Abstract
Immune activation post-myocardial infarction is an orchestrated sequence of cellular responses to effect tissue repair and healing. However, excessive and dysregulated inflammation can result in left ventricular remodeling and pathological alterations in the structural and mechanical attributes of the heart. Identification of key pathways and critical cellular mediators of inflammation is thus essential to design immunomodulatory therapies for myocardial infarction and ischemic heart failure. Despite this, the experimental approaches to isolate mononuclear cells from the heart are diverse, and detailed protocols to enable maximum yield of live cells in the shortest time possible are not readily available. Here, we describe optimized protocols for the isolation, fixation, and flow cytometric characterization of cardiac CD45+ leukocytes. These protocols circumvent time-consuming coronary perfusion and density-mediated cell-separation steps, resulting in high cellular yields from cardiac digests devoid of contaminating intravascular cells. Moreover, in contrast to methanol and acetone, we show that cell fixation using 1% paraformaldehyde is most optimal as it does not affect antibody binding or cellular morphology, thereby providing a considerable advantage to study activation/infiltration-associated changes in cellular granularity and size. These are highly versatile methods that can easily be streamlined for studies requiring simultaneous isolation of immune cells from different tissues or deployment in studies containing a large cohort of samples with time-sensitive constraints.
NEW & NOTEWORTHY In this article, we describe optimized protocols for the isolation, fixation, and flow cytometric analysis of immune cells from the ischemic/nonischemic hearts. These protocols are optimized to process several samples/tissues, simultaneously enabling maximal yield of immune cells in the shortest time possible. We show that the low-speed centrifugation can be used as an effective alternative to lengthy coronary perfusion to remove intravascular cells, and sieving through 40-μm filter can replace density-mediated mononuclear cell separation which usually results in 50–70% cell loss in the sedimented pellets. We also show that cell fixation using 1% paraformaldehyde is better than the organic solvents such as methanol and acetone for flow cytometric analysis.
Keywords: cardiac leukocytes, immune cells, immune isolation, myocardial infarction
INTRODUCTION
Myocardial infarction (MI) and ischemic cardiomyopathy are associated with inflammation and immune cell infiltration into the myocardium (17, 19, 28). Studies have shown that both innate and adaptive immune cells are activated and infiltrate the hearts in ischemic (5, 17) and nonischemic cardiomyopathy (26, 29, 30). It is now known that early after infarction, proinflammatory immune responses mediated by Ly6Chi monocytes (13), M1 macrophages (27), dendritic cells (DCs) (42), and B- (43) and Th1 T cells (12) predominate in the heart. These populations are replaced by anti-inflammatory cells such as Ly6Clow monocytes (13), M2 macrophages (18, 37), iNKT cells (38), and Th2 and regulatory T cells (Tregs) between 3 and 7 days post-MI (6, 40). Left ventricular (LV) remodeling and chronic ischemic HF (4–8 wk post-MI) are associated with a second wave of proinflammatory immune activation characterized by increased M1 macrophages and DCs (17) and expansion of all effector T-cell subsets (Th1, Th2, Th17, and Tregs) as well as memory T cells (5, 6).
These and other studies have established that immune cells play an important role in altering the mechanical, structural, and functional attributes of the murine heart after both permanent coronary ligation [a preclinical model of heart failure with reduced ejection fraction (HFrEF)] (5, 17, 28) and transverse aortic constriction [a preclinical model for pressure overload-induced LV hypertrophy that recapitulates characteristics of HF with preserved EF (HFpEF) and HFrEF] (29, 30). Despite this understanding, there is no consensus on the optimum strategy for isolation and characterization of immune cells from the heart. Although most of the immune isolation protocols used in several published studies are comparable, there are nonetheless important differences in the way cardiac mononuclear cells (MNCs) have been isolated. Most published protocols differ in one of the following two aspects: 1) the use of Ficoll-paque/Percoll density-based separation mechanisms to remove myocardial debris from cardiac digests (1, 22, 28, 38–40, 43) and 2) the use of coronary perfusion to remove intravascular cells (4, 8, 34, 43). It was, therefore, our intent to explore the effects of these two factors on the isolation of cardiac leukocytes and suggest an optimized immune cell isolation protocol that can be used to derive consistent yields of infiltrating cells.
MATERIALS AND METHODS
Mouse Model
Permanent ligation of the left anterior descending coronary artery was performed to induce acute MI and late ischemic HF as this model reliably produces functional HF at 8 wk post-MI in male 10–12-wk-old C57BL/6 mice (bred in house) (5, 6, 17). All the surgical and post-op analgesic procedures were consistent with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (Publication no. 85-23, Revised 1996) and were approved by the Institutional Animal Care and Use Committees of either the University of Alabama at Birmingham or The Ohio State University. All the mice were maintained at 12-h:12-h light-dark cycle and had ad libitum access to water and chow diet. Guidelines published by Lindsey et al. (24) were followed for all the in vivo studies.
Solutions
Digestion media.
A solution (1 mg/mL) of collagenase II (~250–300 U/mg activity, Worthington LS004177) was prepared in RPMI 1640 (VWR 02-0105-0500) media, prewarmed to 37°C. The digestion media should be prepared fresh every time.
Digestion neutralization buffer.
Cold phosphate-buffered saline (PBS) contained 2 mM EDTA and either 2% FBS or 2% bovine serum albumin (BSA).
Preparation of Single Cell Suspensions from Hearts
Hearts were harvested and immediately placed in cold PBS. All the connective tissues were carefully removed, and the heart was cut in half at the long axis using a heavy-duty single edge razor blade (Lowe’s, catalog no. 74421) to remove clotted blood from the chambers. Some of the isolated hearts underwent retrograde coronary perfusion Ringer's solution to remove intravascular cells (see Effect of Coronary Perfusion for detailed method). The hearts were weighed and transferred to a 10-cm polystyrene dish kept on ice and were finely minced using a heavy-duty single edge razor blade. The minced tissue was transferred to a 50-mL conical tube, and 2-mL ice-cold PBS was added. These tubes were stored on ice until all the hearts were harvested and minced. Once all the hearts were minced, the tubes were centrifuged at 50 g for 2 min, and the supernatant was discarded.
If needed, supernatant can also be collected separately to measure the number of intravascular cells. For comparison, control samples can be centrifuged at 350 g to demonstrate the efficiency of low centrifugation step in removing most of the intravascular cells.
Note: Centrifugation at 50 g along with fine mincing ensures removal of intravascular cells from the cardiac tissue. Alternatively, one can cannulate the aorta and retrograde perfuse the heart with normal saline (see Effect of Coronary Perfusion to Remove Intravascular Cells: Intravascular Cell Staining for detailed method). Digestion media can be prepared at this step.
After discarding the supernatant, 7 to 8 mL of digestion media was added to each tube followed by incubation at 37°C for 20–25 min. Tubes were mixed every 5 min to resuspend the tissue chunks. Alternatively, tubes can also be placed on a rocker for constant shaking during the digestion. In our experience, both approaches are comparable for cardiac digestion. While the tissues were being digested, 10 mL of cold digestion neutralization buffer was taken in clean 50-mL conical tubes and a 40-µm cell strainer (Fisher; catalog no. 22-363-547) was placed on top. After 20–25 min of tissue digestion, the digested contents were transferred onto these cell strainers for filtering the digested tissues. Small tissue chunks collected on the cell strainers were slowly triturated using the rough end of a 3-mL syringe plunger to dissociate all the cells. Once all the tissue chunks were digested, 4 to 5 mL of digestion neutralization media was added onto the strainers using a 1-mL pipette to ensure complete inhibition of digestion enzymes. Cells were pelleted by centrifugation at 500 g for 8–10 min (4–8°C), and the supernatant was discarded. Pellets were either resuspended in 200–300 µL of staining buffer for flow cytometric analysis, or mononuclear cells were isolated from the cardiac digests using Ficoll-Paque density gradient.
Effect of Ficoll-Paque Density Gradient
The pellet was resuspended in 5 mL PBS containing 2 mM EDTA and carefully layered on top of 5 mL prewarmed (18–20°C) Ficoll-Paque density media (premium, 1.085 g/mL, GE Healthcare Life Sciences) in a 15-mL conical tube. Tubes were then centrifuged at 400 g for 30 min, following manufacturer’s instructions. The interphase layer (along with top clear supernatant) containing all mononuclear cells was carefully transferred to a separate tube. The pellet at the bottom of the tubes containing myocyte debris was also collected to measure, if any, leukocytes (CD45+), T cells (CD45+CD4+), neutrophils (CD45+CD4−CD11b+Ly6G+), Ly6Clow and Ly6Chi monocytes/macrophages (CD45+CD4−CD11b+Ly6G−), and B-cells (CD45+CD4−CD11b+Ly6G−B220+) in the pellet. Both the fractions were diluted with three times the volume of PBS (1 to 4 dilution) and centrifuged at 500 g for 10 mins (4-8°C) to pellet all cells. The supernatant was discarded, and both sets of tubes were either fixed (see Effect of Fixation Agents for detailed method) for staining at a later time or stained immediately for flow cytometric analysis by resuspending the pellets obtained from the monolayer and sedimented fractions in 100 and 500 µL staining buffer, respectively.
Effect of Coronary Perfusion to Remove Intravascular Cells: Intravascular Cell Staining
Mice (1-day post-MI) were anesthetized with 3% (vol/vol) isoflurane in O2 or room air in an induction chamber. The animals were transferred to a surgical pad equipped with a nose cone, and the isoflurane concentration was decreased to 1.5%. Lack of response to the toe and/or tail pinch was used to ensure an appropriate level of anesthesia. Ethanol (70% vol/vol) was sprayed onto the abdominal area for sanitization. A transverse abdominal incision was made, and underlying abdominal tissues were carefully pushed aside to expose the inferior vena cava. Anti-mouse CD45-BV605 antibody (3 µg, clone 30-F11; BioLegend catalog no. 103139) was diluted to 100 µL with PBS (15 µL of 200 µg/mL antibody was added to 85 µL of PBS) and injected into the inferior vena cava using a 1-mL disposable insulin syringe. After 3 min, ~100 µL blood was collected via cardiac puncture and transferred to EDTA containing blood collection tubes (BD, catalog no. 365974, purple top). The heart, mediastinal lymph nodes, and spleen were harvested immediately and used for cell isolation.
Note: For intravascular staining, circulating blood cells were used to ensure complete (>95%) staining of intravascular cells, and mediastinal lymph node cells were sampled to ensure <10% staining as negative controls. Spleens were also sampled since several studies have shown that the spleen acts as an extramedullary site to mediate monocytopoiesis and lymphocyte trafficking early after infarction (1-day post-MI) (23).
Processing of Peripheral Blood Mononuclear Cells (PBMCs) for Flow Cytometric Analysis
Blood was collected in EDTA containing blood collection tubes (purple top) and 100 µL blood was transferred to a 15-mL conical tube followed by addition of 7–10 times the volume of RBC lysis buffer (Invitrogen, catalog no. 00-4333-57). Tubes were mixed well by flicking, incubated on ice for 10 min, and the excess lysis buffer was neutralized using 12 to 13 mL cold PBS. Tubes were centrifuged at 500 g for 10 min (4–8°C) to pellet white blood cells. The supernatant was discarded, and the pelleted cells were either fixed (see Effect of Fixation Agents for detailed method) for staining at a later time or stained immediately for flow cytometric analysis by resuspending the pellets in 100–200 µL staining buffer.
Processing of Mononuclear Cells from the Spleen and Lymph Nodes
Tissues were harvested and transferred to a 40-µm cell strainer placed on top of a 50-mL conical tube containing 7 to 8 mL PBS supplemented with 2% BSA and 2 mM EDTA and carefully triturated using the rough end of a 3-mL syringe plunger until only the colorless connective tissue is visible. Cells were pelleted by centrifugation at 500 g for 10 min (4–8°C), and the supernatant was discarded. Cells were either fixed for staining at a later time or stained immediately for flow cytometric analysis by resuspending the pellets in staining buffer.
Note: While the leukocytes from lymph nodes were resuspended in 100-200 µL of staining buffer, cells obtained from spleens were diluted appropriately to obtain a concentration of ~106/100 µL per sample for flow cytometric staining.
Effect of Fixation Agents
Splenocytes were resuspended in 400 μL PBS supplemented with 2% BSA (wt/vol in PBS) and to one-fourth of the sample either 1% paraformaldehyde (PFA; 100 uL), ice-cold methanol (1 mL), or ice-cold acetone (1 mL) was added.
Note: PFA solution can be made in bulk and stored at 4–8°C for 1 to 2 yr.
PFA treated samples were incubated on ice, whereas methanol and acetone tubes were placed in −20˚C for 10 minutes. Staining buffer (800 µL) was added to dilute excess fixing solution and centrifuged at 500 g for 8–10 minutes. Supernatant was discarded by decanting and the pellet was resuspended in the leftover solution (~100-150 µL). Resuspended fixed samples were used immediately for flow cytometric staining.
Note: Samples fixed using 1% PFA can either be used for staining immediately for flow cytometric analysis or can be stored at 4°C for staining at a later time (20, 21).
Staining for Flow Cytometry
Total volume of the sample was measured, and a known volume (~80-100 µL) was aliquoted into a 5-mL round bottom polystyrene FACS tube. An appropriate amount of Fc blocker was added (BioLegend catalog no. 101302), and the samples were incubated on ice for 10 min to block nonspecific binding sites.
Note: BSA contained in the staining buffer (used to neutralize excess fixing solution) also blocks nonspecific binding sites during storage.
Total amount of antibodies needed for all the samples was calculated and added together to prepare a cocktail in a 0.5-mL microfuge tube. The antibodies CD45-PE/Cy7, clone I3/2.3 (catalog no. 147704); CD11b-APC, clone M1/70 (catalog no. 553312); Ly6G-PE, clone 1A8 (catalog no. 127608); and B220-PerCP, clone RA3-6B2 (catalog no. 103234) were obtained from BioLegend (San Diego, CA). The antibody CD4-FITC, clone RM4-5 (catalog no. MCD0401) and Ly6C-eFluor 450 were obtained from Invitrogen (Carlsbad, CA). All the antibody-related staining protocols followed previously published guidelines on antibody use in physiology studies (10). Samples were incubated for 30-45 min on ice, followed by the addition of 4 mL cold PBS to wash excess antibody, and the cells were pelleted by centrifugation at 500 g for 10 min. The supernatant was discarded by decanting, and cells were resuspended in the leftover volume by mild to moderate vortexing. Cell counting beads (2–4 µL; Spherotech, ACBP-100-10, 1 × 106 beads/mL) were added to each sample, and the following equation was used to calculate cell numbers:
Total no. of cells/tissue = (Cells captured by flow × no. of beads added × total sample volume)/(beads captured by flow × sample volume taken for staining)
Total no. of cells/µL blood = (Cells captured by flow × no. of beads added × total sample volume)/(beads captured by flow × sample volume taken for staining × volume of blood taken)
Note: SSC-A was set on a logarithmic scale while collecting data on a flow cytometer since it allowed clear distinction of counting beads from all other cellular components.
For unfixed samples, recommended amounts of 7-aminoactinomycin D (7-AAD; Invitrogen catalog no. A1310) was added 5-10 min before data acquisition for staining dead cells.
Data Acquisition and Analysis
Data were acquired using BD LSRII or BD Fortessa. However, any other suitable flow cytometer equipped with required lasers and channel configurations can be used. Flowjo was used to analyze all data.
Statistical Analysis
All data are reported as means ± SD. Student’s unpaired 2-tailed t-test was used to compare differences between two groups and one-way ANOVA with Dunnet’s multiple comparisons test for more than two groups (as in Effect of Fixing Reagent). Shapiro-Wilk test was used to ensure a normal distribution of data. If the data distribution was not normal, it was log transformed followed by the appropriate statistical test as stated in the figure legends. All analyses were done using GraphPad Prism software following published recommendations (25), and a P value of <0.05 was considered statistically significant.
RESULTS
Effect of Density-Mediated Mononuclear Cell Isolation
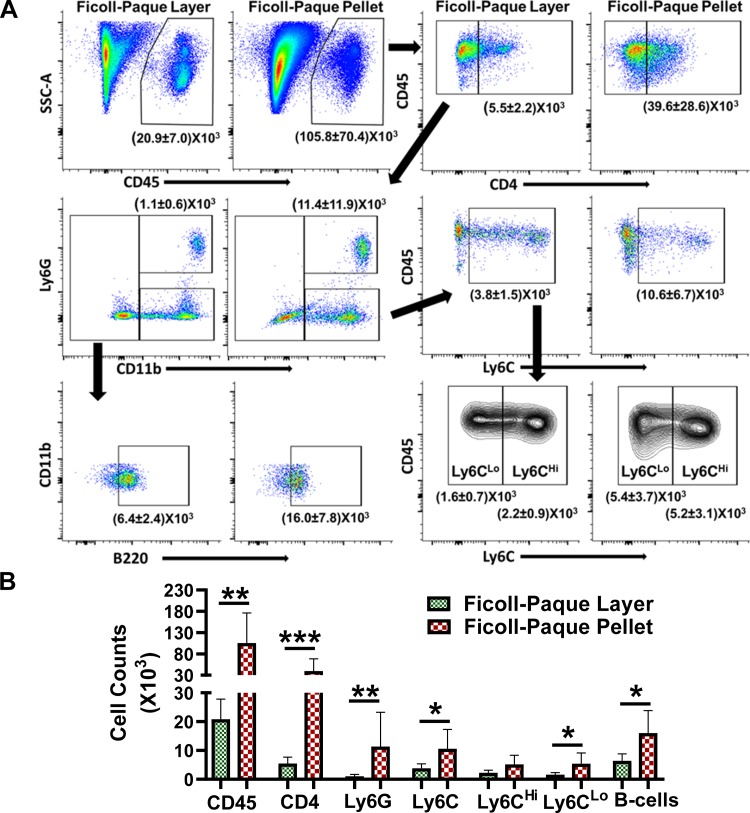
Density-mediated separation protocols are widely used to separate MNCs from the peripheral blood in humans and rodents and has also been adapted to isolate mononuclear cells from tissue digests. Therefore, we tested the efficacy and adaptability of this approach to enrich leukocyte populations and remove myocardial debris, respectively, in cardiac digests by counting total CD45+ leukocytes and different immune cell subsets including CD4+ T cells, CD11b+Ly6G+ neutrophils, Ly6Clow and Ly6Chi monocytes/macrophages, and B220+ B cells in the interphase layer and the pellets after density-mediated separation using Ficoll-Paque. As shown in Fig. 1, the yield of leukocytes from continuous gradient was low and only ~20% of cells were retrievable from the Ficoll-Paque layer as the majority (~80%) of CD45+ cells were present in the pellets. We also observed ~85% of CD4+ cells, 90% neutrophils, and 30–40% Ly6C+ monocytes and B220+ B cells in the pellets, suggesting a significant loss of both innate and adaptive immune cells in the sediments during density-mediated cell separation. The myocardial debris, however, was significantly lower in the monolayers.
Fig. 1.
A: representative flow cytometry scatter plots for CD45+, CD45+CD4+, CD45+CD4−CD11b+Ly6G+, CD45+CD4−CD11b+Ly6G−Ly6C+ (Ly6CLo and Ly6CHi), and CD45+CD4−CD11b−Ly6G−Ly6C−B220+ cells in the Ficoll-Paque layers and the pellets after density mediated cell separation of cardiac digests obtained from heart failure mice. B: quantitative group data from A. All the data except B220+ cell counts were log transformed to ensure normal distribution of data, and the transformed data for each cell type were analyzed using unpaired 2-tailed Student’s t-test. *P < 0.05, **P < 0.01, and ***P < 0.001 was considered significant (n = 5 in both groups).
Effect of Fixing Reagent
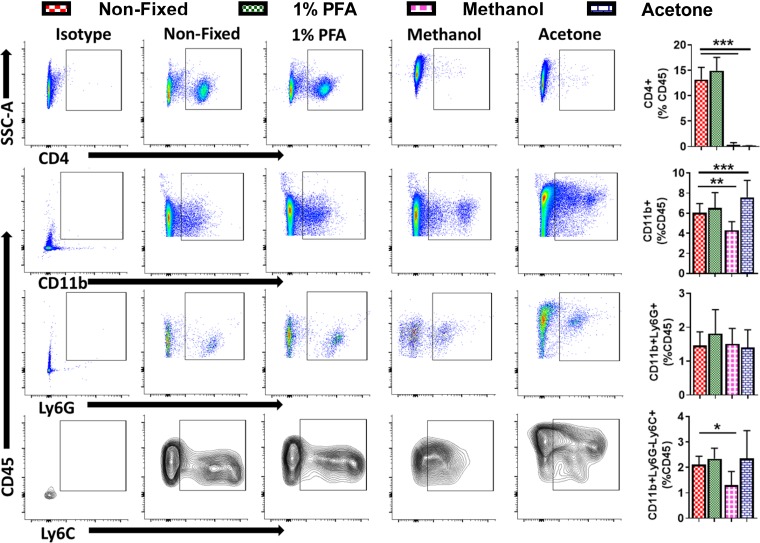
There are several reagents that can be used to fix cells for flow cytometry/immunocytochemistry and tissues for immunohistochemistry. PFA (1–4%), ice-cold methanol, and acetone are all widely used. To test the effect of fixing reagents on antibody binding, cellular morphology, and membrane integrity, we fixed splenocytes with either 1% PFA, ice-cold methanol, or acetone and compared the levels of CD4+ T cells, CD11b+ myeloid cells, CD11b+Ly6G+ neutrophils, and CD11b+Ly6G−Ly6C+ monocytes/macrophages with those of unfixed cells. As shown in Fig. 2, 1% PFA did not affect antibody binding for any of the proteins tested, and the levels for CD4+ T cells, CD11b+ cells, CD11b+Ly6G+ neutrophils, and CD11b+Ly6G−Ly6C+ monocytes were comparable with those in unfixed samples. Ice-cold methanol and acetone, on the other hand, resulted in 1) complete loss of CD4 expression/staining, 2) reduction in CD11b+ and CD11b+Ly6G−LY6C+ monocytes with ice-cold methanol, and 3) increase in CD11b+ cells with ice-cold acetone. No changes in CD11b+Ly6G+ neutrophils were observed with either of the fixing reagents. Unlike 1% PFA, we also observed changes in the cell morphology and staining patterns (as shown in representative images for CD11b, Ly6G, and Ly6C) with ice-cold methanol and acetone when compared with unfixed cells. Analysis of side-scatter area (a measure of granularity) and forward-scatter area (a measure of size) by flow cytometry revealed that there was a significant increase in cellular granularity with both methanol and acetone and an increase in cell size only with methanol, suggesting alterations in cellular morphology (Supplemental Fig. S1; all Supplemental Material is available at https://doi.org/10.5281/zenodo.3269730).
Fig. 2.
Representative flow cytometry scatter plots (left) for splenic CD45+CD4+, CD45+CD4−CD11b+, CD45+CD4−CD11b+Ly6G+, and CD45+CD4−CD11b+Ly6G−Ly6C+ cells either unfixed or fixed with 1% PFA, or cold methanol or acetone, and their quantitative group data (right). Data were analyzed using repeated-measures 1-way ANOVA with Dunnet’s multiple comparisons test. *P <0.05, **P < 0.01, and ***P < 0.001 was considered significant (n = 5 in each group).
Effect of Coronary Perfusion
As shown in Supplemental Fig. S2, A, 3 min of intravascular staining (with 3 µg antibody) was sufficient to label >95% of leukocytes in blood (positive control) and stained only 6–8% splenocytes and <2% leukocytes in the mediastinal lymph nodes (negative controls; Supplemental Fig. S2, B and C) as has been shown previously (3), underscoring the validity and reproducibility of this protocol. Using this strategy, we tested the efficacy of low-speed centrifugation (50 g) as compared with high-speed (350 g) in removing intravascular cells after tissue mincing and found that supernatant from low-speed centrifugation at 50 g contained ~17-fold more cells as compared with centrifugation at 350 g, suggesting efficient removal of contaminating intravascular cells in the supernatant at low-speed centrifugation (Supplemental Fig. S2, D).
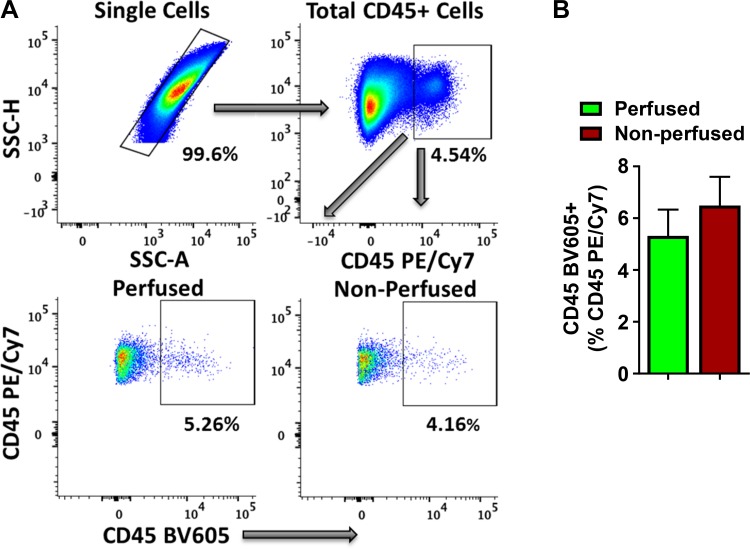
Among total cardiac mononuclear CD45+ (PE/Cy7) cells, we observed that 5.3 ± 2% of intravascular CD45+ (BV605) cells were present in the perfused hearts (Fig. 3). These low levels of circulating cells remaining in tissue digests, even after coronary perfusion for 5 min, suggest either ineffectiveness of tissue perfusion in removing strongly adherent activated immune cells during inflammatory states (such as in MI) (3) or rapid infiltration kinetics of intravascular leukocytes to the ischemic myocardium. The contamination from blood-borne cells in our isolation protocol, without coronary perfusion, was 6.5 ± 2.5%, which was not significantly different than the perfused hearts. Detailed analysis of CD45 BV605+ intravascularly labeled cells amid total CD45 PE/Cy7+ cells in the cardiac digests showed that with the exception of low levels of Ly6Clow cells in the perfused samples, levels of CD4+ T cells, CD11b+Ly6G+ neutrophils, CD11b+Ly6G−Ly6Chi monocytes, and CD11b−Ly6G−LY6C−B220+ B cells were not different between the perfused and nonperfused hearts (Supplemental Fig. S3). Importantly, 7-AAD staining showed that >95% of isolated cardiac single cells, with or without coronary perfusion, were viable and that neither of the protocols resulted in significant cell death (Supplemental Fig. S4).
Fig. 3.
A: representative flow cytometry scatter plots for single cells (top, left), total CD45+ (PE/Cy7+) leukocytes in single cell gate (top, right), and intravascular CD45+ (BV605+) cells in total CD45+ (PE/Cy7+) leukocytes in mouse cardiac digests 1-day post-MI with (bottom, left) or without (bottom, right) ex vivo retrograde coronary perfusion. B: quantitative group data from A. Data were analyzed using 2-tailed unpaired Student’s t-test (n = 4 in perfused and 5 in the nonperfused group). Part of the quantitative data was also shown in Bansal et al. (6).
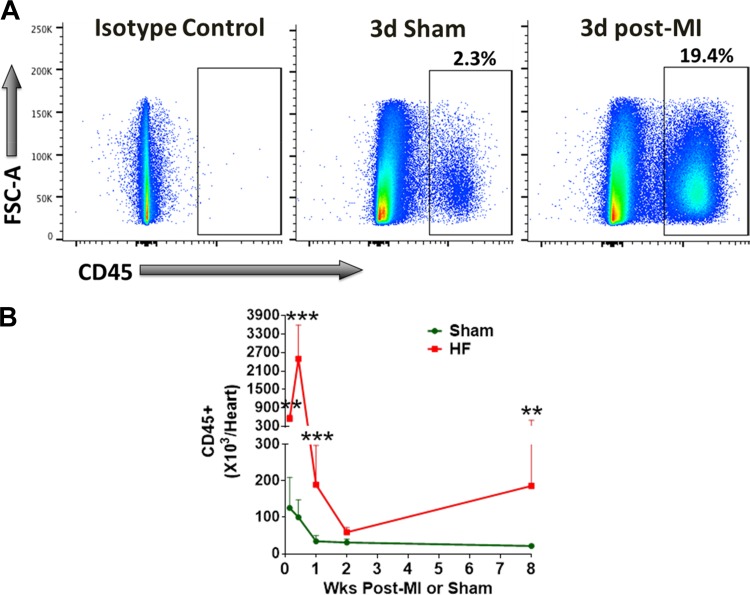
Using this optimized protocol (summarized in Table 1), we measured total CD45+ leukocytes in infarcted hearts at 1-day, 3-days, 1-wk, 2-wk, and 8-wk post-MI. As shown in Fig. 3, CD45+ cells are increased approximately fourfold in infarcted hearts at 1-day post-MI as compared with that in sham-operated hearts. Although leukocyte levels in sham-operated hearts did not change from day 1 (126 × 103 ± 84 × 103) to day 3 (100 × 103 ± 48 × 103), there was a significant increase in CD45+ leukocytes (2,497 × 103 ± 1,099 × 103 at day 3 vs. 550 × 103 ± 122 × 103 at day 1 post-MI) in infarcted hearts, suggesting a continuous influx of cells for the first 3-days post-MI. By week 1, although sham-operated hearts exhibited an observable but nonsignificant decrease in immune cell levels, leukocyte number in the ligated hearts significantly dropped (189.5 × 103 ± 108.1 × 103 at day 7) as compared with day 3 (2,497 × 103 ± 1,099 × 103). However, the levels were significantly higher in infarcted hearts (5 to 6-fold) when compared with sham (34.3 × 103 ± 15.1 × 103). Immune cell levels diminished further by 2 wk and were not different between the sham-operated and infarcted groups. At 8-wk post-MI (Fig. 3), we observed a second wave of immune infiltration with an approximate ninefold higher levels of CD45+ cells in remodeled hearts as compared with sham operation (186.1 × 103 ± 313.4 × 103 vs. 21.6 × 103 ± 4.84 × 103).
Table 1.
Summary of all the experimental variables tested, their results and recommendations
| Objective | Experimental Variable | Results | Recommendation |
|---|---|---|---|
| Density-mediated cell separation | Ficoll-Paque layer vs. Pellet | Reduced myocardial debris | Straining of cardiac digests through 40-μm cell strainers |
| 70–80% loss of leukocytes in pellets | |||
| Cell fixation | 1% PFA | Comparable with unfixed sample | Fixation using 1% PFA for 10 min at 4–8°C |
| Ice-cold methanol/acetone | Decreased antibody binding | ||
| Increased granularity due to protein denaturation | |||
| Intravascular cells | Coronary perfusion | Removal of 90–95% intravascular cells | Low-speed centrifugation at 50 g for 2 min after fine mincing of the tissue, and discard the supernatant containing intravascular cells |
| Low-speed centrifugation post-tissue mincing | Removal of 90–95% intravascular cells | ||
| Majority of intravascular cells are removed in the supernatant. |
DISCUSSION
There are several key findings in this study. First, we show that density-mediated separation protocols cannot be faithfully adapted to separate mononuclear cells from cardiac digests and needs significant optimization to eliminate leukocyte loss in pellets. Second, low-speed centrifugation of finely minced tissues before tissue-digestion is a viable alternative to time-consuming coronary perfusion methods to eliminate contamination of tissue leukocytes with blood-borne cells. Third, chronic ischemic HF 8 wk post-MI is associated with a biphasic immune expansion, i.e., maximal leukocyte infiltration at 3d post-MI decreases to levels similar to sham-operated mice by 2 wk post-MI and increases thereafter coinciding with pathological LV remodeling.
Density mediated cell separation methods using Ficoll-Paque or Percoll are widely used to isolate MNCs from the peripheral blood due to homogenous size and granularity of steady-state circulating cells. This strategy has also been used to isolate MNCs from cardiac digests in several studies (15, 17, 24). Although this method removes a significant amount of cardiac debris and other non-immune cells, it also leads to a significant loss of CD45+ leukocytes in the pellet including CD4+ T cells, CD11b+Ly6G+ Neutrophils, CD11b+Ly6G−Ly6C+ monocytes/macrophages and B220+ B cells. In contrast to circulating cells, tissue infiltrated monocytes/macrophages and T cells respond to a variety of activation stimuli to undergo maturation, proliferation or transmigration that increases their cytoplasmic complexity and granularity (33). Extensive cell divisions (such as in tumors) also induce signaling imbalances associated with increased size and granularity (33), an attribute shared by rapidly proliferating monocytes, macrophages, dendritic cells and T cells in the failing hearts (39). Indeed, it has been shown that cardiac resident (CCR2−HLA-DRhi) and infiltrated (CCR2+HLA-DRhi) macrophages in failing hearts exhibit higher granularity and density as compared with CCR2+HLA-DRlow monocytes (4). Other studies have shown that median lymphocyte volume in circulating cells is higher in patients with MI as compared with those without (11), suggesting dynamic alterations related to the size and granularity of circulating immune cells during immune-activation states.
In these studies, we used AccuCount beads to calculate an absolute number of target cells in our samples. This is an alternative technique to the classic volumetric enumeration of target cells either via hemocytometer counting or cell marker percentages (frequencies) derived from the flow cytometric gating analysis of a known volume of sample. Although both the methods are widely used across different laboratories, we chose the bead-counting method due to several reasons. First, the volumetric enumeration may introduce errors in highly concentrated samples that contain large amounts of tissue debris, and differ due to sampling variations by different independent operators and day-to-day laboratory activities (7, 14). A small sampling error during early stages can expand exponentially through the multiplication of dilution factors in subsequent steps. Reference particles have been shown to mitigate sampling errors across laboratory groups and within laboratory environments by using the same number of particles per volume in each sample run (36). Second, as shown in Fig. 4, the absolute number of cells isolated from sham mice is 3.3 × 104 ± 1.5 × 104 cells/heart after 1 wk of sham-operation. This is a relatively small number of cells and will require the majority of the sample to reach the gold standard of 10,000 events/sample for the accurate evaluation of flow cytometry data. As a consequence, any volumetric amount used to perform cell counts can hinder the ability to obtain the minimum events during flow cytometry sessions. Third, cell counting using reference beads has been validated by multiple groups in diverse tissues such as blood CD4+ lymphocytes (9), mucosal mononuclear cells from macaque colorectal biopsies (35), and mouse lung macrophages (31) and platelets (2). Therefore, given low leukocyte numbers and significant levels of cardiac debris, cell counting using reference beads is a viable alternative to ensure consistency in cell counting across different users and laboratories.
Fig. 4.
A: representative flow cytometry scatter plots for isotype controls (left) and CD45+ cells in the hearts isolated from mice 3 days after the sham surgery (middle) or myocardial infarction (MI, right). B: quantitative group data for total CD45+ cells/heart at the indicated time points. Log-transformed data were analyzed using 2-way ANOVA with Bonferroni post hoc test. N = 5–10/group; **P < 0.01, ***P < 0.001 vs. sham.
Although live cell flow-cytometric imaging is highly desirable, cell fixation (coupled with permeabilization) is often required to measure the expression of intracellular cytokines, transcription-factors and phospho-proteins. There are several fixing agents reported in the literature such as aldehydes (formaldehyde/paraformaldehyde and glutaraldehyde), alcohols (cold ethanol or methanol) and acetone that use different mechanisms to achieve this goal. A comparative analysis of all these methods showed that fixation using low PFA concentrations (0.5–1%) at 4–8°C for 10 min is the most appropriate fixing method that not only preserves cell structure and shape but also prevents loss of antibody binding for flow cytometric analysis. With ice-cold methanol and acetone, we observed a significant loss of CD4+ antibody binding along with significantly increased cellular granularity and size (with methanol) due to protein precipitation resulting from their denaturing properties (15). Loss of antibody binding post-fixation is usually associated with a particular clone and hence can readily be corrected by using an alternate clone. While we did not test the impact of storage on fixed samples, several previous studies have shown that samples fixed with 0.5–1% PFA can be stored for 1–2 wk at 4–8°C providing a considerable advantage over other methods, especially for time-constrained studies (20, 21).
Immune cells continuously recirculate throughout the capillary network and vasculature of every organ. These circulating cells often exhibit different functions owing to their different activation states and phenotypes as compared with tissue localized cells and mostly do not participate in tissue immune responses (41). Therefore, discrimination between the blood-borne cells from those of tissue localized cells is critical to study immunological responses in non-lymphoid tissues. Perfusion mediated removal of intravascular cells is often carried out to rule out contamination from circulating cells from tissue-leukocytes. However, tissue-perfusion has also been shown to deplete leukocytes from the tissue compartments leading to underestimation of immune infiltration kinetics (3). Therefore, we compared and found no differences between the method involving perfusion mediated removal of intravascular cells and our protocol that efficiently removed most of the intravascular cells during the 1st step of centrifugation (see Preparation of Single Cell Suspensions from Hearts). Furthermore, Comparable levels of circulating CD45 (BV605+) leukocytes, CD4+ helper T cells, CD11b+Ly6G+ neutrophils, CD11b+Ly6G−Ly6Chi monocytes, and CD11b−Ly6G−Ly6C−B220+ B cells in the perfused and nonperfused hearts obviates the need for coronary perfusion and suggests that centrifugation mediated removal of circulating cells could be effectively used to eliminate blood-borne cells while isolating tissue leukocytes. Notably, we observed a diffuse staining pattern with high frequencies of CD45+ cells labeled with injected anti-CD45 BV605+ (intravascular cells) among total splenocytes when compared with cells in the lymph nodes. It has been shown that inflammatory conditions alter vascular permeability allowing rapid exudation of antibody or antibody stained leukocytes into the tissues (3). It is also known that spleen acts as an extra-medullary source of monocytopoiesis and monocyte trafficking 1d post-infarction (23). This rapid exchange of antibody stained circulating leukocytes with the splenic populations further substantiates the role of the spleen in mediating immune cell trafficking during the early post-MI period.
It is clear from several studies that the acute inflammatory response post-MI involves rapid (1–3 days post-MI) infiltration of neutrophils (28), CCR2+ proinflammatory Ly6Chi monocytes (28), dendritic cells (42) and T-lymphocytes (16) for prompt clearance of dead and apoptotic cells. This is followed by a reparative phase involving Ly6Clow monocytes (28), iNKT cells (38), B cells (43), and Tregs (40) that lasts from 3 to 14 days post-MI. This initial immune response almost completely subsides by day 14–15 post-MI (18, 28) consistent with the total leukocyte counts observed by us using our optimized protocol. Our total leukocyte analysis also showed the 2nd phase of immune activation and infiltration into the myocardium during chronic HF (8 wk post-MI) that coincides with increased fibrosis and LV remodeling (32). Importantly, this secondary immune activation is associated with i) immune memory as adoptive transfer of splenocytes (17) or activated T cells (5) from HF spleens to naïve animals induces fibrosis, hypertrophy and significant cardiac dysfunction; and ii) a pathological switch to a proinflammatory/profibrotic phenotype that negatively impacts cardiac function as depletion of T cells specifically during chronic HF blunts progressive cardiac dysfunction (5). Nonetheless, this 2nd phase of immune activation is a critical stage for developing and testing immunomodulatory strategies for HF.
In summary, density-mediated mononuclear cell isolation strategies are less precise for quantifying tissue infiltrating immune cells as altered cell activation status upon tissue transmigration alters their granularity and hence density. Loss of mononuclear cells in the pellets underscores the importance of optimizing this step before undertaking detailed quantitative immune cell analysis in non-lymphoid tissues. Additionally, centrifugation of finely minced tissues at a very low speed is a viable alternate strategy to time-consuming tissue perfusion approaches for removing the majority of intravascular blood cells. Our protocol not only improves the cellular yield but also decreases the total processing time for immune cell isolation by optimizing experimental conditions for several critical steps.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants K99 HL132123 and R00 HL132123 (to S. S. Bansal), NIH R01 HL127442 and AHA GIA 17GRNT33700188 (to R. J. Gumina), NIH R01 HL137046 (to T. Hamid), NIH R01 HL136951 (to F. Accornero), NIH R01 HL133050 (to H. Singh), and NIH R01 HL125735, R01 HL147549, and VA I01 BX002706 grants (to S. D. Prabhu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.I., T.H., F.A., R.J.G., S.P., and S.B. conceived and designed research; R.C., G.R., and S.B. performed experiments; R.C., M.A.I., G.R., T.H., R.J.G., and S.B. analyzed data; R.C., M.A.I., G.R., T.H., F.A., H.S., R.J.G., S.P., and S.B. interpreted results of experiments; S.B. prepared figures; R.C. and S.B. drafted manuscript; R.C., F.A., H.S., R.J.G., S.P., and S.B. edited and revised manuscript; R.C., M.A.I., G.R., T.H., F.A., H.S., R.J.G., S.P., and S.B. approved final version of manuscript.
REFERENCES
- 1.Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, Rose NR. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am J Pathol 164: 807–815, 2004. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alugupalli KR, Michelson AD, Barnard MR, Leong JM. Serial determinations of platelet counts in mice by flow cytometry. Thromb Haemost 86: 668–671, 2001. doi: 10.1055/s-0037-1616102. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9: 209–222, 2014. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, Shankar TS, Selzman CH, Drakos SG, Lavine KJ. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 24: 1234–1245, 2018. doi: 10.1038/s41591-018-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail 10: e003688, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 139: 206–221, 2019. doi: 10.1161/CIRCULATIONAHA.118.036065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett D, Granger V, Whitby L, Storie I, Reilly JT. Absolute CD4+ T-lymphocyte and CD34+ stem cell counts by single-platform flow cytometry: the way forward. Br J Haematol 106: 1059–1062, 1999. doi: 10.1046/j.1365-2141.1999.01632.x. [DOI] [PubMed] [Google Scholar]
- 8.Bönner F, Borg N, Burghoff S, Schrader J. Resident cardiac immune cells and expression of the ectonucleotidase enzymes CD39 and CD73 after ischemic injury. PLoS One 7: e34730, 2012. doi: 10.1371/journal.pone.0034730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brando B, Barnett D, Janossy G, Mandy F, Autran B, Rothe G, Scarpati B, D’Avanzo G, D’Hautcourt JL, Lenkei R, Schmitz G, Kunkl A, Chianese R, Papa S, Gratama JW; European Working Group on Clinical Cell Analysis . Cytofluorometric methods for assessing absolute numbers of cell subsets in blood. Cytometry 42: 327–346, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Brooks HL, Lindsey ML. Guidelines for authors and reviewers on antibody use in physiology studies. Am J Physiol Heart Circ Physiol 314: H724–H732, 2018. doi: 10.1152/ajpheart.00512.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhury A, Noiret L, Higgins JM. White blood cell population dynamics for risk stratification of acute coronary syndrome. Proc Natl Acad Sci USA 114: 12344–12349, 2017. doi: 10.1073/pnas.1709228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng X, Liao Y-H, Ge H, Li B, Zhang J, Yuan J, Wang M, Liu Y, Guo Z, Chen J, Zhang J, Zhang L. TH1/TH2 functional imbalance after acute myocardial infarction: coronary arterial inflammation or myocardial inflammation. J Clin Immunol 25: 246–253, 2005. [Erratum in J Clin Immunol 34: 748–749, 2014.] doi: 10.1007/s10875-005-4088-0. [DOI] [PubMed] [Google Scholar]
- 13.Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol 35: 1066–1070, 2015. doi: 10.1161/ATVBAHA.114.304652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratama JW, Kraan J, Keeney M, Granger V, Barnett D. Reduction of variation in T-cell subset enumeration among 55 laboratories using single-platform, three or four-color flow cytometry based on CD45 and SSC-based gating of lymphocytes. Cytometry 50: 92–101, 2002. doi: 10.1002/cyto.10084. [DOI] [PubMed] [Google Scholar]
- 15.Hobro AJ, Smith NI. An evaluation of fixation methods: Spatial and compositional cellular changes observed by Raman imaging. 91: 31–45, 2016. [Google Scholar]
- 16.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125: 1652–1663, 2012. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 17.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 114: 266–282, 2014. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kain V, Halade GV. Big eater macrophages dominate inflammation resolution following myocardial infarction. J Mol Cell Cardiol 87: 225–227, 2015. doi: 10.1016/j.yjmcc.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol 109: 444, 2014. doi: 10.1007/s00395-014-0444-7. [DOI] [PubMed] [Google Scholar]
- 20.Lal RB, Edison LJ, Chused TM. Fixation and long-term storage of human lymphocytes for surface marker analysis by flow cytometry. Cytometry 9: 213–219, 1988. doi: 10.1002/cyto.990090305. [DOI] [PubMed] [Google Scholar]
- 21.Lanier LL, Warner NL. Paraformaldehyde fixation of hematopoietic cells for quantitative flow cytometry (FACS) analysis. J Immunol Methods 47: 25–30, 1981. doi: 10.1016/0022-1759(81)90253-2. [DOI] [PubMed] [Google Scholar]
- 22.Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo J-L, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res 107: 1364–1373, 2010. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 209: 123–137, 2012. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindsey ML, Gray GA, Wood SK, Curran-Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol 315: H303–H313, 2018. doi: 10.1152/ajpheart.00309.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch TL IV, Ismahil MA, Jegga AG, Zilliox MJ, Troidl C, Prabhu SD, Sadayappan S. Cardiac inflammation in genetic dilated cardiomyopathy caused by MYBPC3 mutation. J Mol Cell Cardiol 102: 83–93, 2017. doi: 10.1016/j.yjmcc.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res 191: 15–28, 2018. doi: 10.1016/j.trsl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velázquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM, Alcaide P. Left ventricular T-cell recruitment contributes to the pathogenesis of heart failure. Circ Heart Fail 8: 776–787, 2015. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD. CCR2+ monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic Transl Sci 3: 230–244, 2018. doi: 10.1016/j.jacbts.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poczobutt JM, De S, Yadav VK, Nguyen TT, Li H, Sippel TR, Weiser-Evans MCM, Nemenoff RA. Expression profiling of macrophages reveals multiple populations with distinct biological roles in an immunocompetent orthotopic model of lung cancer. J Immunol 196: 2847–2859, 2016. doi: 10.4049/jimmunol.1502364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119: 91–112, 2016. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raghuram GV, Pathak N, Jain D, Panwar H, Pandey H, Jain SK, Mishra PK. Molecular mechanisms of isocyanate induced oncogenic transformation in ovarian epithelial cells. Reprod Toxicol 30: 377–386, 2010. doi: 10.1016/j.reprotox.2010.05.087. [DOI] [PubMed] [Google Scholar]
- 34.Ramos GC, van den Berg A, Nunes-Silva V, Weirather J, Peters L, Burkard M, Friedrich M, Pinnecker J, Abeßer M, Heinze KG, Schuh K, Beyersdorf N, Kerkau T, Demengeot J, Frantz S, Hofmann U. Myocardial aging as a T-cell-mediated phenomenon. Proc Natl Acad Sci USA 114: E2420–E2429, 2017. doi: 10.1073/pnas.1621047114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves RK, Evans TI, Gillis J, Wong FE, Connole M, Carville A, Johnson RP. Quantification of mucosal mononuclear cells in tissues with a fluorescent bead-based polychromatic flow cytometry assay. J Immunol Methods 367: 95–98, 2011. doi: 10.1016/j.jim.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reimann KA, O’Gorman MR, Spritzler J, Wilkening CL, Sabath DE, Helm K, Campbell DE; The NIAID DAIDS New Technologies Evaluation Group . Multisite comparison of CD4 and CD8 T-lymphocyte counting by single- versus multiple-platform methodologies: evaluation of Beckman Coulter flow-count fluorospheres and the tetraONE system. Clin Diagn Lab Immunol 7: 344–351, 2000. doi: 10.1128/CDLI.7.3.344-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, Adachi H, Yashiro K, Suzuki K. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest 126: 2151–2166, 2016. doi: 10.1172/JCI85782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobirin MA, Kinugawa S, Takahashi M, Fukushima A, Homma T, Ono T, Hirabayashi K, Suga T, Azalia P, Takada S, Taniguchi M, Nakayama T, Ishimori N, Iwabuchi K, Tsutsui H. Activation of natural killer T cells ameliorates postinfarct cardiac remodeling and failure in mice. Circ Res 111: 1037–1047, 2012. doi: 10.1161/CIRCRESAHA.112.270132. [DOI] [PubMed] [Google Scholar]
- 39.Van der Borght K, Scott CL, Nindl V, Bouché A, Martens L, Sichien D, Van Moorleghem J, Vanheerswynghels M, De Prijck S, Saeys Y, Ludewig B, Gillebert T, Guilliams M, Carmeliet P, Lambrecht BN. Myocardial infarction primes autoreactive T cells through activation of dendritic cells. Cell Reports 18: 3005–3017, 2017. doi: 10.1016/j.celrep.2017.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weirather J, Hofmann UDW, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 115: 55–67, 2014. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 41.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 496: 445–455, 2013. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yilmaz A, Dietel B, Cicha I, Schubert K, Hausmann R, Daniel WG, Garlichs CD, Stumpf C. Emergence of dendritic cells in the myocardium after acute myocardial infarction - implications for inflammatory myocardial damage. Int J Biomed Sci 6: 27–36, 2010. [PMC free article] [PubMed] [Google Scholar]
- 43.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guérin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre J-S, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 19: 1273–1280, 2013. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]