Abstract
Motor unit firing times are weakly coupled across a range of frequencies during voluntary contractions. Coherent activity within the beta-band (15–35 Hz) has been linked to oscillatory cortical processes, providing evidence of functional connectivity between the motoneuron pool and motor cortex. The aim of this study was to investigate whether beta-band motor unit coherence is altered with increasing abduction force in the first dorsal interosseous muscle. Coherence between motor unit firing times, extracted from decomposed surface electromyography (EMG) signals, was investigated in 17 subjects at 10, 20, 30, and 40% of maximum voluntary contraction. Corresponding changes in nonlinear surface EMG features (specifically sample entropy and determinism, which are sensitive to motor unit synchronization) were also examined. A reduction in beta-band and alpha-band coherence was observed as force increased [F(3, 151) = 32, P < 0.001 and F(3, 151) = 27, P < 0.001, respectively], accompanied by corresponding changes in nonlinear surface EMG features. A significant relationship between the nonlinear features and motor unit coherence was also detected (r = −0.43 ± 0.1 and r = 0.45 ± 0.1 for sample entropy and determinism, respectively; both P < 0.001). The reduction in beta-band coherence suggests a change in the relative contribution of correlated and uncorrelated presynaptic inputs to the motoneuron pool, and/or a decrease in the responsiveness of the motoneuron pool to synchronous inputs at higher forces. The study highlights the importance of considering muscle activation when investigating changes in motor unit coherence or nonlinear EMG features and examines other factors that can influence coherence estimation.
NEW & NOTEWORTHY Intramuscular alpha- and beta-band coherence decreased as muscle contraction force increased. Beta-band coherence was higher in groups of high-threshold motor units than in simultaneously active lower threshold units. Alterations in motor unit coherence with increases or decreases in force and with the onset of fatigue were accompanied by corresponding changes in surface electromyography sample entropy and determinism. Mixed-model analysis indicated mean firing rate and number of motor units also influenced the coherence estimate.
Keywords: coherence, determinism, EMG, motor unit, sample entropy
INTRODUCTION
During voluntary contraction, the discharge of motor units within a muscle is not completely independent and motor units exhibit a weak tendency to fire within a few milliseconds of one another, with a rate of occurrence above that expected due to chance. Short-term motor unit synchrony is particularly prominent in distal finger and hand muscles, which receive direct monosynaptic connections from corticomotoneuronal cells (Porter and Lemon 1993). Indirect evidence of functional connectivity between the motor cortex and the motoneuron pool is provided by studies investigating corticomuscular coherence, which have shown that motor unit firing is temporally correlated with oscillatory cortical activity within the beta frequency range (15–30 Hz) during steady muscle contractions (Conway et al. 1995). Time and frequency domain analysis of the firing times of pairs of motor units has revealed the presence of two neural components, one responsible for 15–30 Hz coherence and short-term motor unit synchronization and another component in the 1–12 Hz frequency range unrelated to short-term synchronization (Farmer et al. 1993). Coherence between motor unit firing times is likely to be influenced by a number of factors (Kirkwood 2016); these include the strength of shared beta oscillatory activity among presynaptic inputs (functional common input) and the anatomical organization of shared inputs to the motoneuron (structural organization). Although the overall motor unit coherence estimate is not a direct measure of the magnitude of synchronized motoneuron inputs, variations in coherent beta-band activity may reveal concurrent changes in the properties of synaptic inputs to the motoneuron pool.
Although corticomuscular and motor unit coherence have been examined under a range of different conditions, it has not yet been established whether there is a systematic change in the magnitude of beta-band motor unit coherence with increases in the level of muscle activation. Beta-band corticomuscular coherence has been observed to decrease at higher forces during isometric contractions in the tibialis anterior, the first dorsal interosseous (FDI), and knee extensors and flexors (Dal Maso et al. 2017; Perez et al. 2012; Ushiyama et al. 2012). In contrast, it was found to be unchanged with increasing force in the soleus, abductor pollicis brevis, and biceps (Mima and Hallett 1999; Ushiyama et al. 2012) and to increase with contraction strength in the FDI at very low forces (Kilner et al. 2000; Witte et al. 2007). Although several studies have observed a change in corticomuscular coherence, the majority of those investigating coherent beta-band activity in the firing times of motor units within a muscle have not detected a corresponding change with increasing contraction intensity (Castronovo et al. 2015; Christou et al. 2007; Schmied and Descarreaux 2011); however, a higher incidence of significant coherence has been reported (Laine et al. 2015). Conflicting findings have been described in studies assessing variations in motor unit short-term synchronization, which is directly related to beta-band coherence (Farmer et al. 1993; Lowery et al. 2007; Semmler et al. 2002). At higher forces, motor unit synchronization strength has been reported to decrease (Kline and De Luca 2016; Nordstrom et al. 1992), increase (Schmied and Descarreaux 2010), show no systematic trend (Christou et al. 2007), or exhibit disparate changes with different synchrony indices (Fling et al. 2009). These earlier studies on motor unit coherence and synchronization have been largely limited to very low force levels to reliably discriminate motor units as the contraction intensity increases. Recent advances in surface electromyography (EMG) decomposition enable motor unit activity across a wider range of force levels to be investigated and yield information on a greater number of concurrently active motor units than traditional intramuscular EMG methods. Compared with estimates from paired motor unit recordings, coherence analysis of a larger motor unit sample using composite spike trains has the potential to enhance the detection of correlated motor unit discharges (Farina et al. 2014). Furthermore, quantifying coherent motor unit activity within the same muscle may provide a more accurate assessment of synchrony at the whole muscle level when compared with corticomuscular and intermuscular recordings.
The primary aim of this study was to examine whether beta-band motor unit coherence in the FDI muscle changes systematically with increasing index finger abduction force between 10 and 40% maximal voluntary contraction (MVC). Motor unit coherence was also investigated in the lower alpha-band (8–12 Hz), as synchronous motor unit activity has been observed in this range during slow finger movements and under isometric force conditions (Elble and Randall 1976; Farmer et al. 1993; Halliday et al. 1999; Semmler et al. 2003). It is reasonable to assume that the structure of the global surface EMG signal will also be affected by the degree of coherent activity in its constituent motor unit discharges. Thus, a secondary aim of this study was to establish whether changes in the underlying motor unit beta-band coherence are reflected in the nonlinear estimates of surface EMG signal complexity and deterministic structure, specifically the sample entropy (SampEn) and percentage determinism (%DET). These nonlinear measures characterize the degree of similarity and repeating structure within a signal (Richman and Moorman 2000; Webber et al. 1995) and have previously captured differences in surface EMG signals under conditions where normal motor unit synchronization is enhanced (Farina et al. 2002; Fattorini et al. 2005). However, experimental studies have not yet detected a significant relationship between these nonlinear surface EMG parameters and beta-band motor unit coherence (Schmied and Descarreaux 2011).
METHODS
Index finger abduction force and EMG activity of the FDI muscle were recorded during isometric abduction of the index finger in 17 young adults with no neurological conditions (8 female, age 28 ± 5 yr, 2 left-handed, 1 ambidextrous or ambiguously handed). Written informed consent was obtained from all participants, and the experiments were conducted in accordance with the standards set by the Declaration of Helsinki and approved by Human Research Ethics Committee for Sciences at University College Dublin.
Experimental procedure.
Participants were seated upright with their upper arm and hand comfortably resting in pronation on a support, which was securely mounted with magnetic stands to a heavy steel table. To standardize hand position and to minimize contributions of other muscles, the forearm and index finger were cast and the little, ring, and middle fingers were separated from the index finger and strapped to the support surface. The thumb was secured at an ~60-degree angle to the index finger. The proximal phalanx of the index finger was fixed to a ring-mounted interface attached to two load cells (SML-110N; Interface) to record force generated in the abduction/adduction and extension/flexion directions. The magnitude and direction of the force generated and the target force were presented on a screen positioned at eye level. Surface EMG was recorded from the FDI muscle using a surface sensor array (Delsys) that consisted of 5 cylindrical probes located at the corners and center of a 5 × 5 mm square (Nawab et al. 2010) and a reference electrode on the skin surface of olecranon. Pairwise differential recordings of the 5 electrodes yielded 4 channels of surface EMG, which were amplified and filtered between 20 Hz and 450 Hz. The signals were sampled at 20 kHz and stored on a computer for further processing. The force produced by the index finger was band-pass filtered between 0.5 and 30 Hz, and the root mean square error was calculated between the force generated in the abduction/adduction direction and the target force.
MVC during isometric index finger abduction was determined for each subject as the highest force achieved during three short (3 s) maximum contractions, separated by a 1-min rest period, where the maximum force between trials lay within 10% of each other. Subjects then performed a series of isometric voluntary contractions, in which they were required to either maintain a constant abduction force or increase/decrease their force level by 10% MVC midway through the contraction. The constant force trials were performed at 10, 20, 30, and 40% MVC, the first half of the increasing force trials began at either 10 or 20% MVC, and the first half of decreasing force trials began at either 20 or 30% MVC. The total length of the force trajectory for each trial was 45 s, which included a 3 s quiescent period at the beginning and end of the trajectory for baseline noise calculation. Increases and decreases in the required force level were graded at a rate of 10% MVC/s. The protocol consisted of three repetitions of the constant force trials at 10 and 20% MVC and two repetitions of the other constant force (30% MVC, 40% MVC) and the two-force level trials (10→20% MVC, 20→10% MVC, 20→30% MVC, 30→20% MVC). Subjects were occasionally required to perform additional repetitions to ensure that high force accuracy was obtained in at least one trial for each trajectory. Trials were performed in a randomized order, although consecutive high-force contractions (i.e., 30% and 40% MVC) were avoided to minimize the development of fatigue. A minimum of 45 s of rest was provided between trials. Discriminable motor units were extracted from the surface EMG signal using the decomposition EMG system (dEMG Analysis, version 1.1.3). The decomposition algorithm is outlined in detail in Nawab et al. (2010). For each detected motor units, the output of the decomposition algorithm consisted of the motor units firing times and 4 motor unit action potential (MUAP) waveforms corresponding to 4 pairs of bipolar electrode channels.
Motor unit acceptance criteria.
The identified firing times for each motor unit were used to calculate the spike-triggered average (STA) of the surface EMG signal on each channel, resulting in 4 representative STA MUAP waveforms for each motor unit. The variation of the STA MUAP template over time was quantified using a 4-s moving average window with 0.5-s time step. A MUAP template estimate was calculated based on the firing events in each window, and the window was shifted along the length of the surface EMG signal, as performed in Hu et al. (2013). The STA templates were then examined in four-dimensional (4-D) space, with the coordinates of the 4-D trajectory provided by the MUAP waveform samples on each of the four channels. The trajectory of the estimated MUAP template for each window was compared with a reference trajectory, calculated as average template estimate across all windows. For a detected motor unit to be accepted for further analysis, a minimum of 75% of the trajectories obtained from the moving average window were required to lie within a fixed radius of the reference trajectory for that motor unit. Each accepted motor unit was also required to have a waveform trajectory distinct or separate from all other decomposed MUAP waveforms in 4-D space. To evaluate the separation or heterogeneity of the MUAP waveforms, the Euclidean distance between the trajectories of two detected motor units was calculated in 4-D for all possible pair combinations. Motor unit pairs with a distance less than −2.5 standard deviations from the mean were excluded from further analysis. In addition to satisfying the requirements of trajectory stability and heterogeneity, motor units detected during the trials with two force levels were only accepted if their MUAP waveform trajectories were consistent across both force levels, indicating that the same motor unit was detected at both levels. Constant force trials were required to have a minimum of 12 accepted motor units, and two-force trials at least 8 accepted motor units to be included in the subsequent coherence analysis.
Because of the high level of motor unit superposition in surface EMG signals, when two or more motor units fire within a few milliseconds of one another, firing instances may have a higher likelihood of being missed in the decomposition of these signals compared with intramuscular EMG recordings. Missed coincident firings will not be detected with coherence analysis, but a large quantity of missed synchronous firings could conceivably cause a reduction in coherence. Thus, an additional validation step was introduced to assess whether this limitation of surface EMG decomposition influenced the results. Cross-correlation histograms with 2-ms bins were constructed between pairs of firing trains for all forward and reverse times to quantify the level of missed coincident firings in the decomposed motor unit data. A large number of missed coincident firings between two motor units would be expected to manifest as a dip or “trough” at approximately zero lag in the cross-correlogram between their firing trains. The cross-correlogram for each pair was classified as belonging to one of three subgroups: those that exhibited a trough, those that exhibited a broad or narrow peak typical of synchronous motor units, and those that did not show any distinct peaks in the correlogram. To assess whether missed firings could account for changes in coherence across the different force levels, the number of motor unit pairs exhibiting troughs in the cross-correlogram across all force levels was examined. Motor units that exhibited a trough in the cross-correlogram formed with more than three other motor units were removed and the coherence analysis was then repeated for the remaining motor units.
Coherence analysis.
Motor unit activity was examined over 23 s, centered midway through the constant force trials, and during a 10-s period at each force level in the two-force trials. The middle section of each trajectory was chosen to exclude periods of motor unit recruitment and derecruitment during changes in force and at the start and end of each trial. The coherence within the motoneuron pool was estimated from pairs of composite spike trains (Farina et al. 2014; Negro and Farina 2011). The accepted motor units from each trial were divided into two groups, each containing an equal number of randomly chosen motor units. The firing trains in each group were summed to obtain two composite spike trains. The magnitude squared coherence between the two composite spike trains was calculated with 1 s overlapping Hamming windows (nfft = 1,024, 75% overlap, 86 segments for the single force trial and two sets of 36 segments for the two-force trials). This was repeated for 200 randomly chosen combinations of two groups from the same set of motor units, as each combination will generate a slightly different coherence estimate. Each trial was represented by the median coherence spectrum over all 200 combinations.
The Fisher “z-transform” was applied to the magnitude squared coherence estimates C(f) to obtain a normally distributed variable Z(f), Eq. 1 (Enochson and Goodman 1965), with approximate unit variance (Halliday and Rosenberg 2000).
| (1) |
Although the explicit formula for the statistical distribution of magnitude squared coherence with overlapping windows is not known, approximations are available (Gallet and Julien 2011). The number of disjoint segments (L) was substituted for the effective number of segments with 75% overlap () to calculate the variance and significance threshold for the transformed z-scores as described by Gallet and Julien (2011). The window function (wl) and fixed delay (D, equal to 25% of the segment length) were used in the calculation of . The 95% confidence limit (γ), Eq. 5, was z-transformed to determine a significance threshold for the z-scores.
| (2) |
| (3) |
| (4) |
| (5) |
The mean coherence value in the 100–500 Hz range should theoretically be zero if there is no physiological coupling between the motor unit spike trains. However, in practice it has a small numerical value that can vary according to the degree of overlap, the spectral window function, and number of independent segments used. Therefore, for each trial the mean z-score in this range was subtracted from Z(f) at all frequencies to remove this bias, as in Baker et al. (2003). The integral of significant values of Z(f) was then calculated for each trial in the alpha (8–12 Hz) and beta-band frequency ranges (15–35 Hz). The value of the integral of the coherence in each frequency band was divided by the number of integration points in the frequency band.
The amplitude of each MUAP, an approximation of the motor unit size, was estimated as the distance traversed by the action potential in multidimensional space. To investigate whether there was a difference in the beta-band coherent activity of high- and low-threshold motor units, motor units were arranged in ascending order of size. The coherence for the first 8 motor units was calculated between two composite pulse trains comprised of 4 motor units each. This was repeated for all combinations of two groups of 4 units (70 possible combinations), and the median coherence over all combinations was estimated. The coherence estimate for the first 8 motor units was then compared with that of the last 8 units for all trials (with at least 16 accepted motor units).
Nonlinear analysis.
Before analysis, the surface EMG signal was low-pass filtered at 400 Hz (8th order Chebyshev IIR filter) and downsampled to 1 kHz. Recurrence quantification analysis was performed on nonoverlapping 1.5-s segments of the surface EMG signal during the periods of constant force production in each trial. Recurrence quantification analysis involves transforming the single-channel surface EMG signal onto a multidimensional phase space trajectory, with each point on the trajectory representing a different point in time. This result is mapped onto a two-dimensional recurrence plot to provide a visualization of the times at which a phase space trajectory returns to a location in phase space that it has visited before. A recurrence represents a pair of points on the trajectory that are separated by a distance smaller than a specified radius value, and the recurrence plot depicts this result for all possible pairs of time points. The parameters selected in this study were chosen to effectively capture the dynamics of motor unit firing patterns (i.e., time delay = 1, embedding dimension = 15, minimum diagonal line = 10, and radius = 0.2) (Flood et al. 2019; Marwan et al. 2007). Recurrence plots were used to calculate the %DET, (number of diagonal lines of consecutive points on the plot), a feature that illustrates how far the surface EMG signal is from a purely random signal (Webber et al. 1995). The median value over all channels and segments was taken as the representative %DET value. The median frequency and the root-mean-square amplitude of the surface EMG signal were calculated over the same sections of the signal as the %DET.
The surface EMG signals were also assessed using SampEn, a measure of signal complexity and regularity that has been derived specifically for physiological time-series signals (Richman and Moorman 2000). Briefly, SampEn quantifies the degree of uncertainty or randomness in the EMG signal using template matching, whereby a short epoch of the signal is defined as a template and that template is compared with the remainder of the signal to assess the conditional probability of it being repeated. A low value of entropy reflects a high degree of regularity in a signal (e.g., periodicity), with more similarity between each epoch of the signal. SampEn was calculated for each trial over the same period examined in the coherence analysis. SampEn was calculated over three 10-s windows with an overlap of 4.5 s during the constant force trials and over two 10-s segments during the two-force trials (at the higher and lower force levels, respectively). The tolerance r (threshold for similarity between templates) for the SampEn calculation was given by Eq. 6, where MAD is the median absolute deviation of the signal x.
| (6) |
The embedding dimension (length of the template used for comparison) and parameter k were empirically set to 3 and 0.2, respectively (Flood et al. 2019). The tolerance scheme was selected based on each signal section under analysis to focus on the signal structure rather than its amplitude. The median value over all windows and channels in the third dimension was used as the representative value for SampEn.
Statistical analysis.
All statistical analyses were performed in the software R (www.r-project.org, version 3.5.1). The relationship between beta-band coherence (and SampEn/%DET) and the force of the muscle contraction (Force) was investigated with a linear mixed-effects model with maximum likelihood fit using the lme4 library (Bates et al. 2011). A mixed-effects model with unstructured variance covariance structure was used to account for the statistical correlation between multiple coherence values obtained from the same subject and to include the results of each trial in the statistical analysis without averaging. The coherence estimate obtained for each trial (first level) was nested according to Force (second level), which was in turn nested within each Subject (third level). Force was entered as a fixed effect in the model and Subject was included as a random effect, with a random intercept chosen for each subject to account for baseline differences in coherence. Previous simulation studies have shown that both the number of motor units used in the coherence calculation and the mean firing rate (MFR) of these units can influence the estimated coherence (Farina et al. 2014; Lowery et al. 2007; McManus et al. 2016). The number of motor units used in the coherence calculation (MUnum) and the motor unit MFR for each trial were therefore used as predictor variables to assess their relative influence on the coherence estimate. A similar mixed-model format was used to examine the effect of Force on the beta-band motor unit coherence during the two-force trials, with MFR and MUnum as predictor variables.
To examine whether motor unit coherence changed from the first to the second half of each trial, a mixed model was applied to the data, again incorporating predictor variables MFR and MUnum. An additional fixed effect was used to indicate whether the coherence estimate was obtained from the first or second half of the trial (Time), and an interaction term (Force*Time) was included in the model to investigate whether any change in coherence differed over the four force levels. Lastly, motor unit coherence was estimated using the 8 largest and 8 smallest motor units for each trial to assess whether there was any difference in the coherent activity of low- and high-threshold motor units. Differences between the two populations were assessed using a mixed model with a fixed effect to indicate whether the coherence estimate was from a low- or high-threshold motor unit subgroup (Group), in addition to MFR as a predictor variable. An interaction term (Force*Group) was included to examine whether any difference in coherence between the two populations varied over the four force levels. Model diagnostic plots were assessed to check for violations of regression assumptions, i.e., linearity, heteroscedasticity, and normality (of both residuals and random effects). The variance inflation factor (VIF) of each predictor was calculated to ensure that there was no collinearity between the predictors (i.e., VIF < 3). The F-tests and P values in the ANOVA table were generated using Kenward-Roger’s method for denominator degrees-of-freedom. Differences in motor unit coherence and MFR across each force level were examined by pairwise comparisons of least-square means using the Benjamini and Hochberg correction to account for multiple testing. Least-square means assesses the difference between force levels while adjusting for the effect of any predictor variables included in the model (e.g., MUnum, MFR). The intraclass correlation coefficient (ICC) was calculated to report the proportion of variance in the motor unit coherence that could be explained by the grouping structure (i.e., variability due to intersubject differences in baseline coherence). The conditional R2(c) and the marginal R2(m) were also estimated to determine the variance explained by the entire model (i.e., both fixed and random effects) and the variance of just the fixed effects, respectively (Nakagawa and Schielzeth 2013). To assess the relative importance of each fixed effect, semi-partial R2 values were calculated for the effect of Force and for predictors (Edwards et al. 2008; Nakagawa and Schielzeth 2013).
To investigate the relationship between the motor unit coherence and the nonlinear features/force accuracy, a repeated measures correlation analysis was performed for the constant force (n = 171 trials) and two-force trials (n = 142 trials, equivalent to 284 signal segments), as described by Bakdash and Marusich (2017). The relationship between beta-band coherence and the accuracy of the abduction force produced was further investigated with a linear mixed model, with the force level and beta-band coherence estimates included as possible predictors of force accuracy.
In figures depicting data pooled over all subjects, data were normalized per subject to focus on within-subject effects and minimize the contribution of intersubject variance in baseline values to the visual representation of results. Data were normalized for a given subject by subtracting that subject’s mean coherence for the four force levels and adding on the grand mean over all subjects (Loftus and Masson 1994).
RESULTS
The relative effects of contraction force level, motor unit sample size, and motor unit firing rate on estimated motor unit beta-band coherence during constant force isometric contraction are first presented. Motor unit coherence is also presented for trials where the same motor unit sample was tracked across two force levels. The influence of the detected motor unit sample on the coherence estimate is then further highlighted by examining differences in beta-band coherence between low- and high-threshold motor units. To investigate whether the development of fatigue affected the coherence estimate at higher force levels, motor unit coherence during the first and second half of the constant force contractions was compared. Finally, parallel changes in the nonlinear surface EMG features are presented. The full mixed model results can be found in the supplemental material (all supplemental material is available at https://doi.org/10.5281/zenodo.3257376).
The average number of motor units detected during each trial is presented in Table 1, along with the number of units that satisfied the acceptance criteria for further analysis. The mean abduction force over all subjects was 23.6 ± 4.3 N.
Table 1.
Detected and accepted motor units
| Constant Force | Increasing Force | Decreasing Force | ||||||
|---|---|---|---|---|---|---|---|---|
| 10% MVC | 20% MVC | 30% MVC | 40% MVC | 10→ 20% MVC | 20→ 30% MVC | 20→ 10% MVC | 30→ 20% MVC | |
| Total MUs | 27.3 ± 7 | 26.2 ± 6 | 26.6 ± 7 | 28.9 ± 6 | 28.5 ± 8 | 31.3 ± 8 | 26.2 ± 7 | 25 ± 6 |
| Accepted MUs | 20.9 ± 5 | 20.6 ± 5 | 20.9 ± 5 | 22.0 ± 5 | 17.0 ± 5 | 18.3 ± 4 | 16.0 ± 4 | 15.9 ± 4 |
Values are means ± SD. Average number of motor units (MUs) detected during each trial, and the number of units used in further analysis, for the constant force and two-force trials. MVC, maximal voluntary contraction.
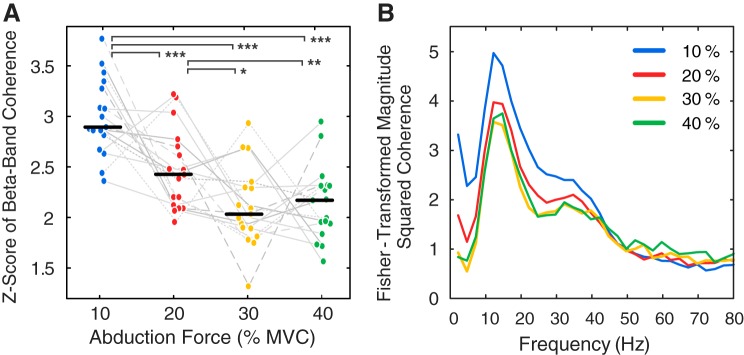
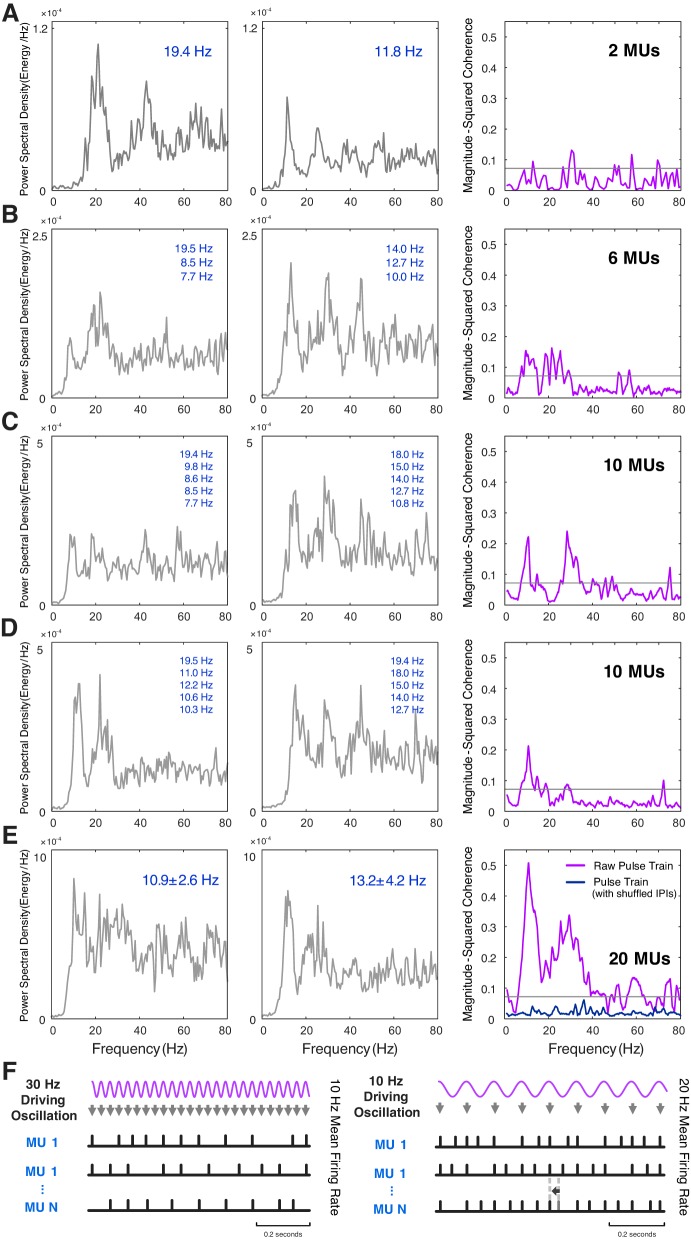
Beta-band motor unit coherence decreased with increasing force during the constant force trials between 10 and 40% MVC (Fig. 1). The motor unit coherence spectrum and motor unit MFRs are shown for a representative subject in Fig. 2. The power spectra of the individual single motor unit pulse trains featured a spectral peak at the mean discharge rate (19.4 Hz and 11.8 Hz in Fig. 3A) and a smaller harmonic component at double the firing frequency. The corresponding coherence spectrum shows a small but significant peak at 30 Hz that was not present in the power spectra (Fig. 3A). The spectral peaks at ~10 Hz and ~30 Hz were not clearly defined in coherence estimates from motor unit pairs (Fig. 3A), but distinct peaks emerged as more motor units were included in the estimation (Fig. 3, B–E). The coherence between motor units disappeared when the estimate was obtained after shuffling the interpulse intervals of the raw pulse trains, a process that removes any correlation between motor unit discharges but maintains the same MFRs (Fig. 3E). The maximum number of available motor units was thus used to calculate the coherence estimate for each trial, as various motor unit subsets could generate differing coherence spectra (Fig. 3, C and D). Motor unit discharge times may be shifted by an oscillatory input, resulting in an increased likelihood of a motor unit firing in response to the stimulus across the population.
Fig. 1.
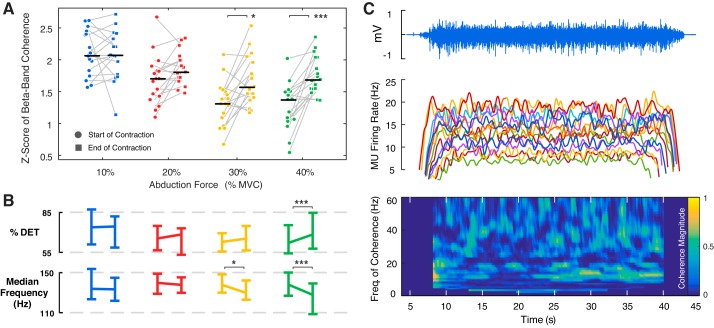
A: median and interquartile range of the Fisher-transformed coherence values in the beta-band range across all subjects. Differences in motor unit (MU) coherence between force levels were tested with pairwise comparisons of least-square means using all trials (n = 171 trials, see Table 1 for average MU number per trial) while adjusting for the effect of the number of MU in the coherence calculation and mean firing rate. B: composite coherence spectrum across all subjects, averaged across all subjects and trials (in 2.5-Hz bins). *P < 0.05; **P < 0.01; ***P < 0.001. MVC, maximal voluntary contraction.
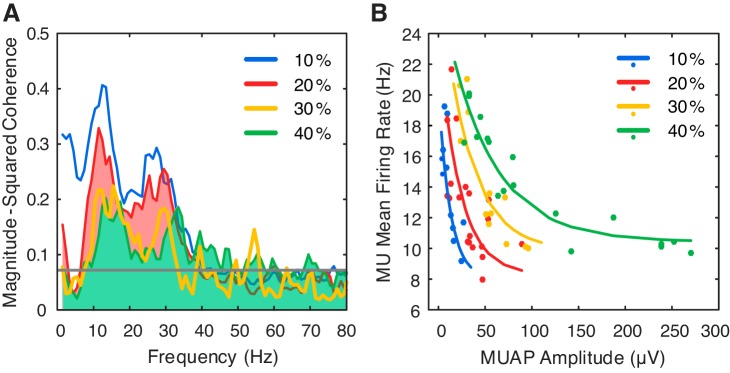
Fig. 2.
Magnitude-squared coherence spectrum for a single subject, averaged across all trials (in 1-Hz bins) (A) and the motor unit (MU) mean firing rates as a function of the MU action potential (MUAP) size within a single representative trial at each force level (fit with a stretched exponential function) (B).
Fig. 3.
Power spectral density of two motor unit (MU) pulse trains for a representative 20% maximal voluntary contraction trial, their respective firing rates [MU 1: interpulse interval (IPI) = 0.052 ± 0.013 s and MU 1: IPI = 0.085 ± 0.026 s], and the coherence estimate obtained for the MU pair are shown (A). Power spectral density is presented for the summed MU pulse trains in the same trial in groups of 6 MUs (B), 10 MUs (C and D), and 20 MUs (E), alongside the composite MU coherence estimate for each group. In (E), interpulse intervals of the raw pulse trains were shuffled to remove any correlation but maintain the same mean firing rates, and the coherence estimate was calculated on the reconstructed pulse trains with shuffled IPIs. A schematic illustrating how a driving oscillation of a particular frequency could modulate MU activity is shown (F) [McAuley and Marsden (2000); reprinted and adapted by permission of Oxford University Press on behalf of the Guarantors of Brain. OUP and the Guarantors of Brain are not responsible or in any way liable for the accuracy of the adaptation. L. McManus is solely responsible for the adaptation in this publication]. A synchronous input can induce an MU to fire earlier than a similar unsynchronized input, so that the MU has a higher probability of firing with each 30 or 10 Hz input. An external periodic signal can thus modulate MU firing patterns to produce coherence peaks at frequencies distinct from the MU mean firing rates.
A reduction in alpha-band motor unit coherence was also detected at higher force levels; however, a mixed-model analysis indicated that a significant portion of the variance in the alpha-band coherence could be explained by differences in average motor unit firing rates. Higher motor unit MFRs resulted in lower alpha-band coherence estimates, but firing rate had no effect on the beta-band (Table 2 and Table S1). Both the number of motor units used in the calculation (see also Fig. 3) and the force level of the contraction influenced the coherence estimates. The mixed model enabled each trial to be included in the statistical analysis without averaging (n = 171 trials), allowing the inherent variability in coherence measurement to be explored and incorporated into all tests of significance. Approximately 40% (adjusted ICC = 0.4) of the total variance in motor unit coherence could be attributed to variance in the coherence estimate across subjects. Even with the inclusion of covariates and fixed factors, differences in subject baseline coherence could still account for more than 20% of the variance in the motor unit coherence estimate (conditional ICC = 0.23).
Table 2.
Mixed-model ANOVA results for the constant force and two-force trials
| Model Term | df1/2 | F-Statistic | P | Semi-Partial R2 |
|---|---|---|---|---|
| Beta-band motor unit coherence during constant force trials | ||||
| Force | 3/151 | 31.89 | <0.001 | 0.22 [0.21, 0.23] |
| MFR | 1/151 | 1.59 | 0.21 | 0.01 [0.00, 0.07] |
| MUnum | 1/165 | 67.7 | <0.001 | 0.28 [0.17, 0.38] |
| Marginal R2 | 0.43 | Conditional R2 | 0.66 | |
| Beta-band motor unit coherence during two-force trials | ||||
| Force | 7/260 | 47.02 | <0.001 | 0.31 [0.30, 0.32] |
| MFR | 1/240 | 2.20 | 0.14 | 0.01 [0.00, 0.04] |
| MUnum | 1/272 | 48.51 | <0.001 | 0.13 [0.06, 0.20] |
| Marginal R2 | 0.50 | Conditional R2 | 0.69 | |
Mixed model ANOVA results (Type III) ANOVAs using the Kenward-Roger approximation for degrees of freedom investigating the effect of each fixed factor on the beta-band motor unit coherence. The degrees of freedom (df) listed under df1/2 were rounded to the next integer. MFR, mean firing rate; MUnum, number of motor units used in the coherence calculation.
Motor unit action potential amplitudes were larger at each consecutive force level [F(3, 151) = 110.7, P < 0.001], indicating the recruitment of larger motor units at higher force levels (10% MVC: 13.0 ± 7.5 µV, 20% MVC: 24.9 ± 12.1 µV, 30% MVC: 37.9 ± 20.2 µV, and 40% MVC: 48.2 ± 19.5 µV, P < 0.001 for all pairwise comparisons). Motor unit MFRs were also significantly affected by changing contraction force level [F(3, 151) = 4.2, P = 0.007], although pairwise comparisons only revealed a significant difference between MFRs at 10% MVC (14.7 ± 2.7 Hz) and both 30% MVC (13.6 ± 2.4 Hz, P = 0.017) and 40% MVC (13.7 ± 1.8 Hz, P = 0.015) and not between 10 and 20% MVC (14.0 ± 2.5 Hz, P = 0.099). Together, these results suggest the recruitment of larger, higher threshold motor units with lower MFRs as force was increased (McManus et al. 2015).
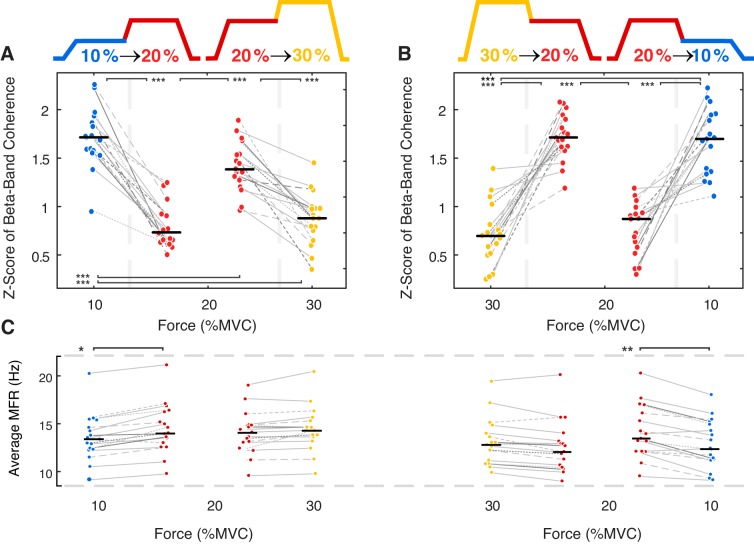
Force level had a significant effect on beta-band motor unit coherence during the constant force trials (semi-partial R2 = 0.19 and 0.22, respectively; Table 2) and an even greater influence on coherence during the two-force trials, where the coherence estimate was calculated on the same sample of motor units across two different force levels (semi-partial R2 = 0.29 and 0.31, respectively; Table 2 and Fig. 4). The decrease/increase in coherence as force was increased/decreased was even more pronounced when analyzing the same motor unit sample across force levels (Fig. 4, A and B, respectively). Although alpha-band coherence exhibited similar changes to those observed in the beta-band, a significant proportion of the variation in alpha-band coherence was again explained by differences in motor unit MFR, with no influence of MFR on beta-band coherence detected (Table S1).
Fig. 4.
Median and interquartile range of the Fisher-transformed coherence values in the beta-band range during the first and second half of a trial with two contraction force levels. Both increasing (A) and decreasing (B) force trials are shown. Changes in motor unit (MU) mean firing rates (MFRs) during the two force level trials are shown (C). Differences in MU coherence and MU MFR between force levels were tested with pairwise comparisons of least-square means using both the low- and high-force sections of all trials (n = 142 trials, see Table 1 for average MU number per trial), with differences in MU coherence adjusted for the effect of the number of MUs used in the coherence calculation and MFR. *P < 0.05; **P < 0.01; ***P < 0.001. MVC, maximal voluntary contraction.
Consistent with the constant force isometric contractions, contraction force also affected motor unit MFRs during the two-force trials [F(7, 260) = 5.8, P < 0.001]. Motor unit MFRs, estimated from a constant motor unit population, were significantly altered when the force was decreased from 20→ 10% MVC (14.1 ± 2.8 Hz to 12.9 ± 2.5 Hz, P = 0.01) and when force was increased from 10→ 20% MVC (13.6 ± 2.5 Hz to 14.5 ± 2.6 Hz, P = 0.047). When the motor unit firing rates during the two-force trials were compared with those recorded during the constant force trials, firing rates were higher when force was increased to 30% MVC (14.1 ± 2.2 Hz, P = 0.019) from 20% MVC when compared with the constant force trial at 30% MVC (Fig. 4C). Similarly, motor unit MFRs were lower when the contraction force was decreased from 30 to 20% MVC (P = 0.027) and from 20 to 10% MVC (P < 0.001), when compared with the trials at a single constant force (Fig. 4C).
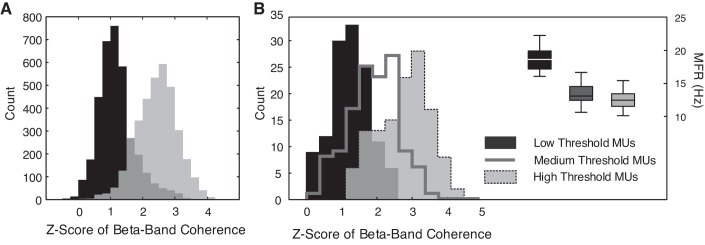
When the beta-band coherence estimate was compared between groups of low- and high-threshold motor units, it was consistently greater in larger, high-threshold motor units [F(1, 287) = 23.38, P < 0.001; Table S2]. Beta-band coherence was larger in high-threshold motor units at all force levels while accounting for increases in the coherence estimate that could be attributed to lower MFRs. The difference in beta-band coherence between low- and high-threshold motor units is shown for 40% MVC across all subjects in Fig. 5A and for a single trial at 20% MVC in a representative subject in Fig. 5B. Although differences between motor unit groups did not vary across the four force levels for the beta-band coherence [F(3, 274) = 1.37, P = 0.25], a significant interaction between Force*Group was detected in the alpha-band coherence [F(3, 273) = 2.98, P = 0.03]. Alpha-band coherence estimates differed between low- and high-threshold motor units in the 10 and 20% MVC trials (P < 0.0001 and P = 0.004, respectively), but not for the trials at 30 and 40% MVC (P = 0.09 and P = 0.39, respectively), when differences in motor unit MFR were considered.
Fig. 5.
A: distribution of the Fisher-transformed coherence values in the beta-band range for the 8 smallest motor units (MUs) (low threshold) and the 8 largest MUs (high threshold) across all subjects at 40% maximal voluntary contraction [MVC; data from each subject was normalized to minimize the contribution of intersubject variance (Loftus and Masson 1994)]. B: distribution of the Fisher-transformed coherence for a single trial in a representative subject at 20% MVC, with the coherence and MU mean firing rates (MFRs) calculated for three groups of 10 MUs arranged in order of size.
Motor unit coherence during the constant force trials was then compared between the first and second half of the contraction, with beta-band coherence shown in Fig. 6A. Motor unit coherence was significantly higher during the second half of the trial in both the alpha- and beta-band [F(1, 316) = 19.86, P < 0.001 and F(1, 316) = 21.0, P < 0.001, respectively; Table S3]. The progressive increase in the beta-band coherence during the contraction is shown for a representative subject at 30% MVC in Fig. 6C. This was accompanied by a decrease in the median frequency of the surface EMG signal during the sustained contraction at 20, 30, and 40% MVC (Fig. 6B), indicating the development of peripheral fatigue, associated with reduced muscle fiber conduction velocity as the contraction progressed at higher force levels. The increase in coherence from the first to the second half of the trials did not vary across the four force levels in the alpha-band [F(3, 316) = 1.35, P = 0.26]. However, alterations in beta-band coherence differed according to force level [F(3, 316) = 4.21, P = 0.006], with a significant increase detected at the higher force levels 30 and 40% MVC (P = 0.008 and P < 0.001, respectively) but no change observed at 10 and 20% MVC (P = 0.92 and P = 0.27, respectively). Motor unit MFRs influenced both the alpha- and beta-band coherence estimate [F(3, 279) = 52.15, P < 0.001 and F(1, 294) = 5.55, P = 0.019, respectively]; however, this effect was stronger in the alpha-band estimates when compared with the beta-band (semi-partial R2 = 0.19 and 0.02, respectively).
Fig. 6.
A: median and interquartile range of the Fisher-transformed coherence values in the beta-band range during the first and second half of the constant force contraction across all subjects. B: median and SD of the percentage determinism (%DET) and the median frequency of the surface electromyography (EMG) signal. C: surface EMG, motor unit (MU) mean firing rates, and the wavelet coherence during a single force trial at 30% maximal voluntary contraction (MVC) in a representative subject. *P < 0.05; ***P < 0.001.
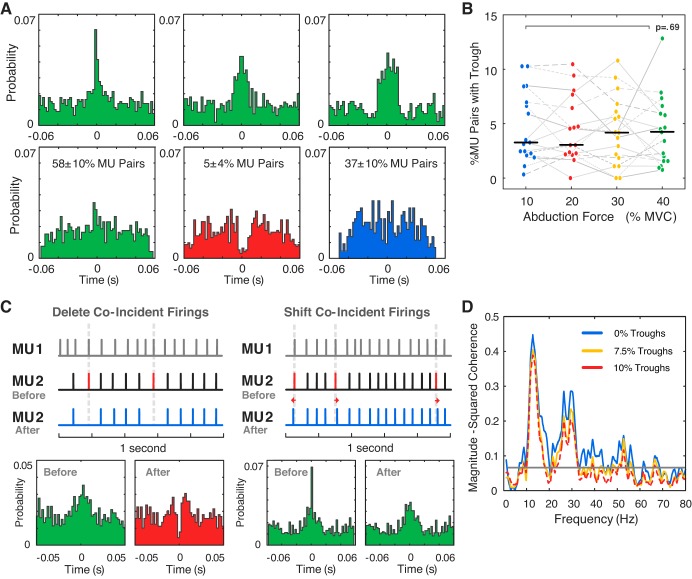
Cross-correlogram analysis revealed the presence of a peak (of varying amplitude) centered around zero lag in ~58 ± 10% of motor unit pairs, indicating an excess of coincident firing between two motor units (Fig. 7A). A relatively small number of motor unit pairs (5 ± 4%) exhibited a dip or “trough” at zero lag (Fig. 7A). It was found that troughs similar to those detected in experimental data could be artificially induced in the cross-correlogram of motor unit pairs by removing coincident firing instances from one of the motor units in the pair (Fig. 7C). Shifting coincident firings (by 3 ms or less) in one motor unit relative to the other motor unit created a wider peak in the cross-correlogram (Fig. 7C). Figure 7C illustrates the effect that such errors in the precise timing of motor unit firing instances could have on the cross-correlogram. The results of the cross-correlogram analysis suggest that missed coincident firings did not significantly influence the observed reduction in motor unit coherence at higher force levels. First, there was no systematic change in the percentage of motor unit pairs that exhibited troughs at zero in the cross-correlogram, which indicates that the number of missed coincident firings did not increase at higher force levels [F(3, 151) = 0.49, P = 0.69; Fig. 7B]. There was, however, a significant decrease in the detection of significant peaks [F(3, 152) = 5.2, P = 0.002], consistent with the reduction in beta-band coherence. Second, a decrease in both alpha and beta-band coherence remained after the removal of motor units that were identified as having high levels of missed coincident firings [F(3, 143) = 24.5, P < 0.001 and F(3, 142) = 33.12, P < 0.001, respectively]. Additionally, higher threshold motor units still exhibited greater alpha- and beta-band coherence than lower threshold units [F(1, 303) = 30.6, P < 0.001 and F(1, 283) = 5.5, P = 0.02, respectively]. There was no difference in the number of motor units removed at each force level [average of 1.4 ± 2.3 motor units per trial, F(3, 143) = 0.5, P = 0.68]. Lastly, artificially introducing missed coincident firings into 10% of the total number of motor unit pairs in experimental data did not have a large effect on the coherence spectrum (Fig. 7D).
Fig. 7.
A: sample cross-correlograms between pairs of motor unit (MU) pulse trains. Across all trials, ~58 ± 10% of MU pairs had a narrow or broad peak (of varying amplitude) centered at approximately zero lag in the cross-correlogram (green), 5 ± 4% had a dip or trough at zero lag (red), and 37 ± 10% showed no distinct peaks or troughs (blue). B: percentage of the MU pairs that exhibited a trough at zero lag in the cross-correlogram at each force level for all subjects (median over all subjects shown in black). C: troughs could be artificially induced in the cross-correlogram for an MU pair by deleting coincident firing times in one MU (MU2) relative to the other reference unit (MU1). A broader, less-distinct peak in the correlogram was observed when firing times were shifted (by 3 ms or less) in MU2 relative to coincident firings in MU1. D: changes in the coherence spectrum after the deletion of coincident firings in selected MUs for a trial in which no troughs were originally detected. Troughs were artificially induced in 1) 7.5% of all MU pairs (2 of 15 MUs indicated for removal) and 2) 10% of all MU pairs (3 of 15 MUs indicated for removal). MVC, maximal voluntary contraction.
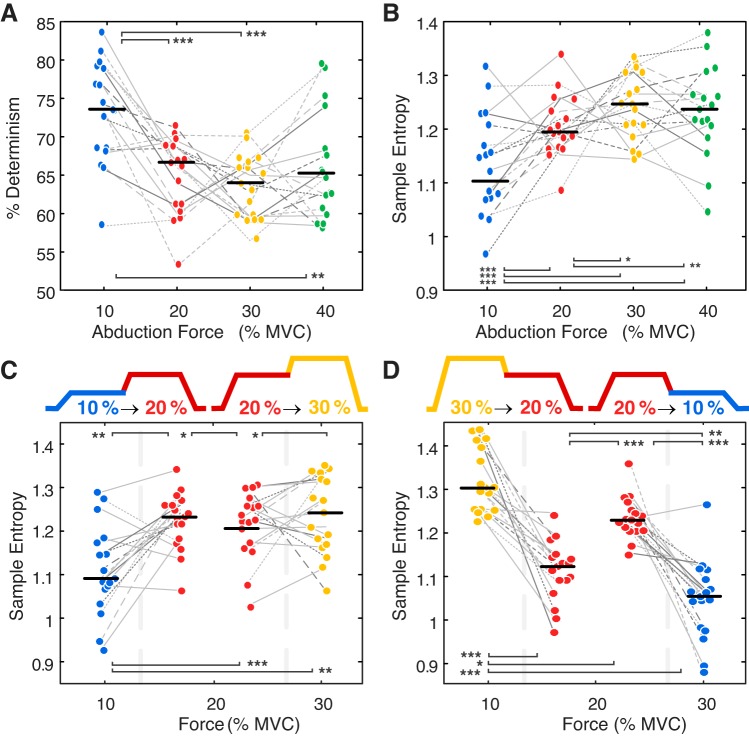
The nonlinear features extracted from the surface EMG signal exhibited comparable changes to those observed in the underlying motor unit coherence. During the constant force contractions, the %DET of the surface EMG signal decreased and the SampEn showed a corresponding increase (Fig. 8, A and B, respectively), indicating an increase in the surface EMG complexity. The changes in the nonlinear features during the two-force trials also mirrored the decrease in coherence observed as force was increased (Fig. 8C) and reciprocal increase as force was decreased (Fig. 8D). An increase in %DET was detected during the second half of the contraction at higher force levels (Fig. 6B), mirroring the observed increase in beta-band motor unit coherence, and the inverse trend was found in the SampEn. SampEn and %DET were more sensitive than motor unit coherence to intersubject differences, which could account for ~70% of the variance in these measures (conditional ICC = 0.76 and 0.62, respectively). The nonlinear parameters were weakly correlated with beta- and alpha-band motor unit coherence obtained during the constant force level trials (Table 3). A stronger correlation was observed between the nonlinear parameters and coherence during the two-force trials (Table 3), where more pronounced changes in motor unit coherence were observed, Fig. 4.
Fig. 8.
Median and interquartile range of the sample entropy (A) and percentage determinism (%DET; B) calculated from the surface electromyography (EMG) signal during the constant force trials at 10, 20, 30, and 40% maximal voluntary contraction (MVC). Both increasing (A) and decreasing (B) force trials are shown. The sample entropy (SampEn) of the surface EMG signal was calculated for the first and second half of the trials with two contraction force levels, with both increasing (C) and decreasing force trials shown (D). Differences in SampEn and %DET between force levels were tested with pairwise comparisons of least-square means using all constant force (n = 171 trials) and two-force trials (n = 142 trials; see Table 1 for average MU number per trial). *P < 0.05; **P < 0.01; ***P < 0.001.
Table 3.
Repeated measures correlation results for the constant force and two-force trials
| Constant Force Trials |
Two-Force Trials |
|||||||
|---|---|---|---|---|---|---|---|---|
| SampEn | %DET | SampEn | %DET | |||||
| r | P | r | P | r | P | r | P | |
| Beta-band MU coherence (15–35 Hz) | −0.29 [−0.43, −0.14] | <0.001 | 0.32 [0.17, 0.46] | <0.001 | −0.53 [−0.61, −0.43] | <0.001 | 0.48 [0.39, 0.57] | <0.001 |
| Alpha-band MU coherence (8–12 Hz) | −0.35 [−0.49, −0.21] | <0.001 | 0.44 [0.31, 0.56] | <0.001 | −0.53 [−0.61, −0.44] | <0.001 | 0.56 [0.47, 0.63] | <0.001 |
The results of a repeated measures correlation between the nonlinear parameters [sample entropy (SampEn)/percentage determinism (%DET)] and the motor unit (MU) coherence (Bakdash and Marusich 2017). Correlations and confidence intervals were obtained for the beta-band (15–35 Hz) and alpha-band (8–12 Hz) motor unit (MU) coherence. Each P value was corrected for multiple comparisons using the Benjamini-Hochberg procedure.
Beta-band motor unit coherence exhibited a significant correlation with the root mean square error (r = −0.34 [−0.47, −0.19], P < 0.001) and the coefficient of variation of the index finger abduction force (r = 0.22 [0.07, 0.36], P = 0.005). However, the results of the mixed-model analysis indicate that differences in level of beta-band coherence across trials were unable to account for any additional variability in force accuracy after changes in force level were considered. The root mean square error of the force produced increased at higher force levels [F(3, 152) = 64.3, P < 0.001] and the beta-band coherence estimate was not a significant predictor of variability in force accuracy [F(1, 165) = 0.008, P = 0.92]. Conversely, the force coefficient of variation decreased at higher force levels [F(3, 153) = 8.4, P < 0.001], but again beta-band coherence did not have a significant effect on the variation independent of changes in force level [F(1, 164) = 0.17, P = 0.68].
DISCUSSION
The present study shows for the first time a progressive reduction in beta-band intramuscular coherence as muscle force increases during index finger abduction (Fig. 1 and Fig. 4). During the 10% MVC contractions, two peaks were generally observed in the coherence spectrum at 10–15 Hz and 25–35 Hz [similar to the peaks observed in pooled coherence between motor unit pairs (Halliday et al. 1999; Semmler et al. 2003)]. The beta-band peak between 25 and 35 Hz was more commonly detected at the lower force levels and was often absent or replaced by broadband coherence at the higher contraction intensities. Coherent beta-band motor unit activity is widely believed to be cortical in origin, arising as a result of synchronized presynaptic inputs to the motoneuron pool (Baker et al. 2003). Such inputs would alter the firing probability of motor units (Fig. 3F), introducing a correlation between motor unit firing trains at the frequency of the shared oscillatory modulation. This component is not present between motor units discharging independently regardless of any similarity in MFRs (Fig. 3E). Motor unit MFRs can, however, influence the expression of coherent activity in the motor unit discharges. Most motor units detected in the current study discharged at rates below 20–35 Hz and would thus only respond intermittently to an external modulation in this frequency range. A relatively large motor unit sample may therefore be necessary to effectively capture the collective beta-band modulation (Fig. 3). An oscillatory input to the motoneuron pool is likely to induce synchronous firings in different combinations of motor units over the course of the muscle contraction. The inclusion of multiple simultaneously active motor units in the composite pulse trains will therefore increase the effective sampling of this modulation at any given point in time and is likely to enhance the detection of coherent activity when compared with alternative methods such as pooled coherence from paired motor unit recordings. The results suggest that the accurate estimation of beta-band coherence using composite pulse trains requires a larger number than the 5-motor unit minimum proposed for examining coherence at lower frequencies (Farina et al. 2014). Accordingly, in the present study, coherence estimates were likely to be greater when more motor units were included in the calculation (Table 2). An inhomogeneous distribution of coherent activity across the motor unit population could also contribute to this effect if more high-threshold motor units were present in the detected motor unit sample (Fig. 5). Changes in motor unit coherence in the present study were thus assessed while aiming to control for some of the variability introduced by using different numbers of motor units for each coherence calculation (MUnum; Table 2). In studies investigating changes in motor unit coherence across conditions, this approach may be preferable to restricting the number of motor units used in the coherence calculation to a constant number across trials, as coherence could vary substantially based on the randomly chosen motor unit sample (Fig. 3, C and D). A large portion of the variability in the coherence estimates could be attributed to motor unit sample size and intersubject differences in baseline coherence (ICC, 20–40%); however, neither of these factors could account for the decrease in motor unit coherence observed with increasing force.
A decrease in alpha-band coherence was also observed alongside the reduction in beta-band coherence, but estimates in this frequency range were disparately affected by variations in motor unit MFR and contraction force level. Although previous studies in humans and primates have provided evidence that the ~10 Hz modulation of motor unit discharges during isometric contractions is cortical or subcortical in origin (Marsden et al. 2001; Williams et al. 2009), the alpha-band component is also influenced by muscle spindle activity and resonance within the afferent feedback loops (Christakos et al. 2006; McManus et al. 2013). The generation of motor unit synchrony in the alpha-band range is thus likely multifactorial, and the results of the present study indicate that the average motor unit firing rate is another contributing component. Higher motor unit MFRs were associated with lower alpha-band coherence estimates (Table 2). This suggests that motor units are more powerfully entrained by ~10 Hz central oscillators and/or peripheral feedback loop resonances when their average firing rates lie closer to this frequency [see Fig. 5A in Lowery et al. (2007)]. The average firing rate in each trial did not significantly influence the beta-band coherence estimate. However, it is likely that the average discharge rate estimated over all motor units does not fully capture the complex interaction between motoneuron firing rate and its responsiveness to a synchronizing input. Changes in average motoneuron firing rates could alter the resulting expression of coherence or synchronization in the motor unit discharges and influence how effectively synaptic inputs can be detected (Kline and De Luca 2016; Lowery and Erim 2005).
The observed decrease in motor unit coherence with increasing force mirrors the reduction in beta-band corticomuscular coherence reported for the FDI muscle during index finger abduction within a similar force range (Perez et al. 2012). It is thus possible that the change in beta-band coherence reflects a collective decrease in coherent beta-band activity within the motor cortex with increasing contraction strength. It has been shown that beta-band oscillations are stronger in the presence of higher intracortical inhibition (Matsuya et al. 2017), which is progressively suppressed as the strength of a muscle contraction increases (Zoghi and Nordstrom 2007). There is also experimental evidence to suggest that cortical beta-band oscillations are reduced during periods of increased neuronal firing rates during brief muscle contractions (<1 s) (Ritter et al. 2009; Spinks et al. 2008); however, this may not extend to longer duration contractions, and it is also unclear whether beta-band activity in the motor cortex is modulated with force (Dal Maso et al. 2017; Mima and Hallett 1999).
Alternatively, it is possible that an increase in other inhibitory or excitatory inputs to the motoneuron pool could dilute or diminish the relative strength of the synchronized input. If the level of asynchronous inputs increases, the efficacy of a synchronous oscillatory input at inducing coherent motor unit firing will be reduced. Although the corticospinal system has a prominent role in the production of weak forces in tasks requiring fine, fractionated control of finger muscles (Lawrence and Kuypers 1968), stronger forces may require excitatory inputs from other descending pathways. In particular, there is evidence to suggest that contributions from the reticulospinal pathway become increasingly important during stronger muscle contractions in intrinsic hand muscles (Baker 2011). Changes in excitation from descending pathways may also influence motoneurons indirectly through local segmental interneurons (Alstermark and Isa 2012). Changes in either descending (Cheney et al. 1991; Riddle et al. 2009) or afferent information (Hultborn and Pierrot-Deseilligny 1979; Macefield et al. 1996; Pierrot-Deseilligny and Burke 2005) at higher muscle forces could generate complex interactions within interneuronal networks, altering their effects on the motoneuron population. Afferent activity could also indirectly influence coherent motor unit firing via supraspinal centers as part of a beta-synchronized feedback loop (Baker et al. 2006; Witham et al. 2007). Experimental evidence indicates that beta-range corticomuscular and intermuscular coupling can be modulated by ascending sensory pathways (Fisher et al. 2002; Kilner et al. 2004; Pohja and Salenius 2003). Consequently, alterations in either afferent and efferent activity could disrupt the bidirectional flow of information that exists between oscillatory beta-band cortical and muscular activities (Witham et al. 2011).
Finally, a decrease in motoneuron responsiveness to an oscillatory input could also arise from increases in background synaptic noise, which can vary with motoneuron background discharge rates [see review by Powers and Türker (2010)]. At higher motoneuron firing rates, increases in membrane conductance reduce the synaptic current reaching the soma from the dendritic synapses, lowering the spike-triggering efficacy of an excitatory postsynaptic potential input. It is possible that an increase in the activity of persistent inward currents in the motoneuron could similarly contribute to a decrease in motor unit coherence (Taylor and Enoka 2004), but this has yet to be systematically explored (Powers and Türker 2010). Lastly, it should be noted that none of the above-mentioned hypotheses are mutually exclusive. All factors could potentially contribute to a reduction in coherence at higher force levels, although it is not possible to draw definitive conclusions on the underlying mechanisms based on the data presented. Additionally, changes in motor unit coherence with increasing force are likely to be muscle and task dependent, as the relative contribution of various descending pathways will differ across muscles and movements. The decrease in motor unit coherence at higher force levels could be specific to muscles involved in fine motor control and may not be observed in larger muscles (Laine et al. 2015). Tasks that require the coordination of several muscles may also yield different results, although the contribution from muscles other than the FDI is likely to be minimal during index finger abduction (Infantolino and Challis 2010).
Because of the nature of surface EMG decomposition, there is a higher likelihood of a motor unit firing being missed when two motor units discharge within a few milliseconds of one another, as compared with intramuscularly recorded motor units. However, the results suggest that missed coincident firings do not account for the observed reduction in motor unit coherence with increasing force, as there was no systematic increase in the number of missed firings at higher force levels (which could contribute to the observed reduction in motor unit coherence; Fig. 7B). Furthermore, when motor units with high levels of missed firings were removed from the analysis, the decrease in coherence remained. The results of the current study also present evidence that motor unit coherence is less sensitive than synchronization measures to the firing time accuracy in motor units (Fig. 7D) and provides a more global measure of population synchrony. The results highlight the importance of reporting time-domain data alongside coherence analysis when presenting results based on motor unit firing trains from decomposed surface EMG.
Differences in beta-band coherence between high- and low-threshold motor units.
Beta-band coherence was consistently larger in high-threshold motor units (Fig. 5). This complements the results of previous studies that have found greater short-term synchronization between high-threshold motor unit pairs (Datta and Stephens 1990; Schmied et al. 2014). High-threshold motor units, but not low-threshold units, also exhibited increased coherent beta-band activity when execution errors were amplified during a force tracking task (Hwang et al. 2017). Large excitatory postsynaptic potentials may be more likely to trigger an action potential in motoneurons firing at low, “subprimary” firing rates (Matthews 1996), as the membrane voltage lies in a plateau phase close to the firing threshold for a larger proportion of the interspike interval (Powers and Türker 2010). Although firing rate differences may contribute to the difference in coherence between high- and low-threshold units (Fig. 5), there is also experimental evidence to suggest that corticospinal inputs are greater in high-threshold motoneurons in decerebrate cats (Binder et al. 1998) and primates (Clough et al. 1968). Alpha-band coherence also differed between high- and low-threshold motor units. However, a significant difference was only detected for the 10 and 20% MVC trials after variations in motor unit MFRs were accounted for.
Variations in beta-band coherence from the start to the end of the contraction.
During the trials performed at a constant force, beta-band motor unit coherence increased during the second half of the 30 and 40% MVC contractions (Fig. 6A), accompanied by a decrease in the surface EMG median frequency (Fig. 6B). In contrast, alpha-band coherence showed a consistent increase during the second half of the trial at all force levels. The indication of peripheral fatigue as the contraction progressed suggests that the parallel increase in beta-band coherence at higher forces also occurred as a result of fatigue. A progressive increase in motor unit synchronization, alpha- and beta-band coherence during a fatiguing contraction and directly postfatigue has been previously demonstrated within the FDI muscle (Kattla and Lowery 2010; McManus et al. 2016). Parallel changes in the EMG signal structure were also observed (Fig. 6B), providing further evidence that these features are influenced by muscular fatigue (Cashaback et al. 2013; Farina et al. 2002; Webber et al. 1995).
Changes in beta-band coherence during the two-force trials.
The effect of contraction strength on beta-band coherence was much more pronounced in the trials where the same motor units were tracked over two force levels (Fig. 4). When abduction force was increased or decreased during the second half of the trial, the beta-band coherence decreased or increased, respectively. As the coherence estimate was calculated over a shorter time period (10 s vs. 23 s), with fewer motor units (~20% less; Table 1), the coherence values during the first half of the two-force trials were lower than those reported for the same contraction strength during the constant force trials. High-threshold motor units that were recruited/de-recruited during the contraction were excluded from the analysis. It is also possible that small, low-threshold motor units were more likely to be missed in the two-force trials as a result of the transition to/from higher force levels. Although a smaller subset of motor units may have been detected during the two-force trials, average motor unit firing rates during the first half of the ramp trials were comparable to those reported during the constant force trials (Fig. 4C). However, during the decreasing force trials, average firing rates during the second half of the two-force trial were significantly lower than for the corresponding constant force trials. Conversely, during the increasing force trials from 20 → 30% MVC, motor units tended to fire faster at the higher force level when compared with the equivalent constant force trials. The higher or lower motor unit MFRs during the second half of the ramp trials may be a response to muscle force depression or enhancement that can occur after active muscle shortening or lengthening, respectively (Herzog 2004). Prior shortening of a muscle has been shown to require greater neural activation to maintain a given isometric force, and conversely, lower surface EMG amplitudes have been observed after lengthening contractions (Jones et al. 2016). In the present study, lower motor unit firing rates at 20% MVC and 10% MVC were accompanied by higher beta-band coherence in contractions that were preceded by a decrease in abduction force (Fig. 4B). A similar difference was observed in the nonlinear surface EMG features (Fig. 8D). Conversely, beta-band coherence was lower at 20% MVC when the contraction was preceded by an increase in force (Fig. 3A).
Relationship between motor unit coherence and the nonlinear parameters and force accuracy.
The changes in motor unit coherence presented in the current study were supported by corresponding changes in nonlinear parameters calculated from the surface EMG signal (Fig. 8). A secondary result of this study was the novel detection of a significant, moderate correlation between nonlinear features based on the surface EMG signal (%DET and SampEn) and the underlying motor unit coherence (Table 3). The estimation of coherence across the motoneuron pool and the inclusion of a greater range of contraction forces may have facilitated the detection of a relationship between these two measures, as this type of protocol and data analysis were not previously possible with intramuscular EMG (Dideriksen et al. 2009; Schmied and Descarreaux 2011). In the present study %DET decreased with increasing force, exhibiting the same trends as the beta-band motor unit coherence (Fig. 8B). SampEn, a measure approximately inversely related to %DET, increased with increasing contraction strength (Fig. 8A). Previous studies using various entropy measures have found similar increases with increasing force (Cashaback et al. 2013; Istenic et al. 2010), although reports on the sensitivity of %DET and SampEn to changes in muscle contraction level vary (Del Santo et al. 2007; Meigal et al. 2009). A stronger relationship between the coherence and the nonlinear features was detected for the two-force trials, where changes in coherence were more pronounced, than for trials at a constant force level (Table 3; Fig. 4, A and B). However, it is important to note that motor unit coherence is unlikely to be the only factor contributing to the observed changes in the nonlinear features, as increases in surface EMG signal density due to increased motor unit recruitment and firing rate could also alter the SampEn and %DET. Collectively, the results suggest that SampEn and %DET could be useful in detecting large differences in motor unit coherence, i.e., during muscular fatigue (Cashaback et al. 2013; Farina et al. 2002; Webber et al. 1995) or in pathological conditions (Fattorini et al. 2005; Flood et al. 2019; Meigal et al. 2009). The results also highlight the sensitivity of these features to intersubject differences in physiology (e.g., muscle size, motor unit distribution) and the need to consider muscle contraction strength in any quantitative analysis.
Finally, variations in the force accuracy of trials performed at the same force could not be explained by differences in beta-band motor unit coherence, although coherence was correlated with force accuracy across different force levels. Studies investigating corticomuscular coherence have reported that motor performance was not associated with the level of beta-band coherence during index finger abduction and ankle dorsi-plantarflexion (Johnson et al. 2011; Perez et al. 2006). Other studies employing isometric force compensation protocols have found that higher beta-band corticomuscular coherence was associated with decreased relative error in force (Kristeva et al. 2007; Witte et al. 2007), possibly reflecting a higher contribution from afferent sensory feedback during this type of task (Lim et al. 2014).
In conclusion, a reduction in beta-band intramuscular coherence was observed with increasing muscle force. The variations in coherence during changes in muscle activation level and with the onset of fatigue were accompanied by parallel changes in the SampEn and %DET of the surface EMG signal, and a significant relationship between the nonlinear features and the underlying motor unit coherence was demonstrated for the first time. The results show that the properties of the detected motor unit sample and level of activation of the muscle are important factors to consider when investigating the modulation or disruption of beta-band activity.
GRANTS
This study was supported by the European Research Council: ERC-2014-CoG-646923_DBSModel.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.M. and M.M.L. conceived and designed research; L.M. performed experiments; L.M., M.W.F. and M.M.L. analyzed data; L.M. and M.M.L. interpreted results of experiments; L.M. prepared figures; L.M. and M.M.L. drafted manuscript; L.M., M.W.F. and M.M.L. edited and revised manuscript; L.M., M.W.F. and M.M.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jérémy Liegey for help with experimental work.
REFERENCES
- Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annu Rev Neurosci 35: 559–578, 2012. doi: 10.1146/annurev-neuro-062111-150527. [DOI] [PubMed] [Google Scholar]
- Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol 8: 456, 2017. [Erratum in Front Psychol 10: 1201, 2019.] doi: 10.3389/fpsyg.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol 589: 5603–5612, 2011. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Chiu M, Fetz EE. Afferent encoding of central oscillations in the monkey arm. J Neurophysiol 95: 3904–3910, 2006. doi: 10.1152/jn.01106.2005. [DOI] [PubMed] [Google Scholar]
- Baker SN, Pinches EM, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. II. Effect of oscillatory activity on corticospinal output. J Neurophysiol 89: 1941–1953, 2003. doi: 10.1152/jn.00832.2002. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear Mixed-Effects Models Using S4 Classes. R Package Version 0.999375-42. 2011. https://cran.r-project.org/web/packages/lme4/index.html.
- Binder MD, Robinson FR, Powers RK. Distribution of effective synaptic currents in cat triceps surae motoneurons. VI. Contralateral pyramidal tract. J Neurophysiol 80: 241–248, 1998. doi: 10.1152/jn.1998.80.1.241. [DOI] [PubMed] [Google Scholar]
- Cashaback JG, Cluff T, Potvin JR. Muscle fatigue and contraction intensity modulates the complexity of surface electromyography. J Electromyogr Kinesiol 23: 78–83, 2013. doi: 10.1016/j.jelekin.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Castronovo AM, Negro F, Conforto S, Farina D. The proportion of common synaptic input to motor neurons increases with an increase in net excitatory input. J Appl Physiol (1985) 119: 1337–1346, 2015. doi: 10.1152/japplphysiol.00255.2015. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Prog Brain Res 87: 213–252, 1991. doi: 10.1016/S0079-6123(08)63054-X. [DOI] [PubMed] [Google Scholar]
- Christakos CN, Papadimitriou NA, Erimaki S. Parallel neuronal mechanisms underlying physiological force tremor in steady muscle contractions of humans. J Neurophysiol 95: 53–66, 2006. doi: 10.1152/jn.00051.2005. [DOI] [PubMed] [Google Scholar]
- Christou EA, Rudroff T, Enoka JA, Meyer F, Enoka RM. Discharge rate during low-force isometric contractions influences motor unit coherence below 15 Hz but not motor unit synchronization. Exp Brain Res 178: 285–295, 2007. doi: 10.1007/s00221-006-0739-5. [DOI] [PubMed] [Google Scholar]
- Clough JF, Kernell D, Phillips CG. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon’s hand and forearm. J Physiol 198: 145–166, 1968. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol 489: 917–924, 1995. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Maso F, Longcamp M, Cremoux S, Amarantini D. Effect of training status on beta-range corticomuscular coherence in agonist vs. antagonist muscles during isometric knee contractions. Exp Brain Res 235: 3023–3031, 2017. doi: 10.1007/s00221-017-5035-z. [DOI] [PubMed] [Google Scholar]
- Datta AK, Stephens JA. Synchronization of motor unit activity during voluntary contraction in man. J Physiol 422: 397–419, 1990. doi: 10.1113/jphysiol.1990.sp017991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Santo F, Gelli F, Ginanneschi F, Popa T, Rossi A. Relation between isometric muscle force and surface EMG in intrinsic hand muscles as function of the arm geometry. Brain Res 1163: 79–85, 2007. doi: 10.1016/j.brainres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Dideriksen JL, Falla D, Bækgaard M, Mogensen ML, Steimle KL, Farina D. Comparison between the degree of motor unit short-term synchronization and recurrence quantification analysis of the surface EMG in two human muscles. Clin Neurophysiol 120: 2086–2092, 2009. doi: 10.1016/j.clinph.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O. An R2 statistic for fixed effects in the linear mixed model. Stat Med 27: 6137–6157, 2008. doi: 10.1002/sim.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble RJ, Randall JE. Motor-unit activity responsible for 8- to 12-Hz component of human physiological finger tremor. J Neurophysiol 39: 370–383, 1976. doi: 10.1152/jn.1976.39.2.370. [DOI] [PubMed] [Google Scholar]
- Enochson LD, Goodman NR. Gaussian Approximations to the Distribution of Sample Coherence. Los Angeles, CA: Measurement Analysis Corp., 1965. [Google Scholar]
- Farina D, Fattorini L, Felici F, Filligoi G. Nonlinear surface EMG analysis to detect changes of motor unit conduction velocity and synchronization. J Appl Physiol (1985) 93: 1753–1763, 2002. doi: 10.1152/japplphysiol.00314.2002. [DOI] [PubMed] [Google Scholar]
- Farina D, Negro F, Dideriksen JL. The effective neural drive to muscles is the common synaptic input to motor neurons. J Physiol 592: 3427–3441, 2014. doi: 10.1113/jphysiol.2014.273581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J Physiol 470: 127–155, 1993. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorini L, Felici F, Filligoi GC, Traballesi M, Farina D. Influence of high motor unit synchronization levels on non-linear and spectral variables of the surface EMG. J Neurosci Methods 143: 133–139, 2005. doi: 10.1016/j.jneumeth.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Galea MP, Brown P, Lemon RN. Digital nerve anaesthesia decreases EMG-EMG coherence in a human precision grip task. Exp Brain Res 145: 207–214, 2002. doi: 10.1007/s00221-002-1113-x. [DOI] [PubMed] [Google Scholar]
- Fling BW, Christie A, Kamen G. Motor unit synchronization in FDI and biceps brachii muscles of strength-trained males. J Electromyogr Kinesiol 19: 800–809, 2009. doi: 10.1016/j.jelekin.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Flood MW, Jensen BR, Malling A-S, Lowery MM. Increased EMG intermuscular coherence and reduced signal complexity in Parkinson’s disease. Clin Neurophysiol 130: 259–269, 2019. doi: 10.1016/j.clinph.2018.10.023. [DOI] [PubMed] [Google Scholar]
- Gallet C, Julien C. The significance threshold for coherence when using the Welch’s periodogram method: effect of overlapping segments. Biomed Signal Process Control 6: 405–409, 2011. doi: 10.1016/j.bspc.2010.11.004. [DOI] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Load-independent contributions from motor-unit synchronization to human physiological tremor. J Neurophysiol 82: 664–675, 1999. doi: 10.1152/jn.1999.82.2.664. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR. On the application, estimation and interpretation of coherence and pooled coherence. J Neurosci Methods 100: 173–174, 2000. doi: 10.1016/S0165-0270(00)00267-3. [DOI] [PubMed] [Google Scholar]
- Herzog W. History dependence of skeletal muscle force production: implications for movement control. Hum Mov Sci 23: 591–604, 2004. doi: 10.1016/j.humov.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Hu X, Rymer WZ, Suresh NL. Reliability of spike triggered averaging of the surface electromyogram for motor unit action potential estimation. Muscle Nerve 48: 557–570, 2013. doi: 10.1002/mus.23819. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol 297: 229–251, 1979. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IS, Lin YT, Huang WM, Yang ZR, Hu CL, Chen YC. Alterations in neural control of constant isometric contraction with the size of error feedback. PLoS One 12: e0170824, 2017. doi: 10.1371/journal.pone.0170824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantolino BW, Challis JH. Architectural properties of the first dorsal interosseous muscle. J Anat 216: 463–469, 2010. doi: 10.1111/j.1469-7580.2009.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istenic R, Kaplanis PA, Pattichis CS, Zazula D. Multiscale entropy-based approach to automated surface EMG classification of neuromuscular disorders. Med Biol Eng Comput 48: 773–781, 2010. doi: 10.1007/s11517-010-0629-7. [DOI] [PubMed] [Google Scholar]
- Johnson AN, Wheaton LA, Shinohara M. Attenuation of corticomuscular coherence with additional motor or non-motor task. Clin Neurophysiol 122: 356–363, 2011. doi: 10.1016/j.clinph.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Jones AA, Power GA, Herzog W. History dependence of the electromyogram: Implications for isometric steady-state EMG parameters following a lengthening or shortening contraction. J Electromyogr Kinesiol 27: 30–38, 2016. doi: 10.1016/j.jelekin.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Kattla S, Lowery MM. Fatigue related changes in electromyographic coherence between synergistic hand muscles. Exp Brain Res 202: 89–99, 2010. doi: 10.1007/s00221-009-2110-0. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci 20: 8838–8845, 2000. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Fisher RJ, Lemon RN. Coupling of oscillatory activity between muscles is strikingly reduced in a deafferented subject compared with normal controls. J Neurophysiol 92: 790–796, 2004. doi: 10.1152/jn.01247.2003. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA. The origin of motoneuron synchronization. J Neurophysiol 115: 1077–1078, 2016. doi: 10.1152/jn.01027.2015. [DOI] [PubMed] [Google Scholar]
- Kline JC, De Luca CJ. Synchronization of motor unit firings: an epiphenomenon of firing rate characteristics not common inputs. J Neurophysiol 115: 178–192, 2016. doi: 10.1152/jn.00452.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeva R, Patino L, Omlor W. Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage 36: 785–792, 2007. doi: 10.1016/j.neuroimage.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Laine CM, Martinez-Valdes E, Falla D, Mayer F, Farina D. Motor neuron pools of synergistic thigh muscles share most of their synaptic input. J Neurosci 35: 12207–12216, 2015. doi: 10.1523/JNEUROSCI.0240-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain 91: 15–36, 1968. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Lim M, Kim JS, Kim M, Chung CK. Ascending beta oscillation from finger muscle to sensorimotor cortex contributes to enhanced steady-state isometric contraction in humans. Clin Neurophysiol 125: 2036–2045, 2014. doi: 10.1016/j.clinph.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson ME. Using confidence intervals in within-subject designs. Psychon Bull Rev 1: 476–490, 1994. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Lowery MM, Erim Z. A simulation study to examine the effect of common motoneuron inputs on correlated patterns of motor unit discharge. J Comput Neurosci 19: 107–124, 2005. doi: 10.1007/s10827-005-0898-6. [DOI] [PubMed] [Google Scholar]
- Lowery MM, Myers LJ, Erim Z. Coherence between motor unit discharges in response to shared neural inputs. J Neurosci Methods 163: 384–391, 2007. doi: 10.1016/j.jneumeth.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Häger-Ross C, Johansson RS. Control of grip force during restraint of an object held between finger and thumb: responses of cutaneous afferents from the digits. Exp Brain Res 108: 155–171, 1996. doi: 10.1007/BF00242913. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Brown P, Salenius S. Involvement of the sensorimotor cortex in physiological force and action tremor. Neuroreport 12: 1937–1941, 2001. doi: 10.1097/00001756-200107030-00033. [DOI] [PubMed] [Google Scholar]
- Marwan N, Romano MC, Thiel M, Kurths J. Recurrence plots for the analysis of complex systems. Phys Rep 438: 237–329, 2007. doi: 10.1016/j.physrep.2006.11.001. [DOI] [Google Scholar]
- Matsuya R, Ushiyama J, Ushiba J. Inhibitory interneuron circuits at cortical and spinal levels are associated with individual differences in corticomuscular coherence during isometric voluntary contraction. Sci Rep 7: 44417, 2017. doi: 10.1038/srep44417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol 492: 597–628, 1996. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley JH, Marsden CD. Physiological and pathological tremors and rhythmic central motor control. Brain 123: 1545–1567, 2000. doi: 10.1093/brain/123.8.1545. [DOI] [PubMed] [Google Scholar]
- McManus L, Hu X, Rymer WZ, Lowery MM, Suresh NL. Changes in motor unit behavior following isometric fatigue of the first dorsal interosseous muscle. J Neurophysiol 113: 3186–3196, 2015. doi: 10.1152/jn.00146.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus L, Hu X, Rymer WZ, Suresh NL, Lowery MM. Muscle fatigue increases beta-band coherence between the firing times of simultaneously active motor units in the first dorsal interosseous muscle. J Neurophysiol 115: 2830–2839, 2016. doi: 10.1152/jn.00097.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus LM, Budini F, Di Russo F, Berchicci M, Menotti F, Macaluso A, De Vito G, Lowery MM. Analysis of the effects of mechanically induced tremor on EEG-EMG coherence using wavelet and partial directed coherence. 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER). 2013: 561–564, 2013. doi: 10.1109/NER.2013.6695996. [DOI] [Google Scholar]
- Meigal AI, Rissanen S, Tarvainen MP, Karjalainen PA, Iudina-Vassel IA, Airaksinen O, Kankaanpää M. Novel parameters of surface EMG in patients with Parkinson’s disease and healthy young and old controls. J Electromyogr Kinesiol 19: e206–e213, 2009. doi: 10.1016/j.jelekin.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Mima T, Hallett M. Corticomuscular coherence: a review. J Clin Neurophysiol 16: 501–511, 1999. doi: 10.1097/00004691-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods Ecol Evol 4: 133–142, 2013. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- Nawab SH, Chang S-S, De Luca CJ. High-yield decomposition of surface EMG signals. Clin Neurophysiol 121: 1602–1615, 2010. doi: 10.1016/j.clinph.2009.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro F, Farina D. Linear transmission of cortical oscillations to the neural drive to muscles is mediated by common projections to populations of motoneurons in humans. J Physiol 589: 629–637, 2011. doi: 10.1113/jphysiol.2010.202473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom MA, Fuglevand AJ, Enoka RM. Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol 453: 547–574, 1992. doi: 10.1113/jphysiol.1992.sp019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Lundbye-Jensen J, Nielsen JB. Changes in corticospinal drive to spinal motoneurones following visuo-motor skill learning in humans. J Physiol 573: 843–855, 2006. doi: 10.1113/jphysiol.2006.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Soteropoulos DS, Baker SN. Corticomuscular coherence during bilateral isometric arm voluntary activity in healthy humans. J Neurophysiol 107: 2154–2162, 2012. doi: 10.1152/jn.00722.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge, UK: Cambridge University Press, 2005. [Google Scholar]
- Pohja M, Salenius S. Modulation of cortex-muscle oscillatory interaction by ischaemia-induced deafferentation. Neuroreport 14: 321–324, 2003. doi: 10.1097/00001756-200303030-00005. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford, UK: Oxford University Press, 1993. [Google Scholar]
- Powers RK, Türker KS. Deciphering the contribution of intrinsic and synaptic currents to the effects of transient synaptic inputs on human motor unit discharge. Clin Neurophysiol 121: 1643–1654, 2010. doi: 10.1016/j.clinph.2009.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 278: H2039–H2049, 2000. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum Brain Mapp 30: 1168–1187, 2009. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmied A, Descarreaux M. Influence of contraction strength on single motor unit synchronous activity. Clin Neurophysiol 121: 1624–1632, 2010. doi: 10.1016/j.clinph.2010.02.165. [DOI] [PubMed] [Google Scholar]
- Schmied A, Descarreaux M. Reliability of EMG determinism to detect changes in motor unit synchrony and coherence during submaximal contraction. J Neurosci Methods 196: 238–246, 2011. doi: 10.1016/j.jneumeth.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Schmied A, Forget R, Vedel J-P. Motor unit firing pattern, synchrony and coherence in a deafferented patient. Front Hum Neurosci 8: 746, 2014. doi: 10.3389/fnhum.2014.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler JG, Kornatz KW, Dinenno DV, Zhou S, Enoka RM. Motor unit synchronisation is enhanced during slow lengthening contractions of a hand muscle. J Physiol 545: 681–695, 2002. doi: 10.1113/jphysiol.2002.026948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler JG, Kornatz KW, Enoka RM. Motor-unit coherence during isometric contractions is greater in a hand muscle of older adults. J Neurophysiol 90: 1346–1349, 2003. doi: 10.1152/jn.00941.2002. [DOI] [PubMed] [Google Scholar]
- Spinks RL, Kraskov A, Brochier T, Umilta MA, Lemon RN. Selectivity for grasp in local field potential and single neuron activity recorded simultaneously from M1 and F5 in the awake macaque monkey. J Neurosci 28: 10961–10971, 2008. doi: 10.1523/JNEUROSCI.1956-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Enoka RM. Quantification of the factors that influence discharge correlation in model motor neurons. J Neurophysiol 91: 796–814, 2004. doi: 10.1152/jn.00802.2003. [DOI] [PubMed] [Google Scholar]
- Ushiyama J, Masakado Y, Fujiwara T, Tsuji T, Hase K, Kimura A, Liu M, Ushiba J. Contraction level-related modulation of corticomuscular coherence differs between the tibialis anterior and soleus muscles in humans. J Appl Physiol (1985) 112: 1258–1267, 2012. doi: 10.1152/japplphysiol.01291.2011. [DOI] [PubMed] [Google Scholar]
- Webber CL Jr, Schmidt MA, Walsh JM. Influence of isometric loading on biceps EMG dynamics as assessed by linear and nonlinear tools. J Appl Physiol (1985) 78: 814–822, 1995. doi: 10.1152/jappl.1995.78.3.814. [DOI] [PubMed] [Google Scholar]
- Williams ER, Soteropoulos DS, Baker SN. Coherence between motor cortical activity and peripheral discontinuities during slow finger movements. J Neurophysiol 102: 1296–1309, 2009. doi: 10.1152/jn.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham CL, Riddle CN, Baker MR, Baker SN. Contributions of descending and ascending pathways to corticomuscular coherence in humans. J Physiol 589: 3789–3800, 2011. doi: 10.1113/jphysiol.2011.211045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham CL, Wang M, Baker SN. Cells in somatosensory areas show synchrony with beta oscillations in monkey motor cortex. Eur J Neurosci 26: 2677–2686, 2007. doi: 10.1111/j.1460-9568.2007.05890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte M, Patino L, Andrykiewicz A, Hepp-Reymond MC, Kristeva R. Modulation of human corticomuscular beta-range coherence with low-level static forces. Eur J Neurosci 26: 3564–3570, 2007. doi: 10.1111/j.1460-9568.2007.05942.x. [DOI] [PubMed] [Google Scholar]
- Zoghi M, Nordstrom MA. Progressive suppression of intracortical inhibition during graded isometric contraction of a hand muscle is not influenced by hand preference. Exp Brain Res 177: 266–274, 2007. doi: 10.1007/s00221-006-0669-2. [DOI] [PubMed] [Google Scholar]