Abstract
Cholinergic receptor activation leads to premature development of hypertension and infiltration of proinflammatory CD161a+/CD68+ M1 macrophages into the renal medulla. Renal inflammation is implicated in renal sodium retention and the development of hypertension. Renal denervation is known to decrease renal inflammation. The objective of this study was to determine the role of CD161a+/CD68+ macrophages and renal sympathetic nerves in cholinergic-hypertension and renal sodium retention. Bilateral renal nerve denervation (RND) and immune ablation of CD161a+ immune cells were performed in young prehypertensive spontaneously hypertensive rat (SHR) followed by infusion of either saline or nicotine (15 mg·kg−1·day−1) for 2 wk. Immune ablation was conducted by injection of unconjugated azide-free antibody targeting rat CD161a+. Blood pressure was monitored by tail cuff plethysmography. Tissues were harvested at the end of infusion. Nicotine induced premature hypertension, renal expression of the sodium-potassium chloride cotransporter (NKCC2), increases in renal sodium retention, and infiltration of CD161a+/CD68+ macrophages into the renal medulla. All of these effects were abrogated by RND and ablation of CD161a+ immune cells. Cholinergic activation of CD161a+ immune cells with nicotine leads to the premature development of hypertension in SHR. The effects of renal sympathetic nerves on chemotaxis of CD161a+ macrophages to the renal medulla, increased renal expression of NKCC2, and renal sodium retention contribute to cholinergic hypertension. The CD161a+ immune cells are necessary and essential for this prohypertensive nicotine-mediated inflammatory response.
NEW & NOTEWORTHY This is the first study that describes a novel integrative physiological interaction between the adrenergic, cholinergic, and renal systems in the development of hypertension, describing data for the role of each in a genetic model of essential hypertension. Noteworthy findings include the prevention of nicotine-mediated hypertension following successful immune ablation of CD161a+ immune cells and the necessary role these cells play in the overexpression of the sodium-potassium-chloride cotransporter (NKCC2) in the renal medulla and renal sodium retention. Renal infiltration of these cells is demonstrated to be dependent on the presence of renal adrenergic innervation. These data offer a fertile ground of therapeutic potential for the treatment of hypertension as well as open the door for further investigation into the mechanism involved in inflammation-mediated renal sodium transporter expression. Taken together, these findings suggest immune therapy, renal denervation, and, possibly, other new molecular targets as having a potential role in the development and maintenance of essential hypertension.
Listen to this article's corresponding podcast at https://ajpheart.podbean.com/e/cd161a-immune-cells-in-cholinergic-hypertension/.
Keywords: adrenergic, CD68, CD161, cholinergic, hypertension, inflammation, innate immunity, macrophage, nicotine, renal, renal denervation
INTRODUCTION
The immune system and inflammation have come to the forefront of our understanding of the pathogenesis of hypertension, complementing the role of the nervous, renal, and vascular systems. Inflammation has been documented in hypertensive patients as early as the 1950s, with prospective studies suggesting a causal role for inflammation in the development of hypertension (28–30). Experimental and genetic models of hypertension have shown a clear pathophysiological role for inflammation in the development of hypertension (14, 35).
Increased renal sympathetic nerve activity contributes to renal inflammation and contributes to the pathogenesis of hypertension through activation of the renin-angiotensin-aldosterone-system (RAAS) and promotion of renal medullary infiltration by inflammatory macrophages (8, 39, 40). As a result, renal nerve denervation (RND) was proposed as a therapy for essential hypertension (11, 20) due to its effects on renal inflammation (37, 38). RND attenuated hypertension and renal immune responses in glomerulonephritis as well as DOCA and salt-sensitive models of hypertension (38, 40, 41). In the genetic model of human essential hypertension, the spontaneously hypertensive rat (SHR), bilateral RND was shown to be effective in delaying the onset and attenuating the severity of hypertension when performed in young, prehypertensive animals (39).
Activation of cholinergic receptors on immune cells is known to mediate inflammation. In the SHR, activation of nicotinic acetylcholine receptors (nAChRs) results in proinflammatory immune responses (15, 16). Specifically, direct activation of cholinergic receptors led to the abnormal expansion and activation of a CD161a+ immune cell population and exaggeration of Toll-like receptor (TLR)-triggered innate immune responses selectively in immune cells isolated from prehypertensive SHR but not their normotensive controls (15). Cholinergic-mediated proinflammatory innate immune responses preceded the migration of these immune cells into the renal medulla and premature development of hypertension in young prehypertensive SHR (16).
CD161a (also known as KLRB1a) is generally associated with expression on natural killer cells. However, it is also expressed on dendritic cells, macrophages, as well as T and B lymphocytes (4, 5, 27, 34). Despite the variety of cells that express CD161a, it is clear that the presence of KLRB1a+ immune cells is associated with pathologic inflammatory immune responses (1, 4, 5, 12, 22, 26, 34). Activation of cholinergic receptors with nicotine infusion not only expands the CD161a+ immune cell population but also triggers the premature development of hypertension in the young prehypertensive SHR, a process we refer to as “cholinergic hypertension.” Based on the effects of renal sympathetic innervation and the association of the cholinergic-activated CD161a+ M1 macrophages with the development of hypertension, we asked 1) does RND have an effect on cholinergic hypertension and/or renal infiltration of CD161a+ macrophages, and 2) is the development of cholinergic hypertension dependent on the presence of CD161a+ immune cells.
MATERIALS AND METHODS
Animals and blood pressure.
Three- and four-week-old male SHR (Charles River) were used. Animals were housed in a room maintained at 23 ± 0.5°C with an alternating 12-h:12-h light-dark cycle and maintained on normal chow. Food and water were available ad libitum. Animal use in all the experiments was approved by the Institutional Animal Care and Use Committee at the University of Iowa and performed in accordance with the institutional guidelines. Blood pressure was measured by tail-cuff plethysmography. Animals were acclimated to the tail-cuff apparatus a minimum of two times on separate days the week before the experiments by introducing them into the holding chamber for 20–40 min, beginning at 3 wk of age. Operators were blinded to the treatment groups with analyses performed by another blinded individual. Tail-cuff apparatus was configured to run 10 trial measurements followed by 20 actual measurements to allow for intra-assay acclimation during the protocol.
Bilateral RND.
Animals were administered buprenorphine (0.05–0.1 mg/kg ip) for analgesia and anesthetized by isoflurane. The retroperitoneal space was accessed via bilateral flank incisions to isolate the renal artery and vein. RND was accomplished by surgical disruption and topical application of a 20% phenol solution (wt/vol in absolute ethanol) to the surface of the renal artery. Sham surgeries were conducted in a similar fashion without surgical disruption or chemical treatments.
CD161a immune cell ablation.
Preservative free KLRB1 monoclonal antibody (clone no. 3.2.3; Thermofisher) was injected into animals via intraperitoneal route. Ablation of CD161a+ immune cells was accomplished by two injections of 500 µg of antibody 48 h apart in 3-wk-old animals, followed by injection of 250 µg of antibody twice weekly during the period of saline/nicotine infusion. Confirmation of successful ablation was conducted by flow cytometry of splenocytes and immunofluorescence of renal tissues as described.
Nicotine infusion.
Following a 1-wk recovery period after the denervation procedure or after the induction period of CD161a+ immune ablation, rats anesthetized with isoflurane were implanted with subcutaneous miniosmotic pumps (Cat. No. 2002; Alzet model). Animals were infused continuously with either saline or nicotine bitartrate salt (15 mg·kg−1·day−1) for 2 wk. Blood pressure measurements were obtained at baseline before surgery/ablation, following the denervation/ablation procedure, and at least twice weekly during the period of infusion. Kidneys, spleens, blood, and urine samples were collected at the end of the infusion period.
Flow cytometry.
Splenocytes and bone marrow cells (1 × 106) were isolated. Following the lysis of red blood cells using hypotonic ACK Lysis buffer, isolated cells were filtered through a 45-μm pore mesh to produce a single cell suspension. After being washed, the single cell suspension of splenocytes were stained with fluorochrome conjugated monoclonal antibodies against CD161a (Cat. No. MR6805, ThermoFisher; Cat. No. 555008, BD Biosciences); CD68 (Cat. No. MCA341A700; Bio-Rad). For each acquisition, a minimum of 100,000 events were recorded. Cellular debris and necrotic/nonviable cells were excluded by size discrimination using forward and side scatter in conjunction with staining with Hoechst 33342 fluorescent stain. All values presented are presented as a percentage of the parent population following exclusion of cellular debris.
Tissue homogenization.
Renal protein lysates were prepared using RIPA buffer (Cat. No. 156034; Abcam) supplemented with protease inhibitor cocktail (Cat. No. 11836153001; Roche). Protein estimation was performed via BCA protein assay kit (Cat. No. 23225; Pierce).
Cytokines, renal toxicity markers/norepinephrine, and sodium assays.
Renal homogenate, urine, and serum were analyzed for inflammatory cytokines/renal toxicity markers by Multiplex assay (inflammatory cytokines: Cat. No. RECYMAG65K27PMX, EMD Millipore or Cat. No. 171-K1001M, Bio-Rad; renal toxicity markers: Cat. No. EPX050-30124-901/EPX050-30125-901, ThermoFisher). Renal norepinephrine levels were quantified by competitive ELISA (Cat. No. NOU39-K010; Eagle Biosciences). All results were normalized to total protein concentration, as determined by BCA assay (Cat. No. 23225; Pierce). Serum and urinary sodium were measured using a colorimetric assay (Cat. No. K391-100; BioVision). Urinary and serum creatinine content were assessed using plate based spectrophotometric assays (Cat. No. KGE005, R&D Systems; and Cat. No. 80340, Crystal Chem). Urinary protein was assessed using an colorimetric assay (Cat. No. ab108789; Abcam). All kits were used as per the manufacturer instructions.
Western blot analysis.
Forty to sixty micrograms of proteins were mixed with 2× Laemmli sample buffer (Cat. No. 1610737; Bio-Rad) and 2-mercaptoethanol (Cat. No. M6250; Sigma-Aldrich), heated and separated on 10% or 4–20% Tris-glycine polyacrylamide gels (Cat. No. XP00105BOX and XP04205BOX; ThermoFisher). Proteins were transferred to a PVDF membrane. After blocking with 5% BSA in TBS-T for an hour, membranes were incubated overnight at 4°C with various antibodies targeted against actin (Cat. No. ab6276; Abcam; 1:8,000); GAPDH (Cat. No. ab9485; Abcam; 1:2,500); monocyte chemoattractant protein-1 (MCP-1; Cat. No. LS-C104751; LSBio; 1:1,000); lectin-like transcript-1 (LLT-1; Cat. No. ab151738; AbCam; 1:1000); very-late antigen-4 (VLA-4; LS-c192282; LSBio; 1:1,000); VCAM-1 (Cat. No. LS-B9104; LSBio; 1:1,000); and sodium-potassium chloride cotransporter 2 (NKCC2; Cat. No. AB3562P; Millipore 1:1,000). Secondary antibodies used were goat anti-mouse IgG (Cat. No. SC-2005; Santa Cruz Biotechnology; 1:5,000) and goat anti-rabbit IgG (Cat. No. SC-2004; Santa Cruz Biotechnology; 1:5,000). Chemiluminescent reactions were performed using ECL Prime Western Blotting Detection Reagent (Cat. No. RPN2236; GE Healthcare) or SuperSignal Chemiluminescent Substrate (Cat. No. 34095; Thermo Fisher), and signals were imaged and quantitated using a PXi4 CCD camera (Syngene). Laboratory personnel performing Western blot analysis were blinded to the sample identities to improve rigor and reproducibility (6).
Immunohistochemistry and immunofluorescence.
Tyrosine hydroxylase and calcitonin-gene related protein immunohistochemistry was performed similar to previous work (24). Immunofluorescence was conducted on air-dried freshly frozen tissue sections. Sections were exposed to mouse anti-rat CD68 and anti-mouse Alexa fluor 555-labeled antibody was used for secondary detection and mouse anti-rat CD161a-FITC monoclonal antibodies for primary detection. Microscopists and pathologists involved in analyzing immunofluorescence and immunohistochemistry sections were blinded to the treatment groups and were not otherwise involved in conducting the experiment to ensure rigor and reproducibility. Antibody specificity was validated independently on tissues in previous assays (6).
RNA extraction cDNA preparation.
Total RNA from rat kidney tissues was initially isolated with TRIzol reagent (no. 15596026; ThermoFisher) and then further purified with RNA Miniprep kit (no. 400800; Agilent Technologies). RNA quantity and quality were assessed using UV-visual spectrophotometry. Equal amounts of RNA were reverse transcribed to generate cDNA with High Capacity cDNA Reverse Transcription Kit (Cat. No. 4374966; ThermoFisher) according to the manufacturer’s recommendation.
Real-time quantitative PCR.
Real-time quantitative PCR was carried out using Power SYBR Green PCR master mix (Cat. No. 4367659; ThermoFisher) with the following cycling conditions: 1 cycle for 2 min at 50°C and then 10-min at 95°C followed by 40 cycles of 30-s denaturation at 95°C and 1-min annealing at 56–60°C. Dissociation curves were generated for all primer pairs and samples. Results were analyzed by the ΔΔCt method using GAPDH as normalizer and presented as relative mRNA expression compared with control. Primers used were as follows: CD163, forward: TCTGTGAAGTGCCTCCCAAGAATGAC, reverse: TCAGAGTCCACAAGAGGAAGGCAATG; KLF9, forward: GGCCAGACTGCCTTAAAAAGTTCTCG, reverse: TGAATCTCTTCTCACACAGCGGACAG; and arginase II (Arg II), forward:GCTCATGCTGACATTAATACACCCCTCAC and reverse: AGAGGCAAGGTTTGATCCAGGAAAATCC.
Statistical analysis.
Results are presented as arithmetic means ± SE. Comparison between groups were performed with a two-tailed Student’s t-test or two-way ANOVA followed by Tukey test upon rejection of the null hypothesis, using GraphPad Prism7 (La Jolla, CA). Statistical significance is indicated with each figure where P < 0.05 is considered statistical significant. Sample sizes and statistical analysis methods for each experiment are indicated in either the legend or figures (23).
RESULTS
RND prevents the development of cholinergic-mediated hypertension.
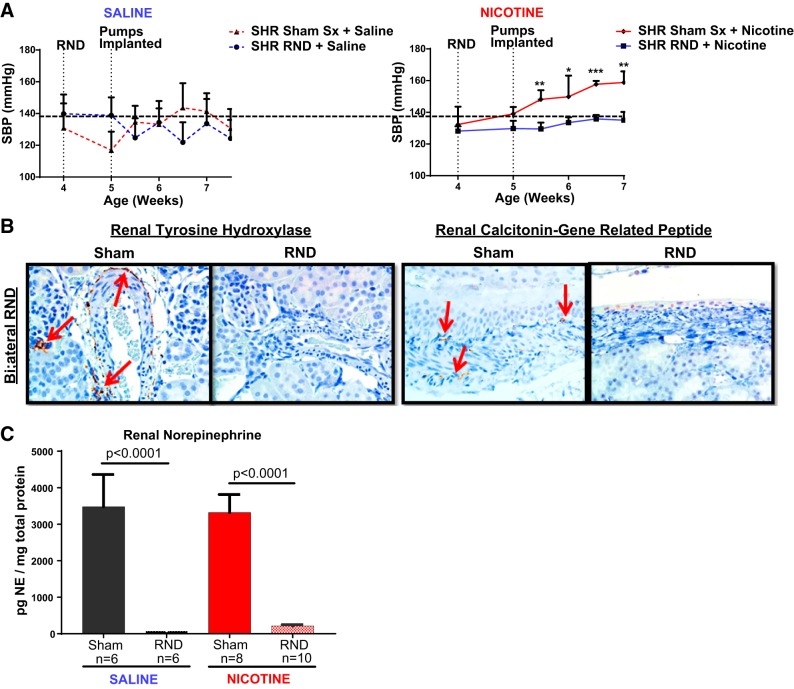
Young prehypertensive SHR underwent either sham surgery or bilateral RND before receiving a 2-wk subcutaneous infusion of either isotonic saline or nicotine. Systolic pressure increased from 132 ± 4 to 159 ± 2 mmHg (P < 0.05) in the sham surgery group in response to nicotine infusion. In contrast, nicotine infusion failed to significantly increase systolic pressure in the group that underwent bilateral RND (128 ± 3 to 135 ± 5 mmHg, P > 0.05; Fig. 1A). Effective RND of both efferent sympathetic nerve and afferent renal nerves was confirmed by the lack of tyrosine hydroxylase and CGRP, respectively, in renal tissues harvested at the end of the infusion period (Fig. 1B) and a >98% decrease in renal norepinephrine in the RND group (Fig. 1C).
Fig. 1.
Bilateral renal nerve denervation (RND) prevents nicotine-induced hypertension in young spontaneously hypertensive rat (SHR). Young 3- to 4-wk-old SHR underwent either sham surgery (Sham Sx; n = 14) or bilateral renal nerve denervation (RND; n = 16), followed by subcutaneous infusion of either saline (n = 12) or nicotine (15 mg·kg−1·day−1) (n = 18) for 2 wk. Renal tissues, obtained at the end of infusion, were sectioned and homogenized. Blood pressure was measured by tail cuff twice weekly. Student’s t-test was conducted between Sham Sx and RND groups for saline and nicotine infusion separately. A: RND prevents nicotine-induced hypertension. SBP, systolic blood pressure. B: renal sections were stained for tyrosine hydroxylase (left, red arrows) and calcitonin gene-related peptide (right, red arrows). C: renal homogenates were analyzed by ELISA for norepinephrine (NE). Error bars represent means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001, RND compared with Sham Sx of same infusion.
RND prevents cholinergic-induced immune cell infiltration in the renal medulla.
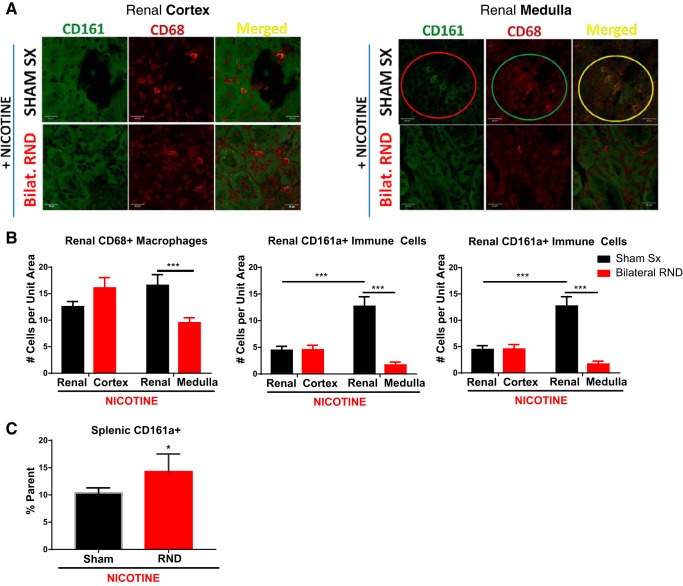
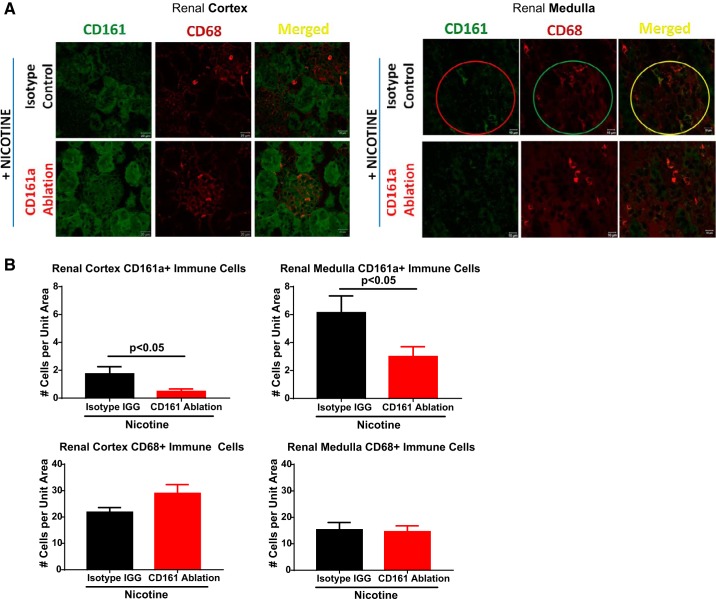
We asked whether bilateral RND may have an effect on the renal infiltration of CD161a+/CD68+ M1 macrophages. When compared with the renal cortex, renal medullary CD161a+ and CD161a+/CD68+ immune cells were 2.8 and 2.2 times, respectively, more prevalent following nicotine infusion in animals with sham surgery (Fig. 2, A and B; P < 0.001). With bilateral RND, there was a 42% decrease in CD68+ macrophages and 87% decrease in CD161a+ immune cells in the renal medulla following nicotine infusion, compared with sham surgery (Fig. 2B; P < 0.001). Consistent with this reduction, there was a 61% decrease in renal medullary CD161a+/CD68+ inflammatory M1 macrophage subset after bilateral RND with nicotine infusion (Fig. 2B; P < 0.001), compared with sham surgery with nicotine infusion. Bilateral RND had no effect on the presence of CD68+, CD161a+, or CD161a+/CD68+ immune cells in the renal cortex (Fig. 2B). In contrast to the renal medulla, there was no decrease in the large prevalent CD161a+ immune cell population in the spleen following bilateral RND (Fig. 2C), demonstrating the localized effects of RND to the kidney.
Fig. 2.
Bilateral renal denervation (RND) prevents renal infiltration of inflammatory CD161a+/CD68+ M1 macrophages. Young 3- to 4-wk-old spontaneously hypertensive rats underwent either sham surgery (Sham Sx; n = 6) or bilateral RND (n = 6), followed by subcutaneous infusion of nicotine (15 mg·kg−1·day−1) for 2 wk. Renal tissues, obtained at the end of infusion, were sectioned and stained with antibodies targeting CD161a (FITC) and CD68 (PE). A: images of the renal cortex (left) and renal medulla (right) were captured using confocal microscopy for CD161a (FITC) and CD68 (Alexa fluor 555). B manual counts from 3 random fields from each animal of CD68+ macrophages (left), CD161a+ immune cells (middle), and CD161a+/CD68+ macrophages (right). C: flow cytometry for CD161a+ immune cells from the spleen, represents peripheral circulation. Two-way ANOVA with Tukey posttest was used to determine significance for renal immune cells between the renal cortex and medulla in the Sham Sx and RND groups. Student’s t-test was used to analyze splenic CD161a+ immune cells between the Sham Sx and RND groups. Error bars represent means ± SE. *P < 0.05 and ***P < 0.001.
Inflammatory cytokines, renal toxicity, and adhesion molecules.
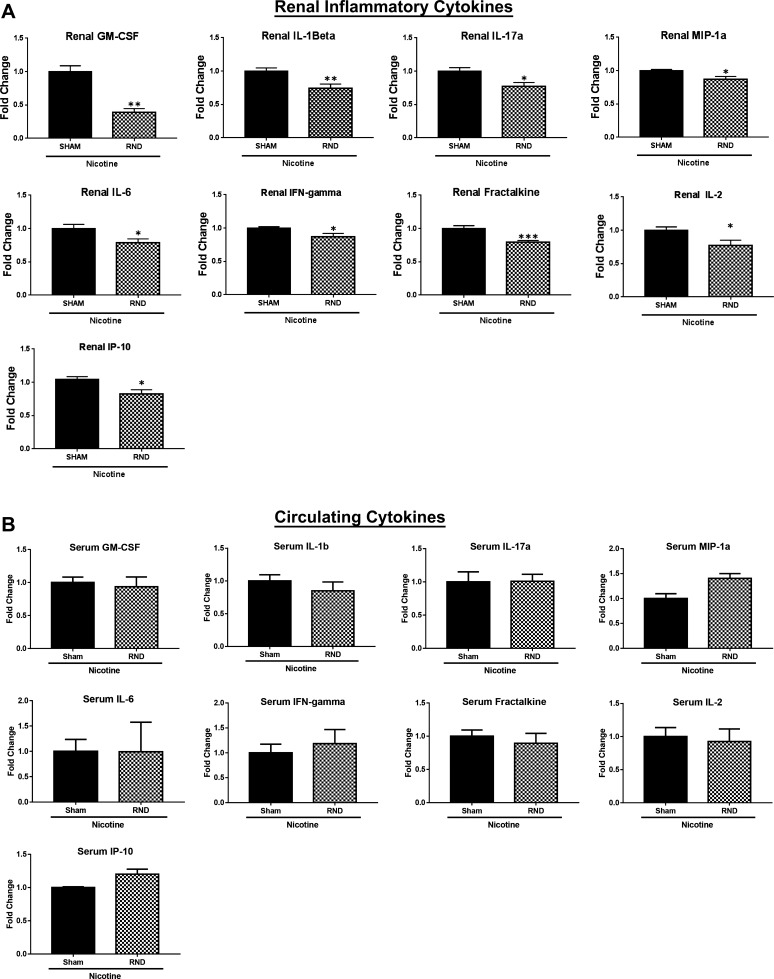
Next, we asked whether bilateral RND had an effect on renal and circulating inflammatory cytokines. In the presence of bilateral RND, there were significant decreases in the levels of renal inflammatory cytokines granulocyte-macrophage colony-stimulating factor (P < 0.01), IL-1β (P < 0.01), IL-17a (P < 0.05), macrophage inflammatory protein-1α (P < 0.05), IL-6 (P < 0.05), fractalkine (P < 0.001), IL-2 (P < 0.05), IFN-γ (P < 0.05), and IP10 (P < 0.05) in response to nicotine infusion, when compared with the sham surgery (Fig. 3A). Although serum levels of IL-17a were reduced following bilateral RND and nicotine infusion, compared with sham surgery, there was no significant change in the serum levels of the remaining inflammatory cytokines (Fig. 3B).
Fig. 3.
Renal nerve denervation (RND) decreases inflammatory renal cytokines. Renal tissue and serum were collected from young 3- to 4-wk-old spontaneously hypertensive rats that underwent sham surgery (Sham Sx; n = 8) or bilateral RND (n = 10), followed by subcutaneous infusion of nicotine (15 mg·kg−1·day−1) for 2 wk. A and B: levels of renal cytokines (A) and serum cytokines (B) were assessed by Luminex. Cytokines levels are reported as fold change above Sham Sx. Student’s t-test was performed to determine significance between RND and Sham Sx groups. GM-CSF, granulocyte-macrophage colony-stimulating factor; MIP-1a, macrophage inflammatory protein-1α. Error bars represent means ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001.
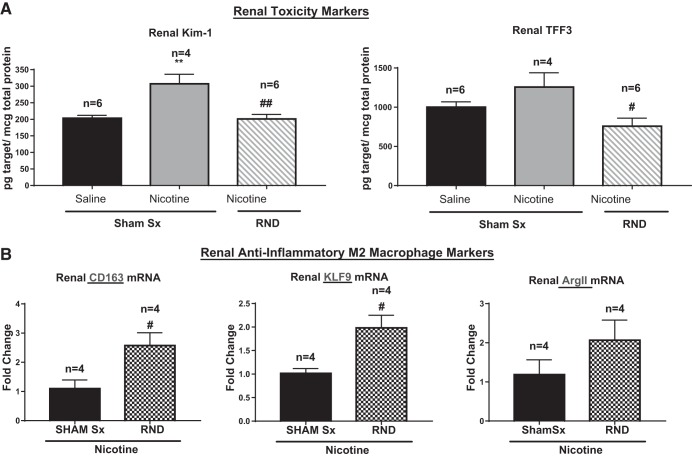
Corresponding with a decrease in inflammatory cytokines within the denervated kidneys, we found a decrease in renal toxicity as marked by a 34% decrease in kidney injury molecule-1 (KIM-1; P < 0.01) and 39% decrease in trefoil factor 3 (TFF3; P < 0.05), compared with sham surgery, in the presence of nicotine infusion (Fig. 4A). Decreases in renal inflammatory cytokines and toxicity markers were correlated with increases in anti-inflammatory M2 markers. Specifically, there was a 95 and 134% increase in CD163 (P < 0.05) and KLF9 (P < 0.05), respectively (Fig. 4B). Similarly, there was an 88% increase in the expression of Arg II (Fig. 4B).
Fig. 4.
Renal nerve denervation (RND) decreases nicotine-induced renal toxicity and activates anti-inflammatory pathways. Renal tissue and serum were collected from young 3- to 4-wk-old spontaneously hypertensive rats that underwent sham surgery (Sham Sx) or bilateral RND, followed by subcutaneous infusion of nicotine (15 mg·kg−1·day−1) for 2 wk. Sample sizes as indicated. A: levels of renal toxicity markers [kidney injury molecule-1 (Kim-1) and trefoil factor 3 (TFF3)]. Levels of toxicity markers were assessed by Luminex assay. B: anti-inflammatory M2-macrophage markers CD163, KLF9, and arginase II (Arg II) were assessed by RT-PCR. Sample sizes: **P < 0.01, compared with Sham Sx treated with saline. #P < 0.05, and ##P < 0.01, compared with Sham Sx treated with nicotine. Groups were analyzed by two-way ANOVA followed by Tukey post hoc test (A) and Student’s t-test between the RND + nicotine and Sham Sx + nicotine groups (B). Error bars represent means ± SD.
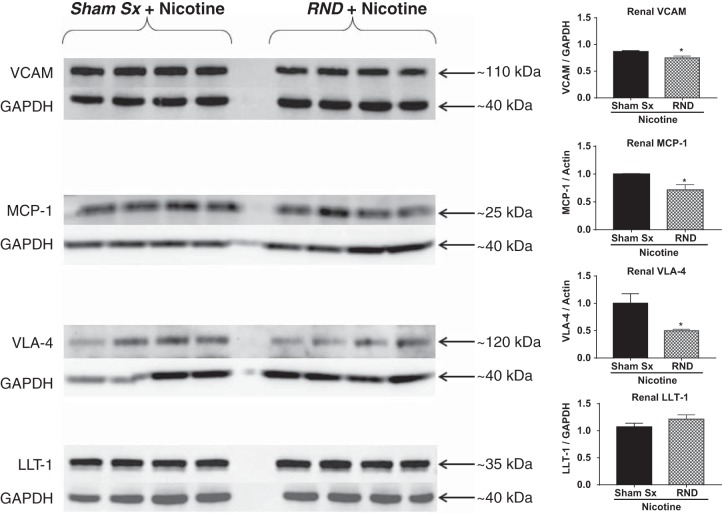
Inflammation is often accompanied by increased expression of adhesion molecules. Previously, we demonstrated that nicotine infusion induced an increase in renal expression of MCP-1 and VLA-4, markers of renal inflammation and damage, in response to nicotine infusion (16). Following bilateral RND, there was a 29 and 50% decrease in the expression of MCP-1 (P < 0.05) and VLA-4 (P < 0.05), respectively, in the context of nicotine infusion, compared with sham surgery (Fig. 5). Following bilateral RND and nicotine infusion, there was a 14% decrease in the renal VCAM-1 (P < 0.05), compared with sham surgery (Fig. 5). However, LLT-1, the innate ligand for CD161a, did not change in the kidney following bilateral RND (Fig. 5).
Fig. 5.
Renal nerve denervation (RND) significant reduces the expression of cellular adhesion/chemotaxis markers. Renal homogenates were prepared from kidneys isolated from young 3- to 4-wk-old spontaneously hypertensive rats (SHR) that underwent sham surgery (Sham Sx, n = 4) or bilateral RND (n = 4), followed by subcutaneous infusion of nicotine (15 mg·kg−1·day−1) for 2 wk. MCP-1, monocyte chemoattractant protein-1; VLA-4, very-late antigen-4; LLT-1, lectin-like transcript-1. Protein levels were determined by Western blot analysis. Student’s t-test was used to determine significance between RND + nicotine and Sham Sx + nicotine groups. Error bars represent means ± SD. *P < 0.05.
Renal sodium retention in cholinergic hypertension.
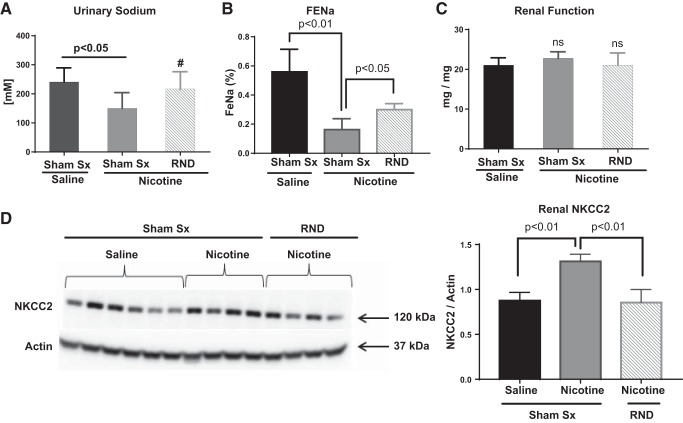
Renal sodium retention contributes to the development of hypertension and can be affected by inflammation and renal sympathetic nerves. There was a 50% decrease in renal sodium excretion, as measured by “spot” urinary sodium, in response to nicotine infusion in animals that underwent sham surgery (Fig. 6A, P < 0.05). This was confirmed by a 71% decrease in fractional excretion of sodium (FENa; Fig. 6B, P < 0.01). Bilateral RND resulted in a 60% increase in FENa, compared with sham surgery with nicotine infusion (Fig. 6B, P < 0.05). No significant alteration in renal function, as measured by protein:creatinine ratio, was observed in response to either nicotine infusion or bilateral RND (Fig. 6C).
Fig. 6.
Renal nerve denervation (RND) decreases cholinergic-induced renal sodium retention and expression of sodium-potassium chloride cotransporter (NKCC2). Young 3- to 4-wk-old spontaneously hypertensive rat underwent either sham surgery (Sham Sx, n = 14) or bilateral RND (n = 16), followed by subcutaneous infusion of either saline (n = 12) or nicotine (15 mg·kg−1·day−1; n = 18) for 2 wk (similar to Fig. 1). Renal tissues and urine were collected at the end of infusion. A–D: spot urine sodium was determined by colorimetric assay (A), fractional excretion of sodium (FeNa) was calculated (B), urine protein to creatinine ratios were calculated as a measure of renal function (C), and renal homogenates (D) were assayed for the presence of sodium-potassium chloride cotransporter (NKCC2) by Western blot analysis. Error bars represent means ± SE. P values are as indicated based on Student’s t-test. #P = 0.16, compared with Sham Sx + nicotine, suggesting trend toward increase in spot urinary sodium.
We next asked whether a change in expression of renal sodium transporters could explain the changes in renal sodium retention. We found a 50% increase in expression of the NKCC2 (Fig. 6D) associated with nicotine infusion in animals that underwent sham surgery, compared with saline infusion. Bilateral RND prevented this nicotine-mediated increase in NKCC2 expression (Fig. 6D).
CD161a+ immune cells are necessary for cholinergic hypertension and involved in renal sodium retention.
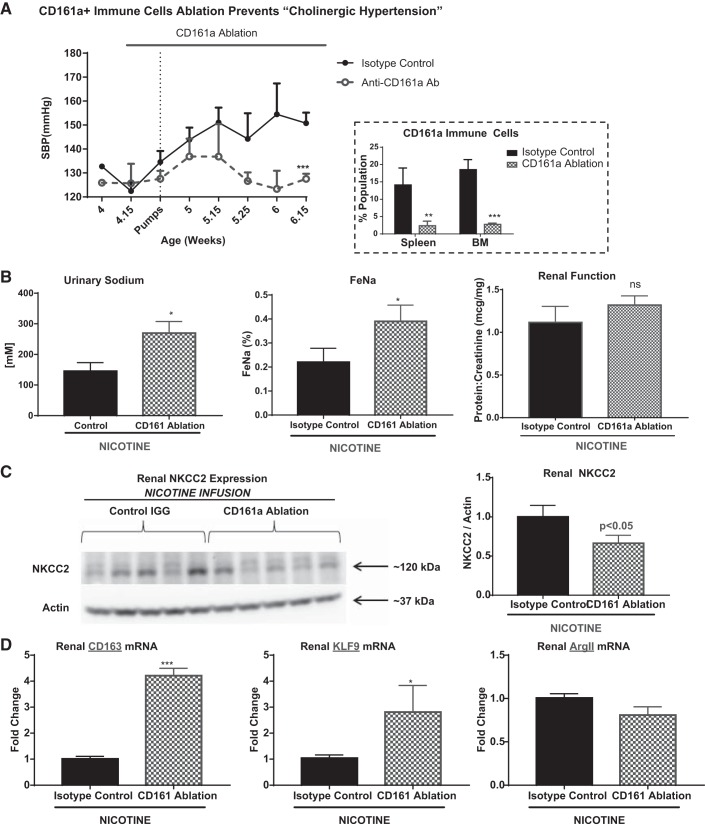
The above studies established a renal nerve-dependent association between the presence of CD161a+/CD68+ M1 macrophages in the renal medulla, renal sodium retention, and increased expression of NKCC2 and the development of cholinergic hypertension. Here we tested the necessity of CD161a+ immune cells for these associations. Confirming our previous results, there was a prevalence of CD161a+ immune cells in the renal medulla (Fig. 7, A and B). Ablation of CD161a+ immune cells resulted in approximately a 62 and 50% decrease in CD161a+ immune cell infiltration into the renal cortex and medulla, respectively, compared with the isotype control (Fig. 7B). It is important to note that the absolute decrease of CD161a+ immune cells was significantly larger in the renal medulla, compared with the renal cortex, based on the predilection of cells to hone into the renal medulla at baseline. Interestingly, there was not a significant change in the CD68+ macrophages within the renal cortex or medulla (Fig. 7B). Similar to bilateral RND, CD161a+ immune cell ablation prevented the development of cholinergic hypertension (123 ± 1.9 to 127.5 ± 2.1 mmHg), compared with the isotype control 132 ± 0.8 to 150.8 ± 4 mmHg; P < 0.05; Fig. 8A). There was a corresponding 83% increase in spot urinary sodium (P < 0.05) and a 50% increase in FENa (P < 0.05; Fig. 8B). Similar to bilateral RND, immune ablation of the CD161a+ immune cells resulted in a 40% decrease in renal expression of NKCC2 (Fig. 8C; P < 0.05) and there was no change in renal function (Fig. 8B). Correlating with a decrease in the inflammatory renal medullary CD161a+/CD68+ M1 macrophages, there was a 311 and 171% increase in the expression of CD163 and KLF9, respectively, markers associated with anti-inflammatory M2 markers.
Fig. 7.
CD161a immune cell ablation prevents renal infiltration of CD161a+ immune cells. Young (3–4 wk) spontaneously hypertensive rat underwent intraperitoneal injection of either isotype control IGG (n = 5)- or anti-CD161a-specific antibodies (n = 5) in conjunction with subcutaneous infusion of nicotine (15 mg·kg−1·day−1) for a 2-wk period. Renal tissues, obtained at the end of infusion, were sectioned and stained antibodies targeting CD161a (FITC) and CD68 (Alexa fluor 555). A: images of the renal cortex (left) and renal medulla (right) were captured using confocal microscopy for CD161a and CD68. B: manual counts from 3 random fields from each animal of CD161a+ immune cells in the renal cortex (left) and renal medulla (right). C: manual counts from 3 random fields from each animal of CD68+ macrophages in the renal cortex (left) and renal medulla (right). Student’s t-test was used to determine significance for renal immune cells between the CD161a Ablation and Isotype IGG groups. P values are as indicated.
Fig. 8.
CD161a+ immune cell ablation prevents cholinergic hypertension, decreases renal sodium retention, and renal expression of sodium-potassium chloride cotransporter (NKCC2). Young 3- to 4-wk-old spontaneously hypertensive rats underwent intraperitoneal injection of either Isotype Control IGG (n = 5) or anti-CD161a specific antibodies (n = 5) in conjunction with subcutaneous infusion of nicotine (15 mg·kg−1·day−1) for a 2-wk period. A: systolic blood pressure (SBP) was measured throughout the infusion period. Urine, serum, and renal tissues were collected at the end of the infusion period with measurements of sodium, creatinine, and protein. BM, bone marrow. B: spot urinary sodium (left), fractional excretion of sodium (FeNa; middle), and renal function (as measured by urinary protein to creatinine ratio; right) were determined by colorimetric assay. C and D: renal expression of NKCC2 was determined by Western blot analysis on renal homogenates (C) and markers of anti-inflammatory M2 markers (D) were determined by RT-PCR on renal tissue. Student’s t-test was conducted to determine significance between the CD161a ablation and isotype IGG-treated groups. Error bars represent means ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001.
DISCUSSION
The main contribution of the current study is that it implicates increased renal sodium absorption as part of the inflammatory response in the development of cholinergic hypertension. We have previously shown that nonneuronal activation of cholinergic receptors with nicotine induces expansion of CD161a+ immune cells and their differentiation into CD161a+/CD68+ M1 inflammatory macrophages. Infiltration of these macrophages into the renal medulla is associated with the premature development of hypertension in response to nicotine infusion, i.e., cholinergic hypertension in the young prehypertensive SHR (15, 16). Results presented in this study demonstrate a propensity toward increased renal sodium reabsorption in response to nicotine infusion, which correlates with increased renal expression of NKCC2. Consistent with previous studies, our results demonstrate an anti-inflammatory effect of bilateral RND that appears to be related to the decreased expression of cell adhesion/chemotactic factors. A decrease in the cholinergic-induced expression of NKCC2 was found in response to RND, suggesting a link between the infiltration of the CD161a+/CD68+ macrophages and renal sodium retention. Immune ablation of CD161a+ immune cells prevented the development of cholingeric hypertension and increase in renal expression of NKCC2, directly implicating the renal infiltration of CD161a+ immune cells in the expression of NKCC2, a key renal sodium transporter in the renal medulla. Taken together, the results of the current study implicate increased renal NKCC2 expression as a possible component of cholinergic hypertension, indicate that CD161a+/CD68+ M1 inflammatory macrophages are necessary and essential for cholinergic hypertension, and indicate that renal nerves are necessary for the migration of these immune cells to the kidney.
Role of the immune system in a genetic model of essential hypertension.
The SHR is a well-accepted genetic model of essential hypertension. In this model, animals develop hypertension as they age, beginning at ~7 wk of age and reach a plateau systolic pressure of approximately 180–200 mmHg at about 15–20 wk of age. SHR mimic many pathophysiological aspects of human essential hypertension, including, but not limited to, the development of left ventricular hypertrophy and increased risk of stroke. Our laboratory identified immunological abnormalities centering on the CD161a+ immune cells and implicated them in the development of hypertension based on increased renal medullary infiltration with advancing age and in response to nicotine infusion (15, 16). Although cholinergic activation of immune cells has been shown to exert anti-inflammatory effects in normotensive animals, exposure of immune cells from the young prehypertensive SHR to nicotine led to the expansion of the CD161a+ immune cell subset and its differentiation into inflammatory CD161a+/CD68+ M1 macrophages. Upon cholinergic activation in vivo, these cells infiltrate the renal medulla, correlating with the premature development of hypertension in the young prehypertensive SHR before 7 wk of age (16) in response to nicotine infusion, i.e., cholinergic hypertension. Of note, renal infiltration of CD161a+/CD68+ M1 macrophages worsens with age natively in the SHR (D. K. Meyerholz and S. C. Harwani, unpublished data), as the CD161a+ population grows. This suggests that native cholinergic activation of these cells may play a role in the pathophysiology of hypertension. As such, our studies focus on the young prehypertensive SHR to better dissect the causative immunological factors involved in the pathogenesis of hypertension in this model.
SHR, NKCC2, and sodium retention.
The development of hypertension in the SHR is complex and appears to encompass interaction between autonomic, immune, vascular, and renal mechanisms. Sodium retention is an important component of renal mechanisms involved in the development of hypertension. Chrysant et al. (7) demonstrated a component of salt sensitivity in the SHR with an additional 20% increase in the mean arterial pressure from 173 to 207 mmHg, 5.5-fold increase in urinary volume, and a 5.6-fold increase in fluid intake over a 24-h period, when adult hypertensive SHR were placed on a 1% sodium diet. Independent observations have complemented these results, showing a sixfold increase in the renal expression of NKCC2 in the renal medulla of the SHR (32, 33). There was an exaggerated increase in fractional excretion of sodium and decreased blood pressure in response to pharmacologic blockade of NKCC2 in SHR, compared with normotensive age-matched WKY controls (32). Increased renal expression of NKCC2 begins in the young prehypertensive SHR and progresses with age as the hypertension develops, correlating with the age-related increase of CD161a+ immune cells (33). Our results confirm previous reports of the involvement of the renal sympathetic nerves in infiltration of the renal medulla with inflammatory macrophages (40) and link the renal infiltration of these cells to mechanisms involved in renal sodium retention. Immune ablation of CD161a+ immune cells prevented the renal infiltration of these cells and the nicotine-mediated increase in renal expression of NKCC2, strengthening a causal link between the renal presence of these immune cells and expression of renal sodium transporters. These data implicate renal medullary infiltration by CD161a+/CD68+ M1 macrophages in the development of hypertension by regulating NKCC2 expression. The mechanism by which these inflammatory CD161a+ macrophages induce the renal expression of NKCC2 may involve the expression of renal inflammatory cytokines, since we show a decrease in renal, but not systemic, levels of inflammatory cytokines in response to RND. Other investigators have demonstrated the ability of IL-17a to mediate expression of the thiazide-sensitive sodium chloride cotransporter (NCC) in an angiotensin II-dependent murine model of hypertension (25). Hence, renal inflammation appears to play a role in the regulation of renal sodium transporters.
Sympathetic-mediated inflammation and renal sodium retention.
Based on the prominent role that renal sympathetic nerves play in hypertension (10), RND was proposed as a potential therapy for “treatment-resistant” essential hypertension (11, 21). Although doubts regarding the clinical efficacy of RND were raised as a result of the Symplicity-HTN 3 Trial, there have been a number of basic science studies, including the current one, and clinical reports that support the role of RND as a potential treatment modality for hypertension (19, 36, 39). Our previous work attempted to investigate the effect of renal nerves in the SHR utilizing unilateral RND and suggested that renal sympathetic nerves may inhibit renal infiltration of CD68+ macrophages (16). These previous experiments involved 24-h nicotine infusion and unilateral denervation (in the presence of an innervated contralateral kidney). In that system, there was an increase in renal CD68+ macrophages in the denervated kidney, compared with the contralateral kidney (16). However, this may be explained by 1) the renorenal reflex due to the presence of the innervated contralateral kidney, 2) incomplete denervation (only 65% reduction in renal norepinephrine was achieved previously), 3) differences in the duration of nicotine infusion (24 h vs. 2 wk), and 4) the fact that the previous results looked at random sections of the corticomedullary junction (as opposed to a specific focus on the renal cortex and medulla). Taken together, the results of the current study support the potential role of RND in the treatment of hypertension based on the prevention of cholinergic hypertension, chemotaxis of inflammatory macrophages into the renal medulla, and decreased renal sodium retention.
The present study confirms the role of renal sympathetic innervation in the renal expression of cellular adhesion molecules such as MCP-1 and fractalkine (17). MCP-1 and fractalkine work together in recruiting activated macrophages to sites of inflammation (13). Thus the renal sympathetic nerve-mediated migration of CD161a+ immune cells to the renal parenchyma appears to involve renal expression of chemotactic factors in the kidney. Although renal sympathetic activity is known to play a role in the increased renal expression of NKCC2 in the SHR before and during the development of hypertension (31), the exact mechanism was not known. The present study demonstrates that renal sympathetic innervation contributes to increased migration of inflammatory CD161a+/CD68+ macrophages to the renal medulla and to the expression of NKCC2 in this genetic model of hypertension. These data provide greater insight into the mechanism behind the attenuation of hypertension following inhibition of MCP-1, which inhibits macrophage migration to the renal parenchyma, in an angiotensin II salt-sensitive model of hypertension (9).
Cholinergic-activated CD161a macrophages and hypertension.
An intriguing finding in our study is the role that CD161a+ inflammatory M1 macrophages play in cholinergic hypertension. CD161a+ is associated with an abnormally prevalent monocyte/macrophage population in the young prehypertensive SHR whose expansion and differentiation is induced by activation of cholinergic receptors (15, 16). Young prehypertensive SHR also demonstrate infiltration of the CD161a+/CD68+ macrophages into the renal medulla before the development of hypertension. Interestingly, a decrease in the renal medullary infiltration by CD161a+/CD68+ macrophages was associated with an increase in markers of anti-inflammatory M2 macrophages. Others have also reported decreased blood pressure in the SHR in the context of increased anti-inflammatory M2 macrophages, suggesting that an imbalance in these two forces is an important factor in the development of hypertension. Based on the renal medullary infiltration and the previous findings regarding overexpression of NKCC2 in SHR, we hypothesized that renal medullary infiltration of CD161a+/CD68+ macrophages played a role in renal expression of NKCC2 in the development of cholinergic hypertension. Immune ablation of CD161a+ immune cells not only prevented the development of cholinergic hypertension but also the nicotine-mediated increase in NKCC2. Others have shown that inflammatory cytokines associated with autoimmune disease, i.e., IL-17a, may enhance expression of renal sodium transporters (25). Interestingly, IL-17a was shown to decrease in the kidney in the absence of the CD161a+ immune cells and this may play a role; however, it is likely that the physical presence of these cells may also play a critical role. Furthermore, studies will be needed to determine the mechanism by which these CD161a+ macrophages enhanced the renal expression of NKCC2.
The “neuro-immune” axis: an integrative physiological model.
Our study clearly demonstrates the manner in which different physiological systems interact with each other in the development of cholinergic hypertension. Nicotine not only directly activates proinflammatory nAChRs on immune cells but also activates central sympathetic efferent signals. Where nAChR activation on immune cells leads to the differentiation and expansion of inflammatory CD161a+/CD68+ macrophages, increased renal sympathetic outflow leads to increased renal expression of cellular adhesion markers, e.g., MCP-1 (Fig. 9). As a result, there is an increase in the renal infiltration of CD161a+/CD68+ inflammatory macrophages, which triggers an increase in the expression of NKCC2. This promotes increased renal sodium retention and the development and maintenance of cholinergic hypertension (Fig. 9). Although it was not the purpose of our study to define the relative contribution of renal afferent versus renal efferent nerves in response to RND, others have demonstrated an important role of the renal afferent nerves in other models of hypertension; however, this has not been the case in the SHR (2, 3, 18, 39). Nonetheless, one can conceive of increased renal inflammation leading to the activation of renal afferent nerves, which may invoke the activation of reflexes such as the reno-renal reflex.
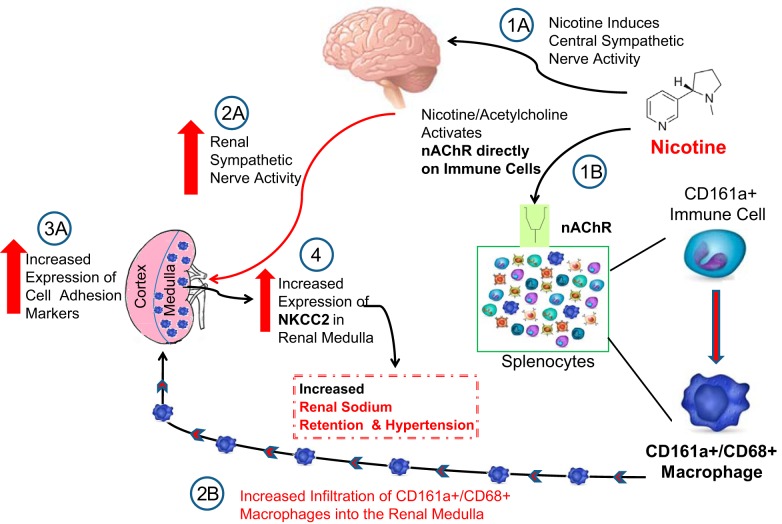
Fig. 9.
Proposed hypothesis of “neuro-immune” axis in cholinergic hypertension and effects of bilateral renal denervation (RND) and CD161a immune cell ablation in young prehypertensive spontaneously hypertensive rat (SHR). A: schematic of the interactions between the cholinergic activation of CD161a+/CD68+ inflammatory macrophages and sympathetic-mediated chemotaxis of these macrophages and their role on renal sodium retention and cholinergic hypertension. 1A: nicotine directly activates efferent central sympathetic pathways. 2A and 3A: this leads to increased renal sympathetic nerve activity (2A) and expression of cellular adhesion molecules (3A) in the renal paranchyma (e.g., monocyte chemoattractant protein-1). 1B: direct activation of nicotinic acetylcholine receptors (nAChRs) on immune cells induces the expansion of the CD161a+ immune cell population and its differentiation into CD161a+/CD68+ inflammatory macrophages that hone into the renal medulla. 2B and 4: infiltration of the renal medulla with CD161a+/CD68+ inflammatory macrophages (2B) induces the renal expression of the sodium-potassium chloride cotransporter 2 (NKCC2; 4) in the thick ascending limb of the loop of Henle. Increased renal expression of NKCC2 in the renal medulla increases renal sodium retention and plays a role in the development of cholinergic hypertension. Ablation of the renal nerve or CD161a+ immune cells in vivo abolishes the development of cholinergic hypertension in young prehypertensive SHR animals.
Significance.
The SHR is a genetic model of essential hypertension that recapitulates many of the pathophysiological characteristics of human essential hypertension. Critical components of this model are the increased sympathetic drive, decreased vascular compliance, immune dysfunction/inflammation, and renal mechanisms (including angiotensin II and NKCC2). Results from this study now begin to uncover the interrelatedness between these components. Specifically, we identify the presence of the abnormally prevalent CD161a+ macrophages as a key component at the intersection of these systems. More importantly, we demonstrate the role activation of the nonneuronal cholinergic receptors in immune cells of the SHR plays in propagating renal inflammation. The renal presence of these immune cells is shown to impact the renal expression of NKCC2 and renal sodium retention. The regulation of renal sodium transporters by immune cells is not well understood, and the exact mechanisms need to be better studied. This places a high level of importance on nicotinic cholinergic receptor regulation of these inflammatory immune cells and the role of nonneuronal cholinergic immune cell regulation in the pathogenesis of hypertension, including regulation of renal sodium transporters.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grant K08-HL-119588. R. A. Fenton was funded by the Fondation Leducq.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.S.R. and S.C.H. conceived and designed research; N.S.R., C.B., F.M.A., and S.C.H. performed experiments; N.S.R., R.A.F., and S.C.H. analyzed data; N.S.R., D.K.M., and S.C.H. interpreted results of experiments; N.S.R., C.B., F.M.A., and S.C.H. prepared figures; N.S.R. and S.C.H. drafted manuscript; N.S.R., P.M.S., R.A.F., F.M.A., and S.C.H. edited and revised manuscript; N.S.R., C.B., P.M.S., R.A.F., D.K.M., F.M.A., and S.C.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mike Cicha and Dr. Chantal Allamargot (Microscopy Core Faciltiy) for assistance in experiments and data gathering. We thank Monica Kopet for administrative assistance. Immunohistochemical and immunofluorescence slides were prepared and stained in the Comparative Pathology Center at University of Iowa. Flow cytometry data presented in this article was obtained at the Flow Cytometry Facility at the University of Iowa, a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility. The facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran's Administration Medical Center.
REFERENCES
- 1.Bai A, Moss A, Kokkotou E, Usheva A, Sun X, Cheifetz A, Zheng Y, Longhi MS, Gao W, Wu Y, Robson SC. CD39 and CD161 modulate Th17 responses in Crohn’s disease. J Immunol 193: 3366–3377, 2014. doi: 10.4049/jimmunol.1400346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banek CT, Gauthier MM, Baumann DC, Van Helden D, Asirvatham-Jeyaraj N, Panoskaltsis-Mortari A, Fink GD, Osborn JW. Targeted afferent renal denervation reduces arterial pressure but not renal inflammation in established DOCA-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol 314: R883–R891, 2018. doi: 10.1152/ajpregu.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B, Ramamurthy N, Zitzmann N, Barnes EJ, Thevanayagam J, Bhagwanani A, Leslie A, Oo YH, Kollnberger S, Bowness P, Drognitz O, Adams DH, Blum HE, Thimme R, Klenerman P. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA 107: 3006–3011, 2010. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brissette-Storkus CS, Kettel JC, Whitham TF, Giezeman-Smits KM, Villa LA, Potter DM, Chambers WH. Flt-3 ligand (FL) drives differentiation of rat bone marrow-derived dendritic cells expressing OX62 and/or CD161 (NKR-P1). J Leukoc Biol 71: 941–949, 2002. [PubMed] [Google Scholar]
- 6.Brooks HL, Lindsey ML. Guidelines for authors and reviewers on antibody use in physiology studies. Am J Physiol Heart Circ Physiol 314: H724–H732, 2018. doi: 10.1152/ajpheart.00512.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrysant SG, Walsh GM, Kem DC, Frohlich ED. Hemodynamic and metabolic evidence of salt sensitivity in spontaneously hypertensive rats. Kidney Int 15: 33–37, 1979. doi: 10.1038/ki.1979.4. [DOI] [PubMed] [Google Scholar]
- 8.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens 11: 197–200, 2002. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in angiotensin II - salt hypertension. Hypertension 50: 1069–1076, 2007. doi: 10.1161/HYPERTENSIONAHA.107.098806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esler M. The sympathetic nervous system through the ages: from Thomas Willis to resistant hypertension. Exp Physiol 96: 611–622, 2011. doi: 10.1113/expphysiol.2010.052332. [DOI] [PubMed] [Google Scholar]
- 11.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators . Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376: 1903–1909, 2010. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 12.Fergusson JR, Fleming VM, Klenerman P. CD161-expressing human T cells. Front Immunol 2: 36, 2011. doi: 10.3389/fimmu.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green SR, Han KH, Chen Y, Almazan F, Charo IF, Miller YI, Quehenberger O. The CC chemokine MCP-1 stimulates surface expression of CX3CR1 and enhances the adhesion of monocytes to fractalkine/CX3CL1 via p38 MAPK. J Immunol 176: 7412–7420, 2006. doi: 10.4049/jimmunol.176.12.7412. [DOI] [PubMed] [Google Scholar]
- 14.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM. Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res 111: 1190–1197, 2012. doi: 10.1161/CIRCRESAHA.112.277475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwani SC, Ratcliff J, Sutterwala FS, Ballas ZK, Meyerholz DK, Chapleau MW, Abboud FM. Nicotine Mediates CD161a+ Renal Macrophage Infiltration and Premature Hypertension in the Spontaneously Hypertensive Rat. Circ Res 119: 1101–1115, 2016. doi: 10.1161/CIRCRESAHA.116.309402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong MN, Li XD, Chen DR, Ruan CC, Xu JZ, Chen J, Wu YJ, Ma Y, Zhu DL, Gao PJ. Renal denervation attenuates aldosterone expression and associated cardiovascular pathophysiology in angiotensin II-induced hypertension. Oncotarget 7: 67828–67840, 2016. doi: 10.18632/oncotarget.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen BJ, van Essen H, Vervoort-Peters LH, Struyker-Boudier HA, Smits JF. Role of afferent renal nerves in spontaneous hypertension in rats. Hypertension 13: 327–333, 1989. doi: 10.1161/01.HYP.13.4.327. [DOI] [PubMed] [Google Scholar]
- 19.Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K, Aoki J, Batson B, Böhm M, Choi JW, Cohen DL, Dangas G, David S, Davies J, Devireddy CM, Kandzari D, Kario K, Lee DP, Lurz PC, Papademetriou V, Patel M, Patel K, Schmieder RE, Sharp ASP, Singh J, Tsioufis K, Walton A, Weber T, Weil J, Zeller T, Ziada K, Tanabe K, Wilkins R, Mahfoud F, East C, Wilensky R, Contreras J, Steigerwalt S, Chapman N, Lea JP, Reedus D, Hoshide S, Ma A, Fengler K, Li P, Svetkey L, Rao A, Schmid A, Watkinson AF, Brown A, Tousoulis D, Hopper I, Suppan M, Agdirlioglu T, Noory E, Chasen C; SPYRAL HTN-ON MED Trial Investigators . Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 391: 2346–2355, 2018. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 20.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373: 1275–1281, 2009. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 21.Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 383: 622–629, 2014. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 22.Kurioka A, Cosgrove C, Simoni Y, van Wilgenburg B, Geremia A, Björkander S, Sverremark-Ekström E, Thurnheer C, Günthard HF, Khanna N, Walker LJ, Arancibia-Cárcamo CV, Newell EW, Willberg CB, Klenerman P; Swiss HIV Cohort Study; Oxford IBD Cohort Investigators . CD161 defines a functionally distinct subset of proinflammatory natural killer cells. Front Immunol 9: 486, 2018. doi: 10.3389/fimmu.2018.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsey ML, Gray GA, Wood SK, Curran-Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol 315: H303–H313, 2018. doi: 10.1152/ajpheart.00309.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyerholz DK, Samuel I. Morphologic characterization of early ligation-induced acute pancreatitis in rats. Am J Surg 194: 652–658, 2007. doi: 10.1016/j.amjsurg.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, Dale BL, Iwakura Y, Hoover RS, McDonough AA, Madhur MS. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin ii-induced hypertension. Hypertension 68: 167–174, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood 121: 2647–2658, 2013. doi: 10.1182/blood-2012-08-443473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pozo D, Valés-Gómez M, Mavaddat N, Williamson SC, Chisholm SE, Reyburn H. CD161 (human NKR-P1A) signaling in NK cells involves the activation of acid sphingomyelinase. J Immunol 176: 2397–2406, 2006. doi: 10.4049/jimmunol.176.4.2397. [DOI] [PubMed] [Google Scholar]
- 28.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA 290: 2945–2951, 2003. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 29.Sesso HD, Jiménez MC, Wang L, Ridker PM, Buring JE, Gaziano JM. Plasma inflammatory markers and the risk of developing hypertension in men. J Am Heart Assoc 4: e001802, 2015. doi: 10.1161/JAHA.115.001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol 34: 685–715, 1958. [PMC free article] [PubMed] [Google Scholar]
- 31.Sonalker PA, Jackson EK. Norepinephrine, via beta-adrenoceptors, regulates bumetanide-sensitive cotransporter type 1 expression in thick ascending limb cells. Hypertension 49: 1351–1357, 2007. doi: 10.1161/HYPERTENSIONAHA.107.088732. [DOI] [PubMed] [Google Scholar]
- 32.Sonalker PA, Tofovic SP, Jackson EK. Increased expression of the sodium transporter BSC-1 in spontaneously hypertensive rats. J Pharmacol Exp Ther 311: 1052–1061, 2004. doi: 10.1124/jpet.104.071209. [DOI] [PubMed] [Google Scholar]
- 33.Sonalker PA, Tofovic SP, Jackson EK. Cellular distribution of the renal bumetanide-sensitive Na-K-2Cl cotransporter BSC-1 in the inner stripe of the outer medulla during the development of hypertension in the spontaneously hypertensive rat. Clin Exp Pharmacol Physiol 34: 1307–1312, 2007. doi: 10.1111/j.1440-1681.2007.04747.x. [DOI] [PubMed] [Google Scholar]
- 34.Steiniger B, Stehling O, Scriba A, Grau V. Monocytes in the rat: phenotype and function during acute allograft rejection. Immunol Rev 184: 38–44, 2001. doi: 10.1034/j.1600-065x.2001.1840104.x. [DOI] [PubMed] [Google Scholar]
- 35.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand [A] 84: 523–528, 1976. doi: 10.1111/j.1699-0463.1976.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 36.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp AS, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Böhm M, Aoki J, Batson B, Böhm M, Choi JW, Cohen DL, Dangas G, David S, Davies J, Devireddy CM, Kandzari D, Kario K, Lee DP, Lurz PC, Patel M, Patel K, Schmieder RE, Sharp AS, Singh J, Tsioufis K, Walton A, Weber T, Weil J, Zeller T, Ziada K, Tanabe K, Wilkins R, Mahfoud F, East C, Wilensky R, Contreras J, Steigerwalt S, Chapman N, Lea JP, Reedus D, Hoshide S, Ma A, Fengler K, Svetkey L, Rao A, Schmid A, Watkinson AF, Brown A, Tousoulis D, Hopper I, Suppan M, Agdirlioglu T, Noory E, Chasen C; SPYRAL HTN-OFF MED trial investigators* . Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 390: 2160–2170, 2017. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 37.Veelken R, Schmieder RE. Renal denervation--implications for chronic kidney disease. Nat Rev Nephrol 10: 305–313, 2014. doi: 10.1038/nrneph.2014.59. [DOI] [PubMed] [Google Scholar]
- 38.Veelken R, Vogel EM, Hilgers K, Amann K, Hartner A, Sass G, Neuhuber W, Tiegs G. Autonomic renal denervation ameliorates experimental glomerulonephritis. J Am Soc Nephrol 19: 1371–1378, 2008. doi: 10.1681/ASN.2007050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winternitz SR, Katholi RE, Oparil S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J Clin Invest 66: 971–978, 1980. doi: 10.1172/JCI109966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J 2nd, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin ii-induced hypertension. Circ Res 117: 547–557, 2015. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaldivia MT, Rivera J, Hering D, Marusic P, Sata Y, Lim B, Eikelis N, Lee R, Lambert GW, Esler MD, Htun NM, Duval J, Hammond L, Eisenhardt SU, Flierl U, Schlaich MP, Peter K. Renal denervation reduces monocyte activation and monocyte-platelet aggregate formation: an anti-inflammatory effect relevant for cardiovascular risk. Hypertension 69: 323–331, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08373. [DOI] [PubMed] [Google Scholar]