Abstract
Background
The prevalence of neuropsychiatric symptoms (NPS) diminishes the quality of life and increases the care burden in patients with dementia. Despite the clinical importance of dementia‐associated NPS, no protocols for treating NPS are already well established. Attention has turned to the effectiveness of nonpharmacological treatments for NPS since their potential safe alternative to pharmacotherapy.
Objective
This study is aimed to compare the effects in older individuals with dementia living in a residential care, of two intervention programs, the gesture‐verbal treatment (GVT), a treatment implemented by us on a previous method for word retrieval in individuals with aphasia, and the better‐known doll therapy (DT). The GVT would act on both receptive and expressive language skills, the DT on attachment and emotional connections.
Methods
We evaluated NPS by the neuropsychiatric inventory in a total of 30 patients divided into 3 groups, the GVT, the DT, and control groups, using a pre‐post design. The treatment groups completed 12‐week nonpharmacological interventions in addition to standard rehabilitative therapies, while the control group participated only in standard rehabilitative therapies.
Results
The DT group showed significant improvements in agitation, irritability, apathy, depression, and delusions relative to controls. The GVT group showed significant improvements in apathy and depression with respect to controls. The DT intervention ameliorated symptoms of agitation compared to the GVT intervention whereas the GVT intervention improved apathy compared to the DT intervention.
Conclusion
Improved understanding of the potential therapeutic benefits of different treatments for neuropsychiatric symptoms is crucial for establishing nonpharmacological interventions in dementia.
Keywords: agitation, apathy, dementia, doll therapy, gesture‐verbal treatment, neuropsychiatric symptoms
Key points.
Efficacy of nonpharmacological treatments on NPS was explored in dementia.
The neuropsychiatric inventory (NPI) was used to assess differences in NPS.
Differences in the effects on NPS were found among the DT and GVT.
The DT was more effective than GVT in reducing agitation and irritability.
The GVT intervention was more effective than DT in reducing apathy.
1. INTRODUCTION
Dementia is a condition caused by neurodegeneration in the absence of other major psychiatric disorders with many different etiologies including Alzheimer's disease (AD) and vascular dementia among others.1 The worldwide prevalence of dementia is estimated ranging between 5% and 7% and is predicted to rise significantly in the next decades,2, 3 especially as the population of older adults increases.
Dementia, no matter what the etiology, is characterized by an inexorably progressive deterioration in cognitive abilities such as memory, language, and executive functions as well as by noncognitive neuropsychiatric symptoms (NPS). Recent reviews reported that 56% to 98% of people with dementia (PwD) had at least 1 NPS with the most prevalent being apathy, irritability, agitation, and depression.4, 5, 6 Neuropsychiatric symptoms are associated with increased caregiver burden,7, 8 poor quality of life,9, 10 greater use of psychotropic drugs,11, 12 and higher rates of institutionalization.13, 14 Interestingly, despite the prevalence and clinical importance of dementia‐associated NPS, there are no established protocols for treating such symptoms.
Existing pharmacological options in NPS treatment include atypical antipsychotics, benzodiazepines, and other drugs (eg, mood stabilizers), which may, however, increase the risk of cognitive decline, cerebrovascular events, or mortality.15, 16, 17 Differently, systematic reviews have demonstrated the effectiveness of nonpharmacological treatments (NPTs) for NPS as well as the increase in perceived quality of life in PwD and their caregivers.14, 18, 19, 20 Consequently, the National Institute for Health and Care Excellence dementia guidelines21 recommend NPTs as first‐line treatments in the management of noncognitive behavioral disturbances in PwD. Thus, studies on NPTs are crucial, and additional research is essential for understanding the benefits, determining the effectiveness of NPTs implemented in routine clinical care outside of research settings and ascertaining which interventions are most effective for behavioral disturbances. Accordingly, recent studies have focused on NPTs such as cognitive stimulation, pet therapy, doll therapy, and caregiver training in problem solving18, 22, 23 to reduce NPS.
Language and communication difficulties are prominent and represent distressing features in PwD. These patients frequently have communication problems including difficulties in word finding (ie, anomia) and in understanding spoken language (ie, auditory comprehension deficits), and discourse typically shows a lack of cohesion and poor information content (eg, “empty speech”).24, 25 In addition, and most importantly, studies have shown that impairment of both receptive and expressive aspects of language skills is associated with the presence of NPS (eg, delusions, aberrant motor behavior).26, 27
Importantly, although language disorders in PwD have been extensively studied, few studies have investigated the impact of language and communication interventions on NPS.28, 29, 30, 31, 32, 33 Whereas communication generally worsens as dementia progresses, some aspects of communication are relatively preserved, including meaningful use of nonverbal communication.34 There is evidence that the use of cospeech gestures could facilitate cognitive functions (eg, episodic memory; spatial working memory)35, 36, 37 as well as speech production and comprehension of spoken language in PwD.38 In particular, some evidences suggest that cognitive‐linguistic interventions that integrate verbal (eg, words) and nonverbal (eg, gestures) forms of communication can ameliorate emotional symptoms (eg, anxiety, irritability).39, 40, 41 However, there is a dearth of research on the impact of gesture‐verbal interventions on NPS in PwD.
In this study, we aimed at evaluating the efficacy of 2 NPTs on NPS. In particular, our protocol implemented an innovative gestural‐verbal treatment (GVT) previously used to facilitate word retrieval in individuals with aphasia.42, 43 Results from studies with aphasic patients suggest that increases in iconic gestures (ie, hand or body movements illustrating the semantic content of speech/words in a pictorial way) as part of the patients communication approach can lead to significantly fewer communication breakdowns that, in turn, may decrease patients' behavioral disturbances. We hypothesized that a GVT may be effective in reducing some NPS of dementia. Differently, other NPTs encourage the use of strategies that engage alternative cognitive‐affective mechanisms to improve NPS. One such strategy is the use of doll therapy (DT), which is a person‐centered therapy that involves behaviors like holding, talking to, feeding, cuddling, or dressing an anthropomorphic doll.44, 45 Many studies demonstrated that DT might lead to a decrease in disruptive behaviors such as agitation in PwD.46, 47, 48 To date, there is no unique explanatory model for this intervention. Researchers assume that Bowlby's theory of attachment49 may represent a possible key to explain the effectiveness of DT.50, 51 Most studies suggest that DT fulfills attachment and nurturing needs by simulating familiar roles and providing comfort and a sense of purpose (eg,52, 53). In addition, several studies have shown that DT can facilitate and encourage communication between demented individuals, other residents, care staff, and families (eg,54, 55). Fraser and James also reported an increase in nonverbal communication, including eye contact and touch.56 Therefore, it is conceivable that DT, by meeting a variety of different needs in individuals with dementia, including the innate human need for communication and attachment, can increase well‐being and thereby reduce challenging behaviors.
Finally, few randomized controlled trials in literature have compared different techniques for the treatment of NPS. This makes the interpretation of the results of those previous studies difficult. In particular, dementia caregivers encounter difficulties in selecting and using effective NPTs for NPS. Thus, the current work aimed to perform a pilot exploratory randomized study to compare the effects of GTV and DT on NPS in institutionalized older individuals with dementia.
2. METHODS
2.1. Participants
We recruited participants from the Residenza Sociosanitaria Assistenziale (RSSA) per Anziani “Storelli” in Bisceglie (Italy). This home care provides permanent services for residents with Alzheimer and other types of dementia. Data were collected from January to March 2018. Participants were potentially eligible if they were 65 years old or older and met criteria for major neurocognitive disorder, previously known as dementia, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5th edition). Inclusion criteria included severe to mild cognitive impairment (Mini‐Mental State Examination [MMSE], score < 15) and the presence of behavioral disorders. Exclusion criteria included current or prior diagnosis of any other axis I DSM‐5 psychiatric disorder other than major neurocognitive disorder (eg, delirium, schizophrenia; bipolar disorder), aphasia, sensory deficits (vision and hearing), or serious neurological, musculoskeletal diagnoses, or terminal somatic illness as diagnosed through a clinical examination and/or patient anamnesis. The psychosocial and caregiver staff at the dementia care home facilitated participant selection by recommending residents who they felt met inclusion criteria. Demographic and psychotropic medication (drug, dose, frequency of antipsychotics, antidepressants, anxiolytics, and sedative/hypnotics) data as well as date of admission to the residence were retrieved through chart review, and any changes in medication during the study were recorded. Psychiatrists or geriatricians using DSM‐5 criteria confirmed dementia diagnoses. The stage of cognitive impairment was assessed with the MMSE.57
The study was carried out in accordance with the Helsinki Declaration. Written informed consent was obtained from both the participant and from their next of kin, who were informed about the aim of the study verbally and in writing before beginning the study. Ethical approval for this study was provided by the local institutional review board and by the RSSA per Anziani “Storelli” Ethic Committee.
2.2. Treatment protocols
Participants (N = 30) who met the inclusion criteria were randomly assigned (1:1:1) to 1 of 3 groups: the GVT group (N = 10), the DT group (N = 10), or the control group (N = 10) that did not receive either intervention. All participants continued to participate in their regularly scheduled programs and therapies including movement activities, artistic activities, and occupational activities, reminiscent therapy and/or any intervention deemed appropriate by the treating physician.
The GVT is a modified version of the protocol used by Raymer and coworkers.42 The protocol was adapted by the therapist (Sergio Racanati) to develop participants' interactive use of gestures, and their ability to integrate gestures with other communication strategies. The protocol consists of 4 steps: (1) The therapist instructs the participants to perform slow‐paced breathing exercises for 5 minutes and then engages a conversation with them for 10 minutes. A conversational cue opens the conversation and suggests a specific direction or subject; (2) the therapist suggests a word (eg, verb = cleaning; noun = spoon) from the selected topic and models the associated gesture for the word. The participants produce the target word and gesture 3 times; (3) the therapist presents the gesture alone and the participants imitate the gesture 3 times; (4) after a 5‐second pause, the therapist prompts the participants to once again show and tell the target word (repeating it 3 times).
Participants were reinforced if correct, while the correct response was modeled if they were incorrect. Twenty noun or verb stimuli were administered in each training session. The verbal‐gesture program included a 1‐hour group session twice weekly for 12 weeks.
The DT envisaged an observation period of 2 weeks during which the staff evaluated the patients/doll interactions. The dolls were placed on a table in the activity room, and the participants were invited to pick a doll and hold it if they wanted. Residents were free to choose any 1 of the dolls. If residents refused or were uncomfortable with taking dolls, they were not forced to choose one until ready. Leading experts in DT trained the operators of the psychosocial and caregiver staff with written material introducing the therapy and practical methods demonstrating the introduction and handling of the doll with the resident. The procedure included 5 steps as described in Pezzati et al with slight modifications: (1) An operator presented the doll to the patient and invited him/her to sit on a chair; (2) the operator interacted with the patients and the doll for 5 to 10 minutes; (3) the operator left the patient alone with the doll; (4) the patient interacts with the doll for about 50 minutes starting from the moment when the nurse left him/her alone with the doll. This phase was interrupted if patients dropped the doll before the time limit; (5) the operator returned and took the doll back. The 1‐hour DT treatment was scheduled at 10 to 12 am or 2 to 5 pm everyday for 12 weeks. The doll was also proposed to patients in other moments during the acute phase of the behavior disturbance to facilitate the therapeutic continuity.
2.3. Outcome measures
Patients' NPSs were monitored using the Neuropsychiatric Inventory Questionnaire (NPI‐Q). The NPI is a widely accepted measure of NPS associated with cognitive disorders. It assesses 12 behavioral domains common in dementia including delusions, hallucinations, agitation/aggression, dysphoria/depression, anxiety, irritability/lability, disinhibition, euphoria, apathy/indifference, aberrant motor behavior, sleep and night‐time behavior change, and appetite/eating disturbances. Clinicians rated the severity and frequency of NPSs based on scripted questions administered to the patient. They rated the severity of each domain (0‐3) on a 3‐point Likert scale and the frequency (0‐4) on a 4‐point Likert scale. Patients were assessed on 2 occasions: before treatment and after 12 weeks. A psychologist blind to the patient's treatment condition performed assessments.
The following endpoints were used: (1) change from baseline in NPI total scores and (2) change from baseline in the NPI scores in each behavioral domain.
2.4. Statistical analysis
Analyses were conducted using the statistical software GraphPad Prism 5 (San Diego, CA, USA). Nonparametric tests and chi‐square tests were performed to investigate if the 3 groups differed with respect to demographic characteristics. Nonparametric tests (Wilcoxon signed‐rank test) were used to examine the difference between the outcome score before the intervention (at preintervention) and the score after the intervention (at postintervention) for all 3 groups. Separate analyses of variance (ANOVA) were performed with NPI total and subdomain scores as dependent variables and interventions as a between‐subject factor. Post hoc analyses were conducted to assess further differences among groups. This analysis only included disturbances reported in at least 50% of participants in the 3 groups. The significance level was set at P < .05.
3. RESULTS
3.1. Characteristics of participants
Table 1 shows demographic characteristics of the participants to the study groups. There were no significant differences in age, sex, types of dementia, education, and neuroleptic medication between the groups. Two participants did not complete the study because of the development of serious physical illness: 1 in the GVT group and 1 in the DT group.
Table 1.
Demographic and clinical patient's characteristics
| GVT Group (n = 10) | DT Group (n = 10) | Control Group (n = 10) | P Value | |
|---|---|---|---|---|
| Age (year) | 82.4 (5.7) | 87.8 (6.6) | 86.9 (5.2) | .12 |
| Education level (year) | 4.3 (5.1) | 6.4 (2.7) | 4.2 (3.4) | .41 |
| Gender M/F | 2/8 | 1/9 | 2/8 | .80 |
| Months in institution | 27.7 (13.8) | 32.3 (20.0) | 29.1 (16.6) | .82 |
| Alzheimer's dementia | 5 | 6 | 6 | .88 |
| Vascular dementia | 5 | 4 | 4 | .88 |
| Neuroleptic medication (n) | 3 | 5 | 5 | .87 |
| MMSE | 10.5 (6.5) | 5.3 (3.5) | 7.1 (3.0) | .07 |
Mean values with standard deviation in brackets are shown. The P values are calculated by 1‐way ANOVA.
Abbreviations: GVT, gesture‐verbal therapy; DT, doll therapy; n, number of person; MMSE, Mini‐Mental State Examination.
All participants exhibited at least 2 NPSs (more than 90% rated as clinically significant ≥4). The most frequent disturbances were agitation/aggression (80%). Major than 50%, as the mean value among the 3 groups, were apathy/indifference (73%), irritability/lability (70%), delusions (63%), and dysphoria/depression (60%).
3.2. Changes in outcomes by respective baseline
Figure 1 shows a frequency distribution of the number of NPI symptoms in the 3 groups. Among the GVT participants, the 70% reported at least 3 disturbances (24% were clinically significant). The most frequent disturbances were apathy/indifference (80%), irritability/lability (70%), dysphoria/depression (70%), and agitation/aggression (60%). Among the DT participants, the 80% reported 3 or more disturbances (38% were clinically significant). The most frequent symptoms were agitation/aggression (90%), aberrant motor behavior (80%), delusions (70%), apathy/indifference (60%), irritability/lability (60%), and dysphoria/depression (60%). In the control group, all participants reported 3 or more symptoms (37.5% were clinically significant). The most frequent symptoms were delusions (90%), agitation/aggression (90%), apathy/indifference (90%), anxiety (80%), irritability/lability (80%), and dysphoria/depression (50%). Significant differences with regard to agitation scores were found between groups at baseline (F(2, 27) = 2.81, P = .07). Post hoc analysis showed that in the DT group, agitation scores were significantly higher compared to the GVT group (P = .02) and the control group (P = .03).
Figure 1.
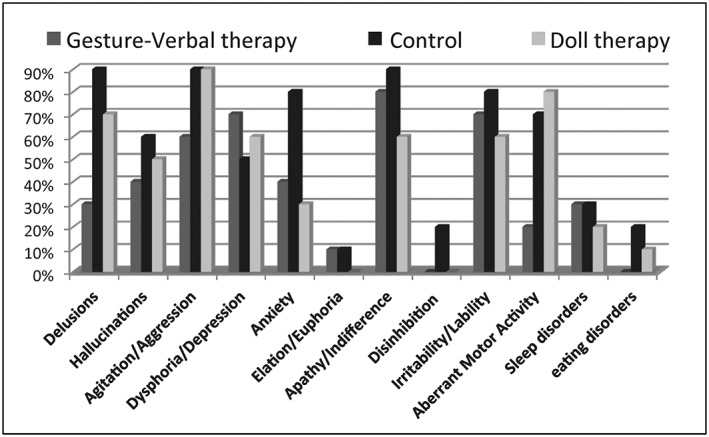
The prevalence of neuropsychiatric symptom subtypes. The percentage of each symptom in the 3 experimental groups are reported
The analysis of the NPI total score between preintervention and postintervention assessments showed a highly significant improvement in the DT group (Z = 2.66, P = .007). Conversely, pre‐post intervention comparisons of the NPI total scores yielded no significant difference in both the GVT group (Z = 1.43, P = .15) and the control group (Z = 1.52, P = .13) (Figure 2).
Figure 2.
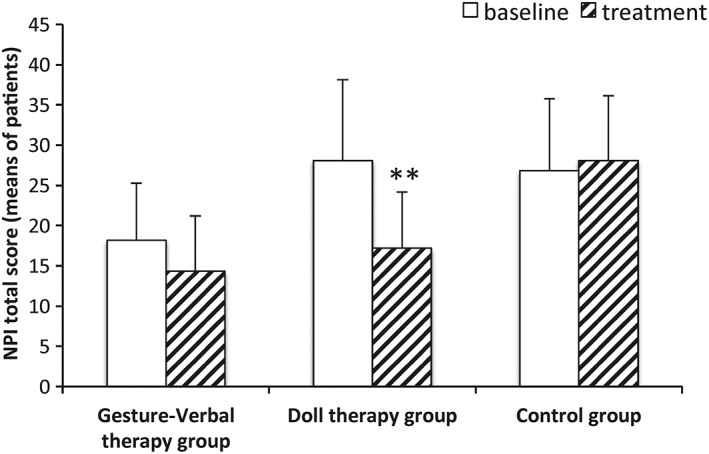
NPI total score preintervention and postintervention assessments. The mean value of the total NPI obtained by NDI‐Q (see Section 2) with standard deviation are shown. Significant differences with respect to the baseline (pretreatment) are indicated with asterisk (**P < .01)
Regarding NPI subdomains, we chose to compare the NPI symptoms present with a frequency of at least 50% in each group, ie, agitation/aggression, dysphoria/depression, apathy/indifference, and irritability/lability. The analysis of NPI subdomain showed in the GVT group a significant decrease in depression (Z = 2.02, P = .04) and apathy (Z = 2.20, P = .02) (Figure 3A). The DT group showed significant decreases in all items analyzed, ie, agitated behaviors (Z = 2.52, P = .01), depression (Z = 2.02, P = .04), apathy (Z = 2.01, P = .04), and irritability (Z = 2.02, P = .04) (Figure 3B). The control group performing the regularly scheduled programs and occupational therapies without the additional treatments under examination showed no significant differences in the NPI subdomain after 12 weeks respect to the baseline (Figure 3C). Significant differences with regards to changes in the preintervention and postintervention scores on agitation (F(2, 25) = 15.71, P = .0004), apathy (F(2, 25) = 12.05, P = .0002), and irritability (F(2, 25) = 6.38, P = .005) were found between the 3 groups.
Figure 3.
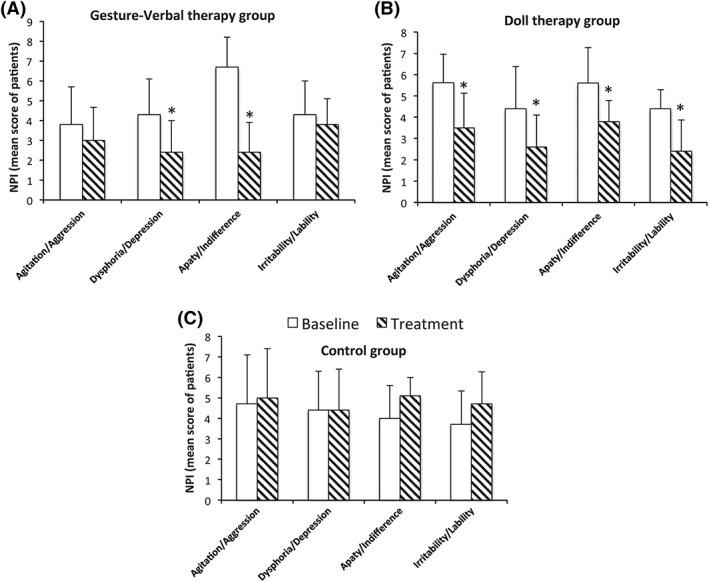
NPI subdomains preintervention and postintervention assessments in (A) gesture‐verbal therapy, (B) doll therapy, and (C) control groups. The mean values of the total NPI in the 3 experiment groups with standard deviation are shown. Significant differences with respect to the baseline (pretreatment) are indicated with asterisk (*P < .05)
3.3. Differences of the efficacy between GTV and DT treatments
The comparison the efficacy of GVT versus DT by post hoc analysis (Figure 4) showed that GVT was more effective than DT in reducing apathy (P = .01, Bonferroni correction), while the DT was more effective than GVT in reducing agitated behaviors (P = .005, Bonferroni correction) as well as irritability (P = .002, Bonferroni correction). The ANCOVA showed significant effects of DT on agitated behaviors even when agitation scores at baseline were taken into account (P = .01, Bonferroni correction).
Figure 4.
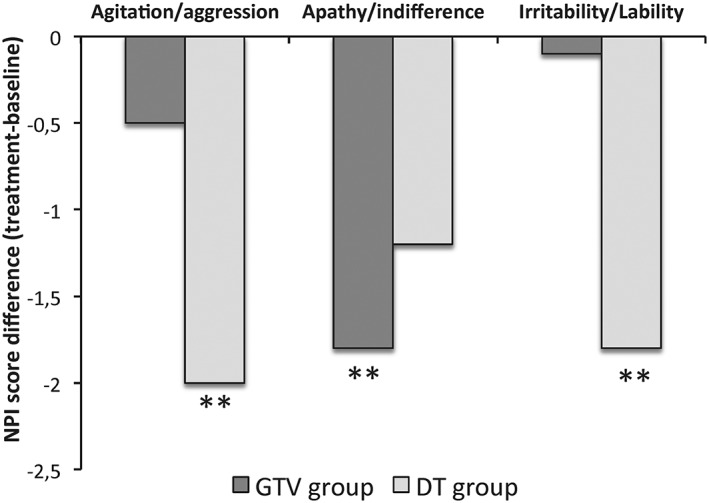
Changes of NPI subdomains in gesture‐verbal therapy (GVT) group vs doll therapy (DT) group. The mean values of the difference between the post‐NPI and pre‐NPI score assessment on agitation/aggression, apathy/indifference, and irritability/liability are shown. Significant differences between GVT and DT groups are indicated with asterisk (**P < .01)
4. DISCUSSION
In the present study, we compared the efficacy in improving NPS of 2 NPTs, the gestural‐verbal and DT in a sample of PwD. To the best of our knowledge, this is the first report that compares the effects of 2 specific cognitive stimulation therapies on NPS in a sample of institutionalized older PwD. The results showed that patients belonging in the DT group exhibited a clear improvement over 12 weeks in NPS (ie, agitation, irritability, apathy, and depression) compared to patients in the control group. A similar decrease in apathy and depression scores was also observed in the GVT group. These findings confirm results of other studies that reported that nonpharmacological treatments were effective in reducing NPS compared to nonintervention in PwD.39, 44 The comparison of the effects of these 2 interventions on NPS revealed that the DT group showed greater improvement in agitated behaviors and irritability than patients in the GVT group. Thus, DT seems to be more effective than GVT for treating agitation and irritability. This finding is consistent with previous studies that underlined the efficacy of DT in managing agitation.47, 48, 53 These studies found that the interaction and familiarity with the doll facilitate communication and the formation of the attachment relationship, which consequently leads to the reduction of behavioral and emotional disturbances. Indeed, it has been suggested that behavioral disturbances may represent forms of attachment requests by patients because of the disruption of their daily existence.51 Therefore, DT can be considered a meaningful therapeutic intervention for the prevention and the treatment of challenging behaviors such as agitation. It might be argued that the benefit we found for DT compared to GVT resulted from differences in methodologies between the psychological treatments (group therapy versus individual therapy) used in this study. In the current study, the DT was individualized, and thus, more attention was paid to the personal needs of each participant. Therefore, it could be argued that the individualized nature of DT intervention may produce greater effects in alleviating agitation and irritability symptoms than a group therapy. Moreover, the findings of this study may be attributed to the number of intervention sessions and the length of sessions, which were slightly different for DT and GVT. Although we cannot rule out this possibility, there is no reason a priori why the difference in the modality and intensity of treatment protocols might show differences in their efficacy in treating NPS. Noteworthy, this design was supported by an earlier study that compared the effects of individualized intervention (ie, aromatherapy) versus group intervention (eg, cognitive stimulation) in PwD.58 In this study, the authors proposed that the differences found between the 2 conditions could be specifically attributed to the specificity of stimulation rather than to more general therapeutic effects such as the 1‐to‐1 attention to the patients, or limited length of intervention. In our study, the decrease in apathy score in the GVT group was larger than the score in the DT group suggesting that GVT improved apathy compared to DT. Psychological disorders such as apathy, lack of interest, and gradual withdrawal from activities are common in dementia and considered to be among the earliest noncognitive expressions of the disease.59 Interestingly, Starr and Lonie reporting a significant association between premorbid mental ability and apathy suggest that the patients with higher premorbid mental ability are less apathic.60 Earlier studies demonstrated that the use of cospeech gestures could facilitate cognitive functions (eg, spatial working memory)35, 36 and comprehension of spoken language in PwD.38 In particular, more recent findings suggest that cospeech gestures may influence verbal processing as well as enhance memory recall.37 Thus, 1 explanation for our findings is that GVT may allow patients to use their limited resources (linguistic and/or cognitive) to participate in conversations and, thus, improve behavioral disturbances such as apathy.
Finally, we underline some limitations of this exploratory study. First, the sample size is small; certainly, the clinical efficacy of both GVT and DT in PwD needs to be rigorously tested with larger sample sizes. Second, we cannot rule out that the psychosocial and caregiver operators may have referred patients to the study whom they believed might particularly profit from such approaches. If so, our sample may have represented a selected subsample of the original population reducing the generalizability of our results. Third, the finding that the effect of DT on agitation was greater than that of GVT may be because such an individualized form of intervention was better able to meet the personal needs of the participants than group therapy. Lastly, other cognitive impairments related to dementia (eg, executive functions) and potentially contributing to the incidence and severity of NPS also need to be taken into consideration. This experimental study confirms the beneficial effects of NPTs on NPS in older PwD.
5. CONCLUSION
We found significant differences in the pretest and posttest scores on agitation, depressive mood, irritability, and apathy in the DT group. Patients in the GVT group showed significant improvements in apathy and depression scores. The DT ameliorated symptoms of agitation compared to GVT whereas GVT showed improvements in apathy compared to DT. In the light of our results, these nonpharmacological protocols could be more deeply refined and explored in future studies.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
MA and RAV conceived and designed the experiments; MF and A Balzotti performed the experiments; CA and BF analyzed the data; A Balzotti and MA RAV wrote the paper; MA, RAV, BF, FD, and A Bellomo discussed the results and provided comments on the paper.
ACKNOWLEDGEMENTS
We thank all the participants to this study and the staff of the RSSA per Anziani “Storelli,” in particular the psychosocial operators and caregivers for their effort and support in the realization of the study. We want to thank Sergio Racanati for the development of an innovative gesture and naming approach for treating neuropsychiatric symptoms in dementia. Finally, we thank Maria Rosa Mirizzi for the assistance in the manuscript editing.
Balzotti A, Filograsso M, Altamura C, et al. Comparison of the efficacy of gesture‐verbal treatment and doll therapy for managing neuropsychiatric symptoms in older patients with dementia. Int J Geriatr Psychiatry. 2019;34:1308–1315. 10.1002/gps.4961
Angela Balzotti and Marianna Filograsso contributed equally to this work.
Contributor Information
Rosa Anna Vacca, Email: r.vacca@ibiom.cnr.it.
Mario Altamura, Email: m_altamura@virgilio.it.
REFERENCES
- 1. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and meta‐analysis. Alzheimers Dement. 2013;9:63‐75.e2. [DOI] [PubMed] [Google Scholar]
- 4. Fernandéz‐Martinez M, Castro J, Molano A, Zarranz JJ, Rodrigo RM, Ortega R. Prevalence of neuropsychiatric symptoms in Alzheimer's disease and vascular dementia. Curr Alzheimer Res. 2008;5(1):61‐69. [DOI] [PubMed] [Google Scholar]
- 5. Selbæk G, Engedal K, Bergh S. The prevalence and course of neuropsychiatric symptoms in nursing home patients with dementia: a systematic review. J Am Med Dir Assoc. 2013;14(3):161‐169. [DOI] [PubMed] [Google Scholar]
- 6. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: systematic review and meta‐analysis. J Affect Disord. 2016;190:264‐271. [DOI] [PubMed] [Google Scholar]
- 7. Lima‐Silva TB, Bahia VS, Carvalho VA, et al. Neuropsychiatric symptoms, caregiver burden and distress in behavioral‐variant frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord. 2015;40(5‐6):268‐275. [DOI] [PubMed] [Google Scholar]
- 8. Caceres BA, Frank MO, Jun J, Martelly MT, Sadarangani T, de Sales PC. Family caregivers of patients with frontotemporal dementia: an integrative review. Int J Nurs Stud. 2016;55:71‐84. [DOI] [PubMed] [Google Scholar]
- 9. Karttunen K, Karppi P, Hiltunen A, et al. Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer's disease. Int J Geriatr Psychiatry. 2011;26(5):473‐482. [DOI] [PubMed] [Google Scholar]
- 10. Hongisto K, Hallikainen I, Selander T, et al. Quality of life in relation to neuropsychiatric symptoms in Alzheimer's disease; 5‐year prospective ALSOVA cohort study. Int J Geriatr Psychiatry. 2018;33(1):47‐57. [DOI] [PubMed] [Google Scholar]
- 11. Nijk RM, Zuidema SU, Koopmans RT. Prevalence and correlates of psychotropic drug use in Dutch nursing‐home patients with dementia. Int Psychogeriatr. 2009;21(03):485‐493. [DOI] [PubMed] [Google Scholar]
- 12. Gulla C, Selbæk G, Flo E, Kjome R, Kirkevold Ø, Husebo BS. Multipsychotropic drug prescription and the association to neuropsychiatric symptoms in three Norwegian nursing home cohorts between 2004 and 2011. BMC Geriatr. 2016;16(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dement. 2011;7(5):532‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Livingston G, Kelly L, Lewis‐Holmes E, et al. A systematic review of the clinical effectiveness and cost‐effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(61):1‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335‐2341. [DOI] [PubMed] [Google Scholar]
- 16. Ballard CG, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease. Cochrane Database Syst Rev. 2006;25:CD003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nowrangi MA, Lykestos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer's dementia. Alzheimers Res Ther. 2015;7(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brodaty H, Arasaratnam C. Meta‐analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry. 2012;169(9):946‐953. [DOI] [PubMed] [Google Scholar]
- 19. Mitchell G, McCormack B, McCance T. Therapeutic use of dolls for people living with dementia: a critical review of the literature. Dementia. 2016;15(5):976‐1001. [DOI] [PubMed] [Google Scholar]
- 20. Legere LE, McNeill S, Martin LS, Acorn M, An D. Non‐pharmacological approaches from behavioral and psychological symptoms of dementia in older adults: a systematic review of reviews. J Clin Nurs. 2018;27(7‐8):e1360‐e1376. [DOI] [PubMed] [Google Scholar]
- 21. NICE: National Institute for Health and Care Excellence (2016). Dementia: supporting people with dementia and their carers in health and social care Clinical guideline [CG42]. https://www.nice.org.uk/guidance/cg42/
- 22. Dourado MC, Laks J. Psychological interventions for neuropsychiatric disturbances in mild and moderate Alzheimer's disease: current evidences and future directions. Curr Alzheimer Res. 2016;13(10):1100‐1111. [DOI] [PubMed] [Google Scholar]
- 23. Theleritis C, Siarkos K, Politis AA, Katirtzoglou E, Politis A. A systematic review of non‐pharmacological treatments for apathy in dementia. Int J Geriatr Psychiatry. 2018;33(2):e177‐e192. [DOI] [PubMed] [Google Scholar]
- 24. Smith ER, Broughton M, Baker R, et al. Memory and communication support in dementia: research‐based strategies for caregivers. Int Psychogeriatr. 2012;24:1927‐1942. [DOI] [PubMed] [Google Scholar]
- 25. Kempler D, Goral M. Language and dementia: neuropsychological aspects. Annu Rev Appl Linguist. 2008;28:73‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brodaty H, Griffin D, Hadzi‐Pavlovic D. A survey of dementia carers: doctor's communications, problem behaviors and institutional care. Aust N Z J Psychiatry. 1990;24(3):362‐370. [DOI] [PubMed] [Google Scholar]
- 27. Potkins D, Myint P, Bannister C, et al. Language impairment in dementia: impact on symptoms and care needs in residential homes. Int J Geriatr Psychiatry. 2003;18(11):1002‐1006. [DOI] [PubMed] [Google Scholar]
- 28. Smith SR, Murdoch BE, Chenery HJ. Language disorders associated with dementia of the Alzheimer type: a review. Aust J Hum Commun Disord. 2014;15:49‐70. [Google Scholar]
- 29. Egan M, Bérubé D, Racine G, Leonard C, Rochon E. Methods to enhance verbal communication between individuals with Alzheimer's disease and their formal informal caregivers: a systematic review. Int J Alzheimers Dis. 2010;2010:906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vasse E, Vernooij‐Dassen M, Spijker A, Rikkert MO, Koopmans R. A systematic review of communication strategies for people with dementia in residential and nursing homes. Int Psychogeriatr. 2010;22(02):189‐200. [DOI] [PubMed] [Google Scholar]
- 31. Mahendra N, Scullion A, Hamerschlag BA. Cognitive‐linguistic interventions from person with dementia. Topics in Geriat Rehabil. 2011;27(4):278‐288. [Google Scholar]
- 32. Murray L. Behavioral/nonpharmacological approaches to addressing cognitive‐linguistic symptoms in individuals with dementia. Perspect ASHA Spec Interest Groups. 2016;1(15):12‐25. [Google Scholar]
- 33. Morello ANDC, Lima TM, Brandão L. Language and communication non‐pharmacological interventions in patients with Alzheimer's disease: a systematic review. Dement Neuropsychol. 2017;11(3):227‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hubbard G, Cook A, Tester S, Downs M. Beyond words: older people with dementia using and interpreting nonverbal behaviour. J. Aging Stud. 2002;16(2):155‐167. [Google Scholar]
- 35. Wesp R, Hesse J, Keutmann D, Wheaton K. Gesture maintain spatial imagery. Am J Psychol. 2001;114(4):591‐600. [PubMed] [Google Scholar]
- 36. Morsella E, Krauss RM. The role of gestures in spatial working memory and speech. Am J Psychol. 2004;117(3):411‐424. [PubMed] [Google Scholar]
- 37. Madan CR, Singhal A. Using actions to enhance memory: effects of enactment, gestures, and exercise on human memory. Front Psych. 2012;3:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pashek GV, DiVenere E. Auditory comprehension in Alzheimer disease: influences of gesture and speech rate. J Med Speech Lang Pathol. 2006;14:143‐155. [Google Scholar]
- 39. Silvestri A, Rosano G, Zannino G, Ricca F, Marigliano V, Fini M. Behavioral disturbances in Alzheimer's disease: a non‐pharmacological therapeutic approach. Arch Gerontol Geriatr Suppl. 2004;9:379‐386. [DOI] [PubMed] [Google Scholar]
- 40. Kindell J, Sage K, Keady J, Wilkinson R. Adapting to conversation with semantic dementia: using enactment as a compensatory strategy in everyday social interaction. Int J Lang Commun Disord. 2013;48(5):497‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stanyon MR, Griffiths A, Thomas SA, Gordon AL. The facilitators of communication with people with dementia in care setting: an interview study with healthcare workers. Age Ageing. 2016;45(1):164‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raymer AM, Singletary F, Rodriguez A, Ciampitti M, Heilman KM, Rothi LJ. Effects of gesture+verbal treatment for noun and verb retrieval in aphasia. J Int Neuropsychol Soc. 2006;12(6):867‐882. [DOI] [PubMed] [Google Scholar]
- 43. Rose ML, Raymer AM, Lanyon LE, Attard MC. A systematic review of gesture treatments fro post‐stroke aphasia. Aphasiology. 2013;27(9):1090‐1127. [Google Scholar]
- 44. Mitchell G. Use of doll therapy for people with dementia: an overview. Nurs Older People. 2014;26(4):24‐26. [DOI] [PubMed] [Google Scholar]
- 45. Ng QX, Wuen CH, Hong SKS, Chuan TW. Doll therapy for dementia suffers: systematic review. Complement Ther Clin Pract. 2017;26:42‐46. [DOI] [PubMed] [Google Scholar]
- 46. James AI, Mackenzie L, Mukætova‐Ladinska E. Doll use in care homes for people with dementia. Int J Geriatr Psychiatry. 2006;21(11):1093‐1098. [DOI] [PubMed] [Google Scholar]
- 47. Mackenzie L, James I, Morse R, Mukaetova‐Ladinska E, Reichelt K. A pilot study on the use of dolls for people with dementia. Age Aging. 2006;35(4):441‐443. [DOI] [PubMed] [Google Scholar]
- 48. Stephens A, Cheston R, Gleeson K. An exploration into the relationships people withdementia have with physical objects: an ethnographic study. Dementia. 2013;12(6):697‐712. [DOI] [PubMed] [Google Scholar]
- 49. Bowlby J. Attachment and Loss (Volume 1) Attachment. London: Hogarth; 1969. [Google Scholar]
- 50. Fernandez R, Arthur B, Fleming R, Perrin C. Effect of doll therapy in managing challenging behaviors in people with dementia: a systematic review. JBI Database System Rev Implement Rep. 2014;12(8):330‐363. [Google Scholar]
- 51. Pezzati R, Molteni V, Bani M, et al. Can doll therapy preserve or promote attachment in people with cognitive, behavioral, and emotional problems? A pilot study in institutionalized patients with dementia. Front Psychol. 2014;5:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miesen B. Alzheimer's disease, the phenomenon of parent fixation and Bowlby's attachment theory. Int J Geriatr Psychiatry. 1993;8(2):147‐153. [Google Scholar]
- 53. Bisiani L, Angus J. Doll therapy: a therapeutic means to meet past attachments needs and diminished behaviors of concern in a person living with dementia‐a case study approach. Dementia. 2012;12:447‐462. [DOI] [PubMed] [Google Scholar]
- 54. Ellingford L, Mackenzie L, Marsland L. Using dolls to alter behavior in people with dementia. Nurs Times. 2007;103:36‐37. [Google Scholar]
- 55. Minshull K. The impact of doll therapy on well‐being of people with dementia. J Dementia Care. 2009;17:35‐38. [Google Scholar]
- 56. Fraser F, James I. Why does doll therapy improve the well‐being of some 188 older adults with dementia. PSIGE Newsletter. 2008;105:55‐63. [Google Scholar]
- 57. Cockrell JR, Folstein MF. Mini‐mental state examination (MMSE). Psychopharmacol Bull. 1988;24(4):689‐692. [PubMed] [Google Scholar]
- 58. Yang YP, Lee FP, Chao HC, Hsu FY, Wang JJ. Comparing the effects of cognitive stimulation, reminiscence, and aroma‐massage on agitation and depressive mood in people with dementia. J Am Med Dir Assoc. 2016;17(8):719‐724. [DOI] [PubMed] [Google Scholar]
- 59. Bierman EJM, Comijs HC, Jonker C, Beekman ATF. Symptoms of anxiety and depression in the course of cognitive decline. Dement Geriatr CognDisord. 2007;24(3):213‐219. [DOI] [PubMed] [Google Scholar]
- 60. Starr JM, Lonie J. Relationship between behavioral and psychological symptoms of dementia and cognition in Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24(5):343‐347. [DOI] [PubMed] [Google Scholar]


