Abstract
In the current study, the potential contributions of ryanodine receptors (RyRs) to intrinsic pumping and responsiveness to substance P (SP) were investigated in isolated rat mesenteric collecting lymphatic vessels. Responses to SP were characterized in lymphatic vessels in the absence or presence of pretreatment with nifedipine to block L-type Ca2+ channels, caffeine to block normal release and uptake of Ca2+ from the sarcoplasmic reticulum, ryanodine to block all RyR isoforms, or dantrolene to more selectively block RyR1 and RyR3. RyR expression and localization in lymphatics was also assessed by quantitative PCR and immunofluorescence confocal microscopy. The results show that SP normally elicits a significant increase in contraction frequency and a decrease in end-diastolic diameter. In the presence of nifedipine, phasic contractions stop, yet subsequent SP treatment still elicits a strong tonic contraction. Caffeine treatment gradually relaxes lymphatics, causing a loss of phasic contractions, and prevents subsequent SP-induced tonic contraction. Ryanodine also gradually diminishes phasic contractions but without causing vessel relaxation and significantly inhibits the SP-induced tonic contraction. Dantrolene treatment did not significantly impair lymphatic contractions nor the response to SP. The mRNA for all RyR isoforms is detectable in isolated lymphatics. RyR2 and RyR3 proteins are found predominantly in the collecting lymphatic smooth muscle layer. Collectively, the data suggest that SP-induced tonic contraction requires both extracellular Ca2+ plus Ca2+ release from internal stores and that RyRs play a role in the normal contractions and responsiveness to SP of rat mesenteric collecting lymphatics.
NEW & NOTEWORTHY The mechanisms that govern contractions of lymphatic vessels remain unclear. Tonic contraction of lymphatic vessels caused by substance P was blocked by caffeine, which prevents normal uptake and release of Ca2+ from internal stores, but not nifedipine, which blocks L-type channel-mediated Ca2+ entry. Ryanodine, which also disrupts normal sarcoplasmic reticulum Ca2+ release and reuptake, significantly inhibited substance P-induced tonic contraction. Ryanodine receptors 2 and 3 were detected within the smooth muscle layer of collecting lymphatic vessels.
Keywords: caffeine, dantrolene, lymphatic, ryanodine receptor, smooth muscle
INTRODUCTION
Lymphatic vessels have critical functions in immunity, fluid homeostasis, and maintenance of normal tissue volume (6). The latter functions are especially evident when lymphatic vessels fail to adequately drain excess interstitial fluid. The resulting lymphedema manifests can range from mild tissue swelling to severe and disfiguring (45). Unlike the blood vessels of the circulatory system that comprise a closed loop with blood flow generated by the pumping of the heart, lymphatic networks are blind ended and primarily move lymph by the intrinsic phasic contractions of the smooth muscle layer of individual collecting lymphatic vessels (54). Bicuspid, intraluminal valves prevent backflow of lymph. The segments between the valves are called lymphangions (6).
Each phasic contraction is coupled one-to-one with a preceding action potential and transient elevation in intracellular free Ca2+ ([Ca2+]i) within the smooth muscle layer. The action potentials are preceded by spontaneous transient depolarizations (STDs) that upon reaching threshold stimulate the opening of voltage-gated ion channels (59, 64, 68, 71). Internal stores of Ca2+ have an important role, as STDs can be abolished by inhibiting reuptake of Ca2+ into the sarcoplasmic reticulum (SR) (13, 69). In addition, activation of IP3 receptor-mediated release of Ca2+ enhances STD amplitude and frequency (71). Ca2+-activated Cl− (CaCl) channels contribute to STDs and oscillation of [Ca2+]i (4, 64, 71, 72). T-type voltage-gated Ca2+ channels and hyperpolarization-activated inward currents mediated by hyperpolarization-activated, cyclic nucleotide-gated channels also appear to contribute to lymphatic vessel pacemaking (39, 43, 46). Opening of voltage-gated Na+ and Ca2+ channels including tetrodotoxin-sensitive channels and L-type Ca2+ channels both contribute to action potentials (22, 59, 61, 71). Transient increases in [Ca2+]i accompany the action potentials and elicit phasic contractions (24, 53, 55, 56, 71, 76). Between phasic contractions, the vessels maintain some tone and typically do not completely relax (11, 16, 17, 35, 36, 57).
Various neurotransmitters and paracrine signals released from local nerve fibers in the walls modulate contractions, for example, substance P (SP) (14, 52). SP generally causes increased tone and contraction frequency (1, 12, 58, 60), yet the mechanistic aspects of how the contractions are modulated remain unclear. One key question is to what extent entry of extracellular Ca2+ versus release of Ca2+ from internal stores contributes to the increase in lymphatic tone caused by SP. To address this question, we designed a series of experiments to block entry of extracellular Ca2+ through L-type Ca2+ channels or deplete internal stores. These two pathways can be blocked using the pharmacological agents nifedipine and caffeine, respectively (55).
Another key question pertaining specifically to SR Ca2+ release and reuptake is whether ryanodine receptors (RyRs) have any functional role in this process in lymphatic vessels and contribute to lymphatic contractions. To directly address this question, the plant alkaloid ryanodine, isolated from Ryania speciose, can be used to modulate RyRs (9). Although RyRs have defined roles in neurons, cardiac muscle, and skeletal muscle (27, 62), studies of the impact of ryanodine on lymphatic pumping have produced mixed results. Application of ryanodine (10−7 and 10−6 M) significantly inhibited contractions of bovine mesenteric lymphatic vessels (2, 3). However, in isolated guinea pig lymphatics, although ryanodine treatment caused a brief increase in [Ca2+]i, it had no effect on the frequency of transients or phasic contractions, nor any impact on the Ca2+ transients or contractions evoked by endothelin-1 (79). Ryanodine treatment also did not affect generation of STDs in guinea pig collecting lymphatics (71).
To what extent lymphatic vessels express RyRs has also not been previously explored to our knowledge. Three isoforms, RyR1, RyR2, and RyR3, have been identified in mammalian tissues. RyR1 is typically found in skeletal muscle, RyR2 is abundant in cardiac myocytes, and RyR3 was originally identified in brain but is found in a variety of different tissues and cell types (18, 20, 37). Whether particular RyR isoforms contribute to lymphatic contractions can be tested with the use of selective pharmacologic agents such as dantrolene, which has been described as selective for RyR1 and RyR3 but not RyR2 (78).
In the current study, we investigated the relative contributions of extracellular entry of Ca2+ versus release from internal stores in SP-induced enhancement of lymphatic vessel tone. We then focused our investigation on the potential role of RyRs. In addition, because we found no previous reports about which RyR isoforms are expressed in lymphatic vessels, we used quantitative PCR (qPCR) to evaluate the presence of the mRNA for RyR1, RyR2, and RyR3 in lysates from isolated lymphatic vessels. We also evaluated localization of RyR isoforms by immunofluorescence confocal microscopy in isolated rat mesenteric collecting lymphatic vessels.
MATERIALS AND METHODS
Materials.
Ryanodine (Cat. No. 1329) and dantrolene (Cat. No. 0507) were purchased from Tocris Bioscience/R&D Systems (Minneapolis, MN). Nifedipine (Cat. No. N7634), caffeine (Cat. No. C0750), and SP (Cat. No. S6883) were purchased from Sigma-Aldrich (St. Louis, MO). Crystallized bovine albumin (purified BSA; Cat. No. J10856) used for preparing physiological salt solutions was purchased from Alfa Aesar-Thermo Fisher Scientific (Tewksbury, MA). All other chemicals and reagents, unless otherwise specified, were purchased from Sigma-Aldrich.
Animal care and use.
All procedures were approved by the Institutional Animal Care and Use Committee at the University of South Florida under protocol number IS00002179. These studies were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (8th ed., 2011) and are reported here in accordance with the ARRIVE Guidelines. A total number of 46 male Sprague-Dawley rats (5–9 wk of age) were purchased from Charles River (Wilmington, MA) and housed in a controlled-temperature (22°C) and controlled-illumination (12-h:12-h light-dark cycle) environment. After arrival, the rats were submitted to a 1-wk acclimation period and were provided standard rat chow (2018 Teklad Global 18% Protein Rodent Diet, Harlan, Indianapolis, IN) and water ad libitum. When possible, multiple lymphatics or tissue samples were obtained from a single rat for multiple experiments to minimize the total number of rats used. All possible measures were taken to minimize pain or suffering, including the administration of general anesthesia before performing experiments (details provided in Mesenteric collecting lymphatic isolation). All rats were euthanized by extending the laparotomy into the chest cavity and injecting Euthasol/Somnasol (0.1 mL/450 g body wt) directly into the cardiac ventricle, in accordance with American Veterinary Medical Association guidelines for the euthanasia of animals.
Mesenteric collecting lymphatic isolation.
Collecting lymphatics were isolated as previously described (35, 65). Briefly, rats were anesthetized (intraperitoneal ketamine and xylazine at 90 and 9 mg/kg, respectively, or isoflurane inhalation), and depth of anesthesia was checked using interdigital pinch and palpebral blink reflex. A midline laparotomy was performed, and the small intestine and mesentery were exteriorized, excised, and placed in ice-cold albumin physiological salt solution (APSS: 120 mM NaCl, 4.7 mM KCl, 2 mM CaCl2·2H2O, 1.2 mM MgSO4·7H2O, 1.2 mM NaH2PO4, 2 mM Na pyruvate, 5mM glucose, 0.02 mM EDTA, 3 mM MOPS, and 1% BSA). Rats were then immediately euthanized as indicated above. In each experiment, a section of mesentery that included the terminal ileum was pinned in a dissection chamber containing ice-cold APSS, and with the aid of a stereomicroscope, a collecting lymphatic vessel (60–150 µm internal diameter and ~0.5 cm length) was carefully dissected from surrounding adipose and connective tissue. The isolated lymphatic was transferred to an isolated vessel chamber (Living Systems Instrumentation, Burlington, VT) and was mounted onto two resistance-matched glass micropipettes and secured with nylon thread. The chamber was transferred to an Accu-Scope 3032 inverted microscope, equipped with a halogen lamp, 10× objective, and charge-coupled device camera for video image acquisition (Living Systems). Intraluminal pressure was imposed using APSS-filled gravity manometers that fed to each micropipette. All experiments were performed with the vessel bathed in 37°C-APSS with the intraluminal pressure set at 2 cm H2O to let the vessel equilibrate for baseline measurements. Only lymphatic vessels that displayed phasic contractions that reduced internal diameter during systole by at least 25% of the diastolic diameter were used for these studies.
Experimental protocols.
After cannulation, the vessels were allowed to equilibrate for 30–45 min to establish baseline contractions. Upon achieving a steady baseline pumping, each protocol started with baseline tracking of diameter for at least 10 min. The impact of SP on lymphatic contractions was assessed by adding it directly to the bath. The SP was prepared initially in a 0.05-M acetic acid solution for storage in aliquots at −20°C (10−3 M SP stock solution). On the day of the experiment, the stock solution was first diluted in APSS to obtain 10−7 M SP, and then 500 µL of APSS was removed from the 5-mL bath and replaced with 500 µL of the 10−7 M SP solution to obtain a final concentration of 10−8 M SP. This concentration was previously reported to produce a positive chronotropic and inotropic response in isolated rat lymphatic vessels (12). A separate experiment in which the vehicle control group (5 × 10−7 M) was compared with baseline was also performed. Nifedipine was used to test the impact of L-type Ca2+ channel blockade on SP-induced lymphatic changes in lymphatic contractions. An initial concentration-response study with nifedipine was performed with n = 5 isolated lymphatic vessels to determine an effective concentration for study. Increasing concentrations of nifedipine were added to the bath every 10–15 min over a final concentration range of 10−10 to 10−6 M, and lymphatic contraction data were recorded for a 2-min period starting 2 min after the addition of each concentration. Because nifedipine was prepared in DMSO for storage in aliquots at −20°C, a concentration-response study with the final DMSO concentrations in the bath was also performed to rule out effects of this vehicle (range 2.8 × 10−7 to 2.8 × 10−3 M). In subsequent studies, nifedipine was applied at 10−7 M based upon the findings from the concentration-response study. The role of intracellular Ca2+ stores in SP-induced lymphatic contractions was tested by adding caffeine to the bath at a final concentration of 10−2 M before SP treatment. Caffeine was dissolved directly in APSS before its addition to the bath. Because caffeine affects multiple targets, including RyRs, the IP3 receptor, and cAMP phosphodiesterase (5, 66), we also directly tested the role of RyRs, using ryanodine at a final concentration of 10−5 M. This concentration has been reported to modify open time of RyRs, generating a “half-open” state that blocks stimulated Ca2+ release from the SR (5, 62). Dantrolene, which is used therapeutically for malignant hyperthermia, was also used at 10−5 M because of its previously described properties to selectively block RyR1 and RyR3 but not RyR2 (78). Ryanodine and dantrolene were both prepared in DMSO, and because the final DMSO concentration applied to the bath was 2.8 × 10−2 M, an additional vehicle control study was performed with this concentration of DMSO. Vessel diameters were tracked throughout each experiment, and the bath solution was changed to Ca2+-free APSS (120 mM NaCl, 4.7 mM KCl, 0.6 mM EDTA, 1.2 mM MgSO4·7H2O, 1.2 mM NaH2PO4, 2 mM Na pyruvate, 5 mM glucose, 3mM MOPS, and 1% BSA) at the end of each experiment to determine the maximal passive diameter (MaxD) at the same luminal pressure imposed earlier in the protocol. Parameters used to characterize lymphatic pump function were calculated from the data (outlined below in Data analyses).
Total RNA isolation and PCR.
Mesenteric collecting lymphatic vessels (30 vessels per rat), hearts, brains, or soleus muscle were freshly isolated, placed on ice in 500 µL QIAzol Lysis Reagent (Qiagen, Valencia, CA), homogenized, snap frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted using chloroform and isopropanol, and the samples were treated with DNase to remove genomic DNA. The RNA was then purified using the RNeasy MinElute Cleanup Kit (Qiagen) according to the manufacturer’s specifications, and the RNA pellet was eluted in 14 µL RNase-free water. RNA concentration and purity were determined using the NanoVue Plus (GE Healthcare, Piscataway, NJ). Total RNA was reverse transcribed into cDNA using the High-Capacity RNA-to-cDNA kit (Applied Biosystems/Thermo Fisher Scientific, Waltham, MA). The cDNA was combined with TaqMan Universal PCR Master Mix (Applied Biosystems/Thermo Fisher Scientific) and primers specific for either rat Ryr1, Ryr2, Ryr3 or rat GAPDH. PCR reactions were performed using a 2-min hold at 50°C, 10-min hold at 95°C, 95°C for 15 s, and 60°C for 1 min (40 cycles) using a QuantStudio 3 System (Thermo Fisher Scientific). The primer sequences used, obtained from Applied Biosystems/Thermo Fisher Scientific, were Rn01545085_m1 (Ryr1), Rn01470303_m1 (Ryr2), Rn01486097_m1 (Ryr3), and Rn01775763_g1 (GAPDH). From the qPCR data obtained, relative expression to GAPDH was determined using the 2-ΔCt method. PCR reaction products were run on a 3% agarose gel, and bands were made detectable with Gelred nucleic acid gel stain (Biotium Inc., Fremont, CA) and visualized under UV, using the Bio-Rad Chemi Doc XRS+ System with Quantity 1-D Analysis Software (Bio-Rad, Hercules, CA).
Immunofluorescence labeling and confocal microscopy.
Rat mesenteric lymphatics were isolated and excised, and for each isolated vessel, one end was mounted onto a glass micropipette filled with APSS in a custom chamber for fixation and labeling as previously described (35, 65). The other end of the lymphatic was left free to float in an APSS bath to allow for APSS to be pulled into or pushed out of the vessel lumen by gently applying negative or positive pressure, respectively, via an attached 1-mL syringe. Each vessel was fixed with 4% paraformaldehyde for 10–15 min at room temperature, followed by two 5-min washes with 100 mM glycine buffer and one 5-min wash with Ca2+/Mg2+-free Dulbecco’s PBS (CMF-DPBS). Ice-cold acetone (−10 to −20°C) was applied for 5 min to permeabilize the cell membranes, followed by three 5-min washes with CMF-DPBS. A blocking solution consisting of 5% normal donkey serum in CMF-DPBS was applied for 30 min at room temperature, followed by overnight incubation with primary antibodies in antibody dilution buffer (151 mM NaCl, 17 mM trisodium citrate, 2% donkey serum, 1% BSA, 0.05% Triton X-100, 0.02% NaN3) at 4°C: 1:500 rabbit anti-RyR2 (AB9080, Chemicon); 1:500 rabbit anti-RyR3 (AB9082, Chemicon); and 1:1,000 mouse anti-α/γ-smooth muscle actin (SMA) (Mab1522, Millipore). All antibodies were validated in previous reports (29, 65, 67, 70, 77). Labeling controls received antibody dilution buffer containing no primary antibody. After overnight incubation, three 10-min rinses with antibody wash solution (151 mM NaCl, 17 mM trisodium citrate, 0.05% Triton X-100) were performed. The vessels were incubated for 30–60 min at room temperature with antibody dilution buffer containing secondary antibodies: 1:400 Alexa-488-donkey anti-rabbit IgG (A21206) and 1:400 Alexa-594, donkey-anti-mouse IgG (A21203). Three 10-min rinses with antibody wash solution were performed, and each vessel was removed from its cannula, placed on a glass slide with a Secure-Seal imaging spacer in 15 µL Prolong Gold Anti-Fade reagent containing DAPI (Life Technologies), and covered with a no. 1 glass coverslip. Care was taken to keep the lymphatic vessel lumen patent. Confocal z-stack images (step size = 1.0 μm) of the vessels were obtained with an Olympus FV1200 spectral inverted laser scanning confocal microscope and a ×40 objective (UPLAN, SAPO, 0.90NA) at the Lisa Muma Weitz Advanced Microscopy and Cell Imaging Core at the University of South Florida. FIJI/ImageJ open-source imaging software (http://fiji.sc) was used to process confocal image stacks into figures.
Data analyses.
Data analyses included collection of lymphatic diameter over time, before and after addition of various drugs and SP. Parameters used to characterize lymphatic pump function were determined from the lymphatic intraluminal diameter measurements. These include contraction frequency (CF), end-diastolic diameter (EDD), end-systolic diameter (ESD), amplitude of contraction (AMP) (EDD − ESD), ejection fraction (EF) [(EDD2 − ESD2)/(EDD2)], and fractional pump flow (FPF) (CF × EF). EDD, ESD, and AMP were normalized to MaxD to account for variability in the resting diameter of different lymphatics. Collecting lymphatic vessels typically relax completely between phasic contractions. This degree of vessel tone is calculated as Tone = 100% × (MaxD − EDD)/MaxD (7). In the current study, when no phasic contractions are apparent, diameter was used in place of EDD to calculate tone. To provide a comprehensive description of how different compounds affect lymphatic tone, both an average value and a maximal value over short periods of time before and after treatments are provided. The maximum tone represents the highest level of tone recorded during a time period and is presented to help capture brief increases in tone that occurred after addition of different compounds. For the studies of the impact of SP on lymphatic pumping, the means presented for each parameter represent the averages for the 2-min periods before addition of SP and starting 30 s after addition of SP. In similar studies in which inhibitors were applied and lymphatic phasic contractions stopped, a 1-min period 30 s before addition of SP and the 2-min period after addition of SP were used. Summarized data are presented as means ± SE. For all experiments, the hypothesis tested was that the various experimental interventions would produce a difference from baseline or the time period before intervention, which served as controls. Statistical analyses were performed using Prism 6 (GraphPad Software, LaJolla, CA). When only two groups were compared (baseline vs. treatment), a paired t-test was used. For three or more treatments over time, a repeated measures ANOVA was used to determine overall significance between groups, followed by Tukey’s test for multiple comparisons. For comparisons that were not repeated measures, a standard ANOVA and Tukey’s test were used. Significance was accepted at P < 0.05. Values of n indicated in each figure are the number of individual lymphatic vessels studied in each experiment.
Online data supplement.
Supplemental Figures published in support of this article are available through Figshare (https://doi.org/10.6084/m9.figshare.8216486.v1).
RESULTS
SP enhances contractions of rat mesenteric collecting lymphatic vessels.
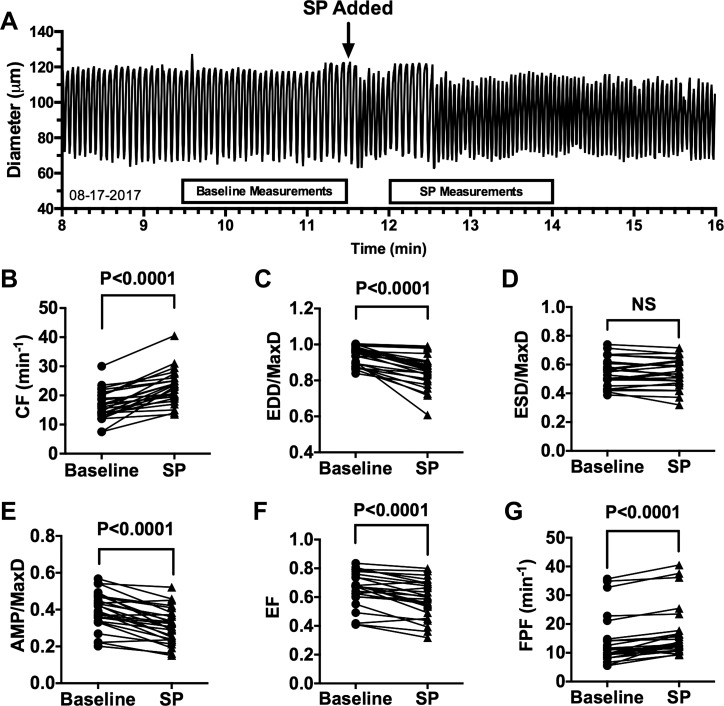
We first verified the activity of SP in our isolated rat lymphatic vessel model (Fig. 1). An example trace of how rapidly SP changes lymphatic pumping is shown in Fig. 1A. As others have reported (1, 12, 58, 60), we found that 10−8 M SP causes a significant increase in mean CF (Fig. 1B) and significant decrease in mean EDD/MaxD (Fig. 1C). Although we did not detect a significant change in ESD/MaxD in response to SP (Fig. 1D), we did find significant decreases in AMP/MaxD (Fig. 1E) and EF (Fig. 1F), plus a significant increase in FPF (Fig. 1G). Exposure of rat mesenteric collecting lymphatics to vehicle alone (5 × 10−8 M acetic acid) did not produce any significant changes in pumping parameters (Supplemental Fig. S1; https://doi.org/10.6084/m9.figshare.8216486.v1). These findings verify that our model reproduces the SP-induced changes in lymphatic contractions reported by others (1, 12, 58, 60).
Fig. 1.
Acute response of isolated rat mesenteric collecting lymphatic vessels to substance P (SP). Representative trace of diameter over time of an isolated rat collecting mesenteric lymphatic vessel before and after addition of 10−8 M SP, indicated by the arrow (A). The inflow and outflow pressures were held at 2 cm H2O throughout the experiment. The boxes above the x-axis indicate the 2-min time periods used for measurements of lymphatic parameters that are provided in B–G. Comparisons between baseline and after addition of SP are shown for CF (B), EDD/MaxD (C), ESD/MaxD (D), AMP/MaxD (E), EF (F), and FPF (G). P values were obtained from paired t-tests and are shown for each comparison considered significant (P < 0.05). N = 25 collecting lymphatic vessels isolated from 25 different rats. CF, contraction frequency; EDD, end-diastolic diameter; EF, ejection fraction; ESD, end-systolic diameter; FPF, fractional pump flow; MaxD, maximal passive diameter; NS, not significant.
Extracellular Ca2+ is required for SP-induced lymphatic contraction.
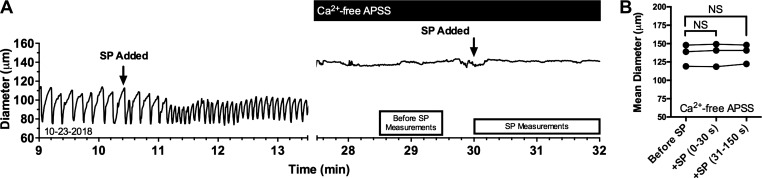
To directly test whether extracellular Ca2+ entry is required for the SP-induced lymphatic contraction, isolated rat mesenteric lymphatic vessels that responded to SP with enhanced contractions were subsequently bathed in a Ca2+-free APSS and then exposed to 10−8 M SP a second time (Fig. 2A). In addition to the cessation of phasic contractions and relaxation of the vessels after removal of extracellular Ca2+, subsequent exposure to 10−8 M SP did not elicit any change in diameter (Fig. 2B). These data suggest that extracellular Ca2+ is required not only for intrinsic phasic contractions and but also for contractions that are elicited by SP.
Fig. 2.
Substance P (SP) fails to elicit contraction of isolated rat mesenteric collecting lymphatic vessels when extracellular Ca2+ is absent. A: representative trace of diameter over time of an isolated rat collecting lymphatic vessel before and after the addition of SP bathed in normal albumin physiological salt solution (APSS; left) and in Ca2+-free APSS (right). The boxes beneath the trace represent the time periods during which diameter was averaged before and after addition of SP, compared in B. Average diameter during a 1-min period between 90 and 30 s before the addition of SP (Before SP), was compared with the 0–30-s and 31–180-s periods after SP was added to the bath by a repeated measures ANOVA followed by Dunnett’s test, which revealed no significant differences. N = 3 lymphatic vessels studied, each obtained from a unique rat. NS, not significant.
SP-induced tonic contraction is not inhibited by nifedipine.
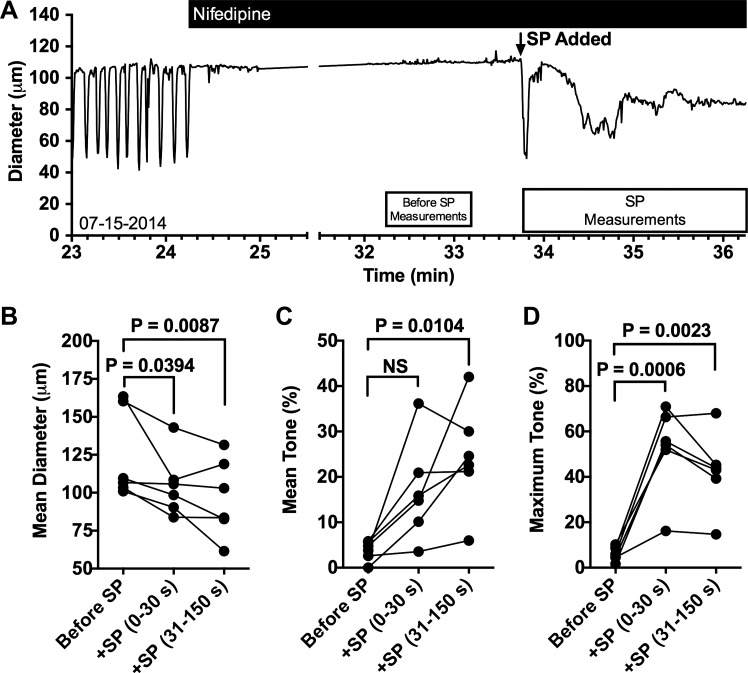
We next investigated how blockade of L-type Ca2+ channel-mediated extracellular Ca2+ entry affects SP-induced contraction of isolated rat mesenteric lymphatic vessels (Fig. 3). In a preliminary concentration-response study we found that 10−7 M nifedipine can effectively stop phasic contractions of isolated rat mesenteric collecting lymphatics, whereas DMSO controls as high as 2.8 × 10−3 M had no effect (Supplemental Fig. S2; https://doi.org/10.6084/m9.figshare.8216486.v1). Based on this data, we applied 10−8 M SP in the presence of 10−7 M nifedipine and typically observed a large contraction and relaxation followed by a period in which the diameter fluctuates but remains smaller than it was before the addition of SP (Fig. 3A). The mean vessel diameter was significantly reduced in the initial 30 s after addition of SP, and this significant reduction was sustained in the period from 31 to 150 s after SP was added (Fig. 3B). When looking at tone, addition of SP caused a significant difference only in the 31–150-s period after its addition (Fig. 3C). However, comparison of the maximum tone, which takes into account strong yet brief contractions such as the one shown immediately after the addition of SP in Fig. 3A, shows that SP elicited significant contraction in both the initial 30-s and the 31–150-s time periods (Fig. 3C). These data suggest that extracellular Ca2+ entry via L-type Ca2+ channels is not required for SP-induced contractions in rat mesenteric collecting lymphatic vessels.
Fig. 3.
Substance P (SP) elicits constriction of isolated rat mesenteric collecting lymphatic vessels during blockade of L-type Ca2+ channels with nifedipine. Representative trace of diameter over time of an isolated rat collecting lymphatic vessel before and after addition of 10−7 M nifedipine, later followed by the addition of 10−8 M SP (A). The boxes beneath the trace represent the time periods during which the quantitative data used for comparisons in B–D were collected. The mean diameter (B), mean tone (C), and maximum tone (D) for the time periods before addition of SP, 0–30 s after SP addition, and 31–150 s after addition of SP were compared using a repeated measures ANOVA model followed by Dunnett’s comparison with control (baseline period). P values for the comparisons are shown. N = 6 isolated lymphatics. Each lymphatic studied was obtained from a unique rat. NS, not significant.
SP-induced tonic contractions are inhibited by caffeine.
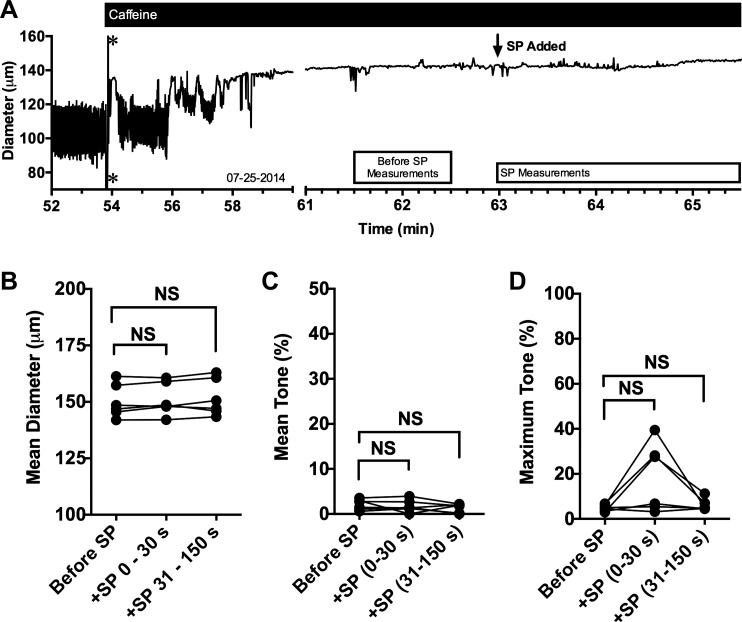
We next tested whether release from internal stores affects SP-induced contraction of isolated rat mesenteric lymphatic vessels (Fig. 2). A trace of an isolated rat mesenteric lymphatic vessel treated with caffeine and subsequently with SP is shown in Fig. 4A. The addition of 10−2 M caffeine typically causes relaxation of the lymphatic vessels (Supplemental Fig. S3; https://doi.org/10.6084/m9.figshare.8216486.v1) and loss of phasic contractions within a few minutes. The subsequent addition of 10−8 M SP in the presence of caffeine in the example trace shows no contractions. When comparing means from multiple vessels, addition of SP in the presence of caffeine caused no significant changes in mean diameter, and no sustained changes in tone were observed (Fig. 4, B and C). Although some vessels displayed a brief contraction in the first 30 s after the addition of SP, which was captured in the analysis of maximum tone during each time period (Fig. 4D), the average maximum tone during the 0–30- and 31–150-s periods after addition of SP were not significantly different from the period before addition of SP (Fig. 4D).
Fig. 4.
Substance P (SP)-induced tonic contraction in isolated rat mesenteric collecting lymphatic vessels is largely inhibited in the presence of caffeine. Representative trace of diameter over time of an isolated rat collecting lymphatic vessel before and after addition of 10−2 M caffeine, later followed by the addition of 10−8 M SP (A). *Artifact in the diameter tracking that occurred when caffeine was added. The boxes beneath the trace represent the time periods during which the quantitative data used for comparisons in B–D were collected. The mean diameter (B), mean tone (C), and maximum tone (D) for the time periods before addition of SP, 0–30 s after SP addition, and 31–150 s after addition of SP were compared using a repeated measures ANOVA model followed by Dunnett’s comparison with control (Before SP). N = 7 isolated lymphatics were studied. Each lymphatic studied was obtained from a unique rat. NS, not significant.
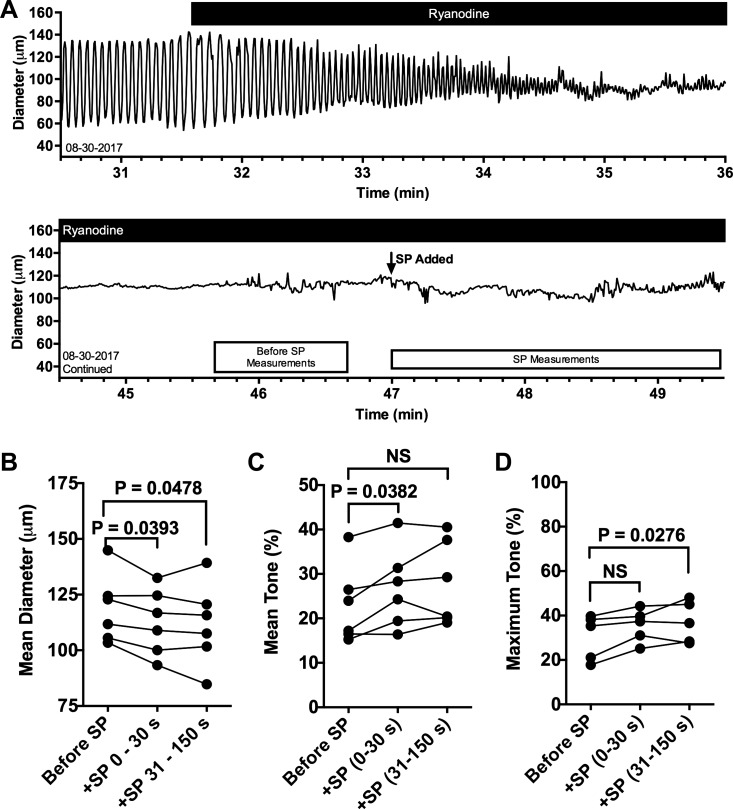
Ryanodine-induced lymphatic tone limits additional SP-induced contraction.
In a similar protocol designed to directly test RyRs, we found that addition of 10−5 M ryanodine gradually leads to a loss of phasic contractions in isolated rat mesenteric lymphatic vessels, with a gradually lower EDD and raised ESD, leading to phasic contractions with a very small AMP and eventual loss of measurable changes in AMP (Fig. 5A). This pattern differs from that of treatment with caffeine in which the vessel becomes relaxed (Fig. 4A), as the diameter measured after addition of ryanodine does not increase (Fig. 5A). The vehicle for ryanodine (2.8 × 10−2 M DMSO) did not alter lymphatic contractions (Supplemental Fig. S4; https://doi.org/10.6084/m9.figshare.8216486.v1). Furthermore, the ryanodine-induced decreases in CF, diameter during tonic contraction, and AMP of phasic contractions were also significantly decreased compared with the time-matched DMSO control (Supplemental Fig. S5; https://doi.org/10.6084/m9.figshare.8216486.v1). With the reduced diameter (and thus increase in tone) in the presence of ryanodine, addition of 10−8 M SP causes small yet significant contractions, as evidenced by the decrease in mean diameter at 0–30 and 31–150 s after addition of SP (Fig. 5B), the increase in mean tone at 0–30 s (Fig. 5C), and the increase in maximal tone at 31–150 s (Fig. 5D). The results suggest that there are functional RyRs present in rat mesenteric lymphatic vessels and that their modulation with ryanodine affects SP-induced contraction.
Fig. 5.
Blockade of ryanodine receptors (RyRs) with ryanodine leads to a loss of phasic contractions and limited substance P (SP)-induced contraction. Representative trace of diameter over time of an isolated rat collecting mesenteric lymphatic vessel treated with 10−5 M ryanodine (A). The continuation of the trace shows a later time period in the same experiment with ryanodine, when 10−8 M SP was added. The boxes beneath the trace represent the time periods during which the quantitative data used for comparisons in B–D were collected. The mean diameter (B), mean tone (C), and maximum tone (D) for the time periods before addition of SP, 0–30 s after SP addition, and 31–150 s after addition of SP were compared using a repeated measures ANOVA model followed by Dunnett’s comparison with control (baseline period). P values for the comparisons are shown. N = 6 lymphatics. Each lymphatic studied was obtained from a unique rat. NS, not significant.
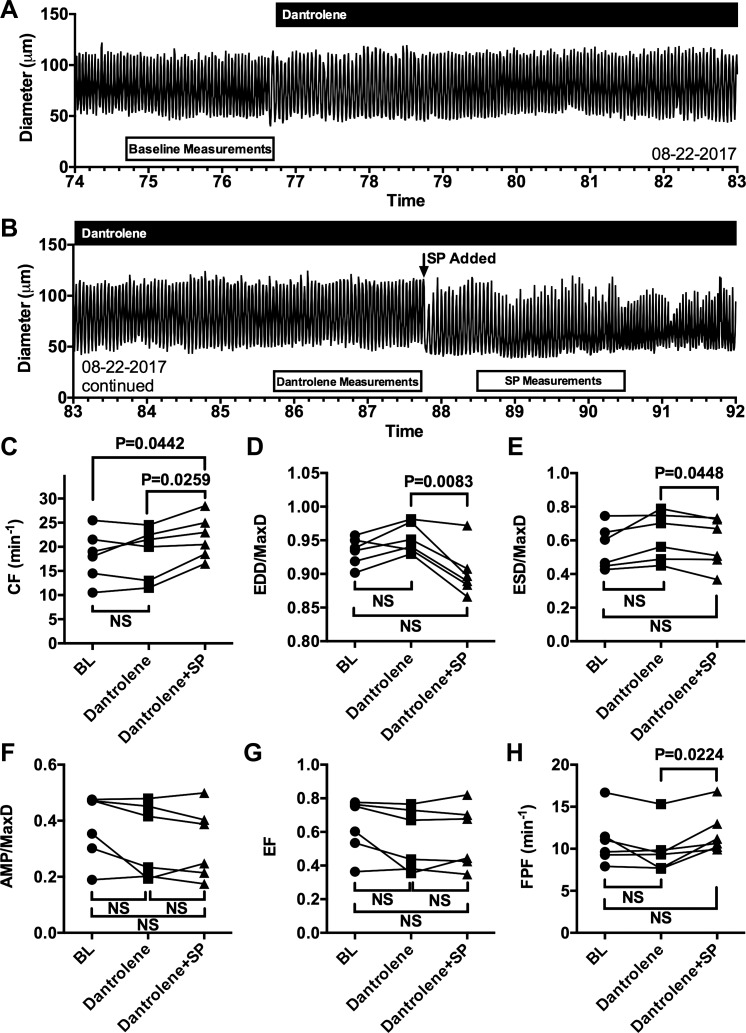
Dantrolene does not affect baseline or SP-induced changes in lymphatic contractions.
Because there are three different RyR isoforms, we investigated whether application of dantrolene, described as a selective antagonist for the RyR1 and RyR3 isoforms, may affect lymphatic vessels and their response to SP in a similar fashion as ryanodine (Fig. 6). As seen in the representative trace in Fig. 6, A and B, addition of 10−5 M dantrolene does not cause any noticeable impact on lymphatic contractions and does not block the typical decrease in EDD or increase in CF elicited by subsequent addition of 10−8 M SP. The summarized data are shown in Fig. 6, C–H. Dantrolene has no impact on any of the parameters studied, while SP causes an increase in mean CF and decrease in mean EDD/MaxD (Fig. 6, C and D) in a similar fashion as in Fig. 1. Unlike the findings in Fig. 1, in this smaller sample there is a significant decrease in mean ESD/MaxD caused by SP in the presence of dantrolene compared with the time period just before SP addition (Fig. 6E). SP also has no significant impact on mean AMP/MaxD (Fig. 6F) or mean EF (Fig. 6G) in the presence of dantrolene, yet does cause a significant increase in mean FPF (Fig. 6H). These data show that dantrolene, described as a selective inhibitor of RyR1/RyR3, does not affect lymphatic contractions or the ability of isolated mesenteric lymphatics to increase CF and lower EDD in response to SP.
Fig. 6.
The ryanodine receptor (RyR)1/RyR3 inhibitor dantrolene does not inhibit rat mesenteric collecting lymphatic contractions or the increased CF and decreased EDD/Max elicited by substance P (SP). Representative trace of diameter over time of an isolated rat mesenteric collecting lymphatic vessel before and after the addition of 10−5 M dantrolene, which remained in the bath (A). Continuation of the same trace, showing the addition of 10−8 M SP in the presence of dantrolene (B). The boxes beneath the traces in A and B indicate 2-min time periods during which the quantitative data used for comparisons in C–H were collected. Comparisons between baseline (BL), after addition of dantrolene, and after addition of SP in the presence of dantrolene were performed for CF (C), EDD/MaxD (D), ESD/MaxD (E), AMP/MaxD (F), EF (G), and FPF (H). P values are shown for comparisons that were found to be significantly different (P < 0.05) using Tukey’s multiple comparison test after an initial one-way repeated measures ANOVA. N = 6 isolated lymphatics studied from 6 different rats. CF, contraction frequency; EDD, end-diastolic diameter; EF, ejection fraction; ESD, end-systolic diameter; FPF, fractional pump flow; MaxD, maximal passive diameter; NS, not significant.
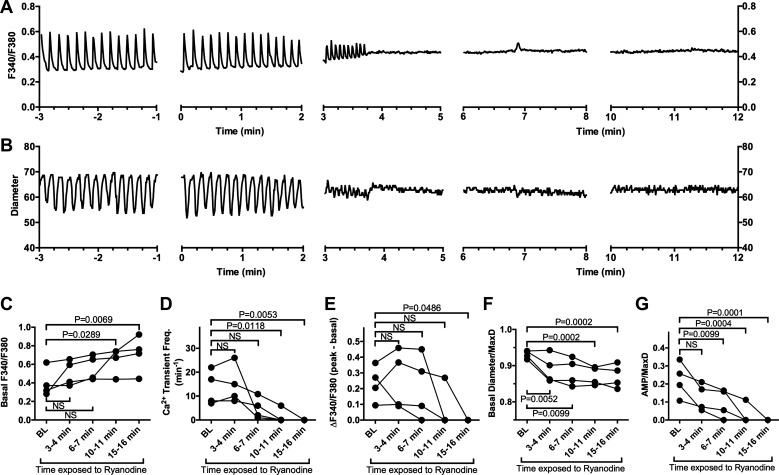
Ryanodine modulates [Ca2+]i in rat mesenteric collecting lymphatics.
To further investigate how ryanodine impacts lymphatic contractions, we evaluated the normal cycle of changes in [Ca2+]i associated with tonic and phasic contractions. This was achieved by measuring the F340/F380 ratio in isolated rat mesenteric collecting lymphatics loaded with the Ca2+ indicator fura-2 AM. Addition of 10−5 M ryanodine gradually reduced the magnitude of Ca2+ transients until they eventually were not detectable (Fig. 7A), in association with smaller phasic contractions that eventually ceased (Fig. 7B). The summarized data from four vessels shows that the mean basal [Ca2+]i between transients gradually became elevated (Fig. 7C), whereas the mean frequency and amplitude of Ca2+ transients decreased (Fig. 7, D and E). In association with these changes, the basal diameter (EDD, or just diameter in the absence of phasic contractions) decreased over time (Fig. 7F), and the mean AMP also decreased (Fig. 7G). Taken together, the data suggest that the ryanodine-induced alterations in lymphatic contractions are connected to an impairment of the retention of Ca2+ within internal stores.
Fig. 7.
Impact of 10−5 M ryanodine on [Ca2+]i in isolated rat mesenteric collecting lymphatic vessels. A: traces of the F340/F380 ratio obtained over time from a single rat mesenteric collecting lymphatic vessel loaded with the Ca2+ indicator dye fura-2 AM before and after exposure to 10−5 M ryanodine (added at time 0 min). Changes in F340/F380 are indicative of changes in [Ca2+]i. B: measurements of luminal diameter obtained from the same lymphatic vessel during the same time periods. C: mean basal F340/F380 between Ca2+ transients from four collecting lymphatics during the baseline (BL) period before ryanodine treatment and the indicated times after ryanodine was added. Basal F340/F380 significantly increased over BL at 10–11 and 15–16 min after addition of ryanodine. D: mean frequency of Ca2+ transients, showing a significant mean reduction at 10–11 and 15–16 min after ryanodine was added. E: mean amplitude of the changes in F340/F380 for Ca2+ transients was significantly decreased at 15–16 min after ryanodine was added, mainly because of the disappearance of Ca2+ transients at this time point. F: basal diameter/MaxD (EDD/MaxD when phasic contractions were present) significantly decreased form BL at all time points after ryanodine was added. G: amplitude of phasic contractions significantly decreased from BL starting at 6–7 min after ryanodine was added. Lymphatics displaying no phasic contractions were assigned an AMP/MaxD value of 0 in this analysis. Data were analyzed by one-way repeated measures ANOVA followed by Dunnett’s test for multiple comparisons with control. P values for all significant difference are shown. N = 4 isolated mesenteric collecting lymphatics obtained from four different rats. EDD, end-diastolic diameter; MaxD, maximal passive diameter; NS, not significant.
RyRs are present in the rat mesenteric collecting lymphatic vessel wall.
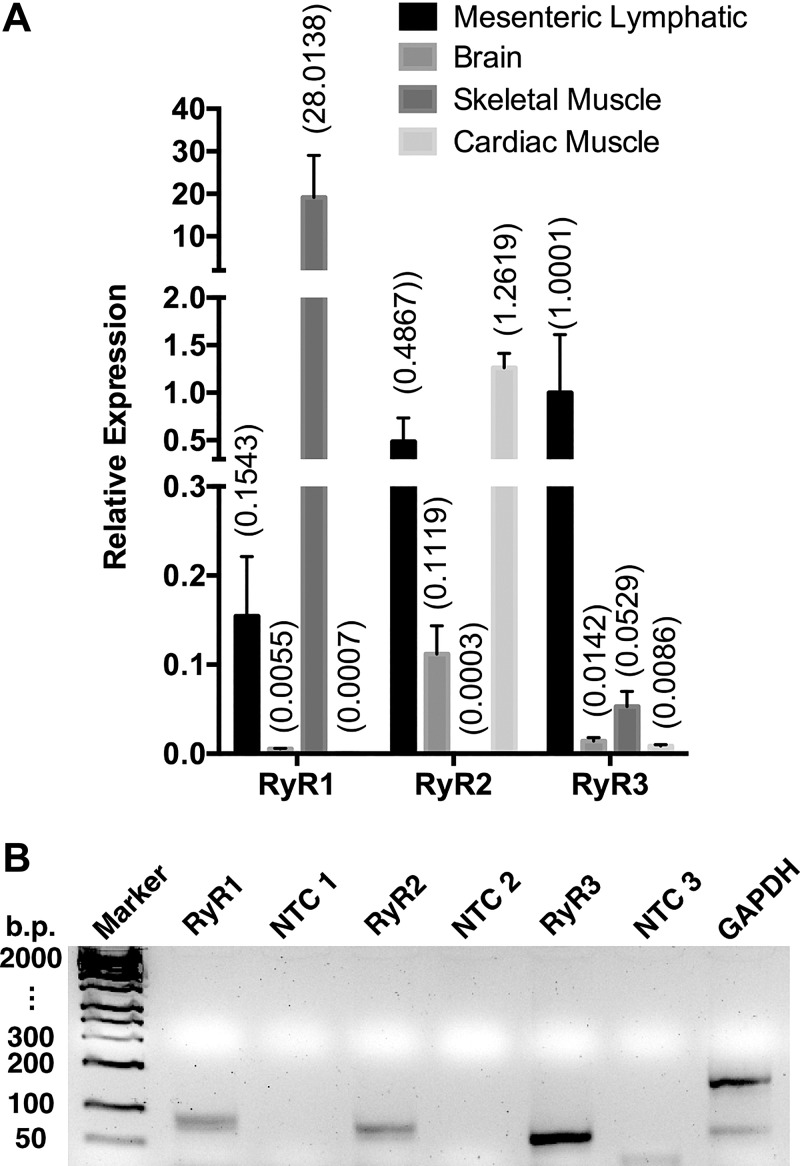
With our finding that ryanodine treatment of lymphatic vessels inhibits pumping and the contractile response to SP, we expected to find that one or more RyRs would be expressed in rat lymphatic vessels. Figure 8 shows representative results from our studies of the mRNA expression of RyR1, RyR2, and RyR3 in isolated mesenteric collecting lymphatic vessels using RT-PCR. Primers for all three isoforms amplified cDNA products of the predicted sizes for the amplicons for RyR1, RyR2, and RyR3 described by the manufacturer (Applied Biosystems) (Fig. 8). The no-template controls did not yield cDNA products.
Fig. 8.
Quantitative PCR (qPCR) detection of ryanodine receptors (RyRs) in rat mesenteric collecting lymphatic vessels. A: means show relative expression to GAPDH determined by qPCR and using the 2-ΔCt method in isolated rat mesenteric collecting lymphatics (N = 7 rats) and rat brain, skeletal muscle, and cardiac muscle (all N = 2 rats). B: gel of the single amplicon products of expected size from the qPCR reactions. Rat mesenteric collecting lymphatics were harvested (30 lymphatic vessels per rat), and total RNA (50 ng) was reverse transcribed into cDNA and qPCR performed with specific primer/probe sets for Ryr1 (Rn01545085_m1), Ryr2 (Rn01470303_m1), Ryr3 (Rn01486097_m1), and GAPDH (Rn01775763_g1). Reactions with no template were also run as controls (NTC). The gel shown is representative of three separate experiments using mesenteric lymphatic samples obtained from three different rats.
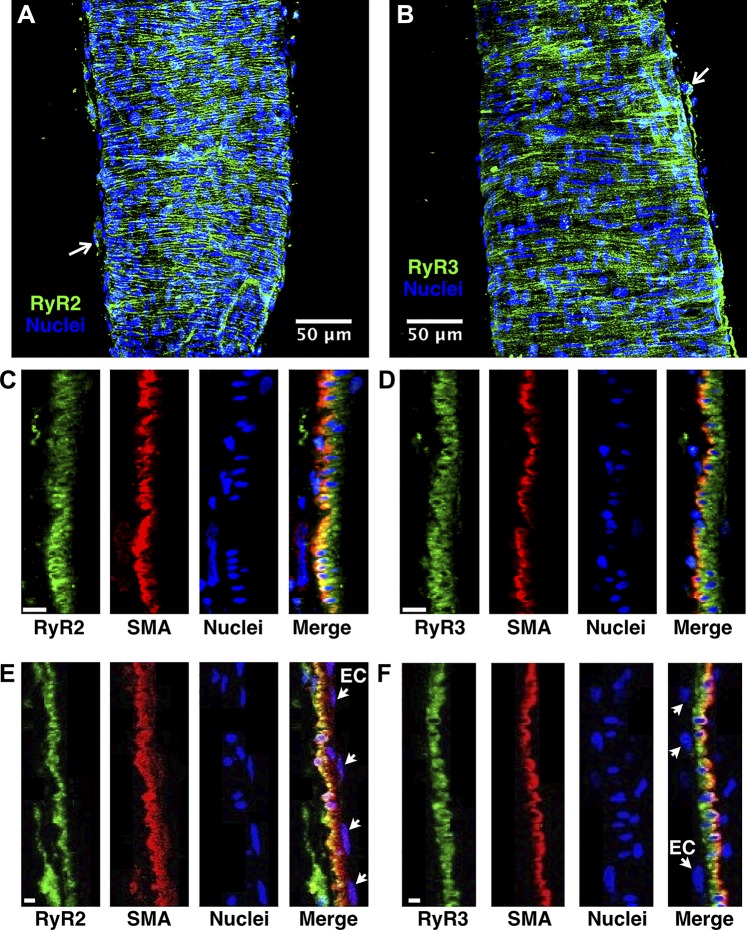
We next evaluated whether RyRs could be detected in the lymphatic wall by immunofluorescence labeling and confocal microscopy. We focused on RyR2 because of its likely functional role and RyR3 because of the relatively strong mRNA signal in our qPCR studies (Fig. 8). In isolated rat mesenteric lymphatics immunolabeled for RyR2 or RyR3, most of the signal appeared in hoop-like structures in the vessel wall, as shown in the z-projections in Fig. 9, A and B. Because this pattern was consistent with the smooth muscle layer, in an additional set of isolated lymphatic vessels we colabeled with anti-SMA antibodies. Figure 9C shows single confocal slices featuring a cross-section of the rat mesenteric collecting lymphatic wall, with the RyR2, SMA, and nuclei channels shown separately, plus a merged image in which partial RyR2 and SMA colocalization can be observed. A similar pattern was observed for RyR3 (Fig. 9D). For both RyR2 and RyR3, some immunolabeling was apparent in the outer parenchymal layer of the vessel (arrows in Fig. 9, A and B). We did not detect labeling of RyR2 or RyR3 in the inner endothelial layer, which was identified by endothelial nuclei on the luminal side of the smooth muscle layer (Fig. 9, E and F). In negative controls with no primary antibody for RyR2 or RyR3, negligible signal was detected (data not shown). These findings suggest that RyR2 and RyR3 are expressed in rat mesenteric collecting lymphatic vessels and are primarily found within the smooth muscle layer.
Fig. 9.
Determination of ryanodine receptor (RyR)2 and RyR3 localization in isolated rat mesenteric collecting lymphatic vessels with immunofluorescence labeling and laser confocal microscopy. A. maximum intensity z-projection (37 confocal slices) of a lymphatic vessel labeled with anti-RyR2 AlexaFluor488-conjugated secondary antibodies and DAPI. The RyR2 labeling appeared mostly in hoop-like patterns in the vessel wall, with some labeling in the adventitial layer (1 example denoted by the small white arrow). B: maximum intensity z-projection (85 confocal slices) of a lymphatic immunolabeled for RyR3 and nuclei. A similar hoop-like pattern was observed with RyR3 immunolabeling, with some adventitial layer labeling (small white arrow). No signal was detected in labeling controls with no primary antibody (data not shown). C and E: labeling of RyR2, smooth muscle actin (SMA), nuclei, and a merge of the three channels in a single confocal slice featuring a cross-section of the collecting lymphatic wall. The orientation is with the adventitial layer on the left and the endothelial layer on the right. RyR2 labeling was predominantly found in the smooth muscle layer, as evidenced by partial colocalization with SMA and RyR2 labeling surrounding the nuclei of smooth muscle cells. D and F: labeling of RyR3, SMA, nuclei, and a merge of the three channels in a single confocal slice featuring a cross-section of the lymphatic wall, oriented with the adventitial layer on the left and endothelium on the right. RyR3 was predominantly found in smooth muscle cells, as evidenced by the partial colocalization with SMA. The small arrows in E and F point toward nuclei of endothelial cells (ECs). A and B: representative of N = 8 labeling experiments each. C–F: representative of N = 4 experiments each. The scale bars in C–F represent 5 µm.
DISCUSSION
The main novel findings from the current study are: 1) SP causes tonic contraction of isolated rat mesenteric collecting lymphatics when phasic contractions are eliminated by nifedipine; 2) SP-induced tonic contraction is blocked when phasic contractions have been stopped by caffeine; 3) SP-induced tonic contraction is significantly reduced (nearly eliminated) when phasic contractions are stopped by ryanodine; 4) dantrolene fails to impair phasic contractions or inhibit SP-induced contraction in the same manner as ryanodine; 5) mRNA for all three RyRs can be detected in isolated rat mesenteric collecting lymphatics; and 6) RyR2 and RyR3 are predominantly found in the smooth muscle layer of the rat mesenteric collecting lymphatic wall. Collectively, the data show for the first time that SP-induced tonic constriction in collecting lymphatic vessels requires normal release of Ca2+ from internal stores. In addition, normal RyR function is required for phasic contractions.
Several previous reports using different protocols have characterized that SP generally causes a positive chronotropic and inotropic response in lymphatic vessels (1, 12, 58, 60). Data from studies of rat mesenteric collecting lymphatic vessels suggest that SP increases pump efficiency (1) independently of preload (12); however, only a few studies have addressed the underlying molecular mechanisms. SP has been shown to act upon the neurokinin receptors NK1R and NK3R in rat mesenteric lymphatic muscle cells (10). Downstream from receptor activation, phosphorylation of regulatory myosin light chains is increased (10, 73). Pharmacologic inhibition of myosin light chain kinase attenuates the SP-induced increases in CF and lymphatic vessel tone in isolated rat mesenteric collecting lymphatics (73). An additional pathway reported in guinea pig mesenteric lymphatics involves SP-induced activation of NK1 receptors on endothelial cells and generation of thromboxane A2 as a paracellular stimulator of the smooth muscle layer (51). To our knowledge, this current study is the first to investigate the relative importance of extracellular Ca2+ entry versus release of Ca2+ from internal stores in SP-induced tonic constriction in collecting lymphatic vessels. In addition to using Ca2+-free APSS to stop phasic contractions, these studies were performed with nifedipine and caffeine, which we previously have shown inhibit both transient increases in [Ca2+]i and phasic contractions (55) through blockade of L-type Ca2+ channels and emptying Ca2+ from the SR, respectively. Our current findings suggest that SP requires extracellular Ca2+ to elicit tonic contraction in rat mesenteric collecting lymphatics but does not specifically require L-type Ca2+ channels. SP also requires release of Ca2+ from internal stores. This mechanism is different from that found in trachea smooth muscle, in which both L-type Ca2+ channels and internal Ca2+ stores are involved in SP-induced contraction (40). The mechanism and outcome in lymphatic vessels are completely different from arteries and arterioles, in which SP causes endothelial-dependent vasodilation (15, 31, 34, 44). Because SP also stimulates increased microvascular permeability and inflammation (23, 28, 63), we speculate that these tissue- and vessel-specific differences may reflect coordination of the overall inflammatory response.
It is worth noting that the concentration of nifedipine we used to stop phasic contractions (10−7 M) in the current study based on our concentration-response data has also been shown to stop pumping of isolated human mesenteric lymphatics and thoracic ducts (61). In that study, a depolarization of ~10 mV in membrane potential was associated with the cessation of pumping in thoracic ducts (61). However, a different study, notably using isolated rat mesenteric collecting lymphatics, reported that this concentration did alter the basal membrane potential and did not stop phasic contractions but rather only decreased the amplitude of the contractions (39). They did observe cessation of phasic contractions with 5 × 10−7 M nifedipine (39). One major difference between the current study and this previous report, which used both wire myograph and pressure myograph protocols, is the method by which nifedipine was applied. In the current study, the drug was pipetted directly to the bath, achieving the final concentration relatively quickly, whereas in the previous study, the drug was washed via a pump system, which would have slowly ramped the concentration upward over time. These differences in the kinetics of administration, which would affect the rate of receptor binding and activation of second messengers, may account for the observed difference in pump function. An additional possibility that might lead to variability across studies is differences in the concentration of albumin in the physiological salt solutions among different studies (39, 55, 61). However, it is unclear whether this accounts for the differences between our current findings using APSS containing 1% albumin and those of the aforementioned study using rat mesenteric collecting lymphatics, which used 0.5% albumin (39), as our findings more closely reflect the previous study evaluating the impact of nifedipine on human mesenteric collecting lymphatics and thoracic ducts (61), in which the bathing solution contained no albumin.
Our finding of the requirement for normal Ca2+ release from internal stores for SP-induced tonic contraction was based upon the use of caffeine, which is known to lower the threshold for Ca2+-induced opening of RyRs (33). Therefore, we also investigated the potential role of RyRs using ryanodine, which inhibits channel opening when applied at micromolar concentrations (5, 62). We found that 10−5 M ryanodine causes a gradual decrease in EDD and rise in ESD so that the AMP decreases with each contraction until phasic contractions eventually become undetectable. We also observed that the gradual changes in pumping parameters were tightly associated with a gradual increase in [Ca2+]i over time and the eventual loss of detectible Ca2+ transients at the moment phasic contractions ceased. These findings suggest that application of 10−5 M ryanodine modifies the normal cycle of Ca2+ release and reuptake from the SR in rat mesenteric collecting lymphatic vessels. Our findings are in general agreement with previous reports using bovine mesenteric lymphatic vessels in which ryanodine reduced flow after a 30-min delay (2, 3). These previous studies used a lower concentration (10−7 M) of ryanodine (2, 3), which would be expected increase open probability of RyRs (5, 62). Also, the parameter measured was outflow of lymph rather than direct examination of contractions (2, 3). Nevertheless, a key similarity between the current findings and these previous studies is the gradual nature of the response to ryanodine (2, 3). Our findings also have similarities to the impaired pump function found in the heart when RyR2 expression is reduced ~50% in cardiac-specific RyR2 knockout mice, which display significantly decreased cardiac output and rate-pressure product (8). The ryanodine-induced reduction in lymphatic EDD has similarities to the ryanodine-induced vasoconstriction observed in arteries (25, 47, 74) and the role of RyRs as negative regulators of tone via Ca2+ spark-mediated activation of KCa channels and membrane hyperpolarization (47, 50). Whether such a mechanism might be involved in the relaxation period of phasic contractions of collecting lymphatic vessels represents a key future direction. A great deal of heterogeneity of RyR function has been reported in different arteries and arterioles (summarized in Ref. 74), and a study of Ca2+ sparks in contractile lymphatic vessels or lymphatic smooth muscle cells would provide new insights into the role of RyRs in lymphatic vessel physiology.
It is worth noting that our findings with ryanodine are in contrast to those from guinea pig mesenteric lymphatics, in which ryanodine had no impact on pumping (71, 79). We speculate that this is a species-specific attribute in guinea pigs because of the responsiveness to ryanodine in the current study and previously reported response in bovine mesenteric collecting lymphatics (2, 3).
Another key finding in the current study is that dantrolene, described as a selective RyR1 and RyR3 antagonist (67), does not significantly impact lymphatic pumping. This finding suggests that these two RyR isoforms may not have a functional role in baseline lymphatic contractions or SP-induced enhancement of contractions. Although this finding may implicate by deduction that RyR2 function plays a role in normal phasic contractions of mesenteric collecting lymphatics and in the SP-induced enhancement of lymphatic contractions, we do acknowledge that there is some controversy about whether dantrolene also may affect RyR2. Dantrolene has been shown to bind to RyR2 and has antiarrhythmic properties in models in which arrhythmias are caused by mutations of RyR2 (26, 32) or heart failure (42). In addition, dantrolene has been shown to reduce open probability of RyR2 in lipid bilayers and decrease the amplitude of calcium waves in permeabilized cardiac myocytes (38, 49).
We also report for the first time that mRNA for RyR1, RyR2, and RyR3 are detectable in rat mesenteric collecting lymphatics and that RyR2 and RyR3 proteins are localized primarily in the smooth muscle layer. RyR2 and RyR3 were not detected in the endothelial layer by immunofluorescence confocal microscopy. These findings are similar to those of vascular smooth muscle, in which all three RyR isoforms have been shown (19, 21, 30, 48, 67, 75). Distinct spatial organization of the three RyR isoforms on the SR or within different subcellular regions has been demonstrated in vascular smooth muscle cells (19, 67). Additional determination of subcellular location of the RyR isoforms in lymphatic smooth muscle cells may provide additional insight into their distinct functional roles. There is also new evidence that a splice variant resulting in a short form of RyR3 may form heterotetramers with RyR2 and full-length RyR3 and suppress their function (41). Although we did not examine RyR1 in the current study by immunofluorescence microscopy, our prediction is that, like RyR2 and RyR3, its protein expression in rat mesenteric collecting lymphatic vessels will likely be found mostly in the smooth muscle layer, with its higher degree of cellularity compared with the endothelium. It is worth noting that there could be levels of RyR2 and RyR3 in the endothelial layer that were simply below our detection threshold.
In summary, our data show for the first time that SR Ca2+ plays a key role in SP-induced tonic contraction of collecting lymphatic vessels. The lymphatic vessels are sensitive to ryanodine, which diminishes phasic contractions, causes elevated tone, and significantly reduces the response to SP. The lymphatic vessels, however, were not sensitive to dantrolene, which may implicate that specific RyRs are of importance. We also provide the novel demonstration that RyRs are present in the smooth muscle layer of lymphatic vessels. These new findings reveal a novel role for RyRs as contributors to the overall mechanisms that control contractions in lymphatic vessels.
GRANTS
This work was supported by the University of South Florida and National Heart, Lung, and Blood Institute Grant L40-HL-097863 (to J. W. Breslin) and Rotary International Global Scholarship Grant GG1746618 (to M. Jo).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J., A.N.T., and J.W.B. conceived and designed the research; M.J., A.N.T., Y.Y., and J.W.B. performed experiments; M.J., A.N.T., Y.Y., and J.W.B. analyzed data; M.J., A.N.T., Y.Y., and J.W.B. interpreted results of the experiments; M.J. and J.W.B. prepared the figures; M.J. drafted the manuscript; A.N.T., Y.Y., and J.W.B. edited and revised the manuscript; M.J., A.N.T., Y.Y., and J.W.B. approved final version of the manuscript.
REFERENCES
- 1.Amerini S, Ziche M, Greiner ST, Zawieja DC. Effects of substance P on mesenteric lymphatic contractility in the rat. Lymphat Res Biol 2: 2–10, 2004. doi: 10.1089/1539685041690409. [DOI] [PubMed] [Google Scholar]
- 2.Atchison DJ, Johnston MG. Role of extra- and intracellular Ca2+ in the lymphatic myogenic response. Am J Physiol 272: R326–R333, 1997. doi: 10.1152/ajpregu.1997.272.1.R326. [DOI] [PubMed] [Google Scholar]
- 3.Atchison DJ, Rodela H, Johnston MG. Intracellular calcium stores modulation in lymph vessels depends on wall stretch. Can J Physiol Pharmacol 76: 367–372, 1998. doi: 10.1139/y98-037. [DOI] [PubMed] [Google Scholar]
- 4.Beckett EA, Hollywood MA, Thornbury KD, McHale NG. Spontaneous electrical activity in sheep mesenteric lymphatics. Lymphat Res Biol 5: 29–43, 2007. doi: 10.1089/lrb.2007.5104. [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature 361: 315–325, 1993. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 6.Breslin JW. Mechanical forces and lymphatic transport. Microvasc Res 96: 46–54, 2014. doi: 10.1016/j.mvr.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. Lymphatic vessel network structure and physiology. Compr Physiol 9: 207–299, 2018. doi: 10.1002/cphy.c180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bround MJ, Asghari P, Wambolt RB, Bohunek L, Smits C, Philit M, Kieffer TJ, Lakatta EG, Boheler KR, Moore ED, Allard MF, Johnson JD. Cardiac ryanodine receptors control heart rate and rhythmicity in adult mice. Cardiovasc Res 96: 372–380, 2012. doi: 10.1093/cvr/cvs260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabras P, Caboni P, Cabras M. Analysis by hplc of ryanodine and dehydroryanodine residues on fruits and in ryania powdery wood. J Agric Food Chem 49: 3161–3163, 2001. doi: 10.1021/jf010224g. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty S, Nepiyushchikh Z, Davis MJ, Zawieja DC, Muthuchamy M. Substance P activates both contractile and inflammatory pathways in lymphatics through the neurokinin receptors NK1R and NK3R. Microcirculation 18: 24–35, 2011. doi: 10.1111/j.1549-8719.2010.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis MJ, Davis AM, Ku CW, Gashev AA. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 296: H293–H302, 2009. doi: 10.1152/ajpheart.01040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, Muthuchamy M, Gashev AA. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol 295: H587–H597, 2008. doi: 10.1152/ajpheart.01029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrusi I, Zhao J, van Helden D, von der Weid PY. Cyclopiazonic acid decreases spontaneous transient depolarizations in guinea pig mesenteric lymphatic vessels in endothelium-dependent and -independent manners. Am J Physiol Heart Circ Physiol 286: H2287–H2295, 2004. doi: 10.1152/ajpheart.00739.2003. [DOI] [PubMed] [Google Scholar]
- 14.Foy WL, Allen JM, McKillop JM, Goldsmith JP, Johnston CF, Buchanan KD. Substance P and gastrin releasing peptide in bovine mesenteric lymphatic vessels: chemical characterization and action. Peptides 10: 533–537, 1989. doi: 10.1016/0196-9781(89)90138-1. [DOI] [PubMed] [Google Scholar]
- 15.Galligan JJ, Jiang MM, Shen KZ, Surprenant A. Substance P mediates neurogenic vasodilatation in extrinsically denervated guinea-pig submucosal arterioles. J Physiol 420: 267–280, 1990. doi: 10.1113/jphysiol.1990.sp017911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasheva OY, Gashev AA, Zawieja DC. Cyclic guanosine monophosphate and the dependent protein kinase regulate lymphatic contractility in rat thoracic duct. J Physiol 591: 4549–4565, 2013. doi: 10.1113/jphysiol.2013.258681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol 128: 893–904, 1995. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert G, Ducret T, Marthan R, Savineau JP, Quignard JF. Stretch-induced Ca2+ signalling in vascular smooth muscle cells depends on Ca2+ store segregation. Cardiovasc Res 103: 313–323, 2014. doi: 10.1093/cvr/cvu069. [DOI] [PubMed] [Google Scholar]
- 20.Hakamata Y, Nakai J, Takeshima H, Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett 312: 229–235, 1992. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- 21.Herrmann-Frank A, Darling E, Meissner G. Functional characterization of the Ca(2+)-gated Ca2+ release channel of vascular smooth muscle sarcoplasmic reticulum. Pflugers Arch 418: 353–359, 1991. doi: 10.1007/BF00550873. [DOI] [PubMed] [Google Scholar]
- 22.Hollywood MA, Cotton KD, Thornbury KD, McHale NG. Tetrodotoxin-sensitive sodium current in sheep lymphatic smooth muscle. J Physiol 503: 13–20, 1997. doi: 10.1111/j.1469-7793.1997.013bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hossen MA, Fujii Y, Sugimoto Y, Kayasuga R, Kamei C. Histamine H3 receptors regulate vascular permeability changes in the skin of mast cell-deficient mice. Int Immunopharmacol 3: 1563–1568, 2003. doi: 10.1016/S1567-5769(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 24.Imtiaz MS, Zhao J, Hosaka K, von der Weid PY, Crowe M, van Helden DF. Pacemaking through Ca2+ stores interacting as coupled oscillators via membrane depolarization. Biophys J 92: 3843–3861, 2007. doi: 10.1529/biophysj.106.095687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson-Weaver O, Osmond JM, Naik JS, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats reduces activation of Ca2+ sparks in mesenteric arteries. Am J Physiol Heart Circ Physiol 309: H1915–H1922, 2015. doi: 10.1152/ajpheart.00179.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, Dorn T, Ruppenthal S, Pfeiffer S, Goedel A, Dirschinger RJ, Seyfarth M, Lam JT, Sinnecker D, Gudermann T, Lipp P, Laugwitz KL. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med 4: 180–191, 2012. doi: 10.1002/emmm.201100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaja S, Payne AJ, Patel KR, Naumchuk Y, Koulen P. Differential subcellular Ca2+ signaling in a highly specialized subpopulation of astrocytes. Exp Neurol 265: 59–68, 2015. doi: 10.1016/j.expneurol.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keeble J, Blades M, Pitzalis C, Castro da Rocha FA, Brain SD. The role of substance P in microvascular responses in murine joint inflammation. Br J Pharmacol 144: 1059–1066, 2005. doi: 10.1038/sj.bjp.0706131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim EE, Shekhar A, Lu J, Lin X, Liu FY, Zhang J, Delmar M, Fishman GI. PCP4 regulates Purkinje cell excitability and cardiac rhythmicity. J Clin Invest 124: 5027–5036, 2014. doi: 10.1172/JCI77495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinnear NP, Wyatt CN, Clark JH, Calcraft PJ, Fleischer S, Jeyakumar LH, Nixon GF, Evans AM. Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium 44: 190–201, 2008. doi: 10.1016/j.ceca.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitamura Y, Okamura T, Kani K, Toda N. Nitric oxide-mediated retinal arteriolar and arterial dilatation induced by substance P. Invest Ophthalmol Vis Sci 34: 2859–2865, 1993. [PubMed] [Google Scholar]
- 32.Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Yamamoto T, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circ J 74: 2579–2584, 2010. doi: 10.1253/circj.CJ-10-0680. [DOI] [PubMed] [Google Scholar]
- 33.Kong H, Jones PP, Koop A, Zhang L, Duff HJ, Chen SR. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J 414: 441–452, 2008. doi: 10.1042/BJ20080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol 255: H1558–H1562, 1988. doi: 10.1152/ajpheart.1988.255.6.H1558. [DOI] [PubMed] [Google Scholar]
- 35.Kurtz KH, Moor AN, Souza-Smith FM, Breslin JW. Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics. Microcirculation 21: 593–605, 2014. doi: 10.1111/micc.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurtz KH, Souza-Smith FM, Moor AN, Breslin JW. Rho kinase enhances contractions of rat mesenteric collecting lymphatics. PLoS One 9: e94082, 2014. doi: 10.1371/journal.pone.0094082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2: a003996, 2010. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laver DR, Attia J, Oldmeadow C, Quail AW. Cardiac calcium release channel (ryanodine receptor 2) regulation by halogenated anesthetics. Anesthesiology 126: 495–506, 2017. doi: 10.1097/ALN.0000000000001519. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Roizes S, von der Weid PY. Distinct roles of L- and T-type voltage-dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. J Physiol 592: 5409–5427, 2014. doi: 10.1113/jphysiol.2014.280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin YR, Kao PC, Chan MH. Involvement of Ca2+ signaling in tachykinin-mediated contractile responses in swine trachea. J Biomed Sci 12: 547–558, 2005. doi: 10.1007/s11373-005-6796-0. [DOI] [PubMed] [Google Scholar]
- 41.Matsuki K, Kato D, Takemoto M, Suzuki Y, Yamamura H, Ohya S, Takeshima H, Imaizumi Y. Negative regulation of cellular Ca2+ mobilization by ryanodine receptor type 3 in mouse mesenteric artery smooth muscle. Am J Physiol Cell Physiol 315: C1–C9, 2018. doi: 10.1152/ajpcell.00006.2018. [DOI] [PubMed] [Google Scholar]
- 42.Maxwell JT, Domeier TL, Blatter LA. Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol Heart Circ Physiol 302: H953–H963, 2012. doi: 10.1152/ajpheart.00936.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCloskey KD, Toland HM, Hollywood MA, Thornbury KD, McHale NG. Hyperpolarisation-activated inward current in isolated sheep mesenteric lymphatic smooth muscle. J Physiol 521: 201–211, 1999. doi: 10.1111/j.1469-7793.1999.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mejia JA, Pernow J, von Holst H, Rudehill A, Lundberg JM. Effects of neuropeptide Y, calcitonin gene-related peptide, substance P, and capsaicin on cerebral arteries in man and animals. J Neurosurg 69: 913–918, 1988. doi: 10.3171/jns.1988.69.6.0913. [DOI] [PubMed] [Google Scholar]
- 45.Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest 124: 915–921, 2014. doi: 10.1172/JCI71608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negrini D, Marcozzi C, Solari E, Bossi E, Cinquetti R, Reguzzoni M, Moriondo A. Hyperpolarization-activated cyclic nucleotide-gated channels in peripheral diaphragmatic lymphatics. Am J Physiol Heart Circ Physiol 311: H892–H903, 2016. doi: 10.1152/ajpheart.00193.2016. [DOI] [PubMed] [Google Scholar]
- 47.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 48.Neylon CB, Richards SM, Larsen MA, Agrotis A, Bobik A. Multiple types of ryanodine receptor/Ca2+ release channels are expressed in vascular smooth muscle. Biochem Biophys Res Commun 215: 814–821, 1995. doi: 10.1006/bbrc.1995.2536. [DOI] [PubMed] [Google Scholar]
- 49.Oo YW, Gomez-Hurtado N, Walweel K, van Helden DF, Imtiaz MS, Knollmann BC, Laver DR. Essential role of calmodulin in RyR inhibition by dantrolene. Mol Pharmacol 88: 57–63, 2015. doi: 10.1124/mol.115.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter VA, Bonev AD, Knot HJ, Heppner TJ, Stevenson AS, Kleppisch T, Lederer WJ, Nelson MT. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. Am J Physiol 274: C1346–C1355, 1998. doi: 10.1152/ajpcell.1998.274.5.C1346. [DOI] [PubMed] [Google Scholar]
- 51.Rayner SE, Van Helden DF. Evidence that the substance P-induced enhancement of pacemaking in lymphatics of the guinea-pig mesentery occurs through endothelial release of thromboxane A2. Br J Pharmacol 121: 1589–1596, 1997. doi: 10.1038/sj.bjp.0701306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sacchi G, Weber E, Aglianó M, Comparini L. Subendothelial nerve fibers in bovine mesenteric lymphatics: an ultrastructural and immunohistochemical study. Lymphology 27: 90–96, 1994. [PubMed] [Google Scholar]
- 53.Shirasawa Y, Benoit JN. Stretch-induced calcium sensitization of rat lymphatic smooth muscle. Am J Physiol Heart Circ Physiol 285: H2573–H2577, 2003. doi: 10.1152/ajpheart.00002.2003. [DOI] [PubMed] [Google Scholar]
- 54.Sloas DC, Stewart SA, Sweat RS, Doggett TM, Alves NG, Breslin JW, Gaver DP, Murfee WL. Estimation of the Pressure Drop Required for Lymph Flow through Initial Lymphatic Networks. Lymphat Res Biol 14: 62–69, 2016. doi: 10.1089/lrb.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Souza-Smith FM, Kerut EK, Breslin JW, Molina PE. Mechanisms of acute alcohol intoxication-induced modulation of cyclic mobilization of [Ca2+] in rat mesenteric lymphatic vessels. Lymphat Res Biol 13: 93–99, 2015. doi: 10.1089/lrb.2014.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Souza-Smith FM, Kurtz KM, Breslin JW. Measurement of cytosolic Ca2+ in isolated contractile lymphatics. J Vis Exp 58: 3438, 2011. doi: 10.3791/3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Souza-Smith FM, Kurtz KM, Molina PE, Breslin JW. Adaptation of mesenteric collecting lymphatic pump function following acute alcohol intoxication. Microcirculation 17: 514–524, 2010. doi: 10.1111/j.1549-8719.2010.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Telinius N, Baandrup U, Rumessen J, Pilegaard H, Hjortdal V, Aalkjaer C, Boedtkjer DB. The human thoracic duct is functionally innervated by adrenergic nerves. Am J Physiol Heart Circ Physiol 306: H206–H213, 2014. doi: 10.1152/ajpheart.00517.2013. [DOI] [PubMed] [Google Scholar]
- 59.Telinius N, Majgaard J, Kim S, Katballe N, Pahle E, Nielsen J, Hjortdal V, Aalkjaer C, Boedtkjer DB. Voltage-gated sodium channels contribute to action potentials and spontaneous contractility in isolated human lymphatic vessels. J Physiol 593: 3109–3122, 2015. doi: 10.1113/JP270166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Telinius N, Majgaard J, Mohanakumar S, Pahle E, Nielsen J, Hjortdal V, Aalkjær C, Boedtkjer DB. Spontaneous and evoked contractility of human intestinal lymphatic vessels. Lymphat Res Biol 15: 17–22, 2017. doi: 10.1089/lrb.2016.0039. [DOI] [PubMed] [Google Scholar]
- 61.Telinius N, Mohanakumar S, Majgaard J, Kim S, Pilegaard H, Pahle E, Nielsen J, de Leval M, Aalkjaer C, Hjortdal V, Boedtkjer DB. Human lymphatic vessel contractile activity is inhibited in vitro but not in vivo by the calcium channel blocker nifedipine. J Physiol 592: 4697–4714, 2014. doi: 10.1113/jphysiol.2014.276683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas NL, Williams AJ. Pharmacology of ryanodine receptors and Ca2+-induced Ca2+release. WIREs Membr Transp Signal 1: 383–397, 2012. doi: 10.1002/wmts.34. [DOI] [Google Scholar]
- 63.Thurston G, Baluk P, Hirata A, McDonald DM. Permeability-related changes revealed at endothelial cell borders in inflamed venules by lectin binding. Am J Physiol 271: H2547–H2562, 1996. doi: 10.1152/ajpheart.1996.271.6.H2547. [DOI] [PubMed] [Google Scholar]
- 64.Toland HM, McCloskey KD, Thornbury KD, McHale NG, Hollywood MA. Ca(2+)-activated Cl(-) current in sheep lymphatic smooth muscle. Am J Physiol Cell Physiol 279: C1327–C1335, 2000. doi: 10.1152/ajpcell.2000.279.5.C1327. [DOI] [PubMed] [Google Scholar]
- 65.Trujillo AN, Katnik C, Cuevas J, Cha BJ, Taylor-Clark TE, Breslin JW. Modulation of mesenteric collecting lymphatic contractions by σ1-receptor activation and nitric oxide production. Am J Physiol Heart Circ Physiol 313: H839–H853, 2017. doi: 10.1152/ajpheart.00702.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Umemura T, Ueda K, Nishioka K, Hidaka T, Takemoto H, Nakamura S, Jitsuiki D, Soga J, Goto C, Chayama K, Yoshizumi M, Higashi Y. Effects of acute administration of caffeine on vascular function. Am J Cardiol 98: 1538–1541, 2006. doi: 10.1016/j.amjcard.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 67.Vaithianathan T, Narayanan D, Asuncion-Chin MT, Jeyakumar LH, Liu J, Fleischer S, Jaggar JH, Dopico AM. Subtype identification and functional characterization of ryanodine receptors in rat cerebral artery myocytes. Am J Physiol Cell Physiol 299: C264–C278, 2010. doi: 10.1152/ajpcell.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Helden DF. The lymphangion: a not so ‘primitive’ heart. J Physiol 592: 5353–5354, 2014. doi: 10.1113/jphysiol.2014.286039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol 471: 465–479, 1993. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanterpool CK, Vanterpool EA, Pearce WJ, Buchholz JN. Advancing age alters the expression of the ryanodine receptor 3 isoform in adult rat superior cervical ganglia. J Appl Physiol (1985) 101: 392–400, 2006. doi: 10.1152/japplphysiol.00167.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von der Weid PY, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol 295: H1989–H2000, 2008. doi: 10.1152/ajpheart.00007.2008. [DOI] [PubMed] [Google Scholar]
- 72.von der Weid PY, Van Helden DF. Functional electrical properties of the endothelium in lymphatic vessels of the guinea-pig mesentery. J Physiol 504: 439–451, 1997. doi: 10.1111/j.1469-7793.1997.439be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang W, Nepiyushchikh Z, Zawieja DC, Chakraborty S, Zawieja SD, Gashev AA, Davis MJ, Muthuchamy M. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol 297: H726–H734, 2009. doi: 10.1152/ajpheart.00312.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol 300: H1616–H1630, 2011. doi: 10.1152/ajpheart.00728.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang XR, Lin MJ, Yip KP, Jeyakumar LH, Fleischer S, Leung GP, Sham JS. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 289: L338–L348, 2005. doi: 10.1152/ajplung.00328.2004. [DOI] [PubMed] [Google Scholar]
- 76.Zawieja SD, Castorena-Gonzalez JA, Scallan JP, Davis MJ. Differences in L-type Ca2+ channel activity partially underlie the regional dichotomy in pumping behavior by murine peripheral and visceral lymphatic vessels. Am J Physiol Heart Circ Physiol 314: H991–H1010, 2018. doi: 10.1152/ajpheart.00499.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L, Liu Y, Song F, Zheng H, Hu L, Lu H, Liu P, Hao X, Zhang W, Chen K. Functional SNP in the microRNA-367 binding site in the 3'UTR of the calcium channel ryanodine receptor gene 3 (RYR3) affects breast cancer risk and calcification. Proc Natl Acad Sci USA 108: 13653–13658, 2011. doi: 10.1073/pnas.1103360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao F, Li P, Chen SR, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J Biol Chem 276: 13810–13816, 2001. doi: 10.1074/jbc.M006104200. [DOI] [PubMed] [Google Scholar]
- 79.Zhao J, van Helden DF. ET-1-associated vasomotion and vasospasm in lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol 140: 1399–1413, 2003. doi: 10.1038/sj.bjp.0705573. [DOI] [PMC free article] [PubMed] [Google Scholar]