Abstract
In the present study, various extracts of C. tricuspidata fruit were prepared with varying ethanol contents and evaluated for their biomarker and biological properties. The 80% ethanolic extract showed the best tyrosinase inhibitory activity, while the 100% ethanolic extract showed the best total phenolics and flavonoids contents. The HPLC method was applied to analyze the chlorogenic acid in C. tricuspidata fruit extracts. The results suggest that the observed antioxidant and tyrosinase inhibitory activity of C. tricuspidata fruit extract could partially be attributed to the presence of marker compounds in the extract. In this study, we present an analytical method for standardization and optimization of C. tricuspidata fruit preparations. Further investigations are warranted to confirm the in vivo pharmacological activity of C. tricuspidata fruit extract and its active constituents and assess the safe use of the plant for the potential development of the extract as a skin depigmentation agent.
Keywords: C.tricuspidata Bureau, HPLC, tyrosinase
1. Introduction
Cudrania tricuspidata (Moraceae) is used as traditional medicine for inflammation, gastritis, cancer, and liver injury [1]. In the previous reports, active constituents from roots and leaves of Cudrania tricuspidata contain pharmaceutically active substances such as neuroprotective [2], anti-inflammatory [3,4], pancreatic lipase inhibitory [5], monoamine oxidase inhibitory [6], and anti-obesity effects [7]. Additively, prenylated isoflavonoids, benzylated flavonoids, xanthones from the fruits displayed potential antioxidant, anti-inflammatory, and neuroprotective activities [8,9,10].
The efficacy of extracts and purified bioactive substances prepared using C. tricuspidata as a medical source has been studied broadly to date. The content of a single compound present in fruits was insufficient for use as biomarkers for pharmaceutical/cosmetic application. Moreover, preparations involving the fruit could be beneficial for productivity purpose as C. tricuspidata is a perennial plant (Table 1).
Table 1.
Chemical constituents and biological activities of C. tricuspidata fruit reported in previous literatures.
| Constituent | Activity | Contents | Effective Dose (mg/kg/day)(route/animal) | Ref. |
|---|---|---|---|---|
| 6,8-diprenylgenistein | Anti-obesity | Single compound | 30 (oral/mouse) | [7] |
| Cudraisoflavones etc | Neuroprotective | *N.D | N.D | [2] |
| Genistein etc | Lipase inhibition | N.D | N.D | [5] |
| 5,7,3′,4′-Tetrahydroxy-6,8-diprenylisoflavone | Antiallergy | N.D | N.D | [11] |
| Water extract | Dermatitis | Rutin was identified | 60 (oral/mouse) | [3] |
| Gancaonin A etc | Monoamine oxidase inhibition | N.D | N.D | [6] |
| Water and etnaolic extract | Tyrosinase inhibition | Chlorogenic acid | N.D | This study |
| Scandenolone | Anti-cancer | Single compound | 5 and 7.5 (intravenous/mouse) | [12] |
*ND; not decribed.
Few studies have been conducted on the fruits of C. tricuspidata and the contents of bioactive substances were observed to be insufficient for use as key compounds for pharmaceutical industrialization. Considerable effort has been focused on developing C. tricuspidata as materials, but no positive results have been achieved.
The aim of this study was to evaluate the fruit extract of C. tricuspudata for tyrosinase inhibitory activity, as well as to characterize the chromatographic profile of its optimized extract to identify the compounds responsible for antioxidant and tyrosinase inhibition. Validation of a High Performance Liquid Chromatography (HPLC) method was preformed for standardize of chlorogenic acid.
In the preliminary study, we purified and identified the main substance, chlorogenic acidwith antioxidant and tyrosinase inhibitory activity from fruits of C. tricuspidata. Previous reports have demonstrated that chlorogenic acid plays important roles in melanogenesis of B16 melanoma cells. Although chlorogenic acid did not exhibit strong tyrosinase inhibitory effect, its metabolic product(s) showed suppression of melanogenesis in B16 melanoma cells by inhibiting tyrosinase activity [13]. Consequently, we set chlorogenic acid as a biomarker for the extract of C. tricuspidata fruit.
Cytotoxicity test was assessed in cell lines to test the cell viability in the presence of the extract of C. tricuspudata fruit with an aim to incorporate the extract in topical form as a skin whitening agent. This is the first study that assess tyrosinase inhibition and quantifythe presence of biomarkers such as chlorogenic acid in C. tricuspudata fruit.
Previously, we had investigated the biological properties of extracts and their biomarkers obtained from C. tricuspidata leaves for the development of medicinal/food sources. In this study, fruit components of C. tricuspidata were screened for cosmetic application. Extracts of C. tricuspidata fruit were prepared for the assessment of chemical composition and biological properties.
2. Results and Discussion
2.1. Chromatographic Conditions for Extract of C. tricuspidata Fruit
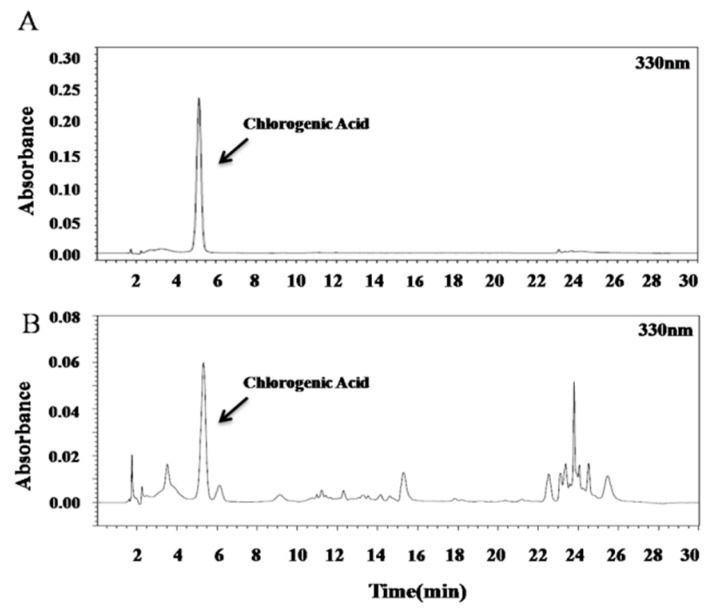
The HPLC conditions were established as follows. A gradient program was used to separate the chlorogenic acid (Table 6). Detection wavelengths were set as 330 nm. As shown in Figure 1, chlorogenic acid was identified as the main component in the extract from C. tricuspidata.
Figure 1.
Analysis of C. tricuspidata fruit extracts by High Performance Liquid Chromatography (HPLC) method. (A) standard; (B) sample extract (fruit).
Lee et al. reported that the extraction yield of water extract of C. tricuspidata fruit was 12.7% and extract contained rutin [3]. However, the content of rutin in the water extract was not described. In the present study, rutin was not found in the extract of C. tricuspidata fruit.
Jiang et al. purified and identified anticancer compound named scandenolone from C. tricuspidata fruit [12]. Jiang described the detailed purification process in the reported study. However, the study lacked a description of the content of active compound in the fruits of C. tricuspidata. Although it has been reported that scandenolone plays an important role in mediating anticancer activity, the potential of the compound to prevent cancer cannot be guaranteed by just consuming C. tricuspidata fruits. In addition, there exists no data on permissible levels of consumption for human. Therefore, scandenolone can be considered as one of the trace components of fruits of C. tricuspidata.
Jo et al. reported about anti-obesity efficacy of 6,8-Diprenylgenisteinusing 70% ethanol extract of C. tricuspidata fruit in their study [7]. The daily intake was set as 10–15 g of fruit. In the present study, 6,8-Diprenylgenistein was analyzed using HPLC, but it was difficult to confirm its presence in the extract of C. tricuspidata fruit. As the species, harvesting time of C. tricuspidata fruit, and the places of cultivation are different, we presumed that the presence of 6,8-Diprenylgenisteinmight also be different.
2.2. Method Validation
2.2.1. Linearity, Limit of Detection (LOD), and Limit of Quantification (LOQ)
In the present study, calibration curves, limit of detection, and quantification were conducted. Calibration curves were set in the range of 3.125–50 μg/mL for chlorogenic acid and exhibited good linear regressions (r2 = 0.998). The LOD was found to be 0.7 μg/mL for chlorogenic acid. The LOQ value for chlorogenic acid was found to be 2.1 μg/mL (Table 2).
Table 2.
HPLC data for the calibration graphs and limit of quantification of the active compound.
| Analyte | Retention Time (min) | R 2 | Linear Range (μg/mL) | LOQ (μg/mL) | LOD (μg/mL) |
|---|---|---|---|---|---|
| Chlorogenic acid | 4.7 | 0.998 | 3.125–50 | 2.11 | 0.7 |
2.2.2. Precision and Accuracy
The results of the intraday and interday precision experiments are shown in Table 3. The overall recovery percentages were in the range of 104.04–107.78% for chlorogenic acid. These results demonstrate that the developed method is reproducible with a good accuracy (Table 3).
Table 3.
Analytical results of intra-day and inter-day precision and accuracy.
| Analyte | Conc (μg/mL) | Intra-Day (n = 3) | Inter-Day (n = 3) | ||
|---|---|---|---|---|---|
| RSD (%) a | Accuracy (%) | RSD (%) | Accuracy (%) | ||
| Chlorogenic acid | 6.25 12.5 25 |
2.65 7.86 2.59 |
105.29 105.32 104.50 |
2.74 5.94 3.17 |
104.04 107.78 105.91 |
a RSD: relative standard deviation.
2.2.3. Repeatability
The results of the repeatability are shown in Table 4. RSD values were below 2.0%. Thus, HPLC method is suitable for analysis of C. tricuspidata fruit.
Table 4.
Analytical data of recovery (n = 6).
| Analyte | Added (μg/mL) | Recovery (%) (Mean ± SD) | RSD (%) a |
|---|---|---|---|
| Cholorogenic acid | 6.25 | 103.39 ± 0.35 | 0.4 |
| 12.5 | 96.79 ± 0.96 | 1.08 | |
| 25 | 98.59 ± 1.20 | 1.26 |
a RSD: relative standard deviation.
2.3. Contents of Marker Compounds from C. tricuspidata Fruit Extracts
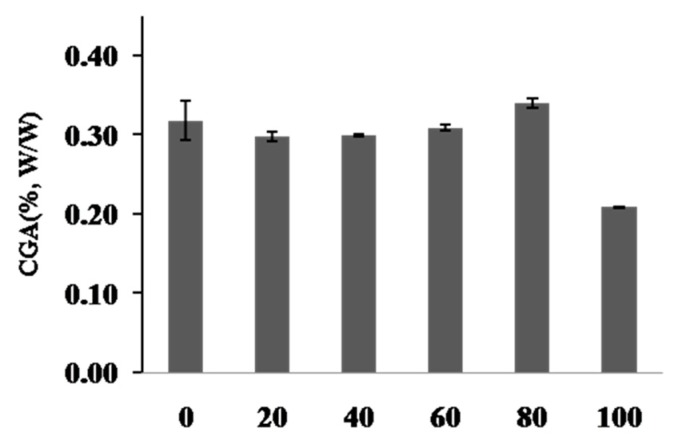
Plant samples were extracted with various solvent compositions to select the best extraction solvent conditions: hot water, 20–100% ethanol (v/v). The validated HPLC method was applied to analyze the samples. The contents (%wt.) of chlorogenic acid is presented in Figure 2. The contents of the chlorogenic acid in the 80% ethanolic extract were greater compared to other ethanolic extracts. Based on these results, the80% ethanol was selected as the most effective extraction solvent (0.34 ± 0.01%, w/w).
Figure 2.
Content of chlorogenic acid (CGA) in hot water and ethanolic extracts from C. tricuspidata fruit. 0 (hot water ex); 20 (20% ethantol ex); 40 (40% ethanol ex); 60 (60% ethanol ex); 80 (80% ethanol ex); 100 (100% ethanol ex). Each value was the mean ± SD (n = 3).
2.4. Cell Viability and Tyrosinase Inhibition of C. tricuspidata Fruit Extracts
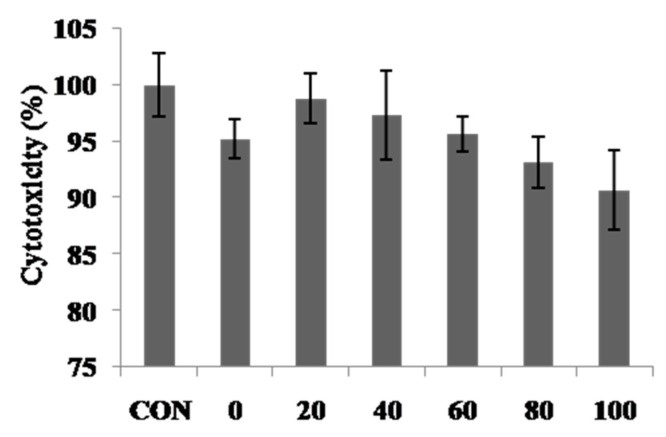
Cytotoxicity of various extracts of C. tricuspidata was determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay [14]. Cytotoxicity was assessed after treatment of B16F10 cells with a various sample concentration of 100 μg/mL for 24 h. For further study, 100 μg/mL or less should be considered as optimal for conducting experiments on unraveling the mechanism of action of C. tricuspidata extract (Figure 3).
Figure 3.
Cell viability of water and ethanolic extracts from C. tricuspidata fruit. 0–100 represent 0–100% ethanolic extract (100 μg/mL). Each value was the mean ± SD (n = 3).
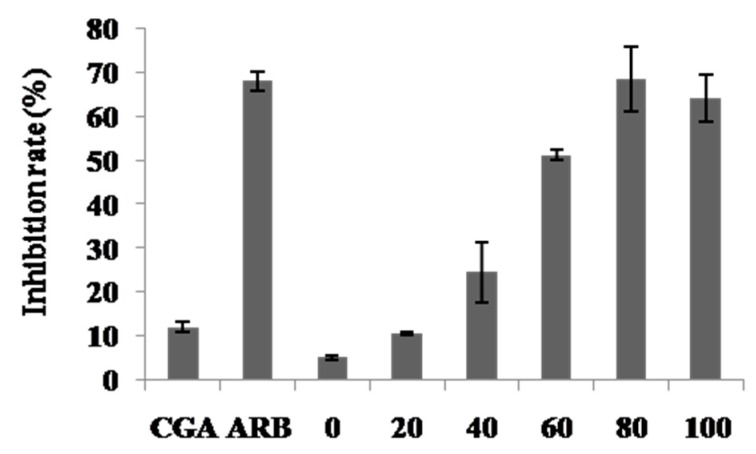
The tyrosinase inhibition of various extracts of C. tricuspidata was determined by the tyrosinase inhibitory assays. The measured tyrosinase inhibitory activity is shown in Figure 4. The tyrosinase inhibition decreased in the following order: 80% ethanol extract (68.3 ± 7.3%) > 100% ethanol extract (64.1 ± 5.2%) > 60% ethanol extract (51.2 ± 1.3%) > 40% ethanol extract (24.5 ± 6.8%) > 20% ethanol extract (10.68 ± 0.4%) > hot water extract (5.22 ± 0.5%).
Figure 4.
Tyrosinate inhibition in hot water and ethanolic extracts from C. tricuspidata fruit.CGA: chlorogenic acid (8 μg/mL); ARB (150 μg/mL): arbutin. 0–100 represent 0–100% ethanolic extract (100 μg/mL). Each value was the mean ± SD (n = 3).
In the present study, we identified chlorogenic acid as one of the tyrosinase inhibitory (anti-whitening related) efficacy factors. Content of chlorogenic acid and the extent of tyrosinase inhibition were the highest in 80% C. tricuspidata fruit extract. In the previous report, chlorogenic acid was reported to affect melanogenesis through tyrosinase inhibition when converted into metabolites in cells [11]. Therefore, we established chlorogenic acid asthe biomarker of C. tricuspidata fruit. HPLC chromatograms revealed chlorogenic acid asthe major component of C. tricuspidata fruit.
In the present study, total phenolic and total flavonoids content of C. tricuspidata fruit extracts were compared. Total phenolic and flavonoids were the highest in 100% ethanolic extracts. Extracts containing phenolic compounds have been reported to exhibit tyrosinase inhibition [15,16,17].
Tyrosinase inhibition was the highest in 80% ethanolic extract. Besides, total phenolic and total flavonoid levels were highest in 100% ethanolic extract, thus indicating that the 80% ethanolic extract contains unknown tyrosinase inhibitors. It is hypothesized that through further studies, unknown tyrosinase inhibitors in 80% ethanolic extracts can be identified (Table 5).
Table 5.
Antioxidant activity and total phenolic contents of C. tricuspidata fruit extracts.
| Extract | Total Flavonoid (Ascorbic Acid eq. μg/100 μg Extract) |
Total Phenolic Content (Gallic Acid eq. mg/g) |
|---|---|---|
| Hot water | 7.9 | 31.9 ± 1.4 |
| 20% EtOH Ex | 14.0 | 36.0 ± 3.0 |
| 40% EtOH Ex | 10.8 | 29.9 ± 1.8 |
| 60% EtOH Ex | 11.2 | 33.9 ± 2.1 |
| 80% EtOH Ex | 19.5 | 35.9 ± 2.2 |
| 100% EtOH Ex | 26.0 | 40.6 ± 2.7 |
3. Experimental Section
3.1. Plant Material and Preparation of the Extract
C. tricuspidata fruit was collected in May 2017 near Naju, Jeonnam Province, Korea. A voucher specimen (MNUCSS-CTF-01) was deposited in the College of Pharmacy, Mokpo National University. Fruits were dried and used for extract preparation. The air-dried and powdered C. tricuspidata fruits (10 g) were subjected to extraction twice with 20–100% ethanol (100 mL) at room temperature for three days. The 0% extract was prepared using hot water extraction (100 °C, 4 h). After filtration, the resultant ethanol solution was evaporated, freeze-dried, and stored at ‒50 °C. The crude extract was resuspended in ethanol and filtered using a 0.4 μm membrane. All samples were used for the optimization of the extraction process and in vitro experiments.
3.2. Instrumentation and Chromatographic Conditions
The HPLC conditions were established as shown in Table 6.
Table 6.
Analytical HPLC conditions for C. tricuspidata fruit extracts.
| Parameters | Conditions | ||
|---|---|---|---|
| Instruments and Column | Alliance 2695 HPLC system (Waters, Millford, MA, USA) Zorbax extended-C18 (C18, 4.6 mm × 150 mm, 5 µm) |
||
| Flow rate | 0.8 mL/min | ||
| Injection volumn | 10 μL | ||
| UV detection | 330 nm | ||
| Run time | 30 min | ||
| Gradient | Time (min) | A (%) | B (%) |
| 0 | 10 | 90 | |
| 7 | 10 | 90 | |
| 8 | 20 | 80 | |
| 20 | 25 | 75 | |
| 21 | 100 | 0 | |
| 25 | 10 | 90 | |
| 30 | 10 | 90 | |
3.3. Preparation of Standards and Sample Solutions
Accurately weighed appropriate amounts of chlorogenic acid was mixed and dissolved in methanol in a 50 mL volumetric flask, to obtain a stock solution of 50 μg/mL. Solutions were subsequently 2-fold serially diluted to 3.125 μg/mL.
Samples (0.5 g) were dissolved in methanol (10 mL). Subsequently, 1 mL was diluted with 9 mL of mobile phase A to obtain a final solution with a known concentration of 25 mg/mL [18].
3.4. Method Validation
The analytical method usedfor the quantification of chlorogenic acid in the various extract of C. tricuspidata fruit was validated in terms of specificity, linearity, sensitivity, accuracy, precision, and recovery. Experiments were performed as previously described [16].
3.5. Analysis of the Extract from C. tricuspidata Fruit
The HPLC method developed herein was used to quantitatively determinate of the amounts of chlorogenic acid in 6 extracts from C. tricuspidata fruit.
3.6. Cell Viability
Human melanoma cells (B16F10) were purchased from the American Tissue Culture Collection (Manassas, VA, USA). Cells were seeded in 96-well plates and treated with 100 μg/mL of sample for 24 h. MTT was added and plates were incubated at 37 °C for 2 h. After dissolving the formazan crystals in 100 μL of DMSO, the absorbance was measured at 490 nm using an Enspire Multimode Plate reader (Perkin-Elmer, Akron, OH) [19].
3.7. Tyrosinase Inhibitory Assay
Tyrosinase inhibition assay was performed following a previously described method with some modification [20]. Briefly, reaction mixtures (total volume of 150 μL) with 49.5 μL of phosphate buffer (pH 6.8, 100 mM), 45 μL of distilled water, and 5 μL of sample dissolved in DMSO (100 μg/mL) were prepared. This was followed by the addition of 0.5 μL of mushroom tyrosinase (10 units) and 50 μL of the substrate, mixed well, and incubated for 10 min at 37 °C. Absorbance was measured at 475nm.
The percent inhibition of the enzyme reaction was calculated as follows:
| Inhibition rate (%) = (B − S)/B × 100 | (1) |
where B and S are the absorbance values for the blank and sample, respectively.
3.8. Determination of Total Phenolic Content
The total phenolic content was determined using Folin-Ciocalteu assay [18]. A 1 mL of sample solution (5 mg/mL) was mixed with 1 mL of 2% (w/v) Na2CO3 solution and 1 mL of 10% Folin-Ciocalteu phenol reagent. After 10 min, the absorbance was measured at 750 nm using a microplate reader (Perkin Elmer, Waltham, MA, USA). The phenolic content was calculated from calibration curve of gallic acid. The results were expressed as mg of gallic acid equivalents per g of sample.
3.9. Determination of Total Flavonoids
The total flavonoid content was determined based ona previously reported colorimetric method [18]. Briefly, a 0.5 mL aliquot of the sample solution was mixed with distilled water (2 mL) and subsequently with 5% NaNO2 solution (0.15 mL). After incubation for 5 min, a 0.15 mL aliquot of 10% AlCl3 solution was added to the mixture and after 5 min, 4% NaOH solution (2 mL) was added to the mixture. Water was added to the sample to bring the final volume to 5 mL, and the mixture was thoroughly mixed and allowed to stand for 15 min. The absorbance of the resultant mixture was measured at 415 nm. Then, the total flavonoid content was calculated as quercetin equivalents (mg quercetin/g extract) by reference to a standard curve (r2 = 0.999).
4. Conclusions
In our preliminary study, we identified chlorogenic acid in the fruits of C. tricuspidata as antioxidant compound using bioassay-guided purification. The validated HPLC method was developed and applied to confirm the presence of chlorogenic acid in C. tricuspidata fruit extracts. Various ethanolic extracts of C. tricuspidata fruit were prepared, and 80% ethanolic extract was found to exhibit the highest tyrosinase inhibitory activity. Besides, 100% ethanolic extract possessed the highest content of total phenolics and flavonoids. Based on the results, it is evident that the 80% ethanolic extract contains unknown tyrosinase inhibitors. Further studies are necessitated to identify the unknown tyrosinase inhibitors in 80% ethanolic extracts. The results suggest that the observed antioxidant and tyrosinase inhibitory activity of C. tricuspidata fruit extract could partially be attributed to the presence of marker compounds in the extract.We report ananalytical method for standardization and extraction optimization of C. tricuspidata fruit for the first time. Further investigations are needed to confirm the biological effect of C. tricuspidata fruit extract for the potential development of the extract as a cosmetic source.
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA)(316007-5), and this research was supported by the funds of the MNU Innovative Programs for National University in 2019.
Author Contributions
Conceptualization, H.-A.O., D.-H.P., J.-H.S., and S.-S.C.; methodology, H.-N.O., D.-H.P., S.-H.L., G.Y., H.-S.M., D.-S.O., S.-H.R., E.-O.I., I.-S.Y., J.-H.S., and S.-S.C.; software, H.-N.O., D.-H.P., S.-H.L., G.Y., H.-S.M., D.-S.O., S.-H.R., E.-O.I., I.-S.Y., J.-H.S., and S.-S.C.; validation, H.-N.O., D.-H.P., J.-Y.P., S.-Y.S., J.-H.S., and S.-S.C.; formal analysis, H.-N.O., D.-H.P., J.-Y.P., S.-Y.S., J.-H.S., and S.-S.C.; investigation, H.-N.O., D.-H.P., J.-Y.P., S.-Y.S., S.-H.L., G.Y., H.-S.M., D.-S.O., S.-H.R., E.-O.I., I.-S.Y., J.-H.S., and S.-S.C.; resources, J.-H.S. and S.-S.C.; data curation, H.-N.O., D.-H.P., J.-Y.P., and S.-Y.S.,; writing—original draft preparation, H.-N.O. and D.-H.P.; writing—review and editing, D.-H.P. S.-H.L., G.Y., H.-S.M., D.-S.O., S.-H.R., E.-O.I., I.-S.Y., J.-H.S., and S.-S.C.; supervision, J.-H.S. and S.-S.C.
Funding
Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries: MAFRA 316007-5, Mokpo National University: MNU Innovative Programs for National University in 2019.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Chang S.H., Jung E.J., Lim D.G., Oyungerel B., Lim K.I., Her E., Choi W.S., Jun M.H., Choi K.D., Han D.J. Anti-inflammatory action of Cudrania tricuspidata on spleen cell and T lymphocyte proliferation. J. Pharm. Pharmacol. 2008;60:1221–1226. doi: 10.1211/jpp.60.9.0015. [DOI] [PubMed] [Google Scholar]
- 2.Hiep N.T., Kwon J., Kim D.-W., Hwang B.Y., Lee H.-J., Mar W., Lee D. Isoflavones with neuroprotective activities from fruits of Cudrania tricuspidata. Phytochemistry. 2015;111:141–148. doi: 10.1016/j.phytochem.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Lee H., Ha H., Lee J.K., Seo C.s., Lee N.h., Jung D.Y., Park S.J., Shin H.K. The fruits of Cudrania tricuspidata suppress development of atopic dermatitis in NC/Nga mice. Phytother. Res. 2012;26:594–599. doi: 10.1002/ptr.3577. [DOI] [PubMed] [Google Scholar]
- 4.Han X.H., Hong S.S., Jin Q., Li D., Kim H.-K., Lee J., Kwon S.H., Lee D., Lee C.-K., Lee M.K. Prenylated and benzylated flavonoids from the fruits of Cudrania tricuspidata. J. Nat. Prod. 2008;72:164–167. doi: 10.1021/np800418j. [DOI] [PubMed] [Google Scholar]
- 5.Jo Y.H., Kim S.B., Liu Q., Do S.-G., Hwang B.Y., Lee M.K. Comparison of pancreatic lipase inhibitory isoflavonoids from unripe and ripe fruits of Cudrania tricuspidata. PLoS ONE. 2017;12:e0172069. doi: 10.1371/journal.pone.0172069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han X.H., Hong S.S., Hwang J.S., Jeong S.H., Hwang J.H., Lee M.H., Lee M.K., Lee D., Ro J.S., Hwang B.Y. Monoamine oxidase inhibitory constituents from the fruits of Cudrania tricuspidata. Arch. Pharm. Res. 2005;28:1324–1327. doi: 10.1007/BF02977895. [DOI] [PubMed] [Google Scholar]
- 7.Jo Y.H., Choi K.-M., Liu Q., Kim S.B., Ji H.-J., Kim M., Shin S.-K., Do S.-G., Shin E., Jung G. Anti-obesity effect of 6, 8-diprenylgenistein, an isoflavonoid of Cudrania tricuspidata fruits in high-fat diet-induced obese mice. Nutrients. 2015;7:10480–10490. doi: 10.3390/nu7125544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin L.-T., Yue S.-J., Fan Y.-C., Wu J.-S., Yan D., Guan H.-S., Wang C.-Y. Cudrania tricuspidata: An updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 2017;7:31807–31832. doi: 10.1039/C7RA04322H. [DOI] [Google Scholar]
- 9.Lee B., Lee J., Lee S.-T., Suk T., Lee W., Jeong T.-S., Hun Park K. Antioxidant and Cytotoxic Activities of Xanthones from Cudrania tricuspidata. Bioorg. Med. Chem. Lett. 2005;15:5548–5552. doi: 10.1016/j.bmcl.2005.08.099. [DOI] [PubMed] [Google Scholar]
- 10.Jeong C.-H., Nam Choi G., Hye Kim J., Hyun Kwak J., Rok Jeong H., Kim D.-O., Heo h.j. Protective Effects of Aqueous Extract from Cudrania tricuspidata on Oxidative Stress-induced Neurotoxicity. Food Sci. Biotechnol. 2010;19:1113–1117. doi: 10.1007/s10068-010-0158-z. [DOI] [Google Scholar]
- 11.Lee T., Kwon J., Lee D., Mar W. Effects of Cudrania tricuspidata Fruit Extract and Its Active Compound, 5, 7, 3′, 4′-Tetrahydroxy-6, 8-diprenylisoflavone, on the High-Affinity IgE Receptor-Mediated Activation of Syk in Mast Cells. J. Agric. Food Chem. 2015;63:5459–5467. doi: 10.1021/acs.jafc.5b00903. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X., Cao C., Sun W., Chen Z., Li X., Nahar L., Sarker S.D., Georgiev M.I., Bai W. Scandenolone from Cudrania tricuspidata fruit extract suppresses the viability of breast cancer cells (MCF-7) in vitro and in vivo. Food. Chem. Toxicol. 2019;126:56–66. doi: 10.1016/j.fct.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Li H.-R., Habasi M., Xie L.-Z., Akber Aisa H. Effect of Chlorogenic Acid on Melanogenesis of B16 Melanoma Cells. Molecules. 2014;19:12940–12948. doi: 10.3390/molecules190912940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stockert J.C., Horobin R.W., Colombo L.L., Blazquez-Castro A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018;120:159–167. doi: 10.1016/j.acthis.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Di Petrillo A., Gonzalez-Paramas A.M., Era B., Medda R., Pintus F., Santos-Buelga C., Fais A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement. Altern. Med. 2016;16:453. doi: 10.1186/s12906-016-1442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karim A.A., Azlan A., Ismail A., Hashim P., Abd Gani S.S., Zainudin B.H., Abdullah N.A. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement. Altern. Med. 2014;14:381. doi: 10.1186/1472-6882-14-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S.B., Jo Y.H., Liu Q., Ahn J.H., Hong I.P., Han S.M., Hwang B.Y., Lee M.K. Optimization of Extraction Condition of Bee Pollen Using Response Surface Methodology: Correlation between Anti-Melanogenesis, Antioxidant Activity, and Phenolic Content. Molecules. 2015;20:19764–19774. doi: 10.3390/molecules201119656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi H.J., Park D.H., Song S.H., Yoon I.S., Cho S.S. Development and Validation of a HPLC-UV Method for Extraction Optimization and Biological Evaluation of Hot-Water and Ethanolic Extracts of Dendropanax morbifera Leaves. Molecules. 2018;23:650. doi: 10.3390/molecules23030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh H.N., Seo J.H., Lee M.H., Kim C., Kim E., Yoon G., Cho S.S., Cho Y.S., Choi H.W., Shim J.H., et al. Licochalcone C induced apoptosis in human oral squamous cell carcinoma cells by regulation of the JAK2/STAT3 signaling pathway. J. Cell. Biochem. 2018;119:10118–10130. doi: 10.1002/jcb.27349. [DOI] [PubMed] [Google Scholar]
- 20.Mirmortazavi S.S., Farvandi M., Ghafouri H., Mohammadi A., Shourian M. Evaluation of novel pyrimidine derivatives as a new class of mushroom tyrosinase inhibitor. Drug. Des. Dev. Ther. 2019;13:2169–2178. doi: 10.2147/DDDT.S209324. [DOI] [PMC free article] [PubMed] [Google Scholar]