditional continuous endurance training (25). However, relatively little is known regarding the influence of exercise intensity, duration, and frequency on the physiological response to interval training (51). Therefore, while SIT is an effective model for muscle plasticity, further work exploring PRMT biology during traditional endurance- and resistance-type training regimes will help increase our understanding of the role these molecules play in muscle plasticity. We performed a secondary analysis of previously published data (52) on ~25,000 genes in human skeletal muscle after 6 wk of high-intensity interval training, which was found at the NCHI Gene Expression Omnibus under the accession number GSE109657. The results indicate that CARM1, PRMT5, and PRMT7 transcripts were significantly decreased following training, which when combined with data from the current study (i.e., increase in PRMT9 mRNA following SIE), suggest that PRMT family gene expression is differentially responsive to acute and chronic cues for muscle plasticity. At the protein level, we observed an increase in CARM1 content after 2 wk (i.e., MID) of SIT, which returned to PRE values after 6 wk (i.e., POST) of exercise. In contrast, PRMT1, PRMT5, PRMT7, and PRMT9 levels remained unchanged. These data align with earlier studies that demonstrate increased protein levels after acute exercise, but not following training, which although highly speculative at this point, may be caused by alterations in transcriptional, posttranscriptional, and/or posttranslational mechanisms. For example, investigators found elevated skeletal muscle PGC-1α content after a single bout of exercise (46), while no change was observed in PGC-1α levels following 2 (47, 72) and 4 wk (28) of aerobic training, as well as following 2 or 6 wk of SIT (21). Interestingly, PGC-1α physically interacts with PRMT1 and CARM1 in mouse skeletal muscle (71); however, the nature of this relationship is currently unknown. Seminal work from Teyssier and colleagues (69) showed that in CV-1 and COS7 cells PRMT1-mediated methylation potentiates the transcriptional coactivator function of PGC-1α. While we found a reduction in global myocellular ADMA marks after 6 wk of SIT, there was evidence of enhanced myonuclear PRMT1 activity at the 2-wk time point. As such, it is reasonable to hypothesize that PRMT1 may be interacting with PGC-1α, and possibly other regulators of muscle phenotype, early in the remodeling process, for instance during an adaptive window following the initiation of training. This is in line with recent work by vanLieshout et al. (71), who observed acute exercise-induced PGC-1α nuclear translocation coincident with augmented local arginine methyltransferase activities in mouse skeletal muscle. Altogether, these enzyme-specific effects on content and function during chronic phenotypic remodeling suggest a sophisticated regulation of PRMT biology in human muscle that warrants continued examination.
In summary, these are the first data demonstrating PRMT expression and function in human skeletal muscle. PRMT1, CARM1, PRMT5, and PRMT7 were the most abundantly expressed arginine methyltransferase transcripts in human muscle, which is consistent with previously established roles for these enzymes in this tissue that have been reported in basic and preclinical cell culture and animal studies. Additionally, the broad subcellular distribution pattern of PRMTs suggests methyltransferase activities throughout human myofibers. A broad spectrum of PRMT-specific inductions, and in some cases decrements, in gene expression and activity were observed in response to acute and chronic cues for muscle plasticity. In all, the complexities revealed here of PRMT-specific biology in human skeletal muscle under basal conditions and during phenotype remodeling necessitates further investigation to advance our understanding of this critical family of enzymes.
GRANTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada and the Canada Research Chairs program. V. Ljubicic is the Canada Research Chair (Tier 2) in Neuromuscular Plasticity in Health and Disease.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.L.v. and V.L. conceived and designed research; T.L.v. and J.T.B. performed experiments; T.L.v., J.T.B., B.J.G., and V.L. analyzed data; T.L.v., J.T.B., B.J.G., and V.L. interpreted results of experiments; T.L.v. prepared figures; T.L.v. drafted manuscript; T.L.v., J.T.B., B.J.G., and V.L. edited and revised manuscript; T.L.v., J.T.B., B.J.G., and V.L. approved final version of manuscript.
REFERENCES
- 1.Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell 33: 1–13, 2009. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanc RS, Richard S. Arginine methylation: the coming of age. Mol Cell 65: 8–24, 2017. doi: 10.1016/j.molcel.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Blanc RS, Richard S. Regenerating muscle with arginine methylation. Transcription 8: 175–178, 2017. doi: 10.1080/21541264.2017.1291083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanc RS, Vogel G, Chen T, Crist C, Richard S. PRMT7 preserves satellite cell regenerative capacity. Cell Reports 14: 1528–1539, 2016. doi: 10.1016/j.celrep.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Blanc RS, Vogel G, Li X, Yu Z, Li S, Richard S. Arginine methylation by PRMT1 regulates muscle stem cell fate. Mol Cell Biol 37: e00457-16, 2017. doi: 10.1128/MCB.00457-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7: e35273, 2012. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boisvert FM, Chénard CA, Richard S. Protein interfaces in signaling regulated by arginine methylation. Sci STKE 2005: re2, 2005. doi: 10.1126/stke.2712005re2. [DOI] [PubMed] [Google Scholar]
- 8.Bonafiglia JT, Edgett BA, Baechler BL, Nelms MW, Simpson CA, Quadrilatero J, Gurd BJ. Acute upregulation of PGC-1α mRNA correlates with training-induced increases in SDH activity in human skeletal muscle. Appl Physiol Nutr Metab 42: 656–666, 2017. doi: 10.1139/apnm-2016-0463. [DOI] [PubMed] [Google Scholar]
- 9.Böttger A, Islam MS, Chowdhury R, Schofield CJ, Wolf A. The oxygenase Jmjd6—a case study in conflicting assignments. Biochem J 468: 191–202, 2015. doi: 10.1042/BJ20150278. [DOI] [PubMed] [Google Scholar]
- 10.Branscombe TL, Frankel A, Lee JH, Cook JR, Yang Z, Pestka S, Clarke S. PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins. J Biol Chem 276: 32971–32976, 2001. doi: 10.1074/jbc.M105412200. [DOI] [PubMed] [Google Scholar]
- 11.Cha B, Jho EH. Protein arginine methyltransferases (PRMTs) as therapeutic targets. Expert Opin Ther Targets 16: 651–664, 2012. doi: 10.1517/14728222.2012.688030. [DOI] [PubMed] [Google Scholar]
- 12.Chang B, Chen Y, Zhao Y, Bruick RK. JMJD6 is a histone arginine demethylase. Science 318: 444–447, 2007. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- 13.Chang YI, Hua WK, Yao CL, Hwang SM, Hung YC, Kuan CJ, Leou JS, Lin WJ. Protein-arginine methyltransferase 1 suppresses megakaryocytic differentiation via modulation of the p38 MAPK pathway in K562 cells. J Biol Chem 285: 20595–20606, 2010. doi: 10.1074/jbc.M109.092411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SL, Loffler KA, Chen D, Stallcup MR, Muscat GE. The coactivator-associated arginine methyltransferase is necessary for muscle differentiation: CARM1 coactivates myocyte enhancer factor-2. J Biol Chem 277: 4324–4333, 2002. doi: 10.1074/jbc.M109835200. [DOI] [PubMed] [Google Scholar]
- 15.Cheng D, Côté J, Shaaban S, Bedford MT. The arginine methyltransferase CARM1 regulates the coupling of transcription and mRNA processing. Mol Cell 25: 71–83, 2007. doi: 10.1016/j.molcel.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Cheng D, Vemulapalli V, Lu Y, Shen J, Aoyagi S, Fry CJ, Yang Y, Foulds CE, Stossi F, Treviño LS, Mancini MA, O’Malley BW, Walker CL, Boyer TG, Bedford MT. CARM1 methylates MED12 to regulate its RNA-binding ability. Life Sci Alliance 1: e201800117, 2018. doi: 10.26508/lsa.201800117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 18.Dacwag CS, Bedford MT, Sif S, Imbalzano AN. Distinct protein arginine methyltransferases promote ATP-dependent chromatin remodeling function at different stages of skeletal muscle differentiation. Mol Cell Biol 29: 1909–1921, 2009. doi: 10.1128/MCB.00742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhar S, Vemulapalli V, Patananan AN, Huang GL, Di Lorenzo A, Richard S, Comb MJ, Guo A, Clarke SG, Bedford MT. Loss of the major Type I arginine methyltransferase PRMT1 causes substrate scavenging by other PRMTs. Sci Rep 3: 1311, 2013. doi: 10.1038/srep01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dial AG, Rooprai P, Lally JS, Bujak AL, Steinberg GR, Ljubicic V. The role of AMP-activated protein kinase in the expression of the dystrophin-associated protein complex in skeletal muscle. FASEB J 32: 2950–2965, 2018. doi: 10.1096/fj.201700868RRR. [DOI] [PubMed] [Google Scholar]
- 21.Edgett BA, Bonafiglia JT, Baechler BL, Quadrilatero J, Gurd BJ. The effect of acute and chronic sprint-interval training on LRP130, SIRT3, and PGC-1α expression in human skeletal muscle. Physiol Rep 4: e12879, 2016. doi: 10.14814/phy2.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Fulton MD, Brown T, Zheng YG. Mechanisms and inhibitors of histone arginine methylation. Chem Rec 18: 1792–1807, 2018. doi: 10.1002/tcr.201800082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galpin AJ, Raue U, Jemiolo B, Trappe TA, Harber MP, Minchev K, Trappe S. Human skeletal muscle fiber type specific protein content. Anal Biochem 425: 175–182, 2012. doi: 10.1016/j.ab.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibala MJ, McGee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc Sport Sci Rev 36: 58–63, 2008. doi: 10.1097/JES.0b013e318168ec1f. [DOI] [PubMed] [Google Scholar]
- 26.Goulet I, Gauvin G, Boisvenue S, Côté J. Alternative splicing yields protein arginine methyltransferase 1 isoforms with distinct activity, substrate specificity, and subcellular localization. J Biol Chem 282: 33009–33021, 2007. doi: 10.1074/jbc.M704349200. [DOI] [PubMed] [Google Scholar]
- 27.Gouspillou G, Sgarioto N, Norris B, Barbat-Artigas S, Aubertin-Leheudre M, Morais JA, Burelle Y, Taivassalo T, Hepple RT. The relationship between muscle fiber type-specific PGC-1α content and mitochondrial content varies between rodent models and humans. PLoS One 9: e103044, 2014. doi: 10.1371/journal.pone.0103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granata C, Oliveira RS, Little JP, Renner K, Bishop DJ. Training intensity modulates changes in PGC-1α and p53 protein content and mitochondrial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J 30: 959–970, 2016. doi: 10.1096/fj.15-276907. [DOI] [PubMed] [Google Scholar]
- 29.Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 13: 343–357, 2012. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo A, Gu H, Zhou J, Mulhern D, Wang Y, Lee KA, Yang V, Aguiar M, Kornhauser J, Jia X, Ren J, Beausoleil SA, Silva JC, Vemulapalli V, Bedford MT, Comb MJ. Immunoaffinity enrichment and mass spectrometry analysis of protein methylation. Mol Cell Proteomics 13: 372–387, 2014. doi: 10.1074/mcp.O113.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrmann F, Pably P, Eckerich C, Bedford MT, Fackelmayer FO. Human protein arginine methyltransferases in vivo–distinct properties of eight canonical members of the PRMT family. J Cell Sci 122: 667–677, 2009. doi: 10.1242/jcs.039933. [DOI] [PubMed] [Google Scholar]
- 32.Hua WK, Chang YI, Yao CL, Hwang SM, Chang CY, Lin WJ. Protein arginine methyltransferase 1 interacts with and activates p38α to facilitate erythroid differentiation. PLoS One 8: e56715, 2013. doi: 10.1371/journal.pone.0056715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyllus D, Stein C, Schnabel K, Schiltz E, Imhof A, Dou Y, Hsieh J, Bauer UM. PRMT6-mediated methylation of R2 in histone H3 antagonizes H3 K4 trimethylation. Genes Dev 21: 3369–3380, 2007. doi: 10.1101/gad.447007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, Dietmann S, Hanna JH, Surani MA. SOX17 is a critical specifier of human primordial germ cell fate. Cell 160: 253–268, 2015. doi: 10.1016/j.cell.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwasaki H, Yada T. Protein arginine methylation regulates insulin signaling in L6 skeletal muscle cells. Biochem Biophys Res Commun 364: 1015–1021, 2007. doi: 10.1016/j.bbrc.2007.10.113. [DOI] [PubMed] [Google Scholar]
- 36.Jeong HJ, Lee HJ, Vuong TA, Choi KS, Choi D, Koo SH, Cho SC, Cho H, Kang JS. PRMT7 deficiency causes reduced skeletal muscle oxidative metabolism and age-related obesity. Diabetes 65: 1868–1882, 2016. doi: 10.2337/db15-1500. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Zhou Z, Jin S, Xu K, Zhang H, Xu J, Sun Q, Wang J, Xu J. PRMT9 promotes hepatocellular carcinoma invasion and metastasis via activating PI3K/Akt/GSK-3β/Snail signaling. Cancer Sci 109: 1414–1427, 2018. doi: 10.1111/cas.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawabe Y, Wang YX, McKinnell IW, Bedford MT, Rudnicki MA. Carm1 regulates Pax7 transcriptional activity through MLL1/2 recruitment during asymmetric satellite stem cell divisions. Cell Stem Cell 11: 333–345, 2012. doi: 10.1016/j.stem.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim C, Lim Y, Yoo BC, Won NH, Kim S, Kim G. Regulation of post-translational protein arginine methylation during HeLa cell cycle. Biochim Biophys Acta 1800: 977–985, 2010. doi: 10.1016/j.bbagen.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Kim D, Lee J, Cheng D, Li J, Carter C, Richie E, Bedford MT. Enzymatic activity is required for the in vivo functions of CARM1. J Biol Chem 285: 1147–1152, 2010. doi: 10.1074/jbc.M109.035865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D, Lim S, Park M, Choi J, Kim J, Han H, Yoon K, Kim K, Lim J, Park S. Ubiquitination-dependent CARM1 degradation facilitates Notch1-mediated podocyte apoptosis in diabetic nephropathy. Cell Signal 26: 1774–1782, 2014. doi: 10.1016/j.cellsig.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Kjøbsted R, Hingst JR, Fentz J, Foretz M, Sanz MN, Pehmøller C, Shum M, Marette A, Mounier R, Treebak JT, Wojtaszewski JFP, Viollet B, Lantier L. AMPK in skeletal muscle function and metabolism. FASEB J 32: 1741–1777, 2018. doi: 10.1096/fj.201700442R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakowski TM, Frankel A. Kinetic analysis of human protein arginine N-methyltransferase 2: formation of monomethyl- and asymmetric dimethyl-arginine residues on histone H4. Biochem J 421: 253–261, 2009. doi: 10.1042/BJ20090268. [DOI] [PubMed] [Google Scholar]
- 44.Larsen SC, Sylvestersen KB, Mund A, Lyon D, Mullari M, Madsen MV, Daniel JA, Jensen LJ, Nielsen ML. Proteome-wide analysis of arginine monomethylation reveals widespread occurrence in human cells. Sci Signal 9: rs9, 2016. doi: 10.1126/scisignal.aaf7329. [DOI] [PubMed] [Google Scholar]
- 45.Lee YH, Stallcup MR. Minireview: protein arginine methylation of nonhistone proteins in transcriptional regulation. Mol Endocrinol 23: 425–433, 2009. doi: 10.1210/me.2008-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R1303–R1310, 2011. doi: 10.1152/ajpregu.00538.2010. [DOI] [PubMed] [Google Scholar]
- 47.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588: 1011–1022, 2010. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Wang T, Ji YJ, Johnson K, Liu H, Johnson K, Bailey S, Suk Y, Lu YN, Liu M, Wang J. A C9orf72-CARM1 axis regulates lipid metabolism under glucose starvation-induced nutrient stress. Genes Dev 32: 1380–1397, 2018. doi: 10.1101/gad.315564.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ljubicic V, Khogali S, Renaud JM, Jasmin BJ. Chronic AMPK stimulation attenuates adaptive signaling in dystrophic skeletal muscle. Am J Physiol Cell Physiol 302: C110–C121, 2012. doi: 10.1152/ajpcell.00183.2011. [DOI] [PubMed] [Google Scholar]
- 50.Lundberg TR, Fernandez-Gonzalo R, Tesch PA, Rullman E, Gustafsson T. Aerobic exercise augments muscle transcriptome profile of resistance exercise. Am J Physiol Regul Integr Comp Physiol 310: R1279–R1287, 2016. doi: 10.1152/ajpregu.00035.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol 595: 2915–2930, 2017. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyamoto-Mikami E, Tsuji K, Horii N, Hasegawa N, Fujie S, Homma T, Uchida M, Hamaoka T, Kanehisa H, Tabata I, Iemitsu M. Gene expression profile of muscle adaptation to high-intensity intermittent exercise training in young men. Sci Rep 8: 16811, 2018. doi: 10.1038/s41598-018-35115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicholson TB, Chen T, Richard S. The physiological and pathophysiological role of PRMT1-mediated protein arginine methylation. Pharmacol Res 60: 466–474, 2009. doi: 10.1016/j.phrs.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Niu Y, Wan C, Zhou B, Wang J, Wang J, Chen X, Li R, Wang X, Liu W, Wang Y. Aerobic exercise relieved vascular cognitive impairment via NF-κB/miR-503/BDNF pathway. Am J Transl Res 10: 753–761, 2018. [PMC free article] [PubMed] [Google Scholar]
- 55.Park MJ, Kim DI, Lim SK, Choi JH, Kim JC, Yoon KC, Lee JB, Lee JH, Han HJ, Choi IP, Kim HC, Park SH. Thioredoxin-interacting protein mediates hepatic lipogenesis and inflammation via PRMT1 and PGC-1α regulation in vitro and in vivo. J Hepatol 61: 1151–1157, 2014. doi: 10.1016/j.jhep.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 56.Pawlak MR, Scherer CA, Chen J, Roshon MJ, Ruley HE. Arginine N-methyltransferase 1 is required for early postimplantation mouse development, but cells deficient in the enzyme are viable. Mol Cell Biol 20: 4859–4869, 2000. doi: 10.1128/MCB.20.13.4859-4869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Porta M, Amione C, Barutta F, Fornengo P, Merlo S, Gruden G, Albano L, Ciccarelli M, Ungaro P, Durazzo M, Beguinot F, Berchialla P, Cavallo F, Trento M. The co-activator-associated arginine methyltransferase 1 (CARM1) gene is overexpressed in type 2 diabetes. Endocrine 63: 284–292, 2019. doi: 10.1007/s12020-018-1740-z. [DOI] [PubMed] [Google Scholar]
- 58.Rehman I, Basu SM, Das SK, Bhattacharjee S, Ghosh A, Pommier Y, Das BB. PRMT5-mediated arginine methylation of TDP1 for the repair of topoisomerase I covalent complexes. Nucleic Acids Res 46: 5601–5617, 2018. doi: 10.1093/nar/gky291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401: 318–320, 2010. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 60.Sakabe K, Hart GW. O-GlcNAc transferase regulates mitotic chromatin dynamics. J Biol Chem 285: 34460–34468, 2010. doi: 10.1074/jbc.M110.158170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scribbans TD, Edgett BA, Vorobej K, Mitchell AS, Joanisse SD, Matusiak JB, Parise G, Quadrilatero J, Gurd BJ. Fibre-specific responses to endurance and low volume high intensity interval training: striking similarities in acute and chronic adaptation. PLoS One 9: e98119, 2014. doi: 10.1371/journal.pone.0098119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen NY, Ng SY, Toepp SL, Ljubicic V. Protein arginine methyltransferase expression and activity during myogenesis. Biosci Rep 38: BSR20171533, 2018. doi: 10.1042/BSR20171533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin HJ, Kim H, Oh S, Lee JG, Kee M, Ko HJ, Kweon MN, Won KJ, Baek SH. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature 534: 553–557, 2016. doi: 10.1038/nature18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simandi Z, Pajer K, Karolyi K, Sieler T, Jiang LL, Kolostyak Z, Sari Z, Fekecs Z, Pap A, Patsalos A, Contreras GA, Reho B, Papp Z, Guo X, Horvath A, Kiss G, Keresztessy Z, Vámosi G, Hickman J, Xu H, Dormann D, Hortobagyi T, Antal M, Nógrádi A, Nagy L. Arginine methyltransferase PRMT8 provides cellular stress tolerance in aging motoneurons. J Neurosci 38: 7683–7700, 2018. doi: 10.1523/JNEUROSCI.3389-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stouth DW, Manta A, Ljubicic V. Protein arginine methyltransferase expression, localization, and activity during disuse-induced skeletal muscle plasticity. Am J Physiol Cell Physiol 314: C177–C190, 2018. doi: 10.1152/ajpcell.00174.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stouth DW, vanLieshout TL, Shen NY, Ljubicic V. Regulation of Skeletal muscle plasticity by protein arginine methyltransferases and their potential roles in neuromuscular disorders. Front Physiol 8: 870, 2017. doi: 10.3389/fphys.2017.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang J, Frankel A, Cook RJ, Kim S, Paik WK, Williams KR, Clarke S, Herschman HR. PRMT1 is the predominant type I protein arginine methyltransferase in mammalian cells. J Biol Chem 275: 7723–7730, 2000. doi: 10.1074/jbc.275.11.7723. [DOI] [PubMed] [Google Scholar]
- 68.Tee WW, Pardo M, Theunissen TW, Yu L, Choudhary JS, Hajkova P, Surani MA. Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev 24: 2772–2777, 2010. doi: 10.1101/gad.606110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teyssier C, Ma H, Emter R, Kralli A, Stallcup MR. Activation of nuclear receptor coactivator PGC-1α by arginine methylation. Genes Dev 19: 1466–1473, 2005. doi: 10.1101/gad.1295005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thandapani P, O’Connor TR, Bailey TL, Richard S. Defining the RGG/RG motif. Mol Cell 50: 613–623, 2013. doi: 10.1016/j.molcel.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 71.Vanlieshout TL, Stouth DW, Tajik T, Ljubicic V. Exercise-induced protein arginine methyltransferase expression in skeletal muscle. Med Sci Sports Exerc 50: 447–457, 2018. doi: 10.1249/MSS.0000000000001476. [DOI] [PubMed] [Google Scholar]
- 72.Vincent G, Lamon S, Gant N, Vincent PJ, MacDonald JR, Markworth JF, Edge JA, Hickey AJ. Changes in mitochondrial function and mitochondria associated protein expression in response to 2-weeks of high intensity interval training. Front Physiol 6: 51, 2015. doi: 10.3389/fphys.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L, Wolgemuth DJ. BET protein BRDT complexes with HDAC1, PRMT5, and TRIM28 and functions in transcriptional repression during spermatogenesis. J Cell Biochem 117: 1429–1438, 2016. doi: 10.1002/jcb.25433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang SC, Dowhan DH, Eriksson NA, Muscat GE. CARM1/PRMT4 is necessary for the glycogen gene expression programme in skeletal muscle cells. Biochem J 444: 323–331, 2012. doi: 10.1042/BJ20112033. [DOI] [PubMed] [Google Scholar]
- 75.Wei H, Mundade R, Lange KC, Lu T. Protein arginine methylation of non-histone proteins and its role in diseases. Cell Cycle 13: 32–41, 2014. doi: 10.4161/cc.27353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yadav N, Lee J, Kim J, Shen J, Hu MC, Aldaz CM, Bedford MT. Specific protein methylation defects and gene expression perturbations in coactivator-associated arginine methyltransferase 1-deficient mice. Proc Natl Acad Sci USA 100: 6464–6468, 2003. doi: 10.1073/pnas.1232272100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Y, Hadjikyriacou A, Xia Z, Gayatri S, Kim D, Zurita-Lopez C, Kelly R, Guo A, Li W, Clarke SG, Bedford MT. PRMT9 is a type II methyltransferase that methylates the splicing factor SAP145. Nat Commun 6: 6428, 2015. doi: 10.1038/ncomms7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu J, Shin B, Park ES, Yang S, Choi S, Kang M, Rho J. Protein arginine methyltransferase 1 regulates herpes simplex virus replication through ICP27 RGG-box methylation. Biochem Biophys Res Commun 391: 322–328, 2010. doi: 10.1016/j.bbrc.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 79.Zhang T, Günther S, Looso M, Künne C, Krüger M, Kim J, Zhou Y, Braun T. Prmt5 is a regulator of muscle stem cell expansion in adult mice. Nat Commun 6: 7140, 2015. doi: 10.1038/ncomms8140. [DOI] [PMC free article] [PubMed] [Google Scholar]