Abstract
Aim
Improved survival rates for premature infants have also increased the population at risk of necrotising enterocolitis (NEC). This study evaluated the outcomes of surgically treated NEC and identified risk factors for mortality, intestinal failure (IF) and IF associated liver disease (IFALD).
Methods
This was a retrospective observational study of 131 infants with surgically treated NEC from 1976 to 2016 in a Swedish tertiary referral centre: 20 in 1976–1996, 33 in 1997–2006 and 78 in 2007–2016. Data were extracted from medical records, and the Cox regression model was used to identify risk factors.
Results
When the first and last periods were compared, they showed decreases in both gestational age, from 30 to 26 weeks, and mortality rates, from 45% to 29%. IF was found in 67 patients (56%), IFALD in 41 patients (34%) and short bowel syndrome (SBS) in 13 (19%). The incidence of IF was high, even in infants without SBS. Low gestational age was an independent risk factor for mortality. No risk factors were identified for IF or IFALD.
Conclusion
Survival rates for NEC improved from 1976–2016, despite a decrease in gestational age. Clinicians should be particularly aware of the risk of infants without SBS developing IF.
Keywords: Intestinal failure, Mortality, Necrotising enterocolitis, Short bowel syndrome, Surgery
Abbreviations
- IFALD
Intestinal failure‐associated liver disease
- IF
Intestinal failure
- NEC
Necrotising enterocolitis
- NICU
Neonatal intensive care unit
- SBS
Short bowel syndrome
Key notes.
We evaluated 131 infants with surgically treated NEC from 1976–2016 to identify risk factors for mortality, intestinal failure (IF) and intestinal failure‐associated liver disease (IFALD).
Gestational age was an independent risk factor for mortality, but no risk factors were identified for IF and IFALD.
NEC survival improved over time, despite decreasing gestational age, but the risk of patients without short bowel syndrome developing IF should be particularly noted.
Introduction
Necrotising enterocolitis (NEC) is a serious gastrointestinal disease that predominantly affects premature infants. The prevalence of NEC in infants with a birth weight of less than 1500 g is 10–15% 1, 2. The condition is characterised by inflammation of the intestines, which can progress to intestinal necrosis and multi‐organ failure 1, 2. NEC can fluctuate from a medical condition treated with gastrointestinal rest, gastric decompression, broad‐spectrum antibiotics and parenteral nutrition to a life‐threatening event requiring emergency surgery 1, 2. About 50% of all infants with NEC will require surgery, and mortality rates of up to 57% have been reported after surgery 3, 4.
Current evidence suggests a multifactorial cause for NEC 2, 5. Prematurity is the main risk factor, presumably due to the immaturity of gastrointestinal motility, the intestinal barrier function and immune defences. Other contributing factors are thought to be genetic predisposition, enteral feeding, intestinal ischaemia and colonisation with pathogenic bacteria 2, 5. Advances in neonatal intensive care have improved survival rates among extremely premature infants, which have increased the population at risk of developing NEC 2.
NEC causes significant morbidity after surgical treatment, with the development of intestinal strictures, neurodevelopmental delays, short bowel syndrome (SBS) and intestinal failure (IF) 6, 7, 8, 9. Meanwhile, IF is a chronic condition characterised by a reduction in the functional intestinal mass that is necessary for the adequate digestion and absorption of nutrients, fluids and growth requirements 10. SBS is a condition that can cause IF, because surgery or congenital conditions can reduce the mucosal surface area 10.
Premature infants have the highest risk of developing IF associated liver disease (IFALD), and the prevalence of this after receiving parenteral nutrition for more than 100 days has been reported to be 85% 11. This study aimed to evaluate the outcomes of surgically treated NEC and to identify risk factors for mortality, IF and IFALD.
Materials and methods
This was a retrospective observational study of all infants with surgically treated NEC at a level three neonatal intensive care unit (NICU) in Sweden from 1976 to 2016. NEC was diagnosed by radiological and clinical features and staged according to Bell et al.'s criteria 12. The indications for surgery were free intraabdominal air or if the infant deteriorated despite optimal medical treatment. All included patients underwent a primary laparotomy, resection of the necrotic intestines and stoma formation or primary anastomosis, depending on the general status of the patient and the perioperative findings. The diagnoses were confirmed by histopathological evaluations of the resected intestines.
Between 1976 and 2000, patients were recorded in a manual register. From 2000 to 2016, digital patient administrative systems were used to identify diagnoses of NEC according to the International Classification of Diseases, ninth and tenth revisions. Each surgical report was reviewed to validate the diagnosis and diminish the risk of faulty registration. Patients with a diagnosis of spontaneous intestinal perforation were excluded. Data were extracted from the patient's medical records and the parameters that were retrieved included their gestational age, sex, date of birth, birth weight and the date of the initial surgery. The data we extracted included the perioperative findings of the NEC localisation, intestinal perforation, enterostomy, anastomosis or both, resection of the colon, resection of the small intestine, more than two episodes of NEC, ileocaecal resection and strictures. The data also included stoma complications – namely prolapse, skin excoriations, stenosis, fluid and electrolyte disturbances – the date of the stoma reversal and the total number of abdominal operations the patient underwent. We discriminated between a focal NEC (<5 cm of affected intestines), multifocal (multiple parts of affected intestines with > 25% viable intestines) and panintestinal necrosis (<25% viable intestines) 13. The surgical methods were not changed during the study period. To compare changes over time, the patients were divided into three groups: 1976–1996, 1997–2006 and 2007–2016. The main outcome measures were death, IF and IFALD. We defined IF as a dependency of parenteral nutrition for more than 60 days or SBS caused by a resection of more than 50% of the small intestines. IFALD was defined as a direct bilirubin level of more than 50 μmol/L. We chose direct bilirubin as a marker for IFALD as it has been the most frequently used measure in studies of IFALD, and it is clearly related to the risk of liver failure 14. Until 2006, our patients were given parenteral nutrition with Intralipid, a 20% soybean‐based lipid emulsion (Fresenius Kabi, Bad Homburg, Germany). From 2006 Intralipid was replaced with Omegaven, a 10% fish oil‐based emulsion (Fresenius Kabi, Bad Homburg, Germany) combined with ClinOleic (Baxter, Maurepas, France), a 20% emulsion based on soy and olive oils, as we earlier reported 14.
Statistics
All statistical analyses were performed using R version 3.1.1 (The R Foundation, Vienna, Austria). The patient population was described at birth using medians and ranges for continuous variables and as frequencies and percentages for categorical variables. The risk of death was analysed using a Cox regression model, where the first step was to analyse a number of preselected potential risk factors. The different risk factors were gender, gestational age, ileocaecal resection, grading of NEC, more than 10 weeks to stoma reversal, age at primary surgery and the time period: 1976–1996, 1997–2006 and 2007–2016. Factors with a p value of <0.1 were then entered into a stepwise model, where the optimal model was selected based on the Akaike information criterion. The risk of IFALD and IF were analysed separately with a logistic regression model, using the same approach as for risk of death, with a univariable analysis in the first instance, followed by a stepwise selection of factors. Possible independent risk factors for mortality, IF or IFALD were presented as hazard ratios (HRs) or odds ratios (OR), both with 95% confidence intervals (95% CI). In addition, the change in gestational age over calendar time was explored and presented in a box plot and both variables were also included in a multivariable Cox regression analysis with regard to time to death. A p value of < 0.05 was considered significant. No p values were adjusted for multiplicity and the findings should be interpreted with that in mind.
This study was approved by the Central Ethical Review Board, registration number 2017/277.
Results
During the study period from 1976–2016, 131 patients were included with surgical NEC: 20 in 1976–1996, 33 in 1997–2006 and 78 in 2007–2016. The increase in the number of infants during the study period, and the decrease in gestational age over time are both demonstrated in Figure 1. Median gestational age was significantly lower (p = 0.004) in 2007–2016 (26 weeks) compared to 1976–1996 (30 weeks). There was no significant difference (p = 0.21) in gestational age between 1997–2006 and 2007–2016.
Figure 1.
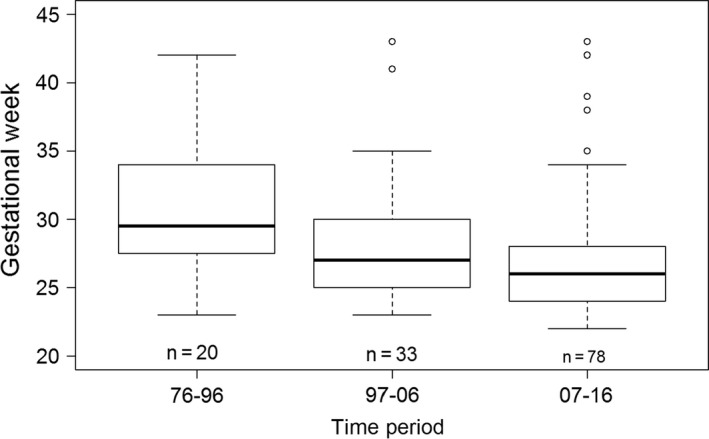
Boxplot illustrating gestational age in respective time‐period.
Characteristics of the 131 patients (61% male) included in the study are shown in Table 1. The median gestational age was 27 weeks (range 22–43), the median birth weight was 788 g (range 404–4855 g), and the median follow‐up time was six years (0–29). The median age at surgery was 10 days (range 2–74), and the median number of abdominal operations was two (range 1–8).
Table 1.
Patient characteristics
| Female gender, n (%) | 51 (39) |
| Median gestational age, weeks (range) | 27 (22–43) |
| Median birth weight, grams (range) | 788 (404–4855) |
| Median follow‐up time, years (range) | 6 (0–29) |
| Median age at surgery, days (range) | 10 (2–74) |
| Number of abdominal surgeries (range) | 2 (1–8) |
| Focal, n (%) | 59 (50) |
| Multifocal, n (%) | 50 (42) |
| Panintestinal, n (%) | 10 (8) |
| Perforation, n (%) | 77 (65) |
| Resection of colon, n (%) | 40 (33) |
| Resection of small intestine, n (%) | 102 (85) |
| Ileocaecal resection, n (%) | 24 (20) |
| Stoma, n (%) | 107 (91) |
| Anastomosis without stoma, n (%) | 11 (9) |
Perioperative grading of NEC was carried out in 119/131 cases, and this revealed 10 cases (8%) of panintestinal NEC, 59 cases (50%) of focal NEC and 50 cases (42%) of multifocal NEC. Information on the extent of the disease at the time of the laparotomy was not available for the other 12 patients: 11 patients from 1976–1996 and one patient from 1997–2006.
Information on intestinal perforation at the time of the initial laparotomy was available for 119/131 cases, and this showed that it was present in 77 cases (65%). The data were missing from the other 12 patients, same as listed above. The intraoperative perforation rates were 8/9 (89%) in 1976–1996, 23/32 (72%) in 1997–2006 and 46/78 (59%) in 2007–2016.
There were also missing values for a number of other procedures and that is why the totals do not reach 131 in some cases. For example, 102/120 patients (85%) were treated with resection of the small intestines and 107/118 patients (91%) had a stoma formation. A primary anastomosis without stoma formation was performed in 11/122 patients (9%). In 40/121 patients (33%), a resection of colon was performed, and 24/120 patients (20%) underwent an ileocaecal resection. Postoperatively, 30/120 patients (25%) developed strictures, 29/131 patients (22%) suffered from ileus requiring surgery and 36/109 patients (33%) had stoma complications. Recurrent episodes of NEC were found in seven patients (5%). IF developed in 67/120 patients (56%), SBS in 13/68 (19%) and IFALD developed in 41/121 patients (34%), as demonstrated in Table 2. The median number of days with parenteral nutrition was 71 days (1–2920). After three months, 60% of the 71/119 patients were off parenteral nutrition, and after two years, 93% were off parenteral nutrition. Again, data were missing for 12/131 patients. Five patients were still on parenteral nutrition at the end of the study: three of them had panintestinal NEC, and the other two were born in the end of the study period. There were 40 deaths, and most of these were a direct consequence of NEC and five deaths were caused by IFALD. Not all deceased patients underwent an autopsy but septicaemia, intracerebral haemorrhage and congenital heart malformation were some of the other causes of death. Gestational age was identified as an independent risk factor for mortality, with an HR of 0.88 p = 0.013, (Tables 3 and 4) but no independent risk factor was identified for IF or IFALD. Mortality decreased from 45% to 29% in the later time period compared to the two earlier time periods after adjustment for gestational age (Table 5).
Table 2.
Morbidity
| Stricture, n (%) | 30 (25) |
| Stoma complications, n (%) | 36 (33) |
| Surgically treated small bowel obstruction, n (%) | 29 (22) |
| NEC recurrence, n (%) | 7 (5) |
| Median number of days with parenteral nutrition (range) | 71 (1–2920) |
| Patients still on PN, n (%) | 5 (4) |
| SBS, n (%) | 13 (19) |
| Intestinal failure, n (%) | 67 (56) |
| IFALD, n (%) | 41 (34) |
| Deaths, n (%) | 40 (31) |
Table 3.
Univariate cox regressions for mortality
| Hazard ratio 95% CI | ρ‐value | |
|---|---|---|
| Gender | 1.40 (0.75–2.61) | 0.290 |
| Gestational age | 0.88 (0.80–0.97) | 0.013 |
| Focal vs panintestinal | 0.37 (0.13–1.04) | 0.060 |
| Multifocal vs panintestinal | 0.44 (0.16–1.23) | 0.115 |
| Age at primary laparotomy | 0.99 (0.97–1.01) | 0.524 |
| 2007–2016 vs 1976–1996 | 0.44 (0.17–1.14) | 0.090 |
| 1997–2006 vs 1976–1996 | 0.64 (0.30–1.39) | 0.262 |
Table 4.
Multiple stepwise cox regression for mortality
| Hazard ratio 95% CI | ρ‐value | |
|---|---|---|
| Gestational age | 0.88 (0.80–0.97) | 0.013 |
Table 5.
Cox regressions for mortality
| Hazard ratio 95% CI | p‐value | |
|---|---|---|
| 97–06 vs < 97 | 0.26 (0.10–0.71) | 0.009 |
| 07–16 vs < 97 | 0.34 (0.14–0.80) | 0.014 |
| Gestational age | 0.84 (0.75–0.94) | 0.003 |
Discussion
This study investigated the outcomes of surgically treated NEC over four decades, from 1976–2016. The number of patients with NEC increased during the study period.
Low gestational age was identified as an independent risk factor for mortality in this group of patients. We found that mortality rates reduced over time, despite a decrease in gestational age, indicating improvements in neonatal intensive care.
Mortality rates after surgically treated NEC have been reported to vary between 20% and 67% 4, 15, 16, 17, 18, 19, 20, and the mortality rate of 31% in our study was within the lower range compared to other studies. In a previous smaller study with 57 patients, the mortality rate was as low as 20% in infants with a birth weight of less than 1000 g 19. The authors explained low mortality with an aggressive approach, where 46% of the patients were operated on without free intraabdominal air. During the whole period covered by the present study, 35% of the patients underwent surgery in the absence of free intraabdominal air. The number of patients who underwent surgery without perforation increased in the later time period. It is possible that the wider indications for surgery in recent years have led to less severely ill patients undergoing surgery, which has probably affected survival rates in a positive way. However, along with the wider indications for surgery, there is a risk that the numbers of NEC patients that could have been managed without surgery might have increased.
In contrast to previous studies, we found no correlation between the grading of NEC during laparotomy and mortality 20, 21. Thyoko et al. found 86% mortality in panintestinal NEC and 21% mortality in focal NEC 21. In a smaller study of 48 surgically treated NEC patients by Hansen et al., the mortality rate was 100% in panintestinal NEC 20. However, mortality rates are difficult to compare, as the number of panintestinal NECs tend to be small and the indications for surgery might be different between institutions.
Gender was not identified as a risk factor for mortality in the present study. This is in contrast to a previous study that found that males with a very low birth weight infants faced a greater risk of death 22.
Stoma complications occurred in 33% of the patients in our study, which was in the same 20–43% range reported by others 23. The standard surgical procedure in our NEC patients was resection of necrotic intestines and a temporary stoma formation. Primary anastomosis can be an alternative for a selected group of patients, as previously reported 24. We only performed anastomosis without a stoma in haemodynamically stable patients with a focal NEC and without a severe peritonitis to avoid dehiscence or obstruction. A 2017 review and meta‐analysis found that patients with NEC who underwent primary anastomosis were associated with less risk of mortality when they were compared to those who underwent enterostomy. This was possibly due to differences in the severity of NEC 25. Unfortunately, there are not any randomised control trials available that enable us to compare outcomes after primary anastomosis and enterostomy in infants with NEC. All 12 studies are retrospective cohort studies with small sample sizes.
In this study, we found that 56% of the patients developed IF, but it was interesting that only 19% were caused by SBS. IF without SBS is likely to be related to inflammation of the intestine, causing impaired absorption of enteral nutrition. This knowledge is important to paediatric surgeons, neonatologists and paediatricians treating infants with NEC after surgery. Unfortunately, we have seen several patients with poor growth due to a hasty increase in enteral nutrition and a decrease in parenteral nutrition after smaller intestinal resections. The incidence of IF in our patients was higher than previously reported with similar numbers of surgically treated infants with NEC 8, 26. However, the median gestational age in our study was lower, 27 weeks compared to 28.8 and 28.6 weeks of gestation in the other two studies. Premature intestines might be more sensitive to damage caused by inflammation, which might explain a longer time on parenteral nutrition.
Infants with SBS, especially those born premature, have a good chance of coming off parenteral nutrition, as they have a great capacity to compensate for lost intestinal length due to intestinal adaptation 27. In our study, 93% of the patients were off parenteral nutrition after two years. IFALD occurred in up to 60% of children with IF and impairs intestinal adaptation, which prolongs the time needed for parenteral nutrition. IFALD is cholestatic in nature. Prematurity, low birth weight, multiple surgical procedures and a diagnosis of NEC are some of the known risk factors for IFALD, which were all represented in our study population and may explain the high rate of IFALD 11. In the present study, no independent risk factors for either IF or IFALD were identified.
The inflammatory process in NEC is considered to be a predisposing factor for formation of intraabdominal adhesions, and the incidence of ileus requiring surgery was similar in this study as in our earlier study 28.
A limitation of this study was its retrospective design and the relatively low number of patients during a long observation period. Low gestational age in itself is a known risk factor for mortality in premature infants. To answer the question about whether low gestational age is an additional risk factor in this specific population a prospective study with age‐matched healthy controls would be needed.
The strengths of this study were the identification and exclusion of patients with spontaneous intestinal perforation who were not at risk of IF and have been shown to have a lower mortality rate than infants with NEC 29. In some datasets on national surgical NEC, up to 20% of the patients who were included only had spontaneous intestinal perforation, resulting into biased results 29.
Conclusion
This study identified low gestational age as an independent risk factor for mortality in patients who were surgically treated for NEC. We found that the mortality rate reduced over time, despite a decrease in gestational age. Mortality was within the lower range when we compared our data to other reports of surgically treated NEC. The incidence of IF was also high in infants without SBS, but 96% resolved over time. Awareness of the risk of developing IF without SBS is important if we are to optimise nutrition and growth after surgery.
Funding
This work received external funding from HRH Crown Princess Lovisa's Association for Child Care.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors are grateful to Marcus Thuresson of Statisticon AB, Uppsala, Sweden, for help with the statistical analysis.
References
- 1. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011; 364: 255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin PW, Stoll BJ. Necrotizing enterocolitis. Lancet 2006; 368: 1271–83. [DOI] [PubMed] [Google Scholar]
- 3. Kastenberg ZJ, Sylvester KG. The surgical management of necrotizing enterocolitis. Clin Perinatol 2013; 40: 135–48. [DOI] [PubMed] [Google Scholar]
- 4. Lakhoo K, Morgan RD, Thakkar H, Gupta A, Grant HW, Wagener S, et al. Exploratory laparotomy in the management of confirmed necrotizing enterocolitis. Ann Pediatr Surg 2015; 11: 123–6. [Google Scholar]
- 5. Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 2016; 13: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stey AM, Kenney BD, Moss RL, Hall BL, Berman L, Cohen ME, et al. A risk calculator predicting postoperative adverse events in neonates undergoing major abdominal or thoracic surgery. J Pediatr Surg 2015; 50: 987–91. [DOI] [PubMed] [Google Scholar]
- 7. Rich BS, Dolgin SE. Necrotizing enterocolitis. Pediatr Rev 2017; 38: 552–9. [DOI] [PubMed] [Google Scholar]
- 8. Elfvin A, Dinsdale E, Wales PW, Moore AM. Low birthweight, gestational age, need for surgical intervention and gram‐negative bacteraemia predict intestinal failure following necrotising enterocolitis. Acta Paediatr 2015; 104: 771–6. [DOI] [PubMed] [Google Scholar]
- 9. Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed 2007; 92: 193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology 2006; 130: 16–28. [DOI] [PubMed] [Google Scholar]
- 11. Christensen RD, Henry E, Wiedmeier SE, Burnett J, Lambert DK. Identifying patients, on the first day of life, at high‐risk of developing parenteral nutrition‐associated liver disease. J Perinatol 2007; 27: 284–90. [DOI] [PubMed] [Google Scholar]
- 12. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978; 187: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O′Neill Pediatric Surgery. Fifth edition. Mosby 1998: 1311
- 14. Angsten G, Finkel Y, Lucas S, Kassa AM, Paulsson M, Lilja HE. Improved outcome in neonatal short bowel syndrome using parenteral fish oil in combination with omega‐6/9 lipid emulsions. J Parenter Enteral Nutr 2012; 36: 587–95. [DOI] [PubMed] [Google Scholar]
- 15. Ricketts RR, Jerles ML. Neonatal necrotizing enterocolitis: experience with 100 consecutive surgical patients. World J Surg 1990; 14: 600–5. [DOI] [PubMed] [Google Scholar]
- 16. de Souza JC, da Motta UI, Ketzer CR. Prognostic factors of mortality in newborns with necrotizing enterocolitis submitted to exploratory laparotomy. J Pediatr Surg 2001; 36: 482–6. [DOI] [PubMed] [Google Scholar]
- 17. Alexander F, Smith A. Mortality in micro‐premature infants with necrotizing enterocolitis treated by primary laparotomy is independent of gestational age and birth weight. Pediatr Surg Int 2008; 24: 415–9. [DOI] [PubMed] [Google Scholar]
- 18. Kelley‐Quon LI, Tseng CH, Scott A, Jen HC, Calkins KL, Shew SB. Does hospital transfer predict mortality in very low birth weight infants requiring surgery for necrotizing enterocolitis? Surgery 2012; 152: 337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gfroerer S, Fiegel H, Schloesser RL, Rolle U. Primary laparotomy is effective and safe in the treatment of necrotizing enterocolitis. World J Surg 2014; 38: 2730–4. [DOI] [PubMed] [Google Scholar]
- 20. Hansen ML, Juhl SM, Fonnest G, Greisen G. Surgical findings during exploratory laparotomy are closely related to mortality in premature infants with necrotising enterocolitis. Acta Paediatr 2017; 106: 399–404. [DOI] [PubMed] [Google Scholar]
- 21. Thyoka M, de Coppi P, Eaton S, Khoo K, Hall NJ, Curry J, et al. Advanced necrotizing enterocolitis part 1: mortality. Eur J Pediatr Surg 2012; 22: 8–12. [DOI] [PubMed] [Google Scholar]
- 22. Holman RC, Stoll BJ, Clarke BJ, Glass RI. The epidermiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health 1997; 87: 2026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aguayo P, Fraser JD, Sharp S, St Peter SD, Ostlie DJ. Stomal complications in the newborn with necrotizing enterocolitis. J Surg Res 2009; 157: 275–8. [DOI] [PubMed] [Google Scholar]
- 24. Hall NJ, Curry J, Drake DP, Spitz L, Kiely EM, Pierro A. Resection and primary anastomosis is a valid surgical option for infants with necrotizing enterocolitis who weigh less than 1000 g. Arch Surg 2005; 140: 1149–51. [DOI] [PubMed] [Google Scholar]
- 25. Haricharan RN, Gallimore JP, Nasr A. Primary anastomosis or ostomy in necrotizing enterocolitis? Pediatr Surg Int 2017; 33: 1139–45. [DOI] [PubMed] [Google Scholar]
- 26. Duro D, Mitchell PD, Kalish LA, Johnston P, Jaksic T, McCarthy M, et al. Risk factors for intestinal failure in infants with necrotizing enterocolitis: a Glaser Pediatric Research Network study. J Pediatr 2010; 157: 203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gambarara M, Ferretti F, Papadatou B, Lucidi V, Diamanti A, Bagolan P, et al. Intestinal adaptation in short bowel syndrome. Transplant Proc 1997; 29: 1862–3. [DOI] [PubMed] [Google Scholar]
- 28. Fredriksson F, Christofferson RH, Lilja HE. Adhesive small bowel obstruction after laparotomy during infancy. Br J Surg 2016; 103: 284–9. [DOI] [PubMed] [Google Scholar]
- 29. Wadhawan R, Oh W, Hintz SR, Blakely ML, Das A, Bell EF, et al. Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J Perinatol 2013; 34: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


