Abstract
The adaptive immune response is key for cardiac wound healing post-myocardial infarction (MI) despite low T-cell numbers. We hypothesized that CD8+ T-cells regulate the inflammatory response, leading to decreased survival and cardiac function post-MI. We performed permanent occlusion of the left anterior descending coronary artery on C57BL/6J and CD8atm1mak mice (deficient in functional CD8+ T-cells). CD8atm1mak mice had increased survival at 7 days post-MI compared with that of the wild-type (WT) and improved cardiac physiology at day 7 post-MI. Despite having less mortality, 100% of the CD8atm1mak group died because of cardiac rupture compared with only 33% of the WT. Picrosirius red staining and collagen immunoblotting indicated an acceleration of fibrosis in the infarct area as well as remote area in the CD8atm1mak mice; however, this increase was due to elevated soluble collagen implicating poor scar formation. Plasma and tissue inflammation were exacerbated as indicated by higher levels of Cxcl1, Ccl11, matrix metalloproteinase (MMP)-2, and MMP-9. Immunohistochemistry and flow cytometry indicated that the CD8atm1mak group had augmented numbers of neutrophils and macrophages at post-MI day 3 and increased mast cell markers at post-MI day 7. Cleavage of tyrosine-protein kinase MER was increased in the CD8atm1mak mice, resulting in delayed removal of necrotic tissue. In conclusion, despite having improved cardiac physiology and overall survival, CD8atm1mak mice had increased innate inflammation and poor scar formation, leading to higher incidence of cardiac rupture. Our data suggest that the role of CD8+ T-cells in post-MI recovery may be both beneficial and detrimental to cardiac remodeling and is mediated via a cell-specific mechanism.
NEW & NOTEWORTHY We identified new mechanisms implicating CD8+ T-cells as regulators of the post-myocardial infarction (MI) wound healing process. Mice without functional CD8+ T-cells had improved cardiac physiology and less mortality 7 days post MI compared with wild-type animals. Despite having better overall survival, animals lacking functional CD8+ T-cells had delayed removal of necrotic tissue, leading to poor scar formation and increased cardiac rupture, suggesting that CD8+ T-cells play a dual role in the cardiac remodeling process.
Keywords: inflammation, myocardial infarction, T-cell, wound healing
INTRODUCTION
Cardiovascular disease is the leading cause of death in the United States. Myocardial infarctions (MIs) are a major cause of heart failure, with ~800,000 Americans alone experiencing an MI each year (5). The adaptive immune response is critical during the healing process; however, until the last decade, the contribution of adaptive immunity to post-MI remodeling has been widely disregarded. Recent studies have highlighted the importance of the adaptive immune response and shown that, in patients and mouse models of MI, lymphocytes are recruited to the infarct and remote myocardium (1, 64). Bansal et al. (3) demonstrated that during heart failure, T-cell alterations are spatiotemporally complex, with a robust T-cell expansion of both CD4+ and CD8+ T-cells within the failing myocardium; Th2 and Th17 were found more predominant than Th1 and regulatory T-cells (Tregs). In a mouse model of MI, CD4+ T-cell deletion was found to have greater leukocyte infiltration and a higher level of proinflammatory monocytes (24). Tregs are considered to have a beneficial role post-MI by modulating macrophage differentiation; however, in a mouse model of heart failure, Tregs have a proinflammatory phenotype and lose immunomodulatory capacity (4, 60). To increase the survival rates of patients with MI in the short and long term, our understanding of these processes needs to be significantly expanded.
Although the majority of research studies have focused on the role of CD4+ T-cells in post-MI remodeling, little is known about the CD8+ T-cells in this context. CD8+ T-cells are activated following MI and can recognize and kill normal cardiomyocytes in vitro, suggesting that they may play a role in infarct expansion and development of heart failure (56, 64). In addition, CD8+ T-cells have been linked to neutrophil and macrophage activation in other disease settings such as obesity and cancer (26a, 44). Both neutrophils and macrophages are responsible for clearing debris, regulating the inflammatory response, and remodeling and repairing the tissue (25, 32, 38, 41, 43). A balance between several competing events that occur during remodeling is needed to form a stable infarct scar. A tip in either direction can result in either a stiff, noncompliant scar or cardiac rupture (12, 13, 57). Based on this, we hypothesized that CD8+ T-cells regulate the innate immune response, which leads to decreased survival and impaired cardiac function post-MI.
METHODS
Animal use.
All animal procedures were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina and the Ralph H. Johnson Veterans Affairs Medical Center in accordance with the “Guide for the Care and Use of Laboratory Animals”and followed the Animal Research: Reporting of In Vivo Experiments guidelines (28). We used two strains of adult mice: C57BL/6J [wild-type (WT); 4.2 ± 0.1 mo of age; n ≥3/sex/post-MI day) or CD8atm1mak (4.4 ± 0.2 mo of age; n ≥3/sex/post-MI day). CD8atm1Mak mice have a mutation on the CD8 receptor that blocks its translocation to the cell surface (19). This results in animals that are deficient in functional CD8+ T-cells. CD8atm1Mak mice were chosen as our mouse model because of the fact that this targeted mutation does not affect CD4+ T-cells in healthy, nondiseased animals. WT (stock no. 000664) and CD8atm1Mak mice (stock no. 002665) were purchased from Jackson Laboratories and maintained within our breeding colony. Mice were kept in a light-controlled environment with a 12-h:12-h light-dark cycle and given free access to standard mouse chow and water.
Coronary artery ligation.
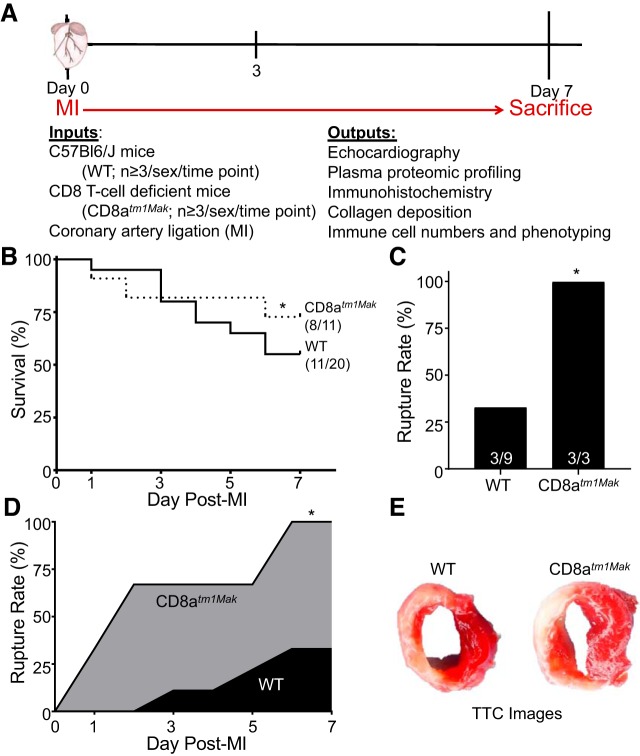
Mice were initially anesthetized with 2% isoflurane followed by the addition of Buprenex (0.1 mg/kg of body wt) before coronary artery ligation. A 1-cm incision was made parallel to the ribs. Blunt dissection of the pectoral muscles revealed the rib cage. Retractors were used to separate the third and fourth rib, exposing the thoracic cavity. The left anterior descending coronary artery was permanently occluded to produce an MI using an 8-0 suture. MI was confirmed by loss of color in the left ventricle (LV). Figure 1A illustrates the overall experimental design.
Fig. 1.
Cd8atm1Mak mice have improved day 7 survival yet increased cardiac rupture compared with wild-type (WT) mice. A: experimental design for all studies focused on post-myocardial infarction (MI) on day 3 and day 7. B: CD8atm1Mak mice had improved overall survival compared with WT animals as assessed by the Kaplan-Meier test. C and D: cause of death was mainly due to cardiac rupture in the CD8atm1Mak group. E: no differences were observed in infarct size as shown by 1% 2,3,5-triphenyltetrazolium chloride (TTC) staining. Rupture rates were analyzed by Fisher’s exact test and multiple group comparisons by one-way ANOVA and Student-Newman-Keuls posttest; *P < 0.05 vs. WT.
Echocardiography and necropsy.
The mice were checked daily for survival. At autopsy, cardiac rupture in nonsurviving mice was confirmed by the presence of coagulated blood in the thoracic cavity or observation of ruptured site on the LV. Left ventricular physiology was determined by echocardiography (Vevo 2100, VisualSonics; Toronto, CA) as recommended by the Guidelines for Measuring Cardiac Physiology in Mice (15, 34, 36). Mice were anesthetized with 1.0–1.5% isoflurane in 100% oxygen. Electrocardiogram, heart rate, and body temperature were monitored during the imaging procedure. Measurements were taken from the LV parasternal long-axis (B-mode) and short-axis (M-mode) views. For each echocardiographic variable, three images from consecutive cardiac cycles were measured and averaged. All images were acquired at heart rates >400 beats/min for physiologically relevant measurements. Strain measurements were performed using the VevoLab software; 10 images from consecutive cardiac cycles were measured and averaged (13).
For euthanasia, mice were anesthetized with 1.5–2.0% isoflurane in an oxygen mix. During tissue collection, heparin (1,000 USP/mL) was injected intraperitoneally, and plasma was collected from the carotid artery. The blood was centrifuged for 5 min to separate out plasma, and proteinase inhibitor (cat. no. 1169749800) was added. The hearts were flushed with cardioplegic solution (69 mM NaCl, 12 mM NaHCO3, 11 mM glucose, 30 mM 2,3-butanedione monoxime, 10 mM EGTA, 0.001 mM Nifedipine, and 50 mM KCl) to arrest the heart in diastole. The hearts were excised, and the LV and right ventricle were separated and weighed individually. The LV was sliced into apex, middle, and base sections, stained with 1% 2,3,5-triphenyltetrazolium chloride (Sigma), and photographed for evaluation of infarct area. Photoshop (Adobe) was used to quantify percentage of infarct area to total LV area. For the apex and base sections, the LV infarct region was separated from noninfarcted remote region, individually snap frozen, and stored at −80°C. Dry and wet lung weights and tibia lengths were recorded.
Plasma proteome profiler.
The Mouse XL Cytokine Array Kit from R&D Systems (ARY028) was used to measure 111 proteins in plasma samples as described by the manufacturer. The Amersham Imager 600 was used for imaging and Image Pro (Media Cybernetics) was used for data analysis.
Protein extraction and analysis.
Protein was extracted from LV tissue by homogenizing the samples sequentially in PBS with 1× protease inhibitor cocktail (16 μL/mg tissue) to remove the soluble protein fraction, followed by protein extraction reagent type 4 (Sigma; 7 M urea, 2 M thiourea, 40 mM Trizma base, and the detergent 1% C7BzO, 15 μL/tissue) with 1× protease inhibitor cocktail for extraction of the insoluble proteins (13, 16). Protein concentrations were determined by the Quick Start Bradford Protein Assay (Bio-Rad). Total protein (10 µg for tissue) was separated on 4%–12% Criterion XT Bis-Tris gels (Bio-Rad), transferred to a nitrocellulose membrane (Bio-Rad), and stained with MemCode Reversible Protein Stain Kit (Thermo Fisher Scientific) to verify protein concentration and loading accuracy. After blocking with 5% nonfat milk (Bio-Rad), the membrane was incubated with primary antibodies against Collagen I (Cedarlane; cat. no. cl50141ap; 1:2,000), fibronectin (Millipore; cat. no. AB1954; 1:5,000), matrix metalloproteinase (MMP)-2 (Cell Signaling; cat. no. D8N9Y; 1:1,000), MMP-9 (Millipore; cat. no. AB19016; 1:1,000), lysyl oxidase (LOX; Novus; cat. no. nb110-41568; 1:1,000), tyrosine-protein kinase MER (MerTK; eBioscience; cat. no. 14-5751-82; 1:500), and tryptase (Abcam; cat. no. ab2378; 1:1,000), followed by secondary anti-rabbit antibody (Abcam; cat. no. ab205718, 1:10,000), anti-rat antibody (Novus; cat. no. NB7115; 1:1,000), or anti-mouse antibody (Promega; cat. no. W402B, 1:5,000) and detected with ECL Prime or Femto Western Blotting Detection Substrate (Amersham) (12, 13, 16, 57). Recombinant proteins for MMP-9 (Millipore; cat. no. PF140) were used as positive controls. The relative expression for each immunoblot was calculated as the densitometry of the protein of interest divided by the densitometry of the entire lane of the total protein-stained membrane. The Amersham Imager 600 was used for imaging, and Image Pro (Media Cybernetics) or Image J was used for data analysis.
ELISA.
ELISAs (Quantikine ELISA, R&D) for IFNγ (cat. no. MIF00), MMP-2 (cat. no. MMP200), Cxcl1 (cat. no. MKC00B), and Ccl11 (cat. no. MME00) were performed using the soluble protein fraction from the infarct tissue as described by the manufacturer. Cardiac troponin I ELISA (DRG Store, cat. no. EIA2952R) was performed using plasma samples as described by the manufacturer.
Immunohistochemistry.
Immunohistochemistry for macrophages (Cedarlane; cat. no. CL8943AP, 1:100), neutrophils (Cedarlane; cat. no. CL8993AP, 1:100), and cKit (Invitrogen; cat. no. 44496G; 1:50) were performed on LV sections (13, 38). The LV middle section was fixed in 10% zinc formalin (Fisher Scientific), paraffin embedded, and sectioned for histological examination as described previously (13). Heat-mediated antigen retrieval (Target Retrieval Solution, Dako) was performed to expose antigen epitopes. Slides were then rinsed, blocking serum was applied (Rabbit Serum; Vectastain), and slides were incubated for 30 min. Afterwards, primary antibody was applied, and the slides were incubated overnight. The following day, slides were incubated with the secondary antibody (Vector Laboratories cat. no. PK-6104 or Abcam cat. no. ab205718) for 30 min. Slides were stained with the HistoMark BLACK Peroxidase Substrate (cat. no. KPL 54-75-00) followed by a counterstain with eosin Y (Sigma; cat. no. H110232).
Picrosirius red (PSR) was used to stain for collagen. Slides were incubated in a solution of 0.2% phosphomolybdic acid (Electron Microscopy Sciences; cat. no. RT 26357-01) for 3 min. The slides were then rinsed and transferred to the solution containing 0.1% Sirius Red in saturated picric acid (Electron Microscopy Sciences; cat. no. RT 26357-02) for 90 min. Slides were placed immediately into acidified water for 2 min. All images were acquired at ×40 and analyzed using Image Pro (Media Cybernetics). Image insets were taken at ×60.
Flow cytometry.
Cells were isolated from the remote and infarct area of post-MI day 3 hearts as previously described (12, 13, 16, 41). The LV tissue was dissociated into single-cell suspension using 600 U/mL collagenase type II (Worthington Biochemicals, cat. no. CLS-2) and 60 U/mL DNase1 (AppliChem, cat. no. A3778.0500). Cells were washed and resuspended in cold PBS supplemented with 0.5% bovine serum albumin (BSA) and 2 mM EDTA. Primary antibodies for T-cell panel (PE anti-CD3, 1:30; APC-Vio770 anti-CD8, 1:50; VioBlue anti-CD4, 1:50; APC anti-IFNγ, 1:50; PE-Cy7 anti-IL4, 1:50; or mix) or neutrophil/macrophage panel (Alexa Fluor 488 anti-F4/80, 1:50; PE anti-CD206, 1:40; APC anti-Ly6G, 1:25; PE-Cy5.5 anti-Ly6C, 1:25; or mix) were added, and cells were incubated for 20 min at 4°C in the dark. All cells were stained with Live/Dead Fix Yellow (Thermo Fisher Scientific, cat. no. L34959). Cells were permeabilized with 3% Tween for intracellular staining. Cells were washed in phosphate-buffered solution with 0.5% BSA and 2 mM EDTA and centrifuged at 300 g for 8 min. Cells underwent filtration using a 5-mL tube with cell-strainer cap and analyzed on the MACS Quant Analyzer (Miltenyi Biotec) and FlowLogic software (Inivai Technologies). Analysis of inflammatory profile was gated for removal of cellular debris and dead cells.
Immunofluorescence.
Heat-mediated antigen retrieval (Target Retrieval Solution, Dako) was performed to expose antigen epitopes. Slides were then rinsed, blocking serum was applied (Rabbit Serum; Vectastain), and slides were incubated for 30 min. Fluorescent-tagged primary antibody for myofibroblast marker, α-smooth muscle actin (AlexaFluor 488-αSMA; eBioscience cat. no. 53-9760-82, 1:100) was added and incubated overnight. TUNEL staining was performed as previously described using the In Situ Cell Death Detection Kit (Sigma-Aldrich, cat. no. 11684809910) (16). To quantify staining, 10 random images from each slide were captured at ×40 magnification. Quantification was performed by Image Pro (Media Cybernetics).
Statistics.
All statistical analyses were performed by investigators blinded to groups. Data are presented as means ± SE. For two-group comparisons, the nonparametric Wilcoxon rank sum test was used. Multiple group comparisons were analyzed using one-way ANOVA, followed by the Student-Newman-Keuls test when the Bartlett’s variation test was passed, or the nonparametric Kruskal-Wallis test, followed by Dunn post hoc test when the Bartlett’s variation test did not pass. The survival rate was analyzed by Kaplan-Meier survival analysis and compared by the log-rank test. Rupture rates were analyzed by Fisher’s exact test. Statistical significance was set at P < 0.05. Volcano plots were constructed using a statistical program available in the Metaboanalyst 3.0 package (www.metaboanalyst.ca/) (58, 62). Pathway analysis of identified proteins was performed using Ingenuity Pathway Analysis (QIAGEN Redwood City; www.qiagen.com/ingenuity).
RESULTS
CD8-deficient animals have improved overall survival and cardiac function despite increased cardiac rupture.
CD8-deficient mice displayed increased 7-day survival at 73% compared with that of WT mice at 55% (Fig. 1B). Consistent with previous reports, cardiac rupture in WT mice accounted for less than half of the deaths (rupture/total deaths: 3/9; Fig. 1, C and D) (13), whereas all mortality (3/3) in the CD8atm1Mak mice was due to cardiac rupture indicative of excessive remodeling. This increase was not due to differences in infarct size (Fig. 1E; Table 1).
Table 1.
CD8-deficient animals have improved cardiac function at day 7 post-MI; cardiac physiology of CD8atm1Mak and WT post-MI measured by echocardiography
| WT |
CD8atm1Mak |
|||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 7 | Day 0 | Day 3 | Day 7 | |
| N | 8 | 6 | 8 | 8 | 8 | 8 |
| Heart rate, beats/min | 471 ± 4 | 475 ± 9 | 503 ± 5 | 445 ± 5 | 509 ± 4 | 454 ± 5 |
| SWTs, mm | 1.27 ± 0.02 | 0.65 ± 0.02 | 0.56 ± 0.03 | 1.17 ± 0.02 | 0.70 ± 0.02 | 0.50 ± 0.04 |
| EDD, mm | 3.64 ± 0.04 | 4.42 ± 0.07* | 5.85 ± 0.10*† | 3.47 ± 0.04 | 4.49 ± 0.06* | 4.99 ± 0.11*‡ |
| ESD, mm | 2.28 ± 0.04 | 4.00 ± 0.08* | 5.59 ± 0.10*† | 2.10 ± 0.04 | 3.97 ± 0.06* | 4.46 ± 0.13*‡ |
| FS, % | 38 ± 1 | 10 ± 1* | 5 ± 1* | 39 ± 1 | 11 ± 1* | 11 ± 1* |
| EDV, μL | 58 ± 1 | 96 ± 4* | 166 ± 6*† | 57 ± 1 | 86 ± 1* | 120 ± 6*‡ |
| ESV, μL | 20 ± 1 | 72 ± 4* | 151 ± 6*† | 19 ± 1 | 65 ± 1* | 101 ± 6*‡ |
| EF (%) | 65 ± 1 | 27 ± 1* | 10 ± 1* | 67 ± 1 | 17 ± 1* | 17 ± 1*‡ |
| Infarct area (%) | NA | 57 ± 1* | 59 ± 1* | NA | 58 ± 1* | 54 ± 1* |
| GLS (%) | −14.8 ± 0.1 | −2.8 ± 0.2* | −3.0 ± 0.3* | −14.5 ± 0.4 | −5.3 ± 0.3*† | −3.8 ± 0.4* |
Values are means ± SE; multiple group comparisons were analyzed by one-way ANOVA with Student-Newman-Keuls post-test. CD8atm1Mak, CD8-deficient animals; EDD, end-diastolic diameter; EDV, end-diastolic volume; EF, ejection fraction; ESD, end-systolic diameter; ESV, end systolic volume; FS, fractional shortening; GLS, global longitudinal strain; MI, myocardial infarction; SWTs, septal wall thickness in systole.; WT, wild-type.
P < 0.05 vs. respective day 0;
P < 0.05 vs. WT day 3;
P < 0.05 vs. WT day 7.
No differences were observed in fractional shortening or ejection fraction between groups at day 3 post-MI (Table 1). Left ventricular strain analysis showed that at day 3, CD8-deficient animals had improved global longitudinal strain (GLS) compared with WT animals, indicating that CD8+ T-cells are affecting longitudinal shortening of the myocardium after MI. GLS has been shown to be a prognostic indicator of all-cause mortality in patients with heart failure, mirroring the improved day 7 survival in the CD8atm1Mak group (45, 49). At day 7 post-MI, CD8-deficient mice had improved ejection fraction and attenuated dilation compared with the WT group. By day 7, GLS was no longer different between groups. Our data suggest that CD8+ T-cells are involved in facilitating LV remodeling and post-MI cardiac physiology.
CD8-deficient animals have impaired scar formation.
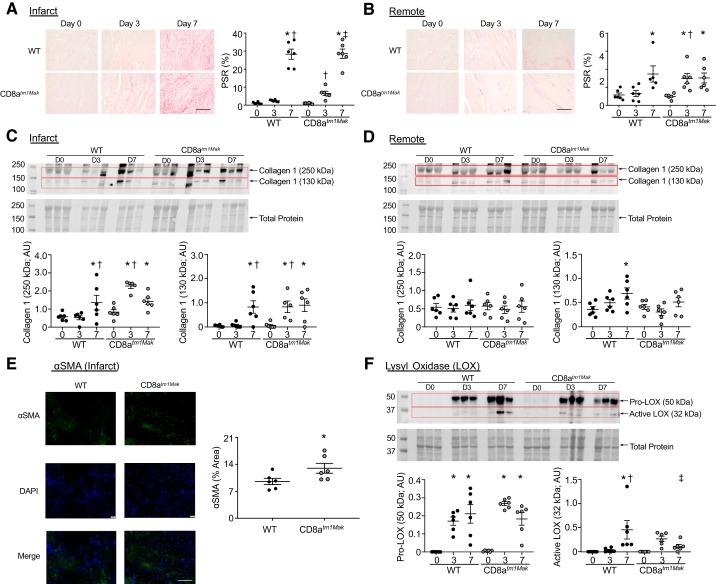
To assess if alteration in cardiac fibrosis in the CD8+ T-cell-deficient animals was linked to the improved cardiac physiology, PSR staining was performed (Fig. 2, A and B). Both WT and CD8atm1Mak animals had an increase in fibrosis of the infarct area reaching around 20% at day 7 post-MI. Immunoblotting of the infarct tissue showed an increase in collagen 1α1, both the cross-linked (250 kDa) form and pro-form (130 kDa), in the WT animals at post-MI day 7. Interestingly, CD8-deficient animals had amplified fibrosis in the infarct at post-MI day 3 compared with WT animals, as shown by PSR (6 ± 1%) and by collagen 1α1 immunoblotting (Fig. 2C). Fibronectin increased post-MI compared with day 0 in both WT and CD8atm1Mak mice (P < 0.0001 for all). Within the infarct, no differences between groups were observed (WT vs. CD8atm1Mak day 3, P = 0.707; WT vs. CD8atm1Mak day 7, P = 0.449), indicating no differences between groups in the provisional fibrin-fibronectin matrix.
Fig. 2.
CD8atm1Mak mice have increased collagen deposition within the infarct and remote regions at post-myocardial infarction (MI) day 3 compared with wild-type (WT) mice. Picrosirius red (PSR) staining shows increased fibrosis in the infarct (A) and remote regions (B) of CD8atm1Mak mice compared with WT mice at day 3 post-MI (scale bar: 100 μm). No significant difference was observed between groups at day 7. Collagen 1α1 immunoblotting indicated an increase in insoluble collagen in the infarct at day 3 post-MI in the CD8atm1Mak mice compared with WT (C). Assessment of the remote tissue showed increased collagen 1α1 at day 7 in the WT mice only compared with day 0 animals (D). Myofibroblast marker, α-smooth muscle actin (αSMA) was elevated in CD8atm1Mak mice at post-MI day 3 compared with WT mice (scale bar, 100 μm) (E). The proform of collagen cross-linking enzyme, lysyl oxidase (LOX), was increased in WT and CD8atm1Mak mice at post-MI day 3 and day 7 (F). The active form of LOX was only increased in WT mice at post-MI day 7 compared with WT at day 0 and CD8atm1Mak at day 7. n = 5–6/day/group; multiple group comparisons were analyzed by one-way ANOVA and Student-Newman-Keuls posttest. For two group comparisons, the nonparametric Wilcoxon rank sum test was used. *P < 0.05 vs. respective baseline (D0); †P < 0.05 vs. WT D3; ‡P < 0.05 vs. CD8atm1Mak D3. AU, arbitrary units; D, day.
Assessment of the remote area also indicated an increase of fibrosis in the CD8+ T-cell-deficient mice at day 3 post-MI compared with WT animals. In the remote area, no differences were found in the cross-linked form of collagen 1 (250 kDa); however, WT animals had a significant elevation of the proform (130 kDa) at post-MI day 7 compared with day 0, and this increase was not observed in the CD8atm1Mak mice (Fig. 2D), indicating that the increase in PSR staining in the CD8atm1Mak mice was not due to changes in collagen 1. Myofibroblast activation was increased in the day 3 infarct of the CD8atm1Mak mice compared with WT mice, consistent with the acceleration of collagen deposition (Fig. 2E).
Collagen cross-linking enzyme LOX has been shown to facilitate formation of the collagen scar after MI (20). LOX is secreted as a proenzyme (pro-LOX) that is proteolytically processed by bone morphogenetic protein-1 and other metalloproteases to release the mature, active LOX (9). Pro-LOX was increased in the infarct of both WT and CD8atm1Mak mice at post-MI day 3 and day 7 (Fig. 2F). Active LOX was elevated in the WT group at post-MI day 7 compared with WT at day 0 and CD8atm1Mak mice at post-MI day 7. Our data suggest that CD8+ T-cells may therefore be affecting scar formation by dysregulating the balance between ECM cleavage and formation.
CD8-deficient animals have an exacerbated post-MI inflammatory response.
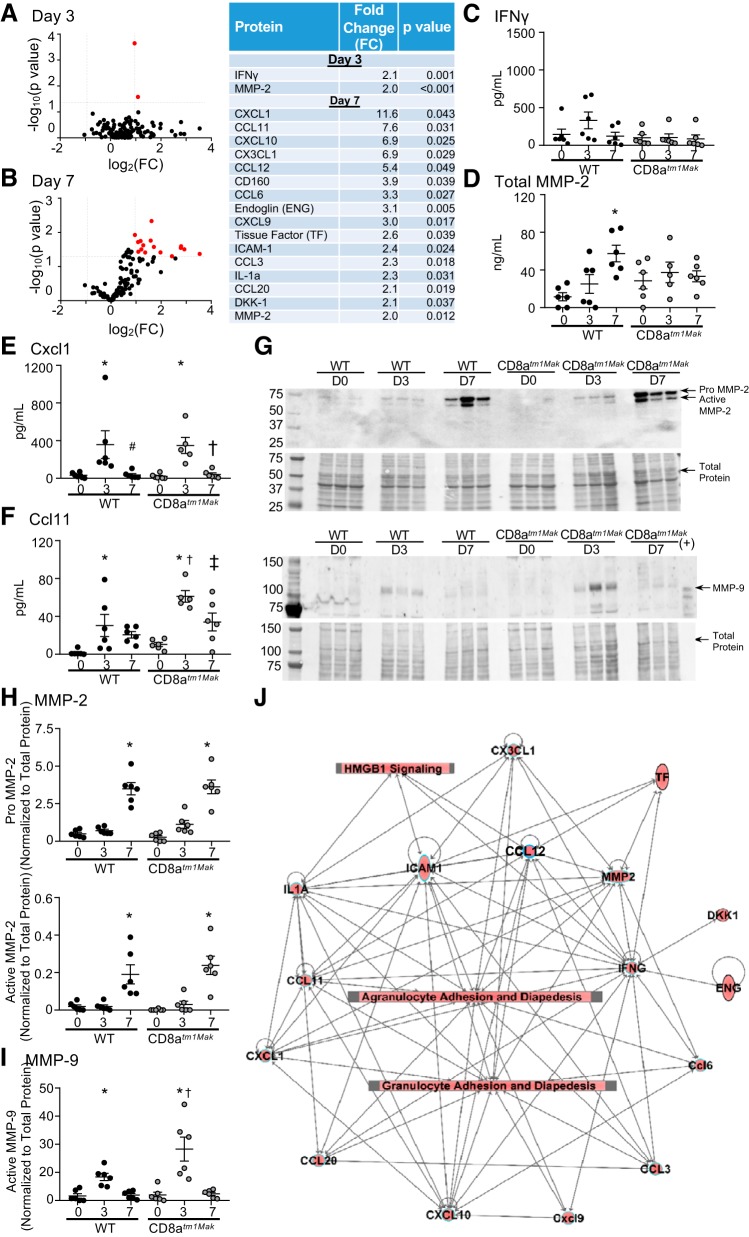
Proteomic plasma profiling of day 3 and day 7 post-MI was used to identify possible mechanisms of CD8+ T-cell effects. Out of the 111 proteins tested, IFNγ and MMP-2 were significantly elevated more than 2-fold at day 3 post-MI in the CD8-deficient mice compared with WT mice (Fig. 3, A and B). At post-MI day 7, 16 proteins (Cxcl1, Ccl11, Cxcl10, Cx3cl1, Ccl12, CD160, Ccl6, CD105, Cxcl9, tissue factor, ICAM-1, Ccl3, IL1α, Ccl20, DKK-1, and MMP-2) were upregulated more than 2-fold in the CD8+ T-cell-deficient animals compared with WT.
Fig. 3.
CD8+ T-cell-deficient animals demonstrate elevated inflammation as shown by plasma proteomic analysis. Volcano plots revealed that CD8atm1Mak mice have increased circulating inflammatory proteins at both post-myocardial infarction (MI) day 3 (A) and day 7 (B) compared with wild-type (WT). Out of 111 proteins tested, Cxcl1, Ccl11, Cxcl10, Cx3cl1, Ccl12, CD160, Ccl6, CD105, Cxcl9, tissue factor, IFNγ, ICAM-1, Ccl3, IL1α, Ccl20, DKK-1, and matrix metalloproteinase (MMP)-2 were found to be more than 2-fold upregulated and P < 0.05 compared with WT mice. ELISA of infarct levels of IFNγ (C) showed no significant differences between groups. Total MMP-2 was elevated in the infarct at post-MI day 7 in the WT but not CD8atm1Mak mice (D). Cxcl1 (E) and Ccl11 (F) peaked at day 3 in both WT and CD8atm1Mak mice. Ccl11 was exacerbated at both day 3 and day 7 compared with WT. Both pro- and active MMP-2 levels increased at day 7 post-MI as confirmed by MMP-2 immunoblot (G and H). MMP-9 level peaked at day 3 in the infarct tissue and was elevated in the CD8atm1Mak animals (G and I). Canonical pathway analysis (J) indicated proteins found to be significantly different between groups to formulate a network that increased high-mobility group box 1 (HMGB1) activation as well as granulocyte and agranulocyte adhesion and diapedesis. Multiple group comparisons were analyzed by one-way ANOVA with Student-Newman-Keuls posttest. For network analysis, multiple-testing corrected P values were calculated using the Benjamini-Hochberg method in Ingenuity Pathway Analysis. The threshold line corresponds to a P value of 0.05. *P < 0.05 vs. respective baseline (D0); †P < 0.05 vs. WT D3; ‡P < 0.05 vs. CD8atm1Mak D3. D, day.
To test whether plasma changes reflected the inflammatory state within the LV, protein assessment of the top two inflammatory mediators at day 3 (IFNγ and MMP-2; Fig. 3, C and D) and day 7 (Cxcl1 and Ccl11; Fig. 3, E and F) were assessed by ELISA. Time course measurements showed no change in IFNγ in the infarct tissue over the post-MI time course. Total MMP-2 levels measured by ELISA were elevated at post-MI day 7 in WT compared with day 0, similar to what was observed by immunoblotting (Fig. 3, G and H). No differences were observed in the CD8atm1Mak mice by ELISA; however, immunoblotting data showed that pro- and active MMP-2 were increased in the infarct at day 7 in both WT and CD8atm1Mak groups compared with unoperated controls (day 0). Infarct levels of Cxcl1 and Ccl11 were increased at post-MI day 3 and had returned to baseline by post-MI day 7. This increase at post-MI day 3 in Ccl11 was intensified in CD8atm1Mak mice.
In addition to MMP-2, MMP-9 is a proteolytic enzyme known to regulate the cardiac wound healing process through cleavage of cytokines such as Cxcl1 and Ccl11 as a mechanism to decrease chemotactic abilities (8, 55). MMP-9 was found to increase in the infarct at post-MI day 3 and return to baseline by day 7 as previously shown (Fig. 3, G and I) (35). This increase was exaggerated in the CD8+ T-cell-deficient animals. Pathway analysis (Fig. 3J) indicated that changes observed in plasma and tissue were linked to high-mobility group box 1 (HMGB1) activation and granulocyte and agranulocyte adhesion and diapedesis, indicative of an increase in immune cell recruitment in CD8+ T-cell-deficient animals. Elevated serum HMGB1 levels have been found to be associated with pump failure, cardiac rupture, and in-hospital cardiac death consistent with the increased cardiac rupture observed in our CD8atm1Mak mice (29).
CD8-deficient animals have accelerated recruitment of innate immune cells within the infarct and remote tissue.
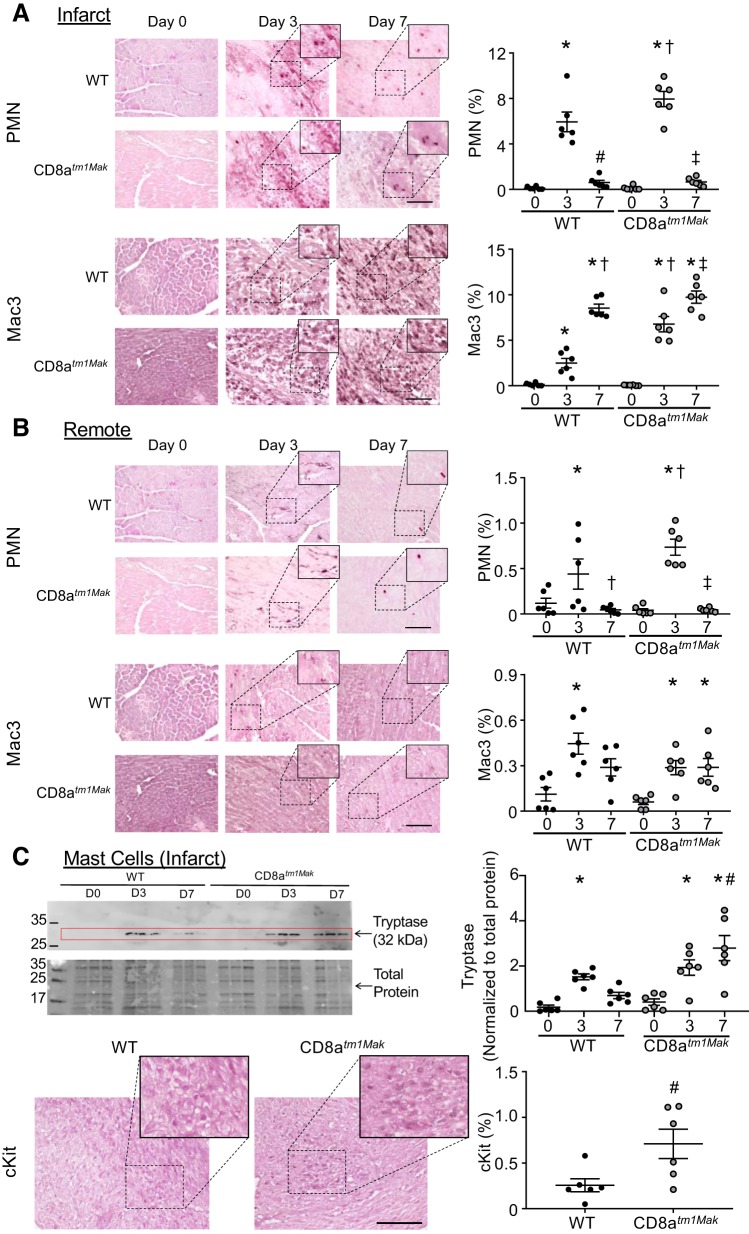
Immunohistochemistry displayed an increase in neutrophils within the infarct of WT animals at day 3 post-MI compared with day 0 (unoperated) hearts (Fig. 4A). At day 7, neutrophil numbers returned to baseline day 0 levels. CD8atm1Mak animals had more neutrophil numbers compared with WT mice at post-MI day 3. No differences were observed at day 7 between groups. Assessment of the remote area showed a similar trend; neutrophil numbers were increased at day 3 post-MI and were exacerbated in the CD8-deficient animals compared with WT (Fig. 4B).
Fig. 4.
CD8atm1Mak have increased neutrophils (PMN) and macrophages within the infarct and remote regions at post-myocardial infarction (MI) day 3 compared with wild-type (WT). A: neutrophil (Ly6b.2) and macrophage (Mac3) staining with eosin counter stain shows increased inflammatory cells at post-MI day 3 in the infarct of CD8atm1Mak mice compared with WT (Scale bar: 100 μm). No significant difference was observed between groups at day 7. B: within the remote region, neutrophils but not macrophages were exaggerated at post-MI day 3 in the CD8atm1Mak mice compared with WT. No differences were observed in neutrophil numbers at post-MI day 7. By day 7, macrophages returned to baseline levels in the WT animals, whereas CD8atm1Mak still had elevated macrophages. C: mast cell markers tryptase and cKit were analyzed by immunoblotting and immunohistochemistry and showed that CD8atm1Mak mice had elevated mast cell markers at post-MI day 7 compared with WT mice. n = 6/day/group; larger images are ×40 with insets at ×60. Multiple group comparisons were analyzed by one-way ANOVA with Student-Newman-Keuls posttest. For two group comparisons, the nonparametric Wilcoxon rank sum test was used. *P < 0.05 vs. respective baseline (D0); †P < 0.05 vs. WT D3; ‡P < 0.05 vs. CD8atm1Mak D3; #P < 0.05 vs. WT D7. D, day.
Assessment of macrophages within the infarct (Fig. 4A) showed an increase in macrophage numbers that peaked at post-MI day 7, consistent with previous reports (13, 15, 32, 43, 53). Compared with WT animals, CD8+ T-cell-deficient animals had increased macrophage numbers in the infarct at post-MI day 3. No significant difference was found between groups in macrophage numbers at day 7. Interestingly, the remote area had increased macrophages at post-MI day 3 in WT and CD8-deficient animals compared with day 0 animals (Fig. 4B). Macrophages remained elevated in the remote areas of the CD8atm1Mak group but not the WT group at post-MI day 7. Mast cell marker tryptase increased in WT and CD8atm1Mak mice at post-MI day 3 compared with day 0 animals (Fig. 4C). In CD8atm1Mak mice, tryptase and cKit levels were exacerbated at post-MI day 7, suggesting prolonged mast cell-mediated inflammation in the absence of CD8+ T-cells. Based on proteomic and immunohistochemistry data, day 3 post-MI seems to be a key point for CD8+ T-cells in regulation of the inflammatory response within the LV tissue.
CD8-deficient animals have increased neutrophils and Th1 cells compared with WT animals.
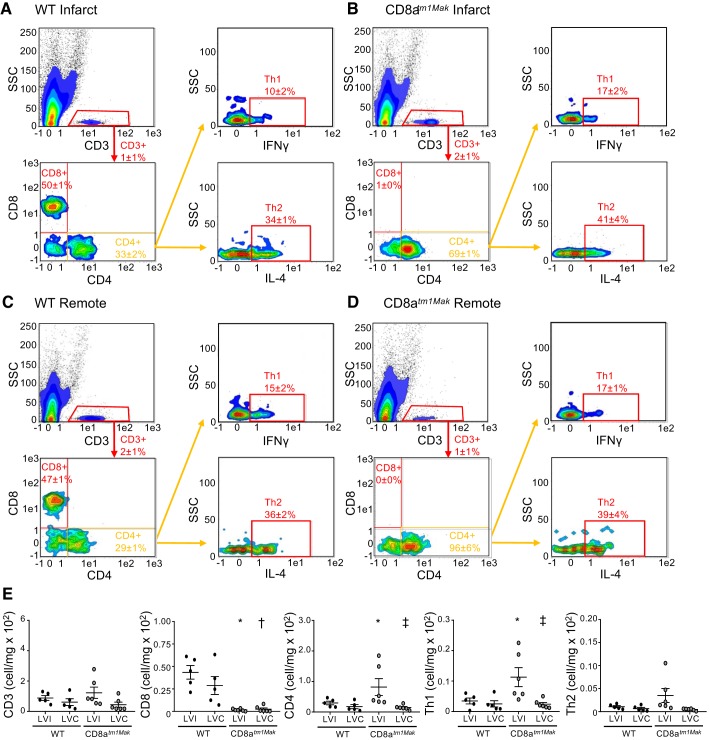
To further classify the effect of CD8+ T-cells on the post-MI immune response, flow cytometry was performed on cells isolated from the infarct (LV infarct) and remote (LV remote) area at day 3. Flow cytometry analysis of infarct and remote tissue showed no significant differences in T-cells at day 3 post-MI (Fig. 5). Interestingly, the majority of T-cells (CD3+) within the infarct of WT animals were CD8+ T-cells (48 ± 2%). As expected, CD8atm1Mak mice had no CD8+ T-cells within the infarct or remote areas. Before ischemic injury, CD8atm1Mak mice have no differences in CD4+ T-cells (19); however, after MI, CD8+ T-cell-deficient animals had more CD4+ T-cells within the infarct but not remote area. This increase in CD4+ T-cells corresponded to an increase in Th1 cells. No differences in Th2 cells were observed between groups in both infarct and remote tissues.
Fig. 5.
CD8atm1Mak mice have elevated Th1 cells within the infarct area compared with wild-type (WT) mice. Flow cytometry analysis of WT infarct (A), CD8atm1Mak infarct (B), WT remote (C), and CD8atm1Mak remote (D) areas at post-myocardial infarction (MI) day 3 showed no differences in T-cell numbers (E) between groups. WT mice had similar levels of CD8+ T-cells within infarct and remote areas. CD8atm1Mak mice had no CD8+ T-cells in infarct or remote areas. Compared with WT mice, CD8atm1Mak mice had an increase in CD4+ T-cells primarily composed of Th1 cells within the infarct area but not remote. No differences were observed in Th2 cells between groups. n = 5–66/day/group; multiple group comparisons were analyzed by one-way ANOVA with Student-Newman-Keuls posttest. *P < 0.05 vs. WT LVI; †P < 0.05 vs. WT LVC; ‡P < 0.05 vs. CD8atm1Mak LVI. LVC, left ventricle remote; LVI, left ventricle infarct; SSC, side scatter.
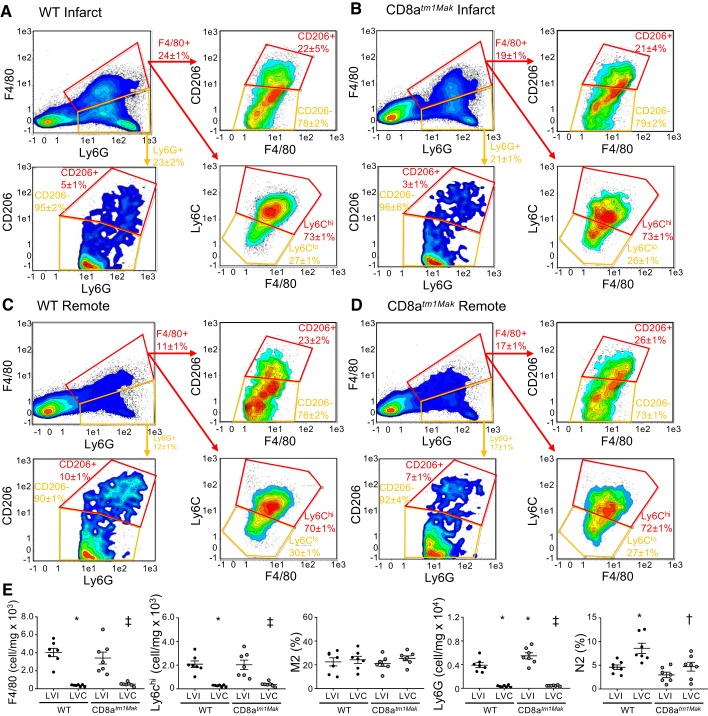
In contrast with the Mac3 staining, F4/80 assessment of the infarct at day 3 post-MI showed no differences between groups (Fig. 6, A–E). Of the F4/80+ cells present in the infarct, majority (70 ± 6%) were Ly6Chi in both WT and CD8+ T-cell-deficient mice. As expected, within the remote area (Fig. 6, C and D) both WT and CD8atm1Mak mice had fewer macrophages (F4/80+ and F4/80+Ly6Chi) compared with infarct tissue. No differences were observed in the M2 (F4/80+CD206+) population between groups or when comparing infarct and remote regions. Spleens were also assessed, and no differences were observed in monocyte numbers, indicating that changes observed in Mac3 may be due to increased proliferation of resident macrophages rather than the splenic reserve.
Fig. 6.
CD8atm1Mak mice have elevated neutrophils within the infarct and remote areas compared with wild-type (WT) mice. Flow cytometry analysis of WT infarct (A), CD8atm1Mak infarct (B), WT remote (C), and CD8atm1Mak remote (D) areas at post-myocardial infarction (MI) day 3 showed increased F4/80+ and F4/80+Ly6Chi macrophages (E) in the infarct areas compared with the remote area. M2 (CD206+) macrophages were not different between groups. Neutrophil (Ly6G+) numbers were increased in the infarct area compared with the remote area and were amplified in the CD8atm1Mak mice. Anti-inflammatory N2 (CD206+) neutrophils were elevated in the remote area of WT mice compared with the infarct area. This increase was not observed in the remote area of the CD8atm1Mak mice. n = 6–7/day/group; multiple group comparisons were analyzed by one-way ANOVA with Student-Newman-Keuls posttest. *P < 0.05 vs. WT LVI; †P < 0.05 vs. WT LVC; ‡P < 0.05 vs. CD8atm1Mak LVI. LVC, left ventricle remote; LVI, left ventricle infarct.
Supporting histological staining, neutrophil numbers (Ly6G+) were elevated in the CD8atm1Mak mice compared with WT mice within the infarct at post-MI day 3 (Fig. 6, A and B). As expected, the remote region had fewer neutrophils compared with the infarct of both WT and CD8atm1Mak mice (Fig. 6, C and D). Previous studies have shown that neutrophils within the infarct are primarily proinflammatory (CD206-) at post-MI day 3 (38). We found for the first time that within the remote area there were more of the N2 anti-inflammatory neutrophils (Ly6G+CD206+) compared with the infarct in WT animals. This effect was attenuated in the CD8+ T-cell-deficient animals.
CD8-deficient animals have decreased cardiomyocyte damage but delayed removal of necrotic tissue.
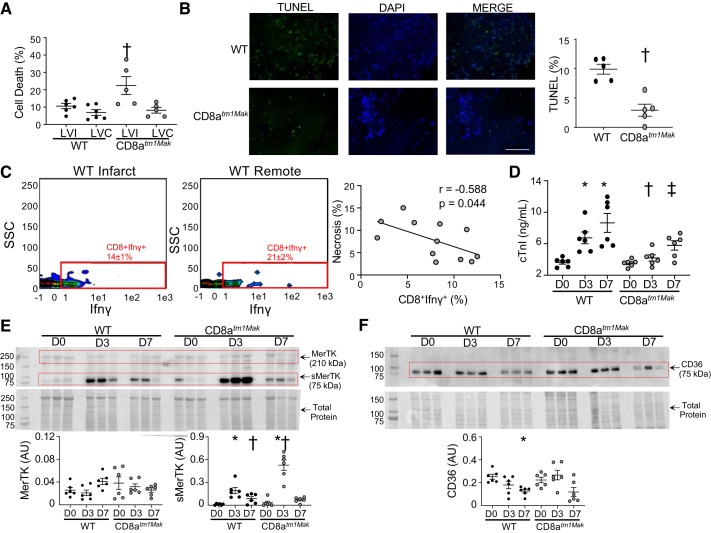
The increase in HMGB1 in our CD8+ T-cell-deficient animals indicates that CD8+ T-cells may be regulating removal of necrotic tissue to facilitate resolution of inflammation. To quantify for necrotic myocytes, cells negative for leukocyte markers (Ly6G−Ly6C−F480−CD3−) were assessed for viability using the live/dead stain. No differences were found in percentage of dead cells within the remote regions between the CD8atm1Mak (8 ± 1%) and WT animals (7 ± 1%; P = 0.599; Fig. 7A). Interestingly, within the infarct, CD8 T-cell-deficient animals had more necrotic myocytes compared with that of the WT animals (22 ± 2% vs. 11 ± 1%; P = 0.036). TUNEL staining (Fig. 7B) showed increased apoptosis in the WT hearts compared with the CD8atm1Mak group, suggesting the cell death observed in our flow data was mainly due to necrosis and not apoptosis. This is in line with our hypothesis that CD8+ T-cells may be regulating removal of necrotic debris in the infarct area.
Fig. 7.
CD8+ T-cells regulate removal of necrotic myocytes. A: flow cytometry of Ly6G−Ly6C−F4/80−CD3− cells showed elevated cell death within the infarct of CD8atm1Mak mice. No differences were observed in remote area. B: TUNEL staining showed decreased apoptotic tissue in the CD8atm1Mak mice compared with wild-type (WT) (scale bar: 100 μm). C: increase in cell death (live/dead stain) negatively correlated with CD8+IFNγ+ T-cells at post-myocardial infarction (MI) day 3. D: plasma levels of cardiac troponin I (cTnI) were increased at post-MI day 3 and day 7 in WT but not the CD8atm1Mak group. E: immunoblotting for tyrosine-protein kinase Mer (MerTK) showed increased levels of soluble MerTK (75 kDa) at post-MI day 3 that was exacerbated in the CD8atm1Mak mice. No significant differences were found in the full-length band (210 kDa) between groups (P = 0.20). F: protein levels of CD36 decreased over the post-MI time course in WT mice but not CD8atm1Mak mice. n = 6/day/group; multiple group comparisons were analyzed by one-way ANOVA with Student-Newman-Keuls posttest. For two group comparisons, the nonparametric Wilcoxon rank sum test was used. *P < 0.05 vs. WT D0; †P < 0.05 vs. WT LVI D3; ‡P < 0.05 vs. WT LVI D7. AU, arbitrary units; D, day; LVC, left ventricle remote; LVI, left ventricle infarct.
Macrophages and neutrophils are key players in the removal of necrotic debris after MI (37, 38, 41). Both CD4 and CD8+ T-cells have been shown to activate macrophages and neutrophils through secretion of IFNγ (10, 23, 46). Our data show that a major source of IFNγ in CD8-deficient animals was from the Th1 cells (Fig. 5E). In addition to Th1 cells, CD8+ T-cells were another source of IFNγ (Fig. 7C) in WT animals. In both infarct and remote tissue, CD8+IFNγ+ T-cells (r = −0.588; P = 0.044) but not Th1 cells (r = −0.493; P = 0.104) correlated with a decrease in necrotic tissue. Our data suggest that removal of necrotic tissue is delayed in the absence of CD8+ T-cells. This would lead to enhanced recruitment of inflammatory cells as an attempt to clear necrotic debris and give reason for the increase in cardiac rupture observed in the CD8atm1Mak group.
To assess if the increase in necrotic tissue was due to increased myocyte damage, cardiac troponin I (cTnI) was measured in plasma samples collected at post-MI day 0, day 3, and day 7. WT mice had increased circulating cTnI at both post-MI day 3 and day 7 compared with post-MI day 0 (Fig. 7D). Plasma levels of cTnI peaks within the first 24 h after initial ischemic injury and returns to baseline levels around 48–62 h after ischemia (18). Increases in cTnI after this period are due to excessive myocardial wall tension (30), reflecting the increased GLS in the WT animals. CD8atm1Mak mice had decreased cTnI at both days 3 and 7 compared with WT mice. Infarct sizes measured by 2,3,5-triphenyltetrazolium chloride staining were the same between groups (Table 1), demonstrating differences in plasma cTnI levels at post-MI day 3 and day 7 are independent of the initial ischemic injury. This is line with previous in vitro studies that have shown that CD8+ T-cells can directly induce cardiac injury, leading to myocyte apoptosis (56, 61). Subsequently, direct actions of the CD8+ T-cells on cardiomyocytes may facilitate infarct expansion and development of heart failure.
Assessment of phagocytic receptor tyrosine-protein kinase Mer (MerTK) showed no significant difference in the full-length form (Fig. 7E) between groups (P = 0.20). Soluble MerTK (sMerTK; 75 kDa) was increased at post-MI day 3 in the WT mice compared with day 0 and returned to baseline levels. In CD8atm1Mak mice, sMerTK was exacerbated at post-MI day 3 compared with WT mice. Increased levels of sMerTK have been shown to inhibit phagocytosis (48). CD36, another receptor critical for macrophage-mediated phagocytosis, decreased over the time course post-MI in WT animals (Fig. 7F) as previously published (16). This decrease was not observed in the CD8atm1Mak mice.
DISCUSSION
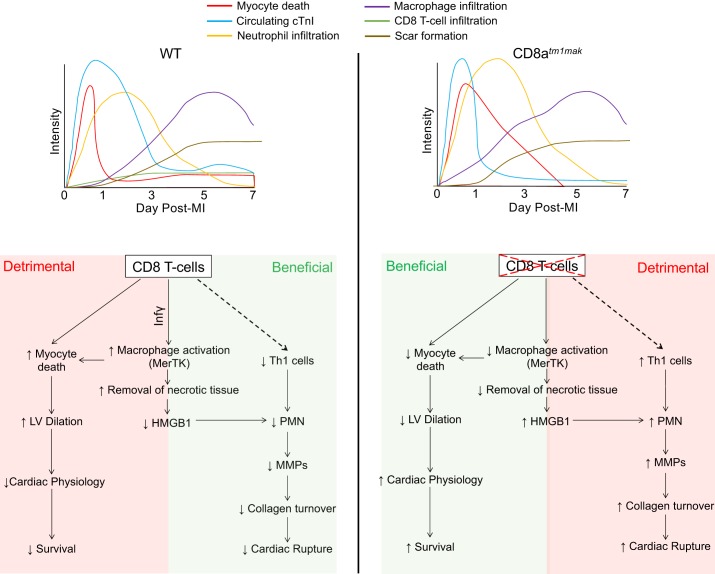
The goal of this study was to determine the effects of CD8+ T-cells on post-MI cardiac remodeling. The key findings were inhibition of CD8+ T-cell activation: 1) delays removal of necrotic tissue despite increased neutrophil and macrophage numbers in the infarct tissue; 2) increases numbers but delays activation of macrophages; 3) accelerates cardiac fibrosis yet displays increased cardiac rupture; and 4) improves overall survival and cardiac physiology. Despite having better overall survival, those that died did so because of cardiac rupture, suggesting that CD8+ T-cells may actually play a dual role in the cardiac remodeling process. In addition, timing of necrotic tissue removal by inflammatory cells was found to be necessary to generate a stable scar (Fig. 8). The role of CD8+ T-cells may be both beneficial and detrimental to cardiac remodeling through a cell-specific mechanism (Table 2).
Fig. 8.
Schematic for CD8+ T-cell regulation of the wound healing environment after myocardial infarction (MI). CD8+ T-cells regulate myocardial healing after MI by activating macrophages to initiate removal of necrotic tissue and facilitate in initiation of the resolution of inflammation. This leads to a beneficial decrease in neutrophil-mediated matrix metalloproteinase (MMP) secretion, collagen turnover, and cardiac rupture. On the other hand, CD8+ T-cells are detrimental both through direct and indirect mechanisms by regulating myocyte death, left ventricular dilation, and cardiac physiology. HMGB1, high-mobility group box 1; LV, left ventricle; MerTK, tyrosine-protein kinase Mer; WT, wild-type.
Table 2.
Summary of protein and cellular changes evaluated in WT and CD8atm1Mak mice
| Post-MI Day 3 | Post-MI Day 7 | |||||
|---|---|---|---|---|---|---|
| Plasma | Infarct | Remote | Plasma | Infarct | Remote | |
| Collagen (PSR) | NA | ↑ (2.5) | ↑ (2.1) | NA | ↔ | ↔ |
| IFNγ | ↑ (2.1) | ↔ | NA | ↔ | ↔ | NA |
| MMP-2 | ↑ (2.0) | ↔ | NA | ↑ (2.0) | ↔ | NA |
| CXCL1 | ↔ | ↔ | NA | ↑ (11.6) | ↔ | NA |
| CCL11 | ↔ | ↑ (2.0) | NA | ↑ (7.6) | ↔ | NA |
| CXCL10 | ↔ | NA | NA | ↑ (6.9) | NA | NA |
| CX3CL1 | ↔ | NA | NA | ↑ (6.9) | NA | NA |
| CCL12 | ↔ | NA | NA | ↑ (5.4) | NA | NA |
| CD160 | ↔ | NA | NA | ↑ (3.9) | NA | NA |
| CCL6 | ↔ | NA | NA | ↑ (3.3) | NA | NA |
| Endoglin | ↔ | NA | NA | ↑ (3.1) | NA | NA |
| CXCL9 | ↔ | NA | NA | ↑ (3.0) | NA | NA |
| Tissue factor | ↔ | NA | NA | ↑ (2.6) | NA | NA |
| ICAM-1 | ↔ | NA | NA | ↑ (2.4) | NA | NA |
| CCL3 | ↔ | NA | NA | ↑ (2.3) | NA | NA |
| IL-1a | ↔ | NA | NA | ↑ (2.3) | NA | NA |
| CCL20 | ↔ | NA | NA | ↑ (2.1) | NA | NA |
| DKK-1 | ↔ | NA | NA | ↑ (2.1) | NA | NA |
| MMP-9 | ↔ | ↑ (2.2) | NA | ↔ | ↔ | NA |
| LOX | NA | ↔ | NA | NA | ↓ (−5.0) | NA |
| Neutrophils (Ly6b.2 and Ly6G) | NA | ↑ (1.4) | V (1.7) | NA | ↔ | ↔ |
| N2 neutrophils (CD206) | NA | ↔ | ↓ (−5.0) | NA | NA | NA |
| Mac3 | NA | ↑ (2.6) | ↔ | NA | ↔ | ↔ |
| F4/80 | NA | ↔ | ↔ | NA | NA | NA |
| CD3 | NA | ↔ | ↔ | NA | NA | NA |
| CD8+ T-cells | NA | ↓ (−83.4) | ↓ (−66.0) | NA | NA | NA |
| CD4+ T-cells | NA | ↑ (2.1) | ↔ | NA | NA | NA |
| Th1 T-cells | NA | ↑ (2.3) | ↔ | NA | NA | NA |
| Necrotic myocytes | NA | ↑ (1.9) | ↔ | NA | NA | NA |
| Cardiac troponin I | ↓ (−1.6) | NA | NA | ↓ (−1.5) | NA | NA |
Arrows represent direction in the CD8-deficient group (CD8atm1Mak) compared with the wild-type (WT) group at respective post-myocardial infarction (MI) day. Parentheses represent fold change of proteins significantly different in CD8atm1Mak mice compared with WT. NA, not measured in that sample. LOX, active lysyl oxidase; MMP, matrix metalloproteinase.
CD8+ T-cell actions were indirectly beneficial by attenuating the recruitment of neutrophils, increasing macrophage activation, and facilitating the removal of necrotic tissue. Our data showed that in the absence of CD8+ T-cells, there was an increase in soluble collagen, most likely because of elevated MMPs, and decreased collagen cross-linking, resulting in cardiac rupture. Neutrophils are the most prevalent immune cell type at post-MI day 3 and are known to secrete multiple MMPs (38, 39). MMP-mediated degradation of collagen promotes disassembly of cellular focal adhesions, thus reducing myofibroblast adherence and initiating cellular apoptosis (6, 47, 63). Therefore, CD8+ T-cell inhibition of neutrophil-mediated inflammation would be beneficial by decreasing MMP-mediated collagen turnover, facilitating scar formation, and decreasing incidence of cardiac rupture.
Mast cells were found to be elevated at post-MI day 7 in the CD8+ T-cell-deficient mice compared with WT mice. Mast cells are known to regulate CD8+ T-cell effector physiology, including proliferation, cytokine secretion, and cytotoxic activity in vitro (51). Therefore, the increase in mast cell numbers within the infarct of the CD8atm1Mak mice may be a compensatory mechanism because of inactivation of the CD8+ T-cell population. In addition, individual mast cell granule products such as tryptase are profibrotic, providing a mechanism for the acceleration of collagen deposition in the CD8atm1Mak mice (21, 54).
CD8+ T-cells were found to be pertinent for removal of necrotic tissue within the infarct, which is necessary for scar formation. Our data indicate that despite having an increase in fibroblast activation, myofibroblasts were not able to form a stable scar without removal of the necrotic debris. This decrease in necrotic tissue removal was mediated by proteolytic cleavage of MerTK, which led to inhibition of macrophage-mediated phagocytosis (31, 48). This was in contrast to CD36, which decreased in WT but not CD8atm1Mak mice at post-MI day 7. CD36 is important for the mitigation of the early remodeling phase and facilitates the transition to MerTK-dependent resolution of inflammation, signifying that CD8+ T-cells are important for this shift in cardiovascular wound healing (7, 11, 59). Deficient removal of the dead myocytes leads to secondary expansion of tissue necrosis, thus prolonging the inflammatory response and weakening the collagen scar (13, 42). This was confirmed by a decrease in LOX found in the CD8+ T-cell-deficient animals. Changes to the LV chamber after an MI depend on multiple factors; therefore, we cannot assume that collagen content alone led to the differences in LV dilation.
In addition to the removal of necrotic myocytes, macrophages are needed for removal of apoptotic neutrophils as a signal for resolution of inflammation (16). Macrophages at post-MI day 3 display a higher capacity for removal of necrotic and apoptotic cells through phagocytosis (41). CD8+ T-cell activation of macrophage phagocytosis may therefore play a beneficial role in cardiac wound healing by laying the foundation by which fibroblasts can enter and deposit the collagen scar as well as begin to resolve neutrophil-mediated inflammation. In addition, our data show an increase in neutrophils, macrophages, mast cells, and Th1 cells within the infarct of the CD8+ T-cell-deficient animals. Although no differences in these inflammatory cells were observed before myocardial injury (day 0) between genotypes, it is possible that this increase is due to the lack of the CD8+ T-cell response. Additional studies performing adoptive transfer of CD8+ T-cells need to be performed to fully understand whether this response was directly due to the loss of CD8+ T-cells or due to a compensatory mechanism.
In our study, we found elevated proteins within the HMGB1 pathway in our CD8atm1Mak mice. HMGB1 levels are associated with adverse clinical outcomes in patients with MI (29). Conversely, in a rat MI model, HMGB1 blockade aggravated LV remodeling, possibly through impairment of the infarct healing process (29). This suggests that both overactivation and loss of the HMGB1-mediated pathways can be detrimental to the wound healing process. An increase in HMGB1 has been shown to induce both CD4 and CD8+ T-cell proliferation (40, 52). This is in line with our study, which revealed an increase in CD4+ T-cells primarily composed of Th1 cells within the infarct of our CD8+ T-cell-deficient animals. Because Th1 cells are known to regulate recruitment and polarization of macrophages, it is possible that the lack of activated CD8+ T-cells may indirectly regulate macrophage recruitment through upregulation of Th1 cells. Future studies dissecting the implication of CD8+ T-cells in macrophage physiology are warranted to fully explore this question.
CD8+ T-cells were detrimental though direct and indirect actions on cardiac myocytes. CD8atm1Mak mice showed improved cardiac physiology and survival compared with WT mice despite exaggerated inflammation. In vitro, CD8+ T-cells are known to directly induce cardiac injury, leading to decreased myocyte contractility and apoptosis and thus facilitating the development of heart failure after MI (27, 56, 61). Similarly, CD8+ T-cells stimulate macrophage nitric oxide production, resulting in increased targeted tissue destruction (22). CD8+ T-cells have also been shown to upregulate CD11b, CD64, and CD62L on neutrophils and delay neutrophil apoptosis in vitro, mainly through secretion of inflammatory factors including IFN‐γ (46). Therefore, CD8+ T-cells may be detrimental to the cardiac remodeling process by inducing direct cytotoxic effects on healthy cardiomyocytes and amplifying neutrophil and macrophage-mediated inflammation, thus resulting in increased LV dilation and decreased cardiac function.
In addition to direct actions on cardiac myocytes, CD8+ T-cells also indirectly injured cardiac myocytes through prolonged and excessive macrophage activation. At day 3 post-MI, CD8-deficient mice showed an increase in Mac3+ cells, yet no changes were observed in F4/80+ cells between the groups. The increase in Mac3 but not F4/80 may be due to differences between resident and monocyte-derived macrophages. Depletion of monocyte-derived macrophages has been shown to be beneficial, whereas resident macrophages were found to be critical for cardiac adaptation and function by initiating angiogenesis (2, 17, 26, 33). CD8+ T-cells may therefore be detrimental to myocardial healing by prolonging activation of monocyte-derived macrophages and reducing resident cardiac macrophage expansion, thus leading to increased myocardial recovery after injury. Expanding the role of CD8+ T-cells on resident versus monocyte-derived macrophages is needed.
Whereas the initial activation of macrophages would be advantageous for the removal of necrotic tissue, prolonged activation would lead to unintended myocardial damage and infarct expansion. In the setting of chronic inflammation, prolonged macrophage activation led to increased cardiac rupture through dysregulation of fibroblast activation (13). Our data suggest that the balance between collagen synthesis and degradation may be tipped in favor of poor cardiovascular scar formation. Future assessment of CD8+ T-cell-mediated regulation of fibroblast physiology is crucial for a better understanding of CD8+ T-cell roles in the post-MI wound healing environment.
In conclusion, the absence of CD8+ T-cells played a vital role in regulating the innate immune response. Early influx of neutrophils and macrophages into the infarcted area of CD8+ T-cell-deficient mice suggests that CD8+ T-cells may have a biphasic role in the post-MI wound healing process. Because our study suggests that CD8+ T-cells have both a beneficial and detrimental role depending on the stage of LV remodeling, future studies that evaluate which stage is best for CD8+ T-cell inhibition would be necessary to test therapeutic potential. These findings give us insight into the role that the adaptive immune response undertakes post-MI and facilitates our understanding of the inflammatory response in this setting.
GRANTS
This work was supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) Grants R00-DK-105160 to D. V. Ilatovskaya and U54-DA-016511 to K. Y. DeLeon-Pennell and the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award IK2BX003922 to K. Y. DeLeon-Pennell. This work was also financially supported in part by the 2019 S&R Foundation Ryuji Ueno Award that was bestowed upon K. Y. DeLeon-Pennell by the American Physiological Society.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Veterans Administration, or the American Physiological Society.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.Y.D.-P. conceived and designed research; C.P., J.C., M.D., M.T., S.P., and K.Y.D.-P. performed experiments; K.Y.D.-P. analyzed data; D.V.I., C.P., M.D., and K.Y.D.-P. interpreted results of experiments; D.V.I., C.P., J.C., and K.Y.D.-P. prepared figures; C.P., J.C., and K.Y.D.-P. drafted manuscript; D.V.I., C.P., J.C., M.D., M.T., S.P., and K.Y.D.-P. edited and revised manuscript; D.V.I., C.P., J.C., M.D., M.T., S.P., and K.Y.D.-P. approved final version of manuscript.
REFERENCES
- 1.Abbate A, Bonanno E, Mauriello A, Bussani R, Biondi-Zoccai GG, Liuzzo G, Leone AM, Silvestri F, Dobrina A, Baldi F, Pandolfi F, Biasucci LM, Baldi A, Spagnoli LG, Crea F. Widespread myocardial inflammation and infarct-related artery patency. Circulation 110: 46–50, 2004. doi: 10.1161/01.CIR.0000133316.92316.81. [DOI] [PubMed] [Google Scholar]
- 2.Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, Kovacs A, Epelman S, Artyomov M, Kreisel D, Lavine KJ. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res 124: 263–278, 2019. doi: 10.1161/CIRCRESAHA.118.314028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail 10: e003688, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 139: 206–221, 2019. doi: 10.1161/CIRCULATIONAHA.118.036065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2017 Update: A report from the American Heart Association. Circulation 135: e146–e603, 2017. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carragher NO, Levkau B, Ross R, Raines EW. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol 147: 619–630, 1999. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi JY, Seo JY, Yoon YS, Lee YJ, Kim HS, Kang JL. Mer signaling increases the abundance of the transcription factor LXR to promote the resolution of acute sterile inflammation. Sci Signal 8: ra21, 2015. doi: 10.1126/scisignal.2005864. [DOI] [PubMed] [Google Scholar]
- 8.Corry DB, Kiss A, Song LZ, Song L, Xu J, Lee SH, Werb Z, Kheradmand F. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J 18: 995–997, 2004. doi: 10.1096/fj.03-1412fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronshaw AD, Fothergill-Gilmore LA, Hulmes DJ. The proteolytic processing site of the precursor of lysyl oxidase. Biochem J 306: 279–284, 1995. doi: 10.1042/bj3060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259: 1739–1742, 1993. doi: 10.1126/science.8456300. . [DOI] [PubMed] [Google Scholar]
- 11.Dehn S, Thorp EB. Myeloid receptor CD36 is required for early phagocytosis of myocardial infarcts and induction of Nr4a1-dependent mechanisms of cardiac repair. FASEB J 32: 254–264, 2018. doi: 10.1096/fj.201700450R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLeon-Pennell KY, de Castro Brás LE, Iyer RP, Bratton DR, Jin YF, Ripplinger CM, Lindsey MLP. P. gingivalis lipopolysaccharide intensifies inflammation post-myocardial infarction through matrix metalloproteinase-9. J Mol Cell Cardiol 76: 218–226, 2014. doi: 10.1016/j.yjmcc.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLeon-Pennell KY, Iyer RP, Ero OK, Cates CA, Flynn ER, Cannon PL, Jung M, Shannon D, Garrett MR, Buchanan W, Hall ME, Ma Y, Lindsey ML. Periodontal-induced chronic inflammation triggers macrophage secretion of Ccl12 to inhibit fibroblast-mediated cardiac wound healing. JCI Insight 2: e94207, 2017. doi: 10.1172/jci.insight.94207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLeon-Pennell KY, Iyer RP, Ma Y, Yabluchanskiy A, Zamilpa R, Chiao YA, Cannon PL, Kaplan A, Cates CA, Flynn ER, Halade GV, de Castro Brás LE, Lindsey ML. The Mouse Heart Attack Research Tool 1.0 database. Am J Physiol Heart Circ Physiol 315: H522–H530, 2018. doi: 10.1152/ajpheart.00172.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLeon-Pennell KY, Tian Y, Zhang B, Cates CA, Iyer RP, Cannon P, Shah P, Aiyetan P, Halade GV, Ma Y, Flynn E, Zhang Z, Jin YF, Zhang H, Lindsey ML. CD36 is a matrix metalloproteinase-9 substrate that stimulates neutrophil apoptosis and removal during cardiac remodeling. Circ Cardiovasc Genet 9: 14–25, 2016. doi: 10.1161/CIRCGENETICS.115.001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40: 91–104, 2014. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frobert A, Valentin J, Magnin JL, Riedo E, Cook S, Giraud MN. Prognostic value of troponin I for infarct size to improve preclinical myocardial infarction small animal models. Front Physiol 6: 353, 2015. doi: 10.3389/fphys.2015.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung-Leung WP, Schilham MW, Rahemtulla A, Kündig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW. CD8 is needed for development of cytotoxic T-cells but not helper T-cells. Cell 65: 443–449, 1991. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 20.González-Santamaría J, Villalba M, Busnadiego O, López-Olañeta MM, Sandoval P, Snabel J, López-Cabrera M, Erler JT, Hanemaaijer R, Lara-Pezzi E, Rodríguez-Pascual F. Matrix cross-linking lysyl oxidases are induced in response to myocardial infarction and promote cardiac dysfunction. Cardiovasc Res 109: 67–78, 2016. doi: 10.1093/cvr/cvv214. [DOI] [PubMed] [Google Scholar]
- 21.Gruber BL, Kew RR, Jelaska A, Marchese MJ, Garlick J, Ren S, Schwartz LB, Korn JH. Human mast cells activate fibroblasts: tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol 158: 2310–2317, 1997. [PubMed] [Google Scholar]
- 22.Gurlo T, von Grafenstein H. Antigen-independent cross-talk between macrophages and CD8+ T-cells facilitates their cooperation during target destruction. Int Immunol 15: 1063–1071, 2003. doi: 10.1093/intimm/dxg106. [DOI] [PubMed] [Google Scholar]
- 23.Han YL, Li YL, Jia LX, Cheng JZ, Qi YF, Zhang HJ, Du J. Reciprocal interaction between macrophages and T-cells stimulates IFN-γ and MCP-1 production in Ang II-induced cardiac inflammation and fibrosis. PLoS One 7: e35506, 2012. doi: 10.1371/journal.pone.0035506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125: 1652–1663, 2012. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 25.Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol 93: 149–155, 2016. doi: 10.1016/j.yjmcc.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol 165: 439–447, 2004. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Kalyan S, Kabelitz D. When neutrophils meet T-cells: beginnings of a tumultuous relationship with underappreciated potential. Eur J Immunol 44: 627–633, 2014. doi: 10.1002/eji.201344195. [DOI] [PubMed] [Google Scholar]
- 27.Kaya Z, Göser S, Buss SJ, Leuschner F, Ottl R, Li J, Völkers M, Zittrich S, Pfitzer G, Rose NR, Katus HA. Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis. Circulation 118: 2063–2072, 2008. doi: 10.1161/CIRCULATIONAHA.108.788711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412, 2010. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohno T, Anzai T, Naito K, Miyasho T, Okamoto M, Yokota H, Yamada S, Maekawa Y, Takahashi T, Yoshikawa T, Ishizaka A, Ogawa S. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res 81: 565–573, 2009. doi: 10.1093/cvr/cvn291. [DOI] [PubMed] [Google Scholar]
- 30.Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart 92: 987–993, 2006. doi: 10.1136/hrt.2005.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law AL, Parinot C, Chatagnon J, Gravez B, Sahel JA, Bhattacharya SS, Nandrot EF. Cleavage of Mer tyrosine kinase (MerTK) from the cell surface contributes to the regulation of retinal phagocytosis. J Biol Chem 290: 4941–4952, 2015. doi: 10.1074/jbc.M114.628297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 209: 123–137, 2012. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao X, Shen Y, Zhang R, Sugi K, Vasudevan NT, Alaiti MA, Sweet DR, Zhou L, Qing Y, Gerson SL, Fu C, Wynshaw-Boris A, Hu R, Schwartz MA, Fujioka H, Richardson B, Cameron MJ, Hayashi H, Stamler JS, Jain MK. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci USA 115: E4661–E4669, 2018. doi: 10.1073/pnas.1720065115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsey ML, Iyer RP, Zamilpa R, Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA, Bratton D, Flynn ER, Cannon PL, Tian Y, Jin YF, Lange RA, Tokmina-Roszyk D, Fields GB, de Castro Brás LE. A novel collagen matricryptin reduces left ventricular dilation post-myocardial infarction by promoting scar formation and angiogenesis. J Am Coll Cardiol 66: 1364–1374, 2015. doi: 10.1016/j.jacc.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol 314: H733–H752, 2018. doi: 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsey ML, Saucerman JJ, DeLeon-Pennell KY. Knowledge gaps to understanding cardiac macrophage polarization following myocardial infarction. Biochim Biophys Acta 1862: 2288–2292, 2016. doi: 10.1016/j.bbadis.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, Henry J, Cates CA, Deleon-Pennell KY, Lindsey ML. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 110: 51–61, 2016. doi: 10.1093/cvr/cvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y, Yabluchanskiy A, Lindsey ML. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair 6: 11, 2013. doi: 10.1186/1755-1536-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol 173: 307–313, 2004. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 41.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, Flynn ER, Freeman TC, Saucerman JJ, Garrett MR, Ma Y, Harmancey R, Lindsey ML. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol 113: 26, 2018. doi: 10.1007/s00395-018-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouton AJ, Ma Y, Rivera Gonzalez OJ, Daseke MJ II, Flynn ER, Freeman TC, Garrett MR, DeLeon-Pennell KY, Lindsey ML. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic Res Cardiol 114: 6, 2019. doi: 10.1007/s00395-019-0715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res 112: 1624–1633, 2013. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T-cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920, 2009. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 45.Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 71: 1947–1957, 2018. doi: 10.1016/j.jacc.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 46.Pelletier M, Micheletti A, Cassatella MA. Modulation of human neutrophil survival and antigen expression by activated CD4+ and CD8+ T-cells. J Leukoc Biol 88: 1163–1170, 2010. doi: 10.1189/jlb.0310172. [DOI] [PubMed] [Google Scholar]
- 47.Sarrazy V, Billet F, Micallef L, Coulomb B, Desmoulière A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen 19, Suppl 1: s10–s15, 2011. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- 48.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109: 1026–1033, 2007. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, Nochioka K, Biering-Sørensen T. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 8: 1351–1359, 2015. doi: 10.1016/j.jcmg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Stelekati E, Bahri R, D’Orlando O, Orinska Z, Mittrücker HW, Langenhaun R, Glatzel M, Bollinger A, Paus R, Bulfone-Paus S. Mast cell-mediated antigen presentation regulates CD8+ T-cell effector functions. Immunity 31: 665–676, 2009. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 52.Sundberg E, Fasth AE, Palmblad K, Harris HE, Andersson U. High mobility group box chromosomal protein 1 acts as a proliferation signal for activated T lymphocytes. Immunobiology 214: 303–309, 2009. doi: 10.1016/j.imbio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616, 2009. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tatler AL, Porte J, Knox A, Jenkins G, Pang L. Tryptase activates TGFbeta in human airway smooth muscle cells via direct proteolysis. Biochem Biophys Res Commun 370: 239–242, 2008. doi: 10.1016/j.bbrc.2008.03.064. [DOI] [PubMed] [Google Scholar]
- 55.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 96: 2673–2681, 2000. [PubMed] [Google Scholar]
- 56.Varda-Bloom N, Leor J, Ohad DG, Hasin Y, Amar M, Fixler R, Battler A, Eldar M, Hasin D. Cytotoxic T lymphocytes are activated following myocardial infarction and can recognize and kill healthy myocytes in vitro. J Mol Cell Cardiol 32: 2141–2149, 2000. doi: 10.1006/jmcc.2000.1261. [DOI] [PubMed] [Google Scholar]
- 57.Voorhees AP, DeLeon-Pennell KY, Ma Y, Halade GV, Yabluchanskiy A, Iyer RP, Flynn E, Cates CA, Lindsey ML, Han HC. Building a better infarct: modulation of collagen cross-linking to increase infarct stiffness and reduce left ventricular dilation post-myocardial infarction. J Mol Cell Cardiol 85: 229–239, 2015. doi: 10.1016/j.yjmcc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93: 411–422, 1998. doi: 10.1016/S0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113: 1004–1012, 2013. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T-cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 115: 55–67, 2014. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 61.Woodley SL, McMillan M, Shelby J, Lynch DH, Roberts LK, Ensley RD, Barry WH. Myocyte injury and contraction abnormalities produced by cytotoxic T lymphocytes. Circulation 83: 1410–1418, 1991. doi: 10.1161/01.CIR.83.4.1410. [DOI] [PubMed] [Google Scholar]
- 62.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res 43: W251–W257, 2015. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xue M, Jackson CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle) 4: 119–136, 2015. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 62: 24–35, 2013. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]