Abstract
It is unknown whether central hemodynamics are impaired during exercise in chronic obstructive pulmonary disease (COPD) patients. We hypothesized that, at a similar absolute V̇o2 during exercise, COPD patients would have a lower stroke volume and cardiac output compared with healthy controls. Furthermore, we hypothesized that greater static hyperinflation [ratio of inspiratory capacity to total lung capacity (IC/TLC)] and expiratory intrathoracic pressure would be significantly related to the lower cardiac output and stroke volume responses in COPD patients. Clinically stable COPD (n = 13; FEV1/FVC: 52 ± 13%) and controls (n = 10) performed constant workload submaximal exercise at an absolute V̇o2 of ~1.3 L/min. During exercise, inspiratory capacity maneuvers were performed to determine operating lung volumes and cardiac output (via open-circuit acetylene rebreathe technique) and esophageal pressure were measured. At similar absolute V̇o2 during exercise (P = 0.81), COPD had lower cardiac output than controls (COPD: 11.0 ± 1.6 vs. control: 12.2 ± 1.2 L/min, P = 0.03) due to a lower stroke volume (COPD: 107 ± 13 vs. control: 119 ± 19 mL, P = 0.04). The heart rate response during exercise was not different between groups (P = 0.66). FEV1 (%predicted) and IC/TLC were positively related to stroke volume (r = 0.68, P = 0.01 and r = 0.77, P < 0.01). Last, esophageal pressure-time integral during inspiration was positively related to cardiac output (r = 0.56, P = 0.047). These data demonstrate that COPD patients have attenuated cardiac output and stroke volume responses during exercise compared with control. Furthermore, these data suggest that the COPD patients with the most severe hyperinflation and more negative inspiratory intrathoracic pressures have the most impaired central hemodynamic responses.
NEW & NOTEWORTHY Chronic obstructive pulmonary disease leads to cardiac structural changes and pulmonary derangements that impact the integrative response to exercise. However, it is unknown whether these pathophysiological alterations influence the cardiac response during exercise. Herein, we demonstrate that COPD patients exhibit impaired central hemodynamics during exercise that are worsened with greater hyperinflation.
Keywords: cardiac output, hyperinflation, intrathoracic pressure, stroke volume, ventilatory constraints
INTRODUCTION
Patients with chronic obstructive pulmonary disease (COPD) exhibit reduced exercise capacity (31). The putative underlying mechanisms responsible are multifactorial and include ventilatory, central hemodynamic, and peripheral limitations (1, 10, 25, 30). One of the strongest predictors of exercise capacity in COPD is peak oxygen pulse, a surrogate for stroke volume (28). Furthermore, previous studies have suggested that COPD patients demonstrate a reduced or similar central hemodynamic response during submaximal exercise (4, 16, 31, 35, 43, 46). However, these studies have either not included a healthy control group or compared COPD with controls at relative intensities (i.e., 40% peak workload). Because of the compromised exercise capacity in COPD, the lower cardiac output and stroke volume in COPD compared with controls reported when relative intensity is matched (16, 35) is consistent with the lower absolute oxygen uptake (V̇o2) and workload. Bogaard et al. (4) compared patients with COPD and controls at matched absolute workloads during exercise and found an attenuated cardiac output (measured via impedance cardiography) and stroke volume in COPD patients; however, the central hemodynamic responses were variable, and the underlying mechanisms contributing to these responses were not investigated.
One of the primary mechanisms suggested to be responsible for the impaired central hemodynamic response to exercise in COPD patients is airway obstruction resulting in hyperinflation and consequently dysfunctional negative intrathoracic pressure swings as well as an augmented expiratory load (23, 24, 29, 42, 43, 45). For example, COPD patients with greater static hyperinflation [i.e., lower ratio of inspiratory capacity to total lung capacity (IC/TLC)] often exhibit a lower cardiac output and stroke volume at peak exercise compared with COPD patients with less static hyperinflation (43), potentially arising from the aberrant negative intrathoracic pressures and/or elevated expiratory load (29, 42). However, it is important to note that these patients exhibiting greater hyperinflation also have lower exercise capacity (43, 45), which may account for the lower cardiac output. Thus, the relationships between static hyperinflation and intrathoracic pressures with cardiac output and stroke volume during exercise remain unclear.
Therefore, the purpose of this study was to 1) compare central hemodynamics between COPD patients and healthy controls and 2) examine the impact of static hyperinflation and elevated intrathoracic pressure on cardiac output and stoke volume in COPD patients. We hypothesized that COPD patients would have lower stroke volume and cardiac output compared with controls at matched V̇o2 during exercise. Furthermore, we hypothesized that lower IC/TLC and increased expiratory intrathoracic pressure would result in lowered cardiac output and stroke volume in COPD patients relative to age-matched healthy controls.
METHODS
Participants.
Thirteen clinically stable COPD patients with moderate to severe COPD [forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC): 52 ± 13% predicted] and 10 healthy control participants matched for age and sex volunteered for this study and provided written informed consent. Exclusion criteria for this study included supplemental oxygen dependence as well as cardiovascular, metabolic, and/or musculoskeletal diseases. All aspects of this study were approved by the Mayo Clinic Institutional Review Board and conformed to the Declaration of Helsinki.
Experimental design.
For this cross-sectional study, participants performed all protocols and measurements during two study visits. On the first study visit, participants were first familiarized with all experimental measurements and protocols, performed resting pulmonary function testing, and then completed an incremental exercise test to volitional fatigue to determine peak oxygen uptake (V̇o2peak). On the second study visit, participants performed constant workload submaximal exercise at an absolute V̇o2 of ~1.3 L/min. During constant workload exercise, inspiratory capacity (IC) maneuvers were performed to determine operating lung volumes, and cardiac output and esophageal pressure were measured.
V̇o2peak.
Participants performed an incremental cycling test to volitional fatigue to determine V̇o2peak, using an electronically braked cycle ergometer (Lode Corival, Groningen, The Netherlands). The incremental test consisted of increasing workloads of 10- to 20- and 20- to 50-W increments for COPD and controls, respectively based on the participants' perceived peak exercise workload. During the incremental test, participants maintained a pedal frequency of 60 rpm and remained seated. Ventilatory and gas exchange variables were collected during the incremental cycling test (MedGraphics CPX/D, St. Paul, MN) and averaged over 30 s. Data acquired included V̇o2, V̇co2, respiratory exchange ratio (RER), ventilation (VE), breathing frequency (fB), and tidal volume (VT). In addition, the ventilatory equivalents for V̇o2 and V̇co2 (VE/V̇o2 and VE/V̇co2, respectively) were calculated at peak exercise. Percent (%)predicted V̇o2peak was calculated from Kaminsky et al. (20). Heart rate was measured continuously via electrocardiogram and recorded at the end of each stage.
Submaximal exercise.
On the second visit, all participants completed a submaximal constant-load exercise test. First, resting data were collected for 3 min, and then participants exercised at a workload resulting in an absolute V̇o2 of ~1.3 L/min. This absolute V̇o2 was chosen as being similar to moderate physical activity in COPD patients (48) and was submaximal for all participants. The initial resistance on the cycle ergometer was determined using data from the incremental test, and then the workload was titrated during minutes 3 to 5 to achieve a V̇o2 of 1.3 L/min. Minutes 5 to 8 were used to achieve steady state (as evident by the heart rate, RER, and V̇o2 responses), at which time an IC maneuver was performed, and cardiac output and blood pressure were measured. Ventilatory and gas exchange variables were collected at rest and during exercise, using the same methodology used during the incremental test, and the 30-s average corresponding to the IC maneuver and cardiac output is reported.
Cardiac output.
Cardiac output was measured using an open-circuit acetylene technique that has previously been validated against direct Fick measurements (17). Previous studies have validated rebreathe techniques (using carbon dioxide and nitrous oxide) against direct Fick, thermodilution, and cardiac magnetic resonance imaging in patients with cardiopulmonary diseases (e.g., COPD, pulmonary hypertension, etc.) (13, 15, 26, 37). Importantly, the Bunsen solubility coefficient of acetylene in blood is approximately two times greater than that of carbon dioxide and nitrous oxide (12). Briefly, participants breathed into a nonrebreathing three-way pneumatic switching valve connected to a pneumotachometer (Hans Rudolph, Kansas City, MO) and gas mass spectrometry (MGA-1100; PerkinElmer Wellesley, MA) integrated with custom analysis software for measurement of cardiac output (17, 32, 44a). The pneumatic switching valve allowed for rapid switching from room air to the gas mixture (0.65% C2H2, 21%O2, 9% He, and balanced N2). Stroke volume was calculated by dividing cardiac output by heart rate. Systemic vascular resistance (SVR) was calculated as the quotient of mean arterial pressure and cardiac output.
Pulmonary function tests.
Pulmonary function tests were performed according to established American Thoracic Society and European Respiratory Society guidelines (27). Total lung capacity (TLC), residual volume (RV), FVC, FEV1, FEV1/FVC ratio, and lung diffusion capacity for carbon monoxide (DLCO) were measured and reported as %predicted values (21, 22, 44).
Respiratory mechanics.
Esophageal pressure was measured using a latex balloon-tip catheter (CooperSurgical, Trumbull, CT) inserted via the nares ~45 cm following local anesthetic. Correct placement of the balloon (i.e., lower one-third of the esophagus) was confirmed using the “occlusion” method (2) following inflation of the balloon with 1 mL of air. The catheter was connected to a differential pressure transducer (MP45; Validyne Engineering, Northridge, CA) and calibrated using a water manometer before each test. Prior to exercise, participants performed multiple IC maneuvers until the maneuvers were valid and consistent. The standard deviation of end-expiratory lung volume (EELV) at rest for the controls and COPD patients were 0.3 and 0.2 L, respectively. At rest and during submaximal exercise, participants performed IC maneuvers from EELV. A “typical” breath was determined if it had similar volume, flow, and pressure characteristics to the previous breaths before the IC maneuver (6, 39–41). A computer program was used to correct for physiological drift during exercise (i.e., when there were unequal inspiratory and expiration volumes) using the IC ratio method (11, 38). TLC was assumed not to significantly change during exercise (18, 19). End-inspiratory lung volume (EILV) was calculated as the sum of VT and EELV and expressed as %TLC. Peak esophageal pressure during inspiration and expiration (Pes,ins and Pes,exp, respectively) and esophageal pressure-time integral during inspiration and expiration (Pes,insTI and Pes,expTI) for each breath were calculated from 8–10 breaths during exercise.
Statistical analyses.
Values are reported as means ± SD. Statistical analyses were performed using SigmaStat 2.0 (Jandel Scientific, San Rafael, CA). Normality and equal variance were assessed using the Shapiro-Wilk and Levene tests, respectively, and nonparametric tests were used when appropriate. Participant characteristics as well as peak exercise, submaximal exercise, and resting data were compared between groups (COPD vs. controls) using unpaired t-tests. Relationships were determined via linear regression. Statistical significance was set at P < 0.05.
RESULTS
Participant characteristics.
COPD patients had a lower %predicted FEV1, FVC, DLCO, and FEV1/FVC than controls but greater body mass index, %predicted RV, and RV/TLC (all, P ≤ 0.03; Table 1). Peak exercise workload, V̇o2, V̇co2, RER, heart rate, VE, and VT were lower in COPD than in controls (Table 2). The %predicted V̇o2peak for the COPD patients was lower than for controls (COPD: 53 ± 15 vs. controls: 83 ± 17%, P < 0.01).
Table 1.
Participant characteristics
| Control | COPD | |
|---|---|---|
| n | 10 | 13 |
| M/W | 8/2 | 11/2 |
| Age, yr | 59 ± 11 | 59 ± 9 |
| Height, cm | 174 ± 8 | 172 ± 5 |
| Weight, kg | 84 ± 10 | 99 ± 25 |
| BMI, kg/m2 | 28 ± 3 | 31 ± 4* |
| FEV1, %pred | 96 ± 13 | 51 ± 10* |
| FVC, %pred | 97 ± 15 | 79 ± 13* |
| FEV1/FVC, % | 79 ± 5 | 52 ± 13* |
| TLC, %pred | 105 ± 16 | 115 ± 23 |
| RV, %pred | 108 ± 17 | 177 ± 58* |
| RV/TLC, % | 35 ± 6 | 52 ± 9* |
| DLCO, %pred | 96 ± 14 | 71 ± 14* |
Values are means ± SD. COPD, chronic obstructive pulmonary disease; M/W, men/women; BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; RV, residual volume; DLCO, difusion capacity for carbon monoxide; pred, predicted.
Significantly different from control.
Table 2.
Peak and submaximal exercise data
| Control | COPD | P Value | |
|---|---|---|---|
| Peak exercise | |||
| Workload, W | 173 ± 50 | 96 ± 21* | <0.01 |
| V̇o2, mL·kg−1·min−1 | 27.1 ± 5.5 | 16.9 ± 4.1* | <0.01 |
| V̇o2, L/min | 2.3 ± 0.5 | 1.6 ± 0.3* | <0.01 |
| V̇co2, L/min | 2.6 ± 0.7 | 1.7 ± 0.5* | <0.01 |
| RER | 1.15 ± 0.08 | 1.04 ± 0.09* | 0.01 |
| Heart rate, beats/min | 157 ± 17 | 125 ± 24* | <0.01 |
| VE, L/min | 93 ± 24 | 54 ± 20* | <0.01 |
| fB, breaths/min | 37 ± 4 | 33 ± 8 | 0.12 |
| VT, L | 2.5 ± 0.8 | 1.6 ± 0.3* | <0.01 |
| VE/V̇co2 | 35 ± 4 | 33 ± 6 | 0.62 |
| VE/V̇o2 | 40 ± 5 | 35 ± 7 | 0.06 |
| Submaximal exercise | |||
| Workload, W | 62 ± 14 | 45 ± 10* | <0.01 |
| V̇o2, mL·kg−1·min−1 | 15.4 ± 2.3 | 13.4 ± 2.8 | 0.08 |
| V̇o2, L/min | 1.3 ± 0.2 | 1.3 ± 0.1 | 0.81 |
| VCO2, L/min | 1.2 ± 0.2 | 1.2 ± 0.1 | 0.63 |
| RER | 0.90 ± 0.04 | 0.93 ± 0.07 | 0.25 |
| Heart rate, beats/min | 102 ± 8 | 103 ± 10 | 0.66 |
| VE, L/min | 33 ± 4 | 37 ± 6 | 0.11 |
| fB, breaths/min | 23 ± 4 | 27 ± 5* | 0.04 |
| VT, L | 1.5 ± 0.3 | 1.4 ± 0.2 | 0.37 |
| IC, L | 3.7 ± 0.9 | 2.5 ± 0.5* | <0.01 |
| IC/TLC, % | 55 ± 7 | 36 ± 10* | <0.01 |
| EILV/TLC, % | 67 ± 8 | 85 ± 7* | <0.01 |
| Peak Pes,ins, cmH2O | −10 ± 3 | −19 ± 6* | <0.01 |
| Peak Pes,exp, cmH2O | 8 ± 7 | 19 ± 7* | <0.01 |
| Pes,inspTI, cmH2O·s | −7 ± 2 | −11 ± 2* | <0.01 |
| Pes,expTI, cmH2O·s | 4 ± 2 | 14 ± 7* | <0.01 |
| SaO2, % | 97 ± 1 | 96 ± 1* | <0.01 |
| RPE | 11 ± 2 | 14 ± 3* | 0.03 |
| Dyspnea | 2 ± 1 | 4 ± 2* | <0.01 |
| MAP, mmHg | 101 ± 8 | 107 ± 15 | 0.40 |
| SVR, mmHg·L−1·min−1 | 8.3 ± 1.3 | 9.9 ± 1.9* | 0.04 |
Mean ± SD. Data from 23 participants (CTL = 10; COPD = 13). Submaximal exercise data were averaged during min 5–8 of the submaximal exercise test. COPD, chronic obstructive pulmonary disease; V̇o2, oxygen uptake; V̇co2, carbon dioxide production; RER, respiratory exchange ratio; VE, ventilation; fB, breathing frequency; VT, tidal volume; IC, inspiratory capacity; TLC, total lung capacity; EILV, end inspiratory lung volume; Pes,ins, inspiratory esophageal pressure; Pes,exp, expiratory esophageal pressure; TI, time integral; RPE, rating of percieved exertion; MAP, mean arterial pressure; SVR, systemic vascular resistance.
Significantly different from control.
Resting cardiopulmonary data.
Resting V̇o2, V̇co2, and VE were greater in the COPD patients compared with the controls (all, P < 0.03), while relative V̇o2, RER, heart rate, VT, fB, and MAP were not different between groups (all P < 0.18). Resting cardiac output (COPD: 5.3 ± 1.1 vs. controls: 5.6 ± 0.6 L/min, P = 0.37), stroke volume (COPD: 77 ± 18 vs. controls: 87 ± 16 mL, P = 0.23), and systemic vascular resistance (COPD: 18.2 ± 4.8 vs. controls: 16.7 ± 3.0 mmHg·L−1·min−1, P = 0.41) were not different between groups. Moreover, resting cardiac output and stroke volume indexed to body surface area were not different for COPD patients compared with controls (COPD: 2.6 ± 0.6 vs. controls: 2.8 ± 0.3 L·min−1·m−2 and COPD: 38 ± 11 vs. controls: 43 ± 9 mL/m2, respectively; both P > 0.20). Last, COPD patients, compared with controls, had more negative peak Pes,insp (COPD: −11 ± 4 vs. controls: −6 ± 2 cmH2O) and Pes,inspTI (COPD: −9 ± 4 vs. controls: −5 ± 1 cmH2O·s) as well as more positive peak Pes,exp (COPD: 5 ± 4 vs. controls: 1 ± 3 cmH2O) and Pes,expTI (COPD: 7 ± 7 vs. controls: 1 ± 1 cmH2O·s) (all P < 0.02).
Submaximal exercise responses.
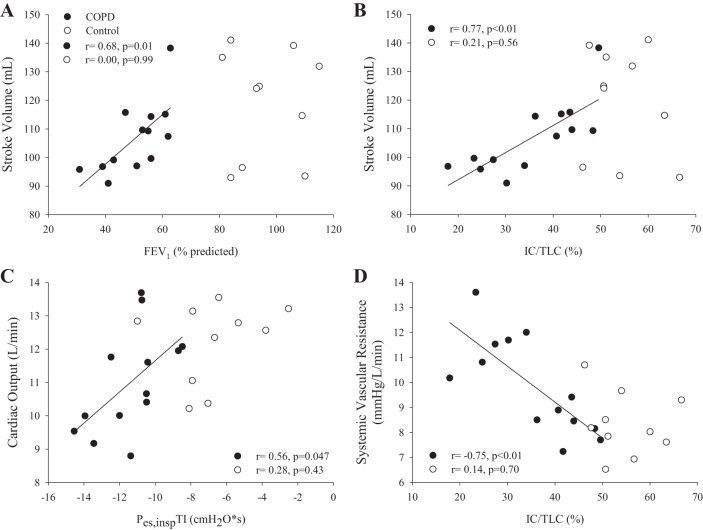
At similar absolute V̇o2, COPD patients had lower workload, IC, and IC/TLC compared with controls (Table 2). Furthermore, COPD patients had greater SVR, EILV/TLC, RPE, and dyspnea than controls. Last, compared with controls, COPD patients had more negative peak Pes,insp and Pes,inspTI as well as more positive peak Pes,exp and Pes,expTI. Cardiac output was lower in COPD than in controls (COPD: 11.0 ± 1.6 vs. controls: 12.2 ± 1.2 L/min, P = 0.03) due to a lower stroke volume (COPD: 107 ± 13 vs. controls: 119 ± 19 mL, P = 0.04), as heart rate was not different (P = 0.66) (Fig. 1). Furthermore, cardiac output and stroke volume indexed to body surface area were significantly lower for COPD patients than for controls (COPD: 5.3 ± 0.6 vs. controls: 6.1 ± 0.6 L·min−1·m−2 and COPD: 51 ± 5 vs. controls: 59 ± 10 mL/m2, respectively; both P ≤ 0.01). In COPD patients, FEV1 (%predicted) was positively related with stroke volume and cardiac output during exercise (r = 0.68, P = 0.01 and r = 0.78, P < 0.01, respectively; Fig. 2). IC/TLC was positively related to stroke volume and negatively related to systemic vascular resistance in COPD patients (r = 0.77, P < 0.01 and r = −0.75, P < 0.01, respectively). Furthermore, Pes,inspTI was positively related to cardiac output (r = 0.56, P = 0.047), whereas Pes,expTI was not related to cardiac output (r = 0.06, P = 0.42). These relationships were not present in controls (P ≥ 0.43). Last, dyspnea was negatively related to cardiac output and stroke volume during exercise in COPD patients (r = −0.61 and r = −0.74, both P < 0.03).
Fig. 1.
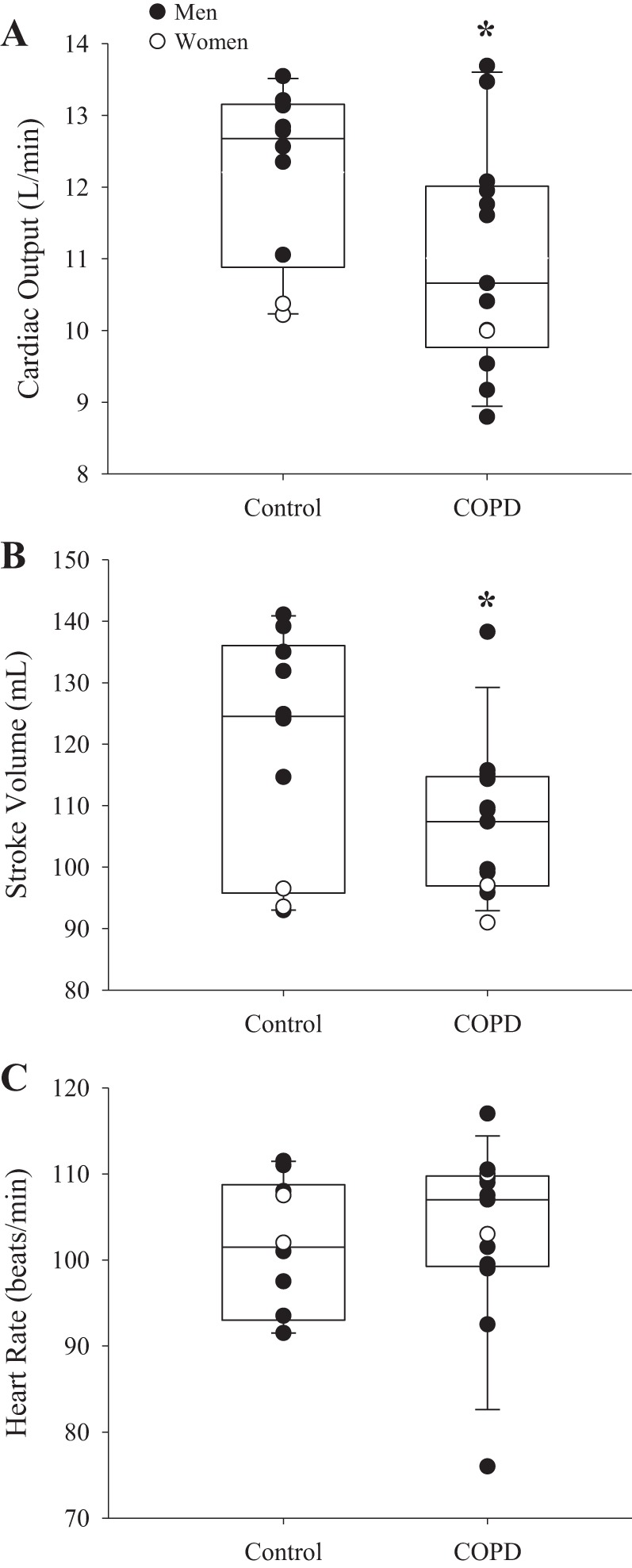
Central hemodynamics in controls and chronic obstructive pulmonary disease (COPD). Cardiac output (A), stroke volume (B), and heart rate (C) in controls (n = 10) and COPD patients (n = 13) during exercise. COPD had lower cardiac output and stroke volume than controls (both P < 0.04), whereas heart rate was not different between groups (P = 0.66). Data are reported as median and 25–75 percentile interquartile range. Significant differences were determined via unpaired t-tests. *Significantly different from control. Men and women are presented in closed and open circles, respectively.
Fig. 2.
Relationships between central hemodynamics and hyperinflation and intrathoracic pressure. Relationships between central hemodynamics and hyperinflation, inspiratory intrathoracic pressure, as well as systemic vascular resistance in controls (○) and chronic obstructive pulmonary disease (COPD) patients (●). There was a positive relationship between FEV1 and stroke volume during exercise in COPD patients (A: r = 0.68, P = 0.01). Furthermore, there were positive and negative relationships between ratio of inspiratory capacity to total lung capacity (IC/TLC) and stroke volume (B) and systemic vascular resistance (D) (r = 0.77, P < 0.01 and r = −0.75, P < 0.01) in COPD patients during exercise. Last, inspiratory esophageal pressure time integral (Pes,inspTI) was positively related to cardiac output (C) (r = 0.56, P = 0.047). No significant relationships were present in the controls (all P ≥ 0.43).
VE-matched submaximal exercise responses.
In a subset of COPD (n = 10) and controls (n = 8), the submaximal exercise responses were compared at similar VE (COPD: 35 ± 4 vs. controls: 34 ± 3 L/min, P = 0.71) and V̇o2 (COPD: 1.3 ± 0.1 vs. controls: 1.3 ± 0.2 L/min, P = 0.27). COPD patients, compared with controls, had more negative peak Pes,insp (COPD: −19 ± 6 vs. controls: −11 ± 3 cmH2O) and Pes,inspTI (COPD: −12 ± 2 vs. controls: −7 ± 3 cmH2O·s) as well as lower IC (COPD: 2.4 ± 0.5 vs. controls: 3.8 ± 0.9 L) and IC/TLC (COPD: 33 ± 10 vs. controls: 55 ± 7%) (all, P < 0.01) during exercise. COPD patients had lower cardiac output (COPD: 10.5 ± 1.5 vs. controls: 12.4 ± 1.0 L/min, P < 0.01) and stroke volume compared with controls during exercise (COPD: 105 ± 15 vs. controls: 122 ± 18 mL, P = 0.03), whereas heart rate was not different between groups (COPD: 101 ± 10 vs. controls: 101 ± 8 beats/min, P = 0.82).
DISCUSSION
Major findings.
The major findings of the present study were threefold. During exercise, COPD patients exhibited lower cardiac output and stroke volume than age-matched healthy adults working at the same absolute V̇o2. Second, stroke volume was positively related to IC/TLC such that the COPD patients with greater hyperinflation (lower IC/TLC) had a lower stroke volume during exercise. Third, and in contrast to our hypothesis, more negative intrathoracic pressure during inspiration was associated with a lower cardiac output during exercise in COPD patients. These findings suggest that hyperinflation and aberrant intrathoracic pressures are likely significant contributors to COPD-related impairments in central hemodynamics.
COPD and cardiac output.
COPD is associated with cardiac structural and functional abnormalities resulting in lower resting stroke volume (16, 35, 47). During exercise, however, the effect of COPD has been highly controversial with studies suggesting cardiac output is similar or reduced compared with controls during exercise (4, 9, 16, 25, 31). Likely explanations for these discrepant findings include exclusion of a control group and comparisons at relative exercise intensities. This latter experimental strategy is problematic, as cardiac output is compared at different absolute V̇o2 and workloads. To circumvent this issue in the present study, we compared COPD and controls at an absolute V̇o2 during exercise. We found that cardiac output was lower in COPD owing to a lower stroke volume during exercise. Importantly, the cardiac output reported herein (i.e., 11.0 L/min) during exercise at a V̇o2 of 1.3 L/min is in line with cardiac output measured via direct Fick (i.e., ~11.7 L/min at a V̇o2 of ~1.1 L/min) (43). Our findings are consistent with those of Bogaard et al. (4) who found an attenuated increase in stroke volume and cardiac output in COPD compared with controls at matched workloads during exercise. It is also important to note that the COPD patients had a greater cost of exercise than the healthy controls, likely resulting from a greater cost of breathing and reduced locomotor mechanical efficiency (33, 34). These findings have important implications during submaximal exercise, as they indicate that COPD patients have a greater reliance on peripheral oxygen utilization than controls.
Previous studies have highlighted the variable cardiac output and stroke volume responses during exercise in COPD (4, 43, 45). For example, cardiac output in COPD has been reported to range between 50 and 190% predicted at a V̇o2 of 800 mL/min during submaximal exercise (43). In line with this, Vassaux et al. (45) found a positive relationship between IC/TLC and oxygen pulse at peak exercise in COPD. However, the interpretation of this latter finding is limited, as oxygen pulse was used as a surrogate for stroke volume, and the COPD patients had different peak exercise capacities (and presumably central hemodynamics). In the present study, we found a positive relationship between stroke volume and IC/TLC at matched V̇o2 during exercise in COPD patients. These findings indicate that stroke volume is reduced in COPD patients during exercise, and this impairment is further exacerbated by greater hyperinflation. Future studies are necessary to determine whether partial alleviation of the hyperinflation during exercise improves cardiac hemodynamics during exercise in COPD patients. Taken together, these data suggest that significant hyperinflation incurs critical cardiovascular consequences in COPD patients during exercise.
Possible mechanisms.
Potential mechanisms affecting stroke volume during exercise in COPD are breathing mechanics and pulmonary vascular alterations (7–9, 29, 43). The impact of respiratory mechanics and, specifically, intrathoracic pressures on central hemodynamics has been controversial. For example, Stark-Leyva et al. (42) found that expiratory loading resulted in reductions in cardiac output and stroke volume during exercise in healthy humans. Furthermore, voluntary hyperinflation (without expiratory loading) increased stroke volume to control levels during exercise due to the more negative intrathoracic pressures (42). In contrast, Montes de Oca et al. (29) found that greater inspiratory intrathoracic pressure was significantly predictive of lower oxygen pulse during peak exercise in COPD patients. Additionally, Cheyne et al. (7, 8) found that more negative intrathoracic pressure during inspiration as well as dynamic hyperinflation were associated with decreased left ventricular stroke volume, likely mediated by direct ventricular interaction in healthy humans at rest. In the present study, we found that more negative intrathoracic pressure (per breath) during inspiration was positively related to cardiac output, and IC/TLC was negatively related to systemic vascular resistance in COPD patients during exercise. These data suggest that the more negative intrathoracic pressure during inspiration in COPD patients impairs the central hemodynamic response, likely due to augmented afterload and/or direct ventricular interaction. Since the COPD patients also exhibited substantially greater expiratory pressures than controls during exercise, the augmented expiratory load likely also contributed to the impaired cardiac response (42). To further elucidate these underlying mechanisms, future studies are required to determine the impact of the intrathoracic pressure swings during a cardiac cycle in COPD patients during exercise. It is possible that pulmonary vascular alterations may have also impacted the stroke volume response in COPD (3, 16, 35). For example, pulmonary vascular resistance has been reported to not change from rest to exercise in COPD patients (with some presenting with pulmonary hypertension), which can significantly influence stroke volume via elevated right ventricular afterload (16, 35). Future studies are needed to investigate the contribution of the pulmonary vasculature in limiting stroke volume in COPD patients with moderate disease severity without pulmonary hypertension.
Methodological considerations.
First, the open-circuit acetylene technique was used to noninvasively measure cardiac output during exercise. COPD patients exhibit inherent ventilation-perfusion mismatch (36), which can influence the mixing of acetylene within the lung. As a result, the cardiac output measurements during exercise may have been underestimated (5). Second, we acknowledge the relatively small sample size in the present investigation. Studies with larger sample sizes may be necessary to confirm our findings. Third, invasive pulmonary hemodynamics were not measured to determine the incidence of pulmonary hypertension or measure pulmonary vascular resistance. However, a recent study found the prevalence of pulmonary hypertension to be low in GOLD (Global Initiative for Chronic. Obstructive Lung Disease) stages II and III (i.e., 5 and 27%) (14). Finally, gastric pressure and thus expiratory muscle pressures were not measured in the present study. Future studies are warranted to determine the contribution of gastric pressure to stroke volume in COPD during exercise.
Conclusions.
During exercise, COPD patients exhibited an attenuated central hemodynamic response during exercise compared with controls. On the basis of these data, we conclude that, in adults with COPD, those with the most severe hyperinflation and more negative inspiratory intrathoracic pressures will exhibit the most significant impairment in exercise-related central hemodynamics. Future studies using chest wall kinematics are necessary to determine whether mitigation of hyperinflation and aberrant intrathoracic pressures will improve central hemodynamics during exercise in COPD patients.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute (HL-126638 to T. P. Olson and HL-071478 to B. D. Johnson) and the American Heart Association (18POST3990251 to J. R. Smith].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R.S., B.D.J., and T.P.O. conceived and designed research; J.R.S., B.D.J., and T.P.O. performed experiments; J.R.S., B.D.J., and T.P.O. analyzed data; J.R.S., B.D.J., and T.P.O. interpreted results of experiments; J.R.S., B.D.J., and T.P.O. prepared figures; J.R.S., B.D.J., and T.P.O. drafted manuscript; J.R.S., B.D.J., and T.P.O. edited and revised manuscript; J.R.S., B.D.J., and T.P.O. approved final version of manuscript.
REFERENCES
- 1.Aliverti A, Macklem PT. Last word on point:counterpoint: the major limitation to exercise performance in COPD is 1) inadequate energy supply to the respiratory and locomotor muscles, 2) lower limb muscle dysfunction, 3) dynamic hyperinflation. J Appl Physiol (1985) 105: 763, 2008. doi: 10.1152/japplphysiol.90745.2008. [DOI] [PubMed] [Google Scholar]
- 2.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126: 788–791, 1982. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- 3.Boerrigter B, Trip P, Bogaard HJ, Groepenhoff H, Oosterveer F, Westerhof N, Vonk Noordegraaf A. Right atrial pressure affects the interaction between lung mechanics and right ventricular function in spontaneously breathing COPD patients. PLoS One 7: e30208, 2012. doi: 10.1371/journal.pone.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogaard HJ, Dekker BM, Arntzen BW, Woltjer HH, van Keimpema AR, Postmus PE, de Vries PM. The haemodynamic response to exercise in chronic obstructive pulmonary disease: assessment by impedance cardiography. Eur Respir J 12: 374–379, 1998. doi: 10.1183/09031936.98.12020374. [DOI] [PubMed] [Google Scholar]
- 5.Bogaard HJ, Wagner PD. Measurement of cardiac output by open-circuit acetylene uptake: a computer model to quantify error caused by ventilation-perfusion inequality. Physiol Meas 27: 1023–1032, 2006. doi: 10.1088/0967-3334/27/10/008. [DOI] [PubMed] [Google Scholar]
- 6.Chenoweth LM, Smith JR, Ferguson CS, Downey AE, Harms CA. The effects of antioxidant vitamin supplementation on expiratory flow rates at rest and during exercise. Eur J Appl Physiol 115: 2049–2058, 2015. doi: 10.1007/s00421-015-3183-z. [DOI] [PubMed] [Google Scholar]
- 7.Cheyne WS, Williams AM, Harper MI, Eves ND. Acute volume loading exacerbates direct ventricular interaction in a model of COPD. J Appl Physiol (1985) 123: 1110–1117, 2017. doi: 10.1152/japplphysiol.01109.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheyne WS, Williams AM, Harper MI, Eves ND. Heart-lung interaction in a model of COPD: importance of lung volume and direct ventricular interaction. Am J Physiol Heart Circ Physiol 311: H1367–H1374, 2016. doi: 10.1152/ajpheart.00458.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiappa GR, Borghi-Silva A, Ferreira LF, Carrascosa C, Oliveira CC, Maia J, Gimenes AC, Queiroga F Jr, Berton D, Ferreira EM, Nery LE, Neder JA. Kinetics of muscle deoxygenation are accelerated at the onset of heavy-intensity exercise in patients with COPD: relationship to central cardiovascular dynamics. J Appl Physiol (1985) 104: 1341–1350, 2008. doi: 10.1152/japplphysiol.01364.2007. [DOI] [PubMed] [Google Scholar]
- 10.Debigaré R, Maltais F. Last word on point:counterpoint: The major limitation to exercise performance in COPD is 1) inadequate energy supply to the respiratory and locomotor muscles, 2) lower limb muscle dysfunction, 3) dynamic hyperinflation. J Appl Physiol (1985) 105: 764, 2008. doi: 10.1152/japplphysiol.90758.2008. [DOI] [PubMed] [Google Scholar]
- 11.Dolmage TE, Goldstein RS. Repeatability of inspiratory capacity during incremental exercise in patients with severe COPD. Chest 121: 708–714, 2002. doi: 10.1378/chest.121.3.708. [DOI] [PubMed] [Google Scholar]
- 13.Farina S, Teruzzi G, Cattadori G, Ferrari C, De Martini S, Bussotti M, Calligaris G, Bartorelli A, Agostoni P. Noninvasive cardiac output measurement by inert gas rebreathing in suspected pulmonary hypertension. Am J Cardiol 113: 546–551, 2014. doi: 10.1016/j.amjcard.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Hilde JM, Skjørten I, Hansteen V, Melsom MN, Hisdal J, Humerfelt S, Steine K. Haemodynamic responses to exercise in patients with COPD. Eur Respir J 41: 1031–1041, 2013. doi: 10.1183/09031936.00085612. [DOI] [PubMed] [Google Scholar]
- 15.Hoeper MM, Maier R, Tongers J, Niedermeyer J, Hohlfeld JM, Hamm M, Fabel H. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am J Respir Crit Care Med 160: 535–541, 1999. doi: 10.1164/ajrccm.160.2.9811062. [DOI] [PubMed] [Google Scholar]
- 16.Holverda S, Rietema H, Westerhof N, Marcus JT, Gan CT, Postmus PE, Vonk-Noordegraaf A. Stroke volume increase to exercise in chronic obstructive pulmonary disease is limited by increased pulmonary artery pressure. Heart 95: 137–141, 2009. doi: 10.1136/hrt.2007.138172. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol (1985) 88: 1650–1658, 2000. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- 18.Johnson BD, Reddan WG, Pegelow DF, Seow KC, Dempsey JA. Flow limitation and regulation of functional residual capacity during exercise in a physically active aging population. Am Rev Respir Dis 143: 960–967, 1991. doi: 10.1164/ajrccm/143.5_Pt_1.960. [DOI] [PubMed] [Google Scholar]
- 19.Johnson BD, Reddan WG, Seow KC, Dempsey JA. Mechanical constraints on exercise hyperpnea in a fit aging population. Am Rev Respir Dis 143: 968–977, 1991. doi: 10.1164/ajrccm/143.5_Pt_1.968. [DOI] [PubMed] [Google Scholar]
- 20.Kaminsky LA, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the Fitness Registry and the Importance of Exercise National Database. Mayo Clin Proc 90: 1515–1523, 2015. doi: 10.1016/j.mayocp.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knudson RJ, Kaltenborn WT, Knudson DE, Burrows B. The single-breath carbon monoxide diffusing capacity. Reference equations derived from a healthy nonsmoking population and effects of hematocrit. Am Rev Respir Dis 135: 805–811, 1987. doi: 10.1164/arrd.1987.135.4.805. [DOI] [PubMed] [Google Scholar]
- 22.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 127: 725–734, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Laveneziana P, Palange P, Ora J, Martolini D, O’Donnell DE. Bronchodilator effect on ventilatory, pulmonary gas exchange, and heart rate kinetics during high-intensity exercise in COPD. Eur J Appl Physiol 107: 633–643, 2009. doi: 10.1007/s00421-009-1169-4. [DOI] [PubMed] [Google Scholar]
- 24.Laveneziana P, Webb KA, Wadell K, Neder JA, O’Donnell DE. Does expiratory muscle activity influence dynamic hyperinflation and exertional dyspnea in COPD? Respir Physiol Neurobiol 199: 24–33, 2014. doi: 10.1016/j.resp.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Light RW, Mintz HM, Linden GS, Brown SE. Hemodynamics of patients with severe chronic obstructive pulmonary disease during progressive upright exercise. Am Rev Respir Dis 130: 391–395, 1984. doi: 10.1164/arrd.1984.130.3.391. [DOI] [PubMed] [Google Scholar]
- 26.Mahler DA, Matthay RA, Snyder PE, Neff RK, Loke J. Determination of cardiac output at rest and during exercise by carbon dioxide rebreathing method in obstructive airway disease. Am Rev Respir Dis 131: 73–78, 1985. doi: 10.1164/arrd.1985.131.1.73. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force . Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 28.Minh VD, Lee HM, Vasquez P, Shepard JW, Bell JW. Relation of VO2max. to cardiopulmonary function in patients with chronic obstructive lung disease. Bull Eur Physiopathol Respir 15: 359–377, 1979. [PubMed] [Google Scholar]
- 29.Montes de Oca M, Rassulo J, Celli BR. Respiratory muscle and cardiopulmonary function during exercise in very severe COPD. Am J Respir Crit Care Med 154: 1284–1289, 1996. doi: 10.1164/ajrccm.154.5.8912737. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell D, Webb K. Last word on point:counterpoint: the major limitation to exercise performance in COPD is 1) inadequate energy supply to the respiratory and locomotor muscles, 2) lower limb muscle dysfunction, 3) dynamic hyperinflation. J Appl Physiol (1985) 105: 765, 2008. doi: 10.1152/japplphysiol.90739.2008. [DOI] [PubMed] [Google Scholar]
- 31.Oelberg DA, Kacmarek RM, Pappagianopoulos PP, Ginns LC, Systrom DM. Ventilatory and cardiovascular responses to inspired He-O2 during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 158: 1876–1882, 1998. doi: 10.1164/ajrccm.158.6.9802015. [DOI] [PubMed] [Google Scholar]
- 32.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 588: 2487–2501, 2010. doi: 10.1113/jphysiol.2009.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potter WA, Olafsson S, Hyatt RE. Ventilatory mechanics and expiratory flow limitation during exercise in patients with obstructive lung disease. J Clin Invest 50: 910–919, 1971. doi: 10.1172/JCI106563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SR, Henry R, Mathieu-Costello O, Wagner PD. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med 169: 89–96, 2004. doi: 10.1164/rccm.200305-627OC. [DOI] [PubMed] [Google Scholar]
- 35.Rietema H, Holverda S, Bogaard HJ, Marcus JT, Smit HJ, Westerhof N, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Sildenafil treatment in COPD does not affect stroke volume or exercise capacity. Eur Respir J 31: 759–764, 2008. doi: 10.1183/09031936.00114207. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez-Roisin R, Drakulovic M, Rodríguez DA, Roca J, Barberà JA, Wagner PD. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol (1985) 106: 1902–1908, 2009. doi: 10.1152/japplphysiol.00085.2009. [DOI] [PubMed] [Google Scholar]
- 37.Saur J, Trinkmann F, Doesch C, Scherhag A, Brade J, Schoenberg SO, Borggrefe M, Kaden JJ, Papavassiliu T. The impact of pulmonary disease on noninvasive measurement of cardiac output by the inert gas rebreathing method. Lung 188: 433–440, 2010. doi: 10.1007/s00408-010-9257-0. [DOI] [PubMed] [Google Scholar]
- 38.Smith JR, Cross TJ, Van Iterson EH, Johnson BD, Olson TP. Resistive and elastic work of breathing in older and younger adults during exercise. J Appl Physiol (1985) 125: 190–197, 2018. doi: 10.1152/japplphysiol.01105.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith JR, Kurti SP, Meskimen K, Harms CA. Expiratory flow limitation and operating lung volumes during exercise in older and younger adults. Respir Physiol Neurobiol 240: 26–31, 2017. doi: 10.1016/j.resp.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Smith JR, Olson TP. Ventilatory constraints influence physiological dead space in heart failure. Exp Physiol 104: 70–80, 2019. doi: 10.1113/EP087183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith JR, Rosenkranz SK, Harms CA. Dysanapsis ratio as a predictor for expiratory flow limitation. Respir Physiol Neurobiol 198: 25–31, 2014. doi: 10.1016/j.resp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol (1985) 96: 1920–1927, 2004. doi: 10.1152/japplphysiol.00756.2003. [DOI] [PubMed] [Google Scholar]
- 43.Stewart RI, Lewis CM. Cardiac output during exercise in patients with COPD. Chest 89: 199–205, 1986. doi: 10.1378/chest.89.2.199. [DOI] [PubMed] [Google Scholar]
- 44.Stocks J, Quanjer PH; Official Statement of The European Respiratory Society . Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Eur Respir J 8: 492–506, 1995. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 44a.Van Iterson EH, Olson TP, Borlaug BA, Johnson BD, Snyder EM. Comparisons of noninvasive methods used to assess exercise stroke volume in heart failure with preserved ejection fraction. Med Sci Sports Exerc 49: 1758–1768, 2017. doi: 10.1249/MSS.0000000000001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vassaux C, Torre-Bouscoulet L, Zeineldine S, Cortopassi F, Paz-Díaz H, Celli BR, Pinto-Plata VM. Effects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPD. Eur Respir J 32: 1275–1282, 2008. doi: 10.1183/09031936.00151707. [DOI] [PubMed] [Google Scholar]
- 46.Vogiatzis I, Athanasopoulos D, Habazettl H, Aliverti A, Louvaris Z, Cherouveim E, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Intercostal muscle blood flow limitation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 1105–1113, 2010. doi: 10.1164/rccm.201002-0172OC. [DOI] [PubMed] [Google Scholar]
- 47.Watz H, Waschki B, Meyer T, Kretschmar G, Kirsten A, Claussen M, Magnussen H. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest 138: 32–38, 2010. doi: 10.1378/chest.09-2810. [DOI] [PubMed] [Google Scholar]
- 48.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J 33: 262–272, 2009. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]