Abstract
Inhalation of organic dust (OD) from swine confinement facilities leads to pulmonary inflammation, airway hyperresponsiveness, and oxidative stress. In mice, pretreatment with a hydroxyl radical scavenger prevents airway inflammation and airway hyperresponsiveness (AHR) induced by OD exposure. We sought to determine a mechanism by which OD could induce oxidative stress in bronchial epithelial cells. Human bronchial epithelial cells (BEAS-2B or NHBE) were treated with various concentrations of OD, followed by evaluation of intracellular oxidative stress using 2′,7′–dichlorofluorescein diacetate (DCFDA). After stimulation with OD, gene expression of antioxidant genes was assessed by real-time quantitative PCR followed by quantification of Nrf2 nuclear translocation using a luciferase reporter assay. Phagocytic markers (CD36 and CD68) were analyzed by FACS. Cells were treated with an actin inhibitor, cytochalasin D, before OD exposure and evaluated for Nrf2 nuclear translocation and DCFDA. Mice were pretreated with sulforaphane, the Nrf2 activator, before OD exposure and evaluated for pulmonary inflammation and airway reactivity. OD induced a time- and concentration-dependent increase in DCFDA. mRNA expression levels of Nrf2-dependent genes and Nrf2 nuclear translocation were increased after OD exposure. OD exposure increased the expression of CD68 and CD36. Cytochalasin D prevented oxidative stress and Nrf2 nuclear translocation after OD. Pretreatment with sulforaphane prevented OD-induced inflammation and AHR while increasing the uptake of OD in bronchial epithelial cells. Bronchial epithelial cells can phagocytose OD, resulting in an increase in endogenous oxidative stress. Nrf2-dependent mechanisms mediate the antioxidant response to OD.
Keywords: airway hyperresponsiveness, bronchial epithelial cells, nuclear factor (erythroid-derived 2)-like 2, organic dust, oxidative stress, phagocytosis
INTRODUCTION
The air in swine confinement facilities has been shown to contain high levels of airborne organic dust (OD) and toxic inhalants, including endotoxin, ammonia, and hydrogen sulfide (7, 8). In consequence, pulmonary dysfunction in swine farmers is not uncommon. Acute exposure induces cough, wheezing, chest tightness, and shortness of breath, flulike symptoms, fever, intense airway inflammation, and bronchial hyperresponsiveness to direct stimuli such as inhaled methacholine while chronic exposure often results in increased prevalence of airway symptoms that meet the criteria for chronic bronchitis (22). In animal studies, innate immune effector cells are recruited into the airways, including macrophages (16) and neutrophils (12) that are likely involved in the in vivo response to OD.
Increased oxidative stress as reflected in elevated exhaled nitric oxide in humans has been associated with airway damage and dysfunction after exposure to OD (7, 8). In mice, pretreatment with a hydroxyl radical scavenger (dimethylthiourea) prevents airway inflammation and airway hyperresponsiveness (AHR) to inhaled methacholine induced by OD exposure (12). We have previously shown that gene expression for the transcription factor Nrf2 and Nrf2-dependent antioxidant enzymes increases after instillation of organic dust intranasally (12), suggesting a specific role for Nrf2 in the pulmonary response to OD. Changes in redox state and associated antioxidant mechanisms have been linked to the phagocytic process in immune cells. Specifically, studies have shown that the involvement of Nrf2, a transcription factor known as the “master regular of the antioxidant response,” enhances phagocytic activity (5, 18, 21). Deficiency of Nrf2 in mice exposed to cigarette smoke resulted in reduced phagocytic activity of alveolar macrophages and increased pulmonary inflammation (3) while in vitro, treatment with sulforaphane, an Nrf2 activator, prior to infection with either Hemophilus influenza or Pseudomonas aeruginosa enhanced macrophage phagocytic activity in macrophages isolated from human chronic obstructive pulmonary disease (COPD) patients (3). While studies exploring the relationship between phagocytic epithelial cells and oxidative stress have been limited, Kokkinaki et al. (6) has shown that upregulation of genes and proteins such as Klotho, an aging-suppressor, lowers the production of reactive oxygen species, resulting in increased phagocytic activity in human retinal epithelial cells.
In the current study, we explored the effects of OD on bronchial epithelial cells after exposure to OD. We sought to determine a mechanism by which OD altered redox activity in bronchial epithelial cells. The specific aims of this study were 1) to explore whether OD alters the phagocytic activity of bronchial epithelial cells in vitro; 2) to determine if OD increases reactive oxygen species generation in human bronchial epithelial cells; 3) to determine if phagocytosis is related to Nrf2 nuclear translocation; and 4) to determine whether increasing Nrf2 activity using sulforaphane affects the capacity for phagocytosis and the effects of this treatment on pulmonary inflammation and AHR in mice exposed to OD.
METHODS
OD collection and preparation.
OD was collected and processed as previously described (12). Briefly, dust was collected from a single barn containing 800–900 pigs and used for all experiments. Dust from horizontal surfaces ~1 m above the floor was placed in polyethylene bags. After collection, dust was gradually aerosolized using a continuous focal air jet at 10 l/min and then diluted to 25 l/min, followed by passage through a separating cyclone with a 50% cutoff at 5 μm. The cyclone effluent was filtered through a 140-mm membrane filter, whereas the dust fraction precipitated in the cyclone was discarded. After filtration, dust was stored immediately at −20°C in an airtight, dark box containing desiccant. Measurements using the PreciseInhale (Inhalation Sciences, Huddinge, Sweden) exposure platform found the mass median aerodynamic diameter of the aerosolized dust to be 2.7 μm. Prior to exposure in mice or on cells, dust was sonicated for 60 min at room temperature in sterile PBS.
While the precise composition of OD is unknown, to determine if LPS within the OD may be of significance, LPS levels in OD were assessed using a Pierce LAL Chromogenic Endotoxin Quantitation Kit (Life Technologies, Burlington, ON, Canada) according to the manufacturer's instructions. The amount of LPS present in OD was determined to be 0.428 μg/100 μg OD.
Observation of organic dust in human bronchial epithelial cells.
A human bronchial epithelial cell line (BEAS-2B) was purchased from ATCC (cat. no. CRL-9609) and used between passages 10 and 16. After expansion of cells in T-75 flasks, cells were trypsinized and cultured in 12-well plates at a density of 70,000 cells/well and grown in DMEM supplemented with 10% FBS and 1% PSA (mix of penicillin, streptomycin, and amphotericin B) for 24 h. Cells were then exposed to 100 µg/ml OD or PBS for 24 h. After exposure, cells were washed five times with cold PBS and trypsinized. Cells were collected in 1.5 ml Eppendorf tubes, spun down at 1,500 g, and resuspended in 500 μl PBS. To determine whether OD was present, 50,000 cells were observed using an ImageStreamX Mk II Imaging Flow Cytometer (MilliporeSigma, Toronto, ON, Canada).
Assessment of phagocytic markers by FACS.
BEAS-2B cells were cultured in 6-well plates at a density of 150,000 cells/well and grown in DMEM supplemented with 10% FBS and 1% PSA for 24 h. Cells were then exposed to 100 µg/ml OD or PBS for 24 h. After exposure, cells were washed five times with cold PBS and trypsinized. Cells were stained with viability dye, eFluor780 (Thermo Fisher Scientific, Mississauga, ON, Canada), followed by surface staining with phycoerythrin-conjugated anti-human CD36 antibody (Biolegend, San Diego, CA). Cells were fixed and permeabilized by fixation/permeabilization solution (BD Biosciences, Mississauga, ON, Canada) and stained with allophycocyanin-conjugated anti-human CD68 antibody (Biolegend, San Diego, CA). Data were acquired by FACSCantoII (BD Biosciences, Mississauga, ON, Canada) and analyzed using FlowJo software.
Assessment of phagocytic markers by ImageStreamX.
To determine whether OD exposure induced changes in phagocytic markers in primary epithelial cells and whether a phagocytic inhibitor could prevent these changes, ImageStreamX analysis was performed on primary bronchial epithelial cells from healthy controls. Primary Bronchial/Tracheal Epithelial Cells; Normal, Human (cat. no. PCS-300-010) (NHBE) cells were purchased from ATCC. After being thawed, cells were initially seeded at a density of 5,000 cells/cm2 in T-25 flasks containing bronchial epithelial basal medium (BEBM) supplemented with growth factors (Lonza, Walkersville, MD) until they reached 80% confluence. After expansion, cells were then passaged or replated into T-75 flasks at a density of 5,000 cells/cm2 for expansion, maintained in BEBM supplemented with growth factors (Lonza), and used for experiments between passages 2 and 5. NHBE cells were cultured in 12-well plates at a density of 70,000 cells/well in BEBM supplemented with growth factors (Lonza) until they reached 80% confluence. Cells were then exposed to either OD (100 µg/ml) or pretreated for 60 min before OD with 5 µg/ml cytochalasin D, an actin inhibitor that prevents phagocytosis (Sigma Aldrich, Ontario, ON, Canada). Twenty-four hours later, cells were collected and stained for phagocytic markers CD36 and CD68, nuclei were stained with Hoechst. Data were acquired by ImageStreamX Mk II Imaging Flow Cytometer (MilliporeSigma) and analyzed by I-DEAs software. We next sought to determine whether repeated OD exposure affects the expression on CD36 and/or CD68 and whether repeated OD exposure affected the amount of dust uptake. For this, we cultured primary human bronchial epithelial cells in 12-well plates at a density of 70,000 cells/well in BEBM supplemented with growth factors (Lonza) until they reached 80% confluence. Cells were exposed to OD (100 µg/ml) either once or twice, 24 h apart. Prior to the second exposure, plates were washed well with PBS to remove any residual OD. Twenty-four hours later, cells were collected and stained for phagocytic markers CD36 and CD68, and nuclei were stained with Hoechst. Data were acquired by ImageStreamX Mark II Imaging Flow Cytometer (MilliporeSigma) and analyzed by IDEAs software.
Assessment of intracellular oxidative stress.
To assess the effect of OD on intracellular oxidative stress, BEAS-2B cells were cultured in a 96-well plate at a density of 20,000 cells per well. Twenty-four hours later, cells were stained with 2',7'-dichlorofluorescein diacetate (DCFDA) according to manufacturer’s instructions (Abcam, Toronto, ON, Canada). Cells were exposed to a range of concentrations of OD (10–400 μg), and DCFDA was evaluated using a plate reader (Tecan Infinite 200 Pro, Tecan, Switzerland) from time 0 until 24 h. We determined an optimal change in intracellular oxidative stress at 100 µg/ml and used this concentration of OD in subsequent experiments to determine whether pretreatment with cytochalasin D (5 µg/ml) could prevent changes in DCFDA. Hydrogen peroxide was used as a positive control. Changes in DCFDA are expressed as % increase over baseline.
Evaluation of gene expression in BEAS-2B cells.
Twenty-four hours after treatment, total RNA was extracted from BEAS-2B cells using RNeasy mini kit (Qiagen, Mississauga, ON, Canada) according to the manufacturer’s instructions. After RNA isolation, cDNA was synthesized with oligo(dT) primers and Super-Script II reverse transcriptase (Invitrogen, Burlington, ON, Canada) according to the manufacturer’s instructions. A total of 250 ng of total RNA was reverse-transcribed into cDNA. Transcripts for the following genes were evaluated: Nuclear factor (erythroid-derived 2)-like 2 (Nrf2), Heme-oxygenase 1 (ho-1), NAD(P)H Quinone Dehydrogenase 1 (nqo-1), Glutamate-cysteine ligase modifier subunit (glcm), Superoxide dismutase 1 (sod-1), and Catalase using real-time quantitative PCR. cDNA was amplified in the StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA). Relative mRNA expression was calculated using the ΔΔ cycle threshold method relative to a sample calibrator. The sample calibrator selected was from RNA isolated from untreated BEAS-2B cells and run in each PCR in lieu of a “housekeeping gene.” A sample calibrator was used to allow for accurate assessment of relative levels of gene expression between experiments and different cell passages conducted on different days.
Nrf2 luciferase reporter assay.
Increase in antioxidant response element (ARE) activity is strongly associated with Nrf2 nuclear translocation. Therefore, to assess whether OD could induce Nrf2 nuclear translocation in epithelial cells, we used human bronchial epithelial cells (BEAS-2B) stably transfected with a pGL4.28 plasmid (Promega, Madison, WI) containing an antioxidant response element (ARE; G TAC CGC AGT CAC AGT GAC TCA GCA GAA TCG CTA G) upstream of a hygromycin B resistance gene and a firefly luciferase construct. Cells were grown in DMEM with 10% FBS, 1% PSA, and 100 µg/ml Hygromycin B and seeded at a density of 70,000 cells/well in 12-well plates for 24 h. Different concentrations (10 or 100 µg/ml) and incubation times (30 min, 2 h, 4 h, 24 h) of OD were used to evaluate whether increases in ARE activity were dose- and time-dependent. In subsequent experiments, cells were pretreated with 5 µg/ml cytochalasin D (Sigma Aldrich) to determine whether inhibition of phagocytosis could prevent changes in Nrf2 nuclear translocation. To assess Nrf2 nuclear translocation after OD treatment, culture medium was removed and cells were gently washed two times with sterile PBS, followed by the addition of 40 µl of reporter lysis buffer (Promega, Madison, WI). Plates were placed on a rocker at 4°C for 5 min followed by collection of whole cell lysates that were spun at 13,000 rpm for 3 min. Supernatant (10 µl) was transferred to a 96-well plate for reading in the Tecan iControl luciferase system in the presence of 470 µM D-Luciferin and 530 µM ATP. Nrf2 nuclear translocation was reported in relative light units.
Semiquantitative assessment of Nrf2 nuclear translocation.
Immunofluorescent staining was performed to assess the proportion of cells that underwent Nrf2 nuclear translocation after OD exposure. BEAS-2B cells were seeded at a density of 160,000 cells/well on sterile glass coverslips in 6-well plates for 24 h. Medium was replaced with 100 µg/ml of OD in DMEM 10% FBS with 1% PSA for 4 or 24 h. Sulforaphane, an Nrf2 activator, was used as a positive control and added to cells at a concentration of 100 µg/ml for 4 h. After incubation with OD or sulforaphane, culture medium was removed and washed one time with cold PBS. Cells were fixed in 4% paraformaldehyde in PBS pH 7.4 for 10 min at room temperature. The coverslips were washed three times with PBS for 5 min each and permeabilized with PBS containing 0.3% Tween 20 for 20 min at room temperature. The coverslips were washed three times with PBS for 5 min each and blocked with 1% BSA in serum-free protein block (Dako). Coverslips were incubated overnight at 4°C in a humidified chamber in 1% BSA in PBS containing Tween 20 with or without primary rabbit antibodies against human Nrf2 (Abcam) at a dilution of 1:200. Cells were washed three times with PBS for 5 min each and incubated with goat anti-rabbit conjugated to Alexa Fluor 488 (Abcam) in 1% BSA in PBS-Tween 20 at a dilution of 1:1,000 in the dark at room temperature. Cells were washed three times with PBS and mounted with Fluoroshield Mounting Medium with DAPI (Abcam) on Colorfrost Plus microscope slides (Fisherbrand) in the dark. Cells were imaged at ×20 magnification under an Olympus BX51 fluorescent microscope using a QI CCD camera (QI Imaging, Surrey, BC, Canada). The percentage of positive cells was determined by counting the number of cells with equal or greater fluorescence at 488 nm compared with adjacent cells when colocalized with the nuclear DAPI stain divided by the total number of nuclei as determined by the DAPI stain. Numbers were averaged from 3–5 fields containing 75–100 cells/field for each slide.
Evaluation of Nrf2 intensity following organic dust uptake in epithelial cells.
After Nrf2 staining, we quantified the intensity of Nrf2 nuclear translocation compared with the amount of OD localized in the cells 24 h after OD exposure. Using ImageJ, we calculated the area, total fluorescence, and integrated density by tracing the complete cell, followed by the area, total florescence, and integrated density of the nucleus. We took background readings in cell-free areas to correct for background fluorescence. We next calculated the corrected total cell fluorescence (CTCF) as integrated density of the nucleus – (area of selected cell × mean fluorescence of background readings). We then calculated the corrected nuclear cell fluorescence (CNCF) as integrated density of the nucleus – (area of selected cell × mean fluorescence of background readings). For each cell, we graphed the CTCF vs. CNCF using GraphPad Prism.
Animal studies.
All animal care and experimental procedures complied with the guidelines of the Canadian Council for Animal Care, and protocols were approved by the Animal Care Committee of McGill University. Animal studies are reported in compliance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Male BALB/c mice (18–22 g; 10 wk old) were purchased from Charles River (Wilmington, MA). Mice were provided with water and food ad libitum throughout the experiment.
Observation of organic dust in mouse bronchial epithelial cells.
Mice were exposed to 100 μg OD once per day for 3 days instilled in 40 μl intranasally in sterile PBS under light isoflurane anesthesia. Twenty-four hours after the third instillation, mice were euthanized using an overdose of sodium pentobarbital (80 mg/kg ip), and bronchoalveolar lavage (BAL) was collected in 1 ml sterile PBS. After BAL, lungs were removed and fixed in 4% formalin overnight using 25 cm water pressure. After collection, cells were spun onto slides using a cytocentrifuge (Cytospin 4, Thermo Fisher Scientific, Waltham, MA). After centrifugation, cells were fixed using 4% paraformaldehyde for 10 min and mounted using Permount mounting medium (Fisher Scientific, Toronto, ON, Canada). The lungs were embedded in paraffin and cut into 5 μm sections. After deparaffination, lungs were mounted using Permount mounting medium (Fisher Scientific). The autofluorescent properties of OD were used to observe the presence of OD in bronchial epithelial cells by fluorescence microscopy on ciliated cells harvested in BAL as well as in intact airways.
Evaluation of pulmonary inflammation and airway physiology after sulforaphane treatment in mice.
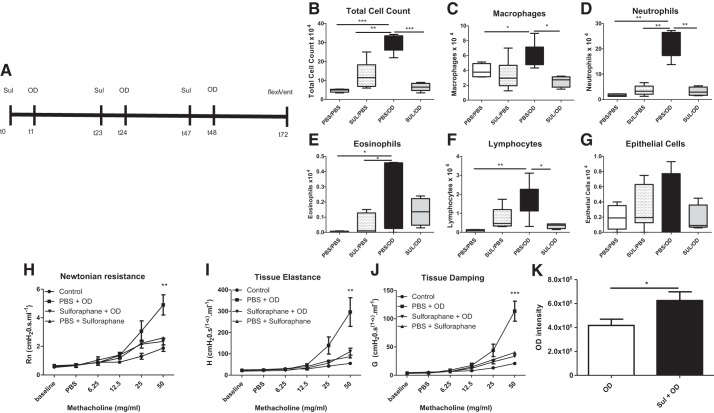
To determine whether increased Nrf2 activity affected airway inflammation and lung function, we used an Nrf2 activator, sulforaphane, 1 h before OD exposure. Mice were exposed to 100 μg OD using a 40 μl intranasal instillation under light isoflurane once per day for 3 days. Some mice were given an intraperitoneal injection of sulforaphane (10 mg/kg) in 200 μl sterile PBS 1 h before each OD exposure. Twenty-four hours after the final OD exposure, airway function was assessed in response to increasing concentrations of inhaled methacholine (6.25–50 mg/ml) using a flexiVent, as previously described (12). After airway function measurements, BAL was performed to assess airway inflammation, as previously described (12).
Treatment with sulforaphane increases epithelial cell engulfment of organic dust.
Epithelial cells, identified as ciliated cells, in BAL from PBS/OD or sulforaphane/OD were evaluated for presence of OD. To quantify the relative amount of OD within the cells, ImageJ was used. First, cell area was determined under light microscopy. Next, fluorescence intensity was measured and background fluorescence measurements taken. Corrected total cell fluorescence was determined by calculating the fluorescence subtracted from the area of the selected cell multiplied by the background readings.
Statistical analysis.
Data were analyzed using a one-way ANOVA for comparison of the means of three or more groups, with a Newman-Keuls post hoc test. For comparison of two means, a two-tailed t-test was used. Airway physiology data were analyzed using a repeated measures ANOVA with a Bonferroni post test. A Spearman test was used to determine the correlation value. Values where P < 0.5 were considered significant.
RESULTS
Observation of organic dust in mouse and human bronchial epithelial cells.
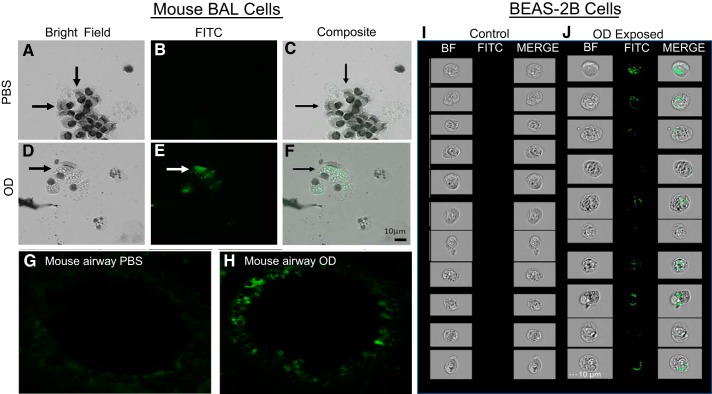
Unstained BAL cells from PBS treated or OD-exposed mice were observed under light microscopy (Fig. 1, A and D). The same cells were observed using fluorescence at 495 nm (FITC) to visualize OD in bronchial epithelial cells, which were identified based on morphology and presence of cilia (Fig. 1E). No fluorescent signal was observed in cells from PBS-treated mice (Fig. 1B). A composite bright field and fluorescent channel confirmed the presence of OD in bronchial epithelial cells from OD mice (Fig. 1F), whereas no signal was observed in PBS-treated mice (Fig. 1C). Illustrative examples of an airway from a control-treated mouse and a similarly sized airway from an OD and OD-treated mouse with a high degree of fluorescence localized to bronchial epithelial cells are shown (Fig. 1, G and H). When studying BEAS-2B using ImageStreamX, cells exposed to 100 μg OD for 24 h showed increased autofluorescent OD intracellularly. Compared with control cells, OD-treated cells clearly demonstrate engulfment of OD, visualized in green (Fig. 1, I and J).
Fig. 1.
Epithelial cells engulf organic dust (OD) in vivo and in vitro. A–C :unstained bronchoalveolar lavage (BAL) cells from PBS-treated mice were observed under light microscopy (A), fluorescence (B), and a composite (C). D: BAL cells from OD-exposed mice under bright field (BF). E: under fluorescence, OD is observed in bronchial epithelial cells. F: composite of bright field and fluorescent channel confirmed the presence of OD in bronchial epithelial cells from OD mice. G: an airway from control-treated mouse, showing no autofluorescence present in bronchial epithelial cells. H: airway from OD-treated mouse, with a high degree of fluorescence localized to bronchial epithelial cells. I: BEAS-2B cells exposed to PBS for 24 h. J: BEAS-2B cells exposed to 100 μg OD for 24 h showed autofluorescent OD intracellularly using ImageStreamX.
Organic dust exposure increases phagocytic markers in bronchial epithelial cells.
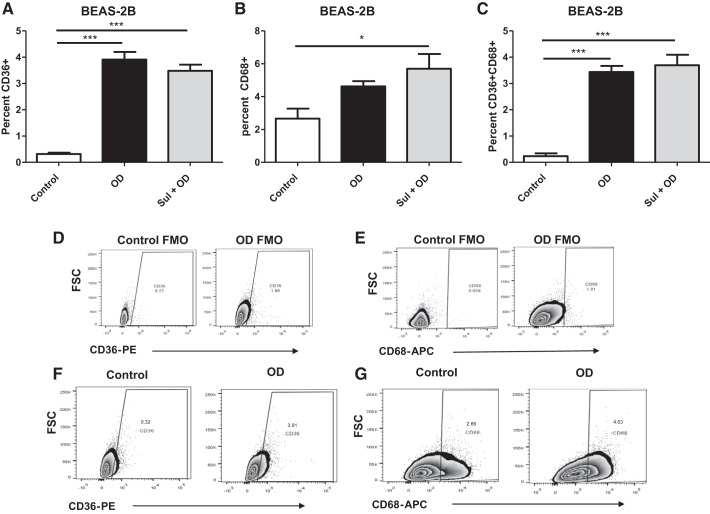
To determine if OD exposure resulted in an increase in phagocytic activity of BEAS-2B cells, two markers of phagocytosis were evaluated by flow cytometry. After 24 h of exposure to OD or pretreated with sulforaphane (10 μM) for 1 h before OD exposure, BEAS-2B cells increased both CD68 and CD36 (Fig. 2, A and B). The number of cells expressing both CD36 and CD68 was also increased (Fig. 2C). Sulforaphane pretreatment increased expression of CD68 compared with control cells but did not increase marker expression in OD-exposed cells (Fig. 2, A–C). The gating strategies for the CD68 and CD36 are shown in Fig. 2, D–G.
Fig. 2.
Organic dust (OD) exposure increases phagocytic markers in BEAS-2B cells. Phagocytic marker expression was assessed after 24 h in OD-exposed cells with or without sulforaphane (Sul) pretreatment. A: increase in the percentage of CD36+ cells after OD or Sul + OD compared with control. B: increase in the percentage of CD68+ cells after OD or Sul + OD compared with control. C: the number of positive cells expressing both CD36 and CD68 was increased after OD exposure compared with control cells. D and E: gating strategies for the CD68 and CD36 are shown. F and G: representative plots showing increase in markers before and after OD. All data were analyzed using ANOVA. *P < 0.5, ***P < 0.001; n = 4 independent experiments. APC, allophycocyanin; FMO, fluorescence minus one; FSC, forward scatter; PE, phycoerythrin.
Inhibition of phagocytosis prevents organic dust engulfment and changes in phagocytic markers.
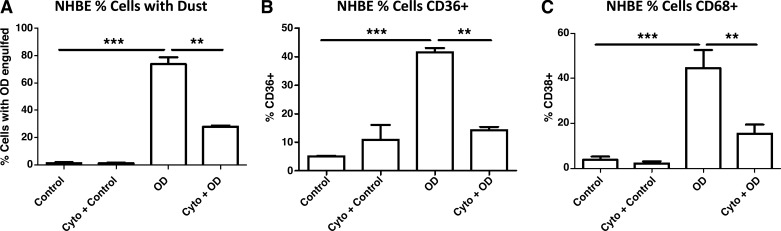
To determine whether primary human cells (NHBE) exhibited the same changes in engulfment ability and upregulation of phagocytic markers and whether inhibition of phagocytosis using cytochalasin D could prevent these changes, NHBE cells were exposed to OD and evaluated by FACS using the ImageStreamX. After 24 h of exposure to OD, 78% of NHBE cells contained OD, compared with 29% treated with cytochalasin D (Fig. 3A). After incubation with OD, 42% of cells had CD36-positive staining, whereas cytochalasin D pretreatment completely prevented this increase (Fig. 3B). Treatment with OD induced a 44% increase in CD68, compared with 17% of cells treated with cytochalasin D before OD exposure (Fig. 3C). We did not observe any change in viability of cells between culture conditions, with viability in all conditions remaining between 75 and 80%.
Fig. 3.
Inhibition of phagocytosis prevents organic dust (OD) engulfment and changes in phagocytic markers in normal human bronchial epithelial (NHBE) cells. Cells were pretreated with cytochalasin D (Cyto) 1 h before OD exposure and evaluated 24 h later for OD engulfment and phagocytic marker expression. A: OD exposure resulted in a robust increase in the number of cells containing OD. Pretreatment with cytochalasin largely prevented this increase in engulfment. B: OD exposure resulted in an increase in the percentage of cells expressing CD36 compared with control. Pretreatment with cytochalasin before OD prevented this increase. C: OD exposure resulted in an increase in the percentage of cells expressing CD68 compared with control cells. Pretreatment with cytochalasin before OD prevented this increase. All data were analyzed using ANOVA. **P < 0.05, ***P < 0.001; n = 3 independent experiments.
Repeated exposure augments organic dust uptake and phagocytic marker expression.
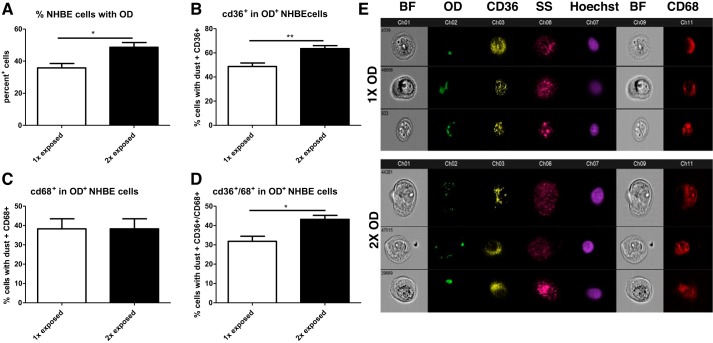
To determine whether OD-treated epithelium was able to increase uptake after a second incubation with OD, cells were treated either once or twice with OD, 24 h apart, and washed between exposures to remove residual OD. Twenty-four hours later, we observed that the percentage of cells containing OD increased in cells that had been exposed twice (Fig. 4A). CD36 expression was also increased after a second exposure in cells that had engulfed OD (Fig. 4B). No change in CD68 expression was observed in OD+ cells after a second OD exposure (Fig. 4C), although OD+ cells expressing both CD36 and CD68 increased after a second OD exposure (Fig. 4D). Figure 4E shows representative cells from the ImageStreamX for the 1× and 2× exposure conditions and demonstrates the engulfment of OD and expression of CD36, CD68, nuclei, and granularity.
Fig. 4.
Repeated organic dust (OD) exposures augment engulfment and receptor expression in normal human bronchial epithelial (NHBE) cells. Cells were exposed either once or twice to OD and evaluated 24 h later to determine if repeated OD exposure affected OD engulfment and phagocytic marker expression. A: after a second exposure, a greater percentage of cells engulfed OD compared with a single exposure. B: a second exposure to OD enhanced CD36 expression in dust-containing (OD+) cells compared with a single OD exposure. C: a second exposure did not affect CD68 expression compared with cells exposed to OD once. D: the number of OD+ cells expressing both CD36 and CD68 was enhanced after a second OD exposure compared with a single OD exposure. E: representative photos of cells exposed to OD once (1×) or twice (2×) showing bright field (BF), OD (green), CD36 (yellow), side scatter (SS; pink), Hoechst (purple), and CD68 (red). All data were analyzed with t-test. *P < 0.5, **P < 0.01, n = 4 independent experiments.
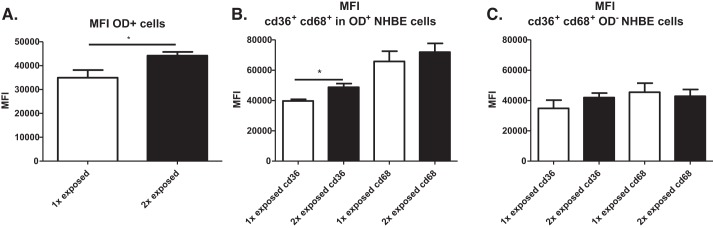
In addition to an increased number of cells containing dust, we also observed a higher median fluorescence intensity (MFI) for cells exposed twice to OD, suggesting that multiple exposures can enhance the amount of dust each NHBE cell can engulf (Fig. 5A). We also observed a higher MFI in cells containing OD for CD36, but not CD68 (Fig. 5B). Interestingly, there was no difference in marker expression for OD-exposed cells, but there was a difference in levels of OD engulfed (Fig. 5C).
Fig. 5.
Repeated organic dust (OD) exposure enhances engulfment and expression of CD36 in normal human bronchial epithelial (NHBE) cells. A: autofluorescent dust in the FITC channel showed that the median fluorescence intensity (MFI) was increased in cells exposed twice to OD compared with one exposure. B: a second exposure to OD increased MFI of CD36 but not CD68 in cells containing dust. C: cells that had no detectable dust engulfed did not upregulate CD36 or CD68 after a second OD exposure. Data in A were analyzed with t-test. Data in B and C were analyzed using ANOVA. *P < 0.5, n = 4 independent experiments.
Organic dust induces increased endogenous oxidative stress in a time- and concentration-dependent manner.
To investigate whether OD causes oxidative stress by activating the epithelial cells, measurement of DCFDA was performed after OD exposure of cultured BEAS-2B cells. OD induced a strong increase in oxidative stress in a time- and concentration-dependent fashion (Fig. 6). Higher concentrations of OD induced an increase in DCFDA within 30 min of exposure compared with control and cells treated with lower concentrations of OD. By 24 h, 100, 200, and 400 µg of OD showed 120, 280, and 405% increases in oxidative stress, respectively. Treated wells (10 and 50 µg) showed a modest increase in oxidative stress levels at 24 h (69 and 75% over baseline levels, respectively).
Fig. 6.
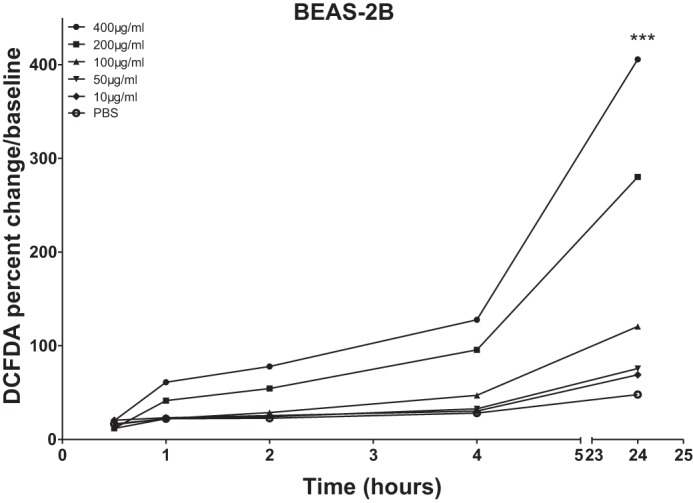
Organic dust (OD) induces increased endogenous oxidative stress in a time- and concentration-dependent fashion. Intracellular reactive oxygen species were determined by evaluating fluorescence levels of 2′,7′-dichlorofluorescein diacetate (DCFDA) after OD exposure. OD induced an increase in oxidative stress assessed in a time- and concentration-dependent fashion. Twenty-four hours after OD exposure, DCFDA was increased compared with control cells. All data were analyzed using ANOVA. ***P < 0.001; n = 4 independent experiments.
Organic dust exposure induces upregulation of Nrf2-dependent genes.
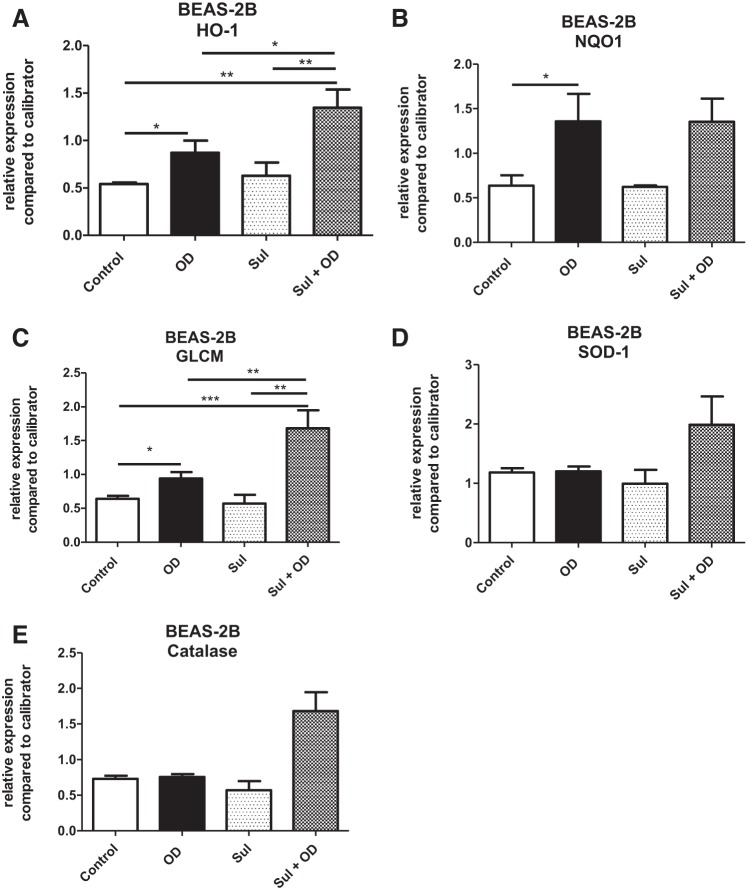
To study the involvement of genes in oxidative stress, gene expression analysis of ho-1, nqo-1, glcm, sod-1, and catalase was performed after 24 h exposure of OD. OD treatment resulted in an increase in expression of Nrf2-dependent genes, nqo-1, glcm, and ho-1 by t-test (Fig. 7, A–C), although OD exposure did not increase Nrf2 gene expression (data not shown). Nrf-2 independent genes, sod-1 and catalase, were also assessed and remained at baseline levels at 24 h (Fig. 7, D and E). To further investigate how Nrf2 activation influenced the same genes, cells were pretreated with sulforaphane 1 h before OD exposure. Pretreatment with sulforaphane increased expression of the Nrf2-dependent genes evaluated, but interestingly only in cells that were also exposed to OD (Fig. 7, A–E). In sulforaphane-only treated cells, no changes in gene expression were observed compared with untreated cells.
Fig. 7.
Organic dust (OD) exposure induces upregulation of Nrf2-dependent genes in BEAS-2B. Expression of antioxidant genes was evaluated 24 h after OD exposure with or without sulforaphane (Sul) pretreatment. A: expression of heme-oxygenase 1 (HO-1) increased after OD exposure compared with control cells. In cells pretreated with Sul, OD exposure resulted in increased HO-1 compared with OD alone. B: expression of NAD(P)H quinone dehydrogenase 1 (NQO-1) increased after OD exposure but not in cells pretreated with Sul. C: expression of Glutamate-cysteine ligase modifier subunit (GLCM) increased after OD exposure compared with control. Pretreatment with Sul before OD increased GLCM expression compared with control, OD alone, and Sul alone treatment. D: expression of superoxide dismutase 1 (SOD-1) was not affected by OD exposure or Sul. E: expression of catalase was not affected by OD exposure or Sul. All data were analyzed using ANOVA. *P < 0.05, **P < 0.05, ***P < 0.001; n = 3–5 independent experiments.
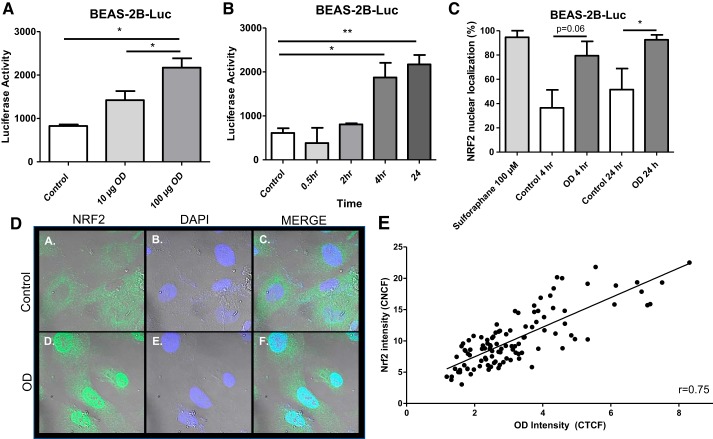
Organic dust induced Nrf2 nuclear translocation in human bronchial epithelial cells.
As Nrf2 nuclear translocation has been shown to be an important action in oxidative stress, we quantified Nrf2 nuclear translocation after OD using BEAS-2B cells stably transfected with an ARE-luciferase reporter (BEAS-2B-Luc). There was a dose-dependent increase in Nrf2 nuclear translocation after OD exposure (Fig. 8A). Next, to establish whether Nrf2 nuclear translocation increased in a time-dependent fashion, BEAS-2B-Luc cells were treated at various time points with 100 µg OD, and ARE activity was evaluated. OD induced a time-dependent increase in Nrf2 nuclear translocation (Fig. 8B). To confirm Nrf2 nuclear translocation, BEAS-2B cells were stained for Nrf2 and the percentage of cells with positive nuclear staining was quantified (Fig. 8C). Figure 8D shows illustrative examples of control versus OD-treated BEAS-2B cells after Nrf2 immunofluorescent staining where OD treatment clearly increases nuclear localization of Nrf2 (green) compared with control wells. Nuclear staining (blue) also appears different between control and OD-treated cells due to overlap between green and blue signals. Figure 8E shows the amount of OD within the cell relative to Nrf2 nuclear translocation. We observed that increased dust engulfment was correlated with greater Nrf2 nuclear translocation.
Fig. 8.
Organic dust (OD) induced Nrf2 nuclear translocation in BEAS-2B cells. BEAS-2B cells stably transfected with a pGL4.28 plasmid containing an antioxidant response element (ARE) sequence upstream of a hygromycin B resistance gene and a luciferase construct with a luciferase reporter cell (BEAS-2B-Luc) were used to evaluate Nrf2 nuclear translocation. A: dose-dependent increase in Nrf2 nuclear translocation after OD exposure in BEAS-2B-Luc cells. B: OD exposure resulted in a time-dependent increase in Nrf2 nuclear translocation in BEAS-2B-Luc cells. C: quantification of the percentage of cells showing positive nuclear staining for Nrf2 shows an increase in Nrf2 nuclear translocation 24 h after OD exposure. D: illustrative examples of control (a–c) vs. OD-treated (d–f) BEAS-2B cells after Nrf2 immunofluorescent staining. Nrf2 = green, nuclear staining = blue. E: individual cells were assessed to show correlation between amount of OD within the cell relative to Nrf2 nuclear translocation. A correlation was found between the signal intensity of Nrf2 nuclear translocation and amount of OD engulfed. Data in A–C were analyzed using ANOVA. Data in E were analyzed using a Spearman test. *P < 0.05, **P < 0.05; n = 3–4 independent experiments. CNCF, corrected nuclear cell fluorescence; CTCF, corrected total cell fluorescence.
Inhibition of phagocytosis prevents Nrf2 nuclear translocation after organic dust.
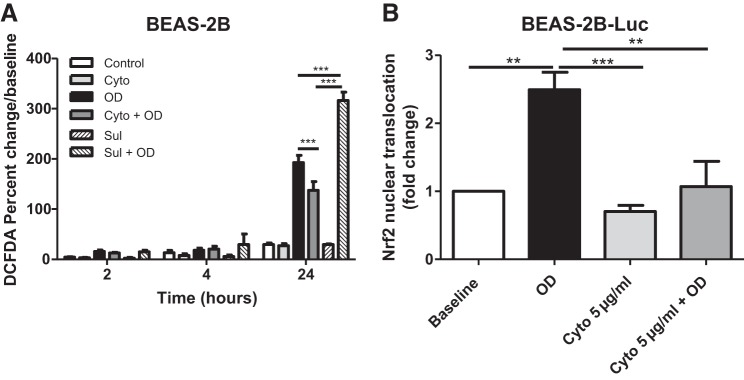
To investigate whether phagocytosis influenced oxidative stress in the epithelial cells, BEAS-2B cells were treated with the phagocytic inhibitor cytochalasin D 1 h before exposure to OD. The treatment with cytochalasin D exposure resulted in a modest decrease in endogenous oxidative stress compared with OD treatment alone at 24 h, although cytochalasin D was not able to reduce OD-induced oxidative stress back to baseline levels (Fig. 9A). Sulforaphane increased DCFDA compared with both OD- and Cyto + OD-treated groups, although sulforaphane alone had no effect (Fig. 9A). Nrf2 nuclear translocation was prevented by pretreatment with cytochalasin D (Fig. 9B).
Fig. 9.
Inhibition of phagocytosis prevents Nrf2 nuclear translocation following organic dust (OD) exposure. A: treatment with cytochalasin D (Cyto) decreased endogenous oxidative stress compared with OD treatment alone at 24 h. Sulforaphane (Sul) increased 2′,7′-dichlorofluorescein diacetate (DCFDA) compared with both OD- and Cyto + OD-treated groups, although Sul alone had no effect by itself. B: Nrf2 nuclear translocation was prevented by pretreatment with cytochalasin. All data were analyzed using ANOVA. **P < 0.05, ***P < 0.001; n = 3 independent experiments. Luc, luciferase.
Sulforaphane treatment prevents organic dust-induced inflammation and airway hyperresponsiveness.
As we earlier have shown that OD causes an increase of AHR in mice (5), we wanted to further study the effects of sulforaphane applied in the same model. A schema of the sulforaphane treatment/OD exposure protocol is shown in Fig. 10A. Pretreatment with sulforaphane prevented OD-induced increases in total inflammatory cells observed in BAL (Fig. 10B). Sulforaphane also prevented OD-induced increases in macrophages (Fig. 10C), neutrophils (Fig. 10D), and lymphocytes (Fig. 10F) but did not prevent an OD-induced increase in eosinophils (Fig. 10E). We did not observe an increase in the number of shed epithelial cells in any group (Fig. 10G). Pretreatment with sulforaphane prevented OD-induced increases in Newtonian Resistance (Fig. 10H), tissue elastance (Fig. 10I), and tissue damping (Fig. 10J). Sulforaphane alone had no effect on AHR for any parameter.
Fig. 10.
Sulforaphane treatment prevents organic dust (OD)-induced inflammation and airway hyperresponsiveness (AHR). Mice were pretreated with sulforaphane (SUL) before OD exposure to determine the effects on airway inflammation and lung mechanics after OD exposure. A: treatment schema for SUL treatments, OD exposures, airway function measurements, and collection of bronchoalveolar lavage (BAL) fluid in mice. B: OD exposure increased total inflammatory cells in BAL compared with control, SUL/PBS, and SUL/OD. Pretreatment with SUL prevented OD-induced increases in total inflammatory cells. C: OD exposure increased the number of macrophages compared with PBS/PBS. Pretreatment with SUL prevented this increase. D: OD exposure increased neutrophil numbers compared with PBS/PBS-, SUL/PBS-, and SUL/OD-treated mice. Pretreatment with SUL before OD prevented increased neutrophilia. E: eosinophils increased after OD exposure compared with PBS/PBS and SUL/PBS. F: lymphocytes increased after OD exposure compared with PBS/PBS mice, an effect prevented by SUL. G: epithelial cell shedding was not different among any of the groups. H: OD exposure induced an increase in Newtonian resistance (Rn) at the highest concentration of methacholine, an effect prevented by SUL. I: OD exposure induced an increase in tissue elastance (H) at the highest concentration of methacholine. Pretreatment with SUL prevented this increase. J: OD exposure induced an increase in tissue damping (G) at the highest concentration of methacholine, an effect prevented by SUL. K: OD localized to bronchial epithelial cells in BAL showed that mice pretreated with SUL had engulfed more OD compared with PBS/OD-treated mice. Data in B–G were analyzed using ANOVA. Data in H–J were analyzed using a repeated measure ANOVA. *P < 0.5, **P < 0.05, ***P < 0.001; A–F: n = 4–8; G–I: n = 6–7 mice per group.
Sulforaphane treatment increases organic dust engulfment of epithelial cells.
To determine whether sulforaphane enhanced phagocytosis in vivo, we quantified the OD within bronchial epithelial cells in BAL. Mice pretreated with sulforaphane engulfed more OD compared with PBS OD-treated mice (Fig. 10K).
DISCUSSION
Organic dust precipitates acute inflammation of the respiratory tract with a predominant neutrophilic inflammation and airway dysfunction mediated by oxidative stress. In the current study, we have addressed the role that airway epithelial cells may have in the response to inhaled OD. We observed that airway epithelial cells readily engulfed OD in vivo in the mouse. We have shown that the phenomenon was also true for human bronchial epithelial cells (BEAS-2B and NHBE) in vitro. Exposure to OD increased expression of phagocytic markers, CD36 and CD68, an effect prevented by inhibiting OD engulfment with cytochalasin D. Repeated exposure to OD in NHBE cells resulted in a greater number of cells that engulfed OD, the amount of OD within the cells, and the expression of CD36 compared with cells exposed only once. The engulfment of OD particles is linked to the generation of oxidative species, and it provoked the nuclear translocation of the master antioxidant transcription factor Nrf2. The degree of Nrf2 nuclear translocation was correlated with the amount of OD engulfed by the cells, and inhibition of engulfment prevented Nrf2 nuclear translocation. Finally, sulforaphane treatment before OD exposure reduced airway inflammation and AHR, suggesting that efficient epithelial cell clearance of OD contributes to lung recovery after OD.
Bronchial epithelial cells exposed to OD underwent a time- and concentration-dependent increase in endogenous oxidative stress in epithelial cells measured by the fluorogenic dye DCFDA that detects a range of reactive oxygen species. The magnitude of the increase in oxidative species was sufficient to cause an upregulation in mRNA levels for antioxidant genes, primarily associated with the translocation of the transcription factor Nrf2. Using an epithelial cell line transfected with a luciferase reporter for antioxidant response element activation, we observed a time- and concentration-dependent increase in Nrf2 nuclear translocation after OD exposure. We also observed a correlation between the intensity of Nrf2 nuclear translocation and OD present within the cells. While no studies have looked at the effect of OD on Nrf2, previous work has shown that exposure to other substances, including particulate matter, diesel exhaust, and nanoparticles, can activate the Nrf2 pathway in epithelial cells.
Interestingly, we observed that the cells that had the greatest degree of Nrf2 nuclear translocation also had OD localized within the cells. The autofluorescent properties of OD allowed for their easy identification within bronchial epithelial cells in vivo as well as in cultured epithelial cells. This observation of dust within the cells led us to hypothesize that epithelial cells were responding to the presence of dust by undergoing a phenotype change to become phagocytic. Therefore, we addressed whether human bronchial epithelial cells upregulated phagocytic markers in response to OD exposure. We documented an increase in CD36 and CD68, markers that are typically found on professional phagocytes but have been observed on epithelial cells (13). We observed that a second OD exposure in NHBE cells led to an increase in the number of cells that engulfed OD compared with one exposure. Furthermore, a second exposure resulted in a greater concentration of OD within the cells, suggesting that multiple exposures enhance the engulfment capacity of NHBE cells. This observation was supported by findings showing that the percentage of CD36+ and CD36+/CD68+ cells was increased in cells containing OD (OD+) compared with cells exposed once. However, repeated exposures did not affect the number of CD68+ cells, which remained elevated but comparable after 1 or 2 exposures. Although the MFI of CD36+ cells increased after a second OD exposure compared with a single exposure, we observed no difference in the MFI for CD68. Why the number of CD36+ cells and expression level of CD36 on those cells was enhanced by a second OD exposure whereas CD68 remained stable remains unknown, and further studies on the kinetics of these receptors in epithelial cells may be of interest. Notably, in cells that were exposed to OD but had failed to engulf it after one or two exposures, we did not observe augmentation of expression in either CD36 or CD68 expression. These data support the relationship between engulfment of OD and changes in these scavenger receptors, and it is likely that efficient uptake requires their upregulation.
Although engulfment of OD by epithelial cells has not previously been observed, studies have found they are capable of phenotypic shift to become phagocytic (6, 15, 17). Mouse mammary epithelial cells engulf surrounding apoptotic cells after weaning during mammary gland involution, expressing many of the same phagocytic-associated markers typically found on macrophages, including phosphatidylserine receptor, the vitronectin receptor αVβ3, CD91, and CD36 (13). CD68, a phagocytic marker associated with macropinocytosis, has been observed on epithelial cells allowing engulfment of latex beads, bacteria, and apoptotic cells (19). Taken together, these results suggest that exposure to OD induces a phenotypic shift in bronchial epithelial cells toward a phagocytic phenotype and engulfment is enhanced through upregulation of CD36 and CD68.
To relate engulfment to Nrf2 directly, we treated cells with the cytochalasin D, an inhibitor of phagocytosis, before OD exposure. Cytochalasin D treatment resulted in a 62% decrease in cells with detectable engulfed dust, prevented increases in CD36 and CD68, and reduced endogenous oxidative stress and Nrf2 nuclear translocation, suggesting that engulfment of dust increases intracellular oxidative stress. Previous studies have also found a relationship between phagocytosis and oxidative stress. Macrophages exposed to oxidative stress through lipid peroxidation increase their expression of CD36 and phagocytic ability (2). Ingestion of asbestos fibers by mesothelial cells increases endogenous oxidative stress, an effect reduced by cytochalasin D (9). Several classes of nanoparticles have been shown to increase oxidative stress through a variety of mechanisms, including Fenton reactions for metal-based nanoparticles, mitochondrial damage from carbon-based nanoparticles, and increased reactive oxygen species as a result of cellular internalization processes that cause increases in NADPH oxidase enzyme activity (10). Excessive oxidative stress may have the opposite effect of slowing phagocytic behavior (6). Whether prevention of phagocytosis in bronchial epithelial cells exposed to OD was beneficial to the cells remains to be explored.
Furthermore, previous studies have shown that in addition to increasing production of antioxidant enzymes, Nrf2 can act as transcription factor for CD36, promoting phagocytosis (4, 11, 14). Nrf2-deficient mice demonstrate reduced CD36 expression on macrophages compared with wild-type mice (4) and impaired clearance and wound healing after hematoma (21). While no direct studies have observed a role for Nrf2 acting as a transcription factor for CD68, Nrf2-deficient mice have fewer CD68 positive cells and show prolonged wound healing compared with wild-type mice at the site of injury after sciatic nerve crush (20). Taken together, these results suggest that ingestion of OD results in an increase in endogenous oxidative stress that results in activation of Nrf2 and subsequent upregulation of phagocytic activity via CD36 and CD68.
To determine how increased Nrf2 signaling could positively affect airway inflammation and AHR in vivo, we treated mice with sulforaphane, a potent Nrf2 activator before OD exposures. We observed that bronchial epithelial cells from mice treated with sulforaphane engulfed more OD compared with vehicle OD-treated mice. Additionally, we observed that sulforaphane treatment prevented OD-induced inflammation as well as AHR. Previous studies have shown that treatment with sulforaphane enhances phagocytic activity (1, 3, 18). While the precise mechanism of enhanced phagocytic activity has not been elucidated, it is plausible that sulforaphane-induced Nrf2 activity could enhance phagocytosis, given that Nrf2 is a transcription factor for CD36. In support of this rationale, we found that bronchial epithelial cells treated with sulforaphane before OD exposure indeed had enhanced expression of both CD36 and CD68. It may be that reduction of oxidative stress and prebuffering the system with sulforaphane increases antioxidant enzymes that could protect the epithelial cells, potentially allowing the cells to engulf more dust, which aids in speed of clearance. If the dust is removed more rapidly, surrounding cells may release fewer inflammatory mediators; however, further studies would need to be done to draw this conclusion definitively. Regardless of the precise mechanism, these results suggest that engulfment of OD by epithelial cells in our model represents an uncommon but useful phenotypic shift, which likely serves to enhance dust clearance within the lung.
In conclusion, OD are phagocytized by bronchial epithelial cells both in vivo and in vitro. A second exposure to OD enhances the number of positive cells and level of expression of the scavenger receptor CD36. Engulfment increases oxidative stress and Nrf2 activity, and this effect can be enhanced using sulforaphane before OD exposure. It appears that increased Nrf2 activity and enhanced engulfment of OD by bronchial epithelial cells is beneficial, leading to reduced AHR and airway inflammation.
GRANTS
This work was supported by Canadian Institutes of Health Research Grant PJT-156322; the Swedish Heart-Lung Foundation Grants 20130532, 20130636, and 20120367; Konsul Th C Berghs research foundation, Karolinska Institutet; COST BM1201; the Swedish Society of Medicine; and the Centre for Allergy Research at Karolinska Institutet.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.K.M., M.C., J.G.M., and M.A. conceived and designed research; T.K.M., S.F., and M.C. performed experiments; T.K.M., S.F., and M.C. analyzed data; T.K.M., M.C., K.L., J.G.M., and M.A. interpreted results of experiments; T.K.M. and M.C. prepared figures; T.K.M. and M.C. drafted manuscript; T.K.M., S.F., M.C., K.L., J.G.M., and M.A. edited and revised manuscript; T.K.M., S.F., M.C., K.L., J.G.M., and M.A. approved final version of manuscript.
REFERENCES
- 1.Biswal S, Thimmulappa RK, Harvey CJ. Experimental therapeutics of Nrf2 as a target for prevention of bacterial exacerbations in COPD. Proc Am Thorac Soc 9: 47–51, 2012. doi: 10.1513/pats.201201-009MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuhrman B, Volkova N, Aviram M. Oxidative stress increases the expression of the CD36 scavenger receptor and the cellular uptake of oxidized low-density lipoprotein in macrophages from atherosclerotic mice: protective role of antioxidants and of paraoxonase. Atherosclerosis 161: 307–316, 2002. doi: 10.1016/S0021-9150(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 3.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, Feller-Kopman D, Wise R, Biswal S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med 3: 78ra32, 2011. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res 94: 609–616, 2004. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi E, Suzuki T, Yamamoto M. Roles nrf2 plays in myeloid cells and related disorders. Oxid Med Cell Longev 2013: 529219, 2013. doi: 10.1155/2013/529219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokkinaki M, Abu-Asab M, Gunawardena N, Ahern G, Javidnia M, Young J, Golestaneh N. Klotho regulates retinal pigment epithelial functions and protects against oxidative stress. J Neurosci 33: 16346–16359, 2013. doi: 10.1523/JNEUROSCI.0402-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kölbeck KGEA, Ehnhage A, Juto JE, Forsberg S, Gyllenhammar H, Palmberg L, Larsson K. Airway reactivity and exhaled NO following swine dust exposure in healthy volunteers. Respir Med 94: 1065–1072, 2000. doi: 10.1053/rmed.2000.0885. [DOI] [PubMed] [Google Scholar]
- 8.Larsson KA, Eklund AG, Hansson LO, Isaksson BM, Malmberg PO. Swine dust causes intense airways inflammation in healthy subjects. Am J Respir Crit Care Med 150: 973–977, 1994. doi: 10.1164/ajrccm.150.4.7921472. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Ernst JD, Broaddus VC. Phagocytosis of crocidolite asbestos induces oxidative stress, DNA damage, and apoptosis in mesothelial cells. Am J Respir Cell Mol Biol 23: 371–378, 2000. doi: 10.1165/ajrcmb.23.3.4094. [DOI] [PubMed] [Google Scholar]
- 10.Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int 2013: 942916, 2013. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama A, Tsukamoto S, Nishikawa K, Yoshida A, Harada N, Motojima K, Ishii T, Nakane A, Yamamoto M, Itoh K. Nrf2 regulates the alternative first exons of CD36 in macrophages through specific antioxidant response elements. Arch Biochem Biophys 477: 139–145, 2008. doi: 10.1016/j.abb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 12.McGovern TK, Chen M, Allard B, Larsson K, Martin JG, Adner M. Neutrophilic oxidative stress mediates organic dust-induced pulmonary inflammation and airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol 310: L155–L165, 2016. doi: 10.1152/ajplung.00172.2015. [DOI] [PubMed] [Google Scholar]
- 13.Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, Fadok VA. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ 12: 107–114, 2005. doi: 10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- 14.Olagnier D, Lavergne RA, Meunier E, Lefèvre L, Dardenne C, Aubouy A, Benoit-Vical F, Ryffel B, Coste A, Berry A, Pipy B. Nrf2, a PPARγ alternative pathway to promote CD36 expression on inflammatory macrophages: implication for malaria. PLoS Pathog 7: e1002254, 2011. doi: 10.1371/journal.ppat.1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penberthy KK, Juncadella IJ, Ravichandran KS. Apoptosis and engulfment by bronchial epithelial cells. Implications for allergic airway inflammation. Ann Am Thorac Soc 11, Suppl 5: S259–S262, 2014. doi: 10.1513/AnnalsATS.201405-200AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole JA, Gleason AM, Bauer C, West WW, Alexis N, van Rooijen N, Reynolds SJ, Romberger DJ, Kielian TL. CD11c(+)/CD11b(+) cells are critical for organic dust-elicited murine lung inflammation. Am J Respir Cell Mol Biol 47: 652–659, 2012. doi: 10.1165/rcmb.2012-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimamura T, Maesaka JK. Phagocytosis of E. coli by renal tubular epithelia. Yale J Biol Med 57: 817–824, 1984. [PMC free article] [PubMed] [Google Scholar]
- 18.Suganuma H, Fahey JW, Bryan KE, Healy ZR, Talalay P. Stimulation of phagocytosis by sulforaphane. Biochem Biophys Res Commun 405: 146–151, 2011. doi: 10.1016/j.bbrc.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travaglione S, Falzano L, Fabbri A, Stringaro A, Fais S, Fiorentini C. Epithelial cells and expression of the phagocytic marker CD68: scavenging of apoptotic bodies following Rho activation. Toxicol In Vitro 16: 405–411, 2002. doi: 10.1016/S0887-2333(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Johnson D, Johnson JA. Deletion of Nrf2 impairs functional recovery, reduces clearance of myelin debris and decreases axonal remyelination after peripheral nerve injury. Neurobiol Dis 54: 329–338, 2013. doi: 10.1016/j.nbd.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Sun G, Ting SM, Song S, Zhang J, Edwards NJ, Aronowski J. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem 133: 144–152, 2015. doi: 10.1111/jnc.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhiping W, Malmberg P, Larsson BM, Larsson K, Larsson L, Saraf A. Exposure to bacteria in swine-house dust and acute inflammatory reactions in humans. Am J Respir Crit Care Med 154: 1261–1266, 1996. doi: 10.1164/ajrccm.154.5.8912733. [DOI] [PubMed] [Google Scholar]