Abstract
Overuse of antibiotic drugs alters the composition of gut microbiota and has detrimental effects on the host. In our study, we investigated association of gut flora and antibiotics in the prognosis of patients with liver cancer who have undergone chemotherapy by analyzing two independent clinical studies. We retrospectively subanalyzed a previously reported randomized controlled trial (RCT) on hepatic arterial infusion chemotherapy in patients with hepatocellular carcinoma (HCC) to investigate the association between use of antibiotics and prognosis. In the other study, we prospectively determined the abundance of specific bacterial genus in patients with HCC by sequencing 16S ribosomal RNA and assessed its association with survival. Subanalysis of the RCT data showed that, of 26 types of antibiotics used, administration of carbapenem before or during chemotherapy was associated with poor progression‐free survival (PFS) and overall survival (OS) of patients with HCC (carbapenem + vs. −; median PFS, 78 days vs. 154 days, p = 0.0053; median OS, 177 days vs. 475 days, p = 0.0003). Multivariate analysis revealed that antianaerobic drug use is an independent predictor of poor prognosis. In the prospective study, the abundance of Blautia in fecal microbiota correlated positively with both PFS and OS of patients with HCC who underwent chemotherapy. Use of antibiotics targeting anaerobes is associated with a poor prognosis in patients with HCC who have undergone chemotherapy, whereas the intestinal anaerobic bacteria, Blautia is associated with a good prognosis. These findings might indicate the need for caution regarding overuse of broad‐spectrum antibiotics targeting anaerobes in patients with HCC.
Keywords: anaerobe, short‐chain fatty acid, microbiota
Short abstract
What's new?
Overuse of antibiotic drugs alters the composition of gut microbiota and can have detrimental effects on the host. However, it remains unclear whether antibiotics impair the anti‐cancer effects of cytotoxic drugs in cancer patients. Using data from two independent clinical studies, here the authors reveal that use of antibiotics targeting anaerobes is associated with a poor prognosis in patients with hepatocellular carcinoma (HCC) who have undergone chemotherapy, whereas the intestinal anaerobic bacteria, Blautia is associated with a good prognosis. These findings might indicate the need for caution regarding overuse of broad‐spectrum antibiotics targeting anaerobes in patients with HCC.
Abbreviations
- AFP
α‐fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- CRP
C‐reactive protein
- CT
computed tomography
- IFN
interferon
- MRI
magnetic resonance imaging
- OS
overall survival
- PCR
polymerase chain reaction
- PFS
progression‐free survival
- RCT
randomized control trial
- rRNA
ribosomal RNA
- SCFA
short‐chain fatty acid
- WBC
white blood cell
Introduction
While intestinal microbiota have changed with their hosts in the process of evolution,1 recent marked alterations in microbiota are attributable to major changes in life‐style.2 One such change, overuse of antibiotic drugs, alters microbiota composition and has both detrimental and beneficial effects on the host.3, 4 Because the intestinal commensal microbiota plays versatile roles in homeostasis of their hosts in the aspects of metabolism, tissue development, inflammation and immunity, dysbiosis of the microbiota can have serious and complex effects on the host.5 Diseases considered to be associated causally with detrimental effects of antibiotic overuse include Clostridium difficile infection, irritable bowel syndrome, childhood obesity and allergic diseases such as asthma and atopic diseases.6, 7, 8 In addition, some harmful effects of antibiotic overuse may still be unclear because the roles of commensal bacteria have not yet been completely determined.
Mouse studies in which gut microbiota were shown to play roles in promoting anticancer effects of various cancer treatments, including cytotoxic chemotherapies and immunotherapies, have recently been reported.9, 10, 11, 12 Conversely, administration of antibiotics impairs anticancer effects by eradicating beneficial bacteria from cancer‐bearing hosts. Depletion of gut flora with combinations of broad antibiotics, including imipenem/cilastatin, reportedly impairs the anticancer effects of the cytotoxic drugs oxaliplatin and cisplatin in mice with subcutaneous cancers.9 However, it remains unclear whether antibiotics impair the anticancer effects of cytotoxic drugs in clinical cancer patients.
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer death and a health concern worldwide because the global incidence of HCC is continuing to increase.13 Administration of cytotoxic drugs via the hepatic artery is an effective form of treatment of patients with advanced HCC.14, 15 Improvements in methods of administering chemotherapy and selection of appropriate patients are needed because the overall survival of patients with HCC who undergo chemotherapy ranges from 5.4 to 8.8 months.14, 16 Recent studies have shown that dysbiosis of gut flora is closely related to liver disease. The composition of the gut microbiota of patients with hepatic cirrhosis17, 18 or HCC18, 19 differs from those of healthy individuals. Changes in intestinal microbiota are significantly associated with severity of nonalcoholic fatty liver disease (NAFLD) and related to the increased permeability of the intestine in these patients.20, 21 The close connection of the liver and gut through the portal vein and lymphatic vessels facilitates an association between altered gut flora and liver disease. Those recent findings prompted us to study associations between gut flora, antibiotics, anticancer effects and prognosis of patients with HCC.
In the present study, we analyzed data from two independent clinical studies with the aim of investigating the roles of gut flora and antibiotics in the prognosis of patients with HCC who undergo chemotherapy. The first study was a retrospective subanalysis of a previously reported16 randomized control trial (RCT) to examine associations between administration of antibiotics and the postchemotherapy prognosis of patients with HCC. In the second study, we collected feces from patients with HCC before they started chemotherapy and prospectively followed up their survival to investigate the relationship between members of gut flora and prognosis.
Methods
Subanalysis of a phase II RCT
We previously reported the results of a randomized phase II trial performed to determine the response rates and survival of patients with HCC treated with systemic interferon (IFN) injections and hepatic arterial infusion of fluorouracil (5‐FU) with or without cisplatin.16 A total of 114 patients with advanced HCC were enrolled in the study and randomized into IFN/fluorouracil or IFN/fluorouracil/cisplatin groups. Only patients with severe vascular invasion (i.e., invasion of the main trunk or second branch of the portal vein, right, left or middle hepatic vein) or five or more intrahepatic lesions were eligible for enrollment. The study was approved by the Internal Review Board of Kanazawa University (Approval number 5169). The patients received a continuous infusion of 5‐FU via the hepatic artery at a dose of 300 mg/m2/day for 5 days in the first and the second week of each cycle. IFN‐α2b (Intron A; Schering‐Plow, Osaka, Japan) was injected i.m. at a dose of 3,000,000 units three times per week for 4 weeks. In the IFN/fluorouracil/cisplatin group, 20 mg/m2 cisplatin (Randa; Nippon Kayaku, Tokyo, Japan) was administered via the hepatic artery on Days 1 and 8. The treatment was administered in 4 weeks cycles and response evaluated at the end of each cycle using computed tomography (CT) or magnetic resonance imaging (MRI) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0. The treatment cycles were continued until there was evidence of progressive disease.
In this subanalysis, we sought information about antibiotic use from the medical records of the 114 participants, and actually 107 of them had complete medical records about antibiotic use. Because the composition of gut microbiota can change considerably even a month after cessation of antibiotics and reverting to the original composition takes 2–4 months,9, 22 all antibiotics administered from 3 months before starting the first cycle of chemotherapy to the end of the final cycle were identified. Associations between use of antibiotics and progression‐free or overall survival (PFS or OS) of the participants were analyzed.
Prospective study to survey correlations between gut microbiota and survival
From June 2013 to May 2015, patients who were admitted to Kanazawa University Hospital to receive treatment for clinically diagnosed HCC were invited to participate in a prospective study to investigate the association between gut microbiota and prognosis. The study was approved by the Ethics Committee of Kanazawa University. After screening with CT or MRI imaging, 32 patients with HCC with vascular invasion, extrahepatic lesions or multiple tumor nodules who were unsuitable for radical treatments such as hepatectomy or radiofrequency ablation were enrolled in our study. All participants received both written and oral information before consenting to participate in the study. Immediately after enrollment, a sample of feces was collected and stored at −80°C. Twenty‐six of the participants started chemotherapy within 3 months after the fecal collection and their survival was followed up to November 2017. The chemotherapy comprised systemic IFN injection and 5‐FU plus cisplatin via hepatic arterial infusion in all cases, as was administered in the previous RCT.16 Progression of HCC during chemotherapy was evaluated by CT or MRI imaging at 4‐week intervals. Spearman's correlation coefficients between abundance of specific bacterial genera in the feces before starting chemotherapy and PFS, OS, tumor number, tumor diameter, serum level of α‐fetoprotein (AFP), C‐reactive protein (CRP) or white blood cell (WBC) counts were calculated. Hierarchical clustering was performed with bacterial genera which included a correlation coefficient with p‐value <0.05 by an algorithm of complete linkage clustering.
Sequencing of 16S ribosomal RNA amplicon
Fecal DNA was extracted from the feces stored at −80°C using a PowerFecal DNA isolation kit according to the manufacturer's instruction (Mo BIO, Carlsbad, CA). For 16S sequencing analysis, polymerase chain reaction (PCR) amplicons were prepared using primers that consisted of sequences targeting the V3 and V4 region of 16S ribosomal RNA (rRNA) and Illumina adapter overhung nucleotide sequences as follows: Forward 5′‐TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG‐3′; Reverse 5′‐GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC‐3′.
PCR reactions were performed using KAPA HiFi HotStart Ready Mix (KAPA Biosystems, Wilmington, MA) with an initial step at 95°C for 3 min, followed by 25 cycles of 30 sec at 95°C, 30 sec at 55°C and 30 sec at 72°C plus a final step at 72°C for 5 min. PCR products were purified from oligo DNA contaminants using AMPure XP beads (Beckman Coulter, Fullerton, CA) and quantified using a 2100 Bioanalyzer and High Sensitivity DNA kit (Agilent Technologies, Santa Clara, CA). Indexing PCR was performed using a Nextera XT index kit (Illumina, San Diego, CA) with an initial step at 95°C for 3 min, followed by eight cycles of 30 sec at 95°C, 30 sec at 55°C and 30 sec at 72°C plus a final step at 72°C for 5 min. Libraries were pooled and mixed with PhiX Control Library (Illumina) and sequencing was performed in Miseq with Miseq Reagent Kit V3 (600 cycles; Illumina). Downstream processing of the sequences was performed using MacQIIME v1.9.1.23 After joining pair‐ends of the sequences and trimming low quality sequences, the sequences obtained had lengths ranging from 442 to 464 bp. Operational taxonomic units (OTU) were picked using UCLUST. For OTU analysis, sequences were clustered and sequences with more than 97% similarity were binned into the same OTUs. Taxonomy of representative sequences in OTU was assigned by RDP classifier using the Greengenes reference database24 clustered at 97% identity. Genus level taxonomy was summarized and used for the subsequent correlation analysis. After the phylogenetic tree had been calculated, unweighted Unifrac distances of gut flora communities were calculated at a depth of 2,000 reads and the resultant distances were visualized by principal coordinate analysis. Significance of the difference between groups after calculation of unweighted Unifrac distances was tested by PERMANOVA.
Measurement of short‐chain fatty acids in feces
Short‐chain fatty acids (SCFAs) in feces were determined by a modified method as previously described.25 Briefly, 100 mg of feces was suspended with 0.9 ml 0.5% phosphoric acid. After centrifugation, the supernatant was mixed with 1 mM 4‐methyl valeric acid as internal standard. SCFA in feces were measured by gas chromatography with a flame ionization detector (7890B; Agilent Technologies) and a capillary column DB‐WAXetr (30 m, 0.25 mm id, 0.25 μm film thickness, Agilent Technologies). Spearman's correlation coefficients between concentration of acetate, iso‐butyrate, n‐butyrate or propionate and PFS or OS were calculated.
Statistical analyses
To compare PFS and OS between groups, cumulative survival proportions were calculated using the Kaplan–Meier method and differences between groups evaluated by the Gehan–Wilcoxon test with GraphPad Prism version 7. Multivariate analysis was performed with Cox's proportional hazard model when variables were significantly associated with survival according to univariate analysis. To assess correlations between microbiota composition and variables in the prospective study, Spearman's rank correlation coefficients were calculated by using SPSS Statics version 24.0.0. A value of p < 0.05 was considered to denote statistical significance.
Availability of data and materials
All sequencing data and metadata were archived in NCBI SRA as BioProject PRJNA445357.
Results
Administration of antibiotics before or during chemotherapy is associated with a poor prognosis in chemotherapy‐treated patients with HCC
To investigate the role of commensal bacteria in effects of chemotherapy treatment of patients with HCC, we retrospectively subanalyzed data from a previously reported RCT on a hepatic arterial infusion chemotherapy. Over half the patients had advanced HCC according to Barcelona Clinic Liver Cancer (BCLC) staging (stage B intermediate, 41.1%; C advanced, 48.6%; D, 10.2%; Supporting Information Table S1).
In all, participants had received 26 kinds of antibiotics from 3 months before starting chemotherapy through to the end of chemotherapy (Supporting Information Table S2). The average number of antibiotics per person was 2.59; 98% of the participants had received multiple antibiotics. First‐generation cephalosporins, third‐generation cephalosporins, new quinolones and carbapenems were the most frequently used classes of antibiotic drugs, having been administered to 86.0, 67.3, 23.3 and 22.4% of participants, respectively, before or during chemotherapy. We had administered the first‐generation cephalosporin, cefazolin, exclusively as prophylaxis to prevent infection after surgery, endoscopic procedures or angiographic procedures whereas we had used third‐generation cephalosporins to treat infection or prophylactically (infection 35.1%; prophylaxis 64.9%). Because use of broad‐spectrum antibiotic drugs was not restricted in early 2000 in general hospitals, new quinolones or carbapenems had been used to treat infection in 76 or 64.5% of cases, respectively, and the other subjects received those drugs prophylactically (Supporting Information Table S2).
Use of cefazolin, third‐generation cephalosporins or new quinolones affected neither PFS nor OS (Figs. 1 a–1 f). However, the PFS of patients who had received carbapenem before or during chemotherapy was significantly worse than that of patients who had not received it (median PFS [mPFS], carbapenem+78 days, carbapenem−154 days, p = 0.0053, Fig. 1 g). Administration of carbapenem was also associated with short OS (median OS [mOS], carbapenem+177 days, carbapenem−475 days, p = 0.0003, Fig. 1 h). Cefazolin, third‐generation cephalosporins and levofloxacin have a limited spectrum for obligate anaerobic bacteria,26 whereas carbapenem has a broad spectrum for obligate anaerobes including Bacteroides genera and some Clostridium genera. We hypothesized that administration of antibiotics targeting obligate anaerobes is associated with a poor prognosis in chemotherapy‐treated patients with HCC. To investigate this hypothesis, we examined the effects of other antianaerobic drugs except carbapenem, including cefmetazole, flomoxef, cefpirome, cefepime, clindamycin and pazufloxacin. Use of these antianaerobic drugs was significantly associated with poor overall survival (mOS, antianaerobics except carbapenem+219 days, antianaerobics except carbapenem−470 days; p = 0.0087, Supporting Information Fig. S1A) but was not significantly associated with PFS (Supporting Information Fig. S1B). Because, like carbapenem, administration of antibiotics targeting anaerobes was associated with OS, we considered that these antianaerobics and carbapenem may have similar effects on the prognosis of patients with HCC who underwent chemotherapy. Patients who had received antianaerobic drugs, including carbapenem, showed significantly worse PFS and OS than those who had not (mPFS, antianaerobics+71 days, antianaerobics−163 days, p = 0.0006; mOS, antianaerobics+179 days, antianaerobics−475 days, p < 0.0001, Figs. 2 a and 2b).
Figure 1.
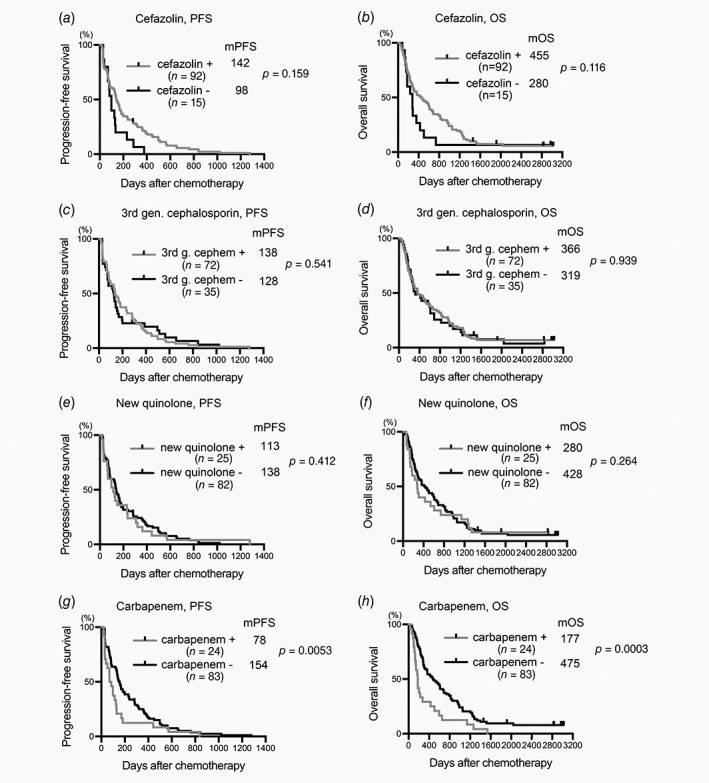
Plots of PFS and OS drawn according to antibiotics received. Plots of PFS (a, c, e and g) and OS (b, d, f and h) were drawn by the Kaplan–Meier method. Median PFS (mPFS, days), mOS time (days), number of patients in each category (n) and p are shown. +, received indicated antibiotic; −, did not receive indicated antibiotic.
Figure 2.
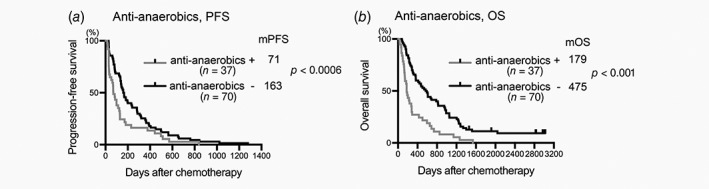
Administration of antianaerobics is associated with poor prognosis in chemotherapy‐treated patients with HCC. Plots of PFS (a) and OS (b) were drawn by the Kaplan–Meier method. Median PFS (mPFS, days), mOS time (days), number of patients in each category (n) and p are shown. +, received indicated antibiotic; −, did not receive indicated antibiotic.
Use of antianaerobics before rather than during chemotherapy is associated with poor prognosis of patients with HCC
If antianaerobic drugs exert direct effects on tumors and hosts or interact with anticancer cytotoxic drugs, their use during rather than before chemotherapy would affect prognosis. To investigate this, we examined associations between period of antibiotic use and prognosis and found that use of antianaerobics before chemotherapy was significantly associated with worse PFS (mPFS, antianaerobics+70 days, antianaerobics−159 days, p = 0.0009, Fig. 3 a); however, use of antianaerobics during chemotherapy did not affect PFS (mPFS, antianaerobics+92 days, antianaerobics−138 days, p = 0.226, Fig. 3 b). OS was worse in patients who received carbapenem either before or during the chemotherapy than in those who did not (Figs. 3 c and 3d). Next, we examined whether both short‐ and long‐term administration of antianaerobics influenced the prognosis. Both use of antianaerobics for fewer than 8 days and long‐term use were associated with poor PFS and OS compared to participants who had not received any antianaerobics (Figs. 3 e and 3f). We therefore hypothesized that impairment of prognosis is attributable to alterations in gut microbiota caused by drugs targeting anaerobes rather than direct effects on tumors and hosts or interactions with cytotoxic drugs; the effect on the prognosis was apparent even after short‐term use of these drugs.
Figure 3.
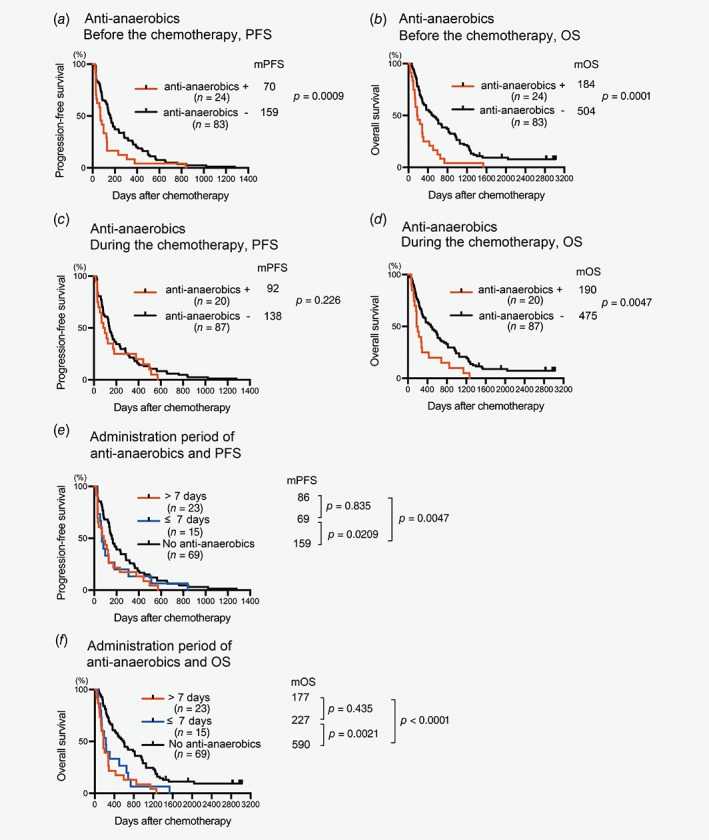
Duration and timing of administration of antianaerobics affects prognosis of chemotherapy‐treated patients with HCC. Plots of PFS (a, b and e) and OS (c, d and f) were drawn by the Kaplan–Meier method. Median PFS time (mPFS, days), mOS time (days), number of patients in each category (n) and p are shown in the graphs+, received indicated antibiotic; −, did not receive indicated antibiotics.
Administration of antianaerobic drugs is an independent predictor of poor prognosis in chemotherapy‐treated patients with HCC
Next, we searched numerous variables for independent factors predicting prognosis, including use of antianaerobics. We found that a higher proportion of patients who had received antianaerobics had extrahepatic lesions than of those who had not received them (p = 0.0080); however, BCLC stage did not differ between the groups (p = 0.129; Supporting Information Table S1). Because we had mostly antianaerobics to treat infections, the group that had received antianaerobics contained a greater proportion of patients with a history of infection (p = 0.022; Supporting Information Table S1). According to univariate analyses, the following six factors were significantly associated with PFS after chemotherapy: use of antianaerobic drugs before chemotherapy, tumor diameter, vascular invasion, extrahepatic lesions, AFP and treatment with cisplatin. Use of antianaerobics before chemotherapy was the only independent unfavorable factor for PFS, whereas treatment with cisplatin was an independent favorable factor according to multivariate analysis with Cox's proportional hazards regression model (Table 1). OS was associated with the following factors: use of antianaerobic drugs before chemotherapy, age, Child–Pugh class, tumor diameter, vascular invasion, extrahepatic lesions and AFP. Of these factors, administration of antianaerobics before chemotherapy was the only independent unfavorable factor for OS according to multivariate analysis (Table 2). Although a part of antianaerobic drugs were used to treat infectious diseases which can occasionally affect survival of the subjects, the presence of history of infection was not associated with both PFS and OS (Tables 1 and 2). While HCC progression was associated with use of antianaerobics according to influence on PFS, course of hepatic reserve after starting chemotherapy was not affected by use of antianaerobics (Supporting Information Fig. S2). In addition, a cause of death of most patients was HCC progression despite use of any antibiotics, and contribution of use of antibiotics such as antianaerobics, third‐generation cephem and new quinolone to the death due to the other causes was marginal (Supporting Information Tables S3–S5). Taken together, antianaerobic drug use before chemotherapy is an independent predictor of poor prognosis of chemotherapy‐treated patients with HCC.
Table 1.
Factors predicting progression‐free survival after the chemotherapy
| Univariate analysis p‐value | Hazard ratio (95% CI) | Multivariate analysis p‐value | ||
|---|---|---|---|---|
| Use of antianaerobic drug before the chemotherapy | Used | 0.001 | 2.105 (1.236–3.587) | 0.006 |
| Age | Years old | 0.162 | ||
| Sex | Male | 0.893 | ||
| ECOG PS | 0 | 0.19 | ||
| Child–Pugh class | C | 0.377 | ||
| HBsAg | Positive | 0.28 | ||
| Anti‐HCV | Positive | 0.326 | ||
| Tumor number | ≥6 | 0.238 | ||
| Tumor diameter | mm | 0.0042 | 1.003 (0.996–1.009) | 0.420 |
| Vascular invasion | Present | 0.002 | 1.367 (0.782–2.388) | 0.272 |
| Extrahepatic lesion | Present | 0.006 | 1.568 (0.792–3.104) | 0.197 |
| WBC | mm3 | 0.153 | ||
| AFP | ng/ml | 0.036 | 1.000 (1.000–1.000) | 0.244 |
| History of infection | Present | 0.526 | ||
| Port trouble | Present | 0.458 | ||
| Treatment with cisplatin | Treated | 0.04 | 0.601 (0.384–0.940) | 0.026 |
Abbreviations: AFP, α‐fetoprotein; Anti‐HCV, antibody to hepatitis C virus; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HBsAg, hepatitis B surface antigen; WBC, white blood cell.
Table 2.
Factors predicting overall survival after the chemotherapy
| Univariate analysis p‐value | Hazard ratio (95% CI) | Multivariate analysis p‐value | ||
|---|---|---|---|---|
| Use of antianaerobic drug before the chemotherapy | Used | <0.001 | 1.878 (1.123–3.142) | 0.016 |
| Age | Years old | 0.031 | 0.985 (0.961–1.010) | 0.234 |
| Sex | Male | 0.341 | ||
| ECOG PS | 0 | 0.447 | ||
| Child–Pugh class C | C | 0.011 | 0.751 (0.346–1.632) | 0.253 |
| HBsAg | Positive | 0.684 | ||
| HCV‐Ab | Positive | 0.734 | ||
| Tumor number | ≥6 | 0.616 | ||
| Tumor diameter | mm | <0.001 | 1.003 (0.996–1.009) | 0.428 |
| Vascular invasion | Present | 0.002 | 1.529 (0.892–2.618) | 0.122 |
| Extrahepatic lesion | Present | <0.001 | 1.803 (0.878–3.702) | 0.109 |
| WBC | mm3 | 0.531 | ||
| AFP | ng/ml | 0.003 | 1.000 (1.000–1.000) | 0.080 |
| History of infection | Present | 0.115 | ||
| Port trouble | Present | 0.82 | ||
| Treatment with cisplatin | Treated | 0.71 |
Abbreviations: AFP, α‐fetoprotein; Anti‐HCV, antibody to hepatitis C virus; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HBsAg, hepatitis B surface antigen; WBC, white blood cell.
Relative abundance of the intestinal anaerobic bacteria, Blautia, is associated with good prognosis in chemotherapy‐treated patients with HCC
We found that use of antianaerobics before chemotherapy is associated with poor prognosis of chemotherapy‐treated patients with HCC, indicating that gut floral composition before starting chemotherapy is an important predictor of prognosis of patients who undergo chemotherapy. Accordingly, we next planned a prospective observational study to examine the composition of the gut microbiota of 26 HCC patients before starting hepatic arterial infusion chemotherapy. Over half these patients had advanced stage HCC according to BCLC staging (Stage B intermediate, 46.1%; C advanced, 50.0%; D, 3.8%; Supporting Information Table S6). Median PFS and OS of all 26 subjects after chemotherapy were 109 and 430 days, respectively.
To determine correlations between composition of gut microbiota before starting chemotherapy and prognosis, we identified gut flora composition by sequencing of 16S rRNA amplicons. Most of the microbiota were consisted of class Bacteroidia, Bacilli and Clostridia (Supporting Information Fig. S3). Relative abundance of Blautia in fecal microbiota was positively correlated with both PFS and OS whereas abundance of Acidaminococcus was negatively correlated with OS. As to tumor status, abundance of Lactobacillus was negatively correlated with number of tumors, whereas abundance of Bilophila, Coprobacillus, Methanobrevibacter and Bulleidia were positively correlated with number of tumors. Abundance of Anaerostipes, Oxalobacter, Bacillus and Corynebacterium were negatively correlated with diameter of tumors. Interestingly, several bacterial genera were significantly associated with serum CRP concentration before starting chemotherapy. Abundance of fecal Lactobacillus, Anaerostipes, Corynebacterium, Parvimonas, Porphyromonas, Ruminococcus and Collinsella were negatively correlated with CRP, whereas Enterococcus and Microbacterium were positively correlated with it (Fig. 4 a). Patients with high proportions of fecal Blautia before starting chemotherapy had significantly better PFS than those with low proportions of Blautia (mPFS, Blautia hi 165 days, Blautia lo 42 days, p = 0.0072, Fig. 4 b). OS was also longer in patients with intestinal Blautia hi than in those with Blautia lo (mOS, Blautia hi 467 days, Blautia lo 113 days, p = 0.0170, Fig. 4 c). No baseline characteristics were significantly different between Blautia lo and Blautia hi groups (Supporting Information Table S6). Patients with intestinal Blautia hi and Acidaminococcus‐negative microbiota showed further longer OS than other groups (mOS, Blautia hi Aminoscidcoccus neg 602 days, Supporting Information Figure S5). Because Blautia is an obligate anaerobic bacteria, these bacteria were less abundant in patients who had received carbapenem before fecal collection (Fig. 4 d). Gut flora communities of patients who had received carbapenem clustered closely in principal coordinate analysis based on unweighted Unifrac matrices (Supporting Information Fig. S4). Difference of microbial communities between patients who had and had not received carbapenem was significant (Supporting Information Fig. S4), indicating that administration of carbapenem alters both composition of gut microbiota and relative abundance of the specific bacterial genus, Blautia. One of the characteristics of Blautia is that they produce the SCFA acetate.27 Measurement of the fecal SCFA revealed that fecal concentrations of acetate and propionate correlated positively with OS but not with PFS (Fig. 4 e). Thus, abundance of intestinal Blautia and SCFA was positively correlated with good prognosis after the chemotherapy.
Figure 4.
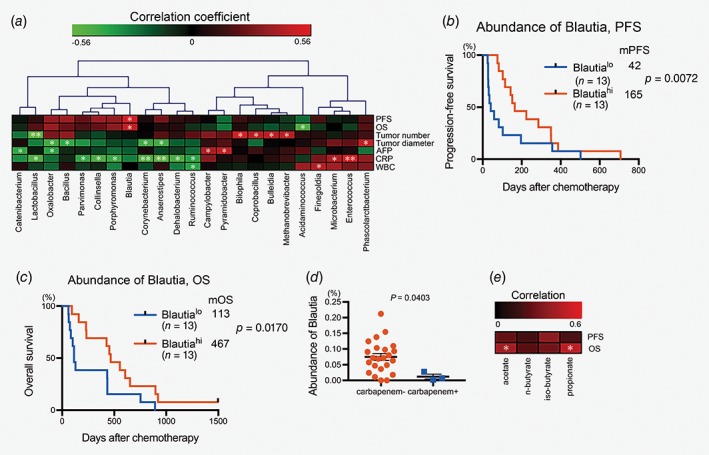
SCFA‐producing the anaerobic bacteria, Blautia, in the gut predict good prognosis in chemotherapy‐treated patients with HCC. (a) Spearman's rank correlation coefficients were calculated to determine correlations between fecal bacterial genera and participants’ characteristics. Hierarchical clustering was performed with bacterial genera that included the correlation coefficient with p‐value <0.05. *p < 0.05; **p < 0.01. (b) The subjects were divided into groups according to abundance of intestinal Blautia. Plots of PFS (b) and OS (c) were drawn by the Kaplan–Meier method. (d) Abundance of intestinal Blautia in patients who did or did not receive carbapenem before fecal collection was analyzed using the Mann–Whitney test. Bars, mean ± SE. (e) Spearman's rank correlation coefficients were calculated to determine correlations between concentration of fecal SCFA and PFS or OS. *p < 0.05.
Discussion
This is the first study to demonstrate that administration of antibiotics targeting anaerobes and alterations in gut flora are associated with poor prognosis after chemotherapy in humans with cancer. Given that improvements in the efficacy of anticancer chemotherapies for hepatic and gastrointestinal cancers are still required, our study provides a novel and important focus on gut flora and antibiotics in chemotherapy‐treated patients with the cancer. Antibiotics are one of the most frequently prescribed categories of drugs worldwide. In the USA, 833 antibiotics per 1,000 outpatients were prescribed in 2010.28 It has been estimated that more than 20% of prescription of antibiotics may be unnecessary.29 In this retrospective study, cefazolin, third‐generation cephalosporins (cefoperazone, ceftriaxone, cefotaxime, ceftazidime, cefdinir, cefcapene and sulbactam/cefoperazone), new quinolones (levofloxacin and pazufloxacin) and carbapenems (meropenem, imipenem/cilastatin, panipenem/betamipron and doripenem) were used for HCC patients. Evidence has recently been emerging that gut microbiota play critical roles in hosts’ physiology and that dysbiosis of microbiota causes pathological abnormalities.5, 30 Administration of antibiotics induces drastic changes in microbiota, and even after cessation of them microbiota do not completely recover to the former composition.31, 32 In this study, use of antibiotics targeting anaerobes before starting chemotherapy in patients with HCC was significantly associated with poor prognosis. Because a history of infection, which was the major reason for use of antibiotics, did not affect PFS or OS, it seems that alterations in gut microbiota might persist even after cessation of antianaerobics use and are directly associated with poor prognosis. The composition of gut flora changes even after short‐term administration of antibiotics and the change persists, at least in part, for a long time.22, 32 Our analyses showed that even patients who received antianaerobics for less than 8 days showed significantly shorter PFS and OS than those who received no antianaerobics. Thus, short‐term administration of antianaerobics can change gut flora composition and prevent prolongation of survival of patients with HCC. These findings suggest that even short‐term inappropriate administration of broad‐spectrum antibiotics to patients with HCC should be avoided.
Favorable roles of gut commensal bacteria in cancer treatments have been demonstrated mainly in mouse studies. Anticancer effects of cisplatin, oxaliplatin and cyclophosphamide on mouse cancers are impaired by depletion of gut flora with antibiotics.9, 10, 33 Tumor‐infiltrating myeloid cells promote apoptosis of platinum‐treated tumors by generating ROS. The presence of intact gut flora promotes apoptosis of tumor cells by activating ROS‐producing myeloid cells.9 The mechanisms underlying promotion of anticancer effects by gut flora differ between cyclophosphamide and platinum‐based drugs. Treatment with cyclophosphamide alters the composition of the microbiota in the small intestine and promotes translocation of gram‐positive bacteria into the second lymphoid organ, resulting in generation of Th17 cell, which in part mediate the anticancer effects of cyclophosphamide. Effects of immunotherapies against cancer are also promoted in mice by specific members of the gut flora.11, 12 Unlike mouse subcutaneous tumor models, bacterial species involved in survival of the clinical liver cancer patients need to be further investigated with detailed mechanisms.
Use of antianaerobics was associated with HCC progression, but not changes of hepatic reserve, indicating that alteration of gut flora might directly influence HCC progression and the subsequent course of prognosis. A possible mechanism underlying the unfavorable association between antianaerobics and HCC progression may be the decreased abundance of Blautia and SCFA production in the gut caused by the antianaerobics. Butyrate, one of the beneficial SCFAs, is produced by the Lachnospiraceae family, including Anaerostipes,34 which correlated negatively with number of tumors and serum CRP concentrations. Butyrate maintains the mucosal barrier by preserving the integrity of the junction with the intestinal epithelia and decreases apoptosis of epithelial cells.35, 36 This barrier is also preserved by T regulatory cells controlling inflammation and IgA generated by plasma cells. Butyrate induces differentiation of colonic T regulatory cells,37, 38 and also promotes differentiation of B cells to plasma cells, which generate IgA type antibodies.39 Blautia is abundant in the gut of healthy people, but not in the gut of older persons and patients with various diseases, including Type 2 diabetes, liver cirrhosis and colon cancer.40, 41, 42, 43 Blautia produces acetate, which is a SCFA and the substrate of butyrate. Acetate also directly strengthens the barrier of colonic epithelial cells, decreasing permeability44 and promoting IgA production from plasma cells like butyrate.39 Blautia also helps growth of other bacterial strain by metabolite cross‐feeding.45 Taken together, SCFA‐producing Lachnospiraceae family members, including Blautia and Anaerostipes may play roles in maintaining the mucosal barrier in the intestines of patients with HCC, thus decreasing local and systemic inflammation. Because systemic inflammation is associated with malignant cancer phenotypes such as growth promotion, epithelial–mesenchymal transition, resistance to cell death and immunosuppression,46 abundant SCFA‐producing intestinal bacteria and low levels of inflammation may be associated with a good prognosis in patients with HCC. More research is required to investigate a possible causal relationship between SCFA‐producing intestinal bacteria and prolonged survival of the patients.
In summary, our two independent clinical studies elucidated possible importance of intestinal anaerobic bacteria including Blautia in the good prognosis of the HCC patients after chemotherapy. Advances in understanding roles of gut commensal bacteria tell us harmful effects of deletion of the flora by broad‐spectrum antibiotics. Choice of appropriate antibiotic drugs under correct diagnosis of infectious diseases is strictly required in the treatments of clinical cancer patients.
Supporting information
Figure S1 Administration of antianaerobics other than carbapenem is associated with poor OS in chemotherapy‐treated patients with HCC. Plots of OS (A) and PFS (B) were drawn by the Kaplan–Meier method. Median PFS time (mPFS, days), mOS time (days), number of patients in each category (n) and P are shown. +, received indicated antibiotic; −, did not receive indicated antibiotics.
Figure S2 Course of Child‐Pugh scores after antianaerobics treatment and chemotherapy. Child‐Pugh scores at week 0, 4, and 8 after starting chemotherapy are shown. A group received antianaerobic drugs before the chemotherapy and the other did not. Mean ± SE. Difference of the scores between two groups at each week point was statistically analyzed by Mann–Whitney test. NS, not significant.
Figure S3 Composition of gut bacterial communities of HCC patients in the prospective study. Composition of fecal bacterial communities at class level of HCC patients before starting chemotherapy is shown. +, received carbapenem; −, did not receive carbapenem.
Figure S4 β‐Diversity analysis of gut floral communities in patients who did and did not receive carbapenem. Principal coordinate analysis was performed on gut floral communities of patients who did and did not receive carbapenem based on unweighted Unifrac matrices. Comparison of groups was tested by PERMANOVA and P is shown.
Figure S5 OS survival curve after chemotherapy according to relative abundance of Blautia and Acidaminococcus in gut. The subjects were divided into groups according to combination of abundance of intestinal Blautia and Acidoaminococcus. Plots of OS were drawn by the Kaplan–Meier method. Median OS time (days), number of patients in each category (n) and P are shown.
Table S1 Characteristics of subjects in sub‐analysis of the RCT
Table S2. List of antibiotics used from 3 months before the start of the chemotherapy through to the end of therapy; number of subjects (%)
Table S3. The cause of death of the patients who received or did not receive antianaerobic drugs; number of subjects (%)
Table S4. The cause of death of the patients who received or did not receive third‐generation cephem; number of subjects (%)
Table S5. The cause of death of the patients who received or did not receive new quinolone; number of subjects (%)
Table S6. Characteristics of subjects in the prospective study
Acknowledgements
We thank Dr Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this article.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1. Ley RE, Lozupone CA, Hamady M, et al. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol 2008;6:776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012;486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 2016;65:1906–15. [DOI] [PubMed] [Google Scholar]
- 4. Lopez CA, Kingsbury DD, Velazquez EM, et al. Collateral damage: microbiota‐derived metabolites and immune function in the antibiotic era. Cell Host Microbe 2014;16:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature 2016;535:75–84. [DOI] [PubMed] [Google Scholar]
- 6. Villarreal AA, Aberger FJ, Benrud R, et al. Use of broad‐spectrum antibiotics and the development of irritable bowel syndrome. WMJ 2012;111:17–20. [PubMed] [Google Scholar]
- 7. Scott FI, Horton DB, Mamtani R, et al. Administration of Antibiotics to children before age 2 years increases risk for childhood obesity. Gastroenterology 2016;151:120–9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun W, Svendsen ER, Karmaus WJ, et al. Early‐life antibiotic use is associated with wheezing among children with high atopic risk: a prospective European study. J Asthma 2015;52:647–52. [DOI] [PubMed] [Google Scholar]
- 9. Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013;342:971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA‐4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti‐PD‐L1 efficacy. Science 2015;350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 14. Song DS, Song MJ, Bae SH, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol 2015;50:445–54. [DOI] [PubMed] [Google Scholar]
- 15. Terashima T, Yamashita T, Arai K, et al. Feasibility and efficacy of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma after sorafenib. Hepatol Res 2014;44:1179–85. [DOI] [PubMed] [Google Scholar]
- 16. Yamashita T, Arai K, Sunagozaka H, et al. Randomized, phase II study comparing interferon combined with hepatic arterial infusion of fluorouracil plus cisplatin and fluorouracil alone in patients with advanced hepatocellular carcinoma. Oncology 2011;81:281–90. [DOI] [PubMed] [Google Scholar]
- 17. Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59–64. [DOI] [PubMed] [Google Scholar]
- 18. Jiang JW, Chen XH, Ren Z, et al. Gut microbial dysbiosis associates hepatocellular carcinoma via the gut‐liver axis. Hepatobiliary Pancreat Dis Int 2019;18:19–27. [DOI] [PubMed] [Google Scholar]
- 19. Liu Q, Li F, Zhuang Y, et al. Alteration in gut microbiota associated with hepatitis B and non‐hepatitis virus related hepatocellular carcinoma. Gut Pathog 2019;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016;63:764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009;49:1877–87. [DOI] [PubMed] [Google Scholar]
- 22. Rashid MU, Zaura E, Buijs MJ, et al. Determining the long‐term effect of antibiotic administration on the human Normal intestinal microbiota using culture and pyrosequencing methods. Clin Infect Dis 2015;60(Suppl 2):S77–84. [DOI] [PubMed] [Google Scholar]
- 23. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high‐throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera‐checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006;72:5069–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia‐Villalba R, Gimenez‐Bastida JA, Garcia‐Conesa MT, et al. Alternative method for gas chromatography‐mass spectrometry analysis of short‐chain fatty acids in faecal samples. J Sep Sci 2012;35:1906–13. [DOI] [PubMed] [Google Scholar]
- 26. Jernberg C, Lofmark S, Edlund C, et al. Long‐term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010;156:3216–23. [DOI] [PubMed] [Google Scholar]
- 27. Sun M, Wu W, Liu Z, et al. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol 2017;52:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013;368:1461–2. [DOI] [PubMed] [Google Scholar]
- 29. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011;128:1053–61. [DOI] [PubMed] [Google Scholar]
- 30. Sharon G, Garg N, Debelius J, et al. Specialized metabolites from the microbiome in health and disease. Cell Metab 2014;20:719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jernberg C, Lofmark S, Edlund C, et al. Long‐term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 2007;1:56–66. [DOI] [PubMed] [Google Scholar]
- 32. Jakobsson HE, Jernberg C, Andersson AF, et al. Short‐term antibiotic treatment has differing long‐term impacts on the human throat and gut microbiome. PLoS One 2010;5:e9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daillere R, Vetizou M, Waldschmitt N, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide‐induced therapeutic immunomodulatory effects. Immunity 2016;45:931–43. [DOI] [PubMed] [Google Scholar]
- 34. Koh A, De Vadder F, Kovatcheva‐Datchary P, et al. From dietary fiber to host physiology: short‐chain fatty acids as key bacterial metabolites. Cell 2016;165:1332–45. [DOI] [PubMed] [Google Scholar]
- 35. Jenq RR, Taur Y, Devlin SM, et al. Intestinal Blautia is associated with reduced death from graft‐versus‐host disease. Biol Blood Marrow Transplant 2015;21:1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome‐derived metabolites modulate intestinal epithelial cell damage and mitigate graft‐versus‐host disease. Nat Immunol 2016;17:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446–50. [DOI] [PubMed] [Google Scholar]
- 38. Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature 2013;504:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim M, Qie Y, Park J, et al. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016;20:202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kakiyama G, Pandak WM, Gillevet PM, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 2013;58:949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non‐diabetic adults. PLoS One 2010;5:e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murri M, Leiva I, Gomez‐Zumaquero JM, et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case‐control study. BMC Med 2013;11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ohigashi S, Sudo K, Kobayashi D, et al. Changes of the intestinal microbiota, short chain fatty acids, and fecal pH in patients with colorectal cancer. Dig Dis Sci 2013;58:1717–26. [DOI] [PubMed] [Google Scholar]
- 44. Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011;469:543–7. [DOI] [PubMed] [Google Scholar]
- 45. Reichardt N, Vollmer M, Holtrop G, et al. Specific substrate‐driven changes in human faecal microbiota composition contrast with functional redundancy in short‐chain fatty acid production. ISME J 2017;12:610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Administration of antianaerobics other than carbapenem is associated with poor OS in chemotherapy‐treated patients with HCC. Plots of OS (A) and PFS (B) were drawn by the Kaplan–Meier method. Median PFS time (mPFS, days), mOS time (days), number of patients in each category (n) and P are shown. +, received indicated antibiotic; −, did not receive indicated antibiotics.
Figure S2 Course of Child‐Pugh scores after antianaerobics treatment and chemotherapy. Child‐Pugh scores at week 0, 4, and 8 after starting chemotherapy are shown. A group received antianaerobic drugs before the chemotherapy and the other did not. Mean ± SE. Difference of the scores between two groups at each week point was statistically analyzed by Mann–Whitney test. NS, not significant.
Figure S3 Composition of gut bacterial communities of HCC patients in the prospective study. Composition of fecal bacterial communities at class level of HCC patients before starting chemotherapy is shown. +, received carbapenem; −, did not receive carbapenem.
Figure S4 β‐Diversity analysis of gut floral communities in patients who did and did not receive carbapenem. Principal coordinate analysis was performed on gut floral communities of patients who did and did not receive carbapenem based on unweighted Unifrac matrices. Comparison of groups was tested by PERMANOVA and P is shown.
Figure S5 OS survival curve after chemotherapy according to relative abundance of Blautia and Acidaminococcus in gut. The subjects were divided into groups according to combination of abundance of intestinal Blautia and Acidoaminococcus. Plots of OS were drawn by the Kaplan–Meier method. Median OS time (days), number of patients in each category (n) and P are shown.
Table S1 Characteristics of subjects in sub‐analysis of the RCT
Table S2. List of antibiotics used from 3 months before the start of the chemotherapy through to the end of therapy; number of subjects (%)
Table S3. The cause of death of the patients who received or did not receive antianaerobic drugs; number of subjects (%)
Table S4. The cause of death of the patients who received or did not receive third‐generation cephem; number of subjects (%)
Table S5. The cause of death of the patients who received or did not receive new quinolone; number of subjects (%)
Table S6. Characteristics of subjects in the prospective study
Data Availability Statement
All sequencing data and metadata were archived in NCBI SRA as BioProject PRJNA445357.


