Abstract
Narragansett Bay is an urban estuary that historically has been impacted by long-term discharge of sanitary wastewater (WW) effluents. High-density water sampling was conducted in Narragansett Bay, RI, USA, in an effort to understand the distribution and behavior of sucralose, an artificial sweetener that has shown utility as a sanitary wastewater tracer. Water samples were collected at sixty-seven sites and analyzed for sucralose, whose performance was compared to other tracers present in wastewater effluents. Concentrations of sucralose were much higher than the other tracers measured, carbamazepine and caffeine, ranging from 18 to 3180 ng/L and corresponded well with salinity (r2 = 0.88), demonstrating conservative behavior throughout the Bay. Mapped interpolation data using an empirical bayesian kriging model clearly show the spatial trends of WW and how estuarine processes influence dilution and dispersion throughout the Bay. These findings provide further evidence of the efficacy of sucralose as a wastewater tracer in large urban estuaries where continuous high-volume discharge of WW occur.
Keywords: Wastewater, tracer, estuary, sucralose
1. Introduction
Sucralose is an artificial sweetener that is used extensively in processed food products and beverages. Sucralose is approximately 600 times sweeter than natural sucrose and is not metabolized or altered by most biological processes, including the human digestive system (Neset et al., 2010). The physical and chemical properties of sucralose also make it resistant to degradation by abiotic processes (Tran et al., 2014). Following human consumption, it is eliminated from the body for the most part unaltered in bodily waste. Recent toxicity studies performed with common standardized test species have frequently yielded no observed effect concentrations well above environmentally relevant concentrations, although some research has indicated there is a potential for adverse effects after chronic exposure (Luo et al., 2019). While the breadth and depth of toxicity data for sucralose is incomplete, the absence of significant effects associated with sucralose exposure, such as minimal bioconcentration factors and absence of bioaccumulation, could suggest low hazard and risk to organisms in the aquatic environment.
In developed countries, most human waste is subjected to some level of sanitary waste treatment. In urbanized locations this generally involves municipal wastewater treatment facilities (WWTFs), whereas residential onsite wastewater treatment systems (e.g., septic systems) are the primary treatment process in less densely populated regions. Previous research has demonstrated that most municipal wastewater treatment processes are ineffective in removing or degrading sucralose (Scheurer et al., 2011). A recent review by Luo et al. 2019 points out that some studies have shown radical-mediated oxidation to be capable of degrading sucralose, but it is not as effective under normal WWTF conditions.
The combination of sucralose’s stability, high aqueous solubility, low affinity for solid phase partitioning and detection at low concentrations supports its suitability as a tracer. Further, its high frequency of detection in WWTF effluents and receiving waters at elevated levels makes it ideal for its use as a wastewater (WW) tracer (Oppenheimer et al., 2011)
Sucralose has been previously evaluated as a WW tracer in groundwater (Buerge et al., 2009), drinking water treatment plants (Mawhinney et al., 2011), surface waters (Oppenheimer et al., 2011), estuaries (Cantwell et al., 2018, 2019) and coastal waters (Baena-Nogueras et al., 2018). For each application, sucralose has shown potential for identifying and tracking sources of WW. For example, sucralose would be well-suited to determine the magnitude and spatial distribution of WW discharges to water bodies used as sources of drinking water (Lange et al., 2012). Similarly, in estuaries sucralose has potential to assess whether WW effluents pose risks to recreational waters as well as areas where wild harvested seafood and aquaculture operations occur. However until recently, its utility as a tracer has not been evaluated under a range of estuarine conditions and locations.
The measurement of artificial sweeteners in coastal marine waters such as sucralose and acesulfame until recently has been limited. While some studies have shown other artificial sweeteners such as acesulfame performing equally as well as sucralose, these studies occurred outside of the US. As opposed to many other countries, sucralose is the most highly consumed artificial sweetener in the U.S., so it is expected that concentrations would be higher here than in other countries. For instance, Subedi and Kannan 2014 found sucralose concentrations in domestic U.S. influents to be 28 times higher than concentrations of acesulfame. This study also found average loadings of sucralose to WWTFs to be significantly higher than in European studies, while loadings of acesulfame were significantly lower. Sucralose also has lower sorption potential than other artificial sweeteners used as WW tracers such as acesulfame (Soh et al., 2011), making it less likely to partition to solids and be removed from the water column.
The conservative nature and range of salinities present in estuarine systems provide an excellent opportunity to assess the performance of a WW tracer. In this study the waters of Narragansett Bay, RI, were sampled for sucralose employing a high density, randomized sampling design, providing higher spatial resolution than those employed in recent studies (Cantwell et al., 2018, 2019). The objectives of this effort were to: (1) illustrate the principal sources and magnitude of WW loadings, assess their spatial distribution and dispersion throughout the Bay and (2) evaluate the sensitivity and performance of sucralose as a WW tracer in a large urban estuary and assess differences in sucralose between other estuaries with different morphologies. Two other compounds previously evaluated as WW tracers, caffeine and carbamazepine (Buerge et al., 2003, Kahle et al., 2009), were also assessed to compare and contrast the performance and behavior of sucralose.
2. Materials and methods
2.1. Study area
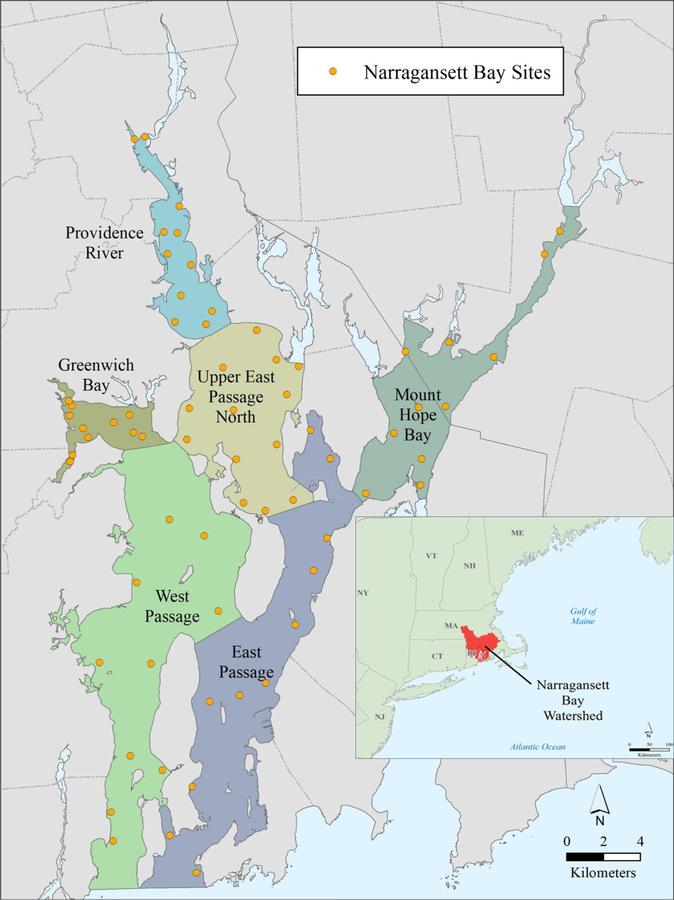
Narragansett Bay is located on the northeastern coast of the United States and is classified as a drowned river estuary (Figure 1). The Bay has an area of 342 km2, an average depth of 9 m (Raposa, 2009) and an estimated flushing time of 26.5 days (Kincaid et al., 2008). The coastline of Narragansett Bay is densely populated, with an estimated population of 1.9 million in the watershed and with most communities connected to WWTFs. A total of thirty-seven WWTFs discharge effluent either directly to Narragansett Bay or into rivers of the watershed, with a total daily effluent discharge to the Bay estimated at 7.7 × 105 m3/d (NBEP, 2017) (Figure S1). Twelve of the 15 WWTFs located within the Narragansett Bay coastal basin discharge more than half of the daily discharge (3.9 × 105 m3/d) directly to the Bay.
Figure 1.
Narragansett Bay sampling sites, sub geographic study areas and Narragansett Bay watershed locus map.
2.2. Sampling
Sixty-seven sites within Narragansett Bay were selected for water sampling and were distributed throughout the main body of the Bay and its major subembayments (Figure 1). The sampling stations were established by using a randomized hexagonal grid and random origin over the study area. The study area is further separated into six sub-areas (Providence River, Upper East Passage, Mount Hope Bay, Greenwich Bay, West Passage, East Passage) that reflect natural geographic boundaries and geomorphic features within the estuary (Figure 1 Table S1).
Water samples (0.5 L total volume) were collected by boat on August 1–3, 2016, through Teflon® tubing from 1 m below the water surface at all sites, pumped via a teflon pump through muffled < 1 µm glass fiber filters (Whatman, GE Healthcare Life Sciences, Pittsburgh, PA) and stored in amber glass bottles. Samples were kept on ice and, upon returning to the lab, adjusted to pH 2 using hydrochloric acid (6N) and stored in the dark at 4°C for subsequent extraction.
2.3. Sample analysis
Sample preparation and analysis was conducted at the USEPA’s Atlantic Ecology Division Research Laboratory. The extraction protocol used Oasis HLB solid phase extraction cartridges (6 cc, 500 mg, Waters Corp., Milford, MA). The full procedure has been described elsewhere (Cantwell et al., 2017). Briefly, samples were fortified with 100 ng of isotopically labeled internal standards, passed through the solid phase cartridge, eluted with methanol, evaporated and reconstituted in 1 mL of mobile phase. Six blanks, four fortified blanks, two field blanks, two matrix evaluation samples, and a duplicate extraction at two sites were also analyzed. A Waters Acquity H-class UPLC and a Waters Xevo TQD MS/MS operated in electrospray ionization (ESI) mode was used for confirmation and quantification of sucralose, caffeine and carbamazepine. Further details on instrumental analysis can be found in the supplemental information.
2.4. Data analysis
In this study, an empirical bayesian kriging (EBK) model was used to map the spatial distribution and concentrations of sucralose and carbamazepine throughout Narragansett Bay. The EBK model is a probabilistic method that uses a geostatistical model to optimize the spatial prediction of the model estimates by minimizing the error or uncertainty between the predicted and measured values. EBK spatial interpolation models do not assume one model fits the entire dataset and instead use local models to capture and account for small scale effects (Krivoruchko, 2012). Using the geostatistical EBK model provides spatial estimations or optimal predictors for WW tracer compounds and their associated errors (uncertainties represented as estimated variances). The approach allows a simple, straightforward technique for spatial predictions of tracer concentrations and salinity. The EBK models were developed using ArcGIS Geostatistical Analyst (Esri, Redlands, CA, USA). Further details on the data analysis is in supplemental information.
3. Results and Discussion
3.1. Salinity and sucralose levels
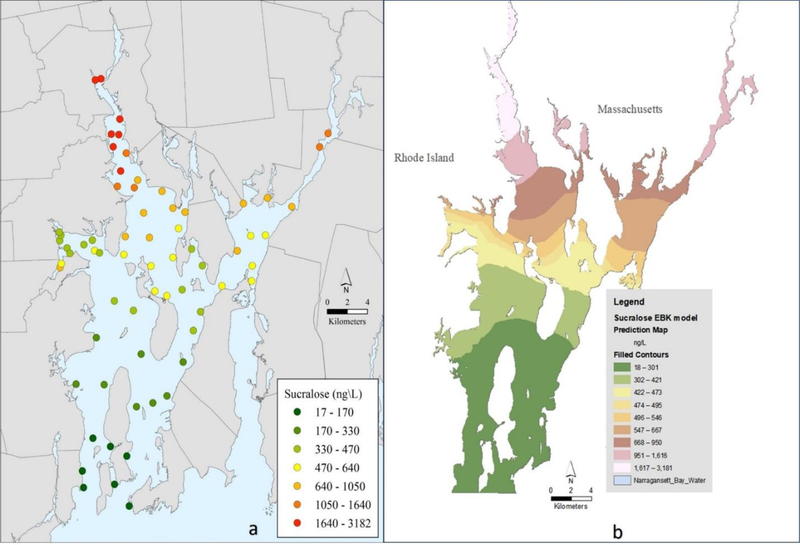
Measured concentrations of sucralose across the Bay ranged widely from 18 to 3180 ng/L, exhibiting a declining trend from north to south (Table S1, Figure 2a). Sucralose concentrations from all sites across Narragansett Bay correlated well with salinity (n = 67, linear model r2 = 0.88), demonstrating its conservative behavior. Sucralose’s inverse relationship and correspondence with salinity is due to several factors, particularly its persistence, which results in near conservative behavior, and the fact that freshwater inputs from WWTFs and rivers with WW discharges are the primary source of sucralose to estuaries. Other potential wastewater sources of sucralose are non-point in origin and include individual and community based residential onsite wastewater treatment systems (e.g., septic systems).
Figure 2.
Maps of sixty-seven measured sampling stations for sucralose (a) and sucralose estimates (b) using Empirical Bayesian Kriging (EBK) spatial interpolation methods
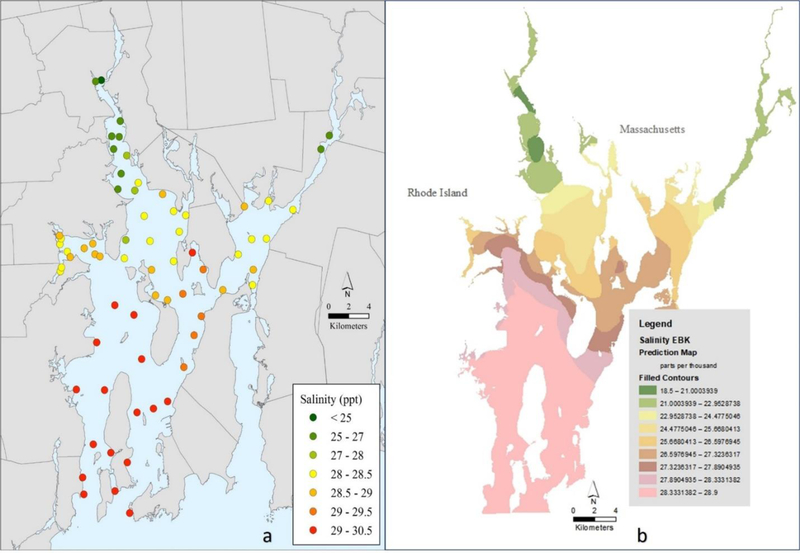
The high-density sampling in this study revealed discrete changes in salinity within and across the six sub-areas of the estuary (Table S1, Figure 3a). In addition to circulation patterns, tides and other hydrologic features, the location and magnitude of WW discharges and freshwater inputs (e.g., rivers) shape the salinity distribution throughout the Bay, especially in the Providence River and Mount Hope Bay sub-areas. This establishes a salinity gradient from the head of the Bay to the entrances to Rhode Island Sound, ranging from 24.1 at the northernmost sampling point in the Providence River to 30.5 at the mouth of the East Passage. The north-south gradient exhibited by sucralose has been reported previously for nutrients, which are a principal component of treated wastewater effluents and have historically been a major environmental issue in Narragansett Bay (Deacutis, 2008). Other WW associated contaminants such as pharmaceuticals have also shown a similar gradient in Narragansett Bay (Cantwell et al., 2017), further establishing the importance of WW discharges in the upper Bay as the primary source of numerous classes of contaminants.
Figure 3.
Maps of sixty-seven measured sampling stations for salinity (a) and salinity estimates (b) using Empirical Bayesian Kriging (EBK) spatial interpolation methods.
In the Providence River sub-area, through which 70% of the treated WW effluent discharged to Narragansett Bay enters, measured concentrations of sucralose were highest, ranging from 821 to 3180 ng/L, while salinities were lowest, ranging from 24.1 to 28.1 (Figures 2a–3a). Sucralose concentrations from the two largest WWTFs discharging effluent to this sub-area averages 77 µg/L (unpublished data). As water exits the Providence River, sucralose in the Upper East Passage sub-area declines to 433–860 ng/L as a result of dilution and mixing, with a corresponding increase in salinity to 28.1–29.2 observed. Salinity increased progressively with distance in the Providence River, as well as in the Taunton River—Mount Hope Bay sub-area, which is also a significant source of fresh water to Narragansett Bay. In the Mount Hope Bay sub-area, WW effluent from the watershed enters via the Taunton River or by direct discharge to the Bay, accounting for approximately 23% of the total discharged to Narragansett Bay. This sub-area had the next highest sucralose concentrations at 499–1400 ng/L and salinity levels ranging from 26.7 to 28.8.
The balance of effluent from ~8 small WWTFs enters the mid to lower Bay, accounting for < 10% of the daily effluent volume discharged to Narragansett Bay. In the main body of the Bay, salinity increased relatively uniformly towards the mouth of the Bay, ranging from 29.1 to 30.5 with some variability due to geomorphic and hydrologic features. Conversely, sucralose concentrations decreased towards the mouth of the Bay due to dilution effects and the absence of large volume, point-source discharges of WW. Similar trends were observed in both the East and West Passage sub-areas of the Bay with measured sucralose concentrations ranging from 18 to 446 ng/L and 124 to 377 ng/L, respectively. In these sub-areas, both salinity and sucralose concentrations were influenced by the proximity of the open ocean and daily tidal exchange. Sucralose concentrations in Greenwich Bay, a small embayment off the West Passage, ranged from 408 to 657 ng/L and were relatively uniform, with the exception of a small cove where there is an outfall from a small WWTF. Salinities in Greenwich Bay varied little across the sub-embayment, ranging from 28.2 to 29, with slightly lower levels in the west end.
3.2. Spatial distributions and models
To illustrate the spatial distributions of salinity and sucralose levels baywide, the measured data was used to generate EBK geostatistical interpolation plots (Figures 2b and 3b, respectively). The spatial patterns of salinity and sucralose are similar baywide, reflecting discharged WW (0 salinity) elevated in sucralose mixing with and being diluted by high salinity seawater (~30) containing only trace concentrations of sucralose entering the Bay. The EBK interpolated map of salinity clearly shows the entry of low salinity water into the Providence River sub-area, confirming it as the major source of freshwater to the estuary. Similarly, the EBK interpolated map of sucralose clearly shows the influence of the Providence River as a major source of sucralose to the estuary due to the volume of WW discharged here and high levels of sucralose present in the effluents.
Direct comparison to sucralose in other northeastern urbanized estuaries showed sucralose levels in Narragansett Bay were somewhat higher. For example, levels were lower in nearby Long Island Sound, ranging from 123 to 671 ng/L (Cantwell et al., 2019), while in New York Harbor concentrations varied from 708 to 1470 ng/L (Cantwell et al., 2018). The differences in these systems reflect several factors, including the number, volume and location of WWTF discharges as well as the estuarine geomorphology and hydrology (e.g., residence times) which regulate dilution and dispersion of effluents. It should be noted that these other systems were not sampled at the same spatial resolution of the current study, limiting comparability. Given the documented persistence of sucralose under estuarine conditions (Baena-Nogueras et al., 2018), the estimated residence time of 26.5 days in Narragansett Bay is likely too short a duration for significant in-situ degradation to occur (Tollefsen et al., 2012), but this may be a factor in systems with lengthy residence times.
3.3. Tracer evaluation
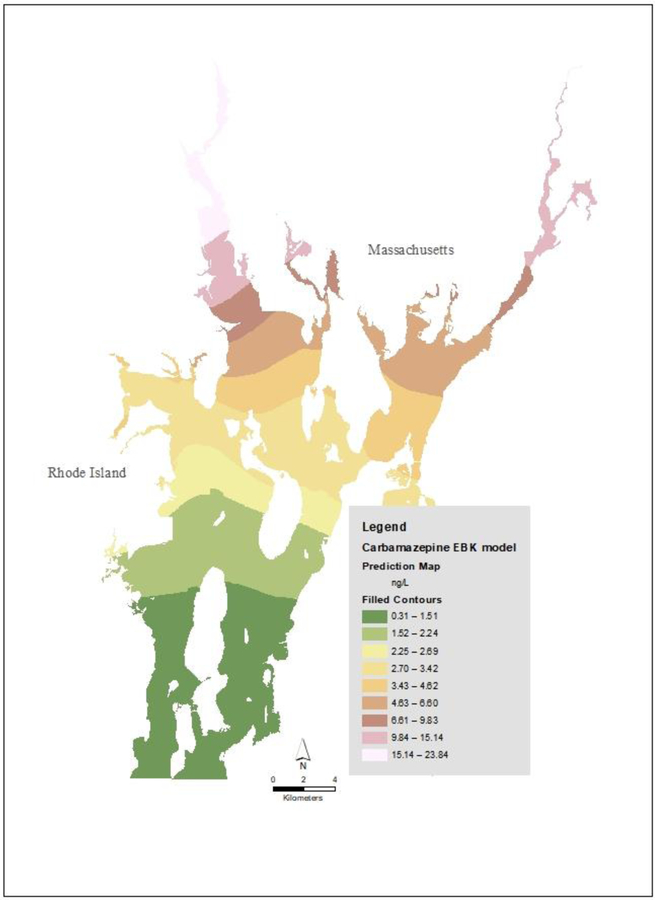
To further evaluate the performance of sucralose as a WW tracer, caffeine and carbamazepine were also measured to compare and contrast their behavior relative to sucralose in Narragansett Bay. Caffeine, like sucralose, is a ubiquitous ingredient in beverages and food products and has been previously employed as a WW tracer under a range of conditions (Buerge et al., 2003, 2006). Regressing concentrations of caffeine against salinity and sucralose using a linear model yielded r2 values of 0.05 and 0.04 (Figure S2, respectively, showing no relationship. The absence of a relationship has been previously observed (Cantwell et al., 2017, 2018), and is likely due to caffeine’s relatively efficient removal during WW treatment and subsequent degradation in receiving waters (Benotti and Brownawell, 2009). Carbamazepine is an anticonvulsant medication that has well-documented persistence and stability in aquatic systems and has previously been employed as a tracer for WW (Kahle et al., 2009; Oppenheimer et al., 2011). Carbamazepine concentrations were regressed against salinity using a linear model producing an identical correlation (r2 = 0.88) to that of sucralose (Figure S2). Regressing sucralose and carbamazepine concentrations with a linear model resulted in a r2 of 0.98, demonstrating almost identical behavior throughout the Bay. A map of carbamazepine prepared using an EBK geostatistical interpolation model shows a spatial distribution highly similar to sucralose throughout the Bay despite its concentration being several orders of magnitude lower (Table S1, Figure 4). Carbamazepine was quantified at most sites in the Bay except for several in the East Passage sub-area which were below detection limits.
Figure 4.
Empirical bayesian Kriging (EBK) interpolation map of carbamazepine concentrations and distribution across Narragansett Bay.
The source specificity of sucralose and carbamazepine makes them both suitable for identifying WW sources; however, the levels of sucralose are highly elevated relative to carbamazepine, providing a distinct advantage for its use as a tracer (Oppenheimer et al., 2011). For example, the dynamic range of sucralose (18–3181 ng/L) compared to carbamazepine (nondetect–25 ng/L) is substantial, highlighting a highly desirable characteristic of a WW tracer (Benotti and Brownawell, 2007), particularly in large aquatic systems. This differential in concentration between sucralose and carbamazepine has been noted in other estuarine studies as well (Cantwell et al., 2018; Silvanima et al., 2018). An estimated daily flux of sucralose to Narragansett Bay from all WWTFs in the watershed was estimated at 59 kg/day based on a daily flow of 7.7 × 105 m3/d (NBEP, 2017). This flux was calculated using average effluent concentrations of the two largest WWTFs measured during this study (Fields Point 74,000 and Bucklin Point 80,000 ng/L) discharging to Narragansett Bay which are representative of WWTF effluent concentrations in the U.S. (Oppenheimer et al., 2011). This daily flux ensures measurable concentrations under most conditions and locations, particularly in estuaries with short hydraulic residence times such as Narragansett Bay. Additionally, carbamazepine has been shown to be susceptible to photolysis under certain conditions, which could lead to an underestimation of WW loadings to water bodies (Badruzzaman et al., 2013). Finally, sucralose is ubiquitous in many foods and beverages, in contrast to carbamazepine, which is a prescribed drug whose use in the US has been declining in recent years (Meador et al., 2009), suggesting environmental concentrations could be declining as well.
The EBK interpolation plot of sucralose provided a system-wide snapshot of WW impacts, the magnitude of their discharge and the dispersion profile of WW and dilution effects throughout Narragansett Bay. The EBK models developed with the two WW tracer compounds evaluated in this study produced reliable estimates of the concentration and spatial distribution of these compounds, based on cross-validation and prediction error statistics (Table 1). Sucralose and carbamazepine both had high r2 values for their respective predicted vs. measured values ranging from 0.89 to 0.96, respectively, while caffeine had a lower r2 of 0.47 (Table 1). The use of caffeine as a tracer of treated WW was not effective in this study with degradation suspected as a major contributor to its poor performance (Benotti and Brownawell, 2009). Carbamazepine was as effective as sucralose despite much lower concentrations, which may limit its effectiveness where dilution effects are considerable, especially in large estuaries or systems minimally impacted by WW discharges.
Table 1.
Prediction error statistics for Empirical Bayesian Kriging (EBK) model results for sucralose, carbamazepine and caffeine.
| Tracer Compound | No. Stations sampled | RMSEa | Standardized Mean | RMSb | ASEc | MREd | Measured vs. Predicted R2 |
|---|---|---|---|---|---|---|---|
| sucralose | 67 | 1.037 | −0.008 | 0.282 | 0.263 | 0.054 | 0.892 |
| carbamazepine | 65 | 0.958 | −0.018 | 0.178 | 0.193 | 0.041 | 0.961 |
| caffeine | 67 | 0.968 | −0.019 | 0.508 | 0.531 | 0.150 | 0.467 |
Standardized Root Mean Square error
Root Mean Squared
Average Standard error
Mean Relative Error
4. Conclusions
The use of sensitive and confirmative tracers to track WW and their associated contaminants is critical to understanding their sources, behavior and fate, particularly in large aquatic systems such as estuaries. Testing these tracers in an estuarine system at high spatial density using salinity as a proxy for conservative behavior allowed for an accurate and high resolution assessment of their performance. While the focus of this study was to evaluate sucralose as a single tracer, utilizing tracers such as sucralose and carbamazepine in tandem may provide enhanced capability in verifying the sources and fate of sanitary WW by incorporating a weight of evidence approach. Another promising approach is to pair tracers that have distinctly different behavior, which may provide enhanced information of WW sources. For example, the use of sucralose and caffeine concentrations as a ratio has previously shown promise to discriminate between sources of treated and untreated (e.g., illegal and CSO) WW discharges in estuaries (Cantwell et al., 2018). The presence of tracers like sucralose at elevated levels in receiving waters suggests that other unmeasured compounds in WW effluents may be present and potentially pose a risk to aquatic life. Future work is needed to assess the performance of tracers such as sucralose for predicting and tracking contaminants of emerging concern that are present in sanitary WW effluents.
Supplementary Material
Acknowledgments
The authors thank Dr. Abigail Joyce, Dr. James Lake, and Mr. Steven Rego for their technical reviews. This is NHEERL Contribution ORD-030803. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Any mention of trade names, products, or services does not imply an endorsement by the U.S. Government or the U.S. Environmental Protection Agency. The EPA does not endorse any commercial products, services, or enterprises.
References
- Badruzzaman M, Oppenheimer JA, & Jacangelo JG (2013). Impact of environmental conditions on the suitability of microconstituents as markers for determining nutrient loading from reclaimed water. Water Research, 47(16): 6198–6210. 10.1016/j.watres.2013.07.029 [DOI] [PubMed] [Google Scholar]
- Baena-Nogueras RM, Traverso-Soto JM, Biel-Maeso M, Villar-Navarro E, & Lara-Martín PA (2018). Sources and trends of artificial sweeteners in coastal waters in the bay of Cadiz (NE Atlantic). Marine Pollution Bulletin, 135: 607–616. 10.1016/j.marpolbul.2018.07.069 [DOI] [PubMed] [Google Scholar]
- Benotti MJ, & Brownawell BJ (2007). Distributions of pharmaceuticals in an urban estuary during both dry-and wet-weather conditions. Environmental Science & Technology, 41(16): 5795–5802. 10.1021/es0629965 [DOI] [PubMed] [Google Scholar]
- Benotti MJ, & Brownawell BJ (2009). Microbial degradation of pharmaceuticals in estuarine and coastal seawater. Environmental Pollution, 157(3): 994–1002. 10.1016/j.envpol.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Buerge IJ, Poiger T, Müller MD, & Buser HR (2003). Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environmental Science & Technology, 37(4): 691–700. 10.1021/es020125z [DOI] [PubMed] [Google Scholar]
- Buerge IJ, Poiger T, Müller MD, Buser HR (2006). Combined sewer overflows to surface waters detected by the anthropogenic marker caffeine. Environmental Science & Technology, 40(13): 4096–4102. https://pubs.acs.org/doi/abs/10.1021/es052553l [DOI] [PubMed] [Google Scholar]
- Buerge IJ, Buser H-R, Kahle M, Muller MD, & Poiger T (2009). Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: an ideal chemical marker of domestic wastewater in groundwater. Environmental Science & Technology, 43(12): 4381–4385. https://pubs.acs.org/doi/abs/10.1021/es900126x [DOI] [PubMed] [Google Scholar]
- Cantwell MG, Katz DR, Sullivan JC, Ho K, & Burgess RM (2017). Temporal and spatial behavior of pharmaceuticals in Narragansett Bay, Rhode Island, United States. Environmental Toxicology and Chemistry, 36(7): 1846–1855. 10.1002/etc.3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell MG, Katz DR, Sullivan JC, Shapley D, Lipscomb J, Epstein J, Juhl AR, Knudson C & O’Mullan GD (2018). Spatial patterns of pharmaceuticals and wastewater tracers in the Hudson River Estuary. Water Research, 137: 335–343. 10.1016/j.watres.2017.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell MG, Katz DR, Sullivan JC, & Lyman M (2019). Evaluation of wastewater tracers to predict pharmaceutical distributions and behavior in the Long Island Sound estuary. Chemosphere, 220: 629–636. 10.1016/j.chemosphere.2018.12.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacutis CF 2008. Evidence of ecological impacts from excess nutrients in upper Narragansett Bay In Desbonnet A, Costa-Pierce BA (Eds.), Science for Ecosystem-based Estuarine Management: Narragansett Bay in the 21st Century (pp 349–381). New York, NY: Springer. [Google Scholar]
- Kahle M, Buerge IJ, Müller MD, & Poiger T (2009). Hydrophilic anthropogenic markers for quantification of wastewater contamination in ground- and surface water. Environmental Toxicology & Chemistry, 28(12): 2528–2536. 10.1897/08-606.1 [DOI] [PubMed] [Google Scholar]
- Kincaid C, Bergondo D, & Rosenberger K (2008). The dynamics of water exchange between Narragansett Bay and Rhode Island Sound In Desbonnet A, Costa-Pierce BA (Eds.), Science for Ecosystem-based Estuarine Management: Narragansett Bay in the 21st Century (pp 301–324). New York, NY: Springer. [Google Scholar]
- Krivoruchko K (2012). Empirical bayesian kriging implemented in ArcGIS geostatistical analyst. ArcUser, 15: 6–10. [Google Scholar]
- Lange FT, Scheurer M, & Brauch HJ (2012). Artificial sweeteners-A recently recognized class of emerging environmental contaminants: A review. Analytical and Bioanalytical Chemistry, 403(9): 2503–2518. 10.1007/s00216-012-5892-z [DOI] [PubMed] [Google Scholar]
- Luo J, Zhang Q, Cao M, Wu L, Cao J, Fang F, Li C, Xue Z, Feng Q, 2019Ecotoxicity and environmental fates of newly recognized contaminants-artificial sweeteners: A review. Science of the Total Environment. 653, 1149e1160. [DOI] [PubMed] [Google Scholar]
- Mawhinney DB, Young RB, Vanderford BJ, Borch T, & Snyder SA (2011). Artificial sweetener sucralose in U.S. drinking water systems. Environmental Science & Technology, 45(20): 8716–8722. 10.1021/es202404c [DOI] [PubMed] [Google Scholar]
- Meador KJ, Penovich P, Baker GA, Pennell PB, Bromfield E, Pack A, & Moore E (2009). Antiepileptic drug use in women of childbearing age. Epilepsy & Behavior, 15(3): 339–343. 10.1016/j.yebeh.2009.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narragansett Bay Estuary Program (NBEP). 2017. State of Narragansett Bay and its Watershed Technical Report. Providence, RI: http://nbep.org/the-state-of-our-watershed/ [Google Scholar]
- Neset TSS, Singer H, Longrée P, Bader HP, Scheidegger R, Wittmer A, & Andersson JCM (2010). Understanding consumption-related sucralose emissions - A conceptual approach combining substance-flow analysis with sampling analysis. Science of the Total Environment, 408(16): 3261–3269. 10.1016/j.scitotenv.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Oppenheimer J, Eaton A, Badruzzaman M, Haghani AW, & Jacangelo JG (2011). Occurrence and suitability of sucralose as an indicator compound of wastewater loading to surface waters in urbanized regions. Water Research, 45(13): 4019–4027. 10.1016/j.watres.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Raposa KB (2009). Ecological Geography of Narragansett Bay In Raposa KB & Schwartz ML (Eds.), An ecological profile of the Narragansett Bay National Estuarine Research Reserve (pp 77–88). Narragansett, RI: Rhode Island Sea Grant. [Google Scholar]
- Scheurer M, Storck FR, Graf C, Brauch HJ, Ruck W, Lev O, & Lange FT (2011). Correlation of six anthropogenic markers in wastewater, surface water, bank filtrate, and soil aquifer treatment. Journal of Environmental Monitoring, 13(4): 966–973. 10.1039/c0em00701c [DOI] [PubMed] [Google Scholar]
- Silvanima J, Woeber A, Sunderman-Barnes S, Copeland R, Sedlacek C, & Seal T (2018). A synoptic survey of select wastewater-tracer compounds and the pesticide imidacloprid in Florida’s ambient freshwaters. Environmental Monitoring and Assessment, 190(7): 435 10.1007/s10661-018-6782-4 [DOI] [PubMed] [Google Scholar]
- Subedi B, Kannan K, 2014. Fate of artificial sweeteners in wastewater treatment plantsin New York State, USA. Environmental. Science and Technology 48, 13668–13674. [DOI] [PubMed] [Google Scholar]
- Tollefsen KE, Nizzetto L, & Huggett DB (2012). Presence, fate and effects of the intense sweetener sucralose in the aquatic environment. Science of the Total Environment, 438: 510–516. 10.1016/j.scitotenv.2012.08.060 [DOI] [PubMed] [Google Scholar]
- Tran NH, Nguyen VT, Urase T, & Ngo HH (2014). Role of nitrification in the biodegradation of selected artificial sweetening agents in biological wastewater treatment process. Bioresource Technology, 161: 40–46. 10.1016/j.biortech.2014.02.116 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.