Abstract
Recently, the development and the application of 3D scaffold able to promote stem cell differentiation represented an essential field of interest in regenerative medicine. In particular, functionalized scaffolds improve bone tissue formation and promote bone defects repair. This research aims to evaluate the role of ascorbic acid (AS) supplementation in an in vitro model, in which a novel 3D-scaffold, bovine pericardium collagen membrane called BioRipar (BioR) was functionalized with human Gingival Mesenchymal Stem Cells (hGMSCs). As extensively reported in the literature, AS is an essential antioxidant molecule involved in the extracellular matrix secretion and in the osteogenic induction. Specifically, hGMSCs were seeded on BioR and treated with 60 and 90 μg/mL of AS in order to assess their growth behavior, the expression of bone specific markers involved in osteogenesis (runtrelated transcription factor 2, RUNX2; collagen1A1, COL1A1; osteopontin, OPN; bone morphogenetic protein2/4, BMP2/4), and de novo deposition of calcium. The expression of COL1A1, RUNX2, BMP2/4 and OPN was evaluated by RT-PCR, Western blotting and immunocytochemistry, and proved to be upregulated. Our results demonstrate that after three weeks of treatment AS at 60 and 90 μg/mL operates as an osteogenic inductor in hGMSCs. These data indicate that the AS supplementation produces an enhancement of osteogenic phenotype commitment in an in vitro environment. For this reason, AS could represent a valid support for basic and translational research in tissue engineering and regenerative medicine.
Keywords: Pericardium membrane, osteogenic differentiation, human gingival derived stem cells, COL1A1, RUNX2, BMP2/4, OPN
Introduction
Tissue engineering is an interesting strategy to promote new bone tissue formation. In bone tissue engineering, the combination of several parameters such as cell type, growth factors or bioactive factors, mechanical stimuli, and 3D-scaffold material, is fundamental in order to obtain positive results.1
The cell source for tissue engineering can be obtained from different tissue niches, one of this is obtained from the oral cavity, and in particular from the gingival tissue. Gingival mesenchymal stem cells (GMSCs) are obtained from stromal tissue with a relatively easy isolation procedure and with ability to produce high level of cells number. 2,3 In tissue engineering field, scaffold materials mimic the role of extracellular matrix (ECM) and provide a mechanical support for cells. An appropriate scaffold should be osteoinductive, osteoconductive and able to enroll MSCs derived from human tissues, and support the growth of the bone tissue.4 Latest study demonstrated that cell proliferation and differentiation can be controlled by bioactive factors inside tissues.5 Recently, to provide a favorable microenvironment for MSCs differentiation, several molecules or ions have been incorporated into 3D scaffolds.
Ascorbic acid (AS) is an essential antioxidant molecule which works as a cofactor for many enzymes. Since humans have lost the ability to synthesize ascorbate due to the increase of mutations in the coding sequence of the L-gulono-1,4-lactone oxidase,6-8 this antioxidant molecule should be included as a supplement in the diet to assure tissue homeostasis. AS is present in the bloodstream at approximately 50-100 μM concentration in plasma of healthy subjects. 9 It can regulate adult stem cell differentiation to some mesenchymal tissues derivatives, such as adipocytes, osteocytes, myocytes, and chondrocytes.10 AS plays a key role as co-factor in post-translational modification of collagen molecules,11 which are components of the ECM of mesenchyme- derived tissues.12 Moreover, this vitamin reduces the detrimental effect induced by methacrylates in clinical dentistry leading to cell growth repair, proinflammatory cytokine reduction, ROS level and NFkB/pERK/ERK signaling pathway down-regulation (data submitted).
In the present study, the role of AS as a bioactive factor for osteogenic differentiation was investigated in an in vitro model in which human GMSCs were grown on a novel 3D-scaffold, i.e. the bovine pericardium collagen membrane called BioRipar (BioR). Cell growth, de novo deposition of calcium as well as the expression of some bone specific markers involved in osteogenesis (namely, the runt-related transcription factor-2, RUNX2; collagen1A1, COL1A1; and osteopontin, OPN) were evaluated.
As extensively reported in the literature, RUNX2 is a transcriptional factor essential for the activation of osteoblast-associated genes and also it has been reported to be an important early indicator of osteoblast differentiation and bone formation.13
OPN codes for one of the most predominant non-collagenous proteins in bone ECM produced by osteoblasts, and it also promotes cell adhesion to the bone surface. 14 This protein regulates cell–matrix interactions and signaling through binding to integrins and CD44 receptors.15 Furthermore, receptors of OPN integrins and CD44 have been described on host stromal cells and in hGMSCs.16 OPN is produced by mature osteoblasts in the process of bone formation and is recognized as a major marker of osteogenic differentiation, it also plays a main role in the regulation of vessel regeneration.17 During bone resorption, OPN plays an important role in the attachment of osteoclasts and in osteogenesis regulating crystal size.18 Upregulation of non-collagenous proteins such as OPN and osteonectin (SPARC), exhibited a vital role in osteogenesis, confirmed by the osteogenesis- promoting effect of the AS on hGMSCs during bone mineralization.19 Collagen 1, known as an early marker of osteoprogenitor cells, fundamental for extracellular matrix synthesis and to promote Bone morphogenetic protein2 (BMP2) release.20 BMP2 is a member of the TGF-β superfamily, detected in cartilage and bone,21 that through activation of the Wnt pathway, promotes bone formation.22-24
Materials and Methods
Cell culture
This research study was approved by the “G. d’Annunzio” University Ethics Committee (n°266 / University of Chieti). Gingival tissue was collected from six healthy patients scheduled to dentistry surgical procedure as previously described,25 in order to obtain hGMSCs. Tissue explants were washed several times with sodium phosphate buffer (PBS, Lonza, Basel, Switzerland), then samples were plated and maintained in Petri dish with Mesenchymal Stem Cells Growth Medium-Chemically Defined (MSCGM-CD) (Lonza). 26 The medium was replaced twice a week with a fresh one. After two weeks of culture, cells spontaneously migrated from tissue biopsies. All experiments were performed using cells at 2nd passage.
Cell characterization
To characterize hGMSCs, used in the present study, Dominici’s criteria have been followed.27 First, to evaluate the mesenchymal features of hGMSCs cytofluorimetric detection and mesengenic differentiation have been performed. Expression of CD13, CD14, CD29, CD34, CD44, CD45, CD73, CD90 and CD105 was evaluated by cytofluorimetric analysis as previously described.28 The analysis was performed by using FACStarPLUS flow cytometry system and the FlowJo™ software (TreeStar, Ashland, OR, USA).
An inverted light microscope Leica DMIL (Leica Microsystem, Milan, Italy) was used to evaluate cell morphology and the capacity to adhere to a plastic substrate. To assess the ability to differentiate into osteogenic and adipogenic commitment hGMSCs were maintained under osteogenic and adipogenic conditions for 21 and 28 days, respectively, as previously reported.29 To evaluate the formation of mineralized precipitates and lipid droplets, after the differentiation period, Alizarin Red S and Adipo Oil Red staining were performed on undifferentiated and differentiated cells. For Alizarin Red S, staining cells were washed with PBS, fixed in 10% (v/v) formaldehyde (Sigma-Aldrich, Milan, Italy) for 30 min, and washed twice with abundant distilled water (dH2O) before addition of 0.5% Alizarin Red S in H2O, pH 4.0, for 1h at room temperature. After cells incubation under gentle shaking, cells were washed with dH2O four times for 5 min. For staining quantification, 800 μL of 10% (v/v) acetic acid were added to each well. Cells were incubated for 30 min with shaking, then scraped from the plate, transferred into a 1.5 mL vial, and vortexed for 30 s. The obtained suspension, overlaid with 500 mL mineral oil (Sigma-Aldrich), was heated to 85°C for 10 min, then transferred to ice for 5 min, carefully avoiding opening of the tubes until fully cooled, and centrifuged at 20,000 g for 15 min. Five hundred μL of the supernatant were placed into a new 1.5 mL vial, and 200 μL of 10% (v/v) ammonium hydroxide were added (pH 4.1-pH 4.5). One hundred fifty μL of the supernatant obtained from differentiated and undifferentiated hPDLSCs were read in triplicate at 405 nm by a spectrophotometer (Synergy HT, BioTek Instruments, Bad Friedrichshall, Germany). For adipogenic specific staining, cells were fixed in 10% formalin for 15 min and washed with dH2O. Subsequently, the cells were stained with Oil Red O working solution (300 mg of Oil Red O/100 mL of isopropanol) for 5 min and counterstained with hematoxylin. The differentiation into adipogenic lineage was evaluated by AdipoRed assay reagent hydrophilic Nile Red fluorescence (Lonza). After differentiation, the plates were rinsed with PBS and 140 mL/well of AdipoRed was added; after 10 min, the fluorescence with an excitation at 485 nm and an emission at 572 nm was measured with a fluorimeter (Synergy HT). To validate the ability to differentiate into osteogenic and adipogenic lineages, the expression of RUNX-2, ALP, FABP4, and PPARγ were evaluated by reverse transcription polymerase chain reaction (RT-PCR) as described by Diomede et al.29 Commercially available TaqMan Gene Expression Assays (RUNX-2 Hs00231692_m1; ALP Hs01029144_m1; FABP4 Hs01086177_m1; PPARγ Hs01115513_m1) and the Taq-Man Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) were used according to standard protocols. Beta- 2 microglobulin (B2M Hs99999907_m1) (Applied Biosystems) was used for template normalization. Real-time PCR was performed in three independent experiments, and duplicate determinations were carried out for each sample.
Figure 4.
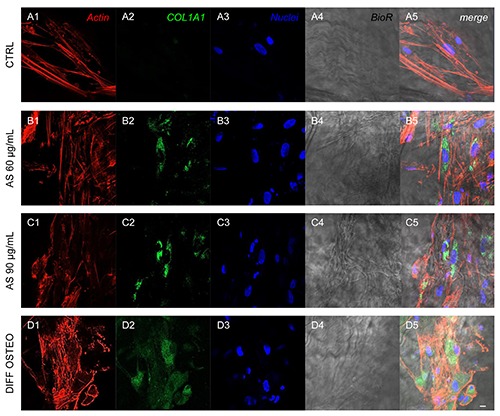
Expression of COL1A1. Immunofluorescence detection of COL1A1 in CTRL (A1-A5), AS 60 μg/mL (B1-B5), AS 90 μg/mL (C1-C5) and DIFF OSTEO (D1-D5). COL1A1 expression were upregulated in AS 90 μg/mL and DIFF OSTEO when compared to the AS 60 μg/mL. No significant differences have been evidenced between AS 90 μg/mL and DIFF OSTEO groups. Red fluorescence, cytoskeleton actin; green fluorescence, specific marker; blue fluorescence, cell nuclei; grey scale, membrane observed at transmission light channel; merge pictures showed the overlapping of abovementioned channels. Scale bar: 10 μm.
Biomaterial
The BioRiparR (BioR; Assut Europe SpA, Magliano dei Marsi, AQ, Italy) is a collagen membrane derived from bovine pericardium. Purified pericardium, composed by type I Collagen and Elastin, represented a new tool for fibroblasts growth and new blood vessel formation. Sterile scissors were used to cut the membrane in small piece size. PBS (Lonza) was used to rehydrate the BioR membrane before use.
Experimental design
Cells at 2nd passage, seeded on BioR, have been divided in four experimental groups:
CTRL: BioR/hGMSCs cultured with basal medium (MSCGM-CD);
AS 60 μg/mL: BioR/hGMSCs treated with ascorbic acid at a concentration of 60 μg/mL;
AS 90 μg/mL: BioR/hGMSCs treated with ascorbic acid at a concentration of 90 μg/mL;
DIFF OSTEO: BioR/hGMSCs cultured with osteogenic differentiation medium as following reported.
Cell proliferation and viability assay
Cell proliferation was evaluated by means of MTT assay as previously described;16 2×103 cells per well were seeded into 96-well plates with BioR in a medium volume of 200 μL to test all experimental groups at different endpoint, 24, 48 and 72 h. Twenty μL of MTT (Promega, Milan, Italy) solution were added to each well. Absorbance at 490 nm was measured with a reference wavelength of 630 nm.30
Cell viability was assessed by trypan blue exclusion test. At this purpose all samples were incubated with trypan blue solution at same endpoint used for MTT test (24, 48 and 72 h) and subsequently analysed with Burker’s chamber at inverted light microscopy as previously described.31
Osteogenic differentiation evaluation
To perform osteogenic differentiation, hGMSCs were seeded in a 6-well plate at approximately 2.5×103 cells/cm2 on BioR membrane. Upon reaching confluence the culture medium was replaced with osteogenic differentiation medium kit (Lonza).32 Osteogenic induction was performed for 21 days.
Evaluation of calcium deposition was obtained by a biochemical analysis, Alizarin Red S (ARS) staining assay, performed after 21 days. Cells were washed with PBS, fixed in 10% (v/v) formaldehyde (Sigma- Aldrich, Milan, Italy) for 30 min and washed twice with abundant dH2O prior to addition 0.5% Alizarin red S in H2O, pH 4.0, for 1 h at room temperature. After cell incubation under gentle shaking, cells were washed with dH2O four times for 5 min. For staining quantification, 800 μL of 10% (v/v) acetic acid was added to each well. Cells incubated for 30 min were scraped from the plate, transferred into a 1.5 mL vial and vortexed for 30 s. The obtained suspension, overlaid with 500 μL of mineral oil (Sigma- Aldrich), was heated to 85 °C for 10 min, then transferred to ice for 5 min, carefully avoiding opening of the tubes until fully cooled, and centrifuged at 20,000× g for 15 min.33 In addition, 500 μL of the supernatant were placed into a new 1.5 mL vial and 200 μL of 10% (v/v) ammonium hydroxide was added (pH 4.1-pH 4.5). Furthermore, 150 μL of the supernatant obtained from cultures were read in triplicate at 405 nm by a spectrophotometer (Synergy HT).
Confocal laser scanning microscope analysis
For immunofluorescence detections BioR/hGMSCs were fixed using 4% paraformaldehyde diluted in 0.1 M PBS (Lonza). After the fixation step, cells were permeabilized with 0.5% Triton X-100 in PBS for 10 min, followed by blocking with 5% skimmed milk in PBS for 30 min.28 Primary antibodies used for immunofluorescence were purchased from Santa Cruz Biotechnology (Santa Cruz Biotechnology, Santa Cruz, CA, USA). COL1A1 (1:200, Santa Cruz Biotechnology), RUNX2 (1:100, Santa Cruz Biotechnology), BMP2/4 (1:200, Santa Cruz Biotechnology) and OPN (1:200, Santa Cruz Biotechnology) were used as primary antibodies. Then cells were incubated by Alexa Fluor 568 red fluorescence conjugated goat anti-rabbit as secondary antibodies (1:200, Molecular Probes, Invitrogen, Eugene, OR, USA). Alexa Fluor 488 phalloidin green fluorescence conjugate (1:400, Molecular Probes) has been used to mark the cytoskeleton actin. After immunofluorescence labelling cells were washed and incubated with TOPRO (1:200, Molecular Probes) for 1 h at 37°C.26 Samples were observed under Zeiss LSM800 confocal system (Zeiss, Jena, Germany). All the experiments were performed in triplicate.
Figure 5.
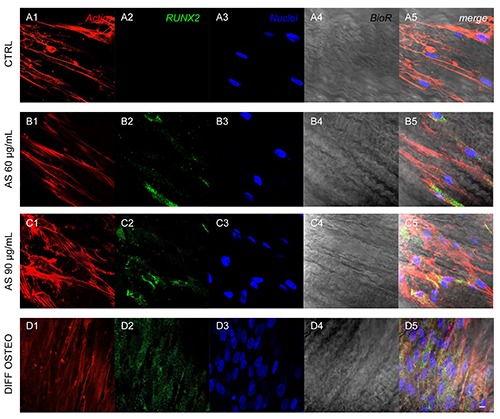
Expression of RUNX2. Immunofluorescence detection of RUNX2 in CTRL (A1-A5), AS 60 μg/mL (B1-B5), AS 90 μg/mL (C1-C5) and DIFF OSTEO (D1-D5). RUNX2 expression were overexpressed in AS 90 μg/mL and DIFF OSTEO samples when compared to the AS 60 μg/mL. No significant differences have been demonstrated when compared AS 90 μg/mL and DIFF OSTEO groups. Red fluorescence, cytoskeleton actin; green fluorescence, specific marker; blue fluorescence, cell nuclei; grey scale, membrane observed at transmission light channel; merge pictures showed the overlapping of abovementioned channels. Scale bar: 10 μm.
Gene expression
Total RNA was isolated from all experimental groups used in the present study through the RNeasy Plus Universal Mini Kit (Qiagen, Valencia, CA, USA). ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) was used for qPCR of studied markers (COL1A1 Hs00164004_m1; RUNX2 Hs00231692_m1; BMP2/4 Hs00154192_m1; OPN Hs00959010_m1; ThermoFischer, Milan, Italy). Beta-2 microglobulin (B2M Hs00187842_m1, Hs99999907_m1; ThermoFischer) was used for template normalization.34 Comparative 2-ΔΔCt relative quantification method has been used to analyze the mRNA expression.
Western blot analysis
Proteins (30 μg) derived from all experimental groups were processed as previously described.35 All antibodies used for western blot procedure were purchased to Santa Cruz Biotechnology. After protein separation, saturated sheets were incubated overnight at 4°C with COL1A1 (1:200, Santa Cruz Biotechnology), RUNX2 (1:1000, Santa Cruz Biotechnology), BMP2/4 (1:750, Santa Cruz Biotechnology), OPN (1:750, Santa Cruz Biotechnology) and β-Actin (1:1000, Santa Cruz Biotechnology).36 Then samples were washed and incubated in secondary antibody diluted 1:1000 in 1x TBS, 5% milk, 0.05% Tween-20. Protein specific bands were visualized by means the electrochemiluminescence method.37
Statistical analysis
Graph Pad Prism 5.0 (GraphPad Software, La Jolla, CA, USA) was used to perform the statistical evaluation. To evaluate the differences in the osteogenic differentiation, gene and protein expression Student’s t-test has been used to analyze the differences between the experimental groups. Obtained results were reported as means ± SEM. A P-value <0.05 was considered statistically significant.
Results
Human gingival mesenchymal stem cells characterization
The expression of different markers as CD13, CD29, CD44, CD73, CD90 and CD105 were analyzed in hGMSCs, whilst, cells were negative for the subsequent molecules CD14, CD34 and CD45 (Figure 1A). Cells were able to adhere on the plastic substrate showing a fibroblast-like morphology. To evaluate the mesenchymal feature cells were induced to adipogenic and osteogenic commitment (Figure 1B). To evaluate the ability to differentiate into osteogenic lineage cells were stained with Alizarin Red S to observe the calcium depositions (Figure 1C). The analysis of transcripts RUNX-2 and ALP confirmed the ability of both cell types to differentiate (Figure 1E). To evaluate the adipogenic differentiation of hGMSCs, cells were stained with Oil Red O and observed at light inverted microscopy. Cells showed several intracellular lipid droplets at cytoplasmic level (Figure 1E). These data were validated by the upregulation of the FABP4 and PPARγ (Figure 1F).
Figure 1.

Cell characterization. A) Cytofluorimetric analysis; −, negative expression (0%); +, moderate expression; ++, positive expression; +++, high expression (100%); MFI ratio is the average of three different biological samples ± standard deviation. B) Plastic adherent hGMSCs observed at inverted light microscopy. C) Alizarin Red S positive staining in hGMSCs culture. D) Oil Red O staining in hGMSCs culture, cells showed a lipid droplet at cytoplasmic level. E) RT-PCR of osteogenic related markers, RUNX2 and ALP. F) RT-PCR of adipogenic related markers, FABP4 and PPAR (undifferentiated vs differentiated). **P<0.01 was considered statistically significant. Scale bars: 20 μm.
Ascorbic acid effects on cell proliferation and viability
MTT and Trypan blue assay were performed to evaluate cell proliferation and viability on all samples. Cells treated with AS 60 μg/mL and AS 90 μg/mL showed no significance differences when compared to the CTRL. DIFF OSTEO showed a decreased in cell proliferation and viability when compared to all others experimental groups (P<0.01) at 48 and 72 h of culture (Figure 2).
Figure 2.
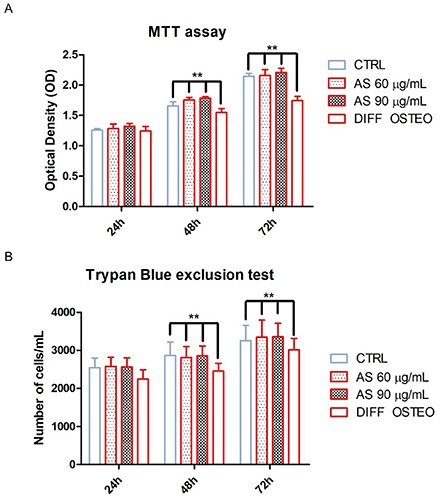
Cell proliferation and viability. A) MTT assay. (B) Trypan blue exclusion test. Treatment with AS 60 μg/mL and AS 90 μg/mL showed no statistical differences in terms of cell proliferation and viability after 24, 48 and 72 h of culture when compared to the CTRL group. DIFF OSTEO group showed a decrease in cell proliferation and viability when compared to all experimental groups (**P<0.01) at 48 and 72 h of culture. The results are expressed as mean ± SD.
Ascorbic acid effects on the osteogenic differentiation
Alizarin Red S staining was performed to evaluate the osteogenic differentiation process on all considered samples after 21 days of culture. Calcium depositions were evident in AS 90 μg/mL and in DIFF OSTEO when compared to the CTRL and to the AS 60 μg/mL. Calcium deposits were stained in red with high levels in AS 90 μg/mL and in DIFF OSTEO samples (Figure 3A-D). The in vitro staining of all samples showed a quantitative result reported in the bar graphs (Figure 3E).
Figure 3.
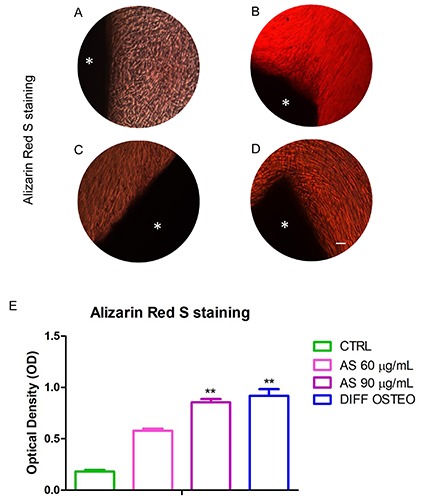
Osteogenic differentiation. Alizarin Red S staining were used to evaluate the calcium depositions (red) in (A) CTRL, (B) AS 60 μg/mL, (C) AS 90 μg/mL and (D) DIFF OSTEO. E) Quantitative analysis of Alizarin Red S staining was performed by measuring the absorbance of Alizarin Red S. *BioR membrane. The results are expressed as mean ± SD (AS 60 μg/mL vs AS 90 μg/mL; 60 μg/mL vs DIFF OSTEO). **P<0.01 was recognized to be significant. Scale bars: 20 μm.
Ascorbic acid treatment modulated the osteogenic markers in hGMSCs
Confocal laser scanning microscopy acquisitions showed the expression of markers related to the osteogenic differentiation, as COL1A1, RUNX2, BMP2/4 and OPN that have been showed an increased expression in DIFF OSTEO and in AS 90 μg/mL, when compared to the CTRL group and with AS 60 μg/mL (Figures 4-7).
Figure 6.

Expression of BMP2/4. Immunofluorescence detection of BMP2/4 in CTRL(A1-A5), AS 60 μg/mL (B1-B5), AS 90 μg/mL (C1-C5) and DIFF OSTEO (D1- D5). BMP2/4 expression showed a higher expression in AS 90 μg/mL and DIFF OSTEO if compared to the AS 60 μg/mL. No significant differences have been demonstrated when compared AS 90 μg/mL and DIFF OSTEO groups. Red fluorescence, cytoskeleton actin; green fluorescence, specific marker; blue fluorescence, cell nuclei; grey scale, membrane observed at transmission light channel; merge pictures showed the overlapping of abovementioned channels. Scale bar: 10 μm.
Ascorbic acid treatment modulates markers related to the osteogenic commitment
Osteogenic differentiation process was regulated in the early stages from COL1A1, RUNX2, BMP2/4 and OPN. RT-PCR analyses have been performed in order to evaluate their expression. The mRNA levels of COL1A1, RUNX2, BMP2/4 and OPN were at similar range in AS 90 μg/mL and DIFF OSTEO (Figure 8A), while the osteogenic markers showed no significance differences in AS 60 μg/mL compared to the CTRL. At the same time, the comparison between AS 90 μg/mL and DIFF OSTEO groups showed an upregulation in mRNA levels of the above-mentioned markers when compared to the AS 60 μg/mL and CTRL groups. The protein expression of COL1A1, RUNX2, BMP2/4 and OPN confirmed the results obtained by RT-PCR (Figure 8 B,C).
Figure 8.
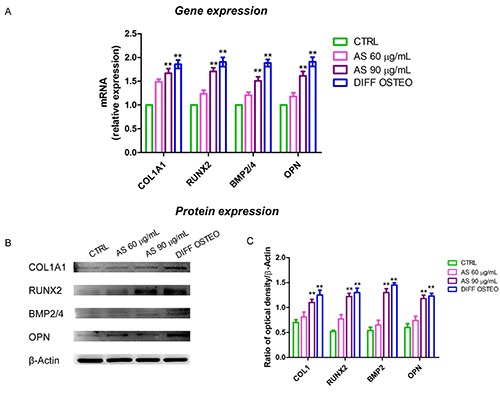
Gene and protein expression. A) Bar graph of RT-PCR showed the expression of mRNA levels in CTRL, AS 60 μg/mL, AS 90 μg/mL and DIFF OSTEO of specific osteogenic related markers. B) COL1A1, RUNX2, BMP2/4 and OPN specific bands of CTRL, AS 60 μg/mL, AS 90 μg/mL and DIFF OSTEO samples. C) Graph bar showed the densitometric analysis of specific band of the markers related to the osteogenic process. The results are expressed as mean ± SD; AS 60 μg/mL vs AS 90 μg/mL; 60 μg/mL vs DIFF OSTEO; **P<0.01.
Discussion
Nowadays, a variety of strategies have been developed to reduce bone loss and increase patients’ life quality. Tissue engineering is an innovative area for tissue regeneration and in particular oral derived MSCs represent a new source of adult stem cells that can be taken from tissue with minimal invasive procedures.38 In the current study, BioR, a biocompatible, bioabsorbable, and osteoconductive collagen membrane from pericardium bovine enriched with hGMSCs, have been used as an in vitro model. Human GMSCs express features such as positivity for CD13, CD29, CD44, CD73, CD90 and CD105, negativity for CD14, CD34 and CD45 markers, as demonstrated by cytofluorimetric detection; moreover, they are able to differentiate into mesenchyme cell lineages with high ability to adhere to a plastic substrate, as evidenced by RT-PCR and morphological observations.39
Collagen is the major structural component of bone matrix and alterations of collagen properties can therefore affect the mechanical properties of bone and increase fracture susceptibility.40 Collagen-base scaffolds have been extensively investigated and used in bone tissue regeneration as a promising approach to achieve the same hierarchical structure of bone.41 Colla - genous membranes were reported to induce osteogenesis in situ (42). Several studies showed that collagen may be efficiently used as a scaffold for 3D cultures of MSCs and for subjecting MSCs to mechanical strains, without inducing cell death.43 In our recent work, it was demonstrated that a 3D coculture platform using a bovine pericardium collagen membrane (BioR) loaded with human periodontal ligament stem cells (hPDLSCs) and endothelial differentiated cells from hPDLSCs (E-hPDLSCs) were able to undergo the osteoangiogenesis differentiation process.39 Moreover, the use of BioR as substrate enriched with hPDLSCs and E-hPDLSCs in coculture, has been considered a resourceful model able to activate the osteoangiogenesis phenotype through ERK1/2 signaling pathway, representing a new potential engineered platform for bone defects repair.39 Actually, functionalized scaffold with conditioned medium, extracellular vesicles, or natural molecules are taking a new role in the regenerative medicine processes.44
Figure 7.
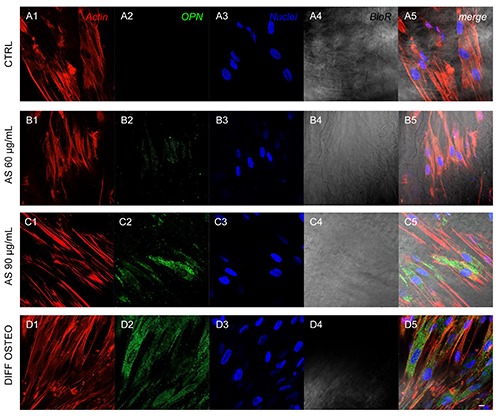
Expression of OPN. Immunofluorescence detection of OPN in CTRL(A1-A5), AS 60 μg/mL (B1-B5), AS 90 μg/mL (C1-C5) and DIFF OSTEO (D1-D5). OPN expression showed a higher expression in AS 90 μg/mL and DIFF OSTEO if compared to the AS 60 μg/mL. No significant differences have been demonstrated when compared AS 90 μg/mL and DIFF OSTEO groups. Red fluorescence, cytoskeleton actin; green fluorescence, specific marker; blue fluorescence, cell nuclei; grey scale, membrane observed at transmission light channel; merge pictures showed the overlapping of abovementioned channels. Scale bar: 10 μm.
AS, a natural molecule and an essential dietary nutrient, required for various biological functions, can be considered a master regulator of the collagen biosynthesis.20 Deficiencies in AS can lead to conditions such as scurvy, which, among other ailments, bone pain and impaired wound healing.45 The role of AS is primarily related to the development and maintenance of bone tissues. Investigations of different epidemiological studies and genetic mouse models regarding the effect of vitamin C exhibit a positive outcome on bone health. Overall, vitamin C exerts a positive outcome on trabecular bone formation by influencing expression of bone matrix genes in osteoblasts.45
In the present study, the biological response of BioR/hGMSCs cultured for three weeks in the presence of 60 and 90 μg/mL of AS, was evaluated in terms of osteogenic differentiation. Alizarin Red S staining indicated a massive mineralization in the living construct BioR/hGMSCs supplemented with 90 μg/mL AS after three weeks of treatment; furthermore, a similar result has been evidenced in BioR/hGMSCs under osteogenic medium (DIFF OSTEO). The mRNA levels of several classic osteogenic genes such as COL1A1, RUNX2, BMP2/4 and OPN, were analyzed.
Mature bone, in vivo, is composed of proteins and minerals, collagen is the most abundant bone protein. AS is able to stimulate cell proliferation, enhance the collagen synthesis, activate alkaline phosphatase, and induce the bone cell differentiation.46,47 AS is also an inhibitor of collagenase and the addition of AS into culture medium will enhance the collagen synthesis to promote the bone formation.48,49 AS has been used in the supplementation of other scaffold types as polyurethane scaffold; the supplementation enhanced bone formation, in vitro and in vivo, and it can be used for bone tissue engineering.50
In literature, it has been reported that the use of AS increased the mRNA level of collagen type I, osteocalcin, bone sialoprotein, and alkaline phosphatase in association with the development of bone nodules in an in vitro system. The expression analysis of COL1A1, BMP2/4 and OPN shows an identical trend of RUNX2 after three weeks in osteogenic living construct BioR/hGMSCs supplemented with 60 and 90 μg/mL of AS. These results supported the critical role played by AS in the in vitro model BioR/hGMSCs to induce osteogenic process. This antioxidant molecule is important for collagen hydroxylation, folding, and secretion; it also increases collagen gene expression and enhances the collagen synthesis stabilizing its tertiary structures. Results obtained through multiparametric analysis evidenced an upregulation of the osteogenic markers involved in the osteogenic process underlying the pivotal and strategic role of AS in living construct BioR/hGMSCs in osteogenesis induction.
In conclusion, given its potential benefits, low cost, and safety profile, AS supplementation could play a key role in the regenerative medicine. In bone tissue regeneration field, biomaterials supplemented with AS could represent an interesting approach in orthopedic and dental surgery procedures.51,52 The proposed in vitro model BioR/hGMSCS supplemented with AS showed an interesting role in the osteogenic process inducing the expression of markers related to the bone formation leading to speed up and ameliorate the regeneration process.
References
- 1.Kock L, van Donkelaar CC, Ito K. Tissue engineering of functional articular cartilage: the current status. Cell Tissue Res 2012;347:613-27. doi: 10.1007/s00441-011-1243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diomede F, Zini N, Pizzicannella J, Merciaro I, Pizzicannella G, D'Orazio M, et al. 5-Aza exposure improves reprogramming process through embryoid body formation in human gingival stem cells. Front Genet 2018;9:419. doi: 10.3389/fgene.2018.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libro R, Scionti D, Diomede F, Marchisio M, Grassi G, Pollastro F, et al. Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Front Physiol 2016;7:559. doi: 10.3389/ fphys.2016.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diomede F, Gugliandolo A, Cardelli P, Merciaro I, Ettorre V, Traini T, et al. Three-dimensional printed PLA scaffold and human gingival stem cellderived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther 2018;9:104. doi: 10.1186/s13287-018-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface 2009;6:S311-24. doi: 10.1098/rsif.2008.0448.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan Y, Shi M, Li L, Liu J, Chen B, Chen Y, et al. Mesenchymal stem cellconditioned media ameliorate diabetic endothelial dysfunction by improving mitochondrial bioenergetics via the Sirt1/AMPK/PGC-1alpha pathway. Clin Sci (Lond) 2016;130:2181-98. [DOI] [PubMed] [Google Scholar]
- 7.Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int 2014;2014: 965849. doi: 10.1155/2014/965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ando Y, Matsubara K, Ishikawa J, Fujio M, Shohara R, Hibi H, et al. Stem cellconditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 2014;61:82-90. doi: 10.1016/j.bone.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A 2012;18: 1479-89. doi: 10.1089/ten.TEA.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlian A, Judawisastra H, Alfarafisa NM, Wibowo UA, Rosadi I. Chondrogenic differentiation of adipose- derived mesenchymal stem cells induced by L-ascorbic acid and platelet rich plasma on silk fibroin scaffold. Peer J 2018;6:e5809. doi: 10.7717/peerj.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geesin JC, Darr D, Kaufman R, Murad S, Pinnell SR. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J Invest Dermatol 1988;90:420-4. doi: 10.1111/1523-1747.ep12460849. [DOI] [PubMed] [Google Scholar]
- 12.De Witte TM, Fratila-Apachitei LE, Zadpoor AA, Peppas NA. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen Biomater 2018;5:197-211. doi: 10.1093/rb/rby013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997;89:747-54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 14.Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med 2000;11:279-303. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Xu WR. Neutrophils diminish T-cell immunity to foster gastric cancer progression: the role of GM-CSF/PDL1/ PD-1 signalling pathway. Gut 2017;66:1878-80. doi: 10.1136/gutjnl- 2017-313923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gugliandolo A, Diomede F, Cardelli P, Bramanti A, Scionti D, Bramanti P, et al. Transcriptomic analysis of gingival mesenchymal stem cells cultured on 3D bioprinted scaffold: A promising strategy for neuroregeneration. J Biomed Mater Res A 2018;106:126-37. doi: 10.1002/jbm.a.36213. [DOI] [PubMed] [Google Scholar]
- 17.Filipowska J, Tomaszewski KA, Niedzwiedzki L, Walocha JA, Niedzwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017;20:291-302. doi: 10.1007/s10456-017-9541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itzstein C, Coxon FP, Rogers MJ. The regulation of osteoclast function and bone resorption by small GTPases. Small GTPases 2011;2:117-30. doi: 10.4161/sgtp.2.3.16453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi AY, Heinayati A, Bao DY, Liu HF, Ding XC, Tong X, et al. Small molecule inhibitor of TGF- signaling enables robust osteogenesis of autologous GMSCs to successfully repair minipig severe maxillofacial bone defects. Stem Cell Res Ther 2019;10:172. doi: 10.1186/s13287-019-1281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrazzo PC, Niccoli S, Khaper N, Rathbone CR, Lees SJ. Ascorbic acid diminishes bone morphogenetic protein 2-induced osteogenic differentiation of muscle precursor cells. Muscle Nerve 2019;59:501-8. doi: 10.1002/mus. 26415. [DOI] [PubMed] [Google Scholar]
- 21.Nomura S, Takano-Yamamoto T. Molecular events caused by mechanical stress in bone. Matrix Biol 2000;19:91-6. [DOI] [PubMed] [Google Scholar]
- 22.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene 2004;341:19-39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 23.Johnson ML, Harnish K, Nusse R, Van Hul W. LRP5 and Wnt signaling: A union made for bone. J Bone Miner Res 2004;19:1749-57. doi: 10.1359/JBMR.040816. [DOI] [PubMed] [Google Scholar]
- 24.van der Horst G, van der Wert SM, Farih-Sips H, van Bezooijen RL, Lowik CWGM, Karperien M. Downregulation of Wnt signaling by increased expression of Dickkopf-1 and-2 is a prerequisite for late-stage osteoblast differentiation of KS483 cells. J Bone Miner Res 2005;20:1867-77. doi: 10.1359/JBMR.050614 [DOI] [PubMed] [Google Scholar]
- 25.Diomede F, Gugliandolo A, Scionti D, Merciaro I, Cavalcanti MF, Mazzon E, et al. Biotherapeutic effect of gingival stem cells conditioned medium in bone tissue restoration. Int J Mol Sci 2018;19 pii:E329. doi: 10.3390/ijms19020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzicannella J, Diomede F, Merciaro I, Caputi S, Tartaro A, Guarnieri S, et al. Endothelial committed oral stem cells as modelling in the relationship between periodontal and cardiovascular disease. J Cell Physiol 2018;233:6734-47. doi: 10.1002/jcp.26515. [DOI] [PubMed] [Google Scholar]
- 27.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. doi: 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 28.Pizzicannella J, Gugliandolo A, Orsini T, Fontana A, Ventrella A, Mazzon E, et al. Engineered extracellular vesicles from human periodontal-ligament stem cells increase VEGF/VEGFR2 expression during bone regeneration. Front Physiol 2019;10:512. doi: 10.3389/ fphys.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diomede F, Rajan TS, Gatta V, D'Aurora M, Merciaro I, Marchisio M, et al. Stemness maintenance properties in human oral stem cells after long-term passage. Stem Cells Int 2017;2017: 5651287. doi: 10.1155/2017/5651287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diomede F, Merciaro I, Martinotti S, Cavalcanti MFXB, Caputi S, Mazzon E, et al. miR-2861 is involved in osteogenic commitment of human periodontal ligament stem cells grown onto 3d scaffold. J Biol Regul Homeost Agents 2016;30:1009-18. [PubMed] [Google Scholar]
- 31.Rajan TS, Giacoppo S, Trubiani O, Diomede F, Piattelli A, Bramanti P, et al. Conditioned medium of periodontal ligament mesenchymal stem cells exert anti-inflammatory effects in lipopolysaccharide-activated mouse motoneurons. Exp Cell Res 2016;349: 152-61. doi: 10.1016/j.yexcr. 2016.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi M, Pisciotta A, Bertoni L, Berni M, Gambardella A, Visani A, et al. Osteogenic differentiation of hDPSCs on biogenic bone apatite thin films. Stem Cells Int 2017;2017: 3579283. 10.1155/2017/3579283. Corrigendum in: Stem Cells Int 2017; 2017:6587384. doi: 10.1155/2017/ 6587384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diomede F, D'Aurora M, Gugliandolo A, Merciaro I, Orsini T, Gatta V, et al. Biofunctionalized scaffold in bone tissue repair. Int J Mol Sci 2018;19 pii: E1022. doi: 10.3390/ijms19041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizzicannella J, Cavalcanti M, Trubiani O, Diomede F. MicroRNA 210 mediates VEGF upregulation in human periodontal ligament stem cells cultured on 3Dhydroxyapatite ceramic scaffold. Int J Mol Sci 2018;19 pii: E3916. doi: 10.3390/ijms19123916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diomede F, D'Aurora M, Gugliandolo A, Merciaro I, Ettorre V, Bramanti A, et al. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int J Nanomedicine 2018;13:3805-25. doi: 10.2147/IJN .S162836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trubiani O, Ballerini P, Murmura G, Pizzicannella J, Giuliani P, Buccella S, et al. Toll-like receptor 4 expression, interleukin-6,-8 and Ccl-20 release, and Nf-Kb translocation in human periodontal ligament mesenchymal stem cells stimulated with Lps-P-gingivalis. Eur J Inflamm 2012;10:81-9. doi: 10.1177/1721727X1201000109 [Google Scholar]
- 37.Mammana S, Gugliandolo A, Cavalli E, Diomede F, Iori R, Zappacosta R, et al. Human gingival mesenchymal stem cells (GMSCs) pre-treated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. J Tissue Eng Regen Med. 2019;13:1109-1121. doi: 10.1002/term.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trubiani O, Toniato E, Di Iorio D, Diomede F, Merciaro I, D'Arcangelo C, et al. Morphological analysis and interleukin release in human gingival fibroblasts seeded on different denture base acrylic resins. Int J Immunopath Ph 2012;25:637-43. doi: 10.1177/039463201202500310. [DOI] [PubMed] [Google Scholar]
- 39.Pizzicannella J, Pierdomenico SD, Piattelli A, Varvara G, Fonticoli L, Trubiani O, et al. 3D human periodontal stem cells and endothelial cells promote bone development in bovine pericardium- based tissue biomaterial. Materials (Basel) 2019;12 pii: E2157 doi: 10.3390/ma12132157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int 2006;17:319-36. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 41.Ferreira AM, Gentile P, Chiono V, Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater 2012;8: 3191-200. doi: 10.1016/j. actbio.2012. 06.014. [DOI] [PubMed] [Google Scholar]
- 42.Taguchi Y, Amizuka N, Nakadate M, Ohnishi H, Fujii N, Oda K, et al. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials 2005;26:6158-66. doi: 10.1016/j.biomaterials.2005. 03.023. [DOI] [PubMed] [Google Scholar]
- 43.Sumanasinghe RD, Osborne JA, Loboa EG. Mesenchymal stem cell-seeded collagen matrices for bone repair: Effects of cyclic tensile strain, cell density, and media conditions on matrix contraction in vitro. J Biomed Mater Res A 2009;88:778-86. doi: 10.1002/ jbm.a.31913. [DOI] [PubMed] [Google Scholar]
- 44.Pizzicannella J, Diomede F, Gugliandolo A, Chiricosta L, Bramanti P, Merciaro I, et al. 3D printing PLA/gingival stem cells/ EVs upregulate miR-2861 and -210 during osteoangiogenesis commitment. Int J Mol Sci. 2019;20). pii: E3256. doi: 10.3390/ ijms20133256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aghajanian P, Hall S, Wongworawat MD, Mohan S. The roles and mechanisms of actions of vitamin C in bone: New developments. J Bone Miner Res 2015;30:1945-55. doi: 10.1002/ jbmr.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys 1972;152:318-28. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- 47.Berg RA, Steinmann B, Rennard SI, Crystal RG. Ascorbate deficiency results in decreased collagen production: under-hydroxylation of proline leads to increased intracellular degradation. Arch Biochem Biophys 1983;226:681-6. doi: 10.1016/0003-9861(83)90338-7. [DOI] [PubMed] [Google Scholar]
- 48.Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and betaglycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther 2013;4:117. doi: 10.1186/scrt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park JB. The effects of dexamethasone, ascorbic acid, and beta-glycerophosphate on osteoblastic differentiation by regulating estrogen receptor and osteopontin expression. J Surg Res. 2012;173:99-104. doi: 10.1016/j.jss.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Wang C, Cao X, Zhang Y. A novel bioactive osteogenesis scaffold delivers ascorbic acid, beta-glycerophosphate, and dexamethasone in vivo to promote bone regeneration. Oncotarget 2017;8:31612-25. doi: 10.18632/oncotarget. 15779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iaquinta MR, Mazzoni E, Manfrini M, D'Agostino A, Trevisiol L, Nocini R, et al. Innovative Biomaterials for Bone Regrowth. Int J Mol Sci. 2019;20 pii: E618. doi: 10.3390/ijms20030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi YJ, Lohman J, Bratlie KM, Peroutka-Bigus N, Bellaire B, Wannemuehler M, et al. Vitamin C and B-3 as new biomaterials to alter intestinal stem cells. J Biomed Mater Res A 2019;107:1886-97. doi: 10.1002/, jbm.a.36715. [DOI] [PMC free article] [PubMed] [Google Scholar]


