Abstract
BACKGROUND
The aim of the study was to rank conventionally used fibre rich feeds for their physicochemical properties and detect possible correlation between analytical fibre determinations. A total of 22 samples were analysed for proximate fibre values, soluble dietary fibre (SDF), insoluble dietary fibre (IDF), crude protein (CP) and crude ash (CA). Physicochemical properties were determined in vitro by hydration capacity (HC) [water holding capacity (WHC), water binding capacity (WBC), swelling property (SwP)] and buffering capacity [linear buffering rate (LBR)].
RESULTS
Fibre content and physicochemical properties varied markedly between the samples. HC was highest for beet pulp and lowest for rice and millet bran. Buffering capacity expressed minimum values for lignocelluloses and maximum values for rape seed hulls. The correlation of methods was positively between WBC, WHC and SwP (r ≤ 0.89; P ≤ 0.003) but not significant for HC and buffering capacity. SwP negatively correlated with crude fibre (CF), neutral detergent fibre (aNDFOM) and IDF (r ≤ −0.48; P ≤ 0.05). WBC and SwP positively correlated with SDF (r ≤ 0.63; P ≤ 0.04). LBR was negatively correlated with CF, aNDFOM, IDF and total dietary fibre (r ≤ −0.55; P ≤ 0.02), but positively with CP (r = 0.53; P = 0.01).
CONCLUSION
The determination of physicochemical properties is applicable to rank fibre rich feeds, some correlations between fibre analytical measurements and physicochemical properties were detected. © 2019 The Authors. Journal of the Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry
Keywords: dietary fibre, physicochemical properties, hydration capacity, buffering capacity
INTRODUCTION
Sustainable utilization of dietary fibre in diets of monogastric animals using industrial by‐products of food production like spelt, husks, hulls, brans and pomaces, which does not compete with food for humans, is necessary. However, the measurement to decide the amount and the source of fibre to be included in the diet is unclear. Fibre is the most poorly understood ‘nutrient’ in the diet. As a part of carbohydrates, and besides the quantitative aspect of fibre in feeds, fibre fulfils a range of functions and interactions in the gastrointestinal tract. One important step is therefore to define fibre, considering that in several studies the solubility and fermentability of fibre demonstrated major impact on the digestibility of various nutrients, digesta transit time, promotion of beneficial microbiota growth and metabolite production, gut heath and histological traits.1, 2, 3, 4 Although several functions are recognized, most attempts to define fibre use quantitative approaches. Analytical methods used to characterize fibre often overlap or may exclude fractions of other distinctly different carbohydrate fractions.5 One common definition coming out from the need for quantitative approaches is that dietary fibre is constituted by those carbohydrates which are not hydrolysed by digestive enzymes in the small intestine but are partly fermented by the gut microbiota.6 Considering those functions and interactions, it becomes clear also that fibre definition in the diet also needs an approach, which may include the physicochemical properties of fibre in addition to the quantitative measurements.
If fibre crosses the whole gastrointestinal tract almost undigested, physicochemical properties of fibre could support a broader definition of dietary fibre. Among physicochemical properties, hydration capacity (HC)5 and buffering capacity7 have been identified to play key roles. Although measured regularly in several publications,8, 9, 10, 11, 12, 13 physicochemical properties are seldom considered for the identification of fibre types, and are rarely used in diet formulation. Therefore, fibre characterization in the diet needs an approach, which may include also physicochemical properties to explain variation in digestion, in competing processes of digestion and in digesta transit.14, 15 Such information may help to close the gap of knowledge in ranking fibre according to their quantitative constitution and according to their physicochemical properties. This may have application, for instance, to choose specific fibre sources for different development stages in swine nutrition.16, 17, 18 This could be the prevention of diarrhoea after weaning,19 the intestine and fermentation development throughout the fattening period20 and the requirement of satiation or prevention of constipation for sows.21, 22 There is currently no standardized recommendation available in monogastric nutrition to include source or amount of fibre in the diet, neither based on physicochemical properties nor based on quantitative approaches.16 Although various studies have been done,11, 12, 23, 24 correlation of a broad range of fibre rich feeds, analysed with the same methods, is rare. In addition, the correlations of physicochemical properties and quantitative measurements of fibre constituents are poorly understood.
The objective of the present study was to determine the variation in HC and linear buffering rate (LBR) of 22 fibre rich feeds showing a broad range in their fibre content. The use of physicochemical properties to characterize fibre is used differently in the literature. Therefore, it was also an objective to establish in vitro methods to evaluate the variability between quantitative approaches and physicochemical properties. In the present study, it was hypothesized that differences among physicochemical properties of fibre rich feeds correlates to proximate fibre analyses, which allows the ranking of fibre rich feeds, contributing in this way to get closer for taking decision about fibre source and amount to be used in monogastric nutrition.
MATERIAL AND METHODS
The study comprised 22 conventionally used fibre rich feeds, including hulls, brans, lignocelluloses and other fibre rich by‐products of food production. Samples were bought or provided from animal feed companies and mills. Details of physical form (PF) and particle size are presented in Table 1. Proximate analyses were performed according to the official methods for nutrient analyses in Germany.25 Dry matter (DM; no. 3.1), crude protein (CP; no. 4.1.2), crude ash (CA; no. 8.1), crude fibre (CF; no. 6.1.1), neutral detergent fibre (aNDFOM; no. 6.5.1), acid detergent fibre (ADFOM; no. 6.5.2, non‐sequential) and acid detergent lignin (ADLOM (sa); no. 6.5.3.) were determined. All fibre analyses were performed using Fibretherm equipment (Gerhardt, Königswinter, Germany). Measurement of soluble dietary fibre (SDF), insoluble dietary fibre (IDF) and total dietary fibre (TDF) were performed according to AOAC (no. 991.43, based on Lee) with a gravimetric procedure (Kit, Merck, Darmstadt, Germany). All analyses were performed at least in duplicate.
Table 1.
Characterization of different fibre sources (g kg−1 DM)
| Feedstuff | PF | PS | DM | ipH | CF | aNDFOM | ADFOM | ADLOM | SDF | IDF | TDF | CA | CP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apple pomace | PF 1 | 1 mm | 905 ± 0.08 | 3.67 | 234 ± 3.44 | 476 ± 2.74 | 368 ± 1.98 | 246 ± 0.16 | 146 ± 1.06 | 530 ± 0.90 | 676 | 20 ± 0.19 | 69 ± 1.09 |
| Beet pulp | PF 2 | 1 mm | 927 ± 0.39 | 5.66 | 153 ± 0.72 | 317 ± 3.39 | 196 ± 2.09 | 87 ± 1.12 | 163 ± 1.20 | 474 ± 2.00 | 637 | 127 ± 1.66 | 100 ± 0.68 |
| Cellulose, pure | PF 3 | <1 mm | 952 ± 0.12 | 7.86 | n.a. | n.a. | n.a. | n.a. | 0 | 1000 ± 0.74 | 1000 | n.a. | 5 ± 0.00 |
| Grape pomace, red | PF 1 | 1 mm | 963 ± 0.79 | 3.47 | 318 ± 0.87 | 455 ± 6.89 | 386 ± 5.96 | 359 ± 5.43 | 42 ± 1.77 | 558 ± 3.64 | 600 | 58 ± 1.24 | 131 ± 2.75 |
| Grape pomace, white | PF 1 | 1 mm | 953 ± 1.18 | 3.70 | 203 ± 2.12 | 298 ± 14.13 | 292 ± 2.30 | 237 ± 3.57 | 50 ± 0.21 | 363 ± 4.10 | 413 | 47 ± 0.16 | 90 ± 1.36 |
| Hemp bran | PF 1 | 1 mm | 922 ± 0.16 | 6.12 | 397 ± 5.32 | 660 ± 1.91 | 506 ± 2.56 | 231 ± 4.11 | n.a. | n.a. | n.a. | 33 ± 1.48 | 175 ± 1.47 |
| Lignocellulose Ia | PF 4 | <1 mm | 919 ± 0.30 | 4.78 | 579 ± 0.63 | 926 ± 4.88 | 728 ± 2.02 | 650 ± 7.85 | 11 ± 0.73 | 942 ± 1.45 | 953 | 5 ± 0.30 | 8 ± 0.04 |
| Lignocellulose II | PF 4 | <1 mm | 904 ± 0.24 | 5.01 | 559 ± 7.04 | 919 ± 3.16 | 757 ± 7.96 | 329 ± 2.43 | 13 ± 0.24 | 933 ± 0.54 | 945 | 10 ± 0.07 | 8 ± 0.52 |
| Lignocellulose III | PF 4 | <1 mm | 923 ± 0.91 | 4.67 | 561 ± 0.84 | 874 ± 8.43 | 737 ± 2.35 | 335 ± 1.92 | 12 ± 0.29 | 938 ± 0.29 | 949 | 7 ± 0.02 | 10 ± 0.94 |
| Lupine hulls | PF 1 | 1 mm | 892 ± 0.02 | 7.05 | 559 ± 7.14 | 870 ± 2.24 | 715 ± 0.47 | 27 ± 5.96 | 27 ± 4.01 | 942 ± 22.70 | 968 | 20 ± 0.04 | 42 ± 0.95 |
| Millet bran | PF 1 | 1 mm | 925 ± 0.33 | 7.67 | 430 ± 1.22 | 810 ± 1.00 | 574 ± 8.64 | 208 ± 1.29 | 6 ± 1.16 | 804 ± 1.84 | 810 | 127 ± 0.33 | 40 ± 7.57 |
| Pectin mixture | PF 3 | <1 mm | 936 ± 0.05 | 3.30 | 174 ± 1.68 | n.a. | n.a. | n.a. | 311 ± 3.29 | 416 ± 1.98 | 726 | 27 ± 0.06 | 52 ± 1.41 |
| Rape seed hulls | PF 1 | 1 mm | 908 ± 0.33 | 6.61 | 334 ± 0.88 | 622 ± 5.27 | 529 ± 0.46 | 363 ± 5.39 | 23 ± 1.25 | 578 ± 8.93 | 601 | 50 ± 0.31 | 169 ± 2.76 |
| Rice bran | PF 1 | 1 mm | 934 ± 0.48 | 6.85 | 460 ± 3.37 | 794 ± 1.19 | 607 ± 2.48 | 249 ± 0.22 | 0 | 785 ± 2.86 | 785 | 179 ± 0.54 | 19 ± 1.53 |
| Soybean hulls I | PF 1 | 1 mm | 911 ± 0.82 | 5.81 | 375 ± 0.68 | 687 ± 2.93 | 507 ± 0.46 | 29 ± 5.08 | 77 ± 1.39 | 713 ± 3.54 | 789 | 49 ± 0.65 | 113 ± 2.94 |
| Soybean hulls II | PF 1 | 1 mm | 935 ± 0.08 | 6.19 | 301 ± 1.52 | 562 ± 1.97 | 388 ± 4.39 | 70 ± 0.70 | 70 ± 3.00 | 585 ± 3.27 | 654 | 52 ± 0.25 | 179 ± 3.59 |
| Spelt hulls I | PF 1 | 1 mm | 934 ± 0.14 | 6.59 | 398 ± 2.33 | 856 ± 3.27 | 494 ± 0.05 | 86 ± 0.19 | 6 ± 0.39 | 866 ± 4.27 | 872 | 60 ± 0.20 | 18 ± 0.68 |
| Spelt hulls II | PF 1 | 1 mm | 926 ± 0.17 | 6.44 | 384 ± 5.10 | 803 ± 10.96 | 477 ± 3.36 | 114 ± 0.34 | 11 ± 0.91 | 830 ± 3.79 | 841 | 57 ± 0.04 | 31 ± 3.12 |
| Sunflower hulls | PF 1 | 1 mm | 919 ± 0.39 | 5.63 | 535 ± 3.33 | 843 ± 2.79 | 679 ± 0.84 | 255 ± 3.83 | 27 ± 0.57 | 871 ± 0.08 | 897 | 26 ± 0.05 | 41 ± 0.02 |
| Vinasse, dried | PF 1 | <1 mm | 911 ± 0.19 | 3.56 | 338 ± 0.78 | 818 ± 0.49 | 565 ± 0.85 | 298 ± 0.61 | 14 ± 0.70 | 749 ± 3.29 | 763 | 7 ± 0.19 | 168 ± 0.71 |
| Wheat bran | PF 1 | 1 mm | 878 ± 1.32 | 6.72 | 145 ± 1.32 | 585 ± 2.70 | 181 ± 0.08 | 70 ± 0.55 | 34 ± 3.10 | 579 ± 2.63 | 612 | 75 ± 0.42 | 159 ± 2.35 |
| Wheat straw | PF 1 | 1 mm | 936 ± 0.16 | 7.41 | 396 ± 0.98 | 826 ± 0.27 | 508 ± 3.33 | 218 ± 17.09 | 15 ± 0.32 | 838 ± 3.82 | 853 | 52 ± 0.33 | 30 ± 1.04 |
I–III identify samples of the same type but received from different batches or companies.
ADFOM, acid detergent fibre; ADLOM, acid detergent lignin; aNDFOM, neutral detergent fibre; CA, crude ash; CF, crude fibre; CP, crude protein; DM, dry matter; IDF, insoluble dietary fibre; ipH, initial pH value; n.a., not analysed; PF, physical form; PF 1, by‐product, bulked material; PF 2, by‐product, pelleted material; PF 3, by‐product, powder material; PF 4, granulate material; PS, particle size; SDF, soluble dietary fibre; TDF, total dietary fibre as calculated vales of SDF + IDF.
Linear buffering capacity
Measurement of LBR was performed using a method of Oliveira et al. 13 with modifications. The method was standardized for each feed sample measuring pH from 8.0 to 2.0. Briefly, 1.0 g of sample (grinded to pass 1.0 mm sieve, Retsch ZM200, Haan, Germany) was soaked in 100 mL distilled water and stirred for 30 min. The initial pH value (pH Meter 766 Calimatic, Knick, Berlin, Germany) was determined, and if necessary pH was raised to 8.0 with sodium hydroxide (NaOH; 0.05 M) and then titrated with hydrochloric acid (HCl) until pH 2.0 (0.05 M HCl to approximately pH 6.0; 0.1 M HCl to pH 2.0) was achieved. For every titration step an equilibrium time for at least one minute was observed. The titratable acidity (TA) was determined as the amount of HCl needed to reduce the pH from 8.0 to 2.0, expressed in milliequivalents per gram of dry matter (mEq g−1 DM) of the sample. The pH data obtained for each titration curve were transformed by the function y = exp(1/pH) and the LBR was calculated as the inverse of the slope of the linear regression between y and the cumulated amount of acid added. All samples were analysed at least in duplicate.
Hydration capacity
To establish the measurements of HC, a combination of different methods was tested and implemented.24, 26, 27, 28 After a series of pre‐trials, two methods were used which were applied for all samples:
1‐ water holding capacity (WHC) using 0.25–0.5 g of whole sample (grinded to pass a 1.0 mm sieve), 24 h pre‐soaking in centrifuge tubes (15 mL, Roth, Karlsruhe, Germany) with 10 mL distilled water and centrifuged (15 min, 6000×g, Centrifuge 5810R, Eppendorf, Wesseling‐Berzdorf, Germany);
2‐ water binding capacity (WBC) by soaking 0.25–0.5 g whole sample (grinded to pass a 1.0 mm sieve) with 10 mL distilled water (24 h) and stirring (first 120 min) in metric cylinder (10:0.2 mL, Isolab, Wertheim, Germany).
Supernatant was assumed as the non‐absorbed water and used to calculate the HC for both methods [mL H2O g−1 DM = ((mL H2O/1000)/weight of DM)*1000]. For method 2, the swelling properties (SwPs) were expressed in percentage, i.e. the difference between the starting volume and the final volume obtained after 24 h, measured in a metric cylinder (10:0.2 mL, Isolab). All samples were analysed at least in duplicates.
Statistical analyses
The correlation of physicochemical properties and quantitative fibre components were statistically analysed with procedure CORR (SAS 6.1 Inst., Inc., Cary, NC, USA). Differences were treated as significant when P ≤ 0.05.
RESULTS
Table 1 highlights the analytical characterization of all tested samples in detail. Next to fibre components, fibre sources were ranked according to their PF and initial pH value. The PF was named PF 1 for by‐products presented as bulked material; PF 2 for by‐product presented as pelleted material; PF 3 for by‐product presented as powder material; and PF 4, for by‐products presented as granulates. This step is important, as after processing (e.g. grinding, pelleting, granulation) the standard grinding for laboratory analyses may generate different particle sizes, including particles with much smaller discrete mean particle sizes (< 1 mm, data not shown). Initial pH values showed the variation among different fibre sources. The values ranged from pH 3.3 up to 7.8 and the ingredient composition displayed their broad distribution.
The fibre content varied greatly in aNDFOM (grape pomace: 298, up to 926 g kg−1 DM for lignocellulose I), ADFOM (wheat bran: 181, up to 757 g kg−1 DM lignocellulose II) and ADLOM (lupine hulls: 27, up to 650 g kg−1 DM for lignocellulose I) as well as SDF (cellulose: 0 up to 311 g kg−1 DM for pectin mixture), IDF (grape pomace: 363 up to 1000 g kg−1 DM for cellulose) and TDF (grape pomace: 413 up to 1000 g kg−1 DM for cellulose). High variation was observed for CF (wheat bran: 145, up to 579 g kg−1 DM for lignocellulose I). CA and CP were rather low (lignocellulose I: 5, up to 179 g CA kg−1 DM for rice bran; cellulose: 5, up to 179 g CP kg−1 DM for soybean hulls II).
Hydration capacity
A large variation of HC for fibre sources was observed (Table 2). On average 4.97 ± 3.14 mL H2O g−1 DM was observed within the method of WHC and 6.22 ± 3.41 mL H2O g−1 DM for WBC. Maximum WBC and WHC were analysed for beet pulp (16.58 and 18.55 mL H2O g−1 DM), minimum amounts for rice and millet bran (WHC: 0.22 and WBC: 2.92; WHC: 2.04 and WBC: 2.90 mL H2O g−1 DM, respectively). Less than half of the samples expressed SwP lower than 100%. Beet pulp showed the highest SwP of 963%, while the lowest value was observed for hemp bran and cellulose with 15% and 31% SwP, respectively.
Table 2.
Means of hydration capacity (HC; mL H2O g−1 DM), swelling properties (%) and linear buffering capacity of fibre rich feeds
| Physicochemical property | ||||
|---|---|---|---|---|
| Feedstuff | WHC | WBC | SwP | LBR |
| Apple pomace | 5.19 ± 0.10 | 11.37 ± 0.36 | 550 ± 25 | 3.69 ± 0.06 |
| Beet pulp | 16.58 ± 0.18 | 18.55 ± 0.88 | 963 ± 13 | 5.08 ± 0.06 |
| Cellulose, pure | 3.04 ± 0.11 | n.a. | 31 ± 5 | 4.79 ± 0.64 |
| Grape pomace, red | 3.82 ± 0.11 | 3.52 ± 0.17 | 96 ± 33 | 6.38 ± 0.21 |
| Grape pomace, white | 3.35 ± 0.62 | 4.42 ± 0.43 | 145 ± 28 | 5.24 ± 0.18 |
| Hemp bran | 2.27 ± 0.11 | 2.91 ± 0.44 | 15 ± 17 | 4.89 ± 0.01 |
| Lignocellulose I | 5.21 ± 0.11 | 7.29 ± 0.13 | 205 ± 5 | 2.47 ± 0.05 |
| Lignocellulose II | 4.63 ± 0.01 | 6.30 ± 0.11 | 150 ± 5 | 2.44 ± 0.11 |
| Lignocellulose III | 7.43 ± 0.01 | 6.35 ± 0.09 | 185 ± 0 | 4.14 ± 0.08 |
| Lupine hulls | 4.48 ± 0.15 | 5.93 ± 0.12 | 236 ± 5 | 5.64 ± 0.24 |
| Millet bran | 2.04 ± 0.01 | 2.90 ± 0.13 | 40 ± 7 | 5.09 ± 0.03 |
| Pectin mixture | 3.80 ± 0.19 | 6.33 ± 0.03 | 350 ± 17 | 6.33 ± 0.44 |
| Rape seed hulls | 5.68 ± 0.20 | 6.25 ± 0.09 | 70 ± 3 | 6.58 ± 0.05 |
| Rice bran | 0.22 ± 0.00 | 2.92 ± 0.25 | 33 ± 4 | 4.77 ± 0.07 |
| Soybean hulls I | 5.55 ± 0.10 | 5.60 ± 0.11 | 178 ± 11 | 4.68 ± 0.16 |
| Soybean hulls II | 4.38 ± 0.57 | 6.40 ± 0.22 | 263 ± 0 | 5.18 ± 0.05 |
| Spelt hulls I | 5.10 ± 0.03 | 5.03 ± 0.10 | 70 ± 1 | 4.51 ± 0.18 |
| Spelt hulls II | 5.34 ± 0.41 | 5.51 ± 0.11 | 67 ± 0 | 2.69 ± 0.08 |
| Sunflower hulls | 4.35 ± 0.00 | 5.88 ± 0.22 | 65 ± 2 | 4.57 ± 0.08 |
| Vinasse, dried | 3.02 ± 0.05 | 4.28 ± 0.11 | 338 ± 13 | 4.37 ± 0.11 |
| Wheat bran | 5.51 ± 0.30 | 5.09 ± 0.38 | 55 ± 0 | 5.74 ± 0.02 |
| Wheat straw | 8.43 ± 0.05 | 7.80 ± 0.05 | 52 ± 0 | 3.55 ± 0.15 |
LBR, linear buffering rate; SwP, swelling property; WBC, water binding capacity; WHC, water holding capacity (centrifugation).
Linear buffering capacity
Samples showed substantial differences in LBR values (Table 2). To decrease the pH value from 8.0 to 2.0 an average of 2.50 mEq H+ g−1 DM was necessary, achieving highest amounts for pectin mixture, grape pomace and rape seed hulls (3.63 mEq H+ g−1 DM) and lowest for lignocellulose (1.34 mEq H+ g−1 DM). Accumulated titrated acid (in mEq H+ g−1 DM) and change in pH value of some representative samples are illustrated in Fig. 1. Correspondingly, the amount of HCl needed to decrease the pH values linearly increased with LBR. Calculated LBR, as a measure of pH change for each sample at any pH interval between 8.0 and 2.0, was highest for rape seed hulls (6.58) and pectin mix (6.33). In contrast, the lowest LBR values were for lignocellulose I and II (2.47 and 2.44, respectively).
Figure 1.
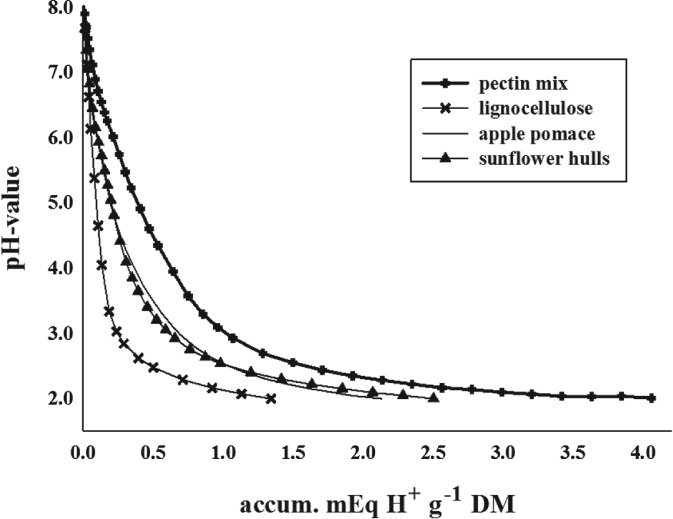
Systematic lowering of pH values and accumulated acid (expressed in milliequivalents H+ per gram of dry matter) of selected fibre rich feeds.
Correlation between physicochemical properties and fibre content
Correlations between the methods of physicochemical properties are presented in Fig. 2. Measurements of WHC and WBC showed the highest positive correlation (r = 0.89, P < 0.0001). High correlation was found also between WBC and SwP (r = 0.88, P < 0.0001) as well as between WHC and SwP (r = 0.70, P = 0.0003). HC and LBR did not correlate.
Figure 2.
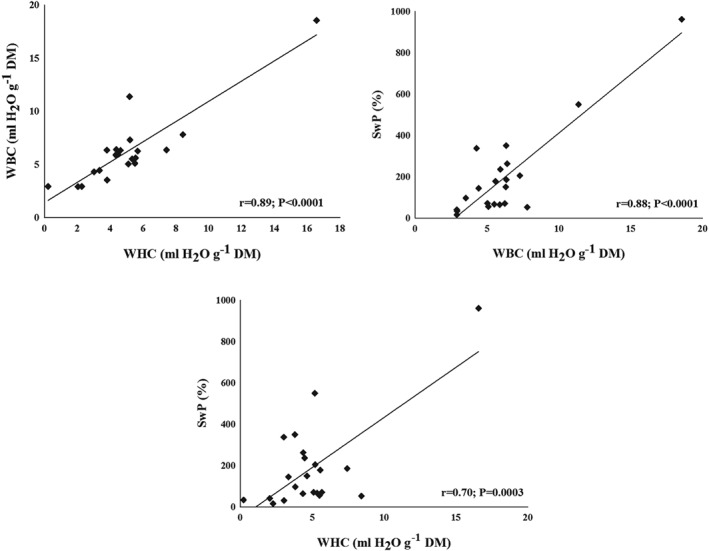
Correlation of water holding capacity (centrifugation) (WHC) and water binding capacity (WBC) as well as swelling property (SwP).
The correlation of nutrient composition of fibre content with their physicochemical properties is presented in Fig. 3. SwP correlated negatively with CF (r = −0.43; P = 0.05), aNDFOM (r = −0.48; P = 0.03) and IDF (r = −0.44; P = 0.04) but positively with SDF (r = 0.63; P = 0.002). WBC correlated positively with SDF (r = 0.47; P = 0.04). LBR was negatively correlated with CF (r = −0.49; P = 0.02), aNDFOM (r = −0.51; P = 0.02), IDF (r = −0.55; P = 0.01) and TDF (r = −0.54; P = 0.01), while it was positively correlated with CP (r = 0.53; P = 0.01).
Figure 3.
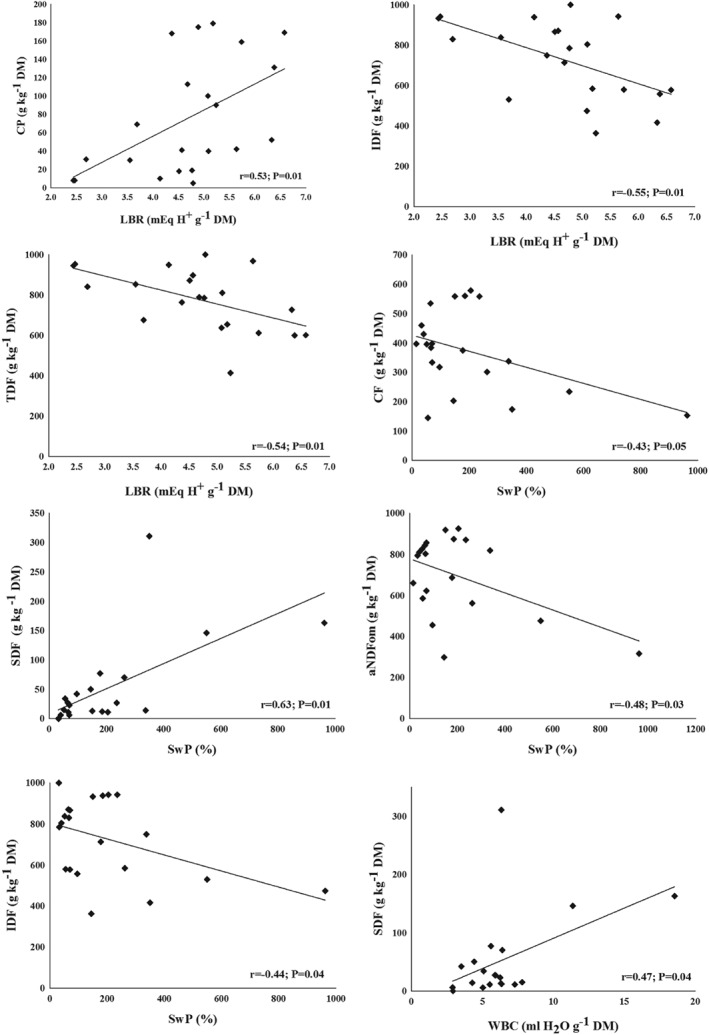
Correlations of physicochemical properties and nutrients of fibre rich feeds. aNDFOM: neutral detergent fibre; CF, crude fibre; CP, crude protein; IDF, insoluble dietary fibre; SwP, swelling property; SDF, soluble dietary fibre; TDF, total dietary fibre; WBC, water binding capacity; WHC, water holding capacity (centrifugation).
DISCUSSION
The present study focused on the determination of fibre rich feeds, including physicochemical properties in addition to quantitative fibre measurements as well as CP and CA. The fibre content differed markedly among fibre rich feeds and the values are in line with several previous studies. For instance, sugar beet pulp showed high amounts of SDF. Lignocellulose samples had approximately 90% aNDFOM, which agrees with the literature.11, 12, 29 Considering that composition of fibre rich by‐products is closely dependent on their previous processing steps, variations between those fibre sources were observed.30 The variation in fibre composition (aNDFOM, ADFOM, ADLOM and TDF) represented the diversity of fibre rich feeds and a variable response in physicochemical properties could be expected.
The values among samples were quite similar for SwP, WHC and WBC, as shown by the correlations. This means SwP, WBC or WHC could be used for fibre characterization. However, as recommendations to analyse the HC are not standardized, values obtained in other studies can hardly be used for comparison. The measurements differ in the application of centrifugal force, pore size of filtration filter or even the implementation of suction pressure during filtration steps. Further the residence time of soaking and the preparation of samples, especially the particle size, may contribute to show diversified values of HC.26, 31, 32 The consequence is that various values for the same fibre rich feed or by‐product were generated in the literature making the comparison difficult among each other. For example, sugar beet pulp expressed high HC in the study of Zhou et al. 11 with WBC: 8.7 kg kg−1 DM; SwP: 12.7 mL g−1 DM but were approximately 50% lower compared to our results. Regarding the ranking of fibre rich feeds, results were close to Brachet et al.,12 who showed high values for sugar beet pulp (5.55 g H2O g−1 DM), lignocellulose (5.14 g H2O g−1 DM) and wheat straw (4.86 g H2O g−1 DM) compared to the values of the present study. However, the values differed considerably for apple pomace (5.15 g H2O g−1 DM), when expressed after centrifugation. Findings in the study of Zhou et al. 11 underline the high correlation of WHC and SwP, whereas Brachet et al. 12 revealed weak correlation (r 2 = 0.52; P < 0.001) between WHC and SwP. In summary, for the present study, the external force during centrifugation influenced the correlation of WHC versus WBC and SwP, even though WHC and WBC seemed to be tightly related. The present data confirmed differences of HC among fibre rich feeds, demonstrating that sources of variation may have its origin in the analytical method steps itself, or differences between similar fibre rich by‐products due to processing (refer to PF in Table 1). The results demonstrated also that there is a need to standardize values using a common analytical method.
Between physicochemical properties and fibre components, variation was observed as well. The lack of significant correlation between WHC and the different quantitative fibre measurements was not expected. With higher SDF, the WBC and SwP increased. SwP decreased when CF, aNDFOM and IDF increased, which is partly in agreement with Brachet et al. 12 The authors observed moderate interactions in fibre rich feeds for the correlation between HC and fibre analytic measurements, i.e. for aNDFOM (r 2 = 0.37), ADF (r 2 = 0.40) and ADL (r 2 = 0.23). In general, it seems to be that HC values are poorly predictable from fibre analytic measurements and should be seen and used as additional information to proximate analyses of fibre rich feeds.
A further point to be mentioned is that the original PF of the samples and further the grinding intensity (e.g. resulting particle size) might alter the HC. Previous experiments emphasized that coarser particles had the ability to bind higher amounts of water, but SwP tended to decrease.11 This underlined the fact that smaller particle size with larger surface area may have higher SwP. In other studies, it was demonstrated that even if there was a larger surface area in small particles, HC values decreased if the matrix collapsed by e.g. fibre rupture.12, 32 This may give a reason to conduct further studies with different particle sizes to understand the correlation to physicochemical properties to obtain optimal grinding. Further, feed processing steps, as shown in PF 1–4 may alter HC because of e.g. starch gelatinization and rupture of fibre.12, 33, 34 Also subsequent modification of physicochemical properties of the fibre sources passing through the gastrointestinal tract might be considered as shown by Zhou et al. 11 Specific estimation of physicochemical properties of diets for the digestion in monogastric animals is a major goal supposing the values can be predicted and additivity of WHC and SwP values for compound feeds is assumed.
Regarding buffering capacity, fibre rich feeds differed considerably in LBR values. In the present study, negative correlation of LBR with results of proximate fibre analyses of feeds was detected. The decision to use LBR, although the linearized form was conducted by Oliveira et al. 13 using NDF‐prepared samples, lay on the fact that LBR allows the use of the buffering capacity rate for a wide range of pH values in feed, mimicking the typical pH variation in different sections of the gastrointestinal tract. It allows also feeds with different initial pH values to be compared. As the present study showed LBR was a practicable tool to rank different samples under similar conditions. Contrasting to the method applied by Oliveira et al.,13 who used sample residues after aNDFOM determination, in the present study whole feed samples were used to avoid possible alterations of feed matrix, which may occur during cooking processes with e.g. neutral detergent solutions. Studies to compare buffering capacity are scarce in the literature, especially as different procedures are available as well.23, 35, 36 Although our results using LBR are not comparable with other studies, the ranking of wheat bran with higher buffering capacity than soybean hulls are in line with other studies.23, 36
No correlation was detected for LBR and CA, which contrasts with observations of Jasaitis et al. 36 One possible explanation is the low amount of CA in the present study (< 1.7% versus 1.17 up to 100%), resulting in poorer buffering capacity. Next to CA, CP generates high buffering capacity, as shown in several other studies.23, 36, 37 Although the fibre rich feeds had low CP content, CP was the main reason for changes in LBR values (r = 0.53). The correction for CP content (e.g. using CP as a co‐variable) may help to understand the effect of fibre constituents on the LBR variation.
Most diets of pigs and poultry are still balanced based on CF, due to the existing large dataset. More detailed information can be obtained from aNDFOM, ADFOM and ADLOM measurements, which show to have application in ruminant nutrition. The main drawback for fibre characterization using proximal analyses is the variation in fibre content due to the filtration procedure. The proximate analyses require particle size reduction to pass a 1 mm sieve and particles smaller than 1 mm may pass through the filter pores. Such very small particles are not recovered in the filtration procedure, although they must be considered chemically as fibre. Although suffering similar restrictions as just mentioned, measurement of IDF and SDF is probably a step forward to improve the understanding of physicochemical properties of fibre rich feeds and by‐products. HC and LBR seem to be important measurements to select appropriate fibre sources when formulating diets, especially considering the contrasting environment along the gastrointestinal tract, like pH values.
CONCLUSIONS
The determination of physicochemical properties of fibre rich feeds is applicable to rank samples for these characteristics. Some correlations between fibre analytical measurements and physico‐chemical properties were detected. The results support the differentiation of samples between HC and LBR as physicochemical properties for fibre rich feeds aimed at diet formulation. However, analytical fibre measurements have still to be additionally analysed to decide on fibre amount and fibre source to be added in the diet. Further investigation is needed to standardize methods to determine HC and LBR to provide a comparable and practicable data base of fibre rich feeds as fibre sources for diet formulation.
ACKNOWLEDGEMENT
The fellowship from the first author was financially supported by the H. Wilhelm Schaumann Foundation (Hamburg, Germany). The authors declare no conflict of interest.
The copyright line for this article was changed on 16 July 2019 after original online publication.
REFERENCES
- 1. Kraler M, Schedle K, Schwarz C, Domig KJ, Pichler M, Oppeneder A et al., Fermented and extruded wheat bran in piglet diets: impact on performance, intestinal morphology, microbial metabolites in chyme and blood lipid radicals. Arch Anim Nutr 69:378–398 (2015). [DOI] [PubMed] [Google Scholar]
- 2. Schedle K, Plitzner C, Ettle T, Zhao L, Domig KJ and Windisch W, Effects of insoluble dietary fibre differing in lignin on performance, gut microbiology, and digestibility in weanling piglets. Arch Anim Nutr 62:141–151 (2008). [DOI] [PubMed] [Google Scholar]
- 3. Jha R and Berrocoso JD, Review: dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 9:1441–1452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bach Knudsen KE, Lærke HN, Ingerslev AK, Hedemann MS, Nielsen TS and Theil PK, Carbohydrates in pig nutrition – recent advances. J Anim Sci 94:1–11 (2016).26812306 [Google Scholar]
- 5. Bach Knudsen KE, The nutritional significance of “dietary fibre” analysis. Anim Feed Sci Technol 90:3–20 (2001). [Google Scholar]
- 6. Cummings JH, Mann JI, Nishida C and Vorster HH, Dietary fibre: an agreed definition. Lancet 373:365–366 (2009). [DOI] [PubMed] [Google Scholar]
- 7. McBurney MI, van Soest PJ and Chase LE, Cation exchange capacity and buffering capacity of neutral‐detergent fibres. J Sci Food Agric 34:910–916 (1983). [Google Scholar]
- 8. Urriola PE and Stein HH, Effects of distillers dried grains with solubles on amino acid, energy, and fiber digestibility and on hindgut fermentation of dietary fiber in a corn‐soybean meal diet fed to growing pigs. J Anim Sci 88:1454–1462 (2010). [DOI] [PubMed] [Google Scholar]
- 9. van Leeuwen P and Jansman AJM, Effects of dietary water holding capacity and level of fermentable organic matter on digesta passage in various parts of the digestive tract in growing pigs. Livest Sci 109:77–80 (2007). [Google Scholar]
- 10. Takahashi T, Furuichi Y, Mizuno T, Kato M, Tabara A, Kawada Y et al., Water‐holding capacity of insoluble fibre decreases free water and elevates digesta viscosity in the rat. J Sci Food Agric 89:245–250 (2009). [Google Scholar]
- 11. Zhou P, Theil PK, Wu D and Knudsen KEB, In vitro digestion methods to characterize the physicochemical properties of diets varying in dietary fibre source and content. Anim Feed Sci Technol 235:87–96 (2018). [Google Scholar]
- 12. Brachet M, Arroyo J, Bannelier C, Cazals A and Fortun‐Lamothe L, Hydration capacity: a new criterion for feed formulation. Anim Feed Sci Technol 209:174–185 (2015). [Google Scholar]
- 13. Oliveira JM, Bockor L, Eggers M, Gierus M, Dittrich JR and Warpechowski MB, Linearização de curvas de titulação para determinação da capacidade tamponante da fibra de alimentos em ampla faixa de pH. Acta Sci Anim Sci 32:55–61 (2010). [Google Scholar]
- 14. Wilfart A, Montagne L, Simmins H, Noblet J and Milgen J, Digesta transit in different segments of the gastrointestinal tract of pigs as affected by insoluble fibre supplied by wheat bran. Br J Nutr 98:54–62 (2007). [DOI] [PubMed] [Google Scholar]
- 15. Wenk C, The role of dietary fibre in the digestive physiology of the pig. Anim Feed Sci Technol 90:21–33 (2001). [Google Scholar]
- 16. de Lange CFM, Pluske J, Gong J and Nyachoti CM, Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest Sci 134:124–134 (2010). [Google Scholar]
- 17. Le Goff G, Jv M and Noblet J, Influence of dietary fibre on digestive utilization and rate of passage in growing pigs, finishing pigs and adult sows. Anim Sci 74:503–515 (2002). [Google Scholar]
- 18. Noblet J and Le Goff G, Effect of dietary fibre on the energy value of feeds for pigs. Anim Feed Sci Technol 90:35–52 (2001). [Google Scholar]
- 19. Bach Knudsen KE, Hedemann MS and Lærke HN, The role of carbohydrates in intestinal health of pigs. Anim Feed Sci Technol 173:41–53 (2012). [Google Scholar]
- 20. Jørgensen H, Serena A, Hedemann MS and Bach Knudsen KE, The fermentative capacity of growing pigs and adult sows fed diets with contrasting type and level of dietary fibre. Livest Sci 109:111–114 (2007). [Google Scholar]
- 21. Oliviero C, Kokkonen T, Heinonen M, Sankari S and Peltoniemi O, Feeding sows with high fibre diet around farrowing and early lactation: impact on intestinal activity, energy balance related parameters and litter performance. Res Vet Sci 86:314–319 (2009). [DOI] [PubMed] [Google Scholar]
- 22. Peltoniemi OAT, Björkman S and Oliviero C, Parturition effects on reproductive health in the gilt and sow. Reprod Domest Anim 51:36–47 (2016). [DOI] [PubMed] [Google Scholar]
- 23. Giger‐Reverdin S, Duvaux‐Ponter C, Sauvant D, Martin O, Nunes do Prado I and Miller R, Intrinsic buffering capacity of feedstuffs. Anim Feed Sci Technol 96:83–102 (2002). [Google Scholar]
- 24. Serena A and Knudsen KEB, Chemical and physicochemical characterisation of co‐products from the vegetable food and agro industries. Anim Feed Sci Technol 139:109–124 (2007). [Google Scholar]
- 25. Verband Deutscher Landwirtschaftlicher Untersuchungs und Forschungsanstalten , Handbuch der Landwirtschaftlichen Versuchs und Untersuchungsmethidik (VDLUFA‐Methodenbuch). VDLUFA‐Verlag, Darmstadt: (2007). [Google Scholar]
- 26. Robertson JA and Eastwood MA, An investigation of the experimental conditions which could affect water‐holding capacity of dietary fibre. J Sci Food Agric 32:819–825 (1981). [Google Scholar]
- 27. Canibe N and Bach Knudsen KE, Degradation and physicochemical changes of barley and pea fibre along the gastrointestinal tract of pigs. J Sci Food Agric 82:27–39 (2002). [Google Scholar]
- 28. McConnell AA, Eastwood MA and Mitchell WD, Physical characteristics of vegetable foodstuffs that could influence bowel function. J Sci Food Agric 25:1457–1464 (1974). [DOI] [PubMed] [Google Scholar]
- 29. Zhang W, Li D, Liu L, Zang J, Duan Q, Yang W et al., The effects of dietary fiber level on nutrient digestibility in growing pigs. J Anim Sci Biotechno 4:17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flis M, Sobotka W and Antoszkiewicz Z, Fiber substrates in the nutrition of weaned piglets – a review. Ann Anim Sci 17:627–644 (2017). [Google Scholar]
- 31. Robertson JA and Eastwood MA, A method to measure the water‐holding properties of dietary fibre using suction pressure. Br J Nutr 46:247–255 (1981). [DOI] [PubMed] [Google Scholar]
- 32. Auffret A, Ralet MC, Guillon F, Barry JL and Thibault JF, Effect of grinding and experimental conditions on the measurement of hydration properties of dietary fibres. LWT – Food Sci Technol 27:166–172 (1994). [Google Scholar]
- 33. de Vries S, Pustjens AM, Schols HA, Hendriks WH and Gerrits WJJ, Improving digestive utilization of fiber‐rich feedstuffs in pigs and poultry by processing and enzyme technologies: a review. Anim Feed Sci Technol 178:123–138 (2012). [Google Scholar]
- 34. Puntigam R, Schedle K, Schwarz C, Wanzenböck E, Eipper J, Lechner EM et al., Very high expander processing of maize on animal performance, digestibility and product quality of finishing pigs and broilers. Animal 12:1536–1546 (2017). [DOI] [PubMed] [Google Scholar]
- 35. Lawlor PG, Lynch PB, Caffrey PJ, O'Reilly JJ and O'Connell MK, Measurements of the acid‐binding capacity of ingredients used in pig diets. Ir Vet J 58:1–6 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jasaitis DK, Wohlt JE and Evans JL, Influence of feed‐ion content on buffering capacity of ruminant feedstuffs in vitro . J Dairy Sci 70:1391–1403 (1987). [Google Scholar]
- 37. Levic J, Prodanovic O and Sredanovic S, Understanding the buffering capacity in feedstuffs. Biotechnol Anim Husb 21:309–313 (2005). [Google Scholar]


