Abstract
Indoor and outdoor airborne pollutants modify our environment and represent a growing threat to human health worldwide. Airborne pollution effects on respiratory and cardiac health and diseases have been well established, but its impact on skin remains poorly described. Nonetheless, the skin is one of the main targets of pollutants, which reach the superficial and deeper skin layers by transcutaneous and systemic routes. In this review, we report the outcomes of basic and clinical research studies monitoring pollutant levels in human tissues including the skin and hair. We present a current understanding of the biochemical and biophysical effects of pollutants on skin metabolism, inflammatory processes and oxidative stress, with a focus on polyaromatic hydrocarbons and ground‐level ozone that are widespread outdoor pollutants whose effects are mostly studied. We reviewed the literature to report the clinical effects of pollutants on skin health and skin ageing and their impact on some chronic inflammatory skin diseases. We also discuss the potential interactions of airborne pollutants with either ultraviolet radiation or human skin microbiota and their specific impact on skin health.
Introduction
Outdoor pollution is associated with about 3.3 million premature deaths per year worldwide, with the Asian continent bearing most of the burden.1 In addition, household pollution, resulting mainly from solid fuel cooking, is a serious threat for human health2 and is deemed responsible for nearly 4 million premature death per year worldwide.3
Airborne pollution is defined as contamination of outdoor and indoor environments by any chemical, physical or biological agent that modifies the natural characteristics of the atmosphere.4
The main outdoor air pollutants, as defined by the United States Environmental Protection Agency (US EPA), derive from gaseous compounds (nitrogen dioxide [NO2], sulphur dioxide [SO2], carbon monoxide [CO]), particulate matter (PM) and heavy metals (Table 1) (for reviews5, 6). In addition, nitrogen oxide compounds interact with volatile organic compounds (VOCs) upon ultraviolet (UV) photoactivation to generate ground‐level ozone (O3) (Table 1). Other classes of air pollutants are persistent organic compounds (POPs), semi‐volatile compounds (SVOCs) and polyaromatic hydrocarbons (PAHs; Table 1). Moreover, some pollutants (e.g. ground‐level O3, PM) and specific PAHs like benzo[a]pyrene (B[a]P) and indeno[1,2,3‐cd]pyrene (I[cd]P) may become more toxic in the presence of UV radiation.7, 8 Airborne PAHs are widespread (Table 1) and several of them are present in cigarette smoke (e.g. B[a]P), which is often used as a surrogate for air pollution in experimental settings.9, 10
Table 1.
Various types of pollutants
| Air pollutant class | Namea , b | Potential sources of pollutants |
|---|---|---|
| Gaseous | Carbon monoxidea | Fossil‐fuel combustion, vehicle emission |
| Nitrogen dioxidea | Fuel combustion, wood burning, vehicle emissions, waste incineration | |
| Ozonea | Formed by interaction of VOCs and NOx compounds upon UV‐photoactivation | |
| Sulphur dioxidea | Fuel combustion, vehicle emissions, maritime transport, electric utilities, industrial facilities, volcanoes | |
| Heavy metals | Leada | Metal refineries, battery manufacturing, waste incineration, industrial facilities, leaded fuel, lead‐based paint, plumbing material |
| Cadmium | Battery manufacturing, aircraft industry, television manufacturing | |
| Nickel | Casting, welding, battery manufacture | |
| Arsenic | Battery manufacture, minerals | |
| Particulate matter (PM) c a | Coarse PM10 (2.5–10 μm) | Road dust, unpaved roads, forest fires, waste degradation including electronic waste, cooking processes |
| Fine PM2.5 (<2.5 μm) | Fossil‐fuel combustion, industrial facilities, maritime transport, biomass burning, waste incineration, cooking | |
| Ultrafine PM0.1 (<0.1 μm) | Vehicle emission, industrial facilities | |
| Persistent organic compounds | Dioxins, dioxin‐like polychlorinated biphenyls | Herbicides, pesticides, industrial processes, forest fires, volcanic eruptions |
| Polyaromatic hydrocarbons (PAHs) d | Examples: acenaphthene, acenaphthylene, anthracene, benz[a]anthracene, benzo[a]pyrene (B[a]P)e, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[g,h,i]perylene, chrysene, dibenz[a,h]anthracene, fluoranthene, fluorene, indenol [1,2,3‐c,d]pyrene (I[cd]P), naphthalene, phenanthrene, pyrene | Incomplete combustion of organic material such as biomass and garbage, vehicle emissions, fumes from asphalt roads, cigarette smoke, forest fires, volcanic eruptions and grilled or charred meats |
| Semivolatile organic compounds (SVOCs) | Examples: Butylated hydroxytoluene, diethyl phthalate, geranyl acetone, nicotine (in free‐base form), parabens | Solvents, fragrances, bactericides, antimicrobial agents, flooring, furniture |
| Volatile organic compounds (VOCs) | Examples: Acetaldehyde, dimethylformamide, formaldehyde, hexane, styrene, toluene, xylene | Fuel combustion, aircraft emission, household products, chemical solvents, paints, varnishes, cigarette smoke |
“6‐criteria” air pollutants as defined by US EPA United States Environmental Pollution Agency https://www.epa.gov/criteria-air-pollutants/naaqs-table
See http://ec.europa.eu/environment/air/quality/standards.htm for the European air quality standards
Mixture between solid particles and liquid droplets found in the air
PAHs may be part of PM: 16 “priority” PAH pollutants defined by US EPA are listed as examples
In Europe, the levels of PAHs in the air are estimated through benzo[a]pyrene monitoring (standard: 1 ng/m3 per year)
NOx, nitrogen oxide compounds; UV, ultraviolet.
The household environment is a wide source of indoor air pollutants, although their contribution is often neglected.3 The concentration of VOCs and SVOCs – emanating from household products (Table 1) and combustion of fuel used for cooking, heating and lighting – has been found to be higher indoors than outdoors.11
At the interface with the air, skin is the target of several environmental stressors.5, 10, 12, 13, 14 Here, we provide an overview of how pollutants penetrate the skin via direct transcutaneous uptake or via indirect systemic distribution of inhaled or ingested pollution through the blood. We report on recent basic research studies investigating the biochemical and molecular changes induced by pollutants in skin and on clinical studies investigating the impact of pollutants on skin ageing and inflammatory skin diseases. Overall, the authors aimed to overview the main topics of the current knowledge on the impact of pollution on skin health and diseases to inform dermatologists so that they could adapt their daily clinical practice and specifically answer to the concerns of their patients.
Methods
A board of 6 dermatologists, E Araviiskaia, E Berardesca, T Bieber, B Dréno, G Gontijo, M Sanchez‐Viera, and 2 scientists from L'Oréal L Marrot and B Chuberre was constituted. Literature review was performed by searching PubMed with the following key words: air pollution, outdoor pollution, indoor pollution, volatile organic compounds, particulate matter, PM2.5, PM10, photosensitizer, oxidative stress, environmental stressors, dermal uptake, skin pigmentation, acne, skin health, skin microbiome, skin ageing, photoaging. The most recent (September 2012 to June 2018) reviews and original articles on basic and clinical research were selected. Were excluded the studies presenting limited confidence in the estimated effect (e.g. low number of subjects enrolled, absence of statistics). Older articles were also included if relevant to the discussion. Furthermore, the design and relevant data of clinical studies have been provided as Table S1 (supporting information).
Pollutant levels in the skin
Skin may be affected by environmental pollutants concentrating at its surface. Levels of uptake of airborne pollutants by the transcutaneous route have been reported to be similar to those measured after the inhalation of some indoor pollutants such as SVOCs and other VOCs15, 16, 17 (Table 1). Dermal exposure and uptake have also been analysed in specific populations, such as in coke oven workers,18 in asphalt‐paving workers,19, 20 asphalt‐roofing workers21 and chimney sweeps22 (Table 2). These studies all indicate that dermal uptake is a direct route of pollutant contamination (Fig. 1).
Table 2.
Detection of PAH levels in human skin and hair
| Human tissue targeted | Number of samples (origin, period) | Pollutant type | Geometric mean, median (range) or mean ± SD concentration | Bioanalytical method | Author, date (country, period) |
|---|---|---|---|---|---|
| Skin surface | 12 (coke oven workers: 5 consecutive 8‐h shifts)a , b | Pyrene | 21–166 μg/8 h leading to an estimated exposure of 4–34 μg/day and 119–893 nmol/weekc | Sonicated extraction in dichloromethane + HPLC‐fluo | Van Rooij et al., 1993 (the Netherlands, September 1990) |
|
24 (nonsmoker asphalt workers: single 10‐h shifts)a
,
b
144 pads in total |
∑16 PAHs | 85.79 μg (30.14–623.66)d | Sonicated extraction in dichloromethane + PTV/GC/MS | Fustinoni et al., 2009 (Milan, Lodi, Italy, spring and summer 2003) | |
| Anthracene | 10.86 μg (3.86–142.19) | ||||
| Fluoranthene | 5.01 μg (1.33–55.12) | ||||
| Fluorene | 5.84 μg (0.48‐9.09) | ||||
| Phenanthrene | 25.21 μg (5.82‐213.88) | ||||
| Pyrene | 7.42 μg (1.32‐55.12) | ||||
| 26 (asphalt‐roofing workers: 2–5 consecutive days)a , b | Benzo[a]pyrene | 3.3 ng/cm2 | DMSO extraction + HPLC | McClean et al., 2007 (Ohio and Kentucky, USA, from April to September 1998) | |
| Pyrene | 11.0 ng/cm2 | ||||
| 12 (asphalt paving workers: 6 smokers, three consecutive working‐days monitored over 4 weeks) | ∑6 PAHs including | POD sampler and sunflower oil hand wash technique post shift | Cavallari et al., 2008 (Wisconsin and Indiana, USA, August to October 2008) | ||
| Phenanthrene | 0.69 ng/cm2 (0.034–15.76) (POD) | ||||
| 1.37 ng/cm2 (–) (HW) | |||||
| Pyrene | 0.30 ng/cm2 (0.087–7.67) (POD) | ||||
| 0.29 ng/cm2 (0.03–6.1) (HW) | |||||
| 5 chimney sweeps (monitored at the end of a working day) | Benzo[a]pyrene | Range: 12.3–40.4 ng (back of the hand)h | Ultrasonic bath + filtration and HPLC‐fluo | Kammer et al., 2011 (Sweden) | |
| Pyrene | Range: 30.9–70.3 ng (back of the hand)h | ||||
| Hair | 50–100 mg of haire (6 smokers, 14 nonsmokers) | ∑14 PAHs including: | n‐hexane washing, alkaline digestion liquid extraction + HPLC‐fluo | Toriba et al., 2003 (Japan) | |
| Benzo[a]pyrene | 1.1 ± 0.7 and 0.7 ± 0.3f pg/mg hair | ||||
| Benzo[k]fluoranthene | 1.2 ± 1.4 and 0.2 ± 0.1f pg/mg hair | ||||
| Chrysene | 2.7 ± 2.0 and 1.5 ± 0.5f pg/mg hair | ||||
| Anthracene | 8.2 ± 7.4 and 3.5 ± 2.4f pg/mg hair | ||||
| Fluoranthene | 24.1 ± 13.5 and 19.4 ± 11.1f pg/mg hair | ||||
| Naphthalene | 511 ± 118 and 628 ± 348f pg/mg hair | ||||
| 105 hair samples (61 smokers, 44 nonsmokers | ∑12 g OH‐PAHs | 24–67 190 pmol/g (median: 118 pmol/g) | Water washing, liquid–liquid extraction with dichloromethane and then cyclohexane + GC‐NCI‐MS | Appenzeller et al., 2012 (Luxembourg, 2007–2008) | |
| 2‐OH‐naphthalene | 213 ± 419 and 102 ± 77f pmol/g | ||||
| 1‐OH‐naphthalene | 5181 ± 17 029 and 1579 ± 1972f pmol/g | ||||
| 9‐OH‐fluorene | 88 ± 5 and 140 ± 161f pmol/g | ||||
| 9‐OH‐phenanthrene | 1570 ± 2182 and 4183 ± 8867f pmol/g | ||||
| 2‐OH‐fluorene | 92 ± 104 and 28 ± 28f pmol/g |
Airborne exposure and urinary metabolites (only the latter in McClean21) were also investigated and were correlated with dermal exposure.
Pollutant exposure and dermal uptake was evaluated by exposure pads (18‐mm to 60‐mm diameter) that were mounted at different body sites (jaw/neck, upper arm, shoulder, wrist, groin and ankle), or wrist only for McClean et al.21
It was estimated that about 75% (28–95%) of the total dose of pyrene on average is absorbed through the skin.
Dermal and airborne exposure were estimated to contribute similarly to the total internal dose of PAHs.
2–3 cm of hair cut near the hair root from the back of the head. Except for naphthalene, all values were higher in smokers than in nonsmokers, with a significant difference (P < 0.05) for benzo[k]fluoranthene, chrysene and anthracene.
Mean ± SD values in smokers and nonsmokers, respectively.
12 PAHs selected on the basis of being the most frequently analysed in urine. Details are provided for the most commonly detected PAHs in hair samples: 2‐OH‐naphthalene (N = 64/105), 1‐OH‐naphthalene (N = 13/105), 9‐OH‐fluorene (N = 13/105), 9‐OH‐phenanthrene (N = 9/105) and 2‐OH‐fluorene (N = 8).
Each number represents the sum of five consecutive tapes (3 × 5 cm2).
BaPeq, benzo[a]pyrene equivalent; DMSO, dimethyl sulfoxide; GC/MS, gas chromatography coupled with mass spectrometry; GC/NCI/MS, gas chromatography‐negative chemical ionization‐mass spectrometry; HPLC‐fluo, high performance liquid chromatography and fluorescence detection; OH‐PAHs, monohydroxy‐PAHs; PAHs, polyaromatic hydrocarbons; POD, passive organic dermal; PTV, programmable temperature vaporisation; (–), missing value.
Figure 1.
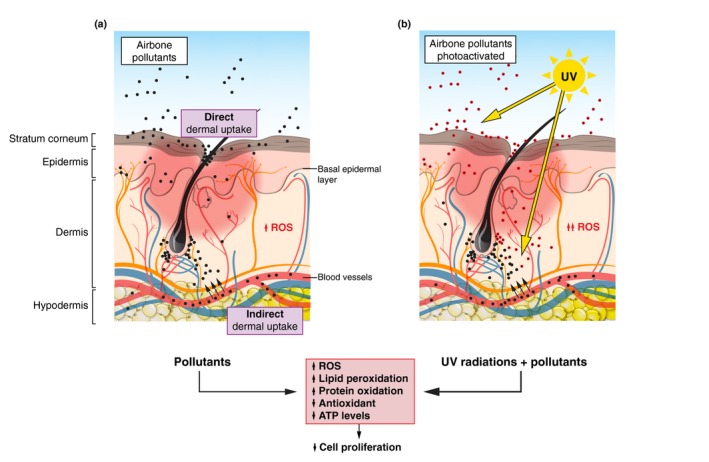
Direct and indirect pollution uptake – Biochemical and clinical effects of pollutants and potential interactions with UV light. Direct dermal uptake with the accumulation of airborne pollutants (PAHs, PM, O3; black dots) on the stratum corneum and subsequent penetration. Indirect dermal uptake in the dermis and basal epidermal layer with the systemic blood distribution of inhaled or ingested pollutants that may have been metabolized (black dots). Left panel: Airborne pollutants (black dots) penetrate the skin directly or indirectly (black arrows) and induce biochemical effects such as an increase in the production of ROS via the aryl hydrocarbon receptor, an elevation of lipid peroxidation, protein oxidation and cell death (apoptosis), and a reduction in cell proliferation and antioxidant and ATP levels. Clinically, the effects of pollutants correspond to the exacerbation of skin ageing processes, the symptoms of inflammatory diseases (e.g. atopic dermatitis) and the deregulation of skin moisture. Right panel: UV radiation penetrates the skin where it might induce the production of ROS. In addition, some pollutants located at the surface or within the skin might induce the production of ROS (red and black filled circles). The combination of UV radiation and pollutants might exacerbate the biochemical and clinical effects of airborne pollutants. ATP, adenosine triphosphate; O3, ground‐level ozone; PAHs, polycyclic aromatic hydrocarbons; PM, particulate matter; ROS, reactive oxygen species; UV, ultraviolet.
Radioactive‐labelling studies established that inhaled ultrafine carbon particles could then be recovered in the blood.23 Advances in bioanalytical methods have now revealed that PAHs and their metabolites can be found in the blood of adults,24, 25, 26, 27 children28, 29 and neonates,26, 30 as well as in urine18, 31, 32, 33 and maternal milk.26 Thus, subjects of all ages and all types of organs can be exposed to pollutants. Once inhaled or ingested (e.g. in grilled meat33, 34), pollutants may be distributed by the systemic circulation to the whole body and reach the dermis and the proliferative epidermal layer. Indeed, integrated PAHs have been detected in hair collected near the root and washed to eliminate externally accumulated pollutants35 (Table 2). PAH concentrations have been found to be higher in hair from smokers than from non‐smokers35 (Table 2). Appenzeller et al.36 found that levels of hair‐incorporated nicotine correlated positively with cigarette consumption and proposed that PAH concentrations of over 1 nmol/g or the detection of more than two PAHs in one hair sample may indicate specific exposure of the subject to PAHs (Table 2).
Overall, these studies show that skin is targeted by pollutants, either by direct accumulation on the skin surface or by indirect distribution by the systemic route after pollutant inhalation or ingestion (Fig. 1). Hence, PAHs (Table 1) can be detected not only in the air, but also in all human bodily fluids and hair (Table 2), making them a good study tool to analyse the effects of airborne pollution on human skin health and diseases.
Biochemical changes and molecular mechanisms induced by pollutants
Metabolism and inflammatory processes
Pollutants may activate cell metabolism and inflammatory processes. In normal human epidermal keratinocytes (NHEKs) exposed to O3, activation of the aryl hydrocarbon receptor (AHR) induced the expression of several cytochrome P450 genes, including CYP1A1.37 AHR activation and increased CYP1A1 expression have also been reported after exposure to B[a]P,38 PM2.5 39 and water‐soluble tobacco smoke extracts.40
Activation of the AHR pathway has also been implicated in the pathology of atopic dermatitis in humans. The neurotrophic factor Artemin (ARTN) has been identified as the missing link between pollution and AHR‐CYP1A1 in an atopic dermatitis mouse model.41 This prospective study also showed that epidermal CYP1A1 and ARTN messenger ribonucleic acid (RNA) levels were significantly higher in human skin samples from patients with atopic dermatitis (N = 20) than in those from patients with contact dermatitis (N = 5) or from healthy subjects (N = 20). AHR‐dependent elevated expression of CYP1A1 and ARTN was also detected in NHEKs and HaCaT cells exposed to diesel exhaust particles. Pollutants may therefore exacerbate atopic dermatitis symptoms via direct activation of AHR and subsequent ARTN overexpression.
Activation of the AHR pathway in NHEKs by PM or B[a]P has been linked to the induction of proinflammatory molecules, such as interleukin‐838 and cyclooxygenase‐242 via the production of reactive oxygen species (ROS) involved in oxidative stress processes.
Oxidative stress and potential interactions of airborne pollutants with UV light
A reduction in vitamin C and E levels after exposure to O3 was first identified in the upper epidermis of mouse skin43 (Table 3). Similar findings were reported in humans (N = 20) when forearm stratum corneum was experimentally exposed to environmental O3 levels44 (Table 3). A 10‐fold increase in lipid peroxidation was also observed in the mouse epidermis after exposure to O3.43 In humans (N = 9), higher levels of oxidization of squalene, a human sebum‐specific unsaturated fatty acid, have been reported in sebum experimentally exposed to cigarette smoke45 (Table 3).
Table 3.
In vitro and in vivo skin changes in oxidation status after exposure to pollutants
| Antioxidant status | Effect of pollutant vs. control | Pollutant or area | Skin or sebum collected from | Author, date |
|---|---|---|---|---|
| Vitamin C (ascorbic acid) |
26% loss P < 0.05 Student's t test |
O3 (10 ppm) 2 h |
Mouse epidermis | Thiele et al., 1997 |
| Vitamin E (α‐tocopherol) |
55% loss P < 0.01 Student's t test |
O3 (10 ppm) 2 h |
Mouse epidermis | Thiele et al., 1997 |
| Vitamin E (α‐tocopherol) | 70% loss |
O3 (0.8 ppm) 2 h |
Human epidermis | He et al., 2006 |
| Lipid peroxidation |
10‐fold increase P < 0.001 Student's t test |
O3 (10 ppm) 2 h |
Mouse epidermis | Thiele et al., 1997 |
| Lipid peroxidation | 2.3‐fold increase |
O3 (0.8 ppm) 2 h |
Human epidermis | He et al., 2006 |
| Squalene peroxidation | 270% increase | Cigarette smoke | Human sebum | Pham et al., 2015 |
| Squalene concentration |
1.8‐fold reduction P < 0.001 Mann–Whitney |
Mexico City vs. Cuernavaca | Human sebum | Lefebvre et al., 2015 |
| % squalene in lipids |
1.4‐fold reduction P < 0.05 Student's t test |
Urban vs. rural Shanghai | Human sebum | Lefebvre et al., 2016 |
| Ratio vitamin E/squalene |
11‐fold reduction P < 0.001 Mann–Whitney |
Mexico City vs. Cuernavaca | Human sebum | Lefebvre et al., 2015 |
| Oxidized proteins |
2.5‐fold reduction P < 0.05 Mann–Whitney |
Mexico City vs. Cuernavaca | Human stratum corneum | Lefebvre et al., 2015 |
| ATP |
2.7‐fold reduction P < 0.001 Mann–Whitney |
Mexico City vs. Cuernavaca | Human stratum corneum | Lefebvre et al., 2015 |
ATP, adenosine triphosphate; O3, ozone; ppm, parts per million.
The direct detection of ROS in NHEKs exposed to B[a]P also illustrated the pollution‐driven induction of oxidative stress processes in human skin cells.38 Cellular ROS production has been shown to mediate PM‐induced cytokine production in cultured primary keratinocytes.46 Oxidative stress responses were also observed in HaCaT cells exposed to concentrated air particles47 and in primary human keratinocytes exposed to cigarette smoke condensate48 or diesel particulate extract.49 Clinical evidence for an oxidative stress response occurring in humans after exposure to pollutants comes from two prospective clinical studies comparing the impact of pollution on subjects living in Mexico City (N = 96) and Cuernavaca (N = 93)50 or in urban (N = 79) and rural (N = 80) areas of Shanghai.51 Among several biochemical changes in sebum and the stratum corneum, vitamin E, squalene and ATP levels decreased. In contrast, oxidized protein levels were augmented (Table 3), indicating that skin antioxidant molecules were depleted in subjects exposed to elevated pollution levels. These studies show that pollutants induce an oxidative stress response in human skin.
Moreover, in vitro experiments showed that the induction of cytotoxic7, 8 and oxidative8, 52 mechanisms by some PAHs was increased when human keratinocytes were exposed to both PAHs and UVA in particular (for a review53). In mouse skin, UVA exposure significantly increased the toxic impact of B[a]P54 or cigarette smoke,55 including the induction of squamous cell carcinoma (SCC).55 In humans, more smokers exposed to sunlight present facial wrinkles (N = 12) than either smoking (N = 9) or sun‐exposed subjects (N = 34) alone.56 An increase in elastosis was found in the sun‐exposed skin of the forehead and cheeks of smokers (N = 17) vs. non‐smokers (N = 14).57 Moreover, a meta‐analysis58 showed that smoking was associated with SCC, whose main risk factor is UV exposure.59 In recent prospective studies on skin cancer, current smoking was associated with SCC,60 but an inverse association was found for basal cell carcinoma (BCC)60 or melanoma.61 However, the authors suspected a bias resulting from the reduced skin cancer screening in current smokers.60, 61 Although pollutants and sunlight seem to interact,62 further clinical investigations are required to understand their specific impact on skin health.
Pollutants and skin ageing
Pollutants are known to be associated with skin ageing.63 Vierkötter et al.64 used data from the SALIA study cohort65 to conduct the first epidemiological study comparing the clinical signs of skin ageing between women exposed to higher (Ruhr area, N = 211) and lower (rural area, N = 189) levels of airborne pollution. A one‐unit increase in traffic‐related particles was associated with increased numbers of lentigines (age‐associated pigment spots) on the forehead and cheeks, as well as with increases in nasolabial fold wrinkles. The risk of lentigines was higher after increased exposure to PM2.5 than after increased exposure to PM10. A specific association between NO2 exposure and the appearance of facial (cheek) lentigines in German Caucasian (N = 806) and Chinese Asian (N = 1072) cohorts has also been reported.66
This skin ageing analysis was then extended to indoor pollution in two cross‐sectional studies assessing the impact of cooking with solid fuels on Chinese women living close to Beijing (Pingding, N = 402) or Shanghai (Taizhou, N = 727).67 The pooled analysis indicated that solid fuel use increased the risk of facial coarse wrinkles and the risk of fine wrinkles on the back of the hands. Further signs of skin ageing included more pronounced laxity of the eyelids and cheeks. In a further study, Ding et al.68 found a direct association between ageing (2 cohorts N = 874 and 1003) and indoor PM2.5 exposure measured in 30 households close to Shanghai.
These studies emphasize the association of outdoor and indoor pollution with signs of ageing in exposed skin. Of note, in a Dutch clinical study (N = 956),69 the risk to develop elastosis was associated with smoking in a cigarette‐dependent way.
Pollutants and skin microbiota
The equilibrium of human skin microbiota plays a key role in skin health (see our previous review70). In a German study, 21 bacterial strains, isolated from the volar forearm and the neck of 11 subjects, could use B[a]P as their only carbon source.71 Four isolates were found to completely degrade B[a]P, possibly preventing dermal B[a]P uptake, while partial B[a]P degradation might generate toxic metabolites. Moreover, He et al.44 observed that the skin microflora of the forearm of 20 women was almost halved after exposure to atmospheric‐equivalent O3 levels for 2 h. These studies suggested interactions between skin microbiota and airborne pollutants. Whether traffic‐related air pollutants can affect the equilibrium of human skin microbiota, as suggested for the gut microbiota of Californian adolescents,72 remains to be investigated.
Pollutants and inflammatory skin diseases
Acne and hyperseborrhea
Krutmann et al.73 recently reviewed three Asian studies that suggested a pollutant‐specific relationship between elevated levels of airborne pollutants and an increased prevalence of acne. Elevated levels of PM2.5, PM10 and NO2 were associated with an increase in the number of acne‐related outpatient visits to a dermatology clinic in Beijing.74 Pollutants were also listed as one of the acne exposome factors in a recent review by Dreno et al.75
The impact of pollution on sebum excretion rates was investigated in the two clinical studies comparing the skin parameters of subjects living in Mexico City and Cuernavaca50 or in urban and rural Shanghai districts.51 The mean sebum excretion rate on the forehead was higher in subjects from Mexico City than in those from Cuernavaca. This effect was not observed in the Shanghai study, either because of the lower level of pollution in Shanghai than in Mexico City or because of differences in sebum between Asian and Caucasian subjects.51 However, a similar increase in facial skin dryness was observed in subjects exposed to pollution in both studies.
Atopic dermatitis and eczema
The link between atopic dermatitis (AD) and pollution has always been very controversially discussed, because the scientific evidence remains scarce in contrast to asthma.10, 76, 77, 78 As for other complex diseases where gene–gene and gene–environment interactions play a role, it is important to distinguish between the potential impact of pollution (including prenatal exposure) on the development of AD79, 80, 81 and the role of pollution as provocation factor for flares, which has been shown in several cross‐sectional studies82 and provocation studies.83 Furthermore, some gaseous pollutants may also contribute to an increase of AD. As previously discussed (see above), the AhR expressed in the skin may link AD and pollution with a mechanism involving the neurotrophic factor Artemin.41 Furthermore, it is assumed that airborne pollutants (e.g. PM10, NO2, SO2 and O3), as well as so‐called xenobiotics, may have a mid‐ to long‐term impact on relevant genes through epigenetic mechanisms (e.g. microRNAs, methylation or acetylation).
In a time‐series analysis conducted from 2011 to 2015 (N = 72 305 outpatient visits for eczema) in a hospital in south‐western China, Li et al.84 found a positive correlation between outpatient visits for eczema and air pollutants (NO2, SO2, PM10), but not relative humidity, suggesting that air pollutants specifically increase the symptoms of eczema. This hypothesis has also been proposed after the multivariable analysis on the data of an Italian survey (N = 10 083 subjects aged 20–44 years)85 that found that the prevalence of eczema was associated with living close to industrial plants and heavy traffic in particular, and in a Belarusian retrospective study conducted in infants aged 0–2 years with AD (N = 1965).86 In their meta‐analysis, Ngoc et al.78 found an association between pollutants and eczema thus reinforcing the hypothesis that living in a polluted environment favours atopy and exacerbates specific inflammatory skin disease symptoms in both children and adults.
In a prospective study on AD patients aged 5 years at most (N = 177), Kim et al.87 investigated the daily short‐term effects of airborne pollutants in young children (aged 2 ± 1.6 years) with AD in the Seoul Metropolitan Area: a 10‐unit increase in PM10, NO2 and O3 was found to aggravate same‐day symptoms. Girls appeared to be specifically affected by PM10, whereas boys were impacted by NO2 and O3 increases. In a further study88 conducted on 125 children aged 6 years at most, the risk of experiencing pollution‐induced disease symptoms was shown to be higher in dry–moderate weather conditions. In contrast, 10‐unit increases in PM2.5 or O3 and 2‐unit increases in NO2 or SO2 did not correlate with worsening of AD symptoms in Japanese school children (N = 339).89 This difference may have been associated with the PM2.5 levels being two‐fold lower in the Japanese study than in the Korean study. The results of their comparative study of skin parameters in adults in Mexico City and Cuernavaca also led Lefebvre et al.50 to suggest that pollution augmented the prevalence of atopic skin diseases: subjects in Mexico City experienced more episodes of atopic eczema or urticaria than those from Cuernavaca.
Overall, the symptoms of chronic inflammatory skin diseases seem exacerbated when adult and paediatric subjects are exposed to high pollution levels.
Conclusion
Outdoor and indoor pollutants are widespread in both urban and rural environments. Inhaled or ingested pollutants can be distributed to the whole body via the systemic circulation, making both the air‐exposed superficial and deep skin layers pollutant targets. Basic and clinical studies have provided growing evidence of the interactions of pollutants with skin. Pollutants may activate cutaneous metabolism and inflammatory pathways and induce oxidative stress by lowering the levels of antioxidants in particular. Skin is also the target of another known source of oxidative stress that is UV radiation. The interactions of pollutants with either UV light or human skin microbiota require further clinical investigations to evaluate their specific impact on skin health. Both outdoor and indoor pollution were found to intensify the signs of skin ageing such as facial lentigines and wrinkles. Living in a polluted environment may also reduce skin moisture, increase the rate of sebum excretion and likely exacerbates the symptoms of chronic inflammatory skin diseases both in children and adults. Home location, type of work and diet all lead to internal and external exposure to various pollutants, with clinical consequences that may accumulate or synergize.90 Pollutants are just one component of the exposome75, 90, 91 meaning that both internal and external factors are to be considered when establishing protecting measures from pollution, which require the development of standardized methods for their evaluation.
Supporting information
Table S1. Summary of the design and of some relevant results of the clinical studies included in the review.
Acknowledgements
The authors are grateful to Céline Zimmer, Marielle Romet and Emma Pilling (Synergy Pharm) who provided medical writing assistance funded by L'Oréal Active Cosmetics division.
Conflicts of interest
The authors declare they have no conflicts of interest that might be relevant to the contents of this manuscript.
Funding sources
Medical writing assistance was funded by L'Oréal Active Cosmetics division.
The authors would like to mention that during the reviewing process of their work, Der Hautartz published an article written by Fuks et al. that focuses on the impact of ozone on skin: Fuks KB, Woodby B, Valacchi G. Skin damage by tropospheric ozone. Der Hautartz. Published online 18 January 2019. https://doi.org/10.1007/s00105-018-4319-y.
References
- 1. Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015; 525: 367–371. [DOI] [PubMed] [Google Scholar]
- 2. Mannucci PM, Franchini M. Health effects of ambient air pollution in developing countries. Int J Environ Res Public Health 2017; 14: 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO . Household air pollution and health 2018. https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health. Accessed October 2018.
- 4. WHO . Air pollution. 2017. http://www.who.int/topics/air_pollution/en/. Accessed December 2017.
- 5. Mancebo SE, Wang SQ. Recognizing the impact of ambient air pollution on skin health. J Eur Acad Dermatol Venereol 2015; 29: 2326–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koohgoli R, Hudson L, Naidoo K, Wilkinson S, Chavan B, Birch‐Machin MA. Bad air gets under your skin. Exp Dermatol 2017; 26: 384–387. [DOI] [PubMed] [Google Scholar]
- 7. Wang S, Sheng Y, Feng M et al Light‐induced cytotoxicity of 16 polycyclic aromatic hydrocarbons on the US EPA priority pollutant list in human skin HaCaT keratinocytes: relationship between phototoxicity and excited state properties. Environ Toxicol 2007; 22: 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soeur J, Belaidi JP, Chollet C et al Photo‐pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J Dermatol Sci 2017; 86: 162–169. [DOI] [PubMed] [Google Scholar]
- 9. Vu AT, Taylor KM, Holman MR, Ding YS, Hearn B, Watson CH. Polycyclic aromatic hydrocarbons in the mainstream smoke of popular U.S. cigarettes. Chem Res Toxicol 2015; 28: 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valacchi G, Sticozzi C, Pecorelli A, Cervellati F, Cervellati C, Maioli E. Cutaneous responses to environmental stressors. Ann N Y Acad Sci 2012; 1271: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. EPA . Air pollutants. 2017. https://www.epa.gov/criteria-air-pollutants. Accessed December 2017.
- 12. English JS, Dawe RS, Ferguson J. Environmental effects and skin disease. Br Med Bull 2003; 68: 129–142. [DOI] [PubMed] [Google Scholar]
- 13. Krutmann J, Liu W, Li L et al Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J Dermatol Sci 2014; 76: 163–168. [DOI] [PubMed] [Google Scholar]
- 14. Puri P, Nandar SK, Kathuria S, Ramesh V. Effects of air pollution on the skin: A review. Indian J Dermatol Venereol Leprol 2017; 83: 415–423. [DOI] [PubMed] [Google Scholar]
- 15. Weschler CJ, Nazaroff WW. SVOC exposure indoors: fresh look at dermal pathways. Indoor Air 2012; 22: 356–377. [DOI] [PubMed] [Google Scholar]
- 16. Weschler CJ, Nazaroff WW. Dermal uptake of organic vapors commonly found in indoor air. Environ Sci Technol 2014; 48: 1230–1237. [DOI] [PubMed] [Google Scholar]
- 17. Weschler CJ, Beko G, Koch HM et al Transdermal uptake of diethyl phthalate and Di(n‐butyl) phthalate directly from air: experimental verification. Environ Health Perspect 2015; 123: 928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. VanRooij JG, Bodelier‐Bade MM, Jongeneelen FJ. Estimation of individual dermal and respiratory uptake of polycyclic aromatic hydrocarbons in 12 coke oven workers. Br J Ind Med 1993; 50: 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fustinoni S, Campo L, Cirla PE et al Dermal exposure to polycyclic aromatic hydrocarbons in asphalt workers. Occup Environ Med 2010; 67: 456–463. [DOI] [PubMed] [Google Scholar]
- 20. Cavallari JM, Osborn LV, Snawder JE et al Predictors of dermal exposures to polycyclic aromatic compounds among hot‐mix asphalt paving workers. Annals Occupat Hygiene 2012; 56: 125–137. [DOI] [PubMed] [Google Scholar]
- 21. McClean MD, Rinehart RD, Sapkota A, Cavallari JM, Herrick RF. Dermal exposure and urinary 1‐hydroxypyrene among asphalt roofing workers. J Occupat Environ Hygiene 2007; 4(Suppl 1): 118–126. [DOI] [PubMed] [Google Scholar]
- 22. Kammer R, Tinnerberg H, Eriksson K. Evaluation of a tape‐stripping technique for measuring dermal exposure to pyrene and benzo(a)pyrene. J Environ Monit 2011; 13: 2165–2171. [DOI] [PubMed] [Google Scholar]
- 23. Nemmar A, Hoet PH, Vanquickenborne B et al Passage of inhaled particles into the blood circulation in humans. Circulation 2002; 105: 411–414. [DOI] [PubMed] [Google Scholar]
- 24. Ramesh A, Kumar A, Aramandla MP, Nyanda AM. Polycyclic aromatic hydrocarbon residues in serum samples of autopsied individuals from Tennessee. Int J Environ Res Public Health 2014; 12: 322–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song XF, Chen ZY, Zang ZJ et al Investigation of polycyclic aromatic hydrocarbon level in blood and semen quality for residents in Pearl River Delta Region in China. Environ Int 2013; 60: 97–105. [DOI] [PubMed] [Google Scholar]
- 26. Tsang HL, Wu S, Leung CK, Tao S, Wong MH. Body burden of POPs of Hong Kong residents, based on human milk, maternal and cord serum. Environ Int 2011; 37: 142–151. [DOI] [PubMed] [Google Scholar]
- 27. Pleil JD, Stiegel MA, Sobus JR, Tabucchi S, Ghio AJ, Madden MC. Cumulative exposure assessment for trace‐level polycyclic aromatic hydrocarbons (PAHs) using human blood and plasma analysis. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 1753–1760. [DOI] [PubMed] [Google Scholar]
- 28. Singh VK, Patel DK, Jyoti Ram S, Mathur N, Siddiqui MK. Blood levels of polycyclic aromatic hydrocarbons in children and their association with oxidative stress indices: an Indian perspective. Clin Biochem 2008; 41: 152–161. [DOI] [PubMed] [Google Scholar]
- 29. Al‐Daghri NM, Alokail MS, Abd‐Alrahman SH, Draz HM. Polycyclic aromatic hydrocarbon distribution in serum of Saudi children using HPLC‐FLD: marker elevations in children with asthma. Environ Sci Pollut Res Int 2014; 21: 12085–12090. [DOI] [PubMed] [Google Scholar]
- 30. Guo Y, Huo X, Wu K, Liu J, Zhang Y, Xu X. Carcinogenic polycyclic aromatic hydrocarbons in umbilical cord blood of human neonates from Guiyu, China. Sci total Environ 2012; 427–428: 35–40. [DOI] [PubMed] [Google Scholar]
- 31. De Craemer S, Croes K, van Larebeke N et al Investigating unmetabolized polycyclic aromatic hydrocarbons in adolescents’ urine as biomarkers of environmental exposure. Chemosphere 2016; 155: 48–56. [DOI] [PubMed] [Google Scholar]
- 32. Jung KH, Liu B, Lovinsky‐Desir S et al Time trends of polycyclic aromatic hydrocarbon exposure in New York City from 2001 to 2012: assessed by repeat air and urine samples. Environ Res 2014; 131: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Maanen JM, Moonen EJ, Maas LM, Kleinjans JC, van Schooten FJ. Formation of aromatic DNA adducts in white blood cells in relation to urinary excretion of 1‐hydroxypyrene during consumption of grilled meat. Carcinogenesis 1994; 15: 2263–2268. [DOI] [PubMed] [Google Scholar]
- 34. Viegas O, Novo P, Pinto E, Pinho O, Ferreira IM. Effect of charcoal types and grilling conditions on formation of heterocyclic aromatic amines (HAs) and polycyclic aromatic hydrocarbons (PAHs) in grilled muscle foods. Food Chem Toxicol 2012; 50: 2128–2134. [DOI] [PubMed] [Google Scholar]
- 35. Toriba A, Kuramae Y, Chetiyanukornkul T et al Quantification of polycyclic aromatic hydrocarbons (PAHs) in human hair by HPLC with fluorescence detection: a biological monitoring method to evaluate the exposure to PAHs. Biomed Chromatogr 2003; 17: 126–132. [DOI] [PubMed] [Google Scholar]
- 36. Appenzeller BM, Mathon C, Schummer C, Alkerwi A, Lair ML. Simultaneous determination of nicotine and PAH metabolites in human hair specimen: a potential methodology to assess tobacco smoke contribution in PAH exposure. Toxicol Lett 2012; 210: 211–219. [DOI] [PubMed] [Google Scholar]
- 37. Afaq F, Zaid MA, Pelle E et al Aryl hydrocarbon receptor is an ozone sensor in human skin. J Invest Dermatol 2009; 129: 2396–2403. [DOI] [PubMed] [Google Scholar]
- 38. Tsuji G, Takahara M, Uchi H et al An environmental contaminant, benzo(a)pyrene, induces oxidative stress‐mediated interleukin‐8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J Dermatol Sci 2011; 62: 42–49. [DOI] [PubMed] [Google Scholar]
- 39. Liu Q, Wu J, Song J et al Particulate matter 2.5 regulates lipid synthesis and inflammatory cytokine production in human SZ95 sebocytes. Int J Mol Med 2017; 40: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ono Y, Torii K, Fritsche E et al Role of the aryl hydrocarbon receptor in tobacco smoke extract‐induced matrix metalloproteinase‐1 expression. Exp Dermatol 2013; 22: 349–353. [DOI] [PubMed] [Google Scholar]
- 41. Hidaka T, Ogawa E, Kobayashi EH et al The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol 2017; 18: 64–73. [DOI] [PubMed] [Google Scholar]
- 42. Lee CW, Lin ZC, Hu SC et al Urban particulate matter down‐regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci Rep 2016; 6: 27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thiele JJ, Traber MG, Tsang K, Cross CE, Packer L. In vivo exposure to ozone depletes vitamins C and E and induces lipid peroxidation in epidermal layers of murine skin. Free Radic Biol Med 1997; 23: 385–391. [DOI] [PubMed] [Google Scholar]
- 44. He QC, Tavakkol A, Wietecha K, Begum‐Gafur R, Ansari SA, Polefka T. Effects of environmentally realistic levels of ozone on stratum corneum function. Int J Cosmet Sci 2006; 28: 349–357. [DOI] [PubMed] [Google Scholar]
- 45. Pham DM, Boussouira B, Moyal D, Nguyen QL. Oxidization of squalene, a human skin lipid: a new and reliable marker of environmental pollution studies. Int J Cosmet Sci 2015; 37: 357–365. [DOI] [PubMed] [Google Scholar]
- 46. Jin SP, Li Z, Choi EK et al Urban particulate matter in air pollution penetrates into the barrier‐disrupted skin and produces ROS‐dependent cutaneous inflammatory response in vivo. J Dermatol Sci 2018; 91: 175–183. [DOI] [PubMed] [Google Scholar]
- 47. Romani A, Cervellati C, Muresan XM et al Keratinocytes oxidative damage mechanisms related to airbone particle matter exposure. Mech Ageing Dev 2018; 172: 86–95. [DOI] [PubMed] [Google Scholar]
- 48. Rajagopalan P, Nanjappa V, Raja R et al How does chronic cigarette smoke exposure affect human skin? A global proteomics study in primary human keratinocytes. Omics: J Integrat Biol 2016; 20: 615–626. [DOI] [PubMed] [Google Scholar]
- 49. Rajagopalan P, Jain AP, Nanjappa V et al Proteome‐wide changes in primary skin keratinocytes exposed to diesel particulate extract‐A role for antioxidants in skin health. J Dermatol Sci 2018; 91: 239–249. [DOI] [PubMed] [Google Scholar]
- 50. Lefebvre MA, Pham DM, Boussouira B, Bernard D, Camus C, Nguyen QL. Evaluation of the impact of urban pollution on the quality of skin: a multicentre study in Mexico. Int J Cosmet Sci 2015; 37: 329–338. [DOI] [PubMed] [Google Scholar]
- 51. Lefebvre MA, Pham DM, Boussouira B et al Consequences of urban pollution upon skin status. A controlled study in Shanghai area. Int J Cosmet Sci 2016; 38: 217–223. [DOI] [PubMed] [Google Scholar]
- 52. Botta C, Di Giorgio C, Sabatier AS, De Meo M. Effects of UVA and visible light on the photogenotoxicity of benzo[a]pyrene and pyrene. Environ Toxicol 2009; 24: 492–505. [DOI] [PubMed] [Google Scholar]
- 53. Fu PP, Xia Q, Sun X, Yu H. Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)‐light‐induced reactive oxygen species, lipid peroxidation, and DNA damage. J Environ Sci Health Part C, Environ Carcinog Ecotoxicol Rev 2012; 30: 1–41. [DOI] [PubMed] [Google Scholar]
- 54. Burke KE, Wei H. Synergistic damage by UVA radiation and pollutants. Toxicol Ind Health 2009; 25: 219–224. [DOI] [PubMed] [Google Scholar]
- 55. Pavlou P, Rallis M, Deliconstantinos G, Papaioannou G, Grando SA. In‐vivo data on the influence of tobacco smoke and UV light on murine skin. Toxicol Ind Health 2009; 25: 231–239. [DOI] [PubMed] [Google Scholar]
- 56. Yin L, Morita A, Tsuji T. Skin aging induced by ultraviolet exposure and tobacco smoking: evidence from epidemiological and molecular studies. Photodermatol Photoimmunol Photomed 2001; 17: 178–183. [DOI] [PubMed] [Google Scholar]
- 57. Boyd AS, Stasko T, King LE Jr, Cameron GS, Pearse AD, Gaskell SA. Cigarette smoking‐associated elastotic changes in the skin. J Am Acad Dermatol 1999; 41: 23–26. [DOI] [PubMed] [Google Scholar]
- 58. Leonardi‐Bee J, Ellison T, Bath‐Hextall F. Smoking and the risk of nonmelanoma skin cancer: systematic review and meta‐analysis. Arch Dermatol 2012; 148: 939–946. [DOI] [PubMed] [Google Scholar]
- 59. Schmitt JV, Miot HA. Actinic keratosis: a clinical and epidemiological revision. An Bras Dermatol 2012; 87: 425–434. [DOI] [PubMed] [Google Scholar]
- 60. Dusingize JC, Olsen CM, Pandeya NP et al Cigarette smoking and the risks of basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol 2017; 137: 1700–1708. [DOI] [PubMed] [Google Scholar]
- 61. Henderson MT, Kubo JT, Desai M et al Smoking behavior and association of melanoma and nonmelanoma skin cancer in the Women's Health Initiative. J Am Acad Dermatol 2015; 72: 190–191.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Marrot L. Pollution and sun exposure: a deleterious synergy. Mechanisms and opportunities for skin protection. Curr Med Chem 2018; 25: 1–18. [DOI] [PubMed] [Google Scholar]
- 63. Burke KE. Mechanisms of aging and development‐A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech Ageing Dev 2018; 172: 123–130. [DOI] [PubMed] [Google Scholar]
- 64. Vierkotter A, Schikowski T, Ranft U et al Airborne particle exposure and extrinsic skin aging. J Invest Dermatol 2010; 130: 2719–2726. [DOI] [PubMed] [Google Scholar]
- 65. Schikowski T, Sugiri D, Ranft U et al Long‐term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res 2005; 6: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huls A, Vierkotter A, Gao W et al Traffic‐related air pollution contributes to development of facial Lentigines: further epidemiological evidence from Caucasians and Asians. J Invest Dermatol 2016; 136: 1053–1056. [DOI] [PubMed] [Google Scholar]
- 67. Li M, Vierkotter A, Schikowski T et al Epidemiological evidence that indoor air pollution from cooking with solid fuels accelerates skin aging in Chinese women. J Dermatol Sci 2015; 79: 148–154. [DOI] [PubMed] [Google Scholar]
- 68. Ding A, Yang Y, Zhao Z et al Indoor PM2.5 exposure affects skin aging manifestation in a Chinese population. Sci Rep 2017; 7: 15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kennedy C, Bastiaens MT, Bajdik CD, Willemze R, Westendorp RG, Bouwes Bavinck JN. Effect of smoking and sun on the aging skin. J Invest Dermatol 2003; 120: 548–554. [DOI] [PubMed] [Google Scholar]
- 70. Dreno B, Araviiskaia E, Berardesca E et al Microbiome in healthy skin, update for dermatologists. J Eur Acad Dermatol Venereol 2016; 30: 2038–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sowada J, Schmalenberger A, Ebner I, Luch A, Tralau T. Degradation of benzo[a]pyrene by bacterial isolates from human skin. FEMS Microbiol Ecol 2014; 88: 129–139. [DOI] [PubMed] [Google Scholar]
- 72. Alderete TL, Jones RB, Chen Z et al Exposure to traffic‐related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environ Res 2017; 161: 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Krutmann J, Moyal D, Liu W et al Pollution and acne: is there a link? Clin Cosmet Investig Dermatol 2017; 10: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu W, Pan X, Vierkotter A et al A time‐series study of the effect of air pollution on outpatient visits for acne vulgaris in Beijing. Skin Pharmacol Physiol 2018; 31: 107–113. [DOI] [PubMed] [Google Scholar]
- 75. Dreno B, Bettoli V, Araviiskaia E, Sanchez Viera M, Bouloc A. The influence of exposome on acne. J Eur Acad Dermatol Venereol 2018; 32: 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol 2014; 134: 993–999; discussion 1000. [DOI] [PubMed] [Google Scholar]
- 77. Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014; 69: 3–16. [DOI] [PubMed] [Google Scholar]
- 78. Ngoc LTN, Park D, Lee Y, Lee YC. Systematic review and meta‐analysis of human skin diseases due to particulate matter. Int J Environ Res Public Health 2017; 14: 1458–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Penard‐Morand C, Raherison C, Charpin D et al Long‐term exposure to close‐proximity air pollution and asthma and allergies in urban children. Eur Respirat J 2010; 36: 33–40. [DOI] [PubMed] [Google Scholar]
- 80. Yi O, Kwon HJ, Kim H et al Effect of environmental tobacco smoke on atopic dermatitis among children in Korea. Environ Res 2012; 113: 40–45. [DOI] [PubMed] [Google Scholar]
- 81. Lee JY, Seo JH, Kwon JW et al Exposure to gene‐environment interactions before 1 year of age may favor the development of atopic dermatitis. Int Arch Allergy Immunol 2012; 157: 363–371. [DOI] [PubMed] [Google Scholar]
- 82. Kim J, Kim EH, Oh I et al Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol 2013; 132: 495–498.e1. [DOI] [PubMed] [Google Scholar]
- 83. Eberlein‐Konig B, Przybilla B, Kuhnl P et al Influence of airborne nitrogen dioxide or formaldehyde on parameters of skin function and cellular activation in patients with atopic eczema and control subjects. J Allergy Clin Immunol 1998; 101: 141–143. [DOI] [PubMed] [Google Scholar]
- 84. Li A, Fan L, Xie L, Ren Y, Li L. Associations between air pollution, climate factors and outpatient visits for eczema in West China Hospital, Chengdu, south‐western China: a time series analysis. J Eur Acad Dermatol Venereol 2018; 32: 486–494. [DOI] [PubMed] [Google Scholar]
- 85. Pesce G, Marcon A, Carosso A et al Adult eczema in Italy: prevalence and associations with environmental factors. J Eur Acad Dermatol Venereol 2015; 29: 1180–1187. [DOI] [PubMed] [Google Scholar]
- 86. Belugina IN, Yagovdik NZ, Belugina OS, Belugin SN. Outdoor environment, ozone, radionuclide‐associated aerosols and incidences of infantile eczema in Minsk, Belarus. J Eur Acad Dermatol Venereol 2018; 32: 1977–1985. [DOI] [PubMed] [Google Scholar]
- 87. Kim YM, Kim J, Han Y, Jeon BH, Cheong HK, Ahn K. Short‐term effects of weather and air pollution on atopic dermatitis symptoms in children: A panel study in Korea. PLoS ONE 2017; 12: e0175229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kim YM, Kim J, Jung K, Eo S, Ahn K. The effects of particulate matter on atopic dermatitis symptoms are influenced by weather type: Application of spatial synoptic classification (SSC). Int J Hyg Environ Health 2018; 221: 823–829. [DOI] [PubMed] [Google Scholar]
- 89. Watanabe M, Noma H, Kurai J et al Association of short‐term exposure to ambient fine particulate matter with skin symptoms in schoolchildren: a panel study in a rural area of Western Japan. Int J Environ Res Public Health 2017; 14: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci 2017; 85: 152–161. [DOI] [PubMed] [Google Scholar]
- 91. Cecchi L, D'Amato G, Annesi‐Maesano I. External exposome and allergic respiratory and skin diseases. J Allergy Clin Immunol 2018; 141: 846–857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of the design and of some relevant results of the clinical studies included in the review.


