Abstract
Redox enzymes modulate intracellular redox balance and are secreted in response to cellular oxidative stress, potentially modulating systemic inflammation. Both aerobic and resistance exercise are known to cause acute systemic oxidative stress and inflammation; however, how redox enzyme concentrations alter in extracellular fluids following bouts of either type of exercise is unknown. Recreationally active men (n = 26, mean ± SD: age 28 ± 8 yr) took part in either: 1) two separate energy-matched cycling bouts: one of moderate intensity (MOD) and a bout of high intensity interval exercise (HIIE) or 2) an eccentric-based resistance exercise protocol (RES). Alterations in plasma (study 1) and serum (study 2) peroxiredoxin (PRDX)-2, PRDX-4, superoxide dismutase-3 (SOD3), thioredoxin (TRX-1), TRX-reductase and interleukin (IL)-6 were assessed before and at various timepoints after exercise. There was a significant increase in SOD3 (+1.5 ng/mL) and PRDX-4 (+5.9 ng/mL) concentration following HIIE only, peaking at 30- and 60-min post-exercise respectively. TRX-R decreased immediately and 60 min following HIIE (−7.3 ng/mL) and MOD (−8.6 ng/mL), respectively. In non-resistance trained men, no significant changes in redox enzyme concentrations were observed up to 48 h following RES, despite significant muscle damage. IL-6 concentration increased in response to all trials, however there was no significant relationship between absolute or exercise-induced changes in redox enzyme concentrations. These results collectively suggest that HIIE, but not MOD or RES increase the extracellular concentration of PRDX-4 and SOD3. Exercise-induced changes in redox enzyme concentrations do not appear to directly relate to systemic changes in IL-6 concentration.
NEW & NOTEWORTHY Two studies were conducted to characterize changes in redox enzyme concentrations after single bouts of exercise to investigate the emerging association between extracellular redox enzymes and inflammation. We provide evidence that SOD3 and PRDX-4 concentration increased following high-intensity aerobic but not eccentric-based resistance exercise. Changes were not associated with IL-6. The results provide a platform to investigate the utility of SOD3 and PRDX-4 as biomarkers of oxidative stress following exercise.
Keywords: antioxidant, oxidative stress, peroxiredoxin, reactive oxygen species, redoxkine
INTRODUCTION
It is well documented that acute exercise perturbs cellular reduction/oxidation (redox) balance through the increased production of reactive oxygen species (ROS) within actively contracting skeletal muscle (34) as well as other infiltrating cell types (35). Evidence suggests that ROS such as hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) have important roles in facilitating muscle contractile activity (25) and regulating the expression of genes involved with metabolism and endogenous antioxidant protection (14, 39). Conversely, heightened levels of exercise-induced H2O2 at the expense of antioxidant defense systems can elicit oxidative stress, which may limit contractile function and promote fatigue (33). Given this biphasic relationship, studies have previously evaluated alterations in redox balance in response to both aerobic and resistance-type exercise. These studies have focused primarily on the quantification of distal markers in extracellular fluids, such as the oxidation biomolecules and/or activity of antioxidant enzymes in plasma (48), serum (31), saliva (11), and urine (41), highlighting exercise duration (3), intensity (17), and muscle-damage (4) as factors governing greater increases. However, criticisms are commonly made with regard to the direct relationship of these markers with the redox state of active tissues during exercise (9). Recent evidence has highlighted that intracellular redox enzymes such as peroxiredoxin (PRDX) can be secreted from skeletal muscle myocytes (28) and immune cells (40) in response to increasing concentrations of H2O2 in vitro. Human studies are also beginning to provide evidence that plasma/serum PRDX-2 and PRDX-4 concentrations could serve as important biomarkers of the intracellular redox state in the context of acute and chronic inflammatory conditions (27, 40).
PRDXs are a major family of ubiquitous redox proteins, which modulate intracellular redox balance through a highly reactive cysteine thiolate group. The reaction rate of this cysteine is markedly greater than any other thiol-containing protein (50), allowing rapid regulation of cellular H2O2, with some evidence to suggest that this may facilitate muscle contraction (26). Therefore, PRDXs are reliable footprints of intracellular redox state, with heightened oxidation of the PRDX cysteine indicative of oxidative stress (37). In addition, upon secretion from immune cells, PRDX can directly bind to Toll-like receptor (TLR)-4 to initiate inflammatory cytokine production [e.g., interleukin (IL)-6] (38), providing some support for the association between PRDX and inflammation (27, 40). Recent work has begun to explore changes in the PRDX catalytic cycle in blood cells isolated from humans before and after acute exercise (6, 46, 47). In parallel with increases in soluble markers of inflammation (e.g., IL-6 and C-reactive protein), an increase in the oxidation of PRDX (i.e., dimer and overoxidized states) has been reported following intensive cycling and running exercise (46, 47). To our knowledge, changes in PRDX have yet to be assessed in the context of exercise in humans and represent a potentially unexplored area of exercise and redox biology. Interestingly, PRDX-2 can be secreted in tandem with its enzymatic reducing partners thioredoxin (TRX-1) and thioredoxin reductase (TRX-R) (20, 40). TRX-1 and TRX-R are cysteine and selenium based-antioxidant enzymes, respectively, with higher reduction potentials than PRDX, thus contributing toward maintaining the antioxidant function of PRDX. In addition, the enzyme superoxide dismutase 3 (SOD3) is an extracellular antioxidant released upon cellular stimulation, providing an immediate change in extracellular antioxidant capacity (15, 20). Given the emerging body of literature supporting a relationship between intracellular oxidative stress, redox enzyme secretion, and soluble inflammatory markers, the quantification of PRDX-2, PRDX-4, TRX-1, TRX-R, and SOD3 in extracellular fluids offers the potential for accurate assessment of changes in oxidative stress and inflammation after different types of exercise.
Based upon existing knowledge of the factors that can impact acute changes in exercise-induced oxidative stress, we sought to perform two experiments to understand how novel markers such as PRDX-2, PRDX-4, TRX-1, TRX-R and SOD3 respond to acute exercise and whether relationships exist between changes in inflammation. Specifically, we aimed to characterize how these markers would be impacted by aerobic exercise intensity and eccentric-based resistance exercise. We tested the hypothesis that both protocols would elicit an increase in the concentrations of redox enzymes within plasma/serum after exercise, with higher exercise intensity causing a larger increase following aerobic exercise.
METHODS
Participants
Healthy, untrained participants were recruited for two independent studies (Table 1) Participants in both studies completed the International Physical Activity Questionnaire, which addresses habitual levels of weekly physical activity. Participants gave their informed, written consent, and all studies were approved by the local ethics review committee in accordance with the Declaration of Helsinki, 2008. Participants were all nonsmokers and had not taken any antioxidant vitamin supplements or anti-inflammatory drugs for 8 wk before the laboratory visits. All participants were required to refrain from any strenuous physical activity, consumption of alcoholic beverages, or caffeine for ≥2days before the experimental sessions.
Table 1.
Demographics for participants in studies 1 and 2
| Energy-Matched Trials (Study 1) | Eccentric-Based Resistance Exercise (Study 2) | Statistical Analysis | |
|---|---|---|---|
| No. of Participants | 9 | 16 | |
| Age, yr | 29 ± 5 | 25 ± 9 | P = NS |
| Body mass index, kg/m2 | 24.2 ± 3.4 | 25.3 ± 4.1 | P = NS |
| IPAQ (METs-min/wk) | 6,683 ± 3,835* | 2,540 ± 2,022 | *P = 0.004 |
| Watt maximum (Watt/kg) | 3.4 ± 0.5 | ||
| V̇o2max, mL·kg−1·min−1 | 44.5 ± 6.4 |
Values are means ± SE. Blank cells indicate missing data. IPAQ, International Physical Activity Questionnaire; NS, not significant.
Significant difference in comparison with study 2; P < 0.05.
Experimental Sessions
The full workflow for this project is detailed in Fig. 1. Experimental sessions took place in the morning (7 to 8 AM start time) under stable climatic conditions (18–20°C and humidity between 45 and 55%) and following at least a 10-h fast. After a period of rest, height (Seca Alpha, Hamburg, Germany) and mass (Tanita, Tokyo, Japan) were determined.
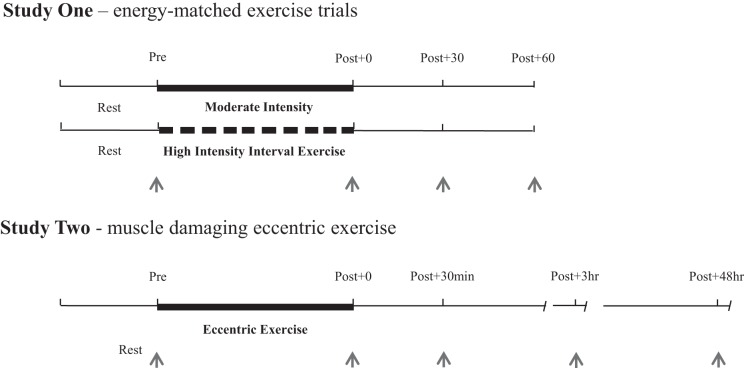
Fig. 1.
Schematic of the 2 exercise studies. Thick lines represent the exercise session, with thin lines indicating pre- and postexercise resting periods. Gaps between dark lines indicate the rest periods during the high-intensity interval exercise trial. Blood samples taken for each study are indicated as arrows.
In study 1, participants first visited the laboratory for an assessment of cardiorespiratory fitness (V̇o2max) using a ramp test to exhaustion on an electromagnetically braked cycle ergometer (Lode Excalibur Sport, Groningen, The Netherlands). The protocol involved commencing pedalling at 100 W, followed by fixed 30-W increments every 4 min. Oxygen uptake was assessed continuously using a breath-by-breath system (Oxycon Pro, Jaeger, Wuerzberg, Germany) and heart rate monitored using a Polar Vantage heart rate monitor (Polar, Kempele, Finland). The test ended when the participant reached volitional exhaustion or when a plateau in oxygen consumption was observed with an increase in workload (49). A final obtained value of rate of oxygen consumption was accepted as V̇o2max and expressed relative to body weight (mL·kg−1·min−1). At least 1 wk later, participants then undertook the first of two energy and time-matched cycling trials in a randomized order ≥1 wk apart: a continuous bout of moderate-intensity cycling at ~60% V̇o2 (%MAX) for 58 min (MOD) and a bout of high-intensity interval exercise (HIIE) consisting of 10 × 4-min intervals at 85% V̇o2 (%MAX), with 2-min rest intervals. In both trials, oxygen uptake was assessed continuously and power output was adjusted where necessary to maintain target and V̇o2 equal energy expenditure between MOD and HIIE (study 1). Rating of perceived exertion (RPE) was monitored every 5 min throughout the trials (5).
In study 2 (n = 16), nonresistance-trained men undertook an eccentric-based resistance exercise protocol adapted from a previous study by Alemany et al. (1). This muscle-damaging protocol was performed on a Humac Norm dynamometer (CSMI). The dynamometer lever arm was programmed to flex the participant’s knee from a start position of 10° of flexion to 90° of flexion, thus allowing a range of motion of 80°. The participants began with their leg at the start position and were asked to maximally contract their quadriceps against a resistance while the lever arm moved to the finish position (90° knee flexion). Once at the finish position, they were advised to relax their leg, and the dynamometer moved them back to the start position to avoid a concentric contraction being performed. The lever arm moved at a set speed of 60°/s. The bout consisted of 20 sets of 10 repetitions, with each set being separated by 1 min of rest. Visual feedback and verbal encouragement were provided to all participants to maximize torque output for each contraction.
Blood sampling and Plasma Isolation
For both studies, a catheter (Appleton Woods, Birmingham, UK) was inserted into the antecubital vein of the arm before exercise to obtain a baseline blood sample after 30 min of rest (Pre). The catheter was continually kept clear with isotonic saline solution (0.9% sodium chloride). As indicated in Fig. 1, blood samples were then taken immediately, 30 min, and 60 min after both HIIE and MOD (study 1: Pre, Post + 0, Post + 30, and Post + 60) and immediately, 30 min, 3 h, and 48 h following the muscle damage protocol (study 2: Pre, Post + 0, Post + 30min, Post +3 h, and Post + 48 h). The post + 48 h (study 2) blood sample was taken via venepuncture. At each time point, 12 mL of blood was drawn into vacutainer tubes containing either potassium ethylene diaminetetraacetic acid in study 1 (Becton, Dickson & Company, Oxford, UK) or no anticoagulant in study 2. In study 1, whole blood was centrifuged at 1, 525 g for 15 min at room temperature. In study 2, whole blood was allowed to clot at room temperature for 20 min and then centrifuged at 1,500 g for 15 min. The resulting plasma (study 1) and serum (study 2) were aliquoted and frozen at −80°C for future analysis of redox enzymes, IL-6, creatine kinase (CK), and lactate dehydrogenase (LDH). Capillary blood samples were obtained from the earlobe after 4 min of exercise and then every 6 min thereafter (i.e., end of each HIIE interval) in study 1. These samples were used for analysis of blood glucose and lactate concentrations to verify intensity-dependent differences between each protocol.
Analytical Procedures
PRDX-2, PRDX-4, TRX-1, TRX-R, and SOD3 ELISAs.
ELISAs for the detection of PRDX-2, PRDX-4, TRX, TRX-R, and SOD3 were developed in-house. Commercially available antigens and antibodies (i.e., PRDX-2, PRDX-4, TRX, and TRX-R) were purchased from either Abcam (Cambridge, UK; ab) or Sigma Aldrich (Dorset, UK; SRP). The human SOD3 antigen and rabbit antiserum directed against human SOD3 were developed as previously described (16, 20). Plasma or serum and standards (100 μL) were loaded onto individual wells of an ELISA plate (Thermo Scientific F8 polysorp immune wells), and protein was left to bind overnight at 4°C. Wells were then prewashed with PBS wash buffer, supplemented with 0.1% casein (PBSwC, 200 μL), and then blocked with 1% casein in PBS (200 μL) for 30 min at room temperature with gentle agitation. Anti-human rabbit antibodies for PRDX-2 (ab133481, 1:2,000), PRDX-4 (ab59542, 1:2,000), and SOD3 (in-house, 1:2,000) and anti-human mouse antibodies for TRX-1 (ab16965, 1:8,000) and TRX-R (ab16847, 1:1,000) were then added to each well and diluted in PBSwC for 45 min at room temperature. Following this, 100 μL of anti-rabbit (1:5,000) or anti-mouse (1:500) IgG biotin antibodies in PBSwC and streptavidin-horseradish peroxidase (1:2,000 in PBSwC) were added separately to each well, both for 45 min, with gentle agitation. Between all stages, all wells were washed three times with PBSwC. Finally, 100 μL of 3,3′,5,5′-tetramethylbenzidine (10 μg) was added per well, and the plate was left to develop in the dark for 15–25 min. Stop solution (1.5 mM H2SO4, 50 μL) was then added to each well and absorption at 450 nm subsequently evaluated by using a plate reader (Multiskan Ascent, Thermo Labsystems). Concentration of each antigen was then determined by comparing absorbance values of recombinant PRDX-2 (ab167977; Abcam), PRDX-4 (ab93947; Abcam), TRX-1 (ab51064; Abcam), TRX-R (SRP6081; Sigma-Aldrich), and SOD3 (in-house) proteins (0–50 ng/mL). ELISA validation experiments showed no cross-reactivity of the PRDX-2, PRDX-4, TRX-1, TRX-R, or SOD3 antibodies with the respective antigens, nor with serum albumin. All values were adjusted for plasma volume according to previous methods (12).
Other analyses.
In both studies, a cytometric bead array was used to quantify plasma (study 1) and serum (study 2) IL-6 concentrations on a BD C6 Accuri Flow Cytometer (BD Biosciences). In study 1, blood lactate and glucose concentrations were determined immediately following collection using an automated lactate and glucose analyzer (Biosen C-Line Clinic, EKF-Diagnostic, Barleben, Germany). In study 2, serum CK and LDH concentrations were determined to monitor muscle damage using an automated ABX Pentra 400 system (Horiba UK). Haematocrit and hemoglobin concentrations were used to ascertain plasma volume changes and make appropriate adjustments in plasma redox enzyme and IL-6 concentrations (Beckman Coulter, London, UK).
Statistical Analysis
The Shapiro-Wilk test was used to test for normality in scale data at all time points. Differences between participant characteristics and the physiological responses to exercise in both studies were assessed using unpaired-samples t-tests or nonparametric Mann-Whitney U-tests. The influence of exercise on plasma/serum PRDX-2, PRDX-4, SOD3, TRX-1, TRX-R, and IL-6 concentration was assessed over time by repeated-measures analysis of variance (ANOVA) or nonparametric Wilcoxon signed-rank tests, depending on variable normality. Post hoc analysis of any significant effect of time or interaction effect (study 1: group × time) was performed by a test of simple effects by pairwise comparisons with Bonferroni correction. Effect sizes for main effects and interaction effects of ANOVA are presented as partial eta2 (η2p), using Cohen’s definition of η2p of 0.01, 0.06, and 0.14 for “small,” “medium,” and “large” effects, respectively (10). Pearson correlation and Spearman rank were used to assess the relationship between parametric and nonparametric data, respectively. All values are presented as means ± SD or error (indicated throughout this article). Statistical significance was accepted at the P < 0.05 level. Statistical analyses were performed using SPSS (PASW Statistics, release 23.0; SPSS, Chicago, IL).
RESULTS
There was no significant difference in age or BMI between the participants taking part in the two studies. Participants in study 1 (P = 0.004) had significantly higher self-reported physical activity than those in study 2.
Acute Physiological Responses to HIIE and MOD
For study 1, the physiological responses during each exercise bout are reported in Table 2. Peak V̇o2 and RPE were significantly greater in HIIE compared with MOD (P < 0.00001), but there were no statistically significant differences in mean V̇o2 and energy expenditure. Whole blood lactate and glucose data are reported in Table 2. Mean lactate concentration was significantly higher during HIIE than MOD (P < 0.0001), but there was no significant difference in average glucose concentration between trials.
Table 2.
Physiological response to aerobic-based exercise (study 1)
| Trial | Energy-matched Cycling Trials (Study 1) |
Statistical Analysis | |
|---|---|---|---|
| Continuous cycling for 58 min, predicted 60% V̇o2max (MOD) |
10 × 4-Min cycling intervals, predicted 85% V̇o2max (2 min rest intervals; total time = 58 min; HIIE) | ||
| Mean V̇o2max, % | 56.5 ± 2.6 | 58.9 ± 4.3 | P = NS |
| Energy expenditure, kJ | 2,077 ± 340 | 2,072 ± 339 | P = NS |
| Average RPE | 12 ± 1 | 16 ± 1* | *P < 0.0001 |
| Mean blood lactate, mmol/L | 1.9 ± 0.6 | 6.8 ± 1.4* | *P < 0.0001 |
| Mean blood glucose, mmol/L | 3.9 ± 0.3 | 4.5 ± 0.6 | P = NS |
Values are means ± SE. MOD, moderate-intensity exercise; HIIE, high-intensity interval exercise; NS, not significant; RPE, rating of perceived exertion.
P < 0.0001, significant difference between MOD and HIIE.
NS, P > 0.05.
Effects of Eccentric-Based Resistance Exercise on Muscle Damage Markers
Changes in markers of muscle damage are reported in Table 3. A stepwise increase (Post + 48 h > Post + 3 h > Post + 30 min > Post + 0 > Pre) in serum CK concentration was observed over time, peaking above Pre at Post + 48 h (P < 0.001). Serum LDH concentration was elevated above Pre at all postexercise time points (P < 0.05), also increasing Post + 3 h and Post + 48 h, relative to Post + 30 min (P < 0.05).
Table 3.
Changes in markers or muscle damage following eccentric-based resistance exercise (study 2)
| Pre | Post + 0 | Post + 30 min | Post + 3 h | Post + 48 h | |
|---|---|---|---|---|---|
| Creatine kinase, U/L | 147.6 ± 27.1 | 236.1 ± 65.5* | 289.9 ± 86.0*† | 560.8 ± 273.5**†# | 575.9 ± 290.8**†#‡ |
| Lactate dehydrogenase, U/L | 254.9 ± 130.6 | 282.7 ± 70.9* | 274.1 ± 77.1* | 290.3 ± 77.8*† | 299.9 ± 165.2*† |
Values are means ± SD.
P < 0.05 and
P < 0.001, significant difference in comparison to Pre.
Significant difference in comparison to Post + 0; P <0.05.
Significant difference in comparison to Post + 30 min, P < 0.05.
Significant difference in comparison to Post + 3 h, P < 0.05.
Effects of Aerobic and Eccentric-Based Resistance Exercise on IL-6 Concentration
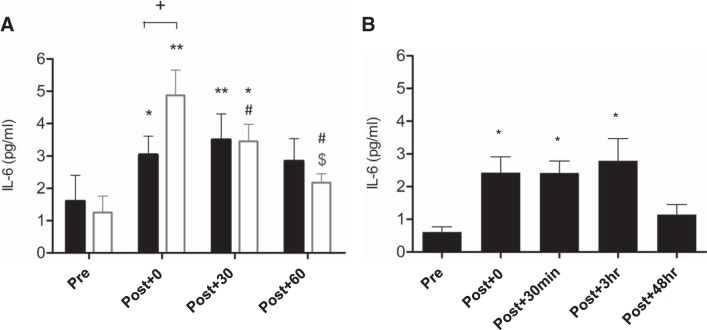
IL-6 data are presented in Fig. 3. In study 1, plasma IL-6 increased in both trials [time effect: F(3) = 15.5, P < 0.0001, η2 = 0.66], being elevated above resting values, both immediately (P = 0.004) and Post + 30 (P = 0.002), but not Post + 60 (Fig. 3A). The magnitude of this increase was significantly greater Post-Ex in HIIE (P = 0.031) than in MOD [time × condition effect: F(3) = 7.0, P < 0.001, η2 = 0.47]. IL-6 concentration decreased Post + 30 (P = 0.004) and Post + 60 (P = 0.007) relative to Post + 0 and Post + 60 and relative to Post + 30 (P = 0.026) in HIIE only. In study 2 (Fig. 3B), IL-6 concentration was significantly higher at all time points ≤3 h, but not 48 h after exercise, relative to Pre [time effect: F(4) = 14.3, P < 0.0001, η2 = 0.30].
Fig. 3.
Changes in plasma interleukin (IL)-6 in response to 2 energy-matched cycling bouts: moderate steady-state (MOD; black bars) and high-intensity interval exercise (HIIE; open bars) (A) and an eccentric-based resistance exercise protocol (B). Values are means ± SE. *Significant differences relative to Pre, P < 0.05; **P < 0.001; #significant difference relative to Post + 0, P < 0.05; $significant difference relative to Post + 30, P < 0.05; +significant difference between MOD and HIIE, P < 0.05.
Effects of Aerobic Exercise on PRDX-2, PRDX-4, TRX-1, TRX-R, and SOD3 Concentration
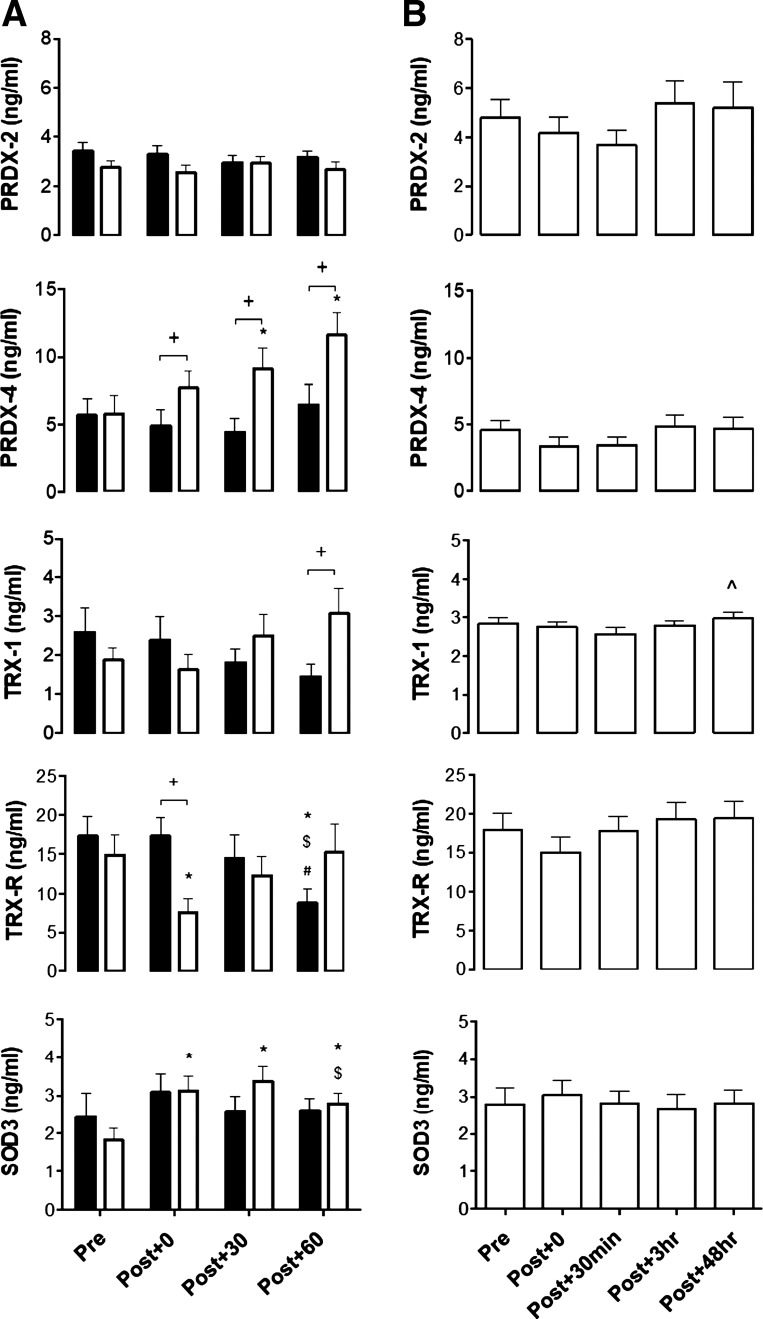
No differences were observed in resting concentrations of PRDX-2, PRDX-4, TRX-1, TRX-R, or SOD3 when quantified in plasma and serum across all trials. Changes in plasma PRDX-2, PRDX-4, TRX-1, TRX-R, and SOD3 in response to MOD and HIIE are reported in Fig. 2A. There was a significant increase in plasma SOD3 [trial × time effect: F(3,1) = 5.3, P = 0.028, η2 = 0.31] and PRDX-4 following HIIE only (nonparametric tests: all P < 0.05). SOD3 concentration was elevated above pre-exercise values at all post-HIIE time points, peaking at Post + 0 (P = 0.015) and Post + 30 (P = 0.013), but only significantly higher than MOD at Post + 30 (P = 0.05). Plasma SOD3 concentration decreased relative to Post + 30 at Post + 60 (P = 0.013). Relative to Pre, PRDX-4 concentration increased at Post + 30 (P = 0.015) and Post + 60 (P = 0.008) following HIIE, with PRDX-4 concentration higher at all postexercise time points compared with MOD (P < 0.038). There was a significant decrease in plasma TRX-R concentration in both MOD and HIIE. Relative to Pre, TRX-R significantly decreased at Post + 0 in HIIE only (P = 0.021), with values significantly less than MOD (P = 0.011). Following MOD, TRX-R was significantly lower at Post + 60, relative to all time points (all P < 0.038). There were no statistically significant changes in PRDX-2 and TRX-1 concentration over time in either trial; however, TRX-1 concentration was significantly higher in HIIE than MOD Post + 60 only (P = 0.021).
Fig. 2.
Changes in redox enzyme concentration in response to 2 energy-matched cycling bouts. A: moderate steady-state (MOD; black bars) and high-intensity interval exercise (HIIE; open bars) and an eccentric-based resistance exercise protocol. B: peroxiredoxin (PRDX)-2, PRDX-4, thioredoxin-1 (TRX-1), thioredoxin-reductase (TRX-R), and superoxide dismutase 3 (SOD3). Values are means ± SE.*Significant differences relative to Pre, P < 0.05; #significant difference relative to Post + 0, P < 0.05; $significant difference relative to Post + 30, P < 0.05; +significant difference between MOD and HIIE, P < 0.05; ^significant difference between Post + 30 min and Post + 48 h time points.
Effects of Eccentric-Based Resistance Exercise on PRDX-2, PRDX-4, TRX-1, TRX-R, and SOD3 Concentration
Serum redox enzyme concentration changes in response to an eccentric-based resistance exercise protocol are presented in Fig. 2B. A trend was observed for a decrease in PRDX-2 concentration Post + 30min (−1.12 ng/mL); however, this did not reach statistical significance [time effect: F(4) = 2.3, P = 0.065, η2 = 0.13]. Similarly, no significant changes were noted in PRDX-4, TRX-R, or SOD3 for ≤48 h following eccentric-based resistance exercise. A significant increase in TRX-1 was shown Post + 48 h, relative to Post + 30min (P = 0.039), but not Pre (P = 0.309).
DISCUSSION
The current results have characterized the kinetic responses of endogenous redox enzymes within the extracellular environment after exercise for the first time. We highlight novel findings that high-intensity aerobic cycling induces a significant increase in SOD3 and PRDX-4 in healthy, untrained men. Similar responses were not observed following moderate-intensity cycling or muscle-damaging resistance exercise. In contrast, plasma TRX-R concentration decreased within 1 h following moderate- and high-intensity cycling exercise but not resistance exercise. Taken together, these findings provide novel insights into the regulation of extracellular redox enzymes in response to exercise.
The current data highlight modality and exercise intensity-specific increases in two abundant redox enzymes. In response to aerobic exercise, PRDX-4 but not PRDX-2 concentration increased 30 min following HIIE and remained elevated until Post + 60. The secretory pathways of PRDXs are isoform specific, with endoplasmic reticulum (ER, i.e., PRDX-4) and cytosolic (i.e., PRDX-2) resident isoforms released via classical and nonclassical secretory pathways, respectively (8). Therefore, the current data suggest that exercise may activate the ER-Golgi pathway to secrete PRDX-4 in an intensity-dependent manner. SOD3, which is also released via this pathway, increased more rapidly than PRDX-4 following HIIE (Post + 0), with levels tailing off Post + 60, relative to Post + 30. SOD3 is an antioxidant enzyme released directly from the cell membrane (15, 20), specifically secreted during exercise to metabolize superoxide anions produced in the extracellular environment to H2O2 (30). The different peak concentrations of SOD3 (i.e., Post + 0) and PRDX-4 (i.e., Post + 30) following HIIE may be explained in part, by 1) the membrane proximity of SOD3 compared with the ER location of PRDX-4 and 2) the appearance of superoxide anions first in the extracellular space following exercise, before their metabolism to H2O2, which then induced PRDX-4 secretion. This may also be reflective of differential secretion rates of SOD3 and PRDX-4 from various tissues during and following exercise. Both proteins are expressed in skeletal muscle (19), a highly redox active tissue (36); however, PRDX-4 is primarily located in pancreas, liver, and heart (21), whereas SOD3 is expressed in the heart and vasculature tissue (42). The association with the vasculature may explain the more rapid increase in plasma SOD3 concentration following HIIE. Aside from these increases, a modest decrease was observed in plasma TRX-R after both MOD and HIIE (study 1), with this change being much more rapid in HIIE (Post + 0) compared with MOD (Post + 60). The mechanisms driving a decrease in TRX-R after exercise are unclear at present. The decrease may represent transient homeostatic fluctuations involving uptake of redox enzymes by neighboring cells and tissues, perhaps to regulate intracellular redox balance (23).
A finding that was in contrast to our hypothesis was that eccentric-based resistance exercise did not induce an increase in the extracellular concentrations of redox enzymes. The measurement of redox enzymes in plasma and serum is an emerging area of biomedical research, particularly in the context of acute (24) and chronic (13, 43) inflammatory conditions, where PRDXs and TRX-1 have been associated with enhanced cytokine and chemokine production (22, 38). The participants in both studies were relatively inactive, with participants in study 2 in particular reporting significantly lower levels of habitual physical activity (Table 2) and being unaccustomed to eccentric-based resistance exercise. Unaccustomed eccentric exercise induces significant amounts of acute muscle damage and inflammation (7), as demonstrated by the stepwise increases in CK and LDH concentrations for ≤48 h following our protocol and IL-6 for ≤3 h postexercise (Fig. 3B). These data suggest that the increase in SOD3 and PRDX-4 observed in study 1 is unlikely due to just a disruption to the plasma membrane, given that no changes were observed following a muscle-damaging bout of resistance exercise. It must be acknowledged that only selective time points were measured following the protocol, and perhaps the secretion of redox enzymes occurs between 3 and 48 h postexercise. Nevertheless, this study has highlighted for the first time that redox enzyme concentrations do not match that of established markers of muscle damage and inflammation when measured in serum samples following an eccentric-based resistance exercise bout. In response to aerobic-based exercise, we have recently demonstrated a positive association between intracellular peroxiredoxin (I–IV) overoxidation in immune cells and plasma IL-6 concentration (47). In the current study, IL-6 concentration increased in an intensity-dependent manner (HIIE > MOD) following aerobic exercise (Fig. 3A); however, there were no statistically significant relationships between absolute or exercise-induced changes in PRDX-4 and SOD3 with IL-6. Therefore, the observations across both studies suggest no relationship between IL-6 and redox enzymes after exercise. A larger sample size may be needed to adequately address these associations and support the previously documented relationship between plasma/serum redox enzymes and soluble inflammatory markers (27, 40).
The results of the current investigation demonstrate clear differences in the changes in SOD3, TRX-R, and PRDX-4 following aerobic versus eccentric-based resistance exercise. With regard to PRDX-2 and TRX-1, no changes were observed following aerobic or eccentric-based resistance exercise. Both PRDX-2 and TRX-1 are cytosolic redox enzymes that contain no NH2-terminal signal peptide for secretion and thus are released via nonclassical pathways and are associated with extracellular vesicles (EVs) such as exosomes and nanoparticles (26a). PRDX-2 and TRX-1 are detectable in plasma/serum samples through their association with the exofacial surface of the EV membrane (18, 44); however, their protein levels may be higher due to protein contained within the EVs. This protein would not be detectable by antibodies when enclosed within the lipid membrane during ELISA quantification, as previously shown (32). Indeed, recent evidence has highlighted that a series of leaderless redox enzymes (i,e, PRDX-1, PRDX-2, PRDX-5, PRDX-6, TRX-1, SOD1, and SOD2) are secreted in EVs via a nonclassical route following exposure to stress, with classically secreted SOD3, TRX-R, and PRDX-4 not detectable within EVs (2). This may explain why plasma/serum PRDX-2 and TRX-1 concentration did not significantly change following muscle-damaging or aerobic exercise. It must be noted that TRX-1 concentration was significantly higher 48 h after the eccentric-based resistance exercise protocol relative to Post + 0 (study 2) and also significantly higher at Post + 60 in HIIE compared with MOD (study 1). These findings again underpin intensity-dependent differences despite concentrations not being higher than pre-exercise values in both cases. In response to a far more extreme bout of exercise, Marumoto et al. (29) reported a marked increase in TRX-1 levels (17.9 ± 1.2 ng/mL at baseline to 70.1 ± 6.9 ng/mL) after a 2-day, 130-km ultra-endurance marathon; however, these exercise bouts were substantially different in nature and thus hard to directly compare. Although an ultramarathon is accompanied by significant amounts of muscle damage, given the findings of study 2, it is unlikely that muscle damage is the primary cause of TRX-1 secretion in this context. Further work is needed to clarify whether TRX-1 and PRDX-2 protein levels alter within EVs after conventional bouts (i.e., not ultra-endurance) of muscle-damaging and aerobic-based exercise.
This study has quantified the responses of antioxidant enzymes in the extracellular environment following acute exercise in age- and BMI-matched individuals from two independent exercise studies (Table 1). We must acknowledge that the studies would have benefited from a direct comparison between redox enzyme concentrations and other established biomarkers of oxidative stress (e.g., protein carbonyls and F2-isoprostanes). However, due to limited sample volume, this analysis was not feasible, and therefore, it should be prioritized as an area of future research. A second limitation is that the quantification of redox enzymes and IL-6 was undertaken in both plasma (study 1) and serum (study 2); however, there were no differences in any of these proteins when quantified in pre-exercise samples.
Conclusion
The results of the present study have highlighted that plasma SOD3 and PRDX-4 concentration increased in response to acute exercise. Importantly, the secretion of these proteins appears to be intensity and modality dependent, with increases observed only in response to high-intensity aerobic cycling in untrained individuals. A decrease in TRX-R was also noted following different aerobic exercise bouts, with exercise intensity driving a more rapid decrease in TRX-R. Future research is required to pinpoint the precise mechanisms governing the secretion and uptake of redox enzymes and their role in regulating redox balance between tissues after exercise.
GRANTS
This research was supported by the University of Worcester and the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.W., T.C., L.J., B.H., D.J.H., S.S.M., M.R.L., and S.J.C. conceived and designed research; A.J.W., G.K., T.C., L.J., J.V., M.D., B.H., D.J.H., and M.R.L. performed experiments; A.J.W., G.K., T.C., L.J., and J.V. analyzed data; A.J.W. interpreted results of experiments; A.J.W. and S.J.C. prepared figures; A.J.W. drafted manuscript; A.J.W., G.K., T.C., L.J., B.H., D.J.H., S.S.M., A.H., S.V.P., N.C.B., M.R.L., and S.J.C. edited and revised manuscript; A.J.W., G.K., T.C., L.J., J.V., M.D., B.H., D.J.H., S.S.M., A.H., S.V.P., N.C.B., M.R.L., and S.J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
This project involved analysis of blood samples from two independent exercise studies run by A. J. Wadley, T. Cullen, J. Vautrinot, G. Keane, M. Davies, and S. J. Coles (study 1) and D. J. Hunter, B. Hussey, L. James, S. Mastana, and M. R. Lindley (study 2). A. J. Wadley and G. Keane carried out ELISA optimization and subsequent analysis.
REFERENCES
- 1.Alemany JA, Delgado-Díaz DC, Mathews H, Davis JM, Kostek MC. Comparison of acute responses to isotonic or isokinetic eccentric muscle action: differential outcomes in skeletal muscle damage and implications for rehabilitation. Int J Sports Med 35: 1–7, 2014. doi: 10.1055/s-0032-1327652. [DOI] [PubMed] [Google Scholar]
- 2.Bekeschus S, Moritz J, Schmidt A, Wende K. Redox regulation of leukocyte-derived microparticle release and protein content in response to cold physical plasma-derived oxidants. Clin Plasma Med 7–8: 24–35, 2017. doi: 10.1016/j.cpme.2017.07.001. [DOI] [Google Scholar]
- 3.Bloomer RJ, Davis PG, Consitt LA, Wideman L. Plasma protein carbonyl response to increasing exercise duration in aerobically trained men and women. Int J Sports Med 28: 21–25, 2007. doi: 10.1055/s-2006-924140. [DOI] [PubMed] [Google Scholar]
- 4.Bloomer RJ, Goldfarb AH, Wideman L, McKenzie MJ, Consitt LA. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J Strength Cond Res 19: 276–285, 2005. doi: 10.1519/14823.1. [DOI] [PubMed] [Google Scholar]
- 5.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann C, Blossfeld J, Pesch M, Krone B, Wiesiollek K, Capin D, Montiel G, Hellmich M, Bloch W, Brixius K. Lipid-peroxidation and peroxiredoxin-overoxidation in the erythrocytes of non-insulin-dependent type 2 diabetic men during acute exercise. Eur J Appl Physiol 112: 2277–2287, 2012. doi: 10.1007/s00421-011-2203-x. [DOI] [PubMed] [Google Scholar]
- 7.Bruunsgaard H, Galbo H, Halkjaer-Kristensen J, Johansen TL, MacLean DA, Pedersen BK. Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J Physiol 499: 833–841, 1997. doi: 10.1113/jphysiol.1997.sp021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JW, Lee SH, Lu Y, Yoo YJ. Transforming growth factor-β1 induces the non-classical secretion of peroxiredoxin-I in A549 cells. Biochem Biophys Res Commun 345: 118–123, 2006. doi: 10.1016/j.bbrc.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 9.Cobley JN, Close GL, Bailey DM, Davison GW. Exercise redox biochemistry: Conceptual, methodological and technical recommendations. Redox Biol 12: 540–548, 2017. doi: 10.1016/j.redox.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen J. Statistical Power Analysis for the Behavioural Sciences (2nd ed.). New York: Taylor & Francis Group, 1988. [Google Scholar]
- 11.Deminice R, Sicchieri T, Payão PO, Jordão AA. Blood and salivary oxidative stress biomarkers following an acute session of resistance exercise in humans. Int J Sports Med 31: 599–603, 2010. doi: 10.1055/s-0030-1255107. [DOI] [PubMed] [Google Scholar]
- 12.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 13.Gerrits EG, Alkhalaf A, Landman GW, van Hateren KJJ, Groenier KH, Struck J, Schulte J, Gans ROB, Bakker SJL, Kleefstra N, Bilo HJG. Serum peroxiredoxin 4: a marker of oxidative stress associated with mortality in type 2 diabetes (ZODIAC-28). PLoS One 9: e89719, 2014. doi: 10.1371/journal.pone.0089719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance [Online]. Am J Clin Nutr 87: 142–149, 2008. doi: 10.1093/ajcn/87.1.142. http://www.ncbi.nlm.nih.gov/pubmed/18175748. [DOI] [PubMed] [Google Scholar]
- 15.Gottfredsen RH, Goldstrohm DA, Hartney JM, Larsen UG, Bowler RP, Petersen SV. The cellular distribution of extracellular superoxide dismutase in macrophages is altered by cellular activation but unaffected by the naturally occurring R213G substitution. Free Radic Biol Med 69: 348–356, 2014. doi: 10.1016/j.freeradbiomed.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottfredsen RH, Tran SM, Larsen UG, Madsen P, Nielsen MS, Enghild JJ, Petersen SV. The C-terminal proteolytic processing of extracellular superoxide dismutase is redox regulated. Free Radic Biol Med 52: 191–197, 2012. doi: 10.1016/j.freeradbiomed.2011.10.443. [DOI] [PubMed] [Google Scholar]
- 17.Groussard C, Rannou-Bekono F, Machefer G, Chevanne M, Vincent S, Sergent O, Cillard J, Gratas-Delamarche A. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur J Appl Physiol 89: 14–20, 2003. doi: 10.1007/s00421-002-0767-1. [DOI] [PubMed] [Google Scholar]
- 18.Hara T, Kondo N, Nakamura H, Okuyama H, Mitsui A, Hoshino Y, Yodoi J. Cell-surface thioredoxin-1: possible involvement in thiol-mediated leukocyte-endothelial cell interaction through lipid rafts. Antioxid Redox Signal 9: 1427–1437, 2007. doi: 10.1089/ars.2007.1661. [DOI] [PubMed] [Google Scholar]
- 19.Hitomi Y, Watanabe S, Kizaki T, Sakurai T, Takemasa T, Haga S, Ookawara T, Suzuki K, Ohno H. Acute exercise increases expression of extracellular superoxide dismutase in skeletal muscle and the aorta. Redox Rep 13: 213–216, 2008. doi: 10.1179/135100008X308894. [DOI] [PubMed] [Google Scholar]
- 20.Iversen MB, Gottfredsen RH, Larsen UG, Enghild JJ, Praetorius J, Borregaard N, Petersen SV. Extracellular superoxide dismutase is present in secretory vesicles of human neutrophils and released upon stimulation. Free Radic Biol Med 97: 478–488, 2016. doi: 10.1016/j.freeradbiomed.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Jin D-Y, Chae HZ, Rhee SG, Jeang K-T. Regulatory role for a novel human thioredoxin peroxidase in NF-κB activation. J Biol Chem 272: 30952–30961, 1997. doi: 10.1074/jbc.272.49.30952. [DOI] [PubMed] [Google Scholar]
- 22.Knoops B, Argyropoulou V, Becker S, Ferté L, Kuznetsova O. Multiple roles of peroxiredoxins in inflammation. Mol Cells 39: 60–64, 2016. doi: 10.14348/molcells.2016.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo N, Ishii Y, Kwon YW, Tanito M, Sakakura-Nishiyama J, Mochizuki M, Maeda M, Suzuki S, Kojima M, Kim YC, Son A, Nakamura H, Yodoi J. Lipid raft-mediated uptake of cysteine-modified thioredoxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1. Antioxid Redox Signal 9: 1439–1448, 2007. doi: 10.1089/ars.2007.1665. [DOI] [PubMed] [Google Scholar]
- 24.Kunze A, Zierath D, Tanzi P, Cain K, Becker K. Peroxiredoxin 5 (PRX5) is correlated inversely to systemic markers of inflammation in acute stroke. Stroke 45: 608–610, 2014. doi: 10.1161/STROKEAHA.113.003813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol 589: 2119–2127, 2011. doi: 10.1113/jphysiol.2010.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KP, Shin YJ, Cho SC, Lee SM, Bahn YJ, Kim JY, Kwon ES, Jeong DY, Park SC, Rhee SG, Woo HA, Kwon KS. Peroxiredoxin 3 has a crucial role in the contractile function of skeletal muscle by regulating mitochondrial homeostasis. Free Radic Biol Med 77: 298–306, 2014. doi: 10.1016/j.freeradbiomed.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 26a.Léveillard T, Aït-Ali N. Cell signaling with extracellular thioredoxin and thioredoxin- like proteins: insight into their mechanisms of action. Oxid Med Cell Longev 2017: 1–11, 2017. doi: 10.1155/2017/8475125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CH, Kuo SW, Hsu LM, Huang SC, Wang CH, Tsai PR, Chen YS, Jou TS, Ko WJ. Peroxiredoxin 1 induces inflammatory cytokine response and predicts outcome of cardiogenic shock patients necessitating extracorporeal membrane oxygenation: an observational cohort study and translational approach. J Transl Med 14: 114, 2016. doi: 10.1186/s12967-016-0869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manabe Y, Takagi M, Nakamura-Yamada M, Goto-Inoue N, Taoka M, Isobe T, Fujii NL. Redox proteins are constitutively secreted by skeletal muscle. J Physiol Sci 64: 401–409, 2014. doi: 10.1007/s12576-014-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marumoto M, Suzuki S, Hosono A, Arakawa K, Shibata K, Fuku M, Goto C, Tokudome Y, Hoshino H, Imaeda N, Kobayashi M, Yodoi J, Tokudome S. Changes in thioredoxin concentrations: an observation in an ultra-marathon race. Environ Health Prev Med 15: 129–134, 2010. doi: 10.1007/s12199-009-0119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McArdle F, Pattwell DM, Vasilaki A, McArdle A, Jackson MJ. Intracellular generation of reactive oxygen species by contracting skeletal muscle cells. Free Radic Biol Med 39: 651–657, 2005. doi: 10.1016/j.freeradbiomed.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Michailidis Y, Jamurtas AZ, Nikolaidis MG, Fatouros IG, Koutedakis Y, Papassotiriou I, Kouretas D. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med Sci Sports Exerc 39: 1107–1113, 2007. doi: 10.1249/01.mss.0b013e318053e7ba. [DOI] [PubMed] [Google Scholar]
- 32.Nameta M, Saijo Y, Ohmoto Y, Katsuragi K, Yamamoto K, Yamamoto T, Ishibashi K, Sasaki S. Disruption of membranes of extracellular vesicles is necessary for ELISA determination of urine AQP2: proof of disruption and epitopes of AQP2 antibodies. Int J Mol Sci 17: 1634, 2016. doi: 10.3390/ijms17101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pattwell D, Ashton T, McArdle A, Griffiths RD, Jackson MJ. Ischemia and reperfusion of skeletal muscle lead to the appearance of a stable lipid free radical in the circulation. Am J Physiol Heart Circ Physiol 284: H2400–H2404, 2003. doi: 10.1152/ajpheart.00931.2002. [DOI] [PubMed] [Google Scholar]
- 34.Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med 37: 1064–1072, 2004. doi: 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med 51: 942–950, 2011. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol 589: 2129–2138, 2011. doi: 10.1113/jphysiol.2010.201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poynton RA, Hampton MB. Peroxiredoxins as biomarkers of oxidative stress. Biochim Biophys Acta 1840: 906–912, 2014. doi: 10.1016/j.bbagen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Riddell JR, Wang XY, Minderman H, Gollnick SO. Peroxiredoxin 1 stimulates secretion of proinflammatory cytokines by binding to TLR4. J Immunol 184: 1022–1030, 2010. doi: 10.4049/jimmunol.0901945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665–8670, 2009. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salzano S, Checconi P, Hanschmann EM, Lillig CH, Bowler LD, Chan P, Vaudry D, Mengozzi M, Coppo L, Sacre S, Atkuri KR, Sahaf B, Herzenberg LA, Herzenberg LA, Mullen L, Ghezzi P. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci USA 111: 12157–12162, 2014. doi: 10.1073/pnas.1401712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shing CM, Peake JM, Ahern SM, Strobel NA, Wilson G, Jenkins DG, Coombes JS. The effect of consecutive days of exercise on markers of oxidative stress. Appl Physiol Nutr Metab 32: 677–685, 2007. doi: 10.1139/H07-051. [DOI] [PubMed] [Google Scholar]
- 42.Strålin P, Karlsson K, Johansson BO, Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol 15: 2032–2036, 1995. doi: 10.1161/01.ATV.15.11.2032. [DOI] [PubMed] [Google Scholar]
- 43.Szabó-Taylor KÉ, Eggleton P, Turner CA, Faro ML, Tarr JM, Tóth S, Whiteman M, Haigh RC, Littlechild JA, Winyard PG. Lymphocytes from rheumatoid arthritis patients have elevated levels of intracellular peroxiredoxin 2, and a greater frequency of cells with exofacial peroxiredoxin 2, compared with healthy human lymphocytes. Int J Biochem Cell Biol 44: 1223–1231, 2012. doi: 10.1016/j.biocel.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 166: 7309–7318, 2001. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 46.Turner JE, Bennett SJ, Campbell JP, Bosch JA, Aldred S, Griffiths HR. The antioxidant enzyme peroxiredoxin-2 is depleted in lymphocytes seven days after ultra-endurance exercise. Free Radic Res 47: 821–828, 2013. doi: 10.3109/10715762.2013.828836. [DOI] [PubMed] [Google Scholar]
- 47.Wadley AJ, Chen YW, Bennett SJ, Lip GYH, Turner JE, Fisher JP, Aldred S. Monitoring changes in thioredoxin and over-oxidised peroxiredoxin in response to exercise in humans. Free Radic Res 49: 290–298, 2015. doi: 10.3109/10715762.2014.1000890. [DOI] [PubMed] [Google Scholar]
- 48.Wadley AJ, Chen YW, Lip GY, Fisher JP, Aldred S. Low volume-high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J Sports Sci 34: 1–9, 2016. doi: 10.1080/02640414.2015.1035666. [DOI] [PubMed] [Google Scholar]
- 49.Wadley AJ, Holliday A, Morgan RG, Heesom KJ, Aldred S, Peters DM, Bueno AA, Coles SJ. Preliminary evidence of reductive stress in human cytotoxic T cells following exercise. J Appl Physiol (1985) 125: 586–595, 2018. doi: 10.1152/japplphysiol.01137.2017. [DOI] [PubMed] [Google Scholar]
- 50.Winterbourn CC. The biological chemistry of hydrogen peroxide. Methods Enzymol 528: 3–25, 2013. doi: 10.1016/B978-0-12-405881-1.00001-X. [DOI] [PubMed] [Google Scholar]