Abstract
Olive paste (OP) is a novel by-product of olive mill industry composed of water, olive pulp, and skin. Due to its richness in bioactive compounds, OP exploitation for human consumption has recently been proposed. Starter driven fermented OP is characterized by a well-balanced lipid profile, rich in mono and polyunsaturated fatty acids, and a very good oxidative stability due to the high concentration of fat-soluble antioxidants. These characteristics make OP particularly suitable as a functional ingredient for food/feed industry, as well as for the formulation of nutraceutical products. New types of taralli were produced by adding 20% of fermented OP from black olives (cv Cellina di Nardò and Leccino) to the dough. The levels of bioactive compounds (polyphenols, triterpenic acids, tocochromanols, and carotenoids), as well as the fatty acid profile, were monitored during 180 days of storage and compared with control taralli produced with the same flour without OP supplementation. Taralli enriched with fermented OP showed significantly higher levels of bioactive compounds than conventional ones. Furthermore, enriched taralli maintained a low amount of saturated fatty acids and high levels of polyphenols, triterpenic acids, tocochromanols, and carotenoids, compared to the initial value, up to about 90 days in the usual conditions of retailer shelves.
Keywords: carotenoids, enriched foods, food by-products, hexanal, polyphenols, tocochromanols, triterpenic acids
1. Introduction
Fruits and vegetables are known to be rich sources of several bioactive compounds, also known as nutraceuticals [1,2]. Agri-food by-products produced during handling and processing of fruits and vegetables, including residual pulp, peels, seeds, leaves, bracts, stems, roots, and bark, represent a major waste disposal problem for industry [3]. Nevertheless, food industry by-products can be good and low-cost sources of valuable bioactive compounds, such as polyphenols, carotenoids, tocochromanols, and phytosterols [4,5]. The bioactive compounds could be extracted, or some by-products could be directly used as ingredients for foods/feed supplementation. Various extraction techniques have been applied from a waste range of plant materials to obtain phytocomplexes rich in high-value components [6,7,8] useful for the industrial preparation of functional foods, dietary supplements, and/or cosmeceuticals. Furthermore, some constituents of the phytocomplex may act as an anticancer agent, anti-inflammatory, and antioxidant or have a role in cell signaling and gene expression regulation, maintaining our health [9]. The olive fruit is an excellent source of unsaturated fatty acids, as well as other nutritionally important health-promoting bioactive compounds [10]. Olive mill industry generates large amounts of different by-products. Among these, olive pomace, olive mill wastewaters, and the olive stones and seeds have attracted attention for their possible exploitation in several industrial sectors. The recently introduced multi-phase decanter technology for olive oil industrial extraction generates large quantities of a novel by-product (olive paste—OP) made up of the partially defatted wet drupe pulp containing very low or any traces of the kernel. Recently, Padalino et al. [11], Cecchi et al. [12], and Tufariello et al. [13] reported that OP is an excellent natural source of unsaturated fatty acids and biologically active substances, including polyphenols, triterpenic acids, tocochromanols, and carotenoids.
Nowadays, the increasing demand for high nutritive value and health-promoting foods encourages the food industry to develop research towards innovative products containing higher concentrations of nutrients and bioactives. Studies have demonstrated that cereal-based food products, such as pasta and bakery products, represent cheap and easy-to-use carriers for phytochemicals. The most recent enrichment of pasta includes a variety of non-traditional ingredients, such as lipophilic (tocochromanols, carotenoids) or hydrophilic/phenolic antioxidants from durum wheat bran by-products, lyophilized tomato containing high levels of lycopene [14], α-cyclodextrin chlatrated pumpkin oil rich in α-, β- carotene and α-tocopherol [15], OP powder rich in maslinic acid and polyphenols, such as tyrosol [11].
Fermentation approaches using microorganisms selected to grow in the presence of high levels of phenolic bioactive compounds have been extensively and successfully applied to olives and derived products [16,17,18]. In a previous study, the biotechnological aptitude, of yeast and lactic acid bacteria selected from different sources, was tested to transform OP in a new fermented product with quality and safety traits suitable to be used as a semi-finished ingredient for human food formulation.
The yeast strain Saccharomyces cerevisiae KI-30-1 and the strain Leuconostoc mesenteroides BC-T3-35 were selected among other candidates as the best performing microorganisms for their metabolic activities able to consume sugars, to produce important organic acids and volatile compounds, and to substantially modify phenolic profiles, by improving nutritional traits of the final product. Indeed, fermented OPs obtained from black olives of the cultivar Cellina di Nardò (OPC) and Leccino (OPL) were shown to represent a good source of bioactive compounds [13]. These strains were used for the first time as starters for pilot-scale fermentations of OP following a sequential inoculation approach, by mimicking the already described process in table olives fermentation, since yeasts are fundamental to modify the product and to prepare the subsequent lactic acid bacteria (LAB) fermentation [13,17,18].
Fermented OP could be then exploited to enrich bakery products, such as taralli, enhancing their nutritional and health-promoting properties. Taralli, are a typical Italian bakery product produced in many Italian regions, mainly in Central and Southern Italy [19], very popular worldwide as a savory snack or bread substitute and characterized by a high friability and long shelf-life. Their formulation includes fats, which confer desirable texture, flavor, friability, and crispy consistency [20]. Fats added to the dough are usually vegetable oils from corn, palm, soybean, sunflower, and, more frequently, olive. Fats also influence the oxidative processes of food and then its shelf life. Caponio et al. [21,22] and Giarnetti et al. [23] reported that oil typology plays an important role in the formation and release of volatile aromas during taralli storage. The authors reported that volatile compounds increased during storage and that the content of individual volatile molecules was higher in taralli made with Extra-Virgin Olive Oil (EVOO) than refined oils.
The aim of this work was the formulation and characterization of innovative enriched taralli containing 20% of fermented OP to improve the nutritional and health value of the product. Furthermore, the content of bioactive compounds, the profiles of fatty acids, and the production of hexanal were monitored over 180 days storage at 25 °C in light conditions in enriched and conventional (control) taralli.
2. Results and Discussion
2.1. Chemical Characterization of Control and Enriched Taralli
Table 1 shows the profile of fatty acids and the chemical composition of polyphenols, triterpenic acids, tocochromanols, and carotenoids in conventional and enriched taralli obtained adding 20% of the fermented OP to the dough. The amount of OP added to the dough was determined by preliminary trials aimed to achieve a significant increase in bioactive compounds without excessively affecting the rheological properties of the taralli compared with the control (CTRL) ones (data not shown). Indeed, the dough containing more than 20% fermented OP presented low specific volume, while the resulting taralli were harder and less leavened than CTRL.
Table 1.
Chemical characterization of control (CTRL) and taralli enriched with fermented olive paste from Cellina di Nardò (OPC) or Leccino (OPL) olive cultivars.
| Taralli | |||
|---|---|---|---|
| CTRL | OPC | OPL | |
| Fatty acids (relative percentage) | |||
| Palmitic acid (C16:0) | 15.41 ± 1.28 a | 15.32 ± 1.50 a | 15.29 ± 0.98 a |
| Palmitoleic acid (C16:1) | 1.60 ± 0.04 a | 1.58 ± 0.03 a | 1.61 ± 0.04 a |
| Heptadecanoic acid (C17:0) | <0.01 | <0.01 | <0.11 |
| Stearic acid (C18:0) | 1.99 ± 0.43 a | 2.03 ± 0.51 a | 2.04 ± 0.44 a |
| Oleic acid (C18:1 n-9) | 66.66 ± 2.91 a | 66.04 ± 2.81 a | 66.54 ± 2.59 a |
| Linoleic acid (C18:2n-6) | 12.46 ± 0.85 a | 13.16 ± 1.05 a | 12.61 ± 0.35 a |
| Linolenic acid(C18:3 n-3) | 0.96 ± 0.05 a | 0.98 ± 0.04 a | 0.97 ± 0.05 a |
| Arachidic acid (C20:0) | 0.36 ± 0.04 a | 0.37 ± 0.05 a | 0.37 ± 0.04 a |
| cis-Eicosenoic acid (C20:1c) | 0.33 ± 0.05 a | 0.34 ± 0.06 a | 0.33 ± 0.07 a |
| Behenic acid (C22:0) | 0.23 ± 0.05 a | 0.18 ± 0.04 a | 0.13 ± 0.04 a |
| SFA | 17.99 ± 1.80 a | 17.90 ± 2.10 a | 17.94 ± 1.50 a |
| MUFA | 68.59 ± 3.00 a | 67.96 ± 2.90 a | 68.48 ± 2.70 a |
| PUFA | 13.42 ± 0.90 a | 14.14 ± 1.09 a | 13.58 ± 0.40 a |
| Polyphenols (µg/g DW) | |||
| Hydroxytyrosol (3,4-DHPEA) | nd | 905 ± 2 | 824 ± 1 |
| Tyrosol (p-HPEA) | nd | 81 ± 2 | 53 ± 3 |
| Verbascoside | nd | 202 ± 2 | 70 ± 3 |
| Isoverbascoside * | nd | nd | 36 ± 1 |
| Oleacin (3,4-DHPEA-EDA) | nd | 146 ± 6 | 33 ± 3 |
| Oleocanthal (p-HPEA-EDA) | nd | 43 ± 1 | 17 ± 1 |
| Total | nd | 1377 ± 13 | 1016 ± 12 |
| Triterpenic acids (µg/g DW) | |||
| Maslinic acid | nd | 63.74 ± 3.01 | 68.93 ± 5.53 |
| Oleanoic acid | nd | 16.52 ± 1.12 | 16.99 ± 1.87 |
| Total | nd | 80.26 ± 4.13 | 80.92 ± 7.40 |
| Tocochromanols (µg/g DW) | |||
| α-T | 10.31 ± 1.06 b | 21.26 ± 1.63 a | 15.63 ± 1.59 a |
| β-T3 | 3.75 ± 0.45 b | 9.01 ± 0.03 a | 6.59 ± 0.96 a |
| Total | 14.06 ± 1.51 b | 30.27 ± 1.66 a | 22.22 ± 2.55 a |
| Carotenoids (µg/g DW) | |||
| Lutein | 0.77 ± 0.06 b | 1.04 ± 0.03 a | 0.93 ± 0.03 a |
| β carotene | 0.06 ± 0.001 b | 0.13 ± 0.01 a | 0.10 ± 0.01 a |
| Total | 0.83 ± 0.06 b | 1.17 ± 0.04 a | 1.03 ± 0.14 b |
Significance: nd, not-detected; * tentative of identification. Data represent the mean ± standard deviation of three replicate measurements (n = 3). DW, dried weight; SFA, saturated fatty acid; MUFA, monosaturated fatty acids; PUFA, polyunsaturated fatty acid; Hydroxytyrosol (3,4-DHPEA), 3,4-(dihydroxyphenyl)ethanol; Tyrosol (p-HPEA), p-(hydroxyphenyl)ethanol; Oleacin (3,4-DHPEA-EDA), dialdehydic form of decarboxymethyl elenolic acid linked to (3,4-dihydroxyphenyl) ethanol; Oleocanthal (p-HPEA-EDA), dialdehydic form of decarboxymethyl elenolic acid linked to (p-hydroxyphenyl)ethanol; α-T, α-tocopherol; β-T3, β-tocotrienol. Different letters indicate significant differences (Student’s test, p < 0.05) within the same row (CTRL taralli vs. enriched taralli).
Lipids play a key role in bakery products, especially biscuits, cookies, and taralli, as they affect the rheological behavior, sensory properties, nutritional value, and shelf life of the final product [21,24]. The fatty acid profiles of CTRL and enriched taralli, expressed as a relative percentage with respect to the total identified molecules, did not show statistically significant differences (p > 0.05). Both comprised mainly mono-unsaturated fatty acids (MUFA; 68%); saturated fatty acids (SFA; 18%), and poly-unsaturated fatty acids (PUFA; 14%). Oleic acid (C18:1 n-9) contributed about 66% of the total fatty acids identified, followed by palmitic (C16:0; 15%) and linoleic (C18:2n-6; 13%) acids. Although the fatty acid profile of CTRL and enriched taralli is influenced by the lipid composition of wheat flour and vegetable oil used as ingredients, it was almost identical to that of taralli made with olive oil, as also reported by Caponio et al. [22].
Taralli obtained adding fermented OP contained several beneficial compounds, including polyphenols (1377 µg/g dried weight (DW) in taralli OPC and 1016 µg/g DW in taralli OPL), triterpenic acids (80.26 µg/g DW in taralli OPC and 80.92 µg/g DW in taralli OPL), typically absent in conventional taralli [13], isoprenoids, such as tocochromanols (30.27 µg/g DW in taralli OPC and 22.22 µg/g DW in taralli OPL) and carotenoids (1.17 µg/g DW in taralli OPC and 1.03 µg/g DW in taralli OPL). In conventional taralli, the amount of these isoprenoids were lower.
Various agri-food by-products rich in polyphenols, including pomegranate, mango, pigeon pea, and apple peels, have been mixed to the dough to prepare bakery products with functional properties. Some researchers reported that the supplementation of polyphenols in bakery foods could decrease starch digestibility and reduce the postprandial glucose levels in the serum, and is thus considered as alternatives to pharmaceutical interventions for the treatment of type II diabetes [25,26,27,28]. In this work, the addition of fermented OP to durum wheat flour led to a significant enrichment of polyphenol compounds.
It is worthwhile noting that although durum wheat is known to be a source of phenolics, most of them are in the form of insoluble phenolic acids bound by covalent cross-linkages to the cell wall polymers (75% of the total phenolic acids) and thus have poor bioavailability [29]. The soluble conjugated and free forms represent about 24% and 1%, respectively [29]. In this work, we focused the attention on the free polyphenol fraction.
The supplementation with the OP strongly increased polyphenols concentrations in the OPC and OPL taralli compared with CTRL sample (Figure S1). This is probably related to the fact that polyphenols content in EVO added to the CTRL dough was low, and it was not detectable in the final product (Table S1). In OP enriched taralli, the main polyphenol compound found was hydroxytyrosol (66% and 77% of the total polyphenols identified in OPC and OPL taralli, respectively) followed by verbascoside. Hydroxytyrosol possesses many beneficial properties for human health, and it is currently used as a therapeutic agent [30]. Recently, Difonzo et al. [31] reported that olive leaf extract (OLE), rich in polyphenols (i.e., oleuropein, verbascoside, luteolin, rutin, hydroxytyrosol, and tyrosol), was also suitable to enhance the quality of bakery products.
Triterpenic acids, from olive by-products, such as maslinic and oleanoic acids, are mainly concentrated in the skin of drupes [11,13,32,33]. These compounds have received great attention because of their functional properties and biological importance [34]. Recently, Sanchez Rodriguez et al. [35] reported that a daily supplementation during three weeks with 30 mL of an enriched virgin olive oil providing 4.7 mg/d of oleanoic acid (171 ppm) and 6 mg/d of maslinic acid (218 ppm) decreased DNA oxidation and plasma inflammatory biomarkers in healthy adults compared to the conventional oil with low levels of triterpenic acids. In our study, taralli enriched with fermented OP showed the presence of both maslinic and oleanoic acids (Table 1).
The fat-soluble micronutrients, tocochromanols, occur in different forms (α-, β-, γ-, and δ-tocopherols and α-, β-, γ-, and δ-tocotrienols) and are known to possess many health benefits, including protection against cancer, cardiovascular, and age-related degenerative diseases [36,37]. The total tocochromanols content of OPC and OPL enriched taralli was about 2.1- and 1.6-times, respectively, higher than CTRL. The main tocochromanols were α-tocopherol (α-T) and β-tocotrienol (β-T3). It is known that α-T is the only form of tocopherols that is actively maintained in the human body [38]. In conventional taralli, α-T content was 10.31 μg/g DW similar to the amount reported by Leenhardt et al. [39] in bread obtained from Triticum monococcum (11.03 μg/g) and durum (9.46 μg/g). In enriched taralli, α-T content ranged from 15.63 μg/g DW (taralli OPL) to 21.26 μg/g DW (taralli OPC), these values are in agreement with the study of Pasias et al. [40] who reported that bakery products (i.e., breadstick) made from Triticum dicoccum wheat and other ingredients, such as vanilla, tomato, sesame, and/or vegetables, gave a high content of α-T that ranged from 18.2 μg/g to 88 μg/g. β-T3 content ranged from 6.59 μg/g DW (taralli OPL) to 9.01 μg/g DW (taralli OPC).
In all samples, lutein was the main carotenoid, followed by β-carotene. Taralli enriched with fermented OP showed a small, but statistically significant, increase in total carotenoids levels with respect to CTRL taralli. In general, bioactive compounds profile in taralli was in agreement with the study of Padalino et al. [11] on spaghetti enriched with olive paste. These authors demonstrated that the innovative spaghetti had high bioactive compounds content. In particular, the results showed that levels of apigenin, luteolin, quercetin, maslinic, and oleanoic acids and α-T, α-, and β-carotene, observed in enriched spaghetti, were higher than in conventional spaghetti.
2.2. Effect of Storage on Fatty Acids Profile and the Content of Hexanal, Polyphenols, Triterpenic Acids, Tocochromanols, and Carotenoids in Conventional and Enriched Taralli
The effect of light exposure, the most important factor influencing taralli shelf-life quality during supermarket storage, was evaluated by determining the fatty acids profile, the amount of hexanal, polyphenols, triterpenic acids, tocochromanols, and carotenoids at 30 day-intervals during storage for 180 days.
Lipid oxidation is the main biochemical process responsible for the deterioration of bakery products and reduction of their shelf life. The oxidation reaction depends on the type of fats used and product composition [20,41]. Table 2 shows the changes (%) in SFA, MUFA, and PUFA in conventional and enriched taralli during storage. In OPC taralli, the SFA percentage did not vary significantly, while some variations were observed in CTRL (p = 0.044) and OPL taralli (p = 0.013) after 90 and 120 days storage, respectively, compared to the initial value. In all tested samples, the results showed no significant variation in MUFA percentage. The percentage of PUFA, instead, underwent a significant decrease after 60 days of storage in CTRL. In enriched taralli, the decrease in the percentage of PUFA was less marked, in particular, it significantly decreased after 120 (p = 0.001; OPC taralli) and 150 days (p = 0.029; OPL taralli) of storage. The PUFA/SFA ratio is one of the main parameters used to assess the nutritional quality of the lipid fraction of foods [42]. Nutritional guidelines recommend a PUFA/SFA ratio above 0.4 [43]. In all samples, PUFA/SFA ratios were significantly reduced during storage; however, the values observed, after 180 days of storage, in OPC (0.39) and OPL taralli (0.43) were higher than the CTRL taralli (0.25). This is in agreement with results reported by Padalino et al. [11] in spaghetti enriched with OP. Also, in that case, the PUFA/SFA ratio resulted higher in enriched spaghetti than in CTRL. The presence of antioxidants in fermented OP, such as polyphenols, tocochromanols, and carotenoids, could play an important role in oxidative protection to the PUFA in enriched taralli. Indeed, some of the natural antioxidants, such as α-T and β-carotene, have been already used to enhance the shelf life of bakery products [44]. Furthermore, recent studies have reported that the OLE, with a high content of phenolic compounds, can reduce the level of oxidation in bakery products, enhancing their quality and shelf-life [27,45].
Table 2.
Changes in fatty acid composition during the storage of CTRL taralli and taralli enriched with fermented olive paste from Cellina di Nardò (OPC) or Leccino (OPL) olive cultivars at 25 °C, 500 lux, and in clear plastic bag for 180 days.
| Time Storage (days) | Taralli | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTRL | OPC | OPL | ||||||||||
| SFA | MUFA | PUFA | PUFA/SFA | SFA | MUFA | PUFA | PUFA/SFA | SFA | MUFA | PUFA | PUFA/SFA | |
| % | % | % | ||||||||||
| 0 | 19.99 ± 1.80 b | 68.59 ± 3.01 a | 13.42 ± 0.90 a | 0.67 | 17.90 ± 2.10 a | 67.96 ± 2.90 a | 14.14 ± 1.09 a | 0.79 | 17.94 ± 1.50 b | 68.48 ± 2.70 a | 13.58 ± 0.40 a | 0.76 |
| 30 | 19.01 ± 1.8 b | 67.75 ± 3.10 a | 13.25 ± 1.10 ab | 0.69 | 18.43 ± 1.99 a | 68.14 ± 2.90 a | 13.43 ± 1.09 a | 0.73 | 17.85 ± 1.90 b | 67.87 ± 2.20 a | 14.28 ± 0.99 a | 0.80 |
| 60 | 19.94 ± 1.87 b | 67.57 ± 4.10a | 11.08 ± 0.99 b | 0.56 | 19.78 ± 1.77 a | 67.01 ± 3.21 a | 13.22 ± 1.01 a | 0.67 | 18.01 ± 1.68 b | 67.90 ± 3.10 a | 14.10 ± 1.10 a | 0.78 |
| 90 | 21.13 ± 2.10 a | 67.87 ± 3.80a | 11.01 ± 0.98 b | 0.52 | 20.51 ± 2.10 a | 67.49 ± 2.90 a | 12.01 ± 0.87 a | 0.58 | 19.21 ± 2.20 b | 67.85 ± 3.10 a | 12.94 ± 0.76 ab | 0.67 |
| 120 | 21.58 ± 1.80 a | 67.57 ± 3.70 a | 10.85 ± 0.88 b | 0.50 | 22.08 ± 2.00 a | 67.47 ± 3.21 a | 10.45 ± 0.79 b | 0.47 | 19.88 ± 2.10 a | 67.81 ± 3.30 a | 12.31 ± 0.65 ab | 0.62 |
| 150 | 22.51 ± 0.90 a | 67.49 ± 4.1 a | 10.01 ± 0.08 b | 0.44 | 22.50 ± 1.80 a | 67.50 ± 4.52 a | 10.01 ± 0.05 b | 0.44 | 21.54 ± 1.20 a | 67.40 ± 5.60 a | 11.06 ± 0.90 bc | 0.51 |
| 180 | 26.01 ± 2.01 a | 67.50 ± 4.50 a | 6.52 ± 0.10 b | 0.25 | 23.01 ± 1.90 a | 68.01 ± 4.69 a | 9.03 ± 0.21 b | 0.39 | 23.08 ± 1.70 a | 67.00 ± 4.71 a | 9.92 ± 0.80 c | 0.43 |
Data are the mean ± standard deviation of three independent replicates (n = 3). Data were submitted to one-way analysis of variance (ANOVA), Tukey’s test was applied to compare the experimental times (0, 30, 60, 90, 120, 150, 180 days) within the same sample for each classification of fatty acids (SFA, MUFA, and PUFA). Different letters mean significant difference at p < 0.05.
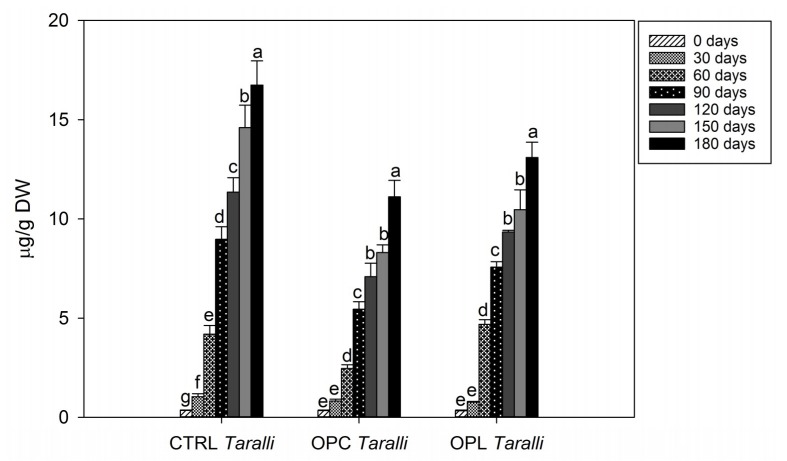
To evaluate the storage stability of taralli samples, we evaluated the increase of hexanal, the most abundant lipid oxidation volatile compound deriving from the oxidation of linoleic acid [46]. Hexanal has been already reported in several cereal-based foods, including pasta, bread, and biscuits. The lipid fraction of semolina, the main ingredient of bakery products, is very susceptible to lipoxygenase activity leading to hydroperoxide productions [47,48]. Hydroperoxides are highly unstable and are converted in volatile compounds, such as hexanal, responsible for rancid off-flavors [49]. Furthermore, hexanal is a representative marker of the oxidative rancidity as an alternative to traditional oxidation indicators (e.g., acidity or peroxide values). In this work, CTRL taralli exhibited a significative increase of hexanal within the first 30 days of storage, whereas, in enriched taralli, hexanal levels increased after 30 days of storage (Figure 1). Moreover, at the end of storage, in CTRL taralli, hexanal was observed at higher levels than enriched taralli (16.75, 11.11, 13.09 µg/g DW in CTRL, OPC, and OPL taralli, respectively). It could be considered that antioxidant compounds in fermented OP added to durum wheat flour were able to reduce the lipid oxidation in enriched taralli products. Similar results were reported by Difonzo et al. [31], who observed that OLE improved lipid stability in baked snacks compared to CTRL.
Figure 1.
Time course of the amounts of hexanal during the storage of control (CTRL) and taralli enriched with fermented olive paste from Cellina di Nardò (OPC) or Leccino (OPL) olive cultivars at 25 °C, 500 lux, and in clear plastic bag for 180 days. Data are the mean ± standard deviation of three independent replicates (n = 3). Data were submitted to one-way analysis of variance (ANOVA), Tukey’s test was applied to compare the experimental times (0, 30, 60, 90, 120, 150, 180 days) within the same sample. Different letters mean significant difference at p < 0.05.
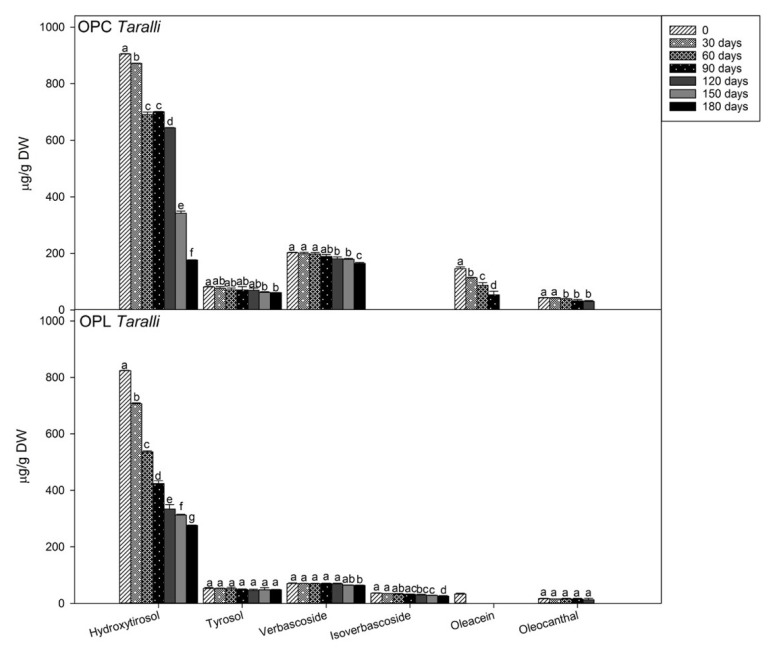
Figure 2 shows the polyphenols profile in enriched taralli during storage. The content of total polyphenols progressively decreased during storage, leading to deterioration as a consequence of oxidative degradations probably due to light exposure. However, individual polyphenols showed a different decrease in trends over the storage period. In OPC and OPL taralli, the difference of hydroxytyrosol content after 30 days of storage was small (although statistically significant, p < 0.001), then a dramatic decrease by about 62% of the initial content occurred between 120 and 180 days of storage. The content of tyrosol, verbascoside, and isoverbascoside was, instead, more stable over time. Tyrosol level significantly decreased only by 23% to the initial value after 150 days of storage in OPC taralli, while it remained stable in OPL taralli. Esposto et al. [50] and Servili et al. [51] reported that tyrosol has lower antioxidant activity than hydroxytyrosol. Thus, hydroxytyrosol presented a higher degradation rate than tyrosol in taralli during storage in the presence of light. A small reduction of verbascoside levels (6–8%) after 120 (p = 0.002) and 180 (p = 0.017) days of storage was also observed in OPC and OPL taralli, respectively. Isoverbascoside content, not detectable in OPC taralli, was observed at low levels in OPL, and its content slowly decreased during storage to 26 µg/g DW. Also, oleacein level in OPL taralli gradually decreased during storage. Oleocanthal, present at the low but detectable amount, resulted undetectable after 120 days of storage both in OPC and OPL taralli.
Figure 2.
Time course of the amounts of polyphenols during the storage of CTRL and taralli enriched with fermented olive paste from Cellina di Nardò (OPC) or Leccino (OPL) olive cultivars at 25 °C, 500 lux, and in clear plastic bag for 180 days. Data are the mean ± standard deviation of three independent replicates (n = 3). Data were submitted to one-way analysis of variance (ANOVA), Tukey’s test was applied to compare the experimental times (0, 30, 60, 90, 120, 150, 180 days) within the same sample for each compound (hydroxytyrosol, tyrosol, verbascoside, isoverbascoside, oleacein, and oleocanthal). Different letters mean significant difference at p < 0.05.
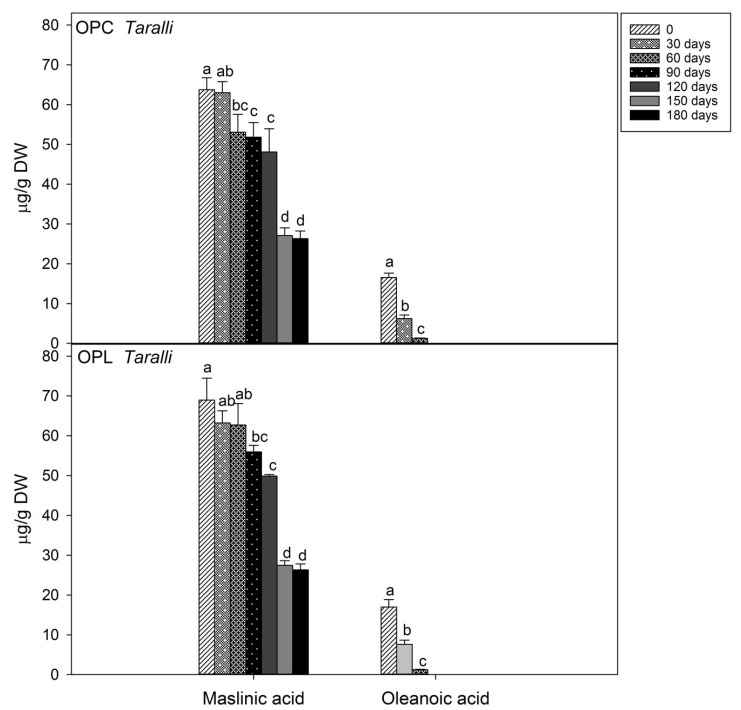
In Figure 3, the effect of storage time on the content of triterpenic acids (maslinic and oleanoic acids) is reported. In taralli enriched with the fermented OP, maslinic acid content decreased slowly by 25 and 20% (OPL and OPC, respectively) after 120 days of storage. A dramatic decrease to less than 59%, in OPL, and 62%, in OPC, of the initial content occurred between 120 and 180 days of storage. In contrast, oleanoic acid rapidly decreased under the condition tested. After 60 days of storage, oleanoic acid level resulted reduced by approximately 93% in both samples.
Figure 3.
Time course of the amounts of triterpenic acids during the storage of CTRL and taralli enriched with fermented olive paste from Cellina di Nardò (OPC) or Leccino (OPL) olive cultivars at 25 °C, 500 lux, and in clear plastic bag for 180 days. Data are the mean ± standard deviation of three independent replicates (n = 3). Data were submitted to one-way analysis of variance (ANOVA), Tukey’s test was applied to compare the experimental times (0, 30, 60, 90, 120, 150, 180 days) within the same sample for each compound (maslinic and oleanoic acid). Different letters mean significant difference at p < 0.05.
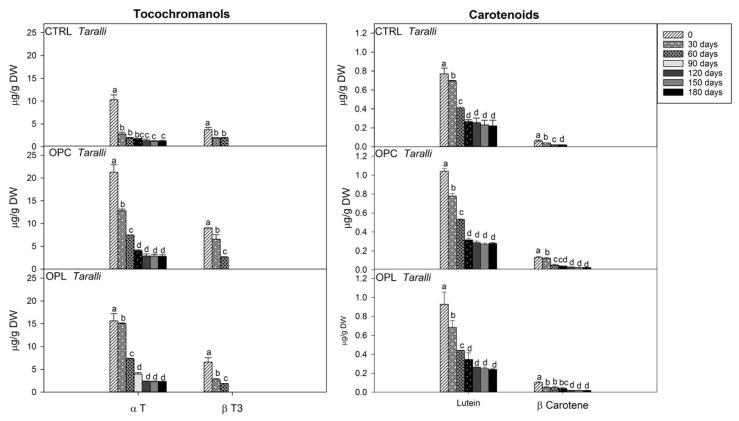
In Figure 4, the time course of the changes in the level of tocochromanols: α-T and β-T3 and carotenoids: β-carotene and lutein in CTRL and enriched taralli is reported.
Figure 4.
Time course of the amounts of tocochromanols and carotenoids during the storage of CTRL and taralli enriched with the fermented olive paste from Cellina di Nardò (OPC) or Leccino (OPL) olive cultivars at 25 °C, 500 lux, and in clear plastic bag for 180 days. Data are the mean ± standard deviation of three independent replicates (n = 3). Data were submitted to one-way analysis of variance (ANOVA), Tukey’s test was applied to compare the experimental times (0, 30, 60, 90, 120, 150, 180 days) within the same sample for each compound (α-T (α-tocopherol), β-T3 (β-tocotrienol), β-carotene, and lutein). Different letters mean significant difference at p < 0.05.
In CTRL taralli, after 30 days of storage, α-T concentration showed a rapid decrease by 72% of the initial value and its content was 1.24 µg/g DW after 180 days of storage. The level of α-T was reduced by 80% after 90 days of storage, then remained almost unchanged in OPC (2.81 µg/g DW) and OPL (2.32 µg/g DW) taralli up to 180 days. β-T3 content progressively decreased in all samples and was undetectable after 90 days of storage. β-T3 is more sensitive and susceptible to oxidation compared to α-T likely in relation to the presence of three, rather than two unsaturations [52]. A similar trend was observed by Durante et al. [6,8], who evaluated the stability of different tocochromanol forms in vegetable oils.
The content of total carotenoids was reduced by more than 70% in all samples after 90 days of storage. In all samples, the amount of lutein progressively decreased over time, and its content was about 0.20 µg/g DW at the end of the storage period. After 90 days of storage, the β-carotene level was completely reduced in CTRL taralli, whereas, in enriched taralli, it remained unchanged up to the end of the storage period (0.018 in taralli OPL and 0.024 µg/g DW in taralli OPC).
3. Materials and Methods
3.1. Raw Material
Semolina from durum wheat (cv Senatore Cappelli) was used to prepare taralli. Fermented OP samples from Cellina di Nardò and Leccino cultivars were produced following the method described by Tufariello et al. [13,16]. Briefly, OP obtained by a local olive mill (Murrone, Caprarica, Lecce, Italy), milled using a Multi-Phase Decanter technology (Leopard, Pieralisi Maip S.p.A., Jesi, AN, Italy), was subjected to heat treatment at 121 °C for 4 min. Then, OP samples were diluted 1:1 with distilled water and added with 0.5% (w/v) yeast extract, 0.5% (w/v) peptone, 0.5% (w/v) glucose in 10 kg capacity vessels. OP fermentations were carried out for 50 days at ambient temperature (18–22 °C) by the sequential inoculum of the yeast Saccharomyces cerevisiae ISPA-LE-KI 30-1 and then (after 30 days) with Leuconostoc mesenteroides ISPA-LE-BT3-35. Fermented OP samples were heat-treated at 90 °C for 2 min.
3.2. Chemicals
High purity standards for the qualitative-quantitative determination of fatty acids (palmitic, palmitoleic, heptadecanoic, stearic, oleic, linoleic, linolenic, arachidic, eicosenoic, behenic), α-T, triterpenic acids (maslinic and oleanoic acids), hexanal, and 2-methylpropyl acetate, as well as all High-Performance Liquid Chromatography (HPLC) grade solvents, were all purchased from Sigma–Aldrich (Milan, Italy). Tyrosol, hydroxytyrosol, and verbascoside were purchased from Fluka (Milan, Italy), Cabru s.a.s. (Arcore, Milan, Italy), and Extrasynthese (Genay Cedex, France), respectively. Oleacein and oleocanthal were obtained from PhytoLab GmbH & Co. (Vestenbergsgreuth, Germany). Carotenoids (lutein and β-carotene) and β-T3 standards were purchased from CaroteNature (Lupsingen, Liestal, Switzerland) and Cayman chemicals (Ann Arbor, MI, USA), respectively.
3.3. Sample Preparation
Taralli were prepared by mixing wheat flour (1 kg), water (0.4 L), salt (0.02 kg), extra virgin olive oil (100 g), and or fermented OP (200 g) with a diving arm kneader for 20 min. Then, the dough was left to rest for 45 min, and after this time, was sheeted in taralli shape, round with a hole in the middle, of about 3 cm in diameter and 8 mm in thickness. Taralli samples were packed in 500 g transparent bags and stored in the real marketplace storage conditions (temperature 25 °C and light exposure, 12 h/day, to an intensity of 500 lux have been electronically controlled), during 180 days with samples analyses repeated every month.
3.4. Polyphenols Extraction and Analysis
Polyphenols were extracted, as reported by Servili et al. [53], with some modifications. Twenty grams of CTRL, OPC, and OPL taralli samples were ground with a blender (Osterizer Blender Cycle Blend Pulse 4153-50, Sunbeam Products, Inc., Boca Raton, FL, USA), and then 5 g was picked and extracted with 50 mL of methanol/water (80:20, v/v) containing 20 mg/L of butylated hydroxytoluene (BHT). The methanol was evaporated, and the aqueous extract was used for solid-phase extraction (SPE) of phenols. The SPE procedure was performed by loading 1 mL of the aqueous extract into a 1000 mg Bond Elut Jr-C18 cartridge (Agilent Technologies, Santa Clara, CA, USA), using 50 mL of methanol as the eluting solvent. The extract was evaporated under vacuum at 30 °C, the residue was dissolved in 1 mL of methanol and analyzed, as described by Servili et al. [53], using an 1100 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA). For the quantitative analysis, an external calibration curve was constructed for each compound, except for isoverbascoside; the isoverbascoside was quantified using the response factor of verbascoside.
3.5. Triterpenic Acids Extraction and Analysis
Triterpenic acids were extracted, as described by Durante et al. [10], from triplicate aliquots (1 g) of CTRL, OPC, and OPL taralli samples. Samples were extracted six times with 4 mL of methanol/ethanol (1:1, v/v) in a Labsonic LBS1-10 ultrasonic bath (Falc Instruments, Treviglio (Bg), Italy). Extracts were combined, evaporated to dryness, redissolved with 1 mL methanol, and analyzed, according to Durante et al. [10], using an 1100 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a Luna column (5 μm, 250 × 4.6mm) (Phenomenex, Torrance, CA, USA).
3.6. Tocochromanols and Carotenoids Extraction and Analysis
Tocochromanols (tocopherols and tocotrienols) and carotenoids were extracted from triplicate aliquots of CTRL, OPC, and OPL taralli samples, as reported by Padalino et al. [11]. The extracts were assayed, as described by Durante et al. [10], using an 1100 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a reverse-phase C30 column (5 µm, 250 × 4.6 mm) (YMC Inc., Wilmington, NC, USA).
3.7. Total Lipid Extraction and Determination
Total lipids were extracted from triplicate aliquots (1 g) of CTRL, OPC, and OPL taralli samples with 5 mL of n-hexane and stirred with a mechanical stirrer (300 rpm) at 4 °C for 16 h. After centrifugation at 6000× g for 5 min, the organic phase was evaporated to dryness under a stream of nitrogen. Lipids extracts were subjected to fatty acid derivatization, as described by Durante et al. [8]. Briefly, a methanolic solution (3 mL) of 0.5 M KOH was added to the samples, after incubation at 100 °C for 5 min and cooling. Boron trifluoride in methanol (2 mL, 12% w/v) was added, and samples were incubated at 100 °C for 30 min. To the reaction mixture, 1 mL of NaCl (0.6% w/v) and 1 mL of n-hexane were added. The organic upper phase was analyzed by GC-MS in accordance to Durante et al. [8] using an Agilent 5977E Series GC/MS system (Agilent Technologies, Santa Clara, CA, USA) equipped with a DB-WAX column (60 m, 0.25 mm i.d., 0.25 mm film thickness) (Agilent Technologies, Santa Clara, CA, USA).
3.8. Headspace Solid-Phase Microextraction (SPME) and GC/MS Analysis of Hexanal
Triplicate aliquots (0.3 g) of CTRL, OPC, and OPL taralli samples with 3 mL saturated aqueous solution of NaCl were placed in a 20 mL vial tightly capped with polytetrafluoroethylene (PTFE) septum. The 2-Methylpropyl acetate as an internal standard at a concentration of 3 µg/g was added, and the mixture was homogenized for 2 min using a laboratory vortex shaker. Hexanal was sampled by SPME. The sample was maintained at 40 °C for 20 min, and after that, the fiber was exposed to the headspace for 30 min at 40 °C. The analyses of hexanal were conducted with an Agilent Technologies 7890B GC, equipped with a “Multimode Injector” (MMI) 7693A injector (Agilent Technologies, Santa Clara, CA, USA) and a thermostated PAL3 RSI 120 autosampler equipped with a fiber conditioning module and agitator (CTC Analytics AG, Zwingen, Switzerland), and the detection system was an Agilent 5977B single quadrupole GC/MSD with EI Extractor (XTR) source (Agilent Technologies, Santa Clara, CA, USA). For the analysis of hexanal, the fiber was thermally desorbed in the hot GC injector port for 5 min at 250 °C with the injector set in splitless mode. The volatile compounds were separated with a DB-WAXetr column (50 m, 0.32 mm i.d., 1 μm film thickness) (Agilent Technologies, Santa Clara, CA, USA) using helium as carrier gas at a constant flow rate of 1.7 mL/min. The GC oven heating program started at 35 °C with this temperature held for 4 min, then increased to 150 °C at a rate of 4 °C/min, increased to 180 °C at a rate of 8 °C/min held for 2 min, and finally increased to 210 °C at a rate of 11 °C/min, this temperature was held for 13.77 min. The temperature of the transfer line was fixed at 215 °C. The MSD was operated in the electron ionization (EI) mode, at ionization energy of 70 eV, in scan mode, with scanning in the mass range of m/z 25–350 a.m.u. at a scan rate of 4.3 scan/s and the MS source and the MS quad temperatures of 190 °C and 150 °C, respectively. The hexanal was identified by comparison of their mass spectrum and retention time with that of the authentic reference compound. This volatile compound was quantified by standardizing the peak area of hexanal with respect to the peak area of the internal standard (2-methylpropyl acetate), according to Lu Xiao et al. [54].
3.9. Statistical Analysis
The data are the mean values of replicate measurements (n = 3) with standard deviation. Student’s t-test was applied to the chemical characterization of CTRL taralli and enriched taralli with fermented olive paste from Cellina di Nardò (OPC) or Leccino (OPL) olive cultivars. A one-way analysis of variance (ANOVA) and the Tukey HSD post hoc test were applied to the time course of fatty acids, polyphenols, triterpenic acids, tocochromanols, and carotenoids. All statistical comparisons were performed using SigmaStat version 11.0 software (Systat Software Inc., Chicago, IL, USA).
4. Conclusions
In this study, the fermented olive paste by-product obtained from black olives (cv Cellina di Nardò and Leccino), due to its richness in bioactive compounds, was exploited to enhance nutritional and health-promoting properties of taralli, a typical Italian bakery product. Our data showed that taralli enriched with 20% of fermented OP were a convenient source of polyphenols, triterpenic acids, tocochromanols, and carotenoids and that the main components of this new types of taralli were hydroxytyrosol, tyrosol, verbascoside, oleacin, oleocanthal, maslinic acid, α-T, and lutein. During storage in the usual conditions of retailer shelves (25 °C and 500 lux), the enriched taralli maintained a low amount of saturated fatty acids and high levels of polyphenols, triterpenic acids, tocochromanols, and carotenoids up to about 90 days of storage. On the other hand, after 180 days of storage, we observed a drastic reduction in the total polyphenols (>60%), triterpenic acids (>67%), tocochromanols (>89%), and carotenoids (>74%). The obtained data highlighted, moreover, that fermented OP addition significantly reduced the level of hexanal compared to CTRL taralli. The results reported in this study encourage further studies to extend shelf-life and maintain the nutritional value of enriched taralli possibly using modified atmosphere packaging technique, in view of fulfilling consumer trend for healthy products. Furthermore, studies are required to assess if the utilization of taralli enriched with fermented OP would enhance human health performance and prevention from certain diseases.
This rather pioneering study is an example of how the circular economy could be applied to depleting food resources. Indeed, the use of microbial fermentation, a well-known strategy for food preservation and nutritional enhancement, is useful to convert a currently considered vegetable waste into functional ingredients and to produce enriched foods for the human diet.
Acknowledgments
The authors wish to acknowledge Federazione Coldiretti Lecce, Coopolio Lecce, Olearia Murrone, Gepra srl, and Panificio Donato Caroppo for their valuable collaboration. The authors also thank Leone D’Amico for his technical assistance.
Supplementary Materials
The following are available online, Figure S1: HPLC chromatogram of polyphenol extracts from Taralli CTRL, OPC, and OPL. Samples were recorded with DAD at 278 nm. Peak numbers: 1, Hydroxytyrosol; 2, Tyrosol; 3, Verbascoside; 4, Oleacin; 5, Isoverbascoside; 6, Oleocanthal, Table S1: Polyphenols composition in virgin olive oil from Cellina di Nardò and Leccino olive cultivars.
Author Contributions
Conceptualization, G.B., G.M., and M.S.; Data curation, M.D., R.S., and G.V.; writing—original draft preparation, M.D.; writing—review and editing, M.D., G.B., G.M., and M.S.
Funding
This work was supported by Regione Puglia (research project PASSATADOLIVA grant number PRS_065).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Durante M., Lenucci M.S., Mita G. Supercritical carbon dioxide extraction of carotenoids from pumpkin (Cucurbita spp.): A review. Int. J. Mol. Sci. 2014;15:6725–6740. doi: 10.3390/ijms15046725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilahy R., Siddiqui M.W., Tlili I., Montefusco A., Piro G., Hdider C., Lenucci M.S. When color really matters: Horticultural performance and functional quality of high-lycopene tomatoes. Crit. Rev. Plant Sci. 2018;37:15–63. doi: 10.1080/07352689.2018.1465631. [DOI] [Google Scholar]
- 3.Ezejiofor T.I.N., Enebaku U.E., Ogueke C. Waste to wealth-value recovery from agro-food processing wastes using biotechnology: A review. Br. Biotechnol. J. 2014;4:418–481. doi: 10.9734/BBJ/2014/7017. [DOI] [Google Scholar]
- 4.Durante M., Lenucci M.S., Rescio L., Mita G., Caretto S. Durum wheat by-products as natural sources of valuable nutrients. Phytochem. Rev. 2012;11:255–262. [Google Scholar]
- 5.Durante M., Montefusco A., Marrese P.P., Soccio M., Pastore D., Piro G., Mita G., Lenucci M.S. Seeds of pomegranate, tomato and grapes: An underestimated source of natural bioactive molecules and antioxidants from agri-food by-products. J. Food Compos. Anal. 2017;63:65–72. doi: 10.1016/j.jfca.2017.07.026. [DOI] [Google Scholar]
- 6.Durante M., Lenucci M.S., Laddomada B., Mita G., Caretto S. Effects of sodium alginate bead encapsulation on the storage stability of durum wheat (Triticum durum Desf.) bran oil extracted by supercritical CO2. J. Agric. Food Chem. 2012;60:10689–10695. doi: 10.1021/jf303162m. [DOI] [PubMed] [Google Scholar]
- 7.Durante M., Lenucci M.S., D’Amico L., Piro G., Mita G. Effect of drying and co-matrix addition on the yield and quality of supercritical CO2 extracted pumpkin (Cucurbita moschata Duch) oil. Food Chem. 2014;148:314–320. doi: 10.1016/j.foodchem.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 8.Durante M., Lenucci M.S., Marrese P.P., Rizzi V., De Caroli M., Piro G., Fini P., Russo G.L., Mita G. α-Cyclodextrin encapsulation of supercritical CO2 extracted oleoresins from different plant matrices: A stability study. Food Chem. 2016;199:684–693. doi: 10.1016/j.foodchem.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 9.Bruno A., Durante M., Marrese P.P., Migoni D., Laus M.N., Pace E., Pastore D., Mita G., Piro G., Lenucci M.S. Shades of red: Comparative study on supercritical CO2 extraction of lycopene- rich oleoresins from gac, tomato and watermelon fruits and effect of the α-cyclodextrin clathrated extracts on cultured lung adenocarcinoma cells’ viability. J. Food Compos. Anal. 2018;65:23–32. doi: 10.1016/j.jfca.2017.08.007. [DOI] [Google Scholar]
- 10.Durante M., Tufariello M., Tommasi M., Lenucci M.S., Bleve G., Mita G. Evaluation of bioactive compounds in black table olives fermented with selected microbial starters. J. Sci. Food Agric. 2017;98:96–103. doi: 10.1002/jsfa.8443. [DOI] [PubMed] [Google Scholar]
- 11.Padalino L., D’Antuono I., Durante M., Conte A., Cardinali A., Linsalata V., Mita G., Logrieco A.F., Del Nobile M.A. Use of Olive Oil Industrial By-Product for Pasta Enrichment. Antioxidants. 2018;7:59. doi: 10.3390/antiox7040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cecchi L., Migliorini M., Cherubini C., Innocenti M., Mulinacci N. Whole lyophilized olives as sources of unexpectedly high amounts of secoiridoids: The case of three tuscan cultivars. J. Agric. Food Chem. 2015;63:1175–1185. doi: 10.1021/jf5051359. [DOI] [PubMed] [Google Scholar]
- 13.Tufariello M., Durante M., Bleve G., Veneziani G., Taticchi A., Servili M., Mita G. Patè olive cake: Possible exploitation of a by-product for food applications. Front. Nutr. 2019;8:83. doi: 10.3389/fnut.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasqualone A., Gambacorta G., Summo C., Caponio F., Di Miceli G., Flagella Z., Marrese P.P., Piro G., Perrotta C., De Bellis L., et al. Functional, textural and sensory properties of dry pasta supplemented with lyophilized tomato matrix or with durum wheat bran extracts produced by supercritical carbon dioxide or ultrasound. Food Chem. 2016;213:545–553. doi: 10.1016/j.foodchem.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Durante M., Lenucci M.S., Gazza L., Taddei F., Nocente F., De Benedetto G., De Caroli M., Piro G., Mita G. Bioactive composition and sensory evaluation of innovative spaghetti supplemented with free or α-cyclodextrin chlatrated pumpkin oil extracted by supercritical CO2. Food Chem. 2019;294:112–122. doi: 10.1016/j.foodchem.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Bleve G., Tufariello M., Durante M., Perbellini E., Ramires F.A., Grieco F., Cappello M.S., De Domenico S., Giovanni M., Tasioula-Margari M., et al. Physico-chemical and microbiological characterization of spontaneous fermentation of Cellina di Nardò and Leccino table olives. Front. Microbiol. 2014;5:570. doi: 10.3389/fmicb.2014.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tufariello M., Durante M., Ramires F.A., Grieco F., Tommasi L., Perbellini E., Falco V., Tasioula-Margari M., Logrieco A.F., Mita G., et al. New process for production of fermented black table olives using selected autochthonous microbial resources. Front. Microbiol. 2015;6:1007. doi: 10.3389/fmicb.2015.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bleve G., Tufariello M., Durante M., Grieco F., Ramires F.A., Mita G., Tasioula-Margari M., Logrieco A.F. Physico-chemical characterization of natural fermentation process of Conservolea and Kalamàta table olives and developement of a protocol for the pre-selection of fermentation starters. Food Microbiol. 2015;46:368–382. doi: 10.1016/j.fm.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Pagani M.A., Lucisano M., Mariotti M. Traditional Italian products from wheat and other starchy flours. In: Hui Y.H., editor. Handbook of Food Products Manufacturing. Principles, Bakery, Beverages, Cereals, Cheese, Confectionery, Fats, Fruits, and Functional Foods. Wiley-Interscience; Mila, Italy: 2007. pp. 327–388. [Google Scholar]
- 20.Caponio F., Cosmai L., Giarnetti M., Gomes T., Summo C., Paradiso V.M. A comparative study on oxidative and hydrolytic stability of monovarietal extra virgin olive oil in bakery products. Food Res. Int. 2013;54:1995–2000. doi: 10.1016/j.foodres.2013.06.022. [DOI] [Google Scholar]
- 21.Caponio F., Summo C., Paradiso V.M., Pasqualone A., Gomes T. Evolution of the oxidative and hydrolytic degradation of biscuits fatty fraction during storage. J. Sci. Food Agric. 2009;89:1392–1396. doi: 10.1002/jsfa.3600. [DOI] [Google Scholar]
- 22.Caponio F., Giarnetti M., Summo C., Gomes T. Influence of the different oils used in dough formulation on the lipid fraction of taralli. J. Food Sci. 2011;76:549–554. doi: 10.1111/j.1750-3841.2011.02113.x. [DOI] [PubMed] [Google Scholar]
- 23.Giarnetti M., Caponio F., Paradiso V.M., Summo C., Gomes T. Effect of the type of oil on the evolution of volatile compounds of taralli during storage. J. Food Sci. 2012;77:326–331. doi: 10.1111/j.1750-3841.2011.02613.x. [DOI] [PubMed] [Google Scholar]
- 24.Caponio F., Delcuratolo D., Summo C., Pasqualone A. Quality of the lipid fraction of Italian biscuits. J. Sci. Food Agric. 2006;86:356–361. doi: 10.1002/jsfa.2357. [DOI] [Google Scholar]
- 25.Colantuono A., Ferracane R., Vitaglione P. In vitro bioaccessibility and functional properties of polyphenols from pomegranate peels and pomegranate peels-enriched cookies. Food Funct. 2016;7:4247–4258. doi: 10.1039/C6FO00942E. [DOI] [PubMed] [Google Scholar]
- 26.Gbenga-Fabusiwa F.J., Oladele E.P., Oboh G., Adefegha S.A., Oshodi A.A. Nutritional properties, sensory qualities and glycemic response of biscuits produced from pigeon pea-wheat composite flour. J. Food Biochem. 2018;42:12505. doi: 10.1111/jfbc.12505. [DOI] [Google Scholar]
- 27.Jun Y., Bae I.Y., Lee S., Lee H.G. Utilisation of preharvest dropped apple peels as a flour substitute for a lower glycaemic index and higher fibre cake. Int. J. Food Sci. Nutr. 2014;65:62–68. doi: 10.3109/09637486.2013.830083. [DOI] [PubMed] [Google Scholar]
- 28.Ramírez-Maganda J., Blancas-Benítez F.J., Zamora-Gasga V.M., García-Magaña M.d.L., Bello-Pérez L.A., Tovar J., Sáyago-Ayerdi S.G. Nutritional properties and phenolic content of a bakery product substituted with a mango (Mangifera indica)‘Ataulfo’processing by-product. Food Res. Int. 2015;73:117–123. doi: 10.1016/j.foodres.2015.03.004. [DOI] [Google Scholar]
- 29.Laddomada B., Durante M., Minervini F., Garbetta A., Cardinali A., D’Antuono I., Caretto S., Blanco A., Mita G. Phytochemical characterization and anti-inflammatory activity of extracts from the whole-meal flour of Italian durum wheat cultivars. Int. J. Mol. Sci. 2015;16:3512–3527. doi: 10.3390/ijms16023512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marković K.A., Torić J., Barbarić M., Jakobušić B.C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules. 2019;24:2001. doi: 10.3390/molecules24102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Difonzo G., Pasqualone A., Silletti R., Cosmai L., Summo C., Paradiso V.M., Caponio F. Use of olive leaf extract to reduce lipid oxidation of baked snacks. Food Res. Int. 2018;108:48–56. doi: 10.1016/j.foodres.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 32.Velasco J., Holgado F., Màrquez-Ruiz G., Ruiz-Méndez M.V. Concentrates of triterpenic acids obtained from crude olive pomace oils: Characterization and evaluation of their potential antioxidant activity. J. Sci. Food Agric. 2018;98:4837–4844. doi: 10.1002/jsfa.9012. [DOI] [PubMed] [Google Scholar]
- 33.Medina E., Romero C., Brenes M. Residual olive paste as a source of phenolic compounds and triterpenic acids. Eur. J. Lipid Sci. Technol. 2018;120:1700368–1700373. doi: 10.1002/ejlt.201700368. [DOI] [Google Scholar]
- 34.Rodríguez-Rodríguez R., Ruiz-Gutiérrez V. Functional properties of pentacyclic triterpenes contained in pomace olive oil. In: Victor R., editor. Olives and Olive Oil in Health and Disease Prevention. Volume 159. Elsevier Inc.; Amsterdam, The Netherlands: 2010. pp. 1431–1438. Preedy and Ronald Ross Watson. [Google Scholar]
- 35.Sanchez-Rodriguez E., Biel-Glesson S., Fernandez-Navarro J.R., Calleja M.A., Espejo-Calvo J.A., Gil-Extremera B., de la Torre R., Fito M., Covas M.I., Vilchez P., et al. Effects of virgin olive oils differing in their bioactive compound contents on biomarkers of oxidative stress and inflammation in healthy adults: A randomized double-blind controlled trial. Nutrients. 2019;11:561. doi: 10.3390/nu11030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reboul E., Richelle M., Perrot E., Desmoulins-Malezet C., Pirisi V., Borel P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006;54:8749–8755. doi: 10.1021/jf061818s. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y., Monahan F.J., McNulty B.A., Gibney M.J., Gibney E.R. Effect of vitamin E intake from food and supplement sources on plasma α- and γ-tocopherol concentrations in a healthy Irish adult population. Br. J. Nutr. 2014;112:1575–1585. doi: 10.1017/S0007114514002438. [DOI] [PubMed] [Google Scholar]
- 38.Traber M.G. Utilization of vitamin E. Biofactors. 1999;10:115–120. doi: 10.1002/biof.5520100205. [DOI] [PubMed] [Google Scholar]
- 39.Leenhardt F., Lyan B., Rock E., Boussard A., Potus J., Chanliaud E., Remesy C. Wheat Lipoxygenase Activity Induces Greater Loss of Carotenoids than Vitamin E during Breadmaking. J. Agric. Food Chem. 2006;54:1710–1715. doi: 10.1021/jf052243m. [DOI] [PubMed] [Google Scholar]
- 40.Pasias I.N., Kiriakou I.K., Papakonstantinou L., Proestos C. Determination of Vitamin E in Cereal Products and Biscuits by GC-FID. Foods. 2018;7:3. doi: 10.3390/foods7010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caruso M.C., Galgano F., Colangelo M.A., Condelli N., Scarpa T., Tolve R., Favati F. Evaluation of the oxidative stability of bakery products by OXITEST method and sensory analysis. Eur. Food Res. Technol. 2017;243:1183–1191. doi: 10.1007/s00217-016-2831-9. [DOI] [Google Scholar]
- 42.Romero M.C., Romero A.M., Doval M.M., Judis M.A. Nutritional value and fatty acid composition of some traditional Argentinean meat sausages. Food Sci. Technol. 2013;33:161–166. doi: 10.1590/S0101-20612013005000007. [DOI] [Google Scholar]
- 43.World Health Organization (WHO) Diet, Nutrition and the Prevention of Chronic Diseases. WHO; Geneva, Switzerland: 2003. pp. 87–88. (Who Technical Report Series No. 916). [PubMed] [Google Scholar]
- 44.Nanditha B., Prabhasankar P. Antioxidants in bakery products: A review. Crit. Rev. Food Sci. Nutr. 2008;49:1–27. doi: 10.1080/10408390701764104. [DOI] [PubMed] [Google Scholar]
- 45.Mateos R., Martínez-López S., Arévalo G.B., Amigo-Benavent M., Sarriá B., Bravo-Clemente L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016;205:248–256. doi: 10.1016/j.foodchem.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Frankel E.N. Volatile lipid oxidation products. Prog. Lipid Res. 1983;22:1–33. doi: 10.1016/0163-7827(83)90002-4. [DOI] [PubMed] [Google Scholar]
- 47.Pasqualone A., Paradiso V.M., Summo C., Caponio F., Gomes T. Influence of drying conditions on volatile compounds of pasta. Food Bioprocess. Technol. 2014;7:719–731. doi: 10.1007/s11947-013-1080-1. [DOI] [Google Scholar]
- 48.Pasqualone A., Bianco A.M., Paradiso V.M., Summo C., Gambacorta G., Caponio F., Blando A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015;180:64–70. doi: 10.1016/j.foodchem.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Choe E., Min D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006;5:169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- 50.Esposto S., Veneziani G., Taticchi A., Selvaggini R., Urbani S., Di Maio I., Sordini B., Minnocci A., Sebastiani L., Servili M. Flash thermal conditioning of olive pastes during the olive oil mechanical extraction process: Impact on the structural modifications of pastes and oil quality. J. Agric. Food Chem. 2013;61:4953–4960. doi: 10.1021/jf400037v. [DOI] [PubMed] [Google Scholar]
- 51.Servili M., Esposto S., Fabiani R., Urbani S., Taticchi A., Mariucci F., Selvaggini R., Montedoro G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology. 2009;17:1–9. doi: 10.1007/s10787-008-8014-y. [DOI] [PubMed] [Google Scholar]
- 52.Sroynak R., Srikalong P., Raviyan P. Radical scavenging capacity and antioxidant activity of the vitamin E extracted from palm fatty acid distillate by sequential cooling hexane. J. Agric. Sci. 2013;5:224–237. doi: 10.5539/jas.v5n4p224. [DOI] [Google Scholar]
- 53.Servili M., Taticchi A., Esposto S., Urbani S., Selvaggini R., Montedoro G.F. Effect of olive stoning on the volatile and phenolic composition of virgin olive oil. J. Agric. Food Chem. 2007;55:7028–7035. doi: 10.1021/jf070600i. [DOI] [PubMed] [Google Scholar]
- 54.Xiao L., Lee J., Gong Z., Ebeler S.E., Wickramasinghe N., Seiber J., Mitchell A.E. HS-SPME GC/MS characterization of volatiles in raw and dry-roasted almonds (Prunus dulcis) Food Chem. 2014;151:31–39. doi: 10.1016/j.foodchem.2013.11.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.