Abstract
Importance
It is important to identify features of polypoidal choroidal vasculopathy (PCV) that differentiate it from typical neovascular age‐related macular degeneration (nAMD) on various imaging modalities, including fluorescein angiography (FA).
Background
PCV was thought to be indistinguishable from nAMD using FA alone. In real‐world practice, indocyanine‐green angiography may often be unavailable or contraindicated.
Design
Analysis of FA images from a prospective, multicentre study.
Participants
Study images of both PCV and nAMD patients from the EVEREST study.
Methods
FA features at baseline were independently graded by masked graders (fellowship‐trained ophthalmologists) using standardized diagnostic algorithms.
Main Outcome Measures
Predictive indicators (sensitivity, specificity, positive and negative predictive values) for PCV.
Results
Of the 95 patients screened, 61 had PCV. Of the 34 screening failures, 15 were diagnosed as nAMD. Hyperfluorescent nodules on FA were observed in 80% of patients with PCV vs 20% with nAMD (P < 0.001). Blocked fluorescence on FA, which corresponded to the presence of subretinal haemorrhage, occurred more frequently among patients with PCV vs nAMD (61.7% vs 13.3%, P = 0.001). Similarly, the leakage characteristic of occult choroidal neovascularization occurred more frequently among patients with PCV vs nAMD (95.0% vs 73.3%, P = 0.026). The positive predictive value for PCV was 94.1% for hyperfluorescent nodules, 94.9% for blocked fluorescence, 83.8% for occult choroidal neovascularization and 82.0% for pigment epithelial detachment.
Conclusions and Relevance
This study demonstrated that certain FA features can be predictive of PCV and may be considered as an indication for retina specialists to perform indocyanine green angiography as confirmatory test.
Keywords: EVEREST, fluorescein angiography, neovascular age‐related macular degeneration, polypoidal choroidal vasculopathy, ranibizumab
1. INTRODUCTION
Neovascular age‐related macular degeneration (nAMD) is a common condition that affects older adults and results in severe impairment of central vision.1 Since Asia accounts for more than 60% of the world population, it is projected that the prevalence of AMD in Asia will experience the fastest growth, and Asia is expected to contribute the most to the global prevalence of AMD by 2040.2 Polypoidal choroidal vasculopathy (PCV) has a higher prevalence among Asian populations compared to Western populations, where occult choroidal neovascularization (CNV) appears to be the more common subtype.3, 4, 5 PCV and nAMD share common clinical features and risk factors; however, their pathophysiology, visual prognosis and clinical course differ considerably.3, 6, 7, 8 While nAMD has been shown to respond well to anti‐vascular endothelial growth factor (anti‐VEGF) monotherapy, studies have demonstrated better rates of polyp regression and improvements in visual acuity among patients receiving combination therapy with photodynamic therapy (PDT) and intravitreal ranibizumab.9, 10 The clinical outcomes of PCV have also been reported to be better than nAMD. Thus, it is clinically relevant to differentiate PCV from nAMD.
PCV presents with clinical features such as aneurysmal orange subretinal nodules (the polypoidal lesions), serosanguineous maculopathy and haemorrhagic or notched pigment epithelial detachment (PED). Multimodal imaging is essential for the accurate diagnosis of both PCV and nAMD, which share some common imaging features. While in many cases, fluorescein angiography (FA) in combination with optical coherence tomography (OCT) is considered sufficient to diagnose nAMD, indocyanine green angiography (ICGA) is considered the gold standard for diagnosing PCV,11, 12 which is based on the detection of polypoidal lesions (or “polyps”) and branching vascular network (BVN). However, in real‐world clinical practice where confirmatory investigations with ICGA are often unavailable or may be contraindicated, alternative imaging methods may be necessary to initially suggest the diagnosis of PCV.
It is believed that PCV may be indistinguishable from typical nAMD using FA alone, and the FA features of PCV may resemble that of nAMD. In real‐world practice in many regions, it is common for patients with features of nAMD to be investigated using FA alone, and hence PCV may be misdiagnosed as nAMD. Given the common occurrence of this clinical scenario, it would be useful for retinal specialists to know the diagnostic value of the features seen on FA for the diagnosis of PCV.
FA is a powerful imaging tool used routinely for clinical diagnosis of various AMD lesions and its CNV subtypes.5 Even though studies have shown that FA may not be as useful in imaging PCV,13 in specific subgroups of PCV such as those with large polypoidal lesions and BVN with overlying atrophy of the retinal pigment epithelium, FA has been shown to be helpful in imaging PCV, although it may not delineate the entire vascular abnormality.13
We performed additional analysis of image sets from the EVEREST study,14 reviewing baseline FA images to identify specific FA features that may serve as biomarkers that differentiate PCV from nAMD cases and determine the predictive values of these features.
2. METHODS
The EVEREST study (NCT00674323) was a prospective, multicentre study conducted in patients with symptomatic macular PCV from April 2008 to May 2009 at seven centres in the Asia‐Pacific region: Hong Kong (1), Republic of Korea (2), Singapore (1), Thailand (1) and Taiwan (2).
The imaging and diagnostic protocols used in the EVEREST study have previously been described in detail.11 Anatomical endpoints assessed during the study included ICGA‐assessed polyp regression based on polyp area and total lesion area, and central retinal thickness measured using OCT.10 PCV was diagnosed using a pre‐specified standardized criteria previously described in detail.11
The angiographic features were independently graded by masked graders (fellowship‐trained ophthalmologists: C.S.T. and T.H.L.) at the Central Reading Centre (Fundus Image Reading Centre [FIRC], National Healthcare Group Eye Institute, Singapore) using standardized diagnostic algorithms.11 The FA images were graded for the presence of leakage and the type of nAMD using the definitions established in the treatment of age‐related macular degeneration with PDT (TAP) and verteporfin in PDT (VIP) studies.15, 16
In this study, additional detailed analysis was performed on the FA image sets, with graders masked to the initial diagnosis (PCV or AMD), and without reference to ICGA. Among the screening failures submitted to the Central Reading Centre, a diagnosis of nAMD was made in 15 patients. Patients diagnosed with retinal angiomatous proliferation were excluded from the analysis. We analysed the FA features of these nAMD patients and compared them with PCV patients who were randomized for the study. These FA features were identified by the senior graders from an a priori review of the FA features seen in PCV in an earlier series of patients with PCV.
Lesion components such as the types and area of CNV, as well as areas of blocked fluorescence were measured using the Heidelberg Eye Explorer software version 1.6 (Heidelberg Engineering, Heidelberg, Germany). In addition, graders identified specific features on FA such as nodular hyperfluorescence (Figure 1), which consisted of a raised, round or oval nodular region, which may also have stippled hyperfluorescence.
Figure 1.
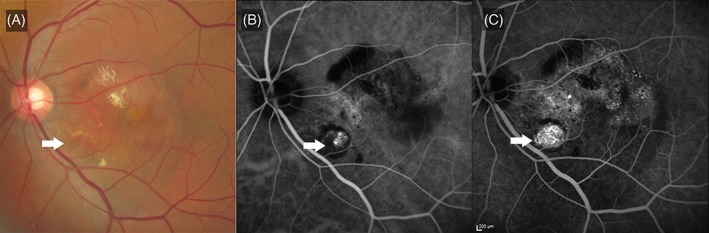
Nodular hyperfluorescence. A, Colour fundus photograph, showing pigment epithelial detachments, subretinal haemorrhages and subretinal fluid. An orange subretinal nodule (white arrow) is seen, corresponding with the location of the polyps. B, Indocyanine green angiogram (ICGA) showing the polypoidal lesion (white arrow), corresponding to the location of the orange subretinal nodule. C, Fluorescein angiogram showing a nodular hyperfluorescence (white arrow) which corresponds to both the orange nodule and the polyp on ICGA. There is stippled hyperfluorescence corresponding to occult choroidal neovascularization
Statistical analysis was performed using the SPSS software version 16 (IBM SPSS, Armonk, New York). Continuous variables were analysed using t tests, while categorical variables using chi‐square tests. The sensitivity, specificity, positive and negative predictive values were calculated for the various diagnostic features.
Ethics approval was obtained from the ethics committee or institutional review board at each study centre of the EVEREST study. Patients provided written informed consent before entering the study. The EVEREST study is registered with ClinicalTrials.gov as NCT00674323.
3. RESULTS
Of the 95 patients screened by the Central Reading Centre, 61 were diagnosed as having PCV (64.2%). The mean (±SD) age of patients with PCV was 65 (±9.2) years and 67.2% were men. All patients were of Asian ethnicity. The right eye was involved in 32 patients (53.5%).11 Of the remaining, 34 patients reported as screen failures, 15 were diagnosed as having nAMD.
Among patients diagnosed with PCV, the FA leakage patterns were occult with no classic (86.9%), minimally classic (6.6%), predominantly classic (0%) and classic with no occult (4.9%). In contrast, patients diagnosed with nAMD had lower rates of occult with no classic (66.7%), with correspondingly higher rates of classic with no occult (20%) and predominantly classic (6.7%) and minimally classic (0%).
3.1. Features on FA associated with PCV
On stereoscopic examination of the FA images, the presence of a raised hyperfluorescent nodule was predictive of PCV (Figures 1 and 2). Among patients with PCV, 80.0% had raised hyperfluorescent nodules on FA, compared to only 20.0% of patients diagnosed as nAMD (P < 0.001; Table 1). As a FA feature predictive of PCV, the presence of a hyperfluorescent nodule had a sensitivity and specificity of 80.0% each, a positive predictive value of 94.1% and a negative predictive value of 50.0% (Table 1).
Figure 2.
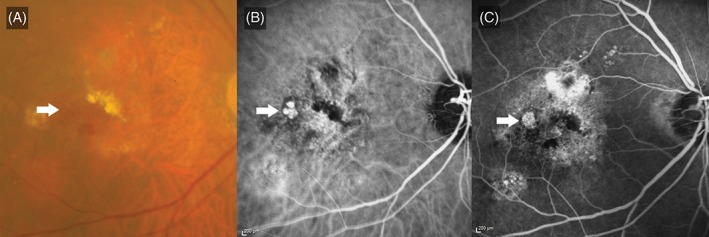
Nodular hyperfluorescence. A, Colour fundus photograph, illustrating subretinal haemorrhage and hard exudates. Orange subretinal nodules (white arrow) are observed temporal to the fovea. B, Indocyanine green angiogram demonstrating a cluster of polyps at the site of the orange nodules. C, Fluorescein angiogram illustrating a hyperfluorescent nodule (white arrow) at the site of the cluster of polyps
Table 1.
Comparison of fluorescein angiography features between polypoidal choroidal vasculopathy and neovascular age‐related macular degeneration
| Frequency among patients with polypoidal choroidal vasculopathy % (proportion) | Frequency among patients with neovascular age‐related macular degeneration % (proportion) | P value | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | |
|---|---|---|---|---|---|---|---|
| Nodular hyperfluorescence | 80.0 (48/61) | 20.0 (3/15) | <0.001 | 80.0 | 80.0 | 94.1 | 50.0 |
| Blocked fluorescence | 61.7 (37/61) | 13.3 (2/15) | 0.001 | 61. 7 | 86. 7 | 94.9 | 36.1 |
| Occult choroidal neovascularization | 95.0 (57/61) | 73.3 (11/15) | 0.010 | 95.0 | 26. 7 | 83.8 | 57.1 |
| Pigment epithelial detachment | 83.3 (50/61) | 73.3 (11/15) | 0.374 | 83.3 | 26. 7 | 82.0 | 28.6 |
The presence of blocked fluorescence on FA, which corresponded to the location of subretinal haemorrhage (Figure 3), occurred more frequently among patients with PCV compared with nAMD (61.7% vs 13.3%, P = 0.001). The specificity of this finding was 86.7%, while the positive predictive value was 94.9% (Table 1). The mean (±SD) size of the region of blocked fluorescence on FA was larger among eyes with PCV than those with nAMD (1.21 ± 2.9 mm2 [range 0‐12 mm2] compared to 0.07 ± 0.3 mm2 [range 0‐1 mm2]; P = 0.132).
Figure 3.
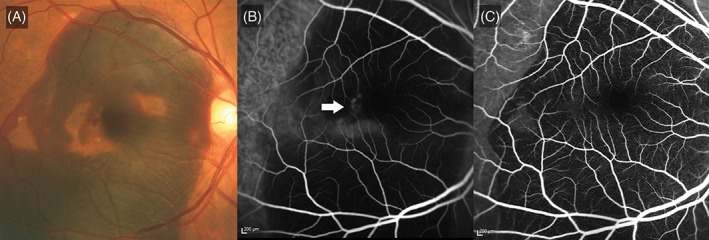
Massive submacular haemorrhage with blocked fluorescence. A, Colour fundus photograph illustrating massive subretinal haemorrhage. Pigment epithelial detachments are seen within the area of haemorrhage. B, Indocyanine green angiogram illustrating hyperfluorescence (white arrow) which is partially masked by the thick layer of haemorrhage. C, Fluorescein angiogram showing blocked fluorescence as a result of the haemorrhage
The pattern of leakage on FA was also predictive of PCV. Cases diagnosed as PCV were more likely to manifest with features characteristic of either occult CNV with no classic or occult with minimally classic on FA (Figure 4), compared with the nAMD cases. Leakage characteristic of occult CNV on FA was observed in a higher proportion of eyes with PCV than those with nAMD (P = 0.010; Table 1). The presence of occult with no classic or minimally classic leakage on FA had a sensitivity of 95.0%, specificity of 26.7% and a positive predictive value of 83.8% as an FA feature predictive of PCV (Table 1). Leakage characteristic of predominantly classic CNV on FA was observed in 5.0% of PCV cases compared with 26.7% of nAMD cases (P = 0.010). In addition, the mean area of the classic CNV component was notably smaller in eyes diagnosed as having PCV compared to those with nAMD (0.09 mm2 vs 0.78 mm2, P = 0.001).
Figure 4.
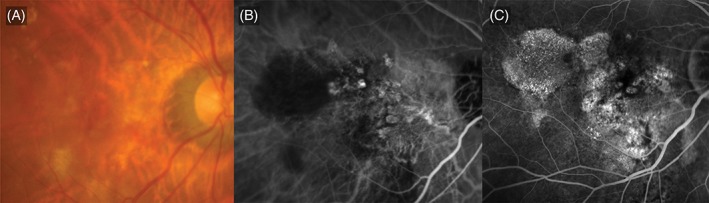
Occult choroidal neovascularization (CNV). A, Colour fundus photograph showing subretinal haemorrhage and pigment epithelial detachments. B, Indocyanine green angiogram showing polypoidal choroidal vasculopathy with multiple polyps and a branching vascular network. C, Fluorescein angiogram showing stippled hyperfluorescence characteristic of occult CNV
4. DISCUSSION
The EVEREST study was the first randomized controlled trial that assessed the efficacy of a combination treatment with ranibizumab and PDT in patients with PCV.10 In this analysis of images from the EVEREST study, we identified the typical features of PCV seen on FA and related the features to findings on ICGA and colour fundus photography.
Nodular hyperfluorescence on FA corresponding to the location of the polyps on ICGA, blocked fluorescence on FA corresponding to submacular haemorrhage on colour fundus photography and leakage characteristic of occult CNV on FA, were all notably higher in eyes with PCV than those with nAMD. Both nodular hyperfluorescence and blocked fluorescence had a positive predictive value for PCV of >90%, while presence of occult CNV and PED had a predictive value of >80.0%. Among eyes with PCV, 95% had leakage characteristic of occult CNV, and the proportion was higher than in eyes with nAMD. Additionally, this occult pattern of leakage observed on FA had high sensitivity for PCV. However, it is important to note the presence of occult CNV had low specificity, as this pattern of leakage also occurs in nAMD patients.
Consistent with the findings in this analysis, studies such as EVEREST II have shown eyes with PCV to mainly have occult CNV (either predominantly occult or occult with no classic) based on FA9; with few eyes (<10%) showing classic CNV.9, 17 In contrast, in studies conducted in typical nAMD, the frequency of occult CNV was 60%‐70%, while classic CNV (minimally and predominantly classic) accounted for 30%‐40% of cases.18, 19, 20 In the few eyes that showed leakage characteristic of classic CNV on FA in this study, the mean area of the classic CNV component was notably smaller in eyes with PCV than those with nAMD. Although few eyes with PCV had classic CNV in this study and in previous studies, it is equally important to correctly diagnose classic CNV, considering the visual prognosis is worse in eyes with PCV and classic CNV.21
Maruko et al. observed that eyes with PCV with an abnormal vascular network but without a polypoidal lesion on ICGA may be misdiagnosed as occult CNV associated with nAMD because the abnormal vascular network of PCV often shows leakage on FA or ICGA.6 It has been suggested that misdiagnosis of nAMD is one of the common clinical factors that affects response to anti‐VEGF therapy, and that nearly 50% of patients with a poor response to anti‐VEGF treatment required revision of the primary diagnosis.22 PCV is reported to occur in 8%‐13% of Caucasian patients with a diagnosis of nAMD.23 The lower prevalence of PCV in Western populations and the lack of ICGA as a routine investigation in many centres might possibly result in PCV initially being diagnosed as nAMD.22 In fact, when both FA and ICGA were included as part of the study assessments, occult CNV on FA was diagnosed as PCV on ICGA in 18.6% of newly diagnosed nAMD patients in a study conducted in the United States,4 and in 20% of newly diagnosed nAMD patients in a study conducted in the United Kingdom.24
The presence of blocked fluorescence correlated with haemorrhage in both PCV and nAMD. In this study, we found that the frequency of haemorrhage, as well as the size of the haemorrhage, was greater among PCV patients compared to nAMD. The observation of haemorrhage, especially large areas of haemorrhage, would be an indication to perform ICGA to detect PCV.
The strength of this study is that this is the first analysis, to our knowledge, conducted to specifically assess differences in FA characteristics between patients who had been diagnosed with PCV or nAMD using stringent, pre‐specified diagnostic criteria, and to determine the sensitivity, specificity and predictive values of these FA features in enabling diagnosis of PCV. All patients underwent standardized imaging,11 and were graded in a masked fashion by experienced graders from a Central Reading Centre. In addition, diagnoses other than nAMD that may mimic PCV on ICGA were also excluded from this analysis.25 The analysis shows that presence of certain FA features would suggest a higher likelihood of PCV. In cases where ICGA is not routinely performed, but is available, identification of these features on FA may be an indication for retina specialists to perform ICGA. In cases where ICGA is not available or contraindicated (eg, in patients with shellfish or iodine allergy), these FA features may be an alternative imaging modality that suggests the diagnosis of PCV.
The limitations of the study are the relatively small patient numbers, unequal sample size between the PCV and nAMD groups, and retrospective nature with potential for selection bias. Also, the EVEREST study specifically aimed to recruit PCV patients, and hence the study design resulted in a study population with a higher prevalence of PCV. It is possible that the PPV may be over‐estimated as the result of the higher frequency of PCV in this series. This may result in the calculation of a non‐normalized PPV and NPV and therefore these values may not be applicable to clinic‐based cohorts or patients from other populations.
In conclusion, results of this analysis of the EVEREST study show the predictive nature of certain FA features in the diagnosis of PCV. The FA features of PCV may also occur in nAMD, and hence FA cannot replace ICGA for the definitive diagnosis of PCV. However, the presence of these FA features can be an indication for retina specialists to perform necessary additional confirmatory tests. The findings from this retrospective analysis need to be confirmed in future prospective trials.
CONFLICTS OF INTEREST
The EVEREST study (NCT00674323) was funded by Novartis Pharma AG, Basel, Switzerland. The sponsor participated in the design and conduct of the EVEREST study, and preparation, review and final approval of this manuscript. However, for this additional analysis, the sponsor did not participate in data collection, management, analysis and interpretation. The sponsor provided medical writing support and also approved the manuscript.
ACKNOWLEDGEMENTS
C.S.T. reports grants from National Medical Research Council (NMRC/TA/0039/2015), grants from National Healthcare Group, honoraria and non‐financial support from Bayer, non‐financial support from Heidelberg Engineering, honoraria and non‐financial support from Novartis, outside the submitted work. W.K.N. reports grant from National Healthcare Group (CSPP‐16008) and non‐financial support from Allergan, outside the submitted work. N.W.T. reports non‐financial support from Allergan, Bayer and Novartis, outside the submitted work. T.H.L. reports non‐financial support from Novartis and Heidelberg Engineering, outside the submitted work. L.W.L. has no financial interests to declare. The authors thank Mayuri Shinde and Lakshmi Venkatraman (Scientific Services Practice—Product Lifecycle Services, Novartis Healthcare Pvt. Ltd., Hyderabad, India) for medical writing and editorial assistance towards the development of this article.
Tan CS, Ngo WK, Lim LW, Tan NW, Lim TH. EVEREST study report 4: Fluorescein angiography features predictive of polypoidal choroidal vasculopathy. Clin. Experiment. Ophthalmol. 2019;47:614–620. 10.1111/ceo.13464
Data from this analysis were previously presented at the American Academy of Ophthalmology Annual Meeting, 15‐18 October 2016, Chicago, Illinois.
Funding information Norvatis Pharma AG
REFERENCES
- 1. Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age‐related macular degeneration. Lancet. 2012;379:1728‐1738. [DOI] [PubMed] [Google Scholar]
- 2. Wong WL, Su X, Li X, et al. Global prevalence of age‐related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta‐analysis. Lancet Glob Health. 2014;2:e106‐e116. [DOI] [PubMed] [Google Scholar]
- 3. Lim LS, Cheung CM, Wong TY. Asian age‐related macular degeneration: current concepts and gaps in knowledge. Asia Pac J Ophthalmol (Phila). 2013;2:32‐41. [DOI] [PubMed] [Google Scholar]
- 4. Iranmanesh R, Eandi CM, Peiretti E, et al. The nature and frequency of neovascular age‐related macular degeneration. Eur J Ophthalmol. 2007;17:75‐83. [DOI] [PubMed] [Google Scholar]
- 5. Jung JJ, Chen CY, Mrejen S, et al. The incidence of neovascular subtypes in newly diagnosed neovascular age‐related macular degeneration. Am J Ophthalmol. 2014;158:769‐779. e2. [DOI] [PubMed] [Google Scholar]
- 6. Maruko I, Iida T, Saito M, Nagayama D. Combined cases of polypoidal choroidal vasculopathy and typical age‐related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2010;248:361‐368. [DOI] [PubMed] [Google Scholar]
- 7. Yannuzzi LA, Wong DW, Sforzolini BS, et al. Polypoidal choroidal vasculopathy and neovascularized age‐related macular degeneration. Arch Ophthalmol. 1999;117:1503‐1510. [DOI] [PubMed] [Google Scholar]
- 8. Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age‐related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29:19‐29. [DOI] [PubMed] [Google Scholar]
- 9. Koh A, Lai TYY, Takahashi K, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453‐1464. [DOI] [PubMed] [Google Scholar]
- 11. Tan CS, Ngo WK, Chen JP, Tan NW, Lim TH. EVEREST study report 2: imaging and grading protocol, and baseline characteristics of a randomised controlled trial of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99:624‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan CS, Ngo WK, Lim LW, et al. A novel classification of the vascular patterns of polypoidal choroidal vasculopathy and its relation to clinical outcomes. Br J Ophthalmol. 2014;98:1528‐1533. [DOI] [PubMed] [Google Scholar]
- 13. Imamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA. Polypoidal choroidal vasculopathy: a review. Surv Ophthalmol. 2010;55:501‐515. [DOI] [PubMed] [Google Scholar]
- 14. ClinicalTrials.gov. Efficacy and Safety of Verteporfin Added to Ranibizumab in the Treatment of Symptomatic Macular Polypoidal Choroidal Vasculopathy (PCV) (Last updated on 2011). https://clinicaltrials.gov/ct2/show/results/NCT00674323?sect=X0156. Accessed on October 31, 2017.
- 15. Verteporfin in Photodynamic Therapy Study Group . Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1‐year results of a randomized clinical trial—VIP report no. 1. Ophthalmology. 2001;108:841‐852. [DOI] [PubMed] [Google Scholar]
- 16. Photodynamic therapy of subfoveal choroidal neovascularization in age‐related macular degeneration with verteporfin: one‐year results of 2 randomized clinical trials—TAP report. Treatment of age‐related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol. 1999;117:1329‐1345. [PubMed] [Google Scholar]
- 17. Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003;121:1392‐1396. [DOI] [PubMed] [Google Scholar]
- 18. Olsen TW, Feng X, Kasper TJ, Rath PP, Steuer ER. Fluorescein angiographic lesion type frequency in neovascular age‐related macular degeneration. Ophthalmology. 2004;111:250‐255. [DOI] [PubMed] [Google Scholar]
- 19. Cohen SY, Creuzot‐Garcher C, Darmon J, et al. Types of choroidal neovascularisation in newly diagnosed exudative age‐related macular degeneration. Br J Ophthalmol. 2007;91:1173‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zawinka C, Ergun E, Stur M. Prevalence of patients presenting with neovascular age‐related macular degeneration in an urban population. Retina. 2005;25:324‐331. [DOI] [PubMed] [Google Scholar]
- 21. Tamura H, Tsujikawa A, Otani A, et al. Polypoidal choroidal vasculopathy appearing as classic choroidal neovascularisation on fluorescein angiography. Br J Ophthalmol. 2007;91:1152‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang S, Zhao J, Sun X. Resistance to anti‐VEGF therapy in neovascular age‐related macular degeneration: a comprehensive review. Drug Des Devel Ther. 2016;10:1857‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004;49:25‐37. [DOI] [PubMed] [Google Scholar]
- 24. Talks J, Koshy Z, Chatzinikolas K. Use of optical coherence tomography, fluorescein angiography and indocyanine green angiography in a screening clinic for wet age‐related macular degeneration. Br J Ophthalmol. 2007;91:600‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan CS, Ngo WK, Lim LW, et al. EVEREST study report 3: diagnostic challenges of polypoidal choroidal vasculopathy. Lessons learnt from screening failures in the EVEREST study. Graefes Arch Clin Exp Ophthalmol. 2016;254:1923‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]


