Abstract
Lung endothelial cell (EC) immune activation during bacterial sepsis contributes to acute lung injury and bronchopulmonary dysplasia in premature infants. The epigenetic regulators of sepsis-induced endothelial immune activation, lung inflammation, and alveolar remodeling remain unclear. Herein, we examined the role of the cytoplasmic histone deacetylase, HDAC6, in regulating EC Toll-like receptor 4 (TLR4) signaling and modulating sepsis-induced lung injury in a neonatal model of sterile sepsis. In human primary microvascular endothelial cells (HPMEC), lipopolysaccharide (LPS)-induced MAPK, IKK-β, and p65 phosphorylation as well as inflammatory cytokine expression were exaggerated with the HDAC6 inhibitor tubastatin A, and by dominant-negative HDAC6 with a mutated catalytic domain 2. Expression of HDAC6 wild-type protein suppressed LPS-induced myeloid differentiation primary response 88 (MyD88) acetylation, p65 (Lys310) acetylation, MyD88/TNF receptor-associated factor 6 (TRAF6) coimmunoprecipitation, and proinflammatory TLR4 signaling in HPMEC. In a neonatal mouse model of sepsis, the HDAC6 inhibitor tubastatin A amplified lung EC TLR4 signaling and vascular permeability. HDAC6 inhibition augmented LPS-induced MyD88 acetylation, MyD88/TRAF6 binding, p65 acetylation, canonical TLR4 signaling, and inflammation in the developing lung. Sepsis-induced decreases in the fibroblast growth factors FGF2 and FGF7 and increase in matrix metalloproteinase-9 were worsened with HDAC6 inhibition, while elastin expression was equally suppressed. Exaggerated sepsis-induced acute lung inflammation observed with HDAC6 inhibition worsened alveolar simplification evidenced by increases in mean linear intercepts and decreased radial alveolar counts. Our studies reveal that HDAC6 is a constitutive negative regulator of cytoplasmic TLR4 signaling in EC and the developing lung. The therapeutic efficacy of augmenting HDAC6 activity in neonatal sepsis to prevent lung injury needs to be evaluated.
Keywords: alveolar remodeling, endothelium, HDAC6, lung inflammation, MyD88 acetylation
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is a chronic form of lung disease that develops in premature infants exposed to hyperoxia, sepsis, and mechanical ventilation (23, 47). Although the pathogenesis of BPD is complex, it is posited that inflammation in the saccular lung disrupts key morphogenic pathways necessary for alveolarization (23, 47). Systemic sepsis is associated with acute lung injury (ALI) in adults and is a major risk factor for the development of BPD in premature infants (30, 47). With the use of both genetic and transgenic approaches, our laboratory has shown that activation of the Toll-like receptor (TLR) family of innate immune receptors in the developing lung directs inflammation and alveolar simplification, akin to that observed in infants with BPD (33, 40, 41). Bacterial ligands, like lipopolysaccharide (LPS) derived from gram-negative bacteria, engage host TLRs, triggering downstream activation of several adapters, effectors, and kinases resulting in transcriptional activation of the NF-κB- and activator protein-1-dependent inflammatory program (1). Posttranslational modifications of TLR signaling pathway adapters, kinases, and effectors via phosphorylation, SUMOylation, methylation, succinylation, and polyubiquitination ensure fine tuning of the host innate immune response (9, 26). Acetylation and deacetylation of TLR signaling components regulate TLR-mediated gene transcription in the nucleus but are increasingly being recognized as modulators of cytoplasmic TLR signaling (9, 20). Herein, we examined the role of reversible acetylation in regulating TLR-mediated lung endothelial immune activation, inflammation, and alveolar remodeling in neonatal mice. Understanding regulation of reversible posttranslational modifications of the TLR pathway in sepsis will provide insights into mitigating sepsis-induced lung injury and BPD in infants.
Acetylation and deacetylation of TLR signaling pathway components are regulated in tandem by histone acetyltransferases and histone deacetylases (HDACs), respectively (20, 45). HDACs are a class of deacetylases that remove acetyl residue from an N-acetyllysine amino acid on a protein that is acetylated by acetyltransferase (39, 44, 45). The class I HDACs, HDAC1, -2, and -3, are mainly localized in the nucleus, but HDAC8 is found in both the nucleus and the cytoplasm. Class II HDACs, HDAC4, -5, -6, -7, -9, and -10, are shuttled in and out of the nucleus, in response to different signals (39, 44, 45). HDAC6, a class IIb HDAC localized in the cytosol, deacetylates target proteins that regulate cell migration, differentiation, and innate immune signaling (19, 20, 51). HDAC inhibitors have been shown to act as anti-inflammatory drugs in disease models of rheumatoid arthritis and septic shock but have been also noted to worsen the development of atherosclerotic lesions by increasing cytokines, indicating potential for isoform-specific roles in regulation of inflammation (3, 27, 28, 60). The lung endothelial cell (EC) is a key component of the innate immune response in sepsis but also primes inflammation and ALI by promoting neutrophil influx and cytokine release (2, 29). While ECs are known to express several HDACs, including class I and class II HDACs that regulate several EC functions (24, 49, 50, 57, 58), the role of cytoplasmic HDAC6 on TLR signaling and inflammation in lung EC has not been investigated, especially in the context of sepsis-induced neonatal lung injury and BPD.
Prior work from our laboratory and others has shown that activation of myeloid differentiation primary response 88 (Myd88)-dependent canonical TLR signaling in lung EC is essential for sepsis-induced endothelial inflammation and ALI in mice (2, 29, 31, 33). While modulation of EC TLR signaling using HDAC inhibitors presents an attractive therapy to suppress sepsis-induced ALI, targeting the correct isoform is necessary because of the differential effects of HDAC inhibition on inflammation (5, 12, 20, 24). HDAC-dependent regulation of MYD88-dependent TLR signaling has primarily focused on acetylation-dependent nuclear export and transcriptional activation of NF-κB components such as p65/RelA (5, 12, 20). Inhibition of nuclear HDACs in vitro or in vivo has been shown to suppress inflammation, whereas the effects of cytoplasmic HDAC inhibition on TLR-mediated inflammation are less clear (5, 20, 50). Chemical inhibition of HDAC6 and HDAC3 has been shown to suppress LPS-induced endothelial permeability (24). In contrast, HDAC6 has also been shown to inhibit the formation of MyD88-TNF receptor-associated factor 6 (TRAF6) signaling complex and represses proinflammatory gene expression in macrophages (22). In this study, we tested the hypothesis that HDAC6 is a constitutive repressor of MyD88-dependent TLR4 signaling in EC and in the lung and examined the ramifications of HDAC6 inhibition on sepsis-induced EC immune activation, lung inflammation, and alveolar remodeling in neonatal mice.
MATERIALS AND METHODS
Ethical approval and animal model.
Care of mice before and during the experimental procedures was conducted in accordance with the policies at the University of Missouri-Kansas City Laboratory Animal Resources Center (Protocol 1510) and the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All protocols had prior approval from the University of Missouri- Kansas City Institutional Animal Care and Use Committee. Wild-type mice, C57BL/6, were obtained commercially from Charles River (Burlington, MA). All animal experiments were performed on mouse pups between day of life 4 and 7. Lipopolysaccharide (LPS) at a dose of 2 mg/kg was given intraperitoneally, with controls receiving sterile saline (Sigma, St. Louis, MO). The HDAC6 inhibitor (tubastatin A, HDAC6-I; Santa Cruz Biotechnology, Santa Cruz, CA) and pan-HDAC inhibitor (trichostatin, pan-HDAC-I; Santa Cruz Biotechnology) were injected intraperitoneally at 1 and 5 mg/kg, respectively, immediately before LPS treatment. Pups were euthanized at 2, 3, 6, 48, and 196 h post-LPS injections. Mice were euthanized using a 100 µl intraperitoneal injection of pentobarbital sodium and exsanguinated with the cessation of heartbeat, and the lungs were harvested and used as described below. Lung lobes were divided as specified: left lobe for histology; right lobes divided for RNA and protein.
Cell culture and reagents.
Human primary pulmonary microvascular endothelial cells (HPMEC) from ScienCell (Carlsbad, CA) were used between passages 3 and 4 for all experiments. Cells are derived from neonatal lungs. HPMEC were grown in endothelial cell medium supplemented with 5% fetal bovine serum (FBS), antibiotics, and endothelial cell growth serum as recommended by the manufacturer (ScienCell) in a humidified incubator containing 5% CO2 at 37°C. Ultrapure LPS (100 ng/mL) was purchased from Invivogen (San Diego, CA). Tubastatin A (HDAC6-I, 100 nM) was purchased commercially from Santa Cruz Biotechnology. For experiments with HDAC6-I in vitro, cells were pretreated for 6 h before the addition of LPS.
Isolation of murine endothelial cells.
For endothelial cell isolation, the lungs from two neonatal C57BL/6 pups (4–5 days old) were pooled per condition. The protocol for the isolation of mouse lung endothelial cells was described previously (48). Briefly, harvested lungs were minced with sterile scissors in ice-cold DMEM and transferred to 15 mL of prewarmed 1 mg/mL collagenase solution in DMEM. The mixture was allowed to rotate for 45 min at 37°C. The digested tissue was then passed through a 14-gauge cannula attached to a 20-mL syringe several times, followed by passage through a 70-µm cell strainer, and washed with 20% FBS + DMEM. Cells were then centrifuged at 400 g for 5 min, and the supernatant was aspirated off. The cell pellet was resuspended with 0.1% BSA in PBS. The suspension was incubated with anti-platelet and endothelial cell adhesion molecule 1 (PECAM-1) antibody-conjugated Dynabeads from Life Technologies (Carlsbad, CA) on a rocker for 15 min at room temperature as per the manufacturer’s instructions. After isolation, cells were washed with PBS three times, and protein or RNA was extracted as described below.
Bronchoalveolar lavage analysis.
Ten-day-old mice were injected with LPS, with or without the HDAC6 or pan-HDAC inhibitors as above, and bronchoalveolar lavage analysis (BAL) was performed at 24 h. Mouse lungs were lavaged 10 times with 200 µL of PBS after endotracheal cannulation. A 10 µL aliquot was loaded on a hemocytometer for cell number counting; 50 µL were used for quantification of total protein by a BCA assay, and a 200 µL aliquot was collected on a cytospin at room temperature; and Shandon Kwik Diff staining (commercially available from Thermo-Fisher) was performed following the manufacturer’s protocol on 300 cells/prep.
Quantification of mRNA expression using real-time PCR.
Total RNA was extracted from HPMEC, mouse lung EC, or lung tissue using the PureLink RNA Mini Kit (Life Technologies) following the manufacturer’s instructions, and cDNA was synthesized from 1 µg of RNA using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Real-time PCR was run on a Bio-Rad IQ5 with SYBR Green Master Mix. Prevalidated primers for mouse and human intercellular adhesion molecule-1 (ICAM-1), IL-1β, IL-8 (keratinocyte chemoattractant), TNF-α, fibroblast growth factor (FGF) 2, FGF7, elastin, matrix metalloproteinase-9 (MMP-9), β-actin, and 18S were purchased commercially from Sigma. 18S or β-actin was used as the housekeeping gene. The relative gene expression was calculated using the Pflaffl method (37).
Immunoblotting for quantifying changes in protein expression.
HPMEC and mouse lung tissue were homogenized in RIPA lysis buffer containing commercially available protease and phosphatase inhibitors (Sigma), with clarified lysates used for Western blotting. Immunoblotting was done following standard protocol. The primary antibodies for mouse anti-acetylated α-tubulin (sc-23950), mouse anti-acetylated p65 (sc-23950), mouse anti-ICAM-1 (sc-8439), rabbit anti-IL-1β (sc-7884), mouse anti-FGF7 (sc-365440), mouse anti-MMP-9 (sc-6841), mouse anti-elastin (sc-58756), mouse anti-TLR4 (sc-293072), mouse anti-PECAM (sc-376764), rabbit anti-p65 (sc-109), and goat anti-HDAC6 (sc-5258) were from Santa Cruz Biotechnology (1:1,000; Dallas, TX). The rabbit anti-acetylated lysine (no. 9814), mouse anti-MyD88 (no. 4283), rabbit anti-acetylated p65 (Lys310, no. 3045), rabbit anti-JNK (no. 9258), rabbit anti-p38 (no. 8690), rabbit anti-inhibitor of NF-κB kinase-β (IKK-β, no. 2370), rabbit anti-phospho-JNK (Tyr185, no. 4668), rabbit anti-phospho-p38 (Thr180/Tyr182, no. 4511), rabbit anti-phospho-IKK-β (Ser177, no. 2078), and rabbit anti-phospho-p65 (Ser536, no. 3033) were from Cell Signaling (1:500; Danvers, MA). The mouse anti-TRAF-6 was from Abcam (ab40675, 1:1,000; Cambridge, MA), and the mouse anti-β-actin was from Sigma (A1978, 1:5,000). Densitometry was performed using ImageJ Software (NIH, Bethesda, MD), and changes were normalized to β-actin or the corresponding nonphosphorylated antibody.
Immunoprecipitation for phosphorylation studies.
HPMEC grown to 90% confluence in 60-mm dishes had various treatments, with lysates used for immunoprecipitation studies. Whole lung homogenates obtained after various timed intraperitoneal treatments were used for studies. The immunoprecipitation protocol was followed as described previously (32, 55). Briefly, 500 µg of protein were incubated with the primary antibody overnight at 4°C and for 2 h with protein A-Sepharose beads. Upon completion, the beads were washed two times with ice-cold Tris-buffered saline, after which 100 µL of Tris-buffered saline and 2× Laemmli buffer were added to each sample, and the beads were boiled for 10 min. Proteins were separated by SDS-PAGE and blotted for phosphorylated proteins. After the blots were developed using enhanced chemiluminescence, they were stripped with Restore Plus stripping buffer (Thermo-Fisher, Rockford, IL) and probed with the primary antibody specific to the protein immunoprecipitated. The primary antibody used for immunoprecipitation was rabbit anti-MyD88 (Cell Signaling). For experiments with MyD88 binding and acetylation, the following antibodies were used: mouse anti-TRAF-6 (Abcam) and rabbit anti-acetylated lysine (Cell Signaling). Densitometry was performed using ImageJ Software (NIH), and changes in acetylation were normalized to MyD88.
Design of wild-type/variant constructs and transfection.
Total RNA was extracted from HPMEC using the PureLink RNA Mini Kit (Life Technologies), and the cDNA library was generated with SuperScript III Reverse Transcriptase (Thermo-Fisher) following the manufacturer’s protocol. Human HDAC6 cDNA was amplified and cloned into pIRES-EGFP-Puro (Addgene, Cambridge, MA) with 5′-ACTGCTAGCCACCATGACCTCAACCGGCCAGGAT-TCCA-3′ and 5′-TGAGAGCTCTTAGTGTGGGTGGGGCATATCCTCCCCAAA-3′. HDAC6 point mutations in the second catalytic domain (D649N, H651V, and H652V) were introduced by two-step PCR strategy and cloned into pIRES-EGFP-Puro (19). HPMEC grown in six-well tissue culture plates were transfected overnight with 2 µg of the indicated plasmids or empty plasmids (mock) with Lipofectamine 3000 (Thermo-Fisher) as per the manufacturer’s protocol. Cells were allowed to recover for 24 h and then treated with LPS for various intervals.
Immunohistochemistry and immunofluorescence staining.
The left lung of the mouse pups was fixed in 4% formalin and blocked in paraffin, and 4-µm sections were used for immunohistochemistry staining using a validated Ets-related gene (ERG, ab92513, 1:100; Abcam) with the corresponding ImmPRESS HRP kit (Vector Laboratories, Burlingame, CA). The nucleus of the cells was stained with Harris Hematoxylin (Sigma). The slides were mounted with Permount mounting media (Fisher Scientific, Waltham, MA). Images were taken at ×40 magnification using an Olympus BX60 microscope with an attached camera. Staining is depicted as brown for ERG and blue for the nuclei. For phosphorylated NF-κB (p-p65) staining, the left lung of mouse pups was fixed in 4% formalin and cryopreserved, and 4-µm sections were used for immunofluorescence staining using validated rabbit anti-phospho-p65 antibody (ab86299, 1:100; Abcam) and rat anti-PECAM antibody (550274, 1:100; BD) with appropriate secondary antibodies. The slides were mounted with anti-fade mounting media with DAPI (Vector Laboratories). Images were taken at ×63 magnification using a Zeiss LSM510 confocal microscope with an attached camera. Staining is indicated with a channel of PECAM as red, p-p65 as green, and DAPI is blue. Five mice were in each group. For HDAC6 in vitro staining, HPMEC cells were fixed in 4% formalin and then stained with a validated rabbit HDAC6 antibody (NBP1–69127, 1:100; Novus Biologicals, Centennial, CO). The slides were mounted with mounting medium with DAPI (Vector Laboratories). Images were taken at ×63 magnification using a Zeiss LSM510 confocal microscope with an attached camera. Staining is indicated with a channel of HDAC6 in green, and DAPI is blue.
Elastic fiber staining.
After LPS and tubastatin A treatments, the left lung of the mouse pups was fixed in formalin and blocked in paraffin, and 4-µm sections were cut on slides. Elastic fiber staining followed the Weigert’s Resorcin Fuchsin protocol (without Weigert’s Iron Hematoxylin) with the pre-Carmine staining protocol commercially available from the manufacturer (Rowley Biochemical, Danvers, MA). At least three mice were used for each experimental group. Images were taken at ×40 magnification using an Olympus BX60 microscope with an attached camera.
Hematoxylin and eosin staining to assess lung development in inflated mouse lungs.
C57BL/6 mouse pups were euthanized 196 h after intraperitoneal injection of LPS or saline. The lungs were inflated using a fixed volume of formalin, as previously described (36). Briefly, with the use of a catheter, 300 µL of formalin fixative were used to inflate the lungs after the mice were euthanized. Precautions were taken not to over- or underinflate the lungs. The lungs were further fixed in formalin before being embedded in paraffin, cut into 4-µm sections on slides, and stained with hematoxylin and eosin (H&E). The H&E slides were scanned using a Leica Slide Scanner, and the scanned images were used at ×40 for assessing radial alveolar count (13, 33) and mean linear intercepts (25) following previously used procedures. Radial alveolar counts were quantified by averaging five measurements per mouse. For mean linear intercepts, the average of five counts per mouse was determined. Five to eight mice were used per experimental condition.
Data analysis.
Data are presented as means ± SD. For cell culture experiments, quantitative data are representative of a minimum of five independent experiments. For animal experiments, five to eight mice were used for each experimental group. Adequate technical and biological replicates were used for all experiments. All data distributions were assessed for departures from the Gaussian distribution (i.e., normality) using the D’Agostino-Pearson omnibus test. If data were normally distributed, then a one-way ANOVA with a post hoc Tukey’s test was used for analysis to compare differences in experimental groups. If data did not meet Gaussian assumptions, a Mann-Whitney U-test was used. For most analysis, fold changes were calculated relative to expression in untreated controls. For changes in phosphorylation, the phosphorylated-to-total protein ratio was calculated for each condition and compared between groups. For determining EC numbers, we quantified ERG+/total cells/high-power field per mouse (sections) across experimental groups and analyzed data using ANOVA. For BAL differential counts, the proportion of three major cell types was quantified on 300 cells/BAL and compared across groups. A P value < 0.05 was considered significant for all statistical tests. Statistical analysis was done using GraphPad Prism 8.0.2 (San Diego, CA). Data from cell culture experiments are expressed as bar charts with mean and standard deviation whisker bars, whereas animal experiments are shown as dot and scatterplots with mean bar and standard deviation whiskers.
RESULTS
HDAC6 inhibition amplifies LPS-induced canonical TLR signaling and inflammation in HPMEC.
We used tubastatin A, a specific HDAC6 inhibitor, to determine whether HDAC6 regulated LPS responsiveness in HPMEC (7, 43). Tubastatin A at concentrations (10–100 nM) selective for HDAC6 suppression increased protein levels of acetylated α-tubulin, a known target of HDAC6 lysine deacetylation (Fig. 1A). Subsequent in vitro experiments were performed with 100 nM of tubastatin A. LPS-induced TNF-α, IL-1β, and IL-8 RNA expression was amplified by approximately twofold in cells pretreated with tubastatin A (Fig. 1B). ICAM-1, a protein necessary for leukocyte endothelial transmigration, and IL-1β protein induced with LPS were further increased with tubastatin A at 24 h (Fig. 1, C and D). We next examined whether HDAC6 inhibition derepressed LPS-mediated canonical TLR4 signaling. LPS activated the TLR pathway canonical kinases, IKK-β, and the mitogen-activated protein kinases (MAPKs), p38 and JNK, as evidenced by increased phosphorylation at 1 h (Fig. 1, E and F). LPS-induced IKK-β, p38, and JNK phosphorylation was significantly increased in HPMEC treated with tubastatin A (Fig. 1, E and F). Tubastatin A or LPS did not alter expression of HDAC6 protein (Fig. 1C) or cellular localization (Fig. 1G). We next examined whether HDAC6 regulates NF-κB activation downstream of LPS-mediated IKK-β and MAPK phosphorylation. p65 (RelA, a subunit of NF-κB) phosphorylation induced with LPS was increased in cells treated with tubastatin A (Fig. 1, E and F). These data demonstrate that HDAC6 inhibition exaggerates LPS-mediated proinflammatory TLR pathway signaling in HPMEC.
Fig. 1.
Histone deacetylase 6 (HDAC6) inhibition amplifies lipopolysaccharide (LPS)-induced canonical Toll-like receptor (TLR) signaling and inflammation in human primary microvascular endothelial cells (HPMEC). HPMEC were treated with LPS (100 ng/mL) with or without HDAC6-I pretreatment (100 nM, 1 h), and cell lysates were used for assays. A: acetylated α-tubulin was quantified by immunoblotting 24 h after LPS after increasing doses of HDAC6-I. B: RNA obtained from cell lysates 18 h after LPS treatment with or without HDAC6-I was used to quantify IL-1β, TNF-α, intercellular adhesion molecule-1 (ICAM-1), and IL-8 expression by qRT-PCR (n = 5/group). C: ICAM-1, IL-1β, and HDAC6 protein was quantified 24 h after LPS and HDAC6-I treatments by immunoblotting cell lysates, with densitometry quantification shown graphically (D); (n = 5/group). E: p65 (RelA), IKK-β, p38, and JNK phosphorylation was quantified by immunoblotting cell lysates obtained 1 h after LPS and HDAC6-I treatments, with densitometry shown (F); (n = 5/group). G: immunofluorescence in HPMEC depicting HDAC6 localization within cells. HDAC6 is green and DAPI (nuclear) is blue. Scale bar is 10 µm. Capped lines, with above significance level (***P < 0.001), denote significant comparison of groups (B, D, and F) using ANOVA and post hoc Tukey test.
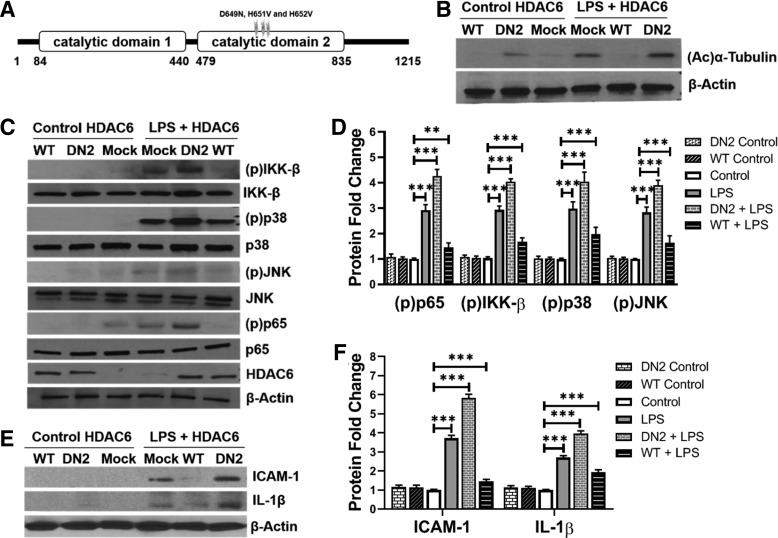
HDAC6 catalytic domain 2 mediates suppression of TLR4 signaling in HPMEC.
To validate that HDAC6 is a negative regulator of EC TLR4 signaling and determine the molecular basis of HDAC6 function, we cloned wild-type human HDAC6 (HDAC6-WT) and HDAC6 with loss of function mutations in the catalytic domain 2 (HDAC6-DN2) in expression vectors (Fig. 2A) (19). HPMEC transfected with HDAC6-WT and HDAC6-DN2 had decreased and increased acetylated α-tubulin expression, respectively, confirming effects on HDAC6 function (Fig. 2B). LPS-induced IKK-β, p38, JNK, and p65 phosphorylation at 1 h was suppressed in cells transfected with HDAC6-WT but was increased in cells transfected with HDAC6-DN2 (Fig. 2, C and D). Consistent with the above results, ICAM-1 and IL-1β expression induced with LPS was suppressed with HDAC6-WT but exaggerated with HDAC6-DN2 (Fig. 2, E and F). These data reveal that HDAC6-DN2 mediates inhibition of TLR signaling in HPMEC.
Fig. 2.
Histone deacetylase 6 (HDAC6) catalytic domain 2 mediates suppression of Toll-like receptor 4 (TLR4) signaling in human primary microvascular endothelial cells (HPMEC). HPMEC transfected with mock (empty plasmid), wild-type HDAC6 (WT), or HDAC6 with a mutated catalytic domain 2 (DN2) plasmids were treated with lipopolysaccharide (LPS, 100 ng/mL) for varying intervals, and cell lysates were used for assays. A: graphical representation of the HDAC6 WT protein with mutations introduced in catalytic domain 2 for a dominant-negative HDAC6 protein (DN2) shown. B: acetylated α-tubulin was quantified by immunoblotting 24 h after LPS in cells transfected with mock, HDAC6 WT, or HDAC6 DN2 plasmids. C: mock, HDAC6 WT, or DN2-transfected HPMEC were treated with LPS for 1 h, and cell lysates were used to examine HDAC6, p65, inhibitor of NF-κB kinase-β (IKK-β), p38, and JNK phosphorylation by immunoblotting, with densitometry shown graphically (D); (n = 5/group). E: 24 h after treatment of mock, HDAC6 WT, or DN2-transfected cells with LPS, intercellular adhesion molecule-1 (ICAM-1) and IL-1β were quantified by immunoblotting lysates, with densitometric quantification shown (F); (n = 5/group). Capped lines, with significance levels (**P < 0.01 and ***P < 0.001), denote significant comparison of groups (D and F) using ANOVA and post hoc Tukey test.
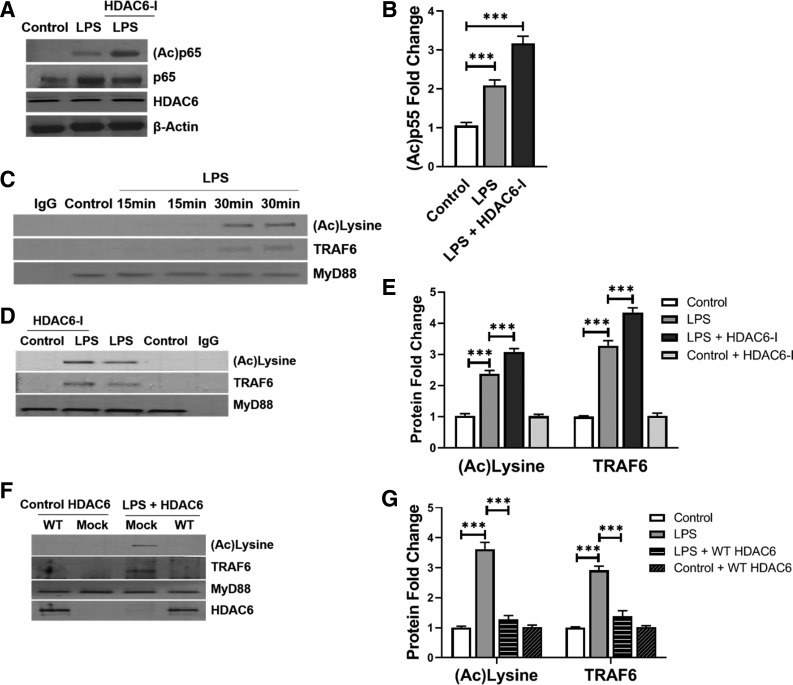
HDAC6 regulates TLR signaling by deacetylating MyD88 and suppressing MyD88-TRAF6 assembly in HPMEC.
We posited that HDAC6 regulates TLR4 signaling by deacetylating key downstream effectors such as MyD88 and NF-κB. NF-κB acetylation facilitates nuclear translocation and activation of its transcriptional program. LPS treatment resulted in increased expression of p65 protein acetylated at lysine residues 310/351 (Fig. 3, A and B). Tubastatin A increased expression of acetylated p65Lys310 in LPS-treated cells at 1 h (Fig. 3, A and B). Because our data with IKK-β and MAPK phosphorylation suggested that events proximal to NF-κB activation in TLR4 signaling were regulated by HDAC6, we examined acetylation of the principal TLR4 adapter, MyD88. MyD88 ligates with TLR4 in LPS-stimulated cells and propagates TLR signaling by binding TNF receptor associated factor 6 (TRAF6), a downstream effector (1, 22). Coimmunoprecipitation studies revealed that MyD88 was acetylated 15 and 30 min after LPS treatment (Fig. 3C). Furthermore, acetylated MyD88 bound with TRAF6 after TLR4 stimulation (Fig. 3C). MyD88 acetylation and assembly with TRAF6 were more pronounced in HPMEC treated with tubastatin A (Fig. 3, D and E), while HDAC6-WT suppressed LPS-induced MyD88 acetylation and binding with TRAF6 (Fig. 3, F and G). These data demonstrate that HDAC6 regulates TLR4 signaling in HPMEC by reversible acetylation of the key adapter MyD88 and the transcription factor NF-κB.
Fig. 3.
Histone deacetylase 6 (HDAC6) regulates Toll-like receptor (TLR) signaling by deacetylating myeloid differentiation primary response 88 (MyD88) and suppressing MyD88-TNF receptor-associated factor 6 (TRAF6) assembly in human primary microvascular endothelial cells (HPMEC). A: acetylated p65 and HDAC6 were quantified in HPMEC lysates obtained at 1 h after lipopolysaccharide (LPS) treatment without or with 100 nM HDAC6-I pretreatment, with densitometry shown (B); (n = 5/group). C: 15 and 30 min after LPS treatment, MyD88 was immunoprecipitated from HPMEC lysates, and TRAF6 and acetylated lysine were probed by immunoblotting MyD88 immunoprecipitates. D: 30 min after LPS treatment with or without 100 nM HDAC6-I pretreatment, MyD88 was immunoprecipitated from HPMEC lysates, and TRAF6 and acetylated lysine were probed by Western blotting, with densitometry shown graphically (E); (n = 5/group). F: cells transfected with mock, HDAC6 WT, or DN2 plasmids were stimulated with LPS for 30 min. MyD88 was subsequently immunoprecipitated from cell lysates, and TRAF6, acetylated lysine, and HDAC6 were probed by Western blotting, with densitometry shown graphically (G); (n = 5/group). Capped lines, with above significance level (***P < 0.001), denote significant comparison of groups (B, E, and G) using ANOVA and post hoc Tukey test.
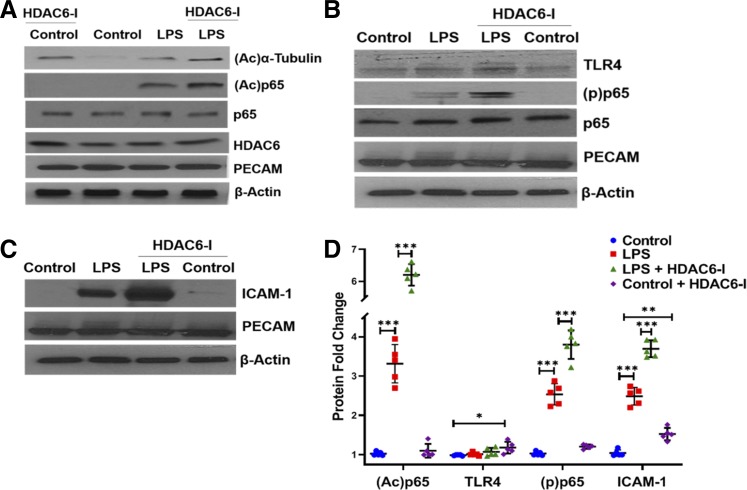
HDAC6 inhibition amplifies LPS-induced mouse lung EC immune activation and inflammation in a neonatal mouse model of sepsis.
To examine the role of HDAC6 in regulating lung EC TLR signaling, we performed experiments in a neonatal mouse model of sepsis that we developed (33). LPS treatment (2 mg/kg ip) induced canonical TLR signaling in mouse lung EC as evidenced by p65 (NF-κB) phosphorylation (Fig. 4, B and D). Tubastatin A (1 mg/kg ip) exaggerated LPS-induced p65 phosphorylation and p65 acetylation in lung EC without altering TLR4 or HDAC6 expression (Fig. 4, B and D). We next examined whether HDAC6 inhibition increased LPS-induced lung EC immune activation in vivo. LPS-induced ICAM-1 expression, a marker of endothelial activation, was amplified with tubastatin A treatment in parallel with increased p65 and α-tubulin acetylation in mouse lung EC in vivo (Fig. 4, A, C, and D). These data demonstrate that HDAC6 inhibition amplifies TLR4-mediated lung EC immune activation in neonatal sepsis.
Fig. 4.
Histone deacetylase 6 (HDAC6) inhibition amplifies lipopolysaccharide (LPS)-induced mouse lung endothelial cell (EC) immune activation and inflammation in a neonatal mouse model of sepsis. A, B, and C: mouse lung endothelial cells extracted from 5-day-old wild-type pups 48 h after treatment with lipopolysaccharide (LPS, 2 mg/kg ip) without or with HDAC6-I (1 mg/kg ip) were homogenized and used to quantify acetylated α-tubulin, acetylated p65, HDAC6, Toll-like receptor 4 (TLR4), phosphorylated (p)-p65, and intercellular adhesion molecule-1 (ICAM-1) expression by Western blotting, with densitometry shown (D);(n = 5/group).PECAM, platelet and endothelial cell adhesion molecule 1. Capped lines, with significance levels (*P < 0.05, **P < 0.01, and ***P < 0.001), denote significant comparison of groups (D) using ANOVA and post hoc Tukey test.
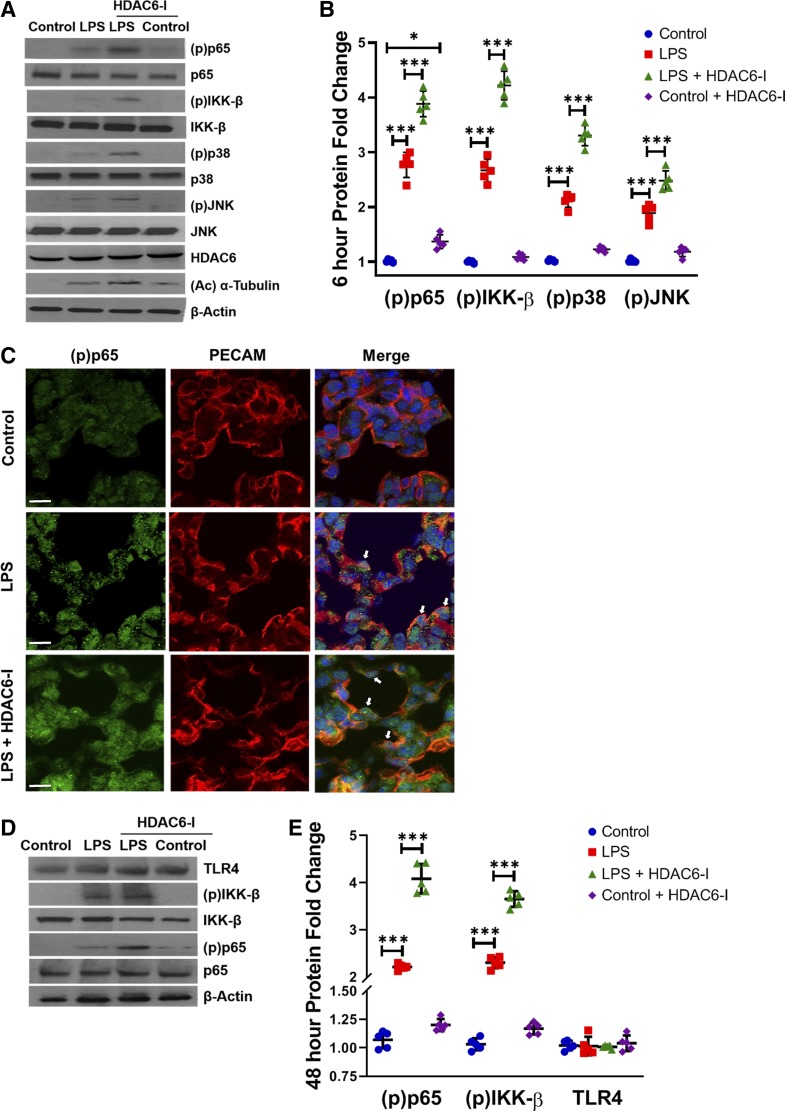
HDAC6 inhibition amplifies LPS-induced canonical TLR signaling in neonatal mouse lungs.
We next investigated whether activation of TLR signaling in the lung after systemic LPS was HDAC6 dependent. Treatment with intraperitoneal tubastatin A (1 mg/kg) in mice increased acetylated α-tubulin expression at 6 h, showing that systemic HDAC6 inhibition modulates acetylation of its downstream target in the lung (Fig. 5, A and B). Intraperitoneal LPS robustly induced TLR signaling in the neonatal mouse lung as evident by IKK-β, p38, JNK, and p65 phosphorylation at 6 h in lung homogenates (Fig. 5, A and B). LPS-induced lung TLR pathway activation evident at 6 h was exaggerated in mice treated with intraperitoneal tubastatin A (Fig. 5, A and B). Immunofluorescence studies revealed that p65 phosphorylation (green fluorescence, Ser536, p-p65, a marker of NF-κB activation) was hardly evident in the nucleus of control pups but was clearly evident in the lung EC (red fluorescence, PECAM1+) of pups treated with LPS or LPS + HDAC6-I at 1 h (Fig. 5C). The effects of HDAC6 inhibition on amplified TLR signaling persisted as evident by increased lung IKK-β and p65 phosphorylation 48 h after tubastatin A and LPS (Fig. 5, D and E). These data suggest that HDAC6 inhibition amplifies LPS-induced activation of canonical TLR4 signaling in the lung.
Fig. 5.
Histone deacetylase 6 (HDAC6) inhibition exaggerates lipopolysaccharide (LPS)-induced canonical Toll-like receptor (TLR) signaling in neonatal mice at 6 and 48 h. A: mouse lungs were harvested from 7-day-old mice 6 h after treatments with LPS (2 mg/kg ip) and HDAC6-I (1 mg/kg ip). Lung homogenates were used to quantify HDAC6, p65, inhibitor of NF-κB kinase-β (IKK-β), p38, and JNK phosphorylation by immunoblotting, with densitometry shown graphically (B); (n = 5/group). C: immunofluorescence was performed for phospho-p65 and platelet and endothelial cell adhesion molecule 1 (PECAM-1) expression and localization on lung sections obtained from day of life 7 mice 1 h after LPS and HDAC6-I treatments. White arrows, nuclear phospho (p)-p65. Scale bar is 10 µm. D: mouse lungs were harvested from 9-day-old mice 48 h after LPS and HDAC6-I treatments, and homogenates were used to quantify TLR4, p65, and IKK-β phosphorylation by immunoblotting, with densitometry shown (E); (n = 5/group). Capped lines, with significance level (*P < 0.05 and ***P < 0.001), denote significant comparison of groups (B and E) using ANOVA and post hoc Tukey test.
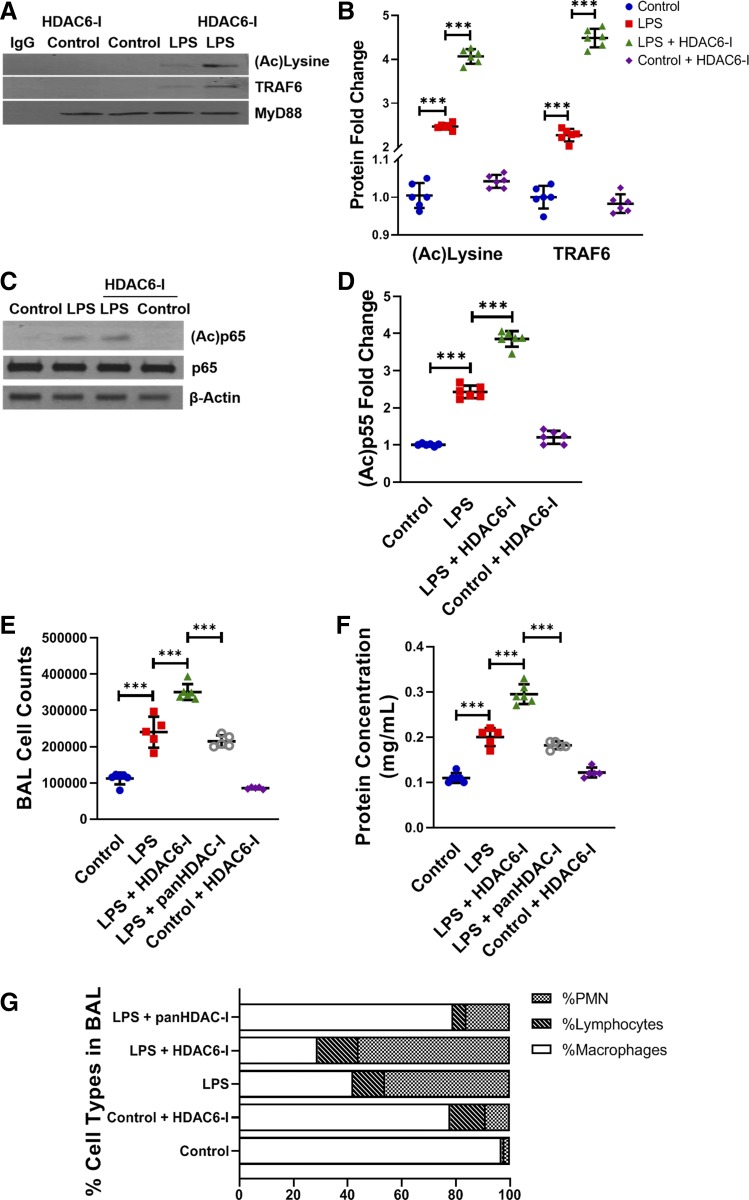
HDAC6 inhibition promotes lung MyD88 acetylation, MyD88-TRAF6 assembly, NF-κB acetylation, and vascular permeability after systemic LPS.
We next examined whether HDAC6-dependent regulation of TLR4 signaling was dependent on acetylation of the key TLR pathway adapter MyD88 in vivo. Immunoprecipitation studies showed that LPS induced acetylation of MyD88 and assembly with TRAF6 in lung homogenates at 6 h (Fig. 6, A and B). Tubastatin A augmented MyD88 acetylation and binding with TRAF6 (Fig. 6, A and B). To determine whether derepression of TLR signaling by HDAC6 inhibition facilitated proinflammatory NF-κB acetylation, we examined p65 acetylation. We noted that LPS induced p65Lys310 acetylation in the lung at 6 h, and tubastatin A treatment increased accumulation of p65Lys310 (Fig. 6, C and D). These data reveal that HDAC inhibition promotes MyD88 and p65 acetylation-dependent TLR4 signaling in vivo during sepsis. To assess whether augmented lung endothelial immune activation (Fig. 4) and TLR activation (Figs. 5 and 6) seen after HDAC6 inhibition increased vascular permeability, we performed BAL. LPS induced a twofold increase in BAL protein and total cell counts (Fig. 6, E and F) at 24 h, indicating vascular leakiness. LPS-induced increases in vascular permeability were exaggerated by >50% in mice treated with tubastatin A (Fig. 6, E and F). Furthermore, while macrophages were the predominant cell type found in control mice, polymorphonuclear cells were the predominant cell type seen with LPS or LPS + HDAC6-I treatment, further confirming increased vascular permeability (Fig. 6G). Interestingly, under similar experimental conditions, the pan-HDAC inhibitor trichostatin A (5 mg/kg) attenuated LPS-induced increases in BAL protein and total cell counts at 24 h. These data suggest that HDAC6 inhibition augments LPS-induced lung vascular permeability, but pan-HDAC inhibition suppresses LPS-mediated vascular permeability.
Fig. 6.
Effect of histone deacetylase 6 (HDAC6) inhibition on lung myeloid differentiation primary response 88 (MyD88) acetylation, MyD88-TNF receptor-associated factor 6 (TRAF6) assembly, NF-κB acetylation, and vascular permeability after lipopolysaccharide (LPS). A: lung homogenates from mice obtained 3 h after LPS (2 mg/kg ip) and HDAC6-I treatments (1 mg/kg ip) were used to immunoprecipitate MyD88. TRAF6, acetylated lysine, and MyD88 were probed in MyD88 immunoprecipitates by immunoblotting, with densitometry shown (B); (n ≥ 5/group). C: acetylated p65 was quantified in lung homogenates of 7-day-old mouse pups 6 h after LPS and HDAC6-I treatments by immunoblotting, with densitometry quantification shown (D). E, F, and G: bronchoalveolar lavage (BAL) fluid from control and LPS-, LPS + HDAC6-I-, and LPS + pan-HDAC-I-treated mice obtained at 24 h was used to quantify the total number of cells (E), protein concentration (F), and macrophage, lymphocyte, and polymorphonuclear leukocyte (PMN) counts (G); (n ≥ 5/group). Capped lines, with significance level (***P < 0.001), denote significant comparison of groups (B, D, and F) using ANOVA and post hoc Tukey test.
LPS-induced lung inflammation and suppression of lung growth factors is worsened with HDAC6 inhibition.
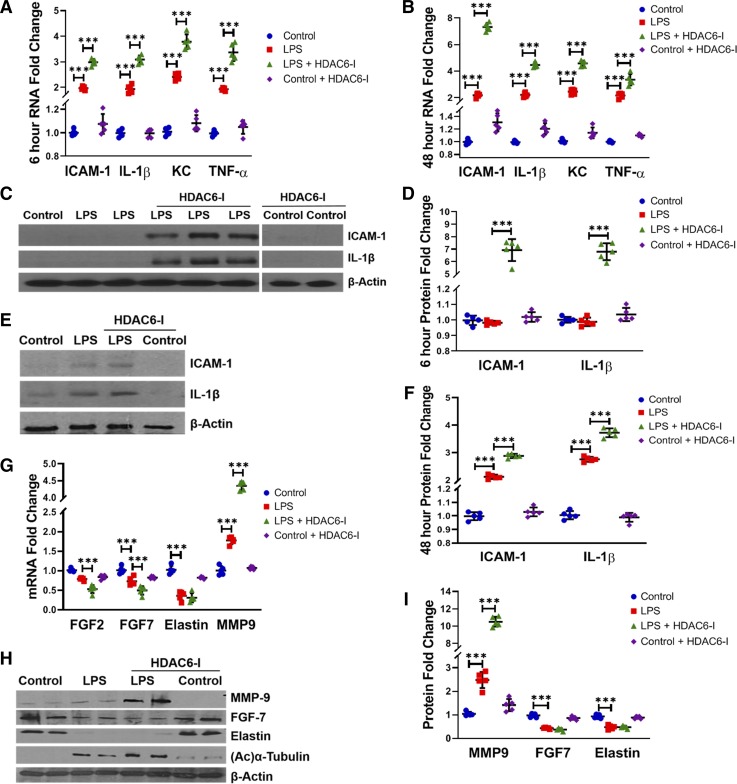
To determine whether increased TLR responsiveness after HDAC6 inhibition worsens severity of LPS-induced lung inflammation, we examined cytokine expression at early (6 h) and late (48 h) time points after systemic LPS. LPS-induced TNF-α, IL-1β, ICAM-1, and keratinocyte chemoattractant RNA expression observed at 6 and 48 h was exaggerated with tubastatin A (Fig. 7, A and B). Interestingly, we noted that, while ICAM-1 and IL-1β gene expression was induced with LPS and LPS + HDAC6-I treatments at 6 h, ICAM-1 and IL-1β protein expression was overtly evident only in mice treated with tubastatin A (Fig. 7, C and D). HDAC6 inhibition also exaggerated LPS-mediated ICAM-1 and IL-1β protein expression at 48 h (Fig. 7, E and F). These data reveal that HDAC6 inhibition magnifies, and potentially accelerates, the kinetics of proinflammatory TLR signaling seen with LPS in the neonatal lung. Inflammation in the developing lung has been associated with BPD in humans and alveolar simplification in animal models of BPD (33, 47). We therefore examined whether HDAC6-dependent LPS-mediated inflammation alters expression of growth factors important for lung development. LPS suppressed FGF7, FGF2, and elastin RNA expression at 48 h (Fig. 7G). Tubastatin A resulted in further decreases in RNA expression of FGF7 and FGF2 but did not further suppress elastin expression (Fig. 7G). LPS-induced MMP-9 protein and RNA expression was exaggerated with tubastatin A (Fig. 7, G, H, and I). Similarly, LPS-induced decrease in FGF7 protein was worse with tubastatin A, while elastin was suppressed equally in both LPS- and LPS + tubastatin A-treated mice (Fig. 7, H and I). These data show that HDAC6 inhibition intensifies inflammation and suppression of lung growth factors seen with LPS.
Fig. 7.
Histone deacetylase 6 (HDAC6) inhibition amplifies lipopolysaccharide (LPS)-induced lung inflammation and suppression of lung growth factors in neonatal mice at 6 and 48 h. A and B: whole lung RNA homogenates obtained at 6 (A) and 48 (B) h posttreatment with LPS (2 mg/kg ip) and HDAC6-I (1 mg/kg ip) were used to quantify intercellular adhesion molecule-1 (ICAM-1), IL-1β, keratinocyte chemoattractant (KC) (mouse IL-8), and TNF-α expression by qRT-PCR (n = 5/group). C: whole lung homogenates obtained 6 h after LPS and HDAC6-I treatments in mice were used to quantify ICAM-1 and IL-1β protein by Western blotting, with densitometry quantization shown (D); (n = 5/group). E: lung homogenates obtained 48 h after LPS and HDAC6-I were used to quantify ICAM-1 and IL-1β by Western blotting, with densitometry quantification shown (F); (n = 5/group). G: lung RNA homogenates obtained 48 h after LPS (2 mg/kg ip) and HDAC6-I (1 mg/kg ip) were used to quantify fibroblast growth factor (FGF) 2, FGF7, elastin, and matrix metalloproteinase-9 (MMP-9) expression by qRT-PCR (n = 5/group). H and I: lung homogenates obtained 48 h after LPS and HDAC6-I treatments were used to quantify MMP-9, FGF7, and elastin protein by Western blotting (H), with densitometry quantification shown graphically (I); (n = 5/group). Capped lines, with above significance level (***P < 0.001), denote significant comparison of groups (A, B, D, F, G, and I) using ANOVA and post hoc Tukey test.
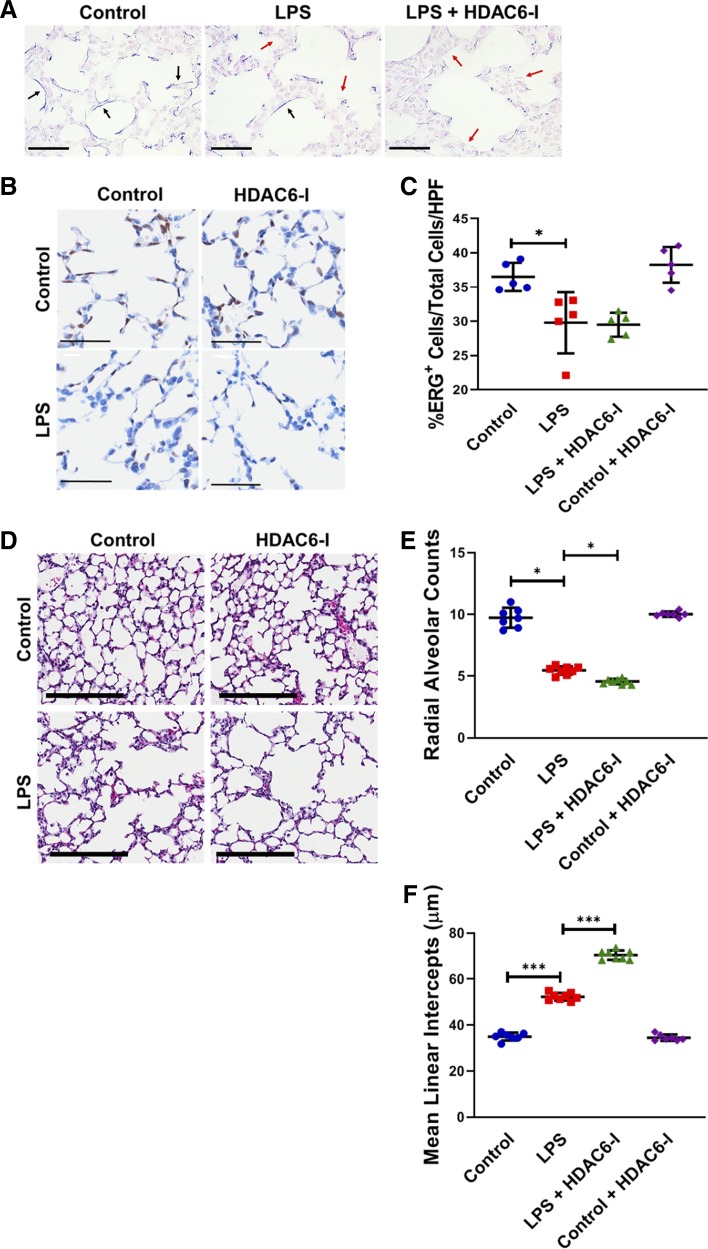
Effect of LPS and HDAC6 inhibition on elastin fiber architecture, lung EC population, and alveolar remodeling.
Elastin fiber deposition and lung EC arborization are critical for lung development. Therefore, we evaluated elastin fiber deposition and lung EC populations in our model using a modified Weigert’s Resorcin Fuchsin protocol with pre-Carmine staining and ETS-related gene (ERG) mapping. Elastic fiber (blue) was clearly visualized in control mice but was thinner, decreased, and disrupted with LPS and even worse with tubastatin A treatment (Fig. 8A). The total of ERG+ cells/high-power field decreased significantly with LPS but did not further decrease with HDAC6-I treatment (Fig. 8, B and C). To determine whether suppression of lung growth factors, decreases in lung EC population, and disruption of elastic fiber deposition seen with LPS and LPS + HDAC6 treatments altered lung architecture, we evaluated radial alveolar counts (RAC) and mean linear intercepts (MLI) on day of life 15 after LPS treatment on day of life 7. LPS treatment decreased RAC (9.8 ± 1.2 vs. 5.6 ± 0.4; P < 0.001, n ≥ 7) and increased MLI (35.76 ± 1.8 vs. 52.0 ± 3.0, P < 0.001, n ≥ 7), indicating alveolar simplification (Fig. 8, D and E). Tubastatin A treatment decreased RAC (LPS vs. LPS + HDAC6-I, 5.6 ± 0.4 vs. 4.5 ± 0.2; P < 0.03, n = 5–6) and increased MLI (LPS vs. LPS + HDAC6-I, 52.0 ± 3.0 vs. 70.5 ± 4.6, P < 0.001) relative to LPS treatment only, indicative of more severe alveolar simplification. These data demonstrate that LPS-induced alveolar simplification is exacerbated with HDAC6 inhibition in association with decreased expression of lung proteins required for alveolar development.
Fig. 8.
Lipopolysaccharide (LPS) and histone deacetylase 6 (HDAC6) inhibition disrupts elastin fiber architecture in conjunction with decreased lung endothelial cell (EC) population and alveolar simplification. A: paraffin sections from control (C) and LPS- and LPS + HDAC6-I-treated mice at 48 h were assessed for elastic fiber structure (blue) using Resorcin-Fuchsin staining with the Carmine counter staining method. Black arrows represent continuous elastic staining and red arrows represent areas where elastic fiber is disrupted/absent. Scale bar is 100 µm. B and C: lung sections obtained from inflation-fixed paraffin sections obtained from control and LPS-, HDAC6-I-, and LPS + HDAC6-I-treated mice on day of life 15 underwent immunohistochemistry studies (B) for Ets-related gene (ERG, brown), a nuclear endothelial cell marker, with cumulative data of ERG+/total cells/high-power field (×40) shown (C); (n ≥ 5/group). D: lung sections obtained from inflation-fixed mice on day of life 15 after LPS and HDAC6-I treatments on day of life 7 were used to quantify radial alveolar counts (E) and mean linear intercepts (F). Image magnification is ×10. Scale bar indicates 200 µm (n ≥ 5–7/group). Capped lines, with above significance level (*P < 0.05 and ***P < 0.001), denote significant comparison of groups (C, E, and F). ANOVA with post hoc Tukey test was used.
DISCUSSION
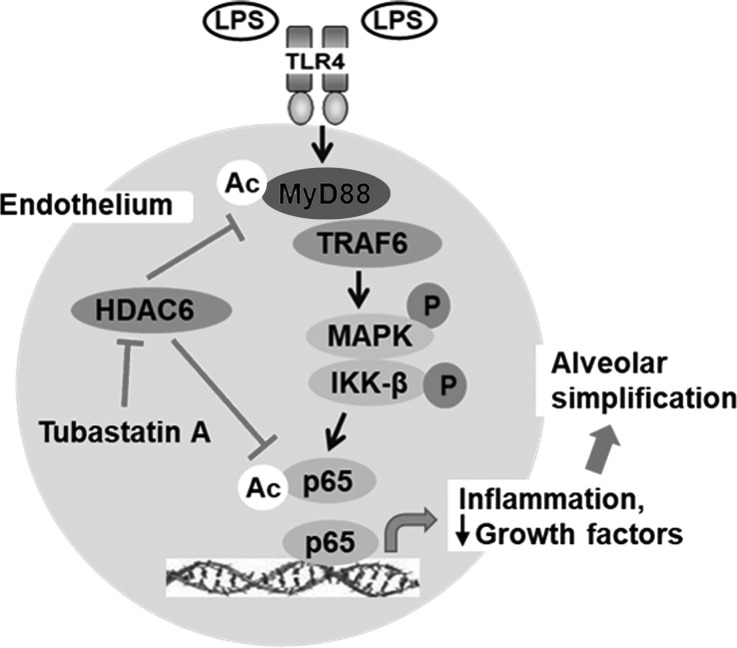
Inflammatory gene expression in response to TLR ligands is regulated by histone acetyltransferases and HDACs (8, 15). Studies using HDAC inhibitors have reported distinct effects depending on the isoform targeted, the cell type, and the stimulus used (3, 20, 22, 57). Specifically, the mechanistic role of HDAC6 in regulating TLR4-mediated EC inflammation and sepsis-induced ALI remains unclear. Using complementary inhibitor and gene-manipulation strategies, we show that HDAC6-dependent deacetylation of the key TLR4 adapter MyD88 suppresses its binding to the downstream effector TRAF6, attenuating LPS-induced MAPK and IKK-β phosphorylation and cytokine expression in primary lung EC (Fig. 9). Furthermore, in a neonatal model of sepsis-induced ALI, we demonstrate that HDAC6 inhibition amplifies MyD88-dependent canonical TLR signaling and inflammation in the lung, which results in exaggerated alveolar simplification akin to BPD in preterm infants. Our data indicate that HDAC6 is a constitutive inhibitor of sepsis-induced proinflammatory TLR signaling in pulmonary EC and in the developing lung.
Fig. 9.
Illustration showing proposed mechanism by which histone deacetylase 6 (HDAC6) regulates canonical Toll-like receptor 4 (TLR4) signaling in lung endothelial cells (EC) and worsens lipopolysaccharide (LPS)-induced alveolar simplification. MyD88, myeloid differentiation primary response 88; TRAF6, TNF receptor-associated factor 6; MAPK, mitogen-activated protein kinases (p38, JNK); IKK-β, inhibitor of NF-κB kinase-β; p65, NFKB subunit RelA; Ac, lysine acetylation; tubastatin A, HDAC6 inhibitor.
Although several HDACs, including 1, 3, 6, and 7, are known to regulate EC differentiation, migration, and angiogenesis, regulation of cytoplasmic TLR signaling by HDACs is not fully characterized (20, 49, 51, 56). Our in vitro studies in HPMEC suggest that HDAC6 represses proinflammatory TLR4 signaling by deacetylating MyD88 and inhibiting formation of MyD88-TRAF6 complexes essential for phosphorylation of IKK-β, MAPK, and p65 and signal transduction. These data are in contrast with Joshi et al. (24) who reported that HDAC6 inhibition with tubastatin A partially attenuated the LPS-mediated decrease in transendothelial permeability in association with suppression of 90-kDa heat shock protein-dependent RhoA activity and signaling. In their experiments, tubastatin A was used in the dose range of 1–2 µM rather than the 30–100 nM range in our study. This difference is significant, since the IC50 for HDAC6 is 15 nM, and, at 1–2 µM doses, the IC50 for other HDACs, especially HDAC8, is exceeded (7, 43). Interestingly, Joshi et al. could demonstrate suppression of LPS-mediated transendothelial permeability only after HDAC3 inhibition, or by combining HDAC3 siRNA with HDAC6 siRNA. To avoid the pitfalls of chemical inhibition, we used plasmid-mediated HDAC6 protein transfection to demonstrate that HDAC6 strongly suppressed LPS-induced canonical TLR signaling and inflammation in HPMEC. Additionally, we specifically mutated the catalytic domain 2 of HDAC6, which results in a dominant-negative form of HDAC6 (DN2), to show that LPS-induced lung EC inflammation is exaggerated with HDAC6 suppression. Similar to Joshi et al., the data of Yu et al. (57) suggest that TNF-α-induced lung EC permeability and microtubule disassembly were suppressed with tubastatin A. However, tubastatin A was used at a dose (3 µM) where other HDACs could have been inhibited, and nonselective off-target effects become likely (7, 43). Our results are consistent with Into et al. (22) who showed that HDAC6 deacetylates MyD88 and is a repressor of TLR4 signaling in RAW 264.7 cells. Similar to our data, Aung et al. (3) reported that trichostatin A (pan-HDAC inhibitor) enhanced LPS-mediated cytokine expression in bone marrow-derived macrophages. Data revealing that HDAC6 suppresses, while HDAC6 inhibition or HDAC6-DN2 amplifies, LPS-induced cytokine expression also supports our conclusion that HDAC6 negatively regulates TLR4 signaling in lung EC.
Posttranslational modification of TLR signaling proteins at lysine residues by acetylation/deacetylation regulates the amplitude of TLR responsiveness (20). Several studies noted that nuclear HDAC inhibitors repress recruitment of transcription factors such as NF-κB to the promoters of cytokine genes and suppress expression (12, 20, 22). However, whether cytoplasmic HDACs regulate TLR signaling by deacetylation of the key TLR4 adapter MyD88 or transcription factor NF-κB has not been examined in EC or in the lung. Our in vitro and in vivo studies show that LPS induces Lys310 acetylation of p65 (RelA) and HDAC6 deacetylates p65 in association with decreased inflammation. Our results are consistent with Chen et al. (11, 12) who indicated that acetylation of Lys310 is important for full transcriptional activation of NF-κB. Both in HPMEC and in the developing lung, we noted that HDAC6 regulated MAPK and IKK-β phosphorylation, suggesting that HDAC6 regulated TLR signaling events proximal to p65. Therefore, we probed MyD88, the key TLR4 adapter, since previous studies have shown that lysine acetylation of MyD88 alters its function (22, 35). Our data suggest that HDAC6 regulates LPS-induced MyD88 acetylation and binding to TRAF6, which is required for TLR4 signal transduction. Our data are consistent with Into et al. (22) who demonstrated that HDAC6 and sequestosome 1 are required for formation of MyD88 aggregates, and HDAC6 suppressed MyD88-dependent cytokine expression in macrophages. New et al. (35) also noted that MyD88 is deacetylated by HDAC6 at Lys132. Coimmunoprecipitation of HDAC6 with MyD88 after LPS in HPMEC also supports a direct role of HDAC6 in regulating MyD88 acetylation and binding with TRAF6 during EC immune activation. Our studies indicate that HDAC6 suppresses TLR4 signaling by deacetylating MyD88 and p65, and inhibition of HDAC6 amplifies LPS-induced MyD88 acetylation, MyD88-TRAF6 binding, p65 acetylation/phosphorylation, and transcriptional activation of inflammation in lung EC and the developing lung. Our studies do not clarify whether HDAC6 directly regulates p65 acetylation independent of MyD88 acetylation, but, in view of prior studies showing HDAC-dependent p65 acetylation, we focused on MyD88 herein.
Pan-HDAC inhibitors have been shown to suppress ALI in models of sepsis in adult mice (24, 50, 59). The role of HDAC6 in regulating sepsis-induced ALI is not well characterized in the developing lung. In our model of sepsis-induced neonatal ALI, we demonstrate that HDAC6 inhibition with tubastatin A (1 mg/kg) results in early (<6 h) and sustained (48 h) increases in proinflammatory TLR signaling in the lung. HDAC6 inhibition also augmented LPS-induced lung vascular permeability as indicated by increased BAL protein and cell counts, whereas pan-HDAC inhibition attenuated alveolar edema. These data are consistent with our in vitro studies in HPMEC that reveal a protective role for HDAC6 in LPS-induced inflammation. Our results differ from Yu et al. (57) and Borgas et al. (6) who showed that tubastatin A (9 or 20 mg/kg) represses TNF-α- and cigarette smoke plus LPS-mediated lung endothelial barrier disruption and lung injury in adult mice, respectively. We used a low-dose regimen (1 mg/kg ip) in contrast to 9 or 20 mg/kg used in other studies to avoid potential nonselective effects of tubastatin A on other HDACs. Even with the low dose used in our study, we found hyperacetylation of α-tubulin, confirming HDAC6 inhibition. Interestingly, in our model, pan-HDAC inhibition suppressed LPS-induced lung alveolar edema, consistent with previous studies showing protective effects of pan-HDAC inhibitors (50, 59). These data suggest that suppression of nuclear HDACs with pan-HDAC inhibitors suppresses inflammation, while selective HDAC6 inhibition (cytoplasmic) enhances TLR signaling.
Chattopadhyay et al. (10) showed that HDAC6−/− mice were protected against LPS-induced mortality but were more vulnerable to Sendai virus-related mortality. Whether differences in neonatal versus adult mice, compensatory changes in other cytoplasmic HDACs, or SIRT2 in HDAC6−/− mice (evidence of tubulin acetylation was not shown in HDAC6−/− mice) and use of a lethal versus a nonlethal LPS dose (our study) contributed to disparate results can only be speculated on. Distinguishing our data from the above studies that only examined downstream markers of ALI like inflammation and permeability, but not TLR signaling, we also demonstrate that tubastatin A (1 mg/kg) enhanced LPS-induced MAPK, IKK-β, and p65 phosphorylation at early and late time points, consistent with the effects of HDAC6 inhibition on lung inflammation. Furthermore, using whole lung lysates, we show that MyD88 acetylation, binding to TRAF6, and p65 Lys310 acetylation induced with LPS were exaggerated with tubastatin A, revealing mechanisms contributing to neonatal ALI seen with tubastatin A. Our data showing that HDAC6 associates with MyD88 in EC and selectively deacetylates lysine residues in MyD88 is consistent with New et al. (35) who revealed using an unbiased mass spectrometry screen and tubastatin A assays that HDAC6 regulates MyD88 function through Lys132 deacetylation, and Myd88 expression modulates sensitivity to HDAC inhibition. Furthermore, Into et al. (22), using siRNA knockdown, showed that HDAC6 and sequestosome 1 negatively regulate TLR4-induced MyD88-dependent p38/JNK activation, and cytokine expression in macrophages mimics our results in lung EC. Wang et al.’s (52) study, however, suggests that HDAC6 inhibition protects against LPS-induced mortality in adult mice. Although the dose of tubastatin used (0.5 mg·kg−1·day−1) was similar to ours, it was unclear why mice were treated daily for 7 days with HDAC-I before exposure to high-dose LPS. Furthermore, evidence of HDAC6 inhibition, i.e., tubulin hyperacetylation, was not presented in contrast to our in vivo data. It is possible that 7 days of HDAC6-I modulate several inflammatory pathways through posttranslational modifications and chromatin remodeling. Consistent with our results, Wang et al. (53) found that tubastatin A enhanced anti-tuberculosis immunity by upregulating TNF-α and inflammatory cytokines. In summary, the divergent results from the above studies suggest that the dose and specificity of HDAC6 inhibitors used, the model used, and maturational age of mice are potential modulators of HDAC6-regulated ALI.
Previous work from our group and others showed that acute inflammation in the developing lung induced with systemic sepsis can remodel the lung, resulting in alveolar simplification, akin to BPD in premature infants (33, 46, 47). To test the postulate that exaggerated inflammation resulting from HDAC6 inhibition will result in more severe sepsis-induced alveolar remodeling, we examined lung development after LPS treatment. We noted that the LPS-induced decrease in radial alveolar count and increase in mean linear intercept were worse with HDAC6 inhibition. To understand how inflammation alters expression of growth factors required for lung development, we examined FGF2, an important mitogen for EC, and FGF7 (KGF), a key growth factor for lung epithelial cells (4, 14, 38, 42). LPS given systemically suppressed FGF2 and FGF7 expression in the lung, and HDAC6-I treatment further decreased expression. Similarly, acute increases in MMP-9 can alter the balance between protease/anti-protease activity, resulting in tissue destruction in BPD (17, 18). Our data reveal that MMP-9 induced with LPS is exaggerated with HDAC6-I treatment. Elastin deposition and fiber formation are essential for lung development, and MMP-9 can cleave elastin (34, 47, 54). In our studies, we found that LPS- and LPS + HDAC-I-treated groups showed comparable reduction in soluble elastin, but elastin fiber destruction tended to be worse with HDAC6-I treatment. While our study examined HDAC6 regulation of inflammatory signaling in the neonatal lung, the effect of HDAC6 on other developmental pathways, including TGF-β and Notch signaling, as well other cell types, including alveolar myofibroblasts, could have contributed to the phenotype (16, 51). In summary, our data suggest that systemic LPS, mimicking sepsis, suppresses lung growth factors and elastin deposition essential for lung development, and HDAC6 inhibition exacerbates these pathological changes.
In conclusion, this is one of the early studies to reveal that HDAC6 is a negative regulator of TLR4-mediated inflammation in EC. Using complementary approaches, we demonstrate that HDAC6 deacetylates MyD88 and NF-κB, resulting in mitigation of LPS-induced inflammation in HPMEC in vitro and in the lung. Enhanced sepsis-induced inflammation seen with HDAC inhibition disrupts lung development, resulting in more severe alveolar remodeling akin to preterm infants who develop BPD. Our results suggest that epigenetic regulation of innate immunity modulates the severity of sepsis-induced lung injury and alveolar remodeling in the developing lung. Augmenting EC HDAC6 levels or suppressing MyD88 acetylation need to be evaluated as potential therapies to mitigate sepsis-induced alveolar injury and remodeling in preclinical models. Whether HDAC6 or other HDACs regulate lung EC and epithelial injury in models of hyperoxia-induced alveolar remodeling are interesting topics for future research (47). While adding to a growing body of literature highlighting the therapeutic potential of HDAC inhibitors on inflammation, cancer, and other diseases, our data suggest that careful consideration of isoform-specific effects and dose ranges is required before clinical translation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant 1R01 HL128374-01 (V. Sampath).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.M., S.X., J.R., and V.S. conceived and designed research; H.M., S.X., S.M.M., S.Q.Y., and V.S. performed experiments; H.M., S.X., S.M.M., J.N.-M., J.R., S.Q.Y., and V.S. analyzed data; H.M., S.X., S.M.M., J.N.-M., J.R., S.Q.Y., and V.S. interpreted results of experiments; H.M., S.X., J.N.-M., and V.S. prepared figures; H.M., S.X., S.M.M., J.N.-M., J.R., S.Q.Y., and V.S. drafted manuscript; H.M., S.X., S.M.M., J.N.-M., J.R., S.Q.Y., and V.S. edited and revised manuscript; H.M., S.X., S.M.M., J.N.-M., J.R., and V.S. approved final version of manuscript.
REFERENCES
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Andonegui G, Zhou H, Bullard D, Kelly MM, Mullaly SC, McDonald B, Long EM, Robbins SM, Kubes P. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J Clin Invest 119: 1921–1930, 2009. doi: 10.1172/JCI36411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aung HT, Schroder K, Himes SR, Brion K, van Zuylen W, Trieu A, Suzuki H, Hayashizaki Y, Hume DA, Sweet MJ, Ravasi T. LPS regulates proinflammatory gene expression in macrophages by altering histone deacetylase expression. FASEB J 20: 1315–1327, 2006. doi: 10.1096/fj.05-5360com. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari A, Bhandari V. Biomarkers in bronchopulmonary dysplasia. Paediatr Respir Rev 14: 173–179, 2013. doi: 10.1016/j.prrv.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Bode KA, Schroder K, Hume DA, Ravasi T, Heeg K, Sweet MJ, Dalpke AH. Histone deacetylase inhibitors decrease Toll-like receptor-mediated activation of proinflammatory gene expression by impairing transcription factor recruitment. Immunology 122: 596–606, 2007. doi: 10.1111/j.1365-2567.2007.02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgas D, Chambers E, Newton J, Ko J, Rivera S, Rounds S, Lu Q. Cigarette smoke disrupted lung endothelial barrier integrity and increased susceptibility to acute lung injury via histone deacetylase 6. Am J Respir Cell Mol Biol 54: 683–696, 2016. doi: 10.1165/rcmb.2015-0149OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc 132: 10842–10846, 2010. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calao M, Burny A, Quivy V, Dekoninck A, Van Lint C. A pervasive role of histone acetyltransferases and deacetylases in an NF-kappaB-signaling code. Trends Biochem Sci 33: 339–349, 2008. doi: 10.1016/j.tibs.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter S, O’Neill LA. Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem J 422: 1–10, 2009. doi: 10.1042/BJ20090616. [DOI] [PubMed] [Google Scholar]
- 10.Chattopadhyay S, Fensterl V, Zhang Y, Veleeparambil M, Wetzel JL, Sen GC. Inhibition of viral pathogenesis and promotion of the septic shock response to bacterial infection by IRF-3 are regulated by the acetylation and phosphorylation of its coactivators. MBio 4: e00636-12, 2013. doi: 10.1128/mBio.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J 21: 6539–6548, 2002. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Lf, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293: 1653–1657, 2001. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 13.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2–intrauterine and early postnatal lung growth. Thorax 37: 580–583, 1982. doi: 10.1136/thx.37.8.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danan C, Franco ML, Jarreau PH, Dassieu G, Chailley-Heu B, Bourbon J, Delacourt C. High concentrations of keratinocyte growth factor in airways of premature infants predicted absence of bronchopulmonary dysplasia. Am J Respir Crit Care Med 165: 1384–1387, 2002. doi: 10.1164/rccm.200112-134BC. [DOI] [PubMed] [Google Scholar]
- 15.Dekker FJ, van den Bosch T, Martin NI. Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases. Drug Discov Today 19: 654–660, 2014. doi: 10.1016/j.drudis.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Deskin B, Lasky J, Zhuang Y, Shan B. Requirement of HDAC6 for activation of Notch1 by TGF-β1. Sci Rep 6: 31086, 2016. doi: 10.1038/srep31086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekekezie II, Thibeault DW, Simon SD, Norberg M, Merrill JD, Ballard RA, Ballard PL, Truog WE. Low levels of tissue inhibitors of metalloproteinases with a high matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio are present in tracheal aspirate fluids of infants who develop chronic lung disease. Pediatrics 113: 1709–1714, 2004. doi: 10.1542/peds.113.6.1709. [DOI] [PubMed] [Google Scholar]
- 18.Fukunaga S, Ichiyama T, Maeba S, Okuda M, Nakata M, Sugino N, Furukawa S. MMP-9 and TIMP-1 in the cord blood of premature infants developing BPD. Pediatr Pulmonol 44: 267–272, 2009. doi: 10.1002/ppul.20993. [DOI] [PubMed] [Google Scholar]
- 19.Hai Y, Christianson DW. Histone deacetylase 6 structure and molecular basis of catalysis and inhibition. Nat Chem Biol 12: 741–747, 2016. doi: 10.1038/nchembio.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Yu Y, Eugene Chin Y, Xia Q. The role of acetylation in TLR4-mediated innate immune responses. Immunol Cell Biol 91: 611–614, 2013. doi: 10.1038/icb.2013.56. [DOI] [PubMed] [Google Scholar]
- 22.Into T, Inomata M, Niida S, Murakami Y, Shibata K. Regulation of MyD88 aggregation and the MyD88-dependent signaling pathway by sequestosome 1 and histone deacetylase 6. J Biol Chem 285: 35759–35769, 2010. doi: 10.1074/jbc.M110.126904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 46: 641–643, 1999. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Joshi AD, Barabutis N, Birmpas C, Dimitropoulou C, Thangjam G, Cherian-Shaw M, Dennison J, Catravas JD. Histone deacetylase inhibitors prevent pulmonary endothelial hyperpermeability and acute lung injury by regulating heat shock protein 90 function. Am J Physiol Lung Cell Mol Physiol 309: L1410–L1419, 2015. doi: 10.1152/ajplung.00180.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudsen L, Weibel ER, Gundersen HJG, Weinstein FV, Ochs M. Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. J Appl Physiol (1985) 108: 412–421, 2010. doi: 10.1152/japplphysiol.01100.2009. [DOI] [PubMed] [Google Scholar]
- 26.Leifer CA, Medvedev AE. Molecular mechanisms of regulation of Toll-like receptor signaling. J Leukoc Biol 100: 927–941, 2016. doi: 10.1189/jlb.2MR0316-117RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Liu B, Fukudome EY, Kochanek AR, Finkelstein RA, Chong W, Jin G, Lu J, deMoya MA, Velmahos GC, Alam HB. Surviving lethal septic shock without fluid resuscitation in a rodent model. Surgery 148: 246–254, 2010. doi: 10.1016/j.surg.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin HS, Hu CY, Chan HY, Liew YY, Huang HP, Lepescheux L, Bastianelli E, Baron R, Rawadi G, Clément-Lacroix P. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br J Pharmacol 150: 862–872, 2007. doi: 10.1038/sj.bjp.0707165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells–conditional innate immune cells. J Hematol Oncol 6: 61, 2013. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maniatis NA, Orfanos SE. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care 14: 22–30, 2008. doi: 10.1097/MCC.0b013e3282f269b9. [DOI] [PubMed] [Google Scholar]
- 31.Menden H, Tate E, Hogg N, Sampath V. LPS-mediated endothelial activation in pulmonary endothelial cells: role of Nox2-dependent IKK-β phosphorylation. Am J Physiol Lung Cell Mol Physiol 304: L445–L455, 2013. doi: 10.1152/ajplung.00261.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menden H, Welak S, Cossette S, Ramchandran R, Sampath V. LPS-mediated Angiopoietin-2 dependent autocrine angiogenesis is regulated by Nox2 in human pulmonary microvascular endothelial cells. J Biol Chem 290: 5449–5461, 2015. doi: 10.1074/jbc.M114.600692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menden HL, Xia S, Mabry SM, Navarro A, Nyp MF, Sampath V. Nicotinamide adenine dinucleotide phosphate oxidase 2 regulates LPS-induced inflammation and alveolar remodeling in the developing lung. Am J Respir Cell Mol Biol 55: 767–778, 2016. doi: 10.1165/rcmb.2016-0006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mižíková I, Morty RE. The extracellular matrix in bronchopulmonary dysplasia: target and source. Front Med (Lausanne) 2: 91, 2015. doi: 10.3389/fmed.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.New M, Sheikh S, Bekheet M, Olzscha H, Thezenas M-L, Care MA, Fotheringham S, Tooze RM, Kessler B, La Thangue NB. TLR adaptor protein MYD88 mediates sensitivity to HDAC inhibitors via a cytokine-dependent mechanism. Cancer Res 76: 6975–6987, 2016. doi: 10.1158/0008-5472.CAN-16-0504. [DOI] [PubMed] [Google Scholar]
- 36.Parkinson CM, O’Brien A, Albers TM, Simon MA, Clifford CB, Pritchett-Corning KR. Diagnostic necropsy and selected tissue and sample collection in rats and mice. J Vis Exp pii: 2966, 2011. doi: 10.3791/2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray P. Protection of epithelial cells by keratinocyte growth factor signaling. Proc Am Thorac Soc 2: 221–225, 2005. doi: 10.1513/pats.200502-012AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Ruijter AJM, van Gennip AH, Caron HN, Kemp S, van Kuilenburg ABP. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737–749, 2003. doi: 10.1042/bj20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sampath V, Davis K, Senft AP, Richardson TR, Kitzmiller JA, Berclaz PY, Korfhagen TR. Altered postnatal lung development in C3H/HeJ mice. Pediatr Res 60: 663–668, 2006. doi: 10.1203/01.pdr.0000246071.50268.51. [DOI] [PubMed] [Google Scholar]
- 41.Sampath V, Garland JS, Le M, Patel AL, Konduri GG, Cohen JD, Simpson PM, Hines RN. A TLR5 (g.1174C > T) variant that encodes a stop codon (R392X) is associated with bronchopulmonary dysplasia. Pediatr Pulmonol 47: 460–468, 2012. doi: 10.1002/ppul.21568. [DOI] [PubMed] [Google Scholar]
- 42.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol 141: 1659–1673, 1998. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seidel C, Schnekenburger M, Dicato M, Diederich M. Histone deacetylase 6 in health and disease. Epigenomics 7: 103–118, 2015. doi: 10.2217/epi.14.69. [DOI] [PubMed] [Google Scholar]
- 44.Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol 6: a018713, 2014. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol 32: 335–343, 2011. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Shrestha AK, Bettini ML, Menon RT, Gopal VYN, Huang S, Edwards DP, Pammi M, Barrios R, Shivanna B. Consequences of early postnatal lipopolysaccharide exposure on developing lungs in mice. Am J Physiol Lung Cell Mol Physiol 316: L229–L244, 2019. doi: 10.1152/ajplung.00560.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva DMG, Nardiello C, Pozarska A, Morty RE. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 309: L1239–L1272, 2015. doi: 10.1152/ajplung.00268.2015. [DOI] [PubMed] [Google Scholar]
- 48.Sobczak M, Dargatz J, Chrzanowska-Wodnicka M. Isolation and culture of pulmonary endothelial cells from neonatal mice. J Vis Exp pii: 2316, 2010. doi: 10.3791/2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spallotta F, Cencioni C, Straino S, Nanni S, Rosati J, Artuso S, Manni I, Colussi C, Piaggio G, Martelli F, Valente S, Mai A, Capogrossi MC, Farsetti A, Gaetano C. A nitric oxide-dependent cross-talk between class I and III histone deacetylases accelerates skin repair. J Biol Chem 288: 11004–11012, 2013. doi: 10.1074/jbc.M112.441816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thangavel J, Samanta S, Rajasingh S, Barani B, Xuan Y-T, Dawn B, Rajasingh J. Epigenetic modifiers reduce inflammation and modulate macrophage phenotype during endotoxemia-induced acute lung injury. J Cell Sci 128: 3094–3105, 2015. doi: 10.1242/jcs.170258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valenzuela-Fernández A, Cabrero JR, Serrador JM, Sánchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol 18: 291–297, 2008. doi: 10.1016/j.tcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Wang B, Rao YH, Inoue M, Hao R, Lai CH, Chen D, McDonald SL, Choi MC, Wang Q, Shinohara ML, Yao TP. Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. Nat Commun 5: 3479, 2014. doi: 10.1038/ncomms4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Tang X, Zhou Z, Huang Q. Histone deacetylase 6 inhibitor enhances resistance to Mycobacterium tuberculosis infection through innate and adaptive immunity in mice. Pathog Dis 76: 6, 2018. doi: 10.1093/femspd/fty064. [DOI] [PubMed] [Google Scholar]
- 54.Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol 23: 320–326, 2000. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- 55.Xia S, Menden HL, Korfhagen TR, Kume T, Sampath V. Endothelial immune activation programs cell-fate decisions and angiogenesis by inducing DLL4 through TLR4-ERK-FOXC2 signalling. J Physiol 596: 1397–1417, 2018. doi: 10.1113/JP275453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang JY, Wang Q, Wang W, Zeng LF. Histone deacetylases and cardiovascular cell lineage commitment. World J Stem Cells 7: 852–858, 2015. doi: 10.4252/wjsc.v7.i5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu J, Ma Z, Shetty S, Ma M, Fu J. Selective HDAC6 inhibition prevents TNF-α-induced lung endothelial cell barrier disruption and endotoxin-induced pulmonary edema. Am J Physiol Lung Cell Mol Physiol 311: L39–L47, 2016. doi: 10.1152/ajplung.00051.2016. [DOI] [PubMed] [Google Scholar]
- 58.Zeng L, Xiao Q, Margariti A, Zhang Z, Zampetaki A, Patel S, Capogrossi MC, Hu Y, Xu Q. HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J Cell Biol 174: 1059–1069, 2006. doi: 10.1083/jcb.200605113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang L, Jin S, Wang C, Jiang R, Wan J. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J Surg 34: 1676–1683, 2010. doi: 10.1007/s00268-010-0493-5. [DOI] [PubMed] [Google Scholar]
- 60.Zheng XX, Zhou T, Wang XA, Tong XH, Ding JW. Histone deacetylases and atherosclerosis. Atherosclerosis 240: 355–366, 2015. doi: 10.1016/j.atherosclerosis.2014.12.048. [DOI] [PubMed] [Google Scholar]