Abstract
We previously described a novel “chemogenetic” animal model of heart failure that recapitulates a characteristic feature commonly found in human heart failure: chronic oxidative stress. This heart failure model uses a chemogenetic approach to activate a recombinant yeast d-amino acid oxidase in rat hearts in vivo to generate oxidative stress, which then rapidly leads to the development of a dilated cardiomyopathy. Here we apply this new model to drug testing by studying its response to treatment with the angiotensin II (ANG II) receptor blocker valsartan, administered either alone or with the neprilysin inhibitor sacubitril. Echocardiographic and [18F]fluorodeoxyglucose positron emission tomographic imaging revealed that valsartan in the presence or absence of sacubitril reverses the anatomical and metabolic remodeling induced by chronic oxidative stress. Markers of oxidative stress, mitochondrial function, and apoptosis, as well as classical heart failure biomarkers, also normalized following drug treatments despite the persistence of cardiac fibrosis. These findings provide evidence that chemogenetic heart failure is rapidly reversible by drug treatment, setting the stage for the study of novel heart failure therapeutics in this model. The ability of ANG II blockade and neprilysin inhibition to reverse heart failure induced by chronic oxidative stress identifies a central role for cardiac myocyte angiotensin receptors in the pathobiology of cardiac dysfunction caused by oxidative stress.
NEW & NOTEWORTHY The chemogenetic approach allows us to distinguish cardiac myocyte-specific pathology from the pleiotropic changes that are characteristic of other “interventional” animal models of heart failure. These features of the chemogenetic heart failure model facilitate the analysis of drug effects on the progression and regression of ventricular remodeling, fibrosis, and dysfunctional signal transduction. Chemogenetic approaches will be highly informative in the study of the roles of redox stress in heart failure providing an opportunity for the identification of novel therapeutic targets.
Keywords: angiotensin receptor, chemogenetics, heart failure, oxidative stress
INTRODUCTION
The term “heart failure” is used to describe a heterogenous clinical syndrome that can result from a diverse range of pathologies, ranging from myocardial ischemia to oxidative stress to genetic lesions in contractile proteins (6). The burden of disease from heart failure is high, and the prevalence of heart failure is growing as the population ages, which is further compounded by the worldwide epidemic of diabetes and obesity (40). There is a large and growing need for novel approaches to identify and validate new drugs and therapeutic targets. Yet many of the present animal models of heart failure either are undermined by methodological complexity or yield only a subtle cardiac phenotype (13). These limitations hamper the development of new therapeutic approaches. There is an urgent need for a tractable animal heart failure model that recapitulates key features of human heart failure to develop and validate new pharmacological targets. We recently developed and characterized a new animal heart failure model using an in vivo chemogenetic approach (32). Oxidative stress has been associated with most forms of heart failure (7), but until recently, establishing a clear causal relationship between oxidative stress and cardiac dysfunction has been elusive. We showed that when oxidative stress is induced in cardiac myocytes by activation of a recombinant d-amino acid oxidase (DAAO), which produces hydrogen peroxide (H2O2), a striking dilated cardiomyopathy results (32). In the present studies, we explore whether the heart failure phenotype in this new heart failure model can be reversed by drug treatment using established heart failure drugs that modulate the renin-angiotensin-aldosterone system (RAAS).
We chose to first study the effects of drugs that target the RAAS because this system modulates critical physiological and pathological pathways that regulate redox balance in cardiovascular tissues (39). Although the proximal cellular processes that precipitate human heart failure may vary, dysregulation of the RAAS has long been recognized as a central feature of the disease (19, 20). This realization inspired the development of multiple generations of drugs that modulate the RAAS, including the widely used angiotensin-converting enzyme (ACE) inhibitors (23) and angiotensin receptor type 1 (AT1R) blockers (ARBs; 8). Recently, an effective new heart failure drug was developed in which the ARB valsartan was combined with the neprilysin inhibitor sacubitril (21), which blocks the degradation of endogenous natriuretic peptides, leading to an increase in cyclic nucleotides in target tissues, particularly in the kidney (15). The combination of these two drugs proved to be more efficacious than RAAS blockade alone in preventing the progression of heart failure (21, 25). Although the effects of AT1R blockade on the vasculature have long been appreciated, the roles of cardiac AT1Rs in development of heart failure are much less well understood. Similarly, natriuretic peptides have an established role in the kidney but, more recently, have been shown to exert pharmacological effects in both the heart and vasculature (36). Although the combination of ARBs and neprilysin inhibitors is known to be efficacious for the treatment of heart failure, a better understanding of the pleiotropic roles of ARBs and neprilysin inhibitors in the heart is needed. Importantly, the roles of these drugs in the modulation of cardiovascular oxidative stress are incompletely understood.
METHODS
Animals.
For animal studies, local approval was granted by the Brigham and Women’s Hospital Institutional Animal Care and Use Committee (approval reference no. 2016N000583).
Construction and chemogenetic DAAO activation.
The HyPer-DAAO-NES DNA construct was previously created by fusing the cytosolic version of DAAO [containing a nuclear exclusion sequence (NES)] to HyPer (1), which was packaged into an adeno-associated virus (AAV) expression vector (Stratagene) with a cardiac troponin T promoter. An AAV expression vector containing HyPer3 was used to generate the control virus. Both the HyPer-DAAO-NES and the HyPer3 constructs were packaged into AAV9 viral particles via a Rep2/Cap9 AAV encapsulation construct by the Boston Children’s Hospital Viral Core. Approximately 1012 viral genome copies were suspended in 100 µL PBS and injected intravenously into ~3-wk-old (40–50 g) male Wistar rats (Charles River Laboratories). For in vivo activation of DAAO, d-alanine (Oakwood Chemical) was added to the drinking water at a concentration of 1 M, which was provided ad libitum.
Administration of sacubitril/valsartan and valsartan.
Sacubitril/valsartan and valsartan (provided by Novartis Pharma) were dissolved in water and administered daily by oral gavage at doses of 68 and 31 mg/kg, respectively. In the control group, an equal amount of water was administered by daily gavage.
Echocardiography.
Echocardiography was performed using a VisualSonics Vevo 3100 system equipped with an MX250 (13–24 MHz) probe. Rats were anesthetized with 1.5–2% isoflurane preceding the acquisitions of standard short-axis and long-axis views of the left ventricle (LV) at the level of the midpapillary muscle. To examine cardiac functional and anatomical parameters, images were analyzed using in vivo laboratory software. Both the operator and analyzer were blinded to the experimental groups (31).
Arterial pressure analysis.
Rats were anesthetized with ~3% isoflurane delivered by a nose cone. The right carotid was dissected from the surrounding structures with care taken to avoid damaging the vagus nerve. The rostral portion of the carotid was ligated with silk suture. Next, either a segment of polyethylene (PE-50) tubing (Instech) filled with 0.9% saline and connected to a pressure transducer (Living Systems) or a 1.2-F solid-state pressure transducer catheter (Transonic) was introduced into the carotid while proximal flow was occluded with a bulldog clamp. Following placement of a distal suture to prevent blood loss, the clamp was removed, and the catheter was advanced to approximately the level of the aortic arch. Pressure signals were recorded through a PowerLab analog-to-digital interface (ADInstruments) and analyzed using LabChart version 8 software. Because of the nonuniform frequency responses that have been observed with fluid-filled catheters in rodents, only mean arterial pressure was measured and reported.
Morphological, histological, and biochemical analysis.
After echocardiography, the animals’ hearts were excised under deep anesthesia with isoflurane, perfused in ice-cold PBS to quickly remove the blood, and then weighed. Heart weights were normalized to tibia length and whole body weight. LV myocardial tissue was fixed in 10% formalin (Sigma) and embedded in paraffin. Histological processing, including Masson’s trichrome staining, was performed by the Pathology Core at Brigham and Women’s Hospital. Images were collected using an upright microscope (Olympus BX63), using a magnification of ×10. Interstitial fibrosis was quantified as a percentage of total tissue area using ImageJ software. For measurements of cross-sectional area of the LV cardiac myocytes, sections of tissue fixed in 10% formalin (Sigma) were stained with hematoxylin-eosin methods, and only areas of tissue with a transverse direction were evaluated. Images were collected using an upright microscope (Olympus BX63) at a magnification of ×10. Acquisition of images and analysis were done by an expert individual who was blinded to the treatments. The biochemical profile of the blood serum collected from the rats was performed by C-Path Comparative Clinical Pathology Services (Columbia, MO; Table 1).
Table 1.
Biochemical profile
| Parameter | Control | DAAO | Sac/Val | Val | Normal Range (Reference) |
|---|---|---|---|---|---|
| n | 3 | 6 | 6 | 5 | |
| Sodium, mEq/L | 191.8 ± 4.4 | 164.5 ± 4.4 | 177.7 ± 5.9 | 176.0 ± 9.9 | 141–149 (8a) |
| Potassium, mEq/L | 4.8 ± 0.5 | 5.2 ± 0.3 | 5.3 ± 0.3 | 5.2 ± 0.3 | 5.2–7.8 (8a) |
| Chloride, mEq/L | 144.8 ± 5.5 | 118.0 ± 4.0 | 132.7 ± 5.4 | 131.3 ± 10.6 | 102–109 (8a) |
| ALT, U/L | 68.3 ± 11.5 | 60.3 ± 9.6 | 58.3 ± 7.4 | 96.8 ± 25.8 | 20–42 (8a) |
| AST, U/L | 97.0 ± 18.2 | 165.2 ± 41.3 | 128.2 ± 30.9 | 223.6 ± 91.5 | 39–84 (8a) |
| Creatinine, mg/dL | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.1** | 0.3 ± 0.0 | 0.3–0.5 (4) |
| BUN, mg/dL | 35.0 ± 3.0 | 34.3 ± 2.3 | 49.7 ± 3.7* | 43.4 ± 4.9 | 16–23 (8a) |
Values are means ± SE; n = no. of rats. Serum chemistry values of control (n = 3), d-amino acid oxidase (DAAO)-expressing rats (n = 6), and DAAO-expressing rats then treated with sacubitril/valsartan (Sac/Val; n = 6) or valsartan (Val; n = 5). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen.
P < 0.05,
P < 0.01 for sacubitril/valsartan vs. DAAO, obtained by 1-way ANOVA with Šídák’s multiple-comparison test or Kruskal-Wallis test followed by Dunn’s multiple-comparison test.
Real-time PCR analysis.
After organ harvest, the LVs were dissected from the rest of the heart, minced, suspended in TRIzol, and homogenized. RNA isolation was performed according to the manufacturer’s instructions (Invitrogen). cDNA synthesis was performed with either Protoscript II First Strand cDNA Synthesis Kit (New England Biolabs) or AzuraQuant cDNA Synthesis Kit (Azura Genomics). cDNA was amplified in a StepOnePlus RT-PCR thermocycler (Applied Biosciences) with AzuraQuant Green Fast qPCR Master Mix (Azura Genomics). Genes of interest were amplified with gene-specific primers (Table 2). To normalize expression levels of the genes of interest, hypoxanthine phosphoribosyltransferase 1 (Hprt1) was used as a housekeeping gene.
Table 2.
Gene-specific primers for RT-PCR amplification
| Transcript | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Hprt1 | TTCCTCCTCAGACCGCTTTTC | ATCACTAATCACGACGCTGGG |
| Nppa | TGAGCGAGCAGACCGATGAA | GAGACGGGTTGACTTCCCCA |
| Nppb | ACAAGAGAGAGCAGGACACCA | TAAGGAAAAGCAGGAGCAGAATCA |
| Myh6 | CTGAAGAGGCGGAGGAACAGG | TATTGTGGGATAGCAACAGCGA |
| Myh7 | CTCCCAGAACACCAGCCTC | CCGCCTCCTCCACCTCT |
| Col1a1 | AAGAACGGAGATGATGGGGAA | ATCCAAACCACTGAAACCTCT |
| Col3a1 | GAATGGTGGCTTTCAGTTCAG | GCAGTGGTATGTAATGTTCTGGG |
| Tgfb1 | CTGAACCAAGGAGACGGAATACA | AGGAGCAGGAAGGGTCGGT |
| Mmp2 | TGCCCAGAGACTGCTATGTCC | GCACACCACACCTTGCCATC |
| Gpx3 | GCTTCCCGTTCCAAATGAGC | GAAGGAGGCTGGTGGCATAG |
| Gpx1 | CCGGGACTACACCGAAATGA | ACCATTCACCTCGCACTTCT |
| Nqo1 | TCCAGAAACGACATCACAGGG | GGCACCCCAAACCAATACAA |
| Txnrd1 | CTCTCTTTATCCTCAGTGTGCTT | CCCGCCGCCCTATGA |
| Srxn1 | AGCAACCTCCTGATACCCCA | GAACGGAACCCCCTCATTCT |
| Hmox1 | CAACATTGAGCTGTTTGAGGAGC | GAGCGGTGTCTGGGATGAAC |
| Idh2 | GAGGCGTTGGTATGGGGATG | CGAAGCCCGAAATGGACTCG |
Col1a1 and Col3a1, collagen α-1(I) chain and collagen α-1(III) chain, respectively; Gpx1 and Gpx3, glutathione peroxidase 1 and 3, respectively; Hmox1, heme oxygenase 1; Hprt1, hypoxanthine phosphoribosyltransferase 1; Idh2, isocitrate dehydrogenase 2; Mmp2, matrix metalloproteinase-2; Myh6 and Myh7, myosin heavy chain 6 and 7, respectively; Nppa and Nppb, natriuretic peptide A and B, respectively; Nqo1, NAD(P)H quinone dehydrogenase 1; Srxn1, sulfiredoxin 1; Tgfb1, transforming growth factor-β1; Txnrd1, thioredoxin reductase 1.
Immunoblot analysis.
After organ harvest, the LVs were dissected from the rest of the heart, minced, and snap-frozen. For protein extraction, the lysates were suspended in lysis buffer (50 mM Tris·HCl, pH 7.4; 150 mM NaCl; 1% Nonidet P-40; 0.25% sodium deoxycholate; 1 mM EDTA; 2 mM Na3VO4; 1 mM NaF; 2 µg/mL leupeptin; 2 µg/mL antipain; 2 µg/mL soybean trypsin inhibitor; and 2 µg/mL lima trypsin inhibitor), homogenized, and centrifuged, and the supernatant was collected. After protein estimation, cell lysates were boiled with SDS (2%) and β-mercaptoethanol (5%). After protein separation by SDS-PAGE, proteins were transferred onto nitrocellulose membranes (tank transfer). For the Western blot blocking step, the blots were incubated for 1 h in a blocking solution (SuperBlock buffer, cat. no. 37515; Thermo Fisher Scientific). Afterward, the membranes were incubated with the specific primary antibody. The isocitrate dehydrogenase 2 (IDH2; cat. no. 60322), caspase-3 (cat. no. 14220), and vinculin (cat. no. 13901) antibodies were from Cell Signaling Technology. The primary antibodies were diluted 1:1,000 and incubated overnight in SuperBlock buffer. Following three washes (15 min each) with Tris-buffered saline-Tween 20 (TBST), the membranes were incubated for 1 h with anti-rabbit or anti-mouse secondary antibody (Cell Signaling Technology) diluted 1:2,000 in SuperBlock buffer. Afterward, the membranes were washed three additional times in TBST and incubated with a chemiluminescent reagent (SuperSignal West Femto, cat. no. 46640), following the manufacturer’s protocols. The membranes were digitally imaged in a Kodak Multispectral Imaging System.
Quantification of 8-hydroxyguanosine.
The quantity of 8-hydroxyguanosine (8-OHG) present in the samples was determined using Oxidative RNA Damage ELISA Kit (cat. no. STA-325; Cell Biolabs) according to the manufacturer’s instructions.
Micro-PET/computed tomography.
Twelve rats were scanned on the Sedecal Argus (formerly GE Healthcare eXplore Vista) micro-PET/computed tomography (CT) scanner after fasting for 16 h. Rats were anesthetized with isofluorane, and CT scans were acquired. Sixty-minute dynamic list-mode PET acquisitions began 5 s before a bolus injection of 15.0–17.0 MBq of [18F]fluorodeoxyglucose (18F-FDG) through a tail vein catheter. PET images were reconstructed into 33 frames (6 × 10 s, 6 × 30 s, 10 × 60 s, 3 × 120 s, and 8 × 300 s) by ordered-subset expectation maximization (16 subsets and 2 iterations) with corrections for random events and scatter, as well as CT-based attenuation correction. Images were processed with the highly constrained backprojection (HYPR) denoising method (9, 12) before analysis. Global myocardial time-activity curves were extracted from the dynamic PET image series using FlowQuant (Ottawa Heart Institute, Ottawa, ON, Canada), with manual intervention by imaging specialists (M. Di Carli and S. Divakaran) in images with very low or absent cardiac uptake. Tissue time-activity curves were imported into PMOD (PMOD Technologies, Zürich, Switzerland) for graphical analysis using the Patlak approach (26), which yielded myocardial glucose uptake rates. Input functions were derived from the aortic blood pool, identified from the early time frames of the PET images, aided by anatomic localization based on CT. Myocardial glucose utilization was calculated by multiplying the myocardial glucose uptake rate by glucose concentration and the lumped constant, assumed to be 1 (17, 29). Late static images, consisting of average uptake between 40 and 60 min after injection, were generated and converted to standardized uptake value (SUV) units. SUV is a unitless metric calculated by dividing activity concentration by the ratio of injected dose to body weight.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 7 software. Data were assessed for normality by the Shapiro-Wilk method. For echocardiographic results, intragroup comparison between two groups was performed using a paired t-test (if data were normally distributed) or by Wilcoxon matched-pairs test (if data were not normally distributed). The intergroup analysis was performed using an unpaired t-test (if normally distributed) or by Mann-Whitney rank sum test (if not normally distributed). Multiple comparisons between groups, for echocardiography, quantitative PCR, Western blot analysis, anatomical and morphological measurements, 18F-FDG PET measurements, and biochemical profile were analyzed by one-way ANOVA with Šídák’s multiple-comparison test (if data were normally distributed) or Kruskal-Wallis test followed by Dunn’s multiple-comparison test (if data were not normally distributed). Interstitial fibrosis was analyzed using ordinary one-way ANOVA followed by uncorrected Fisher’s least significant difference test. All data are presented as means ± SE.
RESULTS
Induction of heart failure by chemogenetics.
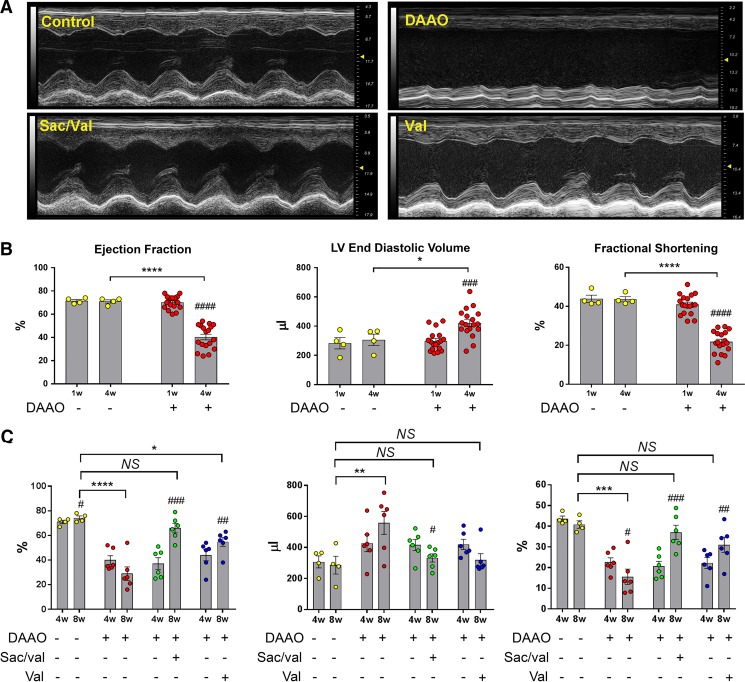
We recently developed and characterized a new in vivo chemogenetic model of heart failure in which we showed that chronic oxidant generation in cardiac myocytes leads to contractile dysfunction and LV dilation (32). The present studies explore whether this severe dilated myopathy can be reversed with drug treatment. For these experiments, we induced oxidative stress in the heart by infecting rats with a cardiotropic AAV9 that expresses a yeast DAAO, which produces H2O2 in the catalytic process of d-amino acid metabolism. Since most mammalian tissues contain nominal levels of d-amino acids (16), the yeast DAAO is quiescent until d-amino acids are provided. In these studies, rats were infected with an AAV9 carrying either the DAAO construct (DAAO virus) or a fluorescent protein (control virus). Four weeks after infection, d-alanine (a substrate for DAAO) was added to the drinking water of DAAO and control groups. Consistent with our previous results (32), a dilated cardiomyopathy developed in DAAO-expressing animals compared with control animals after 4 wk of d-alanine feeding, as evidenced by decreased ejection fraction, fractional shortening, cardiac output, and global longitudinal strain as well as increased LV end-diastolic volumes (Fig. 1B and Supplemental Fig. S2; Supplemental Material for this article is available online at https://doi.org/10.6084/m9.figshare.8852354.v2).
Fig. 1.
Echocardiographic measurements of chemogenetic heart failure after treatment with sacubitril/valsartan or valsartan. A: representative M-mode echocardiographic images acquired from short-axis views of the left ventricle in rats infected with control or d-amino acid oxidase (DAAO) virus, treated as indicated with sacubitril/valsartan (Sac/val) or valsartan alone (Val). B: quantitative measurements of cardiac function in control (n = 4) and DAAO-expressing rats (n = 18) after 4 wk of treatment with d-alanine. *P < 0.05, ****P < 0.0001 for intergroup comparison and ###P < 0.001, ####P < 0.0001 for intragroup comparison. Data are represented as means ± SE. C: echocardiographic measurements describing functional parameters of control virus-infected rats treated with vehicle (water; n = 4), DAAO-expressing rats treated with water (n = 6), sacubitril/valsartan, or valsartan (n = 6 in each group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 obtained by one-way ANOVA with Šídák’s test for multiple comparisons (compared with control) and #P < 0.05, ##P < 0.01, ###P < 0.001 for intragroup comparison. Data are represented as means ± SE. LV, left ventricular; NS, not significant; w, weeks.
Cardiac dysfunction induced by oxidative stress is rescued with valsartan and sacubitril/valsartan.
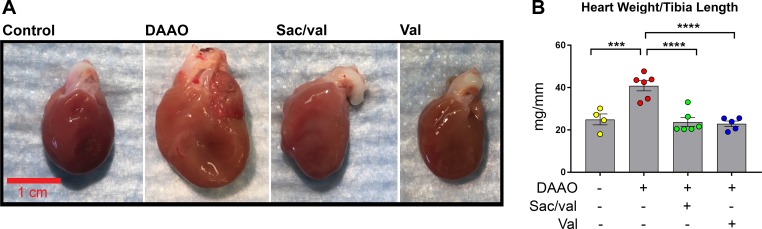
After 4 wk of d-alanine treatment, the animals were treated by gavage daily with either water, sacubitril/valsartan, or valsartan alone (Supplemental Fig. S1); d-alanine treatment was continued for all animals. Over the ensuing 4 wk of drug treatment, echocardiography revealed that both valsartan and sacubitril/valsartan reversed the cardiomyopathic changes observed after the initial 4 wk of DAAO activation (Fig. 1, A and C). Consistent with these echocardiographic results, heart weight was dramatically increased in DAAO animals treated with water but improved to control levels in animals treated with valsartan or sacubitril/valsartan (Fig. 2, A and B, and Supplemental Fig. S4). Whereas cardiac function and anatomic remodeling improved with drug treatment, we found that mean arterial pressure was lower in animals treated with sacubitril/valsartan and valsartan alone, a result consistent with the drugs’ known effects on vascular tone (Supplemental Fig. S3B). We observed no significant differences in myocyte cross-sectional area (a metric for myocyte hypertrophy) between the experimental groups (Supplemental Fig. S4C). Feeding the animals 1 M d-alanine in the drinking water appeared to cause a mild osmotic diuresis: the animals showed hypernatremia and elevated blood urea nitrogen, but serum creatinine and potassium remained in the normal range (Table 1). Neither the induction of oxidative stress nor drug treatment significantly affected these parameters.
Fig. 2.
Morphological evaluation of chemogenetic heart failure after treatment with sacubitril/valsartan or valsartan. A: representative photographs of hearts from control and d-amino acid oxidase (DAAO)-expressing rats following treatments with water, sacubitril/valsartan (Sac/val), or valsartan alone (Val). B: measurements of heart weight to tibia length in control (n = 4) or DAAO-expressing rats (n = 6) treated with water and DAAO-expressing rats treated with sacubitril/valsartan (n = 6) or valsartan (n = 5). ***P < 0.001, ****P < 0.0001 obtained by one-way ANOVA with Šídák’s multiple-comparison test. Data are represented as means ± SE.
Valsartan and sacubitril/valsartan attenuate oxidative damage and improve molecular markers of heart failure.
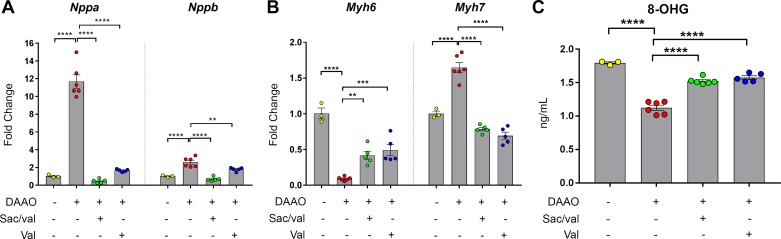
In our previous characterization of this model of chemogenetic heart failure, we demonstrated that DAAO activation reduces the availability of reduced glutathione and increases expression of antioxidant targets of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2; 32). To explore how drug therapy modifies damage from oxidative stress, we measured oxidized RNA guanosine levels in the hearts of animals expressing DAAO, with and without drug treatment. Paralleling the changes in cardiac function, DAAO activation increased guanosine oxidation, but both treatment with valsartan and treatment with sacubitril/valsartan attenuated RNA oxidation (Fig. 3C). Consistent with our previous findings, antioxidant transcriptional responses including targets of Nrf2 and glutathione peroxidase expression were increased with DAAO activation and partially normalized with both valsartan and sacubitril/valsartan (Supplemental Fig. S5).
Fig. 3.
Changes in cardiac tissue biomarkers and oxidative damage after treatments with sacubitril/valsartan or valsartan in chemogenetic heart failure. A: levels of atrial natriuretic peptide (Nppa) and brain natriuretic peptide (Nppb) transcripts in water-treated control (n = 3) or d-amino acid oxidase (DAAO)-expressing rats (n = 6) or in DAAO-expressing rats treated with sacubitril/valsartan (Sac/val) or valsartan alone (Val; n = 5 for each group). B: α-myosin heavy chain (Myh6) and β-myosin heavy chain (Myh7) transcripts in hearts of water-treated control (n = 3) or DAAO-expressing rats (n = 6) or in DAAO-expressing rats treated with sacubitril/valsartan or valsartan (n = 5 for each group). C: measurements of oxidative damage in control (n = 3) or DAAO-expressing rats (n = 6) or in DAAO-expressing rats treated with sacubitril/valsartan (n = 6) or valsartan (n = 5). **P < 0.01, ***P < 0.001, ****P < 0.0001 obtained by one-way ANOVA with Šídák’s multiple-comparison test. Data are represented as means ± SE. 8-OHG, 8-hydroxyguanosine.
We next examined classical molecular markers of heart failure and found that Nppa and Nppb transcripts (coding for atrial and brain/B-type natriuretic peptides, respectively) were elevated in rats expressing DAAO and treated with water (Fig. 3A). Both valsartan and sacubitril/valsartan treatment reduced the levels of these heart failure-associated transcripts, with sacubitril/valsartan being more effective at returning Nppb expression to control levels compared with valsartan alone (Fig. 3A). Similarly, transcript levels for Myh6, which encodes α-myosin heavy chain (α-MHC), were decreased, and Myh7 (coding for β-MHC) transcript levels were increased in DAAO-expressing animals treated with water. Whereas Myh7 expression returned to control levels with both valsartan and sacubitril/valsartan treatment, Myh6 expression increased but did not fully return to levels in control animals (Fig. 3B).
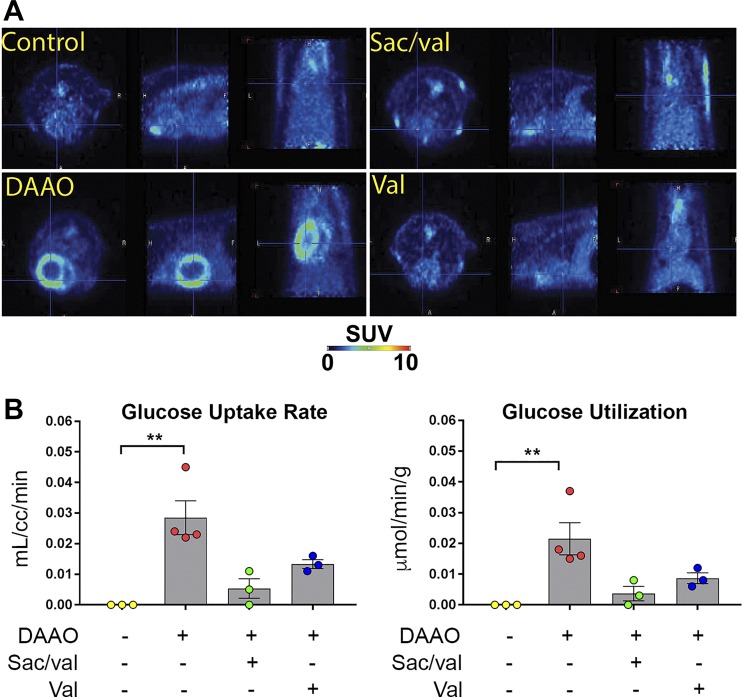
Finally, glucose uptake measured by 18F-FDG PET was dramatically increased in animals expressing DAAO that did not receive any drug treatment, implying a switch to a pattern of enhanced glucose metabolism, which has been observed in both human and animal heart failure (2, 11, 14). Like several of the other molecular markers of heart failure, we found that glucose uptake rates and glucose utilization were decreased by both valsartan and sacubitril/valsartan treatment (Fig. 4, A and B).
Fig. 4.
[18F]fluorodeoxyglucose (18F-FDG) PET imaging analysis of chemogenetic heart failure after treatments with sacubitril/valsartan or valsartan. A: representative 18F-FDG PET images of water-treated control, d-amino acid oxidase (DAAO)-expressing rats treated with water, or DAAO-expressing rats following treatments with either sacubitril/valsartan (Sac/val) or valsartan (Val). B: quantitative data of glucose uptake rate (left) and glucose utilization (right) of control (n = 3), DAAO-expressing rats (n = 4), or DAAO-infected rats following treatments with sacubitril/valsartan (n = 3) or valsartan (n = 3). **P < 0.01 obtained by Kruskal-Wallis test followed by Dunn’s multiple-comparison test. Data are represented as means ± SE. Here, cc, cubic centimeters; SUV, standardized uptake value.
Valsartan and sacubitril/valsartan treatment effects on cardiac fibrosis, mitochondrial protein expression, and apoptosis.
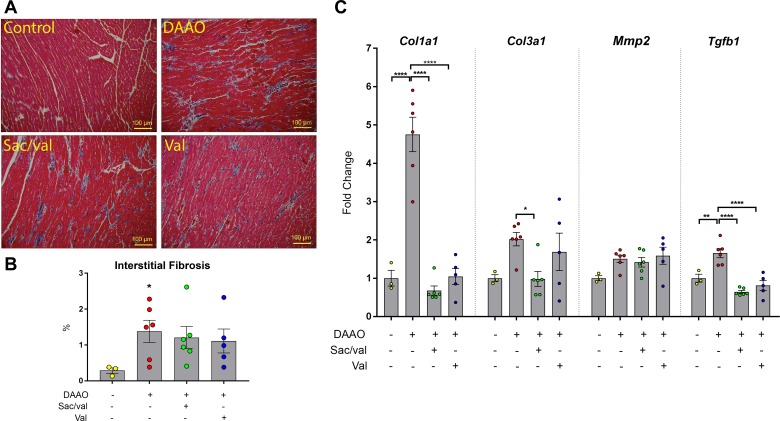
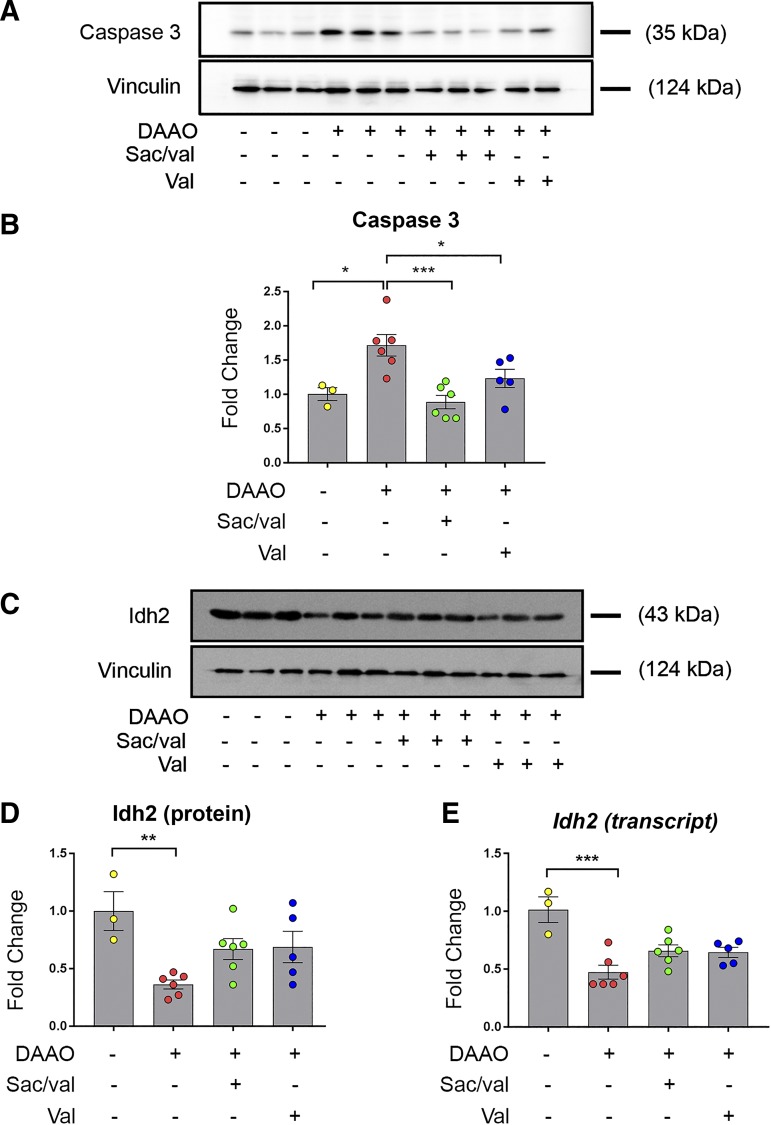
In our previous study of this chemogenetic model of heart failure caused by cardiac myocyte oxidative stress (32), no significant fibrosis was observed after 4 wk of DAAO activation, despite evidence of cardiac dysfunction and ventricular enlargement. However, with the longer-term d-alanine treatment in the present studies, we observed that expression of several of the classical transcriptional markers of fibrosis [collagen α-1(I) chain (Col1a1), collagen α-1(III) chain (Col3a1), and transforming growth factor-β1 (Tgfb1)] increased after 8 wk of chronic activation of oxidative stress. These increases were attenuated by treatment with valsartan and sacubitril/valsartan (Fig. 5C). However, although these transcriptional markers of fibrosis largely resolved in the drug-treated animals, we observed persistent histological evidence of fibrosis in animals treated with valsartan and sacubitril/valsartan (Fig. 5, A and B). To explore the effects of DAAO activation and drug treatment on mitochondrial function, we measured expression of the matrix enzyme Idh2, decreased function of which has previously been associated with cardiac dysfunction (18). Both Idh2 mRNA and total protein were decreased in DAAO animals, with a trend toward restoration of expression that did not achieve statistical significance in the drug treatment groups (Fig. 6, C–E). Finally, we measured levels of total caspase-3, which have been correlated with mitochondrial-induced apoptosis (24, 27). Both valsartan and sacubitril/valsartan reduced the increases in caspase-3 caused by chronic oxidative stress (Fig. 6, A and B).
Fig. 5.
Assessment of cardiac fibrosis after treatments with sacubitril/valsartan or valsartan in chemogenetic heart failure. A: representative cardiac tissue sections stained with Masson’s trichrome stain to detect interstitial fibrosis. B: quantitative data for measurements of interstitial fibrosis [control n = 3, d-amino acid oxidase (DAAO) n = 6, sacubitril/valsartan (Sac/val) n = 6, valsartan (Val) n = 5]. *P < 0.05 obtained by one-way ANOVA followed by Fisher’s least significant difference test. C: fibrosis-associated transcripts collagen α-1(I) chain (Col1a1), collagen α-1(III) chain (Col3a1), matrix metalloproteinase-2 (Mmp2), and transforming growth factor-β1 (Tgfb1) in cardiac tissue collected from rats infected with control virus or with DAAO virus treated with vehicle, sacubitril/valsartan, or valsartan alone (control n = 3, DAAO n = 6, Sac/val n = 6, Val n = 5). *P < 0.05, **P < 0.01, ****P < 0.0001 obtained by one-way ANOVA with Šídák’s multiple-comparison test. Data are represented as means ± SE.
Fig. 6.
Changes in apoptotic and mitochondrial markers after drug treatments in chemogenetic heart failure. A: representative immunoblots of caspase-3 in control or d-amino acid oxidase (DAAO)-expressing rats or in DAAO-expressing rats treated with sacubitril/valsartan (Sac/val) or valsartan (Val). B: quantitative data of caspase-3 protein levels in control (n = 3) or DAAO-expressing rats (n = 6) or in DAAO-expressing rats treated with sacubitril/valsartan (n = 6) or valsartan (n = 5). *P < 0.05, ***P < 0.001 obtained by one-way ANOVA with Šídák’s multiple-comparison test. C: representative immunoblots of isocitrate dehydrogenase 2 (Idh2) in control or DAAO-expressing rats or in DAAO-expressing rats treated with sacubitril/valsartan or valsartan. D: quantitative data of Idh2 protein levels in control (n = 3) or DAAO-expressing rats (n = 6) or in DAAO-expressing rats treated with sacubitril/valsartan (n = 6) or valsartan (n = 5). **P < 0.01 obtained by one-way ANOVA with Šídák’s multiple-comparison test. E: quantitative data of Idh2 transcript levels in control (n = 3) or DAAO-expressing rats (n = 6) or in DAAO-expressing rats treated with sacubitril/valsartan (n = 6) or valsartan (n = 5). ***P < 0.001 obtained by one-way ANOVA with Šídák’s multiple-comparison test. Data are represented as means ± SE.
DISCUSSION
This study represents the first investigation of heart failure therapeutics in a chemogenetic model of oxidative stress. The levels of oxidative stress seen in this model are of a similar magnitude to what has been observed in other models of heart failure (38). Oxidative stress has been associated with nearly every form of human heart failure, including doxorubicin cardiomyopathy, diabetic cardiomyopathy, hypertrophic cardiomyopathy, and cardiomyopathy of aging, and we view as a major strength of our chemogenetic model that this approach isolates the effects of reactive oxygen species from other features of any given pathology. Another major advantage of the chemogenetic animal model is that heart failure develops rapidly and without any need for surgical intervention, as is required in many widely used models of heart failure, including myocardial infarction, aortocaval fistula, and aortic constriction models (13).
An important feature of DAAO catalysis is that both ammonia and pyruvate (the α-ketoacid produced from d-alanine) are generated in equimolar amounts as H2O2 and cannot be entirely excluded as biologically relevant molecules. Although the exact concentration of intracellular H2O2 continues to be debated, H2O2 is generally thought to be present at submicromolar concentrations in most cell types (30). This is in contrast to both ammonia and pyruvate, which are present at concentrations several orders of magnitude greater than H2O2 (3, 5, 22, 28). We therefore believe that the relative increases in ammonia and pyruvate are far less than with H2O2 and that the cardiac phenotype arises from significant increases in H2O2 rather than from nominal increases in ammonia or pyruvate.
We find in the present studies that treatment with either valsartan or sacubitril/valsartan attenuates oxidative damage and partially reverses heart failure caused by oxidative stress, suggesting that these drugs may work in part through modulation of redox signaling pathways in the heart. H2O2 was generated specifically in cardiac myocytes in vivo using chemogenetic approaches, and the animals were treated with either valsartan or sacubitril/valsartan after the development of systolic dysfunction and ventricular enlargement. Echocardiographic indexes of cardiac function improved with both drugs (Fig. 1, A–C, and Supplemental Fig. S3A), as did molecular markers of heart failure, including expression levels of atrial natriuretic peptide, brain/B-type natriuretic peptide, α-MHC, and β-MHC (Fig. 3, A and B). We also found that both 8-OHG levels, a marker of oxidative damage (17), as well as caspase-3 levels, a marker of mitochondrial damage and apoptosis (10, 24, 27), were also reduced by these drugs. The analysis of drug effects in this chemogenetic model of heart failure allows us to distinguish cardiac myocyte-specific responses from the multisystem pathological responses that are seen in many other models of heart failure, including aortic banding, coronary artery ligation, and animal models of diabetes (13). Since DAAO expression is driven by a cardiac-specific promoter, oxidative stress is generated primarily in cardiac myocytes, although we have found that there is also modest expression of DAAO in skeletal muscle in this model (32).
Reductions in cardiac preload and afterload (as evidenced by lower systemic vascular pressures; Supplemental Fig. S3B) may partially explain these drugs’ ability to improve heart failure induced by DAAO. By decreasing systemic arterial tone, these drugs reduce the extrinsic work performed by the heart, thereby decreasing its metabolic rate. The dysfunction induced by DAAO is likely in part due to mitochondrial damage and dysfunction as evidenced by increases in caspase-3 levels and decreased expression of the mitochondrial matrix enzyme Idh2 (Fig. 6). By reducing the heart’s energetic requirements and by attenuating flux through the mitochondrial electron transport chain, decreased cardiac mechanical work may reduce oxidative stress by decreasing the amount of oxygen consumed by damaged and uncoupled mitochondria. However, the reversal of almost all evidence of systolic dysfunction and the normalization of most molecular markers of heart failure suggest a more complex mechanism of these drugs, including the potential for a direct effect on the cardiac myocytes and mitochondrial function.
The ability of ARBs and neprilysin inhibition to reverse heart failure and attenuate the oxidative damage and mitochondrial dysfunction induced by oxidative stress suggests new potential mechanisms of action for these drugs. AT1R is coupled to the enzyme NADPH oxidase 2 (NOX2), which generates superoxide and modulates the cellular redox balance in the heart and other tissues (39). By measuring levels of oxidized and reduced glutathione, we previously demonstrated that DAAO shifts the redox balance in the heart toward an average oxidation state similar to what is seen in other models of heart failure (32). In the present study, we also found increased levels of RNA oxidation in cardiac myocytes (Fig. 3C), which were reversed by AT1R blockade. As previously discussed, part of this resolution in oxidative stress may stem from reduced afterload and decreased cardiac metabolic rate. However, direct AT1R blockade in the heart may also help to explain the reduced oxidative damage seen with valsartan through reduced basal production of reactive oxygen species by the AT1R-coupled NOX2.
Cardiac PET revealed that a metabolic shift toward preferential glucose metabolism induced by chronic oxidative stress was reversed in both drug treatment groups (Fig. 4, A and B). The adult heart preferentially supplies its energetic demands through fatty acid metabolism, but a regression toward “fetal” metabolic pathways has been observed in heart failure, including a preference for glucose over fatty acids. Caspase activation observed in our model and decreased expression of the tricarboxylic acid cycle enzyme Idh2 suggest that mitochondrial dysfunction induced by chronic oxidative stress may be responsible for a shift away from aerobic metabolism. The restoration of a more “physiological” cardiac metabolic state that we observed in both drug treatment groups represents yet an additional effect of AT1R blockade and neprilysin inhibition on cardiac physiology, independent of drug effects on vascular tone and natriuresis.
Whereas treatment with valsartan with or without sacubitril improved cardiac function and normalized many of the molecular markers of heart failure, the drugs’ abilities to protect against fibrosis appear to be less complete. There is a large body of literature demonstrating that AT1R blockade reduces cardiac fibrosis and hypertrophic remodeling, likely by inhibiting cross talk between AT1R and the TGF-β signaling pathway (14, 17, 33–35, 37). Whereas whole heart transcriptional analysis showed that both valsartan and sacubitril/valsartan treatments restore collagen and TGF-β expression to control levels (Fig. 5C), histologic analysis still demonstrated a significantly increased amount of interstitial fibrosis in drug-treated animals compared with control animals (Fig. 5, A and B). These findings may suggest that ARBs are not able to prevent the development of fibrosis but might limit additional fibrosis. We have previously observed that chemogenetic redox stress leads to marked cardiac dysfunction without the development of fibrosis (32), suggesting that fibrosis is not necessary for the development of heart failure in this model. In the present studies of longer-term redox stress, we observed significant recovery of cardiac function with drug treatment, despite the persistence of fibrosis in cardiac tissues. This disconnect between improvement in cardiac function and metabolism in the face of persistent cardiac fibrosis demonstrates that fibrosis alone is insufficient to cause cardiac dysfunction and that cardiac function may normalize with drug treatment despite the persistence of fibrosis.
By isolating the role of cardiac myocyte oxidative stress in heart failure, this model has allowed us to test the effects of established cardiovascular therapeutics on chemogenetic heart failure and to suggest new mechanisms for the actions of contemporary drugs. Since oxidative stress is confined primarily to the heart in this model (32), we hypothesize that the salutary effects of AT1R blockade with or without neprilysin inhibition stem in part from a direct effect of these drugs on cardiac myocytes. These findings suggest that the therapeutic effects of valsartan and sacubitril/valsartan are not limited to the vasculature, and we propose that the cardiac targets of these drugs may play a key role in the pathobiology of heart failure. We anticipate that chemogenetic approaches will open the door to studying many more aspects of cardiovascular pharmacology and identifying new pharmacologic targets not only in heart failure but also in the myriad disease states in which oxidative stress plays a key role in pathophysiology.
GRANTS
This work was supported by funds from Novartis Investigator-Initiated Trial LCZ696BUSNC14T (T. Michel); by National Institutes of Heath Grants R21-AG-063073 (T. Michel) and T32-HL-007604 (S. Badole); by National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-057521 (T. Michel); by National Institute of General Medical Sciences Grant T32-GM-007753 (B. Steinhorn); by Brigham and Women’s Hospital Health and Technology Innovation Award (T. Michel); by Austrian Science Foundation Grant J4113 (E. Eroglu); and by American Diabetes Association Grant 9-17-CMF-012 (A. Sorrentino); S. Divakaran was supported by NHLBI Grants T32-HL-094301 (S. Divakaran) and R01-HL-132021 (M. Di Carli).
DISCLOSURES
These studies were supported in part by funds provided by Novartis as part of an Investigator-Initiated Trial (T. Michel, Principal Investigator). None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.S., B.S., L.T., M.D.C., and T.M. conceived and designed research; A.S., B.S., L.T., S.B., S.S.S.S., E.E., M.F.K., and S.D. performed experiments; A.S., B.S., L.T., S.S.S.S., E.E., M.F.K., S.D., M.D.C., and T.M. analyzed data; A.S., B.S., L.T., S.B., S.S.S.S., E.E., M.F.K., S.D., M.D.C., and T.M. interpreted results of experiments; A.S., B.S., E.E., and T.M. prepared figures; A.S. and B.S. drafted manuscript; A.S., B.S., M.D.C., and T.M. edited and revised manuscript; A.S., B.S., L.T., S.B., S.S.S.S., E.E., M.F.K., S.D., M.D.C., and T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Marc Pfeffer for thoughtful guidance and insightful queries in the course of these studies.
REFERENCES
- 1.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286, 2006. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 2.Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol 15: 457–470, 2018. doi: 10.1038/s41569-018-0044-6. [DOI] [PubMed] [Google Scholar]
- 3.Bjerring PN, Hauerberg J, Frederiksen HJ, Jorgensen L, Hansen BA, Tofteng F, Larsen FS. Cerebral glutamine concentration and lactate-pyruvate ratio in patients with acute liver failure. Neurocrit Care 9: 3–7, 2008. doi: 10.1007/s12028-008-9060-4. [DOI] [PubMed] [Google Scholar]
- 4.Boehm O, Zur B, Koch A, Tran N, Freyenhagen R, Hartmann M, Zacharowski K. Clinical chemistry reference database for Wistar rats and C57/BL6 mice. Biol Chem 388: 547–554, 2007. doi: 10.1515/BC.2007.061. [DOI] [PubMed] [Google Scholar]
- 5.Braissant O, McLin VA, Cudalbu C. Ammonia toxicity to the brain. J Inherit Metab Dis 36: 595–612, 2013. doi: 10.1007/s10545-012-9546-2. [DOI] [PubMed] [Google Scholar]
- 6.Braunwald E. Heart failure. JACC Heart Fail 1: 1–20, 2013. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res 111: 1091–1106, 2012. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 8.Burnier M. Angiotensin II type 1 receptor blockers. Circulation 103: 904–912, 2001. doi: 10.1161/01.CIR.103.6.904. [DOI] [PubMed] [Google Scholar]
- 8a.Charles River Laboratories Baseline Hematology and Clinical Chemistry Values for Charles River Wistar Rats – (CRL:(WI)BR) as a Function of Sex and Age (Online) https://www.criver.com/sites/default/files/resources/BaselineHematologyandClinicalChemistryValuesforCharlesRiverWistarRats%5BCrlWIBR%5DasaFunctionofSexandAgeSpring1998.pdf.
- 9.Christian BT, Vandehey NT, Floberg JM, Mistretta CA. Dynamic PET denoising with HYPR processing. J Nucl Med 51: 1147–1154, 2010. doi: 10.2967/jnumed.109.073999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci USA 99: 6252–6256, 2002. doi: 10.1073/pnas.092022999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doenst T, Abel ED. Spotlight on metabolic remodelling in heart failure. Cardiovasc Res 90: 191–193, 2011. doi: 10.1093/cvr/cvr077. [DOI] [PubMed] [Google Scholar]
- 12.Floberg JM, Mistretta CA, Weichert JP, Hall LT, Holden JE, Christian BT. Improved kinetic analysis of dynamic PET data with optimized HYPR-LR. Med Phys 39: 3319–3331, 2012. doi: 10.1118/1.4718669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ; American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology . Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111: 131–150, 2012. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 14.Jing W, Vaziri ND, Nunes A, Suematsu Y, Farzaneh T, Khazaeli M, Moradi H. LCZ696 (Sacubitril/valsartan) ameliorates oxidative stress, inflammation, fibrosis and improves renal function beyond angiotensin receptor blockade in CKD. Am J Transl Res 9: 5473–5484, 2017. [PMC free article] [PubMed] [Google Scholar]
- 15.Judge P, Haynes R, Landray MJ, Baigent C. Neprilysin inhibition in chronic kidney disease. Nephrol Dial Transplant 30: 738–743, 2015. doi: 10.1093/ndt/gfu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga R, Miyoshi Y, Sakaue H, Hamase K, Konno R. Mouse d-amino-acid oxidase: distribution and physiological substrates. Front Mol Biosci 4: 82, 2017. doi: 10.3389/fmolb.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong Q, Lin CL. Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell Mol Life Sci 67: 1817–1829, 2010. doi: 10.1007/s00018-010-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ku HJ, Ahn Y, Lee JH, Park KM, Park JW. IDH2 deficiency promotes mitochondrial dysfunction and cardiac hypertrophy in mice. Free Radic Biol Med 80: 84–92, 2015. doi: 10.1016/j.freeradbiomed.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Lang CC, Struthers AD. Targeting the renin-angiotensin-aldosterone system in heart failure. Nat Rev Cardiol 10: 125–134, 2013. doi: 10.1038/nrcardio.2012.196. [DOI] [PubMed] [Google Scholar]
- 20.McMurray JJ. Clinical practice. Systolic heart failure. N Engl J Med 362: 228–238, 2010. doi: 10.1056/NEJMcp0909392. [DOI] [PubMed] [Google Scholar]
- 21.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371: 993–1004, 2014. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 22.Merhi A, Delrée P, Marini AM. The metabolic waste ammonium regulates mTORC2 and mTORC1 signaling. Sci Rep 7: 44602, 2017. doi: 10.1038/srep44602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol 71: 1474–1482, 2018. doi: 10.1016/j.jacc.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 24.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW, Israels S, Ballester M, Virmani R, Saxena S, Kharbanda S. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA 96: 8144–8149, 1999. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oatmen KE, Zile MR, Burnett JC Jr, Spinale FG. Bioactive signaling in next-generation pharmacotherapies for heart failure: a review. JAMA Cardiol 3: 1232, 2018. doi: 10.1001/jamacardio.2018.3789. [DOI] [PubMed] [Google Scholar]
- 26.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5: 584–590, 1985. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- 27.Philipp S, Pagel I, Höhnel K, Lutz J, Buttgereit J, Langenickel T, Hamet P, Dietz R, Willenbrock R. Regulation of caspase 3 and Fas in pressure overload-induced left ventricular dysfunction. Eur J Heart Fail 6: 845–851, 2004. doi: 10.1016/j.ejheart.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 28.San Martín A, Ceballo S, Baeza-Lehnert F, Lerchundi R, Valdebenito R, Contreras-Baeza Y, Alegría K, Barros LF. Imaging mitochondrial flux in single cells with a FRET sensor for pyruvate. PLoS One 9: e85780, 2014. doi: 10.1371/journal.pone.0085780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoghi KI, Gropler RJ, Sharp T, Herrero P, Fettig N, Su Y, Mitra MS, Kovacs A, Finck BN, Welch MJ. Time course of alterations in myocardial glucose utilization in the Zucker diabetic fatty rat with correlation to gene expression of glucose transporters: a small-animal PET investigation. J Nucl Med 49: 1320–1327, 2008. doi: 10.2967/jnumed.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem 289: 8735–8741, 2014. doi: 10.1074/jbc.R113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorrentino A, Borghetti G, Zhou Y, Cannata A, Meo M, Signore S, Anversa P, Leri A, Goichberg P, Qanud K, Jacobson JT, Hintze TH, Rota M. Hyperglycemia induces defective Ca2+ homeostasis in cardiomyocytes. Am J Physiol Heart Circ Physiol 312: H150–H161, 2017. doi: 10.1152/ajpheart.00737.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinhorn B, Sorrentino A, Badole S, Bogdanova Y, Belousov V, Michel T. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat Commun 9: 4044, 2018. doi: 10.1038/s41467-018-06533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suematsu Y, Jing W, Nunes A, Kashyap ML, Khazaeli M, Vaziri ND, Moradi H. LCZ696 (sacubitril/valsartan), an angiotensin-receptor neprilysin inhibitor, attenuates cardiac hypertrophy, fibrosis, and vasculopathy in a rat model of chronic kidney disease. J Card Fail 24: 266–275, 2018. doi: 10.1016/j.cardfail.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, Iwata A, Yahiro E, Saku K. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail 18: 386–393, 2016. doi: 10.1002/ejhf.474. [DOI] [PubMed] [Google Scholar]
- 35.Torrado J, Cain C, Mauro AG, Romeo F, Ockaili R, Chau VQ, Nestler JA, Devarakonda T, Ghosh S, Das A, Salloum FN. Sacubitril/valsartan averts adverse post-infarction ventricular remodeling and preserves systolic function in rabbits. J Am Coll Cardiol 72: 2342–2356, 2018. doi: 10.1016/j.jacc.2018.07.102. [DOI] [PubMed] [Google Scholar]
- 36.Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J 35: 419–425, 2014. doi: 10.1093/eurheartj/eht466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, Atar D, Krum H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail 8: 71–78, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001785. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe Y, Watanabe K, Kobayashi T, Saito Y, Fujioka D, Nakamura T, Obata JE, Kawabata K, Mishina H, Kugiyama K. Chronic depletion of glutathione exacerbates ventricular remodelling and dysfunction in the pressure-overloaded heart. Cardiovasc Res 97: 282–292, 2013. doi: 10.1093/cvr/cvs333. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Prosser BL, Bamboye MA, Gondim AN, Santos CX, Martin D, Ghigo A, Perino A, Brewer AC, Ward CW, Hirsch E, Lederer WJ, Shah AM. Contractile function during angiotensin-II activation: increased Nox2 activity modulates cardiac calcium handling via phospholamban phosphorylation. J Am Coll Cardiol 66: 261–272, 2015. doi: 10.1016/j.jacc.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 13: 368–378, 2016. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]