Abstract
In contrast to the once dominant tumour‐centric view of cancer, increasing attention is now being paid to the tumour microenvironment (TME), generally understood as the elements spatially located in the vicinity of the tumour. Thinking in terms of TME has proven extremely useful, in particular because it has helped identify and comprehend the role of nongenetic and noncell‐intrinsic factors in cancer development. Yet some current approaches have led to a TME‐centric view, which is no less problematic than the former tumour‐centric vision of cancer, insofar as it tends to overlook the role of components located beyond the TME, in the ‘tumour organismal environment’ (TOE). In this minireview, we highlight the explanatory and therapeutic shortcomings of the TME‐centric view and insist on the crucial importance of the TOE in cancer progression.
Keywords: tumour microenvironment, tumour organismal environment, immune system, nervous system, microbiome
Abbreviations
- DC
dendritic cell
- FMT
faecal microbial transplantation
- G‐CSF
granulocyte‐colony stimulating factor
- TDLN
tumour‐draining lymph node
- TGF
transforming growth factor
- TLO
tertiary lymphoid organ
- TME
tumour microenvironment
- TOE
tumour organismal environment
Introduction: Why Going Beyond the TME?
Cancer research was dominated by a tumour‐centric view for a long time, which essentially focused on deciphering the intrinsic characteristics of tumour cells with little, if any, attention paid to their surrounding environment. However, since the 1970s, evidence has accumulated that the tumour microenvironment (TME) plays a key role in fostering or restraining tumour development,1, 2 a view that has now become prevalent.3 Indeed, the notion that cancers arise and develop in specific interacting contexts has gained undeniable traction in recent years.4, 5, 6, 7, 8, 9
Thinking in terms of TME has proven extremely useful, in particular because it has helped emphasise the importance of nongenetic and noncell‐intrinsic factors in cancer development. Yet, the recent focus on the TME has led, in parallel, to a ‘TME‐centric’ view of cancer, namely, a view centred on the role of the tumour and its immediate surroundings in cancer progression. In a recent study, we have discussed the spatial boundaries of the TME, and have suggested distinguishing different layers of the tumour environment, from the very local environment to the entire organism. We have dubbed the latter the ‘tumour organismal environment’ (TOE).10
In this minireview, we examine the latest compelling evidence in favour of the causal role of the TOE in tumour development and argue on that basis that the TME‐centric view is too narrow to accurately reflecting the complexities of the interacting networks involved in tumour development. As an important corollary, although TME‐centred anticancer therapies have led to promising results, many of these approaches face shortcomings11 in part because they fail to take into consideration the significant influence of these distant factors commonly excluded from the TME.12 It is therefore essential to look beyond the TME, and consider these distant actors, which are pivotal for cancer progression, and to explore the critical interactions that occur between local and nonlocal components.
The Key Role of the TOE
A few years ago, McAllister and Weinberg reviewed data indicating that tumours can affect distant sites that might in turn exert effects on primary or secondary tumour development, thus providing empirical support to the view of cancer as a systemic disease.13 Here, we expand this discussion by reviewing the latest evidence highlighting the potential involvement of additional distant factors, in particular some systemic immune components, the nervous system and the microbiota. We adopt a broader view with the concept of TOE, which includes elements that might not be modified by the tumour but nonetheless influence it (Fig. 1). We argue that distinguishing the TOE from the TME is important, first because distant elements of the TOE (which for some of them may never enter the TME) can critically affect tumour development, and second because mechanisms operating locally are generally different from those operating at a distance.
Figure 1.
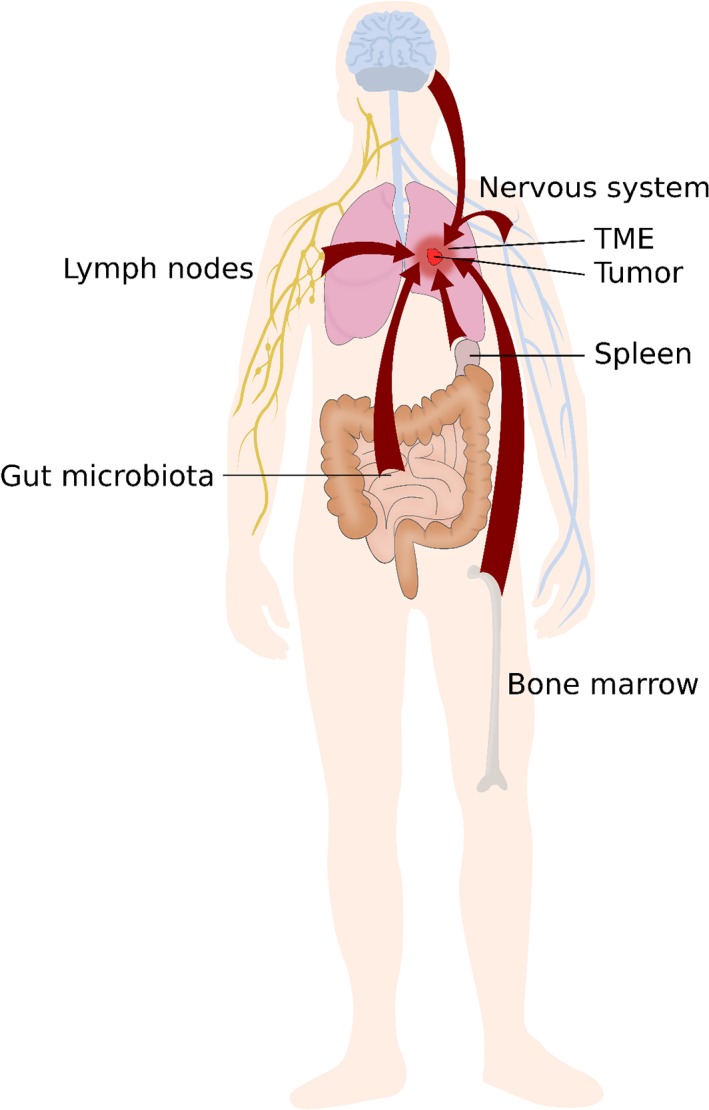
Elements located far from the tumour microenvironment can influence cancer progression. Investigations on cancer growth and dissemination have tended to focus on the causal influence of elements located within the tumour itself or in its immediate vicinity, that is, in the tumour microenvironment (TME). Nevertheless, elements that do not belong to the TME can sometimes have a major impact on cancer progression, including the immune system, the nervous system, and the microbiome. [Color figure can be viewed at wileyonlinelibrary.com]
Role of the immune system beyond the TME
Although many immune cells and soluble factors they produce have been identified within the tumour beds, the immune system constitutes an example of a system that can be localised at a distance from the tumour, and nonetheless exert an important impact on its progression and dissemination. We explore here four major aspects of this phenomenon: the role of lymph nodes, spleen and bone marrow, as well as the truly systemic influence (i.e. whole organism‐level) of some immune processes.
Tumour‐draining lymph nodes (TDLNs) are a case in point of structures involved in antitumour immunity but localised outside tumour boundaries and therefore usually not considered as part of the TME. These sites, in which tumour antigens are first presented to naive T cells, are key determinants in setting the course of immune responses, as they are the primary locations where the mechanisms of both anticancer immunity and tumour‐induced immunosuppression are integrated. For instance, dendritic cell (DC) migration to lymph nodes is critical for the efficiency of immunotherapies such as DC vaccines. As an example, Mitchell and colleagues demonstrated that preconditioning the vaccine site with the potent recall antigen tetanus/diphtheria toxoid generated local inflammatory responses leading to improved DC migration to lymph nodes (vaccine‐site draining LNs as well as more distant LNs), and greatly enhanced the efficacy of their DC vaccine both in a mouse model of glioblastoma and in their patient cohort.14 TDLNs may have a causal effect on what will occur at the tumour site, and they constitute potential therapeutic targets of the microenvironment.15 For instance, accumulation of regulatory T cells in TDLNs of colorectal patients, but their lack of accumulation at the tumour site, has been correlated with disease progression.16 Moreover, TDLNs undergo changes as upstream tumour profoundly alters functional characteristics of downstream lymph nodes.17, 18 For example, the antigen‐presenting cells that migrate from the tumour site to the TDLNs are skewed toward a tolerogenic phenotype.17 Furthermore, no tumour regression was observed after immunotherapy in a spontaneous model of mammary tumour when mice were treated with FTY720, a component blocking the egress of cells from the lymph nodes.19 TDLNs can also have an active role in tumour dissemination, as intralymphatic blood vessels may serve as exit route for tumour metastasis in different organs,20, 21 Furthermore, a premetastatic priming of LNs can occur through the action of VEGF‐C or other factors.22 Thus, this indicates that the immune/lymphatic TOE not only participates in the modulation of the immune tolerance of the primary tumour but also has a role in tumour dissemination.
To better understand the role of TDLNs in tumour progression, it is illuminating to compare them with tertiary lymphoid organs (TLOs). TLOs have a structure similar to that of lymph nodes, as they are composed of T cells and mature dendritic cells in the T‐cell areas close to a B cell follicle and they also have similar functions such as T‐cell priming and activation by antigen‐presenting cells.23, 24 TLOs, described in close vicinity of tumour mass or even within the tumour, are usually considered as an active part of the TME often associated with good prognosis,25 as highlighted in a recent review,26 even though regulatory T cells can also accumulate in TLOs and then suppress antitumour immunity.27 The key point here is that TLOs and TDLNs share many features, structurally and functionally, so much so that the inclusion of TLOs but not TDLNs in the TME is based only on their location, not on their causal influence on tumour progression. If one focuses on how some bodily components causally influence the fate of the tumour, as we do here, then it must be acknowledged that TDLNs are pivotal elements of the TOE and play a major role in tumour progression.
The spleen, as a main secondary lymphoid organ, represents another example of a component of immunity located outside the conventional boundaries of the TME but that still exerts a causal influence on tumour development. Evidence for the role of spleen cells in the control of malignancies has been provided by splenectomy experiments. The outcome of such procedure remains disputed, with some studies indicating that it may promote tumour elimination (in part by removing immunosuppressive cells produced in the spleen) or conversely foster tumour development. For instance, it has been reported that splenectomy enhances antitumour immunity by restoring the effector antitumoural function of T lymphocytes, which effectively improves immunotherapeutic interventions.28 In this latter case, splenectomy is associated with the removal of tumour‐induced immunosuppressive CD11b+Gr1+ cells located outside the microenvironment itself. Importantly, these observations suggest that the distant environment (the spleen in this case) can represent a useful therapeutic target to affect primary tumour progression. Similar findings were reported in other cancer types.29, 30 Along these lines, increased risk of malignancies has been observed in follow‐up studies of patients subjected to splenectomy.31, 32 Of note, in some reports, a tumour‐promoting effects of splenectomy has been observed, associated with increased number of FoxP3+ regulatory T cells at the metastatic sites.33
The bone marrow environment and the TME represent two spatially distant entities. However, the crosstalk between these two compartments significantly affects cancer development, including the metastatic process, at different levels and through a multitude of mechanisms. Many tumour‐derived growth and immunosuppressive factors produced within the TME [for instance, granulocyte‐colony stimulating factor (G‐CSF) and transforming growth factor β (TGFβ), as well as chemokines such as CCL2] can circulate systemically and impact the haematopoietic compartment, resulting in the production of immunosuppressive cells from haematopoietic precursors. These tumour‐promoting cells may home to the TME, where they promote angiogenesis, invasion and metastasis, to the premetastatic sites, or to the secondary lymphoid organs where they impair the priming and development of T‐cell dependent antitumor immune responses.34, 35, 36, 37 In addition, it is noteworthy that the bone marrow has been reported as an important site for the priming of tumour antigen‐specific CD4+ and CD8+ T lymphocytes which are ultimately involved in antitumour immunity and in the control of cancer development.38 Moreover, the bone marrow can play an intermediate causal role between distant tumours, referred to as ‘systemic instigation’. Malignant tumours can stimulate, through the recruitment of haematopoietic cells (Granulin+ Sca1+cKit−CD45+), the growth of distant tumours that would otherwise be indolent.39 Taken together, these observations about lymph nodes, the spleen and bone marrow further advocate for the existence of causal elements not located within the TME but which substantially affect cancer development. Along these lines, it is noteworthy that many immune cells and/or the soluble factors they secrete circulate through the bloodstream from one site to another (e.g. from the bone marrow to the lymph nodes), thus being spatially localised outside the TME but still contributing to tumour development. Immune cells in the circulation also produce proinflammatory or antiinflammatory cytokines, which may affect directly or indirectly tumour growth.
In addition to its activities associated with specific organs, the immune system can have a ‘systemic’ activity, in which case it exerts its functions at the level of the whole organism. Inflammation is considered to promote tumour progression and has even been included in the 2000s among the ‘hallmarks of cancer’.7, 40 Systemic inflammation can even play a role at preneoplastic stages, as highlighted by a recent study showing that patients with atherosclerosis treated using IL‐1β (an inflammatory cytokine) blocking antibodies have lower incidence of lung cancer than patients receiving placebo.41 Moreover, using systems analysis of a mouse model of cancer, Spitzer et al. demonstrated that tumour eradication after immunotherapy requires immune activation in the periphery.19 Thereby, the authors highlighted the importance of a coordinated systemic antitumour immune response for effective immunotherapy.
It is also noteworthy that several tumour‐derived factors can exert cancer‐promoting effects at distance of the TME. For instance, we have already outlined hereinabove that cytokines such as TGFβ or G‐CSF can promote the production of protumoural immunosuppressive cells in the bone marrow.34, 35, 36, 37 Other such examples include tumour‐derived exosomes which can colonise specific organ sites, prepare premetastatic niches,42 and may promote immunosuppression and impair antitumour immune responses in premetastatic organs (notably through their ability to impair T‐cell proliferation and natural killer cell cytotoxic function).43 Tumour‐derived exosomes may also migrate to the lymph nodes and influence tumour development.44
The effects of systemic factors such as exercise, age, diet, adiposity and sex on the immune system are now better understood, and, as shown in a recent review,26 key research is currently underway to determine how these various factors directly affect the quality of the antitumor immune response. Indeed, factors such as inflammation, growth factors, cytokines and chemokines, in addition to their local influence on the TME, are increasingly shown to have important systemic immune‐mediated effects on tumour growth and dissemination.26
Role of the nervous system beyond the TME
The nervous system also connects cancer cells to the whole organism, and recent emphasis has been put on what is now called ‘neurogenic control’ or ‘neural regulation’ of tumour development. Both the sympathetic and the parasympathetic nervous systems have been causally implicated in cancer initiation and progression.45 The interaction of the nervous system with cancer cells can be either direct or mediated by a remodelling of the immune or vascular microenvironment.46
Animal model studies have proved that behavioural stress can accelerate the progression of different cancer types (breast, prostate, ovary, brain, skin, pancreas, as well as some haematopoietic cancers such as leukaemia).47 Stress‐induced biological effects can be efficiently blocked by β‐adrenergic antagonists and mimicked by pharmacologic β‐agonists in many of these models. Moreover, different cellular and molecular mechanisms by which the sympathetic nervous system can influence tumour progression have been identified, including DNA repair, oncogene activation, inflammation and immune response, haematopoiesis, angiogenesis, cell survival and apoptosis.47 Several observational epidemiologic studies have documented associations between exposure to β‐adrenergic antagonists and reduced progression of some cancers,48, 49, 50, 51 though some inconsistencies exist,52, 53 which suggests that randomised controlled studies are needed.
Many investigations have focused on the direct interaction between cancer cells and the local nervous system. However, in the haematopoietic system, the sympathetic nervous system connects haematopoietic stem cells (HSCs) to the external environment: maintenance and trafficking of HSCs show circadian oscillations that are regulated by the sympathetic nervous system through secretion of noradrenaline, activation of β3‐adrenergic receptor, degradation of Sp1 and downregulation of CXCL12, which is modulated by photic cues that are transmitted from the eye to the suprachiasmatic nucleus through the retinal–hypothalamic tract.54, 55 Degradation of the sympathetic nervous system of the bone marrow, through the destruction of Schwann cells by leukemic stem cells and monocyte secretion of IL‐1, promotes the development of myeloproliferative neoplasm by giving mutated cells an advantage over normal HSCs.56 This local degradation disrupts the more systemic regulation of haematopoiesis by the sympathetic nervous system.
Neural control of tumour development is observed not only in haematological malignancies but also in solid tumours. For example, the development of prostate cancers is significantly controlled by the autonomous nervous system.57 Denervation with Botox significantly reduces prostate tumour development and leads to increased tumour cell apoptosis.58 Similar observations have been made in the case of gastric tumorigenesis: in mouse models of gastric cancer, it was shown that surgical or pharmacological denervation of the stomach reduced significantly tumour incidence and progression, but in the denervated portion of the stomach exclusively. Moreover, tumour stage in gastric cancer patients correlated with neural density and activated Wnt signalling, and vagotomy diminished the risk of gastric cancer. The authors propose that vagal innervation contributes to gastric tumorigenesis via M3 receptor‐mediated Wnt signalling in the stem cells, and that denervation could represent a feasible strategy for the control of gastric cancer.59
At a molecular level, β‐adrenergic receptors play an important role in neurogenic effects on cancer,47 and β‐blocking agents are able to inhibit metastasis development. It was shown that tumour cell migration is regulated by signal substances of the environment including chemokines and neurotransmitters. More specifically, after the initial demonstration that the migration of breast, prostate and colon carcinoma cells was enhanced by the stress‐related neurotransmitter norepinephrine in vitro and that this effect could be inhibited by the β‐blocker propranolol, it was later shown that this neurotransmitter‐driven regulation was relevant in vivo.60
Taken together, these results highlight the long‐range effects of the nervous system on tumour development and metastasis.
The increasingly appreciated role of the microbiome
Recent research has revealed that another, largely unexpected, actor can influence cancer development well beyond the site of the TME, namely, the microbiome.61 First, it has been reported that the microbiome plays a role in carcinogenesis not only locally but also at distant sites.12, 62 The influence can be direct, for example when microbes provide toxic metabolites or oncogenic products, or indirect, in particular when microbes induce inflammation or immunosuppression.61 Faecal microbial transplantation (FMT) can transfer the neoplasia‐prone phenotype from knockout mice lacking some immune‐relevant genes.
A second and more recent series of observations indicate that the microbiome can influence how the host responds to anticancer therapies. Several of these therapies, including chemotherapy and anti‐CTLA‐4, anti‐PD1 and anti‐PDL1 immunotherapies, show reduced efficacy in germ‐free mice as well as in mice treated with broad‐spectrum antibiotics (frequently used clinically in cancer patients), or in mice lacking specific bacteria that stimulate the immune system.63, 64, 65, 66, 67 The causal relationship between the dominance of distinct commensals and the efficacy of anticancer therapies in these examples has been significantly strengthened by mouse cohousing experiments or oral gavage with defined species. Studies in both mice and humans suggest that the administration of antibiotics before or during anticancer therapies (which is frequent in the clinic) might well be deleterious. In patients, it was observed that bacteria generally associated with health and/or immune stimulation had a positive impact on responses to some immunotherapies. Initial correlational observations were then confirmed causally, by FMT from patients to mice. Patient faecal samples were transferred into germ‐free or antibiotics‐treated specific‐pathogen‐free mice, that subsequently were inoculated with mouse syngeneic tumours and then treated with monoclonal antibodies to CTLA‐4 and/or PD‐1/PD‐L1. The key observation was that ‘responding’ and ‘nonresponding’ phenotypes could be transferred from humans to mice, and some distinct bacteria playing a major role in this process were identified (Bacteroides species in melanoma treated with ipilimumab, Faecalibacterium in melanoma and Verrucomicrobiacae in lung cancer patients treated with the PD‐1 inhibitors pembrolizumab or nivolumab).66, 67, 68, 69 Mechanistically, several possibilities are being investigated:61 stimulation of T‐cell responses by the microbiome; engagement of pattern recognition receptors inducing proimmune or antiinflammatory effects, including perhaps via the enteric nervous system;70 or production of small metabolites that mediate systemic effects on the host.
Overall, there is a growing consensus that the microbiome can play a major role on both tumour growth and host responses to cancer therapies, which confirms that a distant actor, located well beyond the TME, can causally influence cancer fate.
Collectively, the examples discussed hereinabove clearly demonstrate that, despite the undeniable importance of the TME, elements located beyond the commonly defined spatial boundaries of the TME can also play a crucial role in cancer progression. Together, these remote components belong to the ‘TOE’. An exclusive focus on the components present in the TME could have the unwanted consequence that researchers might tend to neglect the investigation of the causal influence on tumour progression and dissemination of elements situated far from the tumour. Recent research indicates that no element should be excluded a priori, and certainly not on the sole basis of its distance to the tumour. Further investigations are needed (and some are currently underway) to determine whether remote components such as (among several others) the endocrine system, nutrition, exercise and behavioural factors including stress and emotion could play a role in tumour development.71, 72, 73, 74, 75, 76
The Therapeutic Benefits of Considering the TOE
Why does it matter to consider elements that are located beyond the TME and play a role in cancer development? We propose that adopting the notion of TOE is essential not only to apprehend and comprehend the multiple networks of reciprocal interactions and controls between tumours and the host organism but also from a therapeutic point of view. Much anticancer therapeutic research, including immunotherapies and antiangiogenic therapies, aim at targeting the TME.77 Although this is, in many cases, a fruitful strategy, focusing on the TME is problematic for two main reasons.
First, the efficacy of TME‐only centric therapeutics has often been disappointing, as illustrated by the limited impact of antiangiogenic treatments.11 For example, one mechanism that can promote evasive resistance to VEGF pathway inhibitors involves protection of the tumour vasculature by recruiting proangiogenic cells of the immune system (Fig. 2 a).78, 79 This probably reflects a less important causal role of some specific factors than anticipated, and/or a complex network of causal factors, with many interactions between them.80 Another explanation is the plasticity of pathways where the cell machinery adapts to the constraints imposed by therapy (hidden causality), as exemplified by the induction of the c‐Met pathway under antiangiogenesis therapy, which promotes tumour cell invasion.81
Figure 2.
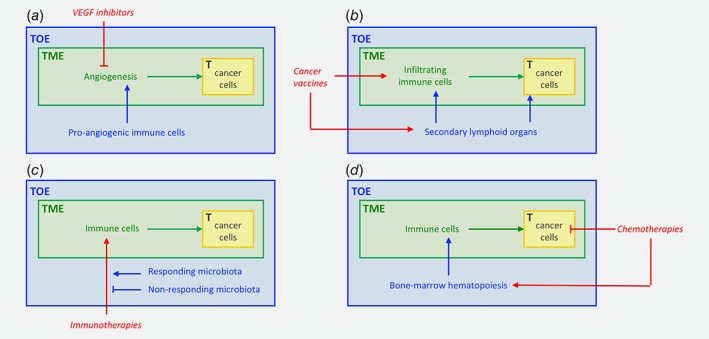
The role of the TOE in the therapeutic response. (a) Recruitment of pro‐angiogenic immune cells by the TOE can promote resistance to VEGF inhibitors; (b) The TOE can play an essential role in the efficacy of some therapies such as cancer vaccines that lead to clonal selection of tumour‐specific T cells in secondary lymphoid structures, away from the tumour site itself; (c) The microbiota, localised within the TOE, may also modulate the efficacy of different therapies such as immunotherapies; (d) Therapies can also exhibit adverse effects on the TOE: for instance, while killing cancer cells, chemotherapies can negatively impact the haematopoietic system, leading to a more pro‐tumorigenic TME. Abbreviations: T, tumour; TME, tumour microenvironment; TOE, tumour organismal environment. The tumour is represented in yellow and the classical TME‐centric view is in green, TME‐centrism overlooks the involvement of the TOE (in blue) in the response to therapies (in red).
Second, TME‐centric approaches tend to overlook the therapeutic importance of other, more distant, components.12 Here we take two examples: the immune system and the microbiome.
In the field of oncoimmunology, strategies aimed at inducing protective anticancer immunity primarily rely on the triggering of antitumour immune responses in the secondary lymphoid organs, where cytotoxic and helper T lymphocytes specific of tumour antigens are produced and activated. In other words, the harnessing and mobilisation of effector immune cells responsible for tumour clearance occur outside the physical perimeter of the TME. Most cancer vaccines such as tumour‐derived peptides, protein–vaccine, RNA or DNA encoding for tumour antigens, or dendritic cells, are administered by subcutaneous, intradermal or intranodal routes, and lead to clonal selection of tumour‐specific T cells in secondary lymphoid structures (in the TOE) away from the tumour site (the TME) itself (Fig. 2 b).82, 83, 84 Along these lines, immunosuppressive cell elimination has been proven to foster anticancer immunity. These beneficial effects result not only from the depletion of suppressive factors within the TME but also at the site of induction of immune responses (the secondary lymphoid organs), where they accumulate in substantial number during tumour progression. In this context, regulatory immune checkpoint blockers (anti‐CTLA4 and anti‐PD1) act not only on immune effectors in tissues but also at the early stages of T‐cell response induction in the secondary lymphoid tissues, thereby targeting elements located outside the TME itself.85 Additionally, different approaches have been considered to maximise vaccine delivery to the draining lymph nodes, one component of the TOE, with the goal of enhancing the priming of tumour‐specific T lymphocytes, and thus antitumoural immunity. Such strategies include the packaging of antigens and/or adjuvants into bioengineered nanoparticles.86 A final point to be considered here concerning the distant action of the immune system from a therapeutic point of view is the fact that current cancer immunotherapies often have significant adverse systemic effects, which could potentially be alleviated therapeutically via the targeting of elements located beyond the TME.
Another particularly illuminating example is the recently documented impact of the microbiome on anticancer therapies, in agreement with mouse experiments already mentioned above. In patients, it was observed that some bacteria had a positive impact on responses to some immunotherapies. Initial correlational observations were then confirmed experimentally by using FMT from patients to mice.61 This demonstrates the possibility of a long‐distance impact on responses to anticancer treatment within the broader context of the TOE, which opens up important avenues for future research (Fig. 2 c).
Crucially, the most promising therapies will certainly be those able to combine the manipulation of both the traditionally defined TME and the TOE. For example, some therapies have deleterious impact on haematopoiesis that can counterproductively participate in tumour progression (Fig. 2 d). To avoid these effects, additional therapies such as 4‐methylcatechol56 or administration of parathyroid hormones87 could be useful.34
These considerations therefore underline the fact that many therapeutic interventions targeting elements clearly located outside the tumour beds ultimately result in the control of cancer development and dissemination. These data emphasise the need to not solely focusing on the microenvironment located at the tumour vicinity when designing anticancer therapies. Ongoing research on potential clinical manipulation of various components of the TOE, including the nervous system, nutrition and different other factors, will determine to what extent therapeutic interventions on distant components are likely to become generalised in the near future.
Conclusion
We have recently suggested a revision of the concept of the TME by distinguishing multiple layers of the tumour environment.10 In our view, the classical TME constitutes only one layer in this multilevel structure. More specifically, it corresponds to the confined (local) tumour environment, while the TOE constitutes the outer layer of the tumour environment.
In this article, we provide mechanistic and clinical arguments showing that many components of the TOE (such as the immune system, the nervous system and the microbiome), and therefore located beyond the traditionally defined TME, can play a key role in promoting or inhibiting tumour development (Figs. 1 and 2). We content that reorienting current research towards the identification and quantification of all causal factors, without overly privileging the elements that surround the tumour, will lead to a much more open‐minded and productive approach to cancer. This will also create new avenues for the exploration of interactions between different layers of the tumour environment, especially between the confined tumour environment and the TOE. Such exploration will undoubtedly be facilitated in the current era of increasing adoption of next‐generation techniques, particularly single‐cell sequencing and high‐resolution data.
References
- 1. Witz IP. The tumor microenvironment: the making of a paradigm. Cancer Microenviron 2009;2:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer 2001;1:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maman S, Witz IP. A history of exploring cancer in context. Nat Rev Cancer 2018;18:359–76. [DOI] [PubMed] [Google Scholar]
- 4. Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev 2008;18:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laconi E. The evolving concept of tumor microenvironments. Bioessays 2007;29:738–44. [DOI] [PubMed] [Google Scholar]
- 6. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008;27:5904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 8. Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem 2007;101:805–15. [DOI] [PubMed] [Google Scholar]
- 9. Witz IP. Tumor‐microenvironment interactions: dangerous liaisons. Adv Cancer Res 2008;100:203–29. [DOI] [PubMed] [Google Scholar]
- 10. Laplane L, Duluc D, Larmonier N, et al. The multiple layers of the tumor environment. Trends Cancer 2018;4:802–9. [DOI] [PubMed] [Google Scholar]
- 11. Jayson GC, Kerbel R, Ellis LM, et al. Antiangiogenic therapy in oncology: current status and future directions. Lancet 2016;388:518–29. [DOI] [PubMed] [Google Scholar]
- 12. Pitt JM, Vétizou M, Daillère R, et al. Resistance mechanisms to immune‐checkpoint blockade in cancer: tumor‐intrinsic and ‐extrinsic factors. Immunity 2016;44:1255–69. [DOI] [PubMed] [Google Scholar]
- 13. McAllister SS, Weinberg RA. The tumour‐induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014;16:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015;519:366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fransen MF, Arens R, Melief CJM. Local targets for immune therapy to cancer: tumor draining lymph nodes and tumor microenvironment. Int J Cancer 2013;132:1971–6. [DOI] [PubMed] [Google Scholar]
- 16. Deng L, Zhang H, Luan Y, et al. Accumulation of foxp3+ T regulatory cells in draining lymph nodes correlates with disease progression and immune suppression in colorectal cancer patients. Clin Cancer Res 2010;16:4105–12. [DOI] [PubMed] [Google Scholar]
- 17. Munn DH, Mellor AL. The tumor‐draining lymph node as an immune‐privileged site. Immunol Rev 2006;213:146–58. [DOI] [PubMed] [Google Scholar]
- 18. Cochran AJ, Huang R‐R, Lee J, et al. Tumour‐induced immune modulation of sentinel lymph nodes. Nat Rev Immunol 2006;6:659–70. [DOI] [PubMed] [Google Scholar]
- 19. Spitzer MH, Carmi Y, Reticker‐Flynn NE, et al. Systemic immunity is required for effective cancer immunotherapy. Cell 2017;168:487–502.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown M, Assen FP, Leithner A, et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 2018;359:1408–11. [DOI] [PubMed] [Google Scholar]
- 21. Pereira ER, Kedrin D, Seano G, et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 2018;359:1403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liersch R, Hirakawa S, Berdel WE, et al. Induced lymphatic sinus hyperplasia in sentinel lymph nodes by VEGF‐C as the earliest premetastatic indicator. Int J Oncol 2012;41:2073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dieu‐Nosjean MC, Goc J, Giraldo NA, et al. Tertiary lymphoid structures in cancer and beyond. Trends Immunol 2014;35:571–80. [DOI] [PubMed] [Google Scholar]
- 24. Engelhard VH, Rodriguez AB, Mauldin IS, et al. Immune cell infiltration and tertiary lymphoid structures as determinants of antitumor immunity. J Immunol 2018;200:432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hiraoka N, Ino Y, Yamazaki‐Itoh R. Tertiary lymphoid organs in cancer tissues. Front Immunol 2016;7:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joshi NS, Akama‐Garren EH, Lu Y, et al. Regulatory T cells in tumor‐associated tertiary lymphoid structures suppress anti‐tumor T cell responses. Immunity 2015;43:579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ugel S, Peranzoni E, Desantis G, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep 2012;2:628–39. [DOI] [PubMed] [Google Scholar]
- 29. Miller MR, Mandell JB, Beatty KM, et al. Splenectomy promotes indirect elimination of intraocular tumors by CD8+ T cells that is associated with IFNγ and Fas/FasL dependent activation of Intratumoral macrophages. Cancer Immunol Res 2014;2:1175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li B, Zhang S, Huang N, et al. Dynamics of the spleen and its significance in a murine H22 orthotopic hepatoma model. Exp Biol Med (Maywood) 2016;241:863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kristinsson SY, Gridley G, Hoover RN, et al. Long‐term risks after splenectomy among 8,149 cancer‐free American veterans: a cohort study with up to 27 years follow‐up. Haematologica 2014;99:392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun L‐M, Chen H‐J, Jeng L‐B, et al. Splenectomy and increased subsequent cancer risk: a nationwide population‐based cohort study. Am J Surg 2015;210:243–51. [DOI] [PubMed] [Google Scholar]
- 33. Higashijima J, Shimada M, Chikakiyo M, et al. Effect of splenectomy on antitumor immune system in mice. Anticancer Res 2009;29:385–93. [PubMed] [Google Scholar]
- 34. Giles AJ, Chien CD, Reid CM, et al. The functional interplay between systemic cancer and the hematopoietic stem cell niche. Pharmacol Ther 2016;168:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balducci L, Hardy CL. High proliferation of early hemopoietic progenitors in tumor‐bearing mice. Pathobiology 1992;60:68–71. [DOI] [PubMed] [Google Scholar]
- 36. Kumar V, Patel S, Tcyganov E, et al. The nature of myeloid‐derived suppressor cells in the tumor microenvironment. Trends Immunol 2016;37:208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu W‐C, Sun H‐W, Chen H‐T, et al. Circulating hematopoietic stem and progenitor cells are myeloid‐biased in cancer patients. Proc Natl Acad Sci USA 2014;111:4221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feuerer M, Beckhove P, Garbi N, et al. Bone marrow as a priming site for T‐cell responses to blood‐borne antigen. Nat Med 2003;9:1151–7. [DOI] [PubMed] [Google Scholar]
- 39. Elkabets M, Gifford AM, Scheel C, et al. Human tumors instigate granulin‐expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J Clin Invest 2011;121:784–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Colotta F, Allavena P, Sica A, et al. Cancer‐related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073–81. [DOI] [PubMed] [Google Scholar]
- 41. Ridker PM, MacFadyen JG, Thuren T, et al. Effect of interleukin‐1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double‐blind, placebo‐controlled trial. Lancet 2017;390:1833–42. [DOI] [PubMed] [Google Scholar]
- 42. Hoshino A, Costa‐Silva B, Shen T‐L, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wen SW, Sceneay J, Lima LG, et al. The biodistribution and immune suppressive effects of breast cancer‐derived Exosomes. Cancer Res 2016;76:6816–27. [DOI] [PubMed] [Google Scholar]
- 44. Ruivo CF, Adem B, Silva M, et al. The biology of cancer Exosomes: insights and new perspectives. Cancer Res 2017;77:6480–8. [DOI] [PubMed] [Google Scholar]
- 45. Simó M, Navarro X, Yuste VJ, et al. Autonomic nervous system and cancer. Clin Auton Res 2018;28:301–14. [DOI] [PubMed] [Google Scholar]
- 46. Hanoun M, Maryanovich M, Arnal‐Estapé A, et al. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 2015;86:360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cole SW, Nagaraja AS, Lutgendorf SK, et al. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 2015;15:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baek MH, Kim DY, Kim SO, et al. Impact of beta blockers on survival outcomes in ovarian cancer: a nationwide population‐based cohort study. J Gynecol Oncol 2018;29:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barron TI, Connolly RM, Sharp L, et al. Beta blockers and breast cancer mortality: a population‐ based study. J Clin Oncol 2011;29:2635–44. [DOI] [PubMed] [Google Scholar]
- 50. Grytli HH, Fagerland MW, Fosså SD, et al. Association between use of β‐blockers and prostate cancer‐specific survival: a cohort study of 3561 prostate cancer patients with high‐risk or metastatic disease. Eur Urol 2014;65:635–41. [DOI] [PubMed] [Google Scholar]
- 51. Hwa YL, Shi Q, Kumar SK, et al. Beta‐blockers improve survival outcomes in patients with multiple myeloma: a retrospective evaluation. Am J Hematol 2017;92:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Na Z, Qiao X, Hao X, et al. The effects of beta‐blocker use on cancer prognosis: a meta‐analysis based on 319,006 patients. OncoTargets Ther 2018;11:4913–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yap A, Lopez‐Olivo MA, Dubowitz J, et al. Effect of beta‐blockers on cancer recurrence and survival: a meta‐analysis of epidemiological and perioperative studies. Br J Anaesth 2018;121:45–57. [DOI] [PubMed] [Google Scholar]
- 54. Méndez‐Ferrer S, Chow A, Merad M, et al. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol 2009;16:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Méndez‐Ferrer S, Lucas D, Battista M, et al. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008;452:442–7. [DOI] [PubMed] [Google Scholar]
- 56. Arranz L, Sánchez‐Aguilera A, Martín‐Pérez D, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 2014;512:78–81. [DOI] [PubMed] [Google Scholar]
- 57. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science 2013;341:1236361. [DOI] [PubMed] [Google Scholar]
- 58. Coarfa C, Florentin D, Putluri N, et al. Influence of the neural microenvironment on prostate cancer. Prostate 2018;78:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao C‐M, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med 2014;6:250ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Palm D, Lang K, Niggemann B, et al. The norepinephrine‐driven metastasis development of PC‐3 human prostate cancer cells in BALB/c nude mice is inhibited by beta‐blockers. Int J Cancer 2006;118:2744–9. [DOI] [PubMed] [Google Scholar]
- 61. Zitvogel L, Ma Y, Raoult D, et al. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science 2018;359:1366–70. [DOI] [PubMed] [Google Scholar]
- 62. Dzutsev A, Badger JH, Perez‐Chanona E, et al. Microbes and cancer. Annu Rev Immunol 2017;35:199–228. [DOI] [PubMed] [Google Scholar]
- 63. Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013;342:971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sivan A, Corrales L, Hubert N, et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti‐PD‐L1 efficacy. Science 2015;350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA‐4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Routy B, Chatelier EL, Derosa L, et al. Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- 68. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti–PD‐1 immunotherapy in melanoma patients. Science 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti–PD‐1 efficacy in metastatic melanoma patients. Science 2018;359:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yissachar N, Zhou Y, Ung L, et al. An intestinal organ culture system uncovers a role for the nervous system in microbe‐immune crosstalk. Cell 2017;168:1135–1148.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol 2017;18:843–50. [DOI] [PubMed] [Google Scholar]
- 72. Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine‐immune pathway from stress to disease? Brain Behav Immun 2003;17:321–8. [DOI] [PubMed] [Google Scholar]
- 73. Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio‐behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 2006;6:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ball LJ, Palesh O, Kriegsfeld LJ. The pathophysiologic role of disrupted circadian and neuroendocrine rhythms in breast carcinogenesis. Endocr Rev 2016;37:450–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res 2014;58:193–210. [DOI] [PubMed] [Google Scholar]
- 76. Ben‐Shaanan TL, Schiller M, Azulay‐Debby H, et al. Modulation of anti‐tumor immunity by the brain's reward system. Nat Commun 2018;9:2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell 2005;7:513–20. [DOI] [PubMed] [Google Scholar]
- 78. Bergers G, Hanahan D. Modes of resistance to anti‐angiogenic therapy. Nat Rev Cancer 2008;8:592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rivera LB, Bergers G. Tumor angiogenesis, from foe to friend. Science 2015;349:694–5. [DOI] [PubMed] [Google Scholar]
- 80. Crawford Y, Ferrara N. Tumor and stromal pathways mediating refractoriness/resistance to anti‐angiogenic therapies. Trends Pharmacol Sci 2009;30:624–30. [DOI] [PubMed] [Google Scholar]
- 81. Lu KV, Chang JP, Parachoniak CA, et al. VEGF inhibits tumor cell invasion and Mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 2012;22:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen DS, Mellman I. Oncology meets immunology: the cancer‐immunity cycle. Immunity 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- 83. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012;12:265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012;12:237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mehta NK, Moynihan KD, Irvine DJ. Engineering new approaches to cancer vaccines. Cancer Immunol Res 2015;3:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li S, Zou D, Li C, et al. Targeting stem cell niche can protect hematopoietic stem cells from chemotherapy and G‐CSF treatment. Stem Cell Res Ther 2015;6:175. [DOI] [PMC free article] [PubMed] [Google Scholar]


