Abstract
Discovery and development of new potentially selective anticancer agents are necessary to prevent a global cancer health crisis. Currently, alternative medicinal agents derived from plants have been extensively investigated to develop anticancer drugs with fewer adverse effects. Among them, steroidal alkaloids are conventional secondary metabolites that comprise an important class of natural products found in plants, marine organisms and invertebrates, and constitute a judicious choice as potential anti‐cancer leads. Traditional medicine and modern science have shown that representatives from this compound group possess potential antimicrobial, analgesic, anticancer and anti‐inflammatory effects. Therefore, systematic and recapitulated information about the bioactivity of these compounds, with special emphasis on the molecular or cellular mechanisms, is of high interest. In this review, we methodically discuss the in vitro and in vivo potential of the anticancer activity of natural steroidal alkaloids and their synthetic and semi‐synthetic derivatives. This review focuses on cumulative and comprehensive molecular mechanisms, which will help researchers understand the molecular pathways involving steroid alkaloids to generate a selective and safe new lead compound with improved therapeutic applications for cancer prevention and therapy. In vitro and in vivo studies provide evidence about the promising therapeutic potential of steroidal alkaloids in various cancer cell lines, but advanced pharmacokinetic and clinical experiments are required to develop more selective and safe drugs for cancer treatment.
Keywords: steroidal alkaloids, anticancer, molecular mechanism, in‐silico drug design
Abbreviations
- Δψm
mitochondrial membrane potential
- AChE
acetylcholinesterase
- APC4
anaphase‐promoting complex 4
- ASA
anticancer steroidal alkaloid
- ATG5
autophagy‐related protein 5
- BChE
butyrylcholinesterase
- bFGF
basic fibroblast growth factor
- Cap‐NMR
capillary nuclear magnetic resonance spectroscopy
- CCND1
cyclin D1
- CDKN2B
cyclin‐dependent kinase inhibitor 2B
- CHOP/GADD153
C/EBP homologous protein/growth arrest‐ and DNA damage‐inducible gene 153
- CNS
central nervous system
- CRPC
castration‐resistant prostate cancer
- EC50
half maximal effective concentration
- ED50
effective dose, for 50% of individuals getting the drug
- EIF‐2
eukaryotic initiation factor‐2
- EP4
E‐prostanoid receptor 4
- ER
endoplasmic reticulum
- ERK
extracellular signal regulating kinase
- ERK1/2
extracellular signal‐regulated kinase 1 and 2
- FAK
focal adhesion kinase
- FDA
Food and Drug Administration
- GADD45α
growth arrest and DNA damage‐inducible protein GADD45 α
- GIT
gastrointestinal tract
- HER2
human epidermal growth factor receptor
- HPLC
high‐performance liquid chromatography
- HPLC‐ESIMS
high‐performance liquid chromatography‐electrospray ionization mass spectrometry
- IκBα
inhibitor of κBα
- LC–MS
liquid chromatography–mass spectrometry
- LC‐NMR‐MS
liquid chromatography–nuclear magnetic resonance spectroscopy‐mass spectrometry
- LC‐SPE‐NMR
liquid chromatography‐solid‐phase extraction‐nuclear magnetic resonance spectroscopy
- LPS
lipopolysaccharide
- MAP‐2
microtubule‐associated protein 2
- Mcl‐1s
Mcl‐1 short form
- MS
mass spectrometry
- MUC1
Mucin 1
- NF‐κB
nuclear factor kappa B
- NMR
nuclear magnetic resonance spectroscopy
- NSCLC
non‐small cell lung cancer
- p38 MAPK
p38mitogen‐activated protein kinase
- PARP‐1
Poli (ADP‐Ribose) Polymerase‐1
- PKCα
protein kinase C‐α
- RECK
reversion‐inducing cysteine‐rich protein with Kazal motifs
- SAs
steroidal alkaloids
- SCUBA
self‐contained underwater breathing apparatus
- SKP2
S‐phase kinase‐assoc. protein 2
- SMAC/DIABLO
second mitochondrion‐derived activator of caspases/direct inhibitor of apoptosis‐binding protein with a low isoelectric point
- SMO
smoothened receptor
- STAT3
signal transducer and activator of transcription 3
- TAR
transactivation‐responsive
- TIMP‐1
tissue inhibitor of metalloproteinase‐1
- TKIs
tyrosine kinase inhibitors
- TNFα
tumor necrosis factor α
- TopoIIα
topoisomerase Iiα
- TXNRD1
thioredoxin reductase 1
- U‐PA
urokinase‐type plasminogen activator
- VEGF
vascular endothelial growth factor
Introduction
Small molecules containing a steroidal structure possess a wide range of pharmacological activities (e.g., antioxidant, neuroprotective and anti‐hypercholesterolemic), irrespective of their highly conserved chemical structures and hormonal action (e.g., glucocorticoids, mineralocorticoids and gonadal steroids).1, 2 Various natural products have traditionally been used to treat or cure different diseases.3, 4, 5, 6, 7, 8, 9 Recently, extensive studies have used natural and synthetically derived compounds to develop novel preventive or therapeutic agents for clinical applications for cancer treatment.10, 11, 12, 13, 14, 15, 16 Over 60% of the available anticancer drugs are derived from natural sources, and this underlies the importance of these studies.17 Understanding the structure–activity relationships and traditional applications of natural product‐derived compounds can facilitate the design and synthesis of a vast array of novel therapeutics.
Prospective natural sources for drug discovery can be terrestrial or marine; those of terrestrial origin (plants, microorganisms, vertebrates and invertebrates) are easily accessible and offer a promising foundation for potentially new chemical entities (NCE). According to the World Health Organization (WHO), approximately 80% of the global population (representing approximately four billion people) living in developing countries depend on plant‐based traditional medicines for their primary health care.18, 19 Ethnomedicinal properties of traditional medicines, along with their diverse medical applications in various diseases, have been well‐characterized in several publications.20, 21, 22, 23, 24, 25, 26 Goat bitter apple or soda apple (Solanum aculeastrum), thorn apple (Solanum incanum L.), tomato (Lycopersicon esculentum M.), false daisy (Eclipta alba L.) and Christmas box (Sarcococca saligna) are well‐known plants possessing anticancer activity. Solamargine, tomatidine, solasonine, α‐solanine and α‐chaconine are natural products isolated from terrestrial plants and exhibit potent anticancer activity (Supporting Information S1, Tables 1–4). In addition to anticancer activity, steroidal alkaloids (SAs) also possess analgesic,27, 28 anti‐inflammatory,27, 29, 30, 31 antimicrobial,32, 33, 34, 35, 36, 37, 38, 39, 40 antithrombotic,41 antiandrogenic42 and antiarrhythmic43, 44, 45 properties, which deem them a subject of high research interest.
Conversely, marine organisms comprise ~50% of the total biodiversity of the Earth, and the marine ecosystem is an exceptional and vast reservoir of bioactive natural products with unique chemical features.46 Although few marine‐derived drug candidates are clinically available, significant numbers of anticancer SAs (ASAs) have been discovered from marine sources.47
Over 10,000 bioactive compounds have been discovered from the marine ecosystem, and hundreds of novel compounds with innumerable pharmacological properties are identified annually.48 Notably, sponges and worms are significant sources of ASAs. Although few ASAs have been obtained from the marine environment to date, among them, cephalostatin 1 is one of the most potent anticancer small molecules ever examined by the U.S. National Cancer Institute (NCI). Cephalostatin I is active at nanomolar concentrations and is noticeably more effective in vitro than paclitaxel in dispelling the mitochondrial membrane potential to induce apoptosis.49, 50, 51
Marine sources show great promise for the discovery of anticancer drugs, and the involvement of interdisciplinary fields, including pharmaceutical chemistry, pharmacology, analytical and organic chemistry, ecology, biology and biochemistry, has established marine anticancer drug discovery as a new field. Approximately seven marine‐derived pharmaceutical elements have been approved by the FDA (Food and Drug Administration) for clinical use as drugs for various diseases. Brentuximab vedotin was approved for the treatment of Hodgkin's lymphoma and anaplastic large T‐cell malignant lymphoma, cytarabine for leukemia, trabectedin for ovarian cancer and soft tissue sarcoma, eribulin mesylate for metastatic breast cancer, Ziconotide for pain, omega‐3‐acid ethyl esters for hypertriglyceridemia, vidarabine for recurrent epithelial keratitis caused by HSV, superficial keratitis and acute kerato‐conjunctivitis; many other compounds are undergoing clinical trials.47, 52, 53, 54, 55, 56 Clinical trials involving cephalostatins 1 and 7 have been hindered because of the complications in harvesting the materials (0.1 g and <60 mg of cephalostatins 1 and 7, respectively, from 450 kg of the marine tube worm (Cephalodiscus gilchristi) from 60 to 80 m deep in sea waters near East Africa.57 Synthesis of cephalostatin will render the bis‐steroidal alkaloid more available and allow clinical trials. To expedite the design and development of cephalostatin, the identification and validation of the biological targets of cephalostatin should be coupled with quantitative structure–activity relationship (QSAR) studies.
To date, various articles have been published regarding the isolation, synthesis and anticancer effects of SAs; however, to the best of our knowledge, a review focusing on the mechanisms of action of SAs and their synthetic/semi‐synthetic derivatives in various cancer cell lines, including their sources and inhibitory concentrations, has not yet been published. Secondary metabolites from natural products exhibit more “drug‐likeness” (a qualitative concept for how “druglike” a compound is with respect to various parameters such as molecular weight, hydrogen bond and bioavailability, used in the field of drug design) properties than wholly synthetic molecules.58 Therefore, we summarized the available information regarding these ASAs to assist future efforts toward anticarcinogenic drug discovery.
In this review, we systematically summarize the in vitro (Supporting Information S1, Tables 1–3) and in vivo (Supporting Information S1, Table 4) anticancer effects of natural, synthetic and semi‐synthetic SAs, with emphasis on their mechanisms of action. Hereafter, we also highlight the molecular mechanisms involved in their anticancer effects (Supporting Information S1, Tables 1–4; Fig. 1), along with various in silico approaches involved in the development of anticancer leads from SAs.
Figure 1.
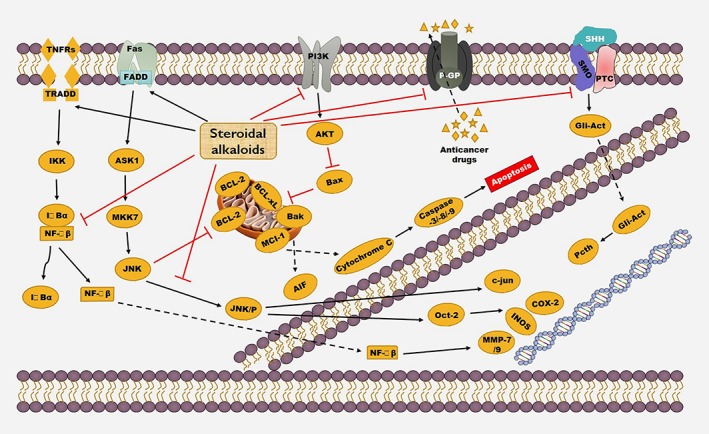
Anticancer mechanism of steroidal alkaloids. Reproduced with permission from the publisher of Ref. 54. Copyright © 2015, John Wiley and Sons.
Steroidal Alkaloids
Pharmacological properties
Steroidal alkaloids are secondary metabolites, which can be defined as organic molecules involved in the defense mechanisms of plants, with no role in growth, development or reproduction. In addition to SAs, terpenes, phenolic compounds, flavonoids and coumarins are known as secondary metabolites.59, 60, 61 They constitute an essential class of alkaloids generally isolated in the glycoalkaloid form, which characteristically occurs in higher plants from the Solanaceae, Liliaceae, Apocynaceae and Buxaceae families, and in amphibians and marine invertebrates (Fig. 2).62, 63, 64 In addition to their different bio‐potentialities, including analgesic,28 antimicrobial,39 and anti‐inflammatory29 activities, their anticancer potentials are being explored.65, 66, 67 Numerous synthetic routes have also been developed for synthesizing SA analogs to achieve desired biological effects. Although there are multiple reports regarding the widespread pharmacological activities of SAs, few systematic reviews regarding the anticancer potentials and mechanisms of action of SAs are currently available.
Figure 2.
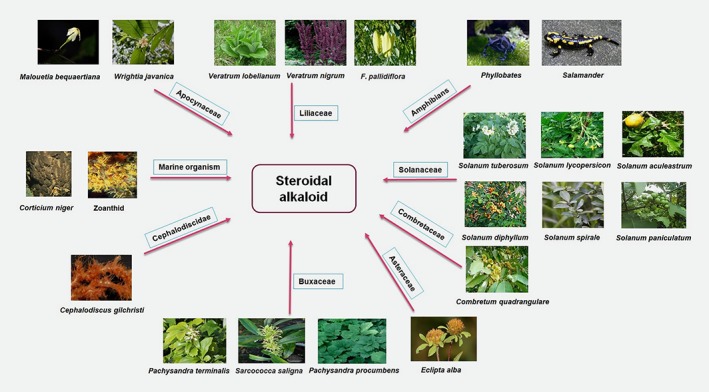
Vital sources of steroidal alkaloids. Reproduced with permission from the publisher of Ref. 54. Copyright © 2015, John Wiley and Sons.
Structural insights and classification
SAs are characterized by a cyclopentanephenanthrene framework (steroidal scaffold), with a nitrogen atom fused as a fundamental fragment of the molecule, or in the ring or side chain.62 Generally, SAs possess a 21‐(pregnane), a 24‐(cyclopregnane), a 27‐carbon heterocyclic ring or combination of these. The C27 heterocyclic ring containing SAs are of two types: cholestane and C‐nor‐D‐homosteroidal alkaloids, mostly occurring in the Solanaceae and Liliaceae families. Table 1 lists the different types of natural SAs.68
Table 1.
Examples of major SAs
| Major types of SAs | Origin | Instances | Brief Chemistry of the representatives |
|---|---|---|---|
| Salamandra | Amphibians | Cycloneosamandione | In this type of steroidal alkaloid, the ring‐A is altered, commonly into aza‐homosteroids, except for Cycloneosamandione. |
| Jerveratrum | Solanaceae, mainly found in food crops like potatoes, tomatoes | Jervine | Here the side‐chain of a C27 steroid has been transformed into a piperidine ring. |
| Spirosolane | Soladulcidine | ||
| Solanidine | Rubijervine | In this type, an extra ring is formed by C‐16 to N bond formation. | |
| Cerveratrum | Monocot families such as the Orchidaceae | Cevane | In this type of steroidal alkaloid, an abeo shift occurs from a procevine‐type precursor, where the D ring has been expanded at the expense of the C ring. A widespread stereochemical difference has been observed within the basic cevane skeleton. |
| Conanine | Apocynaceae | Kurcholessine | Here almost all of the bases comprise an amino or an oxygen function at C‐3 position. Some differences are observed in their skeletons such as methylation at the C‐4 position. |
| Buxus | Cortistatin | This type of steroidal alkaloid is one of the largest groups, where the maximum structures are pentacyclic 4, 4, 14‐trimethyl‐9, 19‐cyclopregnanes. Some of this group posses a tetracyclic system where the 9, 10 bond has been fused to give a seven‐membered ring B. In some representatives, one or both carbon atoms attached to C‐4 have been lost. Presence of a nitrogen function at C‐3 and/or C‐20 has been observed, which may be partially methylated, fully methylated or unmethylated. Slight structural differences remain to be isolated from various Buxus spp. In this group, the suffix letters used in the nomenclature designate the degree of methylation of the nitrogen atom(s). | |
| Pregnane | Apocynaceae, Buxaceae and Didymelaceae | In this type, major representatives contain an amino group or related functional groups at C‐3 or C‐20, and regularly at other positions . | |
| Cephalostatins/ ritterazines |
marine worm Cephalodiscus gilchristi (Cephalostatin 1) and tunicate Ritterella tokioka (Ritterazine A) |
Cephalostatin 1, Ritterazine A | The cephalostatin 1 structure is categorized by an asymmetric blending of the central pyrazine ring with two extremely oxygenated steroidal spiroketal subunits. In ritterazine A, the left half is similar to that of cephalostatin 7, excluding an additional C‐7′ hydroxyl group. In the right half, the steroidal nucleus is totally rearranged. |
| Assorted steroidal alkaloids, including non‐nitrogenous steroids connected by an ester or acetal bond to a nitrogen‐containing unit | Marine sponges, toads, | Plakinamines, bufotoxin | In this group, the ester or acetal bond act as a linker between non‐nitrogenous steroids and the nitrogen containing unit. |
Abbreviation: SA, steroidal alkaloid.
Mode of action of the anticancer activity exerted by SAs
Induction of apoptosis
Induction of apoptosis is one of the essential anticancer mechanisms of SAs. Apoptosis, or programmed cell death, is a highly synchronized and conserved cellular phenomenon maintained by a highly organized network of intrinsic cellular suicide machinery. When the homeostasis between cell proliferation and death is disturbed, apoptosis‐inducing pathways are altered, which results in oncogenesis.69 Receptor‐controlled extrinsic pathways or mitochondrion‐controlled intrinsic pathways can trigger apoptosis.70 Additionally, other cellular organelles, including the endoplasmic reticulum (ER), cytoskeleton, lysosomes and nucleus, might participate in apoptotic communication by detecting impairments or by incorporating pro‐apoptotic signals.71 Various SAs activate apoptosis in specific cancer cell lines, including briofilin [increases Bax protein expression, suppresses Bcl‐2, caspase‐3 initiation and segmentation of poly (ADP‐ribose) polymerase‐1 (PARP‐1) in HeLa cells], the 3‐O‐(β‐d‐glucopyranosyl) etioline‐induced receptor‐mediated extrinsic apoptotic pathway, solamargine (upregulates TNFR‐1), cephalostatin 1 (uses Smac/DIABLO for inducing apoptosis) (Supporting Information S1, Tables 1–4).50, 51, 57
Cell cycle arrest
Disruption of cell cycle progression plays a role in the anti‐oncogenic effect of SA (Supporting Information S1, Tables 1 and 2). Mitogenic signals permit cells to enter controlled pathways, allowing traversing of the cell cycle.72 CDKs are master regulators in the cell cycle.73, 74 Dysregulation or modification of CDKs results in neoplasia.72 Hyperactivation of CDKs results from mutations in CDK‐regulated genes; CDK‐inhibitor genes are linked to several cancer types. Thus, the inhibitors or modulators offer great promise for use in novel anticarcinogenic therapies.75, 76 SAs such as solamargine, ritterazine b and solanidine derivatives arrest the G0/G1 and G2/M checkpoints in cancer cells (Supporting Information S1, Tables 1 and 2).30, 50, 77, 78, 79, 80, 81
Anti‐proliferative and anti‐metastatic effects
The molecular mechanism underlying the antiproliferative effects of SAs might involve the inhibition of various cell signaling pathways and proteins (such as MMP‐2/9 and AKT) that allow the growth of cancer cells.82, 83, 84, 85, 86, 87, 88 Inhibition of MMP‐2 and MMP‐9 by solanine is associated with the suppression of A2058 human melanoma cell migration and invasion, which contributes to the anti‐metastatic activity of SAs.89 Akt (protein kinase B) phosphorylation, which regulates various proteins involved in metastasis and cancer cell proliferation, is inhibited by α‐tomatine.90, 91, 92, 93 The anti‐proliferative activity of α‐tomatine also involves an increase in p21WAF1/CIP1 levels and checkpoint kinase‐2 activation (Supporting Information S1, Tables 1, 3 and 4). Additionally, SAs such as solasodine and cephalostatin 1 can induce DNA fragmentation and inhibit protein synthesis (Supporting Information S1, Tables 1, 2 and 4).50, 51, 57, 80, 94, 95, 96, 97, 98, 99, 100
Anticancer effects of major SAs
Tomatidine
Goat bitter apple or soda apple (S. aculeastrum) and tomato (S. lycopersicon L.) are rich sources of tomatidine and its glycosides such as α‐, β‐, γ‐ and δ‐tomatine.101 Tomatidine exerts cytotoxic activity against HBL‐100 cells.102 MCF‐7, HT‐29 and HeLa cells were blocked at the G0/G1 phase after treatment with tomatidine.103 Tomatidine also obstructs the invasion and migration of A549 cells by upregulating TIMP‐1 and RECK and downregulating MMP‐2/9 expression.66 By inhibiting the function of ABC transporters, tomatidine efficiently sensitizes carcinoma cells.104
Tomatidine glycosides are more cytotoxic against MDA‐MB‐231, KATO III and PC‐3 cells, compared to tomatidine,105 proving that the carbohydrate moiety plays a pivotal role in its cytotoxicity. α‐Tomatine exerts significant cytotoxicity against MCF‐7, HL60, NCI‐H460, AGS, K562 and HT‐29 cells.106, 107, 108 Lee et al., 2011 reported that α‐tomatine treatment for 1 h kills PC‐3 cells, compared to the normal prostate and liver cells.92 The half‐maximal effective concentration (EC50) of α‐tomatine for PC‐3 cancer cells was 1.67 ± 0.3 μM, whereas those for the normal liver cells WRL‐68 and the normal prostate cells RWPE‐1 was higher (>5.0 and 3.85 ± 0.1 μM, respectively) for 24‐h‐long treatment. The anti‐metastatic activity of α‐tomatine has been well characterized through the inhibition of the signaling of the PI3K/Akt pathway, inhibition of FAK phosphorylation and modulation of IκBα protein expression.108 α‐Tomatine can activate cellular apoptosis via caspase‐3/−8/−9, Mcl‐1 and Bak activation.92
Solamargine and solasonine
Solamargine and solasonine are two important spirosalane SAs. Solamargine exhibits selective and significant cytotoxic effects against HepG2, HCT116, MCF‐7, HeLa, K562 and A549 cells, with an IC50 value of 2.5, 3.8, 2.1, 6.0, 5.2 and 8.0 μM, respectively, which are 2–3‐fold lower than that for HL7702 human normal hepatocytes and H9C2 rat normal cardiomyoblasts (IC50, 13.5 and >20 μM, respectively).77 Shiu et al., 2007 reported that solamargine exhibited more cytotoxicity on breast cancer cells, compared to cyclophosphamide, methotrexate, epirubicin, cisplatin and 5‐fluorouracil.78 Furthermore, intravenous injection of solamargine (2.4 mg/kg) inhibited the progression of hepatocellular H22 cancer cells and Ehrlich ascites tumors in mice by 57.37% and 67.55%, respectively.79
Solasodine
Similarly, solasodine and its derivatives exert effective anticancer action on various human cancer cells, including colon, hepatocellular, lung, cervical, gastric, glioblastoma and breast cancer.80, 94, 95, 96, 97 Solasodine hydrochloride monotherapy (intraperitoneal injection; 30 and 50 mg·kg−1·day−1 of total 14 days) can reduce multidrug‐resistant sarcoma S180 tumor growth by 67.4% and 80.1%, respectively, by boosting LAK (lymphokine‐activated killer cell) and NK (Natural Killer) cell activity, stimulating lymphocyte proliferation and improving IL‐2 production.98 In mice, solasodine hydrochloride (15 mg/ml) combined with cyclophosphamide (20 mg/ml) can significantly reduce multidrug‐resistant sarcoma S180 tumor growth, by inducing apoptosis, followed by the downregulation of topoisomerase II and P‐gp expression.99
Apoptosis induction exerts anticancer effects.109 Similarly, the treatment of human osteosarcoma 1,547 cells with solasodine can induce apoptosis.100 Solamargine can also induce apoptosis by modulating tumor necrosis factor receptor (TNFR) expression,80 upregulating caspase‐3/−9 and downregulating Bcl‐2 and Bcl‐xL expression78, 80, 81 in lung cancer cells.
α‐Solanine and α‐chaconine
α‐Solanine and α‐chaconine are important cholestane SAs. They possess almost similar structures, except side chains, where α‐solanine comprises glucose, galactose and rhamnose molecules, and α‐chaconine is constituted of glucose and two rhamnose molecules.110 Tumor growth and metastasis was inhibited by α‐solanine via the downregulation of miR21 expression.111 By inhibiting the phosphorylation of ERK1/2 and activating caspase‐3, α‐chaconine can also induce apoptosis in the HT‐29 cancer cell line.112 PI3K/Akt and MAPK pathways regulate the expression of MMP‐2/−9, and α‐solanine and α‐chaconine inhibit cancer cell invasion and migration by inhibiting Akt and ERK phosphorylation113 and MMP‐2/‐9 activity.89 The synergistic cytotoxic activities of α‐solanine and α‐chaconine are well established in HepG2 and AGS cells.114
Pregnane alkaloids
Various pregnane alkaloids display potent cytotoxic activity against several kinds of cancer cells. The role of sarcovagine D in PANC‐1, SMMC‐7721, HL60 and A549 cells is well established, with an IC50 value of 0.96–16.69 μM.115 The migration of EGF‐induced human breast cancer MB‐MDA‐231 cells was inhibited by the terminamines A–E and H isolated from Japanese pachysandra (Pachysandra terminalis).65 Sarcovagine D and sarcorucinine A1 also exert potent anticancer activity against K562, PANC‐1 and SK‐BR‐3 cells, with an IC50 value of 2.25–5.00 μM. Wrightia javanica contains another pregnane type SA, wrightiamine A, which acts as a cytotoxic agent against vincristine‐resistant P388 cells.116 SMMC‐7721, MCF‐7, HL60, SW480 and A549 cells become susceptible after treatment with veralkamine 3‐(b‐d‐glucopyranoside), 6,7‐epoxyverdine and 3‐O‐acetylveralkamine, isolated from Veratrum taliense.117 Sarsaligenines A and sarsaligenines B also exert cell growth inhibitory activity in human leukemia HL60 cells with an IC50 value of 2.87 and 3.61 μM, respectively.115
Cyclopamine
Many studies have explored the cytotoxic potential of cyclopamine and its derivative in various cancer cell line (in vitro and in vivo) models. The cyclopamine C‐nor‐D‐homosteroidal alkaloid has been characterized as an antagonist of the Hh signaling pathway and is teratogenic in animals.118 In SMMC‐7721, PLC/PRF/5 and Huh7 cells, cyclopamine is responsible for the induction of apoptosis via inhibition of the Shh signaling pathway and downregulation of Bcl‐2 expression.119 The molecular mechanism of apoptosis induction by cyclopamine includes modulation of the AKT and ERK pathways.120
In prostate cancer, veratramine and jervine (structurally related to cyclopamine) exert anti‐proliferative effects.121 Veratramine inhibits cell proliferation and exerts cytotoxicity against PANC‐1, NCI‐H249, SW1990 and A549 cells, with an IC50 value of 14.5, 8.5, 26.1 and 8.9 μM respectively.67 KAAD‐cyclopamine selectively inhibits the Hh signaling pathway by oncogenic mutations,122 and exo‐cyclopamine and its analogs also block the Hh signaling corridor in a Gli‐dependent reporter assay.123 Cyclopamine and KAAD‐cyclopamine act as in vitro and in vivo anti‐proliferative agents by targeting the smoothened (SMO) receptor.124 Cyclopamine selectively targets and reduces Gli1 expression, resulting in the arrest of cell growth and induction of apoptosis in various leukemia and lymphoma cells.125 Moreover, cyclopamine displays noticeable anticancer effects in breast,126 lung,127 GIT/gastric, pancreatic, esophageal and biliary tract,128, 129 and prostate cancer;130 leukemia;125 and oral squamous cell carcinoma.131
Cyclopregnane alkaloids
Cortistatin A‐D,117 E‐H132 and J‐L133 exert antiproliferative activity against HUVECs, KB3‐1, Neuro2A, K562 and NHDF cells; HUVECs; and HUVECs, respectively, with an IC50 value of 2.3–14 μM. Angiogenesis is the process of formation of new blood vessels from former blood vessels. Angiogenesis regulates tumor development and metastasis. Angiogenesis antagonists can be considered potential candidates as antitumor agents.133 Cortistatin, a cyclopregnane alkaloid isolated from the Indonesian marine sponge Corticium simplex exerts cytostatic anti‐proliferative action against HUVECs via inhibition of angiogenesis.133 Among all cortistatins, cortistatin A exhibits discriminating anti‐proliferative action against HUVECs, by inhibiting the migration and tubular development of HUVECs promoted by VEGF or bFGF.117
Other Multifarious Pharmacological Activities of SAs
Apart from anticancer activity, different SAs possess various other pharmacological actions. For example, solasodine, α‐tomatine and neoverataline A and B possess antifungal activity; α‐chaconine, solanidine, α‐solanine and tomatidine exert anti‐inflammatory activity; saligcinnamide, N(a)‐methyl epipachysamine‐D and tomatidine show antibacterial activity; hookerianamide H and hookerianamide, Na‐methylepipachysamine D and sarcovagine C exhibited antiplasmodial activity; solasodine showed anti‐nociceptive and anti‐estrogenic activity; (+)‐(20S)‐3‐(benzoylamino)‐20‐(dimethylamino)‐5α‐pregn‐2‐en‐4β‐ol and [(+)‐(20S)‐20‐(dimethylamino)‐3α‐(methylsenecioylamino)‐5α‐pregn‐12β‐ol possess antiestrogenic activity; and cyclovirobuxine showed cytoprotective effects.43, 44, 45, 134, 135
Toxicity of SAs
The steroidal jerveratrum alkaloid cyclopamine (Fig. 3) and veratrum alkaloid jervine (Fig. 3) are potent teratogens that cause synophthalmia in sheep, rabbits, mice and hamsters.116, 136, 137, 138 Lipinski et al., 2010 reported that cyclopamine and its semi‐synthetic analog AZ75 can cause palate defects, lateral cleft lip and semilobar and alobar holoprosencephaly in mice, respectively.139 Exposure to α‐chaconine might cause significant hepatotoxicity in normal human Chang liver cells.140 In frog embryos, exposure to α‐chaconine and α‐solanine can induce neurological noxiousness (viz. spina bifida and other deformities).141 According to Chaube et al., 1976,141 Smith et al., 1996,142 and Langkilde et al., 2008,143 the inhibition of cholinesterase enzyme activity in the human central nervous system (CNS) and cell membrane disruption in the gastrointestinal tract (GIT) may be attributed to the toxic effects of α‐chaconine and α‐solanine.144 In hamsters, the teratogenic effects of solasodine may be attributed to the induction of spina bifida, exencephaly and cranial blebbing.145 Structure–activity relationship analyses by Friedman et al., 1991,141 showed that the teratogenic effects of jervanes, solanidanes and spirosolanes were caused by C‐5 and C‐6 unsaturation in the steroidal skeleton and the olefinic linkage at C‐5 and C‐6 renders them more toxic. In pregnant and non‐pregnant mice, three steroidal glycoalkaloids (solasodine, solanidine and tomatidine) induced hepatomegaly after being fed a diet containing these aglycones for 2 weeks.146 Solanum potato glycoalkaloids, namely, α‐chaconine and α‐solanine, the aglycones solanidine and solasodine and the veratrum alkaloid jervine exert toxic effects in rainbow trout (Oncorhynchus mykiss) and the Japanese rice fish medaka (Oryzias latipes).147 The SAs from potato peels (solanidine, demissidine, α‐chaconine and α‐solanine) are highly toxic to humans at concentrations of >1 mg/g (dry weight).148, 149
Figure 3.
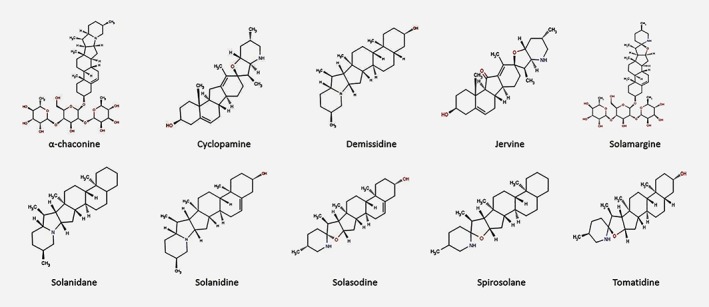
Chemical structure of typical steroidal alkaloids. [Color figure can be viewed at wileyonlinelibrary.com]
In silico approaches for exploiting anticancer leads from SAs
Computer‐aided drug design approaches are a promising area for the development of anticancer drug‐like compounds using SA scaffolds. To avoid the huge costs and labor intensiveness incurred with random screening, different types of in silico approaches, including virtual screening, molecular modeling, molecular docking, QSAR, pharmacophore modeling, high‐throughput screening, and cloud computing, are efficient and effective for anticancer drug discovery campaigns using SAs.150, 151, 152 This technology has changed the scenario of rational design in the anticancer drug discovery process. Various web‐based programs for QSAR [JRC QSAR Model Database, DemQSAR, OCHEM (Online Chemical Modeling Environment), and MC‐3DQSAR] are available for the generation of potential leads.153, 154, 155, 156 For hit identification to lead optimization, structure and ligand‐based virtual screening, other web‐accessible servers and software (commercial/free versions; Supporting Information S2, Tables 1–3) are available. These computational methodologies provide a powerful toolbox for target identification, hit and lead generation and lead optimization. Synergism between dry and wet labs can facilitate the discovery of cost‐effective and reliable personalized medicines to improve anti‐cancer drug discovery. Figure 4 is a flowchart illustrating the generation of smart anti‐cancer drugs from SAs of natural and synthetic origins.
Figure 4.
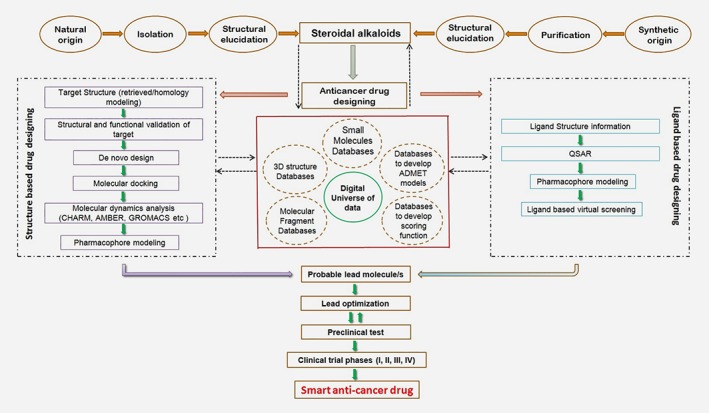
In silico approaches for smart anticancer drug development from steroidal alkaloids. [Color figure can be viewed at wileyonlinelibrary.com]
Recent trends and future directions
Natural or synthetic secondary metabolites possess potent pharmacological activities. Among them, some have cleared the clinical trial parameters and are clinically available.157 For instance, the FDA has approved vincristine, which is isolated from the leaves of the Madagascar periwinkle Catharanthus roseus (L.) G. Don (formerly Vinca rosea L.), for cancer treatment.158 Dactinomycin, produced by Streptomyces parvulus, is administered as an antineoplastic antibiotic for the treatment of Kaposi's sarcoma, infantile fibrosarcoma and testicular cancer.159, 160, 161 In 2005, paclitaxel (isolated from the bark of the Pacific yew (Taxus brevifolia Nutt.) was approved by the FDA for use in the United States for the treatment of pancreatic, breast and non‐small cell lung cancers.162 In recent times, pharmaceutical companies have been focusing on drug discovery from natural and synthetic origins through combinatorial chemistry, which includes the generation of libraries containing millions of compounds,163 in silico molecular docking, structure‐ and target‐based virtual screening, molecular modeling, pharmacophore designing and receptor‐based QSAR studies. Many biotechnological companies also concentrate on lead identification and validation of natural products (or synthetic derivatives), and the development of these leads into drugs.163 Numerous drugs of plant origin are currently undergoing clinical trials.164, 165
Previously, drug discovery using natural sources was a time‐consuming process, in particular, because of the recognition and structural elucidation of the bioactive compounds, which could take months or years. The rapidity of bioassay‐guided fractionation, identification and structural elucidation of compounds can be significantly enhanced by using instruments and methods such as HPLC, MS or LC–MS, higher magnetic field‐strength NMR, cap‐NMR, LC‐NMR‐MS, LC‐solid phase extraction (SPE)‐NMR, LC‐SPE‐NMR in combination with HPLC‐ESIMS, and robotics, to systematize high‐throughput bioassays.166, 167, 168, 169
Prompt screening of plant extracts for their biological activity is no longer a rate‐limiting step because of the improvement of computerized high‐throughput performances. Compounds with features such as autofluorescence or UV absorption often create problems in the screening process because of interference with the output, which can be overcome by prefractionation of the extracts.170 Additionally, computational filtering methods have been applied with most high‐throughput screening methods to minimize false‐positive results.171 In the future, dereplication can be implicated in the routine use of NMR‐hyphenated techniques.168 Furthermore, simple synthetic methodologies can be implicated for bioassays, and novel analogs of the parent molecules can be designed and developed using upgraded combinatorial chemistry.
To meet the continuous demand, a constant and sufficient supply of anticancer drugs is essential. Plant cell culture is an alternative method for compounds with low synthetic yields, as altering the environmental conditions and medium can result in larger yield.172, 173 Paclitaxel and Dioscorea are successfully produced using cell culture techniques.174, 175, 176, 177
Summary and concluding remarks
The increasing incidence of cancer is a global concern. To avoid the toxicity associated with chemotherapy and irradiation, SAs are being investigated as alternative therapeutic measures. Most SAs are from different sources, and their mechanisms of action also vary. They can exert anticancer effects at micromolar or nanomolar concentrations (Supporting Information Files S1 and S2). This review discussed the potential of SAs as anticancer agents, with a focus on their natural and synthetic sources, in vitro or in vivo models for study, inhibitory concentrations and mechanisms of action.
The key molecular targets of SAs are summarized in Figure 5. They exert their anticancer effects by inducing apoptosis or autophagy by upregulating or downregulating apoptotic (Bax, Bcl2, Bcl‐xL, Caspase‐3/8/9, PARP, TNFR I/II, Fas or HER2) and autophagic (LC3, AKT or mTOR) proteins. The inhibition of cell cycle progression at either G0/G1 or G2/M phase by interaction with CDKs is another mechanism underlying their anticancer activity. The antimetastatic action of SAs can be attributed to their interactions with, and the changes in the expression of ERK, RECK, TIMP and MMP‐2/9 proteins. SAs such as solasodine block P‐gp and inhibit multidrug‐resistant cancers. They also disrupt the integrity of the cell membrane by changing cell morphology and DNA content, thereby exerting anticancer activity. SAs such as cyclopamine inhibits xenograft tumor growth via the Hh pathway; α‐tomatine reduces xenograft tumor growth by inducing apoptosis via interactions with survivin, p50 and p65. Apart from the potent anticancer and other pharmacological effects, some SAs exhibit toxic effects such as hepatotoxicity in normal human liver cells (Chang Liver cell line) and teratogenicity, palate defects and lateral cleft lips in sheep, rabbits, mice and hamsters. SAs such as α‐chaconine and α‐solanine are highly toxic to humans at concentrations of >1 mg/g (dry weight).
Figure 5.
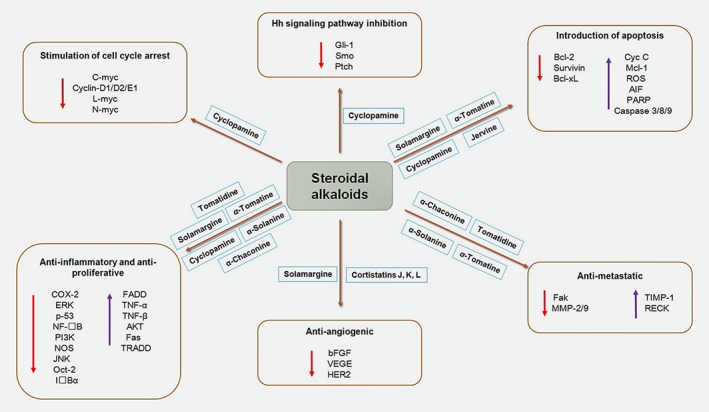
Steroidal alkaloids and major cellular signaling molecules. [Color figure can be viewed at wileyonlinelibrary.com]
Although many plant‐derived and synthetic SAs possess anticancer effects, the underlying molecular mechanisms remain to be established, predominantly with respect to the pharmacokinetics around the active site of the target protein. Such data is essential for clearance of standard clinical trial parameters and confirmation that these drugs are effective and safe. Understanding the crosstalk between SAs and associated signaling molecules will assist improved understanding of their molecular mechanisms of action and the development of anticancer drugs. Various biochemical and biophysical approaches, including co‐crystallization and 3D‐structure determination, can help understand the mechanisms underlying these interactions and develop more selective and less toxic anticancer drugs.
Combining existing chemotherapeutic drugs such as solamargine with cisplatin, MTX or 5‐Fu can elevate the combined anticancer activity, thereby proving that combination therapy can exert synergistic or enhanced anticancer activity. In silico tactics, including molecular docking and QSAR studies, can be introduced to elucidate the molecular mechanisms, which can be validated by in vitro and in vivo experiments to aid significant development toward cancer prevention and treatment.
Cell line details
- A2780
human ovarian cancer cell line
- A431
skin epidermoid carcinoma
- A549
human lung adenocarcinoma cell
- AGS
human Caucasian gastric adenocarcinoma
- AGS
human gastric cancer cell line
- B16F10
murine melanoma
- B16F10
murine melanoma
- B16F2F
mouse melanoma cell line
- BGC‐823
human stomach adenocarcinoma cell line
- BPH‐1
benign prostate hyperplasia epithelial cell line
- C4‐2B
osteotropic prostate cancer cell line
- DU145
human prostate adenocarcinoma cell line
- GM07492A
human lung fibroblasts
- H1650
human lung cancer cells
- H441
human adenocarcinoma
- H460/paclitaxel
multidrug‐resistant counterpart of human NSCLC H460 cells
- H520
squamous cell lung carcinoma
- H661
large cell lung cancer
- H69
small cell lung cancer
- HBE
human bronchial epithelial cell line SV40‐transformed
- HBL‐100
human breast transformed epithelial cell line
- HCT‐116
colon cancer cell line
- HeLa
cervical carcinoma
- HEP2
larynx cancer cell line
- Hep3B
hepatocellular carcinoma
- HEPG2
liver cancer cell line
- HL‐60
human promyelocytic leukemia cell line
- HL7702
normal hepatocyte
- HT‐29
colonic adenocarcinoma
- HUVECs
human umbilical vein endothelial cells
- J16
human leukemia Jurkat T cells
- K562/A02
multidrug‐resistant counterpart of K562 cell line
- K562
human myelogenous leukemia cell line
- KB/VCR
multidrug‐resistant counterpart of KB cell line
- KB
squamous cell carcinoma cell line
- KB3‐1
KB epidermoid carcinoma cells
- LLC
Lewis lung carcinoma cells
- M‐109
Madison lung tumor
- MCF‐7
breast adenocarcinoma
- MDA‐MB‐231
breast cancer cell line
- MEL‐28
human melanoma.
- MG‐63
human osteosarcoma
- MGC‐803
human gastric cancer cell line
- MKN28
gastric cancer cell lines
- MO59J
human glioblastoma
- MOLT‐4
human leukemic T‐lymphocytes cell line
- NCI‐H460
human lung large cell carcinoma
- Neuro2A
murine neuroblastoma cells.
- NHDF
normal human dermal fibroblast.
- NIH3T3
mouse embryonic fibroblast cells
- NSCLC‐N6
human bronchopulmonary nonsmall‐cell lung carcinoma cells
- P388/ADM
parental and the Adriamycin (doxorubicin)‐resistant subline of mouse leukemia cells
- P388/VCR
vincristine‐resistant murine leukemia.
- P‐388
mouse lymphoid neoplasm.
- PC3
human prostate cancer cell line
- RPE‐1
human retinal pigment epithelial‐1 cell line
- RWPE‐1
human normal prostate cell line
- Saos‐2
human osteosarcoma
- SC115
Shionogi carcinoma
- SGC7901
gastric carcinoma
- SK‐BR‐3
breast carcinoma
- SMMC‐7721
human hepatocarcinoma cell line
- U251
human glioblastoma
- U2OS
human osteosarcoma
- U343
human glioblastoma
- U87
human primary glioblastoma cell line
- V79
Chinese hamster lung fibroblasts.
- VERO
African green monkey kidney cell line
- WI‐38
human lung epithelial fibroblast
- WM115
human melanoma cells line
- WM239
human melanoma cells line
- WRL‐68
human normal liver cell line
- ZR‐75‐1
human breast cancer cell line
Supporting information
File S1.
Table 1. The In‐vitro anticancer activity of steroidal alkaloids from natural sources in various cancer cell lines.
S1 Table 2. The in‐vitro anticancer activity of synthetic‐origin steroidal alkaloids in several cancer cell lines.
S1 Table 3. The in‐vitro anticancer activity of steroidal alkaloids from industrial sources in various cell lines.
S1 Table 4. The in‐vivo anticancer activity of steroidal alkaloids in various xenograft animal models.
File S2.
Table 1. Important web‐accessible databases for drug target identification.
S2 Table 2. Important web‐accessible databases for small molecule identification.
S2 Table 3. Important web‐accessible and commercially/freely available databases, and software for molecular docking.
Acknowledgements
This study was supported by the National Research Foundation of Korea grants funded by the Korean Government (grant nos. 2016R1A2B2011071; 2016R1A4A1011189). The authors thank Trent Rogers Ph.D. from Cactus Global for editing a version of our study.
Conflict of interest: The authors declare no conflicts of interest.
References
- 1. Prokai‐Tatrai K, Perjesi P, Rivera‐Portalatin NM, et al. Mechanistic investigations on the antioxidant action of a neuroprotective estrogen derivative. Steroids 2008;73:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rocha M, Banuls C, Bellod L, et al. A review on the role of phytosterols: new insights into cardiovascular risk. Curr Pharm Des 2011;17:4061–75. [DOI] [PubMed] [Google Scholar]
- 3. Dey P, Karuna D, Bhakta T. Medicinal plants used as anti‐acne agents by tribal and non‐tribal people of Tripura. India Am J Phytomed Clin Therap 2014;2:556–70. [Google Scholar]
- 4. Dey P, Bardalai D, Kumar NR, et al. Ethnomedicinal knowledge about various medicinal plants used by the tribes of Tripura. Res J Pharmacogn Phytochem 2012;4:297–302. [Google Scholar]
- 5. Debnath P, Dey P, Chanda A, et al. A survey on pineapple and its medicinal value. Scholars Acad J Pharm 2012;1:24–9. [Google Scholar]
- 6. Dey P, Mukherjee M, Bhakta T, et al. Preliminary phytochemical studies of leaf extracts of Molineria recurvata. J Chem Pharm Res 2012;4:3727–30. [Google Scholar]
- 7. Prasanta Dey TB. Comparative studies on anthelmintic activity of leaf extract of musa acuminate colla and Cajanus cajan (Linn.) leaf extract. Mintage J Pharm Med Sci 2013;2:24–5. [Google Scholar]
- 8. Bera R, Kundu A, Sen T, et al. In vitro metabolic stability and permeability of Gymnemagenin and its in vivo pharmacokinetic correlation in rats–a pilot study. Planta Med 2016;82:544–50. [DOI] [PubMed] [Google Scholar]
- 9. Rammohan B, Samit K, Chinmoy D, et al. Human cytochrome P450 enzyme modulation by Gymnema sylvestre: a predictive safety evaluation by LC‐MS/MS. Pharmacogn Mag 2016;12:S389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahn MY, Jung JH, Na YJ, et al. A natural histone deacetylase inhibitor, Psammaplin A, induces cell cycle arrest and apoptosis in human endometrial cancer cells. Gynecol Oncol 2008;108:27–33. [DOI] [PubMed] [Google Scholar]
- 11. Kim K‐B, Nam YA, Kim HS, et al. α‐Linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem Toxicol 2014;70:163–78. [DOI] [PubMed] [Google Scholar]
- 12. Jeong T, Lee SH, Mishra NK, et al. Synthesis and cytotoxic evaluation of N‐Aroylureas through rhodium (III)‐catalyzed C−H functionalization of Indolines with Isocyanates. Adv Synth Catal 2017;359:2329–36. [Google Scholar]
- 13. Jeong T, Mishra NK, Dey P, et al. C (sp 3)–H amination of 8‐methylquinolines with azodicarboxylates under Rh (iii) catalysis: cytotoxic evaluation of quinolin‐8‐ylmethanamines. Chem Commun 2017;53:11197–200. [DOI] [PubMed] [Google Scholar]
- 14. Jeon M, Park J, Dey P, et al. Site‐selective rhodium(III)‐catalyzed C−H Amination of 7‐Azaindoles with Anthranils: synthesis and anticancer evaluation. Adv Synth Catal 2017;359:3471–8. [Google Scholar]
- 15. Prasanta Dey ND. Carbon nanotubes: its role in modern health care. Int J Pharm Pharmc Sci 2003;5:9–13. [Google Scholar]
- 16. Prasanta Dey TB. Nanotechnology for the delivery of poorly water soluble drugs. Global J Pharmac Res 2012;1:225–50. [Google Scholar]
- 17. Sarfaraj HM, Sheeba F, Saba A, et al. Marine natural products: a lead for anti‐cancer. Indian. J Mar Sci 2012;41:27–39. [Google Scholar]
- 18. Farnsworth NR, Akerele O, Bingel AS, et al. Medicinal plants in therapy. Bull World Health Organ 1985;63:965. [PMC free article] [PubMed] [Google Scholar]
- 19. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 2013;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dey P, Debnath P, Bhakta T. Evaluation of anthelmintic activity of Molineria Recurvata leaf extracts. Int Res J Pharm App Sci 2012;2:16–20. [Google Scholar]
- 21. Dey P, Bhakta T. Evaluation of in‐vitro anticoagulant activity of Molineria recurpata leaf extract. J Nat Prod Plant Resour 2012;2:685–8. [Google Scholar]
- 22. Karuna D, Dey P, Das S, et al. In vitro antioxidant activities of root extract of Asparagus racemosus Linn. J Trad Complem Med 2017;8(1):60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanjoy Das PD, Kundu A, Tejendra Bhakta A. Survey to the extent of spurious drugs in the market of India. J Chem Pharm Res 2017;9:130–8. [Google Scholar]
- 24. Dey P, Das N, Kumar NR, et al. Mineral contents of some commonly consumed tribal foods of Tripura. India Int J Pharm Pharmac Sci 2013;5:388–90. [Google Scholar]
- 25. Prasanta Dey PD, Bhakta T. Evaluation of anthelmintic activity of pineapple fruit extract using Indian earthworm (pheritima posthuma). Mintage journal of pharmaceutical and medical. Sciences 2013;2:26–7. [Google Scholar]
- 26. Ds Karuna PD. Amit Kundu, Vineet Vishal, Tejendra Bhakta evaluation of in vitro antioxidant potential of Aconitum napellus Linn. Root extract. Int J Pharmacogn Chin Med 2018;2:1–8. [Google Scholar]
- 27. Aboyade OM, Grierson DS, Afolayan AJ. Anti‐nociceptive and anti‐inflammatory activities of the aqueous extract of fresh Solanum aculeastrum Dunal. Berries in male Wistar rats. J Med Plants Res 2012;6:5400–5. [Google Scholar]
- 28. Pandurangan A, Khosa RL, Hemalatha S. Antinociceptive activity of steroid alkaloids isolated from Solanum trilobatum Linn. J Asian Nat Prod Res 2010;12:691–5. [DOI] [PubMed] [Google Scholar]
- 29. Emmanuel S, Ignacimuthu S, Perumalsamy R, et al. Antiinflammatory activity of Solanum trilobatum . Fitoterapia 2006;77:611–2. [DOI] [PubMed] [Google Scholar]
- 30. Kenny OM, McCarthy CM, Brunton NP, et al. Anti‐inflammatory properties of potato glycoalkaloids in stimulated Jurkat and Raw 264.7 mouse macrophages. Life Sci 2013;92:775–82. [DOI] [PubMed] [Google Scholar]
- 31. Chiu F‐L, Lin J‐K. Tomatidine inhibits iNOS and COX‐2 through suppression of NF‐κB and JNK pathways in LPS‐stimulated mouse macrophages. FEBS Lett 2008;582:2407–12. [DOI] [PubMed] [Google Scholar]
- 32. Mousseau G, Clementz MA, Bakeman WN, et al. An analog of the natural steroidal alkaloid cortistatin A potently suppresses Tat‐dependent HIV transcription. Cell Host Microbe 2012;12:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou C‐X, Liu J‐Y, Ye W‐C, et al. Neoverataline A and B, two antifungal alkaloids with a novel carbon skeleton from Veratrum taliense . Tetrahedron 2003;59:5743–7. [Google Scholar]
- 34. S‐I I, Ihara T, Tamura H, et al. α‐Tomatine, the major saponin in tomato, induces programmed cell death mediated by reactive oxygen species in the fungal pathogen Fusarium oxysporum . FEBS Lett 2007;581:3217–22. [DOI] [PubMed] [Google Scholar]
- 35. Atta‐Ur‐Rahman AS, Farooq A, Khan MR, et al. Antibacterial steroidal alkaloids from Sarcococca saligna . J Nat Prod 1998;61:202–6. [DOI] [PubMed] [Google Scholar]
- 36. Devkota KP, Lenta BN, Choudhary MI, et al. Cholinesterase inhibiting and antiplasmodial steroidal alkaloids from Sarcococca hookeriana . Chem Pharmac Bull 2007;55:1397–401. [DOI] [PubMed] [Google Scholar]
- 37. Mitchell G, Lafrance M, Boulanger S, et al. Tomatidine acts in synergy with aminoglycoside antibiotics against multiresistant Staphylococcus aureus and prevents virulence gene expression. J Antimicrob Chemotherapy 2011;67:559–68. [DOI] [PubMed] [Google Scholar]
- 38. Devkota KP, Lenta BN, Wansi JD, et al. Bioactive 5α‐pregnane‐type steroidal alkaloids from Sarcococca hookeriana. J Nat Prod 2008;71:1481–4. [DOI] [PubMed] [Google Scholar]
- 39. Wang L, Guo D, Yuan L, et al. Antifungal effect of three natural products on the genetic substance of Saccharomyces cerevisiae GL7 and Prototheca wickerhamii. Acta Pharm Sin 2000;35:860–3. [PubMed] [Google Scholar]
- 40. Fewell AM, Roddick JG. Interactive antifungal activity of the glycoalkaloids α‐solanine and α‐chaconine. Phytochemistry 1993;33:323–8. [Google Scholar]
- 41. Hu D, Liu X, Wang Y, et al. Cyclovirobuxine D ameliorates acute myocardial ischemia by KATP channel opening, nitric oxide release and anti‐thrombosis. Eur J Pharmacol 2007;569:103–9. [DOI] [PubMed] [Google Scholar]
- 42. Gupta R, Dixit V. Effects of short term treatment of solasodine on cauda epididymis in dogs. Indian J Exp Biol 2002;40(2):169–73. [PubMed] [Google Scholar]
- 43. Chen Q, Shan H, Sun H, et al. Effects of cyclovirobuxine D on intracellular Ca2+ and L‐type Ca2+ current in rat ventricular cardiomyocytes. Acta Pharm Sin 2004;39:500–3. [PubMed] [Google Scholar]
- 44. Chen Z, Hu S, Shi W, et al. Electrophysiologic study of the biphasic effects of cyclovirobuxine D on arrhythmias. Chin J Integr Trad West Med 2004;24:1010–3. [PubMed] [Google Scholar]
- 45. Yu B, Fang T‐H, Lü G‐H, et al. Beneficial effect of Cyclovirobuxine D on heart failure rats following myocardial infarction. Fitoterapia 2011;82:868–77. [DOI] [PubMed] [Google Scholar]
- 46. Jimeno JM. A clinical armamentarium of marine‐derived anti‐cancer compounds. Anticancer Drugs 2002;13:S15–9. [PubMed] [Google Scholar]
- 47. Ruiz‐Torres V, Encinar JA, Herranz‐López M, et al. An updated review on marine anticancer compounds: the use of virtual screening for the discovery of small‐molecule cancer drugs. Molecules 2017;22:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Newman DJ, Cragg GM. Advanced preclinical and clinical trials of natural products and related compounds from marine sources. Curr Med Chem 2004;11:1693–713. [DOI] [PubMed] [Google Scholar]
- 49. Imperatore C, Aiello A, D'Aniello F, et al. Alkaloids from marine invertebrates as important leads for anticancer drugs discovery and development. Molecules 2014;19:20391–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moser BR. Review of cytotoxic cephalostatins and ritterazines: isolation and synthesis. J Nat Prod 2008;71:487–91. [DOI] [PubMed] [Google Scholar]
- 51. Dirsch VM, Müller IM, Eichhorst ST, et al. Cephalostatin 1 selectively triggers the release of Smac/DIABLO and subsequent apoptosis that is characterized by an increased density of the mitochondrial matrix. Cancer Res 2003;63:8869–76. [PubMed] [Google Scholar]
- 52. Dyshlovoy SA, Honecker F. Marine compounds and cancer: 2017 updates. Mar. Drugs, 2018;16(2):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Malve H. Exploring the ocean for new drug developments: marine pharmacology. J Pharm Bioallied Sci 2016;8:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lindequist U. Marine‐derived pharmaceuticals–challenges and opportunities. Biomol Therap 2016;24:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mayer AM, Nguyen M, Kalwajtys P, et al. The marine pharmacology and pharmaceuticals pipeline in 2016. FASEB J 2017;31:818.1–1. [Google Scholar]
- 56. Mayer AM, Glaser KB, Cuevas C, et al. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci 2010;31:255–65. [DOI] [PubMed] [Google Scholar]
- 57. Lacour TG, Guo C, Bhandaru S, et al. Interphylal product splicing: the first total syntheses of cephalostatin 1, the north hemisphere of Ritterazine G, and the highly active hybrid analogue, Ritterostatin GN1N1. J Am Chem Soc 1998;120:692–707. [Google Scholar]
- 58. Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov 2005;4:206–20. [DOI] [PubMed] [Google Scholar]
- 59. Agostini C, Vieira RF, Bizzo HR, et al. Secondary metabolites In: Dhanarasu S, ed. Chromatography and its applications. InTech, Croatia: 2012. [Google Scholar]
- 60. Fraenkel GS. The raison d'etre of secondary plant substances. Science 1959;129:1466–70. [DOI] [PubMed] [Google Scholar]
- 61. Tiwari R, Rana C. Plant secondary metabolites: a review. Int J Eng Res Gen Sci 2015;3:661–70. [Google Scholar]
- 62. A‐U R, Choudhary MI. Chemistry and biology of steroidal alkaloids. Alkaloids Chem Biol 1998;50:61–108. [Google Scholar]
- 63. Áiqbal Choudhary M. Diterpenoid and steroidal alkaloids. Nat Prod Rep 1999;16:619–35. [DOI] [PubMed] [Google Scholar]
- 64. Li H‐J, Jiang Y, Li P. Chemistry, bioactivity and geographical diversity of steroidal alkaloids from the Liliaceae family. Nat Prod Rep 2006;23:735–52. [DOI] [PubMed] [Google Scholar]
- 65. Zhai H‐Y, Zhao C, Zhang N, et al. Alkaloids from Pachysandra terminalis inhibit breast cancer invasion and have potential for development as antimetastasis therapeutic agents. J Nat Prod 2012;75:1305–11. [DOI] [PubMed] [Google Scholar]
- 66. Yan K‐H, Lee L‐M, Yan S‐H, et al. Tomatidine inhibits invasion of human lung adenocarcinoma cell A549 by reducing matrix metalloproteinases expression. Chem Biol Interact 2013;203:580–7. [DOI] [PubMed] [Google Scholar]
- 67. Tang J, Li HL, Shen YH, et al. Antitumor activity of extracts and compounds from the rhizomes of Veratrum dahuricum . Phytother Res 2008;22:1093–6. [DOI] [PubMed] [Google Scholar]
- 68. Buckingham J, Baggaley KH, Roberts AD, et al. Dictionary of Alkaloids, with CD‐ROMed. CRC Press, Boca Raton: 2010. [Google Scholar]
- 69. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 70. Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 2004;5:897–907. [DOI] [PubMed] [Google Scholar]
- 71. Ferri KF, Kroemer G. Organelle‐specific initiation of cell death pathways. Nat Cell Biol 2001;3:E255–E63. [DOI] [PubMed] [Google Scholar]
- 72. Ren W, Qiao Z, Wang H, et al. Flavonoids: promising anticancer agents. Med Res Rev 2003;23:519–34. [DOI] [PubMed] [Google Scholar]
- 73. Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene 2005;24:2909–15. [DOI] [PubMed] [Google Scholar]
- 74. Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 2003;36:131–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Senderowicz AM. Flavopiridol: the first cyclin‐dependent kinase inhibitor in human clinical trials. Invest New Drugs 1999;17:313–20. [DOI] [PubMed] [Google Scholar]
- 76. Senderowicz A. Development of cyclin‐dependent kinase modulators as novel therapeutic approaches for hematological malignancies. Leukemia 2001;15:1–9. [DOI] [PubMed] [Google Scholar]
- 77. Wei G, Wang J, Du Y. Total synthesis of solamargine. Bioorg Med Chem Lett 2011;21:2930–3. [DOI] [PubMed] [Google Scholar]
- 78. Shiu L, Chang L, Liang C, et al. Solamargine induces apoptosis and sensitizes breast cancer cells to cisplatin. Food Chem Toxicol 2007;45:2155–64. [DOI] [PubMed] [Google Scholar]
- 79. Tang Z, Zhang Y, Li N, et al. Extraction, purification technology and antineoplastic effects of solamargine. China J Chin Mater Med 2011;36:2192–5. [PubMed] [Google Scholar]
- 80. Liu L‐F, Liang C‐H, Shiu L‐Y, et al. Action of solamargine on human lung cancer cells–enhancement of the susceptibility of cancer cells to TNFs. FEBS Lett 2004;577:67–74. [DOI] [PubMed] [Google Scholar]
- 81. Sun L, Zhao Y, Li X, et al. A lysosomal–mitochondrial death pathway is induced by solamargine in human K562 leukemia cells. Toxicol In Vitro 2010;24:1504–11. [DOI] [PubMed] [Google Scholar]
- 82. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011;278:16–27. [DOI] [PubMed] [Google Scholar]
- 83. Shay G, Lynch CC, Fingleton B. Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol 2015;44:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–74. [DOI] [PubMed] [Google Scholar]
- 85. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cicenas J. The potential role of Akt phosphorylation in human cancers. Int J Biol Markers 2008;23:1–9. [DOI] [PubMed] [Google Scholar]
- 87. Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005;24:7455–64. [DOI] [PubMed] [Google Scholar]
- 88. Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell 2017;169:381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lu M‐K, Shih Y‐W, Chien T‐TC, et al. α‐Solanine inhibits human melanoma cell migration and invasion by reducing matrix metalloproteinase‐2/9 activities. Biol Pharmac Bull 2010;33:1685–91. [DOI] [PubMed] [Google Scholar]
- 90. Lee S‐T, Wong P‐F, He H, et al. Alpha‐tomatine attenuation of in vivo growth of subcutaneous and orthotopic xenograft tumors of human prostate carcinoma PC‐3 cells is accompanied by inactivation of nuclear factor‐kappa B signaling. PloS one 2013;8:e57708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shih Y‐W, Shieh J‐M, Wu P‐F, et al. α‐Tomatine inactivates PI3K/Akt and ERK signaling pathways in human lung adenocarcinoma A549 cells: effect on metastasis. Food Chem Toxicol 2009;47:1985–95. [DOI] [PubMed] [Google Scholar]
- 92. Lee S‐T, Wong P‐F, Cheah S‐C, et al. Alpha‐tomatine induces apoptosis and inhibits nuclear factor‐kappa B activation on human prostatic adenocarcinoma PC‐3 cells. PLoS One 2011;6:e18915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kúdelová J, Seifrtová M, Sucha L, et al. Alpha‐tomatine activates cell cycle checkpoints in the absence of DNA damage in human leukemic MOLT‐4 cells. J Appl Biomed 2013;11:93–103. [Google Scholar]
- 94. Munari CC, de Oliveira PF, Campos JCL, et al. Antiproliferative activity of Solanum lycocarpum alkaloidic extract and their constituents, solamargine and solasonine, in tumor cell lines. J Nat Med 2014;68:236–41. [DOI] [PubMed] [Google Scholar]
- 95. Ikeda T, Tsumagari H, Honbu T, et al. Cytotoxic activity of steroidal glycosides from Solanum plants. Biol Pharmac Bull 2003;26:1198–201. [DOI] [PubMed] [Google Scholar]
- 96. Ding X, Zhu F, Gao S. Purification, antitumour and immunomodulatory activity of water‐extractable and alkali‐extractable polysaccharides from Solanum nigrum L. Food Chem 2012;131:677–84. [Google Scholar]
- 97. Ding X, Zhu F, Yang Y, et al. Purification, antitumor activity in vitro of steroidal glycoalkaloids from black nightshade (Solanum nigrum L.). Food Chem 2013;141:1181–6. [DOI] [PubMed] [Google Scholar]
- 98. Jin‐Xia A. The role of SBHL in tumor growth and immune functions in mice. International Conference on Human Health and Biomedical Engineering (HHBE) 2011 China: IEEE, 2011:521–23.
- 99. Jin‐Xia A. The experimental investigation of SBHL on reversing acquired multi‐drug resistance on S180 mouse tumor bearing model. International Conference on Human Health and Biomedical Engineering (HHBE) 2011 China: IEEE, 2011:518–20.
- 100. Trouillas P, Corbière C, Liagre B, et al. Structure–function relationship for saponin effects on cell cycle arrest and apoptosis in the human 1547 osteosarcoma cells: a molecular modelling approach of natural molecules structurally close to diosgenin. Bioorg Med Chem 2005;13:1141–9. [DOI] [PubMed] [Google Scholar]
- 101. Jiang QW, Chen MW, Cheng KJ, et al. Therapeutic potential of steroidal alkaloids in cancer and other diseases. Med Res Rev 2016;36:119–43. [DOI] [PubMed] [Google Scholar]
- 102. Chiang C‐T, Way T‐D, Tsai S‐J, et al. Diosgenin, a naturally occurring steroid, suppresses fatty acid synthase expression in HER2‐overexpressing breast cancer cells through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett 2007;581:5735–42. [DOI] [PubMed] [Google Scholar]
- 103. Koduru S, Grierson D, Van de Venter M, et al. Anticancer activity of steroid alkaloids isolated from Solanum aculeastrum . Pharm Biol 2007;45:613–8. [Google Scholar]
- 104. Lavie Y, Harel‐Orbital T, Gaffield W, et al. Inhibitory effect of steroidal alkaloids on drug transport and multidrug resistance in human cancer cells. Anticancer Res 2001;21:1189–94. [PubMed] [Google Scholar]
- 105. Choi SH, Ahn J‐B, Kozukue N, et al. Structure–activity relationships of α‐, β1‐, γ‐, and δ‐tomatine and tomatidine against human breast (MDA‐MB‐231), gastric (KATO‐III), and prostate (PC3) cancer cells. J Agric Food Chem 2012;60:3891–9. [DOI] [PubMed] [Google Scholar]
- 106. Chao M‐W, Chen C‐H, Chang Y‐L, et al. α‐Tomatine‐mediated anti‐cancer activity in vitro and in vivo through cell cycle‐and caspase‐independent pathways. PLoS One 2012;7:e44093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Friedman M, Levin CE, Lee S‐U, et al. Tomatine‐containing green tomato extracts inhibit growth of human breast, colon, liver, and stomach cancer cells. J Agric Food Chem 2009;57:5727–33. [DOI] [PubMed] [Google Scholar]
- 108. Shieh J‐M, Cheng T‐H, Shi M‐D, et al. α‐Tomatine suppresses invasion and migration of human non‐small cell lung cancer NCI‐H460 cells through inactivating FAK/PI3K/Akt signaling pathway and reducing binding activity of NF‐κB. Cell Biochem Biophys 2011;60:297–310. [DOI] [PubMed] [Google Scholar]
- 109. Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell 1994;78:539–42. [DOI] [PubMed] [Google Scholar]
- 110. Yamashoji S, Matsuda T. Synergistic cytotoxicity induced by α‐solanine and α‐chaconine. Food Chem 2013;141:669–74. [DOI] [PubMed] [Google Scholar]
- 111. Shen K‐H, Liao AC‐H, Hung J‐H, et al. α‐Solanine inhibits invasion of human prostate cancer cell by suppressing epithelial‐mesenchymal transition and MMPs expression. Molecules 2014;19:11896–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yang S‐A, Paek S‐H, Kozukue N, et al. α‐Chaconine, a potato glycoalkaloid, induces apoptosis of HT‐29 human colon cancer cells through caspase‐3 activation and inhibition of ERK 1/2 phosphorylation. Food Chem Toxicol 2006;44:839–46. [DOI] [PubMed] [Google Scholar]
- 113. Lu M‐K, Chen P‐H, Shih Y‐W, et al. Alpha‐Chaconine inhibits angiogenesis in vitro by reducing matrix Metalloproteinase‐2. Biol Pharmac Bull 2010;33:622–30. [DOI] [PubMed] [Google Scholar]
- 114. Friedman M, Lee K‐R, Kim H‐J, et al. Anticarcinogenic effects of glycoalkaloids from potatoes against human cervical, liver, lymphoma, and stomach cancer cells. J Agric Food Chem 2005;53:6162–9. [DOI] [PubMed] [Google Scholar]
- 115. Sun Y, Yan Y‐X, Chen J‐C, et al. Pregnane alkaloids from Pachysandra axillaris. Steroids 2010;75:818–24. [DOI] [PubMed] [Google Scholar]
- 116. Welch K, Panter K, Lee S, et al. Cyclopamine‐induced synophthalmia in sheep: defining a critical window and toxicokinetic evaluation. J Appl Toxicol 2009;29:414–21. [DOI] [PubMed] [Google Scholar]
- 117. Aoki S, Watanabe Y, Sanagawa M, et al. Cortistatins A, B, C, and D, anti‐angiogenic steroidal alkaloids, from the marine sponge Corticium simplex. J Am Chem Soc 2006;128:3148–9. [DOI] [PubMed] [Google Scholar]
- 118. Cooper MK, Porter JA, Young KE, et al. Teratogen‐mediated inhibition of target tissue response to Shh signaling. Science 1998;280:1603–7. [DOI] [PubMed] [Google Scholar]
- 119. Chen X‐L, Cheng Q‐Y, She M‐R, et al. Expression of sonic hedgehog signaling components in hepatocellular carcinoma and cyclopamine‐induced apoptosis through Bcl‐2 downregulation in vitro. Arch Med Res 2010;41:315–23. [DOI] [PubMed] [Google Scholar]
- 120. Ghezali L, Leger DY, Limami Y, et al. Cyclopamine and jervine induce COX‐2 overexpression in human erythroleukemia cells but only cyclopamine has a pro‐apoptotic effect. Exp Cell Res 2013;319:1043–53. [DOI] [PubMed] [Google Scholar]
- 121. Khanfar MA, El Sayed KA. The Veratrum alkaloids jervine, veratramine, and their analogues as prostate cancer migration and proliferation inhibitors: biological evaluation and pharmacophore modeling. Med Chem Res 2013;22:4775–86. [Google Scholar]
- 122. Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in smoothened and patched can be reversed by cyclopamine. Nature 2000;406:1005–9. [DOI] [PubMed] [Google Scholar]
- 123. Heretsch P, Büttner A, Tzagkaroulaki L, et al. Exo‐cyclopamine—a stable and potent inhibitor of hedgehog‐signaling. Chem Commun 2011;47:7362–4. [DOI] [PubMed] [Google Scholar]
- 124. Berman DM, Karhadkar SS, Hallahan AR, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 2002;297:1559–61. [DOI] [PubMed] [Google Scholar]
- 125. Kawahara T, Kawaguchi‐Ihara N, Okuhashi Y, et al. Cyclopamine and quercetin suppress the growth of leukemia and lymphoma cells. Anticancer Res 2009;29:4629–32. [PubMed] [Google Scholar]
- 126. Zhang X, Harrington N, Moraes RC, et al. Cyclopamine inhibition of human breast cancer cell growth independent of smoothened (Smo). Breast Cancer Res Treat 2009;115:505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Watkins DN, Berman DM, Burkholder SG, et al. Hedgehog signalling within airway epithelial progenitors and in small‐cell lung cancer. Nature 2003;422:313–7. [DOI] [PubMed] [Google Scholar]
- 128. Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res 2007;67:2187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for hedgehog ligand stimulation in growth of digestive tract tumours. Nature 2003;425:846–51. [DOI] [PubMed] [Google Scholar]
- 130. Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 2004;431:707–12. [DOI] [PubMed] [Google Scholar]
- 131. Nishimaki H, Kasai K, Kozaki K‐i, et al. A role of activated sonic hedgehog signaling for the cellular proliferation of oral squamous cell carcinoma cell line. Biochem Biophys Res Commun 2004;314:313–20. [DOI] [PubMed] [Google Scholar]
- 132. Watanabe Y, Aoki S, Tanabe D, et al. Cortistatins E, F, G, and H, four novel steroidal alkaloids from marine sponge Corticium simplex. Tetrahedron 2007;63:4074–9. [Google Scholar]
- 133. Aoki S, Watanabe Y, Tanabe D, et al. Cortistatins J, K, L, novel abeo‐9 (10‐19)‐androstane‐type steroidal alkaloids with isoquinoline unit, from marine sponge Corticium simplex. Tetrahedron Lett 2007;48:4485–8. [Google Scholar]
- 134. Guo Q, Guo J, Yang R, et al. Cyclovirobuxine D attenuates doxorubicin‐induced cardiomyopathy by suppression of oxidative damage and mitochondrial biogenesis impairment. Oxid Med Cell Longev 2015;2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hu D, Liu X, Wang Y, et al. Cyclovirobuxine D ameliorates acute myocardial ischemia by K ATP channel opening, nitric oxide release and anti‐thrombosis. Eur J Pharmacol 2007;569:103–9. [DOI] [PubMed] [Google Scholar]
- 136. Keeler RF. Teratogenic compounds of Veratrum californicum (Durand). X. Cyclopia in rabbits produced by cyclopamine. Teratology 1970;3:175–80. [DOI] [PubMed] [Google Scholar]
- 137. Keeler RF. Teratogenic effects of cyclopamine and jervine in rats, mice and hamsters. Proc Soc Exp Biol Med 1975;149:302–6. [DOI] [PubMed] [Google Scholar]
- 138. Gaffield W. The Veratrum alkaloids: natural tools for studying embryonic development. Stud Nat Prod Chem 2000;23:563–89. [Google Scholar]
- 139. Lipinski RJ, Song C, Sulik KK, et al. Cleft lip and palate results from hedgehog signaling antagonism in the mouse: phenotypic characterization and clinical implications. Birth defects research part a: clinical and molecular. Teratology 2010;88:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lee K‐R, Kozukue N, Han J‐S, et al. Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. J Agric Food Chem 2004;52:2832–9. [DOI] [PubMed] [Google Scholar]
- 141. Friedman M, Rayburn J, Bantle J. Developmental toxicology of potato alkaloids in the frog embryo teratogenesis assay—Xenopus (FETAX). Food Chem Toxicol 1991;29:537–47. [DOI] [PubMed] [Google Scholar]
- 142. Chaube S, Swinyard CA. Teratological and toxicological studies of alkaloidal and phenolic compounds from Solanum tuberosum L. Toxicol Appl Pharmacol 1976;36:227–37. [DOI] [PubMed] [Google Scholar]
- 143. Smith DB, Roddick JG, Jones JL. Potato glycoalkaloids: some unanswered questions. Trends Food Sci Technol 1996;7:126–31. [Google Scholar]
- 144. Langkilde S, Schrøder M, Stewart D, et al. Acute toxicity of high doses of the glycoalkaloids, α‐solanine and α‐chaconine, in the Syrian Golden hamster. J Agric Food Chem 2008;56:8753–60. [DOI] [PubMed] [Google Scholar]
- 145. Keeler R, Young S, Brown D. Spina bifida, exencephaly, and cranial bleb produced in hamsters by the solanum alkaloid solasodine. Res Commun Chem Pathol Pharmacol 1976;13:723–30. [PubMed] [Google Scholar]
- 146. Friedman M, Henika P, Mackey B. Effect of feeding solanidine, solasodine and tomatidine to non‐pregnant and pregnant mice. Food Chem Toxicol 2003;41:61–71. [DOI] [PubMed] [Google Scholar]
- 147. Crawford L, Kocan RM. Steroidal alkaloid toxicity to fish embryos. Toxicol Lett 1993;66:175–81. [DOI] [PubMed] [Google Scholar]
- 148. Morris S, Lee T. The toxicity and teratogenicity of Solanaceae glycoalkaloids, particularly those of the potato (Solanum tuberosum): a review. Food Technol Aust 1984;36:118–124. [Google Scholar]
- 149. Slanina P. Solanine (glycoalkaloids) in potatoes: toxicological evaluation. Food Chem Toxicol 1990;28:759–61. [DOI] [PubMed] [Google Scholar]
- 150. Prada‐Gracia D, Huerta‐Yépez S, Moreno‐Vargas LM. Application of computational methods for anticancer drug discovery, design, and optimization. Bol Med Hosp Infant Mex 2016;73(6):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Dai S‐X, Li W‐X, Han F‐F, et al. In silico identification of anti‐cancer compounds and plants from traditional Chinese medicine database. Sci Rep 2016;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lagunin AA, Goel RK, Gawande DY, et al. Chemo‐ and bioinformatics resources for in silico drug discovery from medicinal plants beyond their traditional use: a critical review. Nat Prod Rep 2014;31:1585–611. [DOI] [PubMed] [Google Scholar]
- 153. Pavan M, Worth A. Publicly‐accessible QSAR software tools developed by the Joint Research Centre. SAR QSAR Environ Res 2008;19:785–99. [DOI] [PubMed] [Google Scholar]
- 154. Demir‐Kavuk O, Bentzien J, Muegge I, et al. DemQSAR: predicting human volume of distribution and clearance of drugs. J Comput Aided Mol Des 2011;25:1121–33. [DOI] [PubMed] [Google Scholar]
- 155. Sushko I, Novotarskyi S, Körner R, et al. Online chemical modeling environment (OCHEM): web platform for data storage, model development and publishing of chemical information. J Comput Aided Mol Des 2011;25:533–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Blanchet M‐F, St‐Onge K, Lisi V, et al. Computational identification of RNA functional determinants by three‐dimensional quantitative structure–activity relationships. Nucleic Acids Res 2014;42:11261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Da Rocha AB, Lopes RM, Schwartsmann G. Natural products in anticancer therapy. Curr Opin Pharmacol 2001;1:364–9. [DOI] [PubMed] [Google Scholar]
- 158. Seca AM, Pinto DC. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int J Mol Sci 2018;19:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Frei E. The clinical use of actinomycin. Cancer Chemoth Rep 1974;58:49–54. [PubMed] [Google Scholar]
- 160. Yoshihara H, Yoshimoto Y, Hosoya Y, et al. Infantile fibrosarcoma treated with postoperative vincristine and dactinomycin. Pediatr Int 2017;59:371–4. [DOI] [PubMed] [Google Scholar]
- 161. Cragg GM, Kingston DG, Newman DJ. Anticancer agents from natural products. CRC Press, Boca Raton: 2011. [Google Scholar]
- 162. Zhang D, Yang R, Wang S, et al. Paclitaxel: new uses for an old drug. Drug Des Devel Ther 2014;8:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod 2004;67:2141–53. [DOI] [PubMed] [Google Scholar]
- 164. Cragg G, Newman D. Natural products as sources of antitumor agents. Ethnopharmacology. Oxford, UK: EOLSS Publishers, 2006. [Google Scholar]
- 165. Butler MS, Robertson AA, Cooper MA. Natural product and natural product derived drugs in clinical trials. Nat Prod Rep 2014;31:1612–61. [DOI] [PubMed] [Google Scholar]
- 166. Martin GE. Small‐volume and high‐sensitivity NMR probes. Annu Rep NMR Spectr 2005;56:1–96. [Google Scholar]
- 167. Schroeder FC, Gronquist M. Extending the scope of NMR spectroscopy with microcoil probes. Angew Chem Int Ed 2006;45:7122–31. [DOI] [PubMed] [Google Scholar]
- 168. Corcoran O, Spraul M. LC–NMR–MS in drug discovery. Drug Discov Today 2003;8:624–31. [DOI] [PubMed] [Google Scholar]
- 169. Lewis RJ, Bernstein MA, Duncan SJ, et al. A comparison of capillary‐scale LC–NMR with alternative techniques: spectroscopic and practical considerations. Magn Reson Chem 2005;43:783–9. [DOI] [PubMed] [Google Scholar]
- 170. Beutler JA. Natural products as a foundation for drug discovery. Curr Protoc Pharmacol 2009;46:9.11.1–9.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Walters WP, Namchuk M. Designing screens: how to make your hits a hit. Nat Rev Drug Discov 2003;2:259–66. [DOI] [PubMed] [Google Scholar]
- 172. Zhong JJ. Biochemical Engineering of the Production of Plant‐Specific Secondary Metabolites by Cell Suspension Cultures. In: Zhong J.J. et al. (eds) Plant Cells. Advances in Biochemical Engineering/Biotechnology. Springer, Berlin, Heidelberg, 2001;72:1–26. [DOI] [PubMed] [Google Scholar]
- 173. Zárate R, El Jaber‐Vazdekis N, Cequier‐Sánchez E, et al. Biotechnology for the production of plant natural products. Stud Nat Prod Chem 2008;34:309–92. [Google Scholar]
- 174. Tabata H. Paclitaxel Production by Plant‐Cell‐Culture Technology. In: Zhong JJ. (eds) Biomanufacturing. Advances in Biochemical Engineering. Springer, Berlin, Heidelberg, 2004; 87: 1–23. [DOI] [PubMed] [Google Scholar]
- 175. Zhong JJ, Yue CJ. Plant Cells: Secondary Metabolite Heterogeneity and Its Manipulation In: Nielsen J. (eds) Biotechnology for the Future. Advances in Biochemical Engineering/Biotechnology. Springer, Berlin, Heidelberg, 2005; 100: 53–88. [DOI] [PubMed] [Google Scholar]
- 176. Tabata H. Production of paclitaxel and the related taxanes by cell suspension cultures of Taxus species. Curr Drug Targets 2006;7:453–61. [DOI] [PubMed] [Google Scholar]
- 177. Rokem J, Tal B, Goldberg I. Methods for increasing diosgenin production by Dioscorea cells in suspension cultures. J Nat Prod 1985;48:210–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1.
Table 1. The In‐vitro anticancer activity of steroidal alkaloids from natural sources in various cancer cell lines.
S1 Table 2. The in‐vitro anticancer activity of synthetic‐origin steroidal alkaloids in several cancer cell lines.
S1 Table 3. The in‐vitro anticancer activity of steroidal alkaloids from industrial sources in various cell lines.
S1 Table 4. The in‐vivo anticancer activity of steroidal alkaloids in various xenograft animal models.
File S2.
Table 1. Important web‐accessible databases for drug target identification.
S2 Table 2. Important web‐accessible databases for small molecule identification.
S2 Table 3. Important web‐accessible and commercially/freely available databases, and software for molecular docking.


