Abstract
Estradiol acutely facilitates sex differences in striatum-dependent behaviors. However, little is understood regarding the underlying mechanism. In striatal regions in adult rodents, estrogen receptors feature exclusively extranuclear expression, suggesting that estradiol rapidly modulates striatal neurons. We tested the hypothesis that estradiol rapidly modulates excitatory synapse properties onto medium spiny neurons (MSNs) of two striatal regions, the nucleus accumbens core and caudate-putamen in adult female and male rats. We predicted there would be sex-specific differences in pre- and postsynaptic locus and sensitivity. We further analyzed whether MSN intrinsic properties are predictive of estrogen sensitivity. Estradiol exhibited sex-specific acute effects in the nucleus accumbens core: miniature excitatory postsynaptic current (mEPSC) frequency robustly decreased in response to estradiol in female MSNs, and mEPSC amplitude moderately increased in response to estradiol in both male and female MSNs. This increase in mEPSC amplitude is associated with MSNs featuring increased intrinsic excitability. No MSN intrinsic electrical property associated with changes in mEPSC frequency. Estradiol did not acutely modulate mEPSC properties in the caudate-putamen of either sex. This is the first demonstration of acute estradiol action on MSN excitatory synapse function. This demonstration of sex and striatal region-specific acute estradiol neuromodulation revises our understanding of sex hormone action on striatal physiology and resulting behaviors.
NEW & NOTEWORTHY This study is the first to demonstrate rapid estradiol neuromodulation of glutamatergic signaling on medium spiny neurons (MSNs), the major output neuron of the striatum. These findings emphasize that sex is a significant biological variable both in MSN sensitivity to estradiol and in pre- and postsynaptic mechanisms of glutamatergic signaling. MSNs in different regions exhibit diverse responses to estradiol. Sex- and region-specific estradiol-induced changes to excitatory signaling on MSNs explain sex differences partially underlying striatum-mediated behaviors and diseases.
Keywords: caudate-putamen, estradiol, medium spiny neuron, miniature excitatory postsynaptic currents, nucleus accumbens, striatum
INTRODUCTION
Sex and steroid sex hormones prominently influence the function of almost every brain region examined to date, including the striatal brain regions such as the nucleus accumbens and caudate-putamen. These striatal regions are highly conserved across species and are critically important for a broad range of cognitive, sensorimotor, and limbic-related functions. Sex and hormone action are significant biological variables for many disorders relevant to the striatum, including Parkinson’s disease, Tourette’s syndrome, anxiety, and drug addiction (Becker and Hu 2008; Nillni et al. 2011; Picillo et al. 2017; Smith and Dahodwala 2014; Wickens et al. 2018). In both humans and animal models, nonpathological behaviors controlled by the striatal circuits are likewise sensitive to sex and hormone action (Gorzek et al. 2007; Hampson 1990; Kent et al. 1991; Marcondes et al. 2001). 17β-Estradiol (estradiol), a potent endogenous estrogen, is particularly implicated in influencing striatum-mediated behaviors on both acute and longer time frames (Becker 1990b; Becker and Rudick 1999; Becker et al. 1987; Cummings et al. 2014; Schultz et al. 2009). Therefore, it is important to understand whether estradiol can rapidly modulate neuronal electrophysiological properties in striatal regions.
The striatum features three broad regions: the caudate-putamen (CP; also called dorsal striatum) and the nucleus accumbens core (AcbC) and shell (collectively called the ventral striatum). The predominant striatal neuron type is the major projection neuron, the medium spiny neuron (MSN; also called spiny projection neuron). The MSN is a GABAergic neuron that integrates inputs from a number of sources to determine the final output of the striatum. The most famous of these inputs is dopaminergic, and years of research from many laboratories has demonstrated that these dopaminergic inputs are sensitive to sex and sex hormones such as estradiol, with estradiol exposure generally augmenting dopaminergic action (Becker 1990a; Becker and Rudick 1999; Bédard et al. 1982; Bossé and DiPaolo 1996; Calipari et al. 2017; Nicola et al. 2000; Xiao and Becker 1994; Yoest et al. in press). The MSN also receives many other inputs as well, most prominently excitatory synaptic inputs from multiple brain regions that are likewise profoundly implicated in mediating striatal function (Russo and Nestler 2013; Scofield et al. 2016). MSNs exhibit developmental period- and region-specific sex differences in both excitatory synapse and intrinsic electrophysiological properties (Cao et al. 2016, 2018b; Dorris et al. 2015). Neuronal anatomy related to excitatory synapse number is sex and hormone sensitive in the AcbC but not in CP (Peterson et al. 2015; Staffend et al. 2011; Wissman et al. 2011, 2012), unlike other neuroanatomical attributes that do not differ by sex, such as MSN soma size, cellular density, and gross striatal region volume (Meitzen et al. 2011; Wong et al. 2016). It has previously been shown that exposure to estradiol during the perinatal developmental period can masculinize/defeminize MSN excitatory synapse properties in the AcbC (Cao et al. 2016), and estrous cycling maintains sex differences of MSN synapse properties in adulthood (Proaño et al. 2018). However, it is unknown whether glutamatergic input onto MSNs is acutely sensitive to estradiol exposure.
To address this critical omission, in this study we tested multiple hypotheses related to whether estradiol rapidly modulates glutamatergic synapse properties of MSNs in the AcbC and CP. Specifically, we tested 1) whether estradiol rapidly changes miniature excitatory postsynaptic current (mEPSC) frequency, amplitude, and decay properties; 2) whether sex and region were mediating variables in estradiol actions; and 3) possible relationships between MSN intrinsic excitability properties and estradiol-induced influences on mEPSC properties.
METHODS
Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee at North Carolina State University. Female (n = 19) and male (n = 29) Sprague-Dawley CD IGS rats were born from timed-pregnant females purchased from Charles River Laboratories. Rats were housed with littermates and dam until weaning. After weaning [postnatal day (P)20–21], animals were group-housed in same-sex cages until experimental collection day (P60–90). Animals were housed in a temperature- and light-controlled room (23°C, 40% humidity, 12:12-h light-dark cycle with lights on 7:00 AM to 7:00 PM) at the Biological Resource Facility of North Carolina State University. All cages are polysulfone bisphenol A (BPA) free and filled with bedding manufactured from virgin hardwood chips (Beta Chip; NEPCO, Warrengsburg, NY) to avoid endocrine disruptors present in corncob bedding (Mani et al. 2005; Markaverich et al. 2002; Villalon Landeros et al. 2012). Glass-bottle water and soy protein-free rodent chow (2020X; Teklad, Madison, WI) were provided ad libitum. All female animals were assessed for estrous cycle stage on the day of experiment. Eight females were in diestrus, two in proestrus and seven in estrus. None of the observed effects differed by estrous cycle stage (P > 0.05 for all). Cycle stage was assessed the morning of each recording day using a wet mount preparation as previously described (Hubscher et al. 2005; Proaño et al. 2018). Specific estrous cycle stages were not targeted a priori, because the aim of this study was to test within-subject changes of acute estradiol modulation on both sexes, rather than detecting differences across cycle stages. For statistical comparison, proestrus and estrus animals were condensed into a single group because two previous studies found no differences in MSN mEPSC and other electrophysiological properties between these stages (Proaño et al. 2018; Wissman et al. 2011). However, given that this biological variable was relatively underpowered, we urge caution in firmly interpreting this result as indicating that MSN estradiol sensitivity is not influenced by estrous cycle stage. For further exploration of estrous-dependent changes in striatal electrophysiology, see Proaño et al. (2018) and Tansey et al. (1983).
Acute brain slice preparation.
Brain slices for electrophysiological recordings were prepared as previously described (Dorris et al. 2015). Rats were deeply anesthetized with isoflurane gas and killed by rapid decapitation. The brain was dissected into ice-cold oxygenated sucrose artificial cerebrospinal fluid (s-ACSF) containing (in mM) 75 sucrose, 1.25 NaH2PO4, 3 MgCl2, 0.5 CaCL2, 2.4 Na pyruvate, 1.3 ascorbic acid, 75 NaCl, 25 NaHCO3, 15 dextrose, and 2 KCl (chemicals obtained from Fisher Scientific, Hampton, NH; Sigma-Aldrich, St. Louis, MO; and Acros, Morris, NJ). The osmolarity of the s-ACSF was 295–305 mOsm with a pH between 7.2 and 7.4. The brain was sectioned coronally at 300 µm using a vibratome (Leica), and left and right hemispheres were separated. Slices were then incubated in ACSF containing (in mM) 126 NaCl, 26 NaHCO3, 10 dextrose, 3 KCl, 1.25 NaH2PO4, 1 MgCl2, and 2 CaCl2. The osmolarity was 295–305 mOsm and the pH between 7.2 and 7.4. Sections were incubated for 30 min at 30–35°C and then for 30 min at room temperature (22–23°C) for a total resting time of 1–5 h before use.
Electrophysiological recording.
After resting for 1 h, each slice was visualized using a Zeiss Axioscope (Zeiss, Oberkochen, Germany) equipped with infrared-differential interference contrast optics, a Dage IR-1000 video camera (Dage, Michigan City, IN), and ×10 and ×40 lenses with optical zoom. The recording chamber had continuously perfused flow of oxygenated ACSF heated to 28–30°C. We employed the whole cell patch-clamp recording technique to record the electrical and synaptic properties of MSNs of two brain regions: the CP and AcbC (Fig. 1). Glass electrodes (10–19 MΩ) contained an internal solution of (in mM) 115 K-d-gluconate, 8 NaCl, 2 EGTA, 2 MgCl2, 2 MgATP, 0.3 NaGTP, 10 phosphocreatine, 0.3% biocytin, and 10 HEPES with osmolarity of 285 mOsm and pH 7.2–7.4 (Sigma-Aldrich). Signals were amplified, filtered (2 kHz), and digitized (10 kHz) with a MultiClamp 700B amplifier (Molecular Devices Axon Instruments, Foster City, CA) attached to a Digidata 1440 system (Molecular Devices Axon Instruments) and a personal computer using pClamp 10.7 software (Molecular Devices Axon Instruments). Membrane potentials were corrected for a calculated liquid junction potential of −13.5 mV. With the use of procedures previously described (Dorris et al. 2015), MSNs were identified by their medium-sized somas, the presence of a slow ramping subthreshold depolarization in response to positive current injections, a hyperpolarized resting potential, inward rectification, and a prominent spike afterhyperpolarization (Belleau and Warren 2000; O’Donnell and Grace 1993). Once electrical access to the MSN was obtained, the MSN rested for ~3 min to allow the resting membrane potential to stabilize (Mu et al. 2010). One series of positive, depolarizing currents to assess action potential properties was performed, followed by two series of negative, hyperpolarizing currents to assess passive and inward rectification properties. After this point, the voltage-gated sodium channel blocker tetrodotoxin (TTX; 1 µM; Abcam, Cambridge, UK) and GABAA receptor antagonist picrotoxin (PTX; 150 µM; Sigma-Aldrich) were bath applied to abolish action potentials and inhibitory postsynaptic current events, respectfully. Once depolarizing positive currents no longer elicited action potentials, the patched MSN was switched to voltage clamp at −70 mV and mEPSCs were recorded. This protocol isolates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-mediated events in MSNs and has been confirmed in our laboratory (Proaño et al. 2018). mEPSCs were first recorded for 5 min before exposure to estradiol. After the 5-min baseline period, 100 nM estradiol (Sigma-Aldrich; dissolved in the same TTX/PTX ACSF solution) was bath applied. This dose has previously been shown to elicit rapid changes in mEPSCs and other attributes of hippocampal neurons (Oberlander and Woolley 2016), and this dose is greater than the minimum required to elicit rapid estradiol changes in L-type calcium signaling and CREB phosphorylation of dissociated and primary cell culture striatal MSNs (Mermelstein et al. 1996). Estradiol was perfused over the tissue for 20 min while mEPSCs were continuously recorded. Series and input resistance were monitored throughout the recording. For control vehicle-only experiment, ACSF containing TTX and PTX was continuously perfused through the bath for the duration of the experiment (25 min). After the recording, MSN location was confirmed and perfusion tubing was rinsed with double-distilled H20 and 70% ethanol to remove estradiol contamination. All surfaces such as the recording chamber and optical lenses were cleaned with 70% ethanol to remove potential estradiol contamination between recordings.
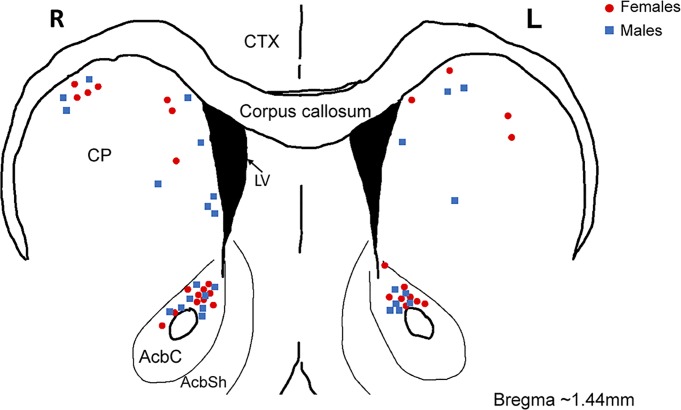
Fig. 1.
Location of whole cell patch-clamped medium spiny neurons (MSNs) in adult female and male rat nucleus accumbens core (AcbC) and caudate-putamen (CP). Blue squares are MSNs recorded from males; red circles are MSNs recorded from females. AbcSh, nucleus accumbens shell; CTX, cortex; L, left hemisphere; LV, lateral ventricle; R, right hemisphere.
Data analysis.
Intrinsic electrophysiological properties and action potential characteristics were analyzed using pClamp Clampfit10.7 (Molecular Devices Axon Instruments). Most properties measured followed the definitions of prior work from our laboratory (Cao et al. 2016; Dorris et al. 2015; Willett et al. 2016, 2019), which were based on those of Perkel and colleagues (Farries et al. 2005; Farries and Perkel 2000, 2002; Meitzen et al. 2009). For action potential measurements, only the first action potential on the current sweep that elicited the action potential was analyzed. Action potential threshold was defined as the first point of sustained positive acceleration of voltage (δ2V/ δt2) that was more than three times the standard deviation of membrane noise before the detected threshold (Baufreton et al. 2005). Action potential halfwidth is the width (ms) of the action potential halfway between the peak and threshold. The action potential amplitude is the change in millivolts between the threshold and the peak. Afterhyperpolarization peak (AHP) amplitude is the difference in millivolts between the threshold and the most hyperpolarized voltage point after the action potential peak. AHP time to peak amplitude is the time (ms) between the threshold point on the descending phase of the action potential and the AHP peak amplitude. Rheobase (nA) is the lowest amplitude of injected positive current required to produce an initial action potential. The slope of the linear range of the evoked action potential firing rate to positive injected current curve (FI slope) was calculated from the first current stimulus that evoked an action potential to the first current stimulus that generated an evoked firing rate that persisted for at least two consecutive current stimuli. Maximum firing rate was defined as the single highest action potential rate generated by any current stimulus. Resting membrane potential was calculated from the average of at least 8,900 ms of recording in the absence of injected current. Rectified range input resistance (RRIR), inward rectification, and percent inward rectification were calculated as described previously, with RRIR measured at the most hyperpolarizing current injected into the MSN (Belleau and Warren 2000). Inward rectification is the input resistance at the −0.02-nA step minus the RRIR. Percent inward rectification is defined as RRIR/IR × 100. Hyperpolarization-induced “sag” (i.e., IH current) was assessed using the “sag index” (Farries et al. 2005). Briefly, sag index is defined as the difference between the minimum voltage measured during the largest hyperpolarizing current pulse and the steady-state voltage deflection of that pulse, divided by the steady-state voltage deflection. All of these measurements occurred before estradiol was introduced to the bath.
Frequency, amplitude, and decay of mEPSCs were analyzed using Mini Analysis (Synaptosoft, Fort Lee, NJ; http://www.synaptosoft.com/MiniAnalysis/). All traces were filtered using a low-pass Gaussian filter at 2,000 Hz. mEPSC threshold was set at a minimum value of 9 pA, and accurate event detection was validated by visual inspection. Frequency is defined as the number of mEPSC events per second (Hz). Amplitude was calculated as the difference between the average baseline 10 ms before initial mEPSC rise and peak mEPSC amplitude (pA). mEPSC decay was calculated as the time required for peak mEPSC amplitude to return to baseline (ms). mEPSC values per cell were normalized as a percent change compared with the baseline period. Percent change was calculated by averaging the values of the first 5 min of the recording period before estradiol or vehicle exposure, and then using that average to normalize each minute of the recording [percent change = (measured value – average of the 5-min baseline)/average of the 5-min baseline) × 100].
Statistics.
Experiments were analyzed as appropriate with either paired two-tailed t-tests for raw values or one-sample t-tests for normalized percent change or linear regression. Cohen’s d was calculated for paired t-tests for effect sizes and is reported numerically. (GraphPad Prism, version 8). P values <0.05 were considered statistically significant and trends as 0.05 < P < 0.10 a priori. Data are means ± SE.
RESULTS
Estradiol rapidly decreases mEPSC frequency in female but not male MSNs in the nucleus accumbens core.
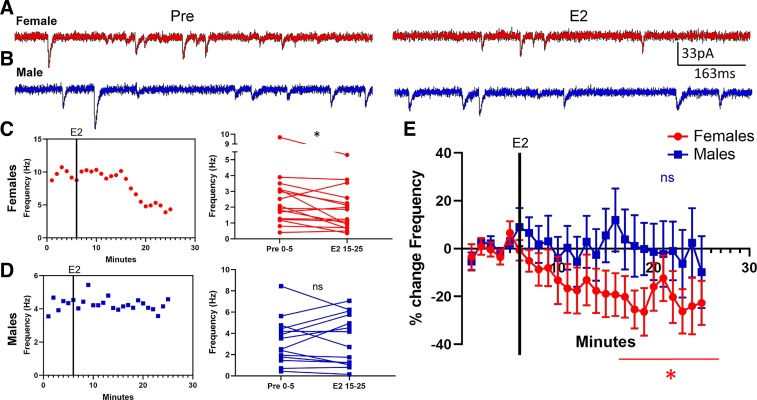
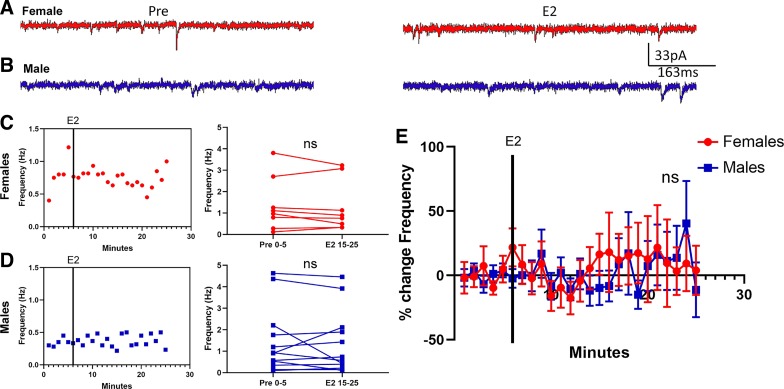
To determine whether estradiol rapidly changes mEPSC properties of AcbC MSNs, we exposed MSNs to estradiol for 20 min following a 5-min baseline period. MSNs were voltage-clamped at −70 mV and exposed to 1 µM TTX and 150 µM PTX to block sodium-dependent action potentials and GABAA receptors, respectively, and mEPSC frequency, amplitude, and decay were analyzed. Data were disaggregated between females (Fig. 2A) and males (Fig. 2B). Overall, estradiol exposure induced a rapid decrease in mEPSC frequency in females (Fig. 2C) but not in males (Fig. 2D). To quantify this decrease, we performed two analyses. First, we found that there was a significant decrease in mEPSC frequency in female MSNs recorded in the presence of estradiol compared with those recorded before estradiol exposure [t15 = 2.30, P = 0.036, mean difference (MD) = −0.71 ± 0.31 Hz, confidence interval (CI) (−1.37, −0.05), d = 0.39; Fig. 2C, right). We did not detect a significant change in mEPSC frequency for male MSNs [t13 = 0.31, P = 0.76, MD = 0.12 ± 0.38 Hz, CI (−0.71, 0.94), d = 0.051; Fig. 2D, right). Second, for each MSN, we normalized the mEPSC frequency recorded per minute during estradiol exposure to the mEPSC frequency recorded across the average of the 5 min before estradiol exposure. We then plotted this normalized average mEPSC frequency per minute as a percent change (Fig. 2E). This population analysis further revealed robust estradiol-induced decrease in mEPSC frequency in female MSNs [−21.42 ± 8.33% from baseline; t15 = 2.57, P = 0.021; CI (−39.19, −3.65)] but not in male MSNs [−0.57 ± 12.01% change from baseline; t13 = 0.048, P = 0.96; CI (−26.51, 25.37)]. These analyses collectively indicate that there is a robust estradiol-induced decrease in mEPSC frequency in AcbC MSNs that is sex-specific to females.
Fig. 2.
Estradiol rapidly decreased miniature excitatory postsynaptic current (mEPSC) frequency in female rat nucleus accumbens core (AcbC) medium spiny neurons (MSNs). A and B: representative examples of mEPSCs recorded in female (A) and male (B) MSNs before (Pre; left) and during exposure to 17β-estradiol (E2; right). C and D: an example experiment in females (C) and males (D) demonstrating the time course of the estradiol-induced decrease in mEPSC frequency (left) and a plot illustrating mean mEPSC frequency during a 5-min baseline before and during estradiol exposure (right). Connected symbols indicate data from an individual neuron. E: plotting the normalized change in mEPSC frequency vs. time indicates that female AcbC MSNs (n = 16) are more sensitive to rapid estradiol action than are male AcbC MSNs (n = 14). Red circles indicate females and blue squares indicate males. Black vertical lines in C–E depict the time point at which estradiol entered the bath. E2 15–25; the last 10 min during 17β-estradiol exposure; Pre 0–5, the 5-min baseline period before estradiol exposure. *P < 0.05; ns, not statistically significant.
Estradiol rapidly increases mEPSC amplitude in both sexes in MSNs in the nucleus accumbens core.
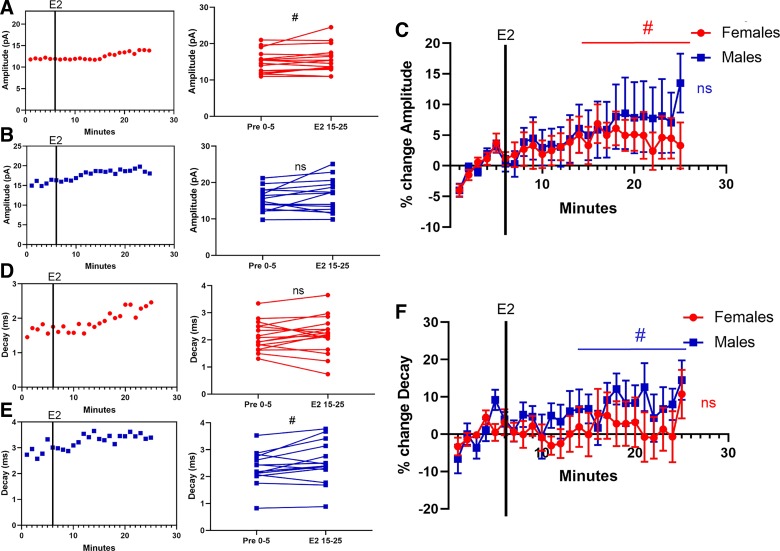
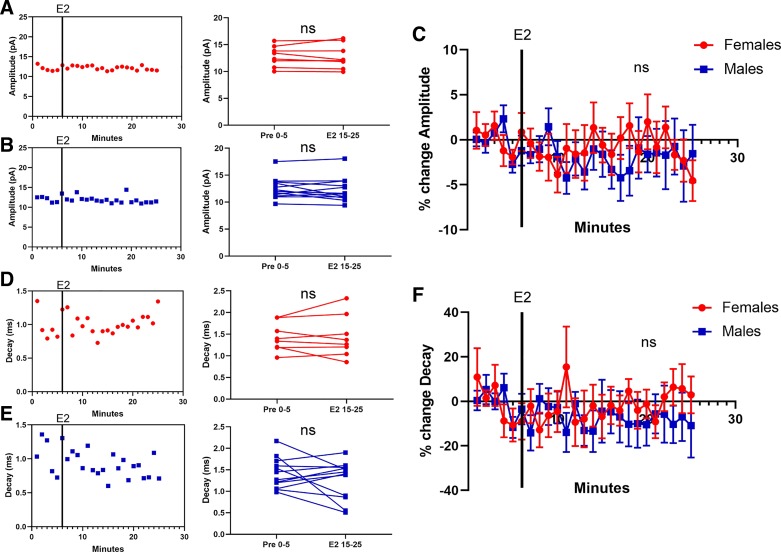
Regarding mEPSC amplitude, female MSNs exhibited a small increase in response to estradiol exposure that trended toward statistical significance [t15 = 1.87, P = 0.08, MD = 0.73 ± 0.39 pA, CI (−0.10, 1.56), d = 0.21; Fig. 3A]. Male MSNs likewise exhibited a small increase in response to estradiol exposure, but this effect did not reach statistical significance [t13 = 1.56, P = 0.14, MD = 1.20 ± 0.77 pA, CI (−0.46, 2.86), d = 0.30; Fig. 3B]. When data were normalized, female MSNs showed a trending estradiol-induced increase in mEPSC amplitude of 4.76 ± 2.35% [t15 = 2.03, P = 0.061, CI (−0.25, 9.76); Fig. 3C], whereas males exhibited an increase of 7.71 ± 5.17% that did not reach significance [t13 = 1.49, P = 0.16, CI (−3.45, 18.9)]. Regarding mEPSC decay, most female MSNs did not exhibit an estradiol-induced change in this metric [t15 = 0.69, P = 0.50, MD = 0.066 ± 0.095 ms, CI (−0.14, 0.27), d = 0.11; Fig. 3D]. Male MSNs exhibited a small estradiol-induced increase in mEPSC decay that trended toward statistical significance [t13 = 2.06, P = 0.06, MD = 0.20 ± 0.10 ms, CI (−0.010, 0.42), d = 0.28; Fig. 3E]. When data were normalized, female MSNs showed a nonsignificant estradiol-induced change in mEPSC decay of 2.31 ± 5.17% [t15 = 0.45, P = 0.66, CI (−8.71, 13.3); Fig. 3F], whereas males exhibited a 8.31 ± 3.91% increase that trended toward statistical significance [t13 = 2.13, P = 0.053, CI (−0.13, 16.76)]. These data suggested to us that estradiol may influence AcbC MSN mEPSC amplitude independently of sex.
Fig. 3.
Estradiol, miniature excitatory postsynaptic current (mEPSC) amplitude, and mEPSC decay in female or male rat nucleus accumbens core (AcbC) medium spiny neurons (MSNs). A and B: an example experiment in females (A) and males (B) demonstrating the time course of the estradiol-induced increase in mEPSC amplitude (left) and a plot illustrating mean mEPSC amplitude before and during estradiol exposure (right). Connected symbols indicate data from an individual neuron. C: plotting the normalized change in mEPSC amplitude disaggregated by sex. D and E: an example experiment in females (D) and males (E) demonstrating the time course of the estradiol-induced increase in mEPSC decay (left) and a plot illustrating mean mEPSC decay before and during estradiol exposure (right). Connected symbols indicate data from an individual neuron. F: normalized changes or lack thereof in mEPSC decay in females (n = 16) or males (n = 14). Red circles indicate females and blue squares indicate males. Black vertical lines depict the time point in which estradiol entered the bath. E2 15–25; the last 10 min during 17β-estradiol exposure; Pre 0–5, the 5-min baseline period before estradiol exposure. #P < 0.10; ns, not statistically significant.
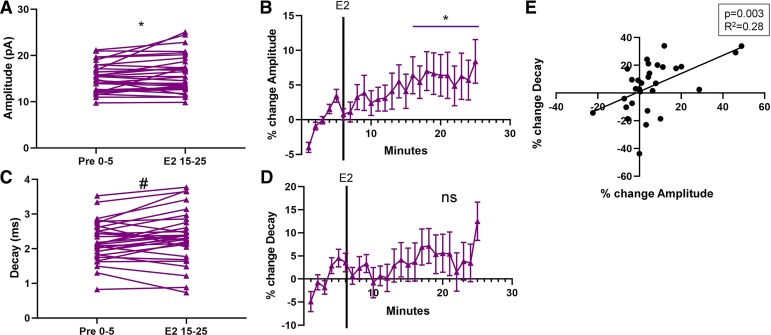
To further test this hypothesis, we aggregated female and male data and analyzed mEPSC amplitude and decay. mEPSC frequency data was not aggregated by sex because of the large sex differences in estradiol-induced changes in mEPSC frequency (females: −21.4%, d = 0.39; males: −0.57%, d = 0.051). Analysis of aggregated female and male mEPSCs amplitudes revealed a sustained estradiol-induced increase in mEPSC amplitude [t29 = 2.32, P = 0.030, MD = 0.95 ± 0.41 pA, CI (0.11, 1.78), d = 0.26; Fig. 4A]. When data were normalized, a significant estradiol-induced increase in mEPSC amplitude was also apparent [t29 = 2.29, P = 0.030, mean (M) = 6.14 ± 2.69%, CI (0.65, 11.6); Fig. 4B]. Thus we conclude that the evidence supports the interpretation that estradiol induces a small increase in mEPSC amplitude in both female and male MSNs.
Fig. 4.
Estradiol rapidly increases miniature excitatory postsynaptic current (mEPSC) amplitude in rat nucleus accumbens core (AcbC) medium spiny neurons (MSNs) independently of sex. A: mean mEPSC amplitude before and during estradiol exposure in both males and females (n = 30). Connected symbols indicate data from an individual neuron. B: plotting the normalized change in mEPSC amplitude in both males and females indicates that both sexes are sensitive to the effects of estradiol. C: mean mEPSC decay before and during estradiol exposure in both males and females. D: plotting the normalized change in mEPSC decay in both males and females indicates little effect of estradiol. E: relationship of %change in amplitude to %change in decay in both male and female AcbC MSNs. Purple triangles indicate both sexes together. Black line is the linear relationship, and there is a significant positive correlation between %change in amplitude and %change in decay. E2 15–25; the last 10 min during 17β-estradiol exposure; Pre 0–5, the 5-min baseline period before estradiol exposure.*P < 0.05; #P < 0.10; ns, not statistically significant.
Regarding mEPSC decay, aggregated analysis of female and male MSNs indicated a trend for an estradiol-induced increase in mEPSC decay [t29 = 1.90, P = 0.068, MD = 0.13 ± 0.068 ms, CI (−0.010, 0.27), d = 0.19; Fig. 4C]. When data were normalized, no significant estradiol-induced increase in mEPSC decay was apparent [t29 = 1.55, P = 0.13, M = 5.11 ± 3.30%, CI (−1.64, 11.9); Fig. 4D]. This analysis indicates that estradiol exerts no or minimal effect on mEPSC decay in this experimental paradigm. Given that mEPSC decay often correlates with mEPSC amplitude, as a control we further tested whether these attributes correlate in the current experiment. Normalized estradiol-induced changes in mEPSC amplitude and decay significantly correlated [F(1,28) = 10.9, P = 0.0026, b = 0.65 ± 0.20, R2 = 0.28; Fig. 4E], indicating that MSNs experiencing moderate increases in amplitude also experienced increases in decay. Both effects were small (amplitude: 6.14%, d = 0.26; decay: 5.11%, d = 0.19) compared with the decrease in female MSN frequency (−21.4%, d = 0.39). Overall, this analysis indicates that estradiol modestly modulates mEPSC amplitude in a select number of MSNs.
Decreased rheobase and action potential threshold identifies estradiol sensitivity of mEPSC amplitude in nucleus accumbens core MSNs.
Next, we wanted to determine if individual action potential, intrinsic excitability, or passive membrane properties of AcbC MSNs could identify estradiol sensitivity in mEPSC frequency, amplitude, or decay. To accomplish this, we measured a comprehensive battery of neuronal electrophysiological properties in current clamp, before estradiol exposure (Table 1). We then tested whether any of these properties correlated with the estradiol-induced percent change in mEPSC frequency, amplitude, and decay. No MSN electrophysiological property significantly correlated with the estradiol-induced changes in mEPSC frequency in either females or males (P > 0.05 for all; Table 1). Considering that mEPSC frequency is primarily associated with changes in presynaptic properties (i.e., number of synapses and/or neurotransmitter release), it is logical that MSN intrinsic properties would not relate to mEPSC frequency. Additionally, no MSN electrophysiological property significantly correlated with the estradiol-induced changes in mEPSC decay (P > 0.05 for all).
Table 1.
Regression statistics
| Frequency, %change |
Amplitude, %change | Decay, %change | ||
|---|---|---|---|---|
| Parameter | Female | Male | Both sexes | Both sexes |
| AP threshold, mV |
F(1,14) = 2.33e-5, P = 0.99 |
F(1,12) = 0.075, P = 0.79 |
F(1,28) = 3.62, P = 0.068† |
F(1,28) = 0.020, P = 0.89 |
| AP amplitude, mV |
F(1,14) = 3.03e-5, P = 0.99 |
F(1,12) = 0.79, P = 0.39 |
F(1,28) = 0.082, P = 0.78 |
F(1,28) = 0.23, P = 0.88 |
| AP halfwidth, ms |
F(1,14) = 0.016, P = 0.90 |
F(1,12) = 0.67, P = 0.43 |
F(1,28) = 0.29, P = 0.59 |
F(1,28) = 0.048, P = 0.83 |
| AHP peak, mV |
F(1,14) = 0.055, P = 0.82 |
F(1,12) = 9.23e-4, P = 0.98 |
F(1,28) = 0.90, P = 0.35 |
F(1,28) = 0.092, P = 0.76 |
| AHP time to peak, ms |
F(1,14) = 1.02, P = 0.33 |
F(1,12) = 3.15, P = 0.10 |
F(1,28) = 1.26, P = 0.27 |
F(1,28) = 1.16, P = 0.29 |
| Rheobase, nA |
F(1,14) = 0.39, P = 0.54 |
F(1,12) = 0.63, P = 0.44 |
F(1,28) = 7.63, P = 0.01* |
F(1,28) = 0.77, P = 0.39 |
| FI slope, Hz/nA |
F(1,14) = 0.20, P = 0.66 |
F(1,12) = 0.35, P = 0.57 |
F(1,28) = 2.31, P = 0.14 |
F(1,28) = 0.58, P = 0.45 |
| Max AP firing rate, Hz |
F(1,14) = 0.40, P = 0.53 |
F(1,12) = 1.16, P = 0.30 |
F(1,28) = 0.37, P = 0.55 |
F(1,28) = 1.50, P = 0.23 |
| RMP, mV |
F(1,14) = 1.02, P = 0.33 |
F(1,12) = 3.24, P = 0.097 |
F(1,28) = 1.32, P = 0.26 |
F(1,28) = 0.66, P = 0.42 |
| IR at 0.02 nA, MΩ |
F(1,14) = 0.11, P = 0.75 |
F(1,12) = 1.85, P = 0.20 |
F(1,28) = 2.85, P = 0.10 |
F(1,28) = 0.49, P = 0.49 |
| Tau at 0.02 nA, ms |
F(1,14) = 1.73, P = 0.21 |
F(1,12) = 0.46, P = 0.51 |
F(1,28) = 0.0030, P = 0.96 |
F(1,27) = 0.034, P = 0.85‡ |
| Cap at 0.02 nA, pF |
F(1,14) = 0.80, P = 0.39 |
F(1,12) = 0.31, P = 0.59 |
F(1,28) = 1.01, P = 0.32 |
F(1,28) = 1.98, P = 0.17 |
| RRIR, MΩ |
F(1,14) = 0.12, P = 0.74 |
F(1,12) = 1.14, P = 0.31 |
F(1,28) = 1.83, P = 0.19 |
F(1,28) = 0.12, P = 0.73 |
| IR, MΩ |
F(1,14) = 0.076, P = 0.79 |
F(1,12) = 0.027, P = 0.87 |
F(1,28) = 2.13, P = 0.16 |
F(1,28) = 0.82, P = 0.37 |
| IR, % |
F(1,14) = 0.089, P = 0.77 |
F(1,12) = 1.28e-4, P = 0.99 |
F(1,28) = 0.78, P = 0.39 |
F(1,28) = 0.069, P = 0.79 |
| Sag index |
F(1,14) = 0.81, P = 0.38 |
F(1,12) = 2.09, P = 0.17 |
F(1,28) = 1.15, P = 0.29 |
F(1,28) = 0.40, P = 0.53 |
AHP, afterhyperpolarization peak; AP, action potential; Cap, Capacitance; FI, frequency of evoked spikes to injected depolarization current; IR, inward rectification; Max, maximum; RMP, resting membrane potential; RRIR, rectified range input resistance.
P < 0.05 (significance);
0.05 < P < 0.10 (trend).
In this analysis, an outlier was removed that was driving a significant relationship.
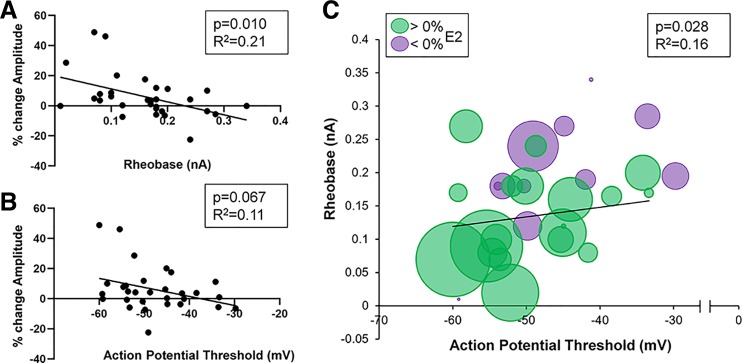
Regarding mEPSC amplitude, select MSN electrophysiological properties significantly correlated with estradiol-induced changes across females and males. There was a significant correlation between estradiol-induced changes in mEPSC amplitude and rheobase [F(1,28) = 7.63, P = 0.010, b = −86.3 ± 31.2, R2 = 0.21; Fig. 5A] and a trending correlation between estradiol-induced changes in mEPSC amplitude and action potential threshold [F(1,28) = 3.62, P = 0.068, b = −0.60 ± 0.32, R2 = 0.11; Fig. 5B]. Given that rheobase and action potential threshold frequently associated with one another in MSNs, as a control we confirmed that rheobase and action potential threshold exhibited a significant relationship [F(1,28) = 5.36, P = 0.028, b = 0.0038, R2 = 0.16; Fig. 5C]. We further tested whether MSNs featuring concurrent low rheobase and action potential threshold exhibited the greatest positive percent change in mEPSC amplitude (Fig. 5C). These features of MSN excitability strongly overlapped with estradiol sensitivity in mEPSC amplitude. Collectively, these data indicate that increased MSN excitability, as indicated by a reduced rheobase and action potential threshold, indicates an increased likelihood of estradiol sensitivity in mEPSC amplitude.
Fig. 5.
Sensitivity of miniature excitatory postsynaptic current (mEPSC) amplitude to estradiol correlates with neuronal excitability in both sexes in rat nucleus accumbens core (AcbC) medium spiny neurons (MSNs). A: relationship of rheobase to the %change in mEPSC amplitude in response to estradiol. B: relationship of action potential threshold to the %change in mEPSC amplitude in response to estradiol. C: combined relationship of rheobase and action potential threshold to the %change in mEPSC amplitude in response to estradiol. Green circles indicate increased %changes of mEPSC amplitude in response to estradiol. Purple circles indicate decreased %changes of mEPSC amplitude in response to estradiol. Increasing circle size indicates larger %change. Black lines indicate the best-fit linear relationship.
mEPSC parameters do not change in response to vehicle exposure in the nucleus accumbens core.
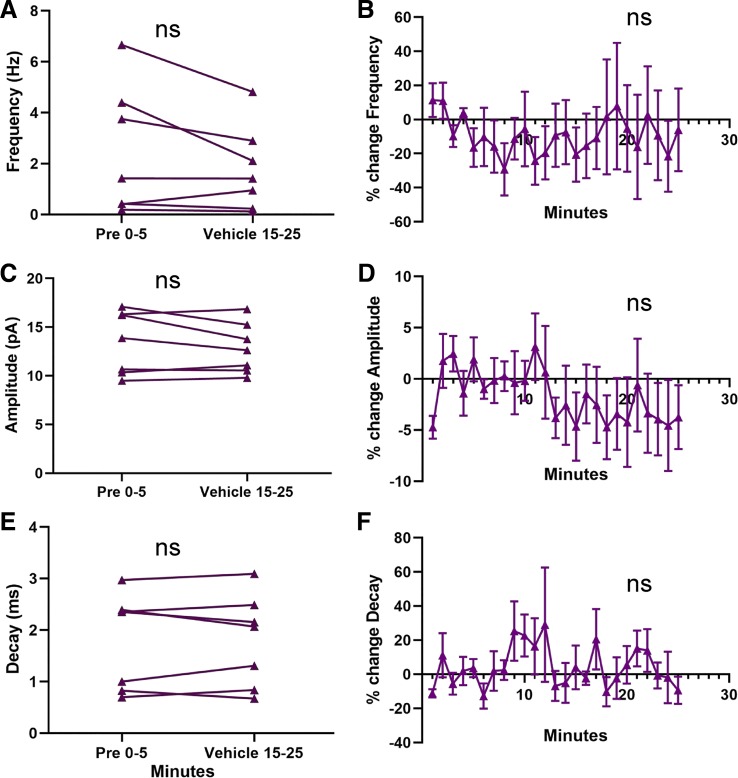
For mEPSC amplitude and decay, a potential upwardly trending baseline period before estradiol exposure was observed (Figs. 4 and 5). Arguing against the presence of an upwardly trending baseline, the percent changes in mEPSC amplitude during the 5 min before bath application were not significantly different from 0 (P > 0.05), and there was an overall drop in mEPSC amplitude between 5 and 6 min of recording. Although this evidence suggests that the effects of estradiol are not due to a general instability in AcbC MSN mEPSC properties, we performed a further control experiment to assess the specificity of estradiol’s actions on mEPSC properties. We recorded mEPSCs from both male and female MSNs in the AcbC using the same parameters as in the estradiol experiments, but instead of bath applying estradiol, we applied vehicle solution (ACSF with TTX/PTX). MSNs from both sexes (females: n = 4; males: n = 3) exhibited no significant change from the first 5 min to the last 10 min of the recording for mEPSC frequency, amplitude, or decay [frequency: t6 = 1.71, P = 0.14, MD = 0.67 ± 0.39 Hz, CI (−1.64, 0.29), d = 0.32, Fig. 6A; amplitude: t6 = 1.24, P = 0.26, MD = 0.59 ± 0.47 pA, CI (−1.76, 0.57), d = 0.20, Fig. 6C; decay: t6 = 0.047, P = 0.96, MD = 0.0040 ± 0.086 ms, CI (−0.21, 0.21), d = 0.004, Fig. 6E] or the normalized percent change [frequency: t6 = 0.29, P = 0.77, MD = −7.24 ± 24.7%, CI (−67.5, 53.1), Fig. 6B; amplitude: t6 = 1.02, P = 0.35, MD = −3.26 ± 3.18%, CI (−11.06, 4.54, Fig. 6D; decay: t6 = 0.43, P = 0.69, MD = 2.88 ± 6.77%, CI (−13.7, 19.4), Fig. 6F]. The results of this control experiment, along with the other analysis discussed above, are consistent with the hypothesis that changes in mEPSC properties are the result of estradiol exposure.
Fig. 6.
Miniature excitatory postsynaptic current (mEPSC) parameters do not change with vehicle exposure in rat nucleus accumbens core (AcbC) medium spiny neurons (MSNs). A: plot illustrating no effect of vehicle exposure on mEPSC frequency in males and females (n = 7). Connected symbols indicate data from an individual neuron. B: normalized mEPSC frequency. C: plot illustrating no effect of vehicle exposure on mEPSC amplitude in males and females. D: normalized mEPSC amplitude. E: plot illustrating no effect of vehicle exposure on mEPSC decay in males and females. F: normalized mEPSC decay. Purple triangles indicate both sexes together. E2 15–25; the last 10 min during 17β-estradiol exposure; ns, not statistically significant; Pre 0–5, the 5-min baseline period before estradiol exposure.
Caudate-putamen MSNs display no evidence of sensitivity to acute estradiol exposure in mEPSC properties in either females or males.
We also determined whether estradiol rapidly changes mEPSC properties of MSNs in the CP. As in the AcbC experiment described above, MSNs were voltage-clamped at −70 mV and exposed to 1 µM TTX and 150 µM PTX, and mEPSC frequency, amplitude, and decay were analyzed. Data were disaggregated between females (Fig. 7A) and males (Fig. 7B). Overall, estradiol exposure did not rapidly modulate mEPSC frequency of MSNs in either female [t7 = 0.87, P = 0.41, MD = −0.10 ± 0.11 Hz, CI (−0.37, 0.17), d = 0.080; Fig. 7C] or male CP [t11 = 0.54, P = 0.60, MD = −0.11 ± 0.20 Hz, CI (−0.54, 0.33), d = 0.071; Fig. 7D]. Normalized mEPSC frequency values likewise demonstrated no estradiol sensitivity in both females and males [females: t7 = 0.57, P = 0.58, M = 14.6 ± 25.5%, CI (−45.6, 74.8); males: t11 = 0.44, P = 0.67, M = 8.01 ± 18.4%, CI (−32.4, 48.4); Fig. 7E]. We conclude that there is no evidence that CP MSN mEPSC frequency is sensitive to rapid estradiol action.
Fig. 7.
Estradiol does not modulate miniature excitatory postsynaptic current (mEPSC) frequency in female or male caudate-putamen (CP) medium spiny neurons (MSNs). A: representative examples of mEPSCs recorded in female MSNs before (Pre; left) and during exposure to 17β-estradiol (E2; right). B: representative examples of mEPSCs recorded in male MSNs before (left) and during exposure to estradiol (right). C and D: an example experiment in females (C) and males (D) demonstrating the time course of no changes in mEPSC frequency in response to estradiol (left) and a plot illustrating mean mEPSC frequency during a 5-min baseline before and during estradiol exposure (right). Connected symbols indicate data from an individual neuron. E: plotting the normalized change in mEPSC frequency vs. time indicates that neither female (n = 8) nor male (n = 12) MSN mEPSC frequency is sensitive to rapid estradiol exposure. Red circles indicate females and blue squares indicate males. Black vertical lines depict the time point at which estradiol entered the bath. E2 15–25; the last 10 min during 17β-estradiol exposure; Pre 0–5, the 5-min baseline period before estradiol exposure; ns, not statistically significant.
Similarly, estradiol exposure did not rapidly modulate MSN mEPSC amplitude in either female [t7 = 0.20, P = 0.84, MD = −0.055 ± 0.27 pA, CI (−0.69, 0.58), d = 0.023; Fig. 8A] or male CP [t11 = 0.85, P = 0.41, MD = −0.28 ± 0.33, CI (−0.99, 0.44), d = 0.13; Fig. 8B]. Normalized mEPSC amplitude values also demonstrated no estradiol sensitivity in both females and males [females: t7 = 0.35, P = 0.74, M = −0.67 ± 1.92%, CI (−5.21, 3.87); males: t11 = 0.85, P = 0.41, M = −2.19 ± 2.56%, CI (−7.83, 3.46); Fig. 8C]. Estradiol exposure likewise did not rapidly modulate mEPSC decay in either female [t7 = 0.15, P = 0.88, MD = 0.013 ± 0.083 ms, CI (−0.18, 0.21), d = 0.031; Fig. 8D] or male CP [t11 = 1.08, P = 0.30, MD = −0.16 ± 0.14 ms, CI (−0.47, 0.16, d = 0.39; Fig. 8E]. Normalized mEPSC decay values also showed no evidence of sensitivity to estradiol exposure [females: t7 = 0.049, P = 0.96, M = −0.27 ± 5.57%, CI (−13.5, 12.9); males: t11 = 0.87, P = 0.40, M = −8.22 ± 9.45%, CI (−29.0, 12.6); Fig. 8F]. Collectively, these data indicate that CP MSN mEPSC properties show no sensitivity to acute estradiol action, at least under these experimental circumstances.
Fig. 8.
Estradiol does not modulate miniature excitatory postsynaptic current (mEPSC) amplitude or decay in female or male caudate-putamen (CP) medium spiny neurons (MSNs). A and B: an example experiment in females (A) and males (B) demonstrating the time course of no changes in mEPSC amplitude in response to estradiol (left) and a plot illustrating mean mEPSC amplitude before and during estradiol exposure (right). Connected symbols indicate data from an individual neuron. C: plotting the normalized change in mEPSC amplitude disaggregated by sex reveals no sensitivity to estradiol exposure. D and E: an example experiment in females (n = 8; D) and males (n = 12; E) demonstrating the time course of no changes in mEPSC decay in response to estradiol (left) and a plot illustrating mean mEPSC decay before and during estradiol exposure (right). F: plotting the normalized change in mEPSC decay disaggregated by sex reveals no sensitivity to estradiol exposure. Red circles indicate females and blue squares indicate males. Black vertical lines depict the time point at which estradiol entered the bath. E2 15–25; the last 10 min during 17β-estradiol exposure; Pre 0–5, the 5-min baseline period before estradiol exposure; ns, not statistically significant.
DISCUSSION
These experiments reveal sex- and region-specific rapid estradiol modulation of glutamatergic synapses in the striatum. Estradiol rapidly modulates glutamatergic signaling in the nucleus accumbens core but not the caudate-putamen. Furthermore, the effects of estradiol are sex specific and bidirectional, in that estradiol rapidly induces a profound decrease in mEPSC frequency in female nucleus accumbens core MSNs, whereas estradiol rapidly induces an increase in mEPSC amplitude in both female and male nucleus accumbens core MSNs. To our knowledge, this is the first study to demonstrate an acute action of estradiol on excitatory synapse action on MSNs in any striatal region.
Regarding sex differences of rapid estradiol regulation of striatal function, one major working model is that estradiol acts acutely and directly on female MSNs via membrane estrogen receptors to decrease electrical activity to in turn directly decrease GABA and indirectly increase dopamine release. This working model posits that estradiol releases an inhibitory “brake” on the overall basal ganglia circuit, ultimately increasing locomotion and drug reinstatement in females (Yoest et al. 2014, 2018). Several key pieces of evidence are consistent with this model. Estradiol rapidly decreases GABA production (Hu et al. 2006), increases immediate-early c-Jun expression (Zhou and Dorsa 1994), and augments dopamine action. Via a membrane estrogen receptor α/β (ERα/β) and metabolic glutamate receptor mechanism, estradiol acutely decreases L-type calcium channel currents in dissociated striatal cells in culture (Mermelstein et al. 1996), and modulates the phosphorylation of the transcription factor CREB (Grove-Strawser et al. 2010). Further supporting this model, adult rat nucleus accumbens and caudate-putamen MSNs solely express membrane-associated ERα/β and G protein-coupled estrogen receptor 1 (GPER1), although some regional differences exist that are not well documented (Almey et al. 2012, 2016; Schultz et al. 2009; Yoest et al. 2018). This lack of nuclear estrogen receptor expression concurrent with this body of knowledge suggested to us that estradiol rapidly modulates electrophysiological properties critical for MSN input/output computations, most prominently those involving glutamatergic neurotransmission in females. However, to date, this key piece of evidence has remained absent until the current study.
Here we demonstrate that estradiol rapidly decreases mEPSC frequency in female but not male MSNs in the nucleus accumbens core. We did not detect rapid estradiol changes in either sex for caudate-putamen, which raises interesting region-specific implications given that the working model for rapid estradiol action in the striatum is largely based on experiments in the caudate-putamen. Further addressing this region-specific question by including the nucleus accumbens shell would be an avenue for future investigation. We note that the current study did not include nucleus accumbens shell because previous experiments from our laboratory and others have suggested that hormone sensitivity and sex differences are less robust in the shell compared with the core (Peterson et al. 2015; Staffend et al. 2011; Willett et al. 2016), although this depends on the metric assessed (Forlano and Woolley 2010) and environmental factors such as stress (Brancato et al. 2017).
A decrease in mEPSC frequency likely indicates a locus of action that ultimately modulates the presynaptic terminal. However, it is possible that estradiol could be either acting directly on the presynaptic glutamatergic synaptic terminals or acting on the MSN or other neuron type to trigger a retrograde messenger. Consistent with the latter possibility, endocannabinoid signaling has been implicated in estradiol modulation of MSN dendritic spine density in the nucleus accumbens core (Peterson et al. 2016). In the nucleus accumbens core, estradiol-induced endocannabinoid signaling is regulated via membrane estrogen receptor signaling associated with metabotropic glutamate receptors (Peterson et al. 2016). We note that GPER1 is also expressed in this brain region and could also potentially play a role (Almey et al. 2016). Indeed, there is evidence from the hippocampus that sex-specific estrogen receptors can play key roles in regulating both presynaptic and postsynaptic glutamatergic transmission (Oberlander and Woolley 2016). Both ERα and GPER1 expression have been described in the caudate-putamen and nucleus accumbens core on terminals and dendrites of striatal neurons of female rats (Almey et al. 2012, 2015, 2016). Sex differences of these receptors expressed on glutamatergic terminals may mediate sex differences in presynaptic estradiol responsiveness; however, to date, these comparisons have not been made. The mechanisms underlying estradiol-induced changes in female nucleus accumbens core glutamatergic signaling is a clear and important avenue for future investigations.
We also observed a small increase in mEPSC amplitude in both sexes, suggesting that there are postsynaptic mechanisms also changing in response to estradiol. In female MSNs, the magnitude of this increase in mEPSC amplitude is much smaller than the change observed in mEPSC frequency; therefore collectively, acute estradiol may still produce an overall decrease in excitatory transmission onto female MSNs. Previously, our laboratory has demonstrated that mEPSCs recorded in adult rat nucleus accumbens core are specifically generated by glutamatergic AMPA channels (Proaño et al. 2018). Thus the measured increase in mEPSC amplitude may be due to increased AMPA receptor trafficking to the membrane, perhaps facilitated directly by MSN dendrite-expressed estrogen receptors. Consistent with a model of decreased synapse number in response to estradiol, multiple-day estradiol injections in ovariectomized female rats and hamsters decrease MSN dendritic spine density in the nucleus accumbens core but not in caudate-putamen (Peterson et al. 2015; Staffend et al. 2011). Conversely, experiments in naturally cycling female rats detected overall increased mEPSC frequency during the late proestrus and early proestrus stages, but rapid estradiol action was not assessed (Proaño et al. 2018). We note that the estrous cycle involves more hormones than just estradiol, perhaps pointing to a role for progesterone or simply that estradiol has diverse actions depending on dose and time frame. Ultimately, these findings illustrate that estradiol exhibits bidirectional effects on glutamatergic signaling in the striatum. The time course of estradiol exposure is of the utmost importance. The impact of estradiol, and perhaps the underlying mechanisms, differ by sex, striatal region, and potentially other factors as well.
Regarding the nucleus accumbens core in both sexes, MSNs that were more excitable (lower rheobase and action potential threshold) exhibited the largest estradiol-induced increase in mEPSC amplitude. Rheobase is a strong predictor of whether a MSN is a D1 or D2 MSN subtype (D1 > D2; as reviewed by Cao et al. 2018a) in the caudate-putamen (Willett et al. 2019). However, these strict definitions of MSN subtypes break down in the nucleus accumbens core (Kupchik et al. 2015), and differences between MSN subtypes in the nucleus accumbens of rats remains an active avenue of research (reviewed in Cao et al. 2018a). Given these important caveats, tentatively, considering that MSNs with a lower rheobase have a stronger response to estradiol, this may predict that these cells are D2 subtype MSNs, although further testing with D1- or D2-labeled neurons in a relevant animal model must be conducted to confirm this prediction.
Although we did not detect rapid changes in mEPSC properties for the caudate-putamen of males and females in response to estradiol, this does not necessarily mean that caudate-putamen MSNs are insensitive to acute estradiol exposure. In fact, most studies of striatal estradiol action have been performed in the caudate-putamen, albeit with different experimental targets. One general finding from this literature that is consistent with the findings presented here is that females are more sensitive to acute estradiol action than are males (Becker et al. 1987; Mermelstein et al. 1996; Yoest et al. 2018). In the 1980s, two different studies demonstrated differences in the in vivo spontaneous action potential generation rates of both unidentified and identified striatonigral MSNs across different phases of the estrous cycle and in response to longer term exposure to estradiol in ovariectomized rat caudate-putamen (Arnauld et al. 1981; Tansey et al. 1983). Following this work, another study has shown in dissociated cultured caudate-putamen MSNs that estradiol decreases L-type calcium currents (Mermelstein et al. 1996). Other studies have demonstrated behavioral changes associated with decreases in GABA release and increases in dopamine transmission in the caudate-putamen after an acute estradiol injection (Becker 1990b; Becker and Rudick 1999; Hu et al. 2006). The coronal brain slices employed by the current study remove the somas (and presumably action potential generation sites) from many glutamatergic inputs, and this study also employed a protocol that abolished action potential generation to assess mEPSCs. Thus it is possible that caudate-putamen (and potentially nucleus accumbens core) MSNs are changing intrinsic and excitability properties in response to estradiol neuromodulation, which could not be assessed in this study. Other brain regions, such as the hypothalamus, have shown estradiol-induced changes to intrinsic properties of neurons (Adams et al. 2019). Furthermore, Grove-Strawser et al. (2010) detected rapid estradiol-induced changes in the phosphorylation of the transcription factor CREB in female but not male caudate-putamen MSNs (demonstrating that estradiol is triggering intracellular signaling pathways that may lead to changes in gene transcription). Alternatively, inhibitory signaling may also be impacted by estradiol, especially given data implicating rapid estradiol modulation of inhibitory signaling in other brain regions (Adams et al. 2018; Huang and Woolley 2012). To synergize the prior work with the current study, estradiol rapidly modulates dopaminergic transmission in caudate-putamen but may not impact glutamatergic signaling onto caudate-putamen MSNs.
Endogenous estradiol production within the striatum may also play a prominent role. In a previous review (Krentzel and Meitzen 2018), we showed that there is sufficient evidence that the striatal neurons are capable of producing estradiol locally. Functionally, Tozzi et al. (2015) detected an aromatase-dependent form of long-term potentiation in adult caudate-putamen MSNs in males, with females not assessed. Especially since the addition of estradiol into the recording bath did not further enhance LTP in male MSNs, this study may suggest that inhibiting aromatase in caudate-putamen may reveal estradiol impacts on MSN physiology. For example, work in the zebra finch has demonstrated that aromatase-enriched regions have rapid responses to estradiol in electrophysiological properties of local neurons (Remage-Healey et al. 2012), as do neurons in other brain regions that lack aromatase, but receive terminal projections from the aromatase rich area (Remage-Healey and Joshi 2012). Estradiol responsiveness is also sex and cell-type specific regarding receptor mechanism (Krentzel et al. 2018). Further complicating this picture, we note that trans-synaptic hormone action has been demonstrated from cortical-like brain regions to striatal regions in songbird brain (Brenowitz and Lent 2001, 2002). Given that multiple types of estrogen receptors have been reported on both the pre- and postsynaptic sides of glutamatergic synapses in MSNs, and also in striatal cholinergic and dopaminergic synapses (Almey et al. 2012, 2015, 2016), there is likely substantial variation in how estradiol modulates striatal electrophysiological function. For instance, there is evidence that estradiol can directly modulate striatal cholinergic systems (Almey et al. 2012; Euvrard et al. 1979). Estradiol likely exhibits a complex mechanism of action in the striatum, and the current study is foundational for elucidating these neuroendocrine mechanisms and their impact on striatal function. For future studies, isolating individual neuronal subtypes or neurotransmitter systems may elucidate the complexities in estradiol neuromodulation of the striatal regions and may explain some of the heterogeneity in mEPSC sensitivity to estradiol.
These findings also generate other potential future avenues of research. Estrogen receptor identity is a paramount question, as are the functional consequences of estradiol modulation of MSN glutamatergic signaling. We note that males and females may utilize different receptor types and that these receptors may differ in mediating either pre- or postsynaptic changes, and that response to the adult female hormone cycle and developmental differences in estrogen receptor expression in the striatum remains largely unexplored. However, given the strong influence of sex and steroid hormones on striatum-relevant behaviors and disorders (Johnson et al. 2019; Lorsch et al. 2018), these and other future avenues of research, especially regarding rapid estradiol action, are critical for understanding striatal and especially nucleus accumbens function. This current study adds to the rich history of the rapid estradiol modulation literature pioneered by Szego, Kelly, Moss, Callard, and others (Callard et al. 1978; Kelly et al. 1976, 1977; Szego and Davis 1967), further illustrating the importance of this hormone’s role in neuromodulation and electrophysiological properties on a variety of timescales (Rudolph et al. 2016).
GRANTS
This work was supported by National Institutes of Health Grants R01MH109471 (J. Meitzen) and P30ES025128 (Center for Human Health and the Environment) and North Carolina State University startup funds.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A.K. and J.M. conceived and designed research; A.A.K. performed experiments; A.A.K., L.R.B., and J.M. analyzed data; A.A.K. and J.M. interpreted results of experiments; A.A.K. and J.M. prepared figures; A.A.K. and J.M. drafted manuscript; A.A.K., L.R.B., and J.M. edited and revised manuscript; A.A.K. and J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank David Dorris for training on patch-clamp electrophysiology and Natalie Truby for assistance in experiment preparation.
REFERENCES
- Adams C, Chen X, Moenter SM. Changes in GABAergic transmission to and intrinsic excitability of gonadotropin-releasing hormone (GnRH) neurons during the estrous cycle in mice. eNeuro 5: ENEURO.0171-18.2018, 2018. doi: 10.1523/ENEURO.0171-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C, DeFazio RA, Christian CA, Milescu LS, Schnell S, Moenter SM. Changes in both neuron intrinsic properties and neurotransmission are needed to drive the increase in GnRH neuron firing rate during estradiol-positive feedback. J Neurosci 39: 2091–2101, 2019. doi: 10.1523/JNEUROSCI.2880-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology 153: 5373–5383, 2012. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav 74: 125–138, 2015. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG. Estrogen receptor α and G-protein coupled estrogen receptor 1 are localized to GABAergic neurons in the dorsal striatum. Neurosci Lett 622: 118–123, 2016. doi: 10.1016/j.neulet.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnauld E, Dufy B, Pestre M, Vincent JD. Effects of estrogens on the responses of caudate neurons to microiontophoretically applied dopamine. Neurosci Lett 21: 325–331, 1981. doi: 10.1016/0304-3940(81)90225-1. [DOI] [PubMed] [Google Scholar]
- Baufreton J, Atherton JF, Surmeier DJ, Bevan MD. Enhancement of excitatory synaptic integration by GABAergic inhibition in the subthalamic nucleus. J Neurosci 25: 8505–8517, 2005. doi: 10.1523/JNEUROSCI.1163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse 5: 157–164, 1990a. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett 118: 169–171, 1990b. doi: 10.1016/0304-3940(90)90618-J. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol 29: 36–47, 2008. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav 64: 53–57, 1999. doi: 10.1016/S0091-3057(99)00091-X. [DOI] [PubMed] [Google Scholar]
- Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav 27: 53–59, 1987. doi: 10.1016/0091-3057(87)90476-X. [DOI] [PubMed] [Google Scholar]
- Bédard PJ, Malouin F, Dipaolo T, Labrie F. Estradiol, TRH and striatal dopaminergic mechanisms. Prog Neuropsychopharmacol Biol Psychiatry 6: 555–561, 1982. doi: 10.1016/S0278-5846(82)80149-8. [DOI] [PubMed] [Google Scholar]
- Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol 84: 2204–2216, 2000. doi: 10.1152/jn.2000.84.5.2204. [DOI] [PubMed] [Google Scholar]
- Bossé R, DiPaolo T. The modulation of brain dopamine and GABAA receptors by estradiol: a clue for CNS changes occurring at menopause. Cell Mol Neurobiol 16: 199–212, 1996. doi: 10.1007/BF02088176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato A, Bregman D, Ahn HF, Pfau ML, Menard C, Cannizzaro C, Russo SJ, Hodes GE. Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience 350: 180–189, 2017. doi: 10.1016/j.neuroscience.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Lent K. Afferent input is necessary for seasonal growth and maintenance of adult avian song control circuits. J Neurosci 21: 2320–2329, 2001. doi: 10.1523/JNEUROSCI.21-07-02320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Lent K. Act locally and think globally: intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc Natl Acad Sci USA 99: 12421–12426, 2002. doi: 10.1073/pnas.192308799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han MH, Nestler EJ. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8: 13877, 2017. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callard GV, Petro Z, Ryan KJ. Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology 103: 2283–2290, 1978. doi: 10.1210/endo-103-6-2283. [DOI] [PubMed] [Google Scholar]
- Cao J, Dorris DM, Meitzen J. Neonatal masculinization blocks increased excitatory synaptic input in female rat nucleus accumbens core. Endocrinology 157: 3181–3196, 2016. doi: 10.1210/en.2016-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Dorris DM, Meitzen J. Electrophysiological properties of medium spiny neurons in the nucleus accumbens core of prepubertal male and female Drd1a-tdTomato line 6 BAC transgenic mice. J Neurophysiol 120: 1712–1727, 2018a. doi: 10.1152/jn.00257.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Willett JA, Dorris DM, Meitzen J. Sex differences in medium spiny neuron excitability and glutamatergic synaptic input: heterogeneity across striatal regions and evidence for estradiol-dependent sexual differentiation. Front Endocrinol (Lausanne) 9: 173, 2018b. doi: 10.3389/fendo.2018.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Jagannathan L, Jackson LR, Becker JB. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend 135: 22–28, 2014. doi: 10.1016/j.drugalcdep.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris DM, Cao J, Willett JA, Hauser CA, Meitzen J. Intrinsic excitability varies by sex in prepubertal striatal medium spiny neurons. J Neurophysiol 113: 720–729, 2015. doi: 10.1152/jn.00687.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euvrard C, Labrie F, Boissier JR. Effect of estrogen on changes in the activity of striatal cholinergic neurons induced by DA drugs. Brain Res 169: 215–220, 1979. doi: 10.1016/0006-8993(79)90392-5. [DOI] [PubMed] [Google Scholar]
- Farries MA, Meitzen J, Perkel DJ. Electrophysiological properties of neurons in the basal ganglia of the domestic chick: conservation and divergence in the evolution of the avian basal ganglia. J Neurophysiol 94: 454–467, 2005. doi: 10.1152/jn.00539.2004. [DOI] [PubMed] [Google Scholar]
- Farries MA, Perkel DJ. Electrophysiological properties of avian basal ganglia neurons recorded in vitro. J Neurophysiol 84: 2502–2513, 2000. doi: 10.1152/jn.2000.84.5.2502. [DOI] [PubMed] [Google Scholar]
- Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci 22: 3776–3787, 2002. doi: 10.1523/JNEUROSCI.22-09-03776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Woolley CS. Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol 518: 1330–1348, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc 39: 248–256, 2007. doi: 10.1249/01.mss.0000241649.15006.b8. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170: 1045–1055, 2010. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology 15: 97–111, 1990. doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse 59: 122–124, 2006. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74: 801–808, 2012. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem 80: 79–87, 2005. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Thibeault KC, Lopez AJ, Peck EG, Sands LP, Sanders CM, Kutlu MG, Calipari ES. Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology 44: 1189–1197, 2019. doi: 10.1038/s41386-019-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res 114: 152–157, 1976. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA, Fawcett CP. The specificity of the response of preoptic-septal area neurons to estrogen: 17α-estradiol versus 17β-estradiol and the response of extrahypothalamic neurons. Exp Brain Res 30: 43–52, 1977. doi: 10.1007/BF00237857. [DOI] [PubMed] [Google Scholar]
- Kent S, Hurd M, Satinoff E. Interactions between body temperature and wheel running over the estrous cycle in rats. Physiol Behav 49: 1079–1084, 1991. doi: 10.1016/0031-9384(91)90334-K. [DOI] [PubMed] [Google Scholar]
- Krentzel AA, Macedo-Lima M, Ikeda MZ, Remage-Healey L. A membrane G-protein coupled estrogen receptor is necessary but not sufficient for sex differences in zebra finch auditory coding. Endocrinology 159: 1360–1376, 2018. doi: 10.1210/en.2017-03102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentzel AA, Meitzen J. Biological sex, estradiol and striatal medium spiny neuron physiology: a mini-review. Front Cell Neurosci 12: 492, 2018. doi: 10.3389/fncel.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci 18: 1230–1232, 2015. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch ZS, Loh YE, Purushothaman I, Walker DM, Parise EM, Salery M, Cahill ME, Hodes GE, Pfau ML, Kronman H, Hamilton PJ, Issler O, Labonté B, Symonds AE, Zucker M, Zhang TY, Meaney MJ, Russo SJ, Shen L, Bagot RC, Nestler EJ. Estrogen receptor α drives pro-resilient transcription in mouse models of depression. Nat Commun 9: 1116, 2018. doi: 10.1038/s41467-018-03567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Reyna AM, Alejandro MA, Crowley J, Markaverich BM. Disruption of male sexual behavior in rats by tetrahydrofurandiols (THF-diols). Steroids 70: 750–754, 2005. doi: 10.1016/j.steroids.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav 74: 435–440, 2001. doi: 10.1016/S0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Markaverich B, Mani S, Alejandro MA, Mitchell A, Markaverich D, Brown T, Velez-Trippe C, Murchison C, O’Malley B, Faith R. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environ Health Perspect 110: 169–177, 2002. doi: 10.1289/ehp.02110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Pflepsen KR, Stern CM, Meisel RL, Mermelstein PG. Measurements of neuron soma size and density in rat dorsal striatum, nucleus accumbens core and nucleus accumbens shell: differences between striatal region and brain hemisphere, but not sex. Neurosci Lett 487: 177–181, 2011. doi: 10.1016/j.neulet.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Weaver AL, Brenowitz EA, Perkel DJ. Plastic and stable electrophysiological properties of adult avian forebrain song-control neurons across changing breeding conditions. J Neurosci 29: 6558–6567, 2009. doi: 10.1523/JNEUROSCI.5571-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16: 595–604, 1996. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA, Schlüter OM, Dong Y. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci 30: 3689–3699, 2010. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23: 185–215, 2000. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nillni YI, Toufexis DJ, Rohan KJ. Anxiety sensitivity, the menstrual cycle, and panic disorder: a putative neuroendocrine and psychological interaction. Clin Psychol Rev 31: 1183–1191, 2011. doi: 10.1016/j.cpr.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse 13: 135–160, 1993. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS. 17β-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci 36: 2677–2690, 2016. doi: 10.1523/JNEUROSCI.4437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Martinez LA, Meisel RL, Mermelstein PG. Estradiol impacts the endocannabinoid system in female rats to influence behavioral and structural responses to cocaine. Neuropharmacology 110: 118–124, 2016. doi: 10.1016/j.neuropharm.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct 220: 2415–2422, 2015. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT. The relevance of gender in Parkinson’s disease: a review. J Neurol 264: 1583–1607, 2017. doi: 10.1007/s00415-016-8384-9. [DOI] [PubMed] [Google Scholar]
- Proaño SB, Morris HJ, Kunz LM, Dorris DM, Meitzen J. Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. J Neurophysiol 120: 1356–1373, 2018. doi: 10.1152/jn.00263.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol 107: 1621–1631, 2012. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci 32: 8231–8241, 2012. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph LM, Cornil CA, Mittelman-Smith MA, Rainville JR, Remage-Healey L, Sinchak K, Micevych PE. Actions of steroids: new neurotransmitters. J Neurosci 36: 11449–11458, 2016. doi: 10.1523/JNEUROSCI.2473-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci 14: 609–625, 2013. [Erratum in Nat Rev Neurosci 14: 736, 2013.] doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-α in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci 29: 1897–1903, 2009. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev 68: 816–871, 2016. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Dahodwala N. Sex differences in Parkinson’s disease and other movement disorders. Exp Neurol 259: 44–56, 2014. doi: 10.1016/j.expneurol.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct 215: 187–194, 2011. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA 58: 1711–1718, 1967. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey EM, Arbuthnott GW, Fink G, Whale D. Oestradiol-17β increases the firing rate of antidromically identified neurones of the rat neostriatum. Neuroendocrinology 37: 106–110, 1983. doi: 10.1159/000123527. [DOI] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Tantucci M, Durante V, Quiroga-Varela A, Giampà C, Di Mauro M, Mazzocchetti P, Costa C, Di Filippo M, Grassi S, Pettorossi VE, Calabresi P. Endogenous 17β-estradiol is required for activity-dependent long-term potentiation in the striatum: interaction with the dopaminergic system. Front Cell Neurosci 9: 192, 2015. doi: 10.3389/fncel.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon Landeros R, Morisseau C, Yoo HJ, Fu SH, Hammock BD, Trainor BC. Corncob bedding alters the effects of estrogens on aggressive behavior and reduces estrogen receptor-α expression in the brain. Endocrinology 153: 949–953, 2012. doi: 10.1210/en.2011-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens MM, Bangasser DA, Briand LA. Sex differences in psychiatric disease: a focus on the glutamate system. Front Mol Neurosci 11: 197, 2018. doi: 10.3389/fnmol.2018.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett JA, Cao J, Dorris DM, Johnson AG, Ginnari LA, Meitzen J. Electrophysiological properties of medium spiny neuron subtypes in the caudate-putamen of prepubertal male and female Drd1a-tdTomato Line 6 BAC transgenic mice. eNeuro 6: ENEURO.0016-19.2019, 2019. doi: 10.1523/ENEURO.0016-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett JA, Will T, Hauser CA, Dorris DM, Cao J, Meitzen J. No evidence for sex differences in the electrophysiological properties and excitatory synaptic input onto nucleus accumbens shell medium spiny neurons. eNeuro 3: ENEURO.0147-15.2016, 2016. doi: 10.1523/ENEURO.0147-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, May RM, Woolley CS. Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct Funct 217: 181–190, 2012. doi: 10.1007/s00429-011-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology 61: 217–227, 2011. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JE, Cao J, Dorris DM, Meitzen J. Genetic sex and the volumes of the caudate-putamen, nucleus accumbens core and shell: original data and a review. Brain Struct Funct 221: 4257–4267, 2016. doi: 10.1007/s00429-015-1158-9. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett 180: 155–158, 1994. doi: 10.1016/0304-3940(94)90510-X. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, Becker JB. Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem 14: 83–89, 2014. doi: 10.2174/1871524914666141226103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, Becker JB. Oestradiol influences on dopamine release from the nucleus accumbens shell: sex differences and the role of selective oestradiol receptor subtypes. Br J Pharmacol. In press. doi: 10.1111/bph.14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoest KE, Quigley JA, Becker JB. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm Behav 104: 119–129, 2018. doi: 10.1016/j.yhbeh.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Dorsa DM. Estrogen rapidly induces c-jun immunoreactivity in rat striatum. Horm Behav 28: 376–382, 1994. doi: 10.1006/hbeh.1994.1034. [DOI] [PubMed] [Google Scholar]