Abstract
Objective
To evaluate the safety and effectiveness of a wrist‐worn peripheral nerve stimulation device in patients with essential tremor (ET) in a single in‐office session.
Methods
This was a randomized controlled study of 77 ET patients who received either treatment stimulation (N = 40) or sham stimulation (N = 37) on the wrist of the hand with more severe tremor. Tremor was evaluated before and immediately after the end of a single 40‐minute stimulation session. The primary endpoint compared spiral drawing in the stimulated hand using the Tremor Research Group Essential Tremor Rating Assessment Scale (TETRAS) Archimedes spiral scores in treatment and sham groups. Additional endpoints included TETRAS upper limb tremor scores, subject‐rated tasks from the Bain and Findley activities of daily living (ADL) scale before and after stimulation as well as clinical global impression‐improvement (CGI‐I) rating after stimulation.
Results
Subjects who received peripheral nerve stimulation did not show significantly larger improvement in the Archimedes spiral task compared to sham but did show significantly greater improvement in upper limb TETRAS tremor scores (p = 0.017) compared to sham. Subject‐rated improvements in ADLs were significantly greater with treatment (49% reduction) than with sham (27% reduction; p = 0.001). A greater percentage of ET patients (88%) reported improvement in the stimulation group as compared to the sham group (62%) according to CGI‐I ratings (p = 0.019). No significant adverse events were reported; 3% of subjects experienced mild adverse events.
Conclusions
Peripheral nerve stimulation in ET may provide a safe, well‐tolerated, and effective treatment for transient relief of hand tremor symptoms.
Keywords: Essential tremor, movement disorders, neurostimulation, noninvasive stimulation, peripheral nerve stimulation, tremor
Introduction
Essential tremor (ET) is one of the most common movement disorders, occurring in 1 out of every 25 people aged 40 years or older, and is estimated to affect up to 7 million people in the United States 1, 2, 3. A majority of ET patients exhibit a postural and kinetic tremor in the upper limbs, and less commonly exhibit tremor of the head, face, voice, trunk, and lower limbs, all of which can impair daily activities 4, 5. Current first‐line pharmacologic treatment options for ET, including propranolol and primidone, are often limited by inadequate effectiveness or intolerable side effects 6. Tremor symptoms are poorly treated or refractory to first‐line treatment options in an estimated 25%‐55% of patients 7, 8.
Though the exact mechanisms are uncertain, ET arises from oscillatory activity within a central tremor network, which involves the ventral intermediate nucleus (VIM) of the thalamus 6, 9, 10. Evidence supports targeting the VIM to treat tremor symptoms in ET patients using methods including deep brain stimulation (DBS), surgical ablation, gamma knife ablation, and focused ultrasound 11, 12, 13. DBS of the VIM is highly effective for tremor suppression, with a reported 68 to 89% reduction in tremor 14, 15, 16, but implantation of the stimulation leads and pulse generator requires an invasive surgical procedure with associated risks 17, 18. Only a small fraction of ET patients who are candidates for DBS surgery receive an implant for various reasons 19. Previous studies have shown electrical median nerve stimulation evokes activity within the VIM and other regions of the central tremor network 20, 21, and that electrical stimulation of these pathways in a synchronized pattern can decrease tremor 22, 23. Based on these observations, we hypothesized that median and radial nerve stimulation at the wrist may reduce hand tremor.
Lin et al. recently reported reductions in hand tremor following noninvasive median and radial nerve stimulation in a small cohort of individuals with ET evaluated at a single site 24. In the current study, we investigated the effect of noninvasive median and radial nerve stimulation in a larger cohort of subjects in a prospective, randomized, sham controlled trial to further assess the safety and effectiveness of a wrist‐worn peripheral nerve stimulation device for treatment of hand tremors in adults with ET.
Methods
Participants
This study was conducted at four sites (see acknowledgements for list of sites). Of the 111 subjects who were screened, 93 subjects (mean age 70.2 ± 10.6 years, 45 males) were randomized to receive either treatment (N = 48) or sham stimulation (N = 45; Fig. 1; Table 1). Subjects who were already taking medications for ET were required to remain on their medications during the study with no changes in medication type or dosage. Key inclusion criteria were 1) at least 22 years of age; 2) diagnosis of ET as confirmed from clinical history and examination by a movement disorder neurologist; 3) signed informed consent; 4) at least one hand exhibiting score ≥2 as assessed by the Tremor Research Group Essential Tremor Rating Assessment Scale (TETRAS) Archimedes spiral task completed during the baseline evaluation, as assessed by the investigator in‐person; and 5) score of 3 or above in any 1 of the items of the Bain and Findley activities of daily living (ADL) scale. Key exclusion criteria were 1) implanted electrical medical device, such as a pacemaker, defibrillator, or deep brain stimulator; 2) history of thalamotomy; 3) suspected or diagnosed epilepsy or other seizure disorder; 4) pregnancy; 5) skin lesions at stimulation site; 6) peripheral neuropathy; 7) alcohol dependence; 8) other possible causes of tremor; 9) neurologic exam not consistent with ET; 10) alcohol or caffeine consumption within 12 hours of study enrollment. ET medications were stable for 1 month before enrollment. Based on prespecified inclusion criteria of a baseline TETRAS spiral rating of ≥2 as assessed by the average score from three blinded central raters, 77 subjects were included in the effectiveness analysis population (EAP).
Figure 1.
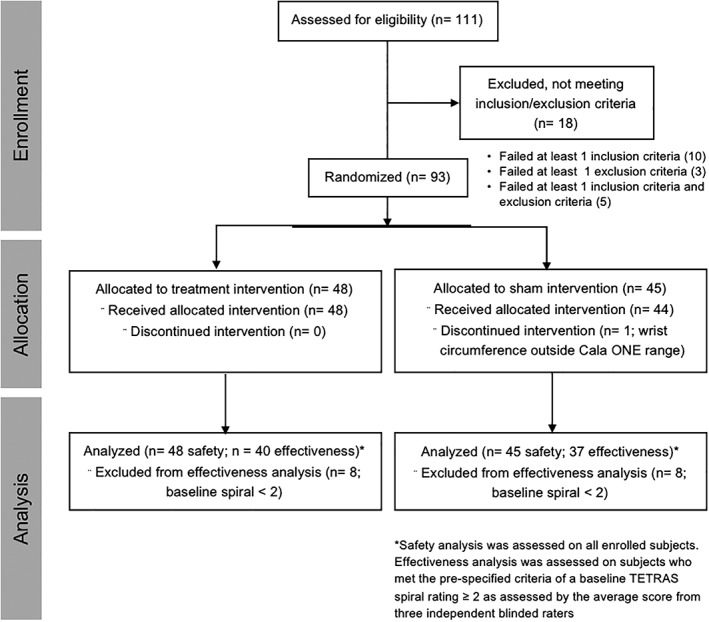
Flow diagram of the study selection.
Table 1.
Subject Demographic and Baseline Severity Information.
| Demographic | Overall (N = 93) | Treatment (N = 48) | Sham (N = 45) |
|---|---|---|---|
| Age––y | 70.2 ± 10.6 | 70.5 ± 11.2 | 69.8 ± 10.1 |
| Male sex––no. (%) | 45 (48) | 23 (48) | 22 (49) |
| Race––no. (%) | |||
| White | 88 (95) | 47 (98) | 41 (91) |
| Asian | 1 (1) | 0 | 1 (2) |
| Black or African American | 0 | 0 | 0 |
| More than one race | 3 (3) | 1 (2) | 2 (4) |
| Onset and Diagnosis––y | |||
| Age of onset | 38.8 ± 21.2 | 41.1 ± 22.2 | 36.2 ± 20.0 |
| Age of diagnosis | 52.8 ± 15.0 | 53.5 ± 15.4 | 52.1 ± 14.6 |
| Family history of ET––no. (%) | 72 (77) | 38 (79) | 34 (76) |
| Current tremor co‐therapy––no. (%) | |||
| None | 36 (39) | 17 (35) | 19 (42) |
| 1 medication | 32 (34) | 16 (33) | 16 (36) |
| > 1 medication | 25 (27) | 15 (31) | 10 (22) |
| Current tremor medications––no. (%) | |||
| Propranolol | 41 (44) | 25 (52) | 16 (36) |
| Primidone | 22 (24) | 13 (27) | 9 (20) |
| Other | 19 (20) | 9 (19) | 10 (22) |
| Duration of current tremor medications––y | 8.2 ± 9.0 | 8.5 ± 8.3 | 7.8 ± 9.8 |
| Prior treatments of ET––no. (%) | |||
| Medication | 54 (58) | 25 (52) | 29 (64) |
| Botulinum toxin | 4 (4.3) | 3 (6.3) | 1 (2.2) |
| Other | 3 (3.2) | 2 (4.2) | 1 (2.2) |
| Bain and Findley ADLs | |||
| Hand subset (range: 7‐28) | 16.1 ± 4.1 | 16.7 ± 4.0 | 15.5 ± 4.1 |
| Total score (range: 25‐100) | 45.4 ± 9.6 | 45.8 ± 9.0 | 45.0 ± 10.3 |
| TETRAS performance subscale | |||
| Archimedes spiral, TETRAS task 6 (range: 0‐4) | 2.6 ± 0.7 | 2.7 ± 0.8 | 2.5 ± 0.6 |
| Upper limb tremor, TETRAS task 4 (range: 0‐12) | 6.1 ± 1.6 | 6.3 ± 1.4 | 6.0 ± 1.7 |
| Total score (range: 0‐64) | 25.3 ± 6.0 | 25.8 ± 6.0 | 24.8 ± 6.0 |
Standard Protocol Approvals, Registrations, and Patient Consent
The clinical investigational protocol and subject informed consent form for this study were reviewed and approved by an Institutional Review Board for each clinical site prior to study initiation. Written informed consent was obtained from all subjects prior to study participation. This study was registered in ClinicalTrials.gov as study number NCT02629614.
Cala ONE Device Fitting, Calibration, and Stimulation Delivery
Following randomization, all subjects were fitted with a Cala ONE device based on the subject's therapy allocation (treatment or sham), wrist circumference (small circumference: 13.5‐15.4 cm, medium: 15.5‐17.4 cm, large 17.5‐19.5 cm), and stimulation hand (right or left). No skin preparation was required prior to application of the device, although subjects were asked to avoid lotion application prior to testing. The stimulation hand was the hand with more severe tremor (or the dominant hand if both hands had equal tremor severity) as determined by the TETRAS Archimedes spiral task completed during the baseline evaluation and assessed in‐person by the study investigator. Cala ONE working electrodes were placed over the median and radial nerves on the anterior surface of the wrist, while a single counter‐electrode was located on the posterior surface of the wrist (Fig. 2a). The electrodes were 2.2 cm × 2.2 cm square hydrogel electrodes spaced according to wrist circumference (small: 1.3 cm between electrodes; medium: 1.8 cm between electrodes; large: 2.3 cm between electrodes). Once the appropriate Cala ONE device was fitted to the subject's hand, the device performed a frequency calibration procedure during which the device measured the subject's tremor frequency while the subject performed a forward postural hold task (Fig. 2b). This frequency was then incorporated into the stimulation waveform (Fig. 2c). Stimulation consisted of a series of charge balanced biphasic pulses, 300 μs biphasic pulses, with a 50 μs interpulse period between pulses, delivered at a frequency of 150 Hz. The stimulation alternated between the median and radial nerve at a frequency equal to tremor frequency as measured by on‐board accelerometers (for example, for a measured 5 Hz tremor frequency, stimulation was applied over the median nerve for 100 msec and then was applied over the radial nerve for 100 msec). Both treatment and sham subjects were exposed to the frequency calibration procedure and to stimulation during an amplitude calibration period, during which study personnel increased the stimulation level by 0.25 mA steps until the subject reported first perceived sensation in the hand or finger area corresponding to distributions of the palmar digital branches of the median nerve and the superficial branch of the radial nerve. Final stimulation amplitude was chosen to be the highest level of tolerable stimulation level (always below muscle contraction) that the subject found comfortable (mean: 5.4 mA ± 2.9). Once final stimulation amplitude was identified, treatment subjects received stimulation at that level during a 40‐minute stimulation session, while sham subjects received no stimulation. Subjects were blinded to whether they were randomized to receive treatment stimulation or sham stimulation. During the stimulation session subjects could request the stimulation amplitude be decreased or discontinued for any reason.
Figure 2.
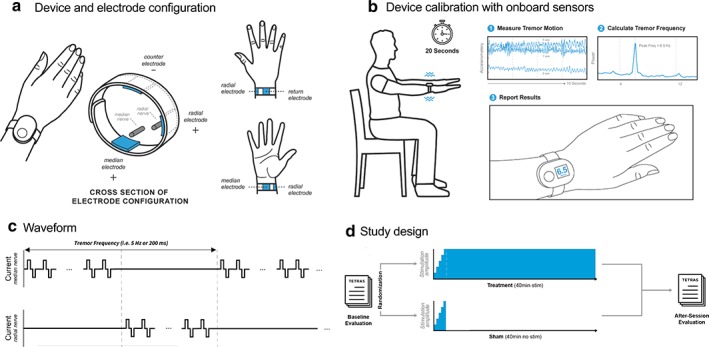
Cala ONE device and study design. a. Electrode placement on subject's wrist to target median and radial nerves, with counter‐electrode positioned on posterior surface of the wrist. b. Subject tremor frequency is captured during a 20 seconds postural hold (middle 10 seconds recorded by the device). The peak tremor frequency is determined on‐board the device and is input into the stimulation waveform to deliver a subject‐specific stimulation. c. Waveform consists of a series of charge balanced biphasic pulses delivered at a frequency of 150 Hz, 300 μs pulse width, and 50 μs interpulse period alternating between the median and radial nerve at a frequency equal to the tremor frequency. d. TETRAS and ADL scores were collected before and after treatment or sham stimulation sessions, and CGI was collected after the session. Both groups underwent the same frequency calibration and stimulation amplitude setting. Treatment consisted of a ramp up of stimulation (typically 1‐2 minutes) followed by a 40‐minute stimulation, whereas sham included a ramp up of 1 minute followed by a rapid ramp down of the stimulation. [Color figure can be viewed at wileyonlinelibrary.com]
Study Outcome Measures
The primary effectiveness measure was predefined as improvement in tremor severity in the dominant hand as measured by the TETRAS task 6 Archimedes spiral score following stimulation compared to sham stimulation for the treated limb. This measure was chosen based on a pilot study which showed a significant improvement in this parameter relative to sham 24. Secondary effectiveness measurements included a subject self‐reported assessment of improvement with the clinical global impressions scale of improvement (CGI‐I) scale. Additional effectiveness endpoints included improvement in TETRAS task 4 upper limb subscores for the treated limb tremor tasks after stimulation, as well as improvement in a subset of Bain and Findley ADLs collected in the office as measured by subject ratings. The effectiveness analyses included only enrolled subjects who met the predefined EAP criteria of having a baseline TETRAS task 6 Archimedes spiral rating ≥ 2, as assessed by average score from the three blinded raters, as predetermined in the study. Percent tremor amplitude reduction was calculated from TETRAS and ADL scores from baseline as described by Elble et al. (α = 0.5) 25, 26. The primary safety endpoint was an analysis of adverse events types and rates for all enrolled subjects, where the adverse event rate was calculated as the percentage of total subjects with an adverse event.
Tremor Rating Assessments
TETRAS ratings were used to assess the effect of stimulation on the treated limb. The TETRAS Archimedes spiral task 6 requires subjects to draw a spiral drawing in a 10‐cm sized square. Spirals were rated on the five‐point (0‐4) TETRAS scale with 0.5‐point resolution (0 = normal, 1 = slight: tremor barely visible, 2 = mild: obvious tremor, 3 = moderate: portions of figure not recognizable, 4 = severe: figure not recognizable). The TETRAS upper limb tremor task 4 assessment included three tasks to assess tremor severity: forward outstretched posture, lateral “wing beating” posture, and kinetic finger‐nose‐finger testing. Each upper limb was assessed and scored individually by the investigator using the following 5‐point (0‐4) TETRAS rating scale: 0 = no tremor, 1 = tremor is barely visible, 1.5 = tremor is visible, but less than 1 cm, 2 = tremor is greater than 1 cm but less than 3 cm amplitude, 2.5 = tremor is greater than 3 cm but less than 5 cm amplitude, 3 = tremor is greater than 5 cm but less than 10 cm amplitude, 3.5 = tremor is greater than 10 cm but less than 20 cm amplitude, 4 = tremor is greater than 20 cm amplitude.
A subset of 7 Bain and Findley ADL tasks that could be performed unilaterally (using one hand), which did not require the dominant hand were performed by the subject at baseline and after the session to evaluate functional improvements in ADLs with stimulation. These tasks included using a spoon to drink soup, holding a cup of tea, pouring milk from a bottle, dialing a phone, picking up change, inserting a plug into a socket, and unlocking a door with a key. The subjects (blinded as to whether they received stimulation or sham) performed the tasks and rated themselves from 1 to 4 on the following Bain and Findley ADL scale: 1 = able to do the activity without difficulty, 2 = able to do the activity with a little effort, 3 = able to do the activity with a lot of effort, 4 = cannot do the activity by yourself. Finally, subjects rated themselves using the CGI‐I scale, which is a seven‐point self‐report scale that required the subject to assess how much their tremor level has improved or worsened relative to their baseline state: 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = much worse, 7 = very much worse. All subjects performed baseline tremor assessments prior to and immediately following a 40‐minute session with the Cala ONE treatment or sham device (Fig. 2d). Adverse events were collected before, during, and after the stimulation session.
Blinding
Subjects and independent raters who provided the ratings for the primary effectiveness endpoint were blinded to therapy allocation. Raters were unblinded to TETRAS upper limb tremor outcome measures. Subjects were blinded throughout the study and during all ratings (including ADL, and CGI‐I ratings). To maintain the blind, all subjects were informed that they “may or may not feel stimulation” during the 40‐minute stimulation session. Visual cues on the device were used to maintain the blind, including a countdown during stimulation shown on the device display. Blinding was assessed with a subject blinding questionnaire after device removal.
Randomization
Subjects were randomized 1:1 to receive the investigational patterned stimulation (“treatment” group) or sham stimulation (“sham” group). The therapy allocation for each subject was determined at the time of enrollment using a randomization approach stratified by site such that therapy allocation was balanced within site. Prior to study initiation, randomization lists were provided by Agility Clinical Corp. for up to five sites, block randomization, with seven blocks of size 6. Within each block, three subjects were randomized to treatment and three to sham stimulation. The order of the spiral images was randomized so that the independent raters were blinded to whether the spirals were before or after treatment and whether a subject was in the treatment group or sham group.
Statistical Analysis
Statistical analyses were predefined in the statistical analysis plan for this study. An analysis of covariance (ANCOVA) model was used to assess the statistical significance of the difference in the mean change between the treatment and sham groups for the primary endpoint which was defined as the change in TETRAS Archimedes spiral drawing score following stimulation. The model included the baseline score for the task as a continuous covariate, and randomization assignment as a classification variable. For secondary endpoints, the statistical significance of the differences in the mean change between the treatment and the sham groups after stimulation was assessed using two‐sample, two‐tailed t‐tests for each individual TETRAS and ADL task as well as for the difference in the composite scores. Changes in CGI‐I between treatment and sham groups were tested for statistical significance using the Wilcoxon Rank Sum test. For all tests, a p‐value of <0.05 was considered statistically significant.
Results
Physician‐Rated Metrics
There was not a significantly larger improvement in the Archimedes spiral rating in treatment (0.55) compared to sham (0.41) (p = 0.26). However, upper limb tremor task 4 TETRAS subscores for the treated limb showed that there was a significantly greater improvement following treatment stimulation compared to sham stimulation. Of the individual improvement in TETRAS scores for subjects following treatment or sham stimulation, there was a significant improvement in forward postural hold rating in the treatment group (0.75) compared to sham (0.35) (p = 0.004) (Fig. 3a), a 46% reduction in tremor amplitude with treatment compared to 24% reduction in tremor amplitude with sham. The average improvement in the treated hand TETRAS upper limb tremor task 4 for treatment (0.61) compared to sham (0.35; p = 0.017; Fig. 3b), a 42% reduction in tremor amplitude with treatment compared to 28% reduction with sham. The total TETRAS performance score (tasks 4 and 6) showed a significant improvement for treatment (2.38) compared to sham (1.45; p = 0.015) (Fig. 3c).
Figure 3.
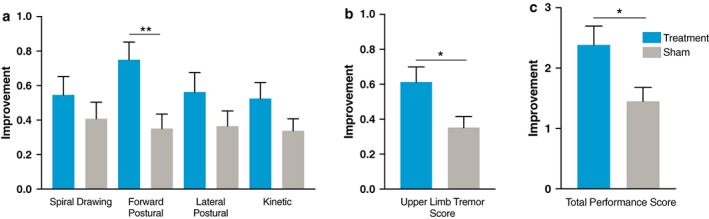
TETRAS improvement with stimulation. a. Average improvement for subjects following treatment (N = 40) or sham (N = 37) stimulation by individual TETRAS Archimedes spiral drawing (task 6) and individual upper limb tremor tasks (task 4). b. TETRAS dominant combined upper limb tremor task (task 4) (p = 0.017). c TETRAS performance subscore (tasks 4 and 6). [Color figure can be viewed at wileyonlinelibrary.com]
Subject‐Rated Metrics
In the Bain and Findley ADLs that were performed and rated by subjects, treatment subjects showed a significant improvement following stimulation compared to sham in tasks including holding a cup of tea (1.03 vs. 0.57; p = 0.011), dialing a telephone (0.70 vs. 0.30; p = 0.015), picking up change (0.53 vs. 0.05; p = 0.002), and unlocking a door with a key (0.6 vs. 0.17; p = 0.010); however, tasks including using a spoon to drink soup, pouring milk from a bottle, and inserting a plug into a socket were not significant compared to sham (Table 2, Fig. 4a). Of note, the treatment group improved significantly compared to baseline on all seven activities (all p‐values <0.05). The sham group improved significantly compared to baseline for five of the seven activities (using a spoon to drink soup, holding a cup of tea, pouring milk from a bottle or carton, dialing a telephone, and inserting an electric plug into a socket). Treatment improved ADLs across all measured tasks by 0.66 while sham improved by 0.36 (p = 0.001) (Fig. 4b), a 49% improvement with treatment compared to a 27% improvement in tremor amplitude with sham.
Table 2.
Bain and Findley Activities of Daily Living.
| Treatment | Sham | |||
|---|---|---|---|---|
| Baseline mean ± SEM | Change mean ± SEM | Baseline mean ± SEM | Change mean ± SEM | |
| Use a spoon to drink soup | 3.18 ± 0.12 | −0.78 ± 0.14 | 3.00 ± 0.14 | −0.59 ± 0.13 |
| Hold a cup of tea | 2.95 ± 0.13 | −1.03 ± 0.12 | 2.65 ± 0.15 | −0.57 ± 0.13 |
| Pour milk from a bottle or carton | 2.78 ± 1.16 | −0.70 ± 0.12 | 2.59 ± 0.14 | −0.59 ± 0.12 |
| Dial a telephone | 2.23 ± 0.14 | −0.70 ± 0.13 | 2.00 ± 0.15 | −0.30 ± 0.10 |
| Pick up your change in a shop | 2.03 ± 0.14 | −0.53 ± 0.11 | 1.92 ± 0.15 | −0.05 ± 0.10 |
| Insert an electric plug into a socket | 1.83 ± 0.12 | −0.33 ± 0.11 | 1.76 ± 0.13 | −0.24 ± 0.09 |
| Unlock your front door with a key | 2.23 ± 0.14 | −0.60 ± 0.14 | 1.86 ± 0.11 | −0.16 ± 0.09 |
| ADL subset total | 17.20 ± 0.65 | −4.65 ± 0.44 | 15.78 ± 0.64 | −2.51 ± 0.45 |
Figure 4.
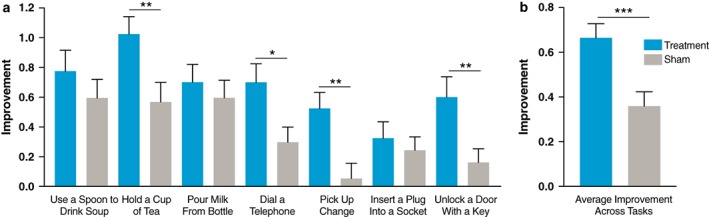
Subject‐rated Bain and Findley activities of daily living improvement with stimulation. a. Average improvement for subjects following treatment (N = 40) or sham (N = 37) stimulation as rated by blinded subjects using the Bain and Findley activities of daily living subscale. b. Average improvement across activities (p = 0.001). (Mean ± SEM) *p < 0.05; **p < 0.01; ***p < 0.001. [Color figure can be viewed at wileyonlinelibrary.com]
Treatment subjects reported improvement in the CGI‐I scale that was significantly greater than sham (p = 0.019. CGI‐I scores showed that a greater percentage of subjects in the treatment group reported an improvement after stimulation, which was a significant improvement compared to the sham group. The mean poststimulation rating on CGI‐I was 2.65 (between minimally and much improved) for the treatment group compared to 3.14 (between no change and minimally improved) for the sham group. Overall, 88% of treated subjects reported improvement in their tremor in the treatment group while 62% of sham subjects reported improvement (Fig. 5).
Figure 5.
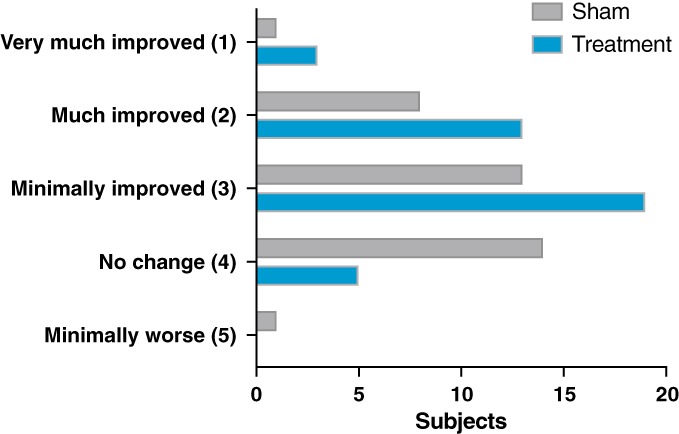
Clinical global impression of improvement scale. The clinical global impression‐improvement scale is a self‐report scale that required the subject to assess how much their tremor level has improved or worsened relative to their baseline state prior to the session. [Color figure can be viewed at wileyonlinelibrary.com]
Blinding assessment showed a blinding index of 0.608 (95% CI: 0.509‐0.708), which indicated a successful blind (0.5 indicates random guessing) 27. This index is a numerical assessment of how well a study blind was maintained 27.
Safety
No significant adverse events or unanticipated adverse device effects were reported in the study. The adverse event rate was low at 3% (two subjects in the treatment group and one subject in the sham group). Observed adverse events included significant and persistent skin irritation (including redness, itchiness, and/or swelling) in two subjects who received treatment and sensation of weakness or stinging pain in the wrist in one subject who received treatment stimulation and one subject who received sham stimulation. All adverse events were mild and resolved within 24 hours without treatment or sequelae. No subjects in this study requested reduction or cessation of stimulation.
Discussion
This study evaluated the safety and effectiveness of a single session of peripheral stimulation of the median and radial nerves on hand tremor symptoms in ET patients. Although the primary endpoint was not met, multiple other endpoints were met. The primary endpoint was specified following a small randomized blinded pilot study that demonstrated a significant difference between treatment and sham measured by the TETRAS Archimedes spiral 24. In the current multisite study, the spirals were not statistically different between treatment and sham because both were significantly improved from baseline. The large sham effect observed in this study is not surprising given the large effect of sham simulation observed in other studies, particularly in neurologic device studies 28, 29. The TETRAS Archimedes spiral rating is a single measure of tremor, and it does not encompass all aspects of hand tremor. Broader measures of tremor in this study showed a significant improvement compared to sham.
The treatment group experienced significantly greater improvements than the sham group in functional outcome measures (TETRAS upper limb tremor scores and ADLs) as well as patient reported outcomes (CGI‐I scores), which are meaningful to both patients and physicians. The magnitude of the improvement in the tasks in the treatment corresponded to a 49% reduction in tremor according to ADLs, and a 42% reduction according to TETRAS upper limb tremor following a single stimulation session 25, 26. These improvements are clinically meaningful, and within the range of tremor amplitude improvement (32%‐75%) reported in controlled studies of the medications most commonly prescribed for ET 26. Additionally, 75% of subjects had a response greater than 30% improvement, and 65% of subjects had a response greater than 40% improvement in TETRAS following a single stimulation session. Further, 70% of subjects reported a greater than 30% improvement in ADLs, and 65% of subjects reported a greater than 40% improvement.
ET continues to cause disabling tremor in a large number of patients who have tried oral medications and are either not candidates for surgical or other invasive interventions, or do not want to consider surgical or invasive interventions. While there have been studies investigating alternative nontherapeutic solutions to aid in specific ADLs, including the use of a real‐time control prosthetic tool, utility is currently limited to eating 30. The observed response in this study was achieved without the risks of surgical or pharmacologic intervention, such as the risk of hemorrhage (5% reported rate) or infection (4% reported rate) with DBS implantation or other invasive procedures 13, 31, 32, or side effects from ET medications 33. One of the more recent invasive FDA approved therapies to treat hand tremor in ET patients is focused ultrasound thalamotomy. While focused ultrasound thalamotomy is demonstrated to significantly reduce hand tremor (47% reduction after 3 months), Elias et al. reported the significant adverse event profile of 56 subjects who received thalamotomy; these adverse events included gait disturbance in 36% of patients and paresthesias or numbness in 38% of patients 13. Although the current study investigated the safety profile following a single stimulation session, there were no serious adverse events, and only 3% of subjects reported adverse events that spontaneously resolved within 24 hours without intervention. This side effect profile is encouraging and may provide an additional treatment option for patients who are interested in a treatment with a more limited side effect profile than current therapy options.
It is important to acknowledge the limitations of this study. First, this study was performed with a small group of subjects in whom safety and effectiveness were evaluated immediately after stimulation in a single in clinic session. As a result, we were unable to measure the effect of stimulation over time or implement automated tools to detect tremor in real‐time to optimize therapy. Future studies should investigate the durability of the therapeutic effect and the effects of chronic use with enabling technologies to automate tremor measurement over time 34, 35, 36. Since the device is worn on the wrist, there is potential to incorporate kinematic measurements to provide feedback regarding tremor burden over time to patients and clinicians. Further, due to the immediate therapeutic effect of stimulation, which is unlike other available pharmacologic interventions, kinematic measurements may provide insight into the effect of stimulation on tremor amplitude over time.
This therapeutic approach was inspired by the observation that peripheral stimulation evokes central activity in brain regions such as the VIM, a target that when effectively stimulated with DBS can improve tremor 18. While the success of the patterned peripheral nerve stimulation tested here is consistent with this hypothesis, other potential mechanisms are possible, including circuitry modulated in previous studies demonstrating tremor reduction by manipulation of sensory input, including with topical anesthesia, cooling, vibration, and electrical stimulation 37, 38, 39, 40. It is also possible that alternative stimulation methods may improve tremor in patients with ET, and determination of optimal treatment for each patient requires further research.
Overall, our data suggest that the subjects who received patterned treatment stimulation experienced a significant reduction in tremor and an improvement in function. These results are encouraging, and future studies are needed to confirm the effectiveness of this noninvasive therapy over time.
Authorship Statement
Rajesh Pahwa, MD and Rohit Dhall, MD served as the principal investigators on the research. They were responsible for the research project execution, manuscript writing, manuscript and statistical analysis review, and critique. Jill Ostrem, MD and Ryder Gwinn, MD also served as principal investigators on the research. They were responsible for research project execution, manuscript and statistical analysis review, and critique. Kelly Lyons, PhD, Nijee Luthra, MD, Cameron Dietiker, MD, and Susie Ro, MD served as sub‐investigators on the research. They were responsible for research project execution, manuscript and statistical analysis review, and critique.
Paula Chidester, MS was responsible for research project execution, manuscript writing, review, and critique. Samuel Hamner, PhD was responsible for research project execution, figure development, manuscript review, and critique. Erika Ross, PhD was responsible for manuscript writing, figure development, statistical analysis and manuscript review, and critique. Scott Delp, PhD was responsible for research project conception, organization, manuscript writing, statistical analysis design, review, and critique.
COMMENT
This study assesses a novel, short‐term peripheral nerve stimulation method to reduce tremor and improve hand function. Further studies may extend the usefulness of this non‐invasive approach for longer‐term symptomatic tremor control, particularly if a feedback system can be developed to modulate the intensity of the stimulation directly in relation to tremor amplitude.
Dennis Turner, MD
Durham, NC, USA
Comments not included in the Early View version of this paper.
Source(s) of financial support: Supported by Cala Health, Inc.
Conflict of Interest: Dr. Pahwa has received consulting fees from Abbvie, Abbott, ACADIA, Acorda, Adamas, Cala Health, Cynapsus, Global Kinetics, Ionis, Lundbeck, Neurocrine, Sunovion, Teva Neuroscience, UCB, and US World Meds. He has received research grants from Abbott, AbbVie, Acorda, Adamas, Biogen, BMS, Boston Scientific, Cala Health, Cavion, Cynapsus, Intec, Jazz, Kyowa, Lilly, Parkinson's Foundation, NIH/NINDS, Parkinson Study Group, Pfizer, Roche, Sunovion, and US WorldMeds. Dr. Dhall is an investigator for Cala Health, Inc., and has served as a consultant for Impax, Merz, Teva, and Acadia Pharmaceuticals. Dr. Ostrem received research support from NIH grants R01NS090913, UH3NS100544, DARPA contract W911NF1420043, ad PCORI contract 782.002 as a co‐investigator. Dr. Ostrem also has received research grant support from The National Parkinson Foundation, Michael J. Fox Foundation, Boston Scientific, St Jude Medical, Cala Health, Google, Sangamo, and Biogen. She has been a consultant for Abbvie, Neurocrine, Medtronic, and Adamas Pharmaceutics. She receives programmatic fellowship training support from Medtronic, Abbvie, Boston Scientific, and Allergan. Dr. Lyons has received consulting fees from ACADIA, Parkinson's Foundation, and Sage Therapeutics. She is also President of the International Essential Tremor Foundation. Dr. Ross is employed by Cala Health, Inc. and receives research support at the Mayo Clinic from DARPA contracts N66001‐17‐2‐4010 and N66001‐17‐2‐4018. Dr. Hamner and Ms. Chidester are employed by Cala Health, Inc. Dr. Delp has received research support from NIH grants U54 EB020405, P2C HD065690, R01 GM107340, R01 NS080954, and DARPA contract W911QX12C0018 on which he is the principal investigator. He also has received support from NIH grant R01 EB009351 as a co‐investigator. Dr. Delp is a consultant, scientific advisor, and board member of Cala Health, Inc., Circuit Therapeutics Inc., and Zebra Medical Technologies, Inc. and receives compensation for this service. Drs. Ro, Gwinn, and Luthra have no potential conflict of interest to report.
References
- 1. Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord 1998;13:5–10. [DOI] [PubMed] [Google Scholar]
- 2. Louis ED, Ottman R. How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet Mov (N Y) 2014;4:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dogu O, Sevim S, Camdeviren H et al. Prevalence of essential tremor: door‐to‐door neurologic exams in Mersin Province, Turkey. Neurology 2003;61:1804–1806. [DOI] [PubMed] [Google Scholar]
- 4. Koller W, Biary N, Cone S. Disability in essential tremor: effect of treatment. Neurology 1986;36:1001–1004. [DOI] [PubMed] [Google Scholar]
- 5. Shalaby S, Indes J, Keung B et al. Public knowledge and attitude toward essential tremor: a questionnaire survey. Front Neurol 2016;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Louis ED. Essential tremor. Lancet Neurol 2005;4:100–110. [DOI] [PubMed] [Google Scholar]
- 7. Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Scientific Committee Mov Disord 1998;13:2–23. [DOI] [PubMed] [Google Scholar]
- 8. Louis ED. Treatment of medically refractory essential tremor. N Engl J Med 2016;375:792–793. [DOI] [PubMed] [Google Scholar]
- 9. Brittain JS, Cagnan H, Mehta AR, Saifee TA, Edwards MJ, Brown P. Distinguishing the central drive to tremor in Parkinson's disease and essential tremor. J Neurosci 2015;35:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haubenberger D, Hallett M. Essential Tremor. N Engl J Med 2018;378:1802–1810. [DOI] [PubMed] [Google Scholar]
- 11. Hassler R, Riechert T. Indications and localization of stereotactic brain operations. Nervenarzt 1954;25:441–447. [PubMed] [Google Scholar]
- 12. Zirh A, Reich SG, Dougherty PM, Lenz FA. Stereotactic thalamotomy in the treatment of essential tremor of the upper extremity: reassessment including a blinded measure of outcome. J Neurol Neurosurg Psychiatry 1999;66:772–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elias WJ, Lipsman N, Ondo WG et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2016;375:730–739. [DOI] [PubMed] [Google Scholar]
- 14. Koller W, Pahwa R, Busenbark K et al. High‐frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann Neurol 1997;42:292–299. [DOI] [PubMed] [Google Scholar]
- 15. Benabid AL, Pollak P, Gervason C et al. Long‐term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991;337:403–406. [DOI] [PubMed] [Google Scholar]
- 16. Schuurman PR, Bosch DA, Bossuyt PM et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 2000;342:461–468. [DOI] [PubMed] [Google Scholar]
- 17. Koller WC, Lyons KE, Wilkinson SB, Pahwa R. Efficacy of unilateral deep brain stimulation of the VIM nucleus of the thalamus for essential head tremor. Mov Disord 1999;14:847–850. [DOI] [PubMed] [Google Scholar]
- 18. Edwards CA, Kouzani A, Lee KH, Ross EK. Neurostimulation devices for the treatment of neurologic disorders. Mayo Clin Proc 2017;92:1427–1444. [DOI] [PubMed] [Google Scholar]
- 19. Kestenbaum M, Ford B, Louis ED. Estimating the proportion of essential tremor and Parkinson's disease patients undergoing deep brain stimulation surgery: five‐year data from Columbia University medical center (2009‐2014). Mov Disord Clin Pract 2015;2:384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanajima R, Chen R, Ashby P et al. Very fast oscillations evoked by median nerve stimulation in the human thalamus and subthalamic nucleus. J Neurophysiol 2004;92:3171–3182. [DOI] [PubMed] [Google Scholar]
- 21. Costa J, Valls‐Sole J, Valldeoriola F, Rumia J. Subcortical interactions between somatosensory stimuli of different modalities and their temporal profile. J Neurophysiol 2008;100:1610–1621. [DOI] [PubMed] [Google Scholar]
- 22. Dideriksen JL, Laine CM, Dosen S et al. Electrical stimulation of afferent pathways for the suppression of pathological tremor. Front Neurosci 2017;11:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gallego JA, Dideriksen JL, Holobar A et al. The phase difference between neural drives to antagonist muscles in essential tremor is associated with the relative strength of supraspinal and afferent input. J Neurosci 2015;35:8925–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin PT, Ross EK, Chidester P et al. Noninvasive neuromodulation in essential tremor demonstrates relief in a sham‐controlled pilot trial. Mov Disord 2018;33:1182–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elble RJ, Pullman SL, Matsumoto JY et al. Tremor amplitude is logarithmically related to 4‐ and 5‐point tremor rating scales. Brain 2006;129:2660–2666. [DOI] [PubMed] [Google Scholar]
- 26. Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol 2011;10:148–161. [DOI] [PubMed] [Google Scholar]
- 27. James KE, Bloch DA, Lee KK, Kraemer HC, Fuller RK. An index for assessing blindness in a multi‐Centre clinical trial: Disulfiram for alcohol cessation–a VA cooperative study. Stat Med 1996;15:1421–1434. [DOI] [PubMed] [Google Scholar]
- 28. Ben‐Menachem E, Manon‐Espaillat R, Ristanovic R et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First international Vagus nerve stimulation study group. Epilepsia 1994;35:616–626. [DOI] [PubMed] [Google Scholar]
- 29. Riederer F, Penning S, Schoenen J. Transcutaneous supraorbital nerve stimulation (t‐SNS) with the Cefaly((R)) device for migraine prevention: a review of the available data. Pain Ther 2015;4:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pathak A, Redmond JA, Allen M, Chou KL. A noninvasive handheld assistive device to accommodate essential tremor: a pilot study. Mov Disord 2014;29:838–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Putzke JD, Wharen RE Jr, Obwegeser AA et al. Thalamic deep brain stimulation for essential tremor: recommendations for long‐term outcome analysis. Can J Neurol Sci 2004;31:333–342. [DOI] [PubMed] [Google Scholar]
- 32. Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg 2014;120:132–139. [DOI] [PubMed] [Google Scholar]
- 33. Koller WC, Vetere‐Overfield B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology 1989;39:1587–1588. [DOI] [PubMed] [Google Scholar]
- 34. Schuhmayer N, Weber C, Kieler M et al. Task‐dependent variability of essential tremor. Parkinsonism Relat Disord 2017;41:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Daneault JF. Could wearable and mobile technology improve the management of essential tremor? Front Neurol 2018;9:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cleeves L, Findley LJ. Variability in amplitude of untreated essential tremor. J Neurol Neurosurg Psychiatry 1987;50:704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Britton TC, Thompson PD, Day BL, Rothwell JC, Findley LJ, Marsden CD. Modulation of postural tremors at the wrist by supramaximal electrical median nerve shocks in essential tremor, Parkinson's disease and normal subjects mimicking tremor. J Neurol Neurosurg Psychiatry 1993;56:1085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooper C, Evidente VG, Hentz JG, Adler CH, Caviness JN, Gwinn‐Hardy K. The effect of temperature on hand function in patients with tremor. J Hand Ther 2000;13:276–288. [DOI] [PubMed] [Google Scholar]
- 39. Pozos RS, Iaizzo PA. Effects of topical anesthesia on essential tremor. Electromyogr Clin Neurophysiol 1992;32:369–372. [PubMed] [Google Scholar]
- 40. Syrkin‐Nikolau J, Neuville R, O'Day J et al. Coordinated reset vibrotactile stimulation shows prolonged improvement in Parkinson's disease. Mov Disord 2017;33:179–180. [DOI] [PMC free article] [PubMed] [Google Scholar]


