Abstract
Subcallosal cingulate cortex deep brain stimulation (SCC-DBS) is an experimental therapy for treatment-resistant depression (TRD). Refinement and optimization of SCC-DBS will benefit from increased study of SCC electrophysiology in context of ongoing high-frequency SCC-DBS therapy. The study objective was a 7-mo observation of frequency-domain 1/f slope in off-stimulation local field potentials (SCC-LFPs) alongside standardized measurements of depression severity in 4 patients undergoing SCC-DBS. SCC was implanted bilaterally with a combined neurostimulation-LFP recording system. Following a 1-mo off-stimulation postoperative phase with multiple daily recordings, patients received bilateral SCC-DBS therapy (130 Hz, 90 μs) and weekly resting-state SCC-LFP recordings over a 6-mo treatment phase. 1/f slopes for each time point were estimated via linear regression of log-transformed Welch periodograms. General linear mixed-effects models were constructed to estimate pretreatment sources of 1/f slope variance, and 95% bootstrap confidence intervals were constructed to estimate treatment phase 1/f slope association with treatment response (50% decrease in preimplantation symptom severity). Results show the time of recording was a prominent source of pretreatment 1/f slope variance bilaterally, with increased 1/f slope magnitude observed during night hours (2300–0659). Increase in right 1/f slope was observed in the setting of treatment response, with bootstrap analysis supporting this observation in 3 of 4 subjects. We conclude that 1/f slope can be measured longitudinally in a combined SCC-DBS/LFP recording system and likely conforms to known 1/f circadian variability. The preliminary evidence of 1/f slope increase during treatment response suggests a potential utility as a candidate biomarker for ongoing development of adaptive TRD-neuromodulation strategies.
NEW & NOTEWORTHY In four patients with treatment-resistant depression undergoing therapeutic deep brain stimulation (DBS), we present the first longitudinal observations of local field potentials (LFP) from the subcallosal cingulate region outside the postoperative period. Specifically, our results demonstrate that frequency-domain 1/f activity is measurable in a combined DBS-LFP recording system and that right hemisphere recordings appear sensitive to mood state, thus suggesting a potential readout suitable for consideration in ongoing efforts to develop adaptive DBS delivery systems.
Keywords: 1/f, BA25, deep brain stimulation, depression, LFP, SCC
INTRODUCTION
Deep brain stimulation of the subcallosal cingulate cortex (SCC-DBS) is an experimental neuromodulation target for refractory major depressive disorder (MDD). In contrast to extensive characterization of chronic DBS effects via PET imaging (Lozano et al. 2008; Martín-Blanco et al. 2015 Mayberg et al. 2005) and recent EEG findings (Broadway et al. 2012; Hilimire et al. 2015; Waters et al. 2018), direct electrophysiological characterization of local SCC activity changes are relatively unexplored beyond limited intra- and perioperative basal state or acute stimulation studies (Clark et al. 2016; Neumann et al. 2014; Smart et al. 2018). Such longitudinal measurements might contribute to the systematic development of a biological framework to assess the strengths and limitations of SCC-DBS treatment (Holtzheimer et al. 2017) and potentially provide clinicians with a biomarker for quantitative monitoring of SCC-DBS efficacy and delivery of adaptive stimulation in individual patients.
Toward development of such a biomarker is a known unifying feature of electrical brain activity across regions and recording scales, the exponential decrease of signal power spectral density (PSD) with frequency, i.e., 1/f activity (El Boustani et al. 2009; He et al. 2010; Miller et al. 2009). Potential sources of neurophysiological 1/f activity are numerous, ranging from a passive filtering property of the extracellular environment (Bédard et al. 2004; Bédard and Destexhe 2009; Gabriel et al. 1996; Halnes et al. 2016; Miceli et al. 2017) to activity-dependent dendritic filtering (Pettersen et al. 2014) and the excitation-inhibition balance of neural networks (Freeman and Zhai 2009; Gao et al. 2017; Voytek and Knight 2015). The latter two properties imply 1/f activity is responsive to neuronal inputs and suggest 1/f activity is sensitive to experimental manipulation. Indeed, 1/f changes are observable between sleeping and waking states and during phases of psychomotor activity (El Boustani et al. 2009; Freeman and Zhai 2009; Podvalny et al. 2015; Sheehan et al. 2018; Voytek et al. 2015). These measurements are frequently conducted through measurement of the slope of the log-transformed PSD (hereafter termed the “1/f slope”). Taken as a putative correlate of excitation-inhibition (E:I) balance, 1/f slope may serve an additional purpose as a field potential metric feasible to compare alongside studies of smaller neuronal populations (Gao et al. 2017), some of which concern preclinical models of SCC highly relevant to mood disorder research (e.g., Ferenczi et al. 2016).
In this observational study of SCC-DBS patients receiving therapeutic DBS with concurrent bilateral local field potential recording (SCC-LFP), we addressed two aims: 1) to explore sources of variance of SCC-LFP 1/f slope magnitude in pre-treatment recordings and 2) to determine if SCC-LFP 1/f slope changes track with changes in depression severity scores measured weekly over 6 mo of SCC-DBS treatment.
METHODS
Subject recruitment.
Six subjects with treatment-resistant major depressive disorder were consecutively enrolled in a study of the safety and efficacy of SCC-DBS for treatment-resistant depression (ClinicalTrials.gov Identifier NCT01984710). The latter four subjects participated in weekly laboratory SCC-LFP recording sessions, with demographic information displayed in Table 1. Inclusion and exclusion criteria are identical to those described in Riva-Posse et al. (2018). All patients provided written informed consent to participate in the study. The protocol was approved by the Emory University Institutional Review Board and the US Food and Drug Administration under a physician-sponsored Investigational Device Exemption (IDE G130107) and is monitored by the Emory University Department of Psychiatry and Behavioral Sciences Data and Safety Monitoring Board.
Table 1.
Subject characteristics
| Subject | Sex | Age at Surgery, yr | Age of MDD Onset, yr | Duration of Current Episode, mo | Lifetime No. of MDD Episodes | Current No. of Medications |
Baseline MADRS Score |
|---|---|---|---|---|---|---|---|
| 1 | F | 66 | 36 | 36 | 4 | 13 | 34.5 |
| 2 | M | 60 | 48 | 132 | 2 | 9 | 27.2 |
| 3 | F | 58 | 16 | 24 | 4 | 8 | 33 |
| 4 | M | 53 | 28 | 24 | 3 | 7 | 27.5 |
Four subjects (2 men) with treatment-resistant major depressive disorder (MDD) participated in a 7-mo study of subcallosal cingulate cortex (SCC) deep brain stimulation with concurrent ambulatory and laboratory SCC-local field potential recording. F, female; M, male; MADRS, Montgomery-Åsburg Depression Rating Scale.
Implantation procedures.
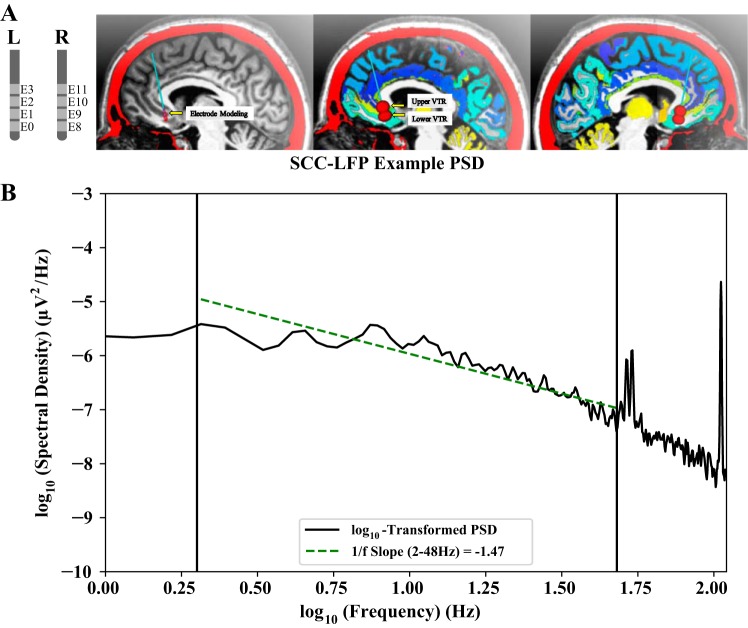
Subjects were implanted with two DBS leads, one in each SCC, and an Activa PC+S pulse generator and recorder (Medtronic, Minneapolis, MN), which combines DBS delivery with the collection of time-stamped LFP recordings during laboratory visits and at preset times outside the laboratory (ambulatory recordings). The model 3387 electrode (E) arrays (Medtronic) were implanted using both MRI and tractography guidance to minimize between-subject spatial variance of electrode placement (Fig. 1A) (Riva-Posse et al. 2018).
Fig. 1.
A: in all subjects, the subcallosal cingulate cortex (SCC) was implanted bilaterally with Medtronic model 3387 electrodes (E) connected to the Activa PC+S combined neural stimulator and local field potential (LFP) recorder with a differential “bipolar” recording configuration (red spheres). Estimated volumes of tissue recorded [VTR; see Lempka and McIntyre (2013) for justification of VTR size] include surrounding anatomical gray matter regions. B: off-stimulation SCC-LFP 1/f slope (green dashed line) was estimated from the log-transformed Welch power spectral density (PSD) over 2–48 Hz [frequency range indicated by vertical lines at log10(2) and log10(48)]. Notably, this range omits peaks present in PC+S saline bath recordings (provided by Medtronic in a personal communication), as well as the low-frequency attenuation from a hardware 0.5-Hz high-pass filter.
Study phases.
Following implantation, stimulation remained off for a 1-mo period while ambulatory SCC-LFPs were recorded (pretreatment phase). One month following implantation, subjects began a 6-mo high-frequency SCC-DBS treatment phase. DBS therapy began with bilateral monopolar stimulation on a single contact per hemisphere at a voltage of 3.5 V in all subjects (except subject 1, who started at 3.0 V). Frequency and pulse width remained constant throughout the study at 130 Hz and 90 μs, respectively. Following a predefined protocol, increases in voltage were made in 0.5-V increments no more frequently than every 4 wk (Holtzheimer et al. 2012).
During the treatment phase, subjects attended weekly laboratory visits to receive Montgomery-Åsburg Depression Rating Scale (MADRS) scores, and stimulation was temporarily interrupted while LFPs were recorded simultaneously from the right and left DBS leads. For the purpose of correlations with depression severity ratings, subjects were considered treatment responders if weekly MADRS scores were ≤50% of pretreatment baseline (Table 1). Three of the four patients (subjects 2–4) responded to treatment by the conclusion of the 6-mo treatment phase.
SCC-LFP recording.
All SCC-LFPs were recorded at a 422-Hz sampling rate from two contacts flanking the stimulus electrode, with the difference calculated between these two contacts to reduce common-mode noise (i.e., a differential recording configuration; see Fig. 1A) and underwent hardware 0.5-Hz high-pass filtering and 100-Hz low-pass filtering (Stanslaski et al. 2012). On-stimulation recordings were omitted from this observational study, because stimulation artifact appears to include a combination of narrowband and broadband artifacts (Supplemental Fig. S1; https://figshare.com/s/44de58e10f2fb519cdca). Recordings were not obtained using the PC+S Nexus-D system. During the off-stimulation pretreatment phase, bilateral time-stamped 20-s ambulatory recordings were obtained with data compression enabled, which increases the roughly 15-min of available SCC-LFP storage to ~30 min (as discussed in Swann et al. 2018a). The recording schedule was set at each laboratory visit to optimize ~30 min of recording space (more than 12 recordings per day, on average). Treatment phase laboratory recordings were obtained weekly in a single 15-min session with stimulation turned off for the final 7.5 min. Data compression was not utilized in these treatment phase laboratory recordings. Table 2 outlines each study phase and the number of available bilateral recordings (e.g., 566 left and 566 right hemisphere pretreatment off-stimulation SCC-LFP recordings were obtained from subject 1).
Table 2.
SCC-LFP recording configurations and number of recordings
| Subject | Pretreatment Phase | Treatment Phase |
||
|---|---|---|---|---|
| Response | Nonresponse | Total | ||
| 1 (L: E1,E3; R: E8,E10) | 566 | 12 | 8 | 20 |
| 2 (L: E1,E3; R: E9,E11) | 487 | 13 | 6 | 19 |
| 3 (L: E0,E2; R: E8,E10) | 374 | 10 | 12 | 22 |
| 4 (L: E1,E3; R: E8,E10) | 443 | 12 | 10 | 22 |
Differential recording electrode (E) configurations in left (L) and (R) hemisphere (see Fig. 1A) and number of available bilateral subcallosal cingulate cortex local field potential (SCC-LFP) recordings in each study phase. Pretreatment phase ambulatory recordings were collected during preset times assigned at each laboratory visit, and treatment phase laboratory recordings were collected during weekly clinical visits. Recordings obtained during weeks when weekly MADRS scores were 50% or less than preimplantation baseline scores (Table 1) were classified as response recordings.
SCC-LFP preprocessing.
Due to amplifier settling, the first 5 s were omitted from each pretreatment ambulatory recording. Welch’s method (parameters: 50% overlapping, 2-s Hanning-windowed segments) was used to estimate the PSD from the available 15-s segment in each left and right SCC-LFP recording. 1/f slope was estimated from the log-transformed PSD using simple linear regression (SLR), with the β1 term indicating the 1/f slope magnitude.
Measurement of 1/f slope requires selection of a frequency range ideally free of artifact and/or oscillatory activity. We selected an optimal 1/f slope frequency range by implementing SLR at various frequency ranges on each pretreatment SCC-LFP PSD and extracting the median R2 value (Fig. 1B). Median R2 value was highest for 2–48 Hz, and this range was selected to calculate SCC-LFP 1/f slope in further analysis.
Resting-state laboratory SCC-LFP 1/f slope estimation.
To calculate 1/f slope for each treatment phase 15-min laboratory recording’s 7.5-min off-stimulation segment, we utilized a 15-s sliding window PSD estimation approach (with 7.5-s overlap) to generate a distribution of within-recording 1/f slope magnitudes in each recording hemisphere. The within-recording distribution median was selected as the representative laboratory recording 1/f slope.
Statistical analysis.
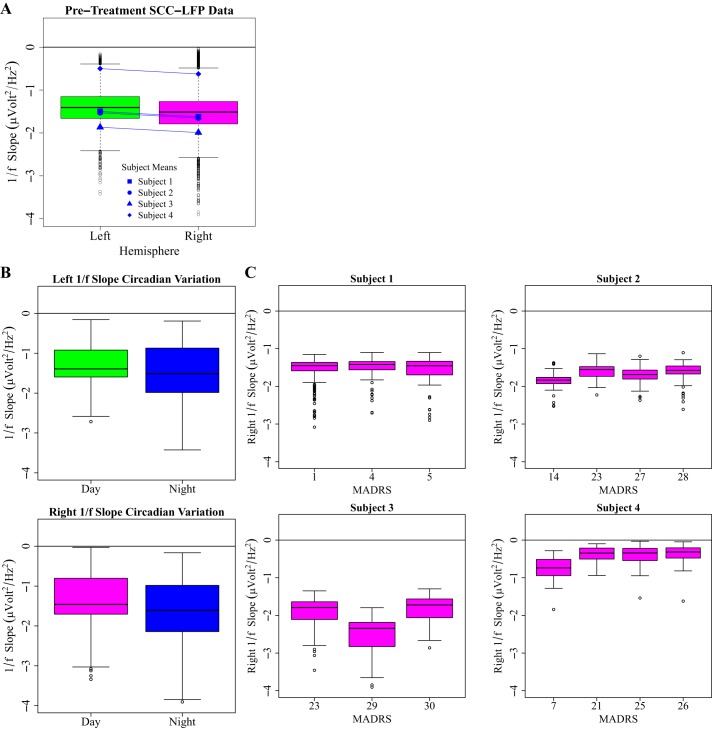
The left- and right-sided recordings were analyzed separately. The investigation into recording hemisphere was not a prespecified hypothesis in this study. During the pretreatment phase, we derived boxplots of 1/f slope magnitude to describe the distribution and range of pretreatment 1/f slope magnitude between hemispheres, as well as between putative day hours (0700–2259) and putative night hours (2300–0659) and by weekly pretreatment MADRS score. To explore sources of pretreatment 1/f slope variance, we constructed random-intercept mixed models comparing 1/f slope across mood and hour of recording [1/fSlope ~Phase (wk) + MoodRating + HourRecording + MoodRating*Phase + HourRecording*Phase, random = 1/subject], and given the known 1/f variance with sleep state (Bédard and Destexhe 2009; Freeman and Zhai 2009), we constructed random-intercept mixed models comparing 1/f slope between putative day (0700h–2259) and night (2300h–0659) hours [1/fSlope ~DayStatus + Phase (wk) + DayStatus *Phase, random = 1/subject]. Random-intercept mixed models were selected because of the substantial differences in recorded 1/f slope values depending on the subject (Fig. 2A).
Fig. 2.
Boxplot descriptions of pre-treatment sources of 1/f slope variation, with negativity on the y-axis (increasing 1/f slope magnitude) indicating 1/f slope steepness. A: pretreatment slopes for all subjects (n = 4), left and right hemisphere, indicating consistently negative 1/f slope values. B: cohort-level pretreatment 1/f slope magnitude appears to be increased during night hours (2300–0659), as suggested by generalized linear mixed-effect models (DF = 1,1220, left 1/f slope: F = 111.98, P < 0.0001, right 1/f Slope: F = 180.34, P < 0.0001). C: pretreatment within-subject right 1/f slope by pretreatment Montgomery-Åsburg Depression Rating Scale (MADRS) score.
During the treatment phase, we generated time-activity curves for responders and the nonresponder case to describe 1/f slope and MADRS behavior over the treatment phase. To describe observations between a binary framework of treatment response (MADRS score 50% or lower than preimplantation baseline), mean PSDs and mean 1/f slope was plotted for each subject and hemisphere. To examine possible 1/f slope association with depressive symptoms with treatment response, bootstrap resampling with replacement was performed within each subject’s response and nonresponse periods, encompassing a total of 1,000 iterations per response status per subject. Utilizing this resampling method allowed us to generate a treatment phase-size data set (Table 2) for each iteration from the individual treatment phase data points. These data sets were then passed into an algorithm to compute the median within each response status and then subtract the two distributions. The final bootstrapped data reflected a 1,000 × n matrix of median response changes. Column mean and 25th lowest/975th highest values were extracted to estimate the within-subject 95% bootstrap confidence interval, and means were calculated, by row, to generate a 1,000 row × 1 data set to estimate the cohort response difference between the two response conditions. To explore possible changes in single canonical oscillatory bands, we repeated the previous cohort-level bootstrap analyses with consideration of absolute power in delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz) and gamma (30–48 Hz) frequency.
In addition to the above analysis of 1/f using a binary framework of treatment response vs. treatment nonresponse, we generated within-subject scatterplots to examine the degree to which 1/f slope exhibits a continuous correlation with MADRS score. 1/f slopes were %baseline corrected to the first treatment phase recording, and MADRS scores were %baseline corrected to a preimplantation average score. To estimate β1 and R2 for each scatterplot, we resample with replacement 1,000 times the residuals of a simple linear regression on the original within-subject data set and construct 95% bootstrap confidence intervals of β1 and R2 by reporting the 25th lowest and 975th highest values.
RESULTS
Subject response to SCC-DBS therapy.
Three of the four subjects met the predefined response criteria at the end of the 6-mo treatment phase. Each treatment responder completed the treatment phase with SCC-DBS voltage of 4.0 V. After the study concluded, subject 1 became a responder following changes in medication and stimulation dose.
SCC-LFP 1/f slope estimation.
The observable decrease in off-stimulation SCC-LFP spectral density with increasing frequency confirms the presence of neurophysiological 1/f activity expected in neural recordings (Fig. 1). The SCC-LFP PSD is notable for high-frequency peaks present in saline bath PC+S recordings (provided in a personal communication from Medtronic), which are likely nonphysiological artifacts generated from a sampling rate-dependent internal clock. As expected, the R2-optimized range of 2–48 Hz appears to avoid the noted PC+S artifacts (Fig. 1). Within the 2- to 48-Hz range, pretreatment 1/f slope values were consistently negative in all subjects in each recording hemisphere (Fig. 2).
Potential sources of pre-treatment SCC-LFP 1/f slope variation.
Random-intercept mixed models suggest a 1/f slope association with hour of recording [degrees of freedom (df) (numerator, denominator) = 1,1116, left 1/f slope: F = 120.060, P < 0.0001, right 1/f slope: F = 193.12, P < 0.0001] and weekly recording session (phase) (df = 1,1116, left 1/f slope: F = 39.35, P < 0.0001, right 1/f slope: F = 51.027, P < 0.0001) with likely little interaction (df = 1,1116, left 1/f slope: F = 0.55, P = 0.45, right 1/f slope: F = 0.38, P = 0.54). Mixed models further suggest a potential circadian source of pretreatment 1/f slope variation (Fig. 2B; df = 1,1120, left 1/f slope: F = 111.98, P < 0.0001, right 1/f slope: F = 180.34, P < 0.0001) with likely little interaction with week of recording (df = 1,1120, left 1/f slope: F = 2.42, P = 0.12, right 1/f slope: F = 0.071, P = 0.79). Pretreatment mixed models additionally suggest pretreatment 1/f slope magnitude is associated with symptom severity (Fig. 2C; df = 1,1116, left 1/f slope: F = 5.93, P = 0.015, right 1/f slope: F = 21.52, P < 0.0001), although terms conveying the interaction of recording week with MADRS score appear relatively prominent in right hemisphere recordings (df = 1,1116, left 1/f slope: F = 0.0036, P = 0.95, right 1/f slope: F = 11.69, P = 0.0007).
SCC-LFP 1/f slope observations over the SCC-DBS treatment phase.
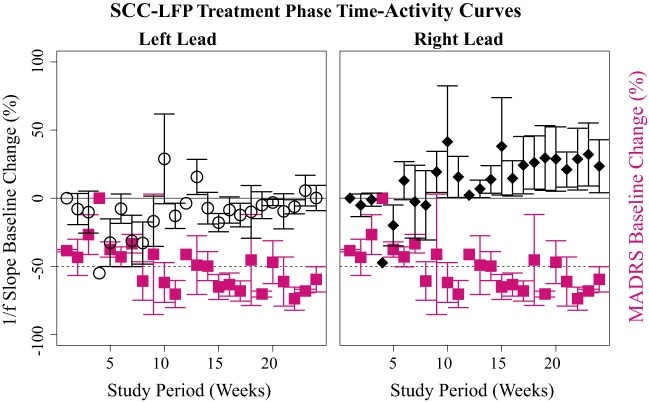
We constructed time-activity curves describing changes in mean weekly 1/f slope and MADRS values over the 6-mo treatment phase in eventual treatment responders (n = 3; Fig. 3). 1/f slope data are baseline-corrected to the first treatment phase laboratory recording, and MADRS to the preimplantation baseline. Each responder entered the treatment response range (MADRS ≤50% of preimplantation baseline MADRS) at different weeks but met response criteria near the end of the treatment phase. Notably, left- and right-lead data appear to exhibit different time activity curves, with right 1/f slope appearing to increase in magnitude as MADRS scores enter the response range (Fig. 3).
Fig. 3.
Trajectory of %baseline-corrected mean weekly left and right off-stimulation subcallosal cingulate cortex local field potential (SCC-LFP) 1/f slope (left hemisphere, open circles; right hemisphere, black diamonds) and Montgomery-Åsburg Depression Rating Scale (MADRS; magenta squares) in 6-mo treatment responders (n = 3 subjects). MADRS scores below the dashed line (−50 on y-axis) indicate a 50% baseline decrease indicative of treatment response. Error bars indicate SE. 1/f slopes were corrected to treatment phase week 1, and MADRS data to preimplantation scores (Table 1).
SCC-LFP 1/f slope between treatment response states.
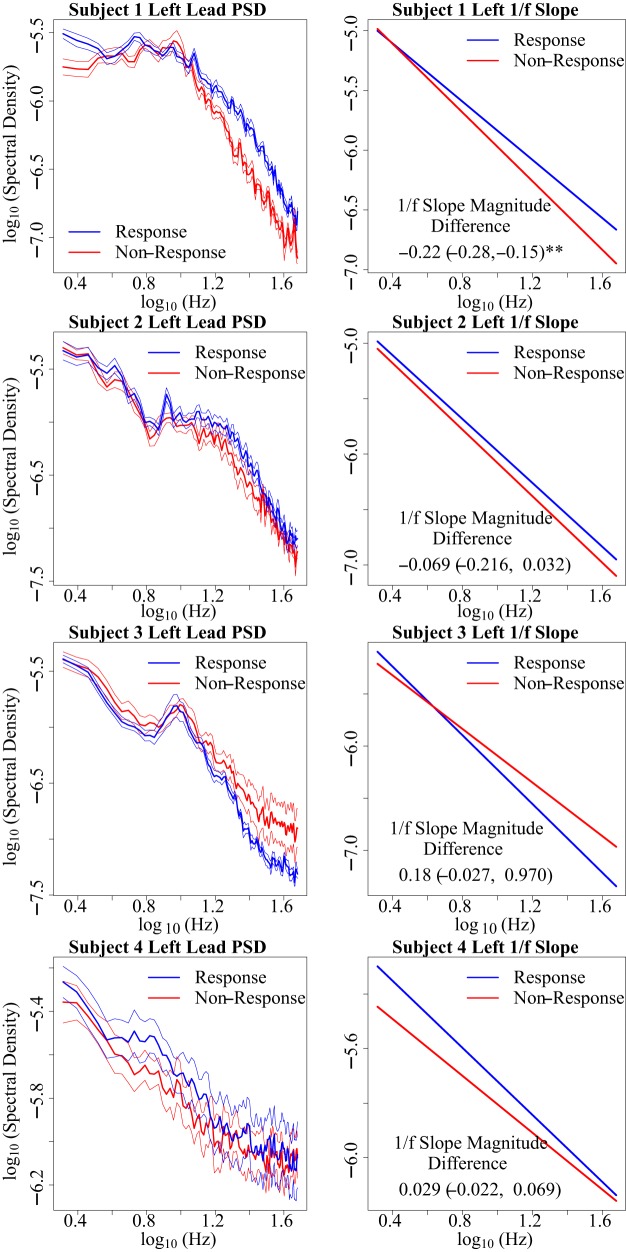
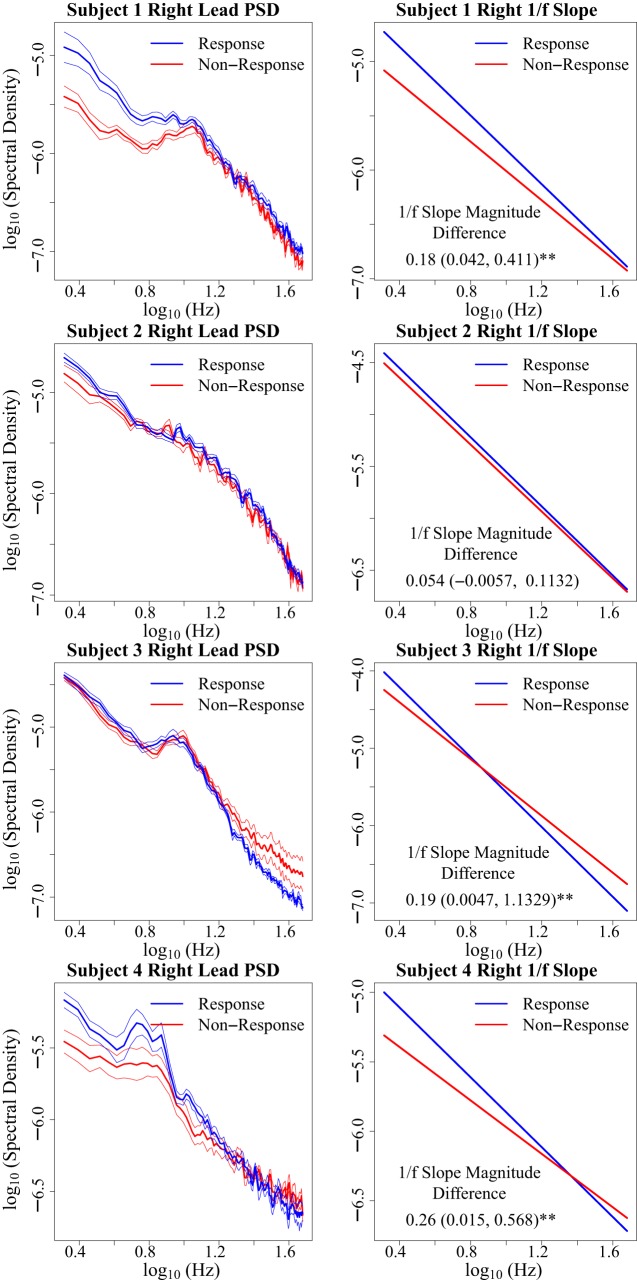
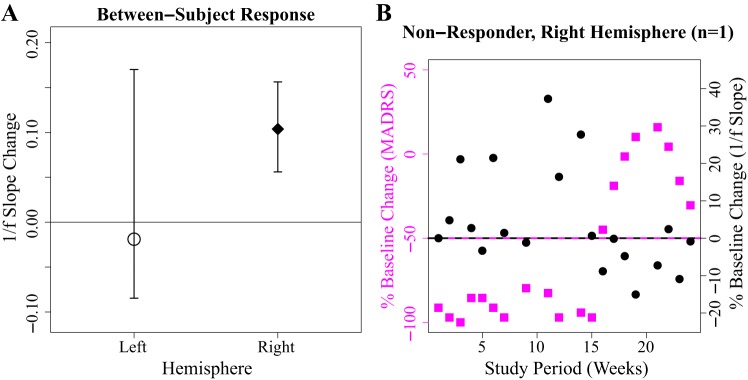
Observations of 1/f slope across treatment response states in left hemisphere (Fig. 4) and right hemisphere (Fig. 5) demonstrate that right l/f slope magnitude appears to be increased in the setting of treatment response [bootstrap 95% confidence interval: 0.17 ± (0.061, 0.44)*] (Fig. 6A). (Asterisks indicate a bootstrap 95% confidence interval that does not include zero.) This observed relationship of right 1/f slope magnitude to treatment response state appears to be absent in left 1/f slope recordings [−0.019 ± (−0.084, 0.17)] (Fig. 6A). Bootstrap analysis of putative right-hemisphere narrowband changes conveys decreases in absolute power changes from lower to higher frequency canonical bands [delta: 1.95e-5 (3.039e-6, 4.15e-5)*, theta: 9.82e-6 (3.62e-6, 1.76e-5)*, alpha: 1.17e-6 (−5.77e-6, 7.69e-6), beta: 1.91e-6 (−7.85e-6, 6.26e-6), gamma: −1.31e-6 (−1.20e-5, 9.12e-7)], which suggests the right-hemisphere response vs. nonresponse observation demonstrates an increase in the broadband 1/f slope magnitude, although does not rule out the possibility of dual delta-theta changes contributing to the observed response vs. nonresponse changes. As with 1/f slope, putative narrowband changes appear absent in the left hemisphere [delta: 1.66e-6 (−2.49e-6, 5.55e-6), theta: 4.20e-6 (−1.00e-6, 9.018e-6), alpha: 2.23e-6 (−2.72e-6, 6.69e-6), beta: 1.10e-5 (−1.093e-6, 2.16e-5), gamma: 4.68e-6 (−6.20e-5, 1.40e-5)]. On an individual level, we observe that right 1/f slope appears increased in the setting of treatment response in three of four subjects [subject 1: 0.18 (0.042, 0.41)*, subject 2: 0.054 (−0.0056, 0.11), subject 3: 0.19 (0.0047, 1.13)*, subject 4: 0.26 (0.015, 0.57)*]. The right-hemisphere SCC-LFP pattern across treatment response conditions is observed across two of the three responders who showed a typical response trajectory (Fig. 3), as well as subject 1, who showed an early but unstable response over the 6 mo of observation with active treatment (Fig. 6B). Although MADRS score was designed to track changes in depression symptom severity (Montgomery and Åsberg 1979), we also explored whether the 1/f slope findings are consistent across other scales such as the 17-item Hamilton Depression Rating Scale (HDRS-17; Hamilton. 1986), which assesses similar but not identical symptom categories (Carmody et al. 2006). We report that HDRS-17 classified response vs. nonresponse slope difference yields a bootstrap 95% confidence interval that includes zero [right: 0.088 ± (−0.0015, 0.36)], suggesting that a mood state change is likely not observable using the HDRS-17.
Fig. 4.
Subject-wise description of treatment phase left-hemisphere off-stimulation subcallosal cingulate cortex local field potential (SCC-LFP) 1/f slope between treatment response states. Within-subject treatment response vs. non-response 1/f slope changes are described using bootstrap 95% confidence intervals (see methods). Asterisks indicate bootstrap 95% confidence intervals that do not include 0. Mean power spectral density (PSD) and 1/f slope plots demonstrate inconsistent left-hemisphere SCC-LFP 1/f slope magnitude differences between subjects. PSD error bars indicate the within-frequency SE.
Fig. 5.
Subject-wise description of treatment phase right-hemisphere off-stimulation subcallosal cingulate cortex local field potential (SCC-LFP) 1/f slope between treatment response states. Within-subject treatment response vs. non-response 1/f slope changes are described using bootstrap 95% confidence intervals (see methods). Asterisks indicate bootstrap 95% confidence intervals that do not include 0. Mean power spectral density (PSD) and 1/f slope plots demonstrate increased right-hemisphere SCC-LFP 1/f slope magnitudes in recordings obtained during periods of treatment response (blue) in 3 of 4 subjects. Increased 1/f slope magnitude is qualitatively observable as a steeper 1/f slope. PSD error bars indicate the within-frequency SE.
Fig. 6.
Right subcallosal cingulate cortex local field potential (SCC-LFP) 1/f slope magnitude is observed to be increased during weeks where Montgomery-Åsburg Depression Rating Scale (MADRS) scores meet treatment response criteria. A: cohort (n = 4 subjects) bootstrap 95% confidence intervals estimate an observed mean increase of right-hemisphere off-stimulation SCC-LFP 1/f slope of 0.17. Horizontal line indicates response vs. nonresponse 1/f slope change of 0. B: time-activity curve of subject 1 weekly %baseline-corrected right-hemisphere off-stimulation SCC-LFP 1/f slope (black circles) and MADRS (magenta squares). MADRS %change of −50 or greater indicates the treatment response threshold. Right 1/f slope association with treatment response (Fig. 3) appears maintained in subject 1 in setting of unstable response trajectory.
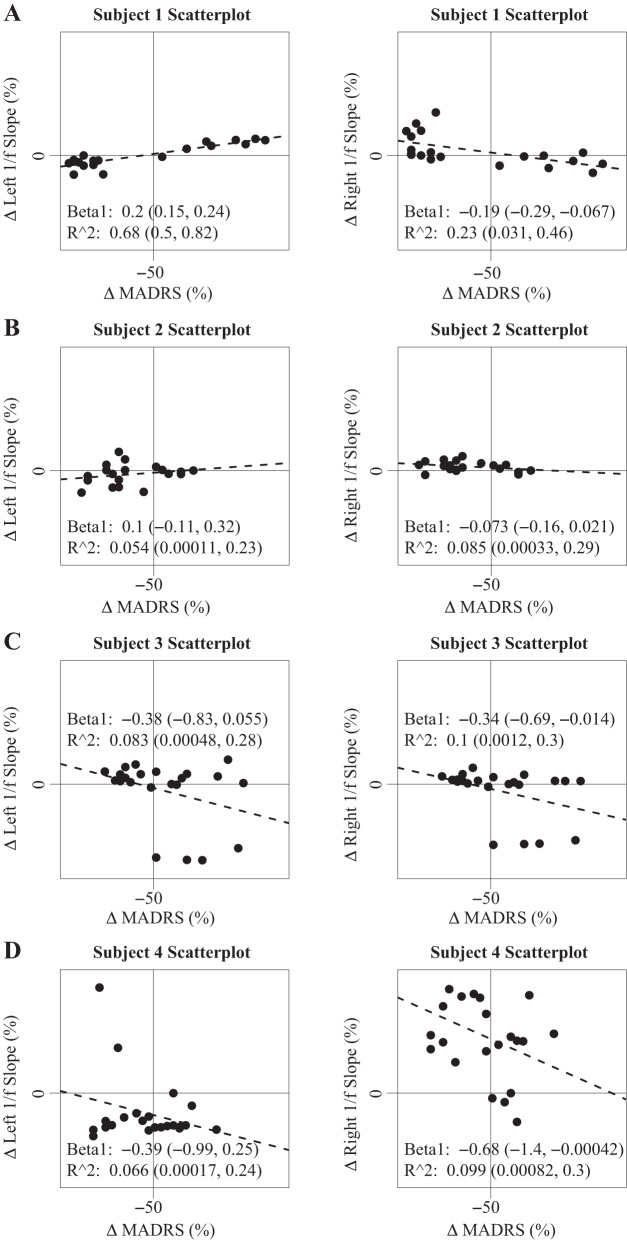
Within-subject scatterplots (Fig. 7) describe negative β1 terms in right-hemisphere recordings in three of four subjects, which matches the pattern observed in bootstrap estimates of response vs. non-response difference, where right SCC-LFP 1/f slope appears to increase during response periods in the same three subjects. A positive β1 correlation is observed in one of four subjects (subject 1) in left-hemisphere recordings. Bilaterally, we observe consistently low R2 estimates (each under 0.4).
Fig. 7.
Within-subject scatterplot comparisons of baseline-corrected 1/f slope and Montgomery-Åsburg Depression Rating Scale (MADRS) scores. Depression severity increases along the x-axis and 1/f slope magnitude increases along the y-axis (Δ, change in values). Vertical line indicates a 50% decline in MADRS, which defines the point of treatment response. Matching the pattern observed following a comparison of 1/f slope values between response and nonresponse states (Fig. 5), right 1/f slope magnitude increase is observed in 2 of 3 responders as well as in a nonresponder case (subject 1), as indicated by bootstrap 95% confidence intervals of the β1 term. R2 values are consistently low in each case.
DISCUSSION
Pretreatment sources of 1/f slope variance.
Mixed-model results suggest that the largest source of pretreatment 1/f slope variance is likely the hour of SCC-LFP recording. To a lesser extent, the pretreatment 1/f slope appears to vary by MADRS score, which appears to show an increase in 1/f slope magnitude in the setting of decreased symptom severity (Fig. 2C), although the notable recording session interaction terms raise concerns that electrode contact impedance fluctuations, recorded weekly, could potentially obscure these findings (Kent and Grill 2014). Mixed models assessing the possibility of a circadian source of pretreatment 1/f slope variation suggest an internal validation of known 1/f sleep-wake trends previously observed in preclinical and epilepsy studies (Bédard and Destexhe 2009; Freeman and Zhai 2009), which supports the capability of the PC+S to detect frequency-domain physiological changes from SCC white matter regions. These observations further suggest that future studies will likely need to control for the time of recording, and/or employ sleep diaries, when attempting to correlate ambulatory recordings with symptoms.
SCC-LFP 1/f slope potentially responds to mood state.
Right-hemisphere SCC-LFP 1/f slope measured in the present study appears to track the contemporaneous depression severity state during SCC-DBS treatment in two treatment responders and also a nonresponder case (3 of 4 subjects). Interestingly, although SCC-DBS treatment is delivered bilaterally, SCC-LFP 1/f slope treatment response state differences are confined to the right hemisphere, with increased right 1/f slope magnitude, as appears to be observed in the pretreatment phase, being associated with decreased depression symptom severity scores comprising treatment response (Fig. 2B, Fig. 6). We further note data from subject 1, where the same right 1/f slope association was observed despite an apparently reversed response trajectory (Fig. 6B). Data from this nonresponder case support that the association between increased right 1/f slope magnitude and decreased symptom severity scores might be time independent and further suggest a case example of SCC-LFP potentially detecting a relapse of MDD symptoms from a state of disease remission. To consider whether the right 1/f slope observations might function better as a detector of wellness or of depressive illness, we retrospectively derived within-subject sensitivity and specificity values using the following putative test: baseline %right 1/f slope magnitude change >0 corresponds with a %MADRS change of −50 or greater (true response); baseline %right 1/f slope magnitude change <0 corresponds with a %MADRS decrease under −50 (i.e., a true nonresponse). From the above pseudoanalysis, we note the following sensitivity and specificity values: subject 1, sensitivity = 0.82, specificity = 0.88; subject 2, sensitivity = 0.85, specificity = 0.40; subject 3, sensitivity = 0.90, specificity = 0.45; and subject 4, sensitivity = 1.00, specificity = 0.33. As supported by scatterplots (Fig. 7), relatively low false negative values (right 1/f slope flattening in context of treatment response) are observed, which suggests that right 1/f slope could function more accurately in detecting relapse (as occurred with subject 1) than in detecting treatment response.
Notably, asymmetric changes in the SCC were previously demonstrated using PET measures of regional blood flow with chronic SCC-DBS (Mayberg et al. 2005). Furthermore, double-blinded intraoperative studies of unilateral SCC-DBS demonstrated lateralized behavioral responses to left- vs. right-sided acute stimulation (Choi et al. 2015), and the distribution of extraoperative 2-Hz DBS-evoked potential components appear sensitive to the hemisphere of stimulation (Waters et al. 2018). These findings lend support for theories of inherent physiological left-right asymmetries previously proposed in various studies of depression and its treatment (as reviewed by Bruder et al. 2017; Hagemann 2004). However, our results suggest increased asymmetry during periods of treatment response (Fig. 3), which are difficult to reconcile with the frontal asymmetry hypothesis, which describes increased frontal alpha-wave asymmetry as a potential mediator of depression susceptibility (Hagemann 2004).
Although response vs. nonresponses changes were not observable at the cohort level with the HDRS-17 scale, we note that although MADRS and HDRS-17 are highly correlated, the scales do not entirely overlap in the types of measured symptom subgroups or in the relative weighting of subgroups (Carmody et al. 2006). Thus the difference in 1/f slope observations across depression scales could be secondary to the 1/f slope tracking certain clinical features of MDD more strongly than others.
Potential sources of apparent SCC-LFP 1/f activity.
Although 1/f activity is currently recognized as a dominant feature of bioelectrical fields across space and scale of recording (El Boustani et al. 2009; He et al. 2010; Miller et al. 2009) and is argued as an E:I correlate (Chaudhuri et al. 2017; Freeman and Zhai 2009; Gao et al. 2017; Voytek and Knight 2015), it is worth noting that the origin of measurable LFP from macroelectrodes and the origin of LFP 1/f activity remain a topic of debate. Because 1/f slope measurements are derived from the PSD, methods are currently under development to best resolve putative oscillations from broadband electrophysiological features such as 1/f slope (Haller et al. 2018; Podvalny et al. 2015). Although bootstrap analyses in this study suggest that right-hemisphere 1/f slope observations are not well explained by a single narrowband change, our observations do not definitively rule out, or rule in, the possibility of concurrent narrowband (Smart et al. 2015) or narrowband plus broadband field potential changes (Podvalny et al. 2015), which could underlie the response vs. nonresponse observations. However, describing field potential changes within the context of a single feature, 1/f slope, may be a more parsimonious and potentially more physiologically grounded way of conveying the results (Haller et al. 2018).
Considering the macroelectrode design of the PC+S, it is also reasonable to propose that contributing SCC-LFP sources, with dependence on the coherence of synaptic inputs, could occur as far as several millimeters away from each recording electrode (Lempka and McIntyre 2013). A spatial reach of this order suggests that whereas SCC-DBS electrodes are implanted in white matter, SCC-LFP recordings are composed of gray matter sources that could include regions outside the SCC (Fig. 1A). As examples, when utilizing FreeSurfer software (Fischl 2012) on postoperative computed tomography images, we observed that cortical regions within 7.5-mm of each PC+S recording electrode potentially include the SCC [Brodmann area 25 (BA25)], anterior cingulate cortex (ACC; BA24), and suborbitofrontal cortex (BA32) (data not shown). Although it remains unclear whether subject-specific electrode implantation trajectories affect the precision of recording from these regions, recent work to resolve white matter networks recruited by DBS (Lujan et al. 2013; Riva-Posse et al. 2014) and forward-modeling studies to quantify the degree of white matter activation (Howell et al. 2019) provide the framework to systematically target and test the contributions of regions of interest through the DBS setting programmer.
Although observable 1/f activity within a given recording could also arise from several sources unrelated to neural activity (Bédard et al. 2004; Bédard and Destexhe 2009; Gabriel et al. 1996; Halnes et al. 2016; Miceli et al. 2017), observable 1/f slope changes between experimental conditions are currently thought to reflect changes in the E:I balance of contributing neural sources (Chaudhuri et al. 2017; Freeman and Zhai 2009; Gao et al. 2017; Voytek and Knight 2015). As an example, increases in 1/f slope magnitude during sleep were previously modeled as neurons driven toward a refractory state (a lower E:I ratio; Freeman and Zhai 2009; Voytek and Knight 2015). Additionally, several recent studies have shown large age-related changes in 1/f slope such that older adults have flatter spectra (i.e., lower 1/f slope magnitude) than younger adults. Within the E:I framework, these results suggest that older adults are generally in an E > I state, a “noisier” tonic state, and that these 1/f changes mediate age-related cognitive decline (Dave et al. 2018; Voytek et al. 2015; Waschke et al. 2017).
That SCC-LFP 1/f slope may serve as a field potential correlate of E:I balance (a metric derived at the scale of small neuronal populations; Gao et al. 2017) is important given E:I balance is a preclinical measure of interest in the pathophysiology of anhedonia (Ferenczi et al. 2016; Yizhar et al. 2011), one of two core components of major depressive disorder (American Psychiatric Association 2013). For example, hyperexcitability of the rodent infralimbic cortex (ILC; the rodent equivalent of the SCC) has been shown to cause impairment in social interaction (Yizhar et al. 2011). In a follow-up study, the same research group reevaluated this question by utilizing LFP recordings in ILC and ventral striatum (VS), finding that hyperexcitability of the ILC reflected an increase in low-gamma coherence between the ILC and VS (Ferenczi et al. 2016). A supplementary study from the same laboratory demonstrated broadband ILC LFP changes in response to optogenetically driven hyperexcitability (Ferenczi et al. 2016, supplementary materials), which could very likely comprise a 1/f slope change.
Although immediate comparison of our results with those of either of the previously noted preclinical studies is complicated by their use of a unilateral recording setup, a steepening of the field potential 1/f slope (i.e., increase in 1/f slope magnitude), if inferred as a shift to a more E < I state (Gao et al. 2017; Voytek and Knight 2015), would support the previous findings of an increase in hyperexcitability causing anhedonic phenotype in animals (Yizhar et al. 2011). With added evidence from a separate rodent study (Veerakumar et al. 2014; not the first author of this article) that demonstrated improvements in the social defeat phenotype following electrical stimulation of the ILC, it is reasonable to propose that DBS could be the direct cause of hyperexcitation decreases in SCC.
Additionally, although our study is one of many 1/f slope investigations within a given region, studies of spatial 1/f correlation (Vallone et al. 2015) could translate to comparisons of 1/f slope between SCC and other regions implicated for MDD, which could aid the investigation of alternative regions that may mediate important endophenotypes comprising the MDD syndrome. Such putative 1/f connectivity work would be guided by recent invasive human electrophysiology studies into the medial temporal lobe, orbitofrontal cortex, and internal capsule, which investigated the variance of perioperative narrowband features and the role of acute neurostimulation in the variance of emotional and cognitive scales (Kirkby et al. 2018, Rao et al. 2018; Widge et al. 2019, Widge and Miller 2019). Additionally, the current work most closely parallels investigations into Parkinson’s disease, which utilize PC+S multiregion recording systems to chronically monitor disease symptoms (Swann et al. 2018b). These works collectively illustrate the current promise in developing electrophysiological hypotheses, potentially utilizing the 1/f slope, surrounding the interplay of distant regions mediating the syndrome of MDD (Mayberg 1997).
Potential application of LFP 1/f slope to guided neuromodulation systems.
The apparent sensitivity of SCC-LFP 1/f slope to concurrent treatment response state raises the question of whether SCC-LFP 1/f slope might be applicable to next-generation neuromodulation systems. The current off-stimulation observations are potentially most applicable in the case of a laboratory diagnostic test to guide changes in stimulation parameters based on weekly 1/f status. Alternatively, next-generation devices could enable preprogrammed short off-stimulation recordings that could be uploaded to the cloud for daily or weekly remote assessments (Stypulkowski et al. 2017). Proceeding from a replication of sleep-wake 1/f findings and/or integrated polysomnography studies to correlate 1/f findings with the EEG sleep architecture, prospective SCC-LFP 1/f slope investigations could further test the potential of generating a PC+S biomarker leveraging previously hypothesized sleep-cycle features of depression (Foster et al. 1976) and/or explore potential common electrophysiological features underlying daytime psychomotor retardation in depression and physiological paralysis during rapid eye movement sleep. However, such systems will likely also require additional neurophysiological readouts of optimal electrode implantation and characterization of 1/f slope changes in response to acute SCC-DBS as well as during ongoing stimulation. Toward these goals, recent studies have tested several potential strategies for confirming optimal target engagement, including PSD with acute intraoperative testing and perturb-and-measure evoked potential measures (Choi et al. 2015; Smart et al. 2018; Waters et al. 2018; Zumsteg et al. 2006). Technical challenges remain for reliably characterizing 1/f slope changes during active ongoing SCC-DBS. In the frequency domain of SCC-LFP recordings, SCC-DBS produces a mixture of narrowband stimulus artifacts and broadband noise floor elevations (Supplemental Fig. S1; https://figshare.com/s/44de58e10f2fb519cdca), possibly due to amplifier gain compression (Gak et al. 2008; Yoshida et al. 2010). These obstacles, although significant, are potentially surmountable with the use of alternative methods of 1/f slope estimation (Haller et al. 2018; Podvalny et al. 2015; Voytek et al. 2015). Additionally, it remains untested whether the filtering properties of neural tissue alter with neuroplastic changes and thus change the 1/f slope independently of neural activity. This remaining question has important implications for the interpretation of 1/f slope comparisons between study phases, which are beyond the scope of the current study. Fortunately, 1/f PSDs remain a useful model of neural activity in theoretical LFP studies (Bédard and Destexhe 2009; Freeman and Zhai 2009; Gao et al. 2017; Łęski et al. 2013; Miller et al. 2009; Pettersen et al. 2014; Reimann et al. 2013; Shirhatti et al. 2016), with in silico models providing a pragmatic path to derive reasonable effect size predictions of 1/f slope changes independent of neural activity.
Limitations.
This study has several limitations. The observation of right-hemisphere SCC-LFP 1/f slope association with binary classification of treatment response vs. treatment nonresponse is not present in all subjects and is limited by low within-subject correlation coefficients. Furthermore, the strength of the association of right 1/f slope with symptom severity appears to vary among different depression rating scales, although this may indicate that SCC-LFP tracks certain symptoms more strongly than others. With four subjects, our observations require replication in larger sample sizes to assess generalizability. 1/f slope values also vary substantially by subject, which implies the need for a within-subject baseline to explore associations with physiological and/or behavioral metrics. Although calculation of 1/f slope between 2 and 48 Hz avoids certain PC+S artifacts, 1/f slope estimation via SLR could be influenced by changes in oscillatory activity. Notably, oscillations in different frequency bands were observed in previous intraoperative/immediate postoperative studies of SCC electrophysiological activity (Clark et al. 2016; Neumann et al. 2014). Should consistent oscillatory activity be found in SCC-LFP recordings, 1/f slope can be reestimated by SLR with omission of the oscillatory band (Voytek et al. 2015), by coarse-grained spectral analysis (Podvalny et al. 2015), or by full PSD parameterization (Haller et al. 2018). Narrowband artifacts, such as cardiac artifacts demonstrated in certain PC+S recordings, may also be present on certain recording electrode configurations (Quinn et al. 2015; Swann et al. 2018a), and hardware improvements mitigating these artifacts can improve confidence in our results.
Conclusions.
The 1/f slope is a measurable quantity in SCC-LFP recordings that likely conforms to known 1/f circadian variability. We observed right-hemisphere 1/f slope changes that appear to discriminate sick/well states during ongoing SCC-DBS treatment in three of four subjects. These preliminary findings suggest the potential utility of SCC-LFP 1/f slope use as a putative biometric of longitudinal DBS-facilitated antidepressant effects. Future investigations will benefit from replication in a larger sample size and model-based analyses of potential secondary sources of 1/f activity. Such replication and modeling efforts will be crucial to further optimize the use of SCC-LFP recordings to guide SCC-DBS treatment.
GRANTS
This study was funded by grants from the Hope for Depression Research Foundation and National Institutes of Health Grants UH3 NS103550, R01 MH102238, R01 MH106173, and F32 NS096839. A. Veerakumar training and mentorship under H. S. Mayberg was funded in part through the Howard Hughes Medical Institute Medical Research Fellows Program.
DISCLAIMERS
This study was performed under physician-sponsored Investigational Device Exemption G130107 (Principal Investigator: H. S. Mayberg) and ClinicalTrials.gov NCT01984710. Medtronic granted Right of Reference to their FDA device file, donated the PC+S devices, and provided technical consultation to engineering and mechanical issues for their use. They had no role in the study design, data analysis, or interpretation of findings.
DISCLOSURES
H. S. Mayberg has a consulting agreement with St. Jude Medical (now Abbott), which has licensed her intellectual property to develop SCC DBS for the treatment of severe depression (US 2005/0033379A1). The terms of this arrangement have been approved by Emory University in accordance with policies to manage conflict of interest. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.V. and H.S.M. conceived and designed research; A.V., V.T., B.H., P.R.-P., and L.D. performed experiments; A.V., V.T., B.H., A.C.W., A.L.C., B.V., P.R.-P., L.D., J.K.R., J.A.E., K.R.B., K.S.C., and H.S.M. analyzed data; A.V., V.T., B.H., A.C.W., A.L.C., B.V., P.R.-P., L.D., J.K.R., J.A.E., K.R.B., K.S.C., and H.S.M. interpreted results of experiments; A.V. and K.S.C. prepared figures; A.V. drafted manuscript; A.V., V.T., B.H., A.C.W., A.L.C., B.V., P.R.-P., K.R.B., K.S.C., and H.S.M. edited and revised manuscript; H.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sinead Quinn and members and staff of the Emory University Depression Biometrics Laboratory for the coordination of subject visits and study administration. We thank members of the Cameron McIntyre, PhD, and Christopher Rozell, PhD, laboratories and Scott Stanslaski of Medtronic for guidance on the collection and analysis of macroelectrode LFP data, and Robert Gross, MD/PhD, for leadership of the neurosurgical team. Most importantly, we thank the research subjects.
REFERENCES
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- Bédard C, Destexhe A. Macroscopic models of local field potentials and the apparent 1/f noise in brain activity. Biophys J 96: 2589–2603, 2009. doi: 10.1016/j.bpj.2008.12.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard C, Kröger H, Destexhe A. Modeling extracellular field potentials and the frequency-filtering properties of extracellular space. Biophys J 86: 1829–1842, 2004. doi: 10.1016/S0006-3495(04)74250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadway JM, Holtzheimer PE, Hilimire MR, Parks NA, Devylder JE, Mayberg HS, Corballis PM. Frontal theta cordance predicts 6-month antidepressant response to subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. Neuropsychopharmacology 37: 1764–1772, 2012. doi: 10.1038/npp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, McGrath PJ. Right brain, left brain in depressive disorders: clinical and theoretical implications of behavioral, electrophysiological and neuroimaging findings. Neurosci Biobehav Rev 78: 178–191, 2017. doi: 10.1016/j.neubiorev.2017.04.021. [DOI] [PubMed] [Google Scholar]
- Carmody TJ, Rush AJ, Bernstein I, Warden D, Brannan S, Burnham D, Woo A, Trivedi MH. The Montgomery Asberg and the Hamilton ratings of depression: a comparison of measures. Eur Neuropsychopharmacol 16: 601–611, 2006. doi: 10.1016/j.euroneuro.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R, He BJ, Wang XJ. Random recurrent networks near criticality capture the broadband power distribution of human ECoG dynamics. Cereb Cortex 28: 3610–3622, 2018. doi: 10.1093/cercor/bhx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Riva-Posse P, Gross RE, Mayberg HS. Mapping the “Depression Switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol 72: 1252–1260, 2015. doi: 10.1001/jamaneurol.2015.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Brown EC, Ramasubbu R, Kiss ZH. Intrinsic local beta oscillations in the subgenual cingulate relate to depressive symptoms in treatment-resistant depression. Biol Psychiatry 80: e93–e94, 2016. doi: 10.1016/j.biopsych.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Dave S, Brothers TA, Swaab TY. 1/f neural noise and electrophysiological indices of contextual prediction in aging. Brain Res 1691: 34–43, 2018. doi: 10.1016/j.brainres.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Boustani S, Marre O, Béhuret S, Baudot P, Yger P, Bal T, Destexhe A, Frégnac Y. Network-state modulation of power-law frequency-scaling in visual cortical neurons. PLoS Comput Biol 5: e1000519, 2009. doi: 10.1371/journal.pcbi.1000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, Kalanithi P, Etkin A, Knutson B, Glover GH, Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351: aac9698, 2016. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage 62: 774–781, 2012. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster FG, Kupfer DJ, Coble P, McPartland RJ. Rapid eye movement sleep density. An objective indicator in severe medical-depressive syndromes. Arch Gen Psychiatry 33: 1119–1123, 1976. doi: 10.1001/archpsyc.1976.01770090109011. [DOI] [PubMed] [Google Scholar]
- Freeman WJ, Zhai J. Simulated power spectral density (PSD) of background electrocorticogram (ECoG). Cogn Neurodyn 3: 97–103, 2009. doi: 10.1007/s11571-008-9064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol 41: 2271–2293, 1996. doi: 10.1088/0031-9155/41/11/003. [DOI] [PubMed] [Google Scholar]
- Gak J, Miguez M, Bremermann M, Arnaud A. On the reduction of thermal and flicker noise in ENG signal recording amplifiers. Analog Integr Circuits Signal Process 57: 39–48, 2008. doi: 10.1007/s10470-008-9187-4. [DOI] [Google Scholar]
- Gao R, Peterson EJ, Voytek B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 158: 70–78, 2017. doi: 10.1016/j.neuroimage.2017.06.078. [DOI] [PubMed] [Google Scholar]
- Hagemann D. Individual differences in anterior EEG asymmetry: methodological problems and solutions. Biol Psychol 67: 157–182, 2004. doi: 10.1016/j.biopsycho.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Haller M, Donoghue D, Peterson E, Varma P, Sebastian P, Gao R, Noto T, Knight RT, Shestyuk A, Voytek B. Parameterizing neural power spectra (Preprint). bioRxiv 299859, 2018. doi: 10.1101/299859. [DOI] [PMC free article] [PubMed]
- Halnes G, Mäki-Marttunen T, Keller D, Pettersen KH, Andreassen OA, Einevoll GT. Effect of ionic diffusion on extracellular potentials in neural tissue. PLoS Comput Biol 12: e1005193, 2016. doi: 10.1371/journal.pcbi.1005193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Assessment of Depression. Berlin: Springer, 1986, p. 143–152. [Google Scholar]
- He BJ, Zempel JM, Snyder AZ, Raichle ME. The temporal structures and functional significance of scale-free brain activity. Neuron 66: 353–369, 2010. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilimire MR, Mayberg HS, Holtzheimer PE, Broadway JM, Parks NA, DeVylder JE, Corballis PM. Effects of subcallosal cingulate deep brain stimulation on negative self-bias in patients with treatment-resistant depression. Brain Stimul 8: 185–191, 2015. doi: 10.1016/j.brs.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, Slavin KV, Berman J, McKhann GM, Patil PG, Rittberg BR, Abosch A, Pandurangi AK, Holloway KL, Lam RW, Honey CR, Neimat JS, Henderson JM, DeBattista C, Rothschild AJ, Pilitsis JG, Espinoza RT, Petrides G, Mogilner AY, Matthews K, Peichel D, Gross RE, Hamani C, Lozano AM, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4: 839–849, 2017. doi: 10.1016/S2215-0366(17)30371-1. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 69: 150–158, 2012. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B, Choi KS, Gunalan K, Rajendra J, Mayberg HS, McIntyre CC. Quantifying the axonal pathways directly stimulated in therapeutic subcallosal cingulate deep brain stimulation. Hum Brain Mapp 40: 889–903, 2019. doi: 10.1002/hbm.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent AR, Grill WM. Analysis of deep brain stimulation electrode characteristics for neural recording. J Neural Eng 11: 046010, 2014. doi: 10.1088/1741-2560/11/4/046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby LA, Luongo FJ, Lee MB, Nahum M, Van Vleet TM, Rao VR, Dawes HE, Chang EF, Sohal VS. An amygdala-hippocampus subnetwork that encodes variation in human mood. Cell 175: 1688–1700.e14, 2018. doi: 10.1016/j.cell.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Lempka SF, McIntyre CC. Theoretical analysis of the local field potential in deep brain stimulation applications. PLoS One 8: e59839, 2013. doi: 10.1371/journal.pone.0059839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łęski S, Lindén H, Tetzlaff T, Pettersen KH, Einevoll GT. Frequency dependence of signal power and spatial reach of the local field potential. PLoS Comput Biol 9: e1003137, 2013. doi: 10.1371/journal.pcbi.1003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry 64: 461–467, 2008. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Lujan JL, Chaturvedi A, Choi KS, Holtzheimer PE, Gross RE, Mayberg HS, McIntyre CC. Tractography-activation models applied to subcallosal cingulate deep brain stimulation. Brain Stimul 6: 737–739, 2013. doi: 10.1016/j.brs.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Blanco A, Serra-Blasco M, Pérez-Egea R, de Diego-Adeliño J, Carceller-Sindreu M, Puigdemont D, Molet J, Álvarez E, Pérez V, Portella MJ. Immediate cerebral metabolic changes induced by discontinuation of deep brain stimulation of subcallosal cingulate gyrus in treatment-resistant depression. J Affect Disord 173: 159–162, 2015. doi: 10.1016/j.jad.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9: 471–481, 1997. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron 45: 651–660, 2005. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Miceli S, Ness TV, Einevoll GT, Schubert D. Impedance spectrum in cortical tissue: Implications for propagation of LFP signals on the microscopic level. eNeuro 4: ENEURO.0291-16.2016, 2017. doi: 10.1523/ENEURO.0291-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Sorensen LB, Ojemann JG, Den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comput Biol 5: e1000609, 2009. doi: 10.1371/journal.pcbi.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 134: 382–389, 1979. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Neumann WJ, Huebl J, Brücke C, Gabriëls L, Bajbouj M, Merkl A, Schneider GH, Nuttin B, Brown P, Kühn AA. Different patterns of local field potentials from limbic DBS targets in patients with major depressive and obsessive compulsive disorder. Mol Psychiatry 19: 1186–1192, 2014. doi: 10.1038/mp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen KH, Lindén H, Tetzlaff T, Einevoll GT. Power laws from linear neuronal cable theory: power spectral densities of the soma potential, soma membrane current and single-neuron contribution to the EEG. PLoS Comput Biol 10: e1003928, 2014. doi: 10.1371/journal.pcbi.1003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podvalny E, Noy N, Harel M, Bickel S, Chechik G, Schroeder CE, Mehta AD, Tsodyks M, Malach R. A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J Neurophysiol 114: 505–519, 2015. doi: 10.1152/jn.00943.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn EJ, Blumenfeld Z, Velisar A, Koop MM, Shreve LA, Trager MH, Hill BC, Kilbane C, Henderson JM, Brontë-Stewart H. Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov Disord 30: 1750–1758, 2015. doi: 10.1002/mds.26376. [DOI] [PubMed] [Google Scholar]
- Rao VR, Sellers KK, Wallace DL, Lee MB, Bijanzadeh M, Sani OG, Yang Y, Shanechi MM, Dawes HE, Chang EF. Direct electrical stimulation of lateral orbitofrontal cortex acutely improves mood in individuals with symptoms of depression. Curr Biol 28: 3893–3902.e4, 2018. doi: 10.1016/j.cub.2018.10.026. [DOI] [PubMed] [Google Scholar]
- Reimann MW, Anastassiou CA, Perin R, Hill SL, Markram H, Koch C. A biophysically detailed model of neocortical local field potentials predicts the critical role of active membrane currents. Neuron 79: 375–390, 2013. doi: 10.1016/j.neuron.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, McIntyre CC, Gross RE, Mayberg HS. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry 23: 843–849, 2018. doi: 10.1038/mp.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, Crowell AL, Garlow SJ, Rajendra JK, Mayberg HS. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry 76: 963–969, 2014. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan TC, Sreekumar V, Inati SK, Zaghloul KA. Signal complexity of human intracranial EEG tracks successful associative memory formation across individuals. J Neurosci 38: 1744–1755, 2018. doi: 10.1523/JNEUROSCI.2389-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirhatti V, Borthakur A, Ray S. Effect of reference scheme on power and phase of the local field potential. Neural Comput 28: 882–913, 2016. doi: 10.1162/NECO_a_00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart O, Choi KS, Riva-Posse P, Tiruvadi V, Rajendra JK, Waters AC, Crowell AL, Edwards J, Gross RE, Mayberg HS. Initial unilateral exposure to deep brain stimulation in treatment-resistant depression patients alters spectral power in the subcallosal cingulate. Front Comput Neurosci 12: 43, 2018. doi: 10.3389/fncom.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart OL, Tiruvadi VR, Mayberg HS. Multimodal approaches to define network oscillations in depression. Biol Psychiatry 77: 1061–1070, 2015. doi: 10.1016/j.biopsych.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanslaski S, Afshar P, Cong P, Giftakis J, Stypulkowski P, Carlson D, Linde D, Ullestad D, Avestruz AT, Denison T. Design and validation of a fully implantable, chronic, closed-loop neuromodulation device with concurrent sensing and stimulation. IEEE Trans Neural Syst Rehabil Eng 20: 410–421, 2012. doi: 10.1109/TNSRE.2012.2183617. [DOI] [PubMed] [Google Scholar]
- Stypulkowski PH, Stanslaski SR, Giftakis JE. Modulation of hippocampal activity with fornix Deep Brain Stimulation. Brain Stimul 10: 1125–1132, 2017. doi: 10.1016/j.brs.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Swann NC, de Hemptinne C, Miocinovic S, Qasim S, Ostrem JL, Galifianakis NB, Luciano MS, Wang SS, Ziman N, Taylor R, Starr PA. Chronic multisite brain recordings from a totally implantable bidirectional neural interface: experience in 5 patients with Parkinson’s disease. J Neurosurg 128: 605–616, 2018a. doi: 10.3171/2016.11.JNS161162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, de Hemptinne C, Thompson MC, Miocinovic S, Miller AM, Gilron R, Ostrem JL, Chizeck HJ, Starr PA. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng 15: 046006, 2018b. doi: 10.1088/1741-2552/aabc9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallone F, Cintio A, Mainardi M, Caleo M, Di Garbo A. Existence of anticorrelations for local field potentials recorded from mice reared in standard condition and environmental enrichment. Phys Rev E Stat Nonlin Soft Matter Phys 91: 012702, 2015. doi: 10.1103/PhysRevE.91.012702. [DOI] [PubMed] [Google Scholar]
- Veerakumar A, Challis C, Gupta P, Da J, Upadhyay A, Beck SG, Berton O. Antidepressant-like effects of cortical deep brain stimulation coincide with pro-neuroplastic adaptations of serotonin systems. Biol Psychiatry 76: 203–212, 2014. doi: 10.1016/j.biopsych.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Knight RT. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol Psychiatry 77: 1089–1097, 2015. doi: 10.1016/j.biopsych.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Kramer MA, Case J, Lepage KQ, Tempesta ZR, Knight RT, Gazzaley A. Age-related changes in 1/f neural electrophysiological noise. J Neurosci 35: 13257–13265, 2015. doi: 10.1523/JNEUROSCI.2332-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke L, Wöstmann M, Obleser J. States and traits of neural irregularity in the age-varying human brain. Sci Rep 7: 17381, 2017. doi: 10.1038/s41598-017-17766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AC, Veerakumar A, Choi KS, Howell B, Tiruvadi V, Bijanki KR, Crowell A, Riva-Posse P, Mayberg HS. Test-retest reliability of a stimulation-locked evoked response to deep brain stimulation in subcallosal cingulate for treatment resistant depression. Hum Brain Mapp 39: 4844–4856, 2018. doi: 10.1002/hbm.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge AS, Miller EK. Targeting cognition and networks through neural oscillations: next-generation clinical brain stimulation. JAMA Psychiatry 76: 671, 2019. doi: 10.1001/jamapsychiatry.2019.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge AS, Zorowitz S, Basu I, Paulk AC, Cash SS, Eskandar EN, Deckersbach T, Miller EK, Dougherty DD. Deep brain stimulation of the internal capsule enhances human cognitive control and prefrontal cortex function. Nat Commun 10: 1536, 2019. doi: 10.1038/s41467-019-09557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477: 171–178, 2011. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Masui Y, Eki R, Iwata A, Yoshida M, Uematsu K. A neural recording amplifier with low-frequency noise suppression. IEICE Trans Electron E93.C: 849–854, 2010. doi: 10.1587/transele.E93.C.849. [DOI] [Google Scholar]
- Zumsteg D, Lozano AM, Wennberg RA. Rhythmic cortical EEG synchronization with low frequency stimulation of the anterior and medial thalamus for epilepsy. Clin Neurophysiol 117: 2272–2278, 2006. doi: 10.1016/j.clinph.2006.06.707. [DOI] [PubMed] [Google Scholar]