Summary
Background
CT‐P13, a biosimilar of the reference product infliximab, has been approved for the treatment of ulcerative colitis on the basis of the results of trials conducted in patients with spondyloarthritis and rheumatoid arthritis.
Aim
To compare the effectiveness and safety of CT‐P13 and the reference product in infliximab‐naive patients with ulcerative colitis
Methods
A comparative real‐life equivalence cohort study was conducted using the French nationwide health administrative database. Infliximab‐naive patients with ulcerative colitis over 15 years of age who started infliximab with no other indications for infliximab were included. The primary outcome was a composite endpoint (death, ulcerative colitis‐related surgery, all‐cause hospitalisation and reimbursement for other biologics). Equivalence was defined as a 95% CI of the hazard ratio (HR) of CT‐P13 vs the reference product, in a multivariable marginal Cox model situated within prespecified margins of (0.80‐1.25).
Results
A total of 3112 patients were included between 1 January 2015 and 30 June 2017: 1434 received the reference product, 1678 received CT‐P13. Overall, 710 patients in the reference product group and 743 patients in the CT‐P13 group met the composite endpoint. In multivariable analysis of the primary outcome, CT‐P13 was equivalent to the reference product (HR 1.04; 95% CI: 0.94‐1.15). The number of serious infections was lower in the CT‐P13 group (HR 0.65; 95% CI: 0.48‐0.88). There was no difference in the incidence of solid or haematologic malignancy (HR 0.81; 95% CI: 0.41‐1.60).
Conclusions
The effectiveness of CT‐P13 is equivalent and the risk of serious infections could be lower than that of the reference product for infliximab‐naive patients with ulcerative colitis.
1. BACKGROUND AND AIMS
Infliximab is an anti‐TNF monoclonal antibody approved for the treatment of ulcerative colitis (UC), Crohn's disease, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis and chronic plaque psoriasis. TNF inhibitors, including infliximab, have improved the management of inflammatory bowel disease, either alone or in combination with thiopurines.1 A biosimilar is a copy of a biological reference product (RP). The patent for the RP infliximab (Remicade, Janssen Biotech, Horsham) expired in 2015 in Europe. Biosimilar infliximab CT‐P13 (Remsima, Celltrion, Incheon, South Korea; Inflectra, Pfizer, New York City, USA) was approved by the European Medicines Agency (EMA) in 2013.
The phase 2 PLANETAS2 study and the phase 3 PLANETRA3 study were conducted in infliximab‐naive patients with ankylosing spondylitis and rheumatoid arthritis, respectively. CT‐P13 has been approved for the treatment of these two diseases and this approval has been extended to other diseases, including UC. The principle of extrapolation has been questioned,4 because of minor structural differences between CT‐P13 and RP and because of the possible differences in the mechanisms of action of infliximab across indications.5 Other prospective studies of CT‐P13 in inflammatory bowel disease patients have been published and provide reassuring results. However, none of these studies,6, 7, 8, 9, 10, 11 except for a subgroup of one study,12 directly compared CT‐P13 and RP. In the light of these results, larger and longer‐term studies are needed.
The study hypothesis was that CT‐P13 and RP are equivalent. The European Medicines Agency and the Food and Drug Administration recommend equivalence trials to demonstrate biosimilarity.13, 14 The study designs of randomised controlled trials conducted with CT‐P13 in rheumatoid arthritis and spondyloarthritis are equivalence trials (PLANETAS et PLANETRA2, 3). This is also the case for adalimumab biosimilars.15 The aim of the present study was to compare the effectiveness and safety of CT‐P13 and RP based on data from a large nationwide observational cohort study of infliximab‐naive patients with UC. In our previous study devoted to Crohn's disease, we showed that the effectiveness of CT‐P13 is equivalent to that of RP in infliximab‐naive patients.16 We used the same methodology in the present study.
2. MATERIALS AND METHODS
The methods have already been described in detail elsewhere.16
2.1. Data source
This study was conducted using the SNDS (Système National des Données de Santé) French nationwide health administrative database.17 This database covers more than 99% of the French population (around 65 000 000 people). Patients with long‐term diseases, such as UC, are 100% reimbursed for their health expenditure, and their diagnosis is recorded in the SNDS. Details are provided in the appendix.
2.2. Study population
This study was designed as a real‐life 17 comparative equivalence cohort study. All patients diagnosed with UC before 30 June 2017 were identified in the SNDS. An individual was considered to have been diagnosed with UC when he/she was eligible for long‐term diseases (since 1 January 2006) or had a hospital discharge diagnosis of UC (since 1 January 2010)18 (Table S1). Infliximab‐naive patients with UC who started infliximab between 1 January 2015 and 31 May 2017 were included in the study. An infliximab‐naive patient was defined as a patient who had not been reimbursed for infliximab during the previous 12 months. A diagnosis of UC had to be reported within 30 days after initiation of infliximab, to take into account longer hospital stays or administrative delays related to long‐term diseases procedures.
Patients under the age of 15 years were excluded due to the very small number of CT‐P13 dispensings. Patients who did not receive out‐patient health care during the 3 years before initiating infliximab were excluded. These patients may have lived outside of France or may not have any out‐patient data entered in the SNDS (less than 1% of the French population). Patients who received anti‐TNF for diagnoses other than UC prior to the first infliximab infusion were also excluded (Table S1). Patients with a diagnosis of cancer during the previous 5 years were excluded from the secondary outcome cancer analysis.
2.3. Exposure definition
The primary exposures of interest were infliximab: CT‐P13 or RP. The other infliximab biosimilar, SB2 was not studied, as it has been marketed in France only since 2017. In France, infliximab is always administered in either public or private hospitals. When the first infliximab reimbursement corresponded to the RP, the patient was included in the RP group, and when the first infliximab reimbursement corresponded to CT‐P13, the patient was included in the CT‐P13 group. Follow‐up started 30 days after the first infusion. Patients were followed until onset of a predefined outcome or censoring. A flare during treatment with infliximab reflects treatment failure (event), but a flare after discontinuation of the treatment reflects the natural history of the disease (censoring). Patients were censored at study end (30 June 2017), switch from RP to CT‐P13 (or vice versa) plus 30 days, or discontinuation of infliximab. In the secondary outcome cancer analysis, patients were censored at study end (30 June 2017), or switch from RP to CT‐P13 (or vice versa) plus 30 days. Discontinuation of infliximab was defined as the absence of drug dispensing for 56 days (theoretical coverage) + 60 days = 116 days.
2.4. Outcomes
The primary outcome was a composite endpoint including all causes of infliximab failure, either due to inadequate efficacy or toxicity: death, UC‐related surgery, all‐cause hospitalisation except childbirth (Table S1) for at least one night or reimbursement of another anti‐TNF (adalimumab/golimumab) or vedolizumab. Only the first event was considered. UC‐related surgery included colectomy and/or rectal resection and intestinal stoma (Table S2). As adalimumab, golimumab and vedolizumab are not co‐administered with infliximab, the use of these biologic therapies indicated failure or toxicity of infliximab. This primary outcome assessed effectiveness, as it includes all‐cause hospitalisation.
Secondary outcomes were UC‐related hospitalisation, UC‐related surgery, or each individual item of the composite endpoint. Serious infection (defined as infection requiring hospitalisation, except for intestinal or anorectal abscess or fistula)19 and solid or hematologic malignancies20 were also assessed (Table S1).
2.5. Covariates
Covariates were time‐fixed at cohort entry and included sociodemographic data: sex, age, Complementary Universal Health Insurance status (free access to health care for people with low income) and a deprivation index expressed in quintiles that was developed in France as the first component of a principal component analysis of four socioeconomic variables.21
The interval since UC‐related long‐term diseases or hospitalisation was used as a proxy for UC duration. Proxies for UC severity were defined during the 12 months before initiation of infliximab and consisted of abdominal or pelvic CT scan, colonoscopy, cumulative duration of UC‐related overnight hospitalisations (excluding UC‐related surgery), UC‐related surgery (Table S2), exposure to antidiarrhoeal drugs, oral or topical aminosalicylates, rectal corticosteroids, cumulative oral prednisone equivalent dose of corticosteroids, thiopurines (azathioprine, mercaptopurine), methotrexate or another biologic therapy. Prior thiopurine exposure was defined by dispensing of thiopurine during the 12 months before infliximab initiation except for the last month. Thiopurine combination therapy was defined by thiopurine dispensing between 1 month before and 1 month after infliximab initiation. The last exposure to other biologic therapies was based on dispensing of another anti‐TNF (adalimumab/golimumab) or vedolizumab, as these drugs are usually used in this order.
Cumulative duration of all‐cause overnight hospitalisations without UC‐related surgery was used as a proxy for general health condition during the 12 months before cohort entry. The type of hospital (university, general or private) in which the first infliximab infusion was administered was also taken into account.
2.6. Statistical analysis
Sample size was determined according to the formula proposed by Chow et al22 based on the therapeutic equivalence of CT‐P13 and RP and an expected event rate of 40% in each group.18 A sample of 2173 patients was required, for a two‐sided α level of 0.05, a power of 90% and a two‐sided equivalence margin of [0.8‐1.25].
In an equivalence trial, two treatments can be considered to be equivalent when the treatment hazard ratio (HR) and CI are situated within the predefined clinical equivalence margins: [Δ−1/Δ]. Equivalence margins in biosimilar arthritis trials were an absolute difference of 15% and the non‐inferiority margin in NOR‐SWITCH was also 15%.3, 12, 23, 24 Equivalence margins of 10% were used in the present study, because such margins can be considered to be more clinically relevant. These 10% margins correspond to relative margins of [0.80‐1.25]. The more stringent confidence interval (95%) recommended by the European Medicines Agency25 was used (90% CI for the Food and Drug Administration26).
Descriptive analysis of covariates at cohort entry was performed: median and interquartile range (IQR) for continuous variables and proportions for dichotomous and class variables. Comparative survival analysis between CT‐P13 and RP was then performed: cumulative incidence plot, log‐rank test and marginal Cox proportional hazards regression model to estimate adjusted HR and their 95% CI. The marginal Cox model is a population average model used for clustered events.27 In this case, the cluster is the hospital, as the choice between CT‐P13 and RP is rarely decided by the clinician, but corresponds to the hospital pharmacy's choice for all hospital patients. This model was used for the primary and secondary outcomes. Details are provided in the appendix.
Although the primary outcome corresponded to a two‐sided equivalence study, two‐sided superiority analysis with an α level of 0.05 was performed to test secondary outcomes. If the primary outcome was in favour of the equivalence of CT‐P13 and RP, the composite endpoint was analysed for heterogeneity according to sex, age, UC duration and exposure to thiopurines by an interaction test.
As the choice of the follow‐up start date (day of first infliximab infusion + 30 days) and end date (56 days + 60 days) was partly arbitrary, various sensitivity analyses were conducted using alternative follow‐up start (day of first infliximab infusion) and end dates (56 days + 30 days, or 56 days + 90 days). Other sensitivity analyses used the primary outcome analysis without a marginal model, or excluding patients who received infliximab between 1 January 2009 and 12 months before initiation of infliximab, or with a more specific definition of UC (excluding patients with eligibility for long‐term disease status or had at least one hospital discharge diagnosis of Crohn's disease before initiation of infliximab), or with the inverse probability of treatment weighting method.28, 29, 30, 31 Details are provided in the appendix.
We also calculated the E‐value, which is the minimum strength of association on the risk ratio scale that an unmeasured confounder would need to have with both the treatment and the outcome, conditional on the measured covariates, to explain away a treatment–outcome association.32
Our public institution has permanent access to SNDS data in application of the provisions of articles R. 1461‐12 et seq. of the French Public Health Code, therefore ethical board approval was not required. All analyses were performed with SAS software version 9.2 (SAS Institute, Inc).
3. RESULTS
3.1. Patient characteristics
A total of 189 406 individuals with UC were identified in the SNDS: 25.1% had a diagnosis of UC based on eligibility for long‐term diseases status, 43.4% had an UC‐related hospitalisation and 31.5% were identified on the basis of both criteria. In this population, 4981 patients initiated infliximab therapy between 1 January 2015 and 31 May 2017. Patients were excluded from this sample for the following reasons: 239 were under the age of 15 years, nine had no prior usage of out‐patient health care during the 3 years before initiating infliximab and 1621 had an additional indication for infliximab (Figure 1). Thus, 3112 individuals were included in the analysis; 1434 (46.1%) in the RP group and 1.678 (53.9%) in the CT‐P13 group. Since January 2015, the proportion of CT‐P13 use over RP increased gradually over the study period: during 2015, the RP was the most prescribed infliximab and during 2016/2017, it was CT‐P13 (Table 1). This rise in prescription explains that the median follow‐up was 423 days (IQR 189‐757) in the RP group and 286 days (IQR 168‐466) in the CT‐P13 group.
Figure 1.
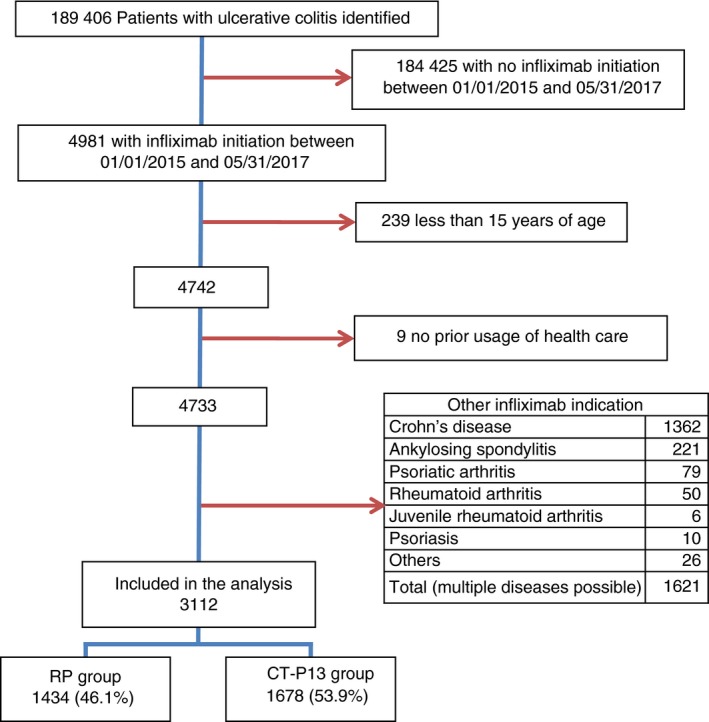
Flow chart. RP, reference product
Table 1.
Demographic and baseline patient characteristics
| RP | CT‐P13 | |
|---|---|---|
| N (%) | 1434 (100.0) | 1678 (100.0) |
| Sex | ||
| Men | 781 (54.5) | 860 (51.3) |
| Women | 653 (45.5) | 818 (48.7) |
| Age (y) | ||
| 15‐29 | 406 (28.3) | 535 (31.9) |
| 30‐44 | 427 (29.8) | 468 (27.9) |
| 45‐59 | 331 (23.1) | 374 (22.3) |
| +60 | 270 (18.8) | 301 (17.9) |
| UC durationa | ||
| <6 mo | 376 (26.2) | 392 (23.4) |
| 6 mo‐2 y | 344 (24.0) | 464 (27.7) |
| 2‐6 y | 411 (28.7) | 468 (27.9) |
| >6 y | 303 (21.1) | 354 (21.1) |
| CMUc status | 142 (9.9) | 150 (8.9) |
| Deprivation index (quintiles) | ||
| Missing | 66 (4.6) | 28 (1.7) |
| 1: Less deprived | 242 (16.9) | 364 (21.7) |
| 2 | 288 (20.1) | 336 (20.0) |
| 3 | 267 (18.6) | 317 (18.9) |
| 4 | 266 (18.5) | 304 (18.1) |
| 5: More deprived | 305 (21.3) | 329 (19.6) |
| Colonoscopyb | 1193 (83.2) | 1451 (86.5) |
| Abdominal or pelvic CT scanb | 472 (32.9) | 590 (35.2) |
| Antidiarrhoeal drugsb | 729 (50.8) | 798 (47.6) |
| Oral aminosalicylatesb | 941 (65.6) | 1179 (70.3) |
| Topical aminosalicylatesb | 752 (52.4) | 943 (56.2) |
| Topical corticosteroidsb | 465 (32.4) | 532 (31.7) |
| Corticosteroidsc | ||
| 0 | 324 (22.6) | 304 (18.1) |
| <2 g | 402 (28.0) | 468 (27.9) |
| 2‐4 g | 348 (24.3) | 428 (25.5) |
| >4 g | 360 (25.1) | 478 (28.5) |
| Thiopurine exposure | ||
| None | 649 (45.3) | 713 (42.5) |
| Prior | 249 (17.4) | 256 (15.3) |
| Combination therapy | 241 (16.8) | 348 (20.7) |
| Prior and combination therapy | 295 (20.6) | 361 (21.5) |
| Methotrexateb | 66 (4.6) | 68 (4.1) |
| Last biologic therapyb | ||
| None | 983 (68.5) | 1085 (64.7) |
| Adalimumab/Golimumab | 441 (30.8) | 556 (33.1) |
| Vedolizumab | 10 (0.7) | 37 (2.2) |
| Duration of all‐cause hospitalisationb , d | ||
| 0 nights | 697 (48.6) | 713 (42.5) |
| < 3 nights | 175 (12.2) | 190 (11.3) |
| 3 nights ‐ 1 wk | 188 (13.1) | 262 (15.6) |
| 1‐2 wk | 199 (13.9) | 277 (16.5) |
| >2 wk | 175 (12.2) | 236 (14.1) |
| Duration of UC‐related hospitalisationb , d | ||
| 0 nights | 814 (56.8) | 846 (50.4) |
| <3 nights | 130 (9.1) | 164 (9.8) |
| 3 nights ‐ 1 wk | 188 (13.1) | 240 (14.3) |
| 1‐2 wk | 172 (12.0) | 261 (15.6) |
| >2 wk | 130 (9.1) | 167 (10.0) |
| UC‐related surgeryb | 12 (0.8) | 19 (1.1) |
| Year of Infliximab initiation | ||
| 2015 | 950 (66.2) | 348 (20.7) |
| 2016 | 350 (24.4) | 909 (54.2) |
| 2017 (until May included) | 134 (9.3) | 421 (25.1) |
| Hospital | ||
| General | 487 (34.0) | 532 (31.7) |
| University | 416 (29.0) | 772 (46.0) |
| Private | 531 (37.0) | 374 (22.3) |
Abbreviations: CMUc, Complementary Universal Health Insurance; RP, reference product; UC, ulcerative colitis.
Time since first diagnosis.
At least once during the 12 mo before cohort entry.
Cumulative prednisone equivalent corticosteroid dose during the 12 mo before cohort entry.
without UC‐related surgery.
Patient characteristics at cohort entry are shown in Table 1. The cohort comprised 47.3% women with a median age of 40 years (IQR 27‐54) and a median UC duration of 2.0 years (IQR 0.5‐5.3), 54.7% of patients had at least one overnight hospitalisation during the previous 12 months and 40.0% initiated infliximab therapy in combination with thiopurines. CT‐P13 was more frequently prescribed in university hospitals (university hospitals 46.0%, general hospitals 31.7%, private hospitals 22.3%), while the RP was more frequently prescribed in private hospitals (29.0%, 34.0%, 37.0% respectively). Patient characteristics at cohort entry were well balanced, but with a trend towards more severe UC in patients who received CT‐P13, who presented more pelvic or abdominal CT scans (35.2% vs 32.9%), UC‐related hospitalisations (49.6% vs 43.2%) and biologic therapy (35.3% vs 31.5%) during the 12 months before inclusion.
During follow‐up, 208 (14.5%) patients discontinued infliximab in the RP group and 187 (11.1%) patients discontinued infliximab in the CT‐P13 group; in addition, 163 (11.4%) and 171 (10.2%) patients, respectively, switched to the other form of infliximab (Table S3).
3.2. Effectiveness
The primary outcome did not differ between the RP and CT‐P13 groups (log‐rank test; P = 0.20). The 12‐ and 24‐month cumulative incidence rates of the primary outcome were 43.0% (95% CI: 40.5‐45.6) and 57.5% (95% CI: 54.9‐60.0), respectively, in the RP group and 45.1% (95% CI: 42.7‐47.5) and 59.8% (95% CI: 57.5‐62.1), respectively, in the CT‐P13 group (Figure 2). Overall, a composite event was reported in 710 patients (49.5%), including 472 hospitalisations (32.9%) in the RP group vs 743 patients (44.3%) including 505 hospitalisations (30.1%) in the CT‐P13 group (Table S3); 60.6% of hospitalisations were UC‐related (Table S4).
Figure 2.
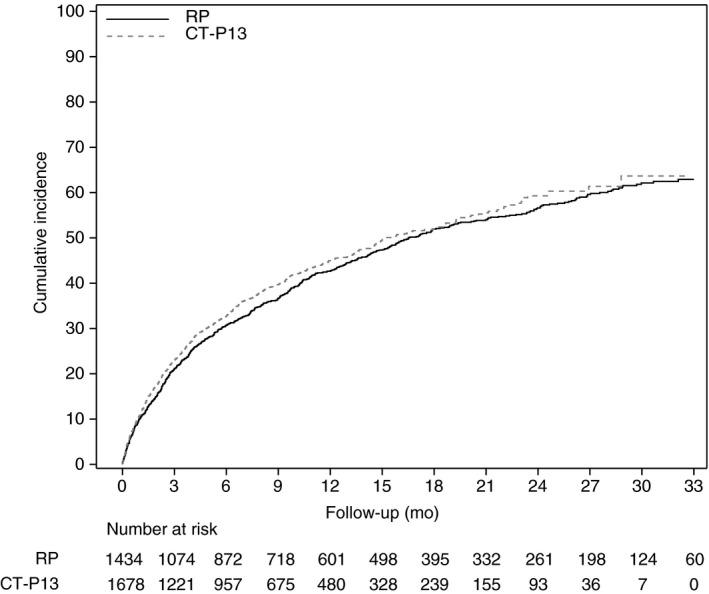
Cumulative incidence plot for event‐free survival (primary outcome). RP, reference product
In multivariable analysis of the primary outcome, CT‐P13 was equivalent to RP (HR 1.04; 95% CI: 0.94‐1.15) (Table 2 and Figure S1). In this multivariable analysis, combination therapy with a thiopurine with (HR 0.79; 95% CI: 0.69 ‐ 0.90) or without (HR 0.59; 95% CI: 0.51‐0.68) prior use of thiopurine were inversely associated with the primary outcome. Prior all‐cause hospitalisations (more than 2 weeks of hospitalisation vs no hospitalisation: HR 2.08; 95% CI: 1.77‐2.45), prior use of vedolizumab (HR 1.57; 95% CI: 1.12‐2.20) and UC‐related surgery (HR 1.87; 95% CI: 1.28‐2.71) were associated with the primary outcome. (Table S5).
Table 2.
Effectiveness
| Events/ N | Incidence rate /1000 PY | Multivariable Cox model | ||||
|---|---|---|---|---|---|---|
| RP | CT‐P13 | RP | CT‐P13 | HR (95% CI) | P | |
| Primary outcome: composite endpointa | 710/1434 | 743/1678 | 497.4 | 613.5 | 1.04 (0.94‐1.15) | |
| All‐cause hospitalisationb | 507/1434 | 536/1678 | 343.8 | 424.5 | 1.01 (0.89‐1.15) | 0.85 |
| UC‐related hospitalisationc | 299/1434 | 353/1678 | 179.3 | 256.9 | 1.10 (0.93‐1.29) | 0.27 |
| UC‐related surgeryd | 70/1434 | 84/1678 | 37.8 | 55.1 | 1.04 (0.74‐1.45) | 0.82 |
| Dispensing of other biotherapye | 359/1434 | 351/1678 | 201.4 | 240.0 | 1.03 (0.89‐1.19) | 0.70 |
Abbreviations: CI, confidence interval; CMUc, Complementary Universal Health Insurance; HR, hazard ratio; PY, person years; RP, reference product; UC, ulcerative colitis.
Multivariable marginal Cox model adjusted for: age, UC duration, CMUc status, topical corticosteroids, antidiarrhoeal drugs, thiopurines, last biologic therapy, all‐cause hospitalisations and UC‐related surgery.
Multivariable marginal Cox model adjusted for: age, UC duration, CMUc status, antidiarrhoeal drugs, thiopurines, last biologic therapy, all‐cause hospitalisations and UC‐related surgery.
Multivariable marginal Cox model adjusted for: age, UC duration, CMUc status, antidiarrhoeal drugs, thiopurines, last biologic therapy, UC‐related hospitalisations and UC‐related surgery.
Multivariable marginal Cox model adjusted for: sex, age, UC duration, antidiarrhoeal drugs, thiopurines, last biologic therapy, all‐cause hospitalisations and UC‐related surgery.
Multivariable marginal Cox model adjusted for: corticosteroids, thiopurines, last biologic therapy and UC‐related surgery.
As the log‐linearity hypothesis was not verified for age, UC duration, nights of UC‐related hospitalisation (without UC‐related surgery) and cumulative corticosteroid dose, these continuous variables were transformed into classes. There was no evidence against the proportional hazards hypothesis.
Multivariable analysis of secondary outcomes did not reveal any significant difference between CT‐P13 and RP for the following events: all‐cause hospitalisation (HR 1.01; 95% CI: 0.89‐1.15), UC‐related hospitalisation (HR 1.10; 95% CI: 0.93‐1.29), UC‐related surgery (HR 1.04; 95% CI: 0.74‐1.45) and reimbursement of another biologic therapy (HR 1.03; 95% CI: 0.89‐1.19) (Table 2 and Figures [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link]).
No heterogeneity of the primary outcome was observed on an interaction test according to sex (P = 0.61), age (P = 0.12), UC duration (P = 0.18) or exposure to thiopurines (P = 0.42) (Figure S2). Sensitivity analyses demonstrated the robustness of the results (Table S6 and S7). The E‐value was 1.3 to move the upper bound of the CI for the HR (1.15) of the primary outcome above the predefined upper equivalence limit [1.25], and 1.5 to move the HR estimate to greater than 1.25.32
3.3. Safety
A total of 157 (47.6/1000 PY) serious infections were identified. There were 42 (12.7/1000 PY) gastrointestinal infections, including 14 (4.2/1000 PY) Clostridium difficile infections, 13 (3.9/1000 PY) cases of cholecystitis or cholangitis and 10 (3.9/1000 PY) cases of Cytomegalovirus colitis. Other serious infections included 32 (9.7/1000 PY) skin and subcutaneous tissue infections and 28 (8.5/1000 PY) lung infections. There were fewer serious infections in the CT‐P13 group (42.4 vs 51.9/1000 PY) including fewer skin and subcutaneous tissue infections (6.6 vs 12.3/1000 PY), fewer lung infections (7.3 vs 9.5/1000 PY) and fewer urinary tract infections (4.6 vs 7.3/1000 PY) (Table S8). Multivariable analysis demonstrated fewer serious infections in the CT‐P13 group (HR 0.65; 95% CI: 0.48‐0.88; Table 3). Forty‐one (8.8/1000 PY) solid or hematologic malignancies were identified, including 13 (2.8/1000 PY) gastrointestinal cancers, nine (1.0/1000 PY) colorectal cancers, six (1.3/1000 PY) breast cancers and five (1.1/1000 PY) skin cancers (Table S9). The median age at cancer diagnosis was 50 years (IQR 40‐64). Multivariable analysis did not demonstrate any significant differences in terms of solid or hematologic malignancies between CT‐P13 and RP (HR 0.81; 95% CI: 0.41‐1.60) (Table 3).
Table 3.
Safety analysis
| Events/N | Incidence rate /1000 PY | Multivariable Cox model | ||||
|---|---|---|---|---|---|---|
| RP | CT‐P13 | RP | CT‐P13 | HR (95% CI) | P | |
| Serious infectiona | 93/ 1434 | 64/ 1678 | 51.9 | 42.4 | 0.65 (0.48 ‐ 0.88) | 0.005 |
| Cancerb | 25/ 1364 | 16/ 1609 | 9.4 | 7.9 | 0.81 (0.41 ‐ 1.60) | 0.54 |
Abbreviations: CI, confidence interval; HR, hazard ratio; PY, person years; RP, reference product; UC, ulcerative colitis.
Multivariable marginal Cox model adjusted for: age, UC duration, all‐cause hospitalisations.
Patients with a diagnosis of cancer during the previous 5 y were excluded from the cancer analysis. Patients were censored at study end (December 31, 2017), or switch from RP to CT‐P13 or from CT‐P13 to RP plus 30 d (no censoring after discontinuation of infliximab for the cancer analysis). Multivariable marginal Cox model adjusted for: age, thiopurine exposure and UC‐related surgery.
4. DISCUSSION
Approval of CT‐P13 for UC was based on extrapolation of the results observed in arthritis. This nationwide real‐life cohort study of infliximab‐naive patients with UC demonstrates equivalent effectiveness of CT‐P13 and RP. The HR and 95% confidence interval (HR 1.04; 95% CI: 0.94‐1.15) were situated within the predefined equivalence margins [0.80‐1.25]. The incidence of serious infection was lower in the CT‐P13 group (HR 0.65; 95% CI: 0.48‐0.88), but there was no significant difference in the incidence of solid or hematologic malignancies was observed between the two groups.
All‐cause and UC‐related hospitalisation rates were 38.1/100 PY and 21.4/100 PY, respectively. These rates are similar to those reported in a real‐life Danish study (all‐cause: 37.4/100 PY; UC‐related: 19.3/100 PY).33 Combination therapy with a thiopurine, with or without previous exposure to thiopurines, was associated with better outcomes. This is consistent with the results of a randomised trial in which combination therapy was associated better outcomes than infliximab monotherapy.1 The incidence rates of serious infections were 42.4 and 51.9 per 1000 person‐years (PY), with CT‐P13 and RP, respectively. The incidence rate of serious infections in UC patients treated with CT‐P13 was similar to that observed in a companion study of patients with Crohn's disease treated with CT‐P13 or RP (39.8 and 42.3 per 1000 PY) and within the expected ranges of 20‐80/1000 PY.16, 19, 34, 35 The incidence of serious infections was higher with RP with greater numbers of skin, lung and urinary tract infections observed in the RP group than in the CT‐P13 group. The reason for this difference remains unclear and deserves further research. A true biological effect between these two drugs (eg a more profound immunosuppression with reference product) cannot be excluded. However, this result can also be explained by a residual confounding or even by chance alone. The cancer incidence rate was 8.7/1000 PY, similar to the expected range of 4 to 8/1000 PY.19, 34, 35, 36
Over the last 40 years, new drug approvals have been based on randomised, double‐blind and placebo‐controlled trials. However, patients included in these trials are highly selected. One study showed that only 26% of UC patients seen in clinical practice would be eligible for inclusion in randomised trials.37 To our knowledge, only one real‐life effectiveness study has been conducted in UC. It used Danish National Patient Registry data for 1719 anti‐TNF‐naive UC patients and showed that the use of adalimumab as first‐line biologic over infliximab was associated with a higher risk of hospitalisation and serious infections.33
We have previously conducted a study on the SNDS database in infliximab‐naïve patients with Crohn's disease, treated with either the RP or CT‐P13. In this previous study, the effectiveness of CT‐P13 was equivalent to that of RP and no difference was observed for safety outcomes.16
The present study has several strengths. First, the SNDS is a comprehensive database for drug dispensing, hospitalisations and surgery in France. Second, this study included a large sample of 3112, unselected UC patients. Third, the equivalence limits were more stringent than those used in randomised controlled trials (10% vs 15% absolute difference). Fourth, and most importantly, the indication bias was minimal; the two groups were well balanced (Table 1) and the choice between CT‐P13 and RP was made by the hospital pharmacy, not by the physician. Additionally, the primary analysis was performed with the inverse probability of treatment weighting method, which did not modify the results.
This study also presents several limitations. First, the SNDS does not contain all relevant clinical data allowing calculation of indices such as Mayo score; additionally, it does not contain relevant biologic data such as C‐reactive protein or faecal calprotectin. We therefore used proxies to estimate disease severity. Second, an algorithm was used to identify UC patients. Other studies have used the same algorithm,18, 20, 38 based on the combination of hospitalisation and long‐term diseases UC codes. The present study also used dispensing of infliximab (excluding other indications for anti‐TNF therapy). Third, only infliximab‐naive patients were included. Further studies are needed to assess the switch from RP to CT‐P13 (or vice versa). Fourth, some hospitals only use the biosimilar, while others only use the RP. This centre effect was taken into account by a marginal model, but was probably minor, as sensitivity analysis with a fixed Cox model (without the marginal model) gave very similar results. Fifth, we observed some Crohn's disease complications in our UC population (Table S4) probably because UC, colonic Crohn's disease and indeterminate colitis may be difficult to differentiate. A sensitivity analysis with a more specific definition of UC (excluding patients who had at least one hospital discharge or long‐term disease diagnosis of Crohn's disease before initiation of infliximab) provided very similar results with almost the same rate of Crohn's disease complications. Sixth, from SNDS data, infliximab dose escalation could not be reliably assessed and trough levels are not available. Seventh, this study did not identify mild disease activity that would not require a new medical treatment or surgery or hospitalisation; and this was true in patients treated with the RP or with CT‐P13. Eighth, an infliximab‐naive patient was defined, as in other infliximab studies,39, 40, 41 as a patient who had not been reimbursed for infliximab during the previous 12 months. Despite this broad definition, only 1.8% of patients had received infliximab since 1 January 2009. Moreover, the results of a sensitivity analysis for the primary outcome excluding these patients were identical.
In conclusion, our observational study of real‐life data suggests that the effectiveness of CT‐P13 is equivalent and the risk of serious infections could be lower than that of the reference product in infliximab‐naive patients with UC. The choice between the two products in patients with inflammatory bowel disease can therefore be mainly based on cost alone.
AUTHORSHIP
Guarantor of the article: Antoine Meyer.
Author contributions: Study concept: Antoine Meyer, Jérémie Rudant, Joël Coste, Franck Carbonnel, Alain Weill. Acquisition of data: Jérôme Drouin, Antoine Meyer. Statistical analysis, interpretation of data and drafting of the manuscript: Antoine Meyer. Critical revision of the manuscript for important intellectual content: every author. Study supervision: Joël Coste, Jérémie Rudant and Alain Weill.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank Anthony Saul, MD, for assistance with English grammar and spelling.
Declaration of personal interests: Dr. Meyer, Mr. Drouin, Dr. Weill and Prof. Coste declared no conflict of interest. Dr. Rudant reports personal fees from CNAM during the conduct of the study. Prof. Franck Carbonnel received board or lecture fees from Enterome, MSD, BMS, Janssen, Pfizer, Abbvie, Mayoly Spindler, Takeda, Pileje.
Meyer A, Rudant J, Drouin J, Coste J, Carbonnel F, Weill A. The effectiveness and safety of infliximab compared with biosimilar CT‐P13, in 3112 patients with ulcerative colitis. Aliment Pharmacol Ther. 2019;50:269–277. 10.1111/apt.15323
The Handling Editor for this article was Dr Nicholas Kennedy, and it was accepted for publication after full peer‐review.
Funding information
This research was funded by French National Health Insurance (CNAM). All authors are employees of a French public organization.
REFERENCES
- 1. Panaccione R, Ghosh S, Middleton S, et al. Combination Therapy With Infliximab and Azathioprine Is Superior to Monotherapy With Either Agent in Ulcerative Colitis. Gastroenterology. 2014;146:392‐400.e3. [DOI] [PubMed] [Google Scholar]
- 2. Park W, Hrycaj P, Jeka S, et al. A randomised, double‐blind, multicentre, parallel‐group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT‐P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double‐blind, parallel‐group study to demonstrate equivalence in efficacy and safety of CT‐P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ben‐Horin S, Vande Casteele N, Schreiber S, Lakatos PL. Biosimilars in inflammatory bowel disease: facts and fears of extrapolation. Clin Gastroenterol Hepatol. 2016;14:1685‐1696. [DOI] [PubMed] [Google Scholar]
- 5. Gámez‐Belmonte R, Hernández‐Chirlaque C, Arredondo‐Amador M, et al. Biosimilars: Concepts and controversies. Pharmacol Res. 2018;133:251‐264. [DOI] [PubMed] [Google Scholar]
- 6. Komaki Y, Yamada A, Komaki F, Micic D, Ido A, Sakuraba A. Systematic review with meta‐analysis: the efficacy and safety of CT‐P13, a biosimilar of anti‐tumour necrosis factor‐α agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:1043‐1057. [DOI] [PubMed] [Google Scholar]
- 7. Fiorino G, Manetti N, Armuzzi A, et al. The PROSIT‐BIO cohort: a prospective observational study of patients with inflammatory bowel disease treated with infliximab biosimilar. Inflamm Bowel Dis. 2017;23:233‐243. [DOI] [PubMed] [Google Scholar]
- 8. Gisbert JP, Chaparro M. Switching from an originator anti‐TNF to a biosimilar in patients with inflammatory bowel disease: Can it be recommended? A systematic review. Gastroenterol Hepatol. 2018;41:389‐405. [DOI] [PubMed] [Google Scholar]
- 9. Argüelles‐Arias F, Guerra Veloz MF, Perea Amarillo R, et al. Switching from reference infliximab to CT‐P13 in patients with inflammatory bowel disease: 12 months results. Eur J Gastroenterol Hepatol. 2017;29:1290‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farkas K, Rutka M, Ferenci T, et al. Infliximab biosimilar CT‐P13 therapy is effective and safe in maintaining remission in Crohn’s disease and ulcerative colitis – experiences from a single center. Expert Opin Biol Ther. 2017;17:269–8. [DOI] [PubMed] [Google Scholar]
- 11. Smits L, Grelack A, Derikx L, et al. Long‐term clinical outcomes after switching from Remicade® to Biosimilar CT‐P13 in inflammatory bowel disease. Dig Dis Sci. 2017;62:3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT‐P13 compared with maintained treatment with originator infliximab (NOR‐SWITCH): a 52‐week, randomised, double‐blind, non‐inferiority trial. The Lancet. 2017;389:2304–2316. [DOI] [PubMed] [Google Scholar]
- 13. European Medicines Agency . Guideline on similar biological medicinal products containing monoclonal antibodies: non‐clinical and clinical issues. [Internet]. 2012. [cited 2018;May:14] http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128686.pdf. Accessed May 14, 2018.
- 14. Food and Drug Administration . Scientific considerations in demonstrating biosimilarity to a reference product. [Internet]. 2015. [cited 2018;May:14] https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed May 14, 2018.
- 15. Jani RH, Gupta R, Bhatia G, et al. A prospective, randomized, double‐blind, multicentre, parallel‐group, active controlled study to compare efficacy and safety of biosimilar adalimumab (Exemptia; ZRC‐3197) and adalimumab (Humira) in patients with rheumatoid arthritis. Int J Rheum Dis. 2016;19:1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer A, Rudant J, Drouin J, Weill A, Carbonnel F, Coste J. Effectiveness and safety of reference infliximab and biosimilar in crohn disease: a French Equivalence Study. Ann Intern Med. 2018;170:99. [DOI] [PubMed] [Google Scholar]
- 17. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: From the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149–S167. [DOI] [PubMed] [Google Scholar]
- 18. Blotière P‐O, Rudant J, Barré A, et al. Conditions of prescription of anti‐TNF agents in newly treated patients with inflammatory bowel disease in France (2011–2013). Dig Liver Dis. 2016;48:620–625. [DOI] [PubMed] [Google Scholar]
- 19. Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray‐Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155:337–346.e10. [DOI] [PubMed] [Google Scholar]
- 20. Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318:1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rey G, Jougla E, Fouillet A, Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997–2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chow S‐C, Shao J, Wang H, Lokhnygina Y. Sample Size Calculations in Clinical Research (3rd edition, 954 p). Boca Raton, Florida: CRC Press; 2017. [Google Scholar]
- 23. Choe J‐Y, Prodanovic N, Niebrzydowski J, et al. A randomised, double‐blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weinblatt ME, Baranauskaite A, Niebrzydowski J, et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate‐to‐severe rheumatoid arthritis. Arthritis Rheumatol. 2018;70:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. European Medicines Agency . Guideline on the choice of the non‐inferiority margin [Internet]. 2005. [cited 2018 May 14]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003636.pdf. Accessed May 14, 2018.
- 26. Food and. Drug Administration . Non‐Inferiority Clinical Trials to Establish Effectiveness. [Internet]. 2016. [cited 2018;May:14] https://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf%20(accessed%206%20Mar%202015). Accessed May 14, 2018.
- 27. Lee EW, Wei LJ, Amato DA. Survival Analysis: State of the Art. Dordrecht, the Netherlands: Springer;1992:237–247. [Google Scholar]
- 28. Rosenbaum PR. Model‐based direct adjustment. J Am Stat Assoc. 1987;82:387. [Google Scholar]
- 29. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268. [DOI] [PubMed] [Google Scholar]
- 33. Singh S, Andersen NN, Andersson M, Loftus EV, Jess T. Comparison of infliximab and adalimumab in biologic‐naive patients with ulcerative colitis: a Nationwide Danish Cohort Study. Clin Gastroenterol Hepatol. 2017;15:1218–1225 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. D’Haens G, Reinisch W, Colombel J‐F, et al. Five‐year safety data from ENCORE, a European Observational Safety registry for adults with Crohn’s disease treated with infliximab [Remicade®] or conventional therapy. J Crohns Colitis. 2017;11:680–689. [DOI] [PubMed] [Google Scholar]
- 35. Fidder H, Schnitzler F, Ferrante M, et al. Long‐term safety of infliximab for the treatment of inflammatory bowel disease: a single‐centre cohort study. Gut. 2009;58:501–508. [DOI] [PubMed] [Google Scholar]
- 36. Lichtenstein GR, Feagan BG, Cohen RD, et al. Drug therapies and the risk of malignancy in Crohn’s disease: results from the TREATTM Registry. Am J Gastroenterol. 2014;109:212–223. [DOI] [PubMed] [Google Scholar]
- 37. Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002–1007. [DOI] [PubMed] [Google Scholar]
- 38. Kirchgesner J, Beaugerie L, Carrat F, Andersen NN, Jess T, Schwarzinger M. Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut. 2017;67:1261–1268. [DOI] [PubMed] [Google Scholar]
- 39. Osterman MT, Haynes K, Delzell E, et al. Effectiveness and safety of immunomodulators with anti‐tumor necrosis factor therapy in Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:1293–1301.e5; quiz e70, e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:811–817.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh S, Heien HC, Sangaralingham LR, et al. Comparative effectiveness and safety of anti‐tumor necrosis factor agents in biologic‐naive patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2016;14:1120–1129.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


