Abstract
Near-infrared diffuse correlation spectroscopy (DCS) is a rapidly evolving optical imaging technique for the assessment of skeletal muscle O2 utilization (mVO2). We compared DCS-derived determinants of mVO2 with conventional measures [blood flow by brachial artery Doppler ultrasound and venous O2 saturation ()] in eight volunteers at rest and during incremental handgrip exercise. Brachial artery blood flow and DCS-derived blood flow index (BFI) were linearly related (R2 = 0.57) and increased with each workload, whereas decreased from 65.3 ± 2.5% (rest) to 39.9 ± 3.0% (light exercise; P < 0.01) with no change thereafter. In contrast, DCS-derived tissue O2 saturation decreased progressively with each incremental stage (P < 0.01), driven almost entirely by an initial steep rise in deoxyhemoglobin/myoglobin, followed by a linear increase thereafter. Whereas seemingly disparate at first glance, we believe these two approaches provide similar information. Indeed, by plotting the mean convective O2 delivery and diffusive O2 conductance, we show that the initial increase in mVO2 during the transition from rest to exercise was achieved by a greater increase in diffusive O2 conductance versus convective O2 delivery (10-fold vs. 4-fold increase, respectively), explaining the initial decline in . In contrast, the increase in mVO2 from light to heavy exercise was achieved by equal increases (1.8-fold) in convective O2 delivery and diffusive O2 conductance, explaining the plateau in . That DCS-derived BFI and deoxyhemoglobin/myoglobin (surrogate measure of O2 extraction) share the same general biphasic pattern suggests that both DCS and conventional approaches provide complementary information regarding the determinants of mVO2.
NEW & NOTEWORTHY Near-infrared diffuse correlation spectroscopy (DCS) is an emerging optical imaging technique for quantifying skeletal muscle O2 delivery and utilization at the microvascular level. Here, we show that DCS provides complementary insight into the determinants of muscle O2 consumption across a wide range of exercise intensities, further establishing the utility of DCS.
Keywords: blood flow, Fick principle, near-infrared spectroscopy, oxygen uptake
INTRODUCTION
Prior research, using invasive hemodynamic measures, has assessed the determinants of skeletal muscle O2 consumption (mVO2) during large (cycling) and small (single leg-knee extension) muscle-mass exercise in healthy individuals and clinical populations (12, 19, 31, 35). Near-infrared diffuse correlation spectroscopy (DCS) is an emerging, noninvasive technique for the simultaneous assessment of O2 delivery and use at the microvascular level (1, 7, 15, 16), with recent application in exercising skeletal muscle (2, 15, 36). Our group has reported that the DCS-derived skeletal muscle blood flow index (BFI; i.e., muscle perfusion) and conventional measures of convective O2 delivery (measured by Doppler ultrasound) are highly related in terms of the magnitude and rate of change during exercise (2, 36). Moreover, we have shown good agreement between DCS-derived measures of mVO2 compared with conventional measurements made at the macrovascular level (36), at least during a single-workload exercise challenge.
Despite the above-mentioned similarities between DCS and conventional approaches, however, we and others have also observed several key differences that highlight the added utility of this technology. For example, when perfusion pressure was experimentally reduced (by raising the exercising limb above the heart), steady-state, Doppler-derived bulk conduit blood flow decreased significantly, whereas DCS-derived, steady-state microvascular perfusion was maintained across conditions, which we interpreted as evidence of microvascular autoregulation (36). A similar observation was also made by Hammer et al. (15), who found DCS-derived microvascular perfusion to be uncoupled from convective O2 delivery during heavy incremental exercise.
Given that our prior observation was limited to a single, moderate-intensity exercise workload (36) and because Hammer et al. (15) did not directly measure skeletal mVO2, we hypothesized that changes in skeletal muscle O2 delivery (related to macro- vs. microvascular uncoupling or otherwise) would be compensated for by greater O2 extraction and that DCS would closely track changes measured directly from the venous effluent. To test this hypothesis, we compared direct macrovascular (Doppler-derived flow and O2 extraction) and indirect microvascular (DCS-derived BFI and tissue saturation) measures across a wide range of exercise intensities using an incremental handgrip exercise test. The data highlight key limitations associated with reliance solely on the arterial-venous O2 difference to reflect changes in O2 extraction and why DCS-derived tissue measures provide important, complementary insight into the determinants of mVO2.
METHODS
Ethical Approval and Subjects
The study was approved by the Institutional Review Board for research involving human subjects at the University of Texas at Arlington. All subjects provided signed consent after receiving a verbal and written description of the experimental protocol and potential risks.
Ten healthy, recreationally active, young (18- to 35-yr-old) men, all of whom volunteered for our previous study (36), participated in the present investigation. Aside from the participant characteristics, none of the data reported previously are herein included. Moreover, unlike the single-workload data presented previously, the data herein evaluate the determinants of mVO2 across a range of exercise intensities (i.e., incremental exercise test). Exclusion criteria included the following: body mass index < 18.5 or > 35 kg/m2; handgrip maximal voluntary contraction (MVC) < 40 kg or > 65 kg (to limit subject heterogeneity); female sex (to avoid large interindividual differences in the relative exercise intensities across the incremental exercise test); history of cardiovascular, pulmonary, or metabolic disease; anemia; current medication or tobacco use; orthopedic limitations; poor venous access; and poor acoustic window for brachial artery imaging by ultrasound.
Experimental Design
Screening and familiarization.
All subjects participated in a separate familiarization visit and underwent dual-energy X-ray absorptiometry (DXA) to quantify fat/lean mass.
Experimental testing visit.
Experimental testing visits were performed on a separate day, after the initial screening/familiarization visit. All studies were performed in a quiet, temperature (~22°C)- and ambient light-controlled room. Subjects were studied in a fasted state, having abstained from alcohol and vigorous exercise for >24 h and caffeine for >12 h. Upon arrival at the laboratory, subjects were positioned supine on a bed and asked to rest quietly while a retrograde intravenous catheter was inserted into a deep vein in the forearm of the exercising (nondominant) arm. Subjects were then instrumented for measurement of continuous blood pressure, heart rate, arterial O2 saturation (), and brachial blood flow (described in detail below). A Smedley handgrip dynamometer was positioned next to the subject’s exercising arm so that the arm could comfortably be extended and supported. Once fully instrumented, subjects performed an incremental rhythmic handgrip exercise test, using a 50% contraction duty cycle (2 s contraction, 2 s relaxation) at a rate of 15 contractions per min. The first workload started at 10 kg and progressed 3 kg every 3 min thereafter. Simultaneous measurements of O2 delivery and use by both approaches (conventional and DCS derived) were performed throughout. Exercise was guided by a monitor that displayed the handgrip force output to give the subject visual feedback on force production and a recorded voice prompt to guide contraction/relaxation. The exercise test was terminated volitionally by the subject or by the investigator when brachial artery image quality was compromised, or the subject was unable to achieve the prescribed force target despite verbal encouragement to do so.
Instrumentation and Measurements
Anthropometrics.
DXA was used to determine whole-body body-fat percent, fat mass, and fat-free mass (DXA Lunar Prodigy; GE Healthcare, Little Chalfont, UK). A DXA-certified radiology technician also constructed a region of interest from the left arm antecubital fossa through the fingertips on the left hand to quantify forearm lean mass. Height and weight were measured with a dual-function stadiometer and weighing scale (Professional 500KL; Health o meter, McCook, IL). Forearm adipose tissue thickness was measured with skinfold calipers (Slim Guide; Creative Health Systems, Plymouth, MI) on the left arm at the location of the flexor digitorum profundus.
Central hemodynamic responses.
Beat-by-beat arterial blood pressure was measured from a small finger cuff placed around the middle finger of the subject’s nonexercising hand using photoplethysmography (Finometer PRO; Finapres Medical Systems, Arnhem, The Netherlands) that was calibrated to an automated brachial artery blood pressure cuff (71WX-B Connex Spot Monitor; Welch Allyn Connex, Skaneateles Falls, NY). Mean arterial pressure (MAP) was calculated as the mean pressure across a continuous average of arterial waveforms. Heart rate was measured via three-lead electrocardiography using standard CM5 placement of electrocardiography electrodes (MLA 0313; ADInstruments, Colorado Springs, CO). was noninvasively obtained by placement of a pulse oximeter (ML 320; ADInstruments) over the index finger of the subject’s nonexercising hand. The analog outputs for arterial blood pressure, heart rate, and modules were connected to a high-performance physiological data acquisition system (PowerLab 16/35; ADInstruments) for simultaneous data recording.
Forearm blood flow.
Brachial artery blood flow velocity and diameter were measured with a duplex ultrasound system (Vivid i; GE Healthcare) on the upper-left portion of the exercising arm (proximal to the antecubital fossa). This ultrasound system had a 12-MHz linear array probe with 60° of insonation. The ultrasound gate was optimized to ensure complete insonation of the entire vessel cross-section with constant intensity. The continuous Doppler audio signal was converted to real-time blood flow velocity waveforms using a validated Doppler audio converter (17) and recorded using a PowerLab data acquisition system (ADInstruments). Brachial artery diameter was measured with B-mode ultrasound imaging, with measurements made during the resting baseline and during the final 30 s of each 3-min exercise stage. The analog outputs from the Doppler ultrasound module, along with the amplified signal from the handgrip dynamometer, were connected to the data acquisition system, detailed above, for simultaneous data recording.
Forearm deep venous blood sampling.
An 18-gauge intravenous catheter was inserted retrograde to venous blood flow into a deep forearm vein. Confirmation that the selected vein drained from the active muscle group of interest (flexor digitorum profundus) was obtained using ultrasound and/or a portable vein illuminator imaging device (VeinViewer Flex; Christie Medical Holdings Inc., Memphis, TN) before insertion of the catheter. The catheter was placed in this manner to improve the likelihood of sampling venous effluent draining from the exercising muscle, whereas also decreasing the potential contribution from the nonexercising muscle and/or the skin. Blood samples were taken during the resting baseline and during the final 30 s of each 3-min exercise stage of the incremental handgrip exercise test to measure venous O2 content (). A 3-mL discard was drawn before the 2-mL blood sample. A 2-mL saline flush followed each sample to prevent the catheter from clotting.
Near-Infrared DCS
Detailed methodology of our in-house DCS system has previously been published (2, 36). Briefly, the system consists of two continuous-wave, long coherence-length laser diodes (785 and 852 nm; CrystaLaser Inc., Reno, NV) that are alternatively switched (Micro-Electro-Mechanical System 2 × 2 Blocking Switch; DiCon Fiberoptics Inc., Richmond, CA) and a single photon-counting avalanche photodiode (APD) as the photon detector (SPCM-AQRH-14-FC; Pacer USA LLC, Palm Beach Gardens, FL). The output of the APD is connected to a computer with a 32-bit, eight-channel data acquisition card (PCI-6602; National Instruments Corp., Austin, TX). A LabVIEW (National Instruments Corp.) program was developed for photon counting. A software autocorrelator calculates the autocorrelation function and absolute intensity (sum of photon counts) of diffused light (10). A three-dimensional printed probe was used to hold a multi-mode fiber (125 μm in core diameter) from the optical switch and a single-mode fiber (5 μm in core diameter) to the APD detector. The probe was affixed to the forearm of the exercise arm, directly over the belly of the flexor digitorum profundus, using Velcro strips. The source-to-detector distance was 2.5 cm. The data sampling rate was 0.25 Hz.
DCS determines a BFI based on the autocorrelation function of light reflectance, measured at each of the two near-infrared wavelengths (785 and 852 nm) and averaged. Then, relative muscle blood flow from the initial baseline at t0 (rMBF) was calculated as the following:
| (1) |
Based on conventional near-infrared spectroscopy (NIRS) methods, the absolute intensity of light reflectance was also used to determine the hemoglobin concentrations. First, the absolute light intensity measured at each wavelength over time, I(t), was converted to the changes in optical density (ΔOD) as the following:
| (2) |
Then two ΔOD outputs measured, respectively, at 785 and 852 nm were used to quantify the relative changes in oxygenated hemoglobin/myoglobin (HbO2) and deoxygenated hemoglobin/myoglobin (Hb) concentrations, ΔHbO2(t) and ΔHb(t), based on the modified Beer-Lambert Law (8). Finally, the change in total hemoglobin concentration [ΔHbT(t)] was derived as the following:
| (3) |
Since conventional NIRS only quantifies the relative changes in hemoglobin concentrations from an initial baseline, the hemoglobin baseline values [HbO2(t0), Hb(t0), and HbT(t0)] were measured before the experiment with a frequency domain near-infrared tissue oximeter (OxiplexTS; ISS Inc., Champaign, IL), and then the real-time absolute hemoglobin concentrations were derived as the following:
| (4) |
Furthermore, the real-time tissue O2 saturation [StO2(t)] was derived as the following:
| (5) |
With the combination of the ΔHbO2 and ΔHb changes from NIRS and the rMBF change from DCS, the relative change of skeletal muscle metabolic rate of O2 (MRO2) was then calculated as the following (6):
| (6) |
Data Analysis
Doppler ultrasound, arterial blood pressure, heart rate, and DCS-derived variables were measured throughout rest, exercise, and postexercise recovery. To minimize the muscle-fiber motion artifact during exercise, DCS data were recorded only during the relaxation phase of the handgrip duty cycle, as previously described (2, 14). Accordingly, brachial artery blood flow measurements were also only made during the relaxation phase of the handgrip duty cycle to match DCS. Venous blood sampling was limited to a single resting baseline and a single sample taken during the final 30 s of each incremental workload. With the exception of data presented continuously in figures, resting baseline values represent a 1-min average of the final minute of a 2-min baseline, and exercise values represent the final 30 s of each 3-min exercise stage.
Venous blood constituents.
An i-STAT analyzer (Abbott Point of Care, Princeton, NJ) was used to analyze the whole blood sample for hemoglobin and forearm venous O2 saturation () using CG8+ test cartridges (Abbott Point of Care). All blood samples were analyzed in duplicate and averaged.
Calculated physiological variables.
Doppler ultrasound, arterial blood pressure, heart rate, and data were analyzed offline by a single observer (R.R.), using the LabChart Pro software environment (ADInstruments). For real-time arterial blood pressure waveforms, a peak-detection algorithm was applied to identify the systolic and diastolic components as the peak and valley of each arterial wave. Then, MAP (in millimeters of mercury) was calculated on a beat-to-beat basis. Similarly, mean blood flow velocity (in centimeters per second) was calculated as the beat-to-beat average from the real-time Doppler ultrasound waveforms. Forearm brachial artery blood flow (FBF) was calculated as [mean blood flow velocity × π (brachial artery diameter/2)2] × 60, where brachial artery diameter (in centimeters) was measured with B-mode ultrasound imaging. FBF was indexed to lean forearm mass and reported as milliliters per minute per 100 grams lean forearm tissue. Forearm vascular conductance was calculated as FBF/MAP × 100 mmHg. Arterial O2 concentration () was calculated as [( × Hb × 1.36) + 0.003 × ], where is the partial pressure of O2 in arterial blood and was assumed to be 100 mmHg (4). Venous blood samples were used to calculate using the formula [( × Hb × 1.36) + 0.003 × ], where is venous O2 partial pressure. mVO2 was calculated using the Fick equation: FBF × ( − ). Arterial and venous O2 transport were calculated as FBF × and , respectively (11).
NIRS and DCS.
All raw DCS and NIRS data were analyzed in MATLAB (Version R2016A; MathWorks Inc., Natick, MA) and exported to Microsoft Excel (Microsoft Corp., Redmond, WA) for subsequent analyses.
Statistics
All statistical analyses were performed with SPSS Software (SPSS 24.0; IBM Corp., Armonk, NY) and Prism, version 8 (GraphPad Software, La Jolla, CA). For all statistical tests, significance was accepted at P < 0.05. All data are presented as means ± SE, unless otherwise noted.
To examine exercise intensity-dependent changes in physiological variables, we used a one-way, repeated-measures ANOVA. As described above, baseline was defined as the 1-min average before exercise, whereas the final 30-s average of each exercise workload was used for comparative purposes. For all repeated-measures ANOVA testing, if the sphericity assumption was violated (Greenhouse-Geisser Ɛ < 0.75), degrees of freedom values were adjusted using the Greenhouse-Geisser correction. When significant overall effects were observed, a Bonferroni correction post hoc analysis was performed to determine where significant differences existed.
To examine the overall relationship between Doppler-derived brachial artery blood flow and DCS-derived BFI, we modeled the kinetic responses illustrated in Fig. 1, A and D, using an exponential growth model with the time constant representing the amount of time required to change 63% of the amplitude or range of the response. In addition, to assess the intra-individual relationship between Doppler-derived brachial artery blood flow and DCS-derived BFI across exercise intensity, we performed intra-individual linear-regression analysis. Similarly, in inter-individual relationships between Doppler-derived blood flow and DCS-derived BFI, deoxyhemaglobin/myoglobin and DCS-derived blood flow index, and DCS-derived relative mVO2 and conventionally measured mVO2, Pearson correlations were performed.
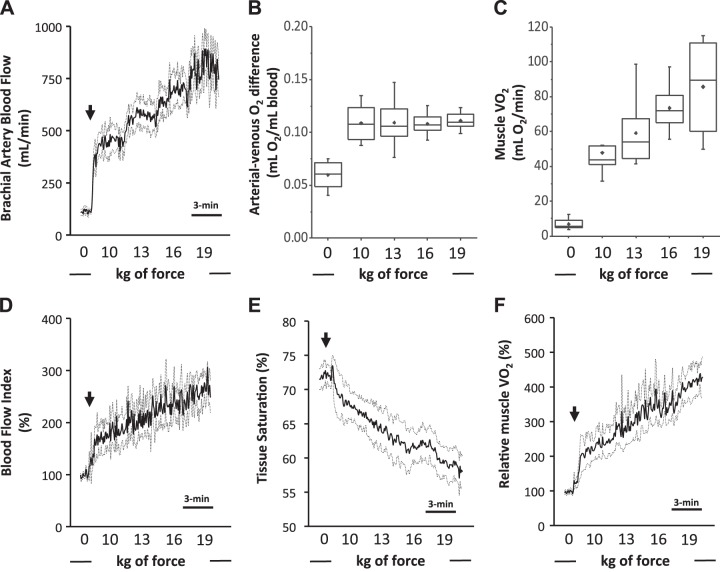
Fig. 1.
A–C: conventional measures of muscle O2 consumption. A: Doppler-derived brachial artery blood flow. B: arterial-venous O2 difference. C: skeletal muscle O2 consumption (V̇o2). D–F: diffuse correlation spectroscopy (DCS)-derived determinants of relative muscle O2 consumption.D: DCS-derived blood flow index. E: near-infrared spectroscopy-derived skeletal muscle tissue saturation. F: relative muscle V̇o2. Rhythmic handgrip began with a fixed workload of 10 kg and progressed by 3 kg every 3 min thereafter. Arrows depict the onset of exercise. Data are reported as means ± SE; n = 8.
RESULTS
Ten subjects volunteered to participate in the study; however, two of these subjects were excluded from the final analysis due to technical difficulties with our DCS device. Subject characteristics for the eight subjects included are presented in Table 1.
Table 1.
Subject characteristics
| Variable | Means ± SD |
|---|---|
| Age, yr | 27 ± 4 |
| Height, cm | 181.0 ± 6.3 |
| Weight, kg | 88.6 ± 16.4 |
| BMI, kg/m2 | 26.9 ± 4.3 |
| Total body fat, % | 23.2 ± 9.4 |
| Left forearm total mass, g | 1,689 ± 195 |
| Left forearm lean mass, g | 1,551 ± 185 |
| Left forearm adipose tissue thickness, mm | 5 ± 1 |
| MVC, kg | 53 ± 8 |
BMI, body mass index; MVC, maximal voluntary contraction; n = 8.
All subjects completed the 19-kg workload; however, only three subjects progressed beyond this workload, with one subject reaching 25 kg and two subjects reaching 22 kg. For comparative purposes, we chose to report data only on the workloads completed by all subjects.
Conventional Determinants of mVO2
Brachial artery blood flow increased twofold from rest to 10 kg rhythmic handgrip exercise (P < 0.01), followed by a linear increase with each subsequent workload (Fig. 1A). Likewise, the arterial-venous O2 difference also increased nearly twofold with the onset of exercise (from rest to 10 kg; P < 0.001), however in contrast to brachial artery blood flow, remained unchanged thereafter (Fig. 1B). Of note, the change in the arterial-venous O2 difference was entirely driven by a decrease in (which changed from 65.3 ± 2.5% at rest to 39.9 ± 3.0% with 10 kg rhythmic handgrip), as we observed no major differences in hemoglobin or arterial saturation. Accordingly, mVO2 increased fourfold with the onset of exercise (P < 0.001), with subsequent linear increases seemingly driven entirely by brachial artery blood flow (Fig. 1C).
DCS-Derived Determinants of mVO2
Similar to Doppler-derived brachial artery blood flow, DCS-derived BFI shared a steep rise with the onset of exercise, followed by a linear increase thereafter (Fig. 1D). Likewise, similar to the directly measured , DCS-derived tissue saturation significantly decreased with the onset of exercise; however, in contrast to the directly measured , tissue saturation continued to decline with each subsequent workload (Fig. 1E). These changes in tissue O2 saturation were driven almost entirely by deoxyhemoglobin/myoglobin (a surrogate measure of O2 extraction), which increased rapidly during the onset of exercise, followed by a progressive linear increase thereafter (Fig. 2B). Oxyhemoglobin/myoglobin remained unchanged throughout exercise (Fig. 2A); as a result, total hemoglobin/myoglobin increased throughout the exercise challenge (Fig. 2C; P < 0.01). Once integrated, relative mVO2 shared a similar increase across the incremental challenge, as conventionally measured mVO2 (Fig. 1F).
Fig. 2.
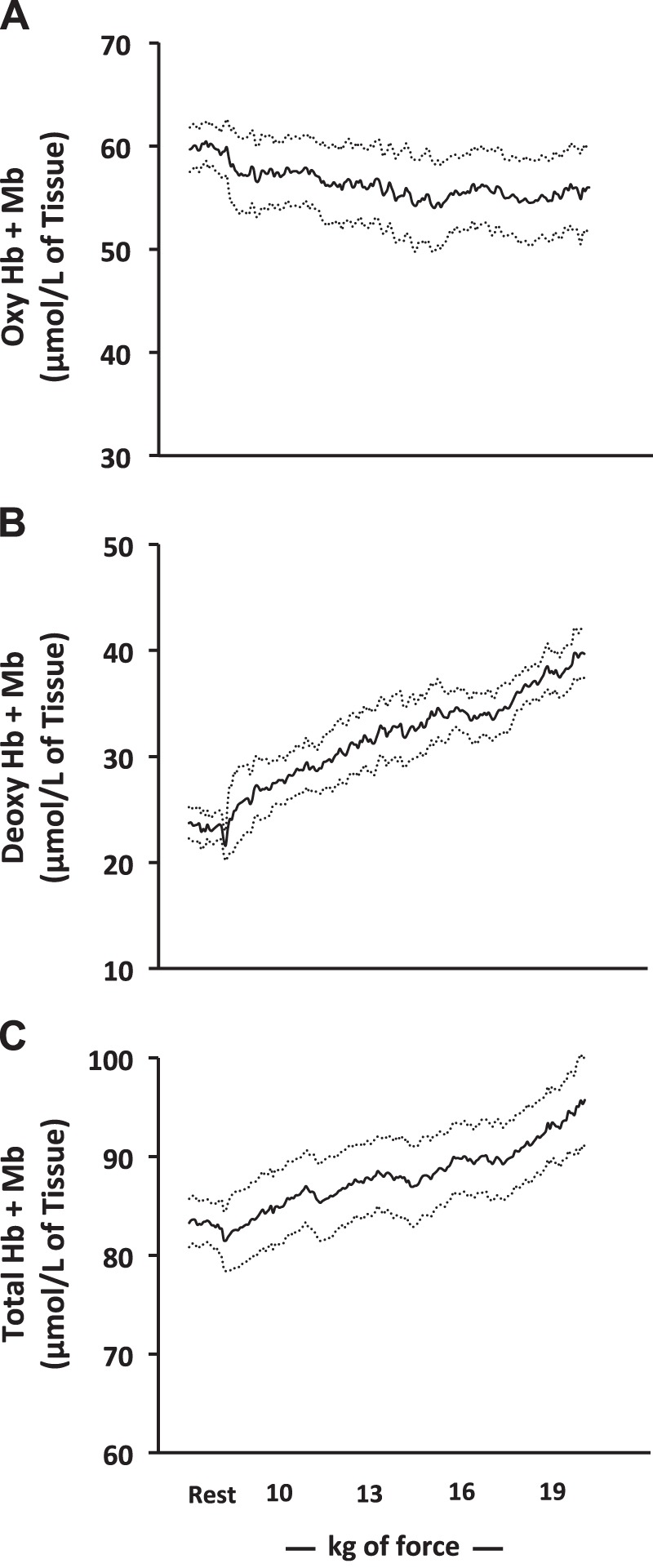
Near-infrared-derived oxygenated (Oxy; A), deoxygenated (Deoxy; B), and total (C) hemoglobin (Hb)/myoglobin (Mb) at rest and throughout the incremental handgrip exercise protocol. Rhythmic handgrip began with a fixed workload of 10 kg and progressed by 3 kg every 3 min thereafter. Data are reported as means ± SE; n = 8. Oxygenated hemoglobin/myoglobin remained unchanged from rest throughout exercise, whereas deoxygenated and total hemoglobin/myoglobin increased with graded exercise (P < 0.01 for both).
Fick Principle and Fick’s Law of Diffusion
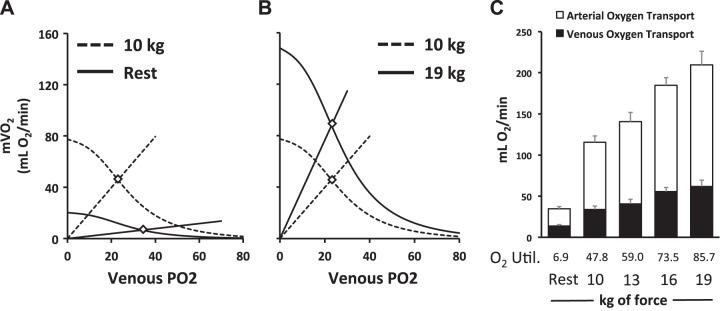
To help interpret the apparent discrepancy between the determinants of mVO2 when assessed by the conventional approach versus DCS-derived MRO2, we plotted the mean convective and diffusive components of O2 transport between two distinct phases in the incremental exercise test: 1) rest to 10 kg of force (Fig. 3A) and 2) 10–19 kg of force (Fig. 3B). The Fick principle curve depicts mVO2 as a function of convective O2 delivery versus venous partial pressure of O2 (Po2. Fick’s law of diffusion depicts O2 utilization (V̇o2) as a function of venous Po2 with the slope representative of muscle O2 diffusive conductance (20). The intersection of these lines determines the V̇o2 achieved. As illustrated, the transition from rest to 10 kg rhythmic handgrip exercise was achieved by a greater increase in diffusive O2 conductance (10-fold increase) compared with convective O2 delivery (fourfold increase) that resulted in increased O2 extraction. The increase in mVO2, from 10 to 19 kg, was achieved by a 1.8-fold increase in both convective O2 delivery and diffusive O2 conductance that resulted in minimal change in the arterial-venous O2 difference. These models are supported by the expression of the proportionate changes in both arterial and venous O2 transport across each exercise workload and the calculation of their respective difference [i.e., V̇o2; Fig. 3C]. Indeed, the greatest increase in V̇o2 occurs during the transition from rest to 10 kg of rhythmic handgrip. During this transition, because diffusive O2 conductance increases to a greater extent than convective O2 delivery, an appreciable increase in O2 extraction (i.e., arterial-venous O2 difference) is observed. However, after this first 10-kg workload, when diffusive and convective O2 conductance are matched, no further changes in O2 extraction (i.e., arterial-venous O2 difference) are observed. This is supported at the microvascular level using DCS. Indeed, during the transition from 10 to 19 kg, absolute O2 extraction increases with each transition (defined by the rise in deoxyhemoglobin/myoglobin; Fig. 2B) but does so at a similar rate as O2 delivery (i.e., DCS-derived BFI; Fig. 1D), thus preserving the arterial-venous O2 difference.
Fig. 3.
The Fick principle curve depicts muscle O2 consumption (mVO2) as a function of convective O2 delivery (curved line) vs. venous partial pressure of O2 (Po2). Fick’s law of diffusion depicts mVO2 as a function of venous Po2, with the slope (straight line) representative of muscle O2 diffusive conductance. The intersection of these lines determines the mVO2 achieved. A: the transition from rest to 10 kg of rhythmic handgrip exercise was achieved by a greater increase in diffusive O2 conductance compared with convective O2 delivery. B: the increase in mVO2 from 10 to 19 kg was achieved by an increase in both convective O2 delivery and diffuse O2 conductance that resulted in a minimal change in venous Po2. C: the arterial (open bars) and venous (closed bars) O2 transport at rest and at the end of each respective exercise workload. O2 utilization (O2 Util.) was calculated as the differences between arterial and venous O2 transport, expressed in milliliters of O2 per minute. Data are reported as means ± SE; n = 8.
Relationships Between Conventional and DCS-Derived Determinants of mVO2
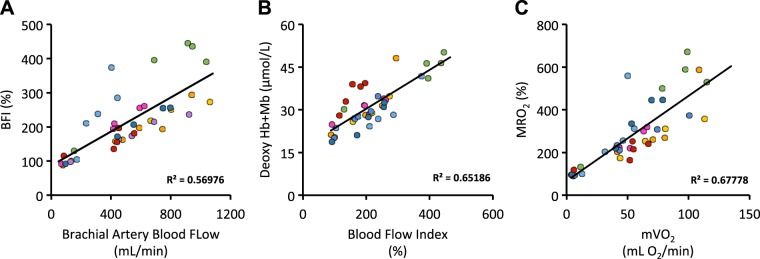
As mentioned, brachial artery blood flow and DCS-derived BFI shared a similar response, increasing sharply with the onset of exercise, followed by a linear increase thereafter (Fig. 1, A and D). To assess the agreement between these two variables, we performed nonlinear-regression modeling. Consistent with our prior reports (2, 36), Doppler-derived brachial blood flow and DCS-derived BFI were very closely related, sharing very similar time constants (264 vs. 270 s, respectively), along with large overlap between their respective 95% confidence intervals (216–324 vs. 192–414 s, Doppler vs. DCS, respectively). In addition to the evaluation of the goodness of fit, we evaluated the within-subject agreement between the two approaches. As illustrated in Fig. 4A, we observed excellent within-subject agreement between Doppler-derived brachial blood flow and DCS-derived BFI (correlation coefficients ranging from 0.79 to 0.99, median = 0.97). We also observed good between-subject agreement between Doppler-derived brachial blood flow and DCS-derived BFI (r2 = 0.57; Fig. 4A).
Fig. 4.
A: relationship between Doppler-derived brachial artery blood flow and diffuse correlation spectroscopy (DCS)-derived blood flow index (BFI) across the incremental exercise test. B: relationship between DCS-derived BFI and near-infrared spectroscopy-derived deoxy hemoglobin (Hb) + myoglobin (Mb; an indicator fractional O2 extraction). C: relationship between conventional muscle O2 utilization (mVO2) and DCS-derived metabolic rate of O2 (MRO2). Individuals are color coded across each panel to highlight intra-individual relationships, which ranged between 0.62 and 0.99 for A, 0.76 and 0.98 for B, and 0.54 and 0.98 for C.
Similar to the comparisons made between Doppler-derived and DCS-derived blood flow, we also assessed relationships between DCS-derived BFI and deoxyhemoglobin/myoglobin [a measure of tissue O2 extraction (9, 13); Fig. 4B]. The tight relationship observed supports the conventionally derived data described immediately above. Together, the data show that when diffusive O2 conductance is greater than convective O2 delivery (i.e., rest to 10 kg), the change in mVO2 is driven largely by O2 extraction (i.e., increased deoxyhemoglobin/myoglobin), whereas when diffusive and convective O2 conductance is similar (i.e., 10–19 kg), the change in mVO2 is driven by seemingly proportional changes in O2 delivery (DCS-derived BFI) and O2 extraction.
Consistent with the close linear relationships already mentioned, we also observed good agreement between conventionally derived mVO2 and DCS-derived MRO2. Indeed, within-subject correlation coefficients ranged from 0.54 and 0.98, with a between-subject agreement of 0.68 (Fig. 4C).
DISCUSSION
DCS is emerging as a noninvasive optical imaging technique for quantifying skeletal muscle O2 delivery and mVO2 at the microvascular level. With the comparison of DCS with conventional measures of convective O2 delivery and mVO2, we show that the two approaches are far more similar than they are different. The data also highlight a common misconception of the Fick principle (25, 28), namely, that increases in skeletal mVO2 are almost entirely dependent on convective O2 delivery, and show instead an equal contribution from diffusive O2 conductance. Taken together, these data highlight the potential role that DCS can play, both clinically and as a research tool, for extending our pathophysiologic understanding of exercise (in)tolerance across the spectrum of health and disease noninvasively.
The observed plateau in and the arterial-venous O2 difference are in line with several recent reports involving handgrip exercise (5, 23, 24). Yet, this observed plateau is in direct contrast with the DCS-derived tissue-saturation measurement, which showed a linear decline throughout the incremental exercise challenge. Each technique therefore provided—at least at first glance—seemingly different interpretations of the determinants of mVO2. That the arterial-venous O2 difference was unchanged during exercise performed at workloads >10 kg suggested that the increase in mVO2 was entirely driven by proportional increases in convective O2 delivery. However, after more detailed analysis incorporating Fick’s law of diffusion, one can appreciate that the increase in mVO2 is also driven by an increase in muscle O2 diffusive conductance (Fig. 3, A and B). Whereas the exact mechanism driving these changes is beyond the scope of the present investigation, the initial increase in diffusive O2 conductance is likely attributable to a rapid decrease in intramuscular Po2 at the onset of exercise, which has been shown to reach its nadir even at submaximal workloads (22, 31, 32). Increases in the diffusive O2 conductance are also likely to be attributable to longitudinal capillary recruitment and an increased capillary hematocrit (26, 27). Given the observed increase in NIRS-derived total hemoglobin in our participants, it is interesting to speculate that capillary hematocrit concentration likely played an important role (3).
Importantly, if diffusive O2 conductance was not matched with the marked linear increase in O2 delivery observed, then the arterial-venous O2 difference would have declined during the transitions from 10 to 19 kg (i.e., an effective shunt). Alternatively, had diffuse O2 conductance increased beyond the rate of O2 delivery (as was the case during the transition from rest to 10 kg), then the arterial-venous O2 difference would have continued to increase throughout exercise. That the arterial-venous O2 difference did not increase with each subsequent workload suggests that muscle O2 extraction was matched with muscle O2 delivery. Indeed, the close linear relationship between DCS-derived BFI and NIRS-derived deoxyhemoglobin/myoglobin [an indirect measure of muscle O2 extraction (9, 13)] supports this interpretation. Because the arterial-venous O2 difference is simply a fractional representation of O2 extraction, it does not provide insight into the absolute amount of O2 being extracted. To determine this, one needs to account for absolute O2 delivery (as illustrated in Fig. 3C). As such, when both convective O2 delivery and diffusive O2 conductance increase proportionately (as was the case in our study between 10 and 19 kg), the arterial-venous O2 difference remains constant. Accordingly, both approaches (conventional vs. DCS) seem to provide complementary insight into the determinants of V̇o2.
That we observed a close relationship and excellent within-subject agreement between DCS-derived muscle BFI and Doppler-derived forearm blood flow is entirely consistent with a growing number of publications (2, 36). Whereas these data support DCS-derived BFI as a viable approach for the measurement of microvascular skeletal muscle O2 delivery, we recognize that others have not found the same tight relationship. For example, Hammer et al. (15) found a clear dissociation between DCS-derived skeletal muscle perfusion and Doppler-derived forearm blood flow during incremental handgrip exercise. However, in the present investigation, the highest workload achieved by all of the subjects was 19 kg, which was ~40% of their MVC. In contrast, Hammer et al. (15) consistently achieved intensities above 50% of MVC. In addition, in the present investigation, data were collected continuously at 0.25 Hz throughout rhythmic handgrip exercise. In contrast, Hammer et al. (15) used a unique postexercise data collection approach, which may have contributed to the differences between these two studies. Caution is therefore warranted with the interpretation of the tight relationship between Doppler-derived blood flow and DCS-derived BFI presented herein, as differences may exist between conduit artery and microcirculatory blood flow kinetics and may differ depending on muscle mass and exercise intensity. It is also interesting to note that differences were observed in the magnitude of change between DCS-derived BFI (~2.5- to 3-fold change at 19 kg) and Doppler-derived brachial artery blood flow (~7- to 10-fold change at 19 kg). We interpret this difference to reflect the indiscriminant nature of conduit blood vessels, which supply blood to the entire limb, compared with the muscle-specific increases reflected by DCS.
Experimental Considerations
This study is not without limitation. We cannot completely rule out the contribution of oxy- and deoxymyoglobin to the changes in the NIRS signal observed throughout exercise. Indeed, NIRS is incapable of differentiating between hemoglobin and myoglobin, and myoglobin has indeed previously been shown to deoxygenate during heavy-intensity rhythmic exercise (33). However, because oxymyoglobin decreases rapidly during the onset of exercise, before reaching a steady plateau (despite greater intensity exercise) (33), we contend that the NIRS changes reported herein were likely driven by deoxy- and oxyhemoglobin; however, since we cannot be sure, we have reported our NIRS measurements as a grouped variable (i.e., Hb + Mb).
Additionally, we only report data for the workloads for which we had a complete data set. In the majority of cases, the primary reason for termination of the test was poor Doppler data quality secondary to excessive upper-arm movement (alternative muscle recruitment). It is possible that we have omitted important micro- versus macrovascular differences in O2 delivery, as previously highlighted by Hammer et al. (15).
Despite efforts to optimize our venous sampling—the selection of a deep-vein draining from the most active region of the forearm during exercise and the insertion of the catheter in a retrograde fashion—we cannot rule out that a portion of the venous sample originated from muscles other than the flexor digitorum profundus. Consequently, the venous blood samples may partly reflect forearm mVO2 from nonexercising tissue. This is in contrast to the DCS-derived measures, which reflect a volume-weighted measure of perfusion and extraction. Attention is also warranted with the consideration of the sample time between our DCS measures and the venous sampling. As stated, DCS data were only taken during the 2-s relaxation period, whereas venous sampling was not limited to a specific phase of the handgrip duty cycle. Indeed, the rhythmic nature of handgrip exercise inevitably imposes a pulsatile pattern on both the blood flow and O2 extraction characteristics, which cannot be accounted for by the approach used herein. Despite this potential consideration, however, the strong relationships between DCS-derived BFI and NIRS-derived deoxyhemoglobin/myoglobin, illustrated in Fig. 4B, and its close approximation of the arterial-venous O2 difference measure by conventional approaches suggest that this confounding factor likely had a minimal impact.
Previous work by McDonough et al. (21) showed that to meet exercise-dependent increases in O2 demand, fast-twitch muscle fibers rely more heavily on increases in fractional O2 extraction, whereas slow-twitch fibers exhibit greater proportional increases in convective O2 delivery. It is important to consider these findings when comparing the forearm model used herein with future DCS-based investigations targeting lower-limb muscles, as the proportional differences in fiber type may influence the results and subsequent interpretation.
NIRS measures change in tissue oxygenation in small arterioles, capillaries, and venules within a specific region of interest. Therefore, the relative contributions of each of these compartments to the optical signal during handgrip exercise are unclear. However, it has been shown that the majority (~84%) of the skeletal muscle microvascular volume comprises capillaries, with the remainder split between the arterioles and venules (4, 31). As such, changes in BFI and/or oxygenated and deoxygenated hemoglobin/myoglobin likely represent changes predominantly in the microcirculation (28). Moreover, DCS reflects only a small fraction of the limb being interrogated (in this case, forearm) and thus may not be representative of changes across the entire limb. In contrast, brachial artery flow measurements and venous sampling yield a much more global view.
We plotted the mean convective and diffusive components of O2 transport between two distinct phases of our incremental exercise test to help explain apparent differences between the conventional and DCS approaches, particularly as they relate to tissue versus . Whereas this approach helped our overall interpretation of the data, it requires that mitochondrial Po2 be negligible. Because we cannot be sure that this was the case, caution is warranted when interpreting these results.
Finally, in addition to our relatively small sample size, we only included male subjects with a relatively narrow range in grip strength. Both exclusion criteria were designed to limit inter-individual variability with respect to the relative exercise intensity performed throughout the incremental test. Future investigations are indeed warranted to assess potential sex differences, as well as to explore unique individual differences across a broader population.
Clinical Implications
Exercise intolerance is a hallmark feature in many diseases and is associated with decreased quality of life and functional performance. The understanding of the mechanisms contributing to exercise intolerance is therefore therapeutically important (20). Indeed, prior work has shown that exercise intolerance may be due to convective or diffusive O2 transport limitations or both (12, 18, 29, 34). Importantly, these works also bring attention to how pharmacological treatment or exercise prescription may or may not be effective in addressing the pathophysiologic basis for the limited ability to exercise.
Exercise intolerance can be objectively measured by O2 consumption, either at the whole-body level or at the level of an individual muscle. The latter case is both technically challenging and requires multiple venous measurements from an indwelling catheter. Moreover, as evidenced by the present data, it may not provide a readily apparent understanding of the determinants of mVO2. DCS offers a noninvasive alternative, with the added benefit of evaluating the microvascular environment in question. This approach could assist clinicians in identifying patients who are more convectively or diffusively limited, which will better define their treatment plan. Application of this technology could therefore have a major impact in the era of precision medicine and help to drive therapeutic discovery.
In conclusion, the data herein provide novel insight into the potential use of DCS for simultaneous, noninvasive evaluation of the microvascular determinants of mVO2. Moreover, DCS appears to provide a more holistic representation of the determinants of mVO2, particularly with regard to O2 extraction, highlighting its added value over traditional approaches measured at the macrovascular level.
GRANTS
Support for this research was funded by a University of Texas at Arlington Nagy Family Endowment Award (to M. D. Nelson) and National Heart, Lung, and Blood Institute Grant R15 HL140989-01 (to M. D. Nelson). Financial support for W. J. Tucker was provided by the American Heart Association (AHA) Postdoctoral Fellowship Grant (AHA Award Number 18POST33990210) and for M. J. Haykowsky by the Moritz Chair in Geriatrics at the University of Texas at Arlington.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.R., W.J.T., J.M.K., F.T., and M.D.N. conceived and designed research; R.R., W.J.T., D.T., H.H.C., C.A.A.-M., Y.Z., and M.D.N. performed experiments; R.R., W.J.T., M.J.H., D.T., H.H.C., Y.Z., J.W., F.T., and M.D.N. analyzed data; R.R., W.J.T., M.J.H., D.T., C.A.A.-M., Y.Z., J.W., J.M.K., F.T., and M.D.N. interpreted results of experiments; R.R., M.J.H., J.W., and M.D.N. prepared figures; R.R., M.J.H., H.H.C., J.W., and M.D.N. drafted manuscript; R.R., W.J.T., M.J.H., D.T., H.H.C., C.A.A.-M., Y.Z., J.W., J.M.K., F.T., and M.D.N. edited and revised manuscript; R.R., W.J.T., M.J.H., D.T., H.H.C., C.A.A.-M., Y.Z., J.W., J.M.K., F.T., and M.D.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors appreciate the time and effort put in by all research subjects. We also thank Dr. Craig G. Crandall (University of Texas Southwestern Medical Center) and Dr. Richard Thompson (University of Alberta) for their constructive feedback on the interpretation of these data.
REFERENCES
- 1.Baker WB, Li Z, Schenkel SS, Chandra M, Busch DR, Englund EK, Schmitz KH, Yodh AG, Floyd TF, Mohler ER III. Effects of exercise training on calf muscle oxygen extraction and blood flow in patients with peripheral artery disease. J Appl Physiol (1985) 123: 1599–1609, 2017. doi: 10.1152/japplphysiol.00585.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangalore-Yogananda CG, Rosenberry R, Soni S, Liu H, Nelson MD, Tian F. Concurrent measurement of skeletal muscle blood flow during exercise with diffuse correlation spectroscopy and Doppler ultrasound. Biomed Opt Express 9: 131–141, 2017. doi: 10.1364/BOE.9.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barstow TJ. Understanding near infrared spectroscopy and its application to skeletal muscle research. J Appl Physiol (1985) 126: 1360–1376, 2019. doi: 10.1152/japplphysiol.00166.2018. [DOI] [PubMed] [Google Scholar]
- 4.Bentley RF, Kellawan JM, Moynes JS, Poitras VJ, Walsh JJ, Tschakovsky ME. Individual susceptibility to hypoperfusion and reductions in exercise performance when perfusion pressure is reduced: evidence for vasodilator phenotypes. J Appl Physiol (1985) 117: 392–405, 2014. doi: 10.1152/japplphysiol.01155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg OK, Nyberg SK, Windedal TM, Wang E. Maximal strength training-induced improvements in forearm work efficiency are associated with reduced blood flow. Am J Physiol Heart Circ Physiol 314: H853–H862, 2018. doi: 10.1152/ajpheart.00435.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boas DA, Strangman G, Culver JP, Hoge RD, Jasdzewski G, Poldrack RA, Rosen BR, Mandeville JB. Can the cerebral metabolic rate of oxygen be estimated with near-infrared spectroscopy? Phys Med Biol 48: 2405–2418, 2003. doi: 10.1088/0031-9155/48/15/311. [DOI] [PubMed] [Google Scholar]
- 7.Carp SA, Farzam P, Redes N, Hueber DM, Franceschini MA. Combined multi-distance frequency domain and diffuse correlation spectroscopy system with simultaneous data acquisition and real-time analysis. Biomed Opt Express 8: 3993–4006, 2017. doi: 10.1364/BOE.8.003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cope M, Delpy DT, Reynolds EO, Wray S, Wyatt J, van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Adv Exp Med Biol 222: 183–189, 1988. doi: 10.1007/978-1-4615-9510-6_21. [DOI] [PubMed] [Google Scholar]
- 9.DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol (1985) 95: 113–120, 2003. doi: 10.1152/japplphysiol.00956.2002. [DOI] [PubMed] [Google Scholar]
- 10.Dong J, Bi R, Ho JH, Thong PS, Soo KC, Lee K. Diffuse correlation spectroscopy with a fast Fourier transform-based software autocorrelator. J Biomed Opt 17: 097004, 2012. doi: 10.1117/1.JBO.17.9.097004. [DOI] [PubMed] [Google Scholar]
- 11.Epstein SE, Beiser GD, Stampfer M, Robinson BF, Braunwald E. Characterization of the circulatory response to maximal upright exercise in normal subjects and patients with heart disease. Circulation 35: 1049–1062, 1967. doi: 10.1161/01.CIR.35.6.1049. [DOI] [PubMed] [Google Scholar]
- 12.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol 55: 1945–1954, 2010. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira LF, Koga S, Barstow TJ. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J Appl Physiol (1985) 103: 1999–2004, 2007. doi: 10.1152/japplphysiol.01414.2006. [DOI] [PubMed] [Google Scholar]
- 14.Gurley K, Shang Y, Yu G. Noninvasive optical quantification of absolute blood flow, blood oxygenation, and oxygen consumption rate in exercising skeletal muscle. J Biomed Opt 17: 075010, 2012. doi: 10.1117/1.JBO.17.7.075010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammer SM, Alexander AM, Didier KD, Smith JR, Caldwell JT, Sutterfield SL, Ade CJ, Barstow TJ. The noninvasive simultaneous measurement of tissue oxygenation and microvascular hemodynamics during incremental handgrip exercise. J Appl Physiol (1985) 124: 604–614, 2018. doi: 10.1152/japplphysiol.00815.2017. [DOI] [PubMed] [Google Scholar]
- 16.Henry B, Zhao M, Shang Y, Uhl T, Thomas DT, Xenos ES, Saha SP, Yu G. Hybrid diffuse optical techniques for continuous hemodynamic measurement in gastrocnemius during plantar flexion exercise. J Biomed Opt 20: 125006, 2015. doi: 10.1117/1.JBO.20.12.125006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol 298: H1626–H1632, 2010. doi: 10.1152/ajpheart.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirai DM, Musch TI, Poole DC. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol 309: H1419–H1439, 2015. doi: 10.1152/ajpheart.00469.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogan MC, Roca J, West JB, Wagner PD. Dissociation of maximal O2 uptake from O2 delivery in canine gastrocnemius in situ. J Appl Physiol (1985) 66: 1219–1226, 1989. doi: 10.1152/jappl.1989.66.3.1219. [DOI] [PubMed] [Google Scholar]
- 20.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, Lewis GD. Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized o2 pathway analysis. Circulation 137: 148–161, 2018. doi: 10.1161/CIRCULATIONAHA.117.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563: 903–913, 2005. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molé PA, Chung Y, Tran TK, Sailasuta N, Hurd R, Jue T. Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol Regul Integr Comp Physiol 277: R173–R180, 1999. doi: 10.1152/ajpregu.1999.277.1.R173. [DOI] [PubMed] [Google Scholar]
- 23.Nyberg SK, Berg OK, Helgerud J, Wang E. Blood flow regulation and oxygen uptake during high-intensity forearm exercise. J Appl Physiol (1985) 122: 907–917, 2017. doi: 10.1152/japplphysiol.00983.2016. [DOI] [PubMed] [Google Scholar]
- 24.Nyberg SK, Berg OK, Helgerud J, Wang E. Reliability of forearm oxygen uptake during handgrip exercise: assessment by ultrasonography and venous blood gas. Physiol Rep 6: e13696, 2018. doi: 10.14814/phy2.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padilla DJ, Musch TI, Poole DC. Abusing the Fick principle. Med Sci Sports Exerc 37: 702, 2005. doi: 10.1249/01.MSS.0000158184.16629.E8. [DOI] [PubMed] [Google Scholar]
- 26.Poole DC, Copp SW, Ferguson SK, Musch TI. Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol 98: 1645–1658, 2013. doi: 10.1113/expphysiol.2013.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poole DC, Copp SW, Hirai DM, Musch TI. Dynamics of muscle microcirculatory and blood-myocyte O2 flux during contractions. Acta Physiol (Oxf) 202: 293–310, 2011. doi: 10.1111/j.1748-1716.2010.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole DC, Musch TI. Solving the Fick principle using whole body measurements does not discriminate “central” and “peripheral” adaptations to training. Eur J Appl Physiol 103: 117–119, 2008. doi: 10.1007/s00421-007-0668-4. [DOI] [PubMed] [Google Scholar]
- 29.Poole DC, Richardson RS, Haykowsky MJ, Hirai DM, Musch TI. Exercise limitations in heart failure with reduced and preserved ejection fraction. J Appl Physiol (1985) 124: 208–224, 2018. doi: 10.1152/japplphysiol.00747.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest 96: 1916–1926, 1995. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson RS, Noyszewski EA, Leigh JS, Wagner PD. Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J Appl Physiol (1985) 85: 627–634, 1998. doi: 10.1152/jappl.1998.85.2.627. [DOI] [PubMed] [Google Scholar]
- 33.Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol (1985) 75: 1911–1916, 1993. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- 34.Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at VO2max. J Appl Physiol (1985) 73: 1067–1076, 1992. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- 35.Roca J, Hogan MC, Story D, Bebout DE, Haab P, Gonzalez R, Ueno O, Wagner PD. Evidence for tissue diffusion limitation of VO2max in normal humans. J Appl Physiol (1985) 67: 291–299, 1989. doi: 10.1152/jappl.1989.67.1.291. [DOI] [PubMed] [Google Scholar]
- 36.Tucker WJ, Rosenberry R, Trojacek D, Chamseddine HH, Arena-Marshall CA, Zhu Y, Wang J, Kellawan JM, Haykowsky MJ, Tian F, Nelson MD. Studies into the determinants of skeletal muscle oxygen consumption: novel insight from near-infrared diffuse correlation spectroscopy. J Physiol 597: 2887–2901, 2019. doi: 10.1113/JP277580. [DOI] [PMC free article] [PubMed] [Google Scholar]