Abstract
Shipping time and shipping delays might affect the quality of the stem cells based engineered “organs.” In our laboratory, we have developed a limbal stem cell deficient (LSCD) rabbit model. To reverse the LSCD, we cultured oral mucosal epithelial cells for 2–3 weeks and engineered cultured autologous oral mucosa epithelial cell sheets (CAOMECS), which were grafted on the LSCD cornea. The purpose of this study was to vitrify CAOMECS and to store it until the CAOMECS can be grafted onto patients. CAOMECS were vitrified in LN2 for up to 204 days. We tested two different methods of vitrification with different solutions; however, CAOMECS were only viable when they were not stored in a vitrification solution; results were only reported from this CAOMECS. On the basis of hematoxylin and eosin staining, we showed that the CAOMECS morphology was well preserved after long‐term storage in LN2. Most of the preservation solutions maintained the CAOMECS phenotype (Ki67, proliferating cell nuclear antigen (PCNA), Beta‐Catenin, ZO‐1, E‐Cadherin, CK3, CK4, CK13). The exception was the solution composed with ethylene glycol and Dimethyl sulfoxide (DMSO): this resulted in loss of DeltaN‐p63 expression. DeltaN‐p63 is an important marker for cell proliferation. The expression of proteins involved in cell–cell connection and the differentiation markers were maintained. Apoptosis was not detected in the thawed CAOMECS. We demonstrated that CAOMECS can be stored long‐term in LN2 without affecting their morphology and phenotype.
Keywords: cell sheets, cold storage, cryoprotective agent, oral mucosa epithelial cells, stem cells, vitrification
1. INTRODUCTION
In the United States, an average of 22 patients die every day waiting for a transplant. In 2016, only 30,970 transplants were performed whereas 121,397 persons were left waiting (data from http://www.organdonor.gov/about/data.html). The gap between donors and patients on the transplant list is increasing. To overcome this problem, two strategies have been developed: (a) cryopreservation of organs for transplantation and (b) preservation of stem cells source to engineer stem cell‐based organs (Fahy & Wowk, 2015). For the first strategy, banking of cryopreserved tissues until they are needed is under development (Garcia‐Dominguez, Vera‐Donoso, Jimenez‐Trigos, Vicente, & Marco‐Jimenez, 2016). As for the second strategy, the cryopreservation of adult stem cells is increasing around the world in anticipation of the need for adult stem cells in research as well as clinical care (Mahla, 2016). On the basis of the database of clinical trials (https://clinicaltrials.gov), as of January 3, 2019, there are currently 7,004 clinical trials involving stem cells, indicating the importance of banking and the use of cells in translational research and clinical application. The production and shipment of organ‐based stem cells must be well controlled and regulated, especially in large geographic regions such as the United States, Europe, Russia, and China. Clinical studies, reporting the use of oral mucosal epithelial cells to treat limbal stem cell deficiencies, were single site studies, as the biopsy, production of cultured autologous oral mucosal epithelial cell sheet (CAOMECS), and grafting onto the patients were conducted on‐site (Burillon et al., 2012; Nishida et al., 2004; Oie & Nishida, 2014). Our long‐term goal is to produce and store CAOMECS at one location and ship to other clinical facilities for corneal transplantation.
It is widely known that engineering organs with stem cells are difficult (Matsa, Burridge, & Wu, 2014), but another important aspect is long‐term storage and transport, and these problems must be addressed. Shipping cell‐based engineered organs to patients at 37°C via airplane is a daunting task. This is due to the following reasons: (a) no electronic devices can be used to maintain the temperature in planes, (b) the nutrients in the culture media will be rapidly depleted and cannot be replaced, (c) the shipping box must be handled with caution to avoid cells detachment from the surface due to agitation, and (d) the acidification of the culture media due to the low atmospheric carbon dioxide levels (Jang, Moon, Hong, & Kim, 2010). At 37°C, the fresh culture media will be rapidly depleted by the cells, resulting in oxidative metabolism and acidification (Kalogeris, Baines, Krenz, & Korthuis, 2012). Additionally, prolonging those culture conditions would increase cellular injury, eventually becoming irreversible and resulting in apoptosis. To avoid the problems of nutrient depletion, temperature control, and acidification of the culture media, cell‐based engineered organs could be vitrified in liquid nitrogen (LN2) for a long‐term storage.
Vitrification is a methodology to cryopreserve organs or cells without the formation of ice, which can damage the cells (during the rapid cooling and thawing steps) by using non‐toxic but high concentrations of cryoprotective agents (CPAs; Fahy & Wowk, 2015). Vitrification requires high concentrations of CPA and rapid cooling; however, this causes the solution in and around the cells to become viscous. Cryopreservation was developed many years ago, and in the past, in vitro fertilization techniques were developed to overcome infertility for the purpose of spermatozoid and oocyte bank preservation (Makar & Toth, 2002). Similarly, the shortage of organs available for transplantation (Abouna, 2008) led to the development of cryopreservation techniques for organs (Pegg, 2015) and organ transport (Mantecchini et al., 2016). Organs are not only preserved for transplantation but also for their stem cells to be used in tissue engineering (Sheikhi, Hultenby, Niklasson, Lundqvist, & Hovatta, 2011). For example, Reubinoff et al. developed a procedure utilizing a straw to cryopreserve embryonic stem cells for future biomedical research (Reubinoff, Pera, Vajta, & Trounson, 2001). In parallel, organs destined for transplantation cannot be preserved for long time. In general, they can be preserved anywhere from a few hours (6 hr for the lungs) to over 1 day (liver) by infusing them with preservation solutions in cold conditions. These infused preservation solutions were developed to maintain the structure and the viability of the organs, but organ reperfusion after preservation in the cold induces organ damage due to the oxidative stress called ischemia–reperfusion injury (Messner, Grahammer, Hautz, Brandacher, & Schneeberger, 2016). Because these organs are available only for a very short time before transplantation and the organ donor shortage, laboratories developed cryopreservation protocols for long‐term organ storage. Fahy et al. have been developing organ vitrification since the 80s to preserve the structure and the function of the organ for long‐term storage (Rall & Fahy, 1985). Cryopreservation of cells and tissues requires LN2, but the formation of ice occurs in the cells during the freezing process and damages cell tissue integrity. CPAs such as dimethyl sulfoxide, 1,2‐propane‐diol, polyvinylpyrrolidone (PVP) are used to reduce ice formation (Asghar, El Assal, Shafiee, Anchan, & Demirci, 2014). Tests on animal organs were performed to develop and to improve the cryopreservation technology. In 2002, Van Den Broecke et al. cryopreserved human ovaries and grafted them back into nude mice. Injection of follicle‐stimulating hormone induced the release of primary and secondary follicles, indicating that the human cryopreserved ovaries were fully functional (Van den Broecke, Liu, Van der Elst, & Dhont, 2002). The cryopreservation technology was tested with success on ovaries, testes, cranial bone, valves, and skeletal muscle tissue (Fan et al., 2018; Jalal & Zidi, 2018; Ming et al., 2018; Wu & Chang, 2018; Yeoman, Wolf, & Lee, 2005). The development of cryopreservation of organs methods and cell‐based engineered organs (such as cell sheets) will increase the availability of organs for patients, an idea mentioned in 1980s by Montefusco and Veith (1985).
For the past few years, we have been developing multilayer CAOMECS by growing isolated oral mucosal epithelial cells to reverse the limbal stem cell deficient (LSCD) of a rabbit model (Bardag‐Gorce et al., 2015). Cell sheets are engineered in the laboratory and transported to the surgery room, where they are then transplanted on the rabbit LSCD cornea (Bardag‐Gorce et al., 2015). The corneal epithelium is renewed by the migration of epithelial cells, coming from the limbus: the limbal stem cells (Zhang et al., 2016). Limbal stem cells migrate from the limbus region to the center of the cornea, following an XYZ migration (Yoon, Ismail, & Sherwin, 2014). In absence of a limbal barrier, the cornea is invaded by blood vessel and by the conjunctival epithelium, resulting in corneal cloudiness (Ebrahimi, Taghi‐Abadi, & Baharvand, 2009). Damaged limbal barrier is also correlated with the absence of limbal stem cells necessary for the renewal of the corneal epithelium. Autoimmune reactions, chemical burns, and infections can cause an LSCD (Sejpal, Bakhtiari, & Deng, 2013). For the majority of the studies (animal and humans), the production of cell sheets and the grafting surgery are usually performed in the same area or facility (Utheim, Utheim, Khan, & Sehic, 2016). The transport of cell sheets over a short distance was uncomplicated, but it becomes challenging when cell sheets must be shipped across the country (Mantecchini et al., 2016; Oie & Nishida, 2014; Shu et al., 2016). One solution is to preserve the CAOMECS through vitrification.
The preservation of cell sheet properties is important for several reasons. Shipping unpreserved CAOMECS in their culture media will continue growing and dividing, consuming all nutrients rapidly, which cannot be replaced or supplemented during the transport. CAOMECS culture media should be replaced daily after they reach confluency. The same problem applies for any shipped cell sheet or other engineered cell‐based organs. In addition to nutrient deprivation, the pH will decrease in the culture media due to their oxidative metabolism and the absence of carbon dioxide (carbon dioxide in the air is only 0.04% when it is 5% in the cell culture incubator). Acidification of the culture media might damage the cells and their extra cellular matrix, which can lead to their apoptosis and loss of regenerative capacity of the CAOMECS (H. J. Park, Lyons, Ohtsubo, & Song, 1999). The additive effect of starvation and acidification of the culture media will affect cell sheet viability and functionality, which could negatively impact the outcome of the grafted cell sheet on the patient's cornea. Vitrification of the cell sheets will protect them and from cell damage due to the cell culture acidification. Other advantages of shipping frozen cell sheets will be the ability to account for delay due to transportation problems (e.g., storm and technical problems) or non‐availability of the patient and/or the surgeon and to limit the number of guanosine monophosphate facilities needed to engineer a cell sheet. The purpose of the study is to store CAOMECS multilayer cell sheets in the liquid nitrogen by preserving their morphology and phenotype.
2. MATERIAL AND METHODS
2.1. Animal studies
New Zealand white rabbits weighting between 2.5 and 3 kg were used. They were maintained according to the Guidelines of Animal Care, as described by the National Academy of Sciences and published by the Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council. This study was approved by the Institutional Animal Care and Use (IACUC) of the Los Angeles Biomedical Research Institute (IACUC no. 20381).
2.2. Isolation of oral mucosal epithelium cells
To perform the interior cheek biopsy, rabbits were lightly sedated, followed by a 6 mm diameter biopsy. The biopsy was then taken to cell culture room to isolate oral mucosal epithelium cells (OMECs). OMECs were isolated based on the protocol of Dr Hayashida (Hayashida et al., 2005). In summary, after incubating the biopsy with Dispase II for 1 hr at 37°C (Roche Diagnostics GmbH, Mannheim, Germany), the epithelium was peeled off from the lamina propria and then was subjected to trypsin digestion in order to isolate the epithelial cells. Isolated cells were then incubated with trypan blue (Invitrogen Corp., Grand Island, NY) and counted using a hemocytometer (Incyto, Covington, GA).
2.3. Engineering cell sheets with oral mucosal epithelium cells on transwell
The isolated epithelial cells were seeded on six‐well plate Transwell® (Corning, Tewksbury, MA), at 65,000 cells/cm2 in coculture with mitomycin C (MMC)‐treated NIH/3 T3 feeder cells (Hayashida et al., 2005). OMECs were seeded in the Transwell® (Corning, Tewsbury, MA) and were cultured with MMC‐treated NIH/3 T3 feeder cells in the following culture media: The culture medium was a mixture of Dulbecco's modified Eagle's medium (Sigma, St Louis, MO) and Ham's F‐12 (Sigma, St Louis, MO) at a ratio of 3:1 supplemented with 10% fetal bovine serum (ThermoFisher Scientific, Chino, CA), 0.4 μg/ml hydrocortisone (Sigma, St Louis, MO), 2 nmol/l triiodothyronine (Sigma, St Louis, MO), 1 nmol/l cholera toxin (List Biological Laboratories, Inc., Campbell, CA), 1X of Insulin‐Transferrin Selenium (100X) (ThermoFisher Scientific, Chino, CA), 10 ng/ml epidermal growth factor (Austral biologicals, San Ramon, Ca), 100 U/ml penicillin (ThermoFisher Scientific, Chino, CA), and 100 μg/ml streptomycin (ThermoFisher Scientific, Chino, CA; Sugiyama, Yamato, Nishida, & Okano, 2014). After 2–3 weeks of growth, engineered multilayer cell sheets were fixed with 10% Neutral Buffered Formalin for future immunocytochemistry analysis.
2.4. Vitrification and thawing of the CAOMECS
CAOMECS were cryopreserved by vitrification procedures in solutions as reported by Sheikhi hereafter named Vitrification Procedure 1 (Sheikhi et al., 2011) and by vitrification procedures reported by Marco‐Jimenez hereafter named Vitrification Procedure 2 (Marco‐Jimenez, Garcia‐Dominguez, Jimenez‐Trigos, Vera‐Donoso, & Vicente, 2015) and following vitrification procedures as reported by Li et al., referred to as Vitrification Procedure 3 (Li, Zhou, Liu, Mai, & Zhuang, 2008). (Scheme 1) Cell sheets and their transwells were vitrified together, and they were placed in small plastic containers using the procedures below. Storage in LN2 was performed in two ways: (a) in the presence of the final vitrification solution of each procedure below (for 18 days in LN2) or (b) “dry” in the absence of the final vitrification solution of each procedure (for 204 days in LN2). The plastic containers were placed in the liquid nitrogen freezer.
Scheme 1.
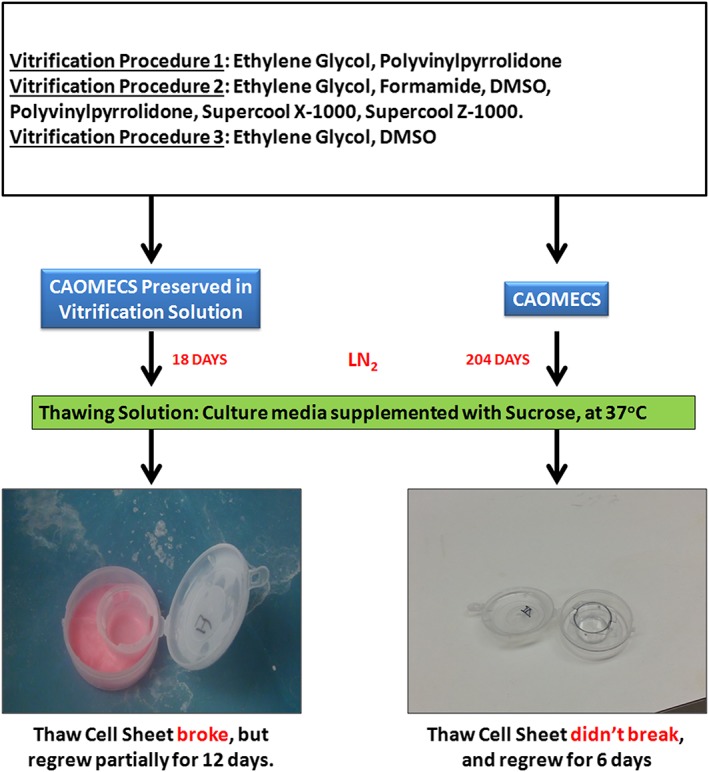
Summary of the cultured autologous oral mucosa epithelial cell sheets (CAOMECS) engineering, vitrification methodology, and thawing steps [Colour figure can be viewed at wileyonlinelibrary.com]
2.4.1. Vitrification Procedure 1
Three solutions were prepared at 2.5%, 5%, and 10% of ethylene glycol (EG; Sigma, St Louis, MO). Only the 10% solution was supplemented with 10% w/v PVP K12. The CAOMECS were immersed in the 2.5% solution for 5 min and for 10 min in the 5% and 10% solutions, before final immersion into the liquid nitrogen. The 2.5% and 5% solutions were maintained at room temperature, whereas the 10% solution was maintained at 4°C.
2.4.2. Vitrification Procedure 2
CAOMECS were immersed in:
1.7% w/v EG (Sigma, St Louis, MO), 1.3% w/v formamide (Sigma, St Louis, MO), 2.2% w/v dimethyl sulfoxide (Sigma, St Louis, MO), 0.7% w/v PVP K12 (PVP of MW 5,000 Da; Sigma, St Louis, MO), and 0.1% w/v final concentrations of SuperCool X‐1000 and SuperCool Z‐1000 (21st Century Medicine, Fontana, CA) for 3 min; then,
4.7% w/v EG, 3.6% w/v formamide, 6.2% w/v DMSO, 1.9% w/v PVP K12, and 0.3% w/v w/v final concentrations of SuperCool X‐1000 and SuperCool Z‐1000 for 1 min; then,
16.84% w/v EG, 12.86% w/v formamide, 22.3% w/v DMSO, 7% w/v PVP K12, and 1% w/v final concentrations w/v final concentrations of SuperCool X‐1000 and SuperCool Z‐1000 followed by direct immersion into LN2 within 1 min.
2.4.3. Vitrification Procedure 3
CAOMECS were immersed in culture media with 10% of DMSO +10% of EG for 1 min; then, they were immersed in culture media +20% of DMSO and 20% of EG (Sigma, St Louis, MO) for 25 s and then immediately immersed in liquid nitrogen.
2.4.4. Thawing procedure
The cell sheets were thawed in four steps using the following solutions at 37°C: (a) 1 min in culture media supplemented with 0.2 M sucrose, (b) 5 min in culture media supplemented with 0.1 M sucrose, (c) and culture media alone for 5 min, and lastly, (d) culture media alone once again for 5 min. After thawing, the CAOMECS were cultured for 6 or 12 days with MMC‐treated NIH/3 T3 feeder cells. All the solutions were filtered before use.
The cell sheets were frozen in the LN2 for 18 days in presence of the vitrification solution or for 204 days in absence of the vitrification solution dry for the tested conditions, with Vitrification Procedures 1, 2, and 3. Results for CAOMECS vitrified for 18 days are shown in Figure 1, and the results for CAOMECS vitrified for 204 days are presented in Figures 1, 2, 3, 4, 5, 6.
Figure 1.
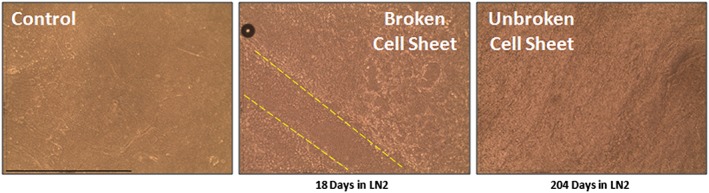
Comparison of the unfrozen and vitrified cultured autologous oral mucosa epithelial cell sheets (CAOMECS). Control CAOMECS, CAOMECS frozen 18 days, and CAOMECS frozen for 204 days are well preserved in LN2. The CAOMECS frozen in the vitrification culture media broke during the thawing step, indicated by the dash lines. Scale bar is 254 μm [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
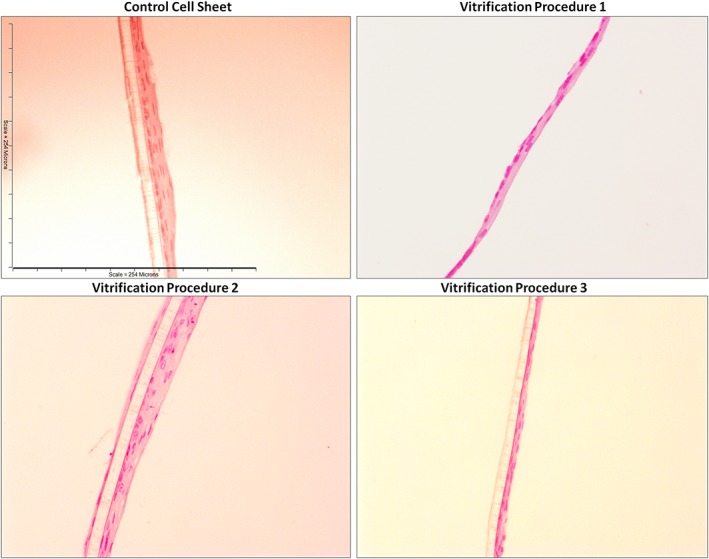
Hematoxylin and eosin on unfrozen cultured autologous oral mucosa epithelial cell sheets (CAOMECS; control cell sheet) and CAOMECS frozen for 204 days using the Vitrification Solutions 1, 2, and 3. Scale bar is 254 μm [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
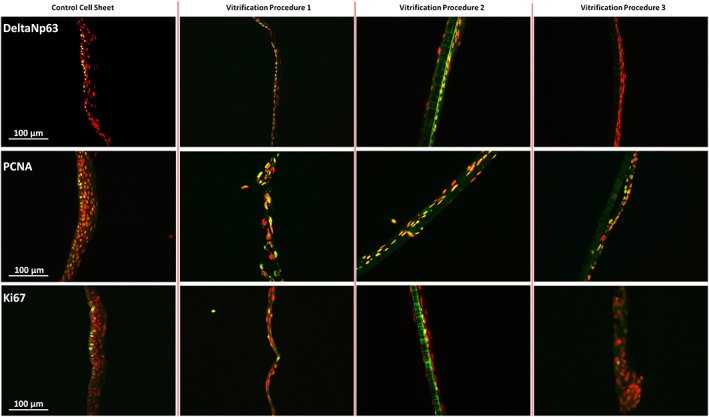
Staining of proliferation markers on unfrozen cultured autologous oral mucosa epithelial cell sheets (CAOMECS; control cell sheet) and CAOMECS frozen for 204 days using the Vitrification Procedures 1, 2, and 3. The expression of proliferating cell nuclear antigen and Ki67 was preserved in any vitrification solution, but DeltaNp63 expression was not preserved in the Vitrification Procedure 3. Scale bar is 100 μm [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.
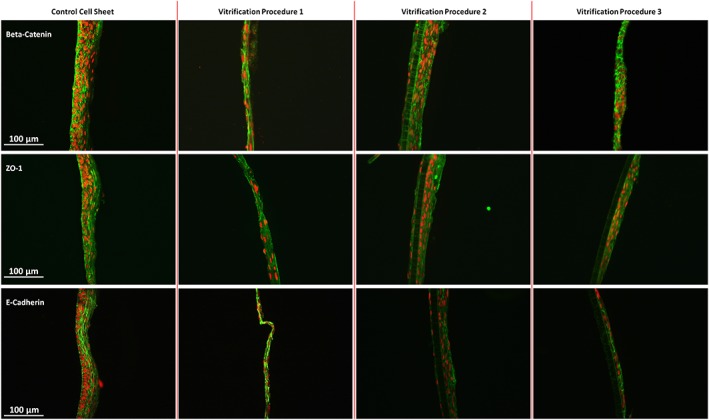
Staining of cell–cell on unfrozen cultured autologous oral mucosa epithelial cell sheets (CAOMECS; control cell sheet) and CAOMECS frozen for 204 days using the Vitrification Procedures 1, 2, and 3. Beta‐Catenin, E‐Cadherin, and ZO‐1 were preserved in all vitrification solution. Scale bar is 100 μm [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 5.
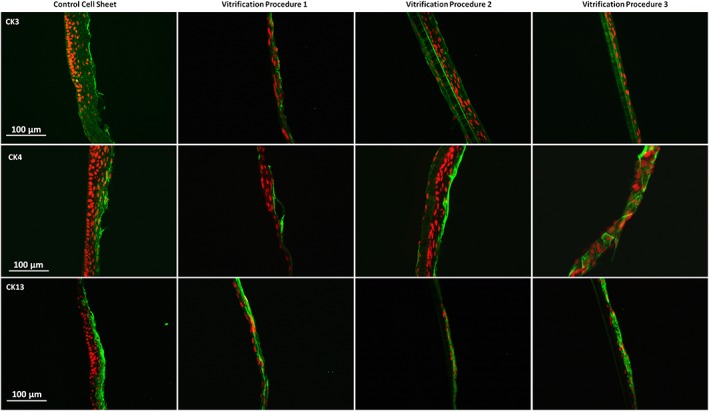
Staining of differentiation markers of the cultured autologous oral mucosa epithelial cell sheets (CAOMECS). For all the vitrification procedure, all the markers CK3, CK4, and CK13 were preserved. Scale bar is 100 μm [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 6.
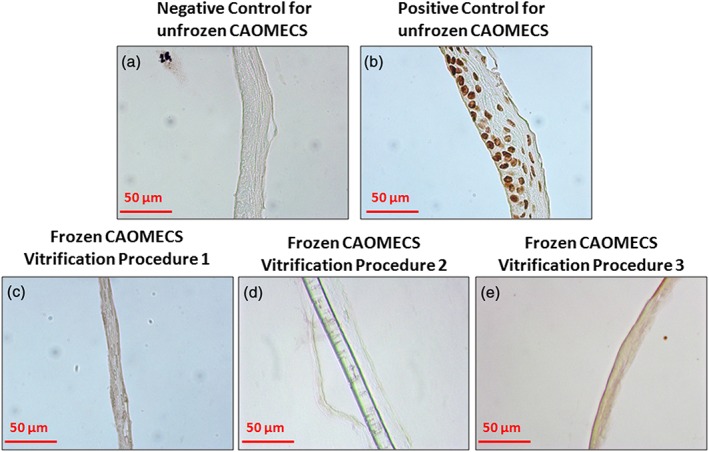
Apoptosis test on frozen (204 days) and unfrozen cultured autologous oral mucosa epithelial cell sheets (CAOMECS). (a) Negative control for the unfrozen CAOMECS. No apoptotic cells were detected. (b) Positive control for the CAOMECS after DNAseI treatment. The whole CAOMECS were positive for the apoptotic staining. Frozen CAOMECS were tested with the apoptotic kit, and all three vitrification procedures did not induce apoptosis (c, d, and e) [Colour figure can be viewed at wileyonlinelibrary.com]
2.5. Hematoxylin and eosin and immunocytochemistry staining
Engineered cell sheets were fixed in 10% neutral buffered formalin, embedded in paraffin, and the tissue sections were then stained with hematoxylin and eosin (H&E) or used for immunofluorescent staining using deltaN‐p63 (Biocare Medical, Concord, CA), proliferating cell nuclear antigen (PCNA; DAKO, Carpinteria, CA), Ki67 (ImmunoTech, Commerce, CA), CK3 (ImmuQuest, North Yorkshire, England), CK4, CK13 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), E‐Cadherin, Beta‐Catenin (BD Biosciences, San Jose, CA), and ZO‐1 (ThermoFisher Scientific, Carlsbad, CA). Alexa Fluor® 488 donkey anti‐mouse conjugated second antibodies or Alexa Fluor® 488 donkey anti‐rabbit conjugated second antibodies (Invitrogen, Eugene, OR) were used. Propidium iodine (Invitrogen, Eugene, OR) was used to stain nuclear DNA. A Nikon 400 fluorescent microscope was used to analyze the slides (Nikon Inc., Melville, NY).
2.6. Apoptosis test
In situ Apoptosis Detection Kit was used to detect apoptotic cells in the CAOMECS (Abcam, Cambridge, MA). We followed the instruction from the manufacturer to detect apoptotic cells on paraffin embedded tissue section. DNase I treatment was used on an unfrozen CAOMECS as a positive control for the methodology.
3. RESULTS
3.1. CAOMECS morphology and vitrification
CAOMECS were observed under an inverted microscope to verify the integrity of the cell sheets prior to vitrification and observed again after thawing. In Figure 1, the control image shows the apical side of the CAOMECS. Uneven surfaces can be observed with 3D‐like structures. CAOMECS morphology, when stored dry for 204 days in the LN2, was similar to the control unfrozen CAOMECS. However, when the CAOMECS were preserved with the vitrification solution in the container (the pink solution in Scheme 1) for 18 days in LN2, the cell sheets broke during the thawing process (Figure 1). Because the CAOMECS were broken, the cell sheets were cultured for a longer time to determine if the broken section of the cell sheets could be repaired; however, the cell sheets were unable to self‐repair the broken sections.
We analyzed and provided results for only those CAOMECS stored in LN2 in the absence of the vitrification solution dry because they were undamaged by the thawing process.
3.2. Morphology of CAOMECS
In Figure 2, H&E showed that the vitrification and thawing procedures preserved the morphology of the CAOMECS when frozen for 204 days in the LN2. The multilayer structures were well preserved as compared with the control cell sheets.
3.3. CAOMECS phenotype
It is important to keep in mind that CAOMECS must replace the corneal epithelium, namely, the cells within the CAOMECS must be renewed over time after corneal grafting (as seen in normal eyes where limbal stem cells replace corneal epithelial cells). Control CAOMECS express proliferative markers such as deltaNp63, PCNA, and Ki67 on the basal side of the cell sheet (Figure 3, first column). PCNA and Ki67 are involved in cell proliferation and also in DNA repair. DeltaNp63 is a key protein and a limbal stem cell marker, which is necessary for the renewal of the epithelium and for the renewal of the CAOMECS. PCNA and Ki67 were preserved and expressed in the vitrified CAOMECS after 204 days in the liquid nitrogen (Figure 3, second and third row). DeltaNp63 expression was preserved in the Vitrification Solutions 1 and 2, but its expression was lost in the Vitrification Solution 3. In the absence of deltaNp63, the CAOMECS may not be capable of self‐renewal in Solution 3.
3.4. Cell–cell interaction in CAOMECS
It was described by our laboratory and by others that the cell–cell connection is a very important biomechanical property of the CAOMECS that is required to preserve the integrity of the layers. Three different proteins have been studied: Beta‐Catenin, E‐Cadherin (adherens junction), and ZO‐1 (tight junction); each of them is part of a cell–cell junction complex. In Figure 4, it is shown that the expression of the three proteins were preserved for all the vitrification solutions compared with the control CAOMECS.
3.5. Stratification and differentiation in CAOMECS
Three CAOMECS markers were studied in the laboratory: CK3, CK4, and CK13. In Figure 5, it is shown that the expression of the three cytokeratins were well preserved, indicating that the cell sheets were able to stratify and differentiate similarly to what is observed with the normal corneal epithelium.
3.6. CAOMECS viability
To ensure that cells from the CAOMECS were viable through the processes, the level of apoptosis in the CAOMECS was verified using an apoptosis kit. No apoptotic cells were detected in the control unfrozen CAOMECS (Figure 6a). As expected, when CAOMECS were treated with DNAse I, apoptotic cells were detected (Figure 6b). No positive apoptotic cells were detected in any CAOMECS vitrified with Solutions 1, 2, or 3 (Figure 6c–e). These results indicate that the cryoprotectant agents were successful in protecting the cells from apoptosis.
4. DISCUSSION
Cell sheet technology has been developing for the past decade to target the delivery of cell sheets onto damaged organs. Success of both cell sheet engineering and transplantation have been reported in numerous articles; however, only three recent publications have reported the vitrification of cell sheets (Maehara et al., 2013; Ohkawara et al., 2018; Tani et al., 2017). Little is known about cell sheet vitrification; more studies are needed to increase our knowledge and to improve the vitrification methodology of cell sheets. In our study, we showed that CAOMECS stored in liquid nitrogen, with vitrification solutions, broke during the thawing process, thus making them not suitable for harvesting nor for cornea transplantation (Bardag‐Gorce et al., 2015; Burillon et al., 2012; Hayashida et al., 2005; Nishida et al., 2004). For this reason, we focused on analyzing CAOMECS stored in the absence of vitrification solutions. In addition, Ohkawara et al. showed that human myoblasts cell sheets vitrified from 2 days to 3 months had the same curative effect on the heart after transplantation, indicating that the time of storage has no effect on the curative properties of the cell sheets (Ohkawara et al., 2018). These authors also mentioned that the cryopreserved cell sheets by slow freezing were not capable of rebuilding the extra cellular matrix upon thawing, confirming the need for a fast freezing of cell sheets. We have shown that CAOMECS can be stored by cryopreservation using Vitrification Procedures 1 and 2 under conditions described for an extended time (204 days) without affecting their morphology and the expression of key proteins. Vitrification Procedure 3 was ineffective because DeltaNp63 expression was lost.
Morphology and protein expression are essential to preserve the physical and biological properties of the CAOMECS. The literature reports that CPAs can affect the expression of genes (Cordeiro, Stirling, Fahy, & de Magalhaes, 2015; Sumida et al., 2011). For example, DMSO was reported to affect the expression of pluripotency genes in human embryonic stem cells. These changes in gene expression can lead to a decrease in stem cell markers (Czysz, Minger, & Thomas, 2015). In our study, Solution 3 affected the expression of deltaN‐p63, which was fully inhibited by an unknown mechanism. However, CAOMECS cell sheet was able to partially repair the broken sections of the stratified cell sheet, possibly through the proliferative function of proliferating cell nuclear antigen (Park, Jeong, Han, Yu, & Jang, 2016). In absence of deltaN‐p63 (Romano et al., 2012), stem cells can still differentiate into epithelial cells, but their renewal capacity was decreased whereas their terminal differentiation was accelerated. The absence of deltaN‐p63 in Solution 3 could be a problem for the corneal epithelium renewal and terminal differentiation after grafting. Solutions 1 and 2 preserved the expression of DeltaN‐p63, a very important protein for the renewal of epithelium such as the corneal epithelium. A recent study reported the vitrification of chondrocyte cell sheet using a mix of DMSO and EG, which were frozen in liquid nitrogen for 4 weeks before being transplanted successfully in rabbits (Tani et al., 2017). Even if the authors showed the efficacy of vitrified cell sheet to decrease the pain and to repair the articular cartilage, the authors did not study the potential effects of the CPAs on cell phenotype (Maehara et al., 2013; Tani et al., 2017). As for our study, immunostaining confirmed the expression and the localization of some specific markers in the cell sheets. However, we do not know if the expression of other important genes related with the renewal and maintenance of the stratified epithelium was affected. RNA sequencing, combined with mass spectrometry, will be the best approach to compare the transcriptome and proteins before and after liquid nitrogen storage. Additionally, the functionality of vitrified CAOMECS needs to be tested in the future to ensure that vitrified CAOMECS can reverse the LSCD on animal models (Bardag‐Gorce et al., 2015; Sugiyama et al., 2014).
The history of cryopreserving cell suspension extends to the early 1950s and 1960s when the first CPAs were used (Lovelock & Bishop, 1959; Polge, Smith, & Parkes, 1949). However, we are less experienced and less successful in utilizing CPAs in more complex tissue. For the past 50 years, many attempts to vitrify complex organs were performed to overcome the lack of organ donors. Due to the difficulty in maintaining the whole tissue structure and function during the vitrification and after thawing, only few positive results were reported on complex organs such as kidneys, ovaries, limbs, and cartilage (Giwa et al., 2017). Cell sheets are an intermediate biological structure between isolated cells and organs, with a simple structure compared with the organs, but they have an organized cell–cell connection (Hayashida et al., 2005). The cell–cell interaction and the extra cellular matrix must be preserved to maintain the morphology of the stratified epithelium (Koster & Roop, 2007). During the freezing steps, cryoprotectant agents will protect the cells against the ice crystal formation. Such a protocol is sufficient when isolated cells are vitrified. However, the protocol is more complex for multistratified tissues because of the formation of ice crystals in the extra cellular space and the disruption of the cell–cell connection by mechanical stresses (Pegg, 2015). Also, if the dehydration of the cells is excessive due to the CPAs, the cell membrane will shrink. The cell–cell connection and the extracellular matrix will be disrupted, negatively affecting the physical cell sheet structure. CAOMECS are a stratified cell sheet, and the multilayer stratification must be preserved during and after the cold storage period in order to be harvested and transplanted onto the cornea. When CAOMECS were stored in the presence of vitrification solutions, the cell sheets were broken during the thawing protocol. These broken CAOMECS cannot be grafted onto corneas; thus, a different storage approach was used. A recent publication reported that slow freezing destroys the extracellular matrix (ECM) and was unable to be repaired confirming the need for our approach for fast freezing in absence of vitrification solutions (dry condition). During the vitrification step, ECM was maintained and preserved the cell sheet structure (Ohkawara et al., 2018). CAOMECS stored in absence of vitrification solution did not break during thawing, and the cell–cell connection was preserved as shown in the immunohistostaining.
Cell‐based engineered organs will become increasingly important in the future. In this study, we demonstrated that our vitrification method preserved the structure of the CAOMECS for long‐term storage in liquid nitrogen. The CAOMECS phenotype was well preserved for Solutions 1 and 2. We also showed that cell sheets must be cryopreserved in the absence of a vitrification solution in the storage box to preserve the physical integrity of CAOMECS. We expect this approach will be further studied to improve the methodology, and we anticipate that this method will become widespread to facilitate the development of cell sheet‐based clinical studies.
CONFLICT OF INTEREST
Dr. Yutaka Niihara is the CEO of Emmaus Medical, Inc, and Joan Oliva is employee of Emmaus Medical, Inc.
ACKNOWLEDGEMENTS
Supported and funded by Emmaus Medical, Inc (Torrance, California). Thank you to Charles Stark, Joseph Becerra, Jason Goodrow, Meiko Mayuzumi, Asia Quan, and Jonathan Aspe for their comments and help for the English.
Oliva J, Florentino A, Bardag‐Gorce F, Niihara Y. Vitrification and storage of oral mucosa epithelial cell sheets. J Tissue Eng Regen Med. 2019;13:1153–1163. 10.1002/term.2864
REFERENCES
- Abouna, G. M. (2008). Organ shortage crisis: Problems and possible solutions. Transplantation Proceedings, 40(1), 34–38. 10.1016/j.transproceed.2007.11.067 [DOI] [PubMed] [Google Scholar]
- Asghar, W. , El Assal, R. , Shafiee, H. , Anchan, R. M. , & Demirci, U. (2014). Preserving human cells for regenerative, reproductive, and transfusion medicine. Biotechnology Journal, 9(7), 895–903. 10.1002/biot.201300074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag‐Gorce, F. , Oliva, J. , Wood, A. , Hoft, R. , Pan, D. , Thropay, J. , … Niihara, Y. (2015). Carrier‐free cultured autologous oral mucosa epithelial cell sheet (CAOMECS) for corneal epithelium reconstruction: A histological study. The Ocular Surface, 13(2), 150–163. 10.1016/j.jtos.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Burillon, C. , Huot, L. , Justin, V. , Nataf, S. , Chapuis, F. , Decullier, E. , & Damour, O. (2012). Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Investigative Ophthalmology & Visual Science, 53(3), 1325–1331. 10.1167/iovs.11-7744 [DOI] [PubMed] [Google Scholar]
- Cordeiro, R. M. , Stirling, S. , Fahy, G. M. , & de Magalhaes, J. P. (2015). Insights on cryoprotectant toxicity from gene expression profiling of endothelial cells exposed to ethylene glycol. Cryobiology, 71(3), 405–412. 10.1016/j.cryobiol.2015.10.142 [DOI] [PubMed] [Google Scholar]
- Czysz, K. , Minger, S. , & Thomas, N. (2015). DMSO efficiently down regulates pluripotency genes in human embryonic stem cells during definitive endoderm derivation and increases the proficiency of hepatic differentiation. PLoS ONE, 10(2), e0117689 10.1371/journal.pone.0117689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi, M. , Taghi‐Abadi, E. , & Baharvand, H. (2009). Limbal stem cells in review. J. Ophthalmic Vis. Res., 4(1), 40–58. [PMC free article] [PubMed] [Google Scholar]
- Fahy, G. M. , & Wowk, B. (2015). Principles of cryopreservation by vitrification. Methods in Molecular Biology, 1257, 21–82. 10.1007/978-1-4939-2193-5_2 [DOI] [PubMed] [Google Scholar]
- Fan, M. C. , Wang, Q. L. , Sun, P. , Zhan, S. H. , Guo, P. , Deng, W. S. , & Dong, Q. (2018). Cryopreservation of autologous cranial bone flaps for cranioplasty: A large sample retrospective study. World Neurosurgery, 109, e853–e859. 10.1016/j.wneu.2017.10.112 [DOI] [PubMed] [Google Scholar]
- Garcia‐Dominguez, X. , Vera‐Donoso, C. D. , Jimenez‐Trigos, E. , Vicente, J. S. , & Marco‐Jimenez, F. (2016). First steps towards organ banks: Vitrification of renal primordial. Cryo Letters, 37(1), 47–52. [PubMed] [Google Scholar]
- Giwa, S. , Lewis, J. K. , Alvarez, L. , Langer, R. , Roth, A. E. , Church, G. M. , … Toner, M. (2017). The promise of organ and tissue preservation to transform medicine. Nature Biotechnology, 35(6), 530–542. 10.1038/nbt.3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida, Y. , Nishida, K. , Yamato, M. , Watanabe, K. , Maeda, N. , Watanabe, H. , … Tano, Y. (2005). Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature‐responsive culture surface. Investigative Ophthalmology & Visual Science, 46(5), 1632–1639. 10.1167/iovs.04-0813 [DOI] [PubMed] [Google Scholar]
- Jalal, N. , & Zidi, M. (2018). Effect of cryopreservation at −80 degrees C on visco‐hyperelastic properties of skeletal muscle tissue. Journal of the Mechanical Behavior of Biomedical Materials, 77, 572–577. 10.1016/j.jmbbm.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Jang, J. , Moon, S. J. , Hong, S. H. , & Kim, I. H. (2010). Colorimetric pH measurement of animal cell culture media. Biotechnology Letters, 32(11), 1599–1607. 10.1007/s10529-010-0341-6 [DOI] [PubMed] [Google Scholar]
- Kalogeris, T. , Baines, C. P. , Krenz, M. , & Korthuis, R. J. (2012). Cell biology of ischemia/reperfusion injury. International Review of Cell and Molecular Biology, 298, 229–317. 10.1016/B978-0-12-394309-5.00006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster, M. I. , & Roop, D. R. (2007). Mechanisms regulating epithelial stratification. Annual Review of Cell and Developmental Biology, 23, 93–113. 10.1146/annurev.cellbio.23.090506.123357 [DOI] [PubMed] [Google Scholar]
- Li, T. , Zhou, C. , Liu, C. , Mai, Q. , & Zhuang, G. (2008). Bulk vitrification of human embryonic stem cells. Human Reproduction, 23(2), 358–364. 10.1093/humrep/dem386 [DOI] [PubMed] [Google Scholar]
- Lovelock, J. E. , & Bishop, M. W. (1959). Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature, 183(4672), 1394–1395. 10.1038/1831394a0 [DOI] [PubMed] [Google Scholar]
- Maehara, M. , Sato, M. , Watanabe, M. , Matsunari, H. , Kokubo, M. , Kanai, T. , … Nagashima, H. (2013). Development of a novel vitrification method for chondrocyte sheets. BMC Biotechnology, 13, 58 10.1186/1472-6750-13-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahla, R. S. (2016). Stem cells applications in regenerative medicine and disease therapeutics. International Journal of Cell Biology, 2016, 6940283 10.1155/2016/6940283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makar, R. S. , & Toth, T. L. (2002). The evaluation of infertility. American Journal of Clinical Pathology, 117 Suppl, S95–S103. [DOI] [PubMed] [Google Scholar]
- Mantecchini, L. , Paganelli, F. , Morabito, V. , Ricci, A. , Peritore, D. , Trapani, S. , … Nanni Costa, A. (2016). Transportation of organs by air: Safety, quality, and sustainability criteria. Transplantation Proceedings, 48(2), 304–308. 10.1016/j.transproceed.2015.12.050 [DOI] [PubMed] [Google Scholar]
- Marco‐Jimenez, F. , Garcia‐Dominguez, X. , Jimenez‐Trigos, E. , Vera‐Donoso, C. D. , & Vicente, J. S. (2015). Vitrification of kidney precursors as a new source for organ transplantation. Cryobiology, 70(3), 278–282. 10.1016/j.cryobiol.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Matsa, E. , Burridge, P. W. , & Wu, J. C. (2014). Human stem cells for modeling heart disease and for drug discovery. Science Translational Medicine, 6(239). 239ps236. 10.1126/scitranslmed.3008921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner, F. , Grahammer, J. , Hautz, T. , Brandacher, G. , & Schneeberger, S. (2016). Ischemia/reperfusion injury in vascularized tissue allotransplantation: Tissue damage and clinical relevance. Current Opinion in Organ Transplantation, 21(5), 503–509. 10.1097/MOT.0000000000000343 [DOI] [PubMed] [Google Scholar]
- Ming, J. M. , Chua, M. E. , Lopes, R. I. , Maloney, A. M. , Gupta, A. A. , & Lorenzo, A. J. (2018). Cryopreservation of testicular tissue in pre‐pubertal and adolescent boys at risk for infertility: A low risk procedure. Journal of Pediatric Urology 10.1016/j.jpurol.2018.02.016, 14, 274.e1–274.e5. [DOI] [PubMed] [Google Scholar]
- Montefusco, C. M. , & Veith, F. J. (1985). Organ selection and preservation for transplantation. Part II: Liver, pancreas, skin, and bone marrow. Hospital Physician, 21(2), 29–33, 36. [PubMed] [Google Scholar]
- Nishida, K. , Yamato, M. , Hayashida, Y. , Watanabe, K. , Yamamoto, K. , Adachi, E. , … Tano, Y. (2004). Corneal reconstruction with tissue‐engineered cell sheets composed of autologous oral mucosal epithelium. The New England Journal of Medicine, 351(12), 1187–1196. 10.1056/NEJMoa040455 [DOI] [PubMed] [Google Scholar]
- Ohkawara, H. , Miyagawa, S. , Fukushima, S. , Yajima, S. , Saito, A. , Nagashima, H. , & Sawa, Y. (2018). Development of a vitrification method for preserving human myoblast cell sheets for myocardial regeneration therapy. BMC Biotechnology, 18(1), 56 10.1186/s12896-018-0467-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie, Y. , & Nishida, K. (2014). Translational research on ocular surface reconstruction using oral mucosal epithelial cell sheets. Cornea, 33(Suppl 11), S47–S52. 10.1097/ICO.0000000000000232 [DOI] [PubMed] [Google Scholar]
- Park, H. J. , Lyons, J. C. , Ohtsubo, T. , & Song, C. W. (1999). Acidic environment causes apoptosis by increasing caspase activity. British Journal of Cancer, 80(12), 1892–1897. 10.1038/sj.bjc.6690617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. Y. , Jeong, M. S. , Han, C. W. , Yu, H. S. , & Jang, S. B. (2016). Structural and functional insight into proliferating cell nuclear antigen. Journal of Microbiology and Biotechnology, 26(4), 637–647. 10.4014/jmb.1509.09051 [DOI] [PubMed] [Google Scholar]
- Pegg, D. E. (2015). Principles of cryopreservation. Methods in Molecular Biology, 1257, 3–19. 10.1007/978-1-4939-2193-5_1 [DOI] [PubMed] [Google Scholar]
- Polge, C. , Smith, A. U. , & Parkes, A. S. (1949). Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature, 164(4172), 666 10.1038/164666a0 [DOI] [PubMed] [Google Scholar]
- Rall, W. F. , & Fahy, G. M. (1985). Ice‐free cryopreservation of mouse embryos at −196 degrees C by vitrification. Nature, 313(6003), 573–575. 10.1038/313573a0 [DOI] [PubMed] [Google Scholar]
- Reubinoff, B. E. , Pera, M. F. , Vajta, G. , & Trounson, A. O. (2001). Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Human Reproduction, 16(10), 2187–2194. 10.1093/humrep/16.10.2187 [DOI] [PubMed] [Google Scholar]
- Romano, R. A. , Smalley, K. , Magraw, C. , Serna, V. A. , Kurita, T. , Raghavan, S. , & Sinha, S. (2012). DeltaNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development, 139(4), 772–782. 10.1242/dev.071191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejpal, K. , Bakhtiari, P. , & Deng, S. X. (2013). Presentation, diagnosis and management of limbal stem cell deficiency. Middle East African Journal of Ophthalmology, 20(1), 5–10. 10.4103/0974-9233.106381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhi, M. , Hultenby, K. , Niklasson, B. , Lundqvist, M. , & Hovatta, O. (2011). Clinical grade vitrification of human ovarian tissue: An ultrastructural analysis of follicles and stroma in vitrified tissue. Human Reproduction, 26(3), 594–603. 10.1093/humrep/deq357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, K. , Kiyama, M. , Nozaki, T. , Nishimura, A. , Suzuki, D. , Kato, M. , … Takeda, S. (2016). Wider adoption of regenerative medicine driven by open innovation. Hitachi Review, 64(10), 6. [Google Scholar]
- Sugiyama, H. , Yamato, M. , Nishida, K. , & Okano, T. (2014). Evidence of the survival of ectopically transplanted oral mucosal epithelial stem cells after repeated wounding of cornea. Molecular Therapy, 22(8), 1544–1555. 10.1038/mt.2014.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida, K. , Igarashi, Y. , Toritsuka, N. , Matsushita, T. , Abe‐Tomizawa, K. , Aoki, M. , … Ohno, Y. (2011). Effects of DMSO on gene expression in human and rat hepatocytes. Human & Experimental Toxicology, 30(10), 1701–1709. 10.1177/0960327111399325 [DOI] [PubMed] [Google Scholar]
- Tani, Y. , Sato, M. , Maehara, M. , Nagashima, H. , Yokoyama, M. , Yamato, M. , … Mochida, J. (2017). The effects of using vitrified chondrocyte sheets on pain alleviation and articular cartilage repair. Journal of Tissue Engineering and Regenerative Medicine 10.1002/term.2257, 11, 3437–3444. [DOI] [PubMed] [Google Scholar]
- Utheim, T. P. , Utheim, O. A. , Khan, Q. E. , & Sehic, A. (2016). Culture of oral mucosal epithelial cells for the purpose of treating limbal stem cell deficiency. Journal of Functional Biomaterials, 7(1). 10.3390/jfb7010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broecke, R. , Liu, J. , Van der Elst, J. , & Dhont, M. (2002). Timing of FSH‐stimulation and follicular development in cryopreserved human ovarian grafts. Reproductive Biomedicine Online, 4(1), 21–26. 10.1016/S1472-6483(10)61910-4 [DOI] [PubMed] [Google Scholar]
- Wu, H. , & Chang, Q. (2018). The cryoprotectant trehalose could inhibit ERS‐induced apoptosis by activating autophagy in cryoprotected rat valves. PLoS ONE, 13(3), e0194078 10.1371/journal.pone.0194078 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yeoman, R. R. , Wolf, D. P. , & Lee, D. M. (2005). Coculture of monkey ovarian tissue increases survival after vitrification and slow‐rate freezing. Fertility and Sterility, 83(Suppl 1), 1248–1254. 10.1016/j.fertnstert.2004.11.036 [DOI] [PubMed] [Google Scholar]
- Yoon, J. J. , Ismail, S. , & Sherwin, T. (2014). Limbal stem cells: Central concepts of corneal epithelial homeostasis. World Journal of Stem Cells, 6(4), 391–403. 10.4252/wjsc.v6.i4.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Sun, H. , Liu, Y. , Chen, S. , Cai, S. , Zhu, Y. , & Guo, P. (2016). The limbal epithelial progenitors in the limbal niche environment. International Journal of Medical Sciences, 13(11), 835–840. 10.7150/ijms.16563 [DOI] [PMC free article] [PubMed] [Google Scholar]


