Abstract
Background
The aim is to describe the association of functional capacity and cognitive functioning with 1‐year mortality in older patients with cancer in the head and neck region.
Methods
We performed a cohort study in which all patients aged 70 years and older received a geriatric screening before treatment. Main outcome was 1‐year mortality.
Results
A total of 102 patients were included. Median age was 78.7 years (interquartile range [IQR], 72.3‐84.5), 25% were cognitive impaired, 40% were malnourished, and 28.4% used a walking device. Overall, 1‐year mortality was 42.3%. Male sex (hazard ratio [HR], 4.30; 95% confidence interval [CI], 1.35‐13.67), malnutrition (HR, 2.55; 95% CI, 1.19‐5.16), and using a walking device (HR, 2.80; 95% CI 1.13‐6.93) were associated with higher mortality risk, independent of stage and comorbidities.
Conclusion
In older patients with head and neck cancer, the mortality rates are high. Nutritional status and mobility are determinants of 1‐year mortality, independent of tumor stage, age, and comorbidity.
Keywords: cognitive disorders, geriatric oncology, head and neck cancer, physical functioning, social functioning
1. INTRODUCTION
Patients diagnosed with head and neck cancer (HNC) are, in case of curative intention, facing major treatment options, such as extensive operation and/or (chemo)radiation therapy. Older patients in general are at higher risk for adverse health outcomes (such as delirium, complications, and longer duration of stay) after treatment, but the risk for patients with HNC is even higher because of a high prevalence of previous excessive alcohol drinking and smoking,1, 2, 3 which makes this group more susceptible for cognitive3, 4 and functional5 decline. It could be important to make a careful selection of the patients who are suitable for the intensive treatment. In the United States, it is expected that between 2010 and 2030, the incidence of oral cavity and pharyngeal cancer in patients aged 65 years and older will increase by 61%.6 Besides, the 5‐year survival is poor with an estimated survival of 50% with a large variation between the different tumor localizations.7, 8 However, limited evidence is available on the association of a geriatric assessment with adverse health outcomes and the role of assisting clinical decision making in older patients with HNC.
Across a variety of (surgical) oncologic population and cancer types, components of the geriatric assessment, such as cognition, functional status, and social status, are predictive for adverse health outcomes such as postoperative complications, institutionalization after discharge, and mortality.9, 10 Several guidelines recommend for a form of geriatric assessments as part of routine preoperative care.11, 12 A recent systematic review in older patients with HNC showed that geriatric conditions were prevalent and in 64% of the included studies there was a statistically significant association of geriatric impairments with a higher risk of adverse outcome.13 However, cognitive function and objectively measured physical capacity were not assessed.
The aim of this study is to describe the association in older patients with cancer in the head and neck region of geriatric measurements, including functional capacity and cognitive functioning, with 1‐year mortality.
2. METHODS
2.1. Study design and setting
We performed a retrospective cohort study (from October 2014 until January 2017) in older patients seen with cancer in the head and neck region in the Leiden University Medical Centre (LUMC). From October 2014, a routine clinical care pathway was implemented in which all older patients with HNC were referred to the Department of Gerontology and Geriatrics for a geriatric screening before treatment. The result of this geriatric screening was discussed in the multidisciplinary team. Patients were referred when aged 70 years and older, or younger but with multiple comorbidities, diagnosed with stage III‐IV HNC, or diagnosed with a lower stage HNC but needing invasive treatment, for geriatric screening before their invasive treatment. In this study, HNC was considered as cancer in the head and neck region needing invasive treatment by the head and neck surgeon. This includes cancer in the sinonasal or oral regions, nasopharynx, oropharynx, hypopharynx, supraglottic, the larynx, the salivary glands, or the proximal esophagus. However, also patients with large or regionally metastasized dermal cancer, lymphoma, an unknown primary, or a recurrent tumor were referred for geriatric assessment. Patients with thyroid cancer are not included in this study, because in the Netherlands thyroid cancer is not treated by a head and neck surgeon. For the retrospective collection and analysis of the data from these patients, the Medical Ethical Committee of the LUMC issued a “certificate of no objection.”
2.2. Determinants
Collected demographics were age, sex, marital status, and level of education. High education level was defined as university or higher vocational training and low education is defined as elementary school, community college, and secondary education. The Adult Comorbidity Evaluation‐27 score (ACE‐27) was calculated.14 The ACE‐27 has specifically been developed for patients with cancer in general. This index contains 27 different comorbidities from various organ systems. Grade 0 corresponds to no comorbidity, grade 1 to mild comorbidity, grade 2 to moderate comorbidity, and grade 3 to severe comorbidity.15, 16 Disease severity indicators consisted of tumor site, tumor stage, and whether the tumor was a new primary tumor. Tumor stage was directly extracted from the medical record.17 Geriatric measurements were the Katz Index of Independence in Activities of Daily Living (Katz ADL),18 the Lawton Instrumental Activities of Daily Living (IADL),19 the 6‐Item Cognitive Impairment Test (6CIT),20 the Mini Nutritional Assessment (MNA),21 and the Identification of Seniors At Risk—Hospitalized Patients questionnaire (ISAR‐HP).22 The Katz ADL score ranges from 0 to 6 and the Lawton IADL score ranges from 0 to 24, a higher score corresponds to more functional dependency. The 6CIT is a short cognition test20 and has a maximum score of 28 points, in this routine clinical care pathway, a score ≥8 is considered as abnormal, suggesting cognitive impairment. Nutritional status was assessed with the MNA questionnaire, a screening tool consisting of 6 questions to estimate the risk of malnutrition,21 a cutoff point of ≤11 was used to define (the risk for) malnutrition. The ISAR‐HP ranges from 0 to 5 and is a screening tool to assess the risk for development of functional decline. A cutoff point of ≥2 points was used to define this risk.22 Furthermore, the use of a walking device was extracted from the medical record.
2.3. Outcome
The main outcome of this study was mortality at 12 months of follow‐up after start of treatment. Mortality data were extracted from the municipal records.
2.4. Statistical methods
Baseline characteristics are presented as mean with SD in case of normal distribution, median with interquartile range (IQR) in case of skewed distribution, or as numbers with percentages. Different groups were compared using the t test for continuous normally distributed data, chi‐square test for categorical data, and the Mann‐Whitney U test for skewed data. To investigate the association between baseline characteristics and mortality, a Cox regression model was used. In the multivariable model (Table 2), treatment intention was not used as a determinant, to avoid overcorrection, because treatment intention is based on all the other determinants. In the multivariable analysis reported in Table 3, we stratified the analysis for curative intention. Hazard ratios with 95% confidence intervals (CI) were calculated, and a P‐value of <0.05 was considered significant. All analyses were performed using SPSS (IBM version 23; IBM Corp., Armonk, New York).
3. RESULTS
A total of 102 older patients with HNC were included in the present study. Table 1 shows the baseline characteristics of this population. The median age was 78.7 years (IQR, 72.3‐84.5) and 71 patients (69.6%) were men. Mild or moderate comorbidity was observed in 71 patients (69.6%) and 25 patients (24.5%) had severe comorbidity. A minority of the patients were diagnosed with skin cancer in the head and neck region (24.5%). Most patients (n = 72) had a newly diagnosed head and neck tumor (70.6%), and 62 patients (65.6%) had stage III‐IV cancer. More than 25% of the patients had cognitive impairment, almost 40% had (risk for) malnutrition, more than 40% had an abnormal ISAR‐HP, and 28.4% of the included patients used a walking device.
Table 1.
Baseline characteristics of the total study population
| Characteristics | No. of participants = 102 |
|---|---|
| Patient characteristics | |
| Age (years), median (IQR) | 78.7 (72.3‐84.5) |
| Male sex, n (%) | 71 (69.6) |
| Married, n (%) | 55 (53.9) |
| Educational level, n (%) | |
| Low | 67 (75.3) |
| High | 22 (24.7) |
| ACE‐27 score, n (%) | |
| No comorbidity | 6 (5.9) |
| Mild comorbidity | 37 (36.3) |
| Moderate comorbidity | 34 (33.3) |
| Severe comorbidity | 25 (24.5) |
| Number of drugs, median (IQR) | 6 (2.3‐8) |
| BMI, median (IQR) | 24.6 (21.6‐26.9) |
| Smoking history, n (%) | 82 (83.7) |
| Alcohol units/week, median (IQR) | 5.0 (0‐14) |
| Disease specific | |
| Tumor site, n (%) | |
| Oral cavity | 24 (23.5) |
| Pharynx | 24 (23.5) |
| Larynx | 9 (8.8) |
| Salivary gland | 8 (7.8) |
| Skin of head and neck region | 24 (24.5) |
| Othera | 13 (12.7) |
| New primary tumor | 72 (70.6) |
| Stage grouping, n (%) | |
| I‐II | 33 (34.4) |
| III‐IV | 62 (65.6) |
| Treatment goal, n (%) | |
| Curative | 67 (65.7) |
| Palliative | 35 (34.3) |
| Geriatric domains | |
| Cognitive impairment, n (%) | 25 (25.3) |
| Functional dependent, n (%) | 14 (13.7) |
| Dependent in IADL function, n (%) | 10 (9.9) |
| Risk of malnutrition or malnourished, n (%) | 40 (39.2) |
| Risk for functional decline after hospitalization, n (%) | 24 (41.4) |
| Use of a walking device, n (%) | 29 (28.4) |
Abbreviations: ACE‐27, Adult Comorbidity Evaluation Score; IADL = Independent Activities in Daily Living; IQR, interquartile range; MNA, Mini Nutritional Assessment; n, number.
Data incomplete for educational level (n = 89), number of drugs (n = 100), BMI (n = 101), smoking history (n = 98), alcohol consumption (n = 97), stage of disease (n = 96), 6‐CIT score (n = 99), IADL score (n = 101).
In the other group were included: unknown primary tumor, sinonasal tumor, proximal esophagus tumors, lymphoma of head and neck and vestibular schwannoma.
Figure 1 shows the cumulative survival curve of all included patients. Within 1 year, 42.3% of the patients were deceased. Table 2 shows the risk of 1‐year mortality for baseline determinants for all included patients. In the univariable analysis, several determinants were associated with an increased mortality; a low BMI with a hazard ratio (HR, 0.89; 95% CI, 0.83‐0.95) compared to a higher BMI, stage III‐IV (HR, 4.12; 95% CI, 1.61‐10.60) compared to stage I‐II, and treatment with palliative intention (HR, 5.16; 95% CI, 2.74‐9.72) compared to curative intention. Also, (risk for) malnutrition was associated with an increased mortality (HR, 3.40; 95% CI, 1.83‐6.33) compared to no (risk for) malnutrition and also dependency in IADL functioning (HR, 1.07; 95% CI, 1.02‐1.12) compared with no dependency. Independent factors for a higher risk for 1‐year mortality were male sex (HR, 4.30; 95% CI, 1.35‐13.67), an abnormal MNA score (HR, 2.55; 95% CI, 1.19‐5.16), and the use of a walking device (HR, 2.80; 95% CI, 1.13‐6.93).
Figure 1.
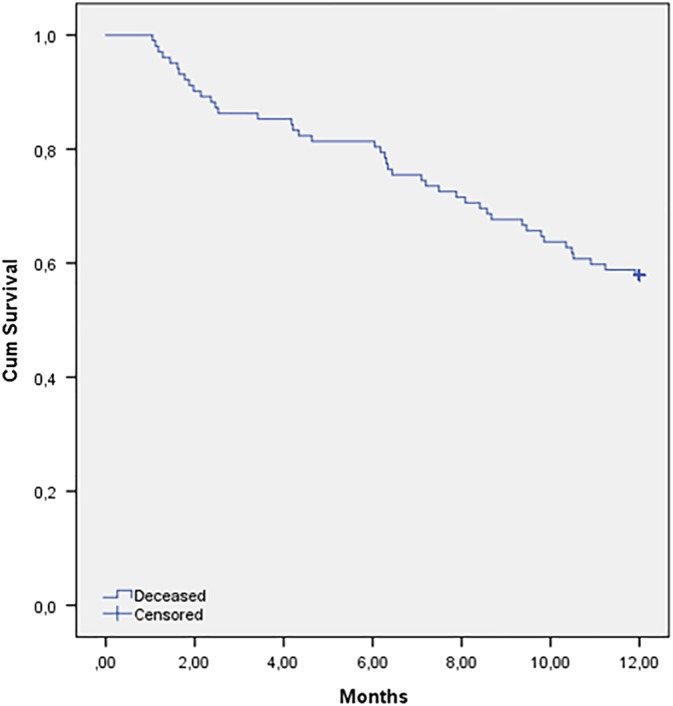
Cumulative survival curve of the total study population [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
The association between baseline characteristics and mortality after 1 year of follow‐up of all included patients
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P‐value | HR | 95% CI | P‐value |
| Age | 0.99 | 0.95‐1.03 | 0.50 | 1.00 | 0.96‐1.05 | 0.91 |
| Male sex | 1.58 | 0.78‐3.20 | 0.21 | 4.30 | 1.35‐13.67 | 0.01 |
| Marital status | ||||||
| Married | Ref | … | … | |||
| Single | 1.00 | 0.58‐1.82 | 0.99 | … | … | … |
| ACE‐27 score | ||||||
| 0‐1 | Ref | … | … | Ref | … | … |
| 2‐3 | 1.77 | 0.91‐3.44 | 0.09 | 1.42 | 0.65‐3.08 | 0.38 |
| Number of drugs | 1.00 | 0.92‐1.07 | 0.79 | 0.97 | 0.89‐1.07 | 0.59 |
| BMI | 0.89 | 0.83‐0.95 | 0.001 | … | … | … |
| Stage of disease | ||||||
| 0‐II | Ref | … | … | Ref | … | … |
| III‐IV | 4.12 | 1.61‐10.60 | 0.003 | 2.03 | 0.79‐5.27 | 0.14 |
| Goal of treatment | ||||||
| Curative | Ref | … | … | |||
| Palliative | 5.16 | 2.74‐9.72 | <0.001 | … | … | … |
| Cognitive impairment | 1.73 | 0.91‐3.29 | 0.09 | 1.76 | 0.61‐5.07 | 0.29 |
| (Risk for) malnutrition | 3.40 | 1.83‐6.33 | <0.001 | 2.55 | 1.23‐5.26 | 0.01 |
| Functional dependent | 1.07 | 0.93‐1.18 | 0.44 | … | … | … |
| Dependent in IADL function | 1.07 | 1.02‐1.12 | 0.01 | 1.07 | 0.97‐1.17 | 0.20 |
| Use of a walking device | 1.77 | 0.95‐3.29 | 0.07 | 2.80 | 1.13‐6.93 | 0.03 |
Abbreviations: 6‐CIT, 6‐Item Cognitive Impairment Test; 95% CI, 95% confidence interval; ACE‐27, Adult Comorbidity Evaluation Score; ADL, Activities in Daily Living; IADL, Independent Activities in Daily Living; HR, hazard ratio; MNA, Mini Nutritional Assessment.
Multivariate analysis was done with complete data for 85 patients.
Figure 2 shows the sensitivity analysis in which we stratified the cumulative survival to treatment intention. After 12 months of follow‐up, 74.3% (n = 26) of the patients treated with palliative intention were deceased in contrast to 25.4% (n = 17) of the patients treated with curative intention. The median survival for the patients treated with palliative intention was 6.3 months. Table 3 shows the risk of 1‐year mortality for baseline determinants for the patients treated with curative intention. Independent factors for a higher risk for 1‐year mortality were male sex (HR, 27.64; 95% CI, 1.56‐490.1), (risk for) malnutrition (HR, 6.81; 95% CI, 1.84‐25.22) compared to no (risk for) malnutrition, and the use of a walking device (HR, 6.93; 95% CI, 1.58‐30.46) compared with no use of a walking device.
Figure 2.
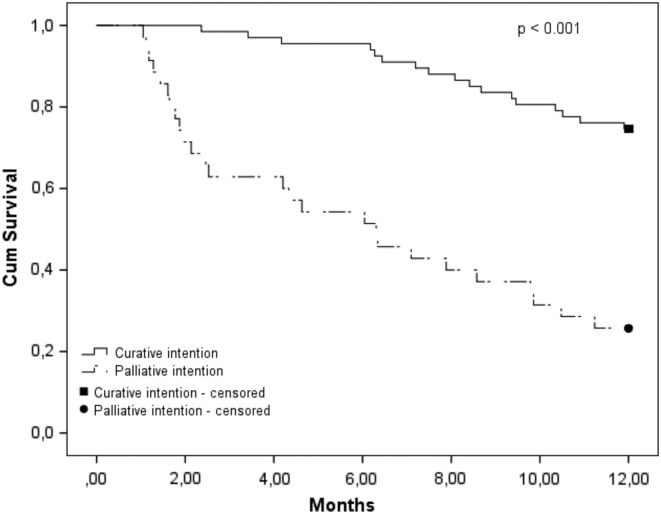
Cumulative survival curve stratified into treatment intention
Table 3.
Independent determinants for 1‐year survival in curative treated patients
| Multivariable analysis | |||
|---|---|---|---|
| Variable | HR | 95% CI | P‐value |
| Age | 1.04 | 0.95‐1.14 | 0.35 |
| Male sex | 27.64 | 1.56‐490.1 | 0.02 |
| ACE‐27 score | |||
| 0‐1 | Ref | … | … |
| 2‐3 | 2.41 | 0.65‐8.95 | 0.19 |
| Number of drugs | 1.00 | 0.86‐1.16 | 0.99 |
| Stage of disease | |||
| 0‐II | Ref | … | … |
| III‐IV | 0.77 | 0.19‐3.02 | 0.70 |
| Cognitive impairment | 2.74 | 0.51‐14.85 | 0.24 |
| (Risk for) malnutrition | 6.81 | 1.84‐25.22 | 0.004 |
| Dependent in IADL functioning | 1.05 | 0.88‐1.24 | 0.59 |
| Use of a walking device | 6.93 | 1.58‐30.46 | 0.01 |
Abbreviations: 6‐CIT, 6‐Item Cognitive Impairment Test; 95% CI, 95% confidence interval; ACE‐27, Adult Comorbidity Evaluation Score; ADL, Activities in Daily Living; IADL, Independent Activities in Daily Living; HR, hazard ratio; MNA, Mini Nutritional Assessment.
Multivariate analysis was done with complete data for 60 patients. The bold values are statistically significant.
4. DISCUSSION
The main findings of this study are that the mortality rate is high, even in the patients treated with curative intent and that (the risk for) malnutrition and mobility were determinants associated with 1‐year mortality, independent of tumor stage, age, and comorbidity in older patients with cancer in the head and neck region.
In our study, several geriatric impairments were associated with 1‐year mortality, but after correcting for sex, age, and disease‐specific determinants, only the use of a walking device was independently associated with 1‐year mortality. Our recently published systematic review reports that in 64% of the reported associations, a decline in functional or cognitive impairment, mood, or social environment was associated with adverse outcomes.13 Very little is known about the use and the predictive value of a geriatric assessment in HNC, because most of the studies included low patient numbers and therefore have a lack of power. In other fields of medicine, a geriatric assessment is well established to guide decision making or to identify unknown geriatric impairments (such as cognitive impairment and functional dependency), which can be taken into account before or during treatment.23, 24 To our knowledge, it is not previously reported that the use of a walking device is associated with 1‐year mortality.
In this cohort, the 1‐year mortality rates are high: 42.3% overall in the included patients, but also 25% of the patients treated with curative intent are deceased within 1 year. In general, the 5‐year survival in patients with HNC is around 50% depending on tumor stage, tumor type, and treatment intention.7, 8 Treatment with curative intention can contain chemoradiation or an operation (depending on the type of HNC) and followed by (chemo)radiation therapy when indicated. In patients aged 70 years and older, adding chemotherapy to radiotherapy does not contribute to higher survival rates.25, 26 Life expectancy is obviously lower when getting older and therefore could be taken into account. The knowledge of the survival rates, the extensiveness of the treatment, and the predictors reported in this study, and in order to personalize the treatment plan for this vulnerable population, more research should be done.
We found a relatively high prevalence of geriatric impairments. For example, a quarter of the included patients were cognitively impaired. Compared to the limited literature available, the proportion of patients who are cognitively impaired reported in our study could potentially even be higher. Williams et al. describe 83 adults with HNC before treatment and report that more than 50% were cognitively impaired.27 The study of Bond et al. describes 70 patients with HNC and reports around 47% of cognitively impaired patients.28 So, probably the cognition test used in our study was not comprehensive enough to recognize subtle cognitive impairment. The clinical implications of cognitive impairment before treatment are not well described in literature, but most likely negatively affect patients with HNC like in other fields of oncologic medicine.29 In these fields, it is known that being cognitively impaired before treatment gives a higher risk for adverse health outcomes such as toxicity, not able to finish treatment, side effects, and mortality.28, 30 Besides, it is probably more difficult for patients with cognitive dysfunction to weigh the risk and benefits for cancer treatment, which impedes good shared decision making, to comply with the treatment plan and to adequately ask for medical attention if necessary. Therefore, it could be informative for the patient as well as the treating specialist to have insight in the cognitive status and to take this information into account.
There are some limitations to our study. First, the included study population was relatively small. Second, the outcome of this study was mortality, whereas remaining functional and cognitive independent and quality of life would be also interesting outcomes to assess. Finally, the tumor types in the present study were heterogeneous. Strengths of this study include the relatively unselected patient cohort which has a result that the included patients in this study were a reflection of the older patients with HNC seen in clinical practice. All included participants underwent a comprehensive geriatric assessment. And this study complements the, until now limited, available literature.
5. CONCLUSIONS
In older patients with HNC, the mortality rates are high. Nutritional status and mobility are determinants of 1‐year mortality, independent of tumor stage, age, and comorbidity.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
ACKNOWLEDGMENTS
The Institute for Evidence‐based Medicine in Old Age (IEMO) is funded by the Dutch Ministry of Health and Welfare and supported by ZonMw (project number 62700.3001). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
van Deudekom FJ, van der Velden L‐A, Zijl WH, et al. Geriatric assessment and 1‐year mortality in older patients with cancer in the head and neck region: A cohort study. Head & Neck. 2019;41:2477–2483. 10.1002/hed.25714
Funding information The Institute for Evidence‐Based Medicine in Old Age (IEMO) is supported by the Dutch Ministry of Health, Welfare and Sport and supported by the Netherlands Organisation for Health Research and Development ZonMW, Grant/Award Number: 62700.3001
REFERENCES
- 1. Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777‐789. [DOI] [PubMed] [Google Scholar]
- 2. Franceschi S, Bidoli E, Negri E, Barbone F, La Vecchia C. Alcohol and cancers of the upper aerodigestive tract in men and women. Cancer Epidemiol Biomarkers Prev. 1994;3(4):299‐304. [PubMed] [Google Scholar]
- 3. Anstey KJ, von Sanden C, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta‐analysis of prospective studies. Am J Epidemiol. 2007;166(4):367‐378. [DOI] [PubMed] [Google Scholar]
- 4. Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am J Public Health. 2003;93(6):994‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. North TL, Palmer TM, Lewis SJ, et al. Effect of smoking on physical and cognitive capability in later life: a multicohort study using observational and genetic approaches. BMJ Open. 2015;5(12):e008393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758‐2765. [DOI] [PubMed] [Google Scholar]
- 7. Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15(9):994‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michiels S, Le Maitre A, Buyse M, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: meta‐analyses of individual patient data. Lancet Oncol. 2009;10(4):341‐350. [DOI] [PubMed] [Google Scholar]
- 9. Feng MA, McMillan DT, Crowell K, Muss H, Nielsen ME, Smith AB. Geriatric assessment in surgical oncology: a systematic review. J Surg Res. 2015;193(1):265‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091‐1101. [DOI] [PubMed] [Google Scholar]
- 11. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288‐300. [DOI] [PubMed] [Google Scholar]
- 12. Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005;55(3):241‐252. [DOI] [PubMed] [Google Scholar]
- 13. van Deudekom FJ, Schimberg AS, Kallenberg MH, Slingerland M, van der Velden LA, Mooijaart SP. Functional and cognitive impairment, social environment, frailty and adverse health outcomes in older patients with head and neck cancer: a systematic review. Oral Oncol. 2017;64:27‐36. [DOI] [PubMed] [Google Scholar]
- 14. Piccirillo JF, Creech CM, Zequeira R, Anderson S, Johnston AS. Inclusion of comorbidity into oncology data registries. J Reg Manag. 1999;26(2):66‐70. [Google Scholar]
- 15. Boje CR. Impact of comorbidity on treatment outcome in head and neck squamous cell carcinoma—a systematic review. Radiother Oncol. 2014;110(1):81‐90. [DOI] [PubMed] [Google Scholar]
- 16. Nesic VS, Petrovic ZM, Sipetic SB, Jesic SD, Soldatovic IA, Kastratovic DA. Comparison of the adult comorbidity evaluation 27 and the Charlson comorbidity indices in patients with laryngeal squamous cell carcinoma. J Laryngol Otol. 2012;126(5):516‐524. [DOI] [PubMed] [Google Scholar]
- 17. Patel SG, Shah JP. TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA Cancer J Clin. 2005;55(4):242‐258. quiz 261–242, 264. [DOI] [PubMed] [Google Scholar]
- 18. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of Adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914‐919. [DOI] [PubMed] [Google Scholar]
- 19. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179‐186. [PubMed] [Google Scholar]
- 20. Tuijl JP, Scholte EM, de Craen AJ, van der Mast RC. Screening for cognitive impairment in older general hospital patients: comparison of the Six‐Item Cognitive Impairment Test with the Mini‐Mental State Examination. Int J Geriatr Psychiatry. 2012;27(7):755‐762. [DOI] [PubMed] [Google Scholar]
- 21. Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin Geriatr Med. 2002;18(4):737‐757. [DOI] [PubMed] [Google Scholar]
- 22. Hoogerduijn JG, Buurman BM, Korevaar JC, Grobbee DE, de Rooij SE, Schuurmans MJ. The prediction of functional decline in older hospitalised patients. Age Ageing. 2012;41(3):381‐387. [DOI] [PubMed] [Google Scholar]
- 23. Antonio M, Saldana J, Linares J, et al. Geriatric assessment may help decision‐making in elderly patients with inoperable, locally advanced non‐small‐cell lung cancer. Br J Cancer. 2018;118:639‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4‐5):330‐339. 10.1016/j.euf.2017.10.010. Epub 2018 Jan 10. [DOI] [PubMed] [Google Scholar]
- 25. Amini A, Jones BL, McDermott JD, et al. Survival outcomes with concurrent chemoradiation for elderly patients with locally advanced head and neck cancer according to the National Cancer Data Base. Cancer. 2016;122(10):1533‐1543. [DOI] [PubMed] [Google Scholar]
- 26. Lacas B, Bourhis J, Overgaard J, et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta‐analysis. Lancet Oncol. 2017;18(9):1221‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams AM, Lindholm J, Siddiqui F, Ghanem TA, Chang SS. Clinical assessment of cognitive function in patients with head and neck cancer: prevalence and correlates. Otolaryngology Head Neck Surg. 2017;157(5):808‐815. [DOI] [PubMed] [Google Scholar]
- 28. Bond SM, Dietrich MS, Murphy BA. Neurocognitive function in head and neck cancer patients prior to treatment. Support Care Cancer. 2012;20(1):149‐157. [DOI] [PubMed] [Google Scholar]
- 29. Libert Y, Dubruille S, Borghgraef C, et al. Vulnerabilities in older patients when cancer treatment is initiated: does a cognitive impairment impact the two‐year survival? PLoS One. 2016;11(8):e0159734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamaker ME, Vos AG, Smorenburg CH, de Rooij SE, van Munster BC. The value of geriatric assessments in predicting treatment tolerance and all‐cause mortality in older patients with cancer. Oncologist. 2012;17(11):1439‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]


