Abstract
Background
Although several risk factors for erectile dysfunction may be present in patients with cirrhosis, data on the actual prevalence and cause of erectile dysfunction is limited. The International Index of Erectile Function‐5 (IIEF‐5) is a well‐validated survey to determine the presence and severity of erectile dysfunction in men. We assessed (i) the prevalence and severity of erectile dysfunction, and (ii) risk factors for erectile dysfunction in patients with cirrhosis.
Methods
In this prospective study, erectile dysfunction was defined as: absent (>21 IIEF‐5‐points), mild (12‐21) and severe (5‐11). Patients with overt hepatic encephalopathy, active alcohol abuse, extrahepatic malignancy, previous urologic surgery, previous liver transplantation and severe cardiac conditions were excluded.
Results
Among n = 151 screened patients, n = 41 met exclusion criteria and n = 30 were sexually inactive. Thus, a final number of n = 80 male patients with cirrhosis were included. Patient characteristics: age: 53 ± 9 years; model for end‐stage liver disease score (MELD): 12.7 ± 3.9; Child‐Pugh score (CPS) A: 30 (37.5%), B: 35 (43.8%), C: 15 (18.7%); alcohol: 38 (47.5%), viral: 25 (31.3%), alcohol/viral: 7 (8.8%) and others: 10 (12.5%). The presence of erectile dysfunction was found in 51 (63.8%) patients with 44 (55%) and 7 (8.8%) suffering from mild‐to‐moderate and moderate‐to‐severe erectile dysfunction. Mean MELD and hepatic venous pressure gradient (HVPG) were significantly higher in patients with erectile dysfunction (P = .021; P = .028). Child‐Pugh score C, MELD, creatinine, age, arterial hypertension, diabetes, low libido, low testosterone and high HVPG were associated with the presence of erectile dysfunction. Interestingly, beta‐blocker therapy was not associated with an increased risk. In multivariate models, arterial hypertension (OR: 6.36 [1.16‐34.85]; P = .033), diabetes (OR: 7.40 [1.31‐41.75]; P = .023), MELD (OR: 1.19 [1.03‐1.36]; P = .015) and increasing HVPG (n = 48; OR: 1.11 [1.002‐1.23]; P = .045) were independent risk factors for the presence of erectile dysfunction.
Conclusion
About two‐thirds of male patients with cirrhosis show erectile dysfunction. Severity of liver dysfunction, portal hypertension, arterial hypertension and diabetes were identified as risk factors for erectile dysfunction.
Keywords: cirrhosis, erectile dysfunction, portal hypertension, sexuality
Abbreviations
- ALD
alcoholic liver disease
- BB
beta blocker
- BT
bioavailable testosterone
- CLD
chronic liver disease
- COPD
chronic obstructive pulmonary disease
- CPS
Child‐Pugh score
- CSPH
clinically significant portal hypertension
- EASL
European Association for the Study of the Liver
- ED
erectile dysfunction
- FSH
follicle‐stimulating hormone
- HBV
hepatitis‐B virus
- HRQOL
health‐related quality of life
- HVPG
hepatic venous pressure gradient
- IIEF‐5
international index of erectile function‐5
- LH
luteinizing hormone
- NAFLD
non‐alcoholic fatty liver disease
- OLT
orthotopic liver transplantation
- PDE‐5
phosphodiesterase‐5
- PRL
prolactin
- SHBG
sex hormone binding globulin
- TT
total testosterone
Key points.
Erectile dysfunction is highly prevalent in male cirrhotics.
Liver dysfunction is significantly associated with erectile dysfunction.
Comorbidities such as arterial hypertension and diabetes mellitus play a key role in its evolvement.
Liver dysfunction and portal hypertension as well as arterial hypertension and diabetes mellitus are significant independent markers associated with the presence of erectile dysfunction in multivariate analysis.
1. INTRODUCTION
Cirrhosis is associated with complications such as development of ascites, hepatic encephalopathy (HE) and variceal bleeding.1, 2 Multiple unplanned outpatient visits, high hospitalization rates, and significant morbidity and mortality contribute to the significant socio‐economic burden of cirrhosis. Although health‐related quality of life (HRQOL) is compromised already in the early course of chronic liver disease,3, 4 the presence of ascites, HE and hyponatraemia are associated with further substantial impairments in HRQOL.5 It has also been shown that targeting specific symptoms improves HRQOL.5 Erectile dysfunction (ED) is defined as the inability to attain or maintain a penile erection of sufficient quality to permit satisfactory sexual intercourse.6 Erectile dysfunction itself is an established determinant of HRQOL in men.7, 8, 9 The reported prevalence of ED ranges between 5% and 50% in the general population and is related to age, overall health status and emotional function.7, 8, 9 Multiple endocrine (hypogonadism) and non‐endocrine (vasculogenic, neurogenic and iatrogenic) abnormalities may contribute to the pathogenesis of ED.7, 8, 10 Few studies have assessed the prevalence and risk factors for ED in patients with chronic liver disease.11 High prevalence of ED was shown in alcoholic liver disease (ALD)12, 13 compared to non‐alcoholic liver disease12 suggesting alcohol as a major aetiological factor. However, Wang et al14 found no difference in ED between alcohol vs hepatitis‐B virus‐related cirrhosis and suggested liver disease itself as the driver of ED. Toda et al15 found an increasing prevalence of ED with higher CPS in 53 cirrhotic patients, but only low albumin level and higher age remained significantly associated with ED. Another study16 found no difference in the prevalence of ED between patients with chronic viral hepatitis vs patients with established cirrhosis. In a French study,17 prevalence of sexual impairment in 146 men with chronic hepatitis C (HCV) was 39.7% compared to 6.5% in healthy controls. Multivariate analysis found the presence of HCV infection, age, no sexual intercourse and unemployment as independent predictors of sexual impairment. In cirrhosis, increasing prevalence of ED could be explained through hypogonadism and low testosterone levels, haemodynamic alterations and decreased quality of life although clarifying studies are lacking.
We therefore aimed (i) to evaluate the prevalence and severity of ED and (ii) to assess independent risk factors for ED in a thoroughly documented cohort of male patients with cirrhosis.
2. METHODS
2.1. Patients
A total of 151 inpatients and outpatients between December 2010 and December 2012 were prospectively screened for this study. Inclusion criteria were male sex and cirrhosis (based on either clinical/radiological parameters or liver histology). Exclusion criteria were overt HE, active alcohol abuse (within the previous 3 months), extrahepatic malignancy, previous urologic surgery, previous liver transplantation and severe cardiac conditions. Overt HE was defined by the current practice guidelines and ruled out by the physician performing the screening visit.18 Concomitant diseases (depression, current or history of arterial hypertension (art. HTN), diabetes mellitus (DM) and coronary heart disease), concomitant medication (antidepressants and beta blockers [BB]), sexual hormones (bioavailable testosterone [BT] and total [TT], follicle‐stimulating hormone [FSH], luteinizing hormone [LH], sex hormone binding globulin [SHBG], prolactin [PRL]), CPS and model for end‐stage liver disease (MELD) (calculated as stated in the UNOS 2016 update19) were recorded. Hepatic venous pressure gradient (HVPG) measurement was performed outside of the study. Thus, HVPG data were not available for all patients. Ascites grades were defined according to the European Association for the Study of the Liver guidelines.20
2.2. IIEF‐5 questionnaire
The IIEF‐5 is an abridged 5‐item version of the 15‐item International Index of Erectile Function (IIEF)21 and was developed to diagnose the presence and severity of ED. The 5 items are selected based on the ability to identify the presence or absence of ED in accordance to the definitions of the National Institute of Health for ED. The maximum score is 25 points and the minimum is 5. Erectile dysfunction severity was classified into the following 3 categories based on IIEF‐5 scores: no ED (22‐25 points), mild‐to‐moderate ED (12‐21 points) and moderate‐to‐severe ED (5‐11 points). (See Appendix S1 for IIEF‐5 questionnaire). We additionally evaluated “general sexual desire” by questioning frequency of sexual desire during the last few months. In total, 5 answering options were available and ranged from “almost never or never” (worst possible answer) to “almost always or always” (best possible answer). We then summarized the answers into group “low libido” (answers: “almost never or never”, “rarely”, “sometimes”) and “normal libido” (answers: “most of the times”, “almost always or always”).
2.3. Laboratory analysis
Haematology, blood coagulation, clinical chemistry and hormone analysis (plasma testosterone [free and bioavailable T], LH, FSH, PRL and SHBG levels) were carried out according to standard procedures by the Clinical Institute for Medical and Chemical Laboratory Diagnostics, General Hospital of Vienna.
2.4. HVPG measurement
The right internal jugular vein was accessed under ultrasound guidance and local anaesthesia with the Seldinger technique using a catheter introducer set (8.5 F, Arrow International, Reading, PA, USA). Then, a balloon catheter (7F, Ferlitsch HVPG catheter, Pejcl Medizintechnik, Austria) was used to cannulate the liver vein via the transjugular access as described previously.22, 23 Clinically significant portal hypertension was defined as an HVPG ≥10 mm Hg.24
2.5. Statistics
Continuous variables were reported as mean ± standard deviation (SD) or median (95%CI), and categorical variables were reported as number (n) of patients with the certain characteristic (proportion of patients with the certain characteristics[%]). Student's t test was used for group comparisons of normally distributed data, and Mann‐Whitney U test was used when data were not normally distributed. Kruskal‐Wallis H test with post hoc comparisons were used to compare medians in groups of 3 or more. The significance level was adjusted using the Bonferroni method. Pearson's chi‐square test or Fisher's exact test was performed to calculate group comparisons between patients with and without ED. Multivariate binary logistic stepwise‐backwards regression models were used to determine independent risk factors for presence of ED. Firstly, univariate binary regression analysis was used for each relevant variable. Then, a multivariate model was calculated including all significant variables from the univariate analysis (MELD was chosen over CPS to avoid multicollinearity). Given the small sample size of patients with available HVPG (n = 48), a second model including HVPG and the variables that were significant in Model 1 was used. HVPG was chosen over MELD as the variable to reflect liver function to avoid multicollinearity. Model 1 included MELD, albumin, age, art. HTN, DM, libido, previous hepatic decompensation and BT. Model 2 included art. HTN, DM and HVPG. Since a stepwise‐backwards binary logistic regression was used, we showed odds ratios and P values of all variables that were initially included in the models (“first step”) and of variables that remained significant after backward elimination of all non‐significant variables (“last step”). Two‐sided P values <.05 were considered as statistically significant. The IBM SPSS 24.0 statistic software (SPSS Inc., Armonk, NY, USA) was used for all statistical analysis.
2.6. Ethics
This study was approved by the Ethics committee of the Medical University of Vienna (Study Number: 450/2010) and performed in accordance to the current version of the Helsinki Declaration. All patients signed an informed consent form prior to study inclusion.
3. RESULTS
Among 110 patients included in the study, 30 reported sexual inactivity; 13/30 patients stated that a missing partner and/or unwillingness for sexual intercourse by their partner was the reason for sexual inactivity and 17/30 patients were sexually inactive. Sexual inactivity was not significantly different between CPS stages (CPS A: 3 [17.6%], B: 8 [47.1%] and C: 6 [35.3%]; P = .180).
Eighty sexually active patients were therefore included in the final analysis. Erectile dysfunction (≤21 points) was present in 51 (63.8%) of patients, and within those 44 (55%) were classified as mild‐to‐moderate and 7 (8.8%) as moderate‐to‐severe. The main patient characteristics are presented in Table 1. The study flow chart is presented in Figure 1. Given the exploratory character of the study and conflicting data on phosphodiesterase‐5 (PDE‐5) inhibitors in cirrhosis, therapy of ED was not part of the study.
Table 1.
General study population
| Overall (n = 80) | CPS A (n = 30) | CPS B (n = 35) | CPS C (n = 15) | P‐value | |
|---|---|---|---|---|---|
| IIEF‐5, median, (95% CI) | 20 (10‐24) | 21 (11‐24.5) | 19 (10.6‐25) | 16 (9‐16) | .038 |
| Erectile dysfunction, n (%) | 51 (63.8%) | 16 (53.5%) | 22 (62.9%) | 13 (86.7%) | .089 |
| ED severity, n (%) | |||||
| No ED | 29 (36.3%) | 14 (46.7%) | 13 (37.1%) | 2 (13.3%) | .037 |
| Mild‐to‐moderate ED | 44 (55%) | 15 (50%) | 20 (57.1%) | 9 (60%) | |
| Moderate‐to‐severe ED | 7 (8.8%) | 1 (3.3%) | 2 (5.7%) | 4 (26.7%) | |
| Active Relationship, n (%) | 64 (80%) | 24 (80%) | 27 (77%) | 13 (87%) | .593 |
| BT, median (95%CI) | 1.31 (0.14‐2.59) | 1.68 (1.16‐3.51) | 1.2 (0.2‐2.38) | 0.48 (0.12‐0.48) | <.001 |
| TT, median (95% CI) | 5.98 (0.57‐12.46) | 7.32 (4.20‐13.94) | 4.66 (1.14‐9.94) | 2.41 (0.25‐2.41) | <.001 |
| Libido, n (%) | |||||
| Normal | 26 (32.5%) | 13 (50%) | 11 (42.3%) | 2 (7.7%) | .126 |
| Impaired | 54 (67.5%) | 17 (31.5%) | 24 (44.4%) | 13 (24.1%) | |
| Beta‐blockera, n (%) | 40 (53.3%) | 14/28 (46.7%) | 18/33 (51.4%) | 8/14 (53.3%) | .893 |
| Depression, n (%) | 13 (16.3%) | 5 (16.7%) | 7 (20%) | 1 (6.7%) | .502 |
| Anti depressive medication, n (%) | 7 (8.8%) | 4 (13.3%) | 3 (8.6%) | 0 (0%) | .328 |
| Active smoker, n (%) | No: 47 (58.8%) | 14 (29.8%) | 23 (48.9%) | 10 (21.3%) | .235 |
| Yes: 33 (41.3%) | 16 (48.5%) | 12 (36.4%) | 5 (15.2%) | ||
| Art. HTN, n (%) | 26 (32.5%) | 11 (36.7%) | 12 (34.3%) | 3 (20%) | .507 |
| Diabetes, n (%) | 25 (31.3%) | 7 (23.3%) | 14 (40%) | 4 (26.7%) | .322 |
| CVD, n (%) | 4 (5%) | 1 (3.3%) | 3 (8.6%) | 0 (0%) | .386 |
| BMI, mean ± SD | 26.7 ± 4.2 | 26.5 ± 4.7 | 26.7 ± 4.1 | 26.8 ± 3.6 | 1.0 |
| Age, mean ± SD | 53 ± 9 | 50 ± 9 | 55 ± 9 | 53 ± 10 | A vs B: 0.138 A vs C: 0.852 B vs C: 1.0 |
BT, bioavailable testosterone; CPS, Child‐Pugh score; ED, erectile dysfunction;; IIEF‐5, international index of erectile function‐5; TT, total testosterone.
BB status was available in 75 patients; in 5 patients, no information on BB intake was available owing to uncertain duration and frequency of BB intake.
Figure 1.
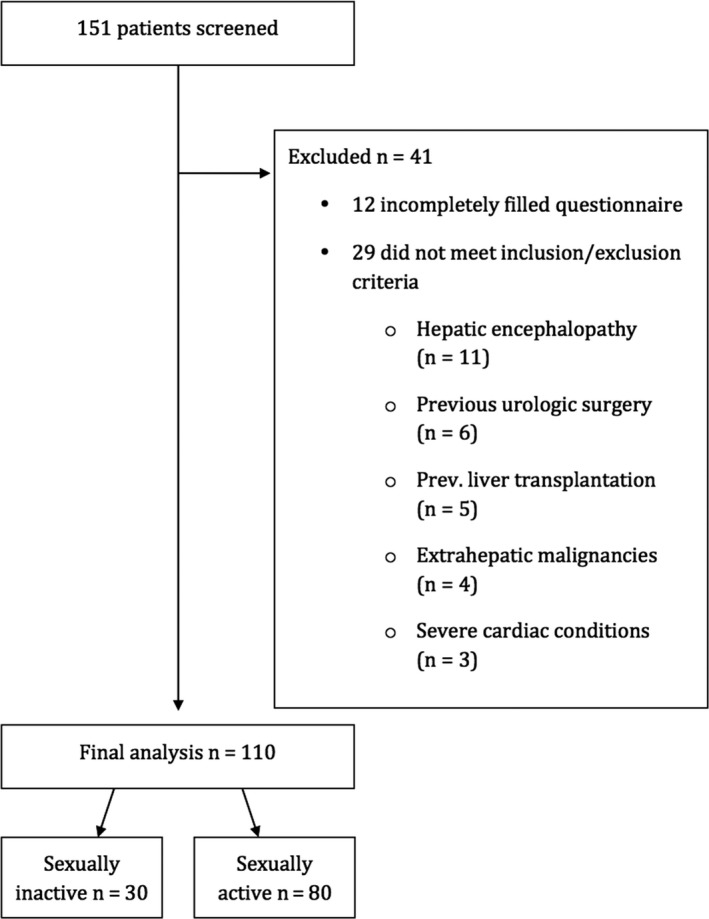
Flow chart diagram
3.1. Erectile dysfunction and cirrhosis
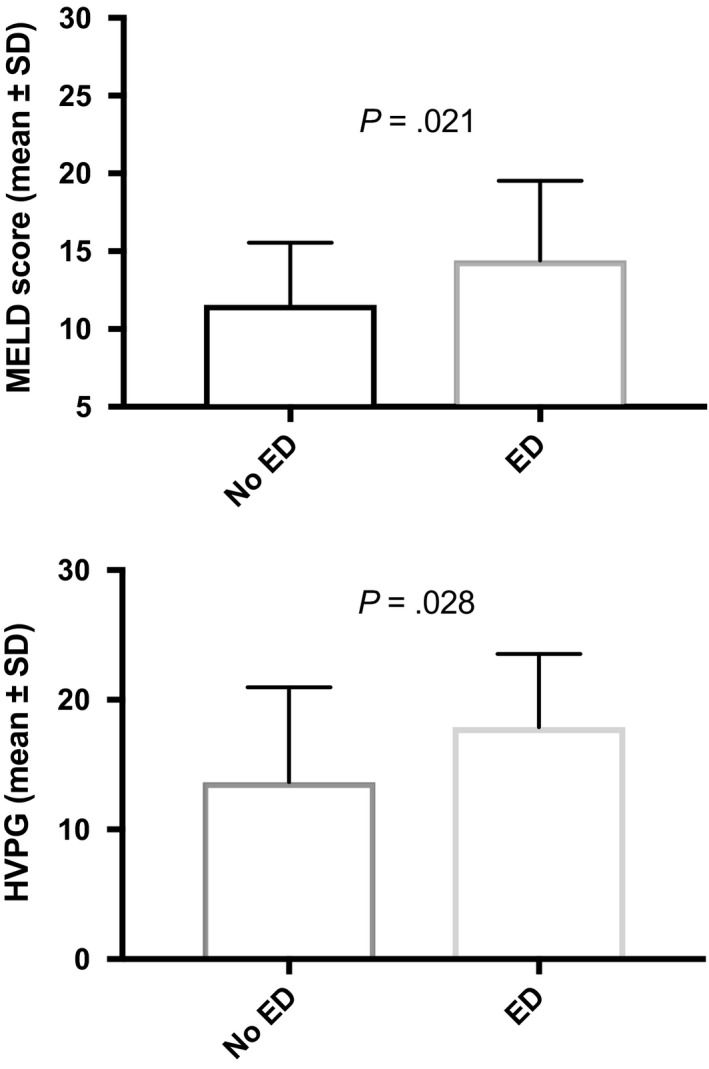
Erectile dysfunction severity was significantly increasing throughout the CPS stages (P = .037, Table 1) and median IIEF‐5 scores were significantly decreasing with worsening of liver function (P = .038). The mean MELD was significantly higher in patients with ED (Figure 3). Also, the MELD increased significantly with severity of ED (no ED: 11.3 ± 3.7; mild‐to‐moderate ED 13 ± 3.9 and moderate‐to‐severe 16.2 ± 2.7 [no vs mild‐to‐moderate: P = .071; no vs moderate‐to‐severe: P = .003; mild‐to‐moderate vs moderate‐to‐severe: P = .037]). Causes of cirrhosis were ALD 38 (47.5%), viral 25 (31.3%), viral/ALD 7 (8.8%) and others 10 (12.5%), and distribution was similar between groups (P = .491).
Figure 3.
Distribution of comorbidities over erectile dysfunction
3.2. Erectile dysfunction and portal hypertension
Thirty‐seven patients had previous portal hypertension‐related complications (18 [22.5%] variceal bleeding, 6 [7.5%] episodes of HE, 12 [15%] ascites and/or spontaneous bacterial peritonitis and 1 [1.3%] jaundice). Previous episodes of hepatic decompensation were significantly more common in patients with ED (55% vs 31%); P = .040, Table 2). Information on oesophageal varices was available in 74 patients (6 had unknown variceal status and had no endoscopy performed in our hospital records). Forty‐five (60.8%) patients had oesophageal varices and no significant difference was found between groups (P = .483; Table 2).
Table 2.
Main characteristics of patients with and without erectile dysfunction
| No ED (>21 pts) (n = 29) | ED (≤21 pts) (n = 51) | P‐value | |
|---|---|---|---|
| Child‐Pugh score, n (%) | |||
| A: 30 (37.5%) | 14 (48.3%) | 16 (31.4%) | .089 |
| B: 35 (43.75%) | 13 (44.8%) | 22 (43.1%) | |
| C: 15 (18.75%) | 2 (6.9%) | 13 (25.5%) | |
| MELD, mean ± SD | 11.3 ± 3.6 | 13.4 ± 3.9 | .021 |
| Aetiologies, n (%) | |||
| ALD: 38 (47.5%) | 11 (37.9%) | 27 (52.9%) | .491 |
| Viral: 25 (31.3%) | 10 (34.5%) | 15 (29.4%) | |
| Viral/ALD: 7 (8.8%) | 4 (13.8%) | 3 (5.9%) | |
| Others: 10 (12.5%) | 4 (13.8%) | 6 (11.8%) | |
| HVPG, mean ± SDa | 13.6 ± 7.3 | 17.9 ± 5.6 | .028 |
| Previous hepatic decompensation, n (%) | 9 (31%) | 28 (55%) | .040 |
| Presence of oesophageal varices, n (%)b | 15 (57%) | 30 (64%) | .483 |
| HCC baseline, n (%) | |||
| No: 71 (88.8%) | 27 (93.1%) | 44 (86.3%) | .353 |
| Yes: 9 (11.2%) | 2 (6.9%) | 7 (13.7%) | |
| Age, mean ± SD | 49 (±10.4) | 55 (±8) | .006 |
| BMI, mean ± SD | 25.6 (±4) | 27.2 (±4.2) | .091 |
| Art. HTN, n (%) | |||
| No: 54 (67.5%) | 25 (86.2%) | 29 (56.9%) | .007 |
| Yes: 26 (32.5%) | 4 (13.8%) | 22 (43.1%) | |
| Diabetes, n (%) | |||
| No: 55 (68.8%) | 25 (82.8%) | 30 (58.8%) | .011 |
| Yes: 25 (31.2%) | 4 (17.2%) | 21 (41.2%) | |
| Depression, n (%) | |||
| No: 67 (83.8%) | 25 (86.2%) | 42 (82.4%) | .653 |
| Yes: 13 (16.3%) | 4 (13.8%) | 9 (17.6%) | |
| Antidepressive medication, n (%) | |||
| No: 73 (91.3%) | 27 (93.1%) | 46 (90.2%) | .658 |
| Yes: 7 (8.8%) | 2 (6.9%) | 5 (9.8%) | |
| Beta‐blockerc (n = 75), n (%) | |||
| No: 35 (46.7%) | 16 (57.1%) | 19 (40.4%) | .160 |
| Yes: 40 (53.3%) | 12 (42.9%) | 28 (59.6%) | |
| Active Smoker, n (%) | |||
| No: 47 (58.8%) | 13 (44.8%) | 34 (66.7%) | .056 |
| Yes: 33 (41.3%) | 16 (55.2%) | 17 (33.3%) | |
| Libido, n (%) | |||
| Normal | 17 (58.6%) | 9 (17.6%) | <.001 |
| Impaired | 12 (41.4%) | 42 (82.4%) | |
| BT, median (95%CI) | 1.47 (0.18‐3.68) | 1.2 (0.13‐1.97) | .051 |
| TT, median (95%CI) | 7.32 (1‐12.87) | 4.73 (0.52‐10.92) | .026 |
| PRL, median (95%CI) | 12.6 (5.13‐38.9) | 11.4 (4.3‐40.9) | .993 |
| LH, median (95%CI) | 6.25 (1.44‐12.3) | 6.3 (1.42‐16.64) | .580 |
| FSH, median (95%CI) | 6.85 (2.82‐27.93) | 7.1 (2‐28.64) | .857 |
| SHBG, mean ± SD | 108.18 ± 48.2 | 93.43 ± 36.8 | .204 |
BT, bioavailable testosterone; ED, erectile dysfunction; FSH, follicle‐stimulating hormone; HVPG, hepatic venous pressure gradient; LH, luteinizing hormone; MELD, model for end‐stage liver disease score; PRL, prolactin; SHBG, sex hormone binding globulin; TT, total testosterone.
HVPG was available in 48 patients.
Status on oesophageal varices was available in 74 patients.
BB status was available in 75 patients, in 5 patients no information on BB intake was available owing to uncertain duration and frequency of BB intake.
Information on HVPG values was available in a subgroup of 48 patients. Mean HVPG was 16.2 ± 6.6 mm Hg. Absolute HVPG was significantly higher in patients with ED (17.9 ± 5.6 vs 13.6 ± 7.3, P = .028, Figure 3). HVPG was independently associated with presence of ED in our multivariate model (OR: 1.11 (1.002‐1.23); P = .045; Table 3).
Table 3.
Univariate and multivariate binary regression analysis to determine independent risk factors for presence of erectile dysfunction
| Univariate OR (95% CI) | Univariate P‐value | MV Model 1, First‐Step OR (95% CI); P‐value | MV Model 1, Last‐Step OR (95% CI); P‐value | MV Model 2, First‐Step OR (95% CI); P‐value | MV Model 2, Last‐Step OR (95% CI); P‐value | |
|---|---|---|---|---|---|---|
| CPS | B:1.48 (0.55‐3.99) | .438 | ||||
| C: 5.687 (1.09‐29.7) | .039 | |||||
| MELD | 1.15 (1.02‐1.28) | .019 | 1.2 (0.99‐1.46); P = .059 | 1.19 (1.03‐1.36); P = .015 | ||
| HVPGa | 1.11 (1.007‐1.232) | .036 | 1.10 (0.99‐1.22); P = .068 | 1.11 (1.002‐1.23); P = .045 | ||
| Previous hepatic decompensation | 2.71 (1.04‐7.07) | .042 | 2.5 (0.59‐10.9); P = .207 | |||
| Presence of esophageal varicesb | 1.41 (0.54‐3.7) | .483 | ||||
| Creatinine | 22.29 (1.4‐354.6) | .028 | ||||
| Albumin | 0.915 (0.84‐0.99) | .038 | 1.07 (0.89‐1.28); P = .48 | |||
| Age | 1.078 (1.018‐1.141) | .010 | 1.07 (0.98‐1.18); P = .129 | 1.08 (0.99‐1.18); P = .086 | ||
| BMI | 1.106 (0.982‐1.246) | .096 | ||||
| Art. HTN | 4.74 (1.44‐15.62) | .011 | 4.86 (0.83‐26.6); P = .080 | 6.36 (1.16‐34.85); P = .033 | 2.94 (0.64‐13.58); P = .167 | |
| DM | 4.37 (1.33‐14.44) | .015 | 7.98 (1.22‐52.31); P = .030 | 7.4 (1.31‐41.75); P = .023 | 3.42 (0.59‐19.87); P = .172 | 4.29 (0.79‐23.37); P = .092 |
| BBc | 1.97 (0.76‐5.07) | .163 | ||||
| HCC | 2.15 (0.42‐11.1) | .362 | ||||
| Anti‐Depressive Medication | 1.47 (0.27‐8.09) | .660 | ||||
| Depression | 1.34 (0.37‐4.81) | .654 | ||||
| Active Smoker | 0.41 (0.16‐1.035) | .059 | ||||
| Impaired Libido | 6.61 (2.36‐18.55) | <.001 | 2.44 (0.57‐10.51); P = .23 | |||
| BT | 0.401 (0.18‐0.91) | .028 | 1.09 (0.28‐4.25); P = .90 | |||
| TT | 0.837(0.71‐0.98) | .029 | ||||
| PRL | 1.01 (0.959‐1.06) | .715 | ||||
| LH | 1.06 (0.93‐1.21) | .379 | ||||
| FSH | 1.009 (0.945‐1.077) | .785 | ||||
| SHBG | 0.991 (0.979‐1.004) | .163 |
MV Model 1 First‐step: Odds‐ratio for all variables included in the multivariate binary regression model (stepwise‐backwards; variables: MELD, Albumin, Age, art. HTN, DM, Libido, previous hepatic decompensation and BT).
MV Model 1 Last‐step: Odds‐ratio for variables still significantly associated with presence of ED in the last‐step of the multivariate binary regression model (stepwise‐backwards).
MV Model 2 First‐step: Odds‐ratio for all variables included in the multivariate binary regression model in the subgroup of patients with available HVPG (stepwise‐backwards; variables: art. HTN, DM, HVPG).
MV Model 2 Last‐step: Odds‐ratio for variables still significantly associated with present of ED in the last‐step of the multivariate binary regression model in the subgroup of patients with available HVPG.
HVPG was available in 48 patients.
Status on esophageal varices was available in 74 patients.
BB status was available in 75 patients, in 5 patients no information on BB intake was available due to uncertain duration and frequency of BB intake.
3.3. Erectile dysfunction and comorbidities
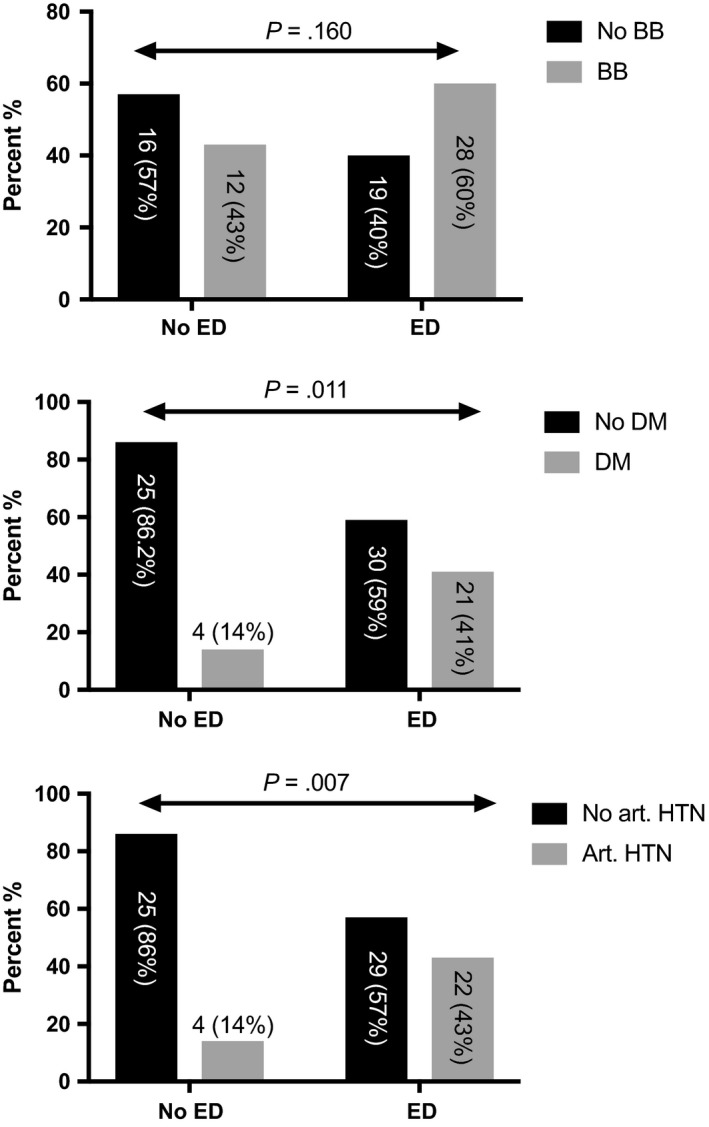
Twenty‐five (31.2%) patients had diabetes and 26 (32.5%) had a history/current arterial hypertension at study inclusion and both factors were significantly associated with presence of ED (OR: 4.38 [1.33‐14.44] P = .011; OR: 4.74 [1.44‐15.62] P = .007; Table 2, Figure 4). The mean age was significantly higher in patients with ED (49 vs 55 years; P = .006). BB status was available in 75 patients. In 5 patients, no information on BB intake was available owing to uncertainties regarding the duration and frequency of BB intake. Importantly, BB intake was not associated with ED (P = .160, Figure 4). Other comorbidities such as depression, intake of antidepressive medication, smoking and high BMI were not associated with the presence of ED (Table 2), while we observed an association between low libido and ED (P < .001).
Figure 4.
Distribution of model for end‐stage liver disease score (mean ± SD) and hepatic venous pressure gradient (mean ± SD) over erectile dysfunction
3.4. Erectile dysfunction and sexual hormones
PRL, LH, FSH and SHBG did not differ between patients with ED, or without ED (Table 2). TT (P = .026) was significantly lower in ED, whereas BT marginally missed significance (P = .051). Both BT and TT significantly decreased over CPS stages (P < .001, Table 1).
3.5. Uni‐ and multivariate binary regression analysis
Child‐Pugh score C, MELD, previous hepatic decompensation, creatinine, age, arterial hypertension, diabetes, low libido, low BT and increasing HVPG were associated with the presence of ED in univariate binary regression analysis (Table 3). In multivariate models, arterial hypertension (OR: 6.36 [1.16‐34.85]; P = .033), diabetes (OR: 7.4 [1.31‐41.75]; P = .023), increasing MELD score (OR: 1.19 [1.03‐1.36]; P = .015) and increasing HVPG (n = 48; OR: 1.11 [1.002‐1.23]; P = .045) were independent risk factors for the presence of ED (Table 3, Models 1 and 2).
4. DISCUSSION
In this study, we investigated the prevalence of ED, and moreover, whether cirrhosis itself, comorbidities or the combination of both drive the development of ED. Hence, we could show that liver dysfunction, an increasing HVPG as well as arterial hypertension and diabetes mellitus seem to be the key risk factors for ED in male cirrhotics (Table 3). Since our data suggests history and/or current arterial hypertension as a significant risk factor for ED, one could argue that our fairly well cohort (none with HE, mean MELD 12.7) explains the 33% classified with arterial hypertension. Significantly, higher MELD scores and absolute HVPG values were found in patients with ED (Figure 3). In the subgroup of patients (n = 48) with available information on HVPG, increasing absolute HVPG levels were independently predictive of ED, suggesting that portal hypertension is of relevance. This association could be explained because of the altered haemodynamic state in splanchnic circulation in patients with portal hypertension. Since non‐endocrine vasculogenic disorders can cause ED and this is attributed to either arterial inflow or venous outflow disorders7, one could argue that these haemodynamic alterations directly impair physiological penile erection. Previous studies found conflicting results with regard to the impact of chronic liver disease (CLD)/cirrhosis on prevalence and severity of ED. Whether cirrhosis is the major driver of ED in those patients has not yet been clarified. The presence of comorbidities and type of CLD was not significantly associated with ED in 1 study,16 whereas alcohol intake, tobacco use and cardiovascular disease was found to be the only significant risk factor for ED in OLT candidates.25 Serum albumin and age have been described as significant independent factors for ED (64 chronic hepatitis and 53 cirrhosis).15Another study compared ED in alcoholics with vs without cirrhosis vs diabetics and found no difference between the groups.13 Only 1 study reported multivariate data in a relatively small cohort of 69 patients (34 with cirrhosis) and found cirrhosis, arterial hypertension, depression and serum albumin as significant independent factors.26 However, a different IIEF‐5 cut‐off than suggested in the literature was used. We found multifactorial disorders significantly associated with the presence of ED indicating that not only comorbidities, but also liver disease contributes towards evolvement (Table 3, Figures 2, 3, 4). No significant difference in the distribution of BB intake was seen in patients with or without ED (Figure 4). This is especially interesting, since a majority of patients with cirrhosis receive BB to decrease portal pressure and prevent variceal (re)bleeding.27, 28 However, a large meta‐analysis29 did not support the conventional judgement of clinicians that BB therapy is associated with a relevant risk of sexual dysfunction and our data is in line with that conclusion.
Figure 2.
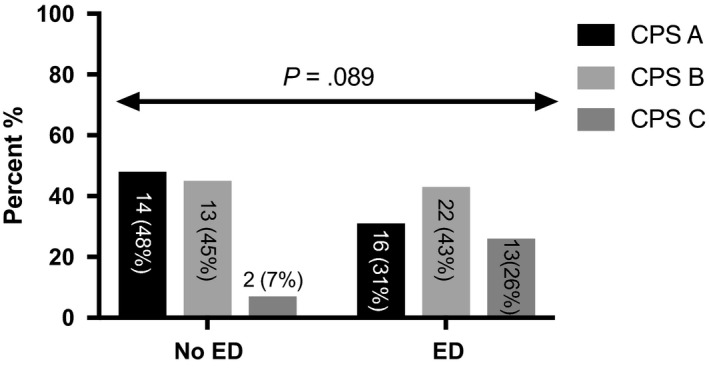
Distribution of Child‐Pugh score over erectile dysfunction
Age, overall health status and emotional function have been strongly related to the presence of ED in a large epidemiological study.30 Furthermore, hypogonadism and especially low testosterone levels have been described in patients with cirrhosis and it is thought that this could be an explanation for the high prevalence of ED.11 However, oral testosterone supplementation seemed to have no effect on sexual dysfunction in cirrhosis.11, 31 Intramuscular testosterone supplementation showed positive effects on muscle and bone mass32 but data on sexual function was not described, and thus, further studies on the possible improvement of ED are warranted. Another hypothesis suggests that reduced serum albumin may affect the ratio of free albumin to bound testosterone with a possible altered testosterone response.11 Even though we found significantly lower testosterone values in patients with ED, this was not significantly associated with the presence of ED in multivariate analysis. About 45% of testosterone is bound to SHBG, another 50% to albumin and approximately 2% circulates as free testosterone. BT sums up free testosterone and albumin‐bound testosterone. Thus, testosterone in the blood stream is highly protein‐bound.33 There is controversy whether TT or BT is the better way to measure bioactive testosterone in cirrhotic men. Hyperoestrogenism and low androgens, as part of a negative‐feedback loop, contribute towards increased production of SHBG, which then leads to reduction in biologically active free testosterone because of increased binding,33, 34 and hence one could argue that free testosterone is therefore underestimating the “real” testosterone status. Nevertheless, it has been suggested that free testosterone /BT is a better marker of bioactive androgens than TT especially in states of altered albumin and SHBG production.33 Regarding measurement of overall testosterone in cirrhosis, TT seems to be the more effective marker because it measures testosterone levels independently of alterations in protein production (SHBG‐bound, albumin‐bound and free). Studies evaluating sexual dysfunction before and after orthotopic liver transplantation (OLT) found controversial results35, 36, 37, 38 and the prevalence seems to be similar to prior OLT.39 Arterial hypertension and diabetes have been previously associated with ED10, 40, 41 and also studies in other chronic disease such as chronic obstructive pulmonary disease found high prevalence rates.42 Depression43 and low HRQOL44 were associated with ED in chronic hepatitis C patients and we could show in our cohort that low libido, most likely owing to the burden of the disease, is significantly decreased in patients with ED. With regard to aetiology, we found no difference in the distribution of ED (Table 2). The prevalence of ED in alcoholic cirrhosis is described with 50%‐70%12, 13, 14 and with 40%‐92% in viral cirrhosis.14, 15, 16, 26 In chronic hepatitis, the prevalence ranged between 9% and 78%15, 26 with most studies reporting prevalence rates around 40%‐50%.16, 17, 43, 44, 45 Hence, it seems that once cirrhosis develops, the prevalence of ED is high irrespective of aetiology and this is also confirmed by our data. To our best knowledge, only 2 studies investigated ED in non‐alcoholic fatty liver disease and found a prevalence of 45%46 and 67.5%47 respectively. It was interesting to note that in the latter study metabolic syndrome was present in 57.5% of patients and was diagnosed more common in patients with ED and this is in line with the results presented in our study. PDE‐5 inhibitors have been studied to lower portal pressure in cirrhosis but results are conflicting.48, 49, 50, 51 Furthermore, decrease in arterial blood pressure was reported.51 No studies investigated the effect of PDE‐5 inhibitors on ED in cirrhosis. Such studies are highly warranted although the previously reported negative effect on systemic blood pressure advises caution.
A substantial bias of studies investigating subjective matters such as ED is always underreporting and/or sugar‐coated information. Talking about sexuality per se is often an unsaid taboo in modern society and ED is associated with stigma. Furthermore, a selection bias regarding patients with very severe disease, mostly CPS C, cannot be avoided since most of those patients either present with overt hepatic encephalopathy (and were not included in our study because of potentially unreliable answers) and/or sexual inactivity. Owing to the social stigma of severe impaired sexual function, one could argue that patients with most severe liver disease suffer from sexual inactivity because of their severe disease and declined to participate in the study. Although this might be true, sexual inactivity is hard to account for because the IIEF‐5 was not designed to measure sexual inactivity and secondly because patients might sugarcoat information. Nevertheless, in our cohort, 30 patients reported sexual inactivity but no association with severity of liver disease was seen, though further evaluation of sexual inactivity should be part of future studies.
In conclusion, ED in patients with cirrhosis is highly prevalent. Our results indicate that along with commonly known risk factors such as arterial hypertension and diabetes mellitus, liver dysfunction and portal hypertension too play a key role in the evolvement of ED. Studies evaluating the effect of PDE‐5 inhibitors on ED in cirrhosis are highly warranted.
CONFLICT OF INTEREST
The authors do not have any disclosures to report.
Supporting information
Paternostro R, Heinisch BB, Reiberger T, et al. Erectile dysfunction in cirrhosis is impacted by liver dysfunction, portal hypertension, diabetes and arterial hypertension. Liver Int. 2018;38:1427–1436. 10.1111/liv.13704
Funding information
The authors have nothing to disclose regarding the work under consideration for publication.
Handling Editor: Christophe Bureau
Rafael Paternostro and Birgit B. Heinisch have contributed equally to the preparation of the manuscript.
REFERENCES
- 1. de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743‐752. [DOI] [PubMed] [Google Scholar]
- 2. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749‐1761. [DOI] [PubMed] [Google Scholar]
- 3. Mandorfer M, Payer BA, Scheiner B, et al. Health‐related quality of life and severity of fatigue in HIV/HCV co‐infected patients before, during, and after antiviral therapy with pegylated interferon plus ribavirin. Liver Int. 2014;34:69‐77. [DOI] [PubMed] [Google Scholar]
- 4. Scheiner B, Schwabl P, Steiner S, et al. Interferon‐free regimens improve health‐related quality of life and fatigue in HIV/HCV‐coinfected patients with advanced liver disease: a retrospective study. Medicine. 2016;95:e4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orr JG, Homer T, Ternent L, et al. Health related quality of life in people with advanced chronic liver disease. J Hepatol. 2014;61:1158‐1165. [DOI] [PubMed] [Google Scholar]
- 6. NIH Consensus Conference . Impotence. NIH consensus development panel on impotence. JAMA. 1993;270:83‐90. [PubMed] [Google Scholar]
- 7. Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McVary KT. Clinical practice. Erectile dysfunction. N Engl J Med. 2007;357:2472‐2481. [DOI] [PubMed] [Google Scholar]
- 9. Montorsi F, Briganti A, Salonia A, et al. The ageing male and erectile dysfunction. BJU Int. 2003;92:516‐520. [DOI] [PubMed] [Google Scholar]
- 10. Lue TF. Erectile dysfunction. The New England Journal of Medicine. 2000;342:1802‐1813. [DOI] [PubMed] [Google Scholar]
- 11. Durazzo M, Premoli A, Di Bisceglie C, et al. Male sexual disturbances in liver diseases: what do we know? J Endocrinol Invest. 2010;33:501‐505. [DOI] [PubMed] [Google Scholar]
- 12. Cornely CM, Schade RR, Van Thiel DH, Gavaler JS. Chronic advanced liver disease and impotence: cause and effect? Hepatology. (Baltimore, Md). 1984;4:1227‐1230. [DOI] [PubMed] [Google Scholar]
- 13. Jensen SB, Gluud C. Sexual dysfunction in men with alcoholic liver cirrhosis. A Comparative Study. Liver. 1985;5:94‐100. [DOI] [PubMed] [Google Scholar]
- 14. Wang YJ, Wu JC, Lee SD, Tsai YT, Lo KJ. Gonadal dysfunction and changes in sex hormones in postnecrotic cirrhotic men: a matched study with alcoholic cirrhotic men. Hepatogastroenterology. 1991;38:531‐534. [PubMed] [Google Scholar]
- 15. Toda K, Miwa Y, Kuriyama S, et al. Erectile dysfunction in patients with chronic viral liver disease: its relevance to protein malnutrition. J Gastroenterol. 2005;40:894‐900. [DOI] [PubMed] [Google Scholar]
- 16. Simsek I, Aslan G, Akarsu M, Koseoglu H, Esen A. Assessment of sexual functions in patients with chronic liver disease. Int J Impot Res. 2005;17:343‐345. [DOI] [PubMed] [Google Scholar]
- 17. Vergniol J, Duc S, Hou G, et al. Sexual quality of life is impaired in patients with chronic hepatitis C. Int J Impot Res. 2016;28:68‐73. [DOI] [PubMed] [Google Scholar]
- 18. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology (Baltimore, Md). 2014;60:715‐735. [DOI] [PubMed] [Google Scholar]
- 19. United Network for Organ Sharing (UNOS) . https://optn.transplant.hrsa.gov/media/1575/policynotice_20151101.pdf.
- 20. EASL . Clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397‐417. [DOI] [PubMed] [Google Scholar]
- 21. Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822‐830. [DOI] [PubMed] [Google Scholar]
- 22. Ferlitsch M, Reiberger T, Hoke M, et al. von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology (Baltimore, Md). 2012;56:1439‐1447. [DOI] [PubMed] [Google Scholar]
- 23. Ferlitsch A, Bota S, Paternostro R, et al. Evaluation of a new balloon occlusion catheter specifically designed for measurement of hepatic venous pressure gradient. Liver Int. 2015;35:2115‐2120. [DOI] [PubMed] [Google Scholar]
- 24. Peck‐Radosavljevic M, Angermayr B, Datz C, et al. Austrian consensus on the definition and treatment of portal hypertension and its complications (Billroth II). Wien Klin Wochenschr. 2013;125:200‐219. [DOI] [PubMed] [Google Scholar]
- 25. Huyghe E, Kamar N, Wagner F, et al. Erectile dysfunction in end‐stage liver disease men. J Sex Med. 2009;6:1395‐1401. [DOI] [PubMed] [Google Scholar]
- 26. Kim M, Kim SY, Rou WS, Hwang SW, Lee BS. Erectile dysfunction in patients with liver disease related to chronic hepatitis B. Clin Mol Hepatol. 2015;21:352‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mandorfer M, Reiberger T. Beta blockers and cirrhosis, 2016. Dig Liver Dis. 2017;49:3‐10. [DOI] [PubMed] [Google Scholar]
- 28. Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J Hepatol. 2017;66:849‐859. [DOI] [PubMed] [Google Scholar]
- 29. Ko DT, Hebert PR, Coffey CS, et al. Beta‐blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA. 2002;288:351‐357. [DOI] [PubMed] [Google Scholar]
- 30. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;151:54‐61. [DOI] [PubMed] [Google Scholar]
- 31. Gluud C, Wantzin P, Eriksen J. No effect of oral testosterone treatment on sexual dysfunction in alcoholic cirrhotic men. Gastroenterology. 1988;95:1582‐1587. [DOI] [PubMed] [Google Scholar]
- 32. Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol. 2016;65:906‐913. [DOI] [PubMed] [Google Scholar]
- 33. Sinclair M, Grossmann M, Gow PJ, Angus PW. Testosterone in men with advanced liver disease: abnormalities and implications. J Gastroenterol Hepatol. 2015;30:244‐251. [DOI] [PubMed] [Google Scholar]
- 34. Foresta C, Schipilliti M, Ciarleglio FA, Lenzi A, D'Amico D. Male hypogonadism in cirrhosis and after liver transplantation. J Endocrinol Invest. 2008;31:470‐478. [DOI] [PubMed] [Google Scholar]
- 35. Park ES, Villanueva CA, Viers BR, Siref AB, Feloney MP. Assessment of sexual dysfunction and sexually related personal distress in patients who have undergone orthotopic liver transplantation for end‐stage liver disease. J Sex Med. 2011;8:2292‐2298. [DOI] [PubMed] [Google Scholar]
- 36. Burra P, Germani G, Masier A, et al. Sexual dysfunction in chronic liver disease: is liver transplantation an effective cure? Transplantation. 2010;89:1425‐1429. [DOI] [PubMed] [Google Scholar]
- 37. Sorrell JH, Brown JR. Sexual functioning in patients with end‐stage liver disease before and after transplantation. Liver Transpl. 2006;12:1473‐1477. [DOI] [PubMed] [Google Scholar]
- 38. Burra P. Sexual dysfunction after liver transplantation. Liver Transpl. 2009;15(Suppl 2):S50‐S56. [DOI] [PubMed] [Google Scholar]
- 39. Huyghe E, Kamar N, Wagner F, et al. Erectile dysfunction in liver transplant patients. Am J Transplant. 2008;8:2580‐2589. [DOI] [PubMed] [Google Scholar]
- 40. Bjerggaard M, Charles M, Kristensen E, et al. Prevalence of sexual concerns and sexual dysfunction among sexually active and inactive men and women with screen‐detected type 2 diabetes. Sex Med. 2015;3:302‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nunes KP, Labazi H, Webb RC. New insights into hypertension‐associated erectile dysfunction. Curr Opin Nephrol Hypertens. 2012;21:163‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Turan O, Ure I, Turan PA. Erectile dysfunction in COPD patients. Chron Respir Dis. 2016;13:5‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma BO, Shim SG, Yang HJ. Association of erectile dysfunction with depression in patients with chronic viral hepatitis. World J Gastroenterol. 2015;21:5641‐5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Danoff A, Khan O, Wan DW, et al. Sexual dysfunction is highly prevalent among men with chronic hepatitis C virus infection and negatively impacts health‐related quality of life. Am J Gastroenterol. 2006;101:1235‐1243. [DOI] [PubMed] [Google Scholar]
- 45. Ferri C, Bertozzi MA, Zignego AL. Erectile dysfunction and hepatitis C virus infection. JAMA. 2002;288:698‐699. [DOI] [PubMed] [Google Scholar]
- 46. Hasanain AFA, Mahdy RE, Mahran AMA, et al. Erectile dysfunction in patients with nonalcoholic fatty liver disease. Arab J Gastroenterol. 2017;18:21‐24. [DOI] [PubMed] [Google Scholar]
- 47. Duman DG, Bicakci E, Celikel CA, Akbal C. Nonalcoholic fatty liver disease is associated with erectile dysfunction: a prospective pilot study. J Sex Med. 2016;13:383‐388. [DOI] [PubMed] [Google Scholar]
- 48. Kreisel W, Deibert P, Kupcinskas L, et al. The phosphodiesterase‐5‐inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose‐finding phase‐II‐study. Dig Liver Dis. 2015;47:144‐150. [DOI] [PubMed] [Google Scholar]
- 49. Clemmesen JO, Giraldi A, Ott P, et al. Sildenafil does not influence hepatic venous pressure gradient in patients with cirrhosis. World J Gastroenterol. 2008;14:6208‐6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deibert P, Schumacher YO, Ruecker G, et al. Effect of vardenafil, an inhibitor of phosphodiesterase‐5, on portal haemodynamics in normal and cirrhotic liver – results of a pilot study. Aliment Pharmacol Ther. 2006;23:121‐128. [DOI] [PubMed] [Google Scholar]
- 51. Tandon P, Inayat I, Tal M, et al. Sildenafil has no effect on portal pressure but lowers arterial pressure in patients with compensated cirrhosis. Clin Gastroenterol Hepatol. 2010;8:546‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


