Abstract
Purpose
To evaluate treatment outcome at 12 months in eyes with neovascular age‐related macular degeneration (nAMD) treated with antivascular endothelial growth factor (anti‐VEGF) injections according to either pro re nata (PRN)‐ or treat‐and‐extend (TE)‐regimen in one clinical setting in Sweden.
Methods
Data were obtained retrospectively from the Swedish Macula Register, optical coherence tomography‐database and electronic patient charts. The study included 443 eyes; 223 PRN‐ and 220 TE‐treated eyes. Baseline (BL) characteristics and follow‐up data at 6 and 12 months were collected. Statistical regression analysis was performed to evaluate association between treatment strategy and visual outcome at 12 months.
Results
Baseline (BL) characteristics were well balanced between cohorts. Visual acuity at 12 months was higher in TE‐cohort 66.5 (13.1) compared to PRN‐cohort 60.1 (17.6) (p = 0.000). Visual improvement at 12 months was +5.2 (11.8) and +1.2 (12.7) letters Early Treatment Diabetic Retinopathy Study (ETDRS) in TE‐ and PRN‐cohorts, respectively (p = 0.002). Number of administered injections at 12 months was 10.2 (2.1) and 6.3 (2.1) in the two cohorts (p = 0.000). Statistical analysis demonstrated a strong association between TE treatment strategy and improvement in visual acuity at 12 months.
Conclusion
Eyes treated according to TE had better visual outcome at 12 months. The results indicate that treatment according to proactive TE‐regimen is superior to treatment according to PRN‐regimen in clinical routine care of nAMD.
Keywords: anti‐VEGF, neovascular AMD, pro re nata, real‐world data, treat‐and‐extend, treatment regimen, visual acuity
Introduction
Age‐related macular degeneration (AMD) is a leading cause of severe visual impairment and blindness among the elderly population in developed countries (Resnikoff et al. 2004; Bourne et al. 2014). Neovascular AMD (nAMD) is characterized by abnormal angiogenesis in the macular region (Nowak 2006). Current treatment for nAMD with intravitreal antivascular endothelial growth factor (anti‐VEGF) injections was established in 2006 and significantly improved treatment outcome compared to earlier treatment strategies photodynamic therapy (PDT) with visudyne (Brown et al. 2006; Rosenfeld et al. 2006).
In the pivotal clinical trials, anti‐VEGF injections were given regularly on a monthly basis generating a high treatment burden on both patients and the healthcare system. To reduce treatment burden, a pro re nata (PRN)‐regimen with treatment‐as‐needed and monthly disease monitoring was introduced. Similar visual results after 1 year of treatment was obtained for both monthly treatment and treatment according to PRN‐regimen. The injection frequency was reduced from 12 injections to on average 7 injections with PRN‐regimen (CATT Research Group et al. 2011).
Further development has lead to a treat‐and‐extend (TE)‐regimen in which treatment is given at every visit to the eye clinic. The interval between visits for disease monitoring is gradually prolonged when there is no sign of disease activity. Treatment outcome with both PRN‐regimen and TE‐regimen show good results with improved visual acuity (CATT Research Group et al. 2011; Berg et al. 2015, 2017). Both treatment regimens are used in today′s clinical practice (Tuuminen et al. 2017). A review of studies, in which either PRN‐ or TE‐regimen was used in treatment of nAMD, indicates better visual outcome in eyes treated according to a TE‐regimen (Chin‐Yee et al. 2016). Similar results have been found in a recent study comparing outcome in patients treated in the UK and Australia, respectively (Johnston et al. 2017). However, uncertainty still exists regarding a possible superiority of one of the two treatment regimens.
The aim of the present study was to use real‐life data from a Swedish county hospital to compare treatment outcome and treatment frequency in two cohorts of eyes with nAMD treated according to PRN‐regimen or TE‐regimen in the same clinical setting.
Materials and Methods
Data were obtained retrospectively from the Swedish Macula Register (SMR), optical coherence tomography (OCT)‐database Imagenet (Topcon Corporation, Tokyo, Japan) and from electronic patient records. The study was performed in accordance within the tenets of the Declaration of Helsinki and was approved by the Regional Ethical Review Board at Uppsala University (Dnr 2015/331 September 16, 2015).
The SMR was used to identify 678 eyes with nAMD. Of those, 272 eyes started treatment according to PRN‐regimen during the period November 1, 2010 until August 28, 2012. The remaining 406 eyes started treatment according to TE‐regimen during the period April 1, 2014 until October 20, 2016. Eyes with other diagnosis than nAMD (PRN 12+ TE 9), eyes that had received PDT (PRN 10+ TE 1), eyes that were diagnosed after November 11, 2015 (TE 159) and eyes with unavailable visual acuity Early Treatment Diabetic Retinopathy Study (ETDRS) data at baseline (BL) (PRN 27+ TE 17) were excluded.
In total, 443 eyes treated with intravitreal anti‐VEGF injections due to nAMD were included in this study, 223 eyes treated according to PRN‐regimen and 220 eyes treated according to TE‐regimen (Fig. 1). All eyes included in the study were treatment‐naïve and had not received any previous treatment for nAMD. The majority of the eyes were treated with anti‐VEGF ranibizumab (Lucentis) exclusively (monotherapy). However, few occasional anti‐VEGF injections were given with aflibercept (Eylea) to a minimal number of eyes.
Figure 1.
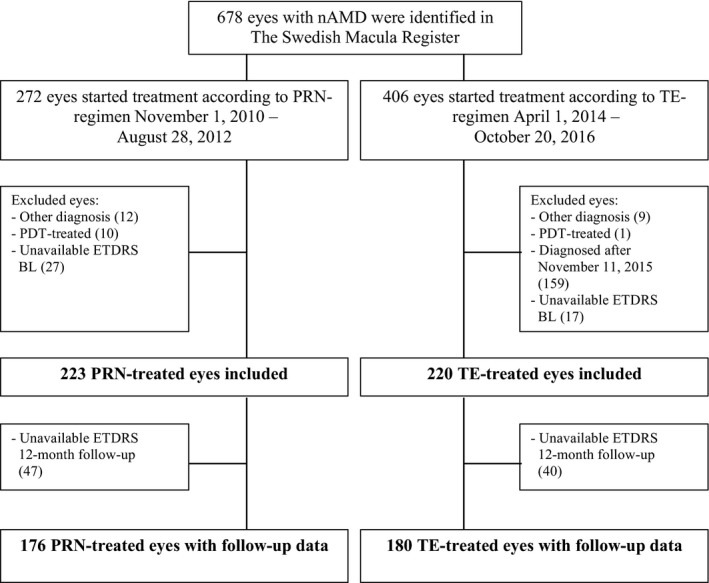
Inclusion of study‐eyes. ETDRS = Early Treatment Diabetic Retinopathy Study, PDT = photodynamic therapy.
From the SMR data regarding BL characteristics such as gender, age at diagnosis, day of diagnosis, affected eye (right/left), duration of subjective symptoms at BL, visual acuity in affected eye (ETDRS) and visual acuity in fellow eye (Snellen) at BL was obtained. Day of diagnosis was considered as BL (= day 0). Visual acuity in the affected eye was measured using the ETDRS letter chart. In the SMR, visual acuity in fellow eye was measured using the Snellen letter chart. The Snellen visual acuity was converted into logMAR‐units using the equation logMAR = −10 log(Snellen) (Holladay 1997). Visual acuity of hand motion (HM) was converted into logMAR 3 and amaurosis into logMAR 4.
Visual acuity ETDRS data were unavailable in 87 eyes (PRN 47+ TE 40) at 12‐month follow‐up. Follow‐up data at 6 months and 12 months was available in 356 eyes, 176 eyes in the PRN‐cohort and 180 eyes in the TE‐cohort.
Follow‐up data were obtained from the SMR regarding visual acuity ETDRS and number of intravitreal anti‐VEGF injections administered. Last observation carried forward (LOCF) visual acuity ETDRS was applied in eyes with non‐available follow‐up at 12 months. Additionally, follow‐up data regarding treatment interval in the TE‐cohort at 12 months were obtained. The 6‐month follow‐up occurred within the range of 160–200 days from BL. The 12‐month follow‐up occurred within the range of 320–400 days from BL.
Validation of data obtained from the SMR regarding the variables visual acuity ETDRS and number of intravitreal anti‐VEGF injections was performed using data from patients′ charts. Data agreement between the SMR and the patients’ charts were evaluated. The agreement of visual acuity ETDRS data was considered satisfactory (97% at BL, 91% at 6‐month follow‐up and 97% at 12‐month follow‐up). Therefore, data from the SMR for the variable visual acuity ETDRS were used in the study. The agreement of data regarding number of intravitreal anti‐VEGF injections administered was considered unacceptable (86% at 6‐month follow‐up and 77% at 12‐month follow‐up). Data agreement <80% for the variable number of intravitreal anti‐VEGF injections administered was considered unacceptable since the study aimed to evaluate two different treatment regimens, including the key variable treatment intensity. Therefore, number of intravitreal anti‐VEGF injections was collected from patients′ charts.
Treatment
In all patients, treatment was initiated with three monthly anti‐VEGF‐injections. Patients treated according to PRN were then monitored every 4–6 week. Anti‐VEGF‐injections were administered based on disease activity, i.e. reduction of visual acuity >3 letters ETDRS and/or occurrence of intra‐ or subretinal fluid on OCT and/or appearance of new or persistent hemorrhage on biomicroscopic fundus examination. Patients treated according to TE were examined and treated every 4 weeks until no signs of active AMD as determined by OCT were found. In eyes without signs of active disease, anti‐VEGF was administered. The interval to the next treatment was extended by 2 weeks at a time, up to a maximum interval of 12 weeks. In case of signs of recurrent disease, defined as occurrence of intra‐or subretinal fluid on OCT and/or new or persistent hemorrhage, treatment interval was shortened by 2 weeks at a time until the disease was again considered to be inactive. Patients then remained on this interval for at least 6 months before treatment interval again was extended as described above.
OCT
OCT‐images were collected from the OCT‐database Imagenet (Topcon Corporation). The fast macular thickness map protocol was used. The images were interpreted by a retina specialist (E.G.). Retinal morphology was evaluated at BL, 6‐, and 12‐month follow‐up. Baseline (BL) and 12‐month follow‐up data were presented. The presence or absence of subretinal fluid (SRF), intraretinal fluid (IRF) and pigment epithelial detachment (PED) was evaluated. These variables were defined according to Schmidt‐Erfurth et al. (2015). Subretinal fluid (SRF) was defined as a nonreflective space between the posterior boundary of the neurosensory retina and the retinal pigment epithelium (RPE)/choriocapillaris signal. Intraretinal fluid (IRF) was defined as round, minimally reflective spaces (cysts) within the neurosensory retina. Pigment epithelial detachment (PED) was defined as a focal elevation of the reflective RPE band over an optically clear or moderately reflective space with a minimum width of 400 μm at the base or a minimum height of 200 μm from the surface of the RPE band to the surface of the choriocapillaris. In addition, central retinal thickness (CRT) was measured at BL, 6‐, and 12‐month follow‐up.
Statistical methods
Sample size
Sample size was calculated using a non‐inferiority limit of 4 letters ETDRS and 13 letters ETDRS standard deviation for change in visual acuity at 12 months (Ho et al. 2014). To demonstrate non‐inferiority between treatment strategies with 80% power and 95% confidence interval (two‐sided test) 174 eyes in each group was required.
Statistical analyses
spss 22 (IBM Corporation, Armonk, NY, USA) was used for the statistical analyses. Day of diagnosis was considered as BL. Student′s t‐test for unpaired data was used for comparison of data between PRN‐cohort and TE‐cohort. Student′s t‐test for paired data was used for comparison within cohorts. Linear regression analysis was performed using change in visual acuity ETDRS at 12 months as the dependent variable. Association between treatment regimen (PRN/TE) and change in visual acuity ETDRS at 12 months was analysed separately. Subsequently, the analysis was adjusted (multiple regression analysis) for the following variables: duration of subjective symptoms at BL, visual acuity ETDRS at BL, age at diagnosis, OCT‐findings at BL (presence of IRF, SRF and PED), number of intravitreal anti‐VEGF injections over 12 months and visual acuity of fellow eye at BL.
Results
In total, 443 treatment‐naïve eyes (402 patients) were identified in the SMR and included in the study. All eyes included started treatment of nAMD with anti‐VEGF injections within the years 2010–2015. Before May 1, 2014, 223 eyes started treatment according to PRN‐regimen; after May 1, 2014, 220 eyes started treatment according to TE‐regimen.
Follow‐up data at 6 and 12 months was available for 356 eyes (80%), 176 eyes in the PRN‐cohort and 180 eyes in the TE‐cohort. Reasons for unavailable follow‐up data were: treatment discontinued due to low visual acuity (47%), treatment discontinued due to patient's request (20%), treatment discontinued due to other morbidity/death (18%) and treatment discontinued due to unknown/other reasons (15%).
The 6‐month follow‐up occurred 179 (17) and 181 (16) days (mean, SD) from BL in the PRN‐cohort and in the TE‐cohort, respectively. The 12‐month follow‐up occurred 369 (31) and 365 (21) days from BL in the PRN‐cohort and in the TE‐cohort, respectively.
Bilateral disease was seen in 41 patients. Both eyes were diagnosed on the same occasion in 19 patients and on separate occasions in 22 patients.
Baseline characteristics
Baseline (BL) characteristics are presented in Table 1. Characteristics were well balanced between cohorts. Fellow eyes with visual acuity HM or worse at BL were 13 (HM 11+ amaurosis 2) in the PRN‐cohort and 5 (HM 3+ amaurosis 2) in the TE‐cohort.
Table 1.
Baseline (BL) characteristics
| PRN‐cohort | TE‐cohort | p value | |
|---|---|---|---|
| Number of eyes | 223 | 220 | |
| Mean age | 78.2 | 78.3 | 0.849 ns |
| Gender (%) | |||
| Men | 33 | 38 | |
| Women | 67 | 62 | |
| Duration of subjective symptoms (%) | |||
| 0–<2 months | 33 | 26 | |
| 2–<4 months | 22 | 24 | |
| 4–6 months | 20 | 17 | |
| >6 months | 25 | 33 | |
| Mean visual acuity affected eye, ETDRS letters (SD) | 55.4 (16.1) | 58.3 (15.6) | 0.052 ns |
| Mean visual acuity fellow eye, logMAR (SD), appr. Snellen |
(n = 202) 0.78 (0.88) 20/125 |
(n = 199) 0.52 (0.71) 20/70 |
|
PRN = pro re nata, TE = treat‐and‐extend, Appr. = approximately, ETDRS = Early Treatment Diabetic Retinopathy Study.
Visual acuity data
Visual acuity data are presented in Table 2. Visual acuity at BL was similar in the PRN‐cohort and in the TE‐cohort. Mean visual acuity at 12 months was significantly higher (p < 0.001, unpaired t‐test) in the TE‐cohort compared to the PRN‐cohort. Improvement in visual acuity from BL to 12‐month follow‐up was significantly higher (p = 0.002) in the TE‐cohort: +1.2 (12.7) ETDRS letters in the PRN‐cohort and +5.2 (11.8) ETDRS letters in the TE‐cohort, respectively. At 12 months, mean visual acuity in all eyes (LOCF ETDRS) was inferior to mean visual acuity in eyes with available follow‐up data.
Table 2.
Mean visual acuity ETDRS in pro re nata (PRN)‐cohort and treat‐and‐extend (TE)‐cohort at baseline (BL), 6‐ and 12‐month follow‐up in eyes with available follow‐up data
| PRN‐cohort (ETDRS) | TE‐cohort (ETDRS) | p value | |
|---|---|---|---|
| Follow‐up eyes | (n = 176) | (n = 180) | |
| BL | 58.9 (13.9) | 61.3 (13.7) | 0.105 ns |
| 6 months | 61.6 (15.3) | 66.8 (13.2) | 0.001 |
| 12 months | 60.1 (17.6) | 66.5 (13.1) | <0.001 |
| All eyes included | (n = 223) | (n = 220) | |
| BL | 55.4 (16.1) | 58.3 (15.6) | 0.052 ns |
| 12 months/LOCF | 56.1 (19.3) | 61.9 (18.3) | 0.001 |
In addition, mean visual acuity ETDRS at BL and 12‐month follow‐up/last observation carried forward (LOCF) for all eyes included.
ETDRS = Early Treatment Diabetic Retinopathy Study.
Number of injections
Eyes in the TE‐cohort received a significantly higher (p < 0.001) number of intravitreal anti‐VEGF injections during the first 12 months of treatment: 6.3 (2.1) injections in the PRN‐cohort and 10.2 (2.1) injections in the TE‐cohort, respectively.
Treatment intervals
Treatment intervals for the TE‐cohort at 12‐month follow‐up are presented in Fig. 2. Mean treatment interval was 6.7 (2.8) weeks at 12‐month follow‐up.
Figure 2.
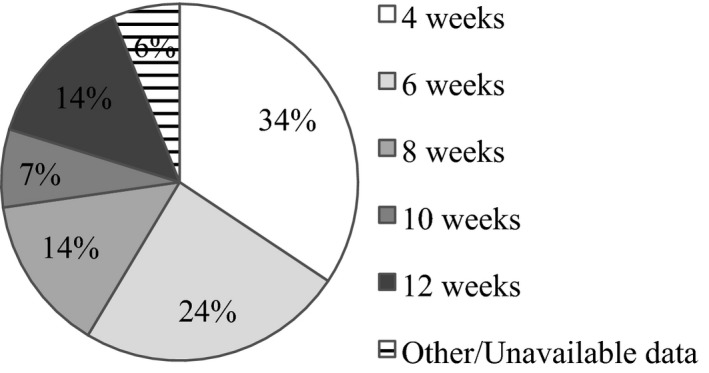
Treatment intervals at 12‐month follow‐up in eyes treated according to treat‐and‐extend‐regimen. (Proportion of eyes, %. Percentages summed to 99% due to rounding.)
OCT‐data
Mean CRT measured in OCT‐imaging at BL, 6‐ and 12‐month follow‐up is presented in Table 3. Central retinal thickness (CRT) decreased after 12 months of treatment in both the PRN‐cohort and the TE‐cohort. For all eyes, reduction in CRT from BL to 6‐month follow‐up and from BL to 12‐month follow‐up was 76.6 (98.1) μm and 85.6 (98.3) μm, respectively. The presence of IRF, SRF and PED at BL and 12‐month follow‐up are presented in Fig. 3.
Table 3.
Mean central retinal thickness (CRT) measured in μm at baseline (BL), 6‐ and 12‐month follow‐up in pro re nata (PRN)‐cohort and treat‐and‐extend (TE)‐cohort
| PRN‐cohort | TE‐cohort | p value | |
|---|---|---|---|
| CRT BL | 313 (101) | 298 (104) | 0.187 ns |
| CRT 6 months | 237 (83) | 219 (61) | 0.025 |
| CRT 12 months | 224 (67) | 216 (52) | 0.236 ns |
Figure 3.
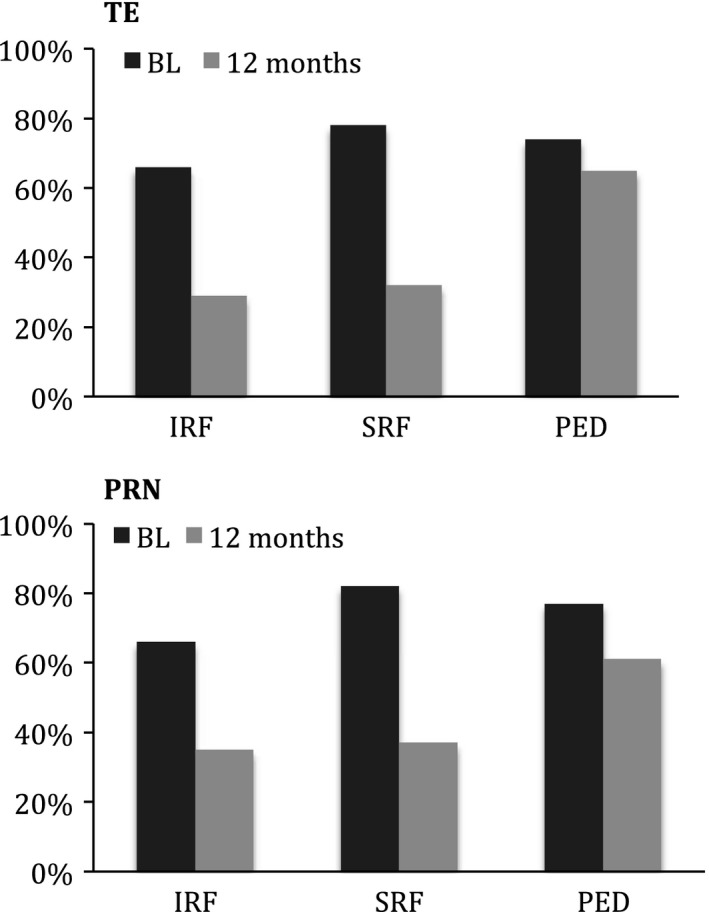
Presence of intraretinal fluid (IRF), subretinal fluid (SRF) and pigment epithelial detachment (PED) at baseline (BL) and at 12‐month follow‐up in pro re nata (PRN)‐cohort and treat‐and‐extend (TE)‐cohort (proportion of eyes, %).
Treatment strategy
Linear regression analysis demonstrated an association between the treatment strategy TE (regression coefficient, mean difference 3,372; CI95: 0.672–6.071; p = 0.014) and improvement in visual acuity at 12 months compared to BL. Following multiple regression analysis adjusted for duration of symptoms at BL, BL visual acuity ETDRS, age at diagnosis, OCT findings at BL, number of intravitreal injections and visual acuity of fellow eye at BL the adjusted mean difference was 6.947 (CI95: 3.588–10.307; p < 0.001) indicating a strong association between the TE treatment strategy and improvement in visual acuity at 12 months.
Discussion
The present study was performed to evaluate the outcome of treatment with anti‐VEGF for nAMD after change of treatment regimen from PRN to TE in a Swedish county hospital. We also addressed the existing uncertainty regarding a possible superiority of one of the two treatment regimens. Our results show that eyes treated according to the TE‐regimen have significantly better visual outcome and was treated with a significantly higher number of anti‐VEGF injections after 12 months of treatment, suggesting that treatment according to a TE‐regimen is superior to a PRN‐regimen in treatment of nAMD in clinical routine care.
According to our results, eyes treated using a TE‐regimen had significantly better visual outcome at 12 months compared to eyes treated according to a PRN‐regimen. Our results are in line with results from a smaller study from Switzerland although in this study, the Snellen chart was used for measuring visual acuity (Hatz & Prünte 2017). The results from our study, performed in one specific clinical setting, are also in accordance with findings in a review of 70 studies (62 PRN and 8 TE) evaluating anti‐VEGF treatment regimens, PRN versus TE (Chin‐Yee et al. 2016).
At 12‐month follow‐up, visual outcome was +1.2 (12.7) ETDRS letters for the eyes treated according to a PRN‐regimen. The magnitude of the improvement in visual acuity in the PRN‐treated eyes was well in line with findings in other studies (Hjelmqvist et al. 2011; Lee et al. 2017). Visual outcome at 12 months in other real‐life studies has been found to vary between +1.1 and +2.7 ETDRS letters. The number of injections given in theses studies varied between 4.3 and 5. 9 (Holz et al. 2015; Westborg et al. 2017). In clinical routine care, monthly monitoring that is required in the PRN‐regimen, is difficult to maintain. If monitoring is delayed, it might lead to significant undertreatment of eyes with nAMD.
Our study shows that eyes treated with a TE‐regimen received a higher number of anti‐VEGF injections during 12 months compared to eyes treated according to a PRN‐regimen. These findings are in line with other studies (Chin‐Yee et al. 2016; Hatz & Prünte 2017; Johnston et al. 2017; Lee et al. 2017). It has been suggested that the more frequent treatment per se is a likely explanation for the greater improvement in visual acuity with a TE‐regimen (Johnston et al. 2017; Lee et al. 2017). However, it can also be hypothesized that the proactive TE model, which aims at maintaining stable disease, may be more efficient in restoring and preserving visual acuity compared to the more reactive PRN regimen.
We compared the effectiveness in treatment strategies with the same anti‐VEGF agent, ranibizumab. Adjusted linear regression analysis based on our data demonstrated an association between the treatment strategy TE and improvement in visual acuity at 12 months compared to treatment start supporting that the TE treatment model itself and not only the number of given anti‐VEGF‐injections is important for the treatment outcome.
Multiple BL characteristics have been investigated for possible impact on visual acuity and treatment outcome in nAMD. Better visual acuity in the affected eye at BL has been associated with less improvement but a more favourable final visual outcome (Ying et al. 2013). Similarly, better visual acuity in both affected and fellow eye at BL has been associated with better final visual acuity (Granstam et al. 2016). Older age at diagnosis has been associated with a less favourable visual outcome and a higher risk for referral to a low vision rehab clinic (Ying et al. 2013; Granstam et al. 2016; Lanzetta et al. 2018). Short duration of symptoms and early treatment start has been associated with better visual outcome (Rasmussen et al. 2015) and avoidance of permanent damage to the retina photoreceptors. The morphology of the retina (i.e. presence of IRF, SRF, and PED) at BL has been associated with visual outcome to various extents (Schmidt‐Erfurth et al. 2015; Waldstein et al. 2016). Similar effects of anti‐VEGF‐treatment on retinal morphology on OCT were observed in our study.
In the present study, the association between the treatment strategy TE and improvement in visual outcome at 12 months remained significant in multiple regression analysis adjusted for factors with assumed impact on visual acuity and also adjusted for the number of treatments during the first treatment year. This strongly indicates that TE‐regimen is superior to PRN‐regimen in treatment of nAMD. We hypothesis that this superiority might be due to the proactive treatment regimen with TE, in which treatment is given before ocular assessment detects an unstable disease and impaired visual acuity.
The strength of our study is that it presents a large set of real‐life data in eyes with nAMD. All study eyes were diagnosed and treated at the same department of ophthalmology in a Swedish county hospital, enabling a uniform clinical assessment and process of treatment. The limitation of the study is its retrospective and observational study design. Analysed data were obtained from eyes that started treatment for nAMD during different time periods (2010–2015).
Approximately one out of five study eyes was lost during the first year of treatment. The magnitude of dropouts is comparable with a previous Swedish study (Hjelmqvist et al. 2011). In the present study, nearly half of the dropouts discontinued treatment due to low visual acuity, which highlights the severity of the disease. One out of five treatment discontinuations was requested by the patient. Further, almost one out of five dropouts was due to other severe morbidity or death of the patient, reflecting that nAMD is a disease that primarily affects older people. For comparison, main reasons reported for discontinuing treatment has been low visual acuity (Swedish Macula Register 2015) or no further follow‐up needed (Hjelmqvist et al. 2011).
In summary, our results demonstrate that in eyes treated according to TE‐regimen visual outcome was better at 12 months compared to eyes treated according to PRN‐regimen. The results indicate that treatment according to proactive TE‐regimen is superior to treatment according to PRN‐regimen in clinical routine care of nAMD. Longer follow‐up is needed to evaluate the impact of treatment regimen on visual outcome in a longer perspective.
Preliminary study results have been presented at the annual conference of the Swedish Ophthalmological Society, Umeå August 23–25, 2017.
The study was supported by grants from: Stiftelsen Kronprinsessan Margaretas Arbetsnämnd för synskadade (KMA; SE), Ögonfonden (SE), Föreningen Synskadades Vänner i Uppsala (SE), Region Västmanland (SE).
Elisabet Granstam: advisory board Bayer and Allergan; advisory board/lecturer Novartis; lecturer Novo Nordisk. Kersti Sjövall: advisory board Bayer.
We wish to thank statistician Philippe Wagner PhD, Region Vastmanland – Uppsala University Centre for Clinical Research, Hospital of Vastmanland Vasteras, for valuable advice.
References
- Berg K, Pedersen TR, Sandvik L & Bragadóttir R (2015): Comparison of ranibizumab and bevacizumab for neovascular age‐related macular degeneration according to LUCAS treat‐and‐extend protocol. Ophthalmology 122: 146–152. [DOI] [PubMed] [Google Scholar]
- Berg K, Roald AB, Navaratnam J & Bragadóttir R (2017): An 8‐year follow‐up of anti‐vascular endothelial growth factor treatment with a treat‐and‐extend modality for neovascular age‐related macular degeneration. Acta Ophthalmol 95: 796–802. [DOI] [PubMed] [Google Scholar]
- Bourne RRA, Jonas JB, Flaxman SR et al. (2014): Prevalence and causes of vision loss in high‐income countries and in Eastern and Central Europe: 1990–2010. Br J Ophthalmol 98: 629–638. [DOI] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP & Schneider S (2006): Ranibizumab versus verteporfin for neovascular age‐related macular degeneration. N Engl J Med 355: 1432–1444. [DOI] [PubMed] [Google Scholar]
- CATT Research Group , Martin DF, Maquire MG, Ying GS, Grunwald JE, Fine SL & Jaffe GJ (2011): Ranibizumab and bevacizumab for neovascular age‐related macular degeneration. N Engl J Med 364: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin‐Yee D, Eck T, Fowler S, Hardi A & Apte RS (2016): A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age‐related macular degeneration. Br J Ophthalmol 100: 914–917. [DOI] [PubMed] [Google Scholar]
- Granstam E, Westborg I, Barkander A et al. (2016): Reduced occurrence of severe visual impairment after introduction of anti‐Vascular Endothelial Growth Factor in wet age‐related macular degeneration ‐ a population‐ and register‐based study from northern Sweden. Acta Ophthalmol 94: 646–651. [DOI] [PubMed] [Google Scholar]
- Hatz K & Prünte C (2017): Treat and extend versus pro re nata regimens of ranibizumab in neovascular age‐related macular degeneration: a comparative 12 Month study. Acta Ophthalmol 95: e67–e72. [DOI] [PubMed] [Google Scholar]
- Hjelmqvist L, Lindberg C, Kanulf P, Dahlgren H, Johansson I & Siewert A (2011): One‐year outcomes using ranibizumab for neovascular age‐related macular degeneration: results of a prospective and retrospective observational multicentre study. J Ophthalmol 2011 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AC, Busbee BG, Regillo CD et al. (2014): Twenty‐four‐month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age‐related macular degeneration. Ophthalmology 121: 2181–2192. [DOI] [PubMed] [Google Scholar]
- Holladay JT (1997): Proper method for calculating average visual acuity. J Refract Surg 13: 388–391. [DOI] [PubMed] [Google Scholar]
- Holz FG, Tadayoni R, Beatty S et al. (2015): Multi‐country real‐life experience of anti‐vascular endothelial growth factor therapy for wet age‐related macular degeneration. Br J Ophthalmol 99: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RL, Carius H‐J, Skelly A, Ferreira A, Milnes F & Mitchell P (2017): A retrospective study of ranibizumab treatment regimens for neovascular age‐related macular degeneration (nAMD) in Australia and the United Kingdom. Adv Ther 34: 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta P, Cruess AF, Cohen SY et al. (2018): Predictors of visual outcomes in patients with neovascular age‐related macular degeneration treated with anti‐vascular endothelial growth factor therapy: post hoc analysis of the VIEW studies. Acta Ophthalmol [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Lee AY, Lee CS, Egan CA et al. (2017): UK AMD/DR EMR REPORT IX: comparative effectiveness of predominantly as needed (PRN) ranibizumab versus continuous aflibercept in UK clinical practice. Br J Ophthalmol 101: 1683–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JZ (2006): Age‐related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep 58: 353–363. [PubMed] [Google Scholar]
- Rasmussen A, Brandi S, Fuchs J, Hansen LH, Lund‐Andersen H, Sander B & Larsen M (2015): Visual outcomes in relation to time to treatment in neovascular age‐related macular degeneration. Acta Ophthalmol 93: 616–620. [DOI] [PubMed] [Google Scholar]
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP & Mariotti SP (2004): Global data on visual impairment in the year 2002. Bull World Health Organ 82: 844–851. [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY & Kim RY (2006): Ranibizumab for neovascular age‐related macular degeneration. N Engl J Med 355: 1419–1431. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Waldstein SM, Deak G‐G, Kundi M & Simader C (2015): Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age‐related macular degeneration. Ophthalmology 122: 822–832. [DOI] [PubMed] [Google Scholar]
- Swedish Macula Register (2015): Annual report. Available at: http://makulareg.se/publikationer/arsrapporter. (Accessed on 15 Nov 2017).
- Tuuminen R, Uusitalo‐Järvinen H, Aaltonen V et al. (2017): The Finnish national guideline for diagnosis, treatment and follow‐up of patients with wet age‐related macular degeneration. Acta Ophthalmol 95: 1–9. [DOI] [PubMed] [Google Scholar]
- Waldstein SM, Simader C, Staurenghi G et al. (2016): Morphology and visual acuity in aflibercept and ranibizumab therapy for neovascular age‐related macular degeneration in the VIEW trials. Ophthalmology 123: 1521–1529. [DOI] [PubMed] [Google Scholar]
- Westborg I, Granstam E, Rosso A, Albrecht S, Karlsson N & Lövestam‐Adrian M (2017): Treatment for neovascular age‐related macular degeneration in Sweden: outcomes at seven years in the Swedish Macula Register. Acta Ophthalmol 95: 787–795. [DOI] [PubMed] [Google Scholar]
- Ying G‐S, Huang J, Maguire MG et al. (2013): Baseline predictors for one‐year visual outcomes with ranibizumab or bevacizumab for neovascular age‐related macular degeneration. Ophthalmology 120: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]


