Abstract
Carnitine plays essential roles in intermediary metabolism. In non-vegetarians, most of carnitine sources (~75%) are obtained from diet whereas endogenous synthesis accounts for around 25%. Renal carnitine reabsorption along with dietary intake and endogenous production maintain carnitine homeostasis. The precursors for carnitine biosynthesis are lysine and methionine. The biosynthetic pathway involves four enzymes: 6-N-trimethyllysine dioxygenase (TMLD), 3-hydroxy-6-N-trimethyllysine aldolase (HTMLA), 4-N-trimethylaminobutyraldehyde dehydrogenase (TMABADH), and γ-butyrobetaine dioxygenase (BBD). OCTN2 (organic cation/carnitine transporter novel type 2) transports carnitine into the cells. One of the major functions of carnitine is shuttling long-chain fatty acids across the mitochondrial membrane from the cytosol into the mitochondrial matrix for β-oxidation. This transport is achieved by mitochondrial carnitine–acylcarnitine cycle, which consists of three enzymes: carnitine palmitoyltransferase I (CPT I), carnitine-acylcarnitine translocase (CACT), and carnitine palmitoyltransferase II (CPT II). Carnitine inborn errors of metabolism could result from defects in carnitine biosynthesis, carnitine transport, or mitochondrial carnitine–acylcarnitine cycle. The presentation of these disorders is variable but common findings include hypoketotic hypoglycemia, cardio(myopathy), and liver disease. In this review, the metabolism and homeostasis of carnitine are discussed. Then we present details of different inborn errors of carnitine metabolism, including clinical presentation, diagnosis, and treatment options. At the end, we discuss some of the causes of secondary carnitine deficiency.
Keywords: carnitine, trimethyllysine (TML) dioxygenase, carnitine transporter, carnitine palmitoyltransferase
1. Introduction
Carnitine (l-3-hydroxy-4-N,N,N-trimethylaminobutyrate), is an essential water soluble molecule that has many biological functions. One of these major functions is shuttling long-chain fatty acids across the mitochondrial membrane from the cytosol into the mitochondrial matrix for β-oxidation, and hence, cellular energy production. Carnitine also modulates the acyl-CoA/CoA ratio, thereby regulating the activity of several mitochondrial enzymes [1]. The acetylated form of carnitine, acetyl-carnitine, is involved in energy storage. Carnitine conjugates with partially metabolized acyl groups and allows their excretion as carnitine esters in urine [2,3,4]. More roles of carnitine being identified include anti-inflammatory and antioxidant properties [5,6,7], and improving insulin resistance [2,8].
Meat, poultry, fish, and dairy products are major sources of carnitine in the diet. On the other hand, the content of carnitine in food of plant origin is low. In non-vegetarians, 75% of carnitine sources are obtained from diet, which provides 2–12 µmol of carnitine per kilogram per day (µmol/Kg/day), and the remaining 25% originates from endogenous production, which provides 1.2 µmol/Kg/day. In contrast, endogenous synthesis provides the majority (>90%) of total carnitine in strict vegetarians, in whom dietary intake provides less than 0.1 µmol/Kg/day of carnitine [9,10,11].
The vast majority (>99%) of body carnitine is situated in the intracellular compartment. Circulating carnitine represents only about 0.5% of body carnitine. Therefore, normal plasma free carnitine levels are low, ranging between 25–50 µmol/L [12]. Normal levels are variable based on age and gender. In one study of 80 healthy volunteers, mean serum-free carnitine levels in males were 41 µmol/L (range 26.4–53.4) whereas in females the mean was 39 µmol/L (range 19.2–44.5) [13]. The same study showed a positive correlation between carnitine levels (free and total) with age, although in males the correlation didn’t reach statistical significance [13]. Normal carnitine levels are maintained by balance between dietary intake, endogenous synthesis, and renal reabsorption. Based on carnitine content in food, bioavailability of dietary carnitine is about 54–86% [14]. In the kidney the majority of carnitine (90–99% of filtered load) is reabsorbed until saturation is reached. The renal threshold for carnitine excretion is around 50 μmol/L. The kidneys are very efficient in maintaining normal levels of plasma carnitine by modulating urinary carnitine excretion according to the intake from diet [14].
Carnitine inborn errors of metabolism (IEM) can be divided into disorders of carnitine biosynthesis, carnitine transport, and mitochondrial carnitine–acylcarnitine cycle. The presentation of these disorders is variable but common findings include hypoketotic hypoglycemia, cardio(myopathy), and liver disease. These manifestations occur due to energy deficiency and accumulation of fatty acids in affected organs. Secondary carnitine deficiency could develop in several IEM, as a side effect of some drugs, or due to increased excretion with tubular dysfunction or dialysis.
2. Carnitine Biosynthesis Disorders
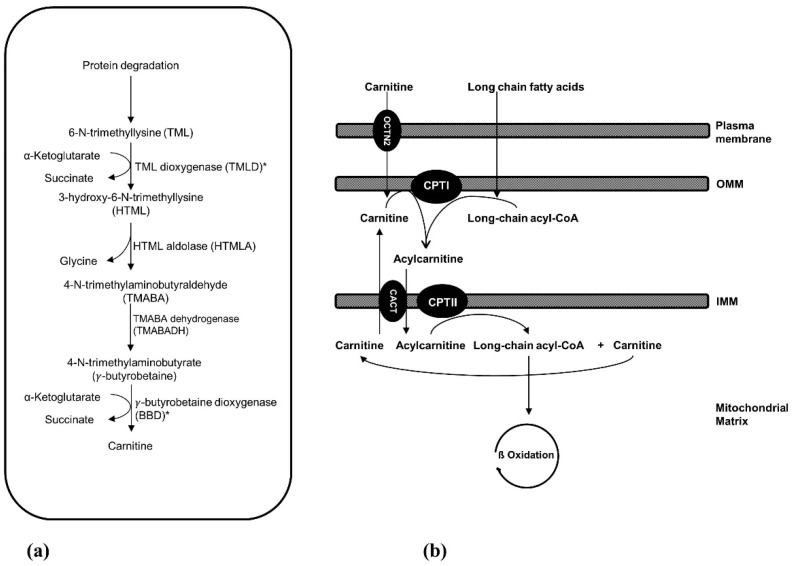
The precursors for carnitine biosynthesis are lysine and methionine which provide the carbon backbone and 4-N-methyl groups of carnitine, respectively. The substrate for carnitine biosynthesis is 6-N-trimethyllysine (TML) (Figure 1a). Certain proteins, like cytochrome c, actin, myosin, and histones, contain N-methylated lysine residues that are produced by post-translational N-methylation modification. This process is catalyzed by methyltransferases which use S-adenosylmethionine as a methyl donor. Subsequently, TML is released by lysosomal or proteasomal degradation of these proteins. The first step of carnitine biosynthesis is hydroxylation of TML by TML dioxygenase (TMLD), producing 3-hydroxy-6-N-trimethyllysine (HTML). The latter undergoes cleavage by HTML aldolase (HTMLA), yielding 4-N-trimethylaminobutyraldehyde (TMABA) and glycine. TMABA is then dehydrogenated to 4-N-trimethylaminobutyrate (γ-butyrobetaine) through action of TMABA dehydrogenase (TMABADH). In the fourth and last reaction of carnitine biosynthesis, γ-butyrobetaine is hydroxylated by γ-butyrobetaine dioxygenase (BBD) to produce carnitine [3,15].
Figure 1.
(a) Carnitine biosynthetic pathway. Only trimethyllysine dioxygenase (TMLD) and γ-butyrobetaine dioxygenase (BBD) deficiencies have been reported; both are denoted with asterisks. (b) Carnitine transport and mitochondrial carnitine–acylcarnitine cycle. IMM: inner mitochondrial membrane; OMM: outer mitochondrial membrane; CPT I: carnitine palmitoyltransferase I; CACT: carnitine-acylcarnitine translocase; CPT II: carnitine palmitoyltransferase II; OCTN2: organic cation/carnitine transporter novel type 2.
Recently, TMLD and BBD deficiencies have been reported. As mentioned earlier, in individuals eating a regular diet, most of carnitine is obtained from the diet whereas carnitine biosynthesis makes a small contribution. Therefore, carnitine deficiency is not observed with carnitine biosynthesis defects, provided dietary intake is normal. The underlying pathophysiologic mechanisms in these disorders then may include abnormal levels of intermediate metabolites in the carnitine biosynthetic pathway and carnitine deficiency early in life [16]. Given the rarity of these disorders, no strictly vegetarian individuals affected with carnitine biosynthesis disorders have been yet reported. However, it is possible that carnitine levels could be low in these circumstances.
2.1. 6-N-Trimethyllysine Dioxygenase (TMLD) Deficiency
TMLD is a mitochondrial enzyme, encoded by TMLHE on the X chromosome. Celestino-Soper et al. performed array CGH (comparative genomic hybridization) in families with autism and identified TMLHE exon 2 deletion in a male child with autism, raising the possibility of a link between TMLD deficiency and autism [17]. Subsequently, the same group evaluated this possibility by testing a cohort of males with autism and comparing them to controls. TMLD deficiency due to exon 2 deletions was found to be common in control males (~0.28%), whereas the percentage in probands with autism was not significantly higher (~0.31%). TMLHE exon 2 deletions were more frequent in probands from families with more than one affected male compared to control subjects suggesting that TMLD deficiency is a risk factor for autism with low penetrance (2–4%). Cultured lymphoblastoid cell lines from males with the exon 2 deletion had low or undetectable TMLD enzyme activity. TMLD deficiency leads to a decrease in the levels of the products of the enzyme, HTML and γ-butyrobetaine, and an increase in the levels of the proximal substrate, TML [18]. In another study, next-generation sequencing in 12 families with males affected with autism identified two affected brothers who had a nonsense variant in TMLHE. The TMLHE coding sequence was then screened in 501 males with autism and 2 additional missense variants were identified [19].
A case report described a young male child with TMLD deficiency due to 2 base pairs deletion in exon 6 of TMLHE who regained his milestones after developmental regression and even gained new skills after initiation carnitine supplementation at 200 mg/kg/day [20]. In this child, TML levels were normal in plasma and high in urine. In contrast, γ-butyrobetaine levels were low in his plasma but within reference range in urine. The TML/γ-butyrobetaine ratio was significantly elevated in plasma and urine. The authors mentioned that this phenomenon was observed in two other individuals with TMLHE variants in their lab, suggesting that the TML/γ-butyrobetaine ratio may be a superior diagnostic marker for TMLD deficiency instead of TML or γ-butyrobetaine levels alone [20]. In contrary to other reports, this child reportedly had low plasma carnitine levels. Given the child was taking a normal diet, it not clear what was the reason for low carnitine levels as this cannot be explained only by TMLD deficiency. There could be a period of poor intake before the levels were measured or the child could have secondary carnitine deficiency due to an unidentified reason [16,20].
The association of TMLD deficiency with autism, and other evidence, could suggest that brain deficiency of carnitine may cause autism (in ~10%–20% of cases) and therefore early carnitine supplementation could be beneficial [21,22].
2.2. γ-Butyrobetaine Dioxygenase (BBD) Deficiency
BBD is encoded by BBOX1 on chromosome 11. A 42-month-old female child with BBD deficiency due to a homozygous 221 kilobases (Kb) deletion at 11p14.2 was reported. This deletion overlaps BBOX1 and FIBIN encoding fibin. The girl presented with microcephaly, speech delay, poor growth and some dysmorphic features. Carnitine levels were normal. With only one case report, it is difficult to conclude causation with any of these genes [23]. In a recent study, Lee et al. showed that BBOX 1 expression was down-regulated in a mouse model with schizophrenia that was induced by maternal immune activation. The authors also did a case control study on 284 subjects with schizophrenia and 409 healthy controls and found that BBOX 1 polymorphisms could be associated with increased schizophrenia susceptibility in the Korean population [24].
3. Carnitine Transport Defect
Systemic primary carnitine deficiency, due to carnitine transport defect, is caused by biallelic variants in SLC22A5 [25]. It has an estimated incidence of 1 in 120,000 newborns in the United states [26]. It is more common in Japan with an estimated incidence of 1 in 40,000 [27]. SLC22A5 encodes organic cation/carnitine transporter novel type 2 (OCTN2). OCTN2 is a high affinity organic cation transporter specific for carnitine that works in Na+ dependent and Na+ independent mechanisms [28]. It is highly expressed in heart, muscle, kidneys, and other tissues. Defects in OCTN2 results in urinary carnitine wasting, low serum carnitine levels, and decreased intracellular carnitine accumulation [12].
As the major function of carnitine is shuttling long-chain fatty acids into the mitochondrial matrix for β-oxidation and energy production, carnitine deficiency results in defective fatty acid oxidation presenting as hypoglycemia due to excessive glucose consumption without regeneration via gluconeogenesis. Fat released from the adipose tissue with fasting will not be utilized and instead will accumulate. Fat accumulation in the liver, skeletal muscle, and heart can impair organs’ function and cause steatosis, cardiomyopathy, and myopathy [12].
The presentation of primary carnitine deficiency is very variable in terms of onset, severity, and involved organs. Inability to utilize fat for energy during stress or fasting can trigger acute metabolic decompensations early in life. Later presentation includes skeletal myopathy, cardiomyopathy, and arrhythmias with sudden death [12]. Still, affected individuals could remain asymptomatic [29].
Around 50% of affected individuals present before the second year of life with acute metabolic decompensations precipitated by fasting or intercurrent illnesses. During these episodes, affected individuals develop lethargy, irritability, and poor feeding. Hepatomegaly is a common sign. Hypoketotic hypoglycemia, elevated liver enzymes, and hyperammonemia, are usually evident on laboratory evaluation. Without early treatment, these episodes could progress to coma and death [16]. The remaining 50% of affected individuals present between 2–4 years of age with a more insidious presentation that includes skeletal myopathy with muscle weakness and hypotonia, elevated CK (creatinine kinase), and dilated cardiomyopathy. Cardiomyopathy in individuals with primary carnitine deficiency responds poorly to standard therapy and without accurate diagnosis and carnitine supplementation, it can be fatal [12,16,30,31].
Adults with primary carnitine deficiency could be asymptomatic or they could have mild symptoms with easy fatigability [16]. While cardiomyopathy is not commonly seen in affected adults, they are still at risk for cardiac arrhythmias and sudden death, even if have been asymptomatic. Affected women with primary carnitine deficiency have been occasionally diagnosed after identifying low carnitine levels in their infants on newborn screening (NBS). Half of these women were asymptomatic whereas the rest complained of fatigability. The metabolic stress associated with pregnancy could trigger symptoms in otherwise asymptomatic women [32,33,34].
There is a correlation between the genotype and the degree of carnitine transport in fibroblasts [35]. Rose et al. showed that carnitine transport was higher in asymptomatic women (diagnosed by abnormal NBS in their babies) compared to symptomatic individuals. They showed higher frequency of nonsense variants in those who were symptomatic [35]. However, there is usually no correlation between genotype and the occurrence of metabolic and cardiac complications as these have been observed in the presence of residual transporter activity [12]. Other factors could contribute to the phenotype in affected individuals including exposure to intercurrent stressors that trigger catabolism (e.g., fasting, infections) or worsening of carnitine deficiency by other factors (e.g., pivalic acid containing antibiotics [36]).
Scaglia et al. showed that heterozygote carriers have partially reduced carnitine transport in fibroblasts with increased urinary losses and borderline plasma carnitine levels compared to controls [37]. Heterozygote carriers are usually asymptomatic although the carrier status could be an independent risk factor for cardiac complications. Xiaofei et al. showed that heterozygote mice developed age-associated left ventricular myocyte hypertrophy with lipid deposition and abnormal mitochondria [38]. Similarly, Takahashi et al. evaluated heterozygote mice in the presence of surgically induced hypertension. These mice showed exaggerated cardiac hypertrophy and increased mortality compared to the wild-type mice. Carnitine supplementation prevented these changes in mice [39]. These findings are further supported by studies in murine models with moderate carnitine deficiency induced by carnitine depleting agents [40,41,42]. In a cohort of Japanese individuals, heterozygote, middle-aged individuals developed mild left ventricular hypertrophy of no clear clinical significance [27]. Another study suggested that heterozygosity for SLC22A5 variants is less likely to be an important cause of cardiomyopathy. In this study, 324 individuals with cardiomyopathy were tested for SLC22A5 variants. The frequency of variants affecting carnitine transport was 0.61% in individuals with cardiomyopathy compared to 1.11% in the general population [43].
Primary carnitine deficiency is suspected in symptomatic individuals who have low free carnitine levels. Affected individuals usually have very low plasma free carnitine levels (<5 μmol/L) [12]. Alternatively, the disorder can be identified by NBS using tandem mass spectrometry [44]. The false positive rate could be high as levels of carnitine in the newborn strongly reflect maternal levels, which could be low as carnitine levels decrease during pregnancy [45]. Also, the mother could have primary or secondary carnitine deficiency (see below) which could be unmasked by abnormal screening in the newborn [46]. In a recent study from California, 1030 out of 3,608,768 newborns screened positive for low free carnitine. Out of these, only 21 cases were confirmed cases of primary carnitine deficiency and a further 27 cases were possible cases [46]. This study also showed that newborns with primary carnitine deficiency could have free carnitine levels close to the cutoff and this raises the possibly that true cases could have been missed by NBS [46]. Diagnosis of primary carnitine deficiency can be confirmed by DNA testing of the SLC22A5 gene. If molecular testing is not conclusive, an alternative option is skin biopsy to assess carnitine transport in cultured fibroblasts [16].
Individuals with primary carnitine deficiency should be treated with carnitine supplementation at a dose of 100–200 mg/kg/day, usually divided three times daily [12]. The dose should be titrated according to the levels of free carnitine in plasma. Carnitine is well tolerated with only few side effects. Diarrhea and abdominal discomfort could be observed with high doses. Bacterial metabolism in the intestine can produce trimethylamine, which has a fishy odor. This side effect may respond to reducing carnitine dose; otherwise, a course of oral metronidazole and/or probiotics may be indicated [12,16]. Primary carnitine deficiency has good outcomes and favorable prognosis provided that affected individuals remain compliant with carnitine supplementation.
4. Mitochondrial Carnitine-Acylcarnitine Cycle Disorders
As mentioned earlier, carnitine is required for the transport of fatty acids into the mitochondrial matrix for β-oxidation. Once inside the cells, fatty acids are first activated by long-chain acyl-CoA synthetase, forming long-chain acyl-CoAs [47]. There are different long-chain acyl-CoA synthetase enzymes specific for different size fatty acids [48]. Once activated, long-chain acyl-CoAs are transported to the mitochondrial matrix through the mitochondrial carnitine–acylcarnitine cycle, which is required to overcome the permeability barrier of the inner mitochondrial membrane. This cycle consists of three steps (Figure 1b), the first of which is conversion of long-chain acyl-CoAs to their acylcarnitine equivalents through the action of carnitine palmitoyltransferase I (CPT I). This enzyme is located on the outer mitochondrial membrane. The second step is the transport of acylcarnitines into the mitochondrial matrix by carnitine-acylcarnitine translocase (CACT). In the last step, carnitine palmitoyltransferase II (CPT II) at the inner mitochondrial membrane reconverts acylcarnitines back to the acyl-CoA species and carnitine [49].
4.1. Carnitine Palmitoyltransferase I (CPT I) Deficiency
There are three isoforms of CPTI. CPT1A is called liver CPT1, but it is also expressed in the brain, kidney, and other organs. CPT1B is the muscle isoform whereas CPT1C is a brain-specific enzyme [50]. Initially, it has been thought that only pathogenic variants in CPT1A, coding CPTIA, are associated with disease in humans. Recently, pathogenic variants in CPT1C, coding CPTIC, were identified to be causative for spastic paraplegia 73 [51,52].
CPT1A deficiency is a rare, autosomal recessive disorder with an estimated incidence from NBS data of about 1:750,000 to 1:2,000,000 [53]. It could be more common in certain populations such as Inuit and Hutterites [54,55]. CPTIA deficiency is characterized by episodes of hepatic encephalopathy that are triggered by fasting or intercurrent illnesses. Affected individuals usually present before 2 years of age with hypoketotic hypoglycemia, hepatomegaly, and elevated liver transaminases. Without treatment, these symptoms can progress to seizures and coma [56,57,58]. Other features include renal tubular acidosis and elevated CK levels. Heart and skeletal muscle involvement is less common [56,59]. There are reports of heterozygous mothers carrying an affected fetus who presented with acute fatty liver of pregnancy (AFLP) [60].
Apart from hypoketotic hypoglycemia and raised transaminases, other suggestive laboratory findings of CPTIA deficiency include mild hyperammonemia and elevated total serum carnitine with elevated C0/C16+C18 ratio. This ratio is in fact very specific to diagnose this disease since it is elevated in both; plasma and direct blood spot (DBS) samples [61]. On the other hand, free carnitine levels are higher in DBS compared to plasma in individuals with CPTIA deficiency and therefore, if only plasma free carnitine levels are measured, the diagnosis can be missed [61,62]. Urine organic acids might show hypoketotic dicarboxylic aciduria, in particular elevated C12 dicarboxylic acid [56,63]. Diagnosis of CPTIA deficiency can be confirmed by DNA testing of the CPT1A gene or by documenting low CPTI enzyme activity on cultured fibroblasts if the DNA testing is not conclusive.
During acute episodes, affected individuals should receive high dextrose fluids to prevent catabolism and lipolysis. Long-term management involves frequent feeding, avoidance of fasting, and uncooked cornstarch at night. The diet should be low in fat, high in carbohydrates, and rich in medium-chain triglycerides (MCT) [56].
4.2. Carnitine-Acylcarnitine Translocase (CACT) Deficiency
Carnitine-acylcarnitine translocase (CACT) deficiency is also a rare autosomal recessive disease caused by defects in SLC25A20. Without the translocase, long-chain fatty acids will not be available for mitochondrial β-oxidation [64]. Most affected individuals present in the neonatal period with a severe phenotype characterized by a rapidly progressive course and a high mortality. Presenting features of CACT deficiency include hypoketotic hypoglycemia that can result in seizures and coma, respiratory distress, arrhythmia, cardiomyopathy, liver disease, and sudden death [65]. In addition to hypoketotic hypoglycemia, other laboratory abnormalities include hyperammonemia, elevated liver enzymes, and elevated CK levels. Late onset presentation with a milder phenotype has been reported but less commonly [66,67]. This phenotype presents in the form of episodes of hypoketotic hypoglycemia that are triggered by fasting or intercurrent illnesses. Milder phenotype is associated with some degree of residual enzyme activity [66].
CACT deficiency is diagnosed based on elevated levels of long-chain fatty acid acylcarnitine esters (especially C16 and C18:1) along with low free carnitine [65]. This biochemical pattern is indistinguishable from CPT II deficiency (see below) and therefore, either DNA testing or measuring enzyme activity in cultured fibroblasts is required to confirm the diagnosis. Urine organic acids can reveal non-specific dicarboxylic aciduria.
Treatment involves avoidance of fasting and frequent meals. The diet should be rich in carbohydrates and low in fat. Most fat intake should come from MCT. Carnitine is commonly used, but it is controversial. There have been concerns about the possible toxicity of acylcarnitine accumulation in long chain fatty acid oxidation disorders (see Section 6).
4.3. Carnitine Palmitoyltransferase II (CPT II) Deficiency
CPT II deficiency is an autosomal recessive disorder caused by biallelic pathogenic variants in CPT2. The presentation is variable in regard to age of onset, severity, and involved organs. Three phenotypes are described including, myopathic form, lethal neonatal form, and severe infantile form [68].
By far, the myopathic form is the most common form of CPT II deficiency and it is a common cause for hereditary rhabdomyolysis [69]. CPT II deficiency is considered as the most common disorder of lipid metabolism affecting skeletal muscles [68]. The myopathic form of CPT II deficiency can present at any age with 70% of cases presented during childhood (0–12 years of age). In one quarter of cases, the disease presented during adolescence (13–22 years) [70,71]. The myopathic form is characterized by recurrent attacks of rhabdomyolysis that are triggered by prolonged exercise in the majority of cases. Other triggers include infections and fasting [71]. During these episodes, myalgia is the most consistent symptom, and it can be associated with muscles weakness and myoglobinuria [68,70,71], and CK levels are elevated. Episodes of rhabdomyolysis can lead to acute renal failure in 7–23% of cases [71]. The myopathic form of CPT II deficiency is more commonly observed in males, although it is an autosomal recessive disorder with expected equal gender distribution [72]. This observation could be due to ascertainment bias as males are more likely to do strenuous exercise and therefore develop myoglobinuria. Hormonal factors were also suggested to be involved in this gender bias [72,73].
The other two forms of CPT II deficiency are very rare. Affected individuals with the neonatal form present soon after birth (hours to a few days) with lethargy, respiratory distress, hypoketotic hypoglycemia, liver failure, cardiac arrhythmias, cardiomyopathy, seizures, and coma [74,75]. Affected neonates could have dysmorphic features, renal dysgenesis, neuronal migration defects, and other brain malformations [68,76]. The prognosis is poor, with death occurring within days to months [68].
The severe infantile form usually presents between 6–24 months of age [57]. It is characterized by hypoketotic hypoglycemia, hepatomegaly, liver failure, cardiomyopathy, and cardiac arrhythmias. Seizures and coma could develop secondary to hypoglycemia [57,68,77]. Episodes of decompensation could be triggered by fasting or intercurrent illnesses. Cardiac arrhythmias can result in sudden death [75]. In both neonatal and infantile forms, laboratory evaluation usually shows hyperammonemia, metabolic acidosis, hypoketotic hypoglycemia, and high CK levels.
CPT II deficiency is diagnosed based on elevation of C12 to C18 acylcarnitines, in particular C16 and C18:1 [68]. Free carnitine levels could be reduced. (C16+C18:1)/C2 ratio is a more sensitive marker and could improve the sensitivity of the NBS [78]. It should be noted that CPT II deficiency is better diagnosed from plasma samples compared to DBS [61]. Diagnosis can be confirmed by DNA testing or enzyme assays in fibroblast. Treatment principles are the same as discussed before for CACT deficiency. Prolonged exercise and other known triggers should be avoided [57,68]. Table 1 summarizes carnitine inborn errors of metabolism.
Table 1.
Summary of inborn errors of carnitine metabolism.
| Category | Disease | Gene | Clinical Features | Diagnostic Markers | Long Term Management | References |
|---|---|---|---|---|---|---|
| Carnitine biosynthesis disorders | TMLD deficiency | TMLHE | • Risk factor for autism | • Low 3-hydroxy-6-N-trimethyllysine (HTML) and γ-butyrobetaine • High TML and TML/γ-butyrobetaine ratio |
• Carnitine supplementation | [16,17,18,19,20] |
| BBD deficiency | BBOX1 | • Microcephaly, speech delay, poor growth and some dysmorphic features a • Schizophrenia susceptibility |
• Not available | • Not available | [23,24] | |
| Carnitine transport defect | Primary carnitine deficiency | SLC22A5 | • Metabolic decompensations precipitated by fasting and intercurrent illnesses • Cardio(myopathy) • Easy fatigability • Asymptomatic |
• Low free carnitine | • Carnitine supplementation (100–200 mg/kg/day) | [12,16,29,30,31,32,33,34,35,56] |
| Mitochondrial carnitine–acylcarnitine cycle disorders | CPT IA deficiency | CPT1A | • Metabolic decompensations precipitated by fasting and intercurrent illnesses • Heart and skeletal muscle involvement is less common • Acute fatty liver of pregnancy (AFLP) reported in heterozygous mothers carrying an affected fetus |
• High free carnitine and C0/C16+C18 ratio | • Frequent feeding, avoidance of fasting • Low fat and high carbohydrates diet • Medium-chain triglycerides (MCT) |
[55,56,57,58,59] |
| CACT deficiency | SLC25A20 | • Neonatal presentation: hypoketotic hypoglycemia, respiratory distress, arrhythmia, cardiomyopathy, liver disease, and sudden death • Milder phenotype with metabolic decompensations precipitated by fasting and intercurrent illnesses |
• Elevated long chain acylcarnitines (especially C16 and C18:1) • Low free carnitine |
• Frequent feeding, avoidance of fasting • Low fat and high carbohydrates diet • MCT • Carnitine supplementation b |
[56,64,65,66] | |
| CPT II deficiency | CPT2 | • Myopathic form: recurrent attacks of rhabdomyolysis triggered by prolonged exercise, infection, fasting, and cold • Neonatal form: hypoketotic hypoglycemia, liver failure, arrhythmias, cardiomyopathy, seizures, dysmorphic features, renal and brain malformations • Infantile form: hypoketotic hypoglycemia, hepatomegaly, liver failure, cardiomyopathy, and arrhythmias |
• Elevated long chain acylcarnitines (especially C16 and C18:1) • Low free carnitine |
• Frequent feeding, avoidance of fasting • Low fat and high carbohydrates diet • MCT • Carnitine supplementation b |
[56,58,67,69,70,71,72,73,74,75] |
See text for abbreviations. a The reported child has a homozygous deletion that includes a BBOX1 gene and FIBIN gene. With only one case report, it is difficult to conclude causation with any of these genes (see text for details). b The use of carnitine is controversial; there have been concerns about possible toxicity of acylcarnitine accumulation in long chain fatty acid oxidations defects (see text for details).
4.4. Carnitine Palmitoyltransferase (CPT) Inhibitors
While the mitochondrial carnitine–acylcarnitine cycle is essential for fatty acid oxidation and energy supply, modulation of these processes, through CPT inhibitors, could be of a potential therapeutic value in several disorders, including diabetes, cancer, and heart diseases [79]. For example, in type 2 diabetes, the underlying reduced insulin sensitivity causes increased fatty acid oxidation which by itself aggravates the hyperglycemia by reducing glucose utilization and increasing production through gluconeogenesis [80]. Selective inhibition of liver CPTI reduces gluconeogenesis and improves glucose homeostasis [81]. In the diseased heart, there is an increase in fatty acid oxidation and a decrease in glucose consumption, which in turn impair energy utilization and decrease cardiac efficiency [82]. Lionetti et al. showed that CPTI inhibition in dogs with heart failure delayed the time to end-stage failure [83]. Etomoxir, an irreversible inhibitor of CPTI, was found to prevent the development of heart failure in rats with pressure overload-induced cardiac hypertrophy [84]. Placebo-controlled trial in humans was stopped prematurely due to hepatotoxicity, although it showed trends for improved cardiac function in treated subjects [85]. Finally, through inhibition of fatty acid oxidation which fuels the tumor cells, and other mechanisms like apoptosis and modulating gene expression, CPT inhibition could be a potential target in cancer [86].
5. Secondary Carnitine Deficiency
Apart from primary carnitine deficiency, which is caused by carnitine transport defect, secondary carnitine deficiency could also develop due to a variety of reasons [87]. As compared to the primary form, secondary carnitine deficiency is less severe and associated with higher carnitine levels that can be treated with small doses of carnitine [16]. Carnitine deficiency is seen in several IEM, in particular, fatty acid oxidation disorders and organic acidemias. In these disorders, carnitine deficiency develops secondary to increased urinary excretion of carnitine in the form of acylcarnitines [88]. It is uncommon to develop carnitine deficiency secondary to poor intake as carnitine biosynthesis along with renal reabsorption are effective in maintaining normal carnitine levels [89]. Still, malnutrition and malabsorption can cause carnitine deficiency. An adult individual with severe malnutrition and history of gastric bypass surgery developed hyperammonemia and was found to have very low carnitine levels. Hyperammonemia was refractory to standard therapy but normalized with carnitine supplementation [90]. Children may be more prone to dietary carnitine deficiency [89]. In particular, preterm infants are born with limited carnitine reserves as placental transfer and biosynthesis of carnitine occurs mainly during the third trimester. They also have limited ability to synthesize carnitine due to immaturity of biosynthetic enzymes [91]. Therefore, carnitine levels may need to be monitored in those neonates, especially with prolonged use of total parental nutrition [92]. However, the evidence for routine administration of carnitine in preterm infants is lacking and more studies are needed [93,94].
Valproic acid (VPA) is one of the well-known drugs causing secondary carnitine deficiency, which can contribute to VPA-induced toxicity [95]. VPA depletes carnitine through different mechanisms including increased urinary excretion in the form valproylcarnitine, reduction in tubular reabsorption and endogenous biosynthesis, and inhibition of the carnitine transporter [95]. Pivalic acid containing antibiotics can also cause carnitine depletion as pivalate conjugates with free carnitine, forming pivaloyl carnitine, which is excreted in the urine [36,96]. Several drugs can cause secondary carnitine deficiency though OCTN2 inhibition including omeprazole [97], zwitterionic drugs (e.g., levofloxacin) [98], and anticancer drugs (e.g., etoposide, vinblastine, actinomycin D) [99].
Secondary carnitine deficiency could also develop from increased urinary loss in Fanconi syndrome or with renal replacement therapy (hemodialysis and peritoneal dialysis). As mentioned earlier, the kidneys are very efficient in maintaining carnitine homeostasis. Several mechanisms contribute to carnitine depletion with chronic dialysis including efficient free carnitine removal through dialysis, decreased intake, and decreased endogenous production [100]. In individuals with end stage renal disease, free carnitine levels are higher than healthy individuals prior to initiation of dialysis. Once dialysis starts, this level declines significantly, about 30% in 4 weeks and 40% in 12 months [101]. This is associated with increased acylcarnitine and acylcarnitine/free carnitine ratio. Dialysis also results in reduction in muscle carnitine content [101]. Dialysis-related carnitine disorder (DLD) is the term used to describe carnitine deficiency in dialysis patients and can be associated with several complications including fatigue, intradialytic hypotension, anemia that is poorly responsive to erythropoietin, and cardiomyopathy [102]. Many clinical trials described the benefits of carnitine to treat these complications although definite evidence is still lacking [103]. To attain desired effects on multiple pathways, higher than physiologic levels of carnitine should be achieved in the plasma, and therefore, in the intracellular compartment. This is possible in individuals with end stage renal disease on dialysis who receive parenteral carnitine supplementation, given their underlying renal disease will impair rapid clearance of carnitine when transporter saturation reached. In this way, carnitine acts as a “conditionally essential nutrient” [104].
6. Carnitine Use in Inborn Errors of Metabolism
Carnitine supplementation is commonly used in several IEM and other disorders. It is the mainstay of treatment in primary carnitine deficiency. Carnitine is also used in other IEM that are associated with secondary carnitine deficiency [105,106], although a Cochrane review concluded that evidence for carnitine supplementation in IEM is lacking [107]. Moreover, there have been concerns about possible toxicity of acylcarnitine accumulation in long chain fatty acid oxidation disorders. In one report, two siblings with very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency developed more frequent episodes of rhabdomyolysis after carnitine supplementation [108]. In a mouse model, accumulating long-chain acylcarnitines in ischemic heart mitochondria inhibited oxidative phosphorylation [109]. Long chain acylcarnitines were found to regulate the hERG (human ether-a-go-go-related gene) channel and contribute to the development of cardiac arrhythmias [110]. Based on this potential risk, the use of carnitine in long chain fatty acid oxidation disorders should be avoided during acute metabolic crises [111]. Carnitine is often used in patients with mitochondrial disorders [112]. In these disorders, plasma free carnitine tends to be lower than normal with increased esterified carnitine due to a partial impairment of β-oxidation [113]. Metabolism of carnitine through gut microbiota produces trimethylamine N-oxide (TMAO). Marked elevation in plasma levels of TMAO were documented in patients with mitochondrial disorders treated with oral carnitine [114]. Recent studies showed a positive correlation between elevated levels of TMAO and an increased risk for cardiovascular disease [115]. Given this potential risk and lack of evidence for the benefit of carnitine in patients with mitochondrial disorders, more studies are needed to evaluate the long-term safety and efficacy of carnitine in these patients.
7. Summery and Conclusions
Carnitine is involved in several biochemical pathways, either directly or indirectly. Carnitine is obtained from the diet, synthesized endogenously, and excreted in the urine. In normal situations, these processes are very efficient in maintaining normal carnitine levels. It is important to recognize inborn errors of carnitine metabolism as early treatment could prevent serious sequelae. Disorders of carnitine biosynthesis were recently identified and are still to be fully elucidated. The association of TMLD deficiency with autism is interesting and could help open venues to better understand the pathophysiology of autism, in a subset of patients. Other inborn errors of carnitine metabolism, carnitine transport defect, and mitochondrial carnitine–acylcarnitine cycle disorders, have been recognized for long time and a common theme in these disorders is energy failure with hypoketotic hypoglycemia, cardio(myopathy), and liver disease. These disorders are part of NBS programs in several areas of the world. The myopathic CPTII deficiency is a common cause for hereditary rhabdomyolysis and should be always considered with such presentations. Carnitine supplementation and the use of compounds to modulate carnitine metabolizing enzymes, like CPT inhibitors and potentiators, have been the focus of many research projects. These agents could be promising targets to treat disorders characterized by alterations in physiologic biochemical pathways, especially those concerning with energy production.
Author Contributions
M.A. (Mohammed Almannai) drafted the initial manuscript and reviewed and revised the manuscript. A.W.E.-H. and M.A. (Majid Alfadhel) reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Pietrocola F., Galluzzi L., Bravo-San Pedro J.M., Madeo F., Kroemer G. Acetyl Coenzyme A: A Central Metabolite and Second Messenger. Cell Metab. 2015;21:805–821. doi: 10.1016/j.cmet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Bene J., Hadzsiev K., Melegh B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr. Diabetes. 2018;8:8. doi: 10.1038/s41387-018-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaz F.M., Wanders R.J.A. Carnitine biosynthesis in mammals. Biochem. J. 2002;361:417–429. doi: 10.1042/bj3610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter A.L., Abney T.O., Lapp D.F. Biosynthesis and metabolism of carnitine. J. Child Neurol. 1995;10(Suppl. 2):S3–S7. doi: 10.1177/0883073895010002S02. [DOI] [PubMed] [Google Scholar]
- 5.Ribas G.S., Vargas C.R., Wajner M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene. 2014;533:469–476. doi: 10.1016/j.gene.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Lee B.-J., Lin J.-S., Lin Y.-C., Lin P.-T. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: A randomized, placebo-controlled trial. Nutr. J. 2014;13:79. doi: 10.1186/1475-2891-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komlósi K., Havasi V., Bene J., Süle N., Pajor L., Nicolai R., Benatti P., Calvani M., Melegh B. Histopathologic abnormalities of the lymphoreticular tissues in organic cation transporter 2 deficiency: Evidence for impaired B cell maturation. J. Pediatr. 2007;150:109–111.e2. doi: 10.1016/j.jpeds.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y., Jiang W., Chen G., Zhu W., Ding W., Ge Z., Tan Y., Ma T., Cui G. L-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2017;26:333–338. doi: 10.17219/acem/61609. [DOI] [PubMed] [Google Scholar]
- 9.Rebouche C.J. Carnitine function and requirements during the life cycle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1992;6:3379–3386. [PubMed] [Google Scholar]
- 10.Stanley C.A. Carnitine deficiency disorders in children. Ann. N. Y. Acad. Sci. 2004;1033:42–51. doi: 10.1196/annals.1320.004. [DOI] [PubMed] [Google Scholar]
- 11.Tein I., Bukovac S.W., Xie Z.W. Characterization of the human plasmalemmal carnitine transporter in cultured skin fibroblasts. Arch. Biochem. Biophys. 1996;329:145–155. doi: 10.1006/abbi.1996.0203. [DOI] [PubMed] [Google Scholar]
- 12.Longo N., Frigeni M., Pasquali M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta. 2016;1863:2422–2435. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opalka J.R., Gellerich F.N., Zierz S. Age and sex dependency of carnitine concentration in human serum and skeletal muscle. Clin. Chem. 2001;47:2150–2153. [PubMed] [Google Scholar]
- 14.Rebouche C.J. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann. N. Y. Acad. Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- 15.Strijbis K., Vaz F.M., Distel B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life. 2010;62:357–362. doi: 10.1002/iub.323. [DOI] [PubMed] [Google Scholar]
- 16.El-Hattab A.W., Scaglia F. Disorders of carnitine biosynthesis and transport. Mol. Genet. Metab. 2015;116:107–112. doi: 10.1016/j.ymgme.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Celestino-Soper P.B.S., Shaw C.A., Sanders S.J., Li J., Murtha M.T., Ercan-Sencicek A.G., Davis L., Thomson S., Gambin T., Chinault A.C., et al. Use of array CGH to detect exonic copy number variants throughout the genome in autism families detects a novel deletion in TMLHE. Hum. Mol. Genet. 2011;20:4360–4370. doi: 10.1093/hmg/ddr363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celestino-Soper P.B.S., Violante S., Crawford E.L., Luo R., Lionel A.C., Delaby E., Cai G., Sadikovic B., Lee K., Lo C., et al. A common X-linked inborn error of carnitine biosynthesis may be a risk factor for nondysmorphic autism. Proc. Natl. Acad. Sci. USA. 2012;109:7974–7981. doi: 10.1073/pnas.1120210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nava C., Lamari F., Héron D., Mignot C., Rastetter A., Keren B., Cohen D., Faudet A., Bouteiller D., Gilleron M., et al. Analysis of the chromosome X exome in patients with autism spectrum disorders identified novel candidate genes, including TMLHE. Transl. Psychiatry. 2012;2:e179. doi: 10.1038/tp.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziats M.N., Comeaux M.S., Yang Y., Scaglia F., Elsea S.H., Sun Q., Beaudet A.L., Schaaf C.P. Improvement of regressive autism symptoms in a child with TMLHE deficiency following carnitine supplementation. Am. J. Med. Genet. A. 2015;167A:2162–2167. doi: 10.1002/ajmg.a.37144. [DOI] [PubMed] [Google Scholar]
- 21.Beaudet A.L. Brain carnitine deficiency causes nonsyndromic autism with an extreme male bias: A hypothesis. BioEssays News Rev. Mol. Cell. Dev. Biol. 2017;39 doi: 10.1002/bies.201700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demarquoy C., Demarquoy J. Autism and carnitine: A possible link. World J. Biol. Chem. 2019;10:7–16. doi: 10.4331/wjbc.v10.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rashidi-Nezhad A., Talebi S., Saebnouri H., Akrami S.M., Reymond A. The effect of homozygous deletion of the BBOX1 and Fibin genes on carnitine level and acyl carnitine profile. BMC Med. Genet. 2014;15:75. doi: 10.1186/1471-2350-15-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H., Kim H.-K., Kwon J.-T., Park S., Park H.J., Kim S.K., Park J.K., Kang W.S., Kim Y.J., Chung J.-H., et al. BBOX1 is down-regulated in maternal immune-activated mice and implicated in genetic susceptibility to human schizophrenia. Psychiatry Res. 2018;259:197–202. doi: 10.1016/j.psychres.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Nezu J., Tamai I., Oku A., Ohashi R., Yabuuchi H., Hashimoto N., Nikaido H., Sai Y., Koizumi A., Shoji Y., et al. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat. Genet. 1999;21:91–94. doi: 10.1038/5030. [DOI] [PubMed] [Google Scholar]
- 26.Therrell B.L., Lloyd-Puryear M.A., Camp K.M., Mann M.Y. Inborn errors of metabolism identified via newborn screening: Ten-year incidence data and costs of nutritional interventions for research agenda planning. Mol. Genet. Metab. 2014;113:14–26. doi: 10.1016/j.ymgme.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koizumi A., Nozaki J., Ohura T., Kayo T., Wada Y., Nezu J., Ohashi R., Tamai I., Shoji Y., Takada G., et al. Genetic epidemiology of the carnitine transporter OCTN2 gene in a Japanese population and phenotypic characterization in Japanese pedigrees with primary systemic carnitine deficiency. Hum. Mol. Genet. 1999;8:2247–2254. doi: 10.1093/hmg/8.12.2247. [DOI] [PubMed] [Google Scholar]
- 28.Ohashi R., Tamai I., Nezu Ji J., Nikaido H., Hashimoto N., Oku A., Sai Y., Shimane M., Tsuji A. Molecular and physiological evidence for multifunctionality of carnitine/organic cation transporter OCTN2. Mol. Pharmacol. 2001;59:358–366. doi: 10.1124/mol.59.2.358. [DOI] [PubMed] [Google Scholar]
- 29.Spiekerkoetter U., Huener G., Baykal T., Demirkol M., Duran M., Wanders R., Nezu J., Mayatepek E. Silent and symptomatic primary carnitine deficiency within the same family due to identical mutations in the organic cation/carnitine transporter OCTN2. J. Inherit. Metab. Dis. 2003;26:613–615. doi: 10.1023/A:1025968502527. [DOI] [PubMed] [Google Scholar]
- 30.El-Hattab A.W. Systemic Primary Carnitine Deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A., editors. GeneReviews®. University of Washington, Seattle; Seattle, WA, USA: 1993. [PubMed] [Google Scholar]
- 31.Magoulas P.L., El-Hattab A.W. Systemic primary carnitine deficiency: An overview of clinical manifestations, diagnosis, and management. Orphanet J. Rare Dis. 2012;7:68. doi: 10.1186/1750-1172-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vijay S., Patterson A., Olpin S., Henderson M.J., Clark S., Day C., Savill G., Walter J.H. Carnitine transporter defect: Diagnosis in asymptomatic adult women following analysis of acylcarnitines in their newborn infants. J. Inherit. Metab. Dis. 2006;29:627–630. doi: 10.1007/s10545-006-0376-y. [DOI] [PubMed] [Google Scholar]
- 33.Schimmenti L.A., Crombez E.A., Schwahn B.C., Heese B.A., Wood T.C., Schroer R.J., Bentler K., Cederbaum S., Sarafoglou K., McCann M., et al. Expanded newborn screening identifies maternal primary carnitine deficiency. Mol. Genet. Metab. 2007;90:441–445. doi: 10.1016/j.ymgme.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 34.El-Hattab A.W., Li F.-Y., Shen J., Powell B.R., Bawle E.V., Adams D.J., Wahl E., Kobori J.A., Graham B., Scaglia F., et al. Maternal systemic primary carnitine deficiency uncovered by newborn screening: Clinical, biochemical, and molecular aspects. Genet. Med. Off. J. Am. Coll. Med. Genet. 2010;12:19–24. doi: 10.1097/GIM.0b013e3181c5e6f7. [DOI] [PubMed] [Google Scholar]
- 35.Rose E.C., di San Filippo C.A., Ndukwe Erlingsson U.C., Ardon O., Pasquali M., Longo N. Genotype-phenotype correlation in primary carnitine deficiency. Hum. Mutat. 2012;33:118–123. doi: 10.1002/humu.21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasmussen J., Nielsen O.W., Lund A.M., Køber L., Djurhuus H. Primary carnitine deficiency and pivalic acid exposure causing encephalopathy and fatal cardiac events. J. Inherit. Metab. Dis. 2013;36:35–41. doi: 10.1007/s10545-012-9488-8. [DOI] [PubMed] [Google Scholar]
- 37.Scaglia F., Wang Y., Singh R.H., Dembure P.P., Pasquali M., Fernhoff P.M., Longo N. Defective urinary carnitine transport in heterozygotes for primary carnitine deficiency. Genet. Med. Off. J. Am. Coll. Med. Genet. 1998;1:34–39. doi: 10.1097/00125817-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Xiaofei E., Wada Y., Dakeishi M., Hirasawa F., Murata K., Masuda H., Sugiyama T., Nikaido H., Koizumi A. Age-associated cardiomyopathy in heterozygous carrier mice of a pathological mutation of carnitine transporter gene, OCTN2. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:B270–B278. doi: 10.1093/gerona/57.7.B270. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi R., Asai T., Murakami H., Murakami R., Tsuzuki M., Numaguchi Y., Matsui H., Murohara T., Okumura K. Pressure overload-induced cardiomyopathy in heterozygous carrier mice of carnitine transporter gene mutation. Hypertens. Dallas Tex. 1979. 2007;50:497–502. doi: 10.1161/HYPERTENSIONAHA.107.088609. [DOI] [PubMed] [Google Scholar]
- 40.Giudice P.L., Bonomini M., Arduini A. A Moderate Carnitine Deficiency Exacerbates Isoproterenol-Induced Myocardial Injury in Rats. Cardiovasc. Drugs Ther. 2016;30:119–127. doi: 10.1007/s10557-016-6647-4. [DOI] [PubMed] [Google Scholar]
- 41.Roussel J., Labarthe F., Thireau J., Ferro F., Farah C., Roy J., Horiuchi M., Tardieu M., Lefort B., François Benoist J., et al. Carnitine deficiency induces a short QT syndrome. Heart Rhythm. 2016;13:165–174. doi: 10.1016/j.hrthm.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Wong G.K., Pehora C., Crawford M.W. L-carnitine reduces susceptibility to bupivacaine-induced cardiotoxicity: An experimental study in rats. Can. J. Anaesth. J. Can. Anesth. 2017;64:270–279. doi: 10.1007/s12630-016-0797-5. [DOI] [PubMed] [Google Scholar]
- 43.Amat di San Filippo C., Taylor M.R.G., Mestroni L., Botto L.D., Longo N. Cardiomyopathy and carnitine deficiency. Mol. Genet. Metab. 2008;94:162–166. doi: 10.1016/j.ymgme.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilcken B., Wiley V., Sim K.G., Carpenter K. Carnitine transporter defect diagnosed by newborn screening with electrospray tandem mass spectrometry. J. Pediatr. 2001;138:581–584. doi: 10.1067/mpd.2001.111813. [DOI] [PubMed] [Google Scholar]
- 45.Winter S.C., Linn L.S., Helton E. Plasma carnitine concentrations in pregnancy, cord blood, and neonates and children. Clin. Chim. Acta Int. J. Clin. Chem. 1995;243:87–93. doi: 10.1016/0009-8981(95)06148-7. [DOI] [PubMed] [Google Scholar]
- 46.Gallant N.M., Leydiker K., Wilnai Y., Lee C., Lorey F., Feuchtbaum L., Tang H., Carter J., Enns G.M., Packman S., et al. Biochemical characteristics of newborns with carnitine transporter defect identified by newborn screening in California. Mol. Genet. Metab. 2017;122:76–84. doi: 10.1016/j.ymgme.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Yan S., Yang X.-F., Liu H.-L., Fu N., Ouyang Y., Qing K. Long-chain acyl-CoA synthetase in fatty acid metabolism involved in liver and other diseases: An update. World J. Gastroenterol. 2015;21:3492–3498. doi: 10.3748/wjg.v21.i12.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watkins P.A., Maiguel D., Jia Z., Pevsner J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J. Lipid Res. 2007;48:2736–2750. doi: 10.1194/jlr.M700378-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.McGarry J.D., Brown N.F. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 50.Houten S.M., Wanders R.J.A. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010;33:469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong D., Cong L., Zhong S., Liu L., Xu Y., Zhang J. A novel CPT1C variant causes pure hereditary spastic paraplegia with benign clinical course. Ann. Clin. Transl. Neurol. 2019;6:610–614. doi: 10.1002/acn3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rinaldi C., Schmidt T., Situ A.J., Johnson J.O., Lee P.R., Chen K.-L., Bott L.C., Fadó R., Harmison G.H., Parodi S., et al. Mutation in CPT1C Associated with Pure Autosomal Dominant Spastic Paraplegia. JAMA Neurol. 2015;72:561–570. doi: 10.1001/jamaneurol.2014.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindner M., Hoffmann G.F., Matern D. Newborn screening for disorders of fatty-acid oxidation: Experience and recommendations from an expert meeting. J. Inherit. Metab. Dis. 2010;33:521–526. doi: 10.1007/s10545-010-9076-8. [DOI] [PubMed] [Google Scholar]
- 54.Collins S.A., Sinclair G., McIntosh S., Bamforth F., Thompson R., Sobol I., Osborne G., Corriveau A., Santos M., Hanley B., et al. Carnitine palmitoyltransferase 1A (CPT1A) P479L prevalence in live newborns in Yukon, Northwest Territories, and Nunavut. Mol. Genet. Metab. 2010;101:200–204. doi: 10.1016/j.ymgme.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Prasad C., Johnson J.P., Bonnefont J.P., Dilling L.A., Innes A.M., Haworth J.C., Beischel L., Thuillier L., Prip-Buus C., Singal R., et al. Hepatic carnitine palmitoyl transferase 1 (CPT1 A) deficiency in North American Hutterites (Canadian and American): Evidence for a founder effect and results of a pilot study on a DNA-based newborn screening program. Mol. Genet. Metab. 2001;73:55–63. doi: 10.1006/mgme.2001.3149. [DOI] [PubMed] [Google Scholar]
- 56.Bennett M.J., Santani A.B. Carnitine Palmitoyltransferase 1A Deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A., editors. GeneReviews®. University of Washington, Seattle; Seattle, WA, USA: 1993. [PubMed] [Google Scholar]
- 57.Longo N., Amat di San Filippo C., Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. C Semin. Med. Genet. 2006;142C:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olpin S.E., Allen J., Bonham J.R., Clark S., Clayton P.T., Calvin J., Downing M., Ives K., Jones S., Manning N.J., et al. Features of carnitine palmitoyltransferase type I deficiency. J. Inherit. Metab. Dis. 2001;24:35–42. doi: 10.1023/A:1005694320063. [DOI] [PubMed] [Google Scholar]
- 59.Bonnefont J.-P., Djouadi F., Prip-Buus C., Gobin S., Munnich A., Bastin J. Carnitine palmitoyltransferases 1 and 2: Biochemical, molecular and medical aspects. Mol. Aspects Med. 2004;25:495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Innes A.M., Seargeant L.E., Balachandra K., Roe C.R., Wanders R.J., Ruiter J.P., Casiro O., Grewar D.A., Greenberg C.R. Hepatic carnitine palmitoyltransferase I deficiency presenting as maternal illness in pregnancy. Pediatr. Res. 2000;47:43–45. doi: 10.1203/00006450-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 61.de Sain-van der Velden M.G.M., Diekman E.F., Jans J.J., van der Ham M., Prinsen B.H.C.M.T., Visser G., Verhoeven-Duif N.M. Differences between acylcarnitine profiles in plasma and bloodspots. Mol. Genet. Metab. 2013;110:116–121. doi: 10.1016/j.ymgme.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Primassin S., Spiekerkoetter U. ESI-MS/MS measurement of free carnitine and its precursor γ-butyrobetaine in plasma and dried blood spots from patients with organic acidurias and fatty acid oxidation disorders. Mol. Genet. Metab. 2010;101:141–145. doi: 10.1016/j.ymgme.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 63.Korman S.H., Waterham H.R., Gutman A., Jakobs C., Wanders R.J.A. Novel metabolic and molecular findings in hepatic carnitine palmitoyltransferase I deficiency. Mol. Genet. Metab. 2005;86:337–343. doi: 10.1016/j.ymgme.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 64.Indiveri C., Iacobazzi V., Tonazzi A., Giangregorio N., Infantino V., Convertini P., Console L., Palmieri F. The mitochondrial carnitine/acylcarnitine carrier: Function, structure and physiopathology. Mol. Aspects Med. 2011;32:223–233. doi: 10.1016/j.mam.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Rubio-Gozalbo M.E., Bakker J.A., Waterham H.R., Wanders R.J.A. Carnitine-acylcarnitine translocase deficiency, clinical, biochemical and genetic aspects. Mol. Aspects Med. 2004;25:521–532. doi: 10.1016/j.mam.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Lopriore E., Gemke R.J., Verhoeven N.M., Jakobs C., Wanders R.J., Roeleveld-Versteeg A.B., Poll-The B.T. Carnitine-acylcarnitine translocase deficiency: Phenotype, residual enzyme activity and outcome. Eur. J. Pediatr. 2001;160:101–104. doi: 10.1007/s004310000644. [DOI] [PubMed] [Google Scholar]
- 67.Morris A.A., Olpin S.E., Brivet M., Turnbull D.M., Jones R.A., Leonard J.V. A patient with carnitine-acylcarnitine translocase deficiency with a mild phenotype. J. Pediatr. 1998;132:514–516. doi: 10.1016/S0022-3476(98)70030-7. [DOI] [PubMed] [Google Scholar]
- 68.Wieser T. Carnitine Palmitoyltransferase II Deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A., editors. GeneReviews®. University of Washington, Seattle; Seattle, WA, USA: 1993. [PubMed] [Google Scholar]
- 69.Nance J.R., Mammen A.L. Diagnostic evaluation of rhabdomyolysis. Muscle Nerve. 2015;51:793–810. doi: 10.1002/mus.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wieser T., Deschauer M., Olek K., Hermann T., Zierz S. Carnitine palmitoyltransferase II deficiency: Molecular and biochemical analysis of 32 patients. Neurology. 2003;60:1351–1353. doi: 10.1212/01.WNL.0000055901.58642.48. [DOI] [PubMed] [Google Scholar]
- 71.Deschauer M., Wieser T., Zierz S. Muscle carnitine palmitoyltransferase II deficiency: Clinical and molecular genetic features and diagnostic aspects. Arch. Neurol. 2005;62:37–41. doi: 10.1001/archneur.62.1.37. [DOI] [PubMed] [Google Scholar]
- 72.Anichini A., Fanin M., Vianey-Saban C., Cassandrini D., Fiorillo C., Bruno C., Angelini C. Genotype-phenotype correlations in a large series of patients with muscle type CPT II deficiency. Neurol. Res. 2011;33:24–32. doi: 10.1179/016164110X12767786356390. [DOI] [PubMed] [Google Scholar]
- 73.Vladutiu G.D., Bennett M.J., Fisher N.M., Smail D., Boriack R., Leddy J., Pendergast D.R. Phenotypic variability among first-degree relatives with carnitine palmitoyltransferase II deficiency. Muscle Nerve. 2002;26:492–498. doi: 10.1002/mus.10217. [DOI] [PubMed] [Google Scholar]
- 74.Malik S., Paldiwal A.A., Korday C.S., Jadhav S.S. Neonatal Carnitine Palmitoyltransferase II Deficiency: A Lethal Entity. J. Clin. Diagn. Res. JCDR. 2015;9:SD01–SD02. doi: 10.7860/JCDR/2015/13600.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vladutiu G.D., Quackenbush E.J., Hainline B.E., Albers S., Smail D.S., Bennett M.J. Lethal neonatal and severe late infantile forms of carnitine palmitoyltransferase II deficiency associated with compound heterozygosity for different protein truncation mutations. J. Pediatr. 2002;141:734–736. doi: 10.1067/mpd.2002.128545. [DOI] [PubMed] [Google Scholar]
- 76.Boemer F., Deberg M., Schoos R., Caberg J.-H., Gaillez S., Dugauquier C., Delbecque K., François A., Maton P., Demonceau N., et al. Diagnostic pitfall in antenatal manifestations of CPT II deficiency. Clin. Genet. 2016;89:193–197. doi: 10.1111/cge.12593. [DOI] [PubMed] [Google Scholar]
- 77.Isackson P.J., Bennett M.J., Lichter-Konecki U., Willis M., Nyhan W.L., Sutton V.R., Tein I., Vladutiu G.D. CPT2 gene mutations resulting in lethal neonatal or severe infantile carnitine palmitoyltransferase II deficiency. Mol. Genet. Metab. 2008;94:422–427. doi: 10.1016/j.ymgme.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Tajima G., Hara K., Tsumura M., Kagawa R., Okada S., Sakura N., Maruyama S., Noguchi A., Awaya T., Ishige M., et al. Newborn screening for carnitine palmitoyltransferase II deficiency using (C16+C18:1)/C2: Evaluation of additional indices for adequate sensitivity and lower false-positivity. Mol. Genet. Metab. 2017;122:67–75. doi: 10.1016/j.ymgme.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 79.Ceccarelli S.M., Chomienne O., Gubler M., Arduini A. Carnitine palmitoyltransferase (CPT) modulators: A medicinal chemistry perspective on 35 years of research. J. Med. Chem. 2011;54:3109–3152. doi: 10.1021/jm100809g. [DOI] [PubMed] [Google Scholar]
- 80.Foley J.E. Rationale and application of fatty acid oxidation inhibitors in treatment of diabetes mellitus. Diabetes Care. 1992;15:773–784. doi: 10.2337/diacare.15.6.773. [DOI] [PubMed] [Google Scholar]
- 81.Conti R., Mannucci E., Pessotto P., Tassoni E., Carminati P., Giannessi F., Arduini A. Selective reversible inhibition of liver carnitine palmitoyl-transferase 1 by teglicar reduces gluconeogenesis and improves glucose homeostasis. Diabetes. 2011;60:644–651. doi: 10.2337/db10-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang W., Lopaschuk G.D. Metabolic therapy for the treatment of ischemic heart disease: Reality and expectations. Expert Rev. Cardiovasc. Ther. 2007;5:1123–1134. doi: 10.1586/14779072.5.6.1123. [DOI] [PubMed] [Google Scholar]
- 83.Lionetti V., Linke A., Chandler M.P., Young M.E., Penn M.S., Gupte S., d’Agostino C., Hintze T.H., Stanley W.C., Recchia F.A. Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc. Res. 2005;66:454–461. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Rupp H., Zarain-Herzberg A., Maisch B. The use of partial fatty acid oxidation inhibitors for metabolic therapy of angina pectoris and heart failure. Herz. 2002;27:621–636. doi: 10.1007/s00059-002-2428-x. [DOI] [PubMed] [Google Scholar]
- 85.Holubarsch C.J.F., Rohrbach M., Karrasch M., Boehm E., Polonski L., Ponikowski P., Rhein S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: The ERGO (etomoxir for the recovery of glucose oxidation) study. Clin. Sci. Lond. Engl. 1979. 2007;113:205–212. doi: 10.1042/CS20060307. [DOI] [PubMed] [Google Scholar]
- 86.Qu Q., Zeng F., Liu X., Wang Q.J., Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: Emerging therapeutic targets in cancer. Cell Death Dis. 2016;7:e2226. doi: 10.1038/cddis.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scaglia F., Longo N. Primary and secondary alterations of neonatal carnitine metabolism. Semin. Perinatol. 1999;23:152–161. doi: 10.1016/S0146-0005(99)80047-0. [DOI] [PubMed] [Google Scholar]
- 88.Flanagan J.L., Simmons P.A., Vehige J., Willcox M.D., Garrett Q. Role of carnitine in disease. Nutr. Metab. 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lombard K.A., Olson A.L., Nelson S.E., Rebouche C.J. Carnitine status of lactoovovegetarians and strict vegetarian adults and children. Am. J. Clin. Nutr. 1989;50:301–306. doi: 10.1093/ajcn/50.2.301. [DOI] [PubMed] [Google Scholar]
- 90.Limketkai B.N., Zucker S.D. Hyperammonemic Encephalopathy Caused by Carnitine Deficiency. J. Gen. Intern. Med. 2008;23:210–213. doi: 10.1007/s11606-007-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clark M.A., Stein R.E.K., Silver E.J., Khalid S., Fuloria M., Esteban-Cruciani N.V. Carnitine deficiency in preterm infants: A national survey of knowledge and practices. J. Neonatal-Perinat. Med. 2017;10:381–386. doi: 10.3233/NPM-16146. [DOI] [PubMed] [Google Scholar]
- 92.Schmidt-Sommerfeld E., Penn D. Carnitine and total parenteral nutrition of the neonate. Biol. Neonate. 1990;58(Suppl. 1):81–88. doi: 10.1159/000243302. [DOI] [PubMed] [Google Scholar]
- 93.Van Aerde J.E. In preterm infants, does the supplementation of carnitine to parenteral nutrition improve the following clinical outcomes: Growth, lipid metabolism and apneic spells? Paediatr. Child Health. 2004;9:573. doi: 10.1093/pch/9.8.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salguero Olid A., Blanco Sánchez G., Alonso Ojembarrena A. A systematic review about prophylactic L-carnitine administration in parenteral nutrition of extremely preterm infants. Farm. Hosp. Organo Expresion Cient. Soc. Espanola Farm. Hosp. 2018;42:168–173. doi: 10.7399/fh.10976. [DOI] [PubMed] [Google Scholar]
- 95.Lheureux P.E.R., Hantson P. Carnitine in the treatment of valproic acid-induced toxicity. Clin. Toxicol. Phila. Pa. 2009;47:101–111. doi: 10.1080/15563650902752376. [DOI] [PubMed] [Google Scholar]
- 96.Kobayashi H., Fukuda S., Yamada K., Hasegawa Y., Takahashi T., Purevsuren J., Yamaguchi S. Clinical Features of Carnitine Deficiency Secondary to Pivalate-Conjugated Antibiotic Therapy. J. Pediatr. 2016;173:183–187. doi: 10.1016/j.jpeds.2016.02.080. [DOI] [PubMed] [Google Scholar]
- 97.Pochini L., Scalise M., Indiveri C. Inactivation by omeprazole of the carnitine transporter (OCTN2) reconstituted in liposomes. Chem. Biol. Interact. 2009;179:394–401. doi: 10.1016/j.cbi.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 98.Hirano T., Yasuda S., Osaka Y., Kobayashi M., Itagaki S., Iseki K. Mechanism of the inhibitory effect of zwitterionic drugs (levofloxacin and grepafloxacin) on carnitine transporter (OCTN2) in Caco-2 cells. Biochim. Biophys. Acta. 2006;1758:1743–1750. doi: 10.1016/j.bbamem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Hu C., Lancaster C.S., Zuo Z., Hu S., Chen Z., Rubnitz J.E., Baker S.D., Sparreboom A. Inhibition of OCTN2-mediated transport of carnitine by etoposide. Mol. Cancer Ther. 2012;11:921–929. doi: 10.1158/1535-7163.MCT-11-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Evans A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2003;41:S13–S26. doi: 10.1016/S0272-6386(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 101.Evans A.M., Faull R.J., Nation R.L., Prasad S., Elias T., Reuter S.E., Fornasini G. Impact of hemodialysis on endogenous plasma and muscle carnitine levels in patients with end-stage renal disease. Kidney Int. 2004;66:1527–1534. doi: 10.1111/j.1523-1755.2004.00916.x. [DOI] [PubMed] [Google Scholar]
- 102.Hedayati S.S. Dialysis-related carnitine disorder. Semin. Dial. 2006;19:323–328. doi: 10.1111/j.1525-139X.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 103.Wasserstein A.G. L-carnitine supplementation in dialysis: Treatment in quest of disease. Semin. Dial. 2013;26:11–15. doi: 10.1111/sdi.12041. [DOI] [PubMed] [Google Scholar]
- 104.Arduini A., Bonomini M., Savica V., Amato A., Zammit V. Carnitine in metabolic disease: Potential for pharmacological intervention. Pharmacol. Ther. 2008;120:149–156. doi: 10.1016/j.pharmthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 105.Merritt J.L., Norris M., Kanungo S. Fatty acid oxidation disorders. Ann. Transl. Med. 2018;6 doi: 10.21037/atm.2018.10.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baumgartner M.R., Hörster F., Dionisi-Vici C., Haliloglu G., Karall D., Chapman K.A., Huemer M., Hochuli M., Assoun M., Ballhausen D., et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J. Rare Dis. 2014;9:130. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nasser M., Javaheri H., Fedorowicz Z., Noorani Z. Carnitine supplementation for inborn errors of metabolism. Cochrane Database Syst. Rev. 2012:CD006659. doi: 10.1002/14651858.CD006659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Watanabe K., Yamada K., Sameshima K., Yamaguchi S. Two siblings with very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency suffered from rhabdomyolysis after l-carnitine supplementation. Mol. Genet. Metab. Rep. 2018;15:121–123. doi: 10.1016/j.ymgmr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liepinsh E., Makrecka-Kuka M., Volska K., Kuka J., Makarova E., Antone U., Sevostjanovs E., Vilskersts R., Strods A., Tars K., et al. Long-chain acylcarnitines determine ischaemia/reperfusion-induced damage in heart mitochondria. Biochem. J. 2016;473:1191–1202. doi: 10.1042/BCJ20160164. [DOI] [PubMed] [Google Scholar]
- 110.Ferro F., Ouillé A., Tran T.-A., Fontanaud P., Bois P., Babuty D., Labarthe F., Le Guennec J.-Y. Long-Chain Acylcarnitines Regulate the hERG Channel. PLoS ONE. 2012;7:e41686. doi: 10.1371/journal.pone.0041686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spiekerkoetter U., Lindner M., Santer R., Grotzke M., Baumgartner M.R., Boehles H., Das A., Haase C., Hennermann J.B., Karall D., et al. Treatment recommendations in long-chain fatty acid oxidation defects: Consensus from a workshop. J. Inherit. Metab. Dis. 2009;32:498–505. doi: 10.1007/s10545-009-1126-8. [DOI] [PubMed] [Google Scholar]
- 112.Parikh S., Saneto R., Falk M.J., Anselm I., Cohen B.H., Haas R. A Modern Approach to the Treatment of Mitochondrial Disease. Curr. Treat. Options Neurol. 2009;11:414–430. doi: 10.1007/s11940-009-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DiMauro S., Hirano M., Schon E.A. Approaches to the treatment of mitochondrial diseases. Muscle Nerve. 2006;34:265–283. doi: 10.1002/mus.20598. [DOI] [PubMed] [Google Scholar]
- 114.Vallance H.D., Koochin A., Branov J., Rosen-Heath A., Bosdet T., Wang Z., Hazen S.L., Horvath G. Marked elevation in plasma trimethylamine-N-oxide (TMAO) in patients with mitochondrial disorders treated with oral l-carnitine. Mol. Genet. Metab. Rep. 2018;15:130–133. doi: 10.1016/j.ymgmr.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Janeiro M.H., Ramírez M.J., Milagro F.I., Martínez J.A., Solas M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients. 2018;10:1398. doi: 10.3390/nu10101398. [DOI] [PMC free article] [PubMed] [Google Scholar]